Abstract
A 50‐year‐old woman with stage IV lung adenocarcinoma received seven cycles of pembrolizumab as third‐line chemotherapy. Following the failure of pembrolizumab, she commenced fourth‐line chemotherapy of docetaxel and ramucirumab. The patient complained of epigastric pain and a computed tomography (CT) scan revealed oedema‐like thickening of the gallbladder wall, dilation of the bile ducts from the common to the intrahepatic bile ducts, and thickening of the common bile duct wall without any visible obstructions. Accumulation of fluorodeoxyglucose (FDG) in the gallbladder wall and bile duct was also detected with positron emission tomography (PET)‐CT. A biopsy of the extrahepatic bile duct showed non‐specific inflammation. Antibiotic treatment was not effective and pathogens were not detected. The patient was diagnosed with secondary sclerosing cholangitis (SSC) by pembrolizumab. She received 80 mg/day of prednisolone (PSL); however, SSC recurred with tapering of PSL. SSC then improved with steroid pulse therapy and subsequently 50 mg/day azathioprine and 80 mg/day PSL.
Keywords: ICI, immune‐related adverse events, non‐small cell lung cancer, pembrolizumab, sclerosing cholangitis
Pembrolizumab, a humanized anti‐programmed cell death‐1 (PD‐1) monoclonal antibody, demonstrates efficacy in treating non‐small cell lung cancer (NSCLC). However, it can cause immune‐related adverse events (irAEs), including inflammatory manifestations. Cholangitis is a rare irAE. Herein, we report on an NSCLC patient who developed pembrolizumab‐induced cholangitis and review the relevant literature.
Introduction
Immune checkpoint inhibitors (ICIs), including pembrolizumab, efficiently treat various malignancies. Pembrolizumab, a humanized anti‐programmed cell death‐1 (PD‐1) monoclonal antibody, demonstrates efficacy in treating non‐small cell lung cancer (NSCLC) 1. However, these inhibitors can cause immune‐related adverse events (irAEs), including inflammatory manifestations. Cholangitis is a rare irAE. Herein, we report on an NSCLC patient who developed pembrolizumab‐induced cholangitis and review the relevant literature.
Case Report
A 50‐year‐old Japanese woman with a past smoking history was diagnosed with lung adenocarcinoma (cT1bN3M1b stage IV) in July 2016. Her lung cancer had a K‐Ras mutation and had metastasized to the pleura, lymph nodes, liver, adrenal glands, and pancreas. She received four cycles of combination chemotherapy with carboplatin (CBDCA), pemetrexed (PEM), and bevacizumab (BEV) in September 2016 followed by 14 cycles of PEM and BEV as maintenance chemotherapy from December 2016 to October 2017, and S‐1 as second‐line chemotherapy from December 2017 to June 2018. Following these treatments, an 18F‐fluorodeoxyglucose (FDG) positron emission tomography (PET)‐computed tomography (CT) scan showed increased accumulation in the left and right neck and supraclavicular and axillary lymph nodes as well as sclerotic changes in the left iliac bone, suggesting bone metastasis. PD‐L1 tumor proportion score (TPS) was 70% positive in a specimen from the left cervical lymph node; therefore, the patient received seven cycles of pembrolizumab as third‐line chemotherapy. Afterward, FDG accumulation was found in the left submandibular region, near the oesophagus, left hilar region, right inguinal region, and mesenteric lymph node, and was judged to be progressive disease (PD). The patient started fourth‐line chemotherapy of docetaxel (DOC) and ramucirumab (RAM) in December 2018. After the second cycle, she complained of epigastric pain and laboratory findings revealed elevated levels of aspartate aminotransferase (AST; 73 IU/L), alanine aminotransferase (ALT; 50 IU/L), alkaline phosphatase (ALP; 1289 IU/L), and γ‐glutamyl transferase (GGT; 370 IU/L). Total bilirubin (0.3 mg/dL), serum immunological markers including antinuclear antibody (ANA), antimitochondrial antibody (AMA), immunoglobulin G4 (IgG4), and myeloperoxidase antineutrophil cytoplasmic antibody were within the normal range. Anti‐SS‐B antibodies were present; however, she had no symptoms of Sjogren's syndrome. A contrast‐enhanced CT scan revealed oedema‐like thickening of the gallbladder wall, dilation of the bile ducts from the common bile duct to intrahepatic bile ducts, and thickening of the common bile duct wall without any visible obstructions, including cholelithiasis or tumours (Fig. 1A). Magnetic resonance cholangiopancreatography showed irregularly narrowed intrahepatic bile ducts and dilation of peripheral bile ducts (Fig. 1B). Accumulation of FDG in the wall of the gallbladder and bile duct was also detected with PET‐CT. A biopsy of the extrahepatic bile duct showed numerous neutrophils and lymphoid infiltrates as non‐specific inflammation (Fig. 1D). Antibiotic treatment with meropenem was not effective and pathogens were not detected from blood and bile cultures. According to these findings and the clinical course of lung adenocarcinoma, the patient was diagnosed with secondary sclerosing cholangitis (SSC) induced by pembrolizumab and began treatment with 80 mg/day prednisolone (PSL). During the PSL tapering, biliary enzymes increased again. Steroid pulse therapy (methyl PSL 1 g for three days) was performed and subsequently, treatment with 50 mg/day azathioprine and 80 mg/day PSL was initiated. The biliary enzymes gradually decreased. Serial examination showed improvement in the wall thickening of the bile duct and gallbladder. FDG accumulation in the wall of the gallbladder and bile duct also disappeared.
Figure 1.
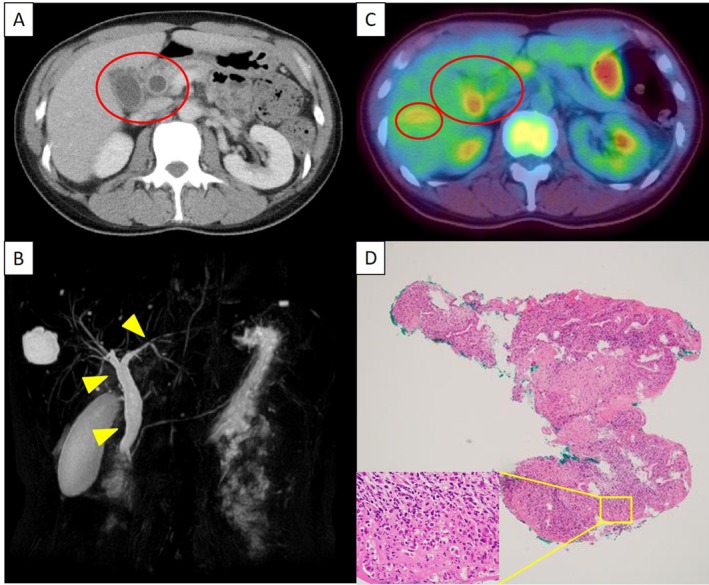
Diagnostic images of a 50‐year‐old Japanese woman diagnosed with lung adenocarcinoma. (A) Enhanced computed tomography (CT) scan revealed oedema‐like thickening of the gallbladder wall, dilation of the bile duct from the common bile duct to the intrahepatic bile ducts, and thickening of the common bile duct wall without any visible obstructions. (B) Magnetic resonance cholangiopancreatography showed irregularly narrowed intrahepatic bile ducts and dilation of peripheral bile ducts. (C) Accumulation of fluorodeoxyglucose (FDG) in the wall of gallbladder and bile duct was also detected with positron emission tomography (PET)‐CT. (D) Biopsy of the extrahepatic bile duct showed numerous neutrophils and lymphoid infiltrates as non‐specific inflammation.
Discussion
ICIs have led to remarkable advances in the treatment of cancers, including NSCLC. However, they can cause irAEs 2. Cholangitis is a rare irAE and four cases of pembrolizumab‐induced SSC in NSCLC patients have been reported 3, 4, 5.
Anti‐PD‐1 antibody‐related cholangitis was mainly reported in NSCLC patients receiving nivolumab (Table 1) 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14. Nivolumab‐related cholangitis was characterized by (1) extrahepatic bile duct dilation without obstruction; (2) diffuse hypertrophy of the extrahepatic bile duct wall; (3) increase in the biliary enzymes ALP and GGT relative to the hepatic enzymes AST and ALT; (4) absence of the serum immunological markers ANA, AMA, anti‐smooth muscle antibody, and IgG4; (5) CD8+ T cell infiltration in the portal area by liver biopsy; and (6) a moderate‐to‐poor response to steroid therapy 6. Some cases responded well to steroid therapy or improved with discontinuation of anti‐PD‐1 antibodies (Table 1). Further study is needed to fully understand cholangitis induced by anti‐PD‐1 antibodies.
Table 1.
Summary of reported cases of PD‐L1 inhibitor‐induced cholangitis in non‐small cell lung cancer.
| Age | Sex | Drugs | Histology | Symptom | Initiation to occurrence | Elevation of ALP/GGT | Elevation of bilirubin | Treatment | Response | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 52 | M | Nivo | Ad | Abdominal pain | 8 cycles | + | − | PSL 0.5 mg/kg | Yes | 3 |
| N/A | M | Durva | Sq | Fever, abdominal pain | 4 cycles | N/A | N/A | PSL 120 mg | Yes | 3 |
| 61 | M | Pem | Ad | None | 17 cycles | N/A | N/A | PSL 1 mg/kg | Yes | 3 |
| 68 | M | Pem | Ad | Abdominal pain, vomiting | 5.5 months | + | − | PSL 50 mg | Yes | 4 |
| 67 | M | Pem | NSCLC | Fever, malaise | 1 month | + | − | PSL 40 mg | Yes | 4 |
| 63 | M | Pem | NSCLC | None | 7 cycles | + | − | PSL 1 mg/kg | Yes | 5 |
| 55 | M | Nivo | NSCLC | Abdominal pain | 11 cycles | + | − | mPSL 2 mg/kg, MMF 2 g | Yes | 5 |
| 81 | F | Nivo | NSCLC | Backache | 25 cycles | + | − | mPSL 2 mg/kg, MMF 2 g | Yes | 5 |
| 82 | F | Nivo | NSCLC | None | 2 cycles | + | − | mPSL 1.6 mg/kg | Yes | 5 |
| 64 | M | Nivo | Ad | Fever, abdominal discomfort | 9 cycles | + | − | PSL 30 mg | No | 6 |
| 73 | F | Nivo | Sq | Fever, vomiting, abdominal discomfort, diarrhoea | 6 cycles | + | + | PSL 30 mg | Yes | 6 |
| 83 | F | Nivo | Sq | Fever, general fatigue | 12 cycles +2 months | + | − | Discontinuation | Yes | 6 |
| 63 | M | Nivo | Ad | Fever, abdominal discomfort | 5 cycles | + | − | PSL 2 mg/kg | Yes | 7 |
| 69 | M | Nivo | Ad | Rash | 3 cycles | − | + | PSL 50 mg, mPSL 500 mg | Yes | 8 |
| 57 | F | Nivo | Sq | Abdominal discomfort |
7 cycles + 7 months |
+ | − | Discontinuation | Yes | 9 |
| 79 | M | Nivo | NSCLC | Itching, jaundice | 62 days | + | + | mPSL 1 mg/kg | Yes | 10 |
| 56 | F | Nivo | Ad | Myalgia, diffuse skin thickening | 16 cycles | + | − | Corticosteroids | N/A | 11 |
| 69 | M | Avel | Ad | Right upper abdominal discomfort | 21 cycles | + | − | mPSL 1 mg/kg | Yes | 12 |
| 71 | M | Nivo | NSCLC | None | 11 months | + | − | mPSL 0.5 mg/kg | Yes | 13 |
| 67 | M | Nivo | Mixed Ad and Sq | Right upper abdominal pain | 8 cycles | + | N/A | mPSL, PSL 50 mg, MMF, Tacrolimus | No | 14 |
| 50 | F | Pem | Ad | Epigastric pain | 7 cycles | + | − | PSL 80 mg, azathioprine 50 mg | Yes | Present case |
Ad, adenocarcinoma; ALP, alkaline phosphatase; Avel, avelumab; Durva, durvalumab; GGT, γ‐glutamyl transferase; MMF, mycophenolate mofetil; mPSL, methyl‐PSL; N/A, not available; Nivo, nivorlumab; NSCLC, non‐small cell lung cancer; PD‐L1, programmed death‐ligand 1; Pem, pembrolizumab; PSL, prednisolone; Ref, reference; Sq, squamous cell lung cancer.
In addition to SSC induced by pembrolizumab, SSC caused from paraneoplastic syndrome should be considered during treatment with ICIs. Milkiewicz et al. reported six patients with neoplastic diseases who acquired autoimmune liver diseases and suggested the possibility of paraneoplastic syndrome 15. Testing for antibodies that are specific to paraneoplastic syndrome, such as anti‐Hu antibodies, could have been useful in this case to elucidate the reason for cholangitis.
Our patient was positive for anti‐SS‐B antibodies before receiving pembrolizumab. Anti‐PD‐1 antibody is efficient and safe for patients with advanced NSCLC 16. Anti‐PD‐1 antibody has also been tested to determine its influence on ANA 16. Although the incidence of irAEs was not significantly different between ANA‐positive and ‐negative cases, the incidence of irAEs tended to increase as the ANA titre increased. Furthermore, the frequency of irAEs including infusion reaction, pneumonitis, and arthralgia after administration of ICIs was high in NSCLC patients positive for ANA and rheumatoid factor (RF), although no patients developed autoimmune disease after ICIs were administered 17. Owing to these reports, our patient's irAE may be related to her positive anti‐SS‐B antibody status.
A PET‐CT showed FDG accumulation in the wall of the gallbladder and bile duct. There are no reports of PET‐CT scans identifying anti‐PD‐L1 antibody‐induced cholangitis; however, PET‐CT is useful for detecting some irAEs and early detection of cholangitis might be possible with PET‐CT 18.
ICIs have clinical benefits; however, they have a risk of very rare adverse events like SSC that may go unreported. Further studies are necessary to establish the risk of SSC in patients receiving ICIs and the optimal management of ICI‐induced SSC.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Matsumoto, S , Watanabe, K , Kobayashi, N , Irie, K , Yamanaka, S , Kaneko, T . (2020) Pembrolizumab‐induced secondary sclerosing cholangitis in a non‐small cell lung cancer patient. Respirology Case Reports, 8(5), e00560 10.1002/rcr2.560
Associate Editor: James Ho
References
- 1. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. 2016. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N. Engl. J. Med. 375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer JR, Lacchetti C, Schneider BJ, et al. 2018. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fouchard M, Jantzem H, Quere G, et al. 2019. Three cases of immune cholangitis related to anti‐programmed cell death and programmed cell death ligand agents for the treatment of non‐small cell lung cancer. Eur. J. Cancer 115:107–110. [DOI] [PubMed] [Google Scholar]
- 4. Zen Y, Chen YY, Jeng YM, et al. 2020. Immune‐related adverse reactions in the hepatobiliary system: second‐generation check‐point inhibitors highlight diverse histological changes. Histopathology 76:470–480. [DOI] [PubMed] [Google Scholar]
- 5. Onoyama T, Takeda Y, Yamashita T, et al. 2020. Programmed cell death‐1 inhibitor‐related sclerosing cholangitis: a systematic review. World J. Gastroenterol. 26:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawakami H, Tanizaki J, Tanaka K, et al. 2017. Imaging and clinicopathological features of nivolumab‐related cholangitis in patients with non‐small cell lung cancer. Invest. New Drugs 35:529–536. [DOI] [PubMed] [Google Scholar]
- 7. Kashima J, Okuma Y, Shimizuguchi R, et al. 2018. Bile duct obstruction in a patient treated with nivolumab as second‐line chemotherapy for advanced non‐small‐cell lung cancer: a case report. Cancer Immunol. Immunother. 67:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuraoka N, Hara K, Terai S, et al. 2018. Peroral cholangioscopy of nivolumab‐related (induced) ulcerative cholangitis in a patient with non‐small cell lung cancer. Endoscopy 50:E259–E261. [DOI] [PubMed] [Google Scholar]
- 9. Noda‐Narita S, Mizuno S, Noguchi S, et al. 2019. Development of mild drug‐induced sclerosing cholangitis after discontinuation of nivolumab. Eur. J. Cancer 107:93–96. [DOI] [PubMed] [Google Scholar]
- 10. Gelsomino F, Vitale G, D'Errico A, et al. 2017. Nivolumab‐induced cholangitic liver disease: a novel form of serious liver injury. Ann. Oncol. 28:671–672. [DOI] [PubMed] [Google Scholar]
- 11. Le Tallec E, Ricordel C, Triquet L, et al. 2019. An original case of an association of eosinophilic fasciitis with cholangitis induced by nivolumab. J. Thorac. Oncol. 14:e13–e15. [DOI] [PubMed] [Google Scholar]
- 12. Cho JH, Sun JM, Lee SH, et al. 2018. Late‐onset cholecystitis with cholangitis after avelumab treatment in non‐small cell lung cancer. J. Thorac. Oncol. 13:e34–e36. [DOI] [PubMed] [Google Scholar]
- 13. Hamoir C, de Vos M, Clinckart F, et al. 2018. Hepatobiliary and pancreatic: nivolumab‐related cholangiopathy. J. Gastroenterol. Hepatol. 33:1695. [DOI] [PubMed] [Google Scholar]
- 14. Anderson B, and Dawe DE. 2019. Nivolumab‐induced secondary sclerosing cholangitis with deterioration despite immunosuppression. J. Thorac. Oncol. 14:e205–e206. [DOI] [PubMed] [Google Scholar]
- 15. Milkiewicz P, Mutimer D, Hubscher SG, et al. 1999. Autoimmune liver disease in patients with neoplastic diseases. Eur. J. Gastroenterol. Hepatol. 11:569–573. [DOI] [PubMed] [Google Scholar]
- 16. Yonezawa Y, Tanaka K, Shiraishi Y, et al. 2019. Safety and efficiency of PD‐1 inhibitors in non‐small cell lung cancer patients positive for antinuclear antibodies. Lung Cancer 130:5–9. [DOI] [PubMed] [Google Scholar]
- 17. Toi Y, Sugawara S, Sugisaka J, et al. 2018. Profiling preexisting antibodies in patients treated with anti‐PD‐1 therapy for advanced non‐small cell lung cancer. JAMA Oncol. 5:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mekki A, Dercle L, Lichtenstein P, et al. 2018. Detection of immune‐related adverse events by medical imaging in patients treated with anti‐programmed cell death 1. Eur. J. Cancer 96:91–104. [DOI] [PubMed] [Google Scholar]


