Abstract
Background
LncRNAs play an important role in tumorigenesis and development in tumors, but the function of lncRNA SOCS2-AS in acute myeloid leukemia (AML) is unknown.
Materials and Methods
In the present study, we used RT-PCR to detect the expression of SOCS2-AS in FLT3-ITD+, FLT3-ITD- AML patients and different AML cell lines. The colony formation and CCK-8 assay were performed to analyze the proliferation ability, and the flow cytometry was performed to analyze the capacity of apoptosis in Molm-13 and MV4-11 cells. The Western blot was applied to detect the expression of STAT5 and p-STAT5. The RNA pull-down and luciferase activity were used to investigate the interaction between SOCS2-AS and miR-221.
Results
The results indicate that SOCS2-AS shows overexpression in FLT3-ITD+ AML patients compared to FLT3-ITD- AML patients. Si-SOCS2-AS can inhibit the proliferation, boost the apoptosis and induce the cycle arrest in Molm-13 cells, and SOCS2-AS overexpression promotes proliferation and colony formation in MV4-11 cells. The miR-221 shows overexpression in FLT3-ITD+ AML patients compared to FLT3-ITD- AML patients. And the expression level of miR-221 and SOCS2-AS shows negative correlation in FLT3-ITD+ AML patients. Functionally, SOCS2-AS could be interacted with miR-221 in AML cells. After SOCS2-AS knockdown, the phosphorylation level of STAT5 was significantly decreased. Moreover, miR-221 inhibitor can rescue the viability in cells after si-SOCS2-AS transfection. And it is stated that SOCS2-AS regulates the STAT5 signal transduction pathway with sponging miR-221.
Conclusion
In conclusion, this study confirms the molecular mechanism of SOCS2-AS in AML by targeting the miR-221/STAT5 signaling pathway. This indicates SOCS2-AS may serve as a potential therapeutic target for the treatment of AML.
Keywords: SOCS2-AS, acute myeloid leukemia, miR-221, FLT3-ITD+
Introduction
Acute myeloid leukemia (AML) is a malignant hematological disease caused by uncontrolled proliferation, blocked apoptosis and dysdifferentiation of bone marrow hematopoietic stem cells.1 The mutation of FMS-like receptor tyrosine kinase 3 (FLT3) occurs in about 1/3 of patients with acute myeloid leukemia, and its internal tandem mutation (ITD) often leads to rapid disease progression and poor prognosis.2,3 To improve the prognosis of FLT3-ITD+patients, many FLT3 inhibitors have been published, but these drugs have limited efficacy. The recurrence rate of patients treated with single drug is close to 100%.4 Therefore, it is urgent to further study the molecular mechanism of FLT3-ITD+ mutant leukemia for the treatment of AML.
Long non-coding RNA (LncRNA) is a type of RNA with molecular weight greater than 200 b, and cannot be translated into protein, but can be used as a molecular sponge to regulate gene expression by binding to miRNAs, thereby affecting gene expression.5,6 More and more studies have found that LncRNA plays an important physiological and pathological role in tumorigenesis and development.7 It is not only widely involved in promoting tumorigenesis and development in solid tumors, but also reported to be of great significance in hematological tumors.8 It is reported that LINC00152 regulated the expression of CDK9 by combining with miR-193a and promoted the occurrence and development of AML.9 Inhibiting LncRNA MALAT1 could promote DNA damage and apoptosis in multiple myeloma cells, thereby inhibiting tumor growth.10
Suppressor of cytokine signaling-2, as one of LncRNAs, was demonstrated that it is related to AML progression.11 Laszlo et al reported that higher SOCS2 expression would have a poor outcome in pediatric acute myeloid leukemia.12 Therefore, exploring the role of SOCS2 in AML will contribute to reveal the molecular mechanism, and provide therapeutic targets for AML treatment. In this study, it is found that the ectopic expression of SOCS2-AS in FLT3-ITD + leukemia and overexpression SOCS2-AS promote cell proliferation and colony formation, repress apoptosis in FLT3-ITD + cell lines. Mechanically, it is demonstrated that SOCS2-AS promotes cell proliferation by regulating STAT5 through miR-221.
Materials and Methods
Clinical Samples
The clinical samples were collected from Xi’an Gaoxin Hospital. The research was approved by Ethics Committee of Xi’an Gaoxin Hospital and written informed consents were obtained from the patients (Clinical trial registration number: CTR20191008). Seventy-one FLT3-ITD+ AML patients, 287 FLT3-ITD- AML patients and 330 healthy donors were enrolled and the bone marrow samples were collected. Obtained samples were quickly frozen at −80°C for storage. All the patients were newly diagnosed. Initial AML patients were alleviated by anthracycline plus cytarabine, while M3 patients were treated with all-trans retinoic acid or arsenic. The clinicopathological features were list in Table 1.
Table 1.
Association Between FLT3-ITD+ and FLT3-ITD- Clinicopathological Features of Newly Diagnosed Patients with AML
| FLT3-ITD+ | FLT3-ITD- | Normal | P | |
|---|---|---|---|---|
| No. | 71 | 287 | 330 | |
| Sex | ||||
| Female | 38 | 140 | 168 | 0.738 |
| Male | 33 | 147 | 162 | |
| Age, Years | ||||
| ≤18 | 12 | 43 | 56 | 0.732 |
| 19–40 | 34 | 132 | 145 | |
| 41–60 | 16 | 66 | 89 | |
| >60 | 9 | 46 | 40 | |
| FAB Type | ||||
| M0 | 2 | 11 | – | 0.805 |
| M1 | 5 | 19 | – | |
| M2 | 21 | 94 | – | |
| M3 | 3 | 17 | – | |
| M4 | 18 | 59 | – | |
| M5 | 21 | 75 | – | |
| M6 | 1 | 8 | – | |
| M7 | 0 | 4 | – |
The Molm-13 (FLT3-ITD+ cell line), MV4-11 (FLT3-ITD+ cell line), THP-1 (FLT-ITD wild type) and U937 (FLT-ITD wild type) cell lines were purchased from ATCC and cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) at 37°C with 5% CO2.
Plasmid Transfection of Molm13, MV-14, U937 and THP-1
Si-SOCS2-AS (sequence: 5’-UAGCAUUUCCAUCAAAUCCUG-3’) was used to silence the expression of SOCS2-AS in MOLM-13 and THP-1 cells. These oligonucleotides were chemically synthesized by Sango Biotech. The pcDNA3.1(+)-SOCS2-AS was transfected into MV4-11 and U937 cells. The transfection was conducted using Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Quantitative Real-Time PCR
Total RNA was isolated using FastKing One-Step RT-PCR Kit (Tiangen) following the manufacturer’s instructions. For mRNA, 1 μg of total RNA was used for the cDNA synthesis using Quant One-Step RT-PCR Kit (Tiangen). For miRNA, the miRNA specific stem-loop primers (Applied Biosystems) was used for cDNA conversion. GAPDH and U6 were set as the internal reference controls for mRNA and miRNA, respectively. The PCR reactions consisted of 2 min at 95°C, followed by 30 cycles at 95°C for 20 sec, 60°C for 15 sec and 68°C for 20 sec. Human primer sequences are as follows: SOCS2-AS forward: 5’- TTAAAAGAGGCACCAGAAGGAAC -3’ and reverse: 5’- AGTCGATCAGATGAACCACACT -3’; miR-221 forward: 5’-AGGGGTGTAACATCCTCGACTG-3’ and reverse: 5’-TATTGCGGTCGTGGAGTCG-3’; GAPDH forward: 5’-AGAAGGCTGGGGCTCATTTG-3’ and reverse: 5’-AGGGGCCATCCACAGTCTTC-3’. U6 forward: 5’-GCTTCGGCAGCACATATACTAAAAT-3’ and reverse: 5’CGCTTCACGAATTTGCGTGTCAT-3’. The data analyzed with the comparative Ct method (2−ΔΔCt).
Colony Formation
Cell suspension was diluted by gradient multiple and inoculated in 6-well plate containing culture medium for 2 weeks. The supernatant was discarded and fixed with 4% paraformaldehyde. The GIMSA staining solution was dyed for 20 min. The clone formation rate was calculated, and the clone formation rate was equal to (clone number/inoculated cell number)*100%.
CCK-8 Assay
Prepared cell suspension was inoculated into 96-well plate and after 0, 1, 2, 3 d cultured with 10 μL CCK-8 for 2 hrs. The absorbance was determined at 450 nm.
Flow Cytometry and Cycle Analysis
The cells were centrifugal collected and washed with PBS. Then, the cells were incubated with FITC Annexin V stain and propidium iodide stain (1μL, Thermo Fisher Scientific) at 4°C, 20 min in the dark. And the cells were collected and washed, finally analyzed with flow cytometry. The cycle was analyzed by BD cycletest plus DNA reagent kit (BD Pharmagen) and the apoptosis was measured using FACS Canto II flow cytometry (BD Biosciences).
Western Blot
The cells lysed with RIPA buffer (Thermo Fisher Scientific) and the lysates were load on SDS-PAGE at 160 v, 1 h. Then, the proteins were transferred to nitrocellulose filter membrane. The proteins were incubated with primary antibodies (GAPDH, 1:1000, Abcam; FLT3, 1:1000, Abcam; p-FLT3; 1:1000, Abcam; STAT5, 1:1000, Abcam; p-STAT5, 1:1000, Abcam) and secondary antibodies. The blots were visualized using ChemiDoc MP (BioRad).
RNA Binding Protein Immunoprecipitation (RIP)
Molm-13 cells were lysed using RIPA buffer, and then the lysate was incubated with magnetic beads conjugated to anti-AGO2 antibody (Millipore, USA) or IgG antibody (Millipore, USA). RIP assay was performed using Magna RIP kit (Millipore, USA). The SOCS2-AS relative expression was detected by RT-PCR. The AGO2 expression was detected by Western blot.
Luciferase Reporter Assay
106 HEK293T cells were cultured in 6-well plates and grown to 90% confluency before cell transfection. SOCS2-AS 3’-UTR wild type or mutant type was cloned into the pGL4 plasmid (Biofeng). miR-221 (or miR-NC) and PGL4-SOCS2-AS-3’-UTR-WT (or PGL4-SOCS2-AS-3’-UTR-MUT) were contransfected to 293T cells using the Lipofectamine 3000 reagent (Invitrogen). After 48 h, the luciferase ability was detected by Dual Luciferase Reporter Assay Kit (Thermo Fisher Scientific). Renilla luciferase activity was set as normalization.
Statistical Analysis
GraphPad Prism was applied to analyze the data. All the data were shown as the mean ±standard deviation (SD). Each experiment was carried out at least three times independently. One-way ANOVA analysis and two-tailed Student’s t-test were used for intragroup significance analysis. χ2 test was used to analyze the calculator's information. P-value <0.05 is considered to be statistically significant.
Results
Expression Levels of SOCS2-AS in Different Types of AML Patients and Cell Line
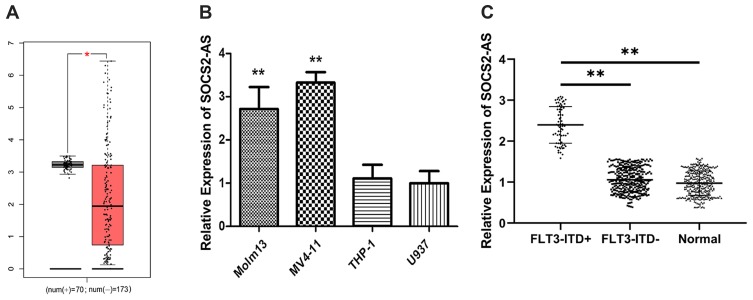
To investigate the expression of SOCS2-AS, the GEPIA database13 was used to analyze and the result showed that the expression levels of SOCS2-AS were higher in FLT3-ITD+ patients compared to FLT3-ITD- patients (Figure 1A). Then, we performed the RT-PCR to measure the expression of SOCS2-AS in different cell lines (Figure 1B). It is showed that the SOCS2-AS expression increased obviously in Molm-13, MV4-11 cells relative to THP-1 and U937 cells. Furthermore, the clinical samples were collected and applied to analyze the levels of SOCS2-AS expression in AML patients (Figure 1C). It is demonstrated that FLT3-ITD + patients had higher SOCS2-AS level compared with those FLT3-ITD- patients and healthy controls. From the above results, our studies provided persuasive evidence that FLT3-ITD+ patients and cell lines (Molm-13, MV4-11) would have higher expression of SOCS2-AS.
Figure 1.
Expression levels of SOCS2-AS in different types of AML patients and cell lines. (A) GEPIA database was used to analyze the expression SOCS2-AS relative between FLT3-ITD+ (70) and FLT3-ITD- (173) AML patients. (B) RT-PCR was used to detect the relative expression of SOCS2-AS in AML cell lines (Molm-13, MV4-11, THP-1 and U937). U937 was used as normal control. (C) SOCS2-AS relative expression was showed by RT-PCR in FLT3-ITD+ (n=71), FLT3-ITD- (n=287) AML patients and healthy controls (n=330). Healthy was used as normal control. The error bars represent the mean ± SD of three independent experiments. *P<0.05, **P<0.01.
Effect of SCOS2-AS Expression on AML Cell Proliferation
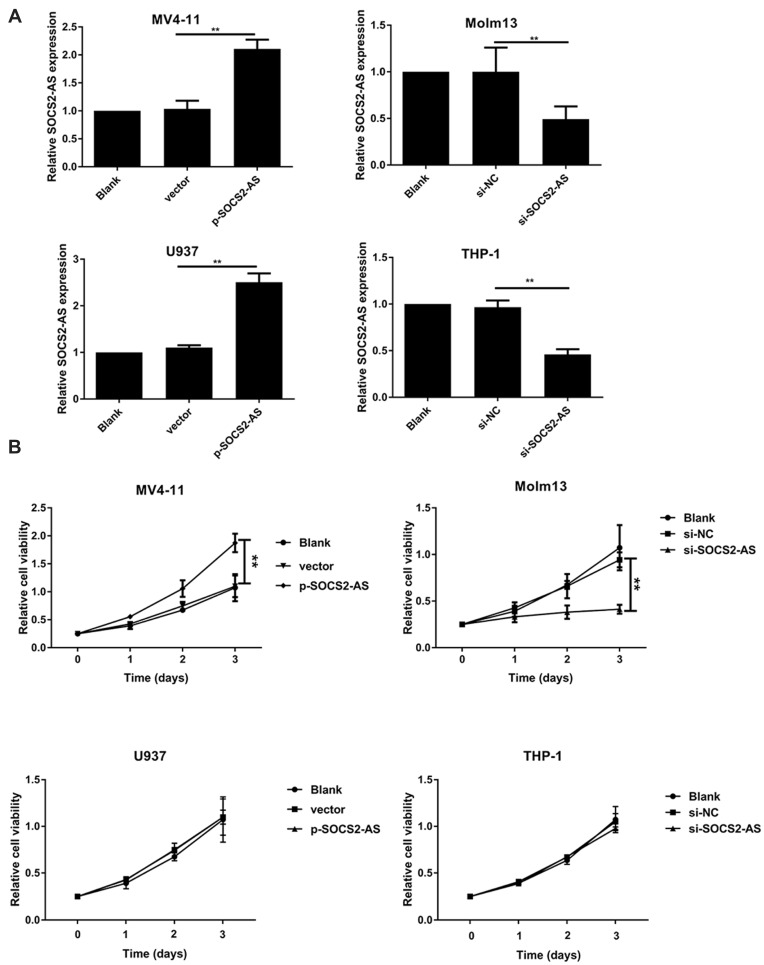
Next, we transfected the overexpression SOCS2-AS plasmid and si-SOCS2-AS into MV4-11 and U937, Molm-13 and THP-1 cells, respectively. RT-PCR was used to validate the high transfection efficiency of SOCS2-AS (Figure 2A). Furthermore, the effect of SCOS2-AS expression on cell viability was carried out in AML cell lines. As shown in Figure 2B, the overexpression of SCOS2-AS would increase the cell viability in MV4-11 cells, and after silencing SOCS2-AS in Molm-13 cells, the cell viability would reduce significantly (P<0.01). But cell activity did not change in THP-1 and U937 cells either the knock-down or overexpression of SCOS2-AS. These results indicated that the abnormal expression of SCOS2-AS in FLT3-ITD+ cell lines caused the change of cell proliferation activity.
Figure 2.
The effect of SOCS2-AS expression on AML cells proliferation. (A) The transfection efficiency of AML cells (MV4-11, Molm-13, U937 and THP-1 cells) with p-SOCS2-AS or si-SOCS2-AS transfection was determined by RT-PCR. (B) The proliferative ability of AML cells (MV4-11, Molm-13, U937 and THP-1 cells) with si-SOCS2-AS or p-SOCS2-AS transfection was determined by CCK-8 at 450 nm. The error bars represent the mean ± SD of three independent experiments. **P<0.01.
The Effect of SOCS2-AS Knockdown on Molm-3 Cell Apoptosis, Cell Cycle
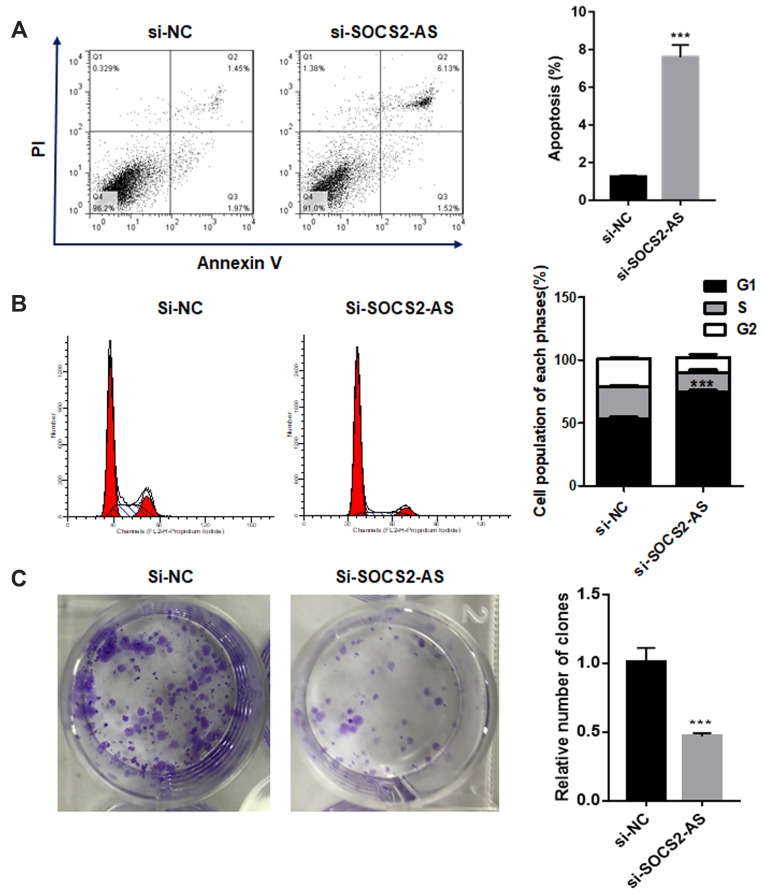
Moreover, the flow cytometry was performed to investigate the apoptosis ability and the results showed that the knockdown of SOCS2-AS would significantly promote the apoptosis of Molm-3 cells (P<0.001) (Figure 3A). And, it is found that si-SOCS2-AS transfection could limit the cell progression from G0 to G1 phase and the cycle arrest occurred in G0/G1 phase (Figure 3B). The colony formation assay was made and it is stated that the proliferation efficiency was reduced after si-SOCS2-AS in Molm-3 cells (Figure 3C). Based on those results, we could reach the following conclusion that si-SOCS2-AS transfection could accelerate the apoptosis and cause the cycle arrest in Molm-13 cells.
Figure 3.
The effect of SOCS2-AS knockdown on Molm-3 cell apoptosis (A), cell cycle (B) and colony formation (C). The error bars represent the mean ± SD of three independent experiments. ***P<0.01.
The Effect of SOCS2-AS Overexpression on MV4-11 Cell Apoptosis, Cell Cycle
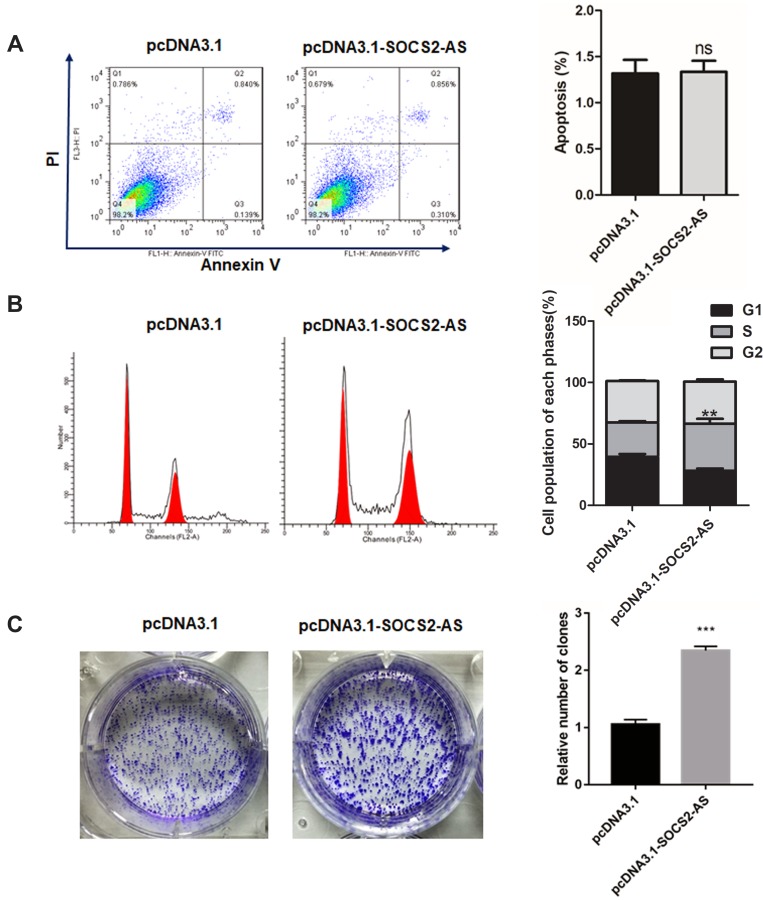
To further the role of SOCS2-AS in FLT3-ITD+ progression, the SOCS2-AS overexpression on MV4-11 cell apoptosis was executed. As shown in Figure 4A, SOCS2-AS overexpression had no effect on MV4-11 cell apoptosis compared with the control group. And, the flow cytometry was used to detect the change of cell cycle, the results showed upregulation of SOCS2-AS would cause more cells in S phase compared to control (Figure 4B) (P<0.01). This result indicated that overexpression SOCS2-AS was beneficial to MV4-11 proliferation. Similarly, the colony formation assay was performed and it is demonstrated that SOCS2-AS overexpression would obviously increase the number of MV4-11 colony (Figure 4C). From these results, it is indicated that SOCS2-AS overexpression accelerates the proliferation and colony formation of MV4-11 cells.
Figure 4.
The effect of SOCS2-AS overexpression on MV4-11 cell apoptosis (A), cell cycle (B) and colony formation (C). The error bars represent the mean ± SD of three independent experiments. **P<0.01, ***P<0.001.
SOCS2-AS Played Functional Role in AML Cells by Sponging miR-221
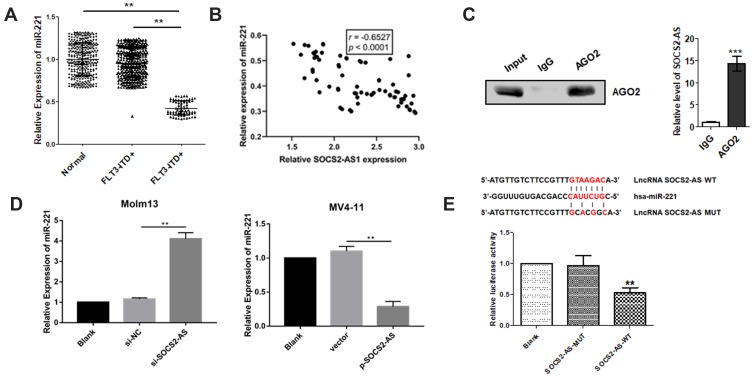
Research had shown that miR-221 regulated the leukemia cell growth through STAT5 signaling pathway. Thus, the miR-221 expression was detected by RT-PCR and it is demonstrated the lower levels exist in FLT3-ITD+ patients compared to FLT3-ITD- patients and healthy controls (Figure 5A). And Pearson correlation analyzation reveals that the miR-221 expression level and SOCS2-AS had negatively correlated (r=−0.6527, P<0.0001) (Figure 5B). To further explore the interaction between SOCS2-AS and miR-221, we performed RNA immunoprecipitation analysis with antibodies against AGO2 using extracts from Molm-13 cells and found that SOCS2-AS was elevated in AGO2-containing miRNAs compared with control IgG immunoprecipitants (Figure 5C). We confirmed that SOCS2- AS could functionally interact with miRNAs in Molm-13 cells. Moreover, it is found that the expression of miR-221 increased significantly when the cells were transfected with si-SOCS2-AS and decreased in overexpression SOCS2-AS MV4-11 cells (Figure 5D). Further, bioinformatics prediction analysis and luciferase reporter assay were used to validate the molecular binding within miR-221 and SOCS2-AS (Figure 5E), showing the function of miRNA sponge. It is speculated that SOCS2-AS played a functional role in AML cells by sponging miR-221.
Figure 5.
The interaction between SOCS2-AS and miR-221 in FLT3-ITD+ patients and cell lines (Molm-13 and MV4-11). (A) The relative expression of miR-221 was determined by RT-PCR in FLT3-ITD+ (n=71) and FLT3-ITD- (n=287) patients compared to healthy controls (n=330) (normal). (B) Pearson correlation analysis was applied to examine the correlation between miR-221 and SOCS2-AS in FLT3-ITD+ (n=71) patients. (C) Immunoprecipitation using anti-AGO2 antibody or IgG followed by Western blot analysis using a mouse monoclonal anti-AGO2. Co-IP with rabbit anti-AGO2 antibody or preimmunized IgG from extracts of Molm-13 cells. The level of SOCS2-AS in immunoprecipitants was analyzed by RT-PCR. (D) The relative expression of miR-221 in Molm-13 and MV4-11 cells were determined by RT-PCR. (E) Luciferase reporter assay determined the interaction SOCS2-AS and miR-221. The error bars represent the mean ± SD of three independent experiments. **P<0.01, ***P<0.001.
SOCS2-AS Regulated the Expression of STAT5 Through miR-221 in Molm-13 Cells
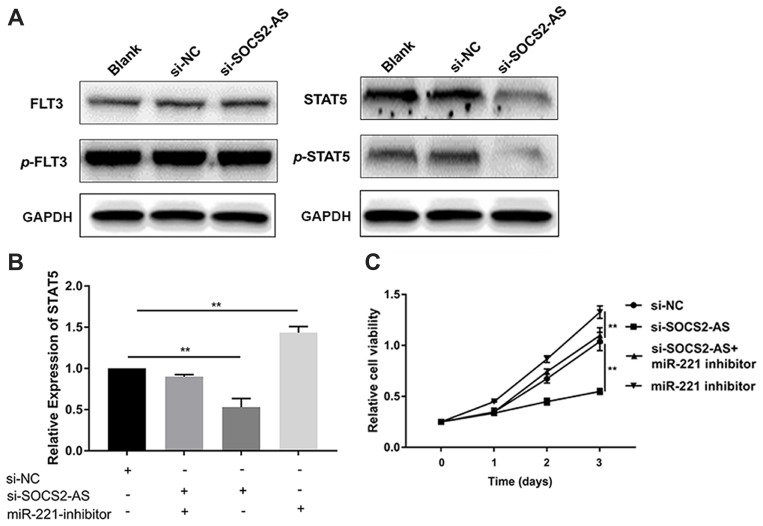
Besides, the expression of STAT5/p-STAT5, not FLT3/p-FLT3, was reduced significantly in si-SOCS2-AS cells relative to controls (Figure 6A). To further uncover the correlation between SOCS2-AS and miR-221, the RT-PCR was carried out and the results showed that compared to si-NC cells, the relative expression level of STAT5 was reduced significantly in si-SOCS2-AS cells, but barely changed in si-SOCS2-AS and miR-221 inhibitor cotransfection cells (Figure 6B). The results indicated that miR-221 inhibitor could rescue the expression of STAT5 when the knockdown of SOCS2-AS and miR-221 inhibitor would increase the expression of STAT5 (Figure 6B). To further prove the idea, the CCK-8 assay was applied and it is showed that the cell viability would increase in si-SOCS2-AS and miR-221 inhibitor cotransfection cells compared to si-SOCS2-AS cells, and miR-221 inhibitor alone treatment markedly increased the cell viability (Figure 6C). These results revealed that SOCS2-AS regulated the expression of STAT5 through miR-221 in FLT3-ITD+ cells.
Figure 6.
SOCS2-AS regulated the expression of STAT5 through miR221 in Molm-13 cells. (A) The phosphorylation level of FLT3 and STAT5 in Molm-13 cells with si-SOCS2-AS transfection was determined by Western blot. (B) The relative expression of STAT5 was determined by RT-PCR in si-NC, si-SOCS2-AS, and miR-221 inhibitor co-transfection Molm-13 cells. (C) The proliferative ability was determined by CCK-8 at 450 nm in si-NC, si-SOCS2-AS, si-SOCS2-AS+ and miR-221 inhibitor co-transfection Molm-13 cells. The error bars represent the mean ± SD of three independent experiments. **P<0.01.
Discussion
FLT3, a proto-oncogene, is reported that it is involved with hematopoiesis progression and survival.14 Particularly, the mutations of FLT3 affect the clinical prognosis, treatment and survival of patients. Internal tandem duplication (ITD), one of FLT3 mutation, is found that it accelerates ligand-independent auto-phosphorylation and receptor constitutive activation.15 FLT3-ITD type of AML patients have poor prognosis, vulnerability to relapse and shorter survival and FLT3-ITD affecting 28% of AML patients.16 Thus, to improve the life quality of FLT3-ITD+ patients, the molecular mechanism is urgent to reveal.
In recent years, reports have found that lncRNAs play an important role in cancer initiation, development and progression.17 For example, the low expression of lncRNA RP11-69I8.3 in acute lymphoblastic leukemia and the overexpression of SNHG5 in AML patients are found.18 And, lncRNA ANRIL is verified to be upregulated in AML and downregulated after AML remission.19 The expression of suppressor of cytokine signaling 2 (SOCS2), one of lncRNAs, would promote leukemogenesis and AML aggressiveness.11 Moreover, it’s reported that SOCS2 can inhibit the carcinogenesis of multiple cancers, such as hepatoma carcinoma, lung cancer, breast carcinoma, and prostate cancer.20 In this study, we explore the role of SOCS2-AS in FLT3-ITD+ patients. And first, it’s found that SOCS2-AS expression had a higher level in FLT3-ITD+ patients and cell lines (Molm-13, MV4-11), but not FLT3-ITD- patients and cell lines. And si-SOCS2-AS-transfected cells would repress the cell proliferation and promote the cell apoptosis, overexpression of SOCS2-AS would accelerate cell proliferation and colony formation. Thus, we deduce that SOCS2-AS was related to FLT3-ITD+ patients’ progression. Previous study has shown that SOCS2-AS expression is higher in castration-resistant prostate cancer cells and the SOCS2-AS represses prostate cancer apoptosis by regulating the epigenetic control for androgen receptor target genes. And, we prove that SOCS2-AS regulate the expression of STAT5 through miR-221 in FLT3-ITD+ progression.
STAT5, a transcription factor, is an important mediator of the JAK/STAT cancer pathway in AML. Activated by FLT3-ITD, STAT5 drives a wide spectrum of the pathogenesis of AML. High pY-STAT5 levels are found to be involved with prognosis in myeloid malignancies and TKI-resistance.21 Several STAT5 inhibitors with encouraging activities have been discovered, such as Pimozide, AC-4-130.22,23 Thus, exploring STAT5 function in FLT3-ITD+ AML of treatment is reasonable and well-founded. In this study, after the knockdown of SOCS2-AS, the expression of STAT5/p-STAT5 reduces obviously, this indicates that SOCS2-AS can regulate the STAT5 signaling pathway. Then, we find that the proliferation capacity significantly increases in si-SOCS2-AS and miR-221 cotransfection cells than si-SOCS2-AS transfection cells. And, miR-221 inhibitor can make up the loss caused by si-SOCS2-AS. The results reveal SOCS2-AS regulates the STAT5 by sponging miR-221. Previous study has proved that SOCS1 is an effective suppressor of NF-κB signaling pathway and a direct target of miR-221, and overexpression of SOCS1 can rescue the miR-221 inhibition-mediated inhibitory effects on inflammation and apoptosis in RAW264.7 cells.24 In addition, the miR-221 dysregulated expression has been implicated in chronic myeloid leukemia progression, and miR-221 can improve the sensitivity of CML to IM by targeting STAT5 and regulating downstream apoptosis signals. Furthermore, it is confirmed that miR-221 can modulate breast carcinoma cell proliferation through STAT5 signaling pathway.25 This result is similar to ours that SOCS2-AS regulated the expression of STAT5 through miR-221.
In the present study, we conformed that the lncRNA SOCS2-AS, as a proto-oncogene, could promote FLT3-ITD+ AML progression by regulating the STAT5 signal transduction pathway with sponging miR-221. Subsequently, the function of SOCS2-AS should be examined in an animal model of AML and the STAT5/miR-221 signaling pathway should be further confirmed. This discovery suggests that SOCS2-AS may serve as a potential therapeutic target for the treatment of FLT3-ITD+ AML.
Funding Statement
This study was funded by the Social Development of Science and Technology Research Project in Shaanxi Province (2015SF135).
Abbreviations
AML, acute myeloid leukemia; FLT3-ITD, FMS-like tyrosine kinase 3-internal tandem duplication.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Harada Y, Nishiwaki S, Sugimoto T, et al. Successful treatment with allogeneic stem cell transplantation followed by DLI and TKIs for e6a2 BCR-ABL-positive acute myeloid leukaemia: a case report and literature review. Medicine. 2017;96(50):e9160. doi: 10.1097/MD.0000000000009160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. doi: 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer M, Schnetzke U, Spies WB, et al. Impact of FLT3-ITD diversity on response to induction chemotherapy in patients with acute myeloid leukemia. Haematologica. 2017. 102(4):e129–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larrosagarcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16(6):991–1001. doi: 10.1158/1535-7163.MCT-16-0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vance KW, Ponting CP. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends Genet. 2014;30(8):348–355. doi: 10.1016/j.tig.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrieri C, Cimatti L, Biagioli M, et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491(7424):454–457. doi: 10.1038/nature11508 [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi: 10.1016/j.bbagrm.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 8.Garzon R, Volinia S, Papaioannou D, et al. Expression and prognostic impact of lncRNAs in acute myeloid leukemia. Proc Natl Acad Sci. 2014;111(52):18679–18684. doi: 10.1073/pnas.1422050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang XX, Tao WG. Long noncoding RNA LINC00152 facilitates the leukemogenesis of acute myeloid leukemia by promoting CDK9 through miR-193a. DNA Cell Biol. 2019;38(3):1–7. doi: 10.1089/dna.2018.4482 [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Cao LX, Luo ZY, et al. LncRNA MALAT1 promotes cell proliferation and imatinib resistance by sponging miR-328 in chronic myelogenous leukemia. Biochem Biophys Res Commun. 2018;507(1):1–8. doi: 10.1016/j.bbrc.2018.09.034 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen CH, Glüxam T, Schlerka A, et al. SOCS2 is part of a highly prognostic 4-gene signature in AML and promotes disease aggressiveness. Sci Rep. 2019;9(1):9139. doi: 10.1038/s41598-019-45579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laszlo GS, Ries RE, Gudgeon CJ, et al. High expression of suppressor of cytokine signaling-2 predicts poor outcome in pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. Leuk Lymphoma. 2014;55(12):2817–2821. doi: 10.3109/10428194.2014.893305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford LB, Kim JH, Collins MD, et al. Human cytomegalovirus encodes a novel FLT3 receptor ligand necessary for hematopoietic cell differentiation and viral reactivation. MBio. 2018;9(2):e00682–18. doi: 10.1128/mBio.00682-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–4380. doi: 10.1182/blood-2002-05-1440 [DOI] [PubMed] [Google Scholar]
- 16.Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Liu Y, Zhang W, et al. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14(19):3112–3123. doi: 10.1080/15384101.2015.1078034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Si JJ, Wang WM, Yu D, et al. Long non coding RNA RP11–69I8.3 expression in acute leukemia and its clinical significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26(4):978–983. doi: 10.7534/j.issn.1009-2137.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 19.Lin-Yu S, Xiao-Juan L, Yu-Meng S, et al. LncRNA ANRIL regulates AML development through modulating the glucose metabolism pathway of AdipoR1/AMPK/SIRT1. Mol Cancer. 2018;17(1):127. doi: 10.1186/s12943-018-0879-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misawa A, Takayama KI, Urano T, et al. Androgen-induced lncRNA SOCS2-AS1 promotes cell growth and inhibits apoptosis in prostate cancer cells. J Biol Chem. 2016;291(34):17861–17880. doi: 10.1074/jbc.M116.718536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–2613. doi: 10.1038/onc.2012.347 [DOI] [PubMed] [Google Scholar]
- 22.Elumalai N, Berg A, Rubner S, et al. Rational development of Stafib-2: a selective, nanomolar inhibitor of the transcription factor STAT5b. Sci Rep. 2017;7(1):819. doi: 10.1038/s41598-017-00920-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingelhofer B, Maurer B, Heyes EC, et al. Pharmacologic inhibition of STAT5 in acute myeloid leukemia. Leukemia. 2018;32(5):1135–1146. doi: 10.1038/s41375-017-0005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Jiang L, Wei X, et al. Inhibition of miR-221 alleviates LPS-induced acute lung injury via inactivation of SOCS1/NF-κB signaling pathway. Cell Cycle. 2019;18(16):1893–1907. doi: 10.1080/15384101.2019.1632136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang XX, Cheng YH, Hu CJ, et al. MicroRNA-221 sensitizes chronic myeloid leukemia cells to imatinib by targeting STAT5. Leuk Lymphoma. 2019;60(7):1709–1720. doi: 10.1080/10428194.2018.1543875 [DOI] [PubMed] [Google Scholar]