Abstract
Multicomponent reactions (MCRs) have become a mainstay in both academic and industrial synthetic organic chemistry due to their step- and atom-economy advantages over traditional synthetic sequences1. Recently, bicyclo[1.1.1]pentane (BCP) motifs have come to the fore as valuable pharmaceutical bioisosteres of benzene rings, and, in particular, 1,3-disubstituted BCP moieties have become widely adopted in medicinal chemistry as para-phenyl ring replacements2. Often these structures are generated from [1.1.1]propellane via opening of the internal C─C bond, either through the addition of radicals or metal-based nucleophiles 3-13. The resulting propellane-addition adducts are subsequently transformed to the requisite polysubstituted BCP compounds via a range of synthetic sequences that traditionally involve multiple chemical steps. While this approach has been effective to date, it is clear that a multicomponent reaction that enables single-step access to complex and diverse polysubstituted BCP products would be synthetically advantageous over the current stepwise approaches. Herein we report a one-step three-component radical coupling of [1.1.1]propellane to afford diverse functionalized bicycles using various radical precursors and heteroatom nucleophiles via a metallaphotoredox catalysis protocol. The reaction operates on short time scales (five minutes to one hour) across multiple (>10) nucleophile classes and can accommodate a diverse array of radical precursors, including those which generate alkyl, α-acyl, trifluoromethyl, and sulfonyl radicals. This method has been used to rapidly prepare BCP analogues of known pharmaceuticals, one of which has substantially different pharmacokinetic properties to those of its commercial progenitor.
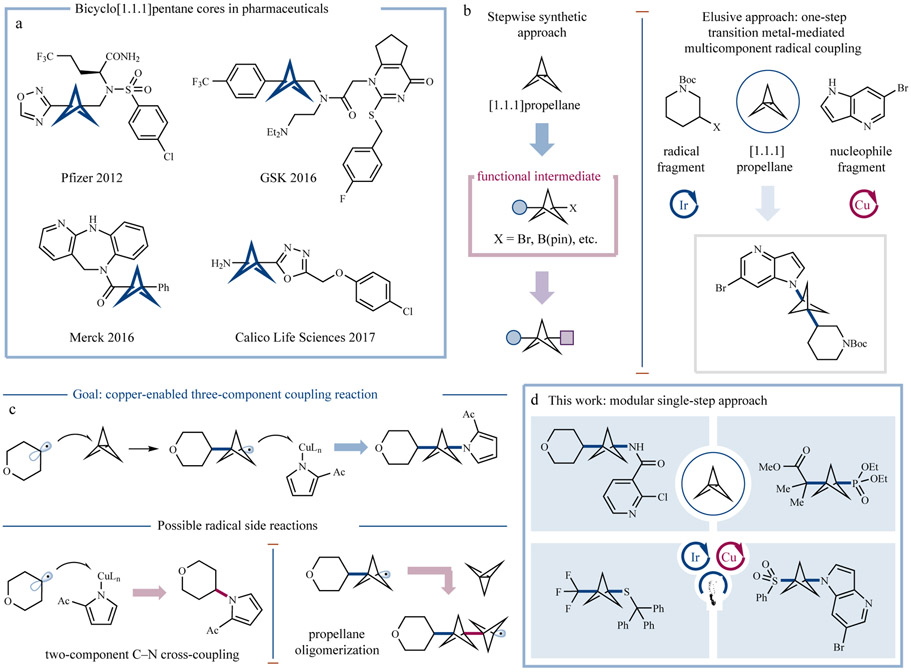
It has been shown that replacement of an aromatic ring with the BCP scaffold can improve the pharmacokinetic profile of many pharmaceutical candidates while providing similar levels of potency (typically via the reduction of metabolic susceptibility and increases in solubility and membrane permeability). It is not surprising, therefore, that the pharmaceutical sector has begun to extensively investigate BCP-containing leads in recent years (Figure 1a)14-17. From a synthetic standpoint, these compounds have largely been prepared by opening of [1.1.1]propellane, most often via multi-step chemical sequences to install and manipulate functional handles at the BCP bridgehead positions (Figure 1b)18. Radical addition to [1.1.1]propellane is well-established and perhaps the most widely leveraged mechanism of BCP functionalization, with numerous examples of chain reactions being reported13. However, it has also been demonstrated that strong nucleophiles, such as turbo Grignard and turbo amide reagents, can be employed to open the internal propellane bond, leading to BCP-organometallic reagents that can be subsequently utilized in transition metal-catalyzed cross-coupling reactions (via a two-step difunctionalization protocol)11,12. While these multi-step approaches have been leveraged to furnish BCP targets with varying levels of synthetic utility, new reaction designs that would enable one-step access to complex and drug-like BCPs would be of significant value to medicinal and process chemists on a community-wide basis. Furthermore, while pioneering work by Uchiyama and coworkers enables a single-step carboamination MCR of [1.1.1]propellane via radical addition to azodicarboxylates4, a modular MCR approach to [1.1.1]propellane difunctionalization that is amenable to a diverse series of structural inputs would allow generic adoption and application across a range of therapeutic areas.
Figure 1 ∣. Direct three-component coupling of [1.1.1]propellane.
a, Examples of the BCP core appearing in bioactive compounds. b, Typical approaches to BCP structures require stepwise synthetic sequences. In contrast, a multicomponent approach might enable single-step access to complex BCP molecules. c, A three-component coupling enabled by a sequence of radical addition and BCP radical capture could be synthetically powerful if selectivity over two-component coupling and oligomerization could be achieved. d, A photoredox-copper platform enables single-step access to an array of diverse products. Ph, phenyl; Et, ethyl; (pin), pinacolato; Boc, tert-butoxycarbonyl; Ac, acetyl; L, ligand; Me, methyl.
Metallaphotoredox catalysis has recently become a valuable platform for the facile generation of radicals from native organic functional groups and their subsequent capture and cross-coupling via a transition metal co-catalyst19-22. Recently, our lab and others have demonstrated that activated carboxylic acids can be leveraged in a photoredox/copper catalysis platform to enable decarboxylative alkylation of N-nucleophiles23-25. These reactions proceed via reductive generation of an alkyl radical that can be subsequently trapped by a copper catalyst, which upon reductive elimination generates the desired C─N fragment-coupled product. Given the susceptibility of [1.1.1]propellane to radical opening with numerous classes of organic radicals13, we recently wondered whether it might be possible to intercept the strained propellane system with photoredox-derived alkyl radicals. Thereafter, subsequent copper-BCP radical trapping/reductive elimination might enable a three-component coupling to yield complex bicyclo[1.1.1]pentane products (Figure 1b). A critical factor to the success of this new MCR pathway would be selective addition of the photo-generated alkyl radical to [1.1.1]propellane in lieu of direct addition to the copper center, which would result in a known, two-component coupling that omits the bicyclopentane framework (Figure 1c). A further complication is the potential for the resultant BCP radical intermediate to add into a second equivalent of the strained [1.1.1]propellane substrate, leading to BCP oligomerization. Although the rates of these elementary steps are not known in the literature, we recognized that the markedly different reactivity of BCP radicals compared to alkyl radicals26 might enable differential reactivity with respect to both [1.1.1]propellane capture (desired in the first bond-forming step, but not the second) and capture of the copper catalyst (desired of the BCP radical but not the photo-generated alkyl radical). Importantly, if selectivity could be achieved, the radicophilic nature of [1.1.1]propellane might enable the use of numerous classes of radical precursors, while the copper catalyst might simultaneously allow several types of N-, P- and S-nucleophiles to be employed, thereby demonstrating the synthetic utility of the transformation to access a diverse array of molecular architectures (Figure 1d).
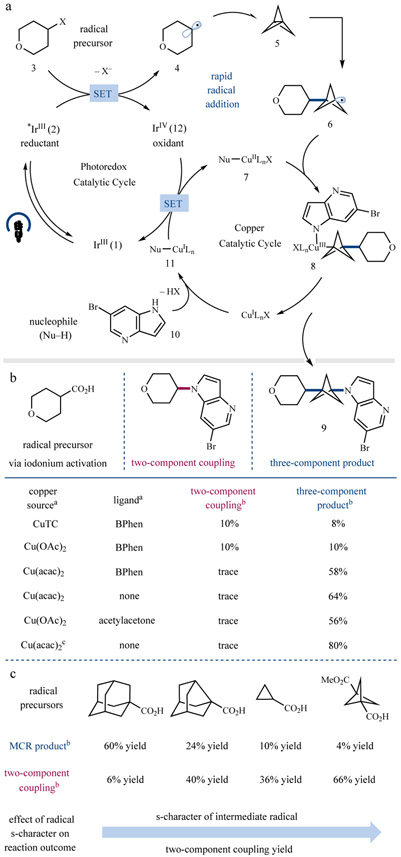
A plausible mechanism for the proposed three-component coupling is shown in Figure 2a. Excitation of photocatalyst Ir(ppy)3 (1) (ppy = 2-phenylpyridinato) is known to generate the long-lived triplet excited state *IrIII complex 2 (lifetime τ = 1.9 μs)27. This excited-state complex is a strong reductant (E1/2 red [IrIV/*IrIII] = −1.81 V vs. SCE in acetonitrile)28 and should readily reduce iodonium dicarboxylate 3 (Epc [3/3•−] = −0.82 V vs. SCE in acetonitrile) to generate alkyl radical 4 upon CO2 extrusion29. This species would then undergo subsequent radical addition to [1.1.1]propellane (5) to generate the resultant BCP radical 6. Radical interception with nucleophile-ligated copper complex 7 would thereafter generate the formal CuIII complex 8, which is poised to undergo reductive elimination22,25 to forge the desired product 9. However, we recognize that the exact electronic configuration of complex 8 may be somewhat more complicated than depicted above based on recent work from Lancaster and coworkers30. Nevertheless, reductive elimination by this complex should still be facile given its electron deficiency. Finally, ligation of another equivalent of N-nucleophile 10 would generate a new CuI species 11, which upon oxidation by the IrIV form of the photocatalyst (E1/2red [IrIV/IrIII] = +0.77 V vs SCE in acetonitrile)28 would simultaneously complete both catalytic cycles.
Figure 2 ∣. Plausible mechanism and catalyst evaluation for three-component coupling.
a, Reductive radical generation gives an alkyl radical (4) which can be intercepted by [1.1.1]propellane (5) to give BCP radical 6. Trapping by an appropriate copper species, such as 7, followed by reductive elimination would give the desired three-component BCP product 9. b, Evaluation of copper salts and ligands to achieve the desired reactivity revealed that copper (II) acetoacetonate (acac) is the optimal catalyst. c, Studies on the effect of radical s-character reveal that selectivity trends with this characteristic. a30 mol% of each copper salt and ligand was used unless otherwise specified. b 1H-NMR yields. c60 mol% Cu(acac)2 used. SET, single-electron transfer; Nu, N-nucleophile; TC, thiophene-2-carboxylate; BPhen, bathophenanthroline.
From the outset, we recognized that controlling the relative rates of radical addition to [1.1.1]propellane versus the copper catalyst would be necessary to enable the desired three-component C─N coupling while minimizing the amount of two-component coupling and/or propellane oligomerization. To this end, we began our studies by evaluating a number of copper salts and ligands (Figure 2b, also see Supplementary Information). To our delight, we found that the use of diketonate ligands, such as acetylacetonate (acac), enabled efficient formation of the desired three-component product with minimal quantities of the two-component decarboxylative C─N coupled product observed. Interestingly, oligomerization does not appear to be a major side reaction in this three-component coupling, with again, only trace amounts of poly-BCP products being observed. The differential reactivity of BCP radical 6 compared to the substrate alkyl radicals (such as 4), which is critical to ensuring this three-component coupling, has been previously documented and might be attributed to the substantial s-character of this and other alkyl bridgehead radicals31-33. To probe this hypothesis, we examined radical precursors that generate alkyl radicals with similar s-character (Figure 2c, also see Supplementary Information). Interestingly, a clear trend is observed demonstrating that as the s-character of the radical increases, the proportion of two-component coupling concomitantly increases (see refs 31-33 for a discussion of radical s-character in pertinent systems). As a corollary, it would further appear that an increase in s-character favors radical addition to copper instead of [1.1.1]propellane. This interesting trend has not, to the best of our knowledge, been documented in the realm of copper catalysis and is under further investigation in our lab.
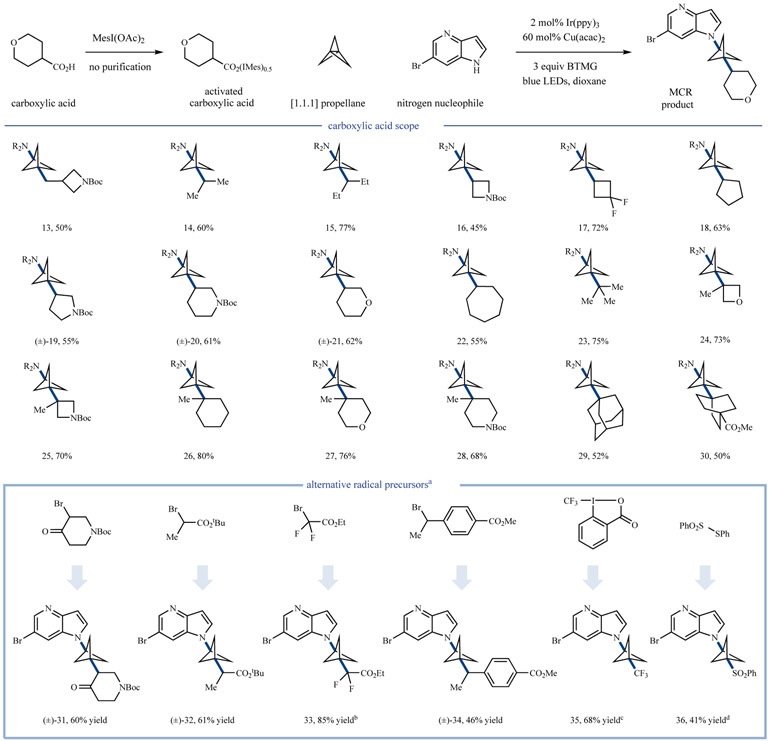
Following our initial optimization studies, we began to evaluate the scope of this three-component coupling for a range of carboxylic acids (via iodonium dicarboxylates, generated without purification) as radical precursors with 7-bromo-4-azaindole as the prototypical N-nucleophile. As can be seen in Figure 3, we found that a variety of alkyl acid structural inputs were amenable to this decarboxylative multi-component coupling, including primary (13, 50% yield) and acyclic secondary (14 and 15, 60% and 77% yield, respectively) substrates, as well as secondary carboxylates appended to cyclic frameworks (4–7 membered rings, 16–22, 45–72% yield). Furthermore, we have found that tertiary carboxylates readily undergo addition to [1.1.1]propellane to give the desired three-component products that bear vicinal quaternary substituted centers in good yields (23–30, 50–80% yield). Notably, this decarboxylative coupling platform enables access to structures bearing pharmaceutically relevant aliphatic heterocycles, such as oxetanes (24), azetidines (13, 16, and 25), pyrrolidines (19), and piperidines (20 and 28). Having established that a wide array of carboxylic acid structural formats are suitable electrophiles for this transformation, we next sought to expand the scope of radical precursors beyond that of carboxylic acids. In so doing, we found that activated alkyl bromides, such as α-bromo carbonyls and benzylic bromides, were viable radical precursors, providing functionalized BCP products in modest to excellent yields (31–34, 46–85% yield), in line with results obtained by both the Anderson5 and Aggarwal34 groups in similar radical additions to strained sigma bonds. Furthermore, we also found that amino-trifluoromethylation of [1.1.1]propellane can be efficiently achieved using commercially available Togni reagent II (35, 68% yield). Notably, while control experiments with other radical precursors revealed that both light and photocatalyst were necessary for efficient product formation, 1,3-amino-trifluoromethylation is achieved efficiently in the dark with the desired product formed in only a matter of minutes (see Supplementary Information for details). Finally, thiosulfonates were also found to be viable electrophiles, generating the amino-sulfone adduct 36 with useful efficiency (41% yield). It is important to consider that the capacity to implement multiple classes of radical precursors should render this three-component coupling protocol valuable across a number of areas of chemical synthesis and medicinal chemistry.
Figure 3 ∣. Radical precursor scope for three-component coupling.
Numerous radical precursors can be utilized in this transformation. All yields are isolated unless otherwise noted. Experiments typically run with 1 eq. of nucleophile, 1.5 eq. of [1.1.1]propellane, and 2 eq. of iodonium dicarboxylate; however, alternative stoichiometry is optimal in some cases. See Supplementary Information for exact experimental conditions. aConditions vary slightly for each class of radical precursor. See Supplementary Information for exact reaction conditions. b 1H-NMR yield. c30 mol% Cu(acac)2, no light (see SI). dTHF as solvent. NR2, 6-bromo-4-azaindole; Mes, mesityl; BTMG, 2-tert-butyl-1,1,3,3- tetramethylguanidine; tBu, tert-butyl.
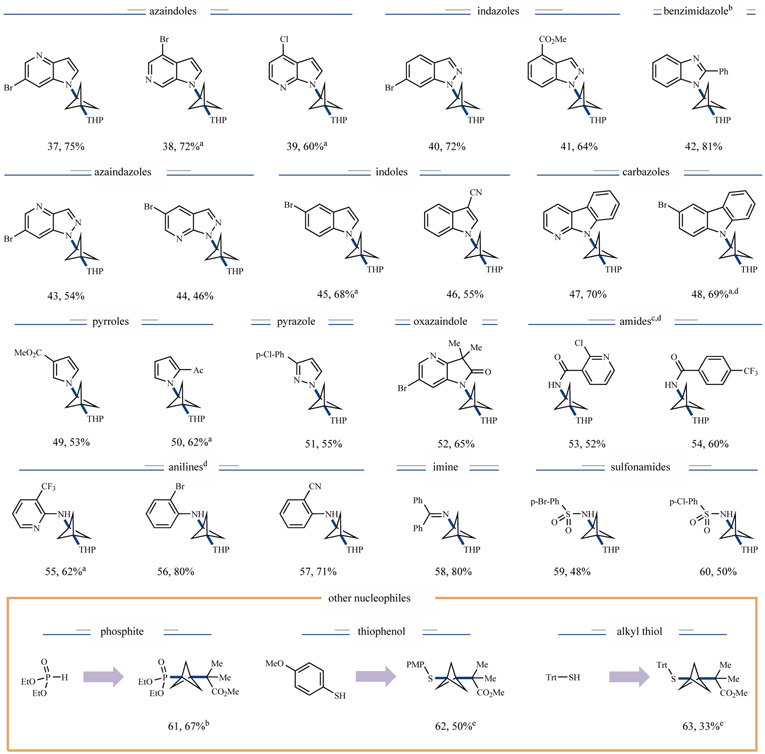
We next turned our attention to the scope of the N-nucleophile component in this new BCP-MCR protocol (Figure 4). To our delight, nearly every class of medicinally relevant N-heterocycles, including azaindoles (37–39, 60–75% yield), indazoles (40 and 41, 72% and 64% yield, respectively), benzimidazoles (42, 81% yield), azaindazoles (43 and 44, 54% and 46% yield, respectively), indoles (45 and 46, 68% and 55% yield, respectively), carbazoles (47 and 48, 70% and 69% yield, respectively), pyrroles (49 and 50, 53% and 62% yield, respectively), pyrazoles (51, 55% yield), and oxazaindoles (52, 65% yield) can be successfully employed to deliver the desired products in good to excellent efficiency. As further shown in Figure 4, this three-component C─N coupling method is not limited to the cross-coupling of N-heterocycles. Under standard or slightly modified conditions, a variety of other N-nucleophiles, including amides (53 and 54, 52% and 60%, respectively), anilines (55–57, 62–80% yield), imines (58, 80% yield), and sulfonamides (59 and 60, 48% and 50% yield, respectively) were found to participate readily in this multi-component reaction. Notably, functional groups including nitriles (46, 55% yield), aryl bromides (37, 75% yield) and ketones (50, 62% yield) were readily tolerated, a useful feature with respect to further synthetic manipulation. Furthermore, regioselectivity could be achieved for a substrate bearing multiple nucleophilic nitrogen sites (see Supplementary Information, substrate S5). Remarkably, we were also able to demonstrate that P- and S-nucleophiles were competent in this three-component platform, allowing access to a broad array of chemical diversity under a single reactivity platform (61–63, 33%–67% yield. See Supplementary Information for further examples).
Figure 4 ∣. Nucleophile scope of three-component coupling.
All yields are isolated, and conditions are similar to those in Figure 3 unless otherwise noted. a 1H-NMR yields. Isolated yields for these compounds typically 10–15% lower, see Supplementary Information for details. bCu(TMHD)2 used instead of Cu(acac)2. cCu(II) bis-(2-isobutyrylcyclohexanone) complex used instead of Cu(acac)2. dBTTP used as base instead of BTMG.eUPLC yields. THP, 4-tetrahydropyranyl; Trt, trityl; TMHD, 2,2,6,6-tetramethyl-3,5-heptanedionate; BTTP, tert-butylimino-tri(pyrrolidino)phosphorane.
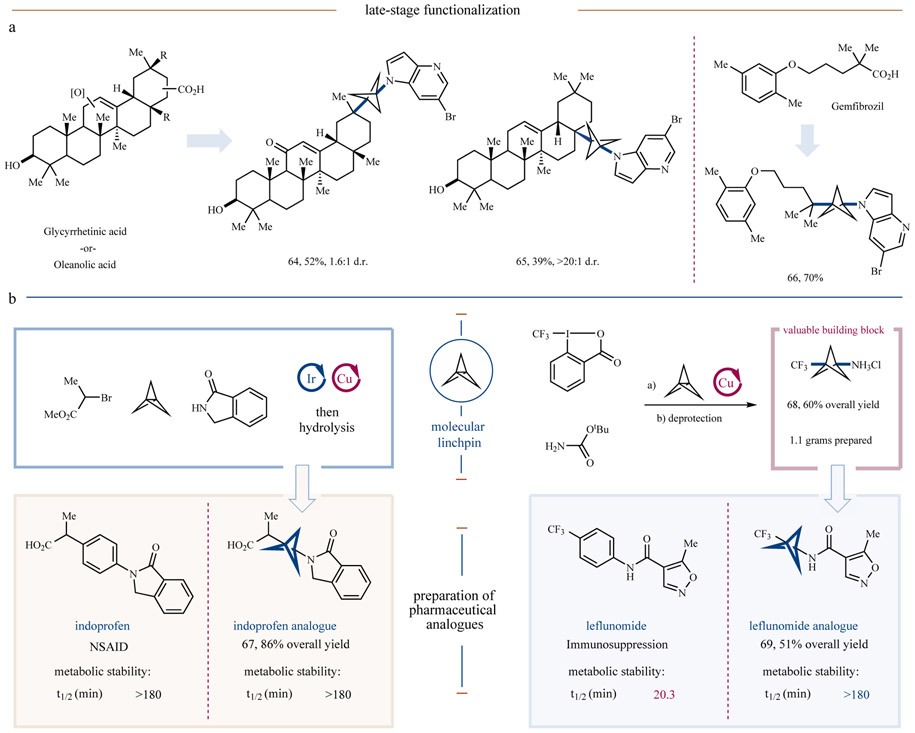
To demonstrate the synthetic utility of this new MCR, we sought to apply it to the late-stage modification of several readily available natural products and pharmaceuticals. As can be seen in Figure 5a, direct installation of an azaindole-bearing BCP unit onto several commercial steroid systems was possible in this context, enabling single-step access to vicinal quaternary centers within multicyclic products in synthetically useful yields (64 and 65, 39 and 52% yield, respectively). Furthermore, modification of the pharmaceutical gemfibrozil was also possible, giving product 66 in 70% yield.
Figure 5 ∣. Rapid functionalization of drugs and natural products and preparation of pharmaceutical analogues.
a, Drug and natural product carboxylic acids can be leveraged in this three-component coupling for rapid diversification. b, This protocol can also be applied to the rapid preparation of pharmaceutical analogues, such as compounds 67 and 69. All yields are isolated, see Supplementary Information for exact conditions.
Carbocyclic aryl rings are major sites of metabolic action by cytochrome P450 (CYP) enzymes, and therefore isosteric replacement of such motifs with BCPs, which are less susceptible to oxidative degradation, has the potential to drastically reduce compound clearance and increase metabolic half-life14. With this in mind, we sought to synthesize and test the in vitro metabolic stability of MCR products 67 and 69, which constitute two bicyclo[1.1.1]pentane analogues of the known pharmaceutical agents, indoprofen and leflunomide respectively. To this end, indoprofen analogue 67 was prepared via our three-component coupling protocol, followed by an ester hydrolysis step to generate the desired carboxylic acid in excellent overall yield (86% yield over two steps). Next, we applied our conditions for amino-trifluoromethylation using tert-butyl carbamate as the nucleophile to enable gram-scale synthesis of trifluoromethyl bicyclo[1.1.1]pentylamine hydrochloride 68 in only two steps and good yield (60% combined yield). Acylation of the amine using a commercially available acid chloride then gave leflunomide analogue 69 in short order, demonstrating the practicality of 68 as a molecular building block. We next assessed the in vitro metabolic stability of analogues 67 and 69 for comparison to their parent pharmaceuticals. Intriguingly, we found that compound 67 has similar pharmacokinetic properties to indoprofen. Remarkably, however, the corresponding leflunomide analogue 69 was found to exhibit markedly improved metabolic stability, with the BCP-variant demonstrating significantly longer half-life in both rat and human liver microsomes than the parent leflunomide.
This methodology is amenable to a variety of radical precursors as well as multiple classes of N-, P-, and S-nucleophiles, allowing single-step access to a diverse array of products. Analogues of known drugs have been prepared in short order and their properties measured in comparison to their aromatic counterparts, demonstrating marked improvements in metabolic stability in the case of leflunomide.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the NIH National Institute of General Medical Sciences (1R35GM134897-01) and gifts from Merck, Bristol-Myers Squibb, Eli Lilly, and Janssen Research and Development LLC. The authors would like to acknowledge Dr. Yufan Liang for helpful discussions.
Footnotes
Competing interests The authors declare no competing interests.
Data availability The data supporting the findings of this study are available within the paper and its Supplementary Information.
References
- 1 ).Dömling A, Wang W & Wang K Chemistry and biology of multicomponent reactions. Chem. Rev. 112, 3083–3135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 ).Mykhailiuk PK Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem 17, 2839–2849 (2019). [DOI] [PubMed] [Google Scholar]
- 3 ).Kanazawa J & Uchiyama M Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 30, 1–11 (2019). [Google Scholar]
- 4 ).Kanazawa J, Maeda K & Uchiyama M Radical multicomponent carboamination of [1.1.1]propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017). [DOI] [PubMed] [Google Scholar]
- 5 ).Nugent J, Arroniz C, Shire BR, Sterling AJ, Pickford HD, Wong MLJ, Mansfield SJ, Caputo DFJ, Owen B, Mousseau JJ, Duarte F & Anderson EA A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal 9, 9568–9574 (2019). [Google Scholar]
- 6 ).Kondo M, Kanazawa J, Ichikawa T, Shimokawa T, Nagashima Y, Miyamoto K & Uchiyama M Silaboration of [1.1.1]propellane to provide a storable feedstock for bicyclo[1.1.1]pentane derivatives. Angew. Chem. Int. Ed (2019) Accepted Author Manuscript. doi: 10.1002/anie.201909655. [DOI] [PubMed] [Google Scholar]
- 7 ).Kaszynski P & Michl J A practical photochemical synthesis of bicyclo[1.1.1]pentane-l,3-dicarboxylic acid J. Org. Chem 53, 4593–4594 (1988). [Google Scholar]
- 8 ).Caputo DFJ, Arroniz C, Dürr AB, Mousseau JJ, Stepan AF, Mansfield SJ & Anderson EA Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci 9, 5295–5300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9 ).Trongsiriwat N, Pu Y, Nieves-Quinones Y, Shelp RA, Kozlowski MC & Walsh PJ Reactions of 2-aryl-1,3-dithianes and [1.1.1]propellane. Angew. Chem. Int. Ed 58, 13416–13420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10 ).Gianatassio R, Lopchuk JM, Wang J, Pan C-M, Malins LR, Prieto L, Brandt TA, Collins MR, Gallego GM, Sach NW, Spangler JE, Zhu H, Zhu J & Baran PS Strain-release amination. Science 351, 241–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11 ).Makarov IS, Brocklehurst CE, Karaghiosoff K, Koch G & Knochel P Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and para-disubstituted benzenes from [1.1.1]propellane. Angew. Chem. Int. Ed 56, 12774–12777 (2017). [DOI] [PubMed] [Google Scholar]
- 12 ).Hughes JME, Scarlata DA, Chen AC-Y, Burch JD & Gleason JL Aminoalkylation of [1.1.1]propellane enables direct access to high-value 3-alkylbicyclo[1.1.1]pentan-1-amines. Org. Lett 21, 6800–6804 (2019). [DOI] [PubMed] [Google Scholar]
- 13 ).Wiberg KB & Waddell ST Reactions of [1.1.1]propellane. J. Am. Chem. Soc 112, 2194–2216 (1990). [Google Scholar]
- 14 ).Stepan AF et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem 55, 3414–3424 (2012). [DOI] [PubMed] [Google Scholar]
- 15 ).Measom ND, Down KD, Hirst DJ, Jamieson C, Manas ES, Patel VK & Somers DO Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an LpPLA2 inhibitor. ACS Med. Chem. Lett 8, 43−48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 ).Fischer C, Bogen S, Childers ML, Llinas FXF, Ellis JM, Esposite S, Hoffman DM, Huang C, Kattar S, Kim AJ, Lampe JW, Machacek MR, Mcmasters DR, Parker DL Jr., Reutershan M, Sciammetta N, Shao PP, Sloman DL, Sun W, Ujjainwalla F, Wu Z, Gibeau C Patent WO/2016/089830 A1 (2016). [Google Scholar]
- 17 ).Sidrauski C, Pliushchev M, Frost JM, Black LA, Xu X, Sweis RF, Shi L, Zhang QI, Tong Y, Hutchins CW, Chung S, Dart MJ Patent WO/2017/193030 A1 (2017). [Google Scholar]
- 18 ).Kaszynski P, McMurdie ND & Michl J Synthesis of doubly bridgehead substituted bicyclo[1.1.1]pentanes. Radical transformations of bridgehead halides and carboxylic acids. J. Org. Chem 56, 307–316 (1991). [Google Scholar]
- 19 ).Twilton J, Le C, Zhang P, Shaw MH, Evans RW & MacMillan DWC The merger of transition metal and photocatalysis. Nat. Rev. Chem 1, 0052 (2017). [Google Scholar]
- 20 ).Kalyani D, McMurtrey KB, Neufeldt SR & Sanford MS Room-temperature C─H arylation: merger of Pd-catalyzed C─H functionalization and visible-light photocatalysis . J. Am. Chem. Soc 133, 18566–18569 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21 ).Primer DN & Molander GA Enabling the cross-coupling of tertiary organoboron nucleophiles through radical-mediated alkyl transfer J. Am. Chem. Soc 139, 9847–9850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 ).Le C, Chen TQ, Liang T, Zhang P & MacMillan DWC A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 ).Mao R, Frey A, Balon J & Hu X Decarboxylative C(sp3)─N cross-coupling via synergetic photoredox and copper catalysis. Nat. Catal. 1, 120–126 (2018). [Google Scholar]
- 24 ).Zhao W, Wurz RP, Peters JC, & Fu GC Photoinduced, copper-catalyzed decarboxylative C─N coupling to generate protected amines: an alternative to the Curtius rearrangement. J. Am. Chem. Soc 139, 12153–12156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25 ).Liang Y, Zhang X & MacMillan DWC Decarboxylative sp3 C─N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26 ).Banks JT, Ingold KU, Della EW & Walton JC Bicyclo[1.1.1]pent-1-yl: a tertiary radical with enhanced reactivity. Tetrahedron Lett 37, 8059–8060 (1996). [Google Scholar]
- 27 ).Dixon IM, Collin J, Sauvage J, Flamigni L, Encinas S & Barigelletti F A family of luminescent coordination compounds: iridium(III) polyimine complexes. Chem. Soc. Rev 29, 385–391 (2000). [Google Scholar]
- 28 ).Nacsa ED & MacMillan DWC Spin-center shift-enabled direct enantioselective α-benzylation of aldehydes with alcohols J. Am. Chem. Soc 140, 3322–3330 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29 ).Minisci F, Vismara E, Fontana F & Barbosa MCN A new general method of homolytic alkylation of protonated heteroaromatic bases by carboxylic acids and iodosobenzene diacetate. Tetrahedron Lett 30, 4569–4572 (1989). [Google Scholar]
- 30 ).DiMucci IM, Lukens JT, Chatterjee S, Carsch KM, Titus CJ, Lee SJ, Nordlund D, Betley TA, MacMillan SN & Lancaster KM The myth of d8 copper(III) J. Am. Chem. Soc 141, 18508–18520 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31 ).Walton JC Bridgehead radicals. Chem. Soc. Rev. 21, 105–112 (1992). [Google Scholar]
- 32 ).Fiorentino M, Testaferri L, Tiecco M & Troisi L Structural effects on the reactivity of carbon radicals in homolytic aromatic substitution. Part 4. The nucleophilicity of bridgehead radicals. J. Chem. Soc., Perkin Trans 2, 87–93 (1977). [Google Scholar]
- 33 ).Della EW, Cotsaris E, Hine PT, & Pigou PE 13C Spectral parameters of some polycyclic hydrocarbons. II Bicyclo[3,1,1]heptane, tricyclo[3,1,1,03,6]heptane, tricyclo[3,3,0,02,6]octane and bicyclo[1,1,1]pentane Aust. J. Chem 34, 913–916 (1981). [Google Scholar]
- 34 ).Silvi M & Aggarwal VK Radical addition to strained σ-bonds enables the stereocontrolled synthesis of cyclobutyl boronic esters J. Am. Chem. Soc 141, 9511–9515 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.