Abstract
TAFRO syndrome, a clinical subtype of idiopathic multicentric Castleman disease (iMCD), consists of a constellation of symptoms/signs including thrombocytopenia, anasarca, fever, reticulin fibrosis/renal dysfunction, and organomegaly. The etiology of iMCD-TAFRO and the basis for cytokine hypersecretion commonly seen in iMCD-TAFRO patients has not been elucidated. Here we identified a somatic MEK2P128L mutation and a germline RUNX1G60C mutation in two patients with iMCD-TAFRO, respectively. The MEK2P128L mutation, which has been identified previously in solid tumor and histiocytosis patients, caused hyperactivated MAP kinase signaling, conferred IL-3 hypersensitivity and sensitized the cells to various MEK inhibitors. The RUNX1G60C mutation abolished the transcriptional activity of wild-type RUNX1 and functioned as a dominant negative form of RUNX1, resulting in enhanced self-renewal activity in hematopoietic stem/progenitor cells. Interestingly, ERK was heavily activated in both patients, highlighting a potential role for activation of MAPK signaling in iMCD-TAFRO pathogenesis and a rationale for exploring inhibition of the MAPK pathway as a therapy for iMCD-TAFRO. Moreover, these data suggest that iMCD-TAFRO might share pathogenetic features with clonal inflammatory disorders bearing MEK and RUNX1 mutations such as histiocytoses and myeloid neoplasms.
Introduction
TAFRO syndrome is a clinical subtype of idiopathic multicentric Castleman disease (iMCD) characterized by thrombocytopenia, anasarca, fever, reticulin fibrosis/renal dysfunction, and organomegaly [1, 2]. iMCD-TAFRO can occur in individuals of all ages, with a median age of 50 and a slight predilection for male gender [3]. Similar to other cases of iMCD, iMCD-TAFRO patients often present with elevated serum cytokines and chemokines including IL-6, IP-10, MDC, and CXCL13 [4, 5]. The etiology of iMCD-TAFRO and the basis for cytokine hypersecretion has not been elucidated, although germline and somatic genetic alterations have been proposed to underlie iMCD-TAFRO [6]. Here we report the identification and functional characterization of a somatic MEK2 mutation and a germline RUNX1 mutation in two iMCD-TAFRO patients, respectively.
Results and Discussion
Identification of somatic MAP2K2 (MEK2) mutation and germline RUNX1 mutation in patients with iMCD-TAFRO
Clinical information was provided by treating physicians and extracted from electronic medical records. Studies were approved by the Institutional Review Boards of Memorial Sloan Kettering Cancer Center. Three patients (male, 32-year-old, 3-year-old and 20-year-old) presented with typical signs and symptoms of iMCD-TAFRO including fever, anasarca, leukocytosis, anemia, thrombocytopenia, renal insufficiency, hepatosplenomegaly, diffuse lymphadenopathy, and evidence of systemic inflammation with elevated ESR, CRP, and IL-6 levels (Table 1). Evaluation for autoantibodies and viruses (HHV-8, EBV) were negative. Lymph node excisional biopsies (neck and inguinal nodes, respectively) were compatible with iMCD-TAFRO (Fig. 1a, Supplementary Fig. S1a) [7]. Bone marrow biopsies revealed moderately increased megakaryocytes with occasional megakaryocytic emperipolesis and increased reticulin fibrosis. Flow cytometric analyses of nodal and marrow specimens were negative for lymphoma or leukemia. The overall findings were compatible with iMCD-TAFRO. All 3 patients were treated with high-dose steroids and anti-IL-6 antibodies (2 Tocilizumab and 1 Siltuximab) and obtained complete remission of symptoms including lymphadenopathy (Fig. 1b, 1c).
Table 1.
Clinicopathologic features of iMCD-TAFRO patients.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (years) | 32 | 3 | 20 |
| Sex | M | M | M |
| Fever | Yes | Yes | Yes |
| Anasarca | Large pleural effusion and ascites | Pleural effusion, ascites, pericardial effusion | Pleural effusion, ascites, pericardial effusion |
| Hb (g/dL) | 10.8 | 7.8 | 5.1 |
| PLT (x103/dL) | 32 | 14 | 100 |
| ALP (U/L) | Not available | 108 | 300 |
| LDH (U/L) | Not available | 481 | 409 |
| Viral workup (EBV/HHV-8/adenovirus/rhinovirus) |
Negative | Negative | Negative |
| Autoantibodies | Negative | Negative | Negative |
| Renal insufficiency | Yes | Yes | Yes (Hemodialysis) |
| Organomegaly | Yes | Yes | Yes |
| Lymphadenopathy | Diffuse | Diffuse | Diffuse |
| Castleman like changes in node | Yes | Yes | Yes |
| Flow cytometry in node | Negative | Negative | Negative |
| Bone marrow atypical megakaryocytes | Yes | Yes | Yes |
| Bone marrow fibrosis | Yes (MF1-2/3) | Yes (MF1/3) | Yes (MF1-2/3) |
| Bone marrow flow cytometry | Negative | Negative | Negative |
| JAK2/MPL/CALR mutations | Negative | Negative | Negative |
| Serum IL-6 level | 43.6 pg/mL | Significantly increased | 138.2 pg/mL |
| Anti-IL-6 treatment | Tocilizumab | Tocilizumab | Siltuximab |
| Steroids treatment | Yes | Yes | Yes |
| Follow-up | Recovered, but relapsed | Recovered, no relapse for 3 years | Recovered |
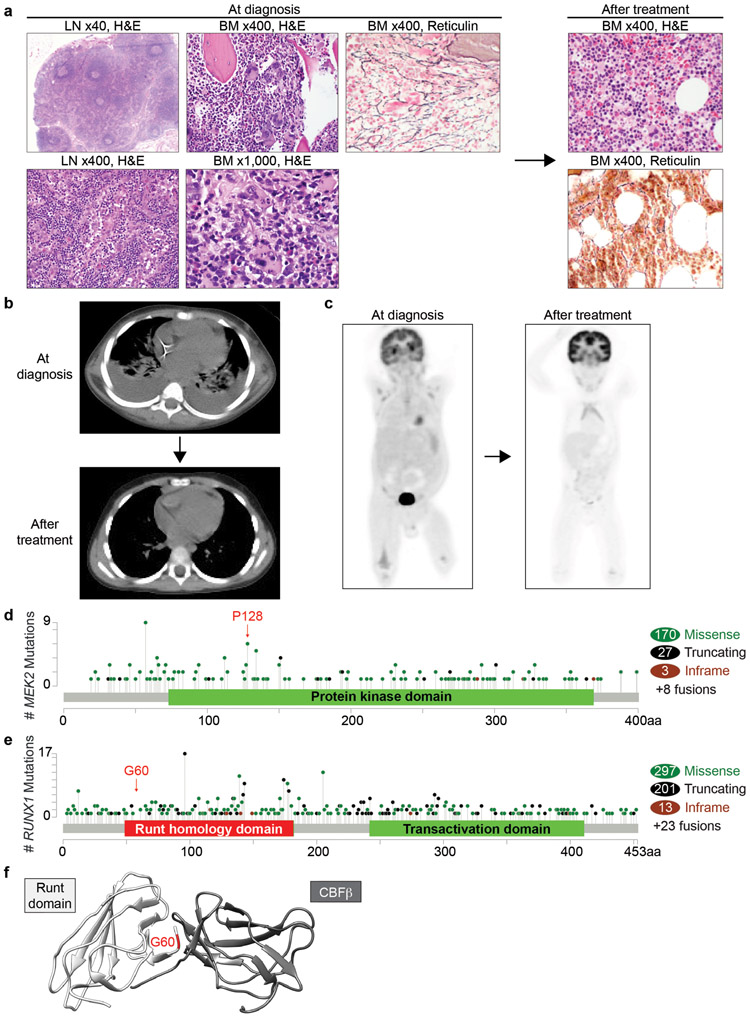
Fig. 1. Clinical presentation and genetic features of patients with iMCD-TAFRO.
a Hematoxylin & eosin (H&E) and reticulin stain of a lymph node (LN) and bone marrow (BM) at diagnosis and after treatment (Patient 2; magnifications are indicated). b, c Representative plain CT (b) and PET scan (c) images of Patient 2 at diagnosis and after treatment. d, e Lollipop graphs showing frequencies of mutations in MEK2 (d) and RUNX1 (e) across cancers. Figures were made based on the cBioPortal data of 42,049 samples derived from TCGA PanCancer Atlas Studies and curated set of non-redundant studies (158 studies in total) and the location of mutations that were identified in Patient 1 and 2 are highlighted. Mutations affecting MEK2P128 were identified in 3 bladder carcinoma, 1 melanoma, and 1 pancreatic ductal adenocarcinoma. f Structure of WT Runt homology domain (white) and CBFβ (gray) with RUNX1G60C position highlighted in red. Figure was made based on PDB ID: 1E50.
In order to evaluate for potential genetic alterations underlying iMCD-TAFRO, the coding region of ~500 genes implicated in cancer was sequenced by a next generation sequencing platform (MSK-IMPACT) on the lymph node biopsy tissue from the three patients. Several germline variants of unclear significance were detected (Table 2). Among these variants, mutations in MEK2 and RUNX1 are commonly identified in a variety of cancers and were predicted to be damaging by both SIFT and Polyphen-2 programs. In addition, sequencing of the lymph node from Patient 1 identified a MAP2K2 (MEK2) p.P128L mutation with a variant allele frequency (VAF) of 10%, which was absent from the patient’s bone marrow, indicative of a somatic mutation. Although the function of this specific mutant has not been characterized previously, it is recurrent across several cancer types and predicted to activate MAPK signaling (Fig. 1d). Sequencing of Patient 2 identified a RUNX1 p.G60C variant with similarly high VAF across nodal (48%), marrow (49%), and nail DNA (49%), indicative of a germline variant. RUNX1 p.G60C, located at the N-terminus of the RUNT DNA binding domain (Fig. 1e), has not been previously reported but is structurally predicted to affect the heterodimerization of RUNX1 with CBFβ, which is essential for RUNX1 function (Fig. 1f). We also examined for possible fusions by targeted next generation RNA sequencing (199-gene Archer panel) in all three patients but no in-frame fusions were detected. Based on these observations, we chose MEK2 and RUNX1 variants for further functional studies, although we cannot completely exclude the possibility that the other variants (such as RARA and SETD2 variants in patient 3) could also be pathogenic.
Table 2.
Variants detected in iMCD-TAFRO patients.
| Gene | Protein change | Allele Freq. |
Chr. | Start Pos. | End pos. | Ref | Var | SIFT prediction |
Polyphen-2 prediction |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | MAP2K2 (MEK2) | P128L | 0.10 | 19 | 4110574 | 4110574 | G | A | Damaging | Probably damaging |
| AR | A646D | 0.99 | X | 66931295 | 66931295 | C | A | Tolerated | Benign | |
| TCF3 | N347T | 0.49 | 19 | 1621020 | 1621020 | T | G | Tolerated | Probably damaging | |
| ARID2 | T1193A | 0.46 | 12 | 46245483 | 46245483 | A | G | Tolerated | Benign | |
| Patient 2 | KMT2B (MLL4) | E373del | 0.40 | 19 | 36211355 | 36211357 | AAG | A | - | - |
| MPEG1 | L221I | 0.50 | 11 | 58979678 | 58979678 | G | T | Tolerated | Benign | |
| RUNX1 | G60C | 0.48 | 21 | 36259232 | 36259232 | C | A | Damaging | Probably damaging | |
| Patient 3 | EGFR | H145R | 0.46 | 7 | 55214308 | 55214308 | A | G | Tolerated | Benign |
| EPHA3 | S662T | 0.52 | 3 | 89462392 | 89462392 | T | G | Tolerated | Benign | |
| RARA | G248D | 0.53 | 17 | 38508695 | 38508695 | G | A | Damaging | Probably damaging | |
| SOX9 | T316A | 0.44 | 17 | 70119944 | 70119944 | A | A | Tolerated | Benign | |
| SESN1 | H47R | 0.48 | 6 | 109415137 | 109415137 | T | C | Tolerated | Benign | |
| SETD2 | G1649E | 0.46 | 3 | 47143017 | 47143017 | C | T | Damaging | Probably damaging | |
| ANKRD11 | A2284V | 0.47 | 16 | 89346099 | 89346099 | G | A | (not scored) | Benign | |
| TET3 | V1137I | 0.49 | 2 | 74327729 | 74327729 | G | A | Tolerated | Benign | |
| ZFHX3 | Q3203_Q3204del | 0.28 | 16 | 72822564 | 72822569 | TGCTGC | - | - | - |
Pathogenetic roles of mutant MEK2 and mutant RUNX1
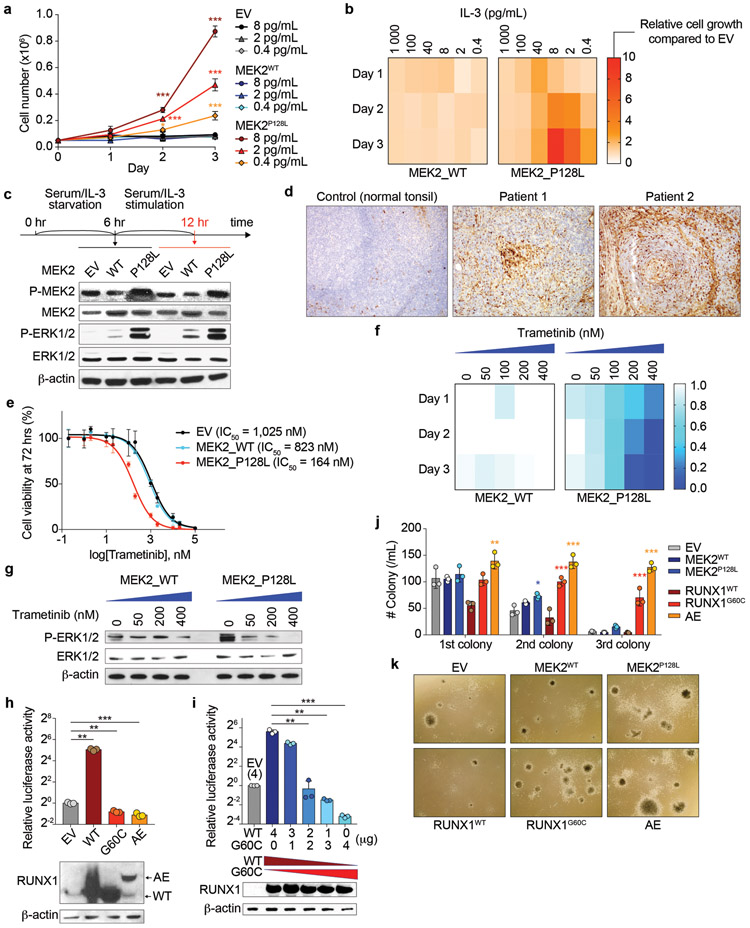
To determine the functional impact of the somatic MEK2 mutation, we expressed wild-type (WT) MEK2 and MEK2P128L into 32D cells and examined the effects on IL-3 dependent growth and downstream signaling. Although MEK2P128L did not confer cytokine independence, 32D cells expressing MEK2P128L had robust growth at very low IL-3 concentrations in which control cells could not survive (Fig. 2a, 2b), demonstrating cytokine hypersensitivity conferred by MEK2P128L. Accordingly, MEK2P128L enhanced phosphorylation of MEK1/2 and downstream ERK1/2 constitutively and after IL-3 stimulation (Fig. 2c). Although, BaseScope technology detected MEK2P128L mRNA in a minor subset of cells in the nodal biopsy (Supplementary Fig. S1b), levels of phospho-ERK1/2 by immunohistochemistry were increased in the biopsy tissue and corresponded to histiocytes and/or follicular dendritic cells (Fig. 2d) while lymphocytes had no detectable phospho-ERK. Consistent with these observations, 32D cells expressing MEK2P128L were sensitive to various MEK inhibitors (Trametinib, Selumetinib, and U0126) (Fig. 2e, Supplementary Fig. S2a, 2b), demonstrating suppressed cell growth and ERK activation in a dose-dependent manner (Fig. 2f, 2g, Supplementary Fig. S2c-e), suggesting that inhibition of MAPK pathway could be a potential therapeutic strategy for iMCD-TAFRO. Interestingly, phospho-ERK expressing cells were also increased in patient 2 with the RUNX1G60C mutation (Fig. 2d). These results suggest that the MAP kinase pathway might be commonly activated in iMCD-TAFRO.
Fig. 2. Pathogenic roles of mutant MEK2 and mutant RUNX1.
a, b Cell growth of isogenic 32D cells cultured with various concentration of mouse IL-3 (a). Heatmaps were made based on relative cell growth of MEK2WT and MEK2P128L expressing cells compared to EV (empty vector)-transduced cells in b (n = 3 per genotype; the mean percentage ± standard deviation (SD); two-way ANOVA with Dunnett’s multiple comparison test). c Representative western blot (WB) analysis of isogenic 32D cells from three biologically independent experiments with similar results. Experimental design is shown above. d Representative immunohistochemistry of phospho-ERK1/2 for the lymph nodes from both patient 1 and 2 as well as a normal tonsil as a control (original magnification x100). e Dose response curves of isogenic 32D cells to trametinib (n = 3; the mean value ± SD is shown). IC50 value for each genotype is indicated. f Cell growth of isogenic 32D cells cultured with various concentration of trametinib. Heatmaps were made based on relative cell growth of MEK2WT and MEK2P128L expressing cells compared to EV (empty vector)-transduced cells in Supplementary Fig. 2c-e. g Representative WB analysis of isogenic 32D cells from three biologically independent experiments with similar results. h Luciferase activity of isogenic THP-1 cells retrovirally transduced with indicated constructs (top) and representative WB analysis of the same cells using an antibody against RUNX1 which recognizes N-terminus of RUNX1 protein (bottom). These cells were transfected with a M-CSF receptor reporter plasmid, and luciferase activities were measured as previously described [18] and presented as the fold change relative to EV-transduced cells (AE: RUNX1/RUNX1T1 (AML1/ETO); n = 3; the mean + SD; one-way ANOVA followed by Holm-Sidek’s multiple comparison test). i Luciferase activity of HEK293T cells transfected with the reporter plasmid and mammalian expression vectors for RUNX1WT and RUNX1G60C at various doses as indicated (data are shown as in h). j, k Results of serial replating assays using primary mouse BM cells transduced with indicated constructs (n = 3 per genotype). Number of colonies (j) (the mean ± SD; two-way ANOVA with Tukey’s multiple comparison test; p-values compared to EV-expressing cells are shown), and representative images of colonies (k) (original magnification x 20) are shown. *p < 0.05; **p < 0.01; ***p < 0.001.
Unlike MEK2P128L, expression of RUNX1G60C did not affect the growth of 32D cells (data not shown). However, given the location of this mutation within the RUNT DNA binding domain, we investigated the effect of mutant RUNX1G60C on RUNX1 transcriptional activity. Using a luciferase reporter assay for a well-known RUNX1 target gene, M-CSFR, RUNX1WT clearly induced RUNX1 transcriptional activity, an effect completely abolished in the presence of RUNX1G60C (Fig. 2h). Furthermore, RUNX1G60C suppressed the activity of its WT counterpart, suggesting a dominant negative effect (Fig. 2i, Supplementary Fig. S2f). As patients with germline RUNX1 mutations are predisposed to myeloid neoplasms, we transduced RUNX1G60C into mouse BM progenitor cells and performed colony-forming assay. MEK2P128L had no effect on number or types of primary colonies and showed mildly increased colonies at second passage with no impact on serial replating capacity, suggesting that MEK2P128L does not affect self-renewal capacity. In contrast, RUNX1G60C significantly enhanced self-renewal capacity of BM progenitors to the same extent as the RUNX1/RUNX1T1 fusion (Fig. 2j, 2k, Supplementary Fig. S2g). Somatic MEK2 mutations occur in histiocytoses [8] while somatic RUNX1 mutations occur in myeloid neoplasms and are associated with predisposition to myeloid neoplasms when present in the germline [9, 10]. These results suggest that iMCD-TAFRO might be related to a wider spectrum of diseases that converge on the same constellation associated with cytokine/chemokine hypersecretion. In fact, expression of both MEK2P128L and RUNX1G60C induced overproduction of some of the key cytokines/chemokines known to be elevated in iMCD-TAFRO including IL-6, IP-10, and MDC [4, 5] (Supplementary Fig. S3a-c).
The data above identify pathogenic genetic variants in two out of three subjects studied with iMCD-TAFRO. Although, a DNMT3A mutation was previously reported in an iMCD-TAFRO patient [11], the causative relationship of this mutation to iMCD-TAFRO is uncertain as DNMT3A is frequently mutated in clonal hematopoiesis [12, 13]. Another study reported a germline FASL mutation in a family with unicentric Castleman disease and iMCD [14]. However, germline FASL mutations are nearly pathognomonic for autoimmune lymphoproliferative syndrome, a condition that can resemble Castleman disease [15]. Instead, here we identified germline and somatic mutations in iMCD-TAFRO patients which have been implicated in clonal hematopoietic disorders and we demonstrate impact on gene function. These results suggest that iMCD-TAFRO might share pathogenetic features with these other clonal inflammatory disorders.
Materials and methods
Patient Samples
Tissues were obtained from three patients with iMCD-TAFRO. Clinical information was provided by treating physicians. Studies were approved by the Institutional Review Boards of Memorial Sloan Kettering Cancer Center (under MSK IRB protocol X17-025). Written informed consent was obtained from the three participants.
Mutational analysis of patient samples
Targeted sequencing for recurrent mutations was done by MSK-IMPACT [16] targeting all coding regions of 585 genes known to be recurrently mutated in leukemias, lymphomas, and solid tumors. Targeted next generation RNA sequencing platform (199-gene Archer panel) was also performed.
Cell culture and cytokine measurement
THP-1 (human acute monocytic leukemia cell line), HEK293T (cell line derived from human embryonic kidney 293 cells), and 32D (32Dcl3; murine myeloblast-like cell line) cells were cultured in IMDM/10% FCS (Fetal Calf Serum, heat inactivated), DMEM/10% FCS, and IMDM/10% FCS + mIL3 (R&D Systems; 1 ng/mL), respectively. None of the cell lines above were listed in the data base of commonly misidentified cell lines maintained by ICLAC and NCBI Biosample. For the cytokine/chemokine measurement, 1 × 106 THP-1 cells were cultured in a 6-well plate and treated with phorbol 12-myristate 13-acetate (PMA; Millipore Sigma) at 10 ng/mL to induce differentiation into macrophage-like cells. The supernatant was collected at 72 hrs and the cytokine concentrations were measured using MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel (Millipore) by following instruction provided by the manufacturer. Standard curves were analyzed using Millipore Milliplex Analyst 5.1 Software.
Retroviral transduction and serial replating assays
Bone marrow (BM) cells from 5-fluoruracil (150 mg/kg) treated C57BL/6 mice were extracted as previously described [17]. RBCs were removed by ACK lysis buffer, and nucleated BM cells were transduced with viral supernatants containing MSCV-RUNX1WT/G60C-IRES-GFP, MSCV-RUNX1/RUNX1T1-IRES-GFP, or MSCV-MEK2WT/P128L-IRES-GFP for 2 days in RPMI/20% FCS supplemented with mouse stem cell factor (mSCF; 25 ng/mL; R&D Systems), mouse IL-3 (mIL3; 10 ng/mL; R&D Systems), and mIL-6 (10 ng/mL; R&D Systems). GFP-sorted cells were used for the following serial replating assays as described [17]. Briefly, single-cell suspension was prepared and 15,000 cells/1.5 mL were plated in triplicate in cytokine supplemented methylcellulose medium (MethoCult™ GF M3434; StemCell Technologies), and colonies were enumerated weekly.
Reporter assay
Analysis of luciferase activity was performed as described previously [18]. Cells were transfected with a M-CSF receptor (M-CSFR) [19] plasmid (and expression vectors for RUNX1WT and RUNX1G60C) using Polyethylenimine (Polysciences, Inc.). Transfected cells were harvested 48 hours later and assayed for luciferase activity with a dual luciferase kit (Promega). Firefly luciferase activity was measured as relative light units. Relative light units from individual transfection were normalized by measurement of Renilla luciferase activity in the same samples. Relative M-CSFR promoter activity was presented as the ratio of normalized luciferase activity of empty vector-transduced cells.
Histological analyses
Bone marrow biopsies from the patients were decalcified and fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Paraffin blocks were sectioned at 4 μm and stained with hematoxylin and eosin (H&E). Immunohistochemical staining was performed using anti-phosphor-ERK antibody (clone D13.14.4E, Cell signaling Technology). Images were acquired using an Olympus microscope.
RNA in situ mutation detection (BaseScope) assay
RNA in situ hybridization experiments were performed using RNAscope®, an RNA in situ hybridization technique described previously [20, 21]. Paired double-Z oligonucleotide probes were designed against target RNA using custom software. The following probes were used: I-BA-Hs-MAP2K2-P128L-Cand1, cat no. 720821, NM_030662.3, 1zz pair, nt 610-643; I-BA-Hs-MAP2K2-P128WT-Cand1, cat no. 720841, NM_030662.3, 1zz pair, nt 611-642. The BaseScope™ Reagent Kit (Advanced Cell Diagnostics, Newark, CA) was used according to the manufacturer’s instructions. FFPE cell and tissue sections were prepared according to manufacturer’s recommendations. Each sample was quality controlled for RNA integrity with a 1zz probe specific to the housekeeping gene PPIB. Negative control background staining was evaluated using a 1zz probe specific to the bacterial dapB gene.
Cell culture
THP-1 (human acute monocytic leukemia cell line), HEK293T (cell line derived from human embryonic kidney 293 cells), and 32D (32Dcl3; murine myeloblast-like cell line) cells were cultured in IMDM/10% FCS (Fetal Calf Serum, heat inactivated), DMEM/10% FCS, and IMDM/10% FCS + mIL3 (R&D Systems; 1 ng/mL), respectively. None of the cell lines above were listed in the database of commonly misidentified cell lines maintained by ICLAC and NCBI Biosample.
Antibodies and reagents
For western blotting, the following antibodies were used: Phospho-MEK1/2 (Ser217/221) (Cell Signaling Technologies; #9154S), MEK1/2 (Cell Signaling Technologies; #9126S), Phospho-p44/42 MAPK (Erk1/2) (thr202/Tyr204) (Cell Signaling Technologies; #4370S), p44/42 MAPK (Erk1/2) (Cell Signaling Technologies; #4695S), β-actin (Sigma-Aldrich; A-5441). Trametinib (GSK1120212) (S2673), Selumetinib (AZD6244) (S1008), and U0126-EtOH (S1102) were perchased from Selleckchem.
Statistics and reproducibility
Statistical significance was determined by (1) unpaired two-sided Student’s t-test after testing for normal distribution, (2) one-way or two-way ANOVA followed by Tukey’s, Holm-Sidak’s, or Dunnett’s multiple comparison test, or (3) Kruskal-Wallis tests with Uncorrected Dunn’s test where multiple comparisons should be adjusted. Representative WB results are shown from three or more than three biologically independent experiments.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Sequencing data have been deposited in NCBI ClinVar under accession number SCV000965590-SCV000965596. Other data that support this study’s findings are available from the authors upon reasonable request.
Supplementary Material
Acknowledgements/Grant Support
This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. A.Y. is a Special Fellow of The Leukemia and Lymphoma Society and supported by grants from the Aplastic Anemia and MDS International Foundation (AA&MDSIF), the Lauri Strauss Leukemia Foundation, the Leukemia and Lymphoma Society Special Fellow Award, and JSPS Overseas Research Fellowships. O.A.-W. is supported by grants from NIH/NHLBI (R01 HL128239), the Dept. of Defense Bone Marrow Failure Research Program (W81XWH-16-1-0059), the Starr Foundation (I8-A8-075), the Henry & Marilyn Taub Foundation, the Edward P. Evans Foundation, the Josie Robertson Investigator Program, the Leukemia and Lymphoma Society, and the Pershing Square Sohn Cancer Research Alliance. W.X. is supported by a grant from Castleman’s Awareness and Research Effort/Castleman Disease Collaborative Network.
Footnotes
Conflict of interest
DCF receives research funding from EUSA Pharma for the ACCELERATE Registry (formerly sponsored by Janssen Pharmaceuticals). A.D. has received personal fees from Roche, Corvus Pharmaceuticals, Physicians’ Education Resource, Seattle Genetics, Peerview Institute, Oncology Specialty Group, Pharmacyclics, Celgene, and Novartis and research grants from National Cancer Institute, Roche. O.A.-W. has served as a consultant for H3 Biomedicine, Foundation Medicine Inc., Merck, and Janssen and serves on the scientific advisory board of Envisagenics Inc.; O.A.-W. has received personal speaking fees from Daiichi Sankyo. O.A.-W. has received prior research funding from H3 Biomedicine unrelated to the current manuscript. O.A.-W. is an inventor on a provisional patent application submitted by Fred Hutchinson Cancer Research Center that covers BRD9 activation in cancer. W.X has received research support from Stemline therapeutics. Other authors have nothing to disclose.
References
- 1.Kawabata H, Takai K, Kojima M, Nakamura N, Aoki S, Nakamura S, et al. Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop. 2013;53. [DOI] [PubMed] [Google Scholar]
- 2.Inoue M, Ankou M, Hua J, Iwaki Y, Hagihara M, Ota Y. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporin A : a case report. J Clin Exp Hematop. 2013;53:95–9. [DOI] [PubMed] [Google Scholar]
- 3.Iwaki N, Fajgenbaum DC, Nabel CS, Gion Y, Kondo E, Kawano M, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91:220–6. [DOI] [PubMed] [Google Scholar]
- 4.Iwaki N, Gion Y, Kondo E, Kawano M, Masunari T, Moro H, et al. Elevated serum interferon gamma-induced protein 10 kDa is associated with TAFRO syndrome. Scientific reports. 2017;7:42316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierson SK, Stonestrom AJ, Shilling D, Ruth J, Nabel CS, Singh A, et al. Plasma proteomics identifies a ‘chemokine storm’ in idiopathic multicentric Castleman disease. Am J Hematol. 2018;93:902–12. [DOI] [PubMed] [Google Scholar]
- 6.Liu AY, Nabel CS, Finkelman BS, Ruth JR, Kurzrock R, van Rhee F, et al. Idiopathic multicentric Castleman’s disease: a systematic literature review. Lancet Haematol. 2016;3:e163–75. [DOI] [PubMed] [Google Scholar]
- 7.Fajgenbaum DC, Uldrick TS, Bagg A, Frank D, Wu D, Srkalovic G, et al. International, evidence-based consensus diagnostic criteria for HHV-8–negative/idiopathic multicentric Castleman disease. Blood. 2017;129:1646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozkaya N, Rosenblum MK, Durham BH, Pichardo JD, Abdel-Wahab O, Hameed MR, et al. The histopathology of Erdheim-Chester disease: a comprehensive review of a molecularly characterized cohort. Mod Pathol. 2018;31:581–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song WJ, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–75. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimi A, Toya T, Nannya Y, Takaoka K, Kirito K, Ito E, et al. Spectrum of clinical and genetic features of patients with inherited platelet disorder with suspected predisposition to hematological malignancies: a nationwide survey in Japan. Ann Oncol. 2016;27:887–95. [DOI] [PubMed] [Google Scholar]
- 11.Nagy A, Bhaduri A, Shahmarvand N, Shahryari J, Zehnder JL, Warnke RA, et al. Next-generation sequencing of idiopathic multicentric and unicentric Castleman disease and follicular dendritic cell sarcomas. Blood Adv. 2018;2:481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker TS, Gambino KJ, Schriefer L, Lim JY, Steinberg KM, Fajgenbaum DC, et al. A novel FAS mutation with variable expressivity in a family with unicentric and idiopathic multicentric Castleman disease. Blood Adv. 2018;2:2959–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolze A, Byun M, McDonald D, Morgan NV, Abhyankar A, Premkumar L, et al. Whole-exome-sequencing-based discovery of human FADD deficiency. Am J Hum Genet. 2010;87:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimi A, Lin KT, Wiseman DH, Rahman MA, Pastore A, Wang B, et al. Coordinated alterations in RNA splicing and epigenetic regulation drive leukaemogenesis. Nature. 2019;574:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimi A, Goyama S, Watanabe-Okochi N, Yoshiki Y, Nannya Y, Nitta E, et al. Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood. 2011;117:3617–28. [DOI] [PubMed] [Google Scholar]
- 19.Michaud J, Wu F, Osato M, Cottles GM, Yanagida M, Asou N, et al. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 2002;99:1364–72. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Lan X, Li C, Zhang Y, Wang Y, Xue W, et al. Recurrent PDGFRB mutations in unicentric Castleman disease. Leukemia. 2019;33:1035–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Sequencing data have been deposited in NCBI ClinVar under accession number SCV000965590-SCV000965596. Other data that support this study’s findings are available from the authors upon reasonable request.