Abstract
Objective:
Suicide prediction, prevention, and intervention are urgent research areas. One barrier for research with high-risk populations is limited resources to manage risk in a research setting. We describe using the University of Washington Risk Assessment Protocol (UWRAP) to assess and manage suicide risk during phone-administered eligibility assessments in two clinical trials.
Method:
Study 1 (N=151) recruited suicidal adults who were not engaged in mental health treatment and Study 2 (N=135) recruited suicidal adults who used alcohol to regulate emotions. Pre- and post-assessment ratings of stress, urge to harm self, urge to use drugs/alcohol, and intent to harm self were compared and strategies to manage increased suicide risk following screening interviews were implemented, as indicated.
Results:
In both studies, average post-assessment ratings were significantly lower than pre-assessment. A minority of participants reported higher ratings on one or more domains; however, following more thorough suicide risk assessment, risk was appropriately managed by providing low-level interventions (e.g., validation).
Conclusions:
Suicide risk in research involving community participants can be managed by using appropriate risk protocols.
Keywords: Suicide risk assessment, Suicide protocol, Risk management
Suicide is a pervasive problem in the United States and worldwide. The most recent report from the Centers for Disease Control and Prevention (CDC, 2018) found that the suicide rate rose in 49 of the 50 United States from 1999 to 2016 and the World Health Organization estimates that 800,000 people die by suicide worldwide annually (WHO, 2018). These statistics highlight the critical importance of suicide research; however, recent large-scale meta-analyses of decades of research point to a discouraging lack of progress toward understanding suicide and suicide risk (e.g., Franklin et al., 2017). Taken together, it is not surprising that the CDC has identified etiology and prediction of suicide, as well as prevention and intervention as research priorities.
Unfortunately, relative to other lines of psychological inquiry, fewer studies focus on suicide-related research questions (Lakeman & Fitgerald, 2009; Mishara and Weisstub, 2005; Wilson & Christensen, 2012). Furthermore, many clinical outcome studies exclude individuals at risk for suicide (Ronconi, Shiner, & Watts, 2014; Wilks, Zeive, & Lessing, 2016; Zimmerman et al., 2017). The ubiquity of suicide coupled with the relative lack of a large body of suicide research ensures that our progress toward understanding and preventing death by suicide will be slow and minimal. Considerable barriers exist in research with individuals at risk for suicide. Both researchers and institutional review boards have expressed concerns about the logistics regarding the assessment and management of suicide risk in research settings (e.g., Lakeman & FitzGerald, 2009; Wilson & Christensen, 2012; Hom et al., 2017). Additionally, given limited availability of training in suicide risk assessment and management, particularly in a research context, researchers are often ill-equipped to design and implement appropriate procedures. Given the difficulty predicting and preventing suicide in clinical settings (Silverman & Berman, 2014), it makes sense why ethical concerns about the safety of research participants would arise (Fisher et al., 2002; Mishara & Weisstub, 2005).
The American Psychological Association (APA) has outlined practice guidelines in assessing and managing suicide risk in clinical settings; these include the need to immediately document risk factors and clinical decision making (APA, 2003). However, encounters with suicidal individuals in a research context, where interactions may be brief and suicide risk may be uncertain, provide considerable challenges to scientists wanting to conduct research with suicidal subjects. As such, factors such as “clinical decision making” may need to be explicitly spelled out to assessors as they may not have the clinical training to manage suicidal crises. One such strategy is the use of protocol checklists, which can prompt or remind individuals to engage in recommended treatment procedures (e.g. Bellg et al., 2004). The University of Washington Risk Assessment Protocol (UWRAP; Linehan, Comtois & Ward-Ciesielski, 2012) is a wrap-around risk assessment procedure as well as a management protocol specifically designed for use in research assessments with suicidal or other high-risk participants. It involves pre- and post-assessment tracking of risk variables (i.e., level of stress, urge to harm self, urge to use drugs/alcohol, intent to harm self) as well as procedures to preemptively plan ways to manage potential increases in risk that may occur during participation. Finally, the UWRAP provides guidance for managing elevated risk during and after study participation and implementing emergency intervention, as needed. The UWRAP distinguishes itself from a suicide risk assessment within a treatment context as it outlines strategies known to reduce acute distress (e.g. identifying coping strategies and validating thoughts and emotions; Linehan, 1997; Stanley & Brown, 2012) which may not be apparent to researchers without clinical training.
One aspect included in the UWRAP is the pre-post assessment procedure, which is designed to evaluate potential distress and suicidal urges that may have been elicited by the assessment. Indeed, while previous research has consistently found that participation in research, even in-depth interviews, may temporarily increase emotional distress for some participants, but does not increase suicide risk (e.g. Reynolds et al., 2006; Jorm, Kelly, & Morgan, 2007; Eynan et al., 2014; Law et al., 2015), there are instances in which individuals may experience elevated suicidal urges, for which suicide risk management procedures should be implemented.
Therefore, given the urgent need to bring more researchers into the field of suicide research, we describe the implementation of a suicide risk and management protocol (the UWRAP) in the context of two different randomized clinical trials. The aims of the current study are 1) to provide applied examples in which the UWRAP was used with suicidal participants, 2) to describe the effects of screening procedures on individuals recruited from the community for suicide-related research, and 3) to describe how suicide risk was managed in individuals who endorsed elevated urges to die. Study 1 sought to recruit adults who were currently experiencing suicidal ideation but not engaged in mental health treatment. Study 2 sought to recruit adults who were suicidal with high emotion dysregulation and engaged in heavy drinking.
Methods1
Study 1
Participants & Recruitment.
Participants for the present analyses are 151 adults recruited from the community of a northwestern metropolitan area via flyers, newspaper, online, and radio advertisements. Advertisements were designed to recruit participants who were currently experiencing suicidal ideation (i.e., “Are you feeling suicidal but resisting harming yourself?”). Data reported include those from individuals who provided consent and met initial eligibility criteria as described below.
Procedures.
Study procedures were reviewed and received institutional review board approval. As part of study eligibility screening, interested individuals who contacted the researchers completed a phone screening interview with a trained assessor. After providing verbal consent to the phone screening assessment, participants (n=298) provided basic demographic information related to study eligibility criteria. Individuals who were under 18 years old, unable to come to the research office for an in-person appointment if accepted, unable to read or understand written English, had significant cognitive impairment, and/or had received face-to-face mental health treatment with a mental health provider (e.g., psychologist, counselor, psychiatrist) in the previous month/year2 were notified that they did not meet initial eligibility requirements for participation. These individuals were provided with referral information and the phone screening was discontinued (n=147). Individuals who met initial eligibility criteria (n=151) continued through the remainder of the phone screening by providing contact information, completing the UWRAP pre-assessment procedures, and answering detailed questions about current suicidal ideation and their mental health treatment history. Following these assessments, the UWRAP post-assessment procedures were completed, including a suicide risk assessment, as indicated. Participants were then notified about whether they met study eligibility requirements and were scheduled for the in-person session (if eligible) or provided with referral information (if ineligible). Finally, more detailed demographic information was obtained.
Study 2
Participants & Recruitment.
Participants for the second study are 135 adults recruited from the U.S. via online advertisements (e.g. Reddit.com, Craigslist.com). Advertisements were designed to recruit participants who were currently experiencing suicidal ideation and engaged in heavy episodic drinking (i.e. “Are you suicidal and drink alcohol to cope?”). Data reported include those from individuals who provided initial consent.
Procedures.
Study procedures were reviewed and received institutional review board approval. As part of study eligibility screening, interested individuals who contacted the researchers completed a phone screening interview with a trained assessor. After providing verbal consent to the phone screening assessment, participants (n=153) provided basic demographic information related to study eligibility criteria. Individuals who were under 18 years old, did not have Internet, were unwilling to discontinue psychological treatment for alcohol use or emotional problems, and/or unable to read or understand written English were notified that they did not meet initial eligibility requirements for participation. These individuals were provided with referral information and the phone screening was discontinued (n=18). Individuals who met initial eligibility criteria (n=135) continued through the remainder of the phone screening by providing contact information, completing the UWRAP pre-assessment procedures, and answering detailed questions about current suicidal ideation, alcohol use, emotion regulation capabilities, and their mental health treatment history. Following these assessments, the UWRAP post-assessment procedures were completed, including a suicide risk assessment, as indicated. Participants were then notified about whether they met study eligibility requirements and were emailed or mailed an informed consent to be returned (if eligible) or provided with referral information (if ineligible).
Measures
Demographics Data Schedule.
Participant demographic information was obtained using the Demographic Data Schedule – Short Version (DDS; Linehan, 1982) which collects a variety of demographic information such as sex, age, ethnicity, race, marital status, income, educational level, and occupation. The DDS has demonstrated high concurrent validity to responses from hospital chart data for a sample of psychiatric inpatients (Linehan, 1982).
The University of Washington Risk Assessment Protocol.
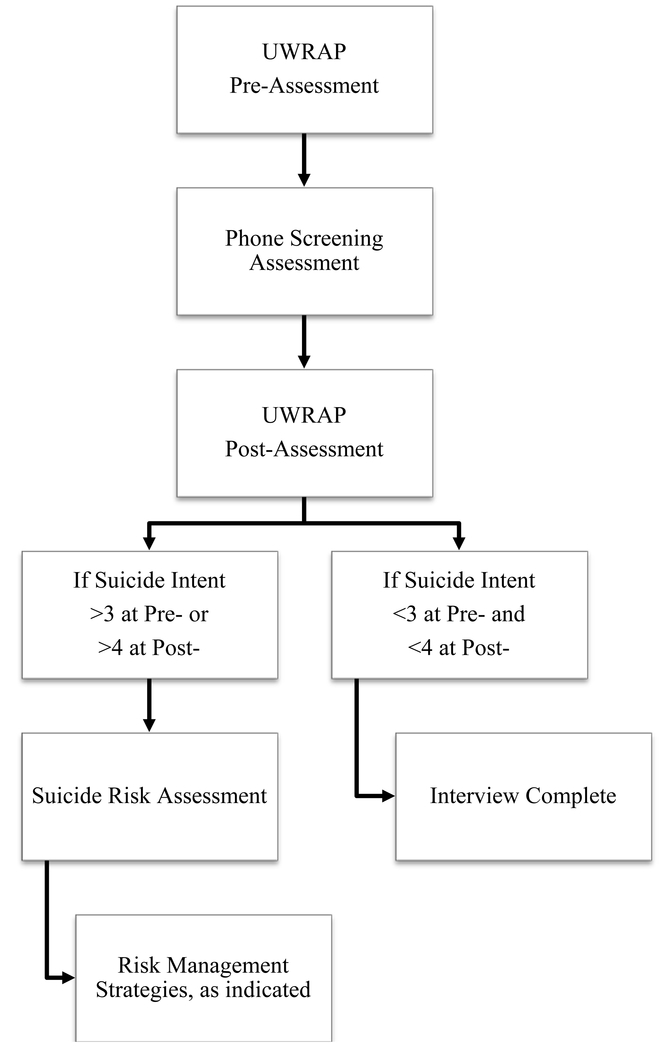
The UWRAP (Linehan et al., 2012) is a structured method to assess and manage suicide risk in the context of research assessments. The UWRAP has been developed for treatment outcome studies for use by masked assessors to manage risk without involving the study therapist. At the beginning of the assessment (i.e., UWRAP pre-assessment), participants are asked whether they have ingested medications, drugs, and/or alcohol, and whether they believe that the substances would affect their performance on the assessment. In addition, participants are asked to rate their level of stress, urge for self-harm, suicide intent, and urge to use drugs or alcohol on a 1–7 point Likert scale (1 = low, 7 = high). Prior to beginning the assessment, participants are queried on how they can manage distress throughout the interview, which the interviewer documents and may implement if participant distress is high. At the end of the assessment (i.e., UWRAP post-assessment), participants are re-assessed on the aforementioned dimensions of stress level, self-harm urge, suicide intent, and drugs/alcohol use urge. At the post-interview assessment, a response on the question, “what is your intent to kill yourself?” of 4 or higher, an increase by 3 or more points (i.e. 1/7 at pre and a 4/7 at post), or a report that they are unsure of their ability to control suicidal urges prompts the assessor to implement a more thorough suicide risk assessment protocol. Regardless of suicide risk, assessors complete a mood improvement protocol, which includes questions such as, “how will you cope with any of the negative emotions that have been prompted by this assessment?” and “do you have any fun plans today?” For those requiring additional suicide risk assessment (SRA), assessors further assess risk by asking more detailed questions about suicide plans, methods, preparation, and other imminent risk factors and then provide appropriate interventions ranging from providing additional support via validation or distraction to using emergency services to get the participant to their nearest emergency department. See Figure 1 for a visual flow of the UWRAP procedures.
Figure 1.
Flow of University of Washington Risk Assessment Protocol in research
Statistical Analysis Strategy
To determine changes in participants’ level of stress as well as urges for self-harm, suicide, and drug and alcohol use pre- to post assessment, we used a paired sample t-tests with a 95% confidence interval. We used Cohen’s d to calculate effect size for the within subject design and was calculated using the following formula: .
Results
On average, participants identified as mostly male (53.3%), Caucasian (89.1%), and single (61.8%). See Table 1 for full participant demographics.
Table 1.
Participant demographics by study
| Study1 | Study 2 | Total | |||
|---|---|---|---|---|---|
| Demographic Variable | M (SD) | # (%) | M (SD) | # (%) | |
| Age | 39.57 (14.84) | 39.03 (11.37) | 39.39 (13.68) | ||
| Sex (% Male) | 110 (51.9) | 67 (56.3) | 177 (52.8) | ||
| Hispanic/Latino | 11 (5.0) | 9 (7.6) | 20 (6.4) | ||
| Race | |||||
| Caucasian | 164 (78.1) | 82 (68.9) | 246 (80.1) | ||
| African American | 23 (11.0) | 12 (10.1) | 35 (10.4) | ||
| Asian | 10 (4.7) | 1 (1.0) | 11 (3.6) | ||
| Other | 13 (6.2) | 2 (0.1) | 15 (4.9) | ||
| Highest level of education | |||||
| Less than high school | 21 (9.8) | 6 (6.1) | 27 (8.6) | ||
| High school | 39 (18.1) | 17 (17.2) | 56 (17.8) | ||
| Some college or tech school | 89 (41.4) | 40 (40.4) | 129 (41.1) | ||
| College graduate | 48 (22.3) | 29 (29.3) | 77 (24.5) | ||
| Grad school or higher | 18 (8.4) | 7 (7.1) | 25 (8.0) | ||
| Income in last year | |||||
| Less than $5,000 | 64 (31.1) | 22 (23.1) | 86 (28.5) | ||
| $5,000–9,999 | 39 (18.8) | 11 (11.6) | 50 (16.6) | ||
| $10,000–14,999 | 37 (17.9) | 11 (11.6) | 48 (15.9) | ||
| $15,000–19,999 | 22 (10.6) | 12 (12.6) | 34 (11.3) | ||
| $20,000–24,999 | 10 (4.8) | 13 (13.7) | 23 (7.6) | ||
| $25,000–29,999 | 6 (2.9) | 4 (4.2) | 10 (3.3) | ||
| $30,000–49,999 | 23 (11.1) | 14 (14.7) | 37 (12.3) | ||
| Over $50,000 | 6 (2.9) | 8 (8.4) | 14 (4.6) | ||
Upon starting the assessment, 42.7% of participants endorsed using drugs, alcohol, and/or medications immediately prior to the assessment; however, only 4.2% of those who endorsed medication, drugs, and/or alcohol use indicated that they believed this use would interfere with their performance, at which point we discontinued the interview and rescheduled for another time. At the beginning of the assessment, the average level of stress was considered moderate (M=4.69, SD=1.64), while urge to engage in self-harm (M=2.78, SD=1.72) and intent to kill self (M=2.13, SD=1.56) were considerably lower on average. The sample’s average urge to use drugs or alcohol was also considered moderately high at the beginning of the assessment (M=4.08, SD=2.28). On average, all subjective reports of stress, self-harm, suicide intent, and urges to use drugs and alcohol decreased after the assessment (see Table 2). Results from a set of paired samples t-test showed that, on average, participants’ scores decreased significantly on stress t[259] = 5.60, p < .001, 95% CI [0.35, 0.73], albeit with a small effect size (d = .35), urge for self-harm, t[262] = 7.852 p < .001, 95% CI [0.49, 0.83], with a medium effect (d = .48), intent to kill self t[262] = 6.00, p < .001, 95% CI [0.30, 0.59], with a small effect (d = .37), and urge to use drugs and alcohol, t[261] = 3.91, p < .001, 95% CI [0.19, 0.57], with a small effect (d = .24).
Table 2.
UWRAP pre- and post-assessment means and standard deviations
| Study 1 | Study 2 | Total | ||||
|---|---|---|---|---|---|---|
| Pre Assessment |
Post Assessment |
Pre Assessment |
Post Assessment |
Pre Assessment |
Post Assessment |
|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Level of Stress | 4.57 (1.64) | 4.34 (1.67) | 4.80 (1.72) | 3.92 (1.93) | 4.68 (1.68) | 4.15 (1.80) |
| Urge to harm self | 2.91 (1.76) | 2.43 (1.58) | 2.73 (1.79) | 1.74 (1.24) | 2.82 (1.77) | 2.12 (1.48) |
| Intent to kill self | 2.23 (1.63) | 1.88 (1.31) | 2.12 (1.61) | 1.45 (1.03) | 2.18 (1.62) | 1.68 (1.21) |
| Urge to use drugs or alcohol | 3.25 (2.25) | 3.11 (2.22) | 5.12 (1.90) | 4.43 (2.21) | 4.17 (2.28) | 3.71 (2.31) |
Note. Scores on each domain range from 1 (low) to 7 (high).
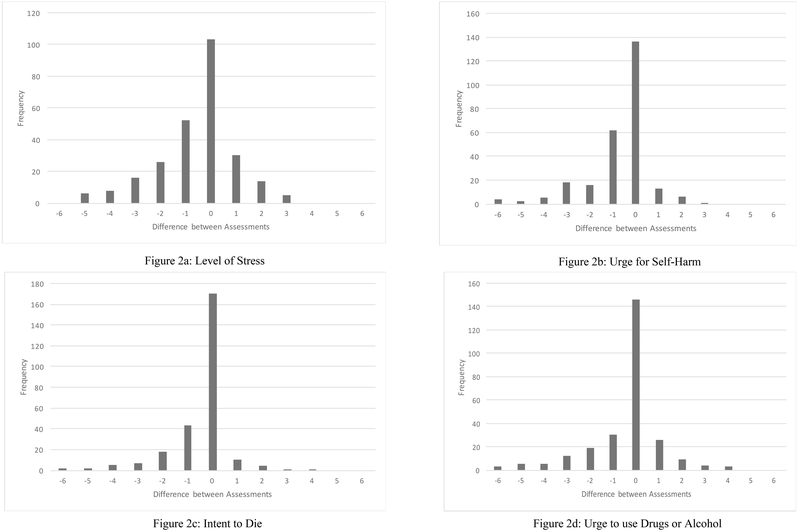
To address the third aim, we also examined what proportion of individuals reported an increase in their levels of stress, self-harm and alcohol/drug use urges, with an emphasis on suicide intent. While the majority of individuals reported the same or lower levels of distress than in the beginning of the assessment, 18.4% reported increased stress, 7.0%, increased urges for self-harm, 5.5% increased suicide intent, and 15.7% increased urges to use drugs or alcohol (see Table 3 and Figures 2a–2d). At the end of the assessment, 4.2% of participants required further assessment and crisis management using the suicide risk assessment (SRA) procedures by the interviewer or supervising clinician. In the event that participants endorsed an elevated urge or prolonged urge for suicide (i.e., increase by 3 or higher than 4 out of 7), assessors were prompted to engage in various strategies from the SRA and would reassess risk. The SRA portion of the UWRAP includes suicide management strategies ranging from “validated thoughts and feelings,” to “arranged hospitalization.” In the event that risk was uncertain, assessors were instructed to consult with licensed clinical psychologists. After completing the SRA portion from the UWRAP, the most common management solution was to provide support and/or validate the participant’s feelings (which included saying things such as, “That was really long; I’m tired too”), talking about other topics until the participant felt more emotionally regulated, and focusing on coping strategies the participant can use or has used in the past in similar situations. When participants were reassessed, most participants denied suicidal intent or plan, but rather endorsed global thoughts of death and dying. No emergency services were contacted (i.e., involuntary hospitalization, police visits).
Table 3.
Percent increase, decrease, and no change from pre- to post-assessment
| % increase | % decrease | % no change | |
|---|---|---|---|
| Level of Stress | 18.4 | 40.6 | 41.0 |
| Urge to harm self | 7.0 | 41.4 | 51.6 |
| Intent to kill self | 5.5 | 64.1 | 30.5 |
| Urge to use drugs or alcohol | 15.7 | 28.2 | 56.1 |
Note. See Figures 2a–2d for corresponding visualization of change between assessments
Figures 2a-2d.
Graphs showing the difference between UWRAP pre- and post-assessments.
Discussion
The current study sought to describe the implementation of an assessment and management protocol with individuals for suicide-related research. In general, participants reported significant reductions in their levels of stress and their urges to self-harm, die by suicide, and use drugs or alcohol. In addition, only a small proportion of individuals reported an increase in their urge to self-injure or level of suicide intent. Therefore, suicide risk was managed by low-level interventions such as providing support and validation, which was sufficient to address the varying levels of risk. While the suicide management strategies were minimal, the management protocol allowed for more intensive intervention including involving friends or family members and arranging hospitalization, although it is notable that none of these strategies were needed.
Recently a meta-analysis was published which punctuated that asking about suicide does not increase suicide risk, nor does assessment in general increase suicidal urges among individuals at risk for suicide (Blades, Stritzke, Page, & Brown, 2018). Indeed, our results add to the mounting research that suggests it is possible to safely assess and manage suicide risk in high-risk research with community samples. While this should be enough to appease IRBs to approve research on individuals at risk for suicide, there is the possibility that some individuals may come away from a screening encounter feeling stronger urges to die than before. As such, implementing a strong-tested protocol to manage varying levels of risk is the silver bullet for IRB concerns.
These findings have important implications for suicide researchers and institutional review boards. While individuals and institutions are understandably cautious about research involving suicidal participants (Hom et al., 2017), the expanding body of literature suggests that evidence-based protocols to assess and manage suicide risk can be flexibly applied across a variety of research contexts. In particular, the UWRAP has demonstrated utility in clinical research trials as well as community-based suicide research (e.g., Reynolds et al., 2006). We hope that this assuages concerns regarding the safety of suicide research that employs evidence-based risk assessment and management protocols. Researchers can reference applications of the UWRAP relevant to their study design when providing rationalization for their study protocols to institutional review boards.
Limitations of these findings warrant discussion. We present the results of two separate studies that recruited suicidal participants from the community who underwent phone-screening assessment. While our intention is to present applications of the UWRAP procedures in community-based data collection, one limitation of the current paper is that it is possible that conducting these assessments by phone had an impact on participants’ willingness to candidly disclose their current levels of stress and urges for self-harm. To our knowledge, there is no prior research examining differences in willingness to disclose suicidality in a research context via phone versus in-person; however, it is possible that participants may have believed they were expected to “feel better” by the end of the assessments or they may have had alternate motivations to report more positive ratings (e.g., wanting to end the call). Since this potential “pressure” to report improvement at the end of the interview would also likely impact in-person ratings of distress, future research is needed to examine the effect of different forms of research-related risk assessment formats on participant disclosure and on the potential role of demand characteristics on participants’ self-report.
Additionally, the variety of procedures researchers may be interested in employing in suicide-focused studies is vast. The results we present here involved eligibility screening interviews required before enrolling participants into randomized trials of experimental interventions. This introduces two additional limitations related to generalizability. First, the types of individuals who responded to study advertisements may not represent the entire population of suicidal participants who had either not engaged in mental health treatment (Study 1) or were drinking to cope with emotions (Study 2). Similar to our limited understanding of the differences between people who do and do not seek mental health treatment during suicidal crises, there is an important, yet unanswered, question about the qualitative and quantitative differences between suicidal individuals who do and do not participate in research when they are suicidal. More research is needed to understand the extent to which these two groups are related. Second, the screening interviews for both studies were relatively short and limited in scope. Thus, it is unclear to what extent the length of the interviews would affect participants’ ratings of distress and suicide-related urges. Although prior research using longer, more in-depth interviews has yielded similar results to those obtained here (e.g., Eynan et al., 2014), additional research that involves longer assessment interviews would help elucidate the effect of longer interviews on these domains. Furthermore, a longitudinal follow-up examination would elucidate any lasting effects in the subgroup of participants who reported stress and/or urges.
Despite these limitations, this study provides additional evidence that it is possible to safely and responsibly assess and manage suicide risk within the research context. In particular, our study expands previous research that has primarily focused on clinical or student samples to demonstrate that the same protocols can effectively manage suicide risk in a wider variety of research contexts. Not only is it possible to conduct suicide research responsibly, participants generally report a decrease in their stress and suicide urges following completion of suicide-related assessment. To have a significant effect on the continually rising suicide rate in the United States, more research on the etiology and treatment of suicide is needed. With resources like the UWRAP available for flexible use in suicide research, our hope is that this will increase interest and willingness to study suicide for both researchers and review boards.
Acknowledgments
Funding Sources
This work was supported by National Research Service Awards from the National Institute of Mental Health (F31 MH095257; PI: Ward-Ciesielski) and the National Institute on Alcohol Abuse and Alcoholism (F31 AA 24658-01; PI: Wilks); a Graduate Research Scholarship from the American Psychological Foundation/Council of Graduate Departments of Psychology/Friedman-Klarreich Family Foundation (PI: Ward-Ciesielski); Dissertation Grant Awards from the American Psychological Association and Division 12 of the American Psychological Association (PI: Ward-Ciesielski); and a pilot grant from the Alcohol Drug Abuse Institute (ADAI-0315-2, PI: Wilks).
Footnotes
The clinical trial methods from each study have been thoroughly described elsewhere (study 1: Ward-Ciesielski, Tidik, Edwards, & Linehan, 2017; study 2: Wilks, Lungu, Ang, Matsumiya, Yin, & Linehan, 2018)
For the first seven months of study recruitment, inclusion criteria required no mental health treatment during the previous year; however, when recruitment was progressing significantly slower than anticipated, this criterion was modified to require no mental health treatment in the previous month.
References
- American Psychiatric Association. (2003). Practice guideline for the assessment and treatment of patients with suicidal behaviors. Retrieved from https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/suicide.pdf [PubMed]
- Biddle L, Cooper J, Owen-Smith A, Klineberg E, Bennewith O, Hawton K, … Gunnell D (2013). Qualitative interviewing with vulnerable populations: Individuals’ experiences of participating in suicide and self-harm based research. Journal of Affective Disorders, 145(3), 356–362. 10.1016/j.jad.2012.08.024 [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, ... & Czajkowski S (2004). Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23(5), 443. [DOI] [PubMed] [Google Scholar]
- Blades CA, Stritzke WG, Page AC, & Brown JD (2018). The benefits and risks of asking research participants about suicide: A meta-analysis of the impact of exposure to suicide-related content. Clinical psychology review. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2018). CDC Vital Sighs: Suicide rising across the US. Retrieved June 19, 2018 from https://www.cdc.gov/vitalsigns/pdf/vs-0618-suicide-H.pdf
- De Leo D, Draper BM, Snowdon J, & Kõlves K (2013). Contacts with health professionals before suicide: Missed opportunities for prevention? Comprehensive Psychiatry, 54(7), 1117–1123. 10.1016/j.comppsych.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Deeley ST, & Love AW (2010). Does asking adolescents about suicidal ideation induce negative mood state? Violence and Victims, 25(5), 677 10.1891/0886-6708.25.5.677 [DOI] [PubMed] [Google Scholar]
- Eynan R, Bergmans Y, Antony J, Cutcliffe JR, Harder HG, Ambreen M, … Links PS (2014). The effects of suicide ideation assessments on urges to self-harm and suicide. Crisis, 35, 123–133. 10.1027/0227-5910/a000233 [DOI] [PubMed] [Google Scholar]
- Fisher CB, Pearson JL, Kim S, & Reynolds CF (2002). Ethical issues in including suicidal individuals in clinical research. IRB: Ethics & Human Research, 24, 9–14. 10.2307/3563804 [DOI] [PubMed] [Google Scholar]
- Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, … Nock MK (2017). Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychological Bulletin, 143(2), 187–232. 10.1037/bul0000084 [DOI] [PubMed] [Google Scholar]
- Hom MA, Podlogar MC, Stanley IH, & Joiner TE (2017). Ethical issues and practical challenges in suicide research: Collaboration with institutional review boards. Crisis, 2(38), 107–114. 10.1027/0227-5910/a000415 [DOI] [PubMed] [Google Scholar]
- Jorm AF, Kelly CM, & Morgan AJ (2007). Participant distress in psychiatric research: A systematic review. Psychological Medicine, 37(7), 917–926. 10.1017/S0033291706009779 [DOI] [PubMed] [Google Scholar]
- Lakeman R, & FitzGerald M (2009). The ethics of suicide research: The views of ethics committee members. Crisis, 30(1), 13–19. 10.1027/0227-5910.30.1.13 [DOI] [PubMed] [Google Scholar]
- Law MK, Furr RM, Arnold EM, Mneimne M, Jaquett C, & Fleeson W (2015). Does assessing suicidality frequently and repeatedly cause harm? A randomized control study. Psychological Assessment, 27(4), 1171 10.1037/pas0000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM (1982). The demographic data schedule (DDS). Unpublished Manuscript, University of Washington. [Google Scholar]
- Linehan MM (1997). Validation and psychotherapy. Empathy reconsidered: New directions in psychotherapy, 353, 392. [Google Scholar]
- Linehan MM, Comtois KA, & Ward-Ciesielski EF (2012). Assessing and Managing Risk With Suicidal Individuals. Cognitive and Behavioral Practice, 19(2), 218–232. 10.1016/j.cbpra.2010.11.008 [DOI] [Google Scholar]
- Luoma JB, Martin CE, & Pearson JL (2002). Contact with mental health and primary care providers before suicide: A review of the evidence. American Journal of Psychiatry. 159(6), 909–916. 10.1176/appi.ajp.159.6.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishara BL, & Weisstub DN (2005). Ethical and legal issues in suicide research. International Journal of Law and Psychiatry, 28(1), 23–41. 10.1016/j.ijlp.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Reynolds SK, Lindenboim N, Comtois KA, Murray A, & Linehan MM (2006). Risky assessments: Participant suicidality and distress associated with research assessments in a treatment study of suicidal behavior. Suicide and Life-Threatening Behavior, 36(1), 19–34. 10.1521/suli.2006.36.1.19 [DOI] [PubMed] [Google Scholar]
- Ribeiro JD, Gutierrez PM, Joiner TE, Kessler RC, Petukhova MV, Sampson NA, … Zaslavsky AM (2017). Health care contact and suicide risk documentation prior to suicide death: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Journal of Consulting and Clinical Psychology, 85(4), 403–408. 10.1037/ccp0000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi JM, Shiner B, & Watts BV (2014). Inclusion and exclusion criteria in randomized controlled trials of psychotherapy for PTSD. Journal of Practical Psychiatry and Behavioral Health, 20, 25–37. doi: 10.1097/01.pra.0000442936.23457.5b [DOI] [PubMed] [Google Scholar]
- Silverman MM, & Berman AL (2014). Suicide risk assessment and risk formulation part I: A focus on suicide ideation in assessing suicide risk. Suicide and Life-Threatening Behavior, 44(4), 420–431. 10.1111/sltb.12065 [DOI] [PubMed] [Google Scholar]
- Stanley B, & Brown GK (2012). Safety planning intervention: a brief intervention to mitigate suicide risk. Cognitive and Behavioral Practice, 19(2), 256–264. [Google Scholar]
- Ward-Ciesielski EF, Tidik JA, Edwards AJ, & Linehan MM (2017). Comparing brief interventions for suicidal individuals not engaged in treatment: A randomized clinical trial. Journal of Affective Disorders, 222, 153–61. 10.1016/j.jad.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J, Pietrusza C, & Purington A (2013). Young adult respondent experiences of disclosing self-injury, suicide-related behavior, and psychological distress in a web-based survey. Archives of Suicide Research, 17(1), 20–32. 10.1080/13811118.2013.748405 [DOI] [PubMed] [Google Scholar]
- Wilks CR, Zieve GG, & Lessing HK (2016). Are trials of computerized therapy generalizable? A multidimensional meta-analysis. Telemedicine and e-Health, 22(5), 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks CR, Lungu A, Ang SY, Matsumiya B, Yin Q, & Linehan MM (2018). A randomized controlled trial of an Internet delivered dialetical behavior therapy skills training for suicidal and heavy episodic drinkers. Journal of Affective Disorders, 232, 219–228. 10.1016/j.jad.2018.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CM, & Christensen BK (2012). Ethical issues relevant to the assessment of suicide risk in nonclinical research settings. Crisis, 33(1), 54 10.1027/0227-5910/a000110 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2018). Suicide. Retrieved August 31, 2018 from https://www.who.int/news-room/fact-sheets/detail/suicide
- Zimmerman M, Multach MD, Clark HL, Walsh E, Rosenstein LK, & Gazarian D (2017). Inclusion/exclusion in late life depression antidepressant efficacy trials. International Journal of Geriatric Psychiatry, 32, 1009–1016. doi: 10.1002/gps.4560 [DOI] [PubMed] [Google Scholar]