Abstract
The understanding of microvascular dysfunction (MVD) without evidence of epicardial coronary artery disease (CAD) pales in comparison to the understanding of obstructive epicardial CAD. A primary limitation in the past had been the lack of development of noninvasive methods of detecting and quantifying MVD. This limitation has particularly affected our ability to study the pathophysiology, morbidity, and treatment of this disease. More recently, almost all of the non-invasive cardiac imaging modalities have been used to quantify blood flow and advance our understanding of MVD.
Keywords: microvascular dysfunction, quantitative perfusion, cardiac magnetic resonance imaging, positron emission tomography, computed tomography, echocardiography
The current understanding of the symptoms, prognosis, and treatment strategies in ischemic heart disease resulting primarily from abnormalities of the coronary microcirculation pales in comparison to that of epicardial coronary artery stenosis. Contemporary consensus statements and guidelines direct assessment and treatment of epicardial coronary artery disease and resultant myocardial ischemia in a variety of clinical situations [1,2,3]. With both invasive and noninvasive testing, the identification of epicardial ischemic disease is better established than for the detection of microvascular disease. Beneficial treatment strategies are available for epicardial stenosis whose significance can be fully evaluated by direct angiographic visualization with functional assessment determined by either perfusion imaging or invasive techniques to obtain fractional flow reserve (FFR), coronary flow reserve (CFR) or indices of microvascular resistance (IMR)[4,5,6]. Recognizing microvascular dysfunction (MVD) has required significant advancements in technology before reliable and reproducible measurements could be made so that prognostic and therapeutic trials could be pursued [7,8]. There is a growing interest in diagnosing and treating MVD as more patients are diagnosed with angina without obstructive coronary disease (ANOCA) or ischemia without obstructive coronary disease (INOCA). Furthermore, MVD may play a vital role in the pathophysiological mechanisms of certain diseases such as heart failure with preserved ejection fraction or takotsubos cardiomyopathy[9; 10]. The diagnosis of MVD is often challenging and can exist in the setting of obstructive epicardial disease and non-obstructive disease[11]. In this manuscript, we will provide a comprehensive review of the multiple imaging modalities that assess coronary MVD in the absence of obstructive epicardial disease.
Defining Coronary Microvascular Dysfunction
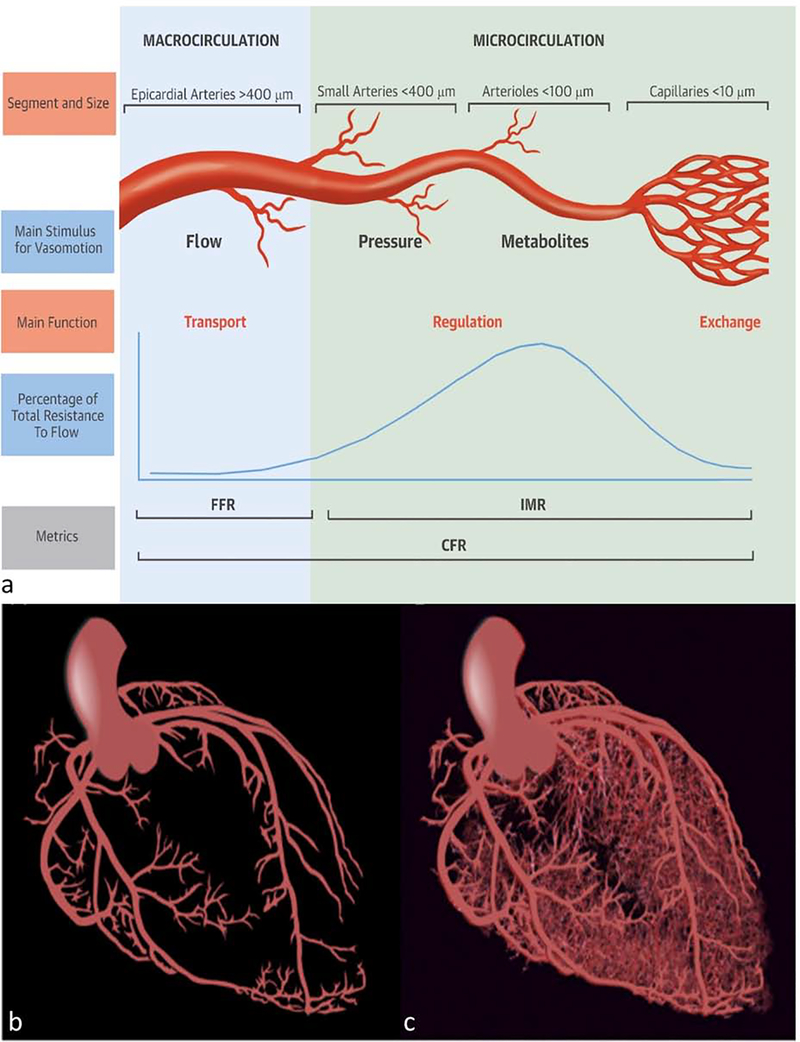
Before understanding the underlying technicalities required to quantify MVD, baseline knowledge of the functional anatomy of the coronary circulation is required (Figure 1). There are three components of the coronary arterial vasculature that are subdivided by the size of the arterial structure, its capacitance, and its resistance to myocardial blood flow (MBF)[12]. The initial component is the epicardial coronary arterial tree (5mm to 400μm in diameter), which have a near negligible coronary resistance in the absence of stenosis and are essentially conductive vessels. The prearteriole vessels follow (100 to 400μm in size); these are still largely extramyocardial and primarily respond to flow and intravascular pressure to deliver a narrow pressure range to the arterioles [12]. The third and distal component is the intramural arterioles (40 to 100μm), which have the primary responsibility of matching blood supply to myocardial oxygen consumption. The distal capillary and venule systems are low resistance capacitance vessels, holding up to 90% of the total intramyocardial blood volume [13]. The pressure gradient between the aortic root and the right atrium is the primary driving force of flow across the myocardium[14]. MBF is defined as the amount of flow through the coronary vessels over time, and is typically expressed as blood flow per gram of myocardium [12].
Figure 1: Coronary circulation schematic.
Components of the coronary circulation (a). Schematic of the macrocirculation (b) and microcirculation(c). FFR-fractional flow reserve, IMR- index of microvasculatory resistance, CFR-coronary flow reserve.
(Permission obtained and adapted from De Bruyne, B et al. Microvascular (Dys)Function and Clinical Outcome in Stable Coronary Disease J Am Coll Cardiol. 2016; 67(10):1170–1172. and Taqueti VR, et al. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018 Nov 27;72(21):2625–2641.)
Under normal conditions, there are elegant mechanisms of autoregulation in the prearteriolar and arteriolar microcirculation that allow for stable coronary blood flow across a large range of perfusion pressures [12,15]. For example, in the setting of hypotension, the driving pressure would decrease and thenautoregulatory mechanisms would subsequently decrease microvascular resistance to attempt to maintain adequate blood flow [14]. There are multiple autoregulatory mechanisms aimed atmanipulating arterial tone. There is myogenic constriction of the distal prearterioles in response to increased pressure. Arterioles can decrease or increase their diameter in response to flow changes, leading to shear stress which induces dilation of larger conductive vessels. In addition, arterioles can regulate blood flow in response to metabolites formed when myocardial oxygen demand increases [16].
In the setting of coronary MVD, there can be a disruption of these adaptive mechanisms, which can be assessed via various noninvasive provocative tests. One such test is sympathetic stimulation using cold pressor testing. The sympathetic response from a cold stimulus will increase myocardial work and thus a proportionate increase in MBF by metabolically-initiated endothelium-related vasodilator forces in the microvasculature [14,17]. Inotropic stimulation with dobutamine can increase myocardial blood flow via increased myocardial oxygen demand[14]. However, these methods are not commonly used and therefore the Coronary Vasomotion Disorders International Study Group created a consensus statement summarizing recommended invasive and noninvasive methods for detecting endothelial-dependent and endoethelial-independent MVD [18]. Many of these noninvasive methods will be discussed within this review. The most common noninvasive method to assess MVD is to administer a vasodilating compound such as intravenous (IV) adenosine to cause maximal hyperemia. It should be noted that this is not a specific test of endothelial function, as adenosine is not an endothelial-mediated vasodilating compound. However, there may be some indirect endothelial activation via shear stress from an increase in the rate-pressure product by the systemic effects of IV adenosine when comparing to intracoronary administration of adenosine[19]. MVD is a broad based term; in the absence of obstructive coronary disease, it includes any pathology that may disrupt the microvasculature, including endothelial dysfunction, coronary spasm, inflammation and atherosclerosis [20]. Changes in arteriolar diameter or microvascular rarefaction with diffuse fibrosis, such as in HFpEF, has been associated with MVD[21, 22]. A reduction in the ratio between maximal hyperemic coronary flow and baseline coronary flow indicates a reduced coronary flow reserve (CFR). CFR can vary based on sex, age, and the modality used for measurement [23,24]. Importantly, CFR is a composite measure of epicardial stenosis severity and microvascular dysfunction; however in the setting of normal epicardial coronaries, an abnormal CFR represents MVD [12,25]. The value that represents an abnormal CFR has differed between studies with values ranging from 1.5 to 2.5 have been used in prognostic studies [20,26,27].
Traditionally, the term CFR has referred to the invasive measurement of flow reserve, while noninvasive measuring methods, as will be discussed in this review, refer to CFR as myocardial perfusion reserve (MPR). This distinction occurs as the measurement is made through changes in myocardial perfusion rather than direct measurement at the level of the coronaries. There are other potentially important imaging parameters also being studied such as microcirculatory blood volume or microvascular blood volume and intramyocardial blood volume[28,29,30]. It has been shown that changes in these parameters or reduction in these parameters can be associated with coronary MVD[31].
Quantifying MBF: Arterial Input Function and Compartmental Kinetics
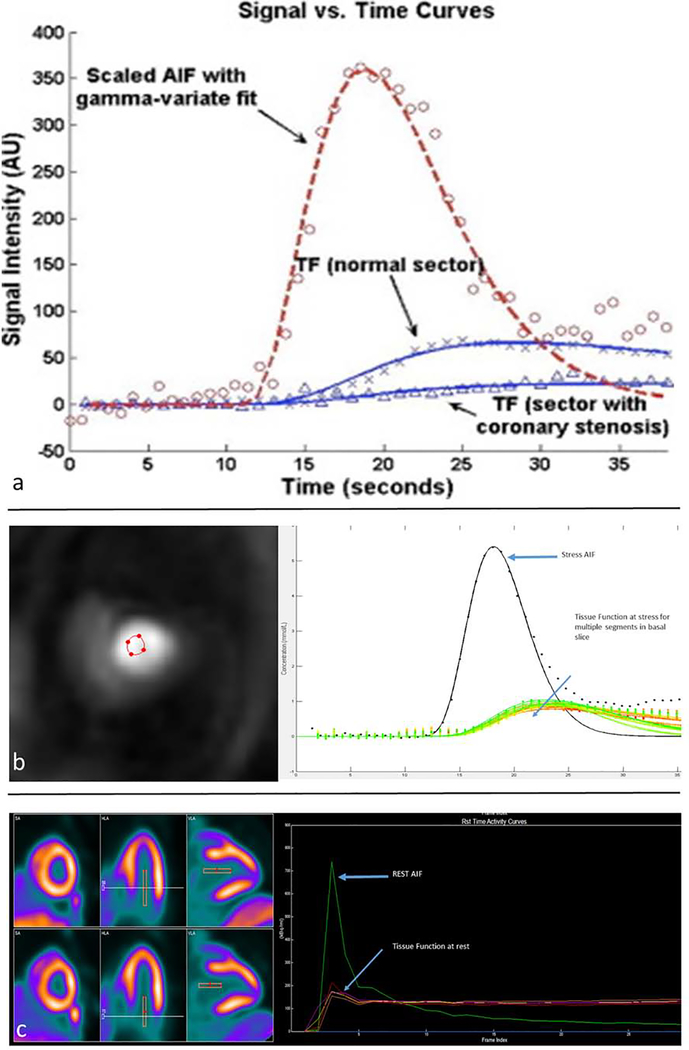
Quantifying MPR with techniques such as PET, first-pass perfusion CMR, and CT perfusion require models that describe the kinetics of the contrast agent as a function of time. The concentration-time curves at rest and with vasodilation can vary due to variables such as contrast bolus timing, contrast injection speed, and cardiac output. These parameters must be accounted for to avoid significant variability in the absolute quantification of MBF. The arterial input function (AIF) is a time-dependent radiotracer or contrast concentration input into the tissue of interest that accounts for variables related to the injection of the contrast or radiotracer that can effect MBF quantification [32]. For absolute quantification of MBF, the AIF can be measured in the left ventricular (LV) cavity or in the left atrium (Figure 2). With kinetic modeling, the concentration of tracer or contrast in the myocardium, also known as the tissue function (TF) is obtained using the AIF and an established kinetic compartmental model to allow fitting of concentration-time curves [33,34].
Figure 2: Time Intensity Curves.
Schematic of time intensity curves for the arterial input function (AIF) and the tissue function (TF) in a normal coronary segment and an abnormal coronary segment (a). CMR obtained stress AIF and TF signal intensity curves (b). PET stress/rest perfusion imaging with resulting AIF and myocardial time intensity curves (c).
(Permission obtained and adapted from Patel, AR et al. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol. 2010; 56(7):561–9.)
Along with the AIF, compartmental modeling, which mathematically describes the kinetics of a contrast agent or radiotracer in a biological tissue of interest, is commonly used to accurately quantify MBF. With radiotracers, different tracers demonstrate different behaviors and each tracer is associated with a particular compartmental model [35]. For example, a two compartmental model (blood and tissue compartments) is typically used for the radiotracers 201Thallium and 13N-Ammonia. Therefore, variability in radiotracer kinetics, along with other specified characteristics to be discussed later, are important to obtain an accurate MPR. Similar methods of compartmental modeling and contrast kinetics are applicable in cardiac magnetic resonance imaging (CMR) derived MPR.
Additionally, blood flow can be derived through several methods without the use of compartmental modeling. Zieler’s central volume principle has been used in CMR as a non-compartmental based method for MBF quantification [36]. It enables quantification of MBF using a simple deconvolution operation using assumptions made from myocardial tracer residue curves and AIF[37]. In addition, other methods that do not require compartmental modeling (discussed below) include the use of freely diffusible tracers such as water in the case of arterial spin labeling (ASL) for CMR and the use of intravascular microbubbles in contrast echocardiography[38,39].
Multimodality Noninvasive Assessment of MVD
Echocardiography
In 1998, Wei et al demonstrated a novel method to quantify both MBF using contrast echocardiography (CE) with the use of a constant venous infusion of air-filled albumin microbubbles [38]. The ability to quantify perfusion was based on two characteristics of the microbubble contrast agent. The “new” generation of microbubbles contained a higher molecular weight gas and thus was non-diffusible and less soluble, allowing for myocardial opacification [40,41]. Secondly, the microbubbles could be destroyed with ultrasound[42]. These properties allowed calculation of MBF by obtaining the mean velocity of myocardial microbubbles and the microvascular cross sectional area. The mean velocity was obtained by measuring the rate of reappearance of microbubbles after destruction with ultrasound in the setting of a constant microbubble venous infusion. The cross sectional area was obtained by measuring the microbubble concentration in the myocardium and is essentially a CE measurement of the myocardial blood volume (MBV) [38]. This method was validated against positron emission tomography (PET) with a correlation coefficient of 0.88 when measuring MBF in healthy volunteers [43].
Potential benefits of the CE approach include that it is a low risk bedside procedure, relatively inexpensive, and has limited adverse effects. The potential adverse effects from contrast microbubbles are minimal, and there is no radiation exposure as compared to PET [44]. However a number of limitations have prevented its widespread use. Echocardiography is operator-dependent and demonstrates considerable intra-observer and inter-observer variability [45]. Echocardiography can be hindered by artifacts, particularly in the setting of obesity and lung disease. Another technical issue is movement of the imaging frame during replenishment of microbubbles, which can lead to difficulty with post-processing. The use of microbubbles for myocardial perfusion assessment is currently not reimbursed in the United States, further hampering its clinical adoption. Finally, these modalities have had success in the evaluation of obstructive disease or post-PCI microvascular assessment, but in the setting of normal coronaries and anginal symptoms they have not shown widespread clinical utility [46,47].
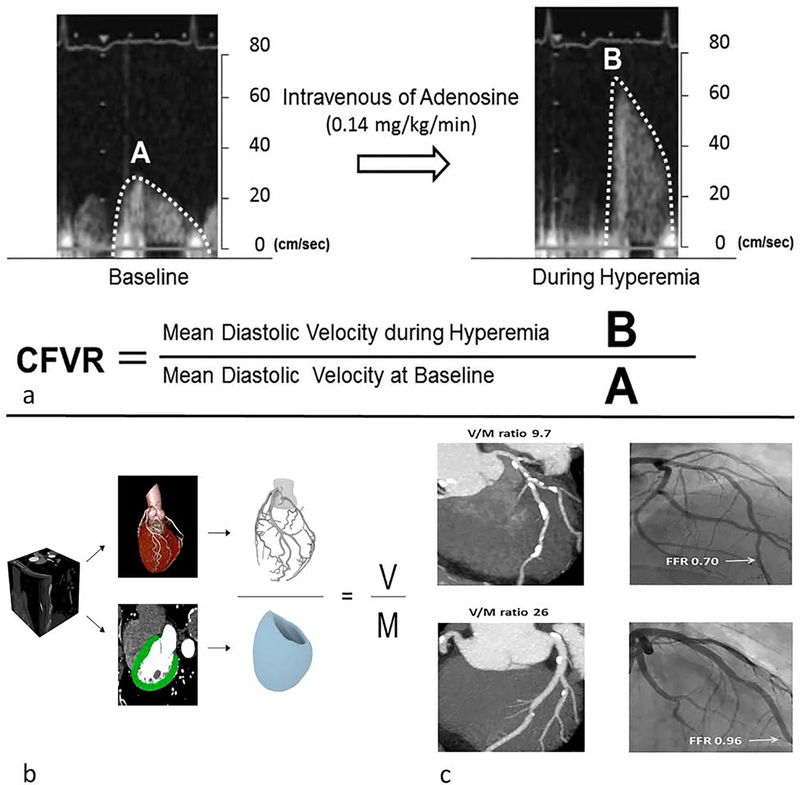
Another echocardiographic method of MBF assessment uses transthoracic Doppler echocardiography (TTDE) to calculate the coronary flow velocity reserve (CFVR) [48,49]. CFVR is obtained by the ratio of coronary flow velocity at stress and rest obtained by the use of pulse wave Doppler sampling of the proximal left anterior descending artery (Figure 3a). This method correlates well with flow acquired from an intracoronary Doppler wire [48,50]. In addition, abnormal CFVR is associated with adverse cardiovascular events [27,51]. However, TTDE CFVR measurement was poorly correlated (r=0.3) with MPR calculated by PET in an evaluation of women with angina and no obstructive coronary artery disease (CAD) [52]. In the prospective multicenter international PROMIS-HFpEF study, TTDE identified a high prevalence of MVD in patients with heart failure with preserved ejection fraction [53].
Figure 3: Echocardiography derived coronary flow volume reserve and computed tomography of derived coronary luminal volume to myocardial mass.
Using transthoracic doppler echocardiography to obtain mean diastolic velocities at rest and stress allows for the derivation of coronary flow volume reserve (a). Computational modeling allows for calculation of luminal volume to myocardial mass (V/M) ratio (b). Example of V/M ratio and fractional flow reserve in 2 patients with non-obstructive CAD (c). The patient with the reduced FFR has a corresponding reduced V/M ratio. CFVR-coronary flow volume reserve, V-luminal volume, M-myocardial mass, FFR-fractional flow reserve.
(Permission obtained from Kakuta, K et al. Comparison of Coronary Flow Velocity Reserve Measurement by Transthoracic Doppler Echocardiography With 320-Row Multidetector Computed Tomographic Coronary Angiography in the Detection of In-Stent Restenosis in the Three Major Coronary Arteries. Am J Cardiol. 2012;110(1):13–20. and Taylor CA, et al. Effect of the ratio of coronary arterial lumen volume to left ventricle myocardial mass derived from coronary CT angiography on fractional flow reserve. J Cardiovasc Comput Tomogr. 2017; 11(6):429–436.)
Computed Tomography
Computed tomography angiography (CTA) in combination with CT perfusion (CTP) has the potential to be a robust modality for the assessment of microvascular disease. Both coronary anatomical and myocardial perfusion information can be obtained in the same study [54]. There are two primary techniques utilized in CT perfusion (CTP) imaging: static CTP and dynamic CTP. Static CTP only requires a single image at peak myocardial contrast opacification, which is then compared to a single rest image, thus lowering the amount of radiation. However, only semi-quantitative or qualitative perfusion assessment can be performed with this technique. Therefore, dynamic CTP involves obtaining several sequential images over time from first pass to wash to allow for quantitative perfusion[55]. CTP imaging is performed using either retrospective or prospective ECG-triggered image acquisition for approximately 30 seconds after contrast injection both at rest and with vasodilator stress. These images are then analyzed using post-processing software to obtain AIFs and time attenuation curves for the quantification of MBF[56]. Even though this technology can potentially identify MVD, CTA does not have any significant advantages over other imaging modalities and is not currently used in regular clinical practice to assess MVD.
Further functional data regarding the hemodynamic effects of epicardial stenosis can be obtained without stress perfusion imaging through measurement of CTA-derived fractional flow reserve (FFRCT). Using proprietary software, HeartFlow (Redwood City, CA) derives a 3-dimensional coronary model with advanced mathematics to simulate maximal hyperemia and quantify MBF and FFRCT at a specified point in the coronary tree [57,58]. FFRCT showed significantly better diagnostic sensitivity when compared to single photon-emission computed tomography (SPECT) in stable CAD [59]. The relationship between FFRCT and MVD is not well defined. However, with the same computational mathematical modeling, HeartFlow can derive other potentially useful parameters for assessment of MVD. Interestingly, Nørgaard et al. showed that a low ratio of CTA-derived coronary luminal volume to myocardial mass (V/M) was an independent predictor of ischemia in non-obstructive coronary disease (Figure 3b and c)[60]. To expand on this concept, Grover et al. compared the V/M ratio of 30 patients with ESC guideline-defined microvascular angina [61] to 32 age-matched asymptomatic controls [62]. They showed that both the mean total coronary lumen volume and the mean myocardial mass were lower in the MVD cohort. The mean V/M ratio was also significantly lower in the MVD group (25.6 mm3/g ± 5.9 vs 30.0 mm3/g ± 6.5, p = 0.007) [62].
Although CTA can provide a comprehensive cardiac exam, it has limitations. Radiation exposure is high for a stress/rest perfusion CTA protocol, with similar effective radiation dose as a SPECT rest/stress protocol of 12.7mSv [63]. In addition, the risk of contrast-induced nephropathy restricts use of this technique in chronic kidney disease. There is data suggesting that iodinated contrast may cause vasodilation, leading to the overestimation of coronary blood flow [64,65].
Nuclear Imaging
Cardiac PET is the imaging modality most validated for the quantification of MBF and assessment of MVD [66]. There are several radiotracers used in PET imaging, each with unique characteristics. Ideally, a radiotracer would be freely diffusible with high first pass uptake, rapid clearance rate, insignificant roll off at elevated blood flows, and kinetics that are unaffected by extrinsic factors[44,67]. In addition, the radiotracer should be safe and without side effects and it should not affect flow hemodynamics. The most commonly used PET radiotracers are 13N-Ammonia, 82Rubidium, and 15O-water. 15O-water is an excellent agent for MBF calculation due to its exceptional first pass uptake of nearly 100% and minimal roll off at higher flows[68,69]. However, due to its low counts and short half-life of 122 seconds, visual assessment of perfusion abnormalities with 15O-water is extremely limited and not approved for clinical use in perfusion imaging by the FDA[70]. 13N-Ammonia has a longer half-life of 2.8 minutes and is better for myocardial perfusion stress imaging. Additionally, it has high first pass uptake, relative low radiation exposure (2mSv), and high myocardial retention, but is limited by the roll off that occurs at high coronary blood flow [67,71]. Unfortunately, 82Rubidium has a lower extraction fraction, more significant roll off at high flows and is associated with higher radiation, making 13N-Ammonia the more preferred agent for accurate MBF quantification particularly at high flows [71]. However, 82Rubidium is more commonly used as it requires only an on-site generator as opposed to a cyclotron [71]. It also has been validated against 13N-Ammonia[72]. The assessment of MBF by PET is primarily performed by post-processing software that performs automated segmentation and AIF measurements during dynamic first pass scanning (Central Illustration A) [72]. Depending on the radiotracer used, the post processing software will perform the kinetic modeling on the dynamic data to compute the regional and global stress and rest MBF [66,73,74]. The intra-software reproducibility of MBF measurements is reasonable, ranging from 4% to 15%[74,75,76].
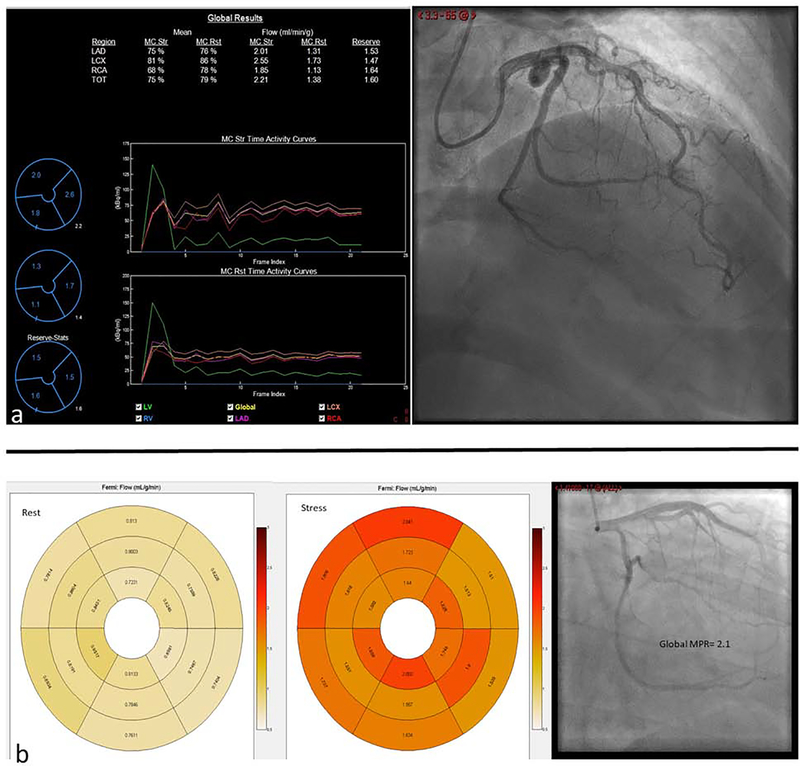
Central Illustration: Abnormal MPR in non-obstructive coronary artery disease.
PET quantification of MBF shows reduced MPR of 1.6 in this 48 year old female with non-obstructive CAD (a). Rest and stress CMR derived segmental quantification of myocardial blood flow with a global MPR of 2.1 in a 59 year old male with non-obstructive CAD. LAD-left anterior descending artery, LCX-left circumflex artery, RCA-right coronary artery, TOT-total, MC-motion corrected, Str-Stress, Rst-Rest (b).
Some prospective PET studies correlate microvascular dysfunction, defined as abnormal MPR, with adverse prognosis[77,78]. Using 82Rubidium PET perfusion scanning, Ziadi et al. found incremental prognostic value of MPR over the more routinely used summed stress score (SSS)[78]. They found that the adverse event rate more than doubled with MPR<2 and normal SSS compared to MPR>2[78]. In addition, a minimal increase in troponin without overt obstructive CAD has also been correlated to PET derived abnormal flow reserves and is a poor prognostic indicator [79]. This data has been replicated by other studies showing MBF and MPR to be predictive of adverse outcomes; these parameters may be utilized for risk stratification depending on normal and abnormal stress perfusion[80,81]. PET-derived MPR and MBF have been particularly useful in analyzing different subsets of populations[82]. Abnormal flow reserve in patients without overt CAD was independently associated with diastolic dysfunction and increased risk of HFpEF hospitalizations [83]. There is evidence of abnormal PET-derived MBF in patients with metabolic syndrome and non-insulin-dependent diabetes[84,85]. These findings are particularly profound in women, indicating the potential of important gender-related differences in MVD[82]. Women, despite having less obstructive CAD when compared to men, are burdened by increased symptoms and similar or worse outcomes[86,87]. Also, as recently described, comprehensive quantitative perfusion analysis by PET that includes regional absolute stress flow, relative stress flow, coronary flow reserve, and quantitative subendocardial perfusion gradients can lead to better diagnostic certainty for microvascular dysfunction[88]. It should be noted that clinically used PET protocols are not capable of accurately quantifying vasodilator–induced subepicardial to subendocaridal perfusion gradients due to limitations in spatial resolution. Despite the vast amount of prognostic data in PET-derived blood flows, PET is not without its limitations which include radiation exposure and cost, depending on which radiotracer is utilized[72].
Single-photon emission computed tomography (SPECT) is the most common nuclear cardiovascular imaging modality, but has been limited to date in quantification of myocardial blood flow due to poor camera sensitivity and temporal resolution with the more common Sodium-Iodide (NaI) cameras[44]. However, new cadmium-zinc-telluride detectors have better sensitivity and resolution that will allow for dynamic SPECT imaging and thus quantification of MBF. Early studies show encouraging flow estimates; however, larger multicenter trials are needed to improve the technical aspects of SPECT processing and to compare it to more traditional imaging methods for flow reserve quantification[89,90].
Cardiac MRI
The utility of MBF and MPR quantification by CMR imaging has been demonstrated in several studies with regards to both epicardial stenosis and microvascular angina[91–95]. Similar to PET, CMR stress first-pass perfusion is performed typically with adenosine infusion or following regadenoson bolus injection. Due to the non-linear signal response of CMR perfusion imaging as a function of gadolinium concentration, care must be taken to measure the AIF accurately using either a dual-contrast or dual-bolus approach, and the signal intensity needs to be converted into gadolinium concentration units before modeling. However, development of perfusion mapping techniques may make the conversion of signal intensity curves to gadolinium concentration units unecessary[96]. A number of approaches have been used to determine MBF including Fermi-function deconvolution, compartmental modeling, and distributed parameter models (Central Illustration B) [97].
Initial canine and porcine models assessing CMR-derived MBF showed excellent correlation (r>0.9) with gold standard microsphere analysis[98,99]. Since then, a number of human studies have been performed. In stable CAD, there was good agreement in global MBF measurements between CMR and 13N-Ammonia PET with r=0.92[100]. However correlation worsened when comparing regional MBF [101]. When comparing patients with chest pain and risk factors for MVD to normal patients, a significant reduction in stress MBF and global MPR was noted in the MVD group[102]. Liu et al showed reductions in stress MBF and global MPR in patients with non-obstructive CAD, specifically shown in the group with an elevated IMR[102]. IMR is an invasive thermodilutional method to assess for microvascular obstruction and has been shown to effect prognosis after an acute coronary occlusion, and is being used as a measure of MVD in non-obstructive CAD [6,103]. CMR-derived myocardial perfusion reserve index (MPRi) detected MVD defined by invasive coronary reactivity testing with sensitivity and specificity of 73–74% in symptomatic women without CAD [104]. In a similar cohort, impaired MPRi correlated with elevation in native T1 suggesting a possible connection between microvascular disease and diffuse fibrosis [105].
Previously, one of the major limitations of CMR-derived MBF was the amount of time required for post processing due to the lack of automated pipelines. Recently there have been a number of studies assessing automated perfusion mapping. Use of automated inline perfusion mapping showed excellent intrastudy and interstudy repeatability when compared to PET quantitation of myocardial blood flow[106]. Kotecha et al. successfully used automated pixel-wise perfusion mapping to diffentiate MVD from multivessel CAD[107].Hsu et al. recently showed that automated MBF measurements made at the time of first pass perfusion imaging reveal similar results to other studies with regard to reductions in stress MBF and global MPR[96].
There are limitations to CMR-derived MVD assessment, including imaging artifacts, exam length, and lack of widespread availability of quantitative first-pass sequences [44]. In addition, gadolinium has restricted use in class 4 and 5 chronic kidney disease and there is a reduction in the extraction of gadolinium with increasing flow rates, altering the quantification of MBF [44]. However, a recent study revealed promising results with stress T1 parametric mapping as a non-contrast method to identify patients with MVD[107]. In addition, ASL is a non-contrast MRI sequence that imparts a magnetic tag on the freely diffusible water protons of arterial blood that differ from the magnetization of surrounding tissue, thus allowing measurement of the “tagged” flow[108]. Currently ASL is in the technological developmental stage as a non-contrast method for myocardial blood flow quantification[39,108,109].Despite its limitations, one clear advantage with CMR is the lack of radiation exposure. In addition, ongoing advancements in technology will shorten the exam, improve patient tolerability, and likely reduce costs. More studies are needed to show the prognostic benefit and clinical utility of CMR assessment of MVD.
Future Directions
The ultimate goal of identifying coronary microvascular dysfunction is to define prognostic differences, therapeutic interventions, and treatment approaches. Currently, studies that address treatment and interventions are limited by variability in defining MVD and small sample size. There are no studies that define any prognostic benefits from the treatment of MVD [110]. As described earlier, there is a worse prognosis for symptomatic MVD, and therefore, many practioners are using interventions similar to the treatment of non-obstructive single-vessel CAD. This includes lifestyle changes by encouraging diet, exercise, and smoking cessation[111]. In addition, treatment of modifiable risk factors such as hyperlipidemia, hypertension, and diabetes is also pursued.
There is one clinical randomized control trial (NCT#03417388) currently recruiting that will assess the prognostic and symptomatic benefit of intensive treatment with statin, ACEi/ARB, and aspirin versus usual care in women with suspected MVD. This is a proper initial step as a treatment approach, however MVD is more complex and mediated via multiple pathways. Now with noninvasive MPR quantification, accurately identifying patients with MVD should be less intensive; in addition, improvements in MPR can be correlated with symptoms to determine success of a particular treatment. Further studies are needed assessing the targeted treatment of MVD in other pathologies such as HFpEF, which has no clear prognostically beneficial treatments.
Conclusions
A number of imaging methods are presently available to measure MBF in the setting of MVD. MBF assessment in obstructive CAD has demonstrated prognostic and clinical benefit [82]. Recently, more studies have been performed using MBF measures to diagnose symptomatic MVD. Many of the modalities have been validated in the quantification of MBF, an important first step. Quantifiable endpoints will help with future clinical studies. Recent studies have shown success in correlating abnormal MPR to symptoms in non-obstructive CAD. PET imaging has the most clinical and prognostic data compared to the other modalities, but CMR-derived MBF measures are being increasingly used to assess clinical utility and response to treatment. A few algorithms have been proposed in the clinical evaluation of microvascular angina and diagnosis of MVD with the use of PET as the diagnostic imaging modality for the evaluation of perfusion abnormalities and MBF quantification [70,112]. CMR measures of MBF remain in the research realm and are not yet fully vetted for clinical use. Clinically, the type of imaging modality used is dependent on local availability of the technology, as well as risk/benefit analysis and cost to the patient. Additional studies are currently examining integrative imaging approaches and regional versus global MPR assessments; this approach shows promise for improving measurement precision [88]. Even though our understanding of mechanisms and therapy of MVD is significantly less than that for obstructive epicardial disease, advances in cardiac imaging will soon allow improved identification of this disease, ultimately leading to improved therapeutic approaches.
Table 1:
The various cardiac imaging modalities and their advantages and disadvantages
| Modality | Technique | Advantages | Disadvantages |
|---|---|---|---|
| Contrast Echocardiography | Constant infusion of echo contrast microbubbles until the cavity is filled, followed by ultrasound destruction of microbubbles. | • Bedside procedure • Minimal risk • No radiation • Relatively inexpensive |
• Microbubble use not FDA approved for perfusion (no reimbursement) • Operator dependent • Poor images related to obesity or the presence of lung disease • Very few validation studies for MVD |
| Transthoracic Doppler echocardiography | Pulsed wave Doppler performed on the proximal left anterior descending artery | • Bedside procedure • Minimal risk • No radiation • Relatively inexpensive • Correlated well with intracoronary Doppler wire |
• Operator dependent • Difficult imaging due to obesity or the presence of lung disease • Poor correlation with PET • Very limited data with use in non-obstructive CAD |
| Computed tomography | Dynamic first pass vasodilator stress and then rest perfusion imaging. | • Anatomical coronary data and perfusion data with the same study | • Perfusion quantification only allowed in high radiation dynamic perfusion imaging • Radiation exposure • Risk of contrast induced nephropathy and contrast allergic reactions • Limited in renal failure • Limited validation in non-obstructive CAD • Limited availability • Iodinated contrast can cause vasodilation leading to overestimation of MBF |
| Positron emission tomography | Dynamic first pass vasodilator stress and then rest perfusion images. | • Most validated modality for MBF quantification in non-obstructive CAD • Extensive prognostic data • Segmented myocardial blood flow • Relatively low radiation exposure due to radiotracers with short life • Not effected by renal dysfunction • Good reproducibility and accuracy • CT can allow for some anatomic assessment of coronaries |
• Radiation exposure • Expensive • Technology is not widely available |
| SPECT | Dynamic first pass vasodilator stress and then rest perfusion images. | • More widely available than PET and CMR | • Requires new generation cameras • Minimal validation in non-obstructive CAD • Radiation exposure is high |
| Cardiac Magnetic Resonance Imaging | Dynamic first pass vasodilator stress and then rest perfusion images. | • No radiation exposure • Excellent spatial resolution • Allows for tissue characterization with the same study • Validated against invasive measurements and PET |
• Expensive • Technology is not widely available • Very minimal prognostic data • Difficult for patients due to frequent breatholds and length of time of the exam • Limited in renal failure |
Table 2:
Suggested future studies for symptomatic MVD without obstructive CAD
| • Comparison of all noninvasive methods of MBF quantification to screen for patients with symptomatic MVD in the absence of CAD |
| • Comparison of CMR versus PET in MVD screening using invasive methods as a gold standard |
| • Randomized controlled trials assessing the prognostic and symptomatic impact of various pharmacological treatment strategies on symptomatic MVD defined by reduced MPR |
| • Randomized controlled trials assessing the impact of lifestyle changes including diet, exercise, and smoking cessation on symptomatic MVD defined by reduced MPR |
| • Assess if specific therapeutic interventions improve quantifiable MPR and determine if any correlation exists with improvement in symptoms, prognosis, or quality of life |
| • Examine improvements in microcirculation relative to prognosis and symptoms in HFpEF |
Highlights.
In this review we discuss, the nuances of myocardial blood flow and myocardial perfusion reserve quantification in echocardiography, cardiac computed tomography, nuclear imaging, and cardiac magnetic resonance imaging.
Each modality, with its own advantages and disadvantages, has played a role in the detection of MVD.
Positron emission tomography derived myocardial blood flow presents the most prognostic data to date related to the impact of MVD.
Research into MBF quantification by cardiac magnetic resonance imaging is growing as the imaging modality becomes increasingly more accessible.
Acknowledgments
Financial support
Roshin C Mathew, MD has declared this paper was supported by T32 EB003841
Jamieson M Bourque, MD receives support from NIH 5K23HL119620-02
Michael Salerno, MD, PhD receives research support from AstraZeneca and Siemens Healthineers. Also support for NIH 5R01HL131919-02.
Christopher M Kramer, MD receives support from R01 HL075792 and U01HL117006-01A1.
Abbreviations list
- FFR
fractional flow reserve
- CFR
coronary flow reserve
- IMR
index of microvascular resistance
- MVD
microvascular dysfunction
- MBF
myocardial blood flow
- ASL
arterial spin labeling
- MBV
myocardial blood volume
- CFVR
coronary flow volume reserve
- CTP
computed tomography perfusion
- FFRct
Computed tomography angiography derived fractional flow reserve
- SPECT
single photon emission computed tomography
- V/M
luminal volume to myocardial mass ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol 2014;64:1929–49. [DOI] [PubMed] [Google Scholar]
- 2.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol 2013;61:e78–140. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes. J Am Coll Cardiol 2014;64:e139–228. [DOI] [PubMed] [Google Scholar]
- 4.Pijls NHJ, Fearon WF, Tonino PAL, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME study. J Am Coll Cardiol 2010;56:177–84 [DOI] [PubMed] [Google Scholar]
- 5.De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 6.Fearon WF, Balsam LB, Farouque HM, et al. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 7.Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33(6):734–44. [DOI] [PubMed] [Google Scholar]
- 8.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312(17):1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elgendy IY, Pepine CJ. Heart Failure With Preserved Ejection Fraction: Is Ischemia Due to Coronary Microvascular Dysfunction a Mechanistic Factor? Am J Med. 2019;132(6):692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galiuto L, De Caterina AR, Porfidia A, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical ballooning or Tako-Tsubo syndrome. Eur Heart J 2010; 31:1319–27. [DOI] [PubMed] [Google Scholar]
- 11.Mejía-Rentería H, van der Hoeven N, van de Hoef TP, et al. Targeting the dominant mechanism of coronary microvascular dysfunction with intracoronary physiology tests. Int J Cardiovasc Imaging 2017; 33(7):1041–1059. [DOI] [PubMed] [Google Scholar]
- 12.Camici PG, Crea F. Coronary Microvascular dysfunction. N Engl J Med 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 13.Kassab GS, Lin DH, Fung YC. Morphometry of pig coronary venous system. Am J Physiol 1994;267(6 Pt 2):H2100–13. [DOI] [PubMed] [Google Scholar]
- 14.Schelbert HR. Anatomy and physiology of coronary blood flow. J Nucl Cardiol 2010;17(4):545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circ Res 1990;66:860–6. [DOI] [PubMed] [Google Scholar]
- 16.Chilian WM. Coronary microcirculation in health and disease: summary of an NHLBI workshop. Circulation 1997;95:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prior JO, Schindler TH, Facta AD, et al. Determinants of myocardial blood flow response to cold pressor testing and pharmacologic vasodilation in healthy humans. Eur J Nucl Med Mol Imaging 2007; 34(1):20–7. [DOI] [PubMed] [Google Scholar]
- 18.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 19.AlBadri A, Behzad S, Wei J, et al. Intracoronary Bolus Injection Versus Intravenous Infusion of Adenosine for Assessment of Coronary Flow Velocity Reserve in Women With Signs and Symptoms of Myocardial Ischemia and No Obstructive Coronary Artery Disease JACC Cardiovasc Interventions 2018;11(20):2125–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinescu MA, Loffler AI, Oullette M, Smith L, Kramer CM, Bourque JM. Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. J Am Coll Cardiol Img 2015;8:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015; 131(6):550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mygind ND, Michelsen MM, Pena A, et al. Coronary microvascular function and myocardial fibrosis in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Cardiovasc Magn Reson 2016;18(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann PA, Camici PG. Myocardial blood flow by PET: technical aspects and clinical applications. J Nucl Med 2005;46:291. [PubMed] [Google Scholar]
- 24.Gerber BL. Quantification of myocardial perfusion and myocardial perfusion reserve by positron emission tomography and cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2012;60:1556–7. [DOI] [PubMed] [Google Scholar]
- 25.Geltman EM, Henes CG, Senneff MJ, Sobel BE, Bergmann SR. Increased myocardial perfusion at rest and diminished perfusion reserve in patients with angina and angiographically normal coronary eries. J Am Coll Cardiol 1990;16: 586–95. [DOI] [PubMed] [Google Scholar]
- 26.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia. J Am Coll Cardiol 2010;55: 2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124: 2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res 1994; 74(6):1157–65. [DOI] [PubMed] [Google Scholar]
- 29.McCommis KS, Zhang H, Goldstein TA, et al. Myocardial blood volume is associated with myocardial oxygen consumption: an experimental study with cardiac magnetic resonance in a canine model.. J Am Coll Cardiol Img 2009;2(11):1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behrenbeck TR, McCollough CH, Miller WL, et al. Early changes in myocardial microcirculation in asymptomatic hypercholesterolemic subjects: as detected by perfusion CT. Ann Biomed Eng 2014; 42(3):515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indermühle A, Vogel R, Meier P, et al. The relative myocardial blood volume differentiates between hypertensive heart disease and athlete’s heart in humans. Eur Heart J 2006;27(13):1571–8. [DOI] [PubMed] [Google Scholar]
- 32.Calamante F. Arterial input function in perfusion MRI: A comprehensive review. Progress in Nuclear Magnetic Resonance Spectroscopy 74 (2013) 1–32 [DOI] [PubMed] [Google Scholar]
- 33.Waller AH, Blankstein R, Kwong RY, Di Carli MF. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr Cardiol Rep 2014;16(5):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson HB, Fritz-Hansen T, Rostrup E, Søndergaard L, Ring P, Henriksen O. Myocardial perfusion modeling using MRI. Magn Reson Med 1996;35(5):716–26. [DOI] [PubMed] [Google Scholar]
- 35.Gullberg GT, Reutter BW, Sitek A, Maltz JS, Budinger TF. Dynamic single photon emission computed tomography–basic principles and cardiac applications. Phys Med Biol. 2010;55:R111–R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zierler KL. Equations for measuring blood flow by external monitoring of radioisotopes. Circ Research 1965; 16(4) 309–321 [DOI] [PubMed] [Google Scholar]
- 37.Goldstein TA, Jerosch-Herold M, Misselwitz B, Zhang H, Gropler RJ, Zheng J. Fast mapping of myocardial blood flow with MR first-pass perfusion imaging. Magn Reson Med 2008;59(6):1394–400. [DOI] [PubMed] [Google Scholar]
- 38.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of Myocardial Blood Flow With Ultrasound-Induced Destruction of Microbubbles Administered as a Constant Venous Infusion. Circulation 1998;97:473–483. [DOI] [PubMed] [Google Scholar]
- 39.Zun Z, Varadarajan P, Pai RG, Wong EC, Nayak KS. Arterial spin labeled CMR detects clinically relevant increase in myocardial blood flow with vasodilation. J Am Coll Cardiol Img 2011. December;4(12):1253–61. [DOI] [PubMed] [Google Scholar]
- 40.Firschke C, Lindner JR, Wei K, Goodman NC, Skyba DM, Kaul S. Myocardial perfusion imaging in the setting of coronary artery stenosis and acute myocardial infarction using venous injection of a second-generation echocardiographic contrast agent. Circulation 1997;96:959–967. [PubMed] [Google Scholar]
- 41.Porter TR, Xie F, Kricsfeld D, Armbruster RW. Improved myocardial contrast with second harmonic transient response imaging in humans using intravenous perfluorocarbonexposed sonicated dextrose albumin. J Am Coll Cardiol 1996;27:1497–1501. [DOI] [PubMed] [Google Scholar]
- 42.Wei K, Skyba DM, Firschke C, Lindner JR, Jayaweera AR, Kaul S. Interaction between microbubbles and ultrasound: in vitro and in vivo observations. J Am Coll Cardiol 1997;29:1081–1088. [DOI] [PubMed] [Google Scholar]
- 43.Vogel R, Indermühle A, Reinhardt J, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J Am Coll Cardiol 2005; 45: 754–762. [DOI] [PubMed] [Google Scholar]
- 44.Feher A, Sinusas AJ. Quantitative Assessment of Coronary Microvascular Function Dynamic Single-Photon Emission Computed Tomography, Positron Emission Tomography, Ultrasound, Computed Tomography, and Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2017;10:e006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigo F et al. Usefulness of coronary flow reserve over regional wall motion when added to dual-imaging dipyridamole echocardiography. Am. J. Cardiol. 91, 269–273 (2003) [DOI] [PubMed] [Google Scholar]
- 46.Mygind ND, Michelsen MM, Pena A, et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women With Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. J Am Heart Assoc 2016;5(3):e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senior R, Janardhanan R, Jeetley P, Burden L. Myocardial contrast echocardiography for distinguishing ischemic from nonischemic first-onset acute heart failure: insights into the mechanism of acute heart failure.Circulation 2005;112(11):1587–93. [DOI] [PubMed] [Google Scholar]
- 48.Caiati C,Montaldo C, Zedda N, et al. Validation of a new noninvasive method (contrastenhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol 1999;34:1193–1200. [DOI] [PubMed] [Google Scholar]
- 49.Saraste M, Koskenvuo J, Knuuti J, et al. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol 2001;21:114–122. [DOI] [PubMed] [Google Scholar]
- 50.Hozumi T, Yoshida K, Akasaka T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol 1998;32(5):1251–9. [DOI] [PubMed] [Google Scholar]
- 51.Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009. March 1;103(5):626–31. [DOI] [PubMed] [Google Scholar]
- 52.Michelsen MM, Mygind ND, Pena A, et al. Transthoracic Doppler echocardiography compared with positron emission tomography for assessment of coronary microvascular dysfunction: The iPOWER study. Int J Cardiol 2017; 228(1):435–443. [DOI] [PubMed] [Google Scholar]
- 53.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39(37):3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danad I, Szymonifka J, Schulman-Marcus J, Min JK. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging 2016;17(8):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Branch KR, Haley RD, Bittencourt MS, Patel AR, Hulten E, Blankstein R. Myocardial computed tomography perfusion. Cardiovasc Diagn Ther 2017;7(5):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.George RT, Jerosch-Herold M, Silva C, et al. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest. Radiol 2007;42: 815–822. [DOI] [PubMed] [Google Scholar]
- 57.Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- 58.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–2241. [DOI] [PubMed] [Google Scholar]
- 59.Sand NPR, Veien KT, Nielsen SS,et al. PRrospEctive Comparison of FFR Derived From Coronary CT Angiography With SPECT PerfuSion Imaging in Stable Coronary ArtEry DiSeaSe: The ReASSESS Study. J Am Coll Cardiol Img 2018;11(11):1640–1650. [DOI] [PubMed] [Google Scholar]
- 60.Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of non-invasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145–55. [DOI] [PubMed] [Google Scholar]
- 61.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J. 2013;34(38):2949–3003. [DOI] [PubMed] [Google Scholar]
- 62.Grover R, Leipsic JA, Mooney J, et al. Coronary lumen volume to myocardial mass ratio in primary microvascular angina. J Cardiovasc Comput Tomogr 2017;11(6):423–428. [DOI] [PubMed] [Google Scholar]
- 63.Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol 2009;54(12):1072–84. [DOI] [PubMed] [Google Scholar]
- 64.Tatineni S, Kern MJ, Deligonul U, Aguirre F. The effects of ionic and nonionic radiographic contrast media on coronary hyperemia in patients during coronary angiography. Am Heart J 1992;123:621–627. [DOI] [PubMed] [Google Scholar]
- 65.Canty JM Jr, Judd RM, Brody AS, Klocke FJ. First-pass entry of nonionic contrast agent into the myocardial extravascular space. Effects on radiographic estimates of transit time and blood volume. Circulation 1991;84:2071–2078. [DOI] [PubMed] [Google Scholar]
- 66.Slomka P, Berman DS, Alexanderson, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging 2014; 2:343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salerno M, Beller GA. Noninvasive Assessment of Myocardial Perfusion. Circ Cardiovasc Imaging 2009;2:412–424. [DOI] [PubMed] [Google Scholar]
- 68.Bergmann SR, Fox KAA, Rand AL, et al. Quantification of regional myocardial blood flow in vivo with H215O. Circulation 1984;70:724–733. [DOI] [PubMed] [Google Scholar]
- 69.Bol A, Melin JA, Vanoverschelde JL, et al. Direct comparison of [13N]ammonia and [15O]water estimates of perfusion with quantification of regional myocardial blood flow by microspheres. Circulation. 1993. February;87(2):512–25. [DOI] [PubMed] [Google Scholar]
- 70.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. J Am Coll Cardiol Img 2010. June;3(6):623–40. [DOI] [PubMed] [Google Scholar]
- 71.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290–1305. [DOI] [PubMed] [Google Scholar]
- 72.Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med 2007;34:1765–1774. [DOI] [PubMed] [Google Scholar]
- 73.Choi Y, Huang SC, Hawkins RA, et al. Quantification of myocardial blood flow using N-13-ammonia and PET: comparison of tracer models. J Nucl Med 1999;40:1045–1055. [PubMed] [Google Scholar]
- 74.El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009; 50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15Olabeled water and PET. J Nucl Med 1999;40:1848–56. [PubMed] [Google Scholar]
- 76.Nagamachi S, Czernin J, Kim AS, et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med 1996;37:1626–31. [PubMed] [Google Scholar]
- 77.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169(9):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58(7):740–8. [DOI] [PubMed] [Google Scholar]
- 79.Taqueti VR, Everett BM, Murthy VL, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131(6):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fukushima K, Javadi MS, Higuchi T, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging J Nucl Med. 2011;52(5):726–32. [DOI] [PubMed] [Google Scholar]
- 81.Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging 2013;14(12):1203–10. [DOI] [PubMed] [Google Scholar]
- 82.Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(21):2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med 2011;52:1369–77. [DOI] [PubMed] [Google Scholar]
- 85.Yokoyama I, Momomura S, Ohtake T, et al. Reduced myocardial flow reserve in noninsulin- dependent diabetes mellitus. J Am Coll Cardiol 1997;30:1472–7 [DOI] [PubMed] [Google Scholar]
- 86.Taqueti VR, Shaw LJ, Cook NR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taqueti VR, Dorbala S, Wolinsky D, et al. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state- of-the-evidence and clinical recommendations. J Nucl Cardiol 2017;24:1402–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gould KL, Johnson NP. Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(21):2642–2662. [DOI] [PubMed] [Google Scholar]
- 89.Han S, Kim YH, Ahn JM, et al. Feasibility of dynamic stress 201Tl/rest 99mTctetrofosmin single photon emission computed tomography for quantification of myocardial perfusion reserve in patients with stable coronary artery disease. Eur J Nucl Med Mol Imaging 2018;45(12):2173–2180. [DOI] [PubMed] [Google Scholar]
- 90.Hyafil F, Rouzet F, Le Guludec D. Quantification of myocardial blood flow with dynamic SPECT acquisitions: ready for prime time? Eur J Nucl Med Mol Imaging 2018; 45: 2170 10.1007/s00259-018-4127-8 [DOI] [PubMed] [Google Scholar]
- 91.Ritter C, Brackertz A, Sandstede J, Beer M, Hahn D, Köstler H. Absolute quantification of myocardial perfusion under adenosine stress. Magn Reson Med 2006;56(4):844–849. [DOI] [PubMed] [Google Scholar]
- 92.Costa MA, Shoemaker S, Futamatsu H, et al. Quantitative magnetic resonance perfusion imaging detects anatomic and physiologic coronary artery disease as measured by coronary angiography and fractional flow reserve. J Am Coll Cardiol 2007;50(6):514–522. [DOI] [PubMed] [Google Scholar]
- 93.Patel AR, Antkowiak PF, Nandalur KR, et al. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol 2010;56(7):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346(25):1948–53. [DOI] [PubMed] [Google Scholar]
- 95.Jerosch-Herold M. Quantification of myocardial perusion by cardiovascular magnetic resonance. JCMR 2010;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hsu LY, Jacobs M, Benovoy M, et al. Diagnostic Performance of Fully Automated Pixel-Wise Quantitative Myocardial Perfusion Imaging by Cardiovascular Magnetic Resonance. J Am Coll Cardiol Img 2018;11(5):697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Biglands JD, Magee DR, Sourbron SP, Plein S, Greenwood JP, Radjenovic A. Comparison of the Diagnostic Performance of Four Quantitative Myocardial Perfusion Estimation Methods Used in Cardiac MR Imaging: CE-MARC Substudy. Radiology 2015;275(2):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Christian TF, Rettmann DW, Aletras AH,et al. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging.Radiology 2004;232(3):677–84. [DOI] [PubMed] [Google Scholar]
- 99.Schuster A, Zarinabad N, Ishida M,et al. Quantitative assessment of magnetic resonance derived myocardial perfusion measurements using advanced techniques: microsphere validation in an explanted pig heart system. J Cardiovasc Magn Reson 2014;14;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engblom H, Xue H, Akil S,et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: a comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J Cardiovasc Magn Reson 2017;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zorach B, Shaw PW, Bourque J, et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J Cardiovasc Magn Reson 2018;20(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu A, Wijesurendra RS, Liu JM, et al. Diagnosis of Microvascular Angina Using Cardiac Magnetic Resonance. J Am Coll Cardiol 2018;71(9):969–979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Carrick D, Haig C, Ahmed N, et al. Comparative prognostic utility of indices of microvascular function alone or in combination in patients with an acute ST-segment elevation myocardial infarction. Circulation 2016;134:1833–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomson LEJ, Wei J, Agarwal M, et al. Cardiac Magnetic Resonance Myocardial Perfusion Reserve Index Is Reduced in Women With Coronary Microvascular Dysfunction. Circ Cardiovasc Imaging 2015;8(4):e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shaw JL, Nelson MD, Wei J, et al. Inverse association of MRI-derived native myocardial T1 and perfusion reserve index in women with evidence of ischemia and no obstructive CAD: A pilot study. Int J Cardiol 2018;270:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown LAE, Onciul SC, Broadbent DA, et al. Fully automated, inline quantification of myocardial blood flow with cardiovascular magnetic resonance: repeatability of measurements in healthy subjects. J Cardiovasc Magn Reson 2018;20(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kotecha T, Martinez-Naharro A, Boldrini M, et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction: Validation Against Invasive Coronary Physiology. JACC Cardiovasc Imaging. 2019. February 11. doi: 10.1016/j.jcmg.2018.12.022. [Epub ahead of print]Liu A, Wijesurendra RS, Liu JM, et al. Gadolinium-Free Cardiac MR Stress T1-Mapping to Distinguish Epicardial From Microvascular Coronary Disease. J Am Coll Cardiol. 2018;71(9):957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mathew RC, Kramer CM. Recent advances in magnetic resonance imaging for peripheral artery disease. Vasc Med 2018;23(2):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Do HP, Jao TR, Nayak KS. Myocardial arterial spin labeling perfusion imaging with improved sensitivity. J Cardiovasc Magn Reson. 2014. January 27;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Löffler AI, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and management. Curr Cardiol Rep 2016;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olsen RH, Pedersen LR, Jurs A, et al. A randomised trial comparing the effect of exercise training and weight loss on microvascular function in coronary artery disease. Int J Cardiol 2015; 185:229–235. [DOI] [PubMed] [Google Scholar]
- 112.Leucker TM, Valenta I, Schindler TH. Positron Emission Tomography-Determined Hyperemic Flow, Myocardial Flow Reserve, and Flow Gradient-Quo Vadis? Front Cardiovasc Med. 2017. July;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]