Abstract
Background:
Intracorporeal anastomosis is associated with several short-term benefits. However, it is a technically challenging procedure with potential risks.
Objective:
To investigate differences in short-term complications and long-term incisional hernia rates after robotic right colectomy with intracorporeal versus extracorporeal anastomoses and standardized extraction sites.
Design:
Historical cohort study.
Setting:
Single surgeon, single institution.
Patients:
All patients undergoing robotic right colectomy with intracorporeal anastomosis and a Pfannenstiel extraction site or extracorporeal anastomosis with a vertical midline extraction site from 2013–2017 were eligible. Exclusion criteria were conversion to laparotomy for tumor-related reasons or lack of follow-up.
Intervention:
Intracorporeal or extracorporeal anastomosis, based on availability of the robotic stapler and appropriate bedside assistance.
Main Outcome Measures:
The primary outcome was incisional hernia, diagnosed either clinically or on postoperative imaging, and analyzed using time-to-event analysis. A Cox proportional hazards model was used for multivariable analysis. Secondary outcomes were analyzed using parametric and nonparametric tests. Statistical significance was set at p<0.05.
Results:
Of 164 patients who met all inclusion criteria, 67 had intracorporeal and 97 had extracorporeal anastomoses. Median follow-up time was similar in both groups (14 vs. 15 months, p=0.73). The one-year estimated incisional hernia rate was 12% for extracorporeal and 2% for intracorporeal anastomoses (p=0.007); this difference was confirmed by multivariable modeling. The severity of postoperative complications was similar between the groups, but there was an increase in incisional infections and a shorter length of stay (one day) for intracorporeal cases.
Limitations:
Retrospective, single surgeon.
Conclusions:
Right colectomy with intracorporeal anastomosis and a Pfannenstiel extraction site may reduce the rate of incisional hernias compared to extracorporeal anastomosis with a vertical midline extraction site. The intracorporeal approach was also associated with a decreased length of stay, but an increase in incisional surgical site infections. These findings have implications for healthcare utilization and patient-centered outcomes. See Video Abstract at http://links.lww.com/DCR/Axxx.
Keywords: Colorectal cancer, Hernia, Intracorporeal anastomosis, Robotic colectomy
INTRODUCTION
Minimally invasive techniques are increasingly utilized in colorectal surgery.1 When compared with open surgery, multiple randomized trials in colorectal cancer have shown equivalent oncologic outcomes for minimally invasive surgery, with improved postoperative pain and earlier return of bowel function.2–5
Despite its wide acceptance by the colorectal community, there is ongoing debate regarding the optimal minimally invasive approach. One controversial technique is intracorporeal anastomosis for right hemicolectomy.6 First introduced in 1994, this approach offers several putative benefits. First, it may lead to a quicker return of bowel function and shorter length of hospital stay, given the decreased need to mobilize the transverse colon.7–10 Second, the extraction site can be placed in an optimal location, such as in a transverse orientation and off the midline, which may lead to fewer incisional hernias (IH).11,12 Finally, there may be less wound contamination because the incision is not exposed during the opening of the bowel, potentially reducing the rate of surgical site infections (SSIs).13,14
The greatest disadvantage of the intracorporeal anastomosis is its technical difficulty, which has limited its acceptance to a select group of surgeons.15,16 Other drawbacks that have been reported in some studies include increased operative time, an increase in low-grade postoperative complications, a greater likelihood of compromised anastomotic integrity, and a higher rate of conversion to an open procedure.11,17,18 To date, most comparisons between intracorporeal and extracorporeal anastomoses in right colectomies have focused on short-term outcomes. The IH rate, a long-term adverse outcome with significant impact on healthcare expenditure and patient satisfaction, has rarely been reported in the minimally invasive literature.12,19–25 In this retrospective study, we investigated the differences between IH rates and short-term complications in patients undergoing robotic right colectomy with intracorporeal versus extracorporeal anastomoses with standardized extraction sites.
MATERIALS AND METHODS
We used Current Procedural Terminology (CPT) codes to identify all patients who had undergone a robotic right colectomy by a single surgeon at our institution between 2013 and 2017. We excluded patients who had extraction sites other than Pfannenstiel for intracorporeal cases or vertical midline for extracorporeal cases. Therefore, the two cohorts for analysis were 1) intracorporeal anastomosis with Pfannenstiel extraction site (IA) and 2) extracorporeal anastomosis with a vertical midline extraction site (EA). Patients who had a conversion to laparotomy prior to the creation of the anastomosis due to locally advanced or metastatic tumors and patients who did not have at least one follow-up visit were excluded.
All patients were assessed preoperatively by a single surgeon (JGA) and his clinical team. Mechanical bowel prep and oral antibiotics were routinely used at our institution during the entire study time period. Additional enhanced recovery measures, including preoperative carbohydrate loading the morning of surgery, were adopted institution-wide in 2016.
A single surgeon (JGA) performed all operations using the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California). The decision to perform an IA or EA was based on the availability of the robotic stapler and whether there was adequate bedside assistance to complete the case without significant delay. Typically, adequate assistance was the presence of a minimally invasive robotic surgical physician assistant or experienced resident. The procedures were performed concomitantly over the four-year time interval of this study, except for a period in 2014 when the robotic stapler was not commercially available.
Trocar and extraction site configurations remained consistent over the study period (Fig. 1). For IA cases, the 8-mm camera port was placed in a supraumbilical, off-midline location. Two 8-mm and one 12-mm suprapubic ports were also used during the operation. The robotic stapler was introduced through the suprapubic port site, which was subsequently lengthened to a Pfannenstiel incision for specimen extraction. In EA cases, two 8-mm ports were used for the operation, and the 8-mm camera port was placed in the supraumbilical midline; the camera port incision was lengthened for extracting the specimen. In both operations, a 5-mm AirSeal (ConMed, Utica, NY) was used as the assistant port. Wound protectors were used for specimen extraction in all cases. Fascial incisions greater than 8mm were closed with running PDS sutures, and skin incisions were closed with interrupted 3–0 Nylon.
Figure 1.
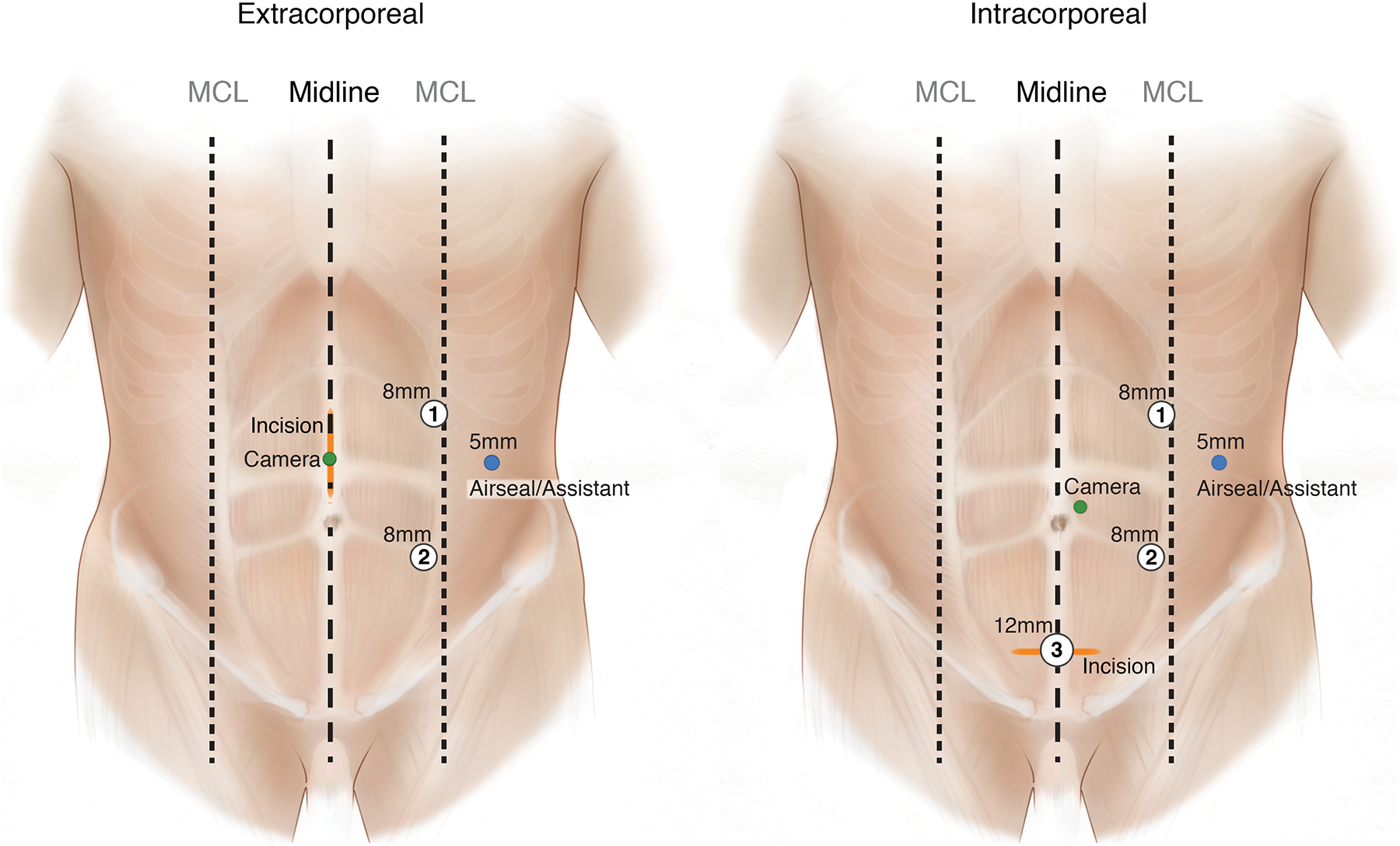
Port placements for IA and EA robotic right colectomies.
Clinical, operative, and postoperative complication data were collected by review of the electronic medical record by two of the authors (MW and MK). Complications were described using the National Surgical Quality Improvement Program descriptions and graded according to the Clavien-Dindo (CD) classification.26,27 SSIs were classified according to the Centers for Disease Control and Prevention criteria as superficial and deep incisional, or organ space infections. At our institution, monitoring for SSIs is also done prospectively as part of an institution-wide initiative and as mandated by state law. Specifically, an infection control specialist conducts both a manual chart review and a telephone survey with surgical patients around postoperative day 30. Survey questions assess the development of cellulitis, drainage, and the need to take antibiotics for a wound infection. All SSI data captured during this process are entered in a searchable institutional database, which was queried for this study.
The primary outcome was the presence of an IH, as defined per the European Hernia Society guidelines as “any abdominal wall gap with or without a bulge in the area of a postoperative scar perceptible or palpable by clinical examination or imaging.”28 When an IH was identified by imaging, all CTs and MRIs were reviewed by three of the authors for agreement (MW, PS, JGA). Secondary outcomes included length of stay, postoperative complications, and SSIs.
We analyzed the primary outcome using time-to-event analysis with a log-rank test. Observations were censored at the time of IH diagnosis, date of last imaging, or date of last clinical follow-up in which an abdominal exam was documented. In addition, if the patient underwent a subsequent laparotomy for tumor progression, they were censored on the date of that surgery. Laparotomy for a complication related to the index surgery was not an indication for censoring. A Cox proportional hazards model was used for multivariable analysis, with stepwise regression to select for possible confounders. Secondary outcomes were analyzed using parametric and nonparametric tests where appropriate. Statistical significance was set at p<0.05. This study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center.
RESULTS
We identified 170 patients who met our initial CPT and operative criteria. Four patients were excluded based on the extraction site incision: in the intracorporeal cases, this included one vaginal and one right lower quadrant transverse incision. In the extracorporeal arm, this included one vertical paramedian incision, and one transverse right upper quadrant incision. Two additional patients were excluded for conversion to laparotomy for locally advanced and unresectable disease prior to creation of an anastomosis.
Of 164 evaluable patients, 67 underwent IA and 97 underwent EA. Table 1 shows baseline demographics and clinical characteristics of these patients. Patients were similar with respect to age, BMI, tumor stage, smoking status, and history of ventral hernia. There were 5 patients on immunosuppressive medications in the IA group and none in the EA group. This included two patients with chronic obstructive pulmonary disease (COPD) on inhaled corticosteroids as needed, two patients with autoimmune disease on long-term steroid treatment, and one patient who was prescribed a high-dose steroid within one week of surgery for bronchitis. The average incision length was longer in the EA group, while the operative time was longer by an average of 30 minutes in the IA group (Table 2).
Table 1.
Demographic and clinical characteristics
| Variable | Extracorporeal (n=97) |
Intracorporeal (n=67) |
p value |
|---|---|---|---|
| Male, n (%) | 47 (48) | 28 (42) | 0.40 |
| Age, years, median (IQR)a | 68 (16) | 62 (20) | 0.07 |
| Body mass index, n (%) | 0.84 | ||
| 18.5 to 24.9 | 26 (27) | 20 (30) | |
| 25 to 29.9 | 29 (30) | 21 (31) | |
| 30+ | 42 (43) | 26 (39) | |
| Diabetes, n (%) | |||
| NIDDM | 13 (13) | 9 (13) | >0.99 |
| IDDMb | 1 (1) | 3 (4) | 0.31 |
| Immunosuppression, n (%)b | nil | 5 (7) | 0.01 |
| COPD/asthma, n (%)b | 3 (3) | 6 (9) | 0.16 |
| Albumin, median (IQR)a | 4.2 (0.4) | 4.2 (0.4) | 0.95 |
| Previous abdominal hernia, n (%)b | 2 (2) | 3 (4) | 0.40 |
| Tobacco use n (%) | 0.62 | ||
| Previous or current | 44 (45) | 33 (49) | |
| AJCC Stage 7th Ed., n (%) | 0.54 | ||
| In situ/adenoma | 15 (15) | 8 (12) | |
| I & II | 50 (52) | 35 (52) | |
| III | 29 (30) | 22 (33) | |
| IV | 3 (3) | 2 (3) |
Wilcoxon
Fisher’s exact test
p < 0.05 denotes statistical significance
AJCC = American Joint Committee on Cancer; COPD = chronic obstructive pulmonary disease; IDDM = insulin-dependent diabetes mellitus; IQR = interquartile range; NIDDM = non-insulin-dependent diabetes mellitus
Table 2.
Operative characteristics
| Variable | Extracorporeal (n=97) |
Intracorporeal (n=67) |
p value |
|---|---|---|---|
| Operative time, min, median (IQR)a | 153 (48) | 186 (55) | <0.01 |
| Conversion to open, n (%)b | 1(1) | nil | >0.99 |
| Incision length, cm, median (Q1-Q3) | 5 (5–6) | 5 (4–5) | <0.01 |
Wilcoxon
Fisher’s exact test
p < 0.05 denotes statistical significance.
IQR = interquartile range
Patients were followed for a median of 14 months from their original surgery. Most patients (131; 80%) had at least one postoperative CT or MRI of the abdomen and pelvis (Table 3), and 12 patients underwent laparotomy for progression of disease. Overall, 18 extraction site IH and two trocar site IH were identified. All of the extraction site IH were in patients who had undergone EA. The two trocar site IH were in the IA cohort and were located near the supraumbilical midline. Of the IH, 75% were identified on CT or MRI without a documented diagnosis from the last available clinical exam. By time-to-event analysis, EA was significantly associated with IH, with an estimated one-year IH rate of 12% (95% CI 7–22%) compared to 2% (95% CI 0–13%) after IA (p=0.007) (Fig. 2). Univariate analyses did not identify associations with IH and other factors, including age, smoking status, or BMI (Table 4). There were no incisional hernias diagnosed in the five patients with known preoperative hernias or prior hernia repairs. The small number of events limited our ability to perform a robust multivariable analysis. In a Cox proportional hazards model using stepwise variable selection, only the IA approach was significantly associated with IH (HR 0.17, p=0.02).
Table 3.
Follow-up and hernia diagnoses
| Variable | Extracorporeal (n=97) |
Intracorporeal (n=67) |
p value |
|---|---|---|---|
| Follow-up, months, median (IQR)a | 14 (28) | 15 (16) | 0.73 |
| At least one post-op CT, n (%) | 75 (77) | 56 (84) | 0.33 |
| IH diagnoses, n (%)d | 18 (19) | 2 (3) | 0.007 |
| Time to IH diagnosis, months, median (IQR)a,c | 12 (14) | 14 (10) | 0.95 |
| Method of diagnosis, n (% of IH)b,c | 0.21 | ||
| Imaging only | 14 (78) | 1 (50) |
Wilcoxon
Fisher’s exact test
n=20
Log-rank test
p < 0.05 denotes statistical significance
CT = computed tomography; IH = incisional hernia; IQR = interquartile range
Figure 2.
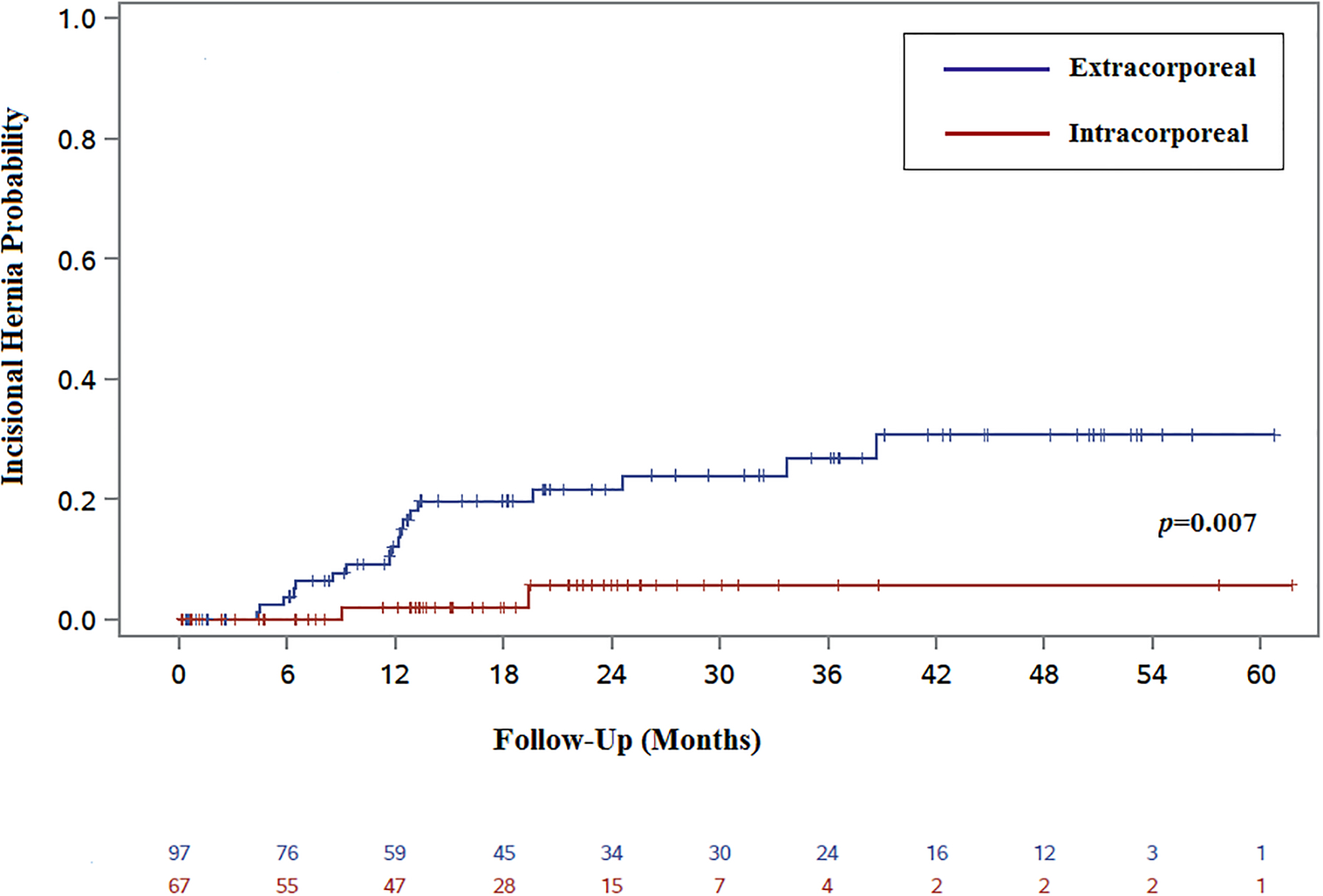
Kaplan-Meier “failure” curve comparing IH after IA and EA anastomoses.
Table 4.
Univariate analysis: incisional hernia
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Intracorporeal anastomosis | 0.17 | 0.04–0.73 | 0.02 |
| Age | 0.98 | 0.95–1.01 | 0.16 |
| Body mass index | |||
| 18.5–24.9 | ref | - | - |
| 25–29.9 | 1.75 | 0.44–6.98 | 0.43 |
| 30+ | 3.01 | 0.84–10.83 | 0.09 |
| Smoking | |||
| Never | ref | - | - |
| Former | 0.42 | 0.15–1.17 | 0.10 |
| Current | 0.50 | 0.07–3.78 | 0.50 |
| AJCC Stage 7th Ed. | |||
| In situ/adenoma | ref | - | - |
| I | 0.75 | 0.07–8.30 | 0.81 |
| II | 1.21 | 0.15–10.07 | 0.86 |
| III | 2.15 | 0.28–16.69 | 0.46 |
| IV | nc | nc | 0.99 |
p < 0.05 denotes statistical significance
AJCC = American Joint Committee on Cancer; CI = confidence interval; HR = hazard ratio; nc = not calculated due to insufficient data
Secondary outcomes are shown in Table 5. The IA and EA cohorts had similar rates of postoperative complications (16% vs. 13%, p=0.59) and a similar number of “high-grade” (CD 3 or greater) complications (3% vs. 4%, p>0.99). The overall rates of SSIs were the same between groups (11% vs. 5%, p=0.23); however, there was a significant association between IA and an increased rate of superficial and deep incisional SSIs (9% vs. 1%, p=0.02). The organ space infection rate was similar in IA and EA cases (1% vs. 4%, p=0.65). When SSIs were classified by severity according to CD classification, the groups again were similar (p=0.16). Finally, there was a statistically significant difference in length of stay between the groups, where IA patients were discharged approximately one day earlier than EA patients (3 vs. 4 days, p<0.01).
Table 5.
Secondary outcomes
| Variable | Extracorporeal (n=97) |
Intracorporeal (n=67) |
p value |
|---|---|---|---|
| 30-day mortality, n (%) | nil | nil | -- |
| Any complication, n (%) | 13 (13) | 11 (16) | 0.59 |
| CD grade 3 or greater, n (%)a | 4 (4) | 2 (3) | >0.99 |
| Surgical site infection, n (%)a | 5 (5) | 7 (11) | 0.23 |
| Superficial and deep incisional | 1 (1) | 6 (9) | 0.02 |
| Organ space | 4 (4) | 1 (1) | 0.65 |
| CD grades 1 and 2 | 3 (3) | 6 (9) | 0.16 |
| CD grades 3+ | 2 (2) | 1 (1) | >0.99 |
| LOS, days, median (IQR)b | 4 (2) | 3 (1) | <0.01 |
| Readmission in 90 days, n (%) | 8 (8) | 5 (7) | 0.85 |
Fisher’s exact test
Wilcoxon
p < 0.05 denotes statistical significance
CD = Clavien-Dindo; IQR = interquartile range; LOS = length of stay
DISCUSSION
In this study, we found that a right colectomy performed with an intracorporeal anastomosis and a Pfannenstiel extraction site was associated with a decrease in IH and a shorter length of stay compared to standardized EA approach. In contrast, we saw an increase in operative time as well as in rates of incisional SSIs after IA, though the severities of SSIs were the same in the two groups. These findings support the benefits of this technically challenging surgical technique while highlighting some of its potential trade-offs.
The true impact of IH on patients and on healthcare utilization is difficult to ascertain and likely underestimated. In a study of clinically detectable IH one year after abdominal surgery, 84% of patients were symptomatic, and there was a significant association between IH and lower scores for measures of health and body image.29 With the five-year survival for all stages of colon cancer increasing, the burden of IH is likely also increasing.30 For patients who are cured after surgery and months of adjuvant therapy, the need to go through an IH repair represents yet another significant healthcare expenditure in what is already an expensive treatment course.31,32 For patients with stage IV or recurrent disease, hernia repair may not be an option, and IH likely impacts quality of life and survivorship.24,25
There is a paucity of data on the true incidence of IH after minimally invasive colectomy. Not only is this long-term outcome rarely reported, but there is a lack of consensus on how to measure it. A 2008 Cochrane review on laparoscopic colectomies included only two trials in which IH rates were even reported and with widely different results (4.7% and 23%).19–22 Meta-analyses of series including robotic and laparoscopic right colectomies included only five studies in which the IH rate was reported. Unfortunately, the data could not be properly analyzed due to heterogeneity not only in the extraction sites but also in the definitions of IH.10,23 The IH rate in our series fall within the range reported in the literature.
In our series, the proportion of IH diagnosed radiologically was 75%. We included both clinically and radiologically diagnosed IH because 23–35% of IH are missed by clinical diagnosis alone, and because this is consistent with society guidelines.33–35 Ultimately, the outcome of interest is symptomatic IH which impair quality of life, regardless of eventual operative intervention. In this retrospective study, we were unable to adequately assess the incidence of symptomatic IH.
In our series, IA was associated with a shorter length of stay. This is consistent with a recent meta-analysis of several case series published between 2010 and 2015 that also reported less postoperative overall morbidity and a quicker return of bowel function.10 Unfortunately, the authors of this meta-analysis were not able to analyze differences in intraabdominal infection rates. At least one publication not included in the meta-analysis reported a higher rate of anastomotic leaks in IA cases (8.2% vs. nil), though this did not reach statistical significance in multivariable analysis.36
In our study, the overall rate and severity of postoperative complications were similar in the two groups. However, contrary to other series, we found a higher rate of superficial and deep incisional, but not organ-space, SSIs in the IA group compared to the EA group.10,11,14 We believe this difference in the rate of incisional SSIs is the consequence of close surveillance of these cancer patients, and the use of a strict definition of SSI as required by the New York State Department of Health. It is also possible that the incisional SSI rate was exaggerated by the fact that we commonly see inflammation around Pfannenstiel incisions in the setting of a large pannus. This was the case for at least one IA patient, for whom the surgeon commented on “irritation” around the incision; the patient was subsequently diagnosed with an SSI by another provider. As this was a retrospective analysis, we were limited in our ability to obtain the actual diagnosis. Finally, in agreement with previous publications, we saw an increase in operative time associated IA, a factor that likely increases the cost of the operation.
The limitations of this study are its retrospective design and the fact that all surgeries were performed by a single surgeon, both of which might limit the generalizability of our findings. However, this design removed the surgeon as a variable in our analysis and ensured that patients received similar postoperative care and surveillance for IH, especially since the techniques were used throughout the four-year time period interchangeably. The standardization of the extraction site limits our ability to discern whether the intracorporeal anastomosis, the extraction site, or both are responsible for the different outcomes in this study. While an intracorporeal anastomosis makes the Pfannenstiel incision feasible without extensive mobilization of the colon, other extraction site incisions are still possible. It is noteworthy that a recent randomized trial comparing transverse and vertical extraction sites in minimally invasive colectomies did not identify a difference in IH rates; however, no intracorporeal anastomoses, and therefore no Pfannenstiel extraction site incisions, were used in this study.37 Finally, while we did not find any specific patient characteristics that were associated with “allocation” to the intracorporeal or extracorporeal technique, selection bias cannot be excluded. Many of these limitations can only be addressed by a prospective, randomized trial.
CONCLUSIONS
Our study suggests that performing an intracorporeal anastomosis with a Pfannenstiel extraction site can reduce the rate of IH and potentially shorten the length of stay after robotic right colectomy. At present, there are several ongoing clinical trials comparing intracorporeal versus extracorporeal anastomoses, with length of stay, recovery, and pain as primary outcomes.38,39 Given the importance of reducing rates of IH for both healthcare utilization and patient-centered outcomes, a randomized trial is needed to validate our findings.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge Margaret McPartland of Memorial Sloan Kettering Cancer Center for her contributions to editing this manuscript.
Funding/Support: This work was funded by the American Society of Colon and Rectal Surgeons—Research in Robotic Surgical Technology Grant and in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Financial Disclaimers: MW and PA have received travel support from Intuitive Surgical, Inc. for robotic training. MK, PS, GMN, and SP have no disclosures. JJS has received travel support from Intuitive Surgical, Inc. for fellow education. JGA has received an honorarium for participating in a webinar by Intuitive Surgical, Inc.
Footnotes
This work was presented as an e-Poster at the American Society of Colon and Rectal Surgeons Annual Scientific Meeting in Nashville, Tennessee, May 19–23, 2018.
REFERENCES
- 1.Moghadamyeghaneh Z, Carmichael JC, Mills S, Pigazzi A, Nguyen NT, Stamos MJ. Variations in laparoscopic colectomy utilization in the United States. Dis Colon Rectum. 2015;58:950–956. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Guillou PJ, Thorpe H, et al. ; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. [DOI] [PubMed] [Google Scholar]
- 3.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75–82. [DOI] [PubMed] [Google Scholar]
- 5.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;(3):CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechaux D. [Intra-corporeal anastomosis in laparoscopic right hemicolectomy]. J Chir (Paris). 2005;142:102–104. [DOI] [PubMed] [Google Scholar]
- 7.Vignali A, Bissolati M, De Nardi P, Di Palo S, Staudacher C. Extracorporeal vs. intracorporeal ileocolic stapled anastomoses in laparoscopic right colectomy: an interim analysis of a randomized clinical trial. J Laparoendosc Adv Surg Tech A. 2016;26:343–348. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Jin C, Hu T, Wei M, Wang Z. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: A systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. 2017;27:348–357. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro R, Keler U, Segev L, Sarna S, Hatib K, Hazzan D. Laparoscopic right hemicolectomy with intracorporeal anastomosis: short- and long-term benefits in comparison with extracorporeal anastomosis. Surg Endosc. 2016;30:3823–3829. [DOI] [PubMed] [Google Scholar]
- 10.van Oostendorp S, Elfrink A, Borstlap W, et al. Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: a systematic review and meta-analysis. Surg Endosc. 2017;31:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna MH, Hwang GS, Phelan MJ, et al. Laparoscopic right hemicolectomy: short- and long-term outcomes of intracorporeal versus extracorporeal anastomosis. Surg Endosc. 2016;30:3933–3942. [DOI] [PubMed] [Google Scholar]
- 12.Widmar M, Keskin M, Beltran P, et al. Incisional hernias after laparoscopic and robotic right colectomy. Hernia. 2016;20:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KH, Ho J, Akmal Y, Nelson R, Pigazzi A. Short- and long-term outcomes of intracorporeal versus extracorporeal ileocolic anastomosis in laparoscopic right hemicolectomy for colon cancer. Surg Endosc. 2013;27:1986–1990. [DOI] [PubMed] [Google Scholar]
- 14.Martinek L, You K, Giuratrabocchetta S, Gachabayov M, Lee K, Bergamaschi R. Does laparoscopic intracorporeal ileocolic anastomosis decreases surgical site infection rate? A propensity score-matched cohort study. Int J Colorectal Dis. 2018;33:291–298. [DOI] [PubMed] [Google Scholar]
- 15.Carnuccio P, Jimeno J, Parés D. Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol. 2014;18:5–12. [DOI] [PubMed] [Google Scholar]
- 16.Jamali FR, Soweid AM, Dimassi H, Bailey C, Leroy J, Marescaux J. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg. 2008;143:762–767. [DOI] [PubMed] [Google Scholar]
- 17.Roscio F, Bertoglio C, De Luca A, Frattini P, Scandroglio I. Totally laparoscopic versus laparoscopic assisted right colectomy for cancer. Int J Surg. 2012;10:290–295. [DOI] [PubMed] [Google Scholar]
- 18.Hellan M, Anderson C, Pigazzi A. Extracorporeal versus intracorporeal anastomosis for laparoscopic right hemicolectomy. JSLS. 2009;13:312–317. [PMC free article] [PubMed] [Google Scholar]
- 19.Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparoscopic vs open colectomy. Surg Endosc. 2002;16:1420–1425. [DOI] [PubMed] [Google Scholar]
- 20.Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34:498–504. [DOI] [PubMed] [Google Scholar]
- 21.Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum. 2005;48:2217–2223. [DOI] [PubMed] [Google Scholar]
- 22.Navaratnam AV, Ariyaratnam R, Smart NJ, Parker M, Motson RW, Arulampalam TH. Incisional hernia rate after laparoscopic colorectal resection is reduced with standardisation of specimen extraction. Ann R Coll Surg Engl. 2015;97:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrucciani N, Sirimarco D, Nigri GR, et al. Robotic right colectomy: A worthwhile procedure? Results of a meta-analysis of trials comparing robotic versus laparoscopic right colectomy. J Minim Access Surg. 2015;11:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baucom RB, Ousley J, Beveridge GB, et al. Cancer survivorship: defining the incidence of incisional hernia after resection for intra-abdominal malignancy. Ann Surg Oncol. 2016;23(suppl 5):764–771. [DOI] [PubMed] [Google Scholar]
- 25.Lee L, Mappin-Kasirer B, Sender Liberman A, et al. High incidence of symptomatic incisional hernia after midline extraction in laparoscopic colon resection. Surg Endosc. 2012;26:3180–3185. [DOI] [PubMed] [Google Scholar]
- 26.Selby LV, Gennarelli RL, Schnorr GC, et al. Association of hospital costs with complications following total gastrectomy for gastric adenocarcinoma. JAMA Surg. 2017;152:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 28.Muysoms FE, Miserez M, Berrevoet F, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Ramshorst GH, Eker HH, Hop WC, Jeekel J, Lange JF. Impact of incisional hernia on health-related quality of life and body image: a prospective cohort study. Am J Surg. 2012;204:144–150. [DOI] [PubMed] [Google Scholar]
- 30.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 31.Veenstra CM, Regenbogen SE, Hawley ST, et al. A composite measure of personal financial burden among patients with stage III colorectal cancer. Med Care. 2014;52:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regenbogen SE, Veenstra CM, Hawley ST, et al. The personal financial burden of complications after colorectal cancer surgery. Cancer. 2014;120:3074–3081. [DOI] [PubMed] [Google Scholar]
- 33.Baucom RB, Beck WC, Holzman MD, Sharp KW, Nealon WH, Poulose BK. The importance of surgeon-reviewed computed tomography for incisional hernia detection: a prospective study. Am Surg. 2014;80:720–722. [PubMed] [Google Scholar]
- 34.Baucom RB, Beck WC, Holzman MD, Sharp KW, Nealon WH, Poulose BK. Prospective evaluation of surgeon physical examination for detection of incisional hernias. J Am Coll Surg. 2014;218:363–366. [DOI] [PubMed] [Google Scholar]
- 35.Claes K, Beckers R, Heindryckx E, et al. Retrospective observational study on the incidence of incisional hernias after colorectal carcinoma resection with follow-up CT scan. Hernia. 2014;18:797–802. [DOI] [PubMed] [Google Scholar]
- 36.Akram WM, Al-Natour RH, Albright J, et al. A propensity score-matched comparison of intracorporeal and extracorporeal techniques for robotic-assisted right colectomy in an Enhanced Recovery Pathway. Am J Surg. 2018;216:1095–1100. [DOI] [PubMed] [Google Scholar]
- 37.Lee L, Mata J, Droeser RA, et al. Incisional hernia after midline versus transverse specimen extraction incision: a randomized trial in patients undergoing laparoscopic colectomy. Ann Surg. 2018;268:41–47. [DOI] [PubMed] [Google Scholar]
- 38.Intracorporeal or extracorporeal anastomosis after laparoscopic right colectomy. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03045107 (ClinicalTrials.gov Identifier: NCT03045107) U.S. National Library of Medicine, 2017. Accessed February 4, 2019. [Google Scholar]
- 39.INtracorporeal Versus EXtracorporeal Anastomosis in Robotic Right Colectomy. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03130166 (ClinicalTrials.gov Identifier: NCT03130166) U.S. National Library of Medicine, 2017. Accessed February 4, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


