Abstract
The loss of fertility and early menopause are common after gonadotoxic therapies and radical pelvic surgery. The strategy of ovarian tissue cryopreservation and auto-transplantation was introduced to prevent this significant quality of health issue. Ovarian transplantation with cryopreserved tissue has gone through remarkable evolution in the last 20 years. In this review, we detail the history and evolution of ovarian transplantation with cryopreserved tissue from its origins to the present. Ovarian cryopreservation and transplantation approach was first tested with animal models. The approach was then validated in human ovarian xenografting models before being applied to patients in pioneering clinical studies. The first orthotopic and heterotopic approaches to ovarian transplantation was developed by Oktay et al. who reported the first successful restoration of ovarian function with these approaches beginning in 2000 with first embryo development in 2004. Controversy remains on when the first live birth occurred after orthotopic ovarian transplantation with cryopreserved tissue as the patient was ovulating with elevated progesterone levels in the case reported in 2004; first live birth is likely to be the one reported by Meirow et al. in 2005. Nevertheless, the technique has evolved to reach a level where most recent live birth rates are exceeding 35% and the procedure is no longer considered experimental by many.
Electronic supplementary material
The online version of this article (10.1007/s43032-019-00066-9) contains supplementary material, which is available to authorized users.
Keywords: Fertility preservation, Cryopreservation, Tissue transplantation, Primary ovarian insufficiency, Drug-related side effects and adverse reactions
Earlier Attempts with Fresh Ovarian Tissue
Though the first successful ovarian auto-transplantation with cryopreserved tissue was not reported until 2000 [1], ovarian transplantation for restoration of fertility has been considered since the end of the nineteenth century. A New York surgeon reported a case of fresh ovarian allografting in 1906 [2] with restoration of endocrinological function confirmed by regular periods after a few months (Fig. 1) [3]. Four years after the grafting, the woman reportedly conceived and delivered a child. However, it is difficult to verify this report because the pregnancy was reported as a “hearsay.” Moreover, an allogeneic ovarian transplant would have required immunosuppressive agents to prevent rejection, which were not available at the time. Nevertheless, this case represents the first report of experimentation with ovarian tissue transplantation prior to the cryopreservation era.
Fig. 1.
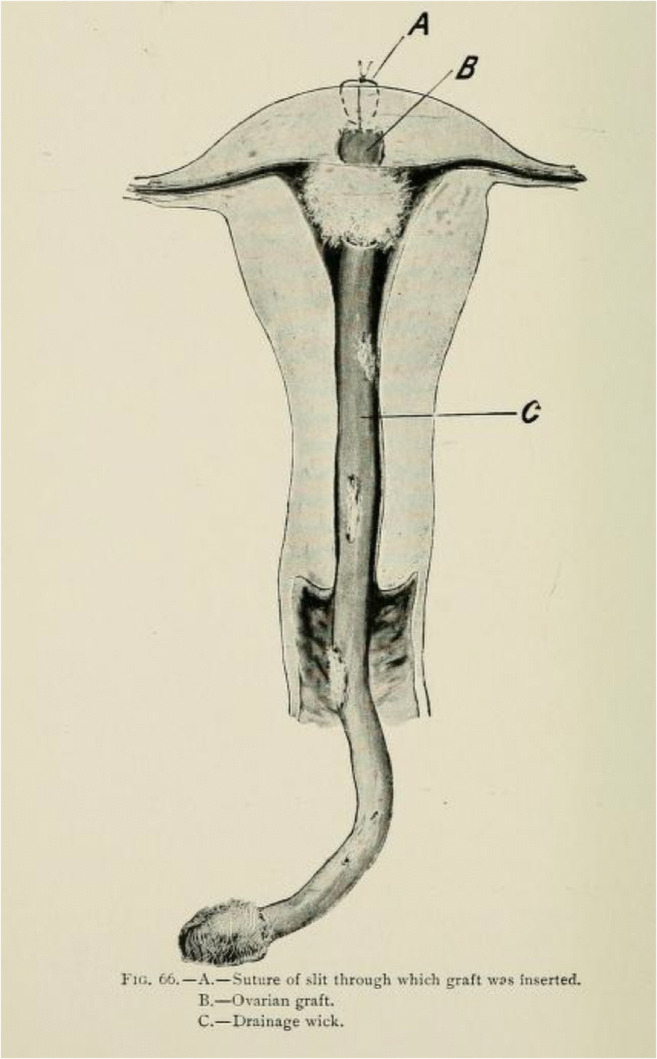
Intrauterine ovarian transplantation as depicted by Morris in 1895. (A) Suture at the site of graft insertion; (B) ovarian graft; (C) drainage wick. In this technique, the surgeon anticipated direct ovulation into the uterus, bypassing fallopian tubes
Evolution of Ovarian Tissue Cryopreservation
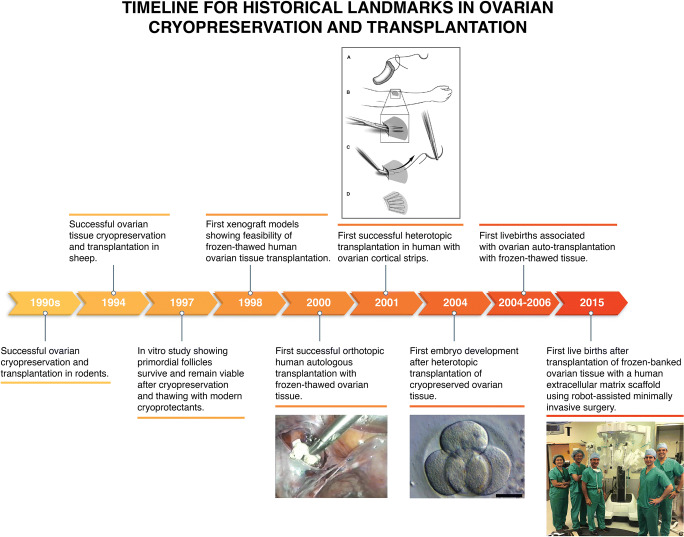
Ovarian transplantation was transformed into a fertility preservation and restoration procedure with the valuable advances made by many animal scientists and researchers (Fig. 2). During the 1950s, numerous researchers studied cryopreservation of ovarian tissue in laboratory animals, rats in particular [4–7]. However, the efforts were largely abandoned until the 1990s because of poor results deriving from the lack of effective cryoprotectants and freezing protocols. In fact, until the 1970s, the only available cryoprotectant was glycerol, which was initially used for sperm freezing in the field of reproduction. When utilized for ovarian tissue freezing, glycerol proved to be ineffective because only about 10% of the primordial follicles survived thawing [7]. In the 1970s, more effective cryoprotectants, including propanediol, ethylene glycol, and dimethyl sulfoxide (DMSO) were discovered, opening the door for advancements in ovarian tissue cryopreservation [8, 9].
Fig. 2.
A brief timeline for the evolution of ovarian auto-transplantation with cryopreserved tissue
In the 1970s, some attempted orthotopic fresh whole-ovary transplantation by means of vascular anastomosis, but this approach could not be used as a fertility preservation approach because it is not technically feasible to efficiently freeze an intact human ovary with its blood vessels. Furthermore, the experiments performed in rabbits [10, 11] and sow [12] resulted in a high incidence of complications such as thrombosis of the anastomotic pedicle and the formation of dense adhesions that encapsulated the ovary. A later study in sheep with frozen whole ovaries also showed limited success and similar complications [13]. Hence at the present time, prospect for whole-ovary cryopreservation and transplantation is unclear in women.
In the 1990s, ovarian tissue cryopreservation and transplantation studies were repeated in rodents using small pieces of ovarian cortex and modern cryoprotectants, resulting in restoration of ovarian endocrine function and fertility [14–16].
Human ovary differs in characteristics from that of mice in that it is much larger and more fibrous. Although not identical to the human counter-part, sheep ovary may serve as a better model. Using the sheep as a model and utilizing DMSO as the cryoprotectant, the first pregnancies after ovarian transplantation were reported in 1994, one from a fresh transplant and another from a frozen-thawed graft [17]. These studies also showed that ovarian tissue frozen as strips can be grafted later, revascularizing spontaneously and producing functional follicle growth; there was no need for a whole-ovary preservation and transplantation, which remains technically infeasible even today.
Though ovarian cortical pieces revascularize spontaneously obviating whole-organ transplants, there is a price to be paid during the ischemic revascularization period in the currency of primordial follicles. Animal studies revealed that ovarian cortical strips revascularize in mice within 48 h of grafting [18]; in sheep, there is a complete revascularization within 1 week [17]. In a xenograft model utilizing sheep ovarian tissue, it was shown that only a small percentage of follicles (7%) was lost during freezing and thawing, while a much larger proportion (68%) was lost during the ischemic phase of tissue revascularization after transplantation [19]. This initial ischemic follicle loss remains to be one of the most significant rate-limiting steps in ovarian transplant success, but as will be discussed later, some promising advances have been made to improve post-ovarian transplant reserve loss.
Determination of the Feasibility of Human Ovarian Tissue Cryopreservation and Transplantation in Laboratory Models
After the establishment of success of ovarian tissue cryopreservation in animal studies using modern cryoprotectants, several groups reported similar success with human ovarian tissue using the same modern cryoprotectants. Hovatta et al. reported in 1996 that human primordial follicles can survive reasonably well with the use of DMSO and propanediol-sucrose as cryoprotectants [20]. Oktay et al. also reported in an in vitro study with human ovarian tissue that primordial follicles survive and remain viable after cryopreservation and thawing with modern cryoprotectants [21].
In 1998, Oktay et al. further demonstrated the feasibility of ovarian cryopreservation and transplantation, this time using human ovarian tissue in a xenograft model. In this model, Oktay et al. showed that the transplanted follicles can grow to the antral stage and can produce estradiol for as long as 6 months [22]. Similarly, Oktay et al. showed that frozen-thawed ovarian xenografts survived and gave rise to follicle growth initiation in the same model [23]. These successful animal models in vivo, as well as human ovarian xenograft and human studies in vitro, suggested that ovarian cryopreservation followed by auto-transplantation could succeed in patients.
Development of the First Successful Human Ovarian Auto-Transplantation Technique with Previously Frozen Tissue: A First Hand Account of the History
Encouraged by the results from animal and human tissue studies [14–18, 24], Dr. Oktay began translating this work to patients as early as in 1996 upon his return from the University of Leeds, where he performed the initial laboratory studies under the tutelage of a pioneer scientist Dr. Roger Gosden; Dr. Oktay obtained the first institutional review board approval for ovarian tissue cryopreservation and transplantation in the USA and began cryopreserving ovarian tissue from cancer patients as early as in 1997.
In 1998, Dr. Oktay received a call from Dr. Gosden who was referring a patient who had her ovarian tissue frozen elsewhere. The patient was looking for a center to have her tissue transplanted back to her, albeit to restore her ovarian endocrine function. Dr. Oktay took on this patient and worked on developing the first ovarian auto-transplantation technique with previously frozen tissue. Dr. Oktay started working on pig models in order to perfect a laparoscopic technique for transplantation. Because this patient had no remaining ovary, the tissues had to be transplanted elsewhere in the pelvis. Inspired by xenograft models, and after experimentation in a pig model, Dr. Oktay decided to graft the tissues under the pelvic peritoneum in the ovarian fossa. Nevertheless, suturing each ovarian piece laparoscopically to the sidewall was technically challenging and time-consuming, likely affecting tissue viability. Dr. Oktay then utilized Surgicel® (Ethicon, Sommerville, N.J.), a polycellulose hemostatic material to form an absorbable scaffold to which he attached the cortical pieces that were strung using a delayed absorbable suture. This triangular scaffold with ovarian cortical pieces attached would be grafted into a pelvic peritoneal pocket laparoscopically. After numerous practice runs with a pig model, the stage was set for the first ovarian transplant with cryopreserved tissue in medical history [1].
Added to this was another innovation, although this has not been reported until now, Dr. Oktay used the first prototype of a surgical robot, the Automated Endoscopic System for Optical Positioning (AESOP), to assist the laparoscopic procedure. This robot was first trained for the surgeon’s voice, who then used voice commands to control the laparoscope from a headset.
In 1999, the procedure went forward. The patient had multiple samples of ovarian cortex, measuring 2 by 2 mm to 5 by 10 mm, which were previously cryopreserved via slow freezing using propanediol as the cryoprotectant. At that time, Dr. Guvenc Karlikaya was a research fellow in Dr. Oktay’s lab. On the day of surgery, Dr. Guvenc Karlikaya thawed the tissues. Dr. Oktay then strung cortical pieces on a delayed absorbable suture and attached 3–4 strings to a scaffold, forming two reconstructed grafts. These were then inserted into the pelvis through an operative port and were sutured under the pelvic peritoneum in the ovarian fossa on one side (See Video 1).
Video 1.
First successful auto-transplantation of frozen thawed ovarian tissue. The video depicts the technique that was used in the first successful ovarian auto-transplantation with frozen-thawed tissue as reported by Oktay & Karlikaya in NEJM 2000. (MP4 58469 kb)
Daily administration of gonadotropins 15 weeks after transplantation resulted in follicular growth and ovulation. Continued function was demonstrated for up to at least 6-month post-transplantation by follicular growth in response to gonadotropin stimulation, confirming the long-term survival of the tissue [1].
After this first successful case, Oktay and colleagues continued to develop additional ovarian transplantation techniques. When transplantation to the pelvis is not technically feasible, alternative transplantation sites may be needed. Drawing on the experience with ovarian xenograft models and based on the successful transplantation of frozen-thawed parathyroid tissue in the forearm [25, 26], Oktay et al. performed the first heterotopic ovarian auto-transplantation techniques, first to the forearm [27, 28] and then to lower abdominal wall, subcutaneously [29]. All of these transplants resulted in restoration of ovarian endocrine function, follicle development, oocyte retrieval, and, in some cases, embryo development, demonstrating the feasibility of the heterotopic ovarian technique [27–29]. Hence the first embryo development from frozen-thawed and transplanted ovarian tissue was reported in 2004 by Oktay et al [29].
Subsequently, live births were reported by Stern et al. with the lower abdominal wall heterotopic ovarian transplantation technique [30, 31]. Interestingly, in one patient that Dr. Oktay performed heterotopic ovarian transplantation to the lower abdomen, four spontaneous pregnancies and three consecutive live births occurred (you can listen to the patient’s account of the story here: https://youtu.be/9XnsrmBIOXM). The patient had undergone bone marrow transplantation and was menopausal for over 2 years. Prior to the ovarian auto-transplantation, multiple ultrasound examinations confirmed that the remaining ovary was atrophic. This report called into question the origins of pregnancies after ovarian transplants and whether the transplanted ovary can help recover the damage in the remaining ovary [32, 33]. There were several speculations to explain the mechanism of conception in this patient. Even though unlikely in the scenario of repetitive pregnancies and prior hematopoietic stem cell transplantation (HSCT), spontaneous recovery of ovarian function in the menopausal ovary, regardless of the ovarian transplant, cannot be ruled out. In fact, there are several reports of spontaneous pregnancies and live births after preconditioning chemotherapy for HSCT [34, 35]. Other speculative explanations for spontaneous pregnancies after heterotopic ovarian transplantation include regenerative signals originating from the transplanted ovary to the damaged ovary to potentially induce recovery and regeneration of primordial germ cells as well as potential germ cell transport from the healthy transplanted ovary to the menopausal ovary [33].
When Did the First Live Births Occur after Auto-Transplantation of Cryopreserved Ovarian Tissue?
In 2004–2005, reports of first live births arrived after laparoscopic transplantation of previously cryopreserved ovarian tissue. Even though a case reported by Donnez et al. was claimed to be the first live birth [36] (Fig. 3A), we and others have expressed concerns about this assessment [37, 38]. Specifically, the patient received low-toxicity gonadotoxic treatment, and she was ovulatory from the residual ovary at the time of ovarian transplantation. The elevated serum progesterone level at the time of ovarian transplantation was later reported by some members associated with that team [39]. Therefore, it is not possible to rule out that the woman would have conceived on her own, regardless of the ovarian cryopreservation or transplantation.
Fig. 3.
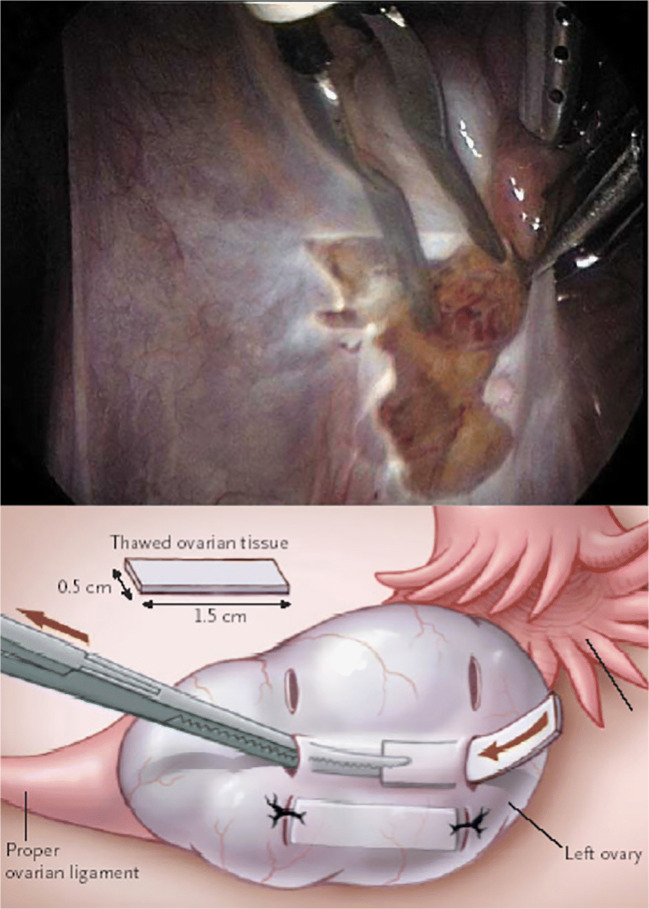
Other techniques of ovarian tissue auto-transplantation. (A) The technique reported by Donnez et al. in 2004 where peritoneal window is created 8 days before the actual deposition of cortical pieces in the same peritoneal pocket without suturing. (B) A technique reported by Meirow et al. in 2005 by inserting thawed ovarian pieces inside the tunnels created under the cortex (left panel) of the remaining menopausal ovary
In 2005, Meirow et al. reported a case where a pregnancy occurred after ovarian auto-transplantation in a patient who experienced premature menopause after high-dose chemotherapy for non-Hodgkin lymphoma. The patient’s ovarian tissue had been harvested after administration of a second-line conventional chemotherapy regimen but before treatment with high-dose chemotherapy with alkylating agents. Patient’s menopausal status was confirmed by 24 months of amenorrhea and elevated levels of endogenous gonadotropins prior to the transplantation [40]. During the surgery, thawed ovarian cortical pieces were transplanted under the cortex of the remaining menopausal ovary (Fig. 3B). Eight months after the auto-transplantation, ultrasound examinations revealed follicle growth and the patient had a menstrual period. Gonadotropin levels returned to the non-menopausal range. She underwent in vitro fertilization and embryo transfer, which resulted in the delivery of a healthy baby at term. Because the patient had received a highly gonadotoxic treatment regimen and was clearly shown to be in ovarian failure prior to ovarian transplantation, to our opinion, the case reported by Meirow et al. is more likely to be the first live birth. Nevertheless, live births in patients who had both ovaries removed prior to ovarian transplantation leave no question that the origin of pregnancies is the transplanted tissue in the majority of the cases [41, 42].
Evolution of Ovarian Transplantation Techniques to Improve Ovarian Tissue Survival and Vascularization
Surgical technique is likely to be a key component in the success of ovarian transplantation with cryopreserved tissue, to reduce the previously discussed loss of primordial follicles that occurs in the first period after transplantation [19, 43]. Several ovarian transplantation techniques have evolved since the first report of the transplantation into pelvic side wall pocket in 2000 [1]. Some suggested improvement of that technique by opening a peritoneal window 1 week prior to the laparoscopic transplantation (Fig. 3A) with the aim of enhancing revascularization [36]. However, there has not been experimental data to support that this approach would improve graft revascularization nor this seems to be practical, as it requires two separate surgeries within a week. Others reported transplantation of frozen-thawed cortical pieces under the ovarian cortex [40, 44] (Fig. 3B) or into the medulla of remaining ovary [45] with or without a fibrin glue, but again, systemic evaluation of these approached have not been reported mainly due to the still rarity of ovarian transplant procedures.
Another key improvement in ovarian transplantation techniques may come from the use of pro-vascularizing agents. Sphingosine-1-phosphate (S1P) is a ceramide-induced death pathway inhibitor with previously proven protective effect against radiation- [46] and chemotherapy-induced [47] apoptotic death. In a human ovarian xenograft model, Oktay laboratory discovered that continuous infusion of S1P can accelerate neovasculogenesis, reduce hypoxia, and maintain primordial follicle density post-ovarian transplantation, thereby improving the likelihood of ovarian transplant success and longevity [43]. While S1P has never been used in humans, its synthetic analog has been approved by FDA for the treatment of multiple sclerosis [48]. Future clinical trials may investigate the role of this synthetic analog in improving ovarian transplant success.
Introduction of Robotic-Assistance and Neovascularizing Human Extracellular Tissue Matrix Scaffold to Improve Ovarian Auto-Transplantation Outcomes
After the first successful ovarian transplantation, Dr. Oktay continued to develop the robotic-assisted techniques which combine the advantages of laparoscopy such as the magnification, reduced post-operative pain, and reduced adhesion formation [49], thereby increasing the possibility of natural conception after ovarian transplantation, with the advantages of open surgery such as the 3D vision and the degrees of freedom of the human wrist. Furthermore, with robotic surgery, more delicate handling of the ovarian tissue, fine anastomosis of the graft to the recipient site, and reduced time from thawing to transplantation may be achieved [50, 51].
Some suggested careful hemostasis to avoid hematoma development that could compromise the attachment of the graft [52]. In the robotic technique, energy use is avoided to control small bleeding as it may compromise the microvascular supply in the recipient site. In addition, to further enhance vascularization and avoid microthrombi, the patients are treated with transdermal estrogen pre-operatively [53]. Prior to transplantation, thawed ovarian tissues are sutured onto a decellularized human extracellular tissue matrix generated from cadaver skin (Alloderm® LifeCell Corp, Branchburg, NJ) which may aid the revascularization process [54] (Video 2). Oktay et al. reported the first pregnancies with robot-assisted ovarian transplantation recently [53]. One woman with hemophagocytic lymphohistiocytosis and another with non-Hodgkin lymphoma underwent ovarian cortical tissue transplantation to the contralateral menopausal ovary with the robot-assisted technique utilizing Alloderm®. In both patients, ovarian tissue harvesting had been performed before preconditioning chemotherapy for hematopoietic stem cell transplantation. The women experienced ovarian failure post-chemotherapy and underwent successful ovarian cortical tissue transplantation 7 and 12 years later. The first patient had multiple embryos cryopreserved and then conceived following her first fresh in vitro fertilization-embryo transfer and delivered a healthy child at term. The patient spontaneously conceived a second child, and delivered at term, as this mansucript was pending publication. She has multiple frozen embryos remaining for future attempts. The second woman underwent multiple IVF cycles to cryopreserve embryos first. She conceived on her first frozen embryo transfer attempt and delivered a healthy child at term. She subsequently underwent another frozen embryo transfer, conceived, and recently delivered her second child. Numerous other patients have undergone successful robot-assisted ovarian transplant procedures, whose results will be reported in the near future. Based on this recently accumulated evidence, robot-assisted approach with Alloderm® scaffold may result in more robust ovarian function compared to conventional ovarian transplant approaches, but data from larger number of women are awaited [48].
Video 2.
Modern technique of frozen-thawed ovarian tissue auto-transplantation. The video depicts the modern technique used for ovarian autotransplantation to the contralateral ovary using robotic assistance and Alloderm. (MP4 189188 kb)
Conclusions
The major advances and progress that have been achieved by numerous researchers in the last 20 years elevated ovarian cryopreservation and transplantation to a level where it may no longer be considered experimental. In fact, American Society of Reproductive Medicine has recently moved to remove this procedure from the experimental category. Based on our recent meta-analysis, 62.3% of women undergoing ovarian cryopreservation and transplantation conceive spontaneously, and 63.9% have prolonged ovarian endocrine function. These are highly encouraging indicators. While the same meta-analysis indicated a 37% cumulative live birth and ongoing pregnancy rate per patient worldwide, with increased experience and improved surgical techniques, current success rates are likely to be higher (Table 1) [55]. Ovarian cryopreservation followed by auto-transplantation offers a unique approach to preserving and restoring natural fertility in both children and women and its true potential is yet to be determined.
Table 1.
Frozen-thawed ovarian tissue transplants outcomes from a recent meta-analysis
| Age at cryopreservation (range) | 29.3 ± 6.5 years (9–44) |
| Age at transplantation (range) | 33.0 ± 5.7 years (13.8–45) |
| Maternal age at delivery | 30.4 ± 4.2 years (23–40) |
| Gestational age at delivery | 38.2 ± 1.8 weeks (33–41.2) |
| Cumulative clinical pregnancy/woman | 57.5% (69/120) |
| Cumulative live birth/woman | 37.7% (65/172) |
| % spontaneously conceiving | 62.3% (48/77) |
| Endocrine function/woman | 63.9% (55/86) |
| Mean graft longevity | 26.9 ± 25.6 months (4–144) |
Funding Information
This report was partially supported by RO1 HD053112 from the Eunice Kennedy Shriver,
National Institute of Child Health and Human Development (NICHD), and National Cancer Institute (KO).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Loris Marin, Email: loris.marin@yale.edu.
Giuliano Bedoschi, Email: giulianobedoschi@gmail.com.
Tai Kawahara, Email: tai.kawahara@yale.edu.
Kutluk H Oktay, Email: info@fertilitypreservation.org.
References
- 1.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. NJEM. 1999;342(25):1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 2.Morris RT. A case of heteroplastic ovarian grafting, followed by pregnancy, and the delivery of a living child. Med Rec. 1906;69:697–698. [PMC free article] [PubMed] [Google Scholar]
- 3.Morris RT. Lectures on appendicitis and notes on other subjects. New York: GR Putnam’s sons; 1985. [Google Scholar]
- 4.Parkes AS, Smith AU. Regeneration of rat ovarian tissue grafted after exposure to low temperature. Proc R Soc Lond B Biol Sci. 1953;140:455–467. doi: 10.1098/rspb.1953.0002. [DOI] [PubMed] [Google Scholar]
- 5.Green SH, Smith AU, Zuckerman S. The number of oocytes in ovarian autografts after freezing and thawing. J Endocrinol. 1956;13:330–334. doi: 10.1677/joe.0.0130330. [DOI] [PubMed] [Google Scholar]
- 6.Deanesly R. Egg survival in immature rat ovaries grafted after freezing and thawing. Proc R Soc Lond B Biol Sci. 1957;147(928):412–421. doi: 10.1098/rspb.1957.0060. [DOI] [PubMed] [Google Scholar]
- 7.Parrot DM. The fertility of mice with orthotopic grafts derived from frozen tissue. J Reprod Fertil. 1960;1:230–241. [Google Scholar]
- 8.Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11:1487–1491. doi: 10.1093/oxfordjournals.humrep.a019423. [DOI] [PubMed] [Google Scholar]
- 9.Oktay K. Ovarian tissue cryopreservation and transplantation: preliminary findings and implications for cancer patients. Hum Reprod Update. 2001;7(6):526–534. doi: 10.1093/humupd/7.6.526. [DOI] [PubMed] [Google Scholar]
- 10.Danjean R, Boeckx W, Gordt S, Brosens I. Ovarian transplantation by selective microvascular-anastomoses in the rabbit. Br J Obstet Gynaecol. 1982;89:652–656. doi: 10.1111/j.1471-0528.1982.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 11.Betteridge KJ. Homotransplantation of ovaries with vascular anastomoses in rabbits: response to transplants to HCG. J Endocrinol. 1970;47:451–461. doi: 10.1677/joe.0.0470451. [DOI] [PubMed] [Google Scholar]
- 12.Harrison FA, Chambers SG, Green EA. Auto-transplantation of the ovary to the neck of the sow. J Endocrinol. 1979;83:46. [PubMed] [Google Scholar]
- 13.Revel A, Elami A, Bor A, Yavin S, Natan Y, Arav A. Whole sheep ovary cryopreservation and transplantation. Fertil Steril. 2004;82(6):1714–1715. doi: 10.1016/j.fertnstert.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Harp R, Leibach J, Black J, Keldahl C, Karow A. Cryopreservation of murine ovarian tissue. Cryobiology. 1994;31:336–343. doi: 10.1006/cryo.1994.1040. [DOI] [PubMed] [Google Scholar]
- 15.Cox SL, Shaw JM, Jenkin G. Transplantation of cryopreserved fetal ovarian tissue to adult recipients in mice. J Reprod Fertil. 1996;107:315–322. doi: 10.1530/jrf.0.1070315. [DOI] [PubMed] [Google Scholar]
- 16.Sztein JM, Sweet H, Farley J, Mobraaten L. Cryopreservation and orthotopic transplantation of mouse ovaries: new approach in gamete banking. Biol Reprod. 1998;58:1071–1074. doi: 10.1095/biolreprod58.4.1071. [DOI] [PubMed] [Google Scholar]
- 17.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at-196 degrees C. Hum Reprod. 1994;9(4):597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 18.Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR. Immature rat ovaries revascularized rapidly after auto-transplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology. 1994;134:1146–1154. doi: 10.1210/endo.134.3.8119153. [DOI] [PubMed] [Google Scholar]
- 19.Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at-196°C. Endocrinology. 1999;140:462–471. doi: 10.1210/endo.140.1.6453. [DOI] [PubMed] [Google Scholar]
- 20.Hovatta O, Silye R, Krausz T. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11:1268–1172. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- 21.Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67(3):481–486. doi: 10.1016/s0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 22.Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13:1133–1138. doi: 10.1093/humrep/13.5.1133. [DOI] [PubMed] [Google Scholar]
- 23.Oktay K, Newton H, Gosden RG. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril. 2000;73(3):599–603. doi: 10.1016/s0015-0282(99)00548-8. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Garcia C, Milenkovic M, Groth K, Dahm-Kahler P, Olausson M, Brannstrom M. Ovarian cortex transplantation in the baboon: comparison of four different intra-abdominal transplantation sites. Hum Reprod. 2011;26(12):3303–3311. doi: 10.1093/humrep/der319. [DOI] [PubMed] [Google Scholar]
- 25.Wells SAJ, Ellis GJ, Gunnells JC, Schneider AB, Sherwood LM. Parathyroid autotransplantation in primary parathyroid hyperplasia. NJEM. 1976;295:57–62. doi: 10.1056/NEJM197607082950201. [DOI] [PubMed] [Google Scholar]
- 26.Wagner PK, Seesko HG, Rothmund M. Replantation of cryopreserved human parathyroid tissue. World J Surg. 1991;15:751–755. doi: 10.1007/BF01665310. [DOI] [PubMed] [Google Scholar]
- 27.Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286(12):1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- 28.Oktay K, Buyuk E, Rosenwaks Z, Rucinski J. A technique for transplantation of ovarian cortical strips to the forearm. Fertil Steril. 2003;80:193–198. doi: 10.1016/s0015-0282(03)00568-5. [DOI] [PubMed] [Google Scholar]
- 29.Oktay K, Buyuk E, Veeck L. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet (London, England) 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 30.Stern CJ, Gook D, Hale LG. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod. 2014;29(8):1828. doi: 10.1093/humrep/deu119. [DOI] [PubMed] [Google Scholar]
- 31.Stern K RG, Gook D, Agresta F, Braat D, Hale L. Are there factors which predict the success of ovarian tissue grafting in onco- fertility patients? Abstract presented at: 32nd annual meeting of the European Society of Human Reproduction and Embryology. 2016:2016. Helsinki, Finland.
- 32.Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod. 2006;21(6):1345–1348. doi: 10.1093/humrep/del007. [DOI] [PubMed] [Google Scholar]
- 33.Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation? Fertil Steril. 2011;95(2):804. doi: 10.1016/j.fertnstert.2010.07.1072. [DOI] [PubMed] [Google Scholar]
- 34.Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R, Appelbaum FR. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87(7):3045–3052. [PubMed] [Google Scholar]
- 35.Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, van Lint M, Powles R, Jackson G, Hinterberger-Fischer M, Kolb HJ, Apperley JF, Late Effects Working Party of the European Group for Blood and Marrow Transplantation Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358(9278):271–276. doi: 10.1016/s0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 36.Donnez J, Dolmans MM, Demylle D. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 37.Oktay K, Tilly J. Livebirth after cryopreserved ovarian tissue autotransplantation. Lancet. 2004;364:2091–2092. doi: 10.1016/S0140-6736(04)17541-7. [DOI] [PubMed] [Google Scholar]
- 38.Wallace WH, Pritchard J. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:2093–2094. doi: 10.1016/S0140-6736(04)17544-2. [DOI] [PubMed] [Google Scholar]
- 39.Hubinont C, Debieve F, Biard JM, Bernard P. Livebirth after cryopreserved ovarian tissue transplantation. Lancet. 2012;380(9837):106. doi: 10.1016/S0140-6736(12)61171-4. [DOI] [PubMed] [Google Scholar]
- 40.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 41.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, Smitz J, Dolmans MM. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–725. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Stern CJ, Gook D, Hale LG, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod (Oxford, England) 2013;28(11):2996–2999. doi: 10.1093/humrep/det360. [DOI] [PubMed] [Google Scholar]
- 43.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen CY, Rosendahl M, Byskov AG, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Human reproduction (Oxford, England) 2008;23(10):2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 45.Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1188–1197. doi: 10.1016/j.bpobgyn.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Paris F, Perez GI, Fuks Z, Haimovitz-Friedman A, Nguyen H, Bose M, Ilagan A, Hunt PA, Morgan WF, Tilly JL, Kolesnick R. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8(9):901–902. doi: 10.1038/nm0902-901. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod. 2014;29(1):107–113. doi: 10.1093/humrep/det391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P, FREEDOMS Study Group A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 49.Jayakumaran J, Patel SD, Gangrade BK, Narasimhulu DM, Pandian SR, Silva C. Robotic-assisted laparoscopy in reproductive surgery: a contemporary review. J Robot Surg. 2017;11(2):97–109. doi: 10.1007/s11701-017-0682-4. [DOI] [PubMed] [Google Scholar]
- 50.Oktay K, Taylan E, Sugishita Y, Goldberg GM. Robot-assisted laparoscopic transplantation of frozen-thawed ovarian tissue. J Minim Invasive Gynecol. 2017;24(6):897–898. doi: 10.1016/j.jmig.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Leal Ghezzi T, Campos CO. 30 years of robotic surgery. World J Surg. 2016;40(10):2550–2557. doi: 10.1007/s00268-016-3543-9. [DOI] [PubMed] [Google Scholar]
- 52.Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, Crawford EC, Gosden RG. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353(1):58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- 53.Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94. doi: 10.1016/j.ajog.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen LA, De Caigny P, Guay NA, Lineaweaver WC, Shokrollahi K. The evidence base for the acellular dermal matrix AlloDerm: a systematic review. Ann Plast Surg. 2013;70(5):587–594. doi: 10.1097/SAP.0b013e31827a2d23. [DOI] [PubMed] [Google Scholar]
- 55.Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24(8):1111–1120. doi: 10.1177/1933719117702251. [DOI] [PubMed] [Google Scholar]