Abstract
Myeloperoxidase (MPO) is positively associated with obesity and diet-induced insulin resistance. Angiopoietin-like protein 6 (ANGPTL6) regulates metabolic processes and counteract obesity through increased energy expenditure. This study aims to evaluate the plasma MPO and ANGPTL6 levels in obese and diabetic individuals as well as MPO association with biochemical markers of obesity. A total of 238 participants were enrolled, including 137 control and 101 type 2 diabetes (T2D) patients. ANGPTL6 and MPO levels and other biomarkers were measured via ELISA. ANGPTL6 levels were significantly higher in the diabetic population and obese individuals. When the group was stratified based on T2D, ANGPTL6 levels were significantly higher in obese-diabetic participants compared with non-obese-diabetics, but obese-non-diabetic individuals had similar ANGPTL6 levels to their controls. MPO levels were higher in obese compared with non-obese participants but did not differ between T2D and control participants. MPO levels were upregulated in obese compared with non-obese in both diabetics and non-diabetics. MPO was positively associated with ANGPTL6, triglyceride, BMI, TNF-alpha, high-sensitivity C-reactive protein, interleukin-6, and plasminogen activator inhibitor-1. Taken together, our findings suggest that both MPO and ANGPTL6 may regulate obesity, although MPO exerts this effect independent of diabetes while ANGPTL6 may have a modulatory role in diabetes.
Subject terms: Obesity, Type 2 diabetes
Introduction
Obesity is a major global health concern that affected over 650 million individuals worldwide in 20161. At 18 years of age, individuals with body mass index (BMI) ≥ 30 kg/m2 have an increased lifetime risk of 55%–74% for diabetes2. In addition to the impact of obesity on the risk of diabetes, it is associated with an increased risk of cardiometabolic disorders, such as dyslipidemia, hypertension, heart failure, and atherosclerotic cardiovascular disease as well as decreased quality of life3,4.
Type 2 diabetes (T2D) occurs due to a progressive decline in the ability of the pancreas to secrete enough insulin as well as insulin resistance in insulin target tissues. The pathophysiology of T2D is characterized by excessive accumulation of ectopic fat in the liver, pancreas, and skeletal muscles, eventually manifesting as insulin resistance in these tissues and pancreatic beta cell dysfunction that ultimately leads to hyperglycemia5.
Obese mice have elevated plasma neutrophil levels6. Neutrophil infiltration into the white adipose tissue is an early event that promotes the development of diet-induced obesity and insulin resistance7. Similarly, patients who lose weight have decreased circulating neutrophil and monocyte levels8. As a result of chemokine production by neutrophils, macrophages are recruited into the adipose tissues where they secrete cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 beta, causing obesity-mediated inflammation and impaired insulin signaling9. Myeloperoxidase (MPO) is the most abundant protein in human neutrophils, and it plays a major role in inflammation, oxidative stress, lipoprotein oxidation, and atherosclerosis10–12. Mice deficient in MPO are resistant to diet-induced obesity and insulin resistance, indicating that MPO may have a regulatory role in obesity13. Furthermore, the inhibition of MPO activity in the neutrophils via a non-specific peroxidase inhibitor decreased diet-induced insulin resistance in obese wild-type mice14. Obesity is one of the most common causes of insulin resistance. The main hallmark of obesity is chronic inflammation in the liver, skeletal muscles, and adipose tissues, which is otherwise known as insulin target tissues15. Moreover, MPO was associated with the increased risk of cardiovascular disease in prediabetic participants16. Furthermore, MPO and its reactive oxidants mediate the pathogenesis of numerous acute and chronic inflammatory conditions17,18.
Angiopoietin-like proteins (ANGPTLs) are a family of eight secreted glycoproteins with structural homology to angiopoietins, which are mainly involved in angiogenesis19. ANGPTL3, ANGPTL4, and ANGPTL8 are the most well-studied members of this family due to their important role in regulating lipid metabolism via inhibiting lipoprotein lipase activity20–22. Several ANGPTL proteins have been associated with obesity, insulin resistance, and diabetes23–25. ANGPTL6, also known as angiopoietin-related growth factor, is a liver-derived secreted protein that is initially involved in promoting angiogenesis, epidermal cell proliferation, and wound healing26,27. Animal knockout and transgenic studies have shown that ANGPTL6 counteracts diet-induced obesity and insulin resistance via increasing energy expenditure28. Furthermore, patients with T2D have elevated serum ANGPTL6 levels, and a positive association was observed between elevated serum ANGPTL6 levels and fasting blood glucose levels29–31. However, the role of both ANGPTL6 and MPO in both obesity and diabetes in humans has not been determined.
Thus, this study aimed to evaluate the plasma MPO and ANGPTL6 levels in both obese and T2D patients and to assess their association with the biochemical markers of obesity, inflammation, and atherosclerosis.
Materials and Methods
Study population and ethical approval
The study cohort comprised 238 participants, including 101 patients diagnosed with T2D and 137 participants without T2D. The participants were stratified according to BMI and were classified as either non-obese (19.5 ≤ BMI < 30 kg/m2) or obese (30 ≤ BMI < 40 kg/m2). Based on this stratification, the non-diabetic participants included 77 non-obese and 60 obese participants, and the T2D patients included 36 non-obese and 65 obese patients. The study was approved by the ethical review board of Dasman Diabetes Institute and was conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all participants before the initiation of the study. The exclusion criteria were as follows: patients with type 1 diabetes, morbidly obese participants (BMI ≥ 40 kg/m2), those with a history of major illness or taking any medication and/or supplements known to influence body composition or bone mass, and those who engaged in any physical exercise within the last 6 months.
Blood collection and biochemical measurements
Blood samples were collected from 238 study participants, and plasma was prepared using vacutainer EDTA tubes. Plasma samples were aliquoted and stored at −80 °C until assayed, as described previously32. Fasting plasma glucose, triglyceride, total cholesterol, low-density lipoprotein, and high-density lipoprotein were measured with Siemens Dimension RXL chemistry analyzer (Diamond Diagnostics, Holliston, MA). HbA1c levels were measured using the Variant device (Bio-Rad Laboratories, Hercules, CA). Hypertension and hyperlipidemia were self-reported by patients when they were asked if they suffered from other conditions.
Plasma ANGPTL6 and MPO levels
The plasma ANGPTL6 and MPO levels were measured using enzyme-linked immunosorbent assay (ELISA) kits purchased from EIAab. The plasma samples were thawed on ice and centrifuged at 10,000 g for 5 min at 4 °C to remove any debris32. The ELISA kit was validated using recombinant ANGPTL6 and MPO at a known concentration in the plasma. A plasma dilution of 1:25 showed linearity and was used in the assay. The intra-assay coefficients of variation ranged from 7.5% to 9.2%, and the inter-assay coefficients of variation ranged from 7.9% to 9.6%.
Plasma biochemical marker levels
The plasma leptin, adiponectin, and PAI-1 levels were measured using multiplexing immunobead array according to the manufacturer’s instructions (R&D Systems). The data were processed using the Bio-Plex Manager Software version 6 (Bio-Rad), with five parametric curve fitting. hsCRP was measured using ELISA, as previously reported33.
Statistical analysis
Some of the continuous parameters measured in Tables 1–3 are normally distributed while some are not, therefore, data are expressed as Median (IQR) and Mann-Whitney U test was used to compare between the different groups. Otherwise, a student’s t-test was utilized and data are expressed as mean ± standard deviation (SD) when normally distributed. Pearson’s partial correlation coefficients were calculated to determine the association between MPO levels and some biochemical markers for obesity, inflammation, and atherosclerosis, such as ANGPTL6, hsCRP, TNF-α, IL-6, PAI-1, and triglyceride levels, and other variables after adjusting for age, BMI and gender. Multivariate logistic regression analysis was performed to evaluate the association between ANGPTL6 or MPO and the outcome of diabetes and obesity. Two sets models were created for the outcomes, Diabetes and Obesity. Both the models were adjusted for demographic factors such as age as a continuous variable and gender as a dichotomous variable. Statistical assessments were two-sided, and a P value <0.05 was considered significant. All analyses were performed using R Software.
Table 1.
Demographics and characteristics of the study subjects.
| Non-Diabetes (n = 137) | Diabetes (n = 101) | p-value | |
|---|---|---|---|
| Age | 43.00 (31.00–53.00) | 54.00 (49.00–57.00) | <0.001 |
| Gender (Male) | 52 (38.0%) | 54 (53.47%) | <0.05 |
| BMI | 28.70 (24.56–33.08) | 32.47 (28.52–34.78) | <0.001 |
| Waist/Hip Ratio | 0.86 (0.80–0.92) | 0.94 (0.89–1.00) | <0.001 |
| TC (mmol/L) | 5.16 (4.50–5.90) | 4.80 (3.85–5.80) | <0.05 |
| HDL (mmol/L) | 1.33 (1.08–1.52) | 1.09 (0.89–1.29) | <0.001 |
| LDL (mmol/L) | 3.30 (2.60–3.90) | 2.90 (2.07–3.90) | <0.05 |
| TGL (mmol/L) | 0.92 (0.63–1.42) | 1.38 (1.06–1.90) | <0.001 |
| FPG (mmol/L) | 5.10 (4.80–5.60) | 6.95 (6.00–9.38) | <0.001 |
| HbA1c (DCCT %) | 5.60 (5.30–5.90) | 7.60 (6.40–8.80) | <0.001 |
| Insulin (mIU/L) | 10.23 (6.21–22.84) | 16.75 (7.88–27.24) | 0.08 |
| Adiponectin (μg/mL) | 4.46 (2.70–6.82) | 3.02 (1.91–4.83) | <0.001 |
| Leptin (ng/mL) | 6.98 (3.85–10.17) | 6.35 (4.34–10.29) | 0.89 |
| PAI-1 (ng/mL) | 13.67 (11.17–18.38) | 15.12 (12.81–18.65) | 0.10 |
| IL-6 (pg/mL) | 16.56 (13.62–20.12) | 17.22 (14.92–20.98) | 0.30 |
| TNF alpha (pg/mL) | 123.45 (103.15–153.18) | 128.09 (108.70–159.69) | 0.46 |
| hsCRP(μg/mL) | 1.89 (0.76–4.43) | 2.53 (1.24–6.65) | <0.01 |
| Hypertension | 16 (11.7%) | 57 (56.4%) | <0.001 |
| Hyperlipidemia | 13 (9.5%) | 34 (33.7%) | <0.001 |
Median (IQR) presented for continuous variables and N (%) presented for categorical variables. P values were calculated using Mann–Whitney U test for continuous variables and chi-squared test for categorical variables. BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; hsCRP, high sensitivity c-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; PAI-1, plasminogen activator inhibitor-1; TGL, triglycerides; TNF alpha, tumor necrosis factor alpha.
Table 3.
Demographics and characteristics of patients with type 2 diabetes.
| Non-obese (n = 36) | Obese (n = 65) | p-value | |
|---|---|---|---|
| Age | 55.00 (47.75–58.00) | 53.00 (49.00–57.00) | 0.95 |
| Gender (Male) | 20 (55.6%) | 34 (52.3%) | 0.92 |
| BMI | 27.21 (25.21–28.95) | 34.19 (32.67–35.72) | <0.001 |
| Waist/Hip Ratio | 0.93 (0.87–0.97) | 0.95 (0.91–1.02) | 0.08 |
| TC (mmol/L) | 4.50 (3.85–5.65) | 4.90 (4.02–5.82) | 0.47 |
| HDL (mmol/L) | 1.04 (0.90–1.21) | 1.11 (0.88–1.33) | 0.56 |
| LDL (mmol/L) | 2.75 (2.02–3.70) | 3.05 (2.09–3.90) | 0.91 |
| TGL (mmol/L) | 1.32 (0.80–1.90) | 1.38 (1.10–1.88) | 0.43 |
| FPG (mmol/L) | 6.70 (5.70–8.70) | 7.30 (6.40–10.40) | <0.05 |
| HbA1c (DCCT %) | 6.40 (5.90–7.60) | 8.15 (6.80–9.15) | <0.001 |
| Insulin (mIU/L) | 17.51 (8.95–31.03) | 15.49 (7.88–26.16) | 0.69 |
| Adiponectin (μg/mL) | 2.93 (1.94–3.87) | 3.33 (1.91–4.98) | 0.43 |
| Leptin (ng/mL) | 5.81 (3.14–8.10) | 7.85 (5.36–10.88) | <0.05 |
| PAI-1 (ng/mL) | 16.37 (14.92–20.98) | 13.85 (11.69–17.43) | <0.05 |
| IL-6 (pg/mL) | 18.47 (15.95–21.95) | 16.54 (13.96–20.27) | 0.15 |
| TNF alpha (pg/mL) | 140.41 (125.35–168.66) | 117.61 (105.84–146.38) | <0.01 |
| hsCRP(μg/mL) | 1.67 (0.74–4.75) | 3.59 (2.07–7.79) | <0.001 |
| Hypertension | 18 (50.0%) | 39 (60.0%) | 0.45 |
| Hyperlipidemia | 13 (36.1%) | 21 (32.3%) | 0.87 |
Median (IQR) presented for continuous variables and N (%) presented for categorical variables. P values were calculated using Mann–Whitney U test for continuous variables and chi-squared test for categorical variables. BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; hsCRP, high sensitivity c-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; PAI-1, plasminogen activator inhibitor-1; TGL, triglycerides; TNF alpha, tumor necrosis factor alpha.
Results
Characteristics of the study population
Table 1 shows the demographic and clinical characteristics of our study population. Patients with T2D had significantly higher age, BMI, waist/hip ratio, triglyceride (TGL), fasting blood glucose (FBG) and HbA1c but lower levels of adiponectin, total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) than normal participants (P value <0.05). Furthermore, patients with T2D had significantly higher plasma high-sensitivity C-reactive protein (hsCRP), hypertension, and hyperlipidemia. Tables 2 and 3 show the characteristics of obese patients with or without T2D. Obese participants were more likely to have a significantly higher BMI, FPG, leptin, and hsCRP levels irrespective of diabetes status than non-obese participants. In patients without T2D, obese participants had significantly higher waist/hip ratio, triglyceride (TGL), insulin, and PAI-1, but lower high-density lipoprotein (HDL) levels than non-obese participants. Meanwhile, in patients with T2D, obese patients had significantly higher glycated hemoglobin (HbA1c) levels but lower PAI-1 and TNF-α levels than non-obese patients.
Table 2.
Demographics and characteristics of the non-diabetic subjects.
| Non-Obese (n = 77) | Obese (n = 60) | p-value | |
|---|---|---|---|
| Age | 41.00 (31.00–52.00) | 46.00 (33.75–56.00) | 0.09 |
| Gender (Male) | 28 (36.4%) | 24 (40.0%) | 0.79 |
| BMI | 24.71 (22.46–26.86) | 34.02 (31.60–36.16) | <0.001 |
| Waist/Hip Ratio | 0.84 (0.79–0.89) | 0.90 (0.82–0.97) | <0.01 |
| TC (mmol/L) | 5.00 (4.35–5.75) | 5.20 (4.60–5.90) | 0.41 |
| HDL (mmol/L) | 1.36 (1.09–1.66) | 1.17 (1.01–1.44) | <0.05 |
| LDL (mmol/L) | 3.10 (2.50–3.90) | 3.50 (2.80–3.85) | 0.24 |
| TGL (mmol/L) | 0.81 (0.60–1.09) | 1.12 (0.66–1.55) | <0.05 |
| FPG (mmol/L) | 5.07 (4.80–5.47) | 5.20 (4.90–5.80) | <0.05 |
| HbA1c (DCCT %) | 5.50 (5.30–5.80) | 5.70 (5.38–5.93) | 0.08 |
| Insulin (mIU/L) | 7.71 (5.21–15.14) | 15.43 (8.10–29.18) | <0.001 |
| Adiponectin (μg/mL) | 4.88 (3.21–7.40) | 4.07 (2.45–5.57) | 0.06 |
| Leptin (ng/mL) | 5.19 (2.69–7.84) | 10.07 (6.79–13.51) | <0.001 |
| PAI-1 (ng/mL) | 13.05 (10.01–16.73) | 15.65 (12.55–19.13) | <0.01 |
| IL-6 (pg/mL) | 16.56 (13.19–19.77) | 16.52 (14.17–20.89) | 0.56 |
| TNF alpha (pg/mL) | 122.25 (104.67–160.75) | 125.95 (102.36–141.80) | 0.54 |
| hsCRP(μg/mL) | 1.06 (0.55–3.04) | 3.07 (1.69–6.53) | <0.001 |
| Hypertension | 8 (10.4%) | 8 (13.3%) | 0.79 |
| Hyperlipidemia | 6 (7.8%) | 7 (11.7%) | 0.64 |
Median (IQR) presented for continuous variables and N (%) presented for categorical variables. P values were calculated using Mann–Whitney U test for continuous variables and chi-squared test for categorical variables. BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; hsCRP, high sensitivity c-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; PAI-1, plasminogen activator inhibitor-1; TGL, triglycerides; TNF alpha, tumor necrosis factor alpha.
Differential expression levels of ANGPTL6 and MPO in patients with T2D or obesity
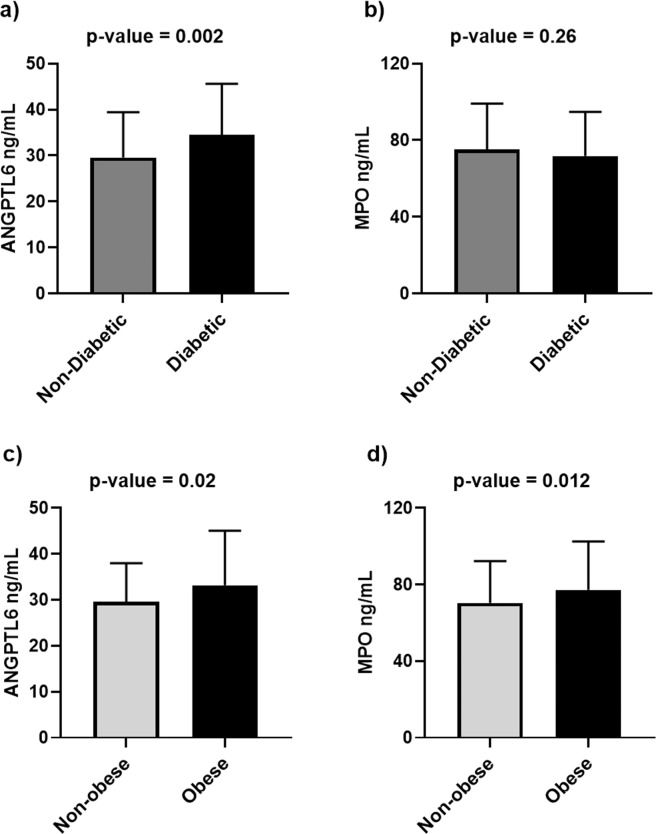
As shown in Fig. 1, patients with T2D had significantly elevated plasma ANGPTL6 levels (34.45 ± 11.16 ng/mL) compared to non-diabetic participants (29.57 ± 9.86 ng/mL) (Fig. 1a). By contrast, the MPO plasma levels did not differ between patients with T2D and healthy participants (Fig. 1b). When the population was stratified based on obesity status, both the plasma ANGPTL6 and MPO levels were significantly higher in obese participants (ANGPTL6 level: 33.19 ± 11.89 ng/mL and MPO level: 77.29 ± 25.22 ng/mL) than in non-obese controls (ANGPTL6 level: 29.58 ± 8.4 ng/mL and MPO level: 70.48 ± 21.76 ng/mL) (Fig. 1c,d).
Figure 1.
Plasma levels of ANGPTL6 and MPO in the population based on their diabetes or obesity status. (a) Plasma levels of ANGPTL6 in non-diabetic and diabetic subjects. (b) Plasma levels of MPO in non-diabetic and diabetic subjects. (c) Plasma levels of ANGPTL6 in non-obese and obese subjects. (d) Plasma levels of MPO in non-obese and obese subjects. Data are presented as mean ± SD. P values were determined using student’s t-test.
Increased levels of ANGPTL6 and MPO in obese participants with T2D
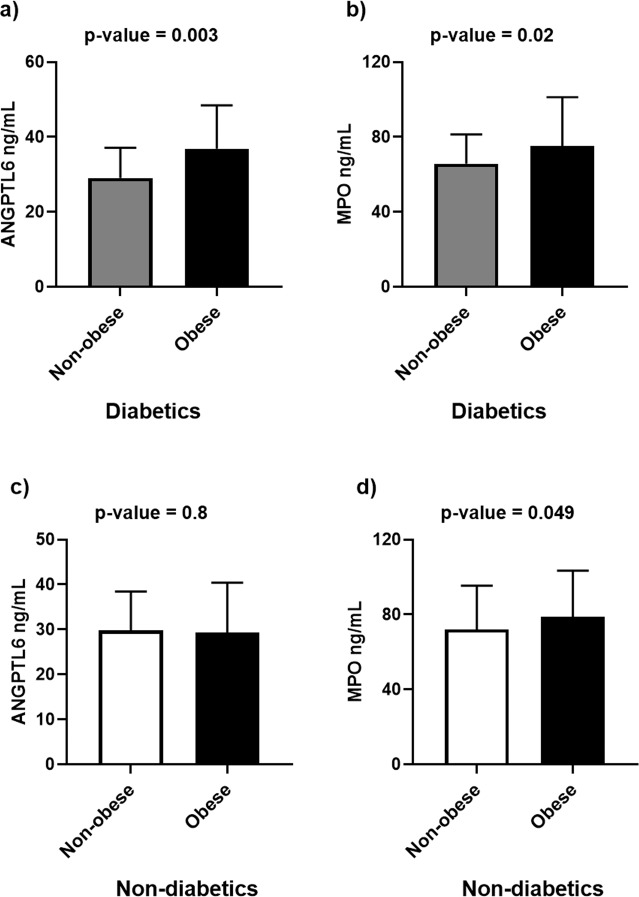
In patients with T2D, both the plasma ANGPTL6 and MPO levels were significantly higher in obese participants than in non-obese participants (ANGPTL6 level: 36.86 ± 11.55 ng/mL vs. 29.05 ± 8.08 ng/mL; P value = 0.003 and MPO level: 75.28 ± 25.94 ng/mL vs. 65.71 ± 15.64 ng/mL; P value = 0.042) (Fig. 2a,b). In contrast, in patients without diabetes, the ANGPTL6 plasma levels did not differ between obese and non-obese participants (Fig. 2c). However, the MPO plasma levels were significantly higher in the obese non-diabetic participants (78.76 ± 24.73 ng/mL) than in non-obese participants (72.08 ± 23.29 ng/mL) (Fig. 2d).
Figure 2.
Plasma levels of ANGPTL6 and MPO in diabetic patients and non-diabetic subjects based on obesity status. (a) Plasma levels of ANGPTL6 in non-obese and obese diabetic patients. (b) Plasma levels of MPO in non-obese and obese diabetic patients. (c) Plasma levels of ANGPTL6 in non-obese and obese non-diabetic subjects. (d) Plasma levels of MPO in non-obese and obese non-diabetic subjects. Data are presented as mean ± SD. P values were determined using student’s t-test.
Correlation between levels of MPO and ANGPTL6 as well as some metabolic markers
We assessed the correlation between MPO and various markers in the non-diabetic population (Table 4). The plasma MPO levels were positively associated with ANGPTL6 (r = 0.17; P < 0.05), TGL (r = 0.16; P < 0.05), TNF alpha (r = 0.19; P < 0.05), hsCRP (r = 0.15; P < 0.05), IL-6 (r = 0.22; P < 0.01), and PAI-1 levels (r = 0.21; P < 0.05) after adjusting for age and BMI. When the analysis was performed after adjusting for age and gender, this association was still observed with TNF alpha (r = 0.22; P < 0.05), hsCRP (r = 0.25; P < 0.01), IL-6 (r = 0.30; P < 0.01), and PAI-1 levels (r = 0.22; P < 0.05) but was lost for ANGPTL6 and TGL.
Table 4.
Partial correlation between MPO plasma levels and biochemical markers for obesity, inflammation, and atherosclerosis.
| Biochemical Marker | Correlation (r) (Adjusted for Age and BMI) | P-value | Correlation (r) (Adjusted for Age and Gender) | P-value |
|---|---|---|---|---|
| ANGPTL6 | 0.17 | <0.05 | 0.11 | 0.27 |
| BMI | 0.20# | <0.01 | 0.19 | <0.05 |
| TGL | 0.16 | <0.05 | 0.042 | 0.64 |
| TNF alpha | 0.19 | <0.05 | 0.22 | <0.05 |
| HsCRP | 0.15 | <0.05 | 0.25 | <0.01 |
| IL-6 | 0.22 | <0.01 | 0.30 | <0.01 |
| PAI-1 | 0.21 | <0.05 | 0.22 | <0.05 |
#Adjusted for Age.
Association between ANGPTL6 or MPO levels and T2D and obesity outcomes
Multivariate logistic regression analysis was performed to ascertain the association between MPO, ANGPTL6 and the outcome of T2D and obesity. ANGPTL6 was associated with an increased risk of T2D (adjusted OR [95% CI] = 1.04 [1.01–1.08]) and obesity (adjusted OR [95% CI]= 1.03 [1.01–1.06]). The association between MPO and T2D and obesity was not statistically significant (Supplementary Table S1).
Discussion
The present study aimed to determine the plasma ANGPTL6 and MPO levels in both obese and diabetic individuals to validate their role in these conditions and to assess any association between MPO levels and the markers of obesity, inflammation, and atherosclerosis. Although the MPO level is upregulated in obese individuals irrespective of the diabetes status, the ANGPTL6 level is elevated in diabetic patients regardless of their BMI, which reflects the unique physiological function of each of these markers and emphasizes the need for comprehensive studies to better understand these functions and utilize them for diagnosis and therapeutic purposes.
Interestingly, our findings indicated that patients with T2D had higher plasma ANGPTL6 levels, but not MPO levels, than normal participants. Such finding was in accordance with that of two other studies conducted in Germany29,34 on patients with T2D, with one study showing a positive correlation between ANGPTL6 and fasting blood glucose. Similarly, serum ANGPTL6 levels were significantly higher in Asian participants who had impaired glucose tolerance than in healthy controls31. This finding was also observed in patients with gestational diabetes35. In contrast, in one study about obesity (BMI > 40 kg/m2), no difference was observed in terms of ANGPTL6 levels between patients with T2D and control participants30. This discrepancy may be attributed to the small number of diabetic patients enrolled in the study. An animal knockout model provided further evidence highlighting the association between ANGPTL6 levels and diabetes, where the targeted disruption of the ANGPTL6 gene led to obesity and insulin resistance in mice28. Similarly, in previous studies, an association between other ANGPTLs and T2D is observed. For example, patients with T2D had elevated serum ANGPTL4, ANGPTL5, and ANGPTL8 levels, and a positive correlation was noted between ANGPTL4, ANGPTL5, and ANGPTL8 levels and fasting blood glucose level23,32,36.
The association between ANGPTL6 level and glucose metabolism is not fully understood. In DIO mice, treatment with ANGPTL6 increases adenosine monophosphate-activated protein kinase activity, which can ultimately improve insulin signaling in the skeletal muscles. Similarly, treatment of C2C12 myoblasts with ANGPTL6 can also enhance the insulin signaling pathways37. Another study on hepatocytes has shown that ANGPTL6 can reduce liver gluconeogenesis since it can decrease the expression of glucose-6-phosphatase via reducing the transcriptional activity of FoxO1 that results from the activation of PI3K/Akt signaling cascades38. Furthermore, another study has shown an increase in both hepatic and circulating ANGPTL6 levels in an obese diabetic mouse model with liver-specific deletion of glucose-6-phosphatase39. Taken together, our data and previous findings propose that the increase in ANGPTL6 levels in diabetes is a compensation mechanism in response to insulin resistance. In fact, a recent study has indicated that the increase in serum ANGPTL6 levels may be a predictor of metabolic syndrome40. In this prospective cohort study, serum ANGPTL6 levels in 221 participants without metabolic syndrome were assessed at baseline and were then followed-up after an average of 3 years. ANGPTL6 levels significantly increased before the development of metabolic syndrome and its complications; therefore, measuring ANGPTL6 levels can significantly improve the prediction of metabolic syndrome.
Our findings showing that the MPO levels did not differ between type 2 diabetic patients and control participants were in accordance with the findings of three clinical studies41–43. Nonetheless, the role of MPO in diabetes is still ambiguous as the findings are contrasting. For example, other clinical studies have shown elevated MPO levels in individuals with T2D44–48. Animal studies have shown a higher MPO activity in the vessels of diabetic Zucker rats than in non-diabetic rats49 and that mice deficient in MPO had improved insulin signaling and were resistant to diet-induced obesity13. In addition to the differences in the methodology in these studies, variations in MPO levels have been reported in a variety of conditions, such as cancer, impaired leukocyte function in diabetes, cardiovascular diseases, endothelial dysfunctions, and pregnancy (based on glycemic control) and in patients taking fenofibrate drugs50–55.
Inflammation plays a pathogenic role in the development of T2D. Our findings showing the elevated levels of hsCRP in patients with T2D are in accordance with those of previous studies56–59. It was also not surprising to have significantly higher numbers of T2D patients who have hypertension or hyperlipidemia compared with non-diabetic participants (Table 1). Obesity is associated with chronic inflammation due to the infiltration of macrophages in the adipose tissue. When a higher number of macrophages is infiltrated, a higher number of pro-inflammatory cytokines and acute-phase molecules is produced by the visceral fat60. A constant increase in inflammatory markers contributes to the development of chronic inflammation, atherosclerosis, and macrovascular disease in obese and diabetic patients61–63. Understanding the function of these inflammatory molecules can reveal the molecular association between obesity and metabolic complications.
Although ANGPTL6 is known to play a role in increasing energy expenditure and protecting against diet-induced obesity in mice28, its role in obesity among humans has rarely been assessed. After classifying patients with T2D according to obesity status, our findings showed that both plasma ANGPTL6 and MPO levels were significantly higher in obese than in non-obese diabetic patients. Interestingly, one study has reported that patients with metabolic syndrome who had higher waist circumference and BMI had higher serum ANGPTL6 levels than healthy controls31. However, considering the lack of sample segmentation based on obesity status, a clear conclusion cannot be obtained based on such findings. In this study, the high ANGPTL6 levels in patients with T2D may either indicate that this protein has a protective role against obesity, as highlighted in a previous animal study28, or that obesity may induce ANGPTL6 resistance in humans, as previously reported in a study about leptin64. Interestingly, other ANGPTLs have also been associated with obesity. For instance, serum ANGPTL4 levels were significantly higher in obese patients with impaired glucose tolerance than in lean healthy participants, and a positive correlation was observed between serum ANGPTL4 levels as well as BMI, waist circumference, fat mass, and triglyceride levels25. Furthermore among non-diabetic participants, serum ANGPTL3, ANGPTL4, and ANGPTL8 levels were higher in obese than in non-obese participants23.
By contrast, only one study has assessed the correlation between obesity and BMI with MPO levels in diabetic patients47 and has shown that obese diabetic patients had higher MPO levels than normal weight or underweight diabetic patients. However, the same pattern for elevated MPO levels was observed in normal participants as they become obese. This result is expected as several studies have shown that circulating MPO levels were more likely to be higher in obese participants, including children, than in normal weight participants11,12,47,65,66. The present data showed that MPO levels are elevated in obesity irrespective of diabetes status. Interestingly, after classifying healthy participants (non-diabetics) according to obesity status in this study, the plasma ANGPTL6 levels did not significantly differ between obese and non-obese participants, indicating that it may be dependent on the diabetes status. This result is in accordance with the findings of another study conducted in Czech Republic30.
Finally, a significant positive correlation was observed between MPO level and other biochemical markers, such as ANGPTL6, triglyceride, BMI, TNF-α, hsCRP, IL-6, and PAI-1. Furthermore, ANGPTL6 was associated with an increased risk of T2D and obesity. In relation to these findings, previous studies have reported an association between MPO as well as some pro-inflammatory markers and insulin resistance12,16,55,67–69. However, no study has reported any association between MPO and ANGPTL6. In addition, the inhibition of MPO was found useful in preventing the production of pro-oxidants and insulin resistance; therefore, it can be a potential treatment for obesity and insulin resistance. However, whether reducing the expression of MPO or just inhibiting its activity is sufficient remains to be fully elucidated.
The present study had several limitations that must be discussed. First, the mechanism of ANGPTL6 and MPO in obesity or T2D was not identified due to the cross-sectional design of the study. Thus, further longitudinal studies about the causal relationship between ANGPTL6 and MPO in T2D and obesity must be conducted. Second, our study did not include data on diet and lifestyle changes in obese or T2D patients, thus making it difficult to evaluate the effect of confounding variables. Finally, the possible effects of anti-diabetic medications on ANGPTL6 and MPO levels were not assessed, even though fenofibrates are known to lower MPO levels.
Conclusions
In this study, the role of MPO and ANGPTL6 on diabetes and obesity was assessed, and results showed that although both markers are elevated in obese participants, diabetes status can affect their physiological function. Thus, future studies should evaluate the beneficial effects of targeting MPO and ANGPTL6 simultaneously for the treatment of both obesity and T2D by inhibiting the activity of MPO and overexpression of ANGPTL6. In addition, the two markers may be affected at an earlier time before the development of the disease; therefore, it could be targeted using a preventative approach.
Supplementary information
Acknowledgements
We are grateful to the Clinical Laboratory and the Biobank Core Facility at DDI for their contribution in handling samples. This research was funded by Kuwait Foundation for the Advancement of Sciences (KFAS) and Dasman Diabetes Institute (RA-2016-025).
Author contributions
M.Q.: Data analysis and interpretation and manuscript writing. I.A., P.C., & M.H.: ELISA assay, sample processing and storage, and immunohistochemistry analysis. M.A.L.: Data interpretation and critical revision of the manuscript. A.C. and T.A.T.: Statistical Analysis. F.A.: Clinical data collection and critical revision of the manuscript. J.A.: Study design, data interpretation and critical revision of the manuscript. M.A.: Study design, data interpretation, directed the laboratory investigation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamed Abu-Farha, Email: mohamed.abufarha@dasmaninstitute.org.
Jehad Abubaker, Email: jehad.abubakr@dasmaninstitute.org.
Supplementary information
is available for this paper at 10.1038/s41598-020-63149-7.
References
- 1.World Health Organization. Obesity and overweight fact sheet. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Published 2017. Accessed May 5, 2019.
- 2.Narayan KM, Boyle J, Thompson T, Gregg EW, Williamson D. Effect of bmi on lifetime risk for diabetes research design and. Diabetes Care. 2007;30(6):1562–1566. doi: 10.2337/dc06-2544.Additional. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Rodriguez E, Guillen-Grima F, Martí A, Brugos-Larumbe A. Comorbidity associated with obesity in a large population: The APNA study. Obes Res Clin Pract. 2015;9(5):435–447. doi: 10.1016/j.orcp.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Sirtori A, et al. Patients with obesity-related comorbidities have higher disability compared with those without obesity-related comorbidities. Int J Rehabil Res. 2016;39(1):63–69. doi: 10.1097/MRR.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 5.Skyler JS, et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Immunol Metab Endocrinol. 2017;66:10–12. doi: 10.2337/db16-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagareddy PR, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19(5):821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talukdar S, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18(9):1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49(9):1894–1903. doi: 10.1194/jlr.m800132-jlr200. [DOI] [PubMed] [Google Scholar]
- 9.Nijhuis J, et al. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity. 2009;17(11):2014–2018. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 10.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase Targets Apolipoprotein A-I, the Major High Density Lipoprotein Protein, for Site-Specific Oxidation in Human Atherosclerotic Lesions *. J Biol Chem. 2012;287(9):6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez García A, Rivera Rodríguez M, Gómez Alonso C, Yazmin Rodríguez Ochoa D, Alvarez Aguilar C. Myeloperoxidase Is Associated with Insulin Resistance and Inflammation in Overweight Subjects with First-Degree Relatives with Type 2 Diabetes Mellitus. Diabetes Metab J. 2015;39:59–65. doi: 10.4093/dmj.2015.39.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olza J, et al. Myeloperoxidase Is an Early Biomarker of Inflammation and Cardiovascular Risk in Prepubertal Obese Children. Diabetes Care. 2012;35:2373–2376. doi: 10.2337/dc12-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, et al. Myeloperoxidase Deletion Prevents High-Fat Diet-Induced Obesity and Insulin Resistance. Diabetes. 2014;63:4172–4185. doi: 10.2337/db14-0026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Heinecke JW, Goldberg IJ. Myeloperoxidase: A Therapeutic Target for Preventing Insulin Resistance and the Metabolic Sequelae of Obesity? Diabetes. 2014;63:4001–4003. doi: 10.2337/db14-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisberg S, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahat RK, Singh N, Rathore V. Association of myeloperoxidase with cardiovascular disease risk factors in prediabetic subjects. Diabetes Metab Syndr Clin Res Rev. 2019;13(1):396–400. doi: 10.1016/j.dsx.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Nauseef W. M. Contributions of myeloperoxidase to proinflammatory events: more than an antimicrobial system. Int J Hematol. 74(2):125–133. http://www.ncbi.nlm.nih.gov/pubmed/11594511. 2001. [DOI] [PubMed]
- 18.Nicholls SJ, Hazen SL. Myeloperoxidase and Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 19.Santulli G, Sweeney G, Korea P, Thorin E, Choi KM. Angiopoietin-like proteins: a comprehensive look. Front Endocrinol. 2014;5(4):1–6. doi: 10.3389/fendo.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tikka A., Jauhiainen M. The role of ANGPTL3 in controlling lipoprotein metabolism. Endocrine.;52. 10.1007/s12020-015-0838-9 2020. [DOI] [PMC free article] [PubMed]
- 21.Dijk W, Kersten S. Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol Metab. 2014;25(3):146–155. doi: 10.1016/j.tem.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Haller JF, et al. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res. 2017;58(6):1166–1173. doi: 10.1194/jlr.m075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Farha M, et al. Increased ANGPTL3, 4 and ANGPTL8/ betatrophin expression levels in obesity and T2D. Lipids Health Dis. 2016;15:181. doi: 10.1186/s12944-016-0337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Farha M., et al. Plasma and adipose tissue level of angiopoietin-like 7 (ANGPTL7) are increased in obesity and reduced after physical exercise. PLos ONE.; 12(3). 10.1371/journal.pone.0173024 2017. [DOI] [PMC free article] [PubMed]
- 25.Barja-fernandez S, et al. Plasma ANGPTL-4 is Associated with Obesity and Glucose Tolerance: Cross-Sectional and Longitudinal Findings. Mol Nutr Food Res. 2018;62(1800060):1–8. doi: 10.1002/mnfr.201800060. [DOI] [PubMed] [Google Scholar]
- 26.Oike Y., et al. Angiopoietin-Related Growth Factor (AGF) Promotes Epidermal Proliferation, Remodeling, and Regeneration. PNAS.; 100(16). 10.1073/pnas.1531901100 2003. [DOI] [PMC free article] [PubMed]
- 27.Oike Y, et al. Angiopoietin-related growth factor (AGF) promotes angiogenesis. Blood. 2004;103(10):3760–3765. doi: 10.1182/blood-2003-04-1272. [DOI] [PubMed] [Google Scholar]
- 28.Oike Y, et al. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11(4):400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 29.Ebert T, et al. Serum levels of angiopoietin-related growth factor in diabetes mellitus and chronic hemodialysis. Metabolism. 2009;58(4):547–551. doi: 10.1016/j.metabol.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Cinkajzlova A, et al. Angiopoietin-like protein 6 in patients with obesity, type 2 diabetes mellitus, and anorexia nervosa: The influence of very low-calorie diet, bariatric surgery, and partial realimentation. Endocr Res. 2017;42(1):22–30. doi: 10.3109/07435800.2016.1169544. [DOI] [PubMed] [Google Scholar]
- 31.Namkung J, Koh SB, Kong ID, Choi J-W, Yeh B-I. Serum levels of angiopoietin-related growth factor are increased in metabolic syndrome. Metabolism. 2011;60(4):564–568. doi: 10.1016/j.metabol.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Alghanim G, et al. Higher Levels of ANGPTL5 in the Circulation of Subjects With Obesity and Type 2 Diabetes Are Associated With Insulin Resistance. Front Endocrinol (Lausanne). 2019;10(July):1–10. doi: 10.3389/fendo.2019.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abu-Farha M, Behbehani K, Elkum N. Comprehensive Analysis of Circulating Adipokines and HsCRP Association with Cardiovascular Disease Risk Factors and Metabolic Syndrome in Arabs. Cardiovasc Diabetology. 2014;13:76. doi: 10.1186/1475-2840-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebert T, et al. Relationship between serum levels of angiopoietin-related growth factor and metabolic risk factors. Horm Metab Res. 2014;46(10):685–690. doi: 10.1055/s-0034-1382078. [DOI] [PubMed] [Google Scholar]
- 35.Abdullah B, Deveci K, Atilgan R, Kiliçli F. & Söylemez M. S. Serum angiopoietin-related growth factor (AGF) levels are elevated in gestational diabetes mellitus and associated with insulin resistance. Ginekol Pol.;83(10):749–753. http://www.ncbi.nlm.nih.gov/pubmed/23383560. 2012. [PubMed]
- 36.Tjeerdema N, et al. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2. diabetes. Care. 2014;2:34. doi: 10.1136/bmjdrc-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hato T, Tabata M, Oike Y. The Role of Angiopoietin-Like Proteins in Angiogenesis and Metabolism. Trends Cardiovasc Med. 2008;18(1):6–14. doi: 10.1016/j.tcm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Kitazawa M, Ohizumi Y, Oike Y, Hishinuma T, Hashimoto S. Angiopoietin-Related Growth Factor Suppresses Gluconeogenesis through the Akt/Forkhead Box Class O1-Dependent Pathway in Hepatocytes. J Pharmacol Exp Ther. 2007;323(3):787–793. doi: 10.1124/jpet.107.127530. [DOI] [PubMed] [Google Scholar]
- 39.Abdul-Wahed A, et al. A link between hepatic glucose production and peripheral energy metabolism via hepatokines. Mol Metab. 2014;3:531–543. doi: 10.1016/j.molmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namkung J, et al. Increased Serum Angiopoietin-Like 6 Ahead of Metabolic Syndrome in a Prospective Cohort Study. Diabetes Metab J. 2019;43:521–529. doi: 10.4093/dmj.2018.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindhelm RK, et al. Comparison of two consecutive fat-rich and carbohydrate-rich meals on postprandial myeloperoxidase response in women with and without type 2 diabetes mellitus. Metabolism. 2008;57(2):262–267. doi: 10.1016/j.metabol.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Jornayvaz FR, Brulhart-Meynet MC, James RW. Myeloperoxidase and paraoxonase-1 in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2009;19(9):613–619. doi: 10.1016/j.numecd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Gorudko IV, et al. Functional Activity of Neutrophils in Diabetes Mellitus and Coronary Heart Disease: Role of Myeloperoxidase in the Development of Oxidative Stress. Bull Exp Biol Med. 2012;154(1):23–26. doi: 10.1007/s10517-012-1865-7. [DOI] [PubMed] [Google Scholar]
- 44.Moldoveanu E, et al. Plasma markers of endothelial dysfunction in type 2 diabetics. Eur J Intern Med. 2006;17(1):38–42. doi: 10.1016/j.ejim.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Baldus S, et al. Myeloperoxidase Serum Levels Predict Risk in Patients With Acute Coronary Syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 46.Vita JA, et al. Serum Myeloperoxidase Levels Independently Predict Endothelial Dysfunction in Humans. Circulation. 2004;110(9):1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shetty S, Suchetha K, Ln M. Variations in Serum Myeloperoxidase Levels With Respect To Hyperglycemia, Duration of Diabetes, Bmi, Sex And Aging in Type 2 Diabetes Mellitus. Int J Res Pharm Biomed Sci ISSN. 2012;3(2):652–655. [Google Scholar]
- 48.Wiersma JJ, et al. Diabetes mellitus type 2 is associated with higher levels of myeloperoxidase. Med Sci Monit. 2008;14(8):406–410. [PubMed] [Google Scholar]
- 49.Zhang C, Yang J, Jennings LK. Leukocyte-derived myeloperoxidase amplifies high-glucose-induced endothelial dysfunction through interaction with high-glucose-stimulated, vascular non-leukocyte-derived reactive oxygen species. Diabetes. 2004;53(11):2950–2959. doi: 10.2337/diabetes.53.11.2950. [DOI] [PubMed] [Google Scholar]
- 50.Lanza F. Clinical manifestation of myeloperoxidase deficiency. J Mol Med (Berl). 76(10):676–681. http://www.ncbi.nlm.nih.gov/pubmed/9766845. 1998. [DOI] [PubMed]
- 51.Nita C, Bala C, Porojan M, Hancu N. Fenofibrate Improves Endothelial Function and Plasma Myeloperoxidase in Patients with Type 2 Diabetes Mellitus: An Open-Label Interventional Study. Diabetology & Metab Syndrome. 2014;6:30. doi: 10.1186/1758-5996-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato N, et al. MPO activity and generation of active O2 species in leukocytes from poorly controlled diabetic patients. Diabetes Care. 1992;15(8):1050–1052. doi: 10.2337/diacare.15.8.1050. [DOI] [PubMed] [Google Scholar]
- 53.Unubol M, et al. Relationship between glycemic control and histochemical myeloperoxidase activity in neutrophils in patients with type 2 diabetes. Diabetol Metab Syndr. 2015;7:119. doi: 10.1186/s13098-015-0115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song P., et al. Association of Plasma Myeloperoxidase Level with Risk of Coronary Artery Disease in Patients with Type 2 Diabetes. Dis Markers.;1-5. 10.1155/2015/761939 2015. [DOI] [PMC free article] [PubMed]
- 55.Meuwese MC, et al. Serum Myeloperoxidase Levels Are Associated With the Future Risk of Coronary Artery Disease in Apparently Healthy Individuals. J Am Coll Cardiol. 2007;50(2):159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 56.Xu L, et al. Serum Levels of Progranulin Are Closely Associated with Microvascular Complication in Type 2 Diabetes. Dis Markers. 2015;2015:1–9. doi: 10.1155/2015/357279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donath M. Y, Shoelson S. E. Type 2 diabetes as an inflammatory disease. Nat Publ Gr.;11. 10.1038/nri2925 2011. [DOI] [PubMed]
- 58.King G. L. The Role of Inflammatory Cytokines in Diabetes and Its Complications. J Periodontol. (August):1527–1534. 10.1902/jop.2008.080246 2008. [DOI] [PubMed]
- 59.Sommer G, et al. Secretory products from human adipocytes stimulate proinflammatory cytokine secretion from human endothelial cells. J Cell Biochem. 2009;106(4):729–737. doi: 10.1002/jcb.22068. [DOI] [PubMed] [Google Scholar]
- 60.Yang X, Smith U. Adipose tissue distribution and risk of metabolic disease: Does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer? Diabetologia. 2007;50(6):1127–1139. doi: 10.1007/s00125-007-0640-1. [DOI] [PubMed] [Google Scholar]
- 61.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Systemic Inflammation in Obese Humans. Diabetes. 2007;56(April):1010–1013. doi: 10.2337/db06-1656.CRP. [DOI] [PubMed] [Google Scholar]
- 62.Nevado J, et al. Amadori adducts activate nuclear factor-κB-related proinflammatory genes in cultured human peritoneal mesothelial cells. Br J Pharmacol. 2005;146(2):268–279. doi: 10.1038/sj.bjp.0706309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2009;17(4):332–341. doi: 10.2217/clp.13.80. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88(3):249–256. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 65.Zur B, Look M, Holdenrieder S, Stoffel-Wagner B. Elevated plasma myeloperoxidase concentration in adults with obesity. Clin Chim Acta. 2011;412(19-20):1891–1892. doi: 10.1016/j.cca.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Sladoje DP, Kisi} B, Miri} D, Kisic B. The monitoring of protein markers of inflammation and serum lipid concentration in obese subjects with metabolic syndrome. J Med Biochem. 2017;36(4):366–374. doi: 10.1515/jomb-2017-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal A, et al. Assessment of oxidative stress and inflammation in prediabetes—A hospital based cross-sectional study. Diabetes Metab Syndr Clin Res Rev. 2016;10(2):S123–S126. doi: 10.1016/j.dsx.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Andreou I, et al. Effects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: A randomized placebo-controlled study. Atherosclerosis. 2010;210(1):194–198. doi: 10.1016/j.atherosclerosis.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 69.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum Myeloperoxidase and Mortality in Maintenance Hemodialysis Patients. Am J Kidney Dis. 2006;48(1):59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.