Abstract
The ABCB1 gene belongs to ATP binding cassette (ABC) transporter genes that has been previously implicated in cancer progression and drug response. This study aimed to evaluate the association between the SNP 3435 and the expression of the ABCB1 gene in lung cancer patients in the Polish population in comparison to clinicopathological parameters and treatment. 150 RNA and 47 DNA samples were isolated from 49 lung cancer cases including both tissue samples and blood taken from the same patients at three time points: diagnosis, 100 days and one year after the surgical intervention. Qualitative and real-time PCR analysis of expression were done, also genotyping by PCR-RFLP. Mutant homozygous TT and allele T are present statistically significantly more frequently in the group of patients with lung cancer. There is no difference with expression level in lung cancer tissue and blood sample taken from the same patients before surgical treatment. On the basis of blood samples analysis it was observed that the expression level of ABCB1 mRNA was growing in time. Higher levels were marked after 100 days and one year after the surgical intervention. The complementary pharmacological treatment induced higher expression levels of ABCB1. The presented data suggest an important role of ABCB1 in lung cancer, the increasing level of ABCB1 mRNA which can be connected with induction of multidrug resistance mechanism is also significant, that observation must be confirmed in further analysis.
Subject terms: Cancer, Genetics, Molecular biology, Biomarkers, Medical research, Molecular medicine
Introduction
Malignant tumors after a cardiovascular disease are the leading cause of death in Poland. Lung cancer is the second most common cancer in men and women, representing approximately 13% of all new cancers. Among all cancers, lung cancer is responsible for the largest number of deaths in oncology patients in highly developed countries. Recent studies suggest that it can overtake breast cancer as the leading cause of cancer deaths among women in Europe by the middle of this decade. According to the estimates of the Polish Oncology Society report “Current state of cancer control in Poland” from 2014, in 10 years’ time the number of deaths from lung cancer may amount to over 30.000 cases annually1.
Despite the alarming statistics of the incidence and mortality due to lung cancer, the development of effective therapy remains unattainable. Most patients diagnosed with lung cancer already have an advanced disease − 40% is stage IV and 30% is stage III2. The five-year survival rate of NSCLC varies from 73% in early detection to 3.7% in advanced metastatic disease3. Understanding the basic biological and molecular mechanisms of developing lung cancer contributed to the development of personalized medicine. A simple binary division of lung cancer into non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) is no longer relevant as knowledge about the human genome is constantly increasing. The ability to genotype creates the possibility of an individual analysis. Lung cancer is currently divided into molecular subtypes and targeted therapies may affect the effectiveness of chemotherapy, and extend the time free from progression and overall survival4.
The ABC transporters are a superfamily of transmembrane proteins that transport many different substrates across lipid extracellular and intracellular membranes metabolites, carcinogens and cytotoxic drugs including anticancer drugs5. In the human genome, 48 different ABC transporters were identified and divided into seven subfamilies A-G based on sequence similarities6.
The ABCB1 gene, encoding the P-glycoprotein, is located on chromosome 7q21.1, consists of 28 introns and 28 exons. ABCB1 mRNA is 4.7 kb and is contained in the coding region of 120 kb. The ABCB1 gene has been extensively studied for characteristic polymorphisms and about 50 SNPs for ABCB1 have been identified7. The most frequently studied polymorphisms in the ABCB1 gene are C1236T (rs1128503), G2677T/A (rs2032582) and C3435T (rs1045642). Genetic variants associated with a change in the amount or activity of transport proteins lead to the loss of the physiological role of these proteins and altered various drugs transporter’s functions8,9. These three most common ABCB1 SNPs in the Caucasian population have been found to be in linkage disequilibrium. The allelic frequencies of these three SNPs are highly variable between ethnic groups10.
The most widely studied variant of ABCB1 is a commonly synonymous C to T transition at nucleotide position 3435 in exon 26 (3435 C > T). Although this transition does not change its encoded amino acid with Ile at position 114522, TT variant has been significantly associated with the decreased mRNA expression and protein stability and may have reduced the drug transport capacity10. So far the effect of synonymous polymorphisms on the protein has not been fully understood. However it is assumed that they can affect the post-transcriptional processing of mRNA by interfering with the process of removing introns or affect the process of alternative transcript splicing. What’s more, silent polymorphisms can be important in the process of protein folding, leading to its abnormal form. In addition, as Kimchi-Sarfaty et al.11 indicate, replacing as a result of silent polymorphisms often used in translation of codons into rare ones can affect the rate of protein folding, and thus change its function or change its substrate specificity. On the other hand, synonymous polymorphisms can change the structure and/or function of a protein by coupling to non-synonymous polymorphisms that directly change the amino acid sequence of a protein11–15.
P-gp is expressed in the apical membranes of many tissues and can be implicated in numerous various processes like differentiation, proliferation, apoptosis and immune response regulation16. In a normal lung, P-gp is expressed on the top surface of the bronchial epithelium, where it can act to remove external compounds from the lung. In lung cancer, initially low P-gp expression level, can change after exposure to chemotherapy as part of acquired drug resistance. P-gp confers resistance to cytotoxic drugs, including etoposide and cisplatin, and polymorphisms may affect the specificity of the substrate17. Research on the influx and efflux mechanisms of drug transporters may be useful to assess the effectiveness of therapy. Various studies have shown that the family of ATP-binding transport proteins (ABC transporters), such as ABCB1 or ABCG2, may be associated with the development of drug resistance18. On the other hand, primary decreased P-gp expression may be associated with accumulation of the metabolites or drugs and as a consequence diseases development.
This study aimed to evaluate the association between the SNP 3435 and the expression of the ABCB1 gene in lung cancer patients in the Polish population in comparison to clinicopathological parameters and treatment.
Results
Genotyping of C3435T of the ABCB1 gene
47 blood samples collected from patients with lung cancer for SNP on position C3435T of the ABCB1 gene were successfully analyzed, for two samples no PCR product was obtained. The polymorphism in both lung cancer patients and healthy individuals were in Hardy-Weinberg equilibrium (Table 1).
Table 1.
Frequencies of the C3435T ABCB1 gene genotypes in lung cancer patients and healthy individuals.
| ABCB1 C3435T | Lung cancer Patients N = 47 | Healthy Individuals N = 96 | p (Chi^2 Pearson) | Odds ratio | 95% Cl |
|---|---|---|---|---|---|
| CC | 4 (8.5 %) | 27 (28.1 %) | 0.0124 | 1 | — |
| CT | 25 (53.2 %) | 48 (50.0 %) | 3.52 | 1.11–11.17 | |
| TT | 18 (38.3 %) | 21 (21.9 %) | 5.78 | 1.70–19.68 | |
| C | 33 (35.1 %) | 102 (53.1 %) | 0.0041 | ||
| T | 61 (64.9 %) | 90 (46.9 %) | |||
| HWE p (Chi^2 Pearson) | 0.6527 | 0.9991 |
Firstly, genotype and allele frequencies for the studied polymorphism between the group of patients with lung cancer and the group of healthy individuals were compared. For SNP at position C3435T, the genotype TT (mutant homozygous) and allele T were statistically significantly more frequent in the group of patients with lung cancer than in the control (p = 0.0124; p = 0.0041, respectively). All data is summarized in Table 1.
Secondly, the lung cancer patients were divided into patients under and equal 67 years old and over 67 years old (average age of the group), and frequencies of SNP C3435T genotypes were compared. No statistical significance was found (p = 0.6578).
Thirdly, the group of patients with cancer was divided according to gender into subgroups of women and men. In this case, the TT genotype tended to be more frequent in the subgroup of men with lung cancer (the subgroup of females TT 10%; the subgroup of men 46%; p = 0.0706).
Next, the investigated group was divided according to their histological type into subgroups of patients with squamous cell carcinoma and adenocarcinoma. The TT genotype (mutant) occurred more frequently in the group of patients with squamous cell lung carcinoma than in the subgroup with lung adenocarcinoma (TT 46.4%; TT 26.3%, respectively). However, no statistical significance was found (p = 0.2252).
Also in the group of patients with lung cancer, based on their medical history, the subgroup of patients who smoked tobacco and a subset of those who did not were listed. There was a tendency for the TT genotype to be more frequent in the subset of tobacco smoking patients (TT 50%) than in the subgroup of non-smokers (TT 17.6%) (p = 0.0902).
After that, dependencies between clinicopathological parameters (TNM stage and grade of histological malignancy) and the C3435T polymorphism in the group of lung cancer patients were verified. Investigated group was divided according to the TNM classification into two subgroups of patients: with less (IA1 or IA2 or IB) and with more advanced clinical stage (IIA or IIB or IIIA). The genotype frequency was then compared between the two subgroups. TT genotype occurred more frequently in patients with less advanced clinical stage (47.8%) than in the group of patients with more advanced clinical stage of cancer (29.2%). No statistically significant difference was observed (p = 0.3929), though. Then, according to the histological malignancy grade, the investigated cohort was divided into a subgroup with G1 and G2 grades combined together and a subgroup of patients with a G3 grade. There was a tendency for the TT genotype to occur more frequently in the subgroup of patients with a G1 or G2 grade than in the subgroup of patients with a G3 grade (TT 42.9%; TT 25%; p = 0.0517). All this data is summarized in Table 2.
Table 2.
Frequency of genotypes and alleles of the SNP C3435T ABCB1 gene according to clinicopathological parameters.
| ABCB1 C3435 | p | ||||
|---|---|---|---|---|---|
| CC | CT | TT | |||
| N (%) | |||||
| Gender | Women | 2 (20) | 7 (70) | 1 (10) | 0.0706 |
| Men | 2 (5.4) | 18 (48.6) | 17 (46) | ||
| Tobacco smoking | Non-smokers | 2 (11.8) | 12 (70.6) | 3 (17.6) | 0.0902 |
| Smokers | 2 (6.7) | 13 (43.3) | 15 (50) | ||
| Histological type of cancer | squamous cell carcinoma | 3 (10.7) | 12 (42.9) | 13 (46.4) | 0.2252 |
| adenocarcionma | 1 (5.3) | 13 (68.4) | 5 (26.3) | ||
| TNM stage | IA1 or IA2 or IB | 2 (8.7) | 10 (43.5) | 11 (47.8) | 0.3929 |
| IIA or IIB or IIIA | 2 (8.3) | 15 (62.5) | 7 (29.2) | ||
| Grade of histological malignancy [G] | G1 or G2 | 1 (2.8) | 19 (54.3) | 15 (42.9) | 0.0517 |
| G3 | 3 (25) | 6 (50) | 3 (25) | ||
The dependence of the genotype on polymorphism at position C3435T of the ABCB1 gene on probability of overall survival time
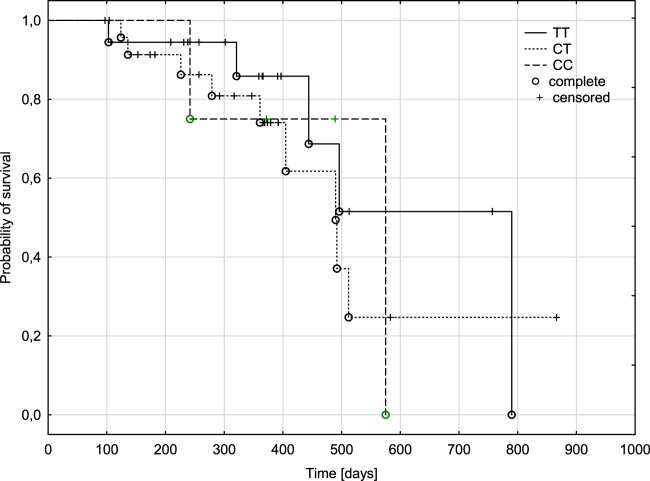
The Kaplan-Meier plot shows the probability of survival in the group of patients with lung cancer from the time of cancer diagnosis up to over 2 years of observation of the patients (Fig. 1). Median survival time was shorter in patients with CT (347 days) or TT (362 days) genotype compared to CC genotype (430 days). However, there was no statistical significance difference in overall survival time according to C3435T genotypes (p = 0.5999).
Figure 1.
Overall survival plots for lung cancer patients with different genotypes for C3435T polymorphism of the ABCB1 gene.
Expression of the ABCB1 gene in blood patients with lung cancer
Relative expression level of the ABCB1 gene was successfully analysed in the same group of patients, blood samples were collected at three points of time.
The mRNA was isolated and its level was analysed in 40 blood samples collected at the time of lung cancer diagnosis, 39 samples 100 days after the surgery and 24 from patients one year after resection (the group is smaller because patients were lost from observation or died). The obtained results were compared with the control group of 56 blood samples from people without cancer. The first comparison involves changing of the relative expression of the ABCB1 gene between all investigated groups: control, patients at the time of diagnosis, 100 days after the surgery and one year after the surgery.
The obtained results showed statistically significant differences in the expression of the ABCB1 gene in the group of patients 100 days after the surgery (p = 0.0000) and one year after the surgery (p = 0.0000) compared to the control group. No statistically significant differences were found for the group of patients at the time of diagnosis compared to control (p = 0.3049). In addition, a statistically significant increased ABCB1 mRNA level was found in the group of patients 100 days after the surgery and one year after the surgery in comparison to samples at the time of the diagnosis (p = 0.0000 and p = 0.0081, respectively). However, there were no differences in the ABCB1 gene expression between the groups of patients 100 days after the surgery and one year after the surgery (p = 0.6622). Data is summarized in Fig. 2.
Figure 2.
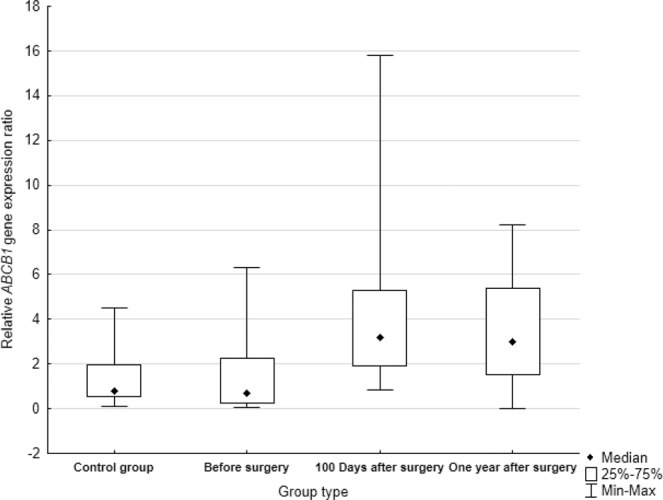
Relative expression levels of ABCB1 in control group and blood taken from patients at three points during the diagnostic-therapeutic procedure.
In the group of patients, correlation between relative mRNA of ABCB1 expression and age of patients was assessed. No statistically significant correlation was found (p = 0.315). After that, patients were divided according to gender. In this case, also no statistical significance between the subgroup of women and men was found (p = 0.7738).
Next, the lung cancer cohort was divided according to histological type of cancer into the patients with squamous cell lung carcinoma (N = 21) and with adenocarcinoma (N = 16). There was no statistically significant difference in relative ABCB1 gene expression between these two subgroups (p = 0.1453).
The group of patients with lung cancer was also divided into the subgroup of tobacco smokers (N = 25) and non-smokers (N = 14). Also in this analysis, no statistically significant differences between ABCB1 mRNA expression level and tested subgroups were found (p = 0.4553).
Then, the group of patients with cancer was divided according to grade of histological malignancy into subgroups of: highly differentiated cancer - G1 or moderate grade - G2 patients and poorly differentiated - G3 patients. Due to a small number of patients who were classified into G1 group (N = 3) the combined G1 and G2 (N = 27) groups with G3 (N = 9) were compared. The analysis showed a lower ABCB1 gene expression in patients with poorly differentiated cancer cells than in patients with G1 and G2 stage (p = 0.0352) Fig. 3.
Figure 3.
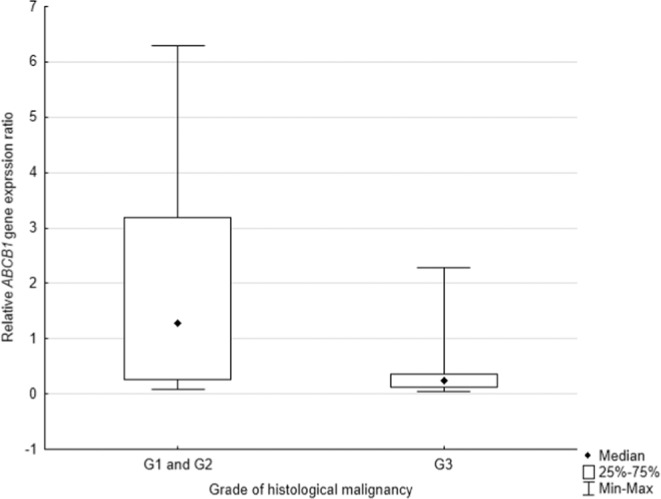
The level of ABCB1 expression in comparison to histological grade of malignancy.
In the next step, the expression of the ABCB1 gene was evaluated according to the use of additional pharmacological treatment after the surgery. Two groups were distinguished, the first one received various types of complementary pharmacological treatment and revealed expression of ABCB1 (N = 12; Table 3), the second with only surgical intervention (N = 27). The results of the conducted analysis showed a statistically significant difference in expression of ABCB1 in people who received pharmacological treatment during 100 days observations between this two groups. (p = 0.0428), Fig. 4. Additionally, the ABCB1 gene expression level was evaluated in the subgroup of patients who received chemotherapy treatment, the analysis also revealed the statistical significant difference in ABCB1 gene expression level. Patients before surgery have lower ABCB1 gene expression than 100 days after surgery and pharmacological treatment (p = 0.0094; Fig. 5). The third time point (one year after surgery) was not included since the data was limited to only five patients.
Table 3.
Scheme of applied chemotherapy in the group of patients with ABCB1 expression.
| Scheme of chemotherapy | Number of patients |
|---|---|
| carboplatin + gemcitabine | 2 |
| cisplatine + etoposide | 2 |
| etoposide | 2 |
| cisplatine + vinorelbine | 6 |
Figure 4.
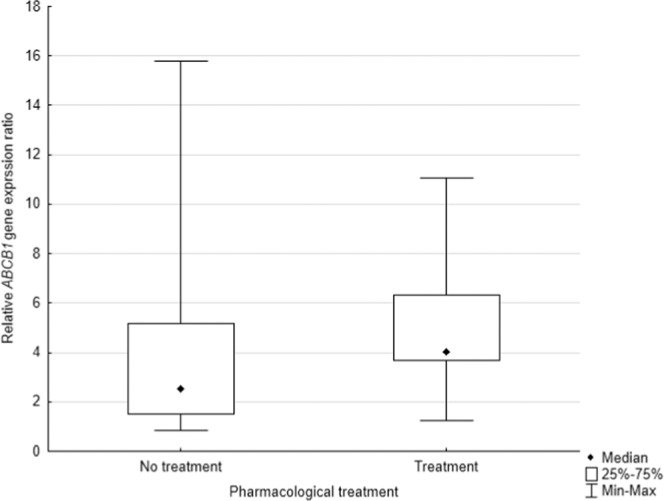
The level of ABCB1 expression in comparison to the scheme of treatment.
Figure 5.
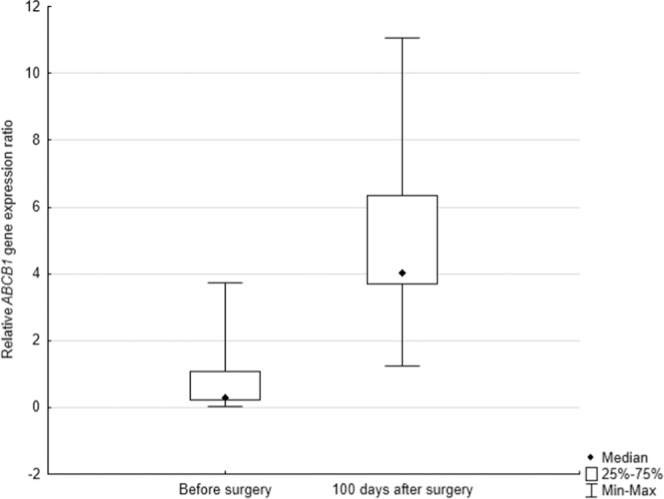
The level of ABCB1 expression in the subgroup of patients who received adjuvant chemotherapy treatment (p = 0.0094).
In further analysis, no significant differences were found among relative ABCB1 gene expression and clinicopathological features like distant metastases (p = 0.2725), the size of primary tumor (p = 0.9841), the involvement of local lymph nodes (p = 0.7493), leukocyte count (p = 0.7753). However, a statistically significant decreased expression of the ABCB1 gene was observed in patients with fibrinogen concentration above the range of reference values (p = 0.04943).
Expression of the ABCB1 gene in lung cancer tissue
In the same group of patients the ABCB1 gene expression was also examined in 47 tumor tissues taken during the surgical procedure and compared to the expression level in the blood before the surgery and then to clinical-pathological features. There were no statistically significant differences between the level of ABCB1 expression in tissue and blood before the surgery (R = −0.0138). Also there were no dependences between the expression of ABCB1 gene cancer altered tissue and clinical-pathological features: tobacco smoking (p = 0.0627), histological type (p = 0.6137), grade of histological malignancy (p = 0.1223), the involvement of local lymph nodes (p = 0.1446), gender (p = 0.7797) and age (R = −0.014).
On the basis of our study we can conclude that mutant homozygous TT and allele T are present significantly more frequent in the group of patients with lung cancer nevertheless are connected with more differentiated tumors (G1/G2). There is no difference with the expression level among lung cancer tissue and blood sample taken from the same patients before surgical treatment. On the basis of blood samples analysis it was observed that the expression level of ABCB1 mRNA was increasing in time, higher levels were determined after 100 days and one year from the surgical intervention. The complementary pharmacological treatment induced higher expression levels which could preliminary suggest the development of multidrug resistance mechanism.
Discussion
In the present study, the incidence of the genotype at position C3435T of the ABCB1 gene in both the test and control groups were consistent with the Hardy-Weinberg equilibrium. The distribution of genotypes prevalence was similar to that in the Caucasian, German-Caucasian or French populations, and different than in the African population, where the CC genotype predominates19–22.
P-glycoprotein, encoded by ABCB1 gene, is present in many normal tissues, including bronchi and lungs. It plays a protective role where, by moving xenobiotics to the extracellular environment, it protects cells from its toxic/carcinogenic activity cell-toxins23,24.
The ABCB1 gene is polymorphic and it has been shown that several of these polymorphisms may be linked with the functioning of P-glycoprotein. Similarly, the occurrence of polymorphisms may be linked with increasing predisposition to various diseases development, including cancer. One of these is polymorphism at position C3435T of the ABCB1 gene, where Hoffmayer et al. have shown that it may change the expression of ABCB1 mRNA and P-glycoprotein function25.
The aim of this study was to assess the potential impact of the ABCB1 gene on the risk of non-small cell lung cancer development. To the best of our knowledge, this is the first such study in the Polish population. To date, most of the worldwide studies on the C3435T polymorphism and lung cancer have focused on the effectiveness of the therapy and not on the risk of developing this cancer.
In this study, by comparing the frequency of genotypes and alleles occurrence of a given polymorphism between the group of patients with non-small cell lung cancer and the control group, it was shown that the TT genotype and allele T of the ABCB1 gene C3435T were significantly more frequent in the group of cancer patients (p = 0.0124; p = 0.0041, respectively). This indicates that the presence of at least one T allele of polymorphism at the C3435T position of the ABCB1 gene is associated with an increased risk of developing non-small cell lung cancer. Additionally, the risk of developing lung cancer was 5.78 times higher in the presence of the TT genotype of the studied polymorphism. These results are similar to those obtained by Subhani et al. where TT genotype of SNP 3435 was associated with 5.23-fold higher risk of lung cancer development26. The obtained results are in contrast to those obtained by Sinues et al. and Gervasini et al., where the relationship between polymorphism at position C3435T and the risk of developing lung cancer has not been demonstrated27,28. The increased efflux of rhodamine 123 from CD56 cells29 may be related to the loss of the protective function of the P-glycoprotein as indicated by Hiltz et al. in the genotype CC of SNP 3435.
Thus, the presence of the TT genotype for this SNP will be associated with the intracellular accumulation of xenobiotics with a potential carcinogenic activity. It is worth noting that the presented study showed a tendency to more frequent occurrence of the TT genotype in the subgroup of people with lung cancer who smoked tobacco compared to the subgroup of non-smokers (p = 0.0902). However, other studies show no association between tobacco smoking and SNP 3435 of ABCB1 gene27,28.
The assessment of genotypes’ frequencies in the group of patients with lung cancer of different clinical stage of cancer (TNM stage) and the grade of histological malignancy of cancer (Grading) allowed to determine the importance of C3435T polymorphism in progression of gastric cancer. Due to the fact that there were no statistically significant correlations between less advanced clinical stage of cancer and patients with more advanced clinical stage of cancer (p = 0.3929) no association between SNP 3435 and the clinical stage of the cancer was demonstrated, which was in contrast to Subhani et al. who showed that TT genotype of C3435T was associated with the advanced stage of lung cancer26. On the other hand, in this study, the TT genotype in a subset of patients with G1 or G2 histologic malignancy of lung cancer (p = 0.0517) tends to occur more frequently, which is additionally confirmed by the result of the study for the mRNA expression of the ABCB1 gene, where it is demonstrated that the expression of mRNA is higher in the subgroup of patients with G1 or G2 (p = 0.0278). This dependence is confirmed by the results of the Subhani et al. who also showed the correlation between the expression of ABCB1 and the intermediate degree of histological malignancy of the cancer26.
However, we did not confirm an observation noted by other authors that during cancer development the expression level is decreased which can be connected with the loss of physiological, protecting function of encoded by the ABCB1 gene protein. Delou et al. reported the loss of constitutive ABCB1 expression in breast cancer, especially in triple-negative tumors that seems to indicate a subgroup of a worse prognosis30. Our data did not show the differences between the expression level in blood and tissue samples in comparison to control group, also with clinicopathological parameters known as a worse prognostic and predictor factors.
In our study, the expression of the ABCB1 gene was checked in cancer tissue and in blood samples taken from patients at three points (at the time of diagnosis, 100 days and one year from the surgical intervention). Data was compared to each other and to the control group. A tendency for the expression level to increase in time was observed after 100 days and one year after the operation, the levels of mRNA were higher in a group to whom additional chemotherapy was administered, which can be related to developing a multidrug resistance process, similar observation was noted by Weissfeld31. This is in agreement with a higher frequency of TT genotype in our investigated group.
The impact of ABCB1 C3435T polymorphism on the function of P-gp can be explained by many hypotheses concerning the influence of a silent polymorphism on features and predispositions revealed phenotypically. One of them assumes the influence of a SNPs on the translation effectiveness. It is also possible that differences in allele specific present in RNA secondary structure could change the splicing process or the translation control. Another one assumes that some of polymorphisms increase the mRNA stability, which in consequence leads to the increased protein level and/or a change of the substrates’ affinity to the P-gp transporter. The modified function of P-gp could be a risk and progression factor of lung cancer32.
We are aware of the limitations of our study, particularly in the restriction of investigated cases number, especially after their classification according to clinical-pathological parameters the resulting groups were small, nonetheless, it still allowed us to perform a statistical analysis. The convergence of the results obtained in post-operative tissues and blood trials should be emphasized.
In our ongoing project, we would like to investigate two other polymorphisms, one leading to amino acid exchange (ABCB1 G2677T/A) and the other one, which has no influence on the amino acid sequence of P-gp but surprisingly, may influence the P-gp function (ABCB1 C1236T). With data on several polymorphisms, it is possible to conduct the haplotype analysis which may provide more useful information than the genotype in case of only one polymorphism16. In our previous study it was proven that the three investigated SNPs of the ABCB1 gene (ABCB1 C1236T, ABCB1 G2677T/A and ABCB1 C3435T) are located in one haploblock32.
The presented data suggests an important role of ABCB1 in lung cancer which we would like to prove in our future research.
Materials and Methods
Investigated group
The investigated group comprised of 49 patients (10 female and 39 male) who were diagnosed with non-small cell lung cancer (squamous cell carcinoma and adenocarcinoma) at the N. Copernicus Regional Specialist Hospital in Lodz, Poland. The mean age at the time of diagnosis was 67.1 years (64.4 for females and 67.8 for males). Peripheral blood of selected patients collected between 2016–2018 was used for research. Samples were collected at three time points: at the time of cancer diagnosis, 100 days after the surgery and one year after the surgery. 47 patients from the entire group underwent a surgical resection, frozen tissue sections were additionally obtained from these patients. In 15 cases adjuvant chemotherapy was included after the surgery (carboplatin + gemcitabine 2, cisplatine + etoposide 2, etoposide 2, cisplatine +vinorelbine 9).
For genotyping at position C3435T of the ABCB1 gene the DNA was successfully isolated from 49 blood samples collected at the time of diagnosis of cancer.
For the expression level analysis of the ABCB1 gene the RNA was successfully isolated from:
40 blood samples collected at time of diagnosis of cancer
39 blood samples collected 100 days after the surgery
24 blood samples collected one year after the surgery
47 frozen tissue sections collected intraoperatively
Control group
The group of healthy individuals (control group) consisted of 96 blood donors from the local blood bank, geographically and ethnically matching the group of patients with non-small cell lung cancer. 96 DNA and 56 RNA samples were successfully isolated from peripheral blood.
The investigation was in accordance with the principles of the Declaration of Helsinki and was approved by the Ethical Committee of the Medical University of Lodz (No RNN/87/16/KE). All individuals included in the study gave their informed consent.
DNA and RNA isolation
DNA and RNA from peripheral blood and frozen tissue sections collected intraoperatively were isolated according to “Blood Mini” and “Total RNA Mini” protocol, respectively (A&A Biotechnology, Poland)33,34. The purity and concentration of DNA and RNA samples were assessed nanospectrophotometrically. Concentration of extracted DNA samples range from 25 to 50 ng/ul, for RNA range from 5,2–80 ng/ul to obtain concentration for reverse transcriptase reaction described in the next subsection. Until the analysis, the DNA and RNA samples were stored at −20 °C and at −76 °C, respectively.
Genotyping C3435T of ABCB1 gene
Polymerase Chain Reaction (PCR)
For studied polymorphism a polymerase chain reaction (PCR) was performed in accordance with 2xPCR Super Master Mix (Biotool.com, USA) protocol. The mixture for PCR reaction consisted of 5 μl of 2xPCR Super Master Mix; 0.5 mM of each primer (Forward 5′TTGATGGCAAAGAAATAAAGC3‘ and Reverse 5′CTTACATTAGGCAGTGACTCG3‘) specific to particular SNP; 50 ng of DNA template and distilled water up to 20 μl. Negative control was included in every experiment. Products of the PCR reactions were assessed using electrophoresis in 2% agarose gel. Reaction products for SNP at position 3435 was the size of 208 bp35.
Restriction Fragment Length Polymorphism (RFLP)
Amplified DNA fragments for SNP on position 3435 were digested by MboI (EURx, Poland) for 16 h at 37 °C. Genotypes were identified using electrophoresis of amplified DNA fragments after digestion by restriction enzyme (one band of 208 bp for genotype TT; two bands of 145 and 63 bp for genotype CC; three bands of 208, 145 and 63 bp for genotype CT).
The results of ABCB1 genotyping of the healthy individuals were described previously36.
Expression of ABCB1 mRNA
Reverse transcription
A total cellular RNA was transcribed into complementary DNA (cDNA) in accordance with High-Capacity cDNA Reverse Transcription Kit protocol (Applied Biosystems; USA)37. The final concentration of RNA in reaction mixture was 0.005 μg/μl. Synthesized cDNA were stored at −20°C until analysis. As reference the GAPDH gene, encoding glyceraldehyde-3-phosphate dehydrogenase, was used38. Only the samples which showed the presence of PCR product for the GAPDH gene (188 bp) were included in further analysis.
Real – time polymerase chain reaction (real-time PCR)
Quantification assessment of ABCB1 (the investigated gene) and GAPDH (the reference gene) mRNA was performed in real-time PCR using the Rotor-Gene 6000 (Corbet Research, Germany) according to “SYBRGreen JumpStart Taq ReadyMix” protocol39. The reaction mixture for both genes consisted of 7.5 μl SYBR-Green ReadyMix, 0.7 μl of each primer (ABCB1 gene: F 5′-GGCCTAATGCCGAACACATT-3′, R 5′-CAGCGTCTGGCCCTTCTTC-3′; GAPDH F 5′-TGGTATCGTGGAAGGACTCAT-3′, R 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′), 1 μl of cDNA and distilled water up to 16 μl final volume. The reactions for ABCB1 and GAPDH were carried out in separate tubes. Samples were tested in triplicates and mean of obtained Ct values for both ABCB1 and GAPDH was calculated. In each experiment, negative control, also tested in triplicates, was included. To calculate relative changes in the gene expression, the ΔΔ Ct method was used40.
Statistical analysis
All statistical analyses were performed using STATISTICA 13 (StatSoft Inc.2018). The Chi^2 Pearson test was applied to evaluate conformity between the observed and expected genotype frequencies according to Hardy-Weinberg rule. To determine the significance of differences in allele and genotype frequencies between the group of lung cancer patients and the group of healthy individuals, as well as analysis of patients data (age, gender, tobacco smoking and clinical and pathological factors) in the lung cancer individuals Chi^2 Pearson was used. To determine the validity between the R-value and genotypes, age, gender, tobacco smoking or clinical and pathological factors the U Mann – Whitney test was used. The Kaplan-Meier analysis was done to estimate overall survival time41,42. In all conducted tests a p value of < 0.05 was assumed as significant.
Ethics approval and consent to participate
The present study was approved by The Ethics Committee of the Medical University of Lodz (No RNN/87/16/KE) and was in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the patients prior to their participation in the research.
Acknowledgements
The present study was supported by the statutory funds of the Department of Pharmaceutical Biochemistry and Molecular Diagnostics, Medical University of Lodz (Grant No. 503/3–015–02/503–31–001) and the funds of the Faculty of Pharmacy, Medical University of Lodz (Grant Nos 502–03/3–015–02/502–34–089 and 502–03/3–015–02/502–34–088). Language correction was done by Headway company, Lodz, Poland.
Author contributions
I.Z. and A.J. planned, conducted experiments, and assisted in the preparation of the manuscript for publication. M.Ł. and J.K. were responsible for collecting and preparing tissues from patients. J.P., M.Ż.-N., K.M., D. Sz.-K. conducted experiments, M.M. assisted in the preparation of the manuscript for publication, E.B. planned, supervised experiments, real-time analysis and assisted in the preparation of the manuscript for publication. All authors read and approved the final manuscript.
Data availability
Correspondence and requests for materials should be addressed to E.B.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Izabela Zawadzka and Agnieszka Jeleń.
References
- 1.Malvezzi M, et al. European cancer mortality predictions for the year. Ann. Oncol. 2013;24:792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- 2.Tockman MSJC. Survival and mortality from lung cancer in a screened population: the Johns Hopkins study. Chest J. 1986;89:324S–325S. doi: 10.1378/chest.89.4_Supplement.324S-a. [DOI] [Google Scholar]
- 3.Vesel M, et al. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir. Res. 2017;18:52. doi: 10.1186/s12931-017-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maltsev N, Rzhetsky A, Conrad Gilliam T. Systems analysis of human multigene disorders. Preface. Adv. Exp. Med. Biol. 2014;799:v–viii. doi: 10.1007/978-1-4614-8778-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhai X, et al. Gene polymorphisms of ABC transporters are associated with clinical outcomes in children with acute lymphoblastic leukemia. Arch. Med. Sci. 2012;8:659. doi: 10.5114/aoms.2012.30290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim. Biophys. Acta. 2009;1794:860–871. doi: 10.1016/j.bbapap.2009.02.014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamidovic A, Hahn K, Kolesar J. Clinical significance of ABCB1 genotyping in oncology. J. Oncol. Pharm. Pract. 2010;16:39–44. doi: 10.1177/1078155209104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, et al. ABCB1 variants confer susceptibility to primary open-angle glaucoma and predict individual differences to latanoprost treatment. Biomed. Pharmacother. 2016;80:115–120. doi: 10.1016/j.biopha.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Au A, et al. Association of genotypes and haplotypes of multi-drug transporter genes ABCB1 and ABCG2 with clinical response to imatinib mesylate in chronic myeloid leukemia patients. Biomed. Pharmacother. 2014;68:343–349. doi: 10.1016/j.biopha.2014.01.009/. [DOI] [PubMed] [Google Scholar]
- 10.Tamura M, et al. Genetic polymorphisms of the adenosine triphosphate-binding cassette transporters (ABCG2, ABCB1) and gefitinib toxicity. Nagoya J. Med. Sci. 2012;74:133–140. [PMC free article] [PubMed] [Google Scholar]
- 11.Kimchi-Sarfaty C, et al. A “Silent” Polymorphism in the MDR1 Gene Changes Substrate Specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmeyer S, et al. Functional polymorphisms of the human multidrugresistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl Acad. Sci. USA. 2000;28:3473–3478. doi: 10.1073/pnas.97.7.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fromm MF. The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans. Adv. Drug. Deliv. Rev. 2002;54:1295–1310. doi: 10.1016/S0169-409X(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 14.Kroetz DL, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–494. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Chamary JV, et al. The price of silent mutation. Sci. Am. 2009;300:46–53. doi: 10.1038/scientificamerican0609-46. [DOI] [PubMed] [Google Scholar]
- 16.Balcerczak E, et al. ABCB1/MDR1 gene polymorphisms as a prognostic factor in colorectal cancer. Int. J. Colorectal Dis. 2010;25:1167–1176. doi: 10.1007/s00384-010-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campa D, et al. A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis. Int. J. Cancer. 2012;131:2920–2928. doi: 10.1002/ijc.27567.. [DOI] [PubMed] [Google Scholar]
- 18.Hodges LM, et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein) Pharmacogenet Genomics. 2011;21:152–161. doi: 10.1097/FPC.0b013e3283385a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komoto, C. et al. MDR1 haplotype frequencies in Japanese and Caucasian, and in Japanese patients with colorectal cancer and esophageal cancer. Drug Metab Pharmacokinet 21, 126-132, https://doi.org/10.2133/dmpk.21.126Get rights and content (2006). [DOI] [PubMed]
- 20.Cascorbi I, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin. Pharmacol. Ther. 2001;69:169–174. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- 21.Jeannesson E, et al. Determination of ABCB1 polymorphisms and haplotypes frequencies in a French population. Fundam. Clin. Pharmacol. 2007;21:411–418. doi: 10.1111/j.1472-8206.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 22.Ameyaw MM, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–221. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Cascorbi I. P-glycoprotein: tissue distribution, substrates, and functional consequences of genetic variations. Handb. Exp. Pharmacol. 2011;201:261–283. doi: 10.1007/978-3-642-14541-4_6.. [DOI] [PubMed] [Google Scholar]
- 24.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmeyer S, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl Acad. Sci. USA. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subhani S, Jamil K, Nirni SS. Association of MDR1 gene (C3435T) polymorphism and gene expression profiling in lung cancer patients treated with platinum-based chemotherapy. Mol. Diagn. Ther. 2015;19:289–297. doi: 10.1007/s40291-015-0154-3. [DOI] [PubMed] [Google Scholar]
- 27.Sinues B. MDR-1 C3435T genetic polymorphism and tobacco-related lung cancer. Oncology. 2003;64:183–185. doi: 10.1159/000067770. [DOI] [PubMed] [Google Scholar]
- 28.Gervasini G, et al. Adenosine triphosphate-binding cassette B1 (ABCB1) (multidrug resistance 1) G2677T/A gene polymorphism is associated with high risk of lung cancer. Cancer. 2006;107:2850–2857. doi: 10.1002/cncr.22332. [DOI] [PubMed] [Google Scholar]
- 29.Hitzl M, et al. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics. 2001;11:293–298. doi: 10.1097/00008571-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Delou JMA, et al. Loss of constitutive ABCB1 expression in breast cancer associated with worse prognosis. Breast cancer. 2017;9:415–428. doi: 10.2147/BCTT.S131284.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissfeld JL, et al. Inherited variation in the ATP-binding cassette transporter ABCB1 and survival after chemotherapy for stage III-IV lung cancer. J. Thorac. Oncol. 2014;9:1264–1271. doi: 10.1097/JTO.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panczyk M, et al. ABCB1 gene polymorphisms and haplotype analysis in colorectal cancer. Int. J. Colorectal Dis. 2009;24:895–905. doi: 10.1007/s00384-009-0724-0. [DOI] [PubMed] [Google Scholar]
- 33.A&A Biotechnology. Blood Mini kit for DNA purification from blood protocol, version 0617, on-line access 20.09.2019 .https://www.aabiot.com/en/download?code=0c019ba95d0dd5fa3a6aa15afcdd507fbd823aea
- 34.A&A Biotechnology. Total RNA Mini kit for total RNA isolation from various type of samples protocol, version 0517, on-line access 20.09.2019. https://www.aabiot.com/en/download?code=d337c000e0f21615bbeda8d9385e753aa36dd648
- 35.Jamroziak K, et al. Distribution of allelic variants of functional C3435T polymorphism of drug transporter MDR1 gene in a sample of Polish population. Pol. J. Pharmacol. 2002;54:495–500. [PubMed] [Google Scholar]
- 36.Salagacka A, et al. C3435T polymorphism of the ABCB1 gene: impact on genetic susceptibility to peptic ulcers. Pharmacol. Rep. 2011;63:992–998. doi: 10.1016/S1734-1140(11)70615-6. [DOI] [PubMed] [Google Scholar]
- 37.Applied Biosystems High Capacity cDNA Reverse Transcription Kits protocol, on-line access 27.11.2019 https://assets.thermofisher.com/TFS-Assets/LSG/manuals/cms_042557.pdf
- 38.Silver N, Bestm S, Jiangm J, Theinm SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006;7:33. doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigma-Aldrich SYBR® Green JumpStart™ Taq ReadyMix™ protocol, on-line access 27.11.2019 https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Bulletin/s4438bul.pdf
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Ali Z, Bhaskar SB. Basic statistical tools in research and data analysis. Indian. J. Anaesth. 2016;60(9):662–669. doi: 10.4103/0019-5049.190623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010;1(4):274–278. doi: 10.4103/0974-7788.76794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Correspondence and requests for materials should be addressed to E.B.