Abstract
An 8-year-old girl with recently diagnosed Systemic Lupus Erythematosus (SLE) (class 4 lupus nephritis with autoimmune hemolytic anemia) presented to the pediatric nephrology clinic with polyuria, tiredness and cramps; laboratory investigations revealed refractory hypokalemia, hypomagnesemia, metabolic alkalosis, hypocalciuria and hyperchloriuria. There was no history of diuretic administration. These features were consistent with the Gitelman syndrome. She required large doses of potassium and magnesium supplementation along with spironolactone, for normalization of the serum potassium and magnesium levels. Immunosuppressive therapy was continued with cyclophosphamide pulses administered on a monthly basis. The doses of potassium and magnesium supplements were tapered off over the next 6 months. The clinical exome sequencing was negative for any mutations in the SLC12A3 gene. An ‘acquired’ form of Gitelman syndrome has been reported earlier in association with Sjogren syndrome and systemic sclerosis. Though tubular disorders such as renal tubular acidosis have been reported in association with SLE, a Gitelman-like syndrome has not been reported earlier. This case adds Gitelman-like tubulopathy to the clinical spectrum of tubular disorders complicating SLE.
Keywords: Lupus, Gitelman syndrome, Nephritis, Tubular disorders
Introduction
Systemic lupus erythematosus (SLE) is a multifaceted autoimmune disorder with protean manifestations. It has a predilection for multiorgan involvement and kidney is the most common visceral organ involved in 50–75% children with SLE [1]. Childhood-onset SLE is more severe and has higher morbidity and mortality compared to adult-onset SLE [2]. Renal tubulopathies are known to occur in lupus nephritis, especially distal renal tubular acidosis (dRTA) [3]. We herein report the occurrence of a Gitelman-like syndrome in a child with SLE.
Case report
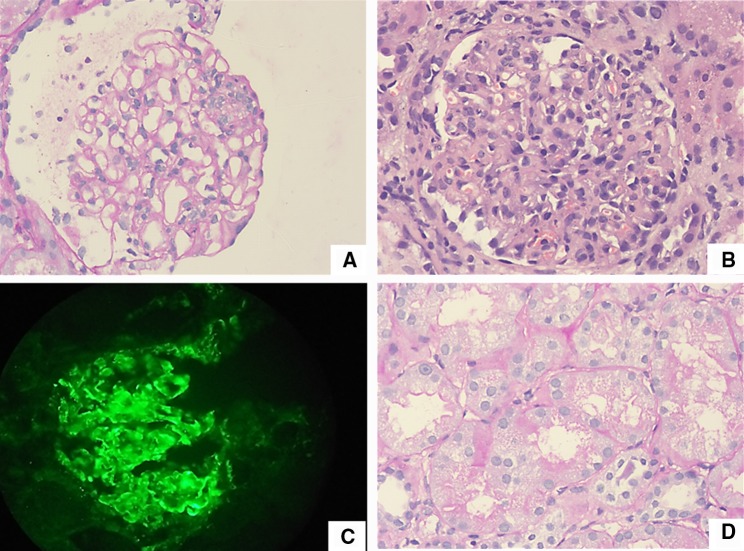
An 8-year-old developmentally normal girl presented with pyrexia of unknown origin in association with pallor and arthralgia. On examination, she had severe pallor, pedal edema, oral ulcers, generalized lymphadenopathy and stage 1 hypertension. She weighed 19.5 kg. Her height was 120 cm (− 1.2 Z). Ophthalmological evaluation was unremarkable. Initial investigations showed Hemoglobin of 4.4 g/dL, TLC 18.24 × 103/mm3, platelet count 2.09 × 105/mm3. Peripheral smear showed normochromic normocytic anemia. The Direct Coomb’s test was positive (4+); serum lactate dehydrogenase (LDH) was 392 U/L. The other investigations were blood urea 67 mg/dL, serum creatinine 0.99 mg/dL (eGFR 50 mL/min/1.73 m2), sodium 136 mEq/L, potassium 4.19 mEq/L, magnesium 1.81 mg/dL, bicarbonate 21 mEq/L, serum calcium (corrected for albumin) 9.5 mg/dL, serum phosphorus 4.5 mg/dL, serum albumin 1.3 g/dL, and cholesterol 143 mg/dL. Urinalysis showed no active sediment and urine albumin was 3+ . Liver function tests, renal ultrasonogram, chest roentgenogram and echocardiogram were normal. The complement levels were low (C3-31.36 mg/dL and C4-32.4 mg/dL). Antinuclear antibodies were positive by immunofluorescence (4+) and anti-dsDNA antibody was also positive. anti-SS-A, anti-SS-B, anti-Scl 70 and anti-U1 RNP antibodies were negative. The child was diagnosed to have SLE as per the Systemic Lupus International Collaborating Clinics (SLICC) criteria [4]. A renal biopsy showed Class IV nephritis (without crescents) as per the Revised ISN-RPS classification [5], with the immunofluorescence showing full-house positivity (Fig. 1). Occasional tubules showed protein reabsorption droplets. The interstitium did not show any significant inflammation, including the subcapsular area and the surrounding arteries (Fig. 1).
Fig. 1.
a Section shows glomerulus with segmental endocapillary proliferation in the 2–3’O clock position and wire loop lesion above the focus of endocapillary proliferation. Periodic Acid Schiff stain, × 200. b Section shows glomerulus with diffuse endocapillary proliferation with dense neutrophilic infiltration, Hematoxylin and Eosin stain, × 200. c Section shows glomerulus with strong deposits of IgG in the glomerular capillary basement membrane, IgG monoclonal antibody from DAKO USA, Fluorescin isothyocyanate stain, × 200. d Section shows tubules with prominent protein reabsorption droplets. There is no significant interstitial inflammatory infiltrate. Periodic Acid Schiff stain, × 400
The child was managed with intravenous methylprednisolone and intravenous cyclophosphamide. She was thereafter discharged on prednisolone, hydroxychloroquine, amlodipine, and calcium supplements. At discharge, her renal functions returned to normal (serum creatinine 0.42 mg/dL) and her hemoglobin had improved after blood transfusion (8 g/dL), with normal urine output (1.5 mL/kg/h). She did not receive any diuretics during hospitalization or at discharge.
On follow-up at the pediatric nephrology clinic after 2 weeks, she complained of tiredness and cramps and increased urine output. The blood pressure was normal (50–90th centile). There was no history of any diuretic intake. The weight at this juncture was 19.4 kg. She was documented to have polyuria (upto 7 mL/kg/h), hypokalemia (1.8 mEq/L) and hypochloremic metabolic alkalosis (serum chloride 90 mEq/L, bicarbonate 28 mEq/L). The serum creatinine (0.41 mEq/L), calcium level (9.2 mg/dL) and phosphorus level (4.3 mg/dL) were normal. The hypokalemia was refractory to intravenous potassium correction (upto 40 mEq/L in the maintenance fluids). At this juncture, the serum magnesium was found to be low (0.6 mEq/L). The urine chloride was 117 mEq/L (high), and the urine calcium: creatinine ratio was 0.01 (low). The fractional excretion of potassium (FeK) was 21% and fractional excretion of magnesium (FeMg) was 15%. Hence, the patient had significant urinary potassium and magnesium loss. The features were suggestive of a Gitelman-like syndrome. She was thereafter treated with oral KCl (10 mEq/kg/day), oral magnesium oxide (upto 100 mg/kg/day) and spironolactone. Gradually, in another 4 weeks, the polyuria resolved, and the magnesium and potassium levels were corrected. The clinical exome sequencing was negative for any mutations in the SLC12A3 gene.
Currently, she has received six pulses of intravenous cyclophosphamide. The potassium and magnesium supplements have been gradually tapered off, and spironolactone has been discontinued. The serum magnesium is currently 2.2 mg/dL, while the serum potassium is 4.1 mg/dL (without any potassium or magnesium supplements). The serum creatinine is 0.39 mg/dL (normal). The urine output is normal. Lupus nephritis is in remission, and DCT is negative. She is receiving prednisolone, hydroxychloroquine and mycophenolate mofetil for SLE.
Discussion
Our patient was diagnosed as lupus nephritis on basis of SLICC criteria. She was started on immunosuppressive therapy as per KDIGO guidelines for lupus nephritis class 4 [6]. At initial presentation, she had normal serum potassium, magnesium and bicarbonate levels. Three weeks after commencement of immunosuppressive therapy, she developed polyuria which was associated with refractory hypokalemia, hypomagnesemia, hyperchloriuria, metabolic alkalosis and hypocalciuria. This constellation of clinical and biochemical findings was consistent with the Gitelman-like syndrome [7].
The development of polyuria in SLE is usually attributed to dRTA [8–10]. This has been postulated to an immunologically mediated tubulitis resulting in a proton secretory defect. Accordingly, when this child, who was recently diagnosed as lupus nephritis presented with polyuria and hypokalemia, an initial possibility of dRTA was considered, which was negated by the presence of metabolic alkalosis and hypocalciuria. dRTA would have been expected to have normal anion gap metabolic acidosis and hypercalciuria which were absent in the present case. There was no concomitant usage of drugs such as thiazides, aminoglycosides, etc. which could have led to a clinical picture mimicking Gitelman syndrome. The authors could not come across any reports of methylprednisolone-induced Gitelman syndrome (a constellation of features like hypokalemia, metabolic alkalosis, hypocalciuria, hyperchloriuria, hypomagnesemia) on literature search. There was no significant weight loss that could have mimicked a pseudo-Gitelman syndrome. The renal histopathology in this case did not have any features suggestive of interstitial nephritis that could have contributed to hypokalemia.
Since the metabolic derangements accompanying the polyuria were suggestive of the Gitelman syndrome, we did a MEDLINE search to look for an association between SLE and Gitelman syndrome or a Gitelman-like tubulopathy. Although Gitelman syndrome has been reported earlier with Sjogren syndrome [11] and systemic sclerosis [12], we could not come across any report of Gitelman syndrome or a Gitelman-like tubulopathy in association with lupus nephritis. It is notable that our patient did not have clinical features associated with Sjogren syndrome or systemic sclerosis and was also negative for their respective auto-antibodies indicating that she did not have features of overlap syndrome. Also, the anecdotal reports of C1q nephropathy in association with Gitelman syndrome [13, 14] might lead one to speculate that lupus too could be associated with a Gitelman-like tubulopathy, as C1q nephropathy is known to precede lupus in some cases.
The occurrence of Gitelman syndrome in Sjogren syndrome and Systemic sclerosis has been attributed to antibodies against the sodium-chloride co-transporter (NCC) as exemplified by renal immunohistochemistry showing absent staining of the NCC in the distal tubules [15], heterozygous mutations involving the NCC [15] or even an incidentally co-inherited homozygous mutation involving the gene encoding the NCC (SLC12A3) [16]. Our case is negative for mutations in the SLC12A3 gene, hinting towards an acquired mechanism, possibly related to antibodies directed against the NCC. Such antibodies could be part of the wide array of antibodies occurring in SLE. We could not demonstrate anti-NCC antibodies by immunohistochemistry in this case due to resource and logistic constraints.
Based on the absence of genetic mutations for the Gitelman syndrome in the index case, and prior reports of ‘acquired’ Gitelman syndrome in other autoimmune disorders such as Sjogren syndrome and systemic sclerosis, we postulate that the same pathophysiology of anti-NCC antibody-induced Gitelman-like tubulopathy can be extrapolated to lupus nephritis. This case expands the clinical manifestations of lupus nephritis by adding Gitelman-like tubulopathy to the spectrum of tubulopathies associated with SLE.
Author contributions
GSB, SK, PK, RR, KR, GD and JGR managed the patient, reviewed the literature and drafted the first version of the manuscript. SK critically revised the manuscript. RNG interpreted the histopathological findings. All authors contributed to review of the literature, drafting of the manuscript and approved the final version of the manuscript. SK shall act as guarantor of the paper.
Funding
None.
Compliance with ethical standards
Conflict of interest
None stated.
Ethical statement
This case report conforms to the World Medical Association (WMA) Declaration of Helsinki ethical principles for medical research involving human subjects. Informed consent has been obtained from the parents for submission of the manuscript for consideration of publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sinha R, Raut S. Pediatric lupus nephritis: management update. World J Nephrol. 2014;3:16–23. doi: 10.5527/wjn.v3.i2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baqi N, Moazami S, Singh A, Ahmad H, Balachandra S, Tejani A. Lupus nephritis in children: a longitudinal study of prognostic factors and therapy. J Am Soc Nephrol. 1996;7:924–929. doi: 10.1681/ASN.V76924. [DOI] [PubMed] [Google Scholar]
- 3.Hataya H, Ikeda M, Ide Y, Kobayashi Y, Kuramochi S, Awazu M. Distal tubular dysfunction in lupus nephritis of childhood and adolescence. Pediatr Nephrol. 1999;13:846–849. doi: 10.1007/s004670050713. [DOI] [PubMed] [Google Scholar]
- 4.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 6.KDIGO-2012-GN-Guideline-English.pdf. https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf. Accessed 2 Sep 2019
- 7.Blanchard A, Bockenhauer D, Bolignano D, Calò LA, Cosyns E, Devuyst O, et al. Gitelman syndrome: consensus and guidance from a Kidney Disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2017;91:24–33. doi: 10.1016/j.kint.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Li SL, Liou LB, Fang JT, Tsai WP. Symptomatic renal tubular acidosis (RTA) in patients with systemic lupus erythematosus: an analysis of six cases with new association of type 4 RTA. Rheumatology (Oxford) 2005;44:1176–1180. doi: 10.1093/rheumatology/keh705. [DOI] [PubMed] [Google Scholar]
- 9.Nandi M, Das MK, Nandi S. Failure to thrive and nephrocalcinosis due to distal renal tubular acidosis: a rare presentation of pediatric lupus nephritis. Saudi J Kidney Dis Transpl. 2016;27:1239–1241. doi: 10.4103/1319-2442.194679. [DOI] [PubMed] [Google Scholar]
- 10.Ter Meulen CG, Pieters GFFM, Huysmans FTM. Flaccid paresis due to distal renal tubular acidosis preceding systemic lupus erythematosus. Neth J Med. 2002;60:29–32. [PubMed] [Google Scholar]
- 11.Kim YK, Song HC, Kim WY, Yoon HE, Choi YJ, Ki CS, et al. Acquired Gitelman syndrome in a patient with primary Sjögren syndrome. Am J Kidney Dis. 2008;52:1163–1167. doi: 10.1053/j.ajkd.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Masab M, Goyal A, Abrol S, Rangaswami J. Acquired Gitelman Syndrome associated with systemic sclerosis. Cureus. 2019;11:e3923. doi: 10.7759/cureus.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanevold C, Mian A, Dalton R. C1q nephropathy in association with Gitelman syndrome: a case report. Pediatr Nephrol. 2006;21:1904–1908. doi: 10.1007/s00467-006-0261-9. [DOI] [PubMed] [Google Scholar]
- 14.Rosado Rubio C, Fraile Gómez P, Gómez Muñoz MA, Garcia-Cosmes P, Lerma Márquez JL. C1q nephropathy in a patient with Gitelman syndrome. NDT Plus. 2011;4:392–393. doi: 10.1093/ndtplus/sfr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu X, Su Z, Chen M, Xu Y, Wang Y. Acquired Gitelman syndrome in a primary Sjögren syndrome patient with a SLC12A3 heterozygous mutation: a case report and literature review. Nephrology (Carlton). 2017;22:652–655. doi: 10.1111/nep.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishima E, Mori T, Sohara E, Uchida S, Abe T, Ito S. Inherited, not acquired, Gitelman syndrome in a patient with Sjögren’s syndrome: importance of genetic testing to distinguish the two forms. CEN Case Rep. 2017;6:180–184. doi: 10.1007/s13730-017-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]