Abstract
Introduction
The present study aimed to explore the role of miR-499a-5p and its molecular mechanism in cervical cancer (CC).
Methods
Quantitative real-time PCR (QRT-PCR) and Western blotting were performed to detect the expression of miR-499a-5p and eukaryotic translation initiation factor 4E (eIF4E) in CC tissues and cell lines. The proliferation, migration, and invasion of CC cells were detected by MTT assay, wound healing assay, and Transwell assay. Apoptosis was evaluated by flow cytometry and alterations of apoptosis-related genes. The effect of miR-499a-5p on epithelial–mesenchymal transition (EMT) was examined by determining the protein levels of EMT-associated genes. Then, colony formation assay was used to determine the radiosensitivity of CC cells. A dual-luciferase reporter assay was performed to confirm the direct target of miR-499a-5p.
Results
MiR-499a-5p was significantly downregulated in CC tissues and cell lines. Overexpression of miR-499a-5p or eIF4E knockdown markedly inhibited cell proliferation, invasion, migration, and EMT, and enhanced apoptosis. eIF4E was predicted and verified as a target gene of miR-499a-5p. The influence of miR-499a-5p upregulation on proliferation, apoptosis, invasion, migration, EMT, and radiosensitivity was abrogated by eIF4E overexpression.
Discussion
MiR-499a-5p promoted the apoptosis and radiosensitivity and inhibited proliferation, invasion, migration, and EMT by directly targeting eIF4E in CC cells.
Keywords: cervical cancer, epithelial–mesenchymal transition, eukaryotic translation initiation factor 4E, miR-499a-5p, radiosensitivity
Introduction
Cervical cancer (CC) is one of the most common gynecological malignant tumors with its high morbidity and mortality. By 2012, it is estimated that 528,000 cases of CC had been diagnosed, of which 266,000 died from CC worldwide.1 Currently, the treatment methods for CC mainly include surgery, radiotherapy, and chemotherapy, among which radiotherapy is the commonly used treatment for patients with the middle and advanced stages.2 However, in clinical practices, a few patients often develop resistance to radiotherapy, resulting in unsatisfactory outcomes and even tumor recurrence and metastasis.3 In addition to the clinicopathological features, the molecular biological characteristics of cancer cells might be the potential influencing factors for efficacy. Therefore, it is of great significance to reveal the molecular mechanism underlying radioresistance in CC patients.
Eukaryotic translation initiation factor 4E (eIF4E) is a crucial regulator of mRNA translation and protein synthesis and functions as a proto-oncogene that participates in cancer-related events such as malignant transformation, proliferation, invasion, and metastasis.4 The aberrant expression of eIF4E was reported to be associated with the occurrence and development of several tumors, such as retinoblastoma,5 esophageal cancer,6 and prostate cancer (PCa).7 With regards to the role of miR-499a-5p in CC, Wang et al found that the restoration of eIF4E promoted CC cell proliferation and migration.8 In recent years, abundant studies provided strong evidence that downregulation of eIF4E significantly sensitizes CC cells to chemotherapy.9,10 Although the role of eIF4E in CC progression has been characterized, little is known about its underlying mechanism.
MicroRNA (miRNA), a class of endogenous non-coding single-strand small molecule RNA of 18–25 nucleotides in length, which is post-transcriptionally regulating eukaryotic gene expression through binding to 3ʹ-untranslated regions (UTR) of target gene.11 In this way, several miRNAs participate in diverse biological processes, including proliferation, apoptosis, invasion, migration, and tumorigenesis. Among these miRNAs, miRNA-499a-5p has been identified as a tumor suppressor gene in a series of cancers. For instance, Liu et al found that the overexpression of miR-499a-5p repressed proliferation and differentiation of osteosarcoma cells by negatively regulating its target gene protein phosphatase 1D (PPM1D) through modulation of Akt/synthase kinase 3β (GSK-3β) signaling.12 Additionally, it has been reported that miR-499a-5p promoted lung cancer cell proliferation, migration, and epithelial–mesenchymal transition (EMT) in vitro and enhanced tumor growth in vivo.13 However, the expression of miR-499a-5p in CC tissues remains unclear, and whether it modulated the radiosensitivity of CC cells is still unknown. Additionally, the interrelation of miR-499a-5p and eIF4E in CC development was also not completely elucidated. In the present study, we aimed to explore the role and mechanism of miR-499a-5p on proliferation, apoptosis, invasion, migration, EMT, and radiosensitivity of CC.
Materials and Methods
Patients and Sample Collection
Between January 2016 and January 2018, we recruited 50 patients with cervical cancer from the First Affiliated Hospital of Zhengzhou University. Informed consent was obtained from each subject before collecting tumor specimens and adjacent normal tissues. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Cell Culture
Human CC cell lines Hela, C-33A, SiHa, ME180, and CaSki and ectocervical epithelial cell line Ect1/E6E7 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin (all from Gibco, Carlsbad, CA, USA) in a humidified atmosphere with 5% CO2 at 37°C.
Cell Transfection and Treatment
The miR-499a-5p mimic, miRNA negative control (miR-NC), siRNA against eIF4E (si-eIF4E), siRNA negative control (si-NC), pcDNA3.1-eIF4E plasmid (eIF4E) and the corresponding negative control (NC) were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). Hela and CaSki cells at the logarithmic growth phase were seeded in 24-well plates in antibiotics-free DMEM and transfected with these plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
Cell Viability Analysis
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (MTT, Sigma-Aldrich, St. Louis, MO, USA) was used to assess the proliferation ability of CC cells. At 0, 24, 48, 72, and 96 post-transfection, viable CC cells were determined by incubation with MTT (5 mg/mL) for 4 hrs at 37°C. The formazan crystals were extracted with dimethylsulfoxide (DMSO; Sigma-Aldrich) for 30 min at room temperature. The absorbance was measured at 540 nm by using a microplate reader (Millipore, Bedford, MA, USA) and the percentage of cell survival was determined with results from corresponding control group set at 100%. Three independent experiments with quadruplicates were repeated for each point.
Cell Apoptosis Assay
Hela and CaSki cells were collected, washed with PBS and fixed with ethyl alcohol at 4°C for 2 hrs. Annexin V-FITC and propidine iodide (PI) double staining was then performed at 4°C under darkness for 30 min according to the manufacturer’s protocol. All cells were examined using flow cytometry (Beckman Coulter, Fullerton, CA, USA).
Transwell Invasion Assay
The invasive ability of CC cells was determined using the transwell system. Hela and CaSki cells in each group were added to the upper surface of the membrane pre-coated with matrigel, while DMEM with 10% FBS was added into the lower chamber. After incubation for 24 hrs, cells that passed through the lower side of the membrane were fixed with paraformaldehyde, stained with crystal violet hydrate solution (JRDUN Biotechnology Co., Ltd., Shanghai, China) and quantified under a light microscope (Leica Microsystems, Wetzlar, Germany).
Wound Healing Assay
Wound-healing assay was performed to measure the cell migration capacity. Briefly, transfected Hela and CaSki cells were scratched with a 10 µL pipette tip, washed with PBS and re-suspended in culture medium for 24 hrs at 37°C. Representative images after wounding were captured with a light microscope.
Colony Formation Assay
Hela and CaSki cells transfected with miR-NC or miR-499a-5p or together with eIF4E or NC were radiated with different radiation doses (0, 2, 4, 6, 8, and 10 Gy) at a dose rate of 2 Gy/min. After incubation for 24 hrs, cells were re-seeded onto 6-well plates, fixed with 4% paraformaldehyde solution for 15 min, and stained with 0.1% crystal violet solutions. The number and size of colonies were analyzed using optical microscope. The experiments were performed in triplicate.
Quantitative Real-Time PCR (QRT-PCR)
Total RNA was extracted from tumor tissues and cultured CC cells by using Trizol reagent (Invitrogen) according to the manufacturer’s instruction. RNA was then reversely transcribed into cDNA using SuperScript III Reverse Transcriptase (Invitrogen) with Oligo (dT) primers. QRT-PCR was performed with an SYBR RT-PCR kit (Takara, Otsu, Japan), with all reactions preceded on the ABI 7500 real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). The quantification of miR-499a-5p and eIF4E was analyzed by using the 2-ΔΔCT method, with U6 and β-actin used as endogenous controls, respectively.
Western Blotting
Cell lysates were obtained with RIPA lysis buffer (Beyotime Biotechnology, Beijing, China), and protein concentrations were determined by the bicinchoninic acid (BCA) method. Equal amounts of protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore). After being blocked, membranes were incubated with specific antibodies from Cell Signaling Technology (Boston, MA, USA) for the detection of eIF4E, N-cadherin, E-cadherin, Vimentin, Snail-1, and GAPDH levels according to the manuscript’s instructions. Immunoreactive bands were detected with an enhanced chemiluminescence kit (Beyotime Biotechnology) and visualized on the ImageQuant LAS 4000 System (GE Healthcare, Milwaukee, Wisconsin, USA).
Luciferase Assays
Hela cells were co-transfected with 200 ng miR-499a-5p mimic or miR-NC and 50 ng recombinant pmirGLO-eIF4E-WT or -MUT reporter plasmids. After 24-hr transfection, luciferase activity was measured with Dual-Luciferase Reporter 1000 Assay System (Promega, Madison, WI, USA), with results expressed as relative fold change of the ratios of firefly luciferase activity normalized to renilla luciferase activity.
Statistical Analysis
Data were presented as the mean ±standard deviation (SD). Statistical analysis was conducted with two-tailed Student’s t-test or analysis of variance (ANOVA) using SPSS 17.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered as statistically significant.
Results
MiR-499a-5p Was Downregulated While eIF4E Was Upregulated in CC Tissues and Cell Lines
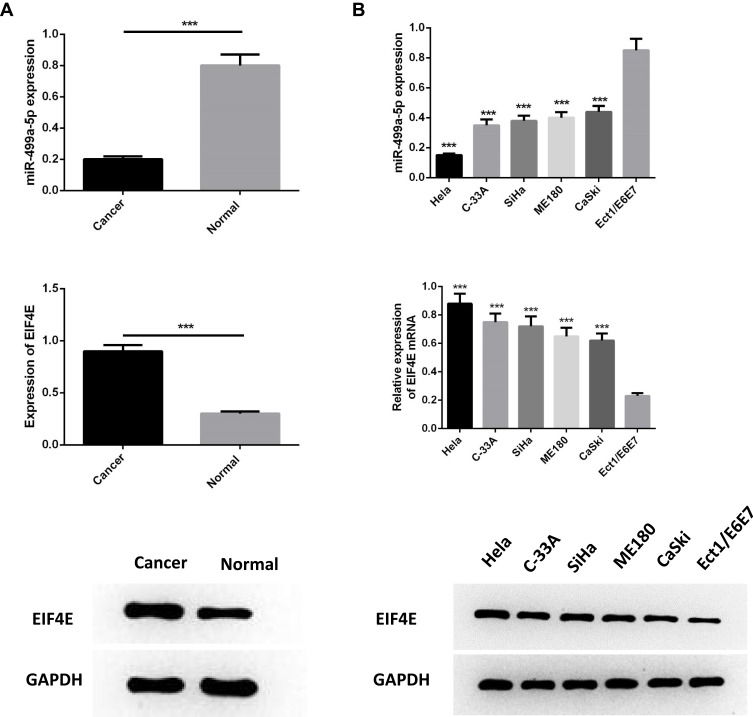
In order to determine the expression of miR-499a-5p and eIF4E in CC progression, qRT-PCR and Western blot were both performed on 50 cases of clinical CC tissues together with adjacent normal tissues, and in five BC cell lines (Hela, C-33A, SiHa, ME180, CaSki) in comparison with ectocervical epithelial Ect1/E6E7 cells. The results manifested that the mRNA expression of miR-499a-5p was significantly decreased, and eIF4E protein levels were upregulated in human CC tissues than those in normal tissues (Figure 1A). Compared with Ect1/E6E7 cells, miR-499a-5p mRNA expression was observably decreased in CC cell lines, while eIF4E protein expression exhibited a contrasting trend. Additionally, miR-499a-5p expression in Hela and CaSki cells was the lowest and highest, respectively (Figure 1B).
Figure 1.
MiR-499a-5p was downregulated while eIF4E was upregulated in CC tissues and cell lines. (A) The expression of miR-499a-5p and protein level of eIF4E were detected by qRT-PCR and Western blotting in CC tissues and normal tissues. (B) The expression of miR-499a-5p and protein level of eIF4E were determined by qRT-PCR and Western blotting in cell lines of Hela, C-33A, SiHa, ME180, CaSki, and ectocervical epithelial cell line Ect1/E6E7. ***P<0.001 compared with Ect1/E6E7.
Upregulated miR-499a-5p or Silenced eIF4E Suppressed CC Cell Proliferation, and Increased Apoptosis
To investigate the functional roles of miR-499a-5p and eIF4E, miR-499a-5p mimics, si-eIF4E, or their corresponding controls were transfected into Hela and CaSki cells, and the expression of miR-499a-5p and EIF4E mRNA was detected after 24 hrs. As illustrated by Figure 2A and B, miR-499a-5p mimic transfection or siRNA-mediated eIF4E knockdown led to increased miR-499a-5p expression. MTT assay was then performed to evaluate the level of proliferation. As presented in Figure 2C and D, the enforced expression of miR-499a-5p or silencing of eIF4E significantly reduced the proliferation of Hela and CaSki cells. Subsequently, the transfected cells were stained with Annexin V and PI. The apoptotic cells were quantified by flow cytometry. The upregulation of miR-499a-5p or downregulation of eIF4E significantly improved the apoptosis rate of Hela and CaSki cells (Figure 2E). The mechanism of transfection of the miR-499a-5p mimic or si-eIF4E on inducing apoptosis in human CC cells was further investigated. The protein levels of apoptosis-related genes caspase-3, Bax, and Bcl-2 were detected by Western blot. Data showed that the upregulation of miR-499a-5p or suppression of eIF4E expression led to significant downregulation of Bcl-2 expression and upregulation of caspase-3 and Bax expressions (Figure 2F).
Figure 2.
Upregulated miR-499a-5p or silenced eIF4E suppressed CC cell proliferation and increased apoptosis. (A, B) qRT-PCR detected the expression of miR-499a-5p and eIF4E mRNA. (C, D) MTT assay calculated proliferation of Hela and CaSki cells. (E) Apoptosis was tested by flow cytometry. (F) The protein levels of caspase-3, Bax, and Bcl-2 were detected by Western blotting in Hela and CaSki cells transfected with miR-499a-5p mimic, miR-NC, si-eIF4E, and si-NC. ***P<0.001.
Overexpression of miR-499a-5p or eIF4E Knockdown Inhibited CC Cell Invasion and Migration
To further determine the effect of miR-499a-5p on invasion, EMT, and migration, we evaluated the invasive capacities, the expressions of EMT markers and migration abilities in transfected Hela and CaSki cells. As shown in Figure 3A, the invasive capabilities of Hela and CaSki cells were remarkably decreased in the miR-499a-5p mimic and si-eIF4E group. Moreover, miR-499 mimic or si-eIF4E could increase the protein expression of E-cadherin, an epithelial marker, and downregulate the expressions of N-cadherin, vimentin, and Snail-1, the mesenchymal markers, in both Hela and CaSki cells. Wound healing assay showed that overexpressing miR-499a-5p or silencing eIF4E inhibited the migratory ability of Hela and CaSki cells (Figure 3B).
Figure 3.
Overexpression of miR-499a-5p or eIF4E knockdown inhibited CC cell invasion and migration. (A) Cell migration capacity was detected by Transwell assay, the protein levels of N-cadherin, E-cadherin, Vimentin, and Snail-1. (B) Cell migration capacity was determined by wound healing assay in Hela and CaSki cells transfected with miR-499a-5p mimic, miR-NC, si-eIF4E, and si-NC. ***P<0.001.
eIF4E Was a Direct Target of miR-499a-5p
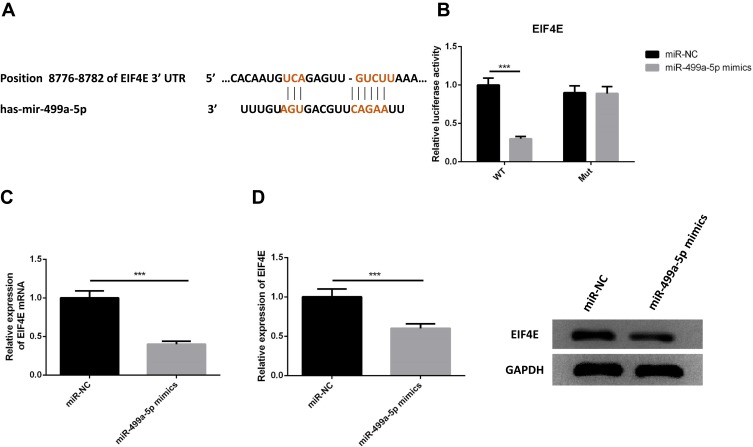
We next examined whether miR-499a-5p could directly regulate eIF4E expression by means of target prediction program. Predicted by the bioinformatics website Targetscan (http://www.targetscan.org), there was several binding sites of miR-499a-5p in eIF4E 3′-UTR, suggesting that miR-499a-5p could directly bind to eIF4E in a targeted manner (Figure 4A). Next, we performed the luciferase assay to confirm whether miR-499a-5p directly targeted eIF4E, and the results showed that miR-499a-5p mimic remarkably down-regulated the luciferase activity of the eIF4E-wt construct in Hela cells, while the luciferase activity of eIF4E-mut was never changed (Figure 4B). Meanwhile, we carried out qRT-PCR and Western blotting to determine the mRNA and protein levels of eIF4E in Hela and CaSki cells transfected with the miR-499a-5p mimic or miR-NC, and found that the mRNA and protein levels of eIF4E (Figure 4C and D) were prominently suppressed after transfection with the miR-499a-5p mimic.
Figure 4.
eIF4E was a direct target of miR-499a-5p. (A) The binding site of miR-499a-5p with eIF4E was confirmed according to bioinformatics analysis. (B) The relative luciferase activity in miR-499a-5p mimic group and miR-NC group. (C, D) The relative expression of eIF4E and protein levels of eIF4E mRNA in miR-499a-5p mimic transfected group and miR-NC group. ***P<0.001.
eIF4E Overexpression Reversed the Effect of miR-499a-5p on CC Cell Proliferation, Apoptosis, Invasion, and Migration
To confirm whether miR-499a-5p regulated the proliferation, apoptosis, invasion, EMT, and migration of CC cells through an eIF4E-dependent mechanism, miR-499a-5p mimic or miR-NC was co-transfected with pcDNA-eIF4E or NC into Hela and CaSki cells. The data showed that miR-499a-5p mimic dramatically decreased eIF4E mRNA and protein expression, whereas the expression of eIF4E was significantly increased after co-transfection of miR-499a-5p mimic and pcDNA-eIF4E (Figure 5A). MTT assay confirmed that miR-499a-5p-induced proliferation inhibition could be rescued by eIF4E overexpression (Figure 5B). Flow cytometry indicated that the induction effect on cell apoptosis caused by miR-499a-5p could be entirely abrogated by the overexpression of eIF4E (Figure 5C). Furthermore, the enhancement of cell invasion ability and E-cadherin expression and downregulation of N-cadherin, vimentin, and Snail-1 induced by miR-499a-5p overexpression were abolished after co-overexpression of miR-499a-5p and eIF4E (Figure 6A). In addition, upregulation of eIF4E also could partially reverse the inhibitory effect of miR-499a-5p overexpression on the migration of Hela and CaSki cells (Figure 6B).
Figure 5.
eIF4E overexpression reversed the effect of miR-499a-5p on CC cell proliferation, apoptosis, invasion, and migration. (A) The expression of miR-499a-5p and expression of eIF4E mRNA and protein levels were tested by qRT-PCR and Western blotting in cells transfected with miR-499a-5p mimics, miR-499a-5p mimics and pcDNA3.1-EIF4E, miR-499a-5p mimics and pcDNA3.1-NC, and miR-NC, respectively. (B) Cell proliferation rate was detected by MTT assay in cells. (C) Apoptosis rate was examined by flow cytometry. ***P<0.001.
Figure 6.
eIF4E overexpression reversed the effect of miR-499a-5p on invasion and migration of CC cell. (A) Cell invasion ability and the protein levels of N-cadherin, E-cadherin, Vimentin, and Snail-1 were detected by transwell invasion assay and Western blotting, respectively. (B) Migration capacity was determined by wound healing assay in Hela and CaSki cells transfected with miR-NC or miR-499a-5p mimic or together with eIF4E or NC. ***P<0.001.
Overexpression of miR-499a-5p Enhanced the Radiosensitivity of CC Cells via Targeting eIF4E
To investigate the potential mechanism behind the role of miR-499a-5p in CC radioresistance, colony formation assay was performed. It was demonstrated that miR-499a-5p overexpression inhibited colony formation in both Hela and CaSki cells after X-ray irradiation, whereas eIF4E upregulation markedly overturned this effect, suggesting that miR-499a-5p targeting eIF4E enhanced the radiosensitivity of CC cells by reducing colony formation (Figure 7).
Figure 7.
Overexpression of miR-499a-5p enhanced the radiosensitivity of CC cells via targeting eIF4E. Colony formation assay was used to calculate the viability of cells in Hela and CaSki cells transfected with miR-499a-5p mimics, miR-499a-5p mimics and pcDNA3.1-EIF4E, miR-499a-5p mimics and pcDNA3.1-NC, and miR-NC, respectively. ***P<0.001.
Discussion
We conducted this research to investigate the expression of miR-499a-5p and its molecular mechanism in the progression of CC. Our results showed that miR-499a-5p expression was declined in CC which inhibited tumor cell proliferation, invasion, EMT, and migration, and facilitated cell apoptosis. Additionally, eIF4E was confirmed as a direct target gene of miR-499a-5p. It was further observed in the current study that overexpression of miR-499a-5p sensitizes CC cells to radiotherapy.
Increasing evidence has revealed that miRNAs are widely involved in the occurrence and development of various tumors, including CC. For example, Shi and Zhang suggested that miR-362 overexpression inhibited the proliferation, migration, and invasion of CC cells through targeting sineoculis homeobox homolog 1 (SIX1).14 Liang et al indicated that miR-187 inhibited the proliferation and promoted apoptosis of CC cells, at least partly through repression of the fibroblast growth factor 9 (FGF9) expression.15 Moreover, Hua et al demonstrated that miR-338-3p suppressed CC cell proliferation and induced apoptosis via downregulating target gene metastasis-associated in colon cancer-1 (MACC1) through mitogen-activated protein kinase (MAPK) signaling pathway.16 MiR-499a-5p has gained increasing attention in cancer research because of its anti-tumor action. The aberrant downregulation of miR-499a-5p expression has been identified in oral squamous cell carcinoma,17 osteosarcoma,12 and lung adenocarcinoma;13 however, little is known regarding its expression in CC. Here, in this study, we firstly determined the expression level of miR-499a-5p in CC tissues and cell lines by qRT-PCR and found that miR-499a-5p was significantly downregulated in CC tissues and cells. MTT and flow cytometry analysis showed that miR-499a-5p reduced proliferation and facilitated apoptosis in CC cells. Transwell assay and Western blotting showed that overexpression of miR-499a-5p dramatically suppressed the invasion of CC cells by increasing E-cadherin expression and inhibiting N-cadherin, vimentin, and Snail-1 expression. Wound healing assay showed that overexpression of miR-499a-5p dramatically suppressed the migration capabilities of CC cells. Then, we further investigated the molecular mechanism by which miR-499a-5p exerted its regulatory effects on CC cells.
eIF4E, a rate-limiting protein, is critical for translational regulation. The highly expressed eIF4E has been demonstrated in various types of cancers, including CC.8 eIF4E could be negatively regulated by upstream miRNAs and contribute to the progression of cancers. For example, miR-34c-3p suppressed non-small cell lung cancer cell proliferation, migration, and invasion by targeting eIF4E.18 MiR-15a downregulated eIF4E to decrease cell proliferation and invasion, and induce cell cycle arrest and apoptosis in renal cell carcinoma.19 MiR-455-3p inhibited the cap-dependent translation and proliferation of prostate cancer cells partly by downregulation of eIF4E expression.20 These findings tempted us to speculate that miR-499a-5p exerted anti-cancer effect via negatively regulating eIF4E expression since upregulated eIF4E was observed in CC tissues and cells. Functional analysis further confirmed that similar to miR-499a-5p overexpression, siRNA-mediated suppression of eIF4E expression repressed proliferation, invasion, migration, EMT, and improved apoptosis of CC cells. Luciferase assay verified that miR-499a-5p directly targeted eIF4E. Additionally, our results showed that eIF4E was negatively regulated by miR-499a-5p and partially reversed miR-499a-5p-induced changes in proliferation, apoptosis, invasion, EMT, and migration.
Recently, accumulating evidence has strongly implied that miRNAs are associated with radiosensitivity of multiple cancers, including CC. For example, Liu et al found that miR-132 was downregulated in radiotherapy-sensitive CC patients and cells, and inhibited proliferation and promoted apoptosis and radiosensitivity of CC cells by targeting Bmi-1.21 Wu et al exhibited that miR-15a-3p was increased in radiation-exposed CC cells and overexpression of miR-15a-3p sensitized CC to irradiation by inhibiting tumor protein D52.22 Our data demonstrated that eIF4E overexpression abolished radiosensitization induced by transfection of miR-499a-5p mimic in CC cells.
In summary, our study for the first time suggested that miR-499a-5p functioned as a tumor suppressor by inhibiting proliferation, invasion, migration, and EMT and enhancing apoptosis and radiosensitivity of CC cells in vitro by downregulating eIF4E. Our research might provide a novel preventive and a therapeutic option for CC.
Highlights
MiR-499a-5p was down-regulated in CC tissues and cell lines.
MiR-499a-5p overexpression or eIF4E knockdown inhibited cell proliferation, invasion, migration, and EMT, and enhanced apoptosis.
eIF4E was predicted and verified as a target gene of miR-499a-5p.
The influence of miR-499a-5p upregulation was abrogated by eIF4E overexpression.
Data Sharing Statement
All data in this study can be obtained by proper request from the authors.
Ethics and Consent Statement
The present study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. Written informed consent was obtained by all participants.
Consent for Publication
All authors agreed the submission and the policy of the journal and copyright.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare there is no conflict of interest in this study.
References
- 1.Manaf RA, Ismail S, Cecilia NC. Global burden of cervical cancer: a literature review. Int J Public Health Clin Sci. 2017;4:2. [Google Scholar]
- 2.Kang DF, Gynaecology DO. Effect analysis of combined treatment of chemotherapy, radiotherapy and surgery to treatⅡb cervical cancer. Chin Mod Med. 2014. [Google Scholar]
- 3.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Re Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miras M, Truniger V, Silva C, Verdaguer N, Aranda MA, Querol J. Structure of eIF4E in transcriptional complex with an eIF4G peptide supports a universal bipartite binding mode for protein translation. Plant Physiol. 2017;174(3):1476–1491. doi: 10.1104/pp.17.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Li Z, Li Z, et al. Targeting eIF4E inhibits growth, survival and angiogenesis in retinoblastoma and enhances efficacy of chemotherapy. Biomed Pharmacother. 2017;96:750–756. doi: 10.1016/j.biopha.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 6.Kai J, Wang Y, Xiong F, Wang S. Genetic and pharmacological inhibition of eIF4E effectively targets esophageal cancer cells and augments 5-FU’s efficacy. J Thorac Dis. 2018;10(7):3983–3991. doi: 10.21037/jtd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Abronzo LS, Ghosh PM. eIF4E phosphorylation in prostate cancer. Neoplasia. 2018;20(6):563–573. doi: 10.1016/j.neo.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Pang T, Gao M, et al. HPV E6 induces eIF4E transcription to promote the proliferation and migration of cervical cancer. FEBS Lett. 2013;587(6):690–697. doi: 10.1016/j.febslet.2013.01.042 [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Wang Z, Xu L, et al. Targeting the eIF4E/beta-catenin axis sensitizes cervical carcinoma squamous cells to chemotherapy. Am J Transl Res. 2017;9(3):1203–1212. [PMC free article] [PubMed] [Google Scholar]
- 10.Xi C, Wang L, Yu J, Ye H, Cao L, Gong Z. Inhibition of eukaryotic translation initiation factor 4E is effective against chemo-resistance in colon and cervical cancer. Biochem Biophys Res Commun. 2018;503(4):2286–2292. doi: 10.1016/j.bbrc.2018.06.150 [DOI] [PubMed] [Google Scholar]
- 11.Hendrickson DG, Hogan DJ, Mccullough HL, et al. Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol. 2009;7(11):e1000238. doi: 10.1371/journal.pbio.1000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Huang L, Su P, et al. MicroRNA-499a-5p inhibits osteosarcoma cell proliferation and differentiation by targeting protein phosphatase 1D through protein kinase B/glycogen synthase kinase 3beta signaling. Oncol Lett. 2018;15(4):4113–4120. doi: 10.3892/ol.2018.7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S, Li Z, Yu Y, et al. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp Cell Res. 2019;379(2):203–213. doi: 10.1016/j.yexcr.2019.03.035 [DOI] [PubMed] [Google Scholar]
- 14.Shi C, Zhang Z. MicroRNA-362 is downregulated in cervical cancer and inhibits cell proliferation, migration and invasion by directly targeting SIX1. Oncol Rep. 2017;37(1):501–509. doi: 10.3892/or.2016.5242 [DOI] [PubMed] [Google Scholar]
- 15.Liang H, Luo R, Chen X, Zhao Y, Tan A. miR-187 inhibits the growth of cervical cancer cells by targeting FGF9. Oncol Rep. 2017;38(4):1977–1984. doi: 10.3892/or.2017.5916 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Hua FF, Liu SS, Zhu LH, et al. MiRNA-338-3p regulates cervical cancer cells proliferation by targeting MACC1 through MAPK signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(23):5342–5352. doi: 10.26355/eurrev_201712_13919 [DOI] [PubMed] [Google Scholar]
- 17.Hou YY, Lee JH, Chen HC, et al. The association between miR-499a polymorphism and oral squamous cell carcinoma progression. Oral Dis. 2015;21(2):195–206. doi: 10.1111/odi.12241 [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Wang X, Li J, et al. miR-34c-3p functions as a tumour suppressor by inhibiting eIF4E expression in non-small cell lung cancer. Cell Prolif. 2015;48(5):582–592. doi: 10.1111/cpr.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Chong T, Xiang X, Yang J, Li H. Downregulation of microRNA-15a suppresses the proliferation and invasion of renal cell carcinoma via direct targeting of eIF4E. Oncol Rep. 2017;38(4):1995–2002. doi: 10.3892/or.2017.5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Yan M, Yun Y, et al. MicroRNA-455-3p functions as a tumor suppressor by targeting eIF4E in prostate cancer. Oncol Rep. 2017;37(4):2449. doi: 10.3892/or.2017.5502 [DOI] [PubMed] [Google Scholar]
- 21.Liu GF, Zhang SH, Li XF, Cao LY, Fu ZZ, Yu SN. Overexpression of microRNA-132 enhances the radiosensitivity of cervical cancer cells by down-regulating Bmi-1. Oncotarget. 2017;8(46):80757–80769. doi: 10.18632/oncotarget.20358 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Wu Y, Huang J, Xu H, Gong Z. Over-expression of miR-15a-3p enhances the radiosensitivity of cervical cancer by targeting tumor protein D52. Biomed Pharmacother. 2018;105:1325–1334. doi: 10.1016/j.biopha.2018.06.033 [DOI] [PubMed] [Google Scholar]