Significance
Oncogenic hotspot mutations in PIK3CA and overexpression of HER2 are known as a driving force for human breast cancer metastasis. AMPK is pivotal in maintaining cellular energy homeostasis. In this study, we demonstrate that transcription inhibition of AMPKα1 is critically important in human advanced breast cancer with poor clinical outcomes, that AMPKα1 is transcriptionally inhibited in response to activation of PI3K/HER2, and that ΔNp63α, a tumor metastasis suppressor, is a direct transcriptional factor mediating oncogenic PI3K/HER2-induced transcriptional suppression of AMPKα1. In addition, inhibition of AMPK leads to disruption of cell–cell adhesion and promotes cancer metastasis. This study highlights a critical role for AMPK in the connection of cell–cell adhesion and cancer metastasis.
Keywords: AMPK, oncogenic signaling, ΔNp63α, cell adhesion, cancer metastasis
Abstract
AMP-activated protein kinase (AMPK) functions as an energy sensor and is pivotal in maintaining cellular metabolic homeostasis. Numerous studies have shown that down-regulation of AMPK kinase activity or protein stability not only lead to abnormality of metabolism but also contribute to tumor development. However, whether transcription regulation of AMPK plays a critical role in cancer metastasis remains unknown. In this study, we demonstrate that AMPKα1 expression is down-regulated in advanced human breast cancer and is associated with poor clinical outcomes. Transcription of AMPKα1 is inhibited on activation of PI3K and HER2 through ΔNp63α. Ablation of AMPKα1 expression or inhibition of AMPK kinase activity leads to disruption of E-cadherin-mediated cell–cell adhesion in vitro and increased tumor metastasis in vivo. Furthermore, restoration of AMPKα1 expression significantly rescues PI3K/HER2-induced disruption of cell–cell adhesion, cell invasion, and cancer metastasis. Together, these results demonstrate that the transcription control is another layer of AMPK regulation and suggest a critical role for AMPK in regulating cell–cell adhesion and cancer metastasis.
AMP-activated protein kinase (AMPK) is critical in maintaining cellular energy homeostasis via regulation of a series of biological processes, including glucose metabolism, lipid biogenesis, and protein synthesis (1). AMPK is a heterotrimer consisting of three subunits (α, β, and γ). The α subunit contains the catalytic kinase domain and the β subunit servers as a scaffold protein important for heterotrimer formation. The γ regulatory subunit binds AMP, resulting in conformation changes of AMPK and exposing T172 for phosphorylation, a critical step for activation of AMPK kinase activity (2). Metabolic stresses, such as glucose deprivation, hypoxia, and other means of accelerating ATP consumption, result in an increased ratio of AMP/ATP, which in turn leads to activation of AMPK by its upstream kinase LKB1 (3, 4). In contrast, calcium flux can activate CaMKK2, which then directly phosphorylates T172 of AMPK (5). Recently, it has been reported that deprivation of fructose-1,6-diphosphate or inactivation of aldolase can promote AMPK-AXIN-LKB1 complex formation to active AMPK in an AMP-independent manner (6).
Down-regulation of AMPK kinase activity has been documented to promote cancer development (7–9). Consistently, inactivation of LKB1 is frequently found in Peutz-Jeghers syndrome, lung cancer, colon cancer, and breast cancers (10–12). In addition, knockout of LKB1 promotes K-Ras-driven lung cancer metastasis in mice (10, 13). However, whether inhibition of AMPK promotes cancer metastasis remains unknown. At this time, several mechanisms have been shown to down-regulate AMPK T172 phosphorylation, including LKB1 defects and activation of AKT, which can directly phosphorylate S485 of AMPKα (14, 15). In addition, it has been reported that AMPKα protein stability can be regulated by ubiquitin ligase UBE2O or MAGE-A3/6-TRIM28 (7, 9).
p63, a p53 family member, plays a critical role in a wide range of biological processes including embryonic development, cell proliferation, apoptosis, survival, senescence, epithelial stem cell regeneration and differentiation, and aging (16). There are multiple p63 protein isoforms, derived from alternative transcription start sites at the N termini and alternative splicing at C termini (16). ΔNp63α, the predominant p63 isoform expressed in epithelia, is a critical transcription factor regulating expression of genes involved in cell adhesion, including E-cadherin, integrin α6, integrin β4, integrin α5, desmoplakin, and fibronectin (17–19). Clinical evidence indicates that expression of ΔNp63α is reduced in advanced cancers (19). Our previous study has demonstrated that ΔNp63α is a common inhibitory target of PI3K/Ras/HER2 and functions as a critical metastasis inhibitor (19).
In this study, we demonstrate that transcriptional inhibition of AMPKα1 is pivotal in cancer metastasis. Suppression of AMPKα1 expression leads to disruption of cell–cell adhesion and facilitates cancer metastasis. ΔNp63α directly transactivates AMPKα1 and is responsible to PI3K/HER2-mediated transcriptional inhibition of AMPKα1. These results highlight another layer of AMPK regulation and a critical role for AMPK in regulating cell–cell adhesion and cancer metastasis.
Results
Down-Regulation of AMPKα1 Expression Is Associated with Advanced Breast Cancer and Poor Clinical Outcomes.
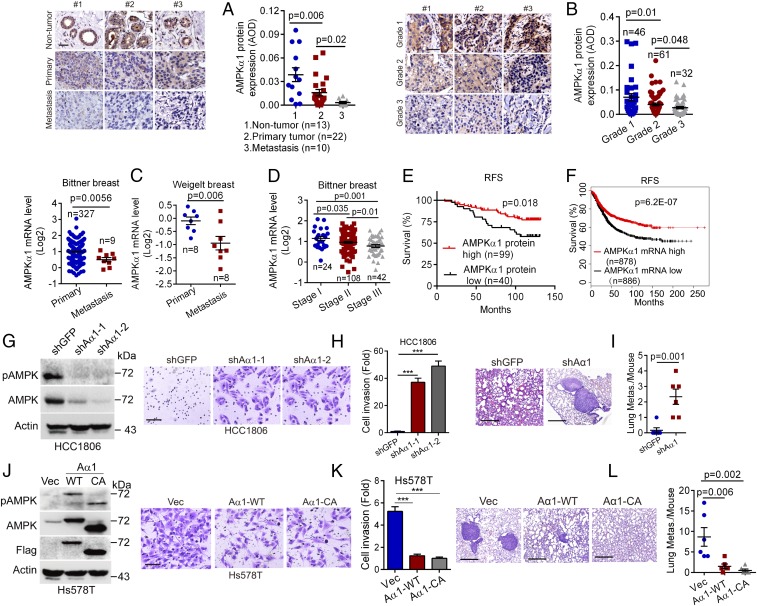
Abundant evidence indicates that AMPK plays a critical role in cancer cell proliferation and tumor growth beyond maintaining energy homeostasis (14, 20). However, whether AMPK plays a role in cancer metastasis is less clear. To address this issue, we first examined the expression of AMPKα1, the catalytic subunit of AMPK, in human breast cancer samples by immunohistochemistry (IHC). As shown in Fig. 1A, AMPKα1 protein levels were reduced in primary breast cancer specimens. In contrast, AMPKα1 protein expression was dramatically decreased in metastasized breast cancer samples. In addition, AMPKα1 protein expression was significantly reduced in a higher degree of breast cancer specimens (Fig. 1B). We then analyzed Oncomine datasets and found that compared with human primary breast tumors, AMPKα1 mRNA levels were significantly decreased in distant metastatic tumors (Fig. 1C). In keeping with the observation derived from protein analyses, AMPKα1 mRNA levels were also significantly decreased in advanced breast tumors (Fig. 1D and SI Appendix, Fig. S1A). Because breast cancer with lymph node metastases is associated with poor patient prognosis, we analyzed expression of AMPKα1 in lymph node-negative or lymph node-positive breast cancer specimens. As shown in SI Appendix, Fig. S1 B and C, both AMPKα1 protein and mRNA expression were dramatically reduced in lymph node-positive breast tumors. Notably, similar to breast cancer, AMPKα1 mRNA levels were also significantly decreased in human lung cancer, colon cancer, and liver cancers (SI Appendix, Fig. S1F). Furthermore, patients with breast cancer with either low AMPKα1 protein or mRNA levels had decreased recurrence-free survival (RFS) (Fig. 1 E and F).
Fig. 1.
Down-regulation of AMPKα1 expression is associated with advanced breast cancers and poor clinical outcomes. (A and B) Human breast cancer tissue microarrays either consisting of primary breast cancer samples (n = 22), breast cancer distant metastasis samples (n = 10), and nontumor breast samples (n = 13) (A) or consisting of breast cancer samples at different stages (grade 1, n = 46; grade 2, n = 61; grade 3, n = 32) (B) were subjected to IHC staining for AMPKα1 (Left, scale bar, 50 μm) with quantitative analyses using average optical density (AOD) (Right), as described in the Materials and Methods. (C and D) The Oncomine Bittner or Weigelt breast cancer dataset was used to analyze AMPKα1 mRNA levels in primary breast cancer samples and distant metastatic breast cancer samples (C) or in breast cancer samples at different stages (D). (E and F) RFS in patients with breast cancer was analyzed using AMPKα1 protein expression (AOD) values derived from B (E) or using AMPKα1 mRNA expression derived from Kaplan-Meier Plotter database (F). (G–L) Alteration of AMPKα1 expression affects breast cancer cell invasion in vitro and cancer metastasis in vivo. HCC1806 cells stably expressing shAMPKα1-1 (shAα1-1), shAMPKα1-2 (shAα1-2), or Hs578T cells stably expressing wild-type flag-AMPKα1 (Aα1-WT), constitutively active mutation flag-AMPKα1-T172D (Aα1-CA) were subjected to Western blot analyses (G and J) or transwell assays for cell invasion (H and K). (Scale bars, H and K, 50 μm.) For the in vivo tumor metastasis assays, 2 × 106 cells (HCC1806 or Hs578T) were i.v. injected into nude mice (n = 6/group). On day 55 (HCC1806) or day 45 (Hs578T), lungs were dissected, fixed, sectioned, and stained by hematoxylin and eosin (HE) for histological analyses. The numbers of metastatic nodules in the lungs per mouse were shown (I and L). (Scale bars, 0.5 mm.) Data are presented as means ± SEM. ***P < 0.001.
We next examined the expression of AMPKα1 in four major subtypes of breast cancers: luminal A, luminal B, HER2 positive (HER2+), and triple-negative breast cancer. As shown in SI Appendix, Fig. S1D, AMPKα1 mRNA levels were significantly reduced in advanced breast cancers of all four subtypes. Moreover, patients with breast cancer in all four subtypes with low AMPKα1 mRNA levels appeared to have short RFS (SI Appendix, Fig. S1E).
Together, these results suggest that reduced expression of AMPKα1 is linked to breast cancer metastasis and poor clinical outcomes.
Alteration of AMPKα1 Expression Impacts Cancer Cell Invasion In Vitro and Tumor Metastasis In Vivo.
To investigate the role for AMPK in cancer metastasis, we silenced AMPKα1 expression in human triple-negative breast cancer HCC1806 cells. As shown in Fig. 1 G–I, silencing of AMPKα1 promoted cell invasion in vitro and tumor metastasis in vivo. In addition, knockdown of AMPKα1 in immortalized human mammary epithelial MCF10A cells also significantly increased cell invasion (SI Appendix, Fig. S1G). Conversely, overexpression of wild-type AMPKα1 (Aα1-WT) or constitutive active mutant, AMPKα1-T172D (Aα1-CA), lacking the 80-amino acid residues of auto-inhibitory domain, significantly inhibited human triple-negative breast cancer Hs578T cell invasion in vitro and tumor metastasis in vivo (Fig. 1 J–L).
Activation of PI3K/HER2 Inhibits AMPKα1 Transcription via Suppression of ΔNp63α.
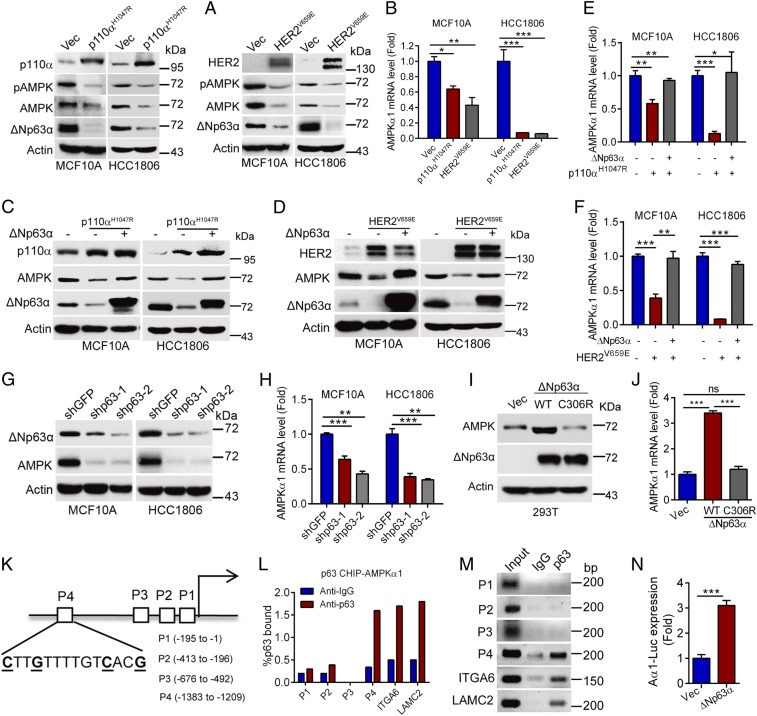
The abovementioned clinical analyses indicate that AMPKα1 protein and mRNA levels were reduced in metastasized breast cancers. Therefore, we hypothesized that AMPKα1 expression is likely inhibited at the transcriptional level. Notably, hotspot constitutive active mutations of PIK3CA, exemplified as p110αH1047R, or overexpression of HER2 are frequently found in human breast cancers, which have been documented to drive breast cancer metastasis (19, 21, 22). To investigate a possible connection between oncogenic PI3K/HER2 and AMPKα1 expression, we expressed p110αH1047R or constitutive active HER2V659E in MCF10A or HCC1806 cells. As shown in Fig. 2A, expression of p110αH1047R or HER2V659E significantly down-regulated AMPKα1 protein expression, concomitant with down-regulation of ΔNp63α, in line with our previous report (19). Apparently, p110αH1047R or HER2V659E inhibited AMPKα1 mRNA expression (Fig. 2B). In addition, H-RasG12V could also suppress AMPKα1 protein and mRNA expression (SI Appendix, Fig. S2A). Importantly, p110αH1047R or HER2V659E-induced down-regulation of AMPKα1 protein and mRNA levels was completely rescued by ectopic expression of ΔNp63α (Fig. 2 C–F).
Fig. 2.
Activation of PI3K/HER2 inhibits AMPKα1 transcription via suppression of ΔNp63α. (A–F) MCF10A or HCC1806 cells stably expressing p110αH1047R or HER2V659E with or without restoration of ΔNp63α expression were subjected to Western blot analyses (A, C, and D) or qPCR analyses (B, E, and F). (G and H) MCF10A or HCC1806 cells stably expressing shp63-1, shp63-2, or shGFP were subjected to Western blot analyses (G) or qPCR analyses (H). (I and J) 293T cells stably overexpressing wild-type ΔNp63α, or DNA-binding mutant (ΔNp63αC306R) were subjected to Western blot (I) or qPCR analyses (J). (K–N) A schematic presentation depicts four putative p63-binding elements (P1-P4) on the AMPKα1 gene promoter (K). Chromatin immunoprecipitation (ChIP) analyses using a p63 antibody or a control IgG were performed in MCF10A cells. Primers specific for P1, P2, P3, P4, integrin α6 (ITGA6), or laminin γ2 (LAMC2) were used. Data derived from qPCR analyses (L) or reverse transcription-PCR (RT-PCR) (M) were shown. (N) 293T cells were cotransfected with AMPKα1-Gluc-SEAP reporter and ΔNp63α expression plasmid. Thirty-six hours posttransfection, AMPKα1-Gluc and SEAP activities in media were measured. Data are presented as means ± SEM. ***P < 0.001; **P < 0.01; *P < 0.05.
We next investigated the effect of ΔNp63α on AMPKα1 transcription. As shown in Fig. 2 G–J and SI Appendix, Fig. S2 B and C, silencing of p63 in MCF10A or HCC1806 cells, both of which predominantly express ΔNp63α protein isoform (SI Appendix, Fig. S2D), inhibited AMPKα1 protein and mRNA expression, whereas overexpression of ΔNp63α, but not the DNA-binding defective mutant ΔNp63αC306R, up-regulated AMPKα1 protein and mRNA expression. Notably, ectopic expression of ΔNp63α, but not TAp63α, TAp63γ, ΔNp63β and ΔNp63γ, up-regulated AMPKα1 protein and mRNA expression (SI Appendix, Fig. S2E).
We then investigated the molecular basis with which ΔNp63α transcriptionally regulates AMPKα1 gene expression. As a transcription factor, ΔNp63α can bind to the conservative binding element (CNNGNNNNNNCNNG) (23). Since there are four putative p63-binding elements (P1: −195 to −1; P2: −413 to −196; P3: −676 to −492; P4: −1383 to −1209) on the AMPKα1 gene promoter (Fig. 2K and SI Appendix, Fig. S2F), we speculated that ΔNp63α may directly transactivate AMPKα1 gene expression. As shown in Fig. 2 L and M, ΔNp63α could directly bind to the P4 element of the AMPKα1 gene promoter in a similar binding strength to the documented ΔNp63α downstream targets, integrin α6 (ITGA6) or laminin γ2 (LAMC2) (17). In addition, luciferase reporter assays showed that ΔNp63α significantly enhanced AMPKα1-Gluc reporter activities (Fig. 2N).
Similar to AMPKα1, AMPKα2 is the other catalytic subunit of AMPK. However, it has been shown that AMPKα2 predominantly expresses in heart and muscle, but not in breast (24). Indeed, protein of AMPKα1, but not AMPKα2, was readily detectable in untransformed breast epithelial MCF10A cells and in breast cancer cells including MCF7, HCC1806, Hs578T, and MDA-MB-231 (SI Appendix, Fig. S2G).
Together, these results indicate that ΔNp63α is most likely a direct transcriptional factor of AMPKα1, mediating the oncogenic PI3K/HER2 signaling in regulation of AMPKα1 expression.
Silencing of AMPKα1 Leads to Disruption of Cell–Cell Adhesion via Twist1-E-Cadherin Axis.
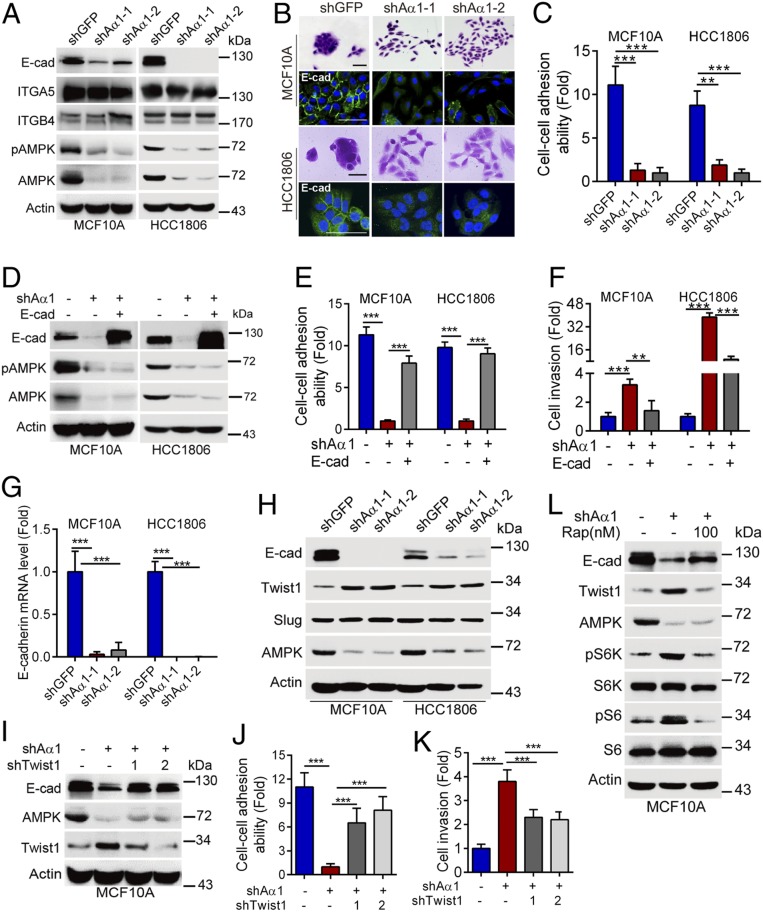
Since silencing of AMPKα1 promotes cancer cell invasion in vitro and tumor metastasis in vivo, we therefore investigated the molecular mechanisms by which AMPK regulates breast cancer metastasis. It is well known that disruption of cell–cell adhesion is critical in cell migration/invasion and cancer metastasis (19, 25). Interestingly, silencing of AMPKα1 dramatically inhibited protein expression of E-cadherin, a key component for cell–cell adhesion, while it had little effect on integrin α5 (ITGA5) or integrin β4 (ITGB4), two well-known cell-matrix adhesion proteins (26, 27) (Fig. 3A). In addition, knockdown of AMPKα1 significantly disrupted cell–cell adhesion, as evidenced by scattering cell growth and reduced cell–cell adhesion ability, defined as forming clustered cells (>4 cells/per colony) (25) (Fig. 3 B and C). Furthermore, expression of a dominant negative mutant, AMPKα1-D139A (Aα1-Dn), inhibited E-cadherin expression and disrupted cell–cell adhesion, similar to that of AMPKα1 ablation (SI Appendix, Fig. S3 A and B), suggesting that the kinase activity of AMPK is critical in the regulation of E-cadherin expression and, consequently, cell–cell adhesion. Indeed, activation of AMPK by AMP or by 2-deoxy-D-glucose up-regulated E-cadherin expression (SI Appendix, Fig. S3 C and D). Importantly, ectopic expression of E-cadherin effectively rescued AMPKα1 ablation-induced disruption of cell–cell adhesion (Fig. 3 D and E and SI Appendix, Fig. S3 E and F) and cell invasion (Fig. 3F). Together, these results indicate that either reduction of AMPKα1 protein expression or inactivation of AMPK kinase function leads to suppression of E-cadherin expression, resulting in disruption of cell–cell adhesion and promoting cell invasion.
Fig. 3.
Silencing of AMPKα1 leads to disruption of cell–cell adhesion via modulation of Twist1-E-cadherin axis. (A–C) MCF10A or HCC1806 cells stably expressing shAMPKα1-1 (shAα1-1), shAMPKα1-2 (shAα1-2) or shGFP were subjected to Western blotting (A), staining with 0.1% crystal violet, or to immunofluorescent staining for E-cadherin (B). Representative images were shown. Cell–cell adhesion ability was presented as described in the Materials and Methods (C). (Scale bars, 50 μm.) (D–F) MCF10A-shAα1-1 or HCC1806-shAα1-1 cells stably expressing E-cadherin were subjected to Western blotting (D), cell–cell adhesion ability analyses (E), or transwell assays for cell invasion (F). (G and H) MCF10A or HCC1806 cells stably expressing shAα1-1, shAα1-2, or shGFP were subjected to qPCR analyses for E-cadherin mRNA levels (G) or to Western blotting (H). (I–K) MCF10A-shAα 1 cells stably expressing shTwist1-1 or shTwist1-2 were subjected to Western blotting (I). Cell–cell adhesion ability (J) and cell invasion (K) were analyzed in parallel. (L) MCF10A-shAα1 cells were treated with rapamycin (Rap) for 24 h. Cells were subjected to Western blot analyses. Data are presented as means ± SEM. ***P < 0.001, **P < 0.01.
To further explore the mechanism by which AMPK regulates E-cadherin expression, we performed qPCR analyses. As shown in Fig. 3G and SI Appendix, Fig. S3G, silencing of AMPKα1 significantly reduced E-cadherin mRNA levels, whereas it imposed little effect on E-cadherin protein stability, suggesting that AMPK likely affects E-cadherin gene transcription. Consistent with this notion, our data showed that silencing of AMPKα1 up-regulated expression of Twist1, a well-known transcriptional suppressor of E-cadherin (28) (Fig. 3H). Notably, simultaneous knockdown of Twist1 markedly rescued AMPKα1 ablation-induced down-regulation of E-cadherin, decreased cell–cell adhesion ability, and increased cell invasion (Fig. 3 I–K and SI Appendix, Fig. S3H). Ablation of AMPKα1 also did not significantly alter steady-state levels of Twist1 mRNA (SI Appendix, Fig. S3I). Since inhibition of AMPK up-regulates mTOR activity, it is possible that ablation of AMPKα1 up-regulates Twist1 expression via activated mTOR. Indeed, our results showed that silencing of AMPKα1 significantly increased pS6K and pS6 protein expression (SI Appendix, Fig. S3J). Importantly, inhibition of mTOR activity by rapamycin significantly suppressed AMPKα1 ablation-induced up-regulation of Twist1 (Fig. 3L). Together, these results indicate that silencing of AMPKα1 inhibits E-cadherin transcription via activation of mTOR-Twist1 axis.
Our abovementioned data indicate that ΔNp63α is a directly transcriptional factor of AMPKα1. It has been reported that loss of p63 leads to decreased cell–cell adhesion and enhanced cell migration and cancer metastasis (17, 19, 29, 30). We examined whether AMPKα1 plays a role in p63-mediated regulation of cell invasion. As shown in SI Appendix, Fig. S3K, silencing of p63 in MCF10A cells significantly led to up-regulation of ZEB1, vimentin, and reduction of E-cadherin and integrin β4 (ITGB4), consistent with previous observations (30, 31). Importantly, ectopic expression of Aα1-WT or Aα1-CA significantly rescued E-cadherin and vimentin expression, but not ZEB1 and ITGB4 (SI Appendix, Fig. S3L). Moreover, silencing of p63-induced cell invasion was markedly rescued by activation of AMPK (SI Appendix, Fig. S3M). These results indicate that AMPK plays a role in silencing of p63-induced cell invasion.
Restoration of AMPKα1 Rescues PIK3CAH1047R/HER2V659E-Induced Disruption of Cell–Cell Adhesion, Increased Cell Invasion, and Tumor Metastasis.
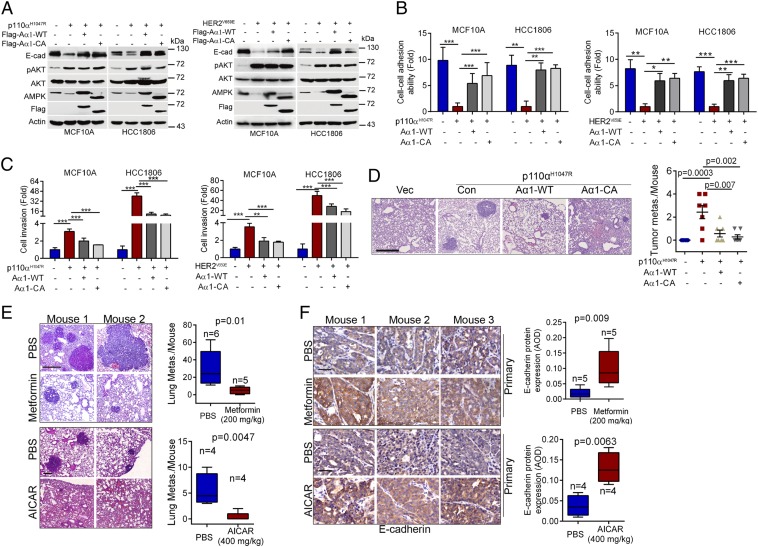
Next, we examined the effects of activated PI3K and HER2 on ΔNp63α-AMPK-E-cadherin pathways. As shown in SI Appendix, Fig. S4 A and B, expression of p110αH1047R or HER2V659E significantly inhibited protein expression of ΔNp63α, AMPK, and E-cadherin, which was markedly rescued by pharmacological inhibition of PI3K, HER2, or AKT. Since activation of PI3K or HER2 has been shown to drive cancer metastasis (19, 21, 22), we therefore investigated whether p110αH1047R- or HER2V659E-mediated suppression of AMPKα1 expression plays a causative role in oncogene-driven breast cancer metastasis. As shown in Fig. 4 A–C, expression of p110αH1047R or HER2V659E in MCF10A or HCC1806 cells promoted cell invasion and enhanced metastatic potential, consistent with our previous results (19). AMPKα1 protein levels, again, were dramatically reduced in these cells, concomitant with reduced expression of E-cadherin, disruption of cell–cell adhesion, and increased cell invasion, which were effectively rescued by ectopic expression of Aα1-WT or Aα1-CA (Fig. 4 A–C and SI Appendix, Fig. S4 C and D). Importantly, expression of Aα1-WT or Aα1-CA significantly suppressed p110αH1047R-induced tumor metastasis in vivo (Fig. 4D).
Fig. 4.
Activation of AMPK inhibits oncogenic PI3K/HER2-induced cell invasion and tumor metastasis. (A–D) MCF10A-p110αH1047R, HCC1806-p110αH1047R, MCF10A-HER2V659E, or HCC1806-HER2V659E cells stably expressing Aα1-WT or Aα1-CA were subjected to Western blotting (A), cell–cell adhesion ability analyses (B) or cell invasion analyses (C). For tumor metastasis assays, indicated stable cells (3 × 106) were i.v. injected into nude mice (n = 7/group) (D). On day 55 after injection, lungs were dissected, fixed, and stained with HE. The numbers of metastatic nodules in the lungs per mouse were shown. (Scale bar, 0.5 mm.) (E and F) Activation of AMPK up-regulates E-cadherin expression and suppresses tumor metastasis in MMTV-PyMT mice. MMTV-PyMT female FVB mice or MMTV-PyMT female C57BL/6 mice were used as described in Materials and Methods. (E) Lung sections were stained with HE. Representative pictures and the numbers of metastasis nodules in the lung per mouse were shown. (Scale bars, 0.5 mm.) (F) Primary tumors were stained for E-cadherin expression, and average optical density (AOD) was calculated. (Scale bars, 50 μm.) Data are presented as means ± SEM. ***P < 0.001; **P < 0.01; *P < 0.05.
Activation of AMPK Inhibits Tumor Metastasis in MMTV-PyMT-Induced Mammary Tumor Model.
To further investigate the role of AMPK in tumor metastasis in vivo, we used a well-established MMTV-PyMT mammary tumor mouse model. As shown in SI Appendix, Fig. S4 E and F, both AMPKα1 and E-cadherin protein levels were significantly reduced in the lung metastasized tumors compared with primary mammary tumors. Administration of either metformin or AICAR, two well-known AMPK activators, significantly inhibited lung metastasis in the MMTV-PyMT mice, concomitant with increased E-cadherin expression in primary mammary tumors (Fig. 4 E and F). Together, these results demonstrate that AMPK-E-cadherin axis plays a pivotal role in regulation of cell adhesion and tumor metastasis.
AMPKα1 Expression Is Linked to Oncogenic Signaling, Expression of p63 and E-Cadherin, as Well as Clinical Outcome in Human Breast Cancer.
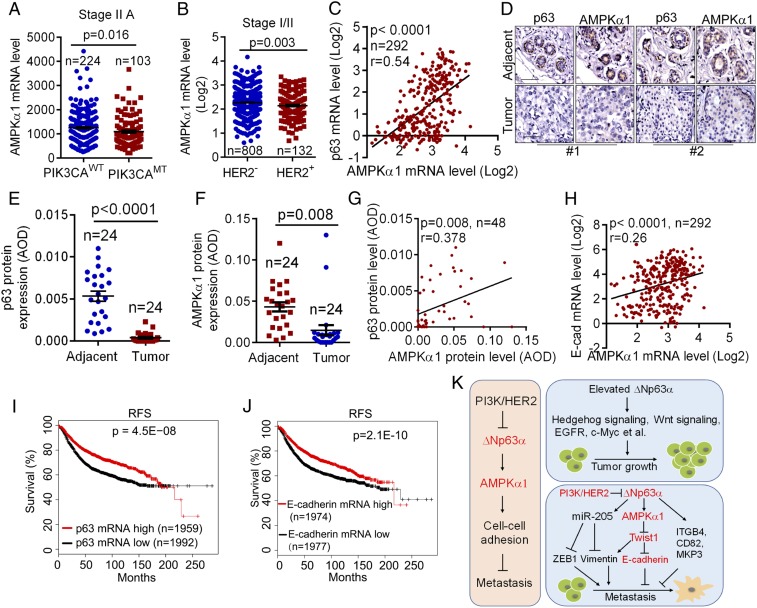
Our data indicate that cancer-associated p110αH1047R or HER2V659E inhibits AMPKα1 transcription. To investigate the clinical relevance, we examined the correlation between cancer-associated PIK3CA gene mutations and AMPKα1 mRNA expression. As shown in Fig. 5A, occurrence of PIK3CA mutations correlated with lower AMPKα1 mRNA levels compared with wild-type PIK3CA in stage II A breast cancers. In addition, HER2+ breast cancers expressed less AMPKα1 mRNA levels than that of HER2− breast cancers (Fig. 5B).
Fig. 5.
Expression of AMPKα1, p63, and E-cadherin is correlated in human breast cancers and is associated with patient outcome. (A) The TCGA database was analyzed for AMPKα1 mRNA levels in human breast cancers bearing wild-type PIK3CA alleles (PIK3CAWT) or PIK3CA mutant alleles (PIK3CAMT). (B) The Oncomine Curtis breast dataset was analyzed for AMPKα1 mRNA levels in human HER2− or HER2+ breast cancers. (C) The Oncomine Curtis breast dataset was analyzed for the correlation of gene expression between AMPKα1 and p63. (D–G) Tissue microarray slides containing consecutive sections derived from human breast carcinoma and adjacent normal tissues were subjected to IHC staining (D) and to quantitative analyses (AOD) for protein expression of p63 (E) and AMPKα1 (F). The correlation between AMPKα1 and p63 protein levels was analyzed (G). (H) The Oncomine Curtis breast dataset was analyzed for the correlation of AMPKα1 and E-cadherin expression. (I and J) The correlation between p63 or E-cadherin mRNA levels and RFS in patients with breast cancer was analyzed using Kaplan-Meier Plotter database. (K) A model depicts the oncogenic PI3K/HER2-mediated transcriptional regulation of AMPKα1 and the role of AMPK in cell–cell adhesion and cancer metastasis. Notably, elevated ΔNp63α promotes tumor growth, whereas suppression of ΔNp63α promotes tumor metastasis.
Furthermore, the expression of both AMPKα1 and p63 mRNA was significantly reduced in breast carcinomas compared with in normal breast samples (SI Appendix, Fig. S5). Notably, the mRNA expression of AMPKα1 and p63 was well correlated (r = 0.54; P < 0.0001; Fig. 5C). IHC analyses showed that both AMPKα1 and p63 protein levels were significantly reduced in breast cancer samples when compared with the adjacent tissues (Fig. 5 D–F). Again, expression of AMPKα1 and p63 proteins exhibited a clear correlation (r = 0.378; P = 0.008; Fig. 5G). Moreover, E-cadherin mRNA expression was significantly reduced in breast cancer samples (SI Appendix, Fig. S5), which was also correlated with AMPKα1 expression (r = 0.26; P < 0.0001; Fig. 5H). Regarding clinical outcomes, Kaplan-Meier analyses showed that patients with breast cancer with a lower mRNA level of p63 or E-cadherin correlated with lower RFS (Fig. 5 I and J), similar to that of AMPKα1 mRNA expression (Fig. 1F).
Together, our study demonstrate that oncogenic PI3K/HER2-mediated down-regulation of AMPKα1 transcription is pivotal in regulation of cell–cell adhesion and cancer metastasis (Fig. 5K).
Discussion
AMPK functions as an energy sensor and is pivotal in maintaining cellular metabolic homeostasis (1). Numerous studies demonstrate that AMPK activities are primarily regulated via T172 phosphorylation by the upstream kinase LKB1 or CaMKK2 (5, 14, 32). AMPKα protein stability can be modulated by ubiquitin ligase UBE2O or MAGE-A3/6-TRIM28 (7, 33). In this study, we demonstrate that transcription of AMPKα1 is suppressed in response to activation of PI3K/HER2, leading to disruption of cell–cell adhesion and promoting cancer metastasis.
This study links the function of AMPK in energy sensing to cancer metastasis. Tumor development needs additional energy, nutrients, and oxygen for cell proliferation and growth (34–36). Indeed, metastatic tumor cells prefer to migrate to lung, liver, or brain, which equip with rich nutrients (37–39). It is well known that tumor microenvironment is usually deprived of glucose (40, 41), which activates AMPK, leading to inhibition of cell proliferation and blockage of tumor growth (42). Thus, it is reasonable that reduced AMPKα1 expression via transcriptional suppression lifts the barrier of tumor growth and, in contrast, leads to disruption of cell–cell adhesion, which consequently promotes metastasis. However, it has been also reported that AMPK can act as a survival factor in response to glucose deprivation (43, 44). Therefore, AMPK can exhibit pleiotropic effects impacting cell growth, survival, and cell mobility.
What is the biological significance that AMPK regulates cell adhesion under normal cellular physiology? Our results indicate that AMP treatment of untransformed MCF10A cells activates AMPK resulting in up-regulation of E-cadherin, raising an interesting possibility that AMPK may link adherent junction to energy homeostasis. Consistent with this notion, it is well known that reduced cellular ATP activates AMPK in promoting glucose metabolism to meet the need for generating ATP under normal cellular physiology (1). In this regard, it is interesting to note that E-cadherin upon mechanical force activates AMPK to facilitate glucose uptake and ATP production (45, 46).
A hallmark of cancer cells is deregulated cellular energetics, as exemplified by the Warburg effect (47), in which AMPK is a key player. Indeed, genetic ablation of AMPKα1 promotes aerobic glycolysis via stabilizing HIF1-α and accelerates Myc-induced lymphomagenesis (8). Similarly, activation of AMPK suppresses mTORC1 activity, leading to inhibition of aerobic glycolysis (48). In addition, activation of AKT, the major downstream target of oncogenic PI3K/HER2, can inactivate AMPK via S485 phosphorylation of AMPKα1 (15). Importantly, oncogenic PI3K/HER2 are known to promote aerobic glycolysis (49–52). In this study, we demonstrate that oncogenic PI3K/HER2 suppresses AMPKα1 mRNA expression. Thus, oncogenic PI3K/HER2 has two modes of AMPK inhibition, resulting in disruption of energy homeostasis.
Accumulating evidence indicate that ΔNp63 is an important tumor metastasis suppressor. Loss of p63 down-regulates miR-205, which in turn promotes expression of ZEB1 and vimentin, two important EMT (epithelial–mesenchymal transition) markers (30). Furthermore, activation of TGFβ signaling or expression of mutant p53 inhibits TAp63 transcriptional activity to promote cell invasion and cancer metastasis via down-regulation of Sharp-1 expression or promoting integrin recycling, respectively (53, 54). Our previous results indicate that oncogenic PI3K/HER2/Ras can inhibit ΔNp63α transcription via AKT-FOXO3a signaling, resulting in increased cell mobility and tumor metastasis (19). Moreover, we demonstrate that knockdown of p63 suppresses cell migration and cancer metastasis via inhibition of CD82, MKP3, or integrin β4 expression (31, 55, 56). In keeping with previous reports, we show that ΔNp63α regulates several important proteins involved in EMT, including ZEB1, vimentin, and E-cadherin. Interestingly, AMPK can markedly rescue effects of silencing of p63 on expression of E-cadherin and vimentin, but not on ZEB1, suggesting that AMPKα1 plays a role in p63-mediated regulation of E-cadherin and vimentin. It has been reported that Twist1 is a major transcriptional suppressor of E-cadherin, whereas Twist1 can promote vimentin expression (28, 57). Importantly, in this study, we show that silencing of AMPKα1 leads to significant increase of Twist1 expression. Together, these results suggest that AMPKα1-Twist1 axis is another layer with which ΔNp63α regulates EMT. Notably, ΔNp63 has been documented as an oncoprotein important for tumor initiation and development. ΔNp63 can sustain self-renewal of mammary cancer stem cells via Sonic Hedgehog signaling (58). ΔNp63 can also promote breast cancer cell stemness via enhancing Fzd7 expression and Wnt signaling (59). Furthermore, it has been reported that ΔNp63α promotes tumor cell growth via increasing EGFR and c-Myc expression (60–62). Our previous results also show that ΔNp63α plays an important role in squamous cell carcinoma cell growth and survival (63, 64). Therefore, ΔNp63α acts as an oncogene to promote tumor growth while it functions as a metastasis suppressor (Fig. 5K).
The down-regulation of AMPKα1 in advanced human cancers has clear clinical implications. Both AMPKα1 mRNA and protein levels are significantly reduced, which is tightly associated not only with the metastatic potential of tumors but also with recurrence-free survival. Interestingly, in keeping with HER2-mediated suppression of AMPKα1 expression, patients with HER2+ breast cancer have shorter recurrence-free survival than patients with luminal A/B breast cancer, which is likely due to low expression of AMPKα1 in HER2+ breast cancer samples. Furthermore, we show that the PI3K/HER2-ΔNp63α-AMPKα1-E-cadherin axis is closely correlated in metastasized cancers. Indeed, PI3K/HER2 is frequently activated in human breast cancers, which, as shown in this study, is tightly associated with low expression of ΔNp63α, AMPKα1, and E-cadherin. Together, these findings suggest that activation of AMPK or restoration of AMPKα1 expression may be a potential strategy for prevention of cancer metastasis.
Materials and Methods
Details are provided in SI Appendix, Materials and Methods for cell culture, transfection, infection, Western blotting, immunofluorescence, immunohistochemistry, chromatin immunoprecipitation, qPCR, luciferase reporter assays, cell–cell adhesion assay, cell invasion, and in vivo metastasis assay.
GraphPad Prism 6.0 (GraphPad Software Inc.) was used for data recording, collection, processing, and calculation. All cell-based experiments were performed at least three times in triplicates. Data were presented as means ± SEM. Quantitative data were analyzed statistically using Student’s t test to assess significance.
All data and associated protocols are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Yujun Zhang, Dr. Zhonghan Li, Dr. Yuanping Han, and Dr. Yan Liu for helpful discussions, and Mr. Kang Han for help on instruments. This work was supported by the National Natural Science Foundation of China (81520108020, 81802951, 81861148031, 81830108, and 31701242 to Z.-X.J.X., Y.Y. or M.N.); National Key R&D Program of China (2018YFC2000100 to Z.-X.J.X.); and China Postdoctoral Science Foundation (2018M631081 to Y.Y.) and Postdoctoral Fellowship of Sichuan University (2018SCU12054 to Y.Y.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914786117/-/DCSupplemental.
References
- 1.Hardie D. G., Ross F. A., Hawley S. A., AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck J. R., et al. , Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic beta and gamma subunits. J. Biol. Chem. 271, 17798–17803 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Hawley S. A., et al. , Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 271, 27879–27887 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Hawley S. A., et al. , Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawley S. A., et al. , Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Zhang C. S., et al. , Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548, 112–116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pineda C. T., et al. , Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 160, 715–728 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faubert B., et al. , AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 17, 113–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vila I. K., et al. , A UBE2O-AMPKα2 axis that promotes tumor initiation and progression offers opportunities for therapy. Cancer Cell 31, 208–224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji H., et al. , LKB1 modulates lung cancer differentiation and metastasis. Nature 448, 807–810 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Shen Z., Wen X. F., Lan F., Shen Z. Z., Shao Z. M., The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin. Cancer Res. 8, 2085–2090 (2002). [PubMed] [Google Scholar]
- 12.Dong S. M., et al. , Frequent somatic mutations in serine/threonine kinase 11/Peutz-Jeghers syndrome gene in left-sided colon cancer. Cancer Res. 58, 3787–3790 (1998). [PubMed] [Google Scholar]
- 13.Gao Y., et al. , LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc. Natl. Acad. Sci. U.S.A. 107, 18892–18897 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shackelford D. B., Shaw R. J., The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 9, 563–575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valentine R. J., Coughlan K. A., Ruderman N. B., Saha A. K., Insulin inhibits AMPK activity and phosphorylates AMPK Ser(4)(8)(5)/(4)(9)(1) through Akt in hepatocytes, myotubes and incubated rat skeletal muscle. Arch. Biochem. Biophys. 562, 62–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergholz J., Xiao Z. X., Role of p63 in development, tumorigenesis and cancer progression. Cancer Microenviron. 5, 311–322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll D. K., et al. , p63 regulates an adhesion programme and cell survival in epithelial cells. Nat. Cell Biol. 8, 551–561 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Testoni B., et al. , Identification of new p63 targets in human keratinocytes. Cell Cycle 5, 2805–2811 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Hu L., et al. , ΔNp63α is a common inhibitory target in oncogenic PI3K/Ras/Her2-induced cell motility and tumor metastasis. Proc. Natl. Acad. Sci. U.S.A. 114, E3964–E3973 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motoshima H., Goldstein B. J., Igata M., Araki E., AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 574, 63–71 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lien E. C., Dibble C. C., Toker A., PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 45, 62–71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day K. C., et al. , HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 77, 74–85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang A., et al. , Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol. Cell 24, 593–602 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Quentin T., et al. , Different expression of the catalytic alpha subunits of the AMP activated protein kinase–An immunohistochemical study in human tissue. Histol. Histopathol. 26, 589–596 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Muller P. A., et al. , Mutant p53 enhances MET trafficking and signalling to drive cell scattering and invasion. Oncogene 32, 1252–1265 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes R. O., Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 (1992). [DOI] [PubMed] [Google Scholar]
- 27.van der Neut R., Krimpenfort P., Calafat J., Niessen C. M., Sonnenberg A., Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat. Genet. 13, 366–369 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Yang J., et al. , Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Yoh K. E., et al. , Repression of p63 and induction of EMT by mutant Ras in mammary epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 113, E6107–E6116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucci P., et al. , Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 109, 15312–15317 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., et al. , Hippo kinases regulate cell junctions to inhibit tumor metastasis in response to oxidative stress. Redox Biol. 26, 101233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen T. E., et al. , Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am. J. Physiol. Endocrinol. Metab. 292, E1308–E1317 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Hardie D. G., An oncogenic role for the ubiquitin ligase UBE2O by targeting AMPK-α2 for degradation. Cancer Cell 31, 163–165 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Vander Heiden M. G., Exploiting tumor metabolism: Challenges for clinical translation. J. Clin. Invest. 123, 3648–3651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaupel P., Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 14, 198–206 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Leo C., Giaccia A. J., Denko N. C., The hypoxic tumor microenvironment and gene expression. Semin. Radiat. Oncol. 14, 207–214 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Kennecke H., et al. , Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Massagué J., Obenauf A. C., Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura T., et al. , Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 3, 217–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaupel P., Kallinowski F., Okunieff P., Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 49, 6449–6465 (1989). [PubMed] [Google Scholar]
- 41.Bergers G., Benjamin L. E., Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3, 401–410 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Mihaylova M. M., Shaw R. J., The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoki K., Zhu T., Guan K. L., TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Jeon S. M., Chandel N. S., Hay N., AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bays J. L., Campbell H. K., Heidema C., Sebbagh M., DeMali K. A., Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat. Cell Biol. 19, 724–731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebbagh M., Santoni M. J., Hall B., Borg J. P., Schwartz M. A., Regulation of LKB1/STRAD localization and function by E-cadherin. Curr. Biol. 19, 37–42 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Kishton R. J., et al. , AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 23, 649–662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elstrom R. L., et al. , Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Makinoshima H., et al. , Signaling through the phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) Axis is responsible for aerobic glycolysis mediated by glucose transporter in epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma. J. Biol. Chem. 290, 17495–17504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng J., Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 4, 1151–1157 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y. H., et al. , Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene 28, 3689–3701 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Adorno M., et al. , A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 137, 87–98 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Muller P. A., et al. , Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327–1341 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Wu J., et al. , ΔNp63α activates CD82 metastasis suppressor to inhibit cancer cell invasion. Cell Death Dis. 5, e1280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergholz J., et al. , ΔNp63α regulates Erk signaling via MKP3 to inhibit cancer metastasis. Oncogene 33, 212–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng J., et al. , Twist1 regulates vimentin through Cul2 circular RNA to promote EMT in hepatocellular carcinoma. Cancer Res. 78, 4150–4162 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Memmi E. M., et al. , p63 Sustains self-renewal of mammary cancer stem cells through regulation of Sonic Hedgehog signaling. Proc. Natl. Acad. Sci. U.S.A. 112, 3499–3504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakrabarti R., et al. , DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat. Cell Biol. 16, 1004–1015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danilov A. V., et al. , DeltaNp63alpha-mediated induction of epidermal growth factor receptor promotes pancreatic cancer cell growth and chemoresistance. PLoS One 6, e26815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee K. B., et al. , p63-Mediated activation of the β-catenin/c-Myc signaling pathway stimulates esophageal squamous carcinoma cell invasion and metastasis. Cancer Lett. 353, 124–132 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Han A., et al. , p63α modulates c-Myc activity via direct interaction and regulation of MM1 protein stability. Oncotarget 7, 44277–44287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C., et al. , Pin1 modulates p63α protein stability in regulation of cell survival, proliferation and tumor formation. Cell Death Dis. 4, e943 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yi Y., et al. , Metformin promotes AMP-activated protein kinase-independent suppression of ΔNp63α protein expression and inhibits cancer cell viability. J. Biol. Chem. 292, 5253–5261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.