Significance
The ability of embryonic stem cells to proliferate and differentiate is influenced by a number of factors. Among them, the metabolism of methionine, an essential amino acid, is considered highly important. However, the detailed regulatory mechanism of methionine metabolism and how the metabolism influences the stem cell fate still remain poorly understood. In this study, we identified AHCY, an important enzyme involved in the methionine metabolism, as a key player in the maintenance of stem cell identity. AHCY is dynamically modified by a single sugar N-acetylglucosamine, which responds to environmental cues to regulate its activity, and further influences stem cell proliferation and differentiation. Our finding expands our current understanding of the role of glycosylation in controlling cell fate decisions.
Keywords: O-GlcNAcylation, stem cell, metabolism
Abstract
Methionine metabolism is critical for the maintenance of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) pluripotency. However, little is known about the regulation of the methionine cycle to sustain ESC pluripotency. Here, we show that adenosylhomocysteinase (AHCY), an important enzyme in the methionine cycle, is critical for the maintenance and differentiation of mouse embryonic stem cells (mESCs). We show that mESCs exhibit high levels of methionine metabolism, whereas decreasing methionine metabolism via depletion of AHCY promotes mESCs to differentiate into the three germ layers. AHCY is posttranslationally modified with an O-linked β-N-acetylglucosamine sugar (O-GlcNAcylation), which is rapidly removed upon differentiation. O-GlcNAcylation of threonine 136 on AHCY increases its activity and is important for the maintenance of trimethylation of histone H3 lysine 4 (H3K4me3) to sustain mESC pluripotency. Blocking glycosylation of AHCY decreases the ratio of S-adenosylmethionine versus S-adenosylhomocysteine (SAM/SAH), reduces the level of H3K4me3, and poises mESC for differentiation. In addition, blocking glycosylation of AHCY reduces somatic cell reprogramming. Thus, our findings reveal a critical role of AHCY and a mechanistic understanding of O-glycosylation in regulating ESC pluripotency and differentiation.
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are in a signature metabolic state that is distinguished from their differentiated counterparts (1, 2). For example, aerobic glycolysis and glutamine oxidation are enhanced in stem cells to provide essential metabolic intermediates to fuel the biosynthesis of macromolecules to support rapid cell growth (3, 4). Besides serving as biosynthetic precursors, certain metabolic intermediates contribute to the maintenance of self-renewal and pluripotency of stem cells (5). The methionine cycle, a major component of the one-carbon metabolism, generates S-adenosylmethionine (SAM), a universal methyl donor for methylation of DNA, RNA, and proteins (6). The intracellular level of SAM influences methyltransferase activities to regulate different histone methylation marks to govern the pluripotency and differentiation of stem cells (4).
Mouse ESCs maintain most of their SAM pools from the catabolism of threonine. Threonine deprivation or knockdown of threonine dehydrogenase in mESCs decreased SAM levels and histone H3 lysine 4 methylation (H3K4me3), leading to slow growth and rapid differentiation (7). Similarly, human ESCs maintain the intracellular SAM levels through exogenous methionine uptake and the methionine cycle. Deprivation of methionine also decreased SAM levels and H3K4me3 and potentiates ESC differentiation (8). Thus, mouse and human ESCs appear to utilize the methionine metabolism to maintain their pluripotency in a similar manner. However, these studies have relied on the removal of essential amino acids from the culture, and the observed phenotype could be attributable to some indirect effects. Despite these elegant studies, direct regulatory mechanisms of the SAM metabolism and the specific effect on gene expressions remain uncharacterized.
Adenosylhomocysteinase (AHCY) catalyzes the hydrolysis of S-adenosylhomocysteine (SAH, or AdoHcy) to generate adenine and homocysteine and constitutes a key step in the methionine cycle (9). It is noted that SAM/SAH ratio is critical for regulating cellular transmethylation because methyltransferases are product-inhibited by SAH (10). Dysfunction of AHCY is closely linked to a number of diseases, such as neurological disorders, myopathy, and cancer (11–13). The chromosomal deletion of AHCY gene leads to embryonic mortality in mice (14), suggesting that AHCY plays an essential role in embryo development and growth control. Despite its important roles in normal development and disease pathology, the cellular regulation of AHCY activity is poorly understood. Notably, the role of AHCY in maintaining SAM levels in cells and how AHCY contributes to the pluripotency of ESCs remain largely uncharacterized.
O-GlcNAc is a simple monosaccharide modification occurring on serine or threonine residues of nucleocytoplasmic proteins in all multicellular eukaryotes (15, 16). Accumulating evidence has shown that O-GlcNAcylation is a nutrient-responsive modification that links metabolic sensing to various biological processes, including cellular signaling, transcriptional regulation, and immune maturation (17–19). More recently, studies have shown that O-GlcNAcylation plays a pivotal role in stem cell biology. For example, a complete loss of OGT (the enzyme responsible for the addition of O-GlcNAc onto proteins) is embryonic lethal in mice (20). O-GlcNAcylation regulates the balance between ESC self-renewal and differentiation via controlling the transcriptional activity and stability of a number of key transcription factors, including Oct4, Sox2, FOXO1, Sp1, and ESRRB (21–25). Hyper-O-GlcNAcylation was shown to restrict the differentiation of human ESCs toward specific lineages and caused a premature neuronal differentiation (26, 27). However, inhibition of O-GlcNAcylation by OGT inhibitor accelerated neuronal differentiation of hESCs (28). However, few studies to date have focused on how O-GlcNAcylation regulates cellular metabolism to impact ESC self-renewal and pluripotency. Specifically, there is no study to investigate the regulation of key metabolic enzymes by O-GlcNAcylation in ESCs.
In the present study, we investigated the role of AHCY and its O-GlcNAcylation in regulating mESC self-renewal and pluripotency. Depletion of AHCY decreased the ratio of SAM/SAH and the level of H3K4me3, which impaired mESC self-renewal and accelerated differentiation. O-GlcNAcylation on T136 of AHCY was shown to increase the enzyme activity via enhancing AHCY oligomerization and its affinity with the reaction substrate. Conversely, blocking O-GlcNAcylation on AHCY decreased the ratio of SAM/SAH, reduced the level of H3K4me3, and poised mESC for differentiation. Finally, blocking glycosylation of AHCY also reduced somatic cell reprogramming. Thus, our findings reveal an unknown mechanism by which O-glycosylation links nutrient sensing with the epigenetic landscape to determine the cell fate of mESCs.
Results
AHCY Is Important for mESC Pluripotency and Self-Renewal.
A high flux of methionine metabolism is crucial for ESC self-renewal and pluripotency, as short-term methionine deprivation was reported to trigger ESC differentiation into the three germ layers (8, 29). We first analyzed mRNA expression levels of the enzymes involved in the methionine cycle upon induction of differentiation (Fig. 1A). mESCs were induced to differentiate by removing leukemia inhibitory factor (LIF) from the culture medium or by adding retinoic acid (RA). The mRNA levels of AHCY, MAT2A, and MAT2B were significantly reduced during RA-induced differentiation (SI Appendix, Fig. S1A). In the LIF-free condition, mRNA levels of AHCY and MAT2A were also decreased significantly (SI Appendix, Fig. S1B). Consistently, in mouse embryonic fibroblasts (MEFs), mRNA expressions of AHCY, MAT2A, DNMT, and MTR were significantly reduced compared to that in mESCs (SI Appendix, Fig. S1C). In line with this, the concentrations of key metabolites in the methionine cycle showed a marked reduction at day 6 of cell differentiation induced by RA (SI Appendix, Fig. S1D). Thus, these results consistently demonstrate that ESC differentiation is associated with a reduced flux of methionine metabolism.
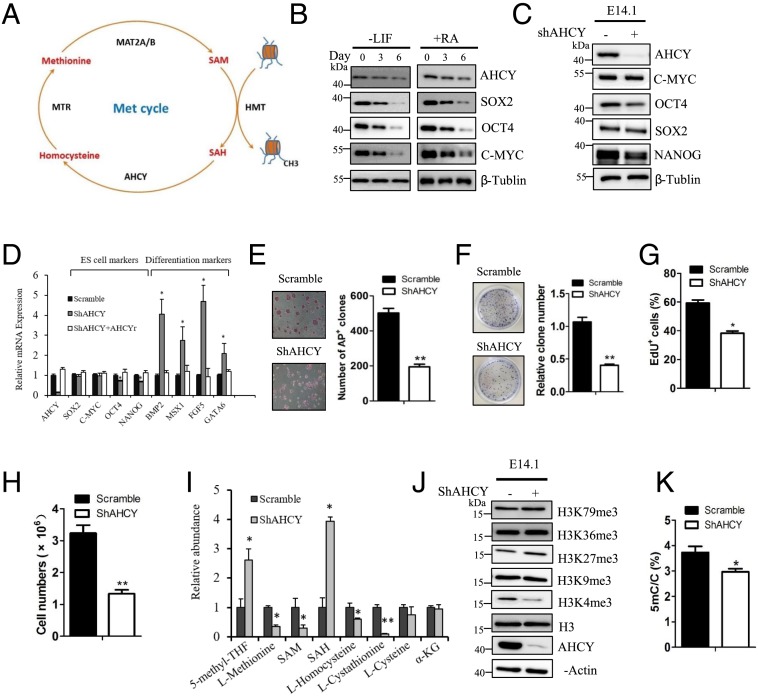
Fig. 1.
AHCY is important for mESC pluripotency and self-renewal. (A) Scheme of the methionine cycle. (B) Immunoblotting of AHCY and pluripotency markers Sox2, Oct4, and C-myc upon induction of mESC differentiation. (C) Immunoblotting of AHCY and pluripotency markers in E14.1 cells upon knocking down AHCY using shRNA. (D) Relative mRNA expression of stem cell markers and differentiation markers in E14.1 cells upon knocking down AHCY and reintroduction of shRNA-resistant AHCY (n = 3 assays). (E) The number of colonies staining positive for AP produced from E14.1 cells infected with scramble shRNA or AHCY-targeting shRNA (n = 3 assays). (F) Relative clone numbers produced from E14.1 cells infected with scramble shRNA or AHCY-targeting shRNA (n = 4 assays). (G) Analysis of DNA synthesis in E14.1 cells infected with scramble shRNA or AHCY-targeting shRNA (n = 3 assays). (H) Cell proliferation of E14.1 cells infected with scramble shRNA or AHCY-targeting shRNA (n = 3 assays). (I) Relative abundance of metabolites in the methionine cycle in E14.1 cells infected with scramble shRNA or AHCY-targeting shRNA (n = 3 assays). (J) Immunoblotting of trimethylation of various histone H3 lysine residues in E14.1 cells infected with scramble shRNA or AHCY-targeting shRNA. (K) Relative DNA methylation levels in E14.1 cells infected with scramble shRNA or AHCY-targeting shRNA (n = 3 assays). Error bars denote the means ± SEM. Statistical analyses were performed by unpaired Student’s t test (*P < 0.05, **P < 0.01).
The protein level of AHCY was reduced upon cell differentiation, as accompanied by the reduction of pluripotency markers C-myc, Oct4, and Sox2 (Fig. 1B). To investigate the role of AHCY in regulating ESC pluripotency, we depleted AHCY expression in E14.1 cells using small hairpin RNA (shRNA) (Fig. 1C). Depletion of AHCY expression was accompanied by the reduction of pluripotency markers, especially Oct4 and Nanog, and with a concomitant increase in the expression of lineage-specific genes, including the ectoderm markers Fgf5 and Bmp2, the mesoderm marker Msx1, and the endoderm marker Gata6 (Fig. 1D). To eliminate the off-target effect of shRNA, we reintroduced shRNA-resistant AHCY into AHCY-depleted cells and observed a complete rescue of mRNA expressions of pluripotency genes and differentiation marker genes (Fig. 1D). Consistent with a recent transcriptome analysis (30), we observed that a number of ribosomal protein genes were transcriptionally down-regulated upon AHCY depletion, while the expressions of Oct4 and Nanog were not reduced during the first 3 d upon small hairpin AHCY (shAHCY) lentiviral infection (SI Appendix, Fig. S2). Cells expressing shAHCY exhibited reduced numbers of colonies staining positive for alkaline phosphatase (AP), less clone numbers, reduced DNA synthesis, and lower cell proliferation, as compared to nontargeting shRNA-expressing cells, suggesting that AHCY depletion caused an impairment in mESC pluripotency and self-renewal ability (Fig. 1 E–H). However, ectopic expression of AHCY in E14.1 cells during RA treatment partially rescued the reduced expressions of pluripotency markers Oct4 and Nanog, while inhibiting the increased expressions of differentiation markers of the three germ layers (SI Appendix, Fig. S3), suggesting an inhibition of differentiation. Thus, these results suggest that AHCY expression is critical for the maintenance of mESC pluripotency and self-renewal. Furthermore, we showed that AHCY plays a similar role in maintaining mouse neural stem cell pluripotency and self-renewal (SI Appendix, Fig. S4). Depletion of AHCY in mouse neural stem cells reduced cell proliferation and induced a marked increase in the expression of the neural-specific marker Tuj1.
Depletion of AHCY resulted in an increased number of cells in the G1 phase, and a concomitant reduction in the S phase (SI Appendix, Fig. S5A). A previous report demonstrated that methionine deprivation up-regulated the p53-dependent signaling pathway to induce human ESC apoptosis (8). Similarly, we observed a significant increase in the percentage of apoptotic cells upon AHCY depletion and a marked change in the levels of p53-dependent gene expression involved in apoptosis, such as MDM2, FAS, BAX, BCL2, and p21 (SI Appendix, Fig. S5 B and C). To confirm the effect of p53, we performed p53 knockdown and observed a partial rescue of gene expression levels and cell apoptosis caused by AHCY depletion (SI Appendix, Fig. S5 C and D). Thus, these results indicate that depletion of AHCY activates p53-dependent signaling pathway to induce apoptosis in mESCs.
It was reported that the intracellular SAM concentrations critically affect ESC pluripotency and self-renewal, and this effect was mediated by trimethylation of H3K4 (7, 8). To investigate the mechanism by which AHCY regulates mESC pluripotency and self-renewal, we analyzed the relative enrichment of metabolites in the methionine cycle upon AHCY depletion in E14.1 cells. Several key metabolites including methionine, SAM, homocysteine, and cystathionine were significantly decreased, whereas 5-methyl-THF and SAH were significantly increased, in AHCY-depleted cells compared to the control cells (Fig. 1I). The decreased SAM/SAH ratio (an index of methylation potential) resulted from AHCY knockdown might lead to impaired transmethylation reactions on histones and DNA. Indeed, the signal of H3K4me3 markedly reduced in AHCY-depleted E14.1 cells, whereas no apparent changes were observed on trimethylation of K9, K27, K36, and K79 (Fig. 1J). Furthermore, reexpression of Flag-tagged AHCY in AHCY-depleted cells rescued the level of H3K4me3 (SI Appendix, Fig. S6). In addition, a modest reduction in global DNA methylation was observed in AHCY-depleted cells (Fig. 1K). To further support the notion that the change of H3K4me3 level drives the expression of pluripotency markers, we performed chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) to analyze the relative enrichment of H3K4me3 in Pou5f1 (Oct4) and Nanog loci in scramble shRNA-infected cells and shAHCY-infected cells (SI Appendix, Fig. S7 A and B). As expected, we detected a reduced enrichment of H3K4me3 at the Pou5f1 and Nanog loci upon AHCY knockdown. Thus, AHCY depletion led to a specific decrease in H3K4me3 to impact the pluripotency of mESCs.
O-GlcNAcylation of AHCY on the Residue T136 Promotes Its Enzyme Activity.
Previous studies have shown that O-GlcNAcylation regulates the activities of several key glycolytic enzymes to impact glucose metabolism in cells (31–33). However, whether O-GlcNAcylation regulates amino acid metabolism has not been investigated. Using a well-established chemoenzymatic labeling method (34), we found that AHCY possesses O-GlcNAcylation, whose level could be markedly enhanced upon overexpression of OGT (Fig. 2 A and B). To quantify the glycosylation stoichiometry on AHCY in mESCs, we employed a previously reported mass tagging approach (35) and obtained a modification ratio of ∼40% on AHCY (Fig. 2C). To identify the sites of O-GlcNAcylation on AHCY, we transiently coexpressed Flag-tagged AHCY and OGT in NIH 3T3 cells. After immunoprecipitation in cell lysates with anti-Flag M2 beads and in-gel trypsin digestion, resulted samples were subjected to mass spectrometry analysis to identify possible glycosylated peptides. We identified four putative O-GlcNAcylation sites (T136, T141, T185, and S187) on AHCY (Fig. 2D and SI Appendix, Fig. S8). We then mutated each of the four residues to alanine and observed that mutation of T136, but not the other three residues, significantly reduced the O-GlcNAcylation level, suggesting that T136 is the major glycosylation site in AHCY (Fig. 2E). Notably, no other forms of modification such as phosphorylation were found at T136 based on the mass spectrum data search. Sequence comparison analysis showed that T136 is conserved in homologs AHCY proteins across different species, indicating a possible functional role for this residue (Fig. 2F).
Fig. 2.
O-GlcNAcylation of AHCY on the residue T136 promotes its enzyme activity. (A) Analysis of AHCY O-GlcNAcylation in cells using a chemoenzymatic labeling method. O-GlcNAcylated proteins in E14.1 cell lysates were first labeled with an azido-N-acetylgalactosamine (GalNAz) sugar. Labeled proteins were then conjugated with biotin via Cu(I)-mediated [3 + 2] azide-alkyne cycloaddition chemistry and further captured with streptavidin-agarose beads. After stringent washing, the eluate was immunoblotted using an antibody against AHCY. The corresponding biotin blot was shown. (B) Analysis of AHCY O-GlcNAcylation upon OGT overexpression. The corresponding biotin blot was shown. (C) Analysis of glycosylation stoichiometry of AHCY by conjugation with alkyne-PEG5k. (D) Tandem mass spectrum of an O-GlcNAcylated peptide on AHCY. (E) Probing the major site of glycosylation on AHCY using various site-directed mutants. (F) Comparison of amino acid sequences containing the glycosylation site among different species. (G) Enzymatic activity of AHCY WT and T136A mutant in the presence or absence of OGT overexpression (n = 4 assays). (H) Analysis of the oligomerization state of AHCY WT or T136A mutant in the presence or absence of TMG treatment using disuccinimidyl suberate (DSS) for protein cross-linking. Error bars denote the means ± SEM. Statistical analyses were performed by unpaired Student’s t test (*P < 0.05, **P < 0.01).
To study the effect of T136 O-GlcNAcylation, we examined the impact on AHCY enzyme activity. Flag-tagged AHCY proteins were immunoprecipitated from NIH 3T3 cells stably expressing Flag-tagged wild type (WT) or T136A AHCY in the presence or absence of OGT overexpression for AHCY activity assays. The result showed that OGT overexpression increased the WT AHCY enzyme activity by 1.5-fold but failed to increase that of the T136A mutant (Fig. 2G). Similar results were obtained when WT or T136A AHCY was ectopically expressed in E14.1 cells (SI Appendix, Fig. S9 A and B). Collectively, these results indicate that T136 O-GlcNAcylation positively regulates AHCY activity.
To further understand the effect of T136 O-GlcNAcylation on AHCY activity, we determined the steady-state kinetics of purified Flag-tagged AHCY proteins with or without T136 glycosylation (SI Appendix, Fig. S9C). WT AHCY had a higher Vmax value and a lower Km value (28.74 µM) for the substrate AdoHcy than T136A mutant (40.15 µM). OGT overexpression further enhanced the Vmax and decreased the Km for WT AHCY but had no effect on those for T136A mutant. Conversely, WT AHCY had a nearly fourfold higher catalytic efficiency (Kcat/Km = 0.057) for AdoHcy than T136A (Kcat/Km = 0.015). OGT overexpression further increased the catalytic efficiency (Kcat/Km = 0.108) by nearly twofold for WT AHCY but had no apparent effect on the Kcat/Km value with T136A mutant. AHCY proteins exist in different oligomeric states, including monomer, dimer, and tetramer, with tetramer possessing the highest activity (36). To test whether T136 glycosylation affects the oligomerization state of AHCY, we performed a protein cross-linking experiment using disuccinimidyl suberate (DSS). The result showed that enhancing O-GlcNAcylation levels by pharmacological inhibition of OGA with a specific inhibitor thiamet-G (TMG) resulted in an increased formation of the tetrameric WT AHCY protein but had no apparent effect on the T136A mutant (Fig. 2H). Collectively, these results suggest that T136 O-GlcNAcylation promotes AHCY activity through increasing AHCY tetramers and the affinity with the reaction substrate AdoHcy.
AHCY T136 O-GlcNAcylation Promotes mESC Pluripotency and Self-Renewal.
Studies have shown that cellular O-GlcNAcylation levels decreased during the course of ESC differentiation (28, 37). We confirmed that inducing mESC differentiation by LIF withdrawal or RA addition resulted in a gradual reduction of cellular O-GlcNAcylation (Fig. 3A). Examination of AHCY O-GlcNAcylation revealed that the glycosylation levels decreased rapidly by day 2, but the total AHCY protein level remained unchanged over the same period (Fig. 3B). Analysis of the enzymatic activity of immunoprecipitated AHCY proteins showed a 50% reduction in activity at day 2 compared to day 0, consistent with our previous observation that O-GlcNAcylation promotes AHCY activity (Fig. 3C).
Fig. 3.
AHCY T136 O-GlcNAcylation promotes mESC pluripotency and self-renewal. (A) Immunoblotting of cellular O-GlcNAcylation levels in E14.1 cells upon induction of differentiation. (B) Immunoblotting of AHCY and its glycosylation levels during the first 2 d of cell differentiation (n = 3 assays). (C) Enzymatic activity of AHCY at indicated time points of cell differentiation (n = 3 assays). (D) The number of colonies staining positive for AP produced from AHCY WT or T136A rescue E14.1 cells (n = 3 assays). (E) Relative clone numbers produced from AHCY WT or T136A rescue E14.1 cells (n = 3 assays). (F) Teratomas formation in nude mice (n = 5 per group) injected with AHCY WT or T136A rescue E14.1 cells. (G) Relative expression levels of the differentiation markers in teratoma derived from WT or T136A AHCY rescue E14.1 cells (n = 3 assays). (H) Relative abundance of metabolites in the methionine cycle in the WT or T136A AHCY rescue E14.1 cells (n = 3 assays). (I) Immunoblotting and quantification of trimethylation of histone H3 lysine residues in AHCY WT or T136A rescue E14.1 cells (n = 3 assays). (J) Expression profiles of selected genes involved in cell stemness and differentiation. Error bars denote the means ± SEM. Statistical analyses were performed by unpaired Student’s t test (*P < 0.05, **P < 0.01).
To further investigate the effect of AHCY T136 O-GlcNAcylation on ESC maintenance, we depleted endogenous AHCY and stably expressed shRNA-resistant Flag-tagged WT or T136A AHCY in E14.1 cells (henceforth referred to as WT AHCY or T136A AHCY rescue cells) (SI Appendix, Fig. S10A). The T136A AHCY rescue cells formed reduced numbers of colonies staining positive for AP, compared to the WT AHCY rescue cells, indicating that AHCY O-GlcNAcylation is important for the maintenance of mESC pluripotency (Fig. 3D). In addition, the WT AHCY rescue cells exhibited significantly higher cell proliferation rate and DNA synthesis compared to the T136A AHCY rescue cells (Fig. 3E and SI Appendix, Fig. S10 B and C). Moreover, the T136A AHCY rescue cells displayed a reduced ability to form teratomas in vivo (Fig. 3F). Teratomas expressing the WT AHCY contained relatively higher expressions of differentiated genes of the three germ layers as compared to teratomas expressing the T136A mutant (Fig. 3G), thus further supporting the role of AHCY O-GlcNAcylation to promote mESC self-renewal and pluripotency. Similar results were obtained in mouse neural stem cells (SI Appendix, Fig. S11). Expressing T136A AHCY in mouse neural stem cells reduced cell proliferation and increased the expression of the neural-specific marker Tuj1.
We next analyzed the relative enrichment of metabolites in the methionine cycle between WT and T136A AHCY rescue cells. The levels of methionine, SAM, homocysteine, and cystathionine were significantly decreased, while the level of SAH was significantly increased, in the T136A AHCY rescue cells compared to the WT AHCY rescue cells (Fig. 3H). Conversely, the signal of H3K4me3 was reduced in the T136A AHCY rescue cells, whereas no apparent changes were observed on trimethylation of K9, K27, K36, and K79 (Fig. 3I). Consistently, ChIP-qPCR experiments showed a relatively lower enrichment of H3K4me3 at Pou5f1 and Nanog loci in the T136A AHCY rescue cells (SI Appendix, Fig. S7 C and D). A modest reduction in global DNA methylation was also observed in the T136A AHCY rescue cells (SI Appendix, Fig. S12).
To further characterize whether the T136A mutation altered global transcript levels, we performed RNA-sequencing (RNA-seq) experiments to compare the gene expression profiles of the WT AHCY rescue cells and the T136A AHCY rescue cells. Significant alterations in gene expression levels were observed, with 1,351 genes up-regulated and 979 genes down-regulated in the T136A AHCY rescue cells (SI Appendix, Fig. S13A) (38). Expectedly, a number of genes associated with pluripotency were down-regulated, while genes associated with differentiation into the three germ layers were up-regulated in the T136A AHCY rescue cells compared to the WT rescue cells (Fig. 3J). Quantitative reverse transcription PCR (qRT-PCR) of selected genes confirmed the differential expression profile (SI Appendix, Fig. S13B). Also consistent with the previous study (30), a number of ribosomal protein genes were significantly down-regulated in the T136A AHCY rescue cells (SI Appendix, Fig. S14). Thus, these data suggest that depletion of AHCY glycosylation alters the balance between self-renewal and differentiation gene expressions in mESCs.
AHCY T136 O-GlcNAcylation Is Required for Efficient Somatic Cell Reprogramming.
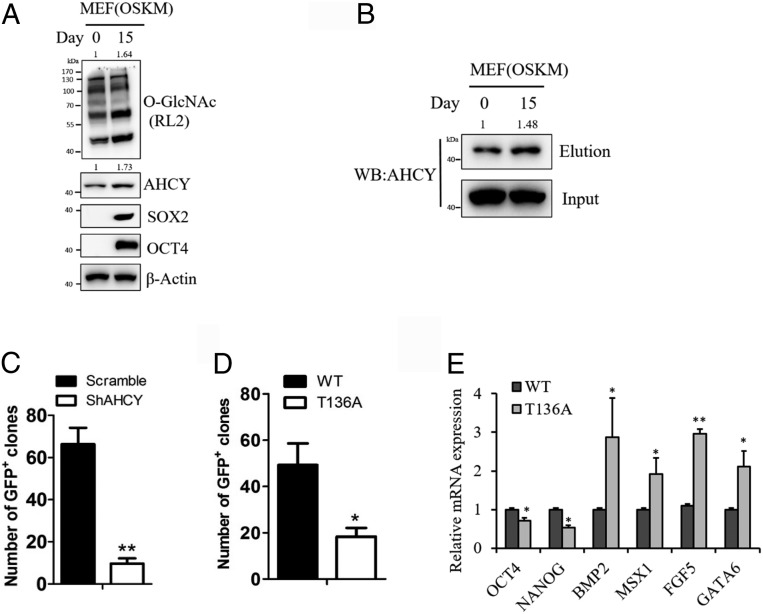
Metabolism has been demonstrated to impact the efficiency of somatic cell reprogramming (39, 40). Thus, we examined the role of AHCY and its T136 O-GlcNAcylation in the generation of iPSCs from MEFs transducing with Yamanaka factors (Oct4, Sox2, Klf4, and c-Myc). On day 15 of MEF reprogramming, we observed an increase in overall O-GlcNAcylation levels, AHCY protein, and AHCY glycosylation levels (Fig. 4 A and B). MEFs carrying an Oct4-GFP reporter were then used to determine the impact of AHCY and T136 O-GlcNAcylation on reprogramming efficiency. Depletion of AHCY markedly reduced the number of GFP+ iPSC colonies compared to the control cells (Fig. 4C). In addition, MEFs expressing T136A AHCY produced significantly less GFP+ iPSC colonies than MEFs expressing WT AHCY (Fig. 4D). We further analyzed the relative expressions of pluripotency and differentiation genes in these cells upon reprogramming (Fig. 4E). Expectedly, iPSCs derived from the T136A AHCY expressing MEFs displayed significantly lower expression levels of pluripotency genes, and higher levels of differentiation genes, as compared to iPSCs from the WT AHCY-expressing cells. Taken together, these results indicate that AHCY and its O-GlcNAcylation are required for efficient somatic cell reprogramming.
Fig. 4.
AHCY T136 O-GlcNAcylation is required for efficient somatic cell reprogramming. (A) Immunoblotting of cellular O-GlcNAcylation, AHCY, and pluripotency markers Sox2 and Oct4 in MEF upon transducing with Yamanaka factors (OSKM) for reprogramming. (B) Analysis of AHCY glycosylation in MEF upon transducing with Yamanaka factors (OSKM) for reprogramming. (C) The number of GFP+ colonies produced from MEFs infected with scramble shRNA or AHCY-targeting shRNA (n = 3 assays). (D) The number of GFP+ colonies produced from MEFs expressing AHCY WT or T136A rescue constructs (n = 3 assays). (E) Relative mRNA expression levels of pluripotency markers and differentiation markers in iPSCs derived from the MEFs expressing WT or T136A AHCY (n = 3 assays). Error bars denote the means ± SEM. Statistical analyses were performed by unpaired Student’s t test (*P < 0.05, **P < 0.01).
Discussion
Recent studies have demonstrated that unique metabolic signatures in ESCs dictate the cell fate of ESCs through regulation of epigenetic changes. For example, intracellular concentrations of acetyl-CoA and SAM, universal donors for histone acetylation and methylation, respectively, are found to be critical in regulating context-dependent cell fate decisions (40–42). α-Ketoglutarate, an important cofactor for histone demethylation, is also shown to maintain naïve PSCs but promote early differentiation at later stages of pluripotency (43). However, the mechanism by which the level of these key metabolites is regulated in ESCs is poorly understood. In this work, we demonstrated that O-GlcNAcylation of AHCY, a key metabolic enzyme in the methionine cycle, regulates intracellular SAM levels in mESCs and, hence, the methylation status of histones and DNA. Being a nutrient-sensitive protein modification, O-GlcNAcylation is rapidly removed in response to differentiation cues to decrease AHCY activity. This event precedes the transcriptional down-regulation of AHCY expression. Thus, deglycosylation of AHCY may serve as a signal to poise mESCs to early differentiation at the later stages of pluripotency.
O-GlcNAcylation is a dynamic modification that is responsive to nutrient status and many forms of cellular stress (44, 45). O-GlcNAcylation levels were shown to oscillate during stem cell development (27, 28). Modulation of O-GlcNAcylation was found to impact cell fate decisions in ESCs via different mechanisms, such as regulating pluripotency-associated transcription factors and epigenetics modifying enzymes (21, 22, 25, 28). Notably, its role in regulating ESC metabolism is just beginning to elucidate. This study provides evidence that O-GlcNAcylation influences the epigenetic profile and the pluripotency of ESCs via regulating the methionine metabolism. Given the dynamics and complexity of stem cell microenvironment, O-GlcNAcylation likely constitutes a molecular mechanism linking the signals/stresses from the stem cell niche with intracellular pathways during stem cell development. Further studies are needed to fully characterize the interplay between stem cell niche, O-GlcNAcylation, and cell fate decisions.
Materials and Methods
Cell Culture.
The E14.1 mESC line was obtained from American type culture collection and was maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco) supplemented with 15% fetal bovine serum (FBS) (Gibco), 2 mM l-glutamine (Gibco), 100 μM nonessential amino acids (Gibco), 0.1 mM β-mercaptoethanol, 1,000 units·ml−1 LIF (Millipore), and penicillin/streptomycin on gelatin-coated plates. HEK293T and NIH 3T3 cells were cultured in DMEM (HyClone) supplemented with 10% FBS (HyClone).
Western Blotting Analysis.
Cells were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with cOmplete protease inhibitors (Roche), and the lysate was resolved on 8–12% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE), transferred to nitrocellulose membrane, and immunoblotted with the indicated antibodies. Antibodies used in this study were obtained from the following sources: anti-O-GlcNAc antibody (RL2, clone 18B10.C7, Thermo Scientific, 1:1,000), anti-Flag antibody (cloneM2, Sigma-Aldrich, 1:2,000), anti-H3, H3K9me3, H3K27me3, H3K36me3, H3K79me3 antibody (PTM Biolabs, 1:2,000), anti-H3K4me3 antibody (Abcam, 1:1,000), anti-AHCY antibody (Abcam, ab134966, 1:1,000), anti-OCT4 antibody (Abcam, ab181557, 1:5,000), anti-SOX2 antibody (Abcam, ab92494, 1:5,000), anti-NANOG antibody (Abcam, ab214549, 1:5,000), anti-C-MYC antibody (Abcam, ab32072, 1:5,000). All protein concentrations were measured using the Bicinchoninic Acid protein assay (Pierce). Western blots were visualized and quantified using an Odyssey Infrared Imaging System (LI-COR Biosciences, Version 2.1).
Immunofluorescence Analysis.
Cells were fixed and incubated with primary antibodies at a dilution of 1:100, fluorescence dye-conjugated secondary antibodies, and 4′,6-diamidino-2-phenylindole, according to standard protocols. Antibodies used in this study were obtained from the following sources: anti-Nestin (BD Pharmingen, 556309), anti-Tuj1 (Promega, G712A), anti-GFAP (DAKO, Z0334), anti-BrdU (Abcam, ab6326). Cells were imaged using confocal microscopy.
Construction of Stable Cell Lines.
To generate the AHCY rescue E14.1 cell lines, Flag-tagged WT AHCY and T136A AHCY were cloned into the expression vector PTY-Lenti-shRNA as described (33). The shRNA sequence targeting AHCY or the corresponding scramble sequence (SI Appendix, Table S1) was inserted into the same vector to allow for the depletion of the endogenous AHCY. Lentiviruses produced from these constructs were used to infect E14.1 cells, and the cells were further selected with 2 μg/mL puromycin for 1 wk.
AHCY O-GlcNAcylation Analysis.
Analysis of AHCY O-GlcNAcylation was conducted as described with minor modifications (46). Briefly, O-GlcNAcylated proteins in cell lysates were first labeled with GalNAz using the Click-iT O-GlcNAc Enzymatic Labeling System (Life Technologies) and conjugated with an alkyne-biotin compound using the Click-iT Protein Analysis Detection System (Life Technologies). Control experiments in the absence of the enzyme GalT (Y289L) or UDP-GalNAz were carried out in parallel. Labeled proteins were then precipitated, resolubilized, neutralized, and further incubated with streptavidin beads at 4 °C overnight. After extensive washing steps, the bound protein fractions were eluted in the boiling elution buffer containing 50 mM Tris⋅HCl pH 6.8, 2.5% SDS, 100 mM DTT, 10% glycerol, and 20 mM biotin. Immunoblotting was carried out using anti-AHCY antibody.
For the analysis the stoichiometry of AHCY O-GlcNAcylation, 200 μg of azido-labeled lysates (from the above protocol) were precipitated using methanol and chloroform, and resolubilized in 50 μL of 50 mM Tris⋅HCl (pH 8.0) containing 1% SDS. The suspensions were conjugated with 100 μM alkyne-PEG-5K (provided by Min Wei, Northeastern Normal University, Chang Chun, China) as per the Click-iT Protein Analysis Detection Kit protocol. Then, the mixture was incubated at 37 °C for 16 h and precipitated using methanol and chloroform. The precipitate was resuspended with 50 μL of SDS loading buffer and detected by immunoblotting with AHCY antibody.
Mapping AHCY Glycosylation Sites.
Flag-tagged AHCY and HA-tagged OGT were coexpressed in NIH 3T3 cells for 48 h. Flag-tagged AHCY was immunoprecipitated from the cell lysates using anti-Flag-M2 magnetic beads and eluted in a buffer containing 4% SDS and 100 mM Tris⋅HCl pH 8.0. After separation and staining in 8–15% Bis-Tris gels, the AHCY protein band was excised and digested with trypsin and chymotrypsin. The extracted mixtures were purified by reverse-phase HPLC (Agilent 1100) using a gradient of 5–30% buffer B (100% MeCN) over 20 min at 4 mL/min and a constant flow of buffer A (0.5% aqueous AcOH). Eluted fractions between 5 and 12 min were manually collected, pooled, lyophilized, and analyzed using nanoLC-LTQ-CID/ETD-MS as previously described (33). Proteome Discovery (MASCOT search engine, version 1.3) was used for data search with O-GlcNAc (Ser/Thr) set as a variable modification.
AHCY Purification and Enzymatic Assay.
Expression constructs of Flag-AHCY were transfected into 293T cells for expression for 48 h. Cells were then collected and lysed in the lysis buffer containing 50 mM Tris⋅HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, and 5 mM thimet-G and further incubated with anti-Flag M2 beads at 4 °C overnight. After washing twice with lysis buffer containing 1% Triton X-100, the Flag-tagged AHCY protein was eluted with phosphate buffer saline (PBS) containing the 3× Flag peptides (Sigma-Aldrich).
Measurement of AHCY activity was performed as shown below. In brief, 200 μL of the enzyme solution containing 0.2 μM SAHH and 0.8 units of adenosine deaminase (Roche Applied Science) in the assay buffer (50 mM potassium phosphate buffer, pH 7.2, 1 mM EDTA, 1 mM NAD+) was added in 50 μL of 100 μM AdoHcy with 250 μM 5,5-dithiobis (nitrobenzoic acid) in the assay buffer. The reaction mixture was mixed rapidly and monitored at 412 nm continuously at 37 °C using a UV-visible spectrophotometer (SHIMADZUUV-2550).
Real-Time qPCR.
Total RNA was TRIzol-extracted, column-purified, and reverse-transcribed using PrimeScript first Strand Cdna Synthesis kit (Takara). All qPCR analyses were performed using Fast SYBR Green (Bio-Rad). Primer sequences are available in SI Appendix, Table S1.
ESC Differentiation Assays.
The differentiation of E14.1 cells was induced by the withdrawal of LIF or treatment with 10 μM RA. Western-blotting analysis was carried out at the indicated time points.
Alkaline Phosphatase and Crystal Violet Staining.
For AP staining, cells were fixed with 4% paraformaldehyde in PBS for 30 s and rinsed once with phosphate buffer saline Tween-100. Detection was performed using a leukocyte AP kit (Millipore) according to the manufacturer’s protocol. For crystal violet staining, cells were fixed with methanol for 30 s and stained with crystal violet solution for 20 min.
Cell Proliferation Assay.
Cell proliferation was measured by the incorporation of EdU into genomic DNA during the S phase of the cell cycle, using the Click-iT EdU Kit (Invitrogen). In brief, E14.1 cells were cultured in the medium supplied with 10 µM EdU for 20 min. The cells were then fixed, conjugated with Alexa594-azide by click reaction, and processed for BrdU immunofluorescent detection. Cells were counterstained with Hoechst and imaged by fluorescence microscopy.
Proliferation and Differentiation Assays of Neural Stem Cells.
In order to prepare neural stem cells (NSCs) culture medium, 20 ng/mL FGF-2, 20 ng/mL EGF, 2% B27 supplement, and 1% antibiotic-antimycotic were added to DMEM/F12 medium. NSCs were cultured in a 6-cm cell culture dish with 4 mL of medium in a 5% CO2 incubator at 37 °C.
For the in vitro proliferation assay, NSCs were cultured on coverslips in the medium supplied with 5 µM BrdU for 8 h, and followed the procedure described above. For the in vitro differentiation assay, NSCs were first cultured on coverslips in the proliferation medium, and then transferred into the differentiation medium containing 1 µM retinoic acid and 5 µM forskolin instead of growth factors for 48 h. Cells were stained using indicated antibodies and imaged with confocal microscopy.
Protein Cross-Linking Assay.
Cells were lysed in RIPA lysis buffer supplemented with cOmplete protease inhibitors. Lysates were then incubated with 100 mM disuccinimidyl suberate with end-to-end rotation at 4 °C for 60 min. Then, lysates were boiled in the elution buffer containing 50 mM Tris⋅HCl pH 6.8, 2.5% SDS, 100 mM DTT, and 10% glycerol for 10 min, followed by SDS/PAGE and Western blotting analysis.
Cell Cycle and Apoptosis Analysis.
For cell cycle analysis, cells were washed three times with ice-cold PBS and then fixed in 70% ethanol in PBS at 4 °C for 12 h. After fixation, cells were washed with cold PBS and stained with 0.5 mL of propidium iodide (PI) staining buffer, which contains 200 mg/mL RNase A, 50 μg/mL PI at 37 °C for 30 min in the dark. Analyses were performed on a CytoFLEX LX flow cytometer. Cell apoptosis analysis was performed using the Annexin V-FITC Apoptosis Detection Kit (Beyotime) according to the manufacturer’s instruction. Early apoptotic cells were defined as Annexin-V-position, PI-negative cells. Analyses were performed on a CytoFLEX LX flow cytometer.
DNA Methylation Assays.
Global DNA methylation status was determined by using the MethylFlash Methylated DNA Quantification Kit (Gepigentek). In brief, genomic DNA was isolated according to Tissue DNA Kit (OMEGA), and 100 ng of sample DNA was added to the designated wells with 1 μL of ME3, 1 μL of diluted ME4. Cover strip plate with plate seal or parafilm M was incubated at 37 °C for 90 min. Then, the binding reaction solution was removed from each well, and each well was washed three times with 150 μL of diluted ME1 1× Wash Buffer. To capture the methylated DNA, a series of steps of incubation with diluted buffers ME5, ME6, ME7, and ME8 were performed according to the manufacturer’s protocol. The reaction was quenched by the addition of 100 μL of ME9 to each well to allow for the color in the positive control wells to turn medium blue. The absorbance was read on a microplate reader at 450 nm within 2–15 min.
Analysis of the Metabolites in the Methionine Cycle.
The relative abundance of the metabolites in the methionine cycle was measured by liquid chromatography-mass spectrometry (LC-MS). Briefly,1 × 107 cells were collected, 1 mL of precooled extractant (70% methanol aqueous solution) was added, and whirled for 1 min. The mixture was frozen for 3 min in liquid nitrogen then thawed on ice for 3 min. This procedure was repeated 3 times. Then the mixture was centrifugated with 12,000 rpm at 4 °C for 10 min. The extracts were analyzed using an high performance liquid chromatography-electrospray tandem mass spectrometry (LC-ESI-MS/MS) system. The analytical conditions were as follows. HPLC: column, Waters ACQUITY UPLC HSS T3 C18 (1.8 µm, 2.1 mm × 100 mm); solvent system, water (0.04% acetic acid): acetonitrile (0.04% acetic acid); gradient program, 100:0V/V at 0 min, 5:95V/V at 11.0 min, 5:95V/V at 12.0 min, 95:5V/V at 12.1 min, 95:5V/V at 15.0 min; flow rate, 0.40 mL/min; temperature, 40 °C; injection volume: 2 μL. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (Q TRAP)-MS. The ESI source operation parameters were as follows: ion source, turbo spray; source temperature 500 °C; ion spray voltage 5500 V; ion source gas I, gas II, curtain gas were set at 55, 60, and 25.0 psi, respectively; the collision gas was high. Instrument tuning and mass calibration were performed with 10 and 100 μmol/L polypropylene glycol solutions in Q TRAP 6500 and LIT modes, respectively. Q TRAP 6500 scans were acquired as MRM experiments with collision gas (nitrogen) set to 5 psi. Declustering potentials (DP) and collision energies (CE) for individual MRM transitions was done with further DP and CE optimization. A specific set of MRM transitions was monitored for each period according to the metabolites eluted within this period.
Somatic Cell Reprogramming.
Mouse embryonic fibroblasts (MEFs) from Oct4–GFP transgenic mice were seeded at a density of 30,000 cells in 12-well plates and infected with either PTY-Lenti-shRNA control, shAHCY, shAHCY+WT, or shAHCY+T136A constructs for 24 h, and then infected with pMXs-based OSKM (Oct4, Sox2, Klf4, and c-Myc) for another 24 h. The virus supernatants were removed, and fresh iPSC culture media were added to the plates. Media were changed every day. After 14 d, iPSC clones were examined by both AP staining and GFP fluorescence.
Teratoma Formation.
The procedure of establishing teratomas in nude mice was approved by the Zhejiang University Laboratory Animal Center. Approximately 1 × 106 E14 cells were suspended in 200 mL of mES medium and injected into the thigh muscle of male 6-wk-old BALB/cA nude mice. The animals were checked two to three times per week. The volume of teratomas was measured twice per week after 2 wk. Five to six weeks after injection, the teratomas were collected and weighed.
RNA Sequencing.
Total RNA was TRIzol-extracted and purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEB. cDNA was synthesized using random hexamer primer and RNase H. The library fragments were purified with QiaQuick PCR kits and elution with elution buffer, then terminal repair A-tailing and adapter added were implemented. The clustering of the index-coded samples was performed on a cBot cluster generation system using HiSeq PE Cluster Kit v4-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on an Illumina platform and 150-bp paired-end reads were generated.
ChIP Assays.
Cells (1 × 107) were digested by trypsin and resuspended in PBS. Twenty-seven microliters of 37% formaldehyde (final concentration 1%) was directly added to the sample and rotated for 10 min at room temperature. Then, 0.125 M glycine solution was added to quench the reaction. Cells were then lysed in 275 μL of ChIP lysis buffer (50 mM Tris⋅HCl pH 8.1, 10 mM EDTA) supplemented with cOmplete protease inhibitors (PIC) for 20 min on ice, and centrifuged at 2,000 rpm for 5 min to get the nuclei pellet. The nuclei pellet was washed in 1 mL of NIM buffer (0.25 mM sucrose, 25 mM KCl, 10 mM Tris⋅HCl pH 7.4, 5 mM MgCl2) twice and lysed in nuclei lysis buffer (50 mM Tris⋅HCl pH 8.1, 10 mM EDTA, 1% SDS) supplemented with PIC. The nuclei lysis was sonicated for 16 min in an Ultrasonic Cell Disruptor (BRANSON SLPe Model) to obtain DNA fragments between 100 and 500 bp. Debris was removed by centrifugation at 12,000 rpm for 10 min at 4 °C, and the supernatant was transferred to new tubes. Anti-H3K4me3 antibody (5 μg) was added and rotated overnight at 4 °C. Corresponding type of IgG antibody (5 μg) was added as a negative control. A volume of 100 µL of Protein G beads was added, and the sample was rotated for a further 3 h at 4 °C. The beads were washed with LiCl wash buffer (Tris⋅HCl 10 mM pH 8.1, 0.25 M LiCl, 1% IGEPAL-CA630/Nonidet P-40, 1% deoxycholic acid [sodium salt], 1 mM EDTA). The DNA was eluted with 300 µL of elution buffer (Tris⋅HCl 20 mM pH 7.5, 20 mM EDTA, 0.5% SDS, 500 µg/mL Proteinase K) and incubated for 4 h at 56 °C. The DNA was extracted with phenol-chloroform followed by purification using a Qiagen MinElute column. qPCR was performed to analyze the enrichment of H3K4me3 in corresponding genes.
Statistical Analysis.
Data are presented as the means ± SEM. Unpaired Student ’s t test was used to determine the differences between two groups; a two-way ANOVA analysis followed by Bonferroni multiple-comparison test was used to determine differences between multiple groups. P < 0.05 was considered statistically significant.
Data Availability.
The data that support the findings of this study are provided in Dataset S1. The RNA-seq data are deposited in the National Center for Biotechnology Information Gene Expression Omnibus Repository under the accession no. GSE143756.
Supplementary Material
Acknowledgments
This work was supported by National Key Research and Development Program of China Grants 2016YFA0100303 (to W.Y.) and 2017YFE0196600 (to X.L.) and National Science Foundation of China Grants 91753125, 31270865, 31322019 (to W.Y.), 31571518, and 31771395 (to X.L.). We thank Dr. Min Wei (Northeastern Normal University, China) and Dr. Xing Chen (Peking University, China) for providing PEGylation reagents.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: RNA-sequencing experiments generated in this article were deposited in the National Center for Biotechnology Information Gene Expression Omnibus repository (accession no. GSE143756).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915582117/-/DCSupplemental.
References
- 1.Intlekofer A. M., Finley L. W. S., Metabolic signatures of cancer cells and stem cells. Nat. Metab. 1, 177–188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martello G., Smith A., The nature of embryonic stem cells. Annu. Rev. Cell Dev. Biol. 30, 647–675 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Tohyama S., et al. , Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 23, 663–674 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Ryall J. G., Cliff T., Dalton S., Sartorelli V., Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell 17, 651–662 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J., Ocampo A., Belmonte J. C. I., Cellular metabolism and induced pluripotency. Cell 166, 1371–1385 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Mentch S. J., Locasale J. W., One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 1363, 91–98 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyh-Chang N., et al. , Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339, 222–226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiraki N., et al. , Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 19, 780–794 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Tehlivets O., Malanovic N., Visram M., Pavkov-Keller T., Keller W., S-adenosyl-L-homocysteine hydrolase and methylation disorders: Yeast as a model system. Biochim. Biophys. Acta 1832, 204–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luka Z., Mudd S. H., Wagner C., Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J. Biol. Chem. 284, 22507–22511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzek A., et al. , Abnormal hypermethylation at imprinting control regions in patients with S-adenosylhomocysteine hydrolase (AHCY) deficiency. PLoS One 11, e0151261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novak E. M., et al. , BLM germline and somatic PKMYT1 and AHCY mutations: Genetic variations beyond MYCN and prognosis in neuroblastoma. Med. Hypotheses 97, 22–25 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Pogribny I. P., et al. , Epigenetically mediated inhibition of S-adenosylhomocysteine hydrolase and the associated dysregulation of 1-carbon metabolism in nonalcoholic steatohepatitis and hepatocellular carcinoma. FASEB J. 32, 1591–1601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller M. W., et al. , The mouse lethal nonagouti (a(x)) mutation deletes the S-adenosylhomocysteine hydrolase (Ahcy) gene. EMBO J. 13, 1806–1816 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart G. W., Housley M. P., Slawson C., Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Fardini Y., Dehennaut V., Lefebvre T., Issad T., O-GlcNAcylation: A new cancer hallmark? Front. Endocrinol. (Lausanne) 4, 99–113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanover J. A., Krause M. W., Love D. C., Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 13, 312–321 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Liu B., et al. , The lineage stability and suppressive program of regulatory T cells require protein O-GlcNAcylation. Nat. Commun. 10, 354–367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells L., Vosseller K., Hart G. W., Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science 291, 2376–2378 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Shafi R., et al. , The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl. Acad. Sci. U.S.A. 97, 5735–5739 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang H., et al. , O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell 11, 62–74 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Myers S. A., et al. , SOX2 O-GlcNAcylation alters its protein-protein interactions and genomic occupancy to modulate gene expression in pluripotent cells. eLife 5, e10647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon J. H., Suh H. N., Kim M. O., Han H. J., Glucosamine-induced reduction of integrin β4 and plectin complex stimulates migration and proliferation in mouse embryonic stem cells. Stem Cells Dev. 22, 2975–2989 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Lee H. J., et al. , Glycerol-3-phosphate acyltransferase-1 upregulation by O-GlcNAcylation of Sp1 protects against hypoxia-induced mouse embryonic stem cell apoptosis via mTOR activation. Cell Death Dis. 7, e2158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao Y., et al. , Next-generation unnatural monosaccharides reveal that ESRRB O-GlcNAcylation regulates pluripotency of mouse embryonic stem cells. Nat. Commun. 10, 4065–4078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parween S., et al. , Higher O-GlcNAc levels are associated with defects in progenitor proliferation and premature neuronal differentiation during in-vitro human embryonic cortical neurogenesis. Front. Cell. Neurosci. 11, 415–433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maury J. J., Chan K. K., Zheng L., Bardor M., Choo A. B., Excess of O-linked N-acetylglucosamine modifies human pluripotent stem cell differentiation. Stem Cell Res. 11, 926–937 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Andres L. M., et al. , Chemical modulation of protein O-GlcNAcylation via OGT inhibition promotes human neural cell differentiation. ACS Chem. Biol. 12, 2030–2039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Locasale J. W., Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aranda S., et al. , Chromatin capture links the metabolic enzyme AHCY to stem cell proliferation. Sci. Adv. 5, eaav2448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., et al. , O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc. Natl. Acad. Sci. U.S.A. 114, 13732–13737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi W., et al. , Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337, 975–980 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao X., et al. , O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat. Commun. 6, 8468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark P. M., et al. , Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J. Am. Chem. Soc. 130, 11576–11577 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rexach J. E., et al. , Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags. Nat. Chem. Biol. 6, 645–651 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner M. A., et al. , Structure determination of selenomethionyl S-adenosylhomocysteine hydrolase using data at a single wavelength. Nat. Struct. Biol. 5, 369–376 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Speakman C. M., et al. , Elevated O-GlcNAc levels activate epigenetically repressed genes and delay mouse ESC differentiation without affecting naïve to primed cell transition. Stem Cells 32, 2605–2615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Q., Next generation sequencing facilitates quantitative analysis of WT AHCY E14.1 cell and T136A AHCY E14.1 cell. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE143756. Deposited 15 January 2020.
- 39.Spyrou J., Gardner D. K., Harvey A. J., Metabolism is a key regulator of induced pluripotent stem cell reprogramming. Stem Cells Int. 2019, 7360121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J., et al. , Metabolism in pluripotent stem cells and early mammalian development. Cell Metab. 27, 332–338 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Mendel M., et al. , Methylation of structured RNA by the M6A writer METTL16 is essential for mouse embryonic development. Mol. Cell 71, 986–1000.e11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietrocola F., Galluzzi L., Bravo-San Pedro J. M., Madeo F., Kroemer G., Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 21, 805–821 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Hwang I. Y., et al. , Psat1-dependent fluctuations in α-ketoglutarate affect the timing of ESC differentiation. Cell Metab. 24, 494–501 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Hart G. W., Nutrient regulation of signaling and transcription. J. Biol. Chem. 294, 2211–2231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrer C. M., Sodi V. L., Reginato M. J., O-GlcNAcylation in cancer biology: Linking metabolism and signaling. J. Mol. Biol. 428, 3282–3294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., et al. , O-GlcNAcylation of core components of the translation initiation machinery regulates protein synthesis. Proc. Natl. Acad. Sci. U.S.A. 116, 7857–7866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are provided in Dataset S1. The RNA-seq data are deposited in the National Center for Biotechnology Information Gene Expression Omnibus Repository under the accession no. GSE143756.