Significance
Fruit-size increase is one of the major changes associated with tomato domestication, and it currently represents an important objective for breeding. Regulatory mutations at the LOCULE NUMBER and FASCIATED loci, the orthologues of the Arabidopsis WUSCHEL and CLAVATA3, have mainly contributed to enlarging fruit size by altering meristem activity. Here, we identify ENO as a tomato fruit regulator, which may function by regulating WUSCHEL gene expression to restrict stem-cell proliferation in a flower-specific manner. Our findings also show that a mutation in the ENO promoter was selected during domestication to establish the background for enhancing fruit size in cultivated tomatoes, denoting that transcriptional changes in key regulators have significant effects on agronomic traits.
Keywords: Solanum lycopersicum, fruit size, floral meristem, CLAVATA-WUSCHEL regulatory network, AP2/ERF transcription factor
Abstract
A dramatic evolution of fruit size has accompanied the domestication and improvement of fruit-bearing crop species. In tomato (Solanum lycopersicum), naturally occurring cis-regulatory mutations in the genes of the CLAVATA-WUSCHEL signaling pathway have led to a significant increase in fruit size generating enlarged meristems that lead to flowers with extra organs and bigger fruits. In this work, by combining mapping-by-sequencing and CRISPR/Cas9 genome editing methods, we isolated EXCESSIVE NUMBER OF FLORAL ORGANS (ENO), an AP2/ERF transcription factor which regulates floral meristem activity. Thus, the ENO gene mutation gives rise to plants that yield larger multilocular fruits due to an increased size of the floral meristem. Genetic analyses indicate that eno exhibits synergistic effects with mutations at the LOCULE NUMBER (encoding SlWUS) and FASCIATED (encoding SlCLV3) loci, two central players in the evolution of fruit size in the domestication of cultivated tomatoes. Our findings reveal that an eno mutation causes a substantial expansion of SlWUS expression domains in a flower-specific manner. In vitro binding results show that ENO is able to interact with the GGC-box cis-regulatory element within the SlWUS promoter region, suggesting that ENO directly regulates SlWUS expression domains to maintain floral stem-cell homeostasis. Furthermore, the study of natural allelic variation of the ENO locus proved that a cis-regulatory mutation in the promoter of ENO had been targeted by positive selection during the domestication process, setting up the background for significant increases in fruit locule number and fruit size in modern tomatoes.
During the domestication process, fruit-bearing crop species have largely increased their fruit size compared with those normally found in progenitor wild species. Accordingly, a large rise in fruit size has been achieved through breeding to increase the final size of floral meristems (FM) in crops such as tomato or maize (1–3). Modification of the CLAVATA (CLV)-WUSCHEL (WUS) negative feedback loop has led to this increase in meristem size. The homeodomain transcription factor WUS specifies stem-cell fate and promotes CLV3 expression, which is a peptide ligand that binds to different plasma membrane-localized receptor complexes to initiate a signaling cascade that subsequently represses WUS activity (4, 5). The core signaling module of the CLV-WUS feedback loop is deeply conserved in diverse plants such as Arabidopsis, tomato, and maize, while dosage compensation mechanisms that operate to buffer stem-cell homeostasis in diverse lineages have diversified (6). In this way, mutations in the CLV-WUS circuit have played a relevant role in crop yield improvement of both dicots and monocots (5, 7). Thus, in tomato, mutations in the CLV3 signal peptide promote stem-cell overproliferation resulting in the development of extra organs in flowers and bigger fruits (8).
Extreme fruit size in tomato (Solanum lycopersicum), which evolved from the small fruited wild ancestor S. pimpinellifolium, is determined mainly by the number of carpels in a flower and, hence, by the final number of locules (seed compartments) forming the mature fruit (9, 10). During tomato breeding, the joint action of fasciated (fas) and locule number (lc) mutations allowed for the development of large-fruited cultivars bearing more than eight locules, in contrast with the bilocular fruits of tomato wild species and most small-fruited varieties (10, 11). The fas mutation is caused by a 294-kb inversion disrupting the tomato CLV3 (SlCLV3) promoter (2), whereas lc is associated with two single-nucleotide polymorphisms (SNPs) in a putative CArG box regulatory element downstream of the tomato WUS (SlWUS) (12, 13). The fas and lc mutations are partial loss-of-function and gain-of-function alleles, respectively, and both mutations positively affect the FM size (14). A tomato mutant, excessive number of floral organs (eno), was recently reported to show alterations in FM size leading to the development of flowers with supernumerary organs and the formation of larger multilocular fruits (15). In this study, ENO was identified as a member of the APETALA2/Ethylene Responsive Factor (AP2/ERF) superfamily of transcription factors. Our findings suggest that ENO regulates SlWUS expression to restrict stem-cell proliferation in a flower-specific manner. Moreover, the analysis of genetic variation in tomato germplasm has shown that ENO played an important role in the increase of fruit size during tomato domestication.
Results
Eno Mutation Affects FM Size, Giving Rise to Plants with Higher Yield.
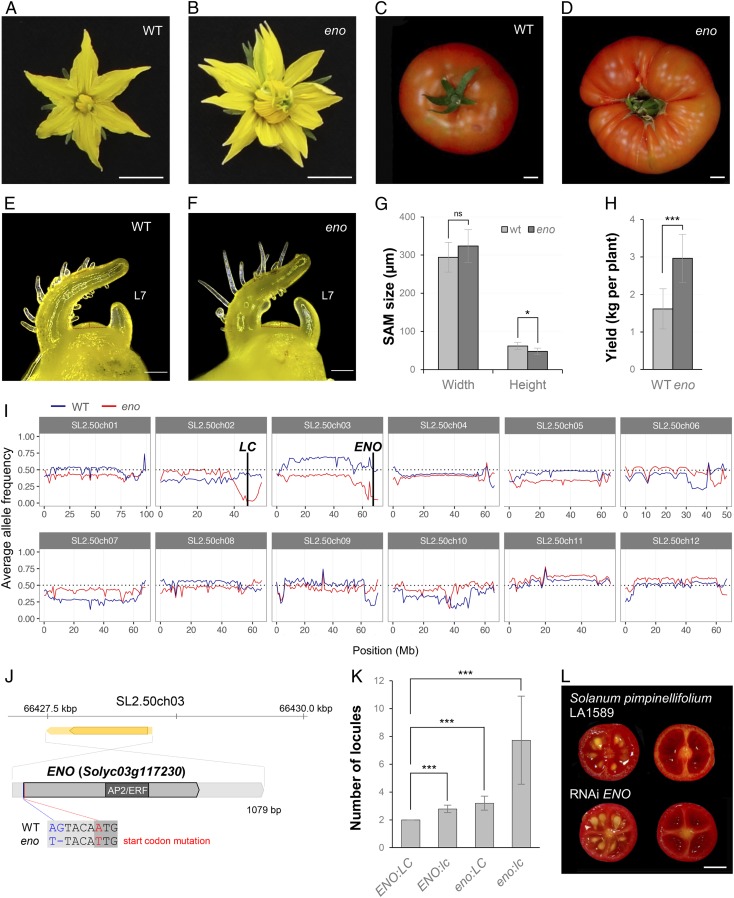
Previously, we reported that eno mutant plants developed an increased number of floral organs and multilocular fruits (Fig. 1 A–D) (15), a phenotype reminiscent of the CLV gene mutants, the shoot apical meristems (SAMs) of which are enlarged (2). Based on this evidence, we examined SAM size at the transition from vegetative to reproductive growth. eno plants showed slightly wider and shorter SAM than the wild type (Fig. 1 E–G), in contrast to the 1.8-fold increase in the size of FM previously detected in the mutant from petal initiation and stamen primordia onward (15). Consistently with this, the increased floral organ number of eno is more evident in the three innermost whorls than in the outermost one (SI Appendix, Table S1). As a consequence of additional carpel development, eno plants produced larger and heavier fruits that resulted in higher yield (Fig. 1H and SI Appendix, Table S2). In addition, eno inflorescences were slightly more branched and contained more flowers than those developed by wild-type plants, although the number of fruits was similar in both genotypes (SI Appendix, Table S2). Hence, the observed phenotypes suggest a role of ENO in reproductive development contributing to regulation of FM size.
Fig. 1.
Characterization and cloning of the eno mutant. Representative flower (A and B) and fruit (C and D) of wild-type (WT) and eno mutant plants. Images of the SAM from WT (E) and eno (F) plants at the transition meristem stage, before forming the first floral bud (L7, leaf 7). (G) Quantification of SAM size from WT and eno plants. (H) Yield performance of WT and eno plants. (I) Distribution of the average allele frequency of WT (blue line) and eno (red line) pools grouped by chromosomes. (J) Positional cloning of the ENO gene (coding and UTRs in dark and light gray, respectively). The SNP mutation in the start codon of the ENO gene is marked in red, and the SNP and the InDel localized in its 5′ UTR region are shown in blue. (K) Number of locules for each genotyped class identified in the interspecific eno × LA1589 (S. pimpinellifolium) F2 mapping population. (L) RNAi-mediated knockdown of ENO gene in S. pimpinellifolium (accession LA1589). Data are means ± SD; n = 20 (G, H, and K). A two-tailed, two-sample Student’s t test was performed, and significant differences are represented by asterisks: ***P < 0.0001; *P < 0.01. ns, no statistically significant differences. (Scale bars, 1 cm [A–D and L] and 200 μm [E and F].)
ENO Encodes an AP2/ERF Transcription Factor.
The eno mutant allele arose from a transfer DNA (T-DNA) insertional mutant collection generated in the genetic background cultivar P73 (16). However, subsequent molecular analyses indicated that somaclonal variation during tissue culture rather than the T-DNA insertion was responsible for the mutant phenotype (15). To identify the mutation that underlies the eno locus, we performed mapping-by-sequencing on an F2 population derived from the cross between eno and a wild tomato S. pimpinellifolium (accession LA1589). Unlike what happened in the original tomato P73 background, where the eno mutant phenotype is inherited as a monogenic recessive trait (15), the 15:1 segregation ratio observed in this interspecific F2 population suggests that the eno phenotype is controlled by two independently segregating recessive genes (468 wild-type plants, 35 mutants, χ2 = 0.43, P value = 0.51). In fact, a genome-wide analysis of the allele frequencies in two pools containing 35 mutant and 50 wild-type plants revealed two genomic regions on chromosome 2 and 3 candidates to harbor the causal mutations (Fig. 1I). Interestingly, the region in the long arm of chromosome 2 harbors the LC locus (12), which is mutated in the P73 cultivar, leading to the hypothesis that lc and eno loci interact synergistically to produce extra organs and locules in flowers and fruits, respectively. Further analysis of the SNP variants on the long arm of chromosome 2 revealed that the wild-type pool was heterozygous for the LC locus (allele frequency 0.59), while the mutant pool was homozygous for the lc mutation.
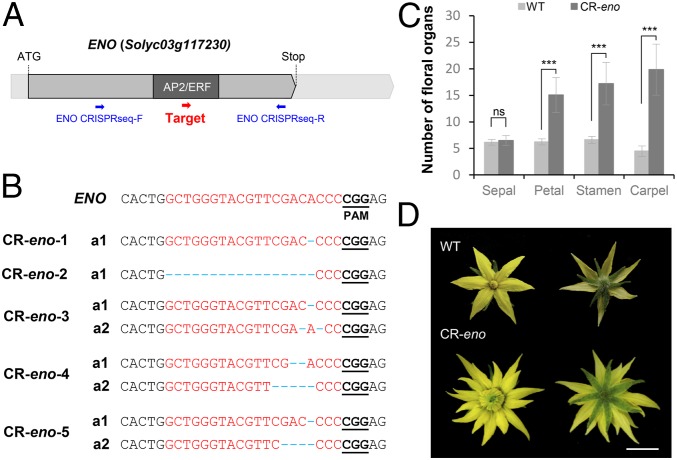
Variant analysis of a 5-Mb interval encompassing the candidate region located at the end of chromosome 3 led to the identification of a SNP in the start codon of the Solyc03g117230 gene, as well as another SNP and one insertion/deletion (InDel) affecting its 5′ untranslated region (UTR) (Fig. 1J). A subsequent phylogenetic analysis showed that Solyc03g117230 encodes a transcription factor of the AP2/ERF superfamily that belongs to the ERF subfamily group VIII (SI Appendix, Fig. S1). To test the identity of Solyc03g117230 as ENO, we engineered knockout mutations by using the CRISPR/Cas9 system with a single guide RNA (Fig. 2A) in the cultivar P73 genetic background. We evaluated five independent first-generation (T0) diploid lines (CR-eno) that were homozygous or biallelic for edited mutant alleles (Fig. 2B). In all cases, CR-eno lines yield fasciated flowers and fruits resembling the phenotype observed in eno mutants (Fig. 2 C and D and SI Appendix, Table S3). Hence, our results revealed that mutations in Solyc03g117230 (hereafter referred to as ENO) in combination with lc are responsible for the fasciation observed in flowers and fruits developed by eno mutant plants.
Fig. 2.
Characterization of CRISPR/Cas9-eno (CR-eno) lines. (A) Schematic illustrating single guide RNA targeting the ENO coding sequence (red arrow). Blue arrows indicate the PCR primers used to evaluate mutation type and efficiency. (B) CR-eno alleles identified by cloning and sequencing PCR products from the ENO targeted region from five T0 plants. Blue dashed lines indicate InDel mutations and black bold and underlined letters indicate protospacer-adjacent motif (PAM) sequences. (C) Quantification and statistical comparisons of floral organ number from wild-type (WT; cv. P73) and CR-eno flowers. Data were collected from five independent T0 lines. Data are means ± SDs; n = 10 flowers per plant. A two-tailed, two-sample Student’s t test was performed, and significant differences are represented by asterisks: ns, no statistically significant differences; ***P < 0.0001. (D) Representative flower from CRISPR/Cas9-eno (CR-eno) lines compared with wild-type (WT) one. (Scale bar, 1 cm.)
eno, lc, and fas Loci Exhibit Synergistic Effects.
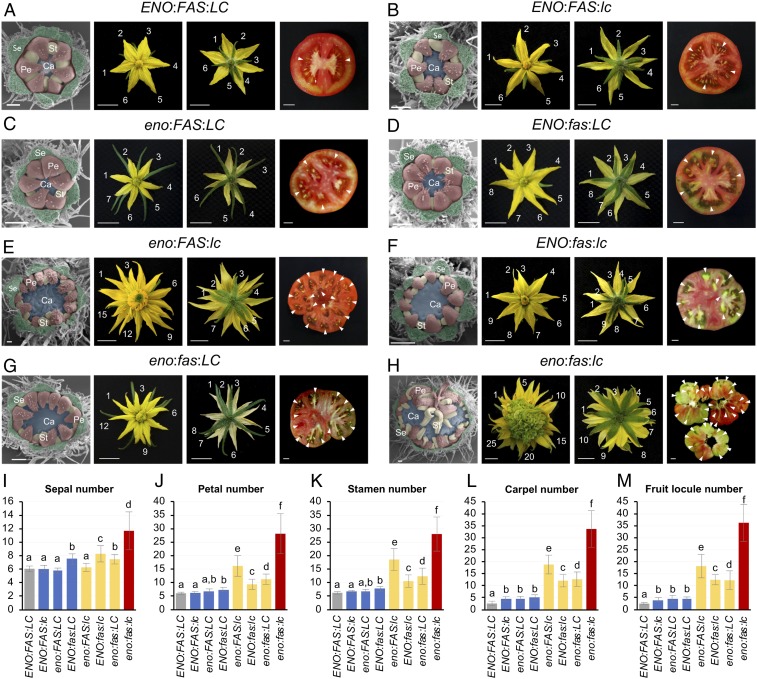
To determine the phenotypic effect of eno locus in a wild-type LC background, allele-specific markers for the ENO and LC loci were evaluated in the interspecific eno × LA1589 F2 mapping population. Thus, plants bearing single lc or eno mutations showed an increased number of locules with respect to wild-type ones, whereas a significant nonadditive increase in the number of locules (determined by a two-way ANOVA; P = 0.004) was observed in plants carrying both the eno and lc mutations (Fig. 1K). The effect of eno on locule number was additionally confirmed by an RNA interference (RNAi)-mediated knockdown of ENO in S. pimpinellifolium (LA1589), which yielded 24% of fruits with three to four locules instead of two-loculed fruits produced by wild-type plants (Fig. 1L and SI Appendix, Table S4). Likewise, in an intraspecific tomato population, eno:LC and ENO:lc genotypes gave rise to an equivalent increase in magnitude for the number of carpels and fruit locules compared with ENO:LC wild-type plants (Fig. 3 A–C, L, and M). These results support that an eno single locus promotes a weak increase in fruit locule number similar to that produced by an lc mutation.
Fig. 3.
Representative floral meristems, flowers, and fruits from the different allelic combinations of ENO, FAS, and LC loci. (A) ENO:FAS:LC. (B) ENO:FAS:lc. (C) eno:FAS:LC. (D) ENO:fas:LC. (E) eno:FAS:lc. (F) ENO:fas:lc. (G) eno:fas:LC. (H) eno:fas:lc. Se, sepals; Pe, petals; Sta, stamens; and Ca, carpels. Note: Sepals were removed in images of floral meristems. Number of petals and sepals are specified, and arrowheads indicate locules. (Scale bars, 200 μm [floral meristems] and 1 cm [flowers and fruits].) Number of sepals (I), petals (J), stamens (K), carpels (L), and fruit locules (M) in wild-type plants (gray) and single (blue), double (yellow), and triple (red) mutant lines for eno, fas, and lc alleles. For each genotype, 10 plants were phenotyped for 10 flowers and 10 fruits (100 measurements). Values are expressed as the mean ± SD. Significant differences were calculated by pairwise comparisons of means using the least significant difference test. Values followed by the same letter (a, b, c, d, e, or f) are not statistically different (P < 0.05).
As lc and fas loci act synergistically to increase fruit size (Fig. 3F) (8), we also wondered whether eno has genetic interaction with fas. To test this hypothesis, we introduced the eno and fas mutations into the wild-type LC background. Thus, unlike eno and fas single mutants the plants of which showed a similar feeble fasciation phenotype (Fig. 3 C and D), fasciation was synergistically enhanced in eno:fas:LC double-mutant plants (Fig. 3 G and I–M). Interestingly, the triple mutant for eno, fas, and lc dramatically increases the size of FM, giving rise to extremely fasciated flowers and fruits (Fig. 3 H and I–M). Although other genetic modifiers may also influence the magnitude of the observed double- and triple-mutant phenotypes, the existence of synergistic interactions indicates that eno, fas, and lc mutations affect different but functionally related genes, which are required to regulate FM size. As fas and lc are cis-regulatory mutations at SlCLV3 and SlWUS loci, respectively (2, 12, 13), these findings suggest that ENO might be a component of the CLV-WUS signaling pathway; alternatively, the possibility that ENO acts in a parallel and convergent pathway to the CLV-WUS network not yet described in tomato cannot be ruled out.
ENO Is Expressed in Shoot and Flower Meristematic Domes.
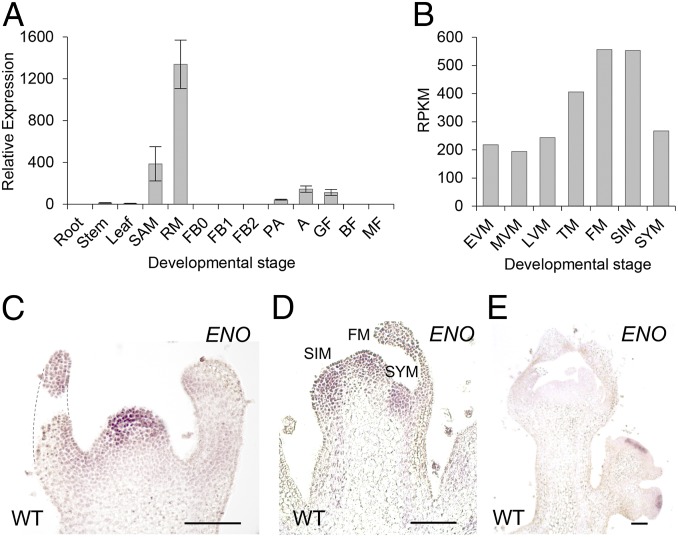
To further understand the function of ENO, we monitored its expression pattern throughout development. As expected from the phenotype of the eno mutation and its genetic interaction with lc and fas, we found high expression levels of ENO in the SAM and reproductive meristems (Fig. 4A). We then used the tomato meristem maturation atlas (17) to deeply assess the expression dynamic of ENO in meristematic tissues, which indicated that ENO is expressed predominantly in FM and sympodial inflorescence meristems (SIM) (Fig. 4B). In situ hybridization further revealed that ENO is expressed in the central zone of the SAM, where putative stem cells are located at the transition to the reproductive phase (Fig. 4C), as well as in the outermost cell layers of FM and SIM domains (Fig. 4D). Once flowers begin to develop, ENO messenger RNA (mRNA) is detected in meristematic cells within the floral buds; later, upon carpel primordia initiation, expression of ENO is no longer detectable (Fig. 4E).
Fig. 4.
Dynamic expression of ENO. (A) qRT-PCR for ENO transcripts in different developmental tissues and stages. Expression was compared to that of the control UBIQUTIN gene. SAM, shoot apical meristem; RM, reproductive meristem; FB0, floral bud of 3.0 to 5.9 mm in length; FB1, floral bud of 6.0 to 8.9 mm in length; FB2, floral bud of 9.0 to 12 mm in length; PA, flower at preanthesis stage; A, flower at anthesis stage; GF, green fruit; BF, breaker fruit; MF, mature fruit. (B) Reads per kilobase per million reads (RPKM) values for ENO across vegetative and reproductive meristem stages: EVM, early vegetative meristem; MVM, middle vegetative meristem; LVM, late vegetative meristem; TM, transition meristem; FM, floral meristem; SIM, sympodial inflorescence meristem; SYM, sympodial meristem. Data were obtained from the tomato meristem maturation atlas (17). (C–E) In situ mRNA hybridization of ENO in vegetative and reproductive meristems of wild-type plants. (Scale bars, 100 µm.)
ENO Acts in the Genetic Network Regulating Floral Meristem Size.
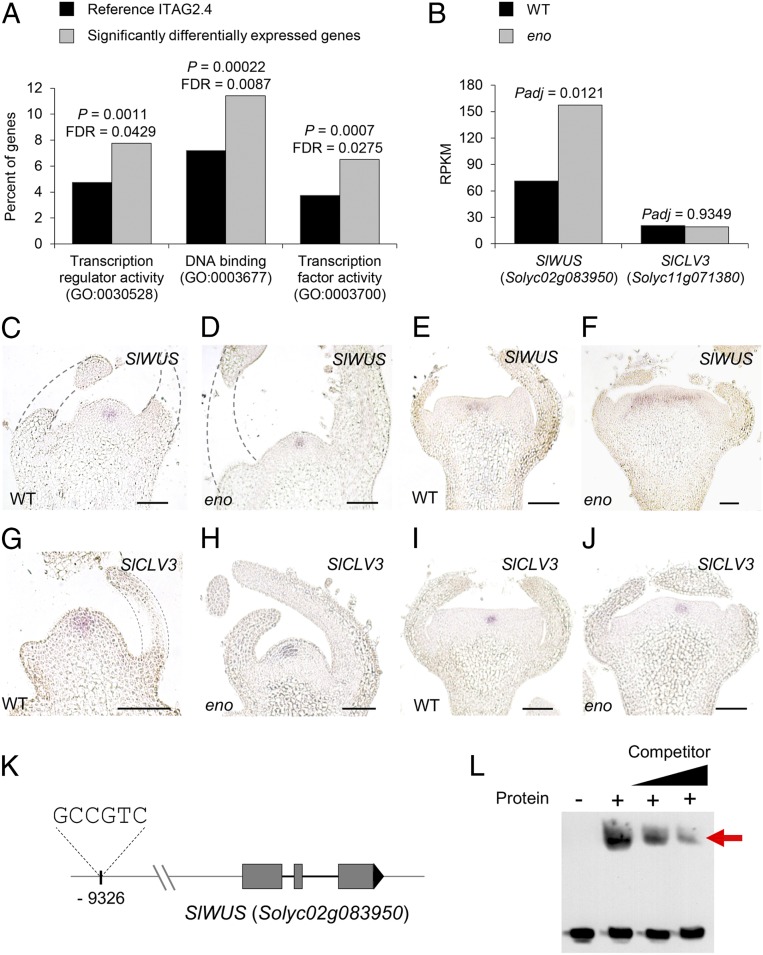
We investigated the molecular signaling cascade downstream of ENO using RNA sequencing in reproductive meristems from eno and wild-type plants. This analysis identified 381 and 397 genes significantly up- and down-regulated, respectively (false discovery rate [FDR] P < 0.05), in the eno mutant relative to wild type (Dataset S1). To gain insight into the functions of these genes, we performed Gene Ontology (GO) term enrichment analysis using agriGO software (18). Particularly, a significant enrichment was found for the molecular function of transcription regulator activity (P = 0.0011, FDR = 0.0429), DNA binding (P = 0.00022, FDR = 0.0087), and transcription factor activity (P = 0.0007, FDR = 0.0275) (Fig. 5A and SI Appendix, Fig. S2), which suggests that ENO functions in a complex transcriptional network that fine-tunes the spatial and temporal regulation of genes controlling meristematic activity.
Fig. 5.
ENO is involved in the transcriptional regulatory network that regulates floral meristem size. (A) GO terms enriched among significantly differentially expressed genes between wild-type and eno mutant reproductive meristems using agriGO v2.0 software. A FDR < 0.05 with the Fisher statistical test and the Bonferroni multitest adjustment was used to determined enriched GO terms. (B) RPKM values for SlWUS and SlCLV3 in wild-type (WT) and eno mutant. Genes with an FDR adjusted P value (Padj) < 0.05 were defined as significantly differentially expressed. (C–J) In situ mRNA hybridization of SlWUS (C–F) and SlCLV3 (G–J) in shoot apical and floral meristems of wild-type and eno plants. (Scale bars, 100 µm.) (K and L) EMSA of ENO protein revealing binding to the SlWUS promoter. Biotinylated probe containing the theoretical ERF binding site (GCCGTC, located at −9,326 bp relative to the translational start site) on the SlWUS promoter (K) incubated with purified ENO protein (L). Black triangle in L indicates the increasing amounts (100 and 1,000) of unlabeled probe used for competition. The specific complex formed is indicated by red arrow.
In addition, functional GO enrichment analysis using ClueGO software (19) for the corresponding Arabidopsis homologs of up- and down-regulated differentially expressed genes revealed 66 and 86 overrepresented GO terms, respectively (Dataset S2). Remarkably, up-regulated genes were highly enriched for GO terms associated with the meristem structural organization and meristem maintenance groups (SI Appendix, Fig. S3A). Among genes included within these groups the homologs of the Arabidopsis WUS (Solyc02g083950) and SHOOT MERISTEMLESS (STM) (Solyc02g081120) stand out, the latter functioning in a parallel and complementary fashion to the CLV-WUS pathway and preventing stem cells from differentiating (20). In contrast, down-regulated genes were strongly enriched for GO terms related to the specification of floral organ identity and floral organ development groups as well as, to a lesser extent, the FM determinacy and regulation of cell differentiation groups (SI Appendix, Fig. S3B). Within these groups, genes were included such as the putative homologs of the Arabidopsis floral homeotic genes APETALA1 (AP1), (Solyc05g056620), AP2 (Solyc03g044300), AP3 (Solyc04g081000), PISTILLATA (PI) (Solyc06g059970), and AGAMOUS (AG) (Solyc02g071730), the latter also involved in FM determinacy (21). Taken together, these findings suggest that ENO loss-of-function results in prolonged FM maintenance leading to an enlargement of FM size.
The role of ENO as a transcription regulator and its genetic interaction with lc and fasciated prompted us to examine expression changes in SlWUS (Solyc02g083950) and SlCLV3 (Solyc11g071380) genes in our RNA sequencing experiment. Notably, SlWUS expression was significantly up-regulated (fold change [FC] = 1.4) in eno reproductive meristems. In contrast, no significant differences were found for SlCLV3 (Fig. 5B and Dataset S1). To further investigate the contribution of ENO to the regulation of the CLV-WUS signaling pathway, expression patterns of SlWUS and SlCLV3 were examined by in situ hybridization. Thus, a similar expression pattern was observed for SlWUS mRNA in wild-type and eno SAMs (Fig. 5 C and D), while substantial expansion of SlWUS expression domains was found in FMs of eno mutants (Fig. 5 E and F). However, the SlCLV3 mRNA domain was found to be comparable in both SAM (Fig. 5 G and H) and FM (Fig. 5 I and J) of wild-type and eno plants. These results suggest that ENO acts by regulating the spatial expression domain of SlWUS specifically in FM and were consistent with the eno mutant phenotype, which mainly shows differences in FM size. Our results also suggest that the increased FM size is produced by stem-cell overproliferation resulting from expanded SlWUS expression. The fact that ENO transcripts were detected not only in reproductive meristem but also in vegetative ones suggests that other tomato genes may have functional redundancy with ENO in vegetative meristems, masking the effects of its loss-of-function. In the proposed CLV-WUS signaling pathway model, WUS promotes the expression of CLV3 peptide to limit its own activity via a kinase signaling cascade mediated by plasma membrane-localized receptor complexes (5, 22). Hence, in contrast to what was observed in FM of eno mutants, the increase of the SlWUS expression domain would lead to an up-regulation of CLV3 transcription. However, recent findings from studies of the SlCLV3 promoter mutant allele collection have revealed a substantial complexity underlying the CLV-WUS pathway as there is not a simple linear relationship between transcriptional changes for SlWUS and SlCLV3 expression levels, which is in agreement with the hypotheses that suggest a nonlinear gene dosage response for developmental regulators involved in complex transcriptional regulatory networks (8, 23).
The gene expression results indicate that ENO might specifically act in developing flowers to spatially regulate SlWUS expression domains. Thus, we wondered whether ENO could bind to the SlWUS promoter to directly regulate its transcriptional activity. The AP2 DNA binding domain of the ERF transcription factors has been shown to target GCC-related elements (GCCGGC and GCCGTC) (24). The analysis of the SlWUS promoter sequence revealed the existence of a GCCGTC element at position −9,326 (Fig. 5K). To examine whether SlWUS may be a direct target of ENO, the capability of ENO protein to bind to this GGC-box cis-regulatory element was tested by using an electrophoretic mobility shift assay (EMSA). A band shift was observed when the purified ENO protein was mixed with the biotin-labeled probe containing the GCCGTC motif. The presence of an excessive amount of the unlabeled probe prevented the formation of DNA-protein complexes, which indicates specific binding of ENO to this cis-regulatory element (Fig. 5L). Therefore, EMSA results showed that the GCCGTC motif encompassed in the SlWUS promoter region is a target of ENO, which indicates that ENO might function by directly regulating SlWUS expression domains within the complex transcriptional machinery that controls FM activity.
Natural Allelic Variation of ENO Locus Affects Fruit Locule Number.
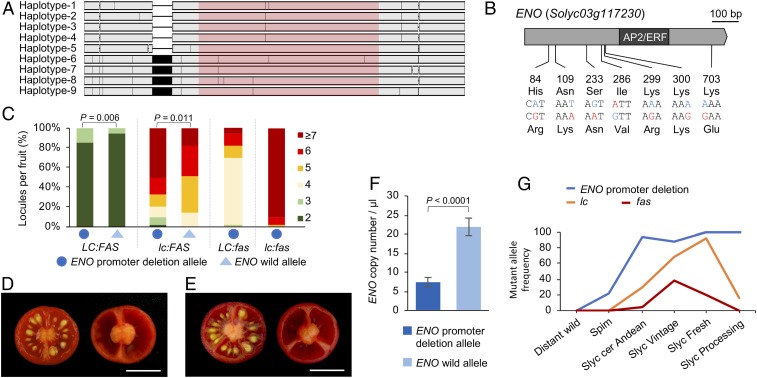
Previous quantitative trait locus (QTL) mapping (25–27) and genome-wide association studies (28) revealed the presence of a QTL contributing to increased fruit locule number (lcn3.1) at the region of the ENO locus. In view of the proximity of both loci, and the fact that mutations in the ENO gene give rise to fruits with extra locules, we hypothesized whether allelic variation at ENO could have contributed to the variability in fruit size present among tomato accessions. For this purpose, 1.6-kb region harboring the full-length ENO coding sequence was sequenced in a set of 103 accessions producing fruits of different sizes, comprising of 92 S. lycopersicum, 7 S. lycopersicum var. cerasiforme, and 4 S. pimpinellifolium accessions (Dataset S3). Sequence analysis identified 24 polymorphic sites and defined 9 haplotypes (SI Appendix, Fig. S4). Seven of these polymorphisms were detected in the ENO coding sequence, which resulted in one synonymous and six nonsynonymous substitutions (Fig. 6 A and B). Furthermore, we identified an 85-bp InDel annotated as a transposon-related element, which is located 107 bp upstream of the ENO start codon that was absent in haplotypes 1 to 5 and present in haplotypes 6 to 9 (Fig. 6A). To thoroughly analyze the functional effect of the detected polymorphic sites on fruit locule number, the set of accessions was additionally genotyped for LC and FAS loci (Dataset S3). Remarkably, we found a significant association between ENO promoter insertion polymorphism and the fruit locule number. Thus, an increase in fruit locule number was significantly associated with the absence of the 85-bp fragment (ENO promoter deletion allele) in both the LC and the lc background (Fig. 6C). It is worth highlighting that, among S. pimpinellifolium accessions, only fruits with two locules were found in plants with the ENO promoter insertion (ENO wild allele) (Fig. 6D), whereas fruits with two to three locules were found in the accession with the ENO promoter deletion allele (Fig. 6E). The functional effect of the promoter insertion polymorphism could not be evaluated in a fas background as tomato accessions bearing ENO wild allele were not found (Fig. 6C). From these results, we wondered about the effect of the promoter insertion polymorphism on ENO expression. To check this effect, allele-specific ENO transcript levels were measured by TaqMan probe using the Droplet Digital PCR (ddPCR) assay F1 hybrids heterozygous for the InDel mutation (haplotype-1 × haplotype-9). Notably, the copy number of the ENO wild allele was significantly higher (FC = 2.96) than the ENO promoter deletion allele (Fig. 6F), indicating that InDel mutation results in ENO expression level variation. Therefore, these results suggest that the ENO promoter deletion allele leads to a decreased expression of ENO which in turn is responsible for the increase in fruit locule number.
Fig. 6.
Natural allelic variation of ENO locus causes phenotypic variation in fruit locule number. (A) Multiple sequence alignment of ENO haplotypes identified in a set of 103 accessions producing fruits of different sizes, comprising of 92 S. lycopersicum, 7 S. lycopersicum var. cerasiforme, and 4 S. pimpinellifolium accessions. The ENO coding sequence is marked in pink. (B) Polymorphisms and deduced amino acid substitutions identified in the ENO coding sequence. (C) Functional effect of ENO promoter deletion allele on fruit locule number on the basis of genotypic information for LC and FAS loci. Fruits of S. pimpinellifolium accessions with the ENO wild allele (D) or the ENO promoter deletion allele (E). (Scale bars, 1 cm.) (F) Allele-specific ENO expression (copy number/μL) determined by TaqMan probe using the ddPCR assay. A two-tailed, two-sample Student’s t test was performed to determine significant differences between genotypes. (G) Frequencies of the ENO promoter, lc, and fas mutant alleles in phylogenetic groups representing sequential domestication steps as defined in Blanca et al. (30). Distant wild: wild tomato species; Spim: wild ancestor S. pimpinellifolium accessions; Slyc cer Andean: Andean accessions of S. lycopersicum var. cerasiforme; Slyc Vintage: S. lycopersicum Vintage varieties; Slyc Fresh: S. lycopersicum accessions for fresh market; Slyc Processing: S. lycopersicum accessions for industrial processing.
To further assess the evolutionary trajectory of the ENO promoter insertion polymorphism during tomato domestication, we analyzed this genomic region in a set of 601 resequenced accessions (29), which were clustered in phylogenetics groups representing sequential domestication steps as defined in Blanca et al. (30) (Dataset S4 and SI Appendix, Materials and Methods and Fig. S5). Results showed that the ENO promoter deletion allele first appeared at low frequencies in S. pimpinellifolium accessions, while it rose to near fixation already in the Andean S. lycopersicum var. cerasiforme group, the next step of domestication (Fig. 6G). Interestingly, all S. lycopersicum accessions tested contained the ENO promoter deletion allele, except for a few Vintage accessions that contained introgressions from wild species in the region of ENO (SI Appendix, Fig. S6). In contrast to the ENO promoter mutation, the lc and fas mutations arose at low frequency in the Andean S. lycopersicum var. cerasiforme group and varied in frequency in the course of tomato breeding depending on the group tested (Fig. 6G). Hence, taken together, the results show that the ENO promoter deletion allele arose prior to tomato domestication and increased in frequency to reach fixation in cultivated tomato, setting up the genetic environment that made significant changes in fruit size possible through selection and breeding of lc and fas mutant alleles.
Discussion
The balance between stem-cell proliferation and differentiation is tightly regulated by a complex transcription factor network that modulates meristematic activity. This equilibrium is achieved by a negative-feedback loop involving WUS and CLV genes, which maintains meristem homeostasis. WUS is known to regulate the CLV3 expression in a concentration-dependent manner (31). CLV3 is a secreted peptide that acts through plasma membrane-localized receptor complexes to activate a kinase signaling cascade leading to the repression of WUS transcription (4, 5). However, little is known about this downstream signaling pathway that finally controls WUS expression domains. In this context, our findings reveal that ENO, encoding a member of the AP2/ERF superfamily of transcription factors, is a component of the transcriptional regulatory network that specifically controls floral meristem activity, which might act to spatially limit the transcription of SlWUS. Overall, genetic and molecular data indicate that the ENO loss-of-function phenotype was due to a failure to properly repress SlWUS expression domains, which would most likely promote stem-cell overproliferation in FMs and finally give rise to an increase in the number of locules in tomato fruits. In agreement with these findings, the ENO (Solyc03g117230) gene has been included in a cluster of 29 genes proposed to regulate stem-cell function, which are also coexpressed with SlWUS. Transcripts of these genes are highly accumulated in FM whereas they diminish as the floral organ primordia are initiated (14). Within this meristematic cluster, SlWUS and ENO were the only ones showing significant genotype by developmental effects. Indeed, both genes showed a different expression pattern along FM developmental stages of lc, fas, and lc:fas mutants (14), which supports the functional role of ENO as a key member of the transcriptional network that regulates FM size. Likewise, the in vitro DNA-protein interaction analysis revealed that ENO is able to bind to the GCCGTC cis-regulatory element located in the SlWUS promoter region. Despite the fact that this DNA-protein interaction needs to be further investigated by in vivo studies, results obtained by in vitro EMSA experiments support that ENO might act directly by regulating SlWUS expression domains to maintain stem-cell homeostasis in a flower-specific manner.
The AP2/ERF superfamily members are classified according to the number of AP2 DNA binding domains that they contain. Thus, AP2 and ERF subfamily genes possess a double tandem-repeat and a single AP2 domain, respectively (32). Genes of the AP2 clade participate primarily in the regulation of developmental programs. For example, mutant studies indicate that the Arabidopsis AP2 gene has many important developmental functions, including stem-cell maintenance (33) and floral development (34), whereas the other members of the AP2 group act redundantly as flowering repressors (35). However, members of the AP2 clade are likely functionally divergent outside Brassicaceae, as they control fruit development and ripening in tomato (36, 37). The ERF subfamily genes are mainly involved in the response to environmental stresses and subdivided in turn into 12 groups (32). This work’s findings revealed that ENO encodes a transcription factor of the ERF subfamily group VIII (SI Appendix, Fig. S1). Within this clade, some members involved in developmental processes have been described such as the Arabidopsis DORNRÖSCHEN (DRN) and DORNRÖSCHEN-LIKE (DRNL) genes, which affect shoot meristem development and participate in the genetic control of embryogenesis (38). Furthermore, DRNL expression marks floral organ founder cells, and it is hypothesized that DRNL contributes to positional determination for floral organ initiation (39). The Arabidopsis PUCHI, an AP2/ERF protein closely related to DRNL, specifies floral meristem identity and bract suppression (40), whereas the PUCHI orthologs BRANCHED SILKLESS1 (BD1) in maize (41) and FRIZZY PANICLE (FZP) in rice (42) function in floral fate determination, revealing a conserved floral function for PUCHI. Hence, the present study provides evidence of the functional role of an ERF transcription factor specifically involved in regulating floral meristematic activity.
Recent research in crop species has substantially expanded knowledge on how the regulation of meristematic activity can lead to developmental alterations with significant implications for crop improvement (5, 7). In tomato, the variation from bilocular fruit to large-fruited cultivars bearing more than eight locules has been achieved by the combinatorial effects of lc and fas loci, which synergistically increase fruit size as a result of mutations in the CLV-WUS circuit (2, 13, 43). The findings in the present work reveal that ENO is a regulator of tomato fruit size, which has been targeted by positive selection during the domestication process. Thus, an increase in fruit locule number was significantly associated with an 85-bp deletion in the ENO promoter region resulting in a reduction of its expression, which supports the important role of cis-regulatory elements in crop improvement (44). In addition, the overall evolutionary trajectory of the ENO promoter and lc and fas mutations during tomato domestication and breeding revealed that, while lc and fas mutations were absent in the wild tomato species, the ENO promoter deletion allele arose in the wild ancestor S. pimpinellifolium and was selected during domestication, setting up the background for significant increases in fruit size in modern tomatoes through mutations in LC and FAS loci.
Collectively, this current work highlights that much still remains to be understood about the factors controlling meristem size and that there are unsuspected regulators of meristematic activity waiting to be discovered. Our findings show the potential to increase crop productivity by tinkering with genes that help to define the expression domains of the WUS stem-cell identity gene. In this respect, future studies for expanding our understanding of the molecular mechanisms governing meristem size maintenance would have far-reaching implications for enhanced agricultural yields. With the availability of genome editing tools, such as CRISPR/Cas9, it is currently possible to generate new customized alleles for crop productivity optimization to meet agricultural and environmental challenges. For example, further characterization of the SlWUS cis-regulatory region or the identification of new components in its transcriptional regulation may provide promising targets to engineer novel weak alleles that will have beneficial effects on tomato crop improvement.
Materials and Methods
A detailed description of plant materials, plant growth conditions, microscopy, gene expression studies, vector construction and plant transformation, bioinformatic sequence analysis, the DNA-protein interaction assay, and any associated references are available in SI Appendix, Materials and Methods.
Data Availability.
The sequencing datasets for this study can be found in the National Center for Biotechnology Information Short Read Archive under the BioProject accession codes PRJNA503558 (45) and PRJNA495568 (46).
Supplementary Material
Acknowledgments
We thank Dr. R. Fernández-Muñoz (Instituto de Hortofruticultura Subtropical y Mediterránea–Universidad de Málaga–Consejo Superior de Investigaciones Científicas) for providing seeds of wild and cultivated tomato accessions, and Dr. R. Micol-Ponce (Universidad de Almería) for help with the EMSA experiments. This research was supported by the Spanish Ministry of Economy and Competitiveness (Grants AGL2015-64991-C3-1-R and AGL2015-64991-C3-3-R) and the Research and Innovation Programme of the European Union Horizon 2020 (Breeding for Resilient, Efficient and Sustainable Organic Vegetable production [BRESOV], Project ID 774244). J.M.J.-G. was funded by Agence Nationale de la Recherche (ANR) Grant tomaTE, ANR-17-CE20-0024-02 and ANR TREMPLIN–European Research Council Grant RITMO, ANR-17-ERC2-0013-01.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The sequencing datasets for this study have been deposited in the National Center for Biotechnology Information Short Read Archive under the BioProject accession codes PRJNA503558 and PRJNA495568.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913688117/-/DCSupplemental.
References
- 1.Bommert P., Nagasawa N. S., Jackson D., Quantitative variation in maize kernel row number is controlled by the FASCIATED EAR2 locus. Nat. Genet. 45, 334–337 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Xu C., et al. , A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 47, 784–792 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Je B. I., et al. , Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat. Genet. 48, 785–791 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Schoof H., et al. , The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Somssich M., Je B. I., Simon R., Jackson D., CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143, 3238–3248 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Leal D., et al. , Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat. Genet. 51, 786–792 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli M., Gallavotti A., Expanding the regulatory network for meristem size in plants. Trends Genet. 32, 372–383 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Leal D., Lemmon Z. H., Man J., Bartlett M. E., Lippman Z. B., Engineering quantitative trait variation for crop improvement by genome editing. Cell 171, 470–480.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Lippman Z., Tanksley S. D., Dissecting the genetic pathway to extreme fruit size in tomato using a cross between the small-fruited wild species Lycopersicon pimpinellifolium and L. esculentum var. Giant Heirloom. Genetics 158, 413–422 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanksley S. D., The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 16 (suppl.), S181–S189 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrero L. S., Cong B., Wu F., Tanksley S. D., Developmental characterization of the fasciated locus and mapping of Arabidopsis candidate genes involved in the control of floral meristem size and carpel number in tomato. Genome 49, 991–1006 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Muños S., et al. , Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiol. 156, 2244–2254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Knaap E., et al. , What lies beyond the eye: The molecular mechanisms regulating tomato fruit weight and shape. Front. Plant Sci. 5, 227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu Y. H., Jang J. C., Huang Z., van der Knaap E., Tomato locule number and fruit size controlled by natural alleles of lc and fas. Plant Direct 3, e00142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Lozano A., et al. , Mutation at the tomato excessive number of floral organs (ENO) locus impairs floral meristem development, thus promoting an increased number of floral organs and fruit size. Plant Sci. 232, 41–48 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Martín F., et al. , A collection of enhancer trap insertional mutants for functional genomics in tomato. Plant Biotechnol. J. 15, 1439–1452 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S. J., Jiang K., Schatz M. C., Lippman Z. B., Rate of meristem maturation determines inflorescence architecture in tomato. Proc. Natl. Acad. Sci. U.S.A. 109, 639–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z., Zhou X., Ling Y., Zhang Z., Su Z., agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38, W64–W70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindea G., et al. , ClueGO: A cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenhard M., Jürgens G., Laux T., The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 129, 3195–3206 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Thomson B., Wellmer F., Molecular regulation of flower development. Curr. Top. Dev. Biol. 131, 185–210 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Yadav R. K., et al. , WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 25, 2025–2030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birchler J. A., Johnson A. F., Veitia R. A., Kinetics genetics: Incorporating the concept of genomic balance into an understanding of quantitative traits. Plant Sci. 245, 128–134 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Franco-Zorrilla J. M., et al. , DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc. Natl. Acad. Sci. U.S.A. 111, 2367–2372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Knaap E., Tanksley S. D., The making of a bell pepper-shaped tomato fruit: Identification of loci controlling fruit morphology in Yellow Stuffer tomato. Theor. Appl. Genet. 107, 139–147 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Gonzalo M. J., et al. , Tomato fruit shape analysis using morphometric and morphology attributes implemented in Tomato Analyzer software program. J. Am. Soc. Hortic. Sci. 134, 77–87 (2009). [Google Scholar]
- 27.Illa-Berenguer E., Van Houten J., Huang Z., van der Knaap E., Rapid and reliable identification of tomato fruit weight and locule number loci by QTL-seq. Theor. Appl. Genet. 128, 1329–1342 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Lin T., et al. , Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 46, 1220–1226 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Zhu G., et al. , Rewiring of the fruit metabolome in tomato breeding. Cell 172, 249–261.e12 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Blanca J., et al. , Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genomics 16, 257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perales M., et al. , Threshold-dependent transcriptional discrimination underlies stem cell homeostasis. Proc. Natl. Acad. Sci. U.S.A. 113, E6298–E6306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licausi F., Ohme-Takagi M., Perata P., APETALA2/ethylene responsive factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 199, 639–649 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Würschum T., Gross-Hardt R., Laux T., APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18, 295–307 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jofuku K. D., den Boer B. G., Van Montagu M., Okamuro J. K., Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6, 1211–1225 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Q. H., Helliwell C. A., Regulation of flowering time and floral patterning by miR172. J. Exp. Bot. 62, 487–495 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Chung M. Y., et al. , A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J. 64, 936–947 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Karlova R., et al. , Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23, 923–941 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandler J. W., Cole M., Flier A., Grewe B., Werr W., The AP2 transcription factors DORNROSCHEN and DORNROSCHEN-LIKE redundantly control Arabidopsis embryo patterning via interaction with PHAVOLUTA. Development 134, 1653–1662 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Chandler J. W., Jacobs B., Cole M., Comelli P., Werr W., DORNRÖSCHEN-LIKE expression marks Arabidopsis floral organ founder cells and precedes auxin response maxima. Plant Mol. Biol. 76, 171–185 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Karim M. R., Hirota A., Kwiatkowska D., Tasaka M., Aida M., A role for Arabidopsis PUCHI in floral meristem identity and bract suppression. Plant Cell 21, 1360–1372 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuck G., Muszynski M., Kellogg E., Hake S., Schmidt R. J., The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298, 1238–1241 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Komatsu M., Chujo A., Nagato Y., Shimamoto K., Kyozuka J., FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 130, 3841–3850 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Barrero L. S., Tanksley S. D., Evaluating the genetic basis of multiple-locule fruit in a broad cross section of tomato cultivars. Theor. Appl. Genet. 109, 669–679 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Swinnen G., Goossens A., Pauwels L., Lessons from domestication: Targeting cis-regulatory elements for crop improvement. Trends Plant Sci. 21, 506–515 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Yuste-Lisbona F. J., Lozano R., Mapping-by-sequencing of the eno mutation. Short Read Archive. https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA503558. Deposited 2 November 2018.
- 46.Yuste-Lisbona F. J., Lozano R., RNA-seq raw data of eno and wild-type tomato reproductive meristems. Short Read Archive. https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA495568. Deposited 10 October 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing datasets for this study can be found in the National Center for Biotechnology Information Short Read Archive under the BioProject accession codes PRJNA503558 (45) and PRJNA495568 (46).