Significance
Although proteins are commonly bound to membranes by hydrophobic transmembrane polypeptide segments, Rab family GTPases are anchored by prenyl groups. Rabs undergo conformational switching, being more active with bound GTP and less active with bound GDP. Rab-binding proteins, termed effectors, bind with higher affinity to Rab:GTP than to Rab:GDP. We now report that the prenylated yeast vacuolar Rab Ypt7 undergoes GTP/GDP switching, which is recognized by its effector HOPS (homotypic fusion and vacuole protein sorting). In contrast, transmembrane-anchored Ypt7 undergoes GTP/GDP switching, but binds and activates HOPS with either bound guanine nucleotide. A function of the Rab prenyl anchor is revealed, allowing an effector to read guanine-nucleotide-regulated Rab conformation.
Keywords: SNARE, Gyp1-46, HOPS, membrane fusion, yeast vacuoles
Abstract
Membrane fusion is catalyzed by conserved proteins R, Qa, Qb, and Qc SNAREs, which form tetrameric RQaQbQc complexes between membranes; SNARE chaperones of the SM, Sec17/αSNAP, and Sec18/NSF families; Rab-GTPases (Rabs); and Rab effectors. Rabs are anchored to membranes by C-terminal prenyl groups, but can also function when anchored by an apolar polypeptide. Rabs are regulated by GTPase-activating proteins (GAPs), activating the hydrolysis of bound GTP. We have reconstituted fusion with pure components from yeast vacuoles including SNAREs, the HOPS (homotypic fusion and vacuole protein sorting) tethering and SNARE-assembly complex, and the Rab Ypt7, bound to membranes by either C-terminal prenyl groups (Ypt7-pr) or a recombinant transmembrane anchor (Ypt7-tm). We now report that HOPS-dependent fusion occurs with Ypt7 anchored by either means, but only Ypt7-pr requires GTP for activation and is inactive either with bound GDP or without bound guanine nucleotide. In contrast, Ypt7-tm is constitutively active for HOPS-dependent fusion, independent of bound guanine nucleotide. Fusion inhibition by the GAP Gyp1-46 is not limited to Ypt7-tm with bound GTP, indicating that this GAP has an additional mode of regulating fusion. Phosphorylation of HOPS by the vacuolar kinase Yck3 renders fusion strictly dependent on GTP-activated Ypt7, whether bound to membranes by prenyl or transmembrane anchor. The binding of GTP or GDP constitutes a selective switch for Ypt7, but with Ypt7-tm, this switch is only read by HOPS after phosphorylation to P-HOPS by its physiological kinase Yck3. The prenyl anchor of Ypt7 allows both HOPS and P-HOPS to be regulated by Ypt7-bound guanine nucleotide.
Membrane fusion during endocytic and exocytic vesicular trafficking is catalyzed by conserved families of proteins (1). SNARE proteins have N domains and conserved heptad repeat SNARE domains, and often have C-terminal apolar transmembrane anchors (2). SNARE domains assemble into 4-membered coiled coils with apolar residues facing inward, with the exception of inward-facing arginyl or glutaminyl central residues, the 0-layer. SNAREs are in 4 conserved subfamilies (3), termed R, Qa, Qb, and Qc, which assemble into RQaQbQc SNARE complexes. These SNARE complexes are in cis if their hydrophobic anchors are in a common bilayer or in trans if anchored to two apposed bilayers before fusion. SM (Sec1/Munc18) proteins have surface binding grooves for the R- and Qa-SNARE domains, which facilitate SNARE complex assembly (4). Before trans-SNARE associations, membranes are tethered together by Rab-family GTPases, which bind tethering effector proteins (5). The complex binding and functional relationships among Rabs, their effector tethers, SM proteins, and SNAREs and their chaperones provide spatiotemporal regulation of fusion (6), although functional assays are lacking for many tethers and SM proteins. Rabs are bound to each membrane by prenyl anchors, and are most active for binding their effectors when the Rab has bound GTP. Rabs are indolent GTPases, but GTPase activating proteins (GAPs) trigger the hydrolysis of their bound GTP. Rabs function as regulated switches, becoming active to bind their effectors when bound with GTP and less active with bound GDP. It is unclear whether the membrane anchoring of Rabs by prenyl groups simply allows for membrane targeting, anchoring, and recycling, or whether prenyl anchors relate directly to how Rabs function as a guanine nucleotide responsive switch.
We study fusion with the vacuoles (lysosomes) of Saccharomyces cerevisiae. Vacuoles undergo continuous homotypic fusion and fission in vivo. Disruption of a fusion gene allows continued fission, resulting in multiple small vacuoles that are readily visualized by light microscopy. This allowed Wada and colleagues to define each VAM (vacuolar morphology) gene that governs vacuole fusion and vacuole protein sorting (VPS): the Rab Ypt7; its effector complex HOPS (homotypic fusion and vacuole protein sorting), which is a large hexameric protein with subunits of Vps 11, 16, 18, 33, 39, and 41; and the Qa-SNARE Vam3 and Qc-SNARE Vam7 (7). The R-SNARE Nyv1 was found separately (8). Other fusion proteins such as the Qb-SNARE Vti1 and the SNARE chaperones Sec17 (α-SNAP) and Sec18 (NSF) are needed for other trafficking steps of the essential exocytic pathway and were thus not part of the VAM gene collection.
Vacuole fusion has been studied in vivo, in vitro with the isolated organelle (9), and with chemically defined reactions that employ proteoliposomes of vacuolar lipid composition and bearing the purified recombinant fusion proteins (10, 11). Fusion requires HOPS and the R-SNARE Nyv1, the Qa-SNARE Vam3, the Qb-SNARE Vti1, and the Qc-SNARE Vam7, termed simply R, Qa, Qb, and Qc hereafter. HOPS tethers membranes by the affinities of two of its subunits, Vps39 and Vps41, for Ypt7 (12, 13). HOPS also has direct affinity for each of the four vacuolar SNAREs and catalyzes their assembly into a trans-complex (14). The direct affinity of HOPS for acidic vacuolar lipid contributes to its tethering function (15). HOPS is negatively regulated through phosphorylation of its Vps41 subunit by the vacuolar membrane kinase Yck3 in response to osmolar shifts in the cell growth medium (16–19). Phosphorylation by Yck3 converts HOPS into phospho-HOPS (P-HOPS), with lower affinity for acidic lipids and more strict dependence on the GTP-bound state of Ypt7 than unphosphorylated HOPS (15–18, 20). Studies of the effects of Yck3 phosphorylation on fusion in vivo or with the isolated organelle are complicated by the fact that this kinase also phosphorylates the Qa-SNARE (17) and the Ypt7 guanine nucleotide exchange factor Ccz1/Mon1 (21). HOPS provides tethering through the product of its affinities for the Rab Ypt7 on each membrane (12), for the SNAREs (4, 14, 22), and for acidic lipids (15). HOPS then engages the SNAREs on tethered membranes to catalyze their assembly into a complex (4, 14, 15, 23). Ypt7 thus serves as the master regulator of fusion, but is regulated by its bound nucleotide and by the phosphorylation of its HOPS effector. Although many elements of HOPS action, such as tethering and catalysis of SNARE assembly, are likely present during fusion at almost all other organelles, the direct physical linkage of dual Rab recognition and SM protein within a single protein complex may be particular to the HOPS family.
Ypt7, similar to other Rabs, is bound to the membrane by C-terminal prenyl (digeranylgeranyl) anchors (24), whose sole known functions are membrane anchoring and permitting its cycling through guanine nucleotide dissociation inhibitor-mediated extraction. A pioneering study by Gallwitz and colleagues (25) showed that the Rab Ypt1 remained active when its prenyl anchor was replaced by a more typical membrane anchor of 20 apolar amino acyl residues. Which aspects of Rab function rely on the prenyl anchor rather than an apolar polypeptide anchor, as is common for other membrane proteins? We now report that the prenyl anchor poises the Rab Ypt7 to require GTP for its active state for HOPS-dependent fusion. Ypt7 remains active with HOPS when its prenyl anchor is replaced with a recombinant transmembrane anchor from Vam3, but this Ypt7-tm is also active with bound GDP or without guanine nucleotide. Ypt7 with a transmembrane anchor (Ypt7-tm) nevertheless binds GTP, as GTPγS confers resistance to the GAP Gyp1-46. GTP (but not GDP) allows Ypt7-tm to support P-HOPS-dependent fusion. The capacity of the Ypt7-effector to mediate fusion is thus a function of the Ypt7 membrane anchor, its bound guanine nucleotide, and the phosphorylation state of HOPS, its effector.
Results
To study the guanine-nucleotide dependence of the vacuolar Rab Ypt7, we prepared complementary sets of test proteoliposomes with vacuolar mixed lipids. One set bore Ypt7 (where indicated) at a 1:8,000 molar ratio to lipids, R-SNARE at a 1:16,000 molar ratio to lipids, and luminal streptavidin derivatized with the fluorophore Cy5. The complementary set of proteoliposomes bore Ypt7 as above, but had the Qa- and Qb-SNAREs at 1:16,000 molar ratios to lipid and had biotinylated phycoerythrin as the luminal fluorescent protein. In mixtures of these proteoliposomes, the Cy5 and phycoerythrin fluorophores are initially separated by at least the thickness of two bilayers, and thus exhibit no FRET (fluorescence resonance energy transfer). After charging Ypt7 with guanine nucleotide, mixing them in the presence of a large molar excess of nonfluorescent streptavidin (to quench any FRET signal from lysis), and incubating with purified HOPS and the soluble Qc-SNARE, fusion mixes their luminal contents. After fusion-mediated luminal content mixing, the binding of biotin to streptavidin brings the Cy5 and phycoerythrin fluorophores into intimate contact, establishing a strong FRET signal (26).
The Membrane Anchor of Ypt7 Regulates the Effect of Bound Guanine Nucleotide.
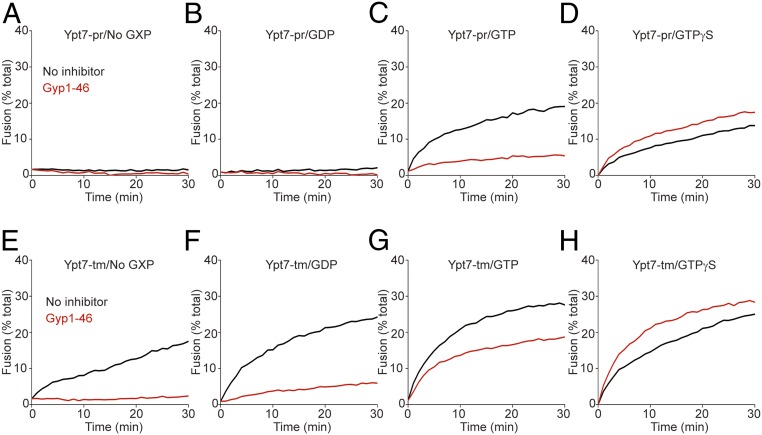
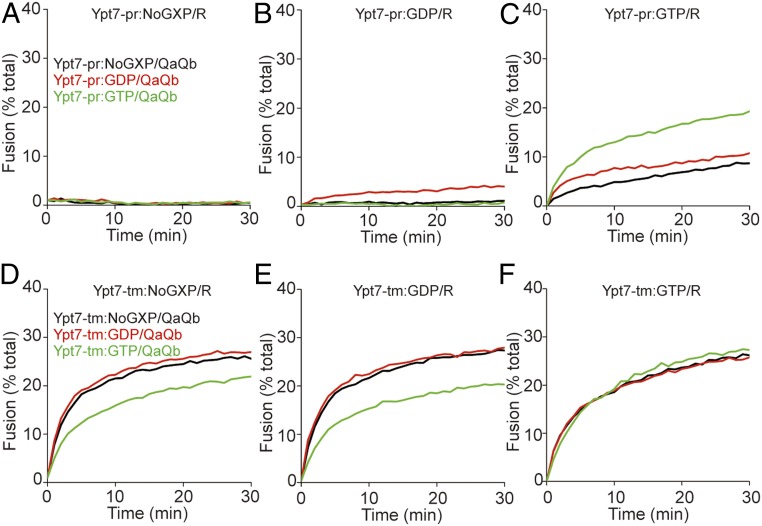
Proteoliposomes bearing Ypt7 with transmembrane anchor (Ypt7-tm) or prenyl-anchored Ypt7 (Ypt7-pr) were charged with GDP, GTP, or GTPγS or without guanine nucleotide by incubation with each nucleotide (or no nucleotide) and EDTA before addition of excess MgCl2 (15, 27, 28). Proteoliposomes with R- or QaQb-SNAREs were then mixed and given HOPS and the Qc-SNARE to initiate fusion. With Ypt7-pr, there was far more fusion with bound GTP (black curve, Fig. 1C; also see SI Appendix, Fig. S1) or GTPγS (Fig. 1D) than without guanine nucleotide (Fig. 1A) or with GDP (Fig. 1B). Fusion with bound GTP was inhibited by the GTPase-activating protein Gyp1-46 (Fig. 1C), but there was no inhibition when Ypt7 bore hydrolysis-resistant GTPγS (Fig. 1D). This pattern of dependence on guanine nucleoside triphosphate, and sensitivity to the activation of GTP hydrolysis by the Gyp1-46 GAP, corresponds well to earlier studies of Ypt7 activity on purified vacuoles (29, 30) and of other Rab proteins (6).
Fig. 1.
The membrane anchor of Ypt7 regulates its response to bound guanine nucleotide. Fusion reactions contained proteoliposomes bearing R- or QaQb-SNAREs at 1:16,000 SNARE to lipid molar ratio with 1:8,000 protein to lipid molar ratio of either Ypt7-pr (A–D) or Ypt7-tm (E–H). R- and QaQb-proteoliposomes were separately charged with No GXP, GDP, GTP, or GTPγS as indicated, and preincubated with buffer alone (black line) or 4 μM Gyp1-46 (red line) for 10 min at 27 °C. R- and QaQb-proteoliposomes were then moved to same well and mixed with 50 nM HOPS and 100 nM Qc. Content mixing of proteoliposomes was assayed by measuring the FRET between luminal Cy5 and phycoerythrin for 30 min at 27 °C. Kinetic curves of content mixing assays in this figure are representative of n ≥ 3 experiments; see SI Appendix, Fig. S1.
Fusion was also supported by Ypt7 anchored by a Vam3-derived transmembrane anchor (Ypt7-tm), as expected (25) and reported (14), but this fusion with Ypt7-tm and HOPS was strikingly independent of bound guanine nucleotide (black curves, Fig. 1 E–H; also see SI Appendix, Fig. S1 E–H). Nonetheless, fusion with Ypt7-tm:GTP remained sensitive to Gyp1-46 (Fig. 1G) unless nonhydrolyzable guanine nucleotide was bound (Fig. 1H). Surprisingly, however, Gyp1-46 even inhibited fusion when Ypt7-tm was charged with GDP or without guanine nucleoside (Fig. 1 E and F). Gyp1-46 had not been added in large excess in this experiment, but rather in the responsive range of its inhibitory activity (SI Appendix, Fig. S2); inhibition was thus not a result of the addition of a large excess of the protein. Gyp1-46 may remain bound in an inhibitory manner to Ypt7 in its conformation with bound GDP or without guanine nucleotide.
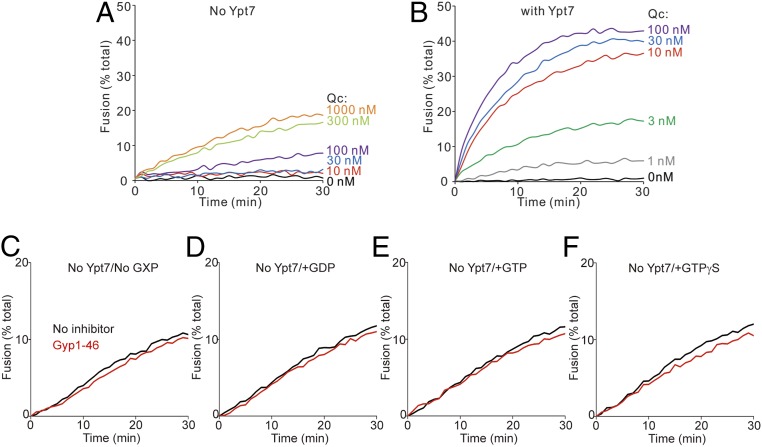
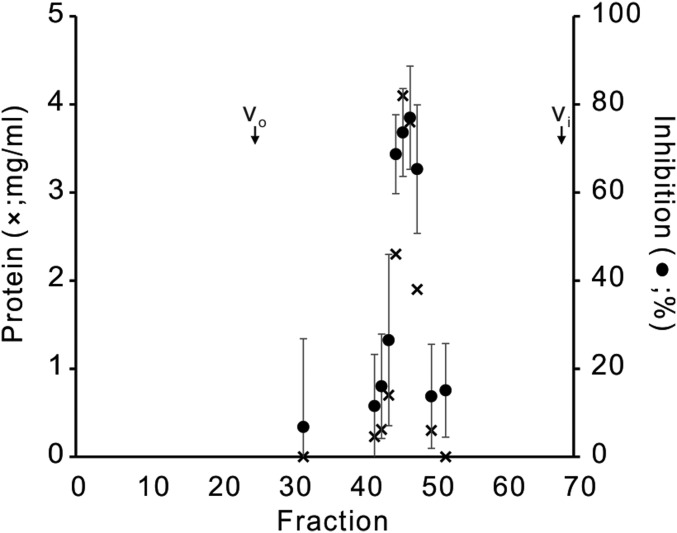
Some of these inhibitions by Gyp1-46 were unexpected. It is unclear how this GAP inhibits fusion with Ypt7-tm that had been charged with GTP (Fig. 1G) when the GDP-charged form of this Rab supports fusion (Fig. 1F), or how it inhibits when Ypt7-tm had been initially charged with GDP. We therefore tested whether this inhibition could be ascribed to the action of this protein on the Rab itself, or whether Gyp1-46, its buffer, or even contaminants might act on the SNAREs, HOPS, or the lipid rearrangements inherent to fusion. To test this, we exploited a limited Rab-bypass fusion. While fusion normally requires Ypt7, a limited bypass of the requirement for Ypt7 is seen at substantially higher levels of the Qc-SNARE (Fig. 2A) than required for fusion with Ypt7 (Fig. 2B). Fusion without Ypt7 at high Qc is independent of guanine nucleotide and is not inhibited by Gyp1-46 (Fig. 2 C–F); therefore, guanine nucleotide and Gyp1-46 regulate fusion through Ypt7. For these studies, Gyp1-46 had been overexpressed in Escherichia coli and affinity-purified to apparent homogeneity. To confirm that the inhibitory activity resided in this protein, a portion was further purified by gel filtration through Superdex 200, which resolves proteins the size of Gyp1-46 (47 kDa). The inhibitory activity cofiltered with the Gyp1-46 protein (Fig. 3). Together, these studies confirm that the novel sensitivity to Gyp1-46 of fusion with Ypt7-tm bearing GDP or without guanine nucleotide was a result of the effect of Gyp1-46 on Ypt7-tm in the fusion reaction. Our working model is that Gyp1-46 can remain bound to Ypt7-tm in its GDP or nucleotide-free conformation and inhibit fusion. Since only a small fraction of Ypt7 and SNAREs assemble into trans prefusion complexes, separating any Gyp1-46 associations with these complexes from Gyp1-46 binding to the bulk (free) Ypt7 will be challenging.
Fig. 2.
Gyp1-46 and guanine nucleotide regulate fusion through Ypt7. (A and B) Fusion reactions contained proteoliposomes bearing R- or QaQb-SNARE at 1:16,000 SNARE to lipid molar ratio. The proteoliposomes either lacked Ypt7 (A) or bore Ypt7-tm at a 1:8,000 protein to lipid molar ratio (B). R- and QaQb-proteoliposomes were charged with GTP, and content mixing was assayed in the presence of 107 nM of HOPS and the indicated concentrations of Qc. (C–F) Fusion reactions contained proteoliposomes without Ypt7, which bore R- or QaQb-SNARE at 1:16,000 SNARE to lipid molar ratio. R- and QaQb-proteoliposomes were mock-charged with No GXP, GDP, GTP, or GTPγS as indicated, and preincubated with either buffer (black line) or 4 μM Gyp1-46 (red line) for 10 min at 27 °C. Proteoliposomes were then mixed with 200 nM HOPS and 600 nM Qc, and content mixing was assayed by measuring FRET between luminal Cy5 and phycoerythrin for 30 min at 27 °C. Kinetic curves of content mixing assays in this figure are representative of n ≥ 3 experiments, see SI Appendix, Fig. S3.
Fig. 3.
Cofiltration of Gyp1-46 protein and fusion inhibition activity. Affinity-purified recombinant Gyp1-46 (1 mL, 138 μM; ref. 35) was applied to an 0.7 × 45 cm column of Superdex 200 (prep grade) in RB150 at 4 °C, eluted by gravity, and 70 fractions of 0.5 mL were collected. Each was assayed for protein by Bradford and, after fivefold dilution in RB150, for inhibition of fusion of proteoliposomes bearing Ypt7-tm charged with GTP.
Added Specificity through HOPS Phosphorylation.
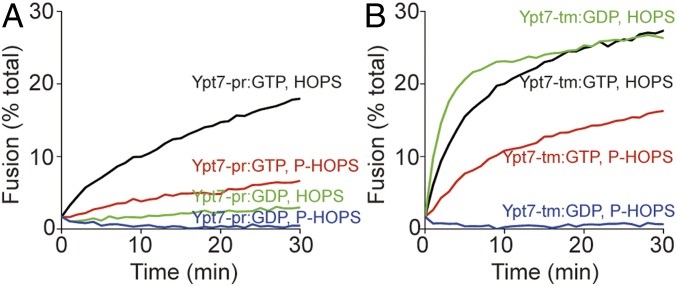
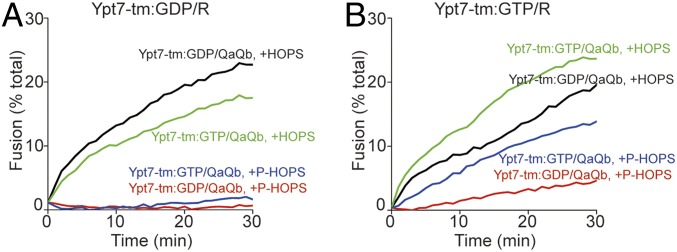
Some portion of HOPS is phosphorylated in vivo by the vacuolar kinase Yck3, conferring diminished fusion activity (16, 20). Prenyl-anchored Ypt7 charged with GTP was active with either HOPS or with purified (12) P-HOPS (Fig. 4A). Ypt7-tm charged with either GTP or GDP was active for fusion with HOPS, but P-HOPS only supported fusion when Ypt7-tm bore GTP (Fig. 4B, red curve), and not when it bore GDP (blue curve). Ypt7-tm thus supports fusion by HOPS or P-HOPS, but does not exhibit guanine nucleotide specificity with HOPS. Only prenyl-anchored Ypt7 shows guanine nucleotide specificity with either HOPS or P-HOPS.
Fig. 4.
Phosphorylated HOPS discriminates between GTP- and GDP-charged Ypt7-tm for proteoliposome fusion. HOPS and phospho-HOPS were purified as described (12). Fusion reactions contained proteoliposomes bearing R- or QaQb-SNARE at 1:16,000 SNARE to lipid molar ratio and either Ypt7-pr (A) or Ypt7-tm (B) at a 1:8,000 protein to lipid molar ratio. R- and QaQb-proteoliposomes were separately charged with GDP or GTP and preincubated for 10 min at 27 °C. R- and QaQb-proteoliposomes were then moved to same well, mixed with 100 nM Qc, and then mixed with either 50 nM HOPS (black and green lines) or 50 nM phospho-HOPS (P-HOPS; red and blue lines). The content mixing between proteoliposomes was assayed as the FRET between luminal Cy5 and phycoerythrin for 30 min at 27 °C. Kinetics in this figure are representative of n ≥ 3 experiments; see SI Appendix, Fig. S4.
Guanine Nucleotide Specificity of the Ypt7 on the R- or Q-SNARE Proteoliposomes.
HOPS-dependent fusion supported by prenyl-anchored Ypt7 requires that the Ypt7-pr on the R-SNARE proteoliposomes (Fig. 5 A–C) is charged with GTP (Fig. 5C), rather than with GDP or without guanine nucleotide (Fig. 5 A and B, respectively). When the R-SNARE proteoliposomal Ypt7-pr is charged with GTP, fusion is optimal with the QaQb-SNARE proteoliposomal Ypt7-pr bearing GTP as well (green curve, Fig. 5C), but is still seen when charged with GDP or no guanine nucleotide (red and black curves, Fig. 5C). In striking contrast, fusion with Ypt7 that is anchored by an apolar polypeptide segment (Fig. 5 D–F) is largely independent of whether the Ypt7-tm on the R- or QaQb-SNARE proteoliposomes bears GTP, GDP, or no guanine nucleotide.
Fig. 5.
Ypt7-pr on R-proteoliposomes must bear GTP for optimal fusion with HOPS. (A–F) Fusion reactions contained proteoliposomes bearing R- or QaQb-SNAREs at 1:16,000 SNARE to lipid molar ratio with 1:8,000 protein to lipid molar ratio of either Ypt7-pr (A–C) or Ypt7-tm (D–F). R-proteoliposomes were charged with either no GXP (A and D), GDP (B and E) or GTP (C and F) and QaQb-proteoliposomes were charged with No GXP (black lines), GDP (red lines), or GTP (green lines). Content mixing was assayed in the presence of 50 nM HOPS and 100 nM Qc by measuring FRET between luminal Cy5 and phycoerythrin for 30 min at 27 °C. Kinetic curves of content mixing assays in this figure are representative of n ≥ 3 experiments; see SI Appendix, Fig. S5.
With Ypt7-tm, HOPS supports comparable fusion, whether the proteoliposome with bound R-SNARE has Ypt7 GDP (Fig. 6A) or GTP (Fig. 6B), and whether the QaQb fusion partner proteoliposomes bear Ypt7-tm with bound GTP (green) or GDP (black). In contrast, with P-HOPS, there is little fusion unless the Ypt7-tm on the R-SNARE proteoliposome bears GTP, but when the Ypt7-tm on the R-SNARE proteoliposomes does bear GTP, there is only a modest preference for GTP on the Ypt7-tm of the QaQb proteoliposomes (red and blue curves, Fig. 6B).
Fig. 6.
Phospho-HOPS only supports fusion of proteoliposomes bearing Ypt7-tm when the R-proteoliposomes are charged with GTP. (A and B) Fusion reactions contained proteoliposomes bearing R- or QaQb-SNAREs at a 1:16,000 SNARE to lipid molar ratio and Ypt7-tm at a 1:8,000 protein to lipid molar ratio. R-proteoliposomes were charged with GDP (A) or GTP (B), and QaQb- proteoliposomes were charged with GDP (black, red lines) or GTP (green and blue lines), followed by preincubation for 10 min at 27 °C. R- and QaQb-proteoliposomes were then moved to the same well, mixed with 100 nM Qc and then with either 50 nM HOPS (black and green lines) or 50 nM phospho-HOPS (red and blue lines). Content mixing of proteoliposomes was assayed by measuring FRET between luminal Cy5 and phycoerythrin for 30 min at 27 °C. Kinetic curves of content mixing assays in this figure are representative of n ≥ 3 experiments.
Discussion
A central tenet of the membrane trafficking paradigm is that Rab GTPases serve as vital markers of organelle identity, active for binding effectors in their GTP-bound conformation and inactive in their GDP-bound state. Rab prenyl anchors affect their organelle targeting (31) and allow guanine nucleotide dissociation inhibitor protein to selectively extract GDP-bound Rabs (28, 32), followed by recycling to membranes as the GDP is exchanged for GTP. Our current studies are in full accord with this model. However, by contrasting the functions of prenyl and a transmembrane anchors, we have found a third role for the prenyl anchor, conferring guanine nucleotide-dependence on the capacity of its effector HOPS to support fusion (Fig. 1). Ypt7-tm, anchored by a recombinant transmembrane domain, does not require bound GTP to support its effector HOPS (Fig. 1 E–H). Ypt7-tm does, however, respond to bound guanine nucleotide, as its inhibition by the GAP Gyp1-46 is blocked by GTPγS (Fig. 1H) and Ypt7-tm requires GTP for fusion supported by P-HOPS (Fig. 4B).
A working model to encompass these findings is that both Ypt7-tm and Ypt7-pr cycle between a GTP-bound state with high affinity for effector and a GDP-bound state with lower effector affinities, but that the Ypt7-tm conformation is inherently more active. In addition, Yck3 kinase and unknown phosphatases cycle the effector HOPS between its higher binding affinity and/or activity state without phosphorylation and its more selective state of lower Rab affinity and fusion catalysis after phosphorylation to P-HOPS. This model is consistent with P-HOPS having a strict requirement for Rab activation by bound GTP, while HOPS only exhibits such selectivity when Ypt7 is prenyl-anchored. Test and refinement of this model will require measurement of the affinities of Ypt7-pr and Ypt7-tm for HOPS and P-HOPS in assembling transassembling prefusion intermediates (14) and assay of the activity of each combination for catalyzing SNARE complex assembly (14).
Our studies have also revealed an unexpected mode of fusion inhibition by the GAP Gyp1-46. GAPs trigger their target Rab to hydrolyze bound GTP, and the Rab with bound GDP is less active (Fig. 1B vs. Fig. 1C). This does not explain the inhibition by Gyp1-46 of the fusion mediated by Ypt7-tm and HOPS. Gyp1-46 is as potent an inhibitor of fusion when Ypt7-tm is without guanine nucleotide, or bears GDP, as when it bears GTP (Fig. 1 E–G). This mode of inhibition cannot be assessed with Ypt7-pr, as the GDP-bound and guanine nucleotide-free forms of Ypt7-pr are inactive (Fig. 1 A and B). Nonetheless, Ypt7-tm retains its capacity to bind guanine nucleotide: the inhibition by Gyp1-46 of fusion supported by Ypt7-tm and HOPS is blocked by GTPγS (Fig. 1H), and fusion supported by P-HOPS and Ypt7-tm is supported by GTP, but not by GDP (Fig. 4B). We suggest that Gyp1-46 activates GTP hydrolysis on Ypt7-tm:GTP, but in addition will bind to Ypt7-tm:GDP or nucleotide-free Ypt7, thereby inhibiting the capacity of Ypt7 to activate HOPS for fusion.
The conformation of Ypt7 may be a function of both its membrane anchor and its bound guanine nucleotide. Ypt7 functions as just one of several receptors for HOPS. HOPS has distinct affinities for each form of Ypt7, modulated by the membrane anchor and by the specific bound guanine nucleotide, but HOPS also has affinity for acidic lipids (15), for phosphoinositides (22), and for membrane-bound SNAREs (14). The combinatorial affinities of HOPS for vacuolar acidic lipids and for SNAREs can suffice for fusion at an artificially high SNARE concentration of 1:1,000 molar ratio to lipids with only Ypt7-pr:GDP, or even without Ypt7 at all (15). P-HOPS has far less affinity for lipids, and therefore more strictly requires Ypt7:GTP (15). At lower, more physiological SNARE levels (11), as employed in the current study, Ypt7 is required for rapid fusion, although a very slow HOPS-dependent fusion can still be detected without Ypt7 at high levels of HOPS and Qc (Fig. 2A). Each HOPS binding affinity of Ypt7-tm in its distinct guanine nucleotide states may, in conjunction with the inherent affinity of HOPS for vacuolar lipids, suffice for fusion, but when the lipid affinity is lost through HOPS phosphorylation, the discrimination of P-HOPS between the GTP and GDP activated forms of Ypt7-tm is revealed. The prenyl anchor of Ypt7, along with its bound nucleotide and the phosphorylation state of its HOPS effector, combine to regulate the rate of organelle fusion.
Experimental Procedures
Most lipids were obtained from Avanti. Ergosterol was purchased from Sigma, and phosphatidylinositol 3-monophosphate from Echelon. Cy5-streptavidin and biotinylated R-phycoerythrin were obtained from SeraCare and from Life Technologies. Nonfluorescent streptavidin was obtained from Thermo Fisher.
Protein Expression and Purification.
HOPS (33), vacuolar SNAREs (10, 26, 34), MBP-Ypt7-tm (14), GST-Ypt7-prenyl tail (33), and Gyp1-46 (35), were purified as described.
Proteoliposome Preparation.
Proteoliposomes were prepared as described (36), with minor modifications. Vacuolar mimic lipid proteoliposomes were prepared from solutions with 50 mM β-octyl glucoside (β-OG), with Ypt7-pr or Ypt7-tm (1:8,000 molar ratio to lipid) and SNAREs (1:16,000 molar ratio to lipid). Lipids in chloroform were mixed with β -OG as before (14). Lipids were mixed in glass vials, and chloroform was removed from samples under a stream of nitrogen for 30 min, followed by 3 h speedvac. The lipid/detergent pellet was solubilized in 400 μL of 2.5×-concentrated RB150 (20 mM Hepes-NaOH at pH 7.4, 150 mM NaCl, 10% [vol/vol] glycerol) by nutation for 2 h at room temperature. The detergent/lipid mixed micelle solution was then mixed with the indicated Rab proteins, SNAREs, TEV protease, and luminal fluorescent probes (Cy5-streptavidin or biotinylated phycoerythrin), and the mixture was dialyzed for 16 h at 4 °C in the dark against RB150 containing 1 mM MgCl2 and BioBeads SM2 (BioRad) with stirring. Proteoliposomes were isolated by flotation through Histodenz (Sigma) density medium as described (33), and assayed for total phosphate (37). Proteoliposomes were diluted with RB150+1 mM MgCl2 to a final concentration of 2 mM and then frozen in 30-μL aliquots in liquid nitrogen and stored at −80 °C.
Fusion Assay.
Fusion was measured by FRET from the mixed proteoliposomal fluorescent contents (26). Fusion incubations were assembled from premixes, (1) Ypt7/R-SNARE and (2) Ypt7/QaQb-SNARE proteoliposomes (0.25 mM lipid), which had been separately charged with guanine nucleotide by incubation (10 min, 27 °C) with 9.6 μM streptavidin, 2 mM EDTA, and 20 μM GxP, followed by addition of MgCl2 to 4 mM (3), Qc (1 μM) (4), HOPS (500 nM) in HOPS buffer (20 mM Hepes-NaOH at pH 7.4, 400 mM NaCl, 200 mM sorbitol, 10% [vol/vol] glycerol, 0.004% Triton X-100, 1 mM β-mercaptoethanol, 10 mM glutathione) (5), 10 mM MgCl2 in RB150 buffer (6), inhibitor test solutions (either RB150 buffer, a no-inhibitor control, or 20 μM Gyp1-46). Premixes were distributed into either PCR strips or in wells of a 384-well plate (Corning 4514) for rapid transfers by multichannel pipettes. The final amounts of these 6 solutions in 20 μL fusion incubations were (1) 5 μL, (2) 5 μL, (3) 2 μL, (4) 2 μL, (5) 2 μL, and (6) 4 μL.
The order of mixing was as follows: Nucleotide exchanged (1) Ypt7/R proteoliposomes and (2) Ypt7/QaQb-proteoliposomes were pipetted into separate wells of a 384-well plate, and each was mixed with half of (6) the inhibitor test solution. MgCl2 solution (5) was added to another row of wells. These were preincubated (10 min, 27 °C) in a Gemini XPS Molecular Devices plate reader. The (3) Qc and (4) HOPS solutions were distributed into separate PCR strips and placed in a 27 °C water bath. After preincubation, the plate was removed from the reader. Multichannel pipettes were used for successive transfer into heretofore empty assay wells of 2 μL of (5) MgCl2, 7 μL of the mixture of (1) Ypt7/R-proteoliposomes plus (6) inhibitor test solution described here, 7 μL of the mixture of (2) Ypt7/QaQb-proteoliposomes plus (6) inhibitor test solution, 2 μL of (3) Qc, and finally 2 μL of (4) HOPS, for a total of 20 μL. The wells contents were mixed, the plate was returned to the plate reader, and FRET was read at Ex: 565, Em: 670, CO: 630. In experiments without Gyp addition, the (1) Ypt7/R-proteoliposomes and (2) Ypt7/QaQb-proteoliposomes were preincubated without inhibitor test solution, and the first addition to the assay wells was 6 μL of (5) 3.33 mM MgCl2, followed by sequential 5-μL additions of nucleotide-exchanged (1) Ypt7/R-proteoliposomes and (2) Ypt7/QaQb-proteoliposomes, then 2 μL of (3) Qc, and finally 2 μL of (4) HOPS.
Complete content mixing levels were assayed by adding 15 μL of 0.14% (wt/vol) Thesit to samples containing 0.25 mM each of R- and QaQb-SNARE proteoliposomes lacking streptavidin.
For the fusion assays containing 4 μM Gyp1-46, Ypt7/R-proteoliposomes and Ypt7/QaQb-proteoliposomes that had been separately nucleotide exchanged were separately mixed with Gyp1-46 and prewarmed for 10 min at 27 °C. For the fusion assay without Ypt7 on the membrane, R- and QaQb-proteoliposomes were mixed together and then mock charged with GTP, GDP, or GTPγS and prewarmed together.
Data Availability.
All of the data are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank Gustav Lienhard for fruitful discussions and Amy Orr for expert assistance. This work was supported by NIH grant R35GM118037.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000923117/-/DCSupplemental.
References
- 1.Wickner W., Rizo J., A cascade of multiple proteins and lipids catalyzes membrane fusion. Mol. Biol. Cell 28, 707–711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizo J., Südhof T. C., The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices–Guilty as charged? Annu. Rev. Cell Dev. Biol. 28, 279–308 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Fasshauer D., Sutton R. B., Brunger A. T., Jahn R., Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. U.S.A. 95, 15781–15786 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker R. W., et al. , A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly. Science 349, 1111–1114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosshans B. L., Ortiz D., Novick P., Rabs and their effectors: Achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. U.S.A. 103, 11821–11827 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutagalung A. H., Novick P. J., Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada Y., Ohsumi Y., Anraku Y., Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J. Biol. Chem. 267, 18665–18670 (1992). [PubMed] [Google Scholar]
- 8.Nichols B. J., Ungermann C., Pelham H. R., Wickner W. T., Haas A., Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387, 199–202 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Wickner W., Membrane fusion: Five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu. Rev. Cell Dev. Biol. 26, 115–136 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Mima J., Hickey C. M., Xu H., Jun Y., Wickner W., Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 27, 2031–2042 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zick M., Wickner W., Improved reconstitution of yeast vacuole fusion with physiological SNARE concentrations reveals an asymmetric Rab(GTP) requirement. Mol. Biol. Cell 27, 2590–2597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickey C. M., Stroupe C., Wickner W., The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J. Biol. Chem. 284, 16118–16125 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lürick A., et al. , Multivalent Rab interactions determine tether-mediated membrane fusion. Mol. Biol. Cell 28, 322–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H., Orr A. S., Lee M., Harner M. E., Wickner W. T., HOPS recognizes each SNARE, assembling ternary trans-complexes for rapid fusion upon engagement with the 4th SNARE. eLife 9, e53559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr A., Wickner W., Rusin S. F., Kettenbach A. N., Zick M., Yeast vacuolar HOPS, regulated by its kinase, exploits affinities for acidic lipids and Rab:GTP for membrane binding and to catalyze tethering and fusion. Mol. Biol. Cell 26, 305–315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaGrassa T. J., Ungermann C., The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J. Cell Biol. 168, 401–414 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brett C. L., et al. , Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J. Cell Biol. 182, 1141–1151 (2008). Erratum in: J. Cell Biol.195, 1061 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera M., et al. , Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol. Biol. Cell 20, 1937–1948 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrera M., et al. , Phosphorylation of a membrane curvature-sensing motif switches function of the HOPS subunit Vps41 in membrane tethering. J. Cell Biol. 191, 845–859 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zick M., Wickner W., Phosphorylation of the effector complex HOPS by the vacuolar kinase Yck3p confers Rab nucleotide specificity for vacuole docking and fusion. Mol. Biol. Cell 23, 3429–3437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence G., et al. , Dynamic association of the PI3P-interacting Mon1-Ccz1 GEF with vacuoles is controlled through its phosphorylation by the type 1 casein kinase Yck3. Mol. Biol. Cell 25, 1608–1619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroupe C., Collins K. M., Fratti R. A., Wickner W., Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 25, 1579–1589 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiao J., et al. , Munc18-1 catalyzes neuronal SNARE assembly by templating SNARE association. eLife 7, 1–32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anant J. S., et al. , Mechanism of Rab geranylgeranylation: formation of the catalytic ternary complex. Biochemistry 37, 12559–12568 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Ossig R., Laufer W., Schmitt H. D., Gallwitz D., Functionality and specific membrane localization of transport GTPases carrying C-terminal membrane anchors of synaptobrevin-like proteins. EMBO J. 14, 3645–3653 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucchi P. C., Zick M., Membrane fusion catalyzed by a Rab, SNAREs, and SNARE chaperones is accompanied by enhanced permeability to small molecules and by lysis. Mol. Biol. Cell 22, 4635–4646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higashijima T., Ferguson K. M., Sternweis P. C., Smigel M. D., Gilman A. G., Effects of Mg2+ and the beta gamma-subunit complex on the interactions of guanine nucleotides with G proteins. J. Biol. Chem. 262, 762–766 (1987). [PubMed] [Google Scholar]
- 28.Araki S., Kikuchi A., Hata Y., Isomura M., Takai Y., Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J. Biol. Chem. 265, 13007–13015 (1990). [PubMed] [Google Scholar]
- 29.Haas A., Scheglmann D., Lazar T., Gallwitz D., Wickner W., The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 14, 5258–5270 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eitzen G., Will E., Gallwitz D., Haas A., Wickner W., Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J. 19, 6713–6720 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calero M., et al. , Dual prenylation is required for Rab protein localization and function. Mol. Biol. Cell 14, 1852–1867 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ullrich O., et al. , Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J. Biol. Chem. 268, 18143–18150 (1993). [PubMed] [Google Scholar]
- 33.Zick M., Wickner W., The tethering complex HOPS catalyzes assembly of the soluble SNARE Vam7 into fusogenic trans-SNARE complexes. Mol. Biol. Cell 24, 3746–3753 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz M. L., Merz A. J., Capture and release of partially zipped trans-SNARE complexes on intact organelles. J. Cell Biol. 185, 535–549 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rak A., et al. , Crystal structure of the GAP domain of Gyp1p: First insights into interaction with Ypt/Rab proteins. EMBO J. 19, 5105–5113 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zick M., Stroupe C., Orr A., Douville D., Wickner W. T., Membranes linked by trans-SNARE complexes require lipids prone to non-bilayer structure for progression to fusion. eLife 3, e01879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen P. S., Toribara T. Y., Warner H., Microdetermination of Phosphorus. Anal. Chem. 28, 1756–1758 (1956). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data are included in the manuscript and SI Appendix.