Significance
Atrial fibrillation (AF) is prevalent in diabetic patients, yet the basis for AF in diabetes is poorly understood. We have used type 1 diabetic Akita mice to study the effects of insulin on atrial electrophysiology in diabetes. We demonstrate that Akita mice are highly susceptible to AF due to impaired electrical conduction and that insulin treatment can reduce the occurrence of this arrhythmia. Atrial action potential morphology was altered in Akita mice in association with reductions in atrial Na+ current (INa) and repolarizing potassium current. Insulin treatment potently increased atrial INa via distinct chronic and acute effects. These experiments identify antiarrhythmic effects of insulin in type 1 diabetes via potent effects on atrial INa.
Keywords: atrial fibrillation, diabetes mellitus, phosphoinositide 3-kinase, action potential, Na+ current
Abstract
Atrial fibrillation (AF) is prevalent in diabetes mellitus (DM); however, the basis for this is unknown. This study investigated AF susceptibility and atrial electrophysiology in type 1 diabetic Akita mice using in vivo intracardiac electrophysiology, high-resolution optical mapping in atrial preparations, and patch clamping in isolated atrial myocytes. qPCR and western blotting were used to assess ion channel expression. Akita mice were highly susceptible to AF in association with increased P-wave duration and slowed atrial conduction velocity. In a second model of type 1 DM, mice treated with streptozotocin (STZ) showed a similar increase in susceptibility to AF. Chronic insulin treatment reduced susceptibility and duration of AF and shortened P-wave duration in Akita mice. Atrial action potential (AP) morphology was altered in Akita mice due to a reduction in upstroke velocity and increases in AP duration. In Akita mice, atrial Na+ current (INa) and repolarizing K+ current (IK) carried by voltage gated K+ (Kv1.5) channels were reduced. The reduction in INa occurred in association with reduced expression of SCN5a and voltage gated Na+ (NaV1.5) channels as well as a shift in INa activation kinetics. Insulin potently and selectively increased INa in Akita mice without affecting IK. Chronic insulin treatment increased INa in association with increased expression of NaV1.5. Acute insulin also increased INa, although to a smaller extent, due to enhanced insulin signaling via phosphatidylinositol 3,4,5-triphosphate (PIP3). Our study reveals a critical, selective role for insulin in regulating atrial INa, which impacts susceptibility to AF in type 1 DM.
Atrial fibrillation (AF), the most common sustained arrhythmia encountered clinically (1, 2), is highly prevalent in diabetes mellitus (DM) (3–6). AF increases the risk of stroke and death and has a substantial negative impact on quality of life. While AF and DM share some common risk factors, it is also clear that DM is an independent risk factor for the development of AF (7, 8). Consistent with this, a longer duration of untreated DM or poor glycemic control independently and significantly increases the risk of developing AF (5, 9). Furthermore, AF is clearly associated with a worse prognosis in diabetic patients, greatly increasing the risk of adverse cardiovascular events and all-cause mortality (3, 6). Despite these links, the mechanisms leading to a substrate for AF in DM are not understood, which likely contributes to the limited efficacy of current AF therapies.
Type 1 DM, which affects up to 10% of the global diabetic population (10), is characterized by a lack of insulin production due to the loss of pancreatic cells that results in hypoinsulinemia and hyperglycemia. Under normal conditions, insulin binds to its receptor and activates the phosphoinositide 3-kinase (PI3K) pathway (11, 12). Specifically, insulin activates PI3K, which phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to produce the second messenger phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 mediates many key effects of insulin, including in the heart (13, 14).
Electrical remodeling in the atria is known to be an important contributor to the creation of a substrate for AF (15, 16). At the cellular level, atrial action potential (AP) morphology is a major determinant of atrial electrophysiology. The upstroke velocity of the AP is determined by the Na+ current (INa), which is a major contributor to atrial conduction velocity (17, 18). Cardiac INa is primarily carried by voltage gated Na+ channels (NaV1.5), which are encoded by the SCN5a gene. Alterations in atrial INa and AP upstroke velocity can affect atrial conduction velocity and the likelihood of electrical reentry, thereby affecting susceptibility to AF (2, 15). Action potential duration (APD) and repolarization of the AP are affected by the balance between several inward and outward currents, including the L-type Ca2+ current (ICa,L) and the late Na+ current (INa,L), and the activity of a number of voltage gated K+ (Kv) channels, including, among others, the transient outward K+ current (Ito; carried by KV4.2/4.3 channels) and the ultrarapid delayed rectifier K+ current (IKur; an atrial-specific current carried by KV1.5 channels).
The goal of the present study was to investigate atrial electrophysiology and the basis for AF in type 1 DM. Our studies were conducted using the Akita mouse model of type 1 DM as well as wild-type mice injected with streptozotocin (STZ) (19, 20). Each of these is a well-validated model of type 1 DM (19). Our data demonstrate that Akita and STZ-injected mice are highly susceptible to induced AF. Furthermore, AF inducibility is reduced by insulin treatment in Akita mice. Our mechanistic studies show that AF in Akita mice is associated with alterations in atrial AP morphology and that insulin has distinct acute and chronic effects on AP morphology and INa in the atria.
Results
Susceptibility to AF in Type 1 Diabetic Mice.
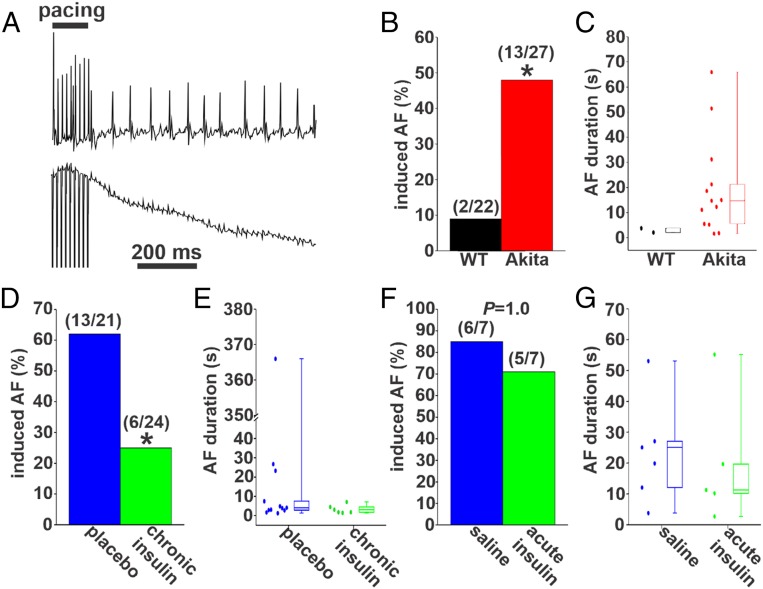
Burst pacing was used to assess susceptibility to AF (Fig. 1A) in anesthetized wild-type and Akita mice. Blood glucose was elevated in Akita mice compared with wild types (SI Appendix, Fig. S1A). Strikingly, Akita mice were highly susceptible to induced AF compared with wild-type mice (Fig. 1B). The episodes of AF induced in two wild-type mice were very brief, lasting less than 5 s (Fig. 1C). In contrast, AF induced in Akita mice was longer in duration. Specifically, 62% of the Akita mice had AF between 5 and 30 s, and 23% of Akita mice were induced into AF lasting longer than 30 s (Fig. 1C and SI Appendix, Table S1). To ensure that these observations were not specific to the Akita model of type 1 DM, we also investigated AF susceptibility in a second model of type 1 DM secondary to STZ injection in wild-type mice. STZ injection led to hyperglycemia as indicated by increases in blood glucose (SI Appendix, Fig. S2A). Furthermore, STZ-injected mice displayed similar increases in AF inducibility (SI Appendix, Fig. S2 B and C) and AF duration (SI Appendix, Fig. S2D and Table S2) to Akita mice. Together, these data illustrate that both mouse models of type 1 DM display increased susceptibility to AF.
Fig. 1.
AF in untreated Akita mice and Akita mice treated with insulin. (A) Representative surface (Upper) and intracardiac atrial (Lower) ECGs showing the induction of AF in an untreated anesthetized Akita mouse following burst pacing. (B) Inducibility of AF in untreated wild-type (WT) and Akita mice. Numbers in parentheses indicate the number of mice induced into AF following burst pacing. *P 0.05 by Fisher’s exact test. (C) Duration of AF in the WT (n = 2) and Akita (n = 13) mice that were induced into AF. (D) Inducibility of AF in Akita mice treated chronically with insulin or placebo for 4 wk. *P 0.05 vs. placebo by Fisher’s exact test. (E) Duration of AF in the placebo- (n = 13) and insulin-treated (n = 6) Akita mice that were induced into AF. (F) Inducibility of AF in Akita mice treated acutely with insulin or saline. Data were analyzed by Fisher’s exact test. (G) Summary data demonstrating the duration of AF in the saline- (n = 7) and insulin-treated (n = 5) Akita mice that were induced into AF. SI Appendix, Tables S1–S4 have additional AF analysis.
Next, we assessed the effects of chronic insulin treatment on AF burden in Akita mice. Insulin pellet implantation reduced blood glucose levels in Akita mice to levels that were similar to wild types, while placebo pellets had no effect on blood glucose in Akita mice (SI Appendix, Fig. S1B). Following chronic insulin treatment, only 25% of Akita mice were induced into AF compared with 62% of placebo-treated Akita mice (Fig. 1D). In addition, when AF was induced in insulin-treated Akita mice, the episodes were brief, lasting less than 5 s in 83% of the mice. No insulin-treated Akita mice had AF lasting longer than 30 s (Fig. 1E and SI Appendix, Table S3). In contrast, 50% of placebo-treated Akita mice demonstrated AF lasting longer than 5 s, and in some cases, it lasted longer than 30 s (Fig. 1E and SI Appendix, Table S3). These data demonstrate that chronic insulin treatment reduced both the susceptibility and the duration of AF in Akita mice.
We also examined the effects of acute insulin treatment on AF in Akita mice. Acute intraperitoneal injection of insulin (5 to 10 U/kg) reduced blood glucose in Akita mice to values similar to wild-type mice within 30 to 45 min, while saline-injected Akita mice were unaffected (SI Appendix, Fig. S1C). Acute insulin injection had no effect on inducibility of AF in Akita mice (Fig. 1F and SI Appendix, Table S4); however, the median AF duration was lower in Akita mice following acute insulin treatment (Fig. 1G).
Atrial Electrophysiology in Type 1 Diabetic Mice.
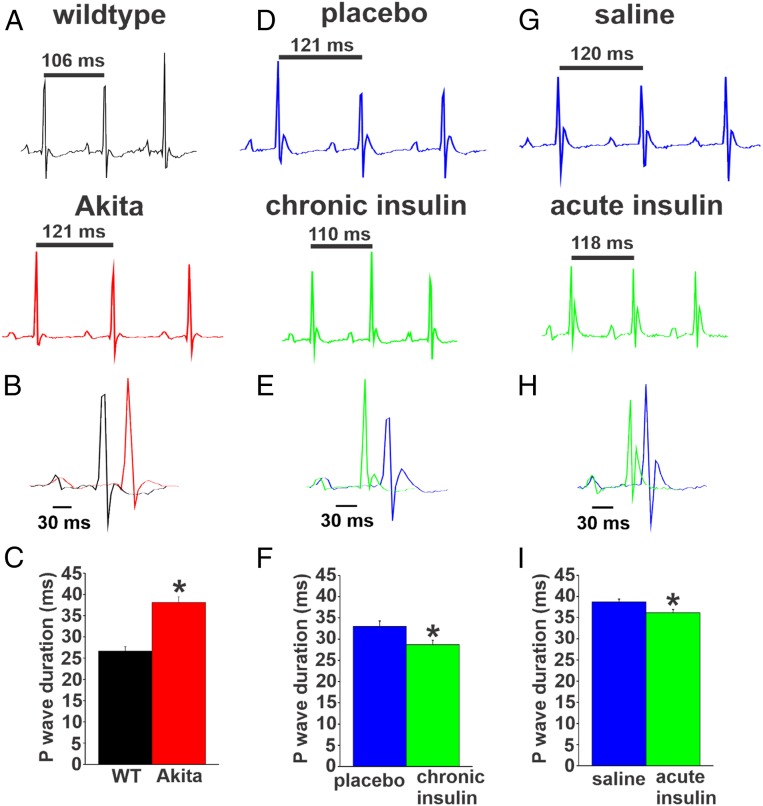
P-wave duration and P wave–R wave (PR) intervals were assessed in Akita mice and STZ-treated mice. Compared with wild types, Akita mice had prolonged P-wave duration (Fig. 2 A–C) and PR intervals (SI Appendix, Table S5). P-wave duration and PR interval were also prolonged in STZ-treated mice (SI Appendix, Fig. S2 E and F and Table S6). Chronic insulin treatment shortened P-wave duration (Fig. 2 D–F) and tended to reduce the PR interval in Akita mice compared with placebo controls (SI Appendix, Table S7). Acute insulin treatment also shortened P-wave duration in Akita mice (Fig. 2 G–I), although this effect was smaller in magnitude compared with chronic insulin treatment. These findings indicate that atrial conduction is impaired in type 1 diabetic mice and improved following chronic as well as acute insulin treatment.
Fig. 2.
P-wave duration in untreated Akita mice and Akita mice treated with insulin. (A) Representative surface ECGs from untreated wild-type (WT) and Akita mice. (B) Overlay of ECGs from WT and Akita mice. (C) Summary of P-wave duration in untreated WT and Akita mice. n = 7 WT mice and 10 Akita mice. *P 0.05 vs. WT by Student’s t test. (D) Representative surface ECGs from Akita mice treated chronically with insulin or placebo pellets for 4 wk. (E) Overlay of ECGs from Akita mice treated chronically with insulin or placebo pellets. (F) Summary of the effects of chronic insulin on P-wave duration in Akita mice. n = 21 placebo-treated mice and 22 insulin-treated mice. *P 0.05 vs. placebo by Student’s t test. (G) Representative surface ECGs from Akita mice treated acutely with insulin or saline by intraperitoneal injection. (H) Overlay of ECGs from Akita mice treated acutely with insulin. (I) Summary of the effects of acute insulin treatment on P-wave duration in Akita mice. n = 8 saline-treated mice and 7 insulin-treated mice. SI Appendix, Tables S5–S8 has additional ECG analysis. *P 0.05 vs. placebo by Student’s t test.
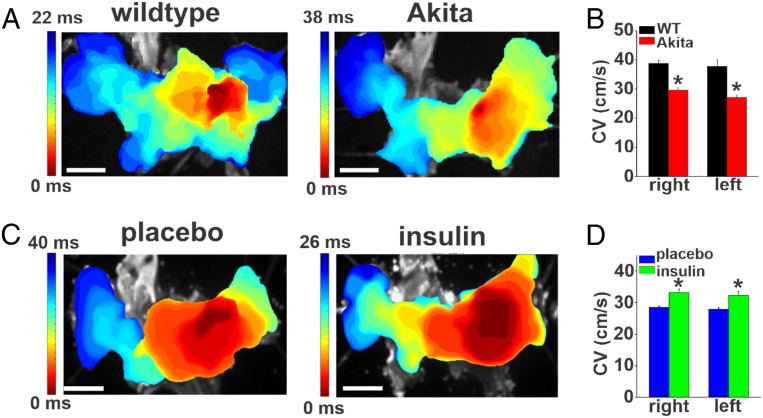
Patterns of atrial conduction in Akita mice were assessed using high-resolution optical mapping in isolated atrial preparations from wild-type and Akita mice. Representative activation maps (Fig. 3A) illustrate that conduction initiates in the right atrial posterior wall and then spreads across the full atrial preparation (21–23). These representative maps show that total conduction time across the full atrial preparation is longer in Akita mice. We also quantified local conduction velocities within the right and left atrial appendage regions. Right and left atrial conduction velocities were both reduced in Akita mice compared with the wild types (Fig. 3B), further confirming that atrial conduction is impaired in Akita mice. Consistent with the changes in P-wave duration measured in vivo, optical mapping studies also demonstrate that chronic insulin treatment in Akita mice resulted in faster conduction time across the atria (Fig. 3C) and increased right and left atrial conduction velocities (Fig. 3D).
Fig. 3.
Patterns of electrical conduction in the atria in untreated Akita mice and Akita mice treated chronically with insulin. (A) Representative activation maps in isolated atrial preparations from wild-type (WT) and untreated Akita mice. The right atrial appendage is on the right side of the image. Red indicates the earliest activation time. The color scale indicates total conduction time across the atrial preparation. (Scale bar: 3 mm.) (B) Summary of local right and left atrial conduction velocities (CV) (SI Appendix, Supplemental Methods) in WT and Akita mice. n = 7 WT and 8 Akita hearts. *P 0.05 vs. WT by two-way ANOVA with Tukey’s post hoc test. (C) Representative activation maps in atrial preparations from Akita mice treated with placebo or chronically with insulin for 4 wk. (D) Summary of local right and left atrial conduction velocities in placebo- and insulin-treated Akita mice. n = 6 placebo- and 5 insulin-treated Akita hearts. *P 0.05 vs. placebo by two-way ANOVA with Tukey’s post hoc test.
Echocardiographic assessment (SI Appendix, Fig. S3 and Table S9) demonstrates that Akita mice did not display substantial changes in ventricular structure or function, which is consistent with prior studies (24). Although maximum left atrial area was modestly increased in Akita mice, other measures of atrial size were similar between wild-type and Akita mice (SI Appendix, Fig. S3 and Table S9). Serum [Na+] and [Cl−] were modestly reduced, while serum [K+] and [Ca2+] were not altered in Akita mice compared with wild-type controls (SI Appendix, Fig. S4).
Alterations in Atrial Myocyte Electrophysiology in Akita Mice.
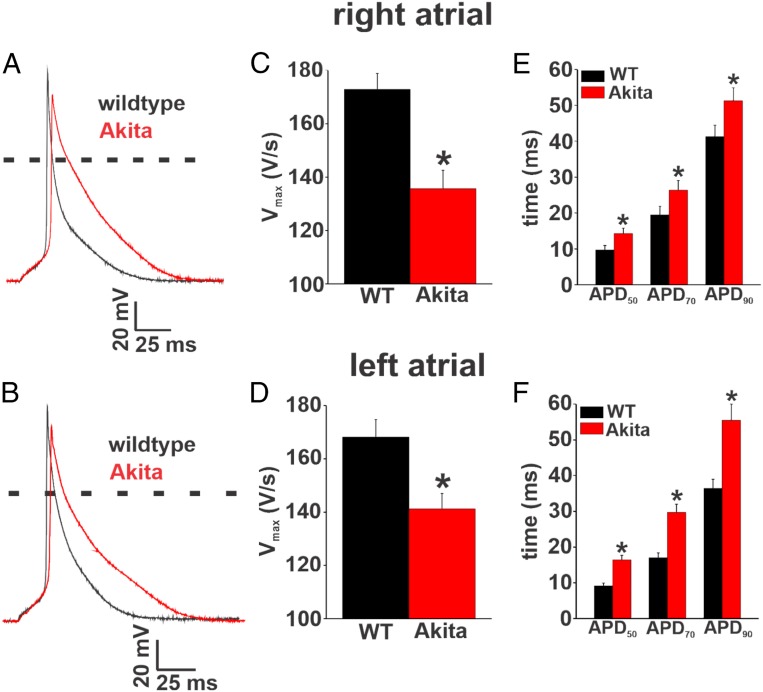
To further investigate the basis for increased AF and impaired atrial conduction in Akita mice, we measured APs in isolated right and left atrial myocytes (Fig. 4 A and B). Consistent with the absence of major changes in atrial size, there were no differences in cell capacitance in right atrial myocytes (50.6 5.1 vs. 52.9 7.3 pF; P = 0.84) or left atrial myocytes (54 4.1 vs. 58.8 5.8 pF; P = 0.50) between wild-type and Akita mice, indicating no differences in cell size. AP upstroke velocity (maximum upstroke velocity [Vmax]) was reduced in right and left atrial myocytes from Akita mice compared with wild-type controls (Fig. 4 C and D). Furthermore, APD was prolonged throughout repolarization in both right and left atrial myocytes from Akita mice (Fig. 4 E and F). These alterations in AP morphology occurred without changes in resting membrane potential (SI Appendix, Tables S10 and S11).
Fig. 4.
Right and left atrial AP morphology in Akita mice. (A and B) Representative stimulated APs in isolated right atrial (A) and left atrial (B) myocytes from wild-type (WT) and Akita mice. (C and D) Summary of AP Vmax in isolated right atrial (C) and left atrial (D) myocytes from WT and Akita mice. (E and F) Summary of APD in isolated right atrial (E) and left atrial (F) myocytes from WT and Akita mice. APD was measured at 50% (APD50), 70% (APD70), and 90% (APD90) repolarization. For right atrial myocytes, n = 18 for the WT and 16 for Akita. For left atrial myocytes, n = 12 for the WT and 11 for Akita. SI Appendix, Tables S10 and S11 has additional AP analysis. *P 0.05 vs. the WT by Student’s t test.
To ensure that the changes in APD observed in isolated cells were not a consequence of cell isolation procedures, we also measured APDs in wild-type and Akita mice using optical mapping (SI Appendix, Fig. S5). Optical APs measured in the right and left atria of intact atrial preparations confirm that APD was prolonged in Akita mice (SI Appendix, Fig. S5).
AP morphology was also assessed in isolated ventricular myocytes from wild-type and Akita mice (SI Appendix, Fig. S6 and Table S12). APD was prolonged in Akita ventricular myocytes; however, in contrast to atrial myocytes, there were no differences in AP Vmax in Akita ventricular myocytes.
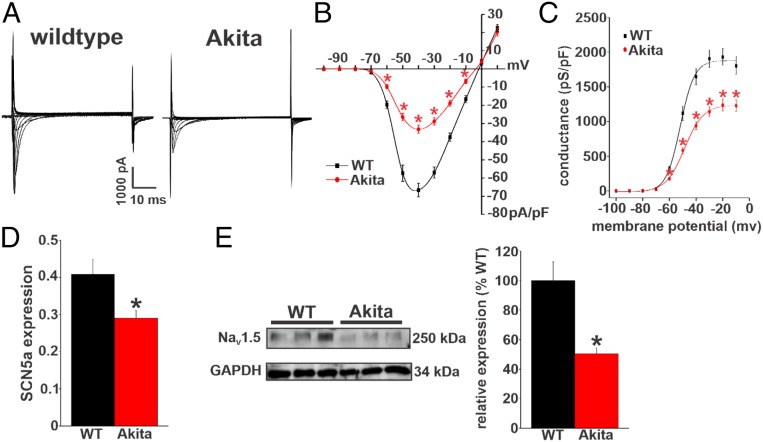
The alterations in atrial conduction (prolonged P-wave duration, reduced atrial conduction velocity) as well as the reduction in atrial AP Vmax in Akita mice are suggestive of changes in atrial INa. Accordingly, we measured INa in isolated right and left atrial myocytes from wild-type and Akita mice (Fig. 5A and SI Appendix, Fig. S7). Summary current–voltage (IV) curves for right atrial myocytes demonstrate that INa is reduced in Akita mice (Fig. 5B). INa activation curves (Fig. 5C) and analysis of INa activation kinetics (SI Appendix, Table S13) demonstrate that the reduction in INa density in Akita mice occurred in association with a reduction in maximum conductance (Gmax) and a modest (∼3-mV) rightward shift in the voltage dependence of activation [V1/2(act)]. Similar alterations in INa density, Gmax, and activation kinetics were observed in left atrial myocytes from Akita mice (SI Appendix, Fig. S7 and Table S13). We also measured steady-state inactivation in right atrial myocytes in Akita mice (SI Appendix, Fig. S8). Voltage dependence of inactivation was not altered in Akita right atrial myocytes.
Fig. 5.
INa is reduced in right atrial myocytes in Akita mice. (A) Representative INa recordings in right atrial myocytes isolated from wild-type (WT) and Akita mice. Cell capacitance for these representative recordings was 44 pF for the WT and 41 pF for Akita. (B) INa IV curves in right atrial myocytes isolated from WT and Akita mice. Current densities are measured in picoamperes/picofarad (pA/pF). (C) Summary INa activation curves in right atrial myocytes from WT and Akita mice. Conductance is measured in picosiemens/picofarad (pS/pF); n = 21 myocytes for the WT and 18 for Akita. INa activation kinetics are summarized in SI Appendix, Table S13. *P 0.05 vs. the WT at each membrane potential by two-way repeated measures ANOVA with Tukey’s post hoc test. (D) SCN5a mRNA expression in the right atrium in WT and Akita mice. n = 23 WT and 15 Akita mice. *P 0.05 vs. the WT by Student’s t test. (E) NaV1.5 protein expression in the right atrium in WT and Akita mice. n = 6 WT and 6 Akita mice. *P 0.05 vs. the WT by Student’s t test.
Because the reductions in INa density and Gmax were large relative to the shift in V1/2(act), we also measured the expression of SCN5a messenger ribonucleic acid (mRNA) as well as NaV1.5 protein in the atria of wild-type and Akita mice. These data show that expressions of SCN5a (Fig. 5D and SI Appendix, Fig. S7D) and NaV1.5 (Fig. 5E and SI Appendix, Fig. S7E) were reduced in the right and left atria of Akita mice compared with wild-type controls.
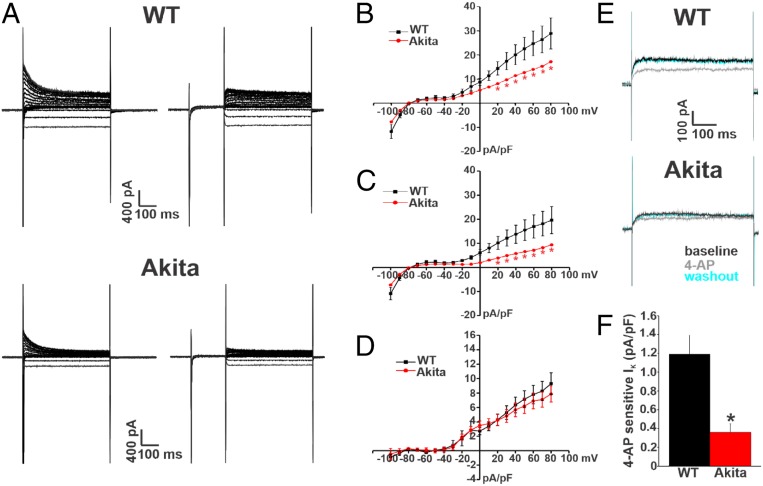
Next, we investigated the properties and expression of repolarizing K+ current (IK) in order to assess the ionic basis for the prolongation in atrial APD observed in Akita mice. We first measured IK in right atrial myocytes from wild-type and Akita mice. IK was measured with and without a prepulse to inactivate Ito (25, 26) (Fig. 6A and SI Appendix, Supplemental Methods). Summary IK IV relationships illustrate that peak total (i.e., no prepulse) IK was reduced in Akita mice at membrane potentials positive to +20 mV (Fig. 6B). Consistent with the lack of differences in resting membrane potential, there were no differences in IK in the region of the IV curves where inward rectifier K+ current (IK1) is active (i.e., negative to −80 mV) between wild-type and Akita mice. IV curves for peak IK measured from the protocols with an inactivating prepulse demonstrate that IK remained reduced in Akita mice at membrane potentials positive to +20 mV (Fig. 6C) when Ito is inactivated. In agreement with these observations, IV relationships for Ito (i.e., the difference current between measurements with and without the inactivating prepulse) illustrate no differences between wild-type and Akita mice (Fig. 6D). Very similar observations were made for IK in left atrial myocytes from wild-type and Akita mice (SI Appendix, Fig. S9). Further evidence that the reduction in repolarizing IK in Akita mice does not involve Ito was obtained from western blot studies, which show no differences in the protein levels of KV4.2 and KV4.3 in right and left atria from wild-type and Akita mice (SI Appendix, Fig. S10).
Fig. 6.
Repolarizing IK is reduced in right atrial myocytes in Akita mice. (A) Representative IK recordings in right atrial myocytes isolated from wild-type (WT) and Akita mice. The recordings on the left represent total IK measured between −100 and +80 mV. The recordings on the right represent IK measured between −100 and +80 mV following a prepulse to −40 mV to inactivate Ito. Cell capacitances for these representative recordings were 33 pF for the WT and 38 pF for Akita. (B) Summary IK IV curves measured at the peak of the IK recordings without the prepulse (recordings on the left in A) for right atrial myocytes isolated from WT and Akita mice. (C) Summary IK IV curves measured at the peak of the IK recordings with the prepulse (recordings on the right in A) for right atrial myocytes isolated from WT and Akita mice. *P 0.05 vs. the WT at each membrane potential by two-way repeated measures ANOVA with Tukey’s post hoc test. (D) Summary IK IV curves for the difference current between B and C, which is a measure of Ito. P = 0.85 for Ito density between the WT and Akita by two-way repeated measures ANOVA. For B–D, n = 19 WT and 16 Akita right atrial myocytes. (E) Representative IK recordings at +30 mV illustrating the effects of 4-AP (100 M), which inhibits Kv1.5-mediated IK, in right atrial myocytes from WT and Akita mice. (F) Summary data illustrating the amplitude of the 4-AP–sensitive IK in right atrial myocytes from WT and Akita mice. n = 9 WT and 10 Akita right atrial myocytes. *P 0.05 vs. the WT by Student’s t test. Current densities are measured in picoamperes/picofarad (pA/pF).
The IKur carried by KV1.5 channels was investigated by measuring the component of IK sensitive to 4-aminopyridine (4-AP) (100 M) (Fig. 6E) (26, 27) in right atrial myocytes from wild-type and Akita mice. From these representative recordings, it is apparent that outward IK is smaller at baseline and that the reduction in IK elicited by 4-AP was smaller in Akita mice. These recordings also demonstrate that the effects of 4-AP were reversible on washout. Summary data confirm that the 4-AP–sensitive current (i.e., KV1.5-mediated IKur) is reduced in Akita mice (Fig. 6F). The expression of the KCNA5 gene (which encodes KV1.5) was not different in Akita mice (SI Appendix, Fig. S11A); however, in agreement with the reduction in current, KV1.5 protein in Akita right atrium was reduced compared with wild-type controls (SI Appendix, Fig. S11B).
APD can also be affected by other currents, such as ICa,L and INa,L, which were both assessed in Akita right atrial myocytes. ICa,L (SI Appendix, Fig. S12) and INa,L (SI Appendix, Fig. S13) were not different between wild-type and Akita mice. There were also no differences in atrial calcium transient morphology between wild-type and Akita mice (SI Appendix, Fig. S14). As atrial electrical function is also affected by connexins, we measured the expression of GJA5 and GJA1 mRNAs, which encode Cx40 and Cx43, in the right atrium. GJA5 and GJA1 expressions were not altered in Akita mice (SI Appendix, Fig. S15).
Increases in APD could lead to the occurrence of triggered activity in the form of early afterdepolarizations (EADs). To assess this possibility, APs were measured at different pacing frequencies at baseline and in the presence of isoproterenol (1 M). No EADs were observed in wild-type or Akita right atrial myocytes in these studies (SI Appendix, Fig. S16).
Effects of Chronic and Acute Insulin Treatment on Atrial Myocyte Electrophysiology in Akita Mice.
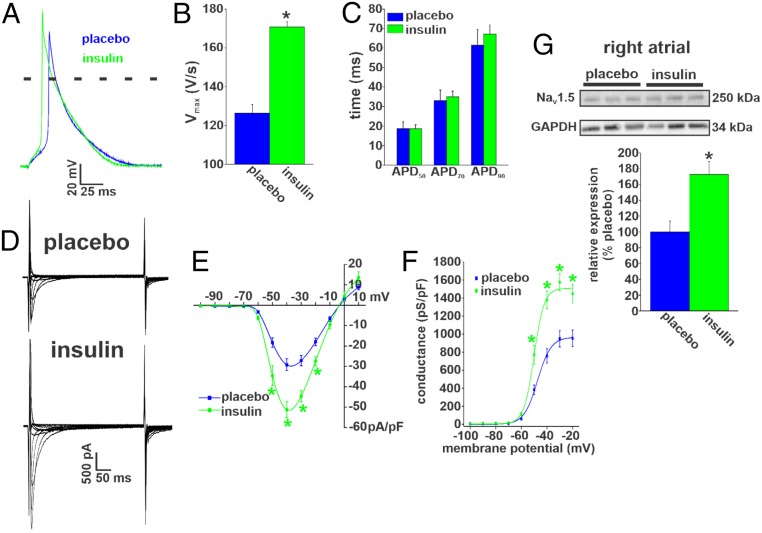
To investigate the basis for the beneficial effects of insulin on AF susceptibility and atrial conduction, we next examined the effects of chronic insulin treatment on atrial myocyte AP morphology (Fig. 7A). AP Vmax was substantially increased following insulin treatment in Akita mice (Fig. 7B). In contrast, insulin treatment had no effect on action potential duration measured at 50% repolarization (APD50), action potential duration measured at 70% repolarization (APD70), or action potential duration measured at 90% repolarization (APD90) compared with placebo controls (Fig. 7C and SI Appendix, Table S14) so that APD remained prolonged in treated Akita mice (compare with wild-type values in Fig. 4).
Fig. 7.
Effects of chronic insulin treatment on atrial AP morphology and INa in Akita mice. (A) Representative right atrial APs in Akita mice treated with insulin or placebo for 4 wk. (B) Summary of the effects of chronic insulin on right atrial AP Vmax in Akita mice. *P 0.05 vs. placebo by Student’s t test. (C) Summary of the effects of chronic insulin on right atrial APD in Akita mice. Chronic insulin had no effect on APD compared with placebo. For AP measurements, n = 19 myocytes for placebo and 14 for chronic insulin treatment. SI Appendix, Table S14 has additional analysis of AP morphology. (D) Representative INa recordings in right atrial myocytes isolated from Akita mice treated chronically with insulin or placebo for 4 wk. Cell capacitances for these representative recordings were 31 pF for the wild type and 30 pF for Akita. (E) Summary INa IV curves for right atrial myocytes from Akita mice treated chronically with insulin or placebo. Current densities are measured in picoamperes/picofarad (pA/pF). (F) Summary INa activation curves for right atrial myocytes isolated from Akita mice treated chronically with insulin or placebo. n = 18 myocytes for placebo and 13 myocytes for chronic insulin treatment. SI Appendix, Table S13 has additional INa kinetic analysis. *P 0.05 vs. placebo by two-way repeated measures ANOVA with Tukey’s post hoc test. Conductance is measured in picosiemens/picofarad (pS/pF). (G) NaV1.5 protein expression in the right atrium of Akita mice treated chronically with insulin or placebo. n = 6 placebo-treated and 6 insulin-treated right atria. *P 0.05 vs. placebo by Student’s t test.
Next, we measured right atrial INa in Akita mice treated chronically with insulin or placebo (Fig. 7D). Summary IV curves demonstrate that atrial INa was increased in Akita mice following chronic insulin treatment (Fig. 7E). Furthermore, INa activation curves (Fig. 7F) and kinetic analysis (SI Appendix, Table S13) demonstrate that chronic insulin increased INa in association with an increase in Gmax, but there were no differences in V1/2(act) or slope factor. Western blot studies (Fig. 7G) illustrate that chronic insulin treatment increased the levels of NaV1.5 in the atria in Akita mice.
Consistent with the lack of effect of insulin on atrial APD, insulin treatment had no effect on IK (with or without an inactivating prepulse) in Akita mice (SI Appendix, Fig. S17). Furthermore, chronic insulin treatment did not affect KV1.5 protein levels in Akita mice compared with placebo-treated controls (SI Appendix, Fig. S18).
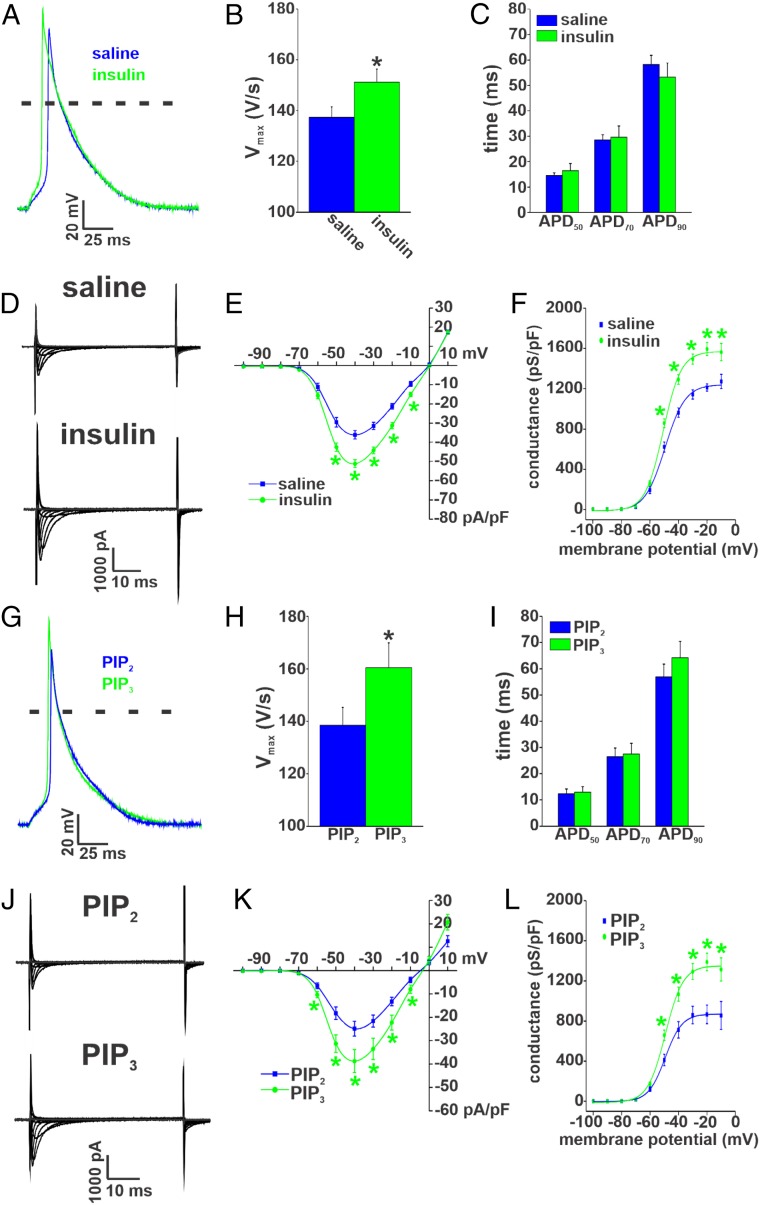
We next sought to assess the effects of enhancing insulin signaling acutely (i.e., intraperitoneal injection of insulin) in Akita mice. Immediately following the reduction in blood glucose from acute insulin injection, right atrial myocytes were isolated, and AP morphology was assessed (Fig. 8A). Summary data illustrate that AP Vmax was increased by acute insulin injection in Akita mice (Fig. 8B) but that APD50, APD70, and APD90 were unaffected compared with saline controls (Fig. 8C and SI Appendix, Table S15). Notably, the increase in Vmax was smaller after acute insulin compared with chronic insulin (SI Appendix, Fig. S19A).
Fig. 8.
Effects of acute insulin treatment and PIP3 on atrial AP morphology and INa in Akita mice. (A) Representative right atrial APs in Akita mice treated acutely with insulin or saline. (B) Summary of the effects of acute insulin on right atrial AP Vmax in Akita mice. *P 0.05 vs. saline by Student’s t test. (C) Summary of the effects of acute insulin on right atrial APD in Akita mice. Acute insulin had no effect on right atrial APD compared with saline. For AP measurements, n = 14 myocytes for saline and 18 myocytes for acute insulin treatment. SI Appendix, Table S15 has additional analysis of AP morphology. (D) Representative INa recordings in right atrial myocytes isolated from Akita mice treated acutely with insulin or saline. Cell capacitances for these representative recordings were 42 pF for the wild type and 45 pF for Akita. (E) Summary INa IV curves for right atrial myocytes from Akita mice treated acutely with insulin or saline. Current densities are measured in picoamperes/picofarad (pA/pF). (F) Summary INa activation curves for right atrial myocytes isolated from Akita mice treated acutely with insulin or saline. n = 8 myocytes for saline and 12 myocytes for acute insulin treatment. SI Appendix, Table S13 has additional analysis of INa activation kinetics. *P 0.05 vs. saline at each membrane potential by two-way repeated measures ANOVA with Tukey’s post hoc test. Conductance is measured in picosiemens/picofarad (pS/pF). (G) Representative APs in right atrial myocytes from Akita mice following dialysis with PIP3 (1 M) or PIP2 (1 M) as a control. Data are from cells dialyzed with phospholipids for 10 min. (H) Summary of the effects of PIP3 on right atrial AP Vmax in Akita mice. *P 0.05 vs. PIP2 by Student’s t test. (I) Summary of the effects of PIP3 on right atrial APD in Akita mice. PIP3 had no effect on right atrial APD. For AP measurements, n = 7 myocytes for PIP2 and 8 myocytes for PIP3. SI Appendix, Table S16 has additional analysis of AP morphology. (J) Representative INa recordings in right atrial myocytes from Akita mice following dialysis with PIP3 (1 M) or PIP2 (1 M) for 10 min. Cell capacitances for these representative recordings were 39 pF for the wild type and 38 pF for Akita. (K) Summary INa IV curves for right atrial myocytes from Akita mice following dialysis with PIP3 or PIP2. (L) Summary INa activation curves for right atrial myocytes from Akita mice following dialysis with PIP3 or PIP2. n = 10 myocytes for PIP2 and 17 myocytes for PIP3. SI Appendix, Table S13 has additional analysis of INa activation kinetics. *P 0.05 vs. PIP2 by two-way repeated measures ANOVA with Tukey’s post hoc test.
Next, we measured INa in Akita right atrial myocytes isolated after acute insulin (or saline) injection (Fig. 8D). INa density was increased following acute insulin injection (Fig. 8E). This increase occurred in association with an increase in Gmax, but there were no changes in V1/2(act) or slope factor (Fig. 8F and SI Appendix, Table S13) compared with saline controls. The increase in INa elicited by acute insulin was smaller than after chronic insulin (SI Appendix, Fig. S19B). Acute insulin injection had no effects on IK in Akita atrial myocytes (SI Appendix, Fig. S20).
The role of acute insulin signaling was further investigated by dialyzing right atrial myocytes from Akita mice with PIP3 [a direct mediator of insulin signaling downstream of the insulin receptor and PI3K (12, 14)] or PIP2 (not a second messenger directly activated by insulin) as a control. Cells were dialyzed with these phospholipids (1 M) for 10 min, and then, atrial AP morphology was assessed (Fig. 8G). Summary data illustrate that PIP3 increased Vmax (Fig. 8H) to a similar extent as acute insulin (SI Appendix, Fig. S19A) but had no effects on APD (Fig. 8I and SI Appendix, Table S16) compared with PIP2-treated myocytes. Akita myocytes dialyzed with PIP2 were very similar to untreated Akita atrial myocytes (compare with Fig. 4).
Consistent with its effects on Vmax, PIP3 also increased atrial INa density in comparison with cells dialyzed with PIP2 (Fig. 8 J and K). This increase in INa was also similar to that seen following acute insulin treatment (SI Appendix, Fig. S19B). In addition, PIP3 increased INa Gmax without affecting V1/2(act) or slope factor in Akita atrial myocytes (Fig. 8L and SI Appendix, Table S13). PIP3 had no effect on IK in Akita atrial myocytes (SI Appendix, Fig. S21). Collectively, these data demonstrate that acutely enhancing insulin signaling selectively increases AP Vmax and INa density in Akita atrial myocytes via PIP3 signaling without affecting APD or repolarizing IK.
Protein Kinase C Inhibition Increases INa in Akita Atrial Myocytes.
The data above demonstrate that enhancing insulin signaling increased atrial INa in Akita mice. These effects of insulin occurred without changes in V1/2(act); however, our data also demonstrate that the reduction in INa in Akita mice is associated with a modest but significant shift in V1/2(act) (SI Appendix, Table S13). It has been shown that protein kinase C (PKC) is elevated in the hearts of Akita mice (28), and PKC is a well-known modulator of the biophysical properties of INa (29). Accordingly, we tested the hypothesis that elevated PKC contributes to the reduction in atrial INa by dialyzing right atrial myocytes from Akita mice with the PKC inhibitor bisindolylmaleimide 1 (30) (BIM1; 1 M) (SI Appendix, Fig. S22). Compared with untreated Akita atrial myocytes, BIM1 increased INa density in Akita atrial myocytes (SI Appendix, Fig. S22B). Furthermore, INa activation curves (SI Appendix, Fig. S22C) and kinetic analysis (SI Appendix, Table S13) illustrate that BIM1 application increased INa Gmax and elicited a left shift in the V1/2(act). BIM1 also tended to reduce the slope factor on the activation curve (SI Appendix, Table S13). These findings indicate that enhanced PKC activity in the atria of Akita mice contributes to the reduction in INa in association with a shift in INa activation kinetics.
Discussion
The present study demonstrates that, consistent with the human diabetic population (3, 5, 7), Akita and STZ-injected mice are highly susceptible to induced AF and that, when it occurred, AF was longer lasting in type 1 diabetic mice compared with wild-type controls. The increase in AF burden was associated with a prolongation of P-wave duration in Akita and STZ-injected mice, indicating that AF in type 1 DM is associated with impaired atrial electrical conduction. Akita and STZ-injected mice are well-established models of nonobese type 1 DM that are known to recapitulate many of the complications that occur in human DM (19, 31). Our observation that Akita and STZ mice also display increased susceptibility to AF establishes that these mice are excellent models of human type 1 DM that can be used for studying the relationships between DM and AF.
In further support of atrial electrical remodeling, we found that atrial AP morphology was substantially altered in Akita mice due to a reduction in Vmax and increases in APD in association with reductions in atrial INa and IKur. While APD was also prolonged in ventricular myocytes in Akita mice, ventricular Vmax was not significantly altered, suggesting that the reduction in Vmax (and hence, the underlying INa) in Akita mice may be restricted to the atria. On the other hand, a previous study did report a reduction in ventricular INa in type 1 diabetic rabbits (alloxan induced) (32). A prior study also demonstrated that ventricular APD is prolonged in Akita mice due, at least in part, to an increase in INa,L (33), whereas we did not observe any changes in INa,L in Akita atrial myocytes. Prior studies have also demonstrated that ICa,L is reduced in ventricular myocytes in Akita mice (34) and PI3K knockout mice (35) (an effect that would presumably lead to APD shortening), which is different from our observations in Akita atrial myocytes. Collectively, our study demonstrates that the changes in AP morphology (especially Vmax) and underlying ionic currents are different in the atria and ventricles in Akita mice and provides important insight into the distinct pattern of ion channel remodeling that occurs in the atria in Akita mice. Further work will be required to determine the basis for differences in atrial and ventricular electrophysiology in type 1 DM.
Chronic insulin treatment substantially reduced AF inducibility and burden and prevented changes in P-wave duration in Akita mice. Furthermore, acute application of insulin reduced the median duration of AF and shortened atrial P-wave duration. These findings suggest an important role for insulin signaling in the pathophysiology of AF in type 1 DM and indicate that this involves atrial electrical remodeling via both acute and chronic effects. In agreement with this, our studies also reveal a previously unknown role for insulin in regulating atrial INa in type 1 DM. We found that a major determinant of reduced INa in the atria of Akita mice was the reduction in atrial expression of SCN5a and NaV1.5. Chronic insulin treatment prevented the declines in atrial INa density and NaV1.5 protein levels, indicating that insulin plays an important role in the de novo synthesis of new NaV1.5 channels due to changes in gene expression. Consistent with this, previous studies have shown that activation of PI3K up-regulates the expression of cardiac Na+ channels (36–38).
Importantly, our investigations revealed additional effects of insulin that could be activated acutely (i.e., on the order of minutes). Specifically, acute insulin injection immediately prior to cell isolation or dialysis of atrial myocytes with PIP3 each increased atrial INa by ∼30%. Consistent with these results, acute insulin also shortened P-wave duration. As these effects were rapid, we infer that they do not involve changes in gene or protein expression. Rather, these results demonstrate that an important component of the effects of insulin are mediated by the acute activation of PIP3 signaling via PI3K.
In addition to the effects of insulin on atrial INa, we found that inhibition of PKC also increases atrial INa (∼38%) in association with a shift in V1/2(act) to values very similar to those measured in wild-type mice. This indicates that PKC activity is increased in the atria in Akita mice and that this can account for the change in INa activation kinetics. Consistent with this, PKC is known to be an important regulator of INa (29, 39), and previous studies have shown that PKC expression is elevated in the heart in Akita mice (28). Thus, our studies demonstrate that atrial INa is reduced in Akita mice in association with both the loss of insulin signaling and enhanced PKC activity. The reductions in INa and AP Vmax in the atria in Akita mice are likely major contributors to the increased susceptibility to AF in type 1 DM. These alterations can explain the impairments in atrial conduction and would be expected to be proarrhythmic by decreasing the wavelength of reentry (15, 40). This would favor of the occurrence and maintenance of AF and is consistent with AF being longer lasting in Akita mice.
Neither insulin or PIP3 had any effect on the reduction in repolarizing IK in Akita atrial myocytes, and APD remained prolonged. A reduction in KV1.5 is known to contribute to atrial electrical remodeling in human chronic AF (41), and our findings indicate this may be the case in Akita diabetic mice as well. Prolongation of the APD in Akita atrial myocytes could contribute to the increased susceptibility to AF by increasing the likelihood of triggered activity in the form of EADs (15); however, we did not observe EADs in our studies, suggesting that this is not a major determinant of AF in Akita mice. Consistent with this, our data demonstrate that chronic insulin treatment reduced AF susceptibility and severity in association with improved atrial conduction, Vmax, and INa, suggesting that these are critical for decreasing the inducibility of AF despite the lack of improvement in IK or APD. Nevertheless, it is also possible that AP prolongation could contribute to impairments in atrial conduction in Akita mice either by contributing to the slowing of conduction at fast atrial rates or increasing the likelihood of conduction block. Additional studies will be required to determine how impaired atrial repolarization contributes to the slowing of atrial conduction and whether preventing AP prolongation can also reduce the susceptibility to AF in type 1 DM.
In addition to electrical remodeling, DM is also associated with structural remodeling due to atrial fibrosis. This has been demonstrated in human DM and in animal models (42–46). Indeed, we have previously demonstrated enhanced atrial fibrosis in the right and left atria in Akita mice (31). Fibrosis can also contribute to a substrate for AF since increased collagen deposition can impede atrial conduction and cause fragmentation of propagating wave fronts, thereby leading to reentry (15, 47–49). We have previously demonstrated that chronic insulin treatment reduces atrial fibrosis in Akita mice (31), and similar findings have been reported in STZ-injected rats treated with insulin (50). Thus, our prior work in combination with our present study demonstrates that insulin can prevent two alterations that are recognized as key contributors to impaired atrial conduction and maintenance of AF—the reduction in Vmax due to reduced INa as well as increased atrial fibrosis—highlighting a previously unappreciated and important role for insulin as an antiarrhythmic agent for AF in type 1 DM. Consistent with these observations, it has been previously demonstrated that enhancing PI3K (activated downstream of the insulin receptor) can mitigate ventricular electrical remodeling in hypertrophy and heart failure (51, 52) and that reduced PI3K activity increases susceptibility to AF (53). Our data suggest that insulin must be delivered for timeframes that are adequate to increase the expression of NaV1.5 and reduce fibrosis in order to maximize its protective effects on atrial electrical and structural remodeling in type 1 DM. While the acute activation of insulin signaling (PI3K/PIP3 dependent) does increase atrial INa, and partially prevents P-wave prolongation, this was not sufficient by itself to fully reduce the AF burden or the prolongation in P-wave duration in vivo.
Some limitations of our study should be acknowledged. Our study was conducted in mice, which exhibit differences in the relative importance of some repolarizing IK compared with humans. Specifically, the rapid and slow delayed rectifier K+ currents (IKr and IKs), which are important in the human heart, do not play a major functional role in the mouse heart (18). Prior studies have shown alterations in some of these K+ currents in canine models of DM (54). Nevertheless, our study shows that IKur (carried by KV1.5 channels) is a major IK that was altered in the atria in Akita mice. KV1.5 is an atrial-specific IK in the human heart (18, 55). It will be insightful to explore the alterations that we have identified in large animal models, tissue samples from human diabetic patients, or possibly, stem cell-derived atrial myocytes. While our study has focused on key ion channels in the atria in type 1 DM, it is possible that there are other alterations in gene and protein expression that are sensitive to insulin regulation. This could be explored in future studies using RNA sequencing. While we did not observe any changes in the mRNA levels of atrial connexins, it is possible that other aspects of connexin function or regulation could be altered in the atria in type 1 diabetes. Similarly, we did not observe changes in atrial calcium transient morphology in Akita mice; however, other aspects of calcium homeostasis could be altered in type 1 diabetes, which could potentially play a role in triggering AF in diabetes: for example, by delayed afterdepolarizations. Based on our findings, it will be important to conduct further studies to examine the links between glycemic control, atrial conduction patterns, and AF occurrence in human diabetic patients. Finally, it will also be important to explore the effects of insulin or of enhancing insulin signaling via PI3K and PIP3 in type 2 DM to determine if similar alterations and effects are present in this condition.
In conclusion, our study provides mechanistic insight into the cellular and molecular basis for AF in type 1 DM. This work demonstrates that AF in this setting is associated with electrical remodeling and changes in AP morphology due to reductions in atrial INa and KV1.5-mediated repolarizing IK. Furthermore, we have discovered that the reduction in INa is multifactorial, involving distinct acute and chronic effects of insulin as well as enhanced PKC activity. Finally, we show that insulin treatment is antiarrhythmic in type 1 DM via effects on atrial Na+ channels but not K+ channels. These data indicate that AF in type 1 DM has distinct features associated with the loss of normal insulin signaling. These findings have important implications for the treatment of AF in type 1 diabetic patients.
Materials and Methods
An expanded methods section is available in SI Appendix.
Mice.
This study used male littermate wild-type and type 1 diabetic Akita mice between the ages of 16 and 22 wk. In some instances, male wild-type mice (16 to 18 wk) were treated with STZ (50 mg/kg intraperitoneal injection) to induce type 1 DM (56). The diabetic phenotype was confirmed by urinalysis to assess urine glucose (using keto-diastix reagent strips) as well as by measuring serum glucose levels (using a glucometer).
In chronic insulin treatment experiments, Akita mice were given insulin pellets (0.2 U/d per pellet) or placebo pellets (LinShin Canada Inc.) subcutaneously for 4 wk beginning at 12 wk of age. For acute insulin studies, Akita mice were given an intraperitoneal injection of insulin (5 to 10 U/kg) or saline, and 30 to 45 min later (after blood glucose was reduced), the mice were used experimentally.
All experimental procedures were approved by the University of Calgary Animal Care and Use Committee and the Dalhousie University Committee for Laboratory Animals and were in accordance with guidelines of the Canadian Council on Animal Care.
Echocardiography and Electrolyte Measurements.
Cardiac structure and function were assessed by echocardiography in mice anesthetized by isoflurane inhalation (2%) using a Vevo 3100 ultrasound machine (Fujifilm VisualSonics) as we have described previously (21). Blood electrolytes were measured using an ePoc blood glass and electrolyte analyzer using blood samples drawn from the jugular vein.
In Vivo Electrophysiology.
Surface electrocardiograms (ECGs) were measured in anesthetized mice (2% isoflurane inhalation) using 30-gauge subdermal needle electrodes (Grass Technologies). In conjunction, a 1.2F octapolar electrophysiology catheter (Transonic) was inserted into the right heart via an incision in the jugular vein and used to assess inducibility of AF during burst pacing as we have done previously (57, 58). Burst pacing protocols were standardized so that all mice were stimulated in identical fashion using the same pacing protocols. AF was defined as a rapid and irregular atrial rhythm (fibrillatory baseline in the ECG) with irregular R wave to R wave (RR) intervals lasting at least 1 s on the surface ECG and rapid irregular activity on the intracardiac atrial electrogram. AF was categorized into three groups: 5 s (brief), 5 to 30 s (nonsustained), and 30 s (sustained). Additional details are available in SI Appendix.
High-Resolution Optical Mapping.
To study activation patterns and electrical conduction in the atria, high-resolution optical mapping was performed in isolated atrial preparations using methods that we have described in detail previously (31, 57, 59, 60). These atrial preparations were studied while in intrinsic sinus rhythm. Atrial preparations were immobilized using blebbistatin (10 M) (61). Changes in fluorescence were captured using the voltage-sensitive dye Di-4-ANEPPS (10 M) and a high-speed electron multiplying charge-coupled device camera at ∼900 frames per 1 s. Spatial resolution was 67 × 67 M per pixel. Conduction velocity was measured locally in the right and left atria using approaches described previously (21, 22, 57, 58). In some instances, optical APs were measured by measuring the change in fluorescence as a function of time at individual pixels in the right and left atria as previously described (21, 22). All experiments were performed at 35 °C. Data were analyzed using custom software written in Matlab. Further details are available in SI Appendix.
Patch Clamping of Isolated Atrial Myocytes.
Right and left atrial myocytes were isolated from mice by enzymatic digestion as we have described previously (62, 63). These myocytes were used to record APs using the perforated patch-clamp technique or the whole-cell patch-clamp technique (so that compounds could be dialyzed into the cells). AP parameters analyzed include resting membrane potential, Vmax, overshoot, and APD. Vmax was measured as the maximum rate of rise of the AP potential (i.e., dV/dtmax) between the resting membrane potential and the peak of the AP. INa, INa,L, IK, and ICa,L were recorded using the whole-cell patch-clamp technique. The solutions and experimental protocols for each of these approaches are available in SI Appendix.
qPCR.
Quantitative gene expression was measured in the right and left atria as we have previously described (21, 22). Intron-spanning primers for SCN5a (NaV1.5), KCNA5 (KV1.5), GJA5 (Cx40), and GJA1 (Cx43) as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (reference gene) were used. Experimental protocols and primer sequences are in SI Appendix.
Western Blotting.
For western blotting, right and left atrial samples were used to measure the protein expression of NaV1.5, KV4.2, KV4.3, and KV1.5 as well as GAPDH as the loading control. The procedures for these experiments are provided in SI Appendix.
Calcium Transients.
Calcium transients were measured using confocal imaging in acutely isolated atrial myocytes. The solutions and experimental protocols are provided in SI Appendix.
Statistical Analysis.
All data are presented as means SEM. Data were analyzed using Fisher’s exact test, Student’s t test, two-way ANOVA with Tukey’s post hoc test, or two-way repeated measures ANOVA with Tukey’s post hoc test as indicated in each figure. P 0.05 was considered significant.
Data Availability.
Relevant data and associated experimental protocols required for interpreting conclusions are included in the manuscript and the SI Appendix. Any additional information, data, or experimental reagents are available from the corresponding author upon request.
Supplementary Material
Acknowledgments
This work was supported by Canadian Institutes of Health Research Operating Grants MOP 142486 and PJT 166105 (to R.A.R.). H.J.J. was the recipient of a Nova Scotia Graduate Scholarship and a Dalhousie Medical Research Foundation MacDonald Graduate Scholarship and holds a Killam Postdoctoral Fellowship. L.J.B. holds a Libin Cardiovascular Institute of Alberta Graduate Studentship. Y.L. holds a Canadian Institutes of Health Research Postdoctoral Fellowship. E.E.E. held a Heart and Stroke Foundation of Canada Fellowship. R.A.R. held a New Investigator Award from the Heart and Stroke Foundation of Canada.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914853117/-/DCSupplemental.
References
- 1.Dobrev D., Carlsson L., Nattel S., Novel molecular targets for atrial fibrillation therapy. Nat. Rev. Drug Discov. 11, 275–291 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Nattel S., Burstein B., Dobrev D., Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol 1, 62–73 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Andrade J., Khairy P., Dobrev D., Nattel S., The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114, 1453–1468 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Dahlqvist S., et al. , Risk of atrial fibrillation in people with type 1 diabetes compared with matched controls from the general population: A prospective case-control study. Lancet Diabetes Endocrinol. 5, 799–807 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Lin Y., et al. , Mechanism of and therapeutic strategy for atrial fibrillation associated with diabetes mellitus. ScientificWorld J. 2013, 209428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes A. R., et al. , Cardiometabolic risk factors and atrial fibrillation. Rev. Cardiovasc. Med. 14, e73–e81 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Bohne L. J., et al. , The association between diabetes mellitus and atrial fibrillation: Clinical and mechanistic insights. Front. Physiol. 10, 135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Z., et al. , A machine learning aided systematic review and meta-analysis of the relative risk of atrial fibrillation in patients with diabetes mellitus. Front. Physiol. 9, 835 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dublin S., et al. , Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J. Gen. Intern. Med. 25, 853–858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ferranti S. D., et al. , Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the American Heart Association and American Diabetes Association. Circulation 130, 1110–1130 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Abel E. D., Insulin signaling in heart muscle: Lessons from genetically engineered mouse models. Curr. Hypertens. Rep. 6, 416–423 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Oudit G. Y., et al. , The role of phosphoinositide-3 kinase and pten in cardiovascular physiology and disease. J. Mol. Cell. Cardiol. 37, 449–471 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B., The emerging mechanisms of isoform-specific pi3k signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B., Stephens L., Hawkins P., Pi3k signalling: The path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 13, 195–203 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Heijman J., Voigt N., Nattel S., Dobrev D., Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 114, 1483–1499 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Nattel S., Atrial electrophysiology and mechanisms of atrial fibrillation. J. Cardiovasc. Pharmacol. Therapeut. 8(suppl. 1), S5–S11 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Kleber A. G., Rudy Y., Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 84, 431–488 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Nerbonne J. M., Kass R. S., Molecular physiology of cardiac repolarization. Physiol. Rev. 85, 1205–1253 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Hsueh W., et al. , Recipes for creating animal models of diabetic cardiovascular disease. Circ. Res. 100, 1415–1427 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka M., Kayo T., Ikeda T., Koizumi A., A novel locus, mody4, distal to d7mit189 on chromosome 7 determines early-onset niddm in nonobese c57bl/6 (Akita) mutant mice. Diabetes 46, 887–894 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Jansen H. J., et al. , Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J. Mol. Cell. Cardiol. 124, 12–25 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Jansen H. J., et al. , Npr-c (natriuretic peptide receptor-c) modulates the progression of angiotensin II-mediated atrial fibrillation and atrial remodeling in mice. Circ. Arrhythm Electrophysiol. 12, e006863 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Mackasey M., et al. , Natriuretic peptide receptor-c protects against angiotensin II-mediated sinoatrial node disease in mice. JACC Basic Transl. Sci. 3, 824–843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bugger H., et al. , Type 1 diabetic Akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes 57, 2924–2932 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo H. C., et al. , A defect in the Kv channel-interacting protein 2 (kchip2) gene leads to a complete loss of i(to) and confers susceptibility to ventricular tachycardia. Cell 107, 801–813 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Lomax A. E., Kondo C. S., Giles W. R., Comparison of time- and voltage-dependent K+ currents in myocytes from left and right atria of adult mice. Am. J. Physiol. Heart Circ. Physiol. 285, H1837–H1848 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Fiset C., Clark R. B., Larsen T. S., Giles W. R., A rapidly activating sustained K+ current modulates repolarization and excitation-contraction coupling in adult mouse ventricle. J. Physiol. 504, 557–563 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel V. B., et al. , Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: A critical role of the angiotensin II/at1 receptor axis. Circ. Res. 110, 1322–1335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marionneau C., Abriel H., Regulation of the cardiac Na+ channel nav1.5 by post-translational modifications. J. Mol. Cell. Cardiol. 82, 36–47 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Toullec D., et al. , The bisindolylmaleimide gf 109203x is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266, 15771–15781 (1991). [PubMed] [Google Scholar]
- 31.Krishnaswamy P. S., et al. , Altered parasympathetic nervous system regulation of the sinoatrial node in Akita diabetic mice. J. Mol. Cell. Cardiol. 82, 125–135 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Stables C. L., et al. , Reduced Na(+) current density underlies impaired propagation in the diabetic rabbit ventricle. J. Mol. Cell. Cardiol. 69, 24–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z., et al. , Increased persistent sodium current due to decreased pi3k signaling contributes to qt prolongation in the diabetic heart. Diabetes 62, 4257–4265 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Z., et al. , Decreased l-type Ca2+ current in cardiac myocytes of type 1 diabetic Akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes 56, 2780–2789 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Lu Z., et al. , Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation 120, 318–325 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballou L. M., Lin R. Z., Cohen I. S., Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ. Res. 116, 127–137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur K., et al. , Tgf-beta1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PLoS One 8, e55391 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang K. C., Tseng Y. T., Nerbonne J. M., Exercise training and pi3kalpha-induced electrical remodeling is independent of cellular hypertrophy and AKT signaling. J. Mol. Cell. Cardiol. 53, 532–541 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brouillette J., Clark R. B., Giles W. R., Fiset C., Functional properties of K+ currents in adult mouse ventricular myocytes. J. Physiol. 559, 777–798 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nattel S., New ideas about atrial fibrillation 50 years on. Nature 415, 219–226 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Van Wagoner D. R., et al. , Outward K+ current densities and kv1.5 expression are reduced in chronic human atrial fibrillation. Circ. Res. 80, 772–781 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Kato T., et al. , Ages-rage system mediates atrial structural remodeling in the diabetic rat. J. Cardiovasc. Electrophysiol. 19, 415–420 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Kato T., et al. , Angiotensin II type 1 receptor blocker attenuates diabetes-induced atrial structural remodeling. J. Cardiol. 58, 131–136 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Lamberts R. R., et al. , Impaired relaxation despite upregulated calcium-handling protein atrial myocardium from type 2 diabetic patients with preserved ejection fraction. Cardiovasc. Diabetol. 13, 72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li B., Pan Y., Li X., Type 2 diabetes induces prolonged p-wave duration without left atrial enlargement. J. Kor. Med. Sci. 31, 525–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe M., et al. , Conduction and refractory disorders in the diabetic atrium. Am. J. Physiol. Heart Circ. Physiol. 303, H86–H95 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Dzeshka M. S., Lip G. Y., Snezhitskiy V., Shantsila E., Cardiac fibrosis in patients with atrial fibrillation: Mechanisms and clinical implications. J. Am. Coll. Cardiol. 66, 943–959 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Rohr S., Myofibroblasts in diseased hearts: New players in cardiac arrhythmias?. Heart Rhythm 6, 848–856 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Wolf R. M., et al. , Atrial fibrillation and sinus node dysfunction in human ankyrin-b syndrome: A computational analysis. Am. J. Physiol. Heart Circ. Physiol. 304, H1253–H1266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito S., et al. , Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc. Res. 104, 5–14 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Yang K. C., Jay P. Y., McMullen J. R., Nerbonne J. M., Enhanced cardiac pi3kalpha signalling mitigates arrhythmogenic electrical remodelling in pathological hypertrophy and heart failure. Cardiovasc. Res. 93, 252–262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang K. C., Ku Y. C., Lovett M., Nerbonne J. M., Combined deep microRNA and mRNA sequencing identifies protective transcriptomal signature of enhanced pi3kalpha signaling in cardiac hypertrophy. J. Mol. Cell. Cardiol. 53, 101–112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pretorius L., et al. , Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am. J. Pathol. 175, 998–1009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lengyel C., et al. , Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovasc. Res. 73, 512–520 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Fedida D., et al. , Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ. Res. 93, 744–751 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Bugger H., et al. , Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. J. Mol. Cell. Cardiol. 52, 1019–1026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egom E. E., et al. , Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor c. J. Physiol. 593, 1127–1146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jansen H. J., et al. , Atrial structure, function and arrhythmogenesis in aged and frail mice. Sci. Rep. 7, 44336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azer J., Hua R., Krishnaswamy P. S., Rose R. A., Effects of natriuretic peptides on electrical conduction in the sinoatrial node and atrial myocardium of the heart. J. Physiol. 592, 1025–1045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moghtadaei M., et al. , The impacts of age and frailty on heart rate and sinoatrial node function. J. Physiol. 594, 7105–7126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fedorov V. V., et al. , Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4, 619–626 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Hua R., et al. , Effects of wild-type and mutant forms of atrial natriuretic peptide on atrial electrophysiology and arrhythmogenesis. Circ. Arrhythm Electrophysiol. 8, 1240–1254 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Springer J., et al. , The natriuretic peptides BNP and CNP increase heart rate and electrical conduction by stimulating ionic currents in the sinoatrial node and atrial myocardium following activation of guanylyl cyclase-linked natriuretic peptide receptors. J. Mol. Cell. Cardiol. 52, 1122–1134 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Relevant data and associated experimental protocols required for interpreting conclusions are included in the manuscript and the SI Appendix. Any additional information, data, or experimental reagents are available from the corresponding author upon request.