Abstract
Concerns have been raised about both the disinfection and the reusability of respiratory protective equipment following a disinfection process. Currently, there is little data available on the effects of disinfection and decontamination on positive pressure respiratory protective hoods (PPRPH). In this study, we evaluated the effect of vaporized hydrogen peroxide (VHP) on the disinfection of PPRPH to determine applicability of this method for disinfection of protective equipment, especially protective equipment with an electric supply system.
A hydrogen peroxide-based fumigation sterilization cabinet was developed particularly for disinfection of protective equipment, and the disinfection experiments were conducted using four PPRPHs hung in the fumigation chamber. The pathogenic microorganism Geobacillus stearothermophilus ATCC 7953 was used as a biological indicator in this study and the relationship between air flow (the amount of VHP) and disinfection was investigated. Both function and the material physical properties of the PPRPH were assessed following the disinfection procedure. No surviving Geobacillus stearothermophilus ATCC 7953, both inside and outside of these disinfected PPRPHs, could be observed after a 60 min treatment with an air flow of 10.5–12.3 m3/h. Both function and material physical properties of these PPRPHs met the working requirements after disinfection.
This study indicates that air flow in the fumigation chamber directly influences the concentration of VHP. The protective equipment fumigation sterilization cabinet developed in this paper achieves the complete sterilization of the PPRPHs when the air flow is at 10.5–12.3 m3/h, and provides a potential solution for the disinfection of various kind of protective equipment.
Keywords: Vaporized hydrogen peroxide, Positive pressure respiratory protective hood, Fumigation sterilization cabinet, Disinfection efficiency
Highlights
Scientific question
Positive pressure respiratory protective hoods (PPRPHs) are used to protect the health of medical staff or researchers dealing with highly infectious pathogens, such as severe acute respiratory syndrome coronavirus (SARS-CoV) or Ebola virus. When there is direct contact with highly infectious pathogens, it is critically important that the PPRPH is thoroughly disinfected following use. This is a crucial issue for the reusability of this protective gear.
Evidence before this study
There are limited reports on the sterilization of respiratory protective equipment. Chlorine disinfectant, autoclaving and dry heat sterilization have been shown to completely sterilize N95 respirators. However, the N95 respirators could not be reused after disinfection. Ethylene oxide and vaporized hydrogen peroxide (VHP) were proposed as ideal chemicals for the disinfection of respiratory protective equipment (Viscusi et al.), but the authors did not carry out a disinfection evaluation.
Highlights of new findings
Compared with ethylene oxide disinfection, VHP disinfection requires less time, and is more environmentally friendly. To understand the effect of VHP decontamination on PPRPHs, a hydrogen peroxide-based fumigation sterilization cabinet was developed. The effect of VHP on the performance of the PPRPHs was investigated in detail. When used in the fumigation cabinet, VHP exhibited excellent disinfection, with no deleterious effect on PPRPH performance. After sterilizations, the PPRPHs remained fully functional.
Significance of the study
We have developed and described reliable disinfection equipment based on VHP, which not only achieves thorough disinfection, but also does not affect the material properties and protective properties of treated PPRPHs. Thus our study provides a universal solution for the reuse of respiratory protective equipment.
Alt-text: Unlabelled Box
1. Introduction
Research on respiratory protection against biologic agents is important to address major concerns such as occupational safety and terrorist attack [1]. Personal protective equipment is known to be a crucial non-pharmaceutical method for preventing influenza pandemics [2], with powered air-purifying respirators (PAPRs) and positive pressure respiratory protective hoods (PPRPHs) commonly used as respiratory protective equipment [3]. It is reported that influenza viruses can survive >8 h on the surface of Dupont Tyvek, surgical masks and so on [4], and Ebola viruses can persist on surfaces for up to five days [5]. Sakaguchi et al. feel that the presence of viruses on the surfaces of personal protective equipment is an important factor in cross-contamination and the spread of infection [4].
Before reuse, respiratory protective equipment has to be decontaminated thoroughly in order to control cross-infection from the pathogenic microorganisms on their surfaces. There are limited reports on sterilization of items such as N95 masks and protective clothing [[6], [7], [8]]. Chlorine disinfectant, autoclaving and dry heating sterilization exhibited good disinfection, and could completely sterilize N95 respirators. However, the N95 respirators were deformed and could not be reused after disinfection [[6], [7], [8]]. Although UVC irradiation and ethanol disinfection had little effect on the properties of the equipment, they were not effective at eliminating pathogens [[7], [8], [9]]. The material of PPRPHs is thermoplastic urethane (TPU) or polyvinyl chloride (PVC) which differs from the N95 respirator. UVC cannot penetrate this material. The electric air supply system of the PPRPH cannot withstand in high temperatures. Therefore, UVC, autoclave and dry heating sterilization are not suitable for the PPRPHs.
Viscusi et al. indicated that ethylene oxide and vaporized hydrogen peroxide (VHP) were potential ideal chemicals that could be used for disinfection of respiratory protective equipment in the future, but unfortunately the author did not carry out the disinfection evaluation [7]. The use of ethylene oxide is limited because of its flammable and explosive properties, and because it needs more than 6 h to achieve an acceptable disinfection effect. VHP can automatically degrade into water and oxygen in nature, and therefore is friendly to the environment. Hydrogen peroxide decontamination systems are widely used to reduce the risk of infection in hospitals, biosafety laboratories, and so on [10,11]. Chou et al. demonstrated that hydrogen peroxide had no effect on the mechanical properties of nylon, polyester, and Nomex [12].
In this paper, the feasibility of VHP as a decontamination chemical for PPRPH was investigated. A hydrogen peroxide-based fumigation sterilization cabinet was particularly designed for the decontamination of protective equipment, and the disinfection efficiency of VHP as well as the performance of the PPRPHs post-disinfection was also evaluated.
2. Materials and methods
2.1. Bacteria and materials
The commercially available biological indicators used in this study were composed of grade 304 stainless steel carriers, each inoculated with 1 × 106 spores of Geobacillus stearothermophilus ATCC7953. The biological indicators sealed in Tyvek™, were manufactured by Mesa Laboratories, Denver, Colorado, USA. Bromocresol peptone medium are commercially available from Rui Chu Biological Technology Corporation in China. Hydrogen peroxide (H2O2, 30%) is mos grade, and other agents are all analytically pure. The air multi-parameter tester (TSI 8386A) used was produced by TSI Company in America. The hydrogen peroxide concentration sensor is HPP270 series products (Vaisala PEROXCAP®) produced by Vaisala company in Finland. The PPRPH used were from the National Bio-protection Engineering Center of China.
2.2. The development of the protective equipment fumigation sterilization cabinet
A hydrogen peroxide-based fumigation sterilization cabinet was developed for protective equipment disinfection. The whole sterilization cabinet was made of welding stainless steel, and consists of two compartments, a VHP generating chamber and a protective equipment fumigation chamber (Figure 1 ), which was connected by a perforated partition in the middle of the protective equipment fumigation sterilization cabinet. The whole disinfection process includes the preparation (hydrogen peroxide injection), the disinfection, and the residual gas removal.
Figure 1.
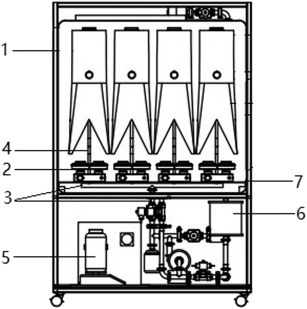
The internal structure of the Fumigation Sterilization Cabinet. Note: 1 - the positive protective respiratory hood, 2 - the electric air supply system for the hood, 3 - the main pipe of the gas disinfectant delivery, 4 - the branch pipe of the gas disinfectant delivery, 5 - the tank of hydrogen peroxide, 6 - the residual elimination module, 7 - the perforated partition between the upper and lower parts of the protective equipment fumigation sterilization chamber.
The VHP generating chamber is composed of a hydrogen peroxide gas generator module, a residual gas elimination module and a pressure relief module. There is a main pipe for transporting VHP, which is divided into 4 branch pipes for delivering VHP to the PPRPHs. The power of the fan in the VHP generating chamber can be changed to alter VHP distribution. The wind speed regulated by the fan power (40%–80%) is measured using a TSI air multi-parameter tester. The method is to place a tube with Φ60 to measure the wind speed supplied for the four branch pipes. The amount of air flow is calculated according to the following (1), (2).
| (1) |
| (2) |
where v, vn, Q, S represent the average wind speed (m/s), the wind speed of each measuring point (m/s), the average air flow speed (m3/h), the cross-sectional area of the test pipeline (m2), respectively.
The parameter monitoring module is integrated into the fumigation chamber and can display real-time temperature, humidity and pressure during the disinfection process. An internal hydrogen peroxide concentration sensor is used to measure the hydrogen peroxide concentration in the fumigation chamber. There are four hooks in the fumigation chamber which are used to hold four sets of PPRPHs or other personal protective equipment. Four sets of electric air supply systems can be placed on the perforated partition. The fumigation chamber is equipped with an airtight door to ensure the air tightness during the disinfection process.
2.3. Determination of VHP disinfection conditions
The disinfection parameter determination was conducted in a cabinet equipped with a door, gloves, injection ports, and connection for electricity. Four electric fans (HSL-2; Emicorcom, Leqing, China) were installed in the corner of the cabinet to mix the fumigant [13]. The rate of hydrogen peroxide injection was adjusted by changing the speed of peristaltic pump connected to the flash vessel. When the speed of peristaltic pump was increased from 6 to 30 rpm, the injection rate of hydrogen peroxide rose correspondingly from 0.8 g/min to 4 g/min. The effect of injection time on disinfection efficiency was also investigated when the total disinfection time remained unchanged. The injection rate was set to 4 g/min, with injection times of 5, 10, 15, and 20 min, respectively, and the consumption of hydrogen peroxide ranged from 20 to 80 g. Before the experiments, three plates (each with a stainless steel slide on it) were placed in the center of the chamber. Following the disinfection experiments, the plates were covered and removed via a pass box to a biological safety cabinet. All of the experiments were repeated three times using three slides.
2.4. Decontamination of PPRPH
The protective equipment fumigation sterilization cabinet was operated according to the instructions. After opening the airtight door, four PPRPHs were placed in the fumigation chamber, and then the breathing tubes and the power supply system (not in operation) were connected to them. The concentration of VHP in different positions of the fumigation chamber was tested by the sensor (Vaisala PEROXCAP®). Four stainless steel slides were placed at different positions inside the PPRPH, and other slides were placed on different positions of the fumigation chamber. After the disinfection, the biological indicators were taken out of the chamber and placed into a sterile beaker.
2.5. Treatment and evaluation of the biological indicators
According to the regulations of the disinfection technical specification (the 2002 edition) [14], the method of qualitative culture was used for the analysis of biological indicators. The stainless steel slides were transferred into a test tube containing sterile bromocresol purple broth medium using sterile forceps and incubated at 55 °C for 48 h. In the event of survival of G. stearothermophilus, the bromocresol purple broth medium will turn yellow, indicating bacterial growth.
2.6. Performance test for PPRPH
2.6.1. Protective performance test
The static pressure difference, noise and air supply volume were tested before and after disinfection of a PPRPH [15]. For the test, a human model wore the PPRPH with the pressure tube placed inside the hood and the noise meter placed near the ear inside the PPRPH. The static pressure and noise of the PPRPH were tested both before stabilization and after stabilization for 10 times, recording the displayed values.
The air supply volume of the power supply air system was tested by measuring the actual flow rate of the four filter inlets. The amount of air supplied was calculated according to the (1), (2) based on testing the wind speed at five points of the cross-sectional area of the pipe with an air multi-parameter tester by clamping the pipe with a length of 300 mm of Φ50 at the air inlet of the filter.
2.6.2. Physical performance test
The physical property of the materials was tested according to the method of the national standard of China (GB 24539-2009) [16] to confirm whether the mechanical properties of the materials after repeated disinfection meet the standard requirements, particularly the seam strength, and the puncture resistance of the material.
3. Results
3.1. The performance of the protective equipment fumigation sterilization cabinet
Figure 2 shows the protective equipment fumigation sterilization cabinet. The complete disinfection procedure includes putting the ready-to-be-disinfected equipment inside the chamber, injection of hydrogen peroxide, disinfection, and removal of residual gas. In the preparation stage, concentrations of VHP were controlled by adjusting both the peristaltic pump and the metering pump (Figure 3a), hydrogen peroxide droplets were added to the flash evaporator at a fixed rate (Figure 3b), which was vaporized instantly. The vaporized hydrogen peroxide vapor was directed into the interior of the hoods using ventilation technology (Figures 3c, 4a). During the injection process, the pressure relief module in the chamber can ensure the pressure is maintained at a favorable range (Figure 4b). After the disinfection, the fan inside the chamber exhausted the vaporized hydrogen peroxide from the catalytic module, and its concentration gradually dropped to 10 ppm through continuous cycles (Figure 4c).
Figure 2.

The fumigation sterilization cabinet.
Figure 3.
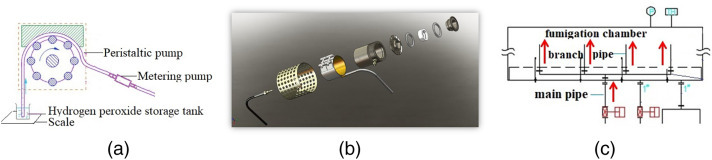
The schematic diagram of different key technologies. Note: (a) Stable addition of hydrogen peroxide; (b) Hydrogen peroxide flash evaporator; (c) Ventilation technology to distribution of hydrogen peroxide injection.
Figure 4.
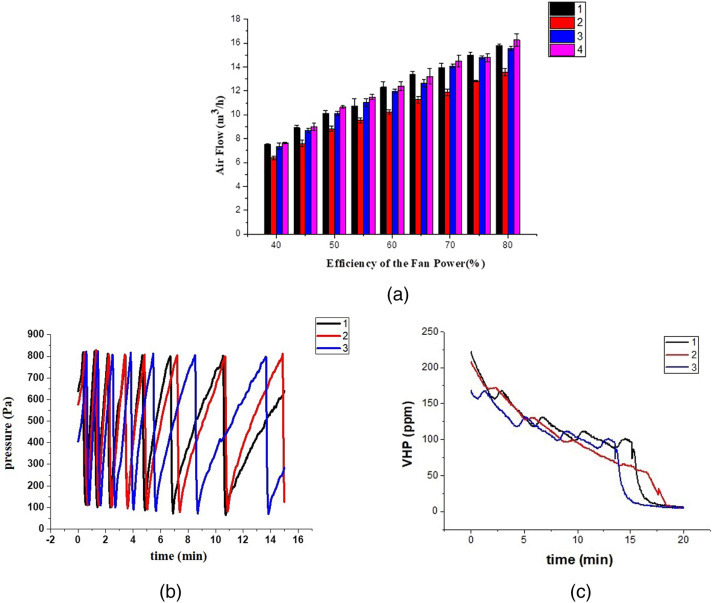
The main performance of the fumigation sterilization cabinet. (a) The relationship between the fan power and the air flow of the four branch pipes; (b) Fluctuations of air pressure in fumigation chamber during hydrogen peroxide injection; (c) Catalytic efficiency of VHP.
3.2. Determination of optimal disinfection conditions using VHP
Survival of Geobacillus stearothermophilus spores was used to verify the disinfection efficiency of VHP in this study [11]. The effects of different injection rates and different injection times were investigated to determine optimal disinfection conditions.
As showed in Table 1 , with the increase of VPH injection rate, the consumption of hydrogen peroxide also increased. When the injection rate was higher than 1.07 g/min which represented the injection amount of VPH higher than 64 g, complete sterilization was achieved. The effect of reducing the injection time on the disinfection was tested using a high injection rate (4 g/min.), with the total disinfection time held constant at 60 min. As the results show in Table 2 , when the injection time was >15 min, and the consumption of hydrogen peroxide was >60 g, complete sterilization can be achieved. The consumption of the hydrogen peroxide was the main factor affecting the disinfection effect. In our results, we obtained two sets of parameters that can completely achieve sterilization: 1 g/min injection rate, 60 min injection time and 4 g/min injection rate, and 15 min injection time. Under these two conditions, a 60 min treatment can achieve complete sterilization.
Table 1.
Effect of different hydrogen peroxide injection rate on disinfection effect.
| Injection rate of hydrogen peroxide (g/min) | Injection amount of hydrogen peroxide (g) | The disinfection efficiency |
Positive control | Negative control | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 4.00 | 240 | − | − | − | + | − |
| 2.60 | 160 | − | − | − | + | − |
| 1.30 | 80 | − | − | − | + | − |
| 1.07 | 64 | − | − | − | + | − |
| 0.80 | 48 | + | + | − | + | − |
Note: 1, 2 and 3 represented that the experiments repeated for three times. ‘−’ means no spores growth; ‘+’ means the spores growth.
Table 2.
Effect of different injection time on disinfection effect.
| Injection time of hydrogen peroxide (min) | Injection amount of hydrogen peroxide (g) | The disinfection efficiency |
Positive control | Negative control | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 20 | 80 | − | − | − | + | − |
| 15 | 60 | − | − | − | + | − |
| 10 | 40 | + | + | + | + | − |
| 5 | 20 | + | + | + | + | − |
Note: 1, 2 and 3 represented that the experiments repeated for three times. ‘−’ means no spores growth; ‘+’ means the spores growth.
3.3. Disinfection effect of VHP on the PPRPH
Four PPRPHs were hung on the top of the fumigation chamber (Figure 2b). When the fan power was set at 60%, the VHP concentration at different position of the fumigation chamber was tested (Figure 5a). The concentration trend at each position was relatively consistent (Figure 5b–d). During the injection process, the VHP concentration continued to increase, and the VHP concentration in the upper position of the fumigation chamber was relatively low (Figure 5b). Then the power of the fan (40%–70%) was changed and the concentration change at the same position was tested. As shown in Figure 5e, the concentration measured at each power level was different, demonstrating the impact of air flow on VHP concentration.
Figure 5.
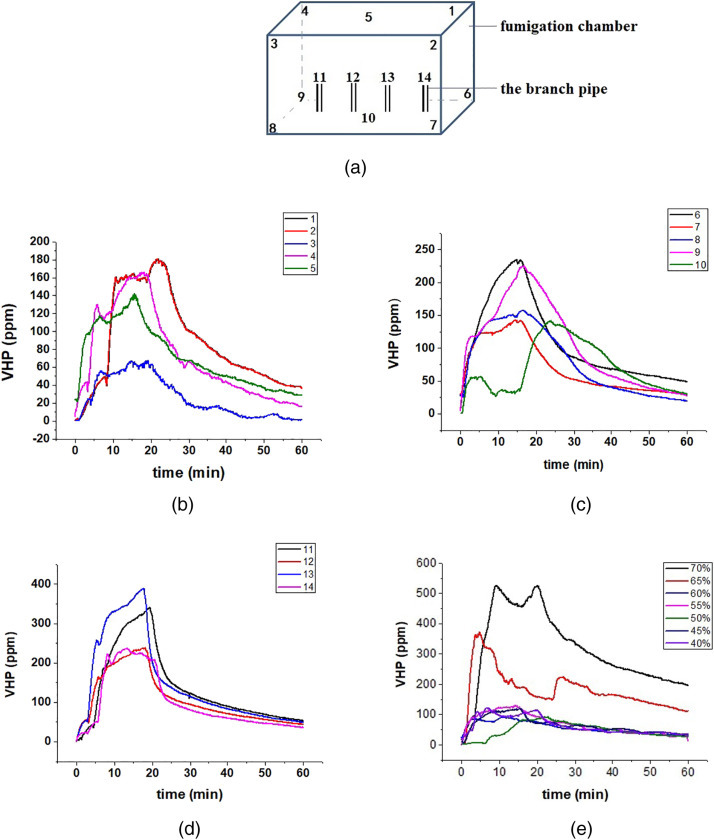
The VHP concentrations in the fumigation chamber. Note: (a) The test location; (b–d) The concentration of VHP at 14 positions when the efficiency of the fan power was 60%; (e) The concentration of VHP at position No. 1 with different efficiency of the fan power.
The relationship between the air flow rate and disinfection efficiency of VHP was investigated in the fumigation chamber. The disinfection experiments were conducted when the efficiency of the fan power changed from 40%–70%. The biological indicators were placed in the appropriate positions (shown in Figure 6 ). In these experiments, only ‘e’ and ‘l’ location were selected to place the slides. When the air flow supplied to the PPRPHs was relatively low (6.4–7.6 m3/h) (Figure 4a), most of the spores both inside and outside of the PPRPHs are not deactivated (Table 3 ). When the air flow supplied to the PPRPHs was relatively high (11.8–14.5 m3/h), the spores outside of the PPRPHs were deactivated, but spores inside the PPRPHs grew after cultivation for 48 h. Complete disinfection was obtained with the fan power at 60% and the air flow of 10.5–12.3m3/h. During the disinfection process, the humidity of the fumigation chamber went up to 90% in 10 min independent of the efficiency of the fan power.
Figure 6.
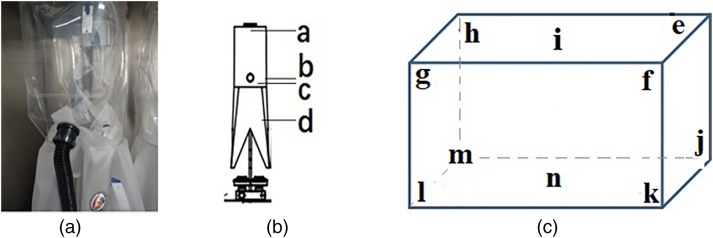
Location of stainless steel slides. Note: (a) Picture of the inside of the disinfection hood; (b) Location of slides inside the hood; (c) Location of slides inside the fumigation chamber.
Table 3.
The disinfection results of the hoods with different air flow.
| Efficiency of fan power (%) | The PPRPH on No. 2 branch pipe |
The PPRPH on No. 4 branch pipe |
Fumigation chamber |
Positive control | Negative control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | A | B | c | d | e | l | |||
| 40 | − | + | + | + | + | + | + | + | + | + | + | − |
| 50 | + | − | − | − | − | − | − | − | + | − | + | − |
| 60 | − | − | − | − | − | − | − | − | − | − | + | − |
| 70 | + | + | − | − | − | + | − | − | − | − | + | − |
Note: The letter represented the position of the stainless steel slides (Figure 6). ‘−’ means no spores growth; ‘+’ means the spores growth.
Multiple replications of the experiments using 60% fan power consistently disinfected the PPRPHs. The biological indicators were placed all the positions indicated in Figure 6. The spores both inside and outside the four PPRPHs did not grow even after cultivation for 72 h (Figure 7 ). The results indicated that the protective equipment fumigation sterilization cabinet can achieve the sterilization of the PPRPHs.
Figure 7.
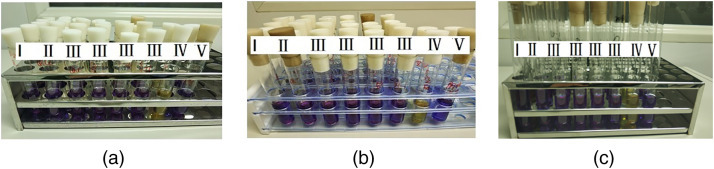
The disinfection results when the fan power was 60%. Note: (a), (b), (c) represented the experiment repeated for three times; I: ‘e’–‘i’ in Figure 6c; II: ‘j’–‘n’ in Figure 6c; III: ‘a’–‘b’ in the PPRPHs; IV: Positive control; V: Negative control.
3.4. Performance test of PPRPH before and after disinfection
It is critical that PPRPH are completely functional after every disinfection. When the PPRPH was sterilized for 10 times, the protective performance and physical properties of the material were tested. There was no significant difference in the protective performance of the PPRPHs disinfected according to the Table 4 , and the physical structure and performance of the disinfected PPRPHs also met the requirements of GB 24539-2009 (Table 5 ). These data indicated that the VHP can achieve complete disinfection of the PPRPHs without affecting their protective performance and physical properties.
Table 4.
Protective performance of PPRPH before and after disinfection.
| No. | Air supply (min/L) | Noise (dB(A)) | Static pressure (Pa) |
|---|---|---|---|
| 1 | 160.37 ± 0.54 | 64.70 ± 0.50 | 56.00 ± 2.55 |
| 2 | 159.00 ± 1.25 | 64.20 ± 0.62 | 56.30 ± 1.74 |
Note: 1 represented before disinfection; 2 represented after 10 rounds disinfections.
Table 5.
Physical performance of PPRPH before and after disinfection.
| Test items | Test location | Before disinfection (N) | After disinfection (N) | Standard rating |
|---|---|---|---|---|
| Breaking strength | Back cover seam | 461.15 ± 102.77 | 531.50 ± 137.9 | ≧250 N (≧ level 4) |
| Hood top seam | 249.50 ± 12.76 | 279.50 ± 63.35 | ≧250 N (≧ level 4) | |
| Seam at the hood window | 601.00 ± 18.97 | 626.50 ± 40.40 | ≧250 N (≧ level 4) | |
| Joint of hood fabric and shawl joint | 245.75 ± 74.10 | 189.25 ± 65.10 | ≧100 N (≧ level 3) | |
| Hood fabric and exhaust valve seam | Intact | Intact | Apply a force of 150 N, complete for 10 s | |
| Hood collar and intake valve seam | Intact | Intact | Apply a force of 150 N, complete for 10 s | |
| Puncture resistance | Hood window | 79.50 ± 21.92 | 76.00 ± 12.72 | >50 N (≧ level 3) |
Note: The top four lines of the table were the average of four test samples, and the last three were the average of two test samples.
4. Discussion
The use of novel ‘no-touch’ automated room disinfection (NTD) systems provides an alternative approach, which removes or reduces reliance on the operator [17]. Automated systems have been adopted widely in many areas such as healthcare, biosafety laboratory, drug product manufacturing, food processing and so on [[18], [19], [20]].The ideal fumigant should leave no residues or should be capable of rapid removal to safe levels following fumigation [21]. A. J. Beswick, et al. have summarized the existing gas disinfection methods in their paper, which includes formaldehyde vaporization, hydrogen peroxide-based fumigation, chlorine dioxide and ozone [22]. Formaldehyde is a toxic chemical and has been classified as a Group 1 human carcinogen. For this reason, a choice of alternative, effective fumigation technologies is desirable. However, none of the fumigants existing are harmless, and all have workplace exposure limits [22]. VHP can decompose into water and oxygen automatically, making it easier to handle after disinfection. Thus VHP was selected to decontaminate the protective equipment in this study.
4.1. Factors of influencing disinfection
A hydrogen peroxide-based fumigation sterilization cabinet was developed for protective equipment disinfection. This cabinet provides hydrogen peroxide injection, vaporization, even distribution, and effective removal. The functional modules were integrated in the lower part of the hydrogen peroxide-based fumigation sterilization cabinet. The VHP transport pipes were connected to the VHP generator with the fumigation chamber. Protective equipment can be placed in the fumigation chamber (Figure 1). B. Unger-Bimczok, et al. found that the hydrogen peroxide concentration was an important factor in the inactivation efficacy [23], which was also verified in this study. The results indicate that the disinfection efficacy of VHP is directly related to the consumption of hydrogen peroxide solution (Table 1, Table 2). VHP can be automatically degraded to the H2O and O2 after relative short time of injection. An effective disinfection time was 60 min, with an initial 15 min injection, followed by a 45 min incubation, during which VHP degradation is occurring.
The concentration of VHP was tested at different positions of the fumigation chamber by a hydrogen peroxide concentration sensor. According to the result seen in Figures 4a and 5e, the air flow changed when the fan power altered. The change of the air flow directly affected the concentration of VHP. The concentration of VHP is the direct factor of influencing the disinfection effect. Thus the air flow and fan power used in the cabinet is of critical importance. Although the distributed ventilation technology was adopted in the development of fumigation cabinet, the concentration of VHP in each position is not uniform, which may be related to the placement of four PPRPHs leading to the creation of four small cavities. Future studies should focus on the influence of airflow patterns on the concentration of VHP in such a nested structure to make the concentration of VHP more uniform.
There are other factors, for example the humidity, which can influence the disinfection efficiency of VHP. B. Unger-Bimczok, et al. reported that higher humidity was found to promote the microbial inactivation rate [23]. The humidity of the fumigation chamber was quite high (>90%) after disinfection for 10min which was consistent with the literature reports.
4.2. Disinfection influence on the performance of hoods
Respiratory protective equipment is widely used to protect wearers against hazardous aerosols [24]. It is a physical barrier for respiratory protection of human being. Reuse of respiratory protective equipment after decontamination is an important area for research.
In this paper, VHP was used to decontaminate the PPRPH. The main material of the hood is TPU, after 10 rounds of disinfection, the physical properties of the TPU material had not changed. The protective performance test results of PPRPH after 10 rounds disinfection remain unchanged, demonstrating that the PPRPH can be reused normally after disinfection.
5. Conclusions
Today the outbreak of severe infectious diseases is a global problem. It is of critical importance to find disinfection methods and disinfection equipment that can be used to avoid cross-contamination and disease transmission when high-grade personal protective equipment is reused. This paper provides a decontamination method which can achieve the complete sterilization of protective equipment, while retaining that equipment's functionality. The protective equipment fumigation sterilization cabinet describe herein can meet the requirements for handling high-grade personal protective equipment, and thus has important economic and social significance.
Acknowledgments
Acknowledgments
We are grateful to Xiu-guo Zhao and Xin-wu Xie for helpful discussions. Thanks for the two funding projects of Chinese Ministry of Science and Technology: National Key research and development plan of China (2016YFC1201404), the Megaproject for Infectious Disease Research of China (2017ZX10304403-004-001).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Jinhui Wu, Email: wujh@npec.org.cn.
Jiancheng Qi, Email: qijch@npec.org.cn.
References
- 1.Rengasamy A., Zhuang Z., Berryann R. Respiratory protection against bioaerosols: literature review and research needs. AJIC. 2004;32(6):345–354. doi: 10.1016/j.ajic.2004.04.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashikura M., Kizu J. Stockpile of personal protective equipment in hospital settings: preparedness for influenza pandemics. AJIC. 2009;37(9):703–707. doi: 10.1016/j.ajic.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer E. Integrated unit performance testing of powered, air-purifying particulate respirators using a DOP challenge aerosol. J. Occup. Environ. Hyg. 2006;3(11):631–641. doi: 10.1080/15459620600954365. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi H., Wada K., Kajioka J., Watanabe M., Nakano R., Hirose T., Hiroshi O., Aizawa Y. Maintenance of influenza virus infectivity on the surfaces of personal protective equipment and clothing used in healthcare settings. Environ. Health Prev. Med. 2010;15(6):344–349. doi: 10.1007/s12199-010-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassir N., Boudjema S., Roux V., Reynier P., Brouqui P. Infectious diseases of high consequence and personal protective equipment: a didactic method to assess the risk of contamination. Infect. Control Hosp. Epidemiol. 2015;36(12):1485–1486. doi: 10.1017/ice.2015.223. [DOI] [PubMed] [Google Scholar]
- 6.Lin T.H., Tang F.C., Hung P.C., Hua Z.C., Lai C.Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;28:754–762. doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann. Occup. Hyg. 2009;53(8):815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessesen M.T., Adams J.C., Radonovich L., Anderson J. Disinfection of reusable elastomeric respirators by health care workers: a feasibility study and development of standard operating procedures. Ajic. 2015;43(6):629–634. doi: 10.1016/j.ajic.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Koganti S., Alhmidi H., Tomas M.E., Cadnum J.L., Sass C., Jencson A.L., Donskey C.J. Evaluation of an ethanol-based spray disinfectant for decontamination of cover gowns prior to removal. Infect. Control Hosp. Epidemiol. 2017;38(3):364–366. doi: 10.1017/ice.2016.295. [DOI] [PubMed] [Google Scholar]
- 10.Ali S., Muzslay M., Bruce M., Jeanes A., Moore G., Wilson A.P.R. Efficacy of two hydrogen peroxide vapour aerial decontamination systems for enhanced disinfection of meticillin-resistant Staphylococcus aureus, Klebsiella pneumoniae and Clostridium difficile in single isolation rooms. J. Hosp. Infect. 2016;93(1):70–77. doi: 10.1016/j.jhin.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Kaspari O., Lemmer K., Becker S., Lochau P., Howaldt S., Nattermann H., Grunow R. Decontamination of a BSL3 laboratory by hydrogen peroxide fumigation using three different surrogates for Bacillus anthracis spores. J. Appl. Microbiol. 2014;117(4):1095–1103. doi: 10.1111/jam.12601. [DOI] [PubMed] [Google Scholar]
- 12.Chou S.F., Gale W.F., Gale H.S., Shannon C.G., Buschle-Diller G., Sofyan N.I. Evaluation of airliner cabin textile materials after vapour phase hydrogen peroxide decontamination. Mater. Sci. Technol. 2010;26(1):66–80. [Google Scholar]
- 13.Wang T., Qi JCh, Wu J.H., Hao L.M., Yi Y., Lin S., Zhang Z.X. Response surface modeling for the inactivation of Bacillus subtilis subsp. niger spores by chlorine dioxide gas in an enclosed space. J. Air Waste Manag. Assoc. 2016;66(5):508–517. doi: 10.1080/10962247.2016.1150365. [DOI] [PubMed] [Google Scholar]
- 14.Technical Standard for Disinfection . 2002 edition. 2002. Ministry of Health of People Republic of China. [Google Scholar]
- 15.GB 30864-2014 Respiratory protection—powered air-purifying respirator. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of China; 2014. [Google Scholar]
- 16.GB 24539-2009 Protective clothing—performance requirements of chemical protective clothing. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of China; 2009. [Google Scholar]
- 17.Otter J.A., Yezli S., Perl T.M., Barbut F., French G.L. A guide to no-touch automated room disinfection (NTD) systems. In: Walker J.T., editor. Decontamination in Hospitals and Healthcare. 2014. pp. 413–460. [Google Scholar]
- 18.Doll M., Morgan D., Anderson D., Bearman G. Touchless technologies for decontamination in the hospital: a review of hydrogen peroxide and UV devices. Curr. Infect. Dis. Rep. 2015;17(9):1–11. doi: 10.1007/s11908-015-0498-1. [DOI] [PubMed] [Google Scholar]
- 19.Keisho Yabuta H.F., Kawasaki Koji, Hirao Masahiko, Sugiyama Hirokazu. Design-oriented regression models for H2O2 decontamination processes in sterile drug product manufacturing considering rapidity and sterility. Int. J. Pharm. 2018;548:466–473. doi: 10.1016/j.ijpharm.2018.06.055. [DOI] [PubMed] [Google Scholar]
- 20.Kirchner P., Oberlaender J., Suso H.-P., Rysstad G., Keusgen M., Schoening M.J. Towards a wireless sensor system for real-time H2O2 monitoring in aseptic food processes. Phys. Status Solidi A. 2013;210(5):877–883. [Google Scholar]
- 21.Joslyn L.J. Lippincott, Williams, & Wilkins; Philadelphia: PA: 2001. Gaseous Chemical Sterilization. [Google Scholar]
- 22.Beswick A.J., Farrant J., Makison C., Gawn J., Frost G., Crook B., Pride J. Comparison of multiple systems for laboratory whole room fumigation. Appl. Biosafety. 2011;16:139–157. [Google Scholar]
- 23.Unger-Bimczok B., Kottke V., Hertel C., Rauschnabel J. The influence of humidity, hydrogen peroxide concentration, and condensation on the inactivation of Geobacillus stearothermophilus spores with hydrogen peroxide vapor. J. Pharm. Innov. 2008;3(2):123–133. [Google Scholar]
- 24.Howie R. Respiratory protective equipment. Occup. Environ. Med. 2005;62:423–428. doi: 10.1136/oem.2002.004424. [DOI] [PMC free article] [PubMed] [Google Scholar]


