Abstract
In cheetah, the captive population has historically been beset by multiple degenerative and infectious diseases that have had an impact on cheetah health and breeding programs. In contrast, the free-ranging population has been relatively free of these same diseases. Although research into feline infectious peritonitis mortalities at a few zoos in the early 1980s suggested a possible genetic susceptibility to infectious disease, these diseases have not been noted in genetically similar populations of wild cheetahs despite evidence of exposure to infectious agents. Multidisciplinary and collaborative research has focused on the role of stress in development of disease in captive cheetahs. Subsequent improvements in husbandry and management have lessened the severity of some diseases; however, others remain intractable. As wild populations become increasingly fragmented and managed, it will be important to use the knowledge gained from captive populations to help safeguard their health and to prevent the development of disease in wild-caught cheetahs.
Keywords: pathology, pathogenesis, infectious disease, degenerative disease
Introduction
Captive cheetahs have been affected by a variety of diseases limiting the sustainability of these populations. To better understand these captive cheetah diseases on a global scale and determine if they impact wild cheetahs, several long-term studies have been conducted (Munson, 1993, Munson et al., 1999, Munson et al., 2005). As a species, cheetahs are considered more susceptible to disease than other carnivores and are carefully managed in captivity (Chapter 24). Thoughts over the causes of disease susceptibility in captive cheetahs have changed over time as new research has been conducted. This chapter will discuss the evolution in our understanding of cheetah diseases and provide details on some of the most significant diseases affecting captive and wild cheetahs. Protocols and forms that are relevant to this chapter can be found at https://www.elsevier.com/books-and-journals/book-companion/9780128040881.
Research from the 1980s had suggested that reduced genetic diversity (Chapter 6) may be the cause of poor health in captivity and high neonatal mortality rates. It was hypothesized that the lack of heterogeneity at major histocompatibility complex (MHC) loci (loci which encode peptides that mediate immune responses to pathogens) was an important factor in a feline infectious peritonitis (FIP) epidemic in one captive population, and it was assumed that cheetahs could be particularly vulnerable to infectious diseases (Evermann et al., 1988). However, in the 1990s, aspects of captive management were shown to affect juvenile mortality (Wielebnowski, 1996), and pathology studies indicated that morbidity and mortality in captive cheetah populations were predominately due to degenerative rather than infectious diseases (Munson, 1993, Munson et al., 1999). Study of wild Namibian cheetahs, a primary source for many founders of captive populations, indicated that these diseases were absent or extremely rare in the wild (Munson et al., 2005). Serology and pathology surveys showed that wild cheetahs were exposed to a wide range of viral pathogens without evident associated disease (Munson et al., 2004a, Munson et al., 2004b, Munson et al., 2005, Thalwitzer et al., 2010). Together, these studies suggest that environmental factors, such as captivity-induced stress, are of equal or greater importance than genetic factors in the development of disease in captive cheetahs (Munson et al., 2005).
Multiple studies have now documented both physiological and behavioral (e.g., stereotypies, such as pacing) chronic stress responses in captive cheetahs (Jurke et al., 1997, Terio et al., 2004, Wells et al., 2004, Wielebnowski, 1999). Captive cheetahs have larger adrenal glands and higher baseline levels of corticoids than wild cheetahs (Terio et al., 2004; Chapter 7). These persistently elevated corticoids can have profound negative effects on many physiological functions, including metabolism, reproductive health, and immune responses. Corticoids can alter immune responses by affecting transcription of mediators that coordinate and determine the type of immune responses (e.g., cytokines and inflammatory mediators). Comparison of these mediators between captive and free-ranging cheetahs has demonstrated that captive, but not free-ranging, cheetahs have decreased expression of TH1-type cytokines (IL-1, IL-2, IFNγ) consistent with elevated corticoids (Terio and Munson, 2005). Because TH1-type cytokines are important in cell-mediated immune responses, stress-induced immune modulation may be associated with the unusual inflammatory reactions observed in captive cheetahs in response to common pathogens.
Corticoid levels differ among institutions and among individual cheetahs residing at the same institution, suggesting that captive management and individual temperament may affect the stress response (Terio et al., 2004, Wells et al., 2004). Gastritis, one of the major cheetah diseases in captivity (see section, “Helicobacter-Associated Gastritis”), has been associated with increased stress levels and has served as a model to study stress in captivity. In cheetahs, severe gastritis correlates with high individual temperament scores for the adjectives “eccentric” and “easy to work with.” Cheetahs with severe gastritis are also more likely to have been exposed to suspected environmental stressors, such as small enclosures, high density of cheetahs, movement to multiple institutions over an individual’s lifetime, and high amounts of exposure to the public. In contrast, gastritis severity is negatively correlated with “excitable” and “aggressive” temperaments, a behavioral phenotype more often seen in wild-caught cheetahs. A negative correlation with “aggressive” is also noted with corticoid concentrations. Cheetahs with opportunities for exercise (running on a lure) have lower fecal glucocorticoid concentrations (Terio et al., 2014), supporting the importance of regular exercise for captive cheetahs.
Understanding the cheetah stress response is important not only for the health and well-being of captive cheetahs, but for management of free-ranging cheetahs. Wild-caught cheetahs temporarily held in captivity develop diseases like those noted in captive-born cheetahs, which may impact the cheetahs’ survival when released back into the wild. One wild cheetah that had previously been in captivity had evidence of “captive cheetah” diseases at the time of death (Munson et al., 2005). As capture, holding, and translocation (Chapter 20) are tools that can be used for mitigating human-wildlife conflict and habitat fragmentation (Chapters 10 and 131013), research aimed at reducing stress in these situations is essential to minimize the impact of capture and captivity on the health of cheetahs worldwide (Teixeira et al., 2007).
Important diseases in the wild
Infectious Disease
Anthrax
There have been multiple published and anecdotal cases of anthrax in wild and captive cheetahs (Ebedes, 1976, Good et al., 2008; Jäger et al., 1990; de Pienaar, 1961). Most outbreaks can be linked to contaminated carcasses fed to captive animals or concurrent outbreaks in other wildlife. Affected cheetahs may have increased respiratory rate and effort and can vomit (Jäger et al., 1990). Similar to other carnivores, cheetahs may have ventral and cervical edema, bloody nasal discharge, scleral congestion, and pulmonary edema (Good et al., 2008; Jäger et al., 1990). Serosurveys after an anthrax outbreak in Botswana found that only 1 of 16 potentially exposed cheetahs had antibodies (Good et al., 2008) and cheetahs in Namibia also do not have antibodies to anthrax (Switzer et al., 2016). These results support claims that cheetahs are susceptible because they have low levels of exposure (as they generally do not scavenge), thus infection is often fatal (Lindeque et al., 1998). Vaccination may be of benefit for certain high-risk populations as cheetahs do mount a potentially protective response to a commercially available anthrax vaccine (Turnbull et al., 2004).
Mycobacterium bovis
Spillover infection of Mycobacterium bovis occurs in cheetahs in areas with infected hoofstock (Keet et al., 1996). Typical granulomatous lesions occur in the lungs of affected cats but the lower airways can also be affected, suggesting that environmental contamination and or direct spread from affected cheetahs is possible. It is unlikely that cheetahs would become maintenance hosts for the disease as their contact rates are relatively low. However, sporadic spill over both to and from cheetahs is a possibility, especially in endemic areas (De Vos et al., 2001).
Sarcoptic Mange
Wild cheetahs in the Masai Mara in East Africa have been diagnosed with mange associated with Sarcoptes scabei infection that is a significant infectious cause of morbidity and mortality (Mwanzia et al., 1995). One study found the prevalence in cheetahs to be 12.8% (Gakuya et al., 2012). Lesions in affected cheetahs include hair loss and thickening of the skin. Infection in cheetahs was associated with the dry season and in particular S. scabei infection in Thompson’s gazelles.
Other Health Problems
Ocular Trauma
Traumatic eye injuries including scratches and lacerations to the lid, nictitans, and cornea, with penetration and secondary uveitis and cataracts, have been documented in free-ranging cheetahs in Namibia (Bauer, 1998). In rare cases, portions of thorns have been found within affected eyes leading to the hypothesis that the trauma is caused by encroachment of thorn bushes due to habitat degradation.
Important diseases in captivity
Infectious Diseases
Helicobacter-Associated Gastritis
The majority of captive and wild cheetahs worldwide are infected with Helicobacter sp., spiral bacteria that colonize the stomach. In wild cheetahs there are numerous bacteria, but little to no associated inflammation (Terio et al., 2005). In contrast, captive cheetahs commonly have some degree of inflammation (gastritis; Fig. 25.1 ) that may be asymptomatic or associated with regurgitation, vomiting, passage of undigested feed, and weight loss (Eaton et al., 1993, Terio et al., 2011). Although gastric colonization with Helicobacter sp. can be confirmed using the urea breath test, antemortem diagnosis of gastritis requires histologic evaluation of endoscopic biopsies (Chapter 24). It should be noted that the appearance of the stomach on endoscopy does not always correlate with the degree of histologic inflammation and damage.
Figure 25.1.

(A) Stomach from a captive cheetah with gastritis. Note the thickened, reddened mucosa. (B) Histologic section of stomach from a captive cheetah with gastritis with large numbers of inflammatory cells in the mucosa, superficial epithelial erosion, and loss of gastric parietal cells. Inset: Normal stomach from a wild cheetah.
The pathogenesis of gastritis is not completely understood. The absence of gastritis in the few cheetahs without evidence of ever being colonized by Helicobacter suggests that the bacteria play a role, but Helicobacter are not the only factor in disease development [Association of Zoos and Aquaria (AZA) Cheetah Species Survival Plan® (SSP) Pathology Archives, unpublished data]. In cheetahs, the distribution of T lymphocytes (T cells) and diffuse upregulation of MHC II expression on gastric epithelium are qualitatively similar to that in other species infected with Helicobacter (Terio et al., 2011). What distinguishes cheetahs with severe gastritis from those with mild disease are large numbers of activated B cells (CD79a+ CD21−) and plasma cells (Fig. 25.1B) which may be due to alterations in the Th1:Th2 cytokine balance and stress-induced modulation of the immune system (Terio et al., 2011).
Longitudinal studies of gastritis in captive cheetahs have documented that while inflammation can wax and wane, in general, it worsens over time irrespective of treatment (Citino and Munson, 2005). Some lesions progress to fibrosis (scarring) and gastric atrophy that limits the ability of cheetahs to eat and digest a complete meal in one sitting (Citino and Munson, 2005, Terio et al., 2011). Although treatment with antibiotic triple therapy may provide short-term decrease in inflammation and eradication of Helicobacter, none of the treatments evaluated to date have had a significant effect on long-term Helicobacter infection or the lifelong progression of inflammation (Citino and Munson, 2005, Lane et al., 2004, Wack et al., 1997). The use of triple therapies may also impact the gut microbiome and potentially predispose to other conditions. Therefore, antibiotic treatment is recommended only in severely affected or symptomatic cheetahs. Diet may contribute to and or ameliorate signs of gastritis, but there are contradictory results on the impact of diet on gastritis severity (Lane et al., 2012, Whitehouse-Tedd et al., 2015).
Herpesvirus
Captive cheetahs are commonly infected with feline herpesvirus and can develop mild sneezing, nasal discharge, and ocular lesions similar to other felids (van Vuuren et al., 1999). In some cases, severe corneal ulcers and/or keratitis and proliferative skin lesions on the face and/or forelimbs are noted (Flacke et al., 2015, Junge et al., 1991, Munson et al., 2004a, Munson et al., 2004b, Witte et al., 2013) (Fig. 25.2 ). Disease has not been noted in wild cheetahs despite evidence of viral exposure (Munson et al., 2004a, Munson et al., 2004b, Munson et al., 2005, Thalwitzer et al., 2010). Studies of the virus have found genetic and antigenic similarity with feline herpesvirus type 1 (FHV-1) (Scherba et al., 1988).
Figure 25.2.
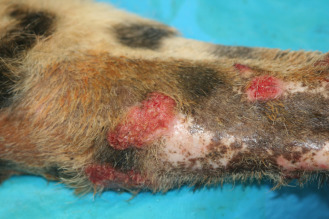
Plaque-like proliferative and ulcerated skin lesion on the forelimb of a cheetah with herpesviral dermatitis.
Diagnosis of FHV-1 infection relies on characteristic clinical signs and/or specific diagnostic tests, such as virus isolation, PCR, histopathology with characteristic herpesviral inclusions, or immunohistochemistry (Witte et al., 2013). Serology alone cannot be used to confirm infection (Witte et al., 2013). Biopsy is essential to differentiate proliferative skin lesions from neoplasms (tumors). Histologically, dense infiltrates of eosinophils and mast cells are present in the dermis, and the intact epithelium adjacent to the ulcer may be hyperplastic with viral inclusions (Munson et al., 2004a, Munson et al., 2004b). Treatment for mild FHV-1 is supportive. Administration of l-lysine and famcyclovir have been helpful in some cases, while skin lesions often require cryotherapy. Vaccination is recommended, although variable responses have been noted in cheetahs and natural exposure is not uncommon (Spencer and Burroughs, 1992, Wack et al., 1993; Chapter 24).
Immune responses to FHV-1 in cheetah have been studied in vitro (Miller-Edge and Worley, 1992). In these studies, cheetah cells (lymphocytes and monocytes) as a group had a significantly lower proliferative response to FHV-1 than domestic cats, although individual responses varied widely. Interestingly, cheetah cellular responses improved when they were first cultured with interleukin-2 (IL-2), a TH-1 cytokine critical to an effective cell-mediated immune response. As previously discussed, low levels of IL-2 have been found in captive but not wild cheetahs (Terio et al., 2005), suggesting that ineffective immune responses, possibly due to stress-induced suppression, may play a role. Disease tends to be more severe if cubs are infected by their dams at a young age (<3 weeks). As cubs at this age should still have maternal antibodies, this finding suggests inadequate colostral antibodies against herpesvirus. Often, these cubs are also more likely to have recurrent lesions throughout their lives.
Feline Infectious Peritonitis/Feline Enteric Coronavirus
Feline enteric coronavirus (FCoV) infection can cause mild enteritis, as well as fatal FIP. Lesions and clinical signs of FIP in cheetahs are similar to those described in domestic cats and include high protein effusions within the abdominal and or thoracic cavities with pyogranulomatous inflammation centered on vessels. In 1983, there was an epidemic of FIP in a captive cheetah population with >60% mortality that resulted in stringent testing and quarantine measures to limit the spread of disease within the North American captive cheetah population (Heeney et al., 1990). Complicating attempts to control spread of the disease was a lack of concordance between serology and PCR identification of virus from feces (Kennedy et al., 2001, Munson and Citino, 2005). Some cheetahs have been found to shed the virus only intermittently to rarely; therefore, captive populations are now managed as endemically infected (Gaffney et al., 2012, Munson and Citino, 2005).
Despite evidence for continued presence of FCoV in the population, fatalities to FIP have been rare (<2.9% total deaths from 1991 to 2016, AZA SSP Pathology Archives). The role of FCoV in enteritis is less clear and an area of needed study. Some cheetahs appear to be persistently infected with FCoV and persistently shed the virus, and could be a source for viral replication and possible mutation to more virulent strains (Kennedy et al., 2006). Some persistently infected cheetahs appear to develop ulcerative colitis with weight loss and lymphoid proliferation.
Fungal Disease
Although fungal disease is generally rare in cheetahs, there have been several individual case reports of Cryptococcus infections in captive cheetahs (Beehler, 1982, Berry et al., 1997, Bolton et al., 1999, Illnait-Zaraozí et al., 2011, Millward and Williams, 2005). Infections present with nasal discharge, neurological deficits, and shortness of breath; lesions are similar to those noted in domestic cats. In these cases, fungi have been classified using varying methods as either Cryptococcus neoformans, C. neoformans var gattii, or C. gattii. C. neoformans var gattii has been recently reclassified as C. gattii, so it is uncertain whether these cases truly represent C. neoformans and C. gattii infections or just C. gattii. There is no evidence to suspect inherent or acquired susceptibility to this infection in cheetah as immune responses are similar to those noted in domestic cats; there has not been any evidence of concurrent infection with immunosuppressive viruses (e.g., feline immunodeficiency virus or feline leukemia virus) (Berry et al., 1997, Bolton et al., 1999, Miller-Edge and Worley, 1992).
Dermatophytosis is common in some captive populations of cheetahs. Affected cheetahs have patchy to generalized alopecia and Microsporum canis can be cultured from affected hair. Young cubs can have more severe infections with involvement of tail tips and nail beds that, if not treated aggressively, can lead to sloughing of nails, digits, and/or the tail tip. Adverse reactions have been noted in cats treated with griseofulvin, including lethargy, diarrhea, bone marrow depletion, and death (Wack et al., 1992). Therefore, topical treatments rather than systemic antifungal therapies are recommended.
Other Infectious Skin and Oral Mucosal Conditions
There have been multiple outbreaks of orthopoxvirus infection resulting in ulcerated skin and oral lesions and, in rare cases, severe acute respiratory disease (Baxby et al., 1982, Marennikova et al., 1977). Histologically, poxviruses were suspected due to large eosinophilic intracytoplasmic inclusions and virus characteristics consistent with cowpox. Rodents used as intended food items, as well as wild rodents caught by cheetahs are the source of infection (Baxby et al., 1982, Marennikova and Shelukhina, 1976).
Sublingual plaques are not uncommon and may be related to papillomavirus infection. A potential cause of oral ulceration is calicivirus. In contrast to herpesvirus, caliciviral infections tend to be self-limiting and more closely follow the course seen in domestic and other nondomestic felids. Treatment is supportive. One notable and important caveat is that caliciviral disease has developed after vaccination of some naïve cheetahs with modified live virus (MLV) vaccine. Similarly, although unproven due to similarities in viral strains, some cheetahs have developed herpesviral lesions after vaccination with MLV vaccines (see also Chapter 24).
Other Infectious Diarrheal Conditions
There are multiple potential causes of diarrhea in cheetah, including infection with parvoviruses, Salmonella spp., Clostridium perfringens, and Plesiomonas shigelloides. Parvoviruses indistinguishable from feline panleukopenia (FPLV) have been reported to cause diarrhea in cheetahs (Steinel et al., 2000, Valícek et al., 1993). Canine parvovirus type 2b has also been identified in cheetahs with chronic diarrhea due to chronic necrotizing enteritis (Steinel et al., 2000). None of these cheetahs had concurrent leukopenia. Molecular phylogeny of outbreaks of FPLV in South Africa has indicated the presence of more than one strain of the virus in captive cheetahs and other felids (Lane et al., 2016). As non-domestic species may be reservoirs of parvoviruses and these viruses readily change host specificity, the risks of FPLV transmission between captive bred and free-ranging carnivores, and domestic cats and dogs, warrants further research. Salmonella infection, including Salmonella typhimurium, is another potential cause of diarrhea, especially in cheetahs fed poultry or horsemeat (Venter et al., 2003). Complicating interpretation of positive fecal cultures are high rates of fecal shedding in seemingly healthy felids fed raw diets (Clyde et al., 1997).
Pancreatitis
In addition to infectious causes, diarrhea and vomiting can be noted in cheetahs with pancreatitis. Pancreatitis is not uncommon in captive cheetahs, especially if fed offal or diets high in fat. There is no association between pancreatitis and pancreatic duct ectasia, a common incidental finding.
Degenerative Disease
Amyloidosis
Cheetahs in captivity can develop amyloidosis when insoluble amyloid fibrils deposit within tissues where they disrupt organ function. Amyloidosis can be due to a genetic predisposition (protein sequence that is more likely to precipitate) or secondary to increased production of SAA which occurs in chronic inflammation or neoplasia. Previous studies identified concurrent inflammation in 100% of the cheetahs with amyloidosis; however, not all cheetahs with inflammation develop amyloidosis. Studies of the amyloid fibrils suggest that these deposits are due to deposition of the full serum amyloid A (SAA) protein, as well as truncated fragments (Bergström et al., 2006, Johnson et al., 1997). Studies of the cheetah AA protein sequence have found it to be similar to those of domestic cats with familial amyloidosis, suggesting a possible genetic predisposition (Chen et al., 2012, Johnson et al., 1997). A genetic role for increased production of the SAA protein was proposed (Zhang et al., 2008b). Subsequent studies, however, have determined that while specific genetic variants were associated with differing SAA serum concentrations, there was no significant association with disease (Franklin et al., 2016). To date, amyloidosis has not been described in a free-ranging cheetah (Munson et al., 2005; Terio and Mitchell, unpublished data).
In affected cheetahs, amyloid deposits most commonly within the kidney, specifically the medullary interstitium, leading to renal failure (Papendick et al., 1997). Amyloid fibrils may also be deposited within the liver, contributing to decreases in hepatic function. Clinical signs are similar to those in other types of renal and hepatic disease and require biopsy for definitive diagnosis. There is no specific treatment for amyloidosis, other than supportive therapy.
In some species AA amyloidosis is thought to be transmissible, and studies have identified AA fibrils within cheetah feces, suggesting a possible mechanism for transmission (Zhang et al., 2008a). If true, this would help explain the high prevalence of the disease in certain collections. As cheetahs are not typically coprophagic and enclosures are cleaned daily in most captive facilities, it is unclear whether this represents a true risk. Ongoing epidemiological studies of amyloidosis that controlled for the presence of concurrent inflammation have not yet found evidence for transmission within North American captive facilities (McLean and Garabed, 2015).
In addition to systemic amyloidosis, beta amyloid deposits have been described in the brains of some cheetahs (Serizawa et al., 2012). Although amyloid deposits can develop in aged animals of many species, some affected cheetahs had concurrent neurofibrillary tangles similar to the pattern identified in humans with Alzheimer’s disease. Declines in cognitive function are difficult to assess in wild animals, and the relative advanced age of affected cats (all were >10 years) suggests that while this may impact the well-being of select captive cheetahs, it is unlikely to be of conservation significance.
Glomerulosclerosis
Glomerulosclerosis is a primary renal disease of cheetahs and, as with many other diseases, occurs primarily in captive cheetahs (Munson, 1993, Munson et al., 1999, Munson et al., 2005). Damage to renal glomeruli consists of thickening of basement membranes due to deposition of collagen, glycoproteins, and advanced glycosylation end products (Bolton and Munson, 1999). Glomerular damage leads to renal inflammation and scarring. The severity and prevalence of glomerulosclerosis increases with age, but there is no sex predilection. Clinically, affected cheetahs have signs of renal disease and, eventually, failure with proteinuria due to leakage through damaged glomeruli. Treatment is supportive. Feeding diets formulated for renal disease or commercial cat diets rather than raw meat may provide some clinical improvement, particularly in serum urea nitrogen (Lane et al., 2012). The cause of glomerulosclerosis is not known. Histologically, lesions resemble that of a diabetic nephropathy, although most affected cheetahs are not diabetic. The higher prevalence in captivity may be due to stress-induced hyperglycemia or another aspect of the captive environment.
Veno-Occlusive Disease
Veno-occlusive disease (VOD) is an unusual liver disease described in cheetahs and snow leopards. Scarring (subendothelial fibrosis) occurs in the central and sublobular hepatic veins with subsequent collapse and loss of the liver tissue surrounding affected vessels. Cirrhosis occurs in severe cases. Affected cheetahs can be asymptomatic or have signs of hepatic failure, including hypoproteinemia, abdominal effusion, elevated liver enzymes (aspartate aminotransferase, alanine aminotransferase), icterus, and in some cases neurologic signs due to hepatic encephalopathy (AZA Cheetah SSP Pathology Archives, unpublished data; Gosselin et al., 1988). In rare cases, effusions have been chylous (Terrell et al., 2003). A few cases of severe acute VOD have occurred in captive cheetahs with centrilobular to massive hepatic necrosis and hemorrhage (AZA Cheetah SSP Pathology Archives).
No specific cause for VOD has been proven in cheetahs. Estrogens are one cause for VOD in humans, and captive cheetah diets may contain high levels of phytoestrogens (Setchell et al., 1987). However, other lesions of excess dietary estrogen are not noted and the disease is seen in North American and South African captive cheetahs fed different diets (Munson et al., 1999). Interestingly, VOD has not been reported in the European captive population (Kotsch et al., 2002). Elevated dietary levels of vitamin A may cause VOD, but findings have been inconsistent (Gosselin et al., 1988). A few wild cheetahs have been diagnosed with VOD which seems to refute the hypothesis that captive diets are causative (Munson et al., 2005). Clinical pathology studies of trapped wild cheetahs have documented elevations in hepatic enzymes (alanine aminotransferase) within the first week of captivity, which could be the result of hepatocellular damage associated with VOD (Munson and Marker, 1997). One hypothesis is that catecholamine release due to the severe acute stress of capture leads to shunting of blood from the liver and hypoxic damage. The potential health impact of stress upon wild cheetahs during capture or periods of temporary captivity warrants further study.
Myelopathy
Myelopathy in cheetahs is a distinct neurological disorder characterized by degenerative lesions of the spinal cord, causing ataxia and paresis (Walzer and Kübber-Heiss, 1995). The disease emerged in the last 20 years and has limited growth of the European captive cheetah population (Walzer et al., 2003). To date, more than 100 cases have been identified in at least 16 different locations in Europe and the United Arab Emirates, resulting in euthanasia of many cheetahs in breeding programs. There is no apparent sex predilection. All affected cheetahs have been captive bred in European, Middle Eastern, or South African institutions from captive-born or wild-caught parents, belonging to the southern African subspecies (Acinonyx jubatus jubatus) or northeastern African subspecies (Acinonyx jubatus soemmeringii). Onset of ataxia ranges from 2.5 months to 12 years, is usually acute, and can occur spontaneously or following a stressful experience for the individual or for the litter. In cubs, clinical signs are often temporally associated with sneezing and ocular discharge typical of FHV-1 infection. The course of the disease is variable; ataxia and paresis may develop rapidly to hind limb dragging or recumbency, or progress slowly and stabilize for several months or years, with possible acute relapsing episodes. Pathologically, the disease is characterized by bilateral symmetrical degeneration of the white matter of the spinal cord, with loss of myelin exceeding axonal loss, suggesting a primary myelin disorder (Robert and Walzer, 2009). The etiology is unknown, but several causes have been considered (Burger et al., 2004, Palmer et al., 2001, Shibly et al., 2006, Walzer et al., 2003). Interestingly, the incidence of myelopathy has decreased, with only a single reported case since 2006.
Leukoencephalopathy (Leukoencephalomyelopathy)
In 1996, a novel neurodegenerative disease was seen in cheetahs at multiple captive breeding facilities throughout North America (Brower et al., 2014). Affected cheetahs were older (median and mean age of 12 years) and presented with ataxia and suspected blindness, leading to inability to follow keepers and locate food. Neurological signs included progressive altered behavior, disorientation, proprioceptive deficits, and, in some cases, seizures. Anesthesia in affected cheetahs was complicated by the need for increased doses of anesthetic agents and prolonged recoveries. Magnetic resonance imaging identified areas with white matter loss and hydrocephalus. Corresponding lesions were observed at necropsy. Testing for possible viruses and epidemiological evaluations failed to identify any potential etiologies. Cases increased with a peak of 22 cases in 1998 after which case numbers declined. No cases have been identified since 2005.
Other Renal Diseases
Cheetahs can also develop kidney disease due to oxalate nephrosis. Although oxalate nephrosis is commonly associated with exposure to ethylene glycol (antifreeze) in other species, exposure to ethylene glycol has not been confirmed despite extensive toxicological evaluations (AZA Cheetah SSP Pathology Survey; Mitchell, unpublished data). Furthermore, this disease has been diagnosed in cheetahs from multiple geographic locations, including North America, Europe, the Middle East, and southern Africa. Affected cheetahs develop acute renal failure due to tubular damage. Crystals can be noted in the urine antemortem. No correlation with other common cheetah diseases has been noted, so genetic and/or dietary causes are targets of ongoing investigations. Pyelonephritis is also noted in cheetahs, commonly females, and associated with coliform bacteria.
Other Conditions of Wild and Captive Cheetahs
In addition to the earlier specifically mentioned diseases and those in Table 25.1, Table 25.2 , cheetahs can be affected by other degenerative and infectious diseases common to the felid taxa. Exposure to environmental toxins (intentional and otherwise), ingestion of foreign bodies, as well as injuries from snake bites and insect bites/stings are possible with signs and lesions similar to those in other species.
Table 25.1.
Common Incidental Findings in Cheetahs
| Condition | Morphology | Occurrence | Reference |
|---|---|---|---|
| Kinked tails | Kink in posterior vertebra of tail | Wild and captive cheetahs | Marker-Kraus (1997) |
| Foam cell foci | Subpleural aggregates of foamy macrophages; appear as 1–2-mm yellow foci on lungs | Wild and captive cheetahs | Munson (1993), Munson and Terio (unpublished data) |
| Pancreatic duct ectasia | Dilation of pancreatic ducts without inflammation; can look like small cysts on the pancreas | Common in captive cheetahs; uncertain in wild cheetahs | Munson (1993) |
| Telangiectasia | Dilation and pooling of blood of hepatic sinusoids; red areas on liver | Wild and captive cheetahs | Munson (1993), Munson and Terio (unpublished data) |
| Parovarian cysts | Cystic structures adjacent to the ovary associated with remnant Wolffian ducts | Wild and wild-caught captive held cheetahs | Munson (1993), Munson and Terio (unpublished data) |
Table 25.2.
Uncommon Conditions of Wild and Captive Cheetahs
| Causes | Clinical signs/findings | Outcome | Reference |
|---|---|---|---|
| VIRAL | |||
| Astrovirus | Diarrhea, regurgitation | Recover with treatment | Atkins et al. (2009) |
| Canine Distemper Virus | Seropositivity in wild cheetahs | Unknown | Munson et al., 2004a, Munson et al., 2004b; Munson et al., 2005; Thalwitzer et al., 2010 |
| Feline leukemia virus (FeLV) | Lethargy, anorexia, lymphadenopathy | Multicentric T-cell lymphoma | Marker et al. (2003) |
| Feline immunodeficiency virus/cheetah immunodeficiency virus | Appears to be rare, incidental infection | None reported | |
| Papillomavirus | None | Incidental plaque-like lesions on tongue and oral cavity | |
| PARASITIC | |||
| Babesia spp. (multiple species) | RBC parasite in southern African cheetah | Uncertain, may be incidental finding | |
| Coccidia (Isospora) | Mild diarrhea | Recover with treatment | Penzhorn et al. (1994) |
| Cytauxzoon felis | Fever, lameness, severe lethargy | Potentially fatal; blood vessels filled with macrophages containing schizonts | |
| Giardia sp. | Mild diarrhea | Recover with treatment | |
| Haemoplasma felis | Rare infection | Incidental finding | Krengel et al. (2013) |
| Ollulanus tricuspis | Vomiting, gastritis | Recover with treatment | Collett et al. (2000) |
| Otodectes cynotis | Ear mite | Recover with treatment | |
| Theileria-like | Occasionally seen in RBC | Incidental finding | |
| Toxocara sp. | Intestinal parasite | Incidental finding except with heavy burdens in young cubs | |
| Toxoplasma sp. | Fever, respiratory distress, abdominal effusion | Multicentric organ necrosis with Toxoplasma zoites | Lloyd and Stidworthy (2007) |
| MISCELLANEOUS | |||
| Diabetes mellitus | Elevated blood sugar, fructosamine | Managed with treatment | Sanchez et al. (2005) |
| Lower esophageal sphincter dysfunction | Prolapse of stomach into esophagus and acquired diaphragmatic hernias | Associated with gastritis leading to gastric esophageal reflux, may be associated with acquired hiatal hernia | Teunissen et al. (1978); Kimber et al. (2001) |
| Prion/Spongiform encephalopathy | Behavioral changes, tremors, ataxia; PrP protein within brain | Fatal, associated with eating contaminated meat (e.g., beef infected with BSE) or vertical transmission | Peet et al. (1992); Kirkwood et al. (1995); Baron et al. (1997); Lezmi et al. (2003); Bencsik et al. (2009); Eiden et al. (2010) |
| Soft tissue mineralization | Renal failure if kidney involved | If renal involvement, can be fatal. No apparent exposure to vitamin D | |
BSE, Bovine Spongiform Encephalopathy; RBC, red blood cell.
Focal Palatine Erosion
Cheetahs have a set of depressions in the hard palate; the most pronounced one being medial to the first upper molar to accommodate the mandibular molar (carnassial tooth) (Steenkamp et al., 2017; Chapter 7). In some wild and captive cheetahs, corresponding depressions are accompanied with mucosal lesions (Fitch and Fagan, 1982). In severe cases, the palatine bone is perforated resulting in communication between the oral and nasal cavities. Both mild and severe lesions were initially coined Focal Palatine Erosion (FPE). When first identified, the soft nature of captive diets and absence of exposure to tougher dietary items like skin and bones were presumed to play a role in the pathogenesis (Phillips et al., 1993). However, studies in Namibia found that over 40% of wild cheetahs had deep palatine depressions with perforation of the palate in 15.3%, suggesting that captive diets are not causative (Marker and Dickman, 2004). In this study, wild cheetahs with FPE were also more likely to have crowded lower incisors and absent upper premolars. Studies of museum skulls identified only rare cases in free-ranging East African cheetahs, suggesting that FPE may be more common in the Namibian population (Zordan et al., 2012).
Neoplasia
Tumors of any organ system are possible in cheetahs, but generally uncommon with the exception of myelolipomas. Myelolipomas are benign, often multiple, tumors of the spleen and occasionally the liver, composed of mature adipocytes (fat cells) mixed with hematopoietic cells (Munson, 1993, Munson et al., 1999). These tumors can be identified by ultrasound and have been mistaken for more malignant tumors resulting in unnecessary removal of the spleen (Walzer et al., 1996). Myelolipomas also occur in wild cheetahs (Mitchell, unpublished data). Cutaneous mast cell tumors and systemic mastocytosis are also noted with some frequency (AZA SSP Pathology Archives).
Diseases of neonatal and juvenile cheetahs
Many of the important noninfectious diseases of neonatal and juvenile cheetahs are nutritionally based (Chapters 24 and 262426). Bilateral carpal valgus deformities that may require surgical repair have been noted in captive cheetahs at multiple facilities (Allan et al., 2008, Bell et al., 2011). In these cases, cubs presented with lameness around 6 months of age or during weaning. Keeping hand-reared kittens on formula too long may predispose to this condition; adding meat early (e.g., 3 weeks) and weaning by 5-6 weeks can be preventative. Excess dietary calcium can inhibit cartilage maturation and contribute to osteochondrosis, an underlying cause of these deformities (Allan et al., 2008). In some cases, however, affected cheetahs were fed the same diet as unaffected littermates, suggesting that other factors may also be involved (Bell et al., 2011). Fractures have also been reported in some cheetahs, especially in king cheetahs, after minimal trauma, raising concerns about inappropriate dietary mineral content.
Conclusions
Much of our understanding of disease in cheetahs comes from long-term pathology surveys of cheetahs in captivity and in the wild. As many of the diseases of cheetahs occur primarily in captivity, it is incumbent upon us to better understand and reduce risk factors that lead to this higher prevalence of disease in captive settings. Using death codes reported in the International Studbook from 1980 to 2009, the relative prevalence of reported infectious disease decreased from 50% to less than 25%, suggesting improvements in infectious disease management (Schmidt-Küntzel et al., in preparation). Excluding infectious, accidental, and congenital causes, diseases impacting the urinary system have been increasing from less than 20% in 1980–84 to over 45% of cases from 2005 to 2009. This likely reflects increasing longevity of the captive cheetah population, with a corresponding increase in chronic renal disease, a common cause of death in many domestic and wild aged felids. Unfortunately, some diseases continue to be intractable. Continued research into management is important not only for improved well-being and medical management of captive populations, but also in limiting development of disease in wild-caught cheetahs that are held in captive settings, either temporarily or permanently, due to human-wildlife conflict (Chapter 13) or other reasons. Although previous studies of wild cheetahs have found them to be generally healthy, it is known that they have exposure to many of the infectious agents that can cause problems in captive cheetahs. It is also possible that pressures including reduced size and quality of free-ranging cheetah habitats (Chapter 10), increased contact with domesticated animals, and climate change (Chapter 12) could change disease dynamics. Continued monitoring of both captive and free-ranging cheetah populations, especially small geographically isolated populations and an improved understanding of normal clinical values, is needed for understanding and management of disease risks to species survival.
References
- Allan G., Portas T., Bryant B., Howlett R., Blyde D. Ulnar metaphyseal osteochondrosis in several captive bred cheetahs (Acinonyx jubatus) Vet. Radiol. Ultrasound. 2008;49:551–556. doi: 10.1111/j.1740-8261.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- Atkins A., Wellehan J.F., Jr., Childress A.L., Archer L.L., Fraser W.A., Citino S.B. Characterization of an outbreak of astroviral diarrhea in a group of cheetahs (Acinonyx jubatus) Vet. Microbiol. 2009;136:160–165. doi: 10.1016/j.vetmic.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron T., Belli P., Madec J.Y., Moutou F., Vitaud C., Savey M. Spongiform encephalopathy in an imported cheetah in France. Vet. Rec. 1997;141:270–271. doi: 10.1136/vr.141.11.270. [DOI] [PubMed] [Google Scholar]
- Bauer, G.A., 1998. Cheetah—running blind. In: Penzhorn, B.L. (Ed.), Proceedings of a Symposium on Cheetahs as Game Ranch Animals. Onderstepoort, pp. 106–108.
- Baxby D., Ashton D.G., Jones D.M., Thomsett L.R. An outbreak of cowpox in captive cheetahs: virological and epidemiological studies. J. Hyg. (Lond.) 1982;89:365–372. doi: 10.1017/s0022172400070935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beehler B.A. Oral therapy for nasal cryptococcosis in a cheetah. J. Am. Vet. Med. Assoc. 1982;181:1400–1401. [PubMed] [Google Scholar]
- Bell K.M., van Zyl M., Ugarte C.E., Hartman A. Bilateral carpal valgus deformity in hand-reared cheetah cubs (Acinonyx jubatus) Zoo Biol. 2011;30:199–204. doi: 10.1002/zoo.20328. [DOI] [PubMed] [Google Scholar]
- Bencsik A., Debeer S., Petit T., Baron T. Possible case of maternal transmission of feline spongiform encephalopathy in a cheetah. PLoS One. 2009;4:e6929. doi: 10.1371/journal.pone.0006929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J., Ueda M., Une Y., Sun X., Misumi S., Shoji S., Ando Y. Analysis of amyloid fibrils in the cheetah (Acinonyx jubatus) Amyloid. 2006;13:93–98. doi: 10.1080/13506120600722621. [DOI] [PubMed] [Google Scholar]
- Berry W.L., Jardine J.E., Espie I.W. Pulmonary cryptococcoma and cryptococcal meningoencephalomyelitis in a king cheetah (Acinonyx jubatus) J. Zoo Wildl. Med. 1997;28:485–490. [PubMed] [Google Scholar]
- Bolton L.A., Lobetti R.G., Evezard D.N., Picard J.A., Nesbit J.W., van Heerden J., Burroughs R.E. Cryptococcocsis in captive cheetah (Acinonyx jubatus): two cases. J. S. Afr. Vet. Assoc. 1999;70:35–39. doi: 10.4102/jsava.v70i1.748. [DOI] [PubMed] [Google Scholar]
- Bolton L.A., Munson L. Glomerulosclerosis in captive cheetahs (Acinonyx jubatus) Vet. Pathol. 1999;36:14–22. doi: 10.1354/vp.36-1-14. [DOI] [PubMed] [Google Scholar]
- Brower A.I., Munson L., Radcliffe R.W., Citino S.B., Bingaman Lackey L., Van Winkle T.J., Stalis I., Terio K.A., Summers B.A., de Lahunta A. Leukoencephalomyelopathy of mature captive cheetahs and other large felids: a novel neurodegenerative disease that came and went? Vet. Pathol. 2014;51:1013–1021. doi: 10.1177/0300985813506917. [DOI] [PubMed] [Google Scholar]
- Burger P.A., Steinborn R., Walzer C., Petit T., Mueller M., Schwarzenberger F. Analysis of the mitochondrial genome of cheetahs (Acinonyx jubatus) with neurodegenerative disease. Gene. 2004:111–119. doi: 10.1016/j.gene.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Une Y., Higuchi K., Mori M. Cheetahs have 4 serum amyloid A genes evolved through repeated duplication events. J. Hered. 2012;103:115–129. doi: 10.1093/jhered/esr105. [DOI] [PubMed] [Google Scholar]
- Citino S.B., Munson L. Efficacy and long-term outcome of gastritis therapy in cheetahs (Acinonyx jubatus) J. Zoo Wildl. Med. 2005;36:401–416. doi: 10.1638/03-117.1. [DOI] [PubMed] [Google Scholar]
- Clyde V.L., Ramsay E.C., Bemis D.A. Fecal shedding of Salmonella in exotic felids. J. Zoo Wildl. Med. 1997;28:148–152. [PubMed] [Google Scholar]
- Collett M.G., Pomroy W.E., Guilford W.G., Johnstone A.C., Blanchard B.J., Mirams S.G. Gastric Ollulanus tricuspis infection identified in captive cheetahs (Acinonyx jubatus) with chronic vomiting. J. S. Afr. Vet. Assoc. 2000;71:251–255. doi: 10.4102/jsava.v71i4.727. [DOI] [PubMed] [Google Scholar]
- de Pienaar U.V. A second outbreak of anthrax amongst game animals in the Kruger National Park. Koedoe. 1961;4:4–16. [Google Scholar]
- De Vos V., Bengis R.G., Kriek N.P., Michel A., Keet D.F., Raath J.P., Huchzermeyer H.F. The epidemiology of tuberculosis in free-ranging African buffalo (Syncerus caffer) in the Kruger National Park, South Africa. Onderstepoort J. Vet. Res. 2001;68:119–130. [PubMed] [Google Scholar]
- Eaton K.A., Radin M.J., Kramer L.W., Wack R.F., Sherding R., Krakowka S., Fox J.G. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus) Vet. Pathol. 1993;30:55–63. doi: 10.1177/030098589303000107. [DOI] [PubMed] [Google Scholar]
- Ebedes H. Anthrax epizoonotics in Etosha National Park. Madoqua. 1976;10:99–118. [Google Scholar]
- Eiden M., Hoffmann C., Balkema-Buschmann A., Müller M., Baumgartner K., Groschup M.H. Biochemical and immunohistochemical characterization of feline spongiform encephalopathy in a German captive cheetah. J. Gen. Virol. 2010;91:2874–2883. doi: 10.1099/vir.0.022103-0. [DOI] [PubMed] [Google Scholar]
- Evermann J.F., Heeney J.L., Roelke M.E., McKeirnan A.J., O’Brien S.J. Biological and pathological consequences of feline infectious peritonitis virus infection in the cheetah. Arch. Virol. 1988;102:155–171. doi: 10.1007/BF01310822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch H.M., Fagan D.A. Focal palatine erosion associated with dental malocclusion in captive cheetahs. Zoo Biol. 1982;1:295–310. [Google Scholar]
- Flacke G.L., Schmidt-Küntzel A., Marker L. Treatment of chronic herpesviral dermatitis in a captive cheetah (Acinonyx jubatus) in Namibia. J. Zoo Wildl. Med. 2015;46:641–646. doi: 10.1638/2014-0206.1. [DOI] [PubMed] [Google Scholar]
- Franklin A.D., Schmidt-Küntzel A., Terio K.A., Marker L., Crosier A.E. Serum amyloid A protein concentration in blood is influenced by genetic differences in the cheetah (Acinonyx jubatus) J. Hered. 2016;117:115–121. doi: 10.1093/jhered/esv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney P.M., Kennedy M., Terio K., Gardner I., Lothamer C., Coleman K., Munson L. Detection of feline coronavirus in cheetah (Acinonyx jubatus) feces by reverse transcription-nested polymerase chain reaction in cheetahs with variable frequency of viral shedding. J. Zoo Wildl. Med. 2012;43:776–786. doi: 10.1638/2011-0110R1.1. [DOI] [PubMed] [Google Scholar]
- Gakuya F., Ombui J., Maingi N., Muchemi G., Ogara W., Soriquer R.C., Alassad S. Sarcoptic mange and cheetah conservation in Masai Mara (Kenya): epidemiological study in a wildlife/livestock system. Parasitology. 2012;139:1587–1595. doi: 10.1017/S0031182012000935. [DOI] [PubMed] [Google Scholar]
- Good K.M., Houser A., Antzen L., Turnbull P.C.B. Naturally acquired anthrax antibodies in a cheetah (Acinonyx jubatus) in Botswana. J. Wildl. Dis. 2008;44:721–723. doi: 10.7589/0090-3558-44.3.721. [DOI] [PubMed] [Google Scholar]
- Gosselin S.J., Loudy D.L., Tarr M.J., Balisteri W.F., Setchell K.D., Johnston J.O., Kramer L.W., Dresser B.L. Veno-occlusive disease of the liver in captive cheetah. Vet. Pathol. 1988;25:48–57. doi: 10.1177/030098588802500107. [DOI] [PubMed] [Google Scholar]
- Heeney J.L., Evermann J.F., McKeirnan A.J., Marker-Kraus L., Roelke M.E., Bush M., Wildt D.E., Meltzer D.G., Colly L., Lukas L., Manton V.J., Caro T., O’Brien S.J. Prevalence and implications of feline coronavirus infections in captive and free-ranging cheetahs (Acinonyx jubatus) J. Virol. 1990;64:1964–1972. doi: 10.1128/jvi.64.5.1964-1972.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illnait-Zaraozí M.T., Hagen F., Fernández-Andreu C.M., Martinez-Machin G.F., Polo-Leal J.L., Boekhout T., Klaassen C.H., Meis J.F. Reactivation of a Cryptococcus gattii infection in a cheetah (Acinonyx jubatus) held in the National Zoo, Havanna, Cuba. Mycoses. 2011;54:e889–e892. doi: 10.1111/j.1439-0507.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- Jäger H.G., Booker H.H., Hubschle O.J. Anthrax in cheetahs (Acinonyx jubatus) in Namibia. J. Wildl. Dis. 1990;26:423–424. doi: 10.7589/0090-3558-26.3.423. [DOI] [PubMed] [Google Scholar]
- Johnson K.H., Sletten K., Munson L., O’Brien T.D., Papendick R., Westermark P. Amino acid sequence analysis of amyloid protein (AA) from cats (captive cheetahs: Acinonyx jubatus) with a high prevalence of AA amyloidosis. Amyloid. 1997;4:171–177. [Google Scholar]
- Junge R.E., Miller R.E., Boever W.J., Scherba G., Sundberg J. Persistent cutaneous ulcers associated with feline herpesvirus 1 infection in a cheetah. J. Am. Vet. Med. Assoc. 1991;198:1057–1058. [PubMed] [Google Scholar]
- Jurke M.H., Czekala N.M., Lindburg D.G., Millard S.E. Fecal corticoid metabolite measurement in the cheetah (Acinonyx jubatus) Zoo Biol. 1997;16:133–147. [Google Scholar]
- Keet D.F., Kriek N.P., Penrith M.L., Michel A., Huchzermeyer H. Tuberculosis in buffaloes (Synverus caffer) in the Kruger National Park: spread of the disease to other species. Onderstepoort J. Vet. Res. 1996;63:239–244. [PubMed] [Google Scholar]
- Kennedy M., Citino S., Dolorico T., McNabb A.H., Moffat A.S., Kania S. Detection of feline coronavirus infection in captive cheetahs (Acinonyx jubatus) by polymerase chain reaction. J. Zoo Wildl. Med. 2001;32:25–30. doi: 10.1638/1042-7260(2001)032[0025:DOFCII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kennedy M.A., Moore E., Wilkes R.P., Citino S.B., Kania S.A. Analysis of genetic mutations in the 7a7b open reading frame of coronavirus of cheetahs (Acinonyx jubatus) Am. J. Vet. Res. 2006;67:627–632. doi: 10.2460/ajvr.67.4.627. [DOI] [PubMed] [Google Scholar]
- Kimber K., Pye G.W., Dennis P.M., Citino S.B., Heart D.J., Bennett R.A. Diaphragmatic hernias in captive cheetahs (Acinonyx jubatus) Proceedings of the AAZV, AAWV, ARAV, NAZWV Joint Conference. 2001;151 [Google Scholar]
- Kirkwood J.K., Cunningham A.A., Flach E.j., Thornton S.M., Wells G.A.H. Spongiform encephalopathy in another captive cheetah (Acinonyx jubatus): Evidence for variation in susceptibility or incubation periods between species? J. Zoo Wildl. Med. 1995;26:577–582. [Google Scholar]
- Kotsch V., Kübber-Heiss A., Url A., Walzer C., Schmidt P. Diseases of captive cheetahs (Acinonyx jubatus) within the European Endangered Species Program (EEP)—a 22-year retrospective histopathological study. Vet. Med. Austria. 2002;89:341–350. [Google Scholar]
- Krengel A., Meli M., Cattori V., Wachter B., Willi B., Thalwitzer S., Melzheimer J., Hofer H., Lutz H., Hofmann-Lehmann R. First evidence of hemoplasma infection in free-ranging Namibian cheetahs (Acinonyx jubatus) Vet. Microbiol. 2013;162:972–976. doi: 10.1016/j.vetmic.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Lane E.P., Brettschneider H., Caldwell P., Oosthuizen A., Dalton D.L., du Plessis L., Steyl J., Kotze A. Feline panleukopaenia virus in captive non-domestic felids in South Africa. Onderstepoort J. Vet. Res. 2016;83:a1099. doi: 10.4102/ojvr.v83i1.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E., Lobetti R., Burroughs R. Treatment with omeprazole, metronidazole, and amoxicillin in captive South African cheetahs (Acinonyx jubatus) with spiral bacterial infection and gastritis. J. Zoo Wildl. Med. 2004;35:15–19. doi: 10.1638/03-050. [DOI] [PubMed] [Google Scholar]
- Lane E.P., Miller S., Lobetti R., Caldwell P., Bertschinger H.J., Burroughs R., Kotze A., van Dyk A. Effect of diet on the incidence of and mortality owing to gastritis and renal disease in captive cheetahs (Acinonyx jubatus) in South Africa. Zoo Biol. 2012;31:669–682. doi: 10.1002/zoo.20431. [DOI] [PubMed] [Google Scholar]
- Lezmi S., Benscik A., Monks E., Petit T., Baron T. First case of feline spongiform encephalopathy in a captive cheetah born in France: PrP9sc analysis in various tissues revealed unexpected targeting of kidney and adrenal gland. Histochem. Cell Biol. 2003;119:415–422. doi: 10.1007/s00418-003-0524-5. [DOI] [PubMed] [Google Scholar]
- Lindeque, P.M., Nowell, K., Preisser, T., Brain, C., Turnbull, P.C.B., 1998. Anthrax in wild cheetahs in the Etosha national Park, Namibia. In: Proceedings ARC-Onderstepoort OIE International Anthrax Congress, Onderstepoort, pp. 9–15.
- Lloyd C., Stidworthy M.F. Acute disseminated toxoplasmosis in a juvenile cheetah (Acinonyx jubatus) J. Zoo Wildl. Med. 2007;38:475–478. doi: 10.1638/2007-0016.1. [DOI] [PubMed] [Google Scholar]
- Marennikova S.S., Maltseva N.N., Korneeva V.I., Garanina N.M. Outbreak of pox disease among carnivore (Felidae) and Edentata. J Infect. Dis. 1977;135:358–366. doi: 10.1093/infdis/135.3.358. [DOI] [PubMed] [Google Scholar]
- Marennikova S.S., Shelukhina E.M. White rats as a source of pox infection in Carnivora of the family Felidae. Acta Virol. 1976;20:442. [PubMed] [Google Scholar]
- Marker-Kraus, L., 1997. Morphological abnormalities reported in Namibian cheetahs (Acinonyx jubatus). In: Proceedings 50th Anniversary Congress of the Veterinary Association of Namibia and the 2cd Africa Congress of the World Veterinary Association, 9.
- Marker L.L., Dickman A.J. Dental anomalies and incidence of palatal erosion in Namibian cheetahs (Acinonyx jubatus jubatus) J. Mammol. 2004;85:19–24. [Google Scholar]
- Marker L., Dickman A.J., Mills M.G.L., Macdonald D.W. Aspects of the management of cheetahs trapped on Namibian farmlands. Biol. Conserv. 2003;114:401–412. [Google Scholar]
- Marker L., Munson L., Basson P.A., Quackenbush S. Multicentric T-cell lymphoma associated with feline leukemia virus infection in a captive Namibian cheetah (Acinonyx jubatus) J. Wildl. Dis. 2003;39:690–695. doi: 10.7589/0090-3558-39.3.690. [DOI] [PubMed] [Google Scholar]
- McLean, K., Garabed, R., 2015. Amyloidosis in cheetahs (Acinonyx jubatus), transmissible? In: Proceedings Annual Meeting of the American Association of Zoo Veterinarians. Portland, Oregon.
- Miller-Edge M.A., Worley M.B. In vitro responses of cheetah mononuclear cells to feline herpesvirus-1 and Cryptococcus neoformans. Vet. Immunol. Immunopathol. 1992;30:261–274. doi: 10.1016/0165-2427(92)90143-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward I.R., Williams M.C. Cryptococcus neoformans granuloma in the lung and spinal cord of a free-ranging cheetah (Acinonyx jubatus). A clinical report and literature review. J. S. Afr. Vet. Assoc. 2005;76:228–232. doi: 10.4102/jsava.v76i4.432. [DOI] [PubMed] [Google Scholar]
- Munson L. Diseases of captive cheetahs (Acinonyx jubatus): results of the Cheetah Research Council pathology survey 1989–1992. Zoo Biol. 1993;12:105–124. [Google Scholar]
- Munson, L., Citino, S., 2005. Major health concerns of cheetahs. Infectious diseases: feline enteric coronavirus/feline infectious peritonitis virus. In: Proceedings of the AZA Cheetah SSP Disease Management Workshop, Yulee Florida, pp. 18–20.
- Munson, L., Marker, L., 1997. The impact of capture and captivity on the health of Namibian farmland cheetahs (Acinonyx jubatus). In: Proceedings of the 50th Namibian Vet Congress.
- Munson L., Marker L., Dubovi E., Spencer J.A., Evermann J.F., O’Brien S.J. Serosurvey of viral infections in free-ranging Namibian cheetahs (Acinonyx jubatus) J. Wildl. Dis. 2004;40:23–31. doi: 10.7589/0090-3558-40.1.23. [DOI] [PubMed] [Google Scholar]
- Munson L., Nesbit J.W., Meltzer D.G.A., Colly L.P., Bolton L., Kriek N.P.K. Diseases of captive cheetahs (Acinonyx jubatus) in South Africa: a 20-year retrospective survey. J. Zoo Wildl. Med. 1999;30:342–347. [PubMed] [Google Scholar]
- Munson L., Terio K.A., Worley M., Jago M., Bagot-Smith A., Marker L. Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J. Wildl. Dis. 2005;41:542–548. doi: 10.7589/0090-3558-41.3.542. [DOI] [PubMed] [Google Scholar]
- Munson L., Wack R., Duncan M., Montali R.J., Boon D., Stalis I., Crawshaw G.J., Cameron K.N., Mortenson J., Citino S., Zuba J., Junge R.E. Chronic eosinophilic dermatitis associated with persistent feline herpes virus infection in cheetahs (Acinonyx jubatus) Vet. Pathol. 2004;41:170–176. doi: 10.1354/vp.41-2-170. [DOI] [PubMed] [Google Scholar]
- Mwanzia, J.M., Kock, R., Wambua, J., Kock, N., Jarret, O., 1995. An outbreak of Sarcoptic mange in the free-living cheetah (Acinonyx jubatus) in the Mara region of Kenya. In: Proceedings of American Association of Zoo Veterinarians and American Association of Wildlife Veterinarians Joint Conference, pp. 105–112.
- Palmer A.C., Callanan J.J., Guerin L.A., Sheanan B.J., Stronach N., Franklin R.J.M. Progressive encephalomyelopathy and cerebellar degeneration in 10-captive bred cheetahs. Vet. Rec. 2001;149:49–54. doi: 10.1136/vr.149.2.49. [DOI] [PubMed] [Google Scholar]
- Papendick R.E., Munson L., O’Brien T.D., Johnson K.H. Systemic AA amyloidosis in captive cheetahs (Acinonyx jubatus) Vet. Pathol. 1997;34:549–556. doi: 10.1177/030098589703400602. [DOI] [PubMed] [Google Scholar]
- Peet R.L., Curran J.M. Spongiform encephalopathy in an imported cheetah (Acinonyx jubatus) Aust. Vet. J. 1992;69:171. doi: 10.1111/j.1751-0813.1992.tb07506.x. [DOI] [PubMed] [Google Scholar]
- Penzhorn B.L., Booth L.M., Meltzer D.G. Isospora rivolta recovered from cheetahs. J. S. Afr. Vet. Assoc. 1994;65:2. [PubMed] [Google Scholar]
- Phillips J.A., Worley M.B., Morsbach D., Williams T.M. Relationship between diet, growth, and occurrence of focal palatine erosion in wild-caught captive cheetahs. Madoqua. 1993;18:79–83. [Google Scholar]
- Robert N., Walzer C. Pathological disorders in captive cheetahs (Patologías de guepardos en cautividad) In: Vargas A., Breitenmoser-Würsten C., Breitenmoser U., editors. Iberian Lynx Ex Situ Conservation: An Interdisciplinary Approach (Conservación Ex Situ del Lince Ibérico: Un Enfoque Multidisciplinar) Fundación Biodiversidad in collaboration with: IUCN Cat Specialist Group; Madrid: 2009. pp. 265–272. [Google Scholar]
- Sanchez C., Bronson E., Deem S., Viner T., Pereira M., Saffoe C., Murray S. Diabetes mellitus in a cheetah: attempting to treat the untreatable? Proceedings of the AAZV, AWV, AZA/NAG Joint Conference. 2005;101 [Google Scholar]
- Scherba G., Hajjar A.M., Pernikoff D.S., Sundberg J.P., Basgall E.J., Leon-Monzon M., Nerukar L., Reichmann M.E. Comparison of a cheetah herpesvirus isolate to feline herpesvirus type 1. Arch. Virol. 1988;100:89–97. doi: 10.1007/BF01310910. [DOI] [PubMed] [Google Scholar]
- Serizawa S., Chambers J.K., Une Y. Beta amyloid deposition and neurofibrillary tangles spontaneously occur in the brains of captive cheetahs (Acinonyx jubatus) Vet. Pathol. 2012;49:304–312. doi: 10.1177/0300985811410719. [DOI] [PubMed] [Google Scholar]
- Setchell K.D., Gosselin S.J., Welsh M.B., Johnston J.O., Balistreri W.F., Kramer L.W., Dresser B.L., Tarr M.J. Dietary estrogens—a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93:225–233. doi: 10.1016/0016-5085(87)91006-7. [DOI] [PubMed] [Google Scholar]
- Shibly S., Schmidt P., Robert N., Walzer C., Url A. Immunohistochemical screening for viral agents in cheetahs (Acinonyx jubatus) with myelopathy. Vet. Rec. 2006;159:557–561. doi: 10.1136/vr.159.17.557. [DOI] [PubMed] [Google Scholar]
- Spencer J.A., Burroughs R. Decline in maternal immunity and antibody response to vaccine in captive cheetah (Acinonyx jubatus) cubs. J. Wildl. Dis. 1992;28:102–104. doi: 10.7589/0090-3558-28.1.102. [DOI] [PubMed] [Google Scholar]
- Steenkamp G., Boy S.C., van Staden P.J., Bester M.N. How the cheetahs’ specialized palate accommodates its abnormally large teeth. J. Zool. 2017;301:290–300. [Google Scholar]
- Steinel A., Munson L., van Vuuren M., Truyen U. Genetic characterization of feline parvovirus sequences from various carnivores. J. Gen. Virol. 2000;81:345–350. doi: 10.1099/0022-1317-81-2-345. [DOI] [PubMed] [Google Scholar]
- Switzer A.D., Munson L., Beesley C., Wilkins P., Blackburn J.K., Marker L. Free-ranging Namibian farmland cheetahs (Acinonyx jubatus) demonstrate immunologic naivety to anthrax (Bacillus anthracis) Afr. J. Wildl. Res. 2016;46:139–143. [Google Scholar]
- Teixeira C.P., Schetini de Azevedo C., Mendl M., Cipreste C.F., Young R.J. Revisiting translocation and reintroduction programmes: importance of considering stress. Anim. Behav. 2007;73:1–13. [Google Scholar]
- Terio, K.A., Munson, L., 2005. Linking stress with altered gastric immune responses in cheetahs. In: Proceedings Annual Meeting of the American Association of Zoo Veterinarians. Omaha, Nebraska.
- Terio K.A., Marker L., Munson L. Evidence for chronic stress in captive but not wild cheetahs (Acinonyx jubatus) based on adrenal morphology and function. J. Wildl. Dis. 2004;40:259–266. doi: 10.7589/0090-3558-40.2.259. [DOI] [PubMed] [Google Scholar]
- Terio K.A., Munson L., Marker L., Aldridge B.M., Solnick J.V. Comparison of Helicobacter spp. in cheetahs (Acinonyx jubatus) with and without gastritis. J. Clin. Microbiol. 2005;43:229–234. doi: 10.1128/JCM.43.1.229-234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terio K.A., Munson L., Moore P.F. Characterization of the gastric immune response in cheetahs (Acinonyx jubatus) with Helicobacter-associated gastritis. Vet. Pathol. 2011;49:824–833. doi: 10.1177/0300985811412620. [DOI] [PubMed] [Google Scholar]
- Terio, K., Whitham, J., Chosy, J., Sanchez, C., Marker, L., Wielebnowski, N., 2014. Associations between gastritis, temperament and management risk factors in captive cheetahs (Acinonyx jubatus). In: Proceedings of the American Association of Zoological Veterinarians Annual Conference. Orlando, Florida.
- Terrell S.P., Fontenot D.K., Miller M.A., Weber M.A. Chylous ascites in a cheetah (Acinonyx jubatus) with venoocclusive liver disease. J. Zoo Wildl. Med. 2003;34:380–384. doi: 10.1638/02-081. [DOI] [PubMed] [Google Scholar]
- Teunissen G.H., Happe R.P., Van Toorenburg J., Wolvekamp W.T. Esophageal hiatal hernia. Case report of a dog and a cheetah. Tijdschr Diergeneeskd. 1978;103:742–749. [PubMed] [Google Scholar]
- Thalwitzer S., Wachter B., Robert N., Wibbelt G., Müller T., Lonzer J., Meli M.L., Bay G., Hofer H., Lutz H. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clin. Vaccine Immunol. 2010;17:232–238. doi: 10.1128/CVI.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P.C.B., Tindall B.W., Coetzee J.D., Conradie C.M., Bull R.L., Lindeque P.M., Huebschle O.J.B. Vaccine-induced protection against anthrax in cheetah (Acinonyx jubatus) and black rhinoceros (Diceros bicornis) Vaccine. 2004;2:3340–3347. doi: 10.1016/j.vaccine.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Valícek L., Smíd B., Váhala J. Demonstration of parvovirus in diarrhoeic African cheetahs (Acinonyx jubatus jubatus, Schreber 1775) Vet. Med. (Praha) 1993;38:245–249. [PubMed] [Google Scholar]
- van Vuuren M., Goosen T., Rogers P. Feline herpesvirus infection in a group of semi-captive cheetahs. J. S. Afr. Vet. Assoc. 1999;70:132–134. doi: 10.4102/jsava.v70i3.774. [DOI] [PubMed] [Google Scholar]
- Venter E.H., van Vuuren M., Carstens J., van der Walt M.L., Nieuwoudt B., Steyn H., Kriek N.P.J. A molecular epidemiologic investigation of Salmonella from a meat source to the feces of captive cheetah (Acinonyx jubatus) J. Zoo Wildl. Med. 2003;34:76–81. doi: 10.1638/1042-7260(2003)34[0076:AMEIOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wack R.F., Eaton K.A., Kramer L.W. Treatment of gastritis in cheetahs (Acinonyx jubatus) J. Zoo Wildl. Med. 1997;28:260–266. [PubMed] [Google Scholar]
- Wack R.F., Kramer L.W., Cupps W. Griseofulvin toxicity in four cheetahs (Acinonyx jubatus) J. Zoo Wildl. Med. 1992;23:442–446. [Google Scholar]
- Wack R.F., Kramer L.W., Cupps W.L., Clawson S., Hustead D.R. The response of cheetahs (Acinonyx jubatus) to routine vaccination. J. Zoo Wildl. Med. 1993;24:109–117. [Google Scholar]
- Walzer C., Hittmayer C., Wagner C. Ultrasonographic identification and characterization of splenic lipomatosis or myelolipomas in cheetahs (Acinonyx jubatus) Vet. Radiol. Ultrasound. 1996;37:267–273. [Google Scholar]
- Walzer C., Kübber-Heiss A. Progressive hind limb paralysis in adult cheetahs (Acinonyx jubatus) J. Zoo Wildl. Med. 1995;26:430–435. [Google Scholar]
- Walzer C., Url A., Robert N., Kübber-Heiss A., Nowotny N., Schmidt P. Idiopathic acute onset myelopathy in cheetah (Acinonyx jubatus) cubs. J. Zoo Wildl. Med. 2003;34:36–46. doi: 10.1638/1042-7260(2003)34[0036:IAOMIC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wells A., Terio K.A., Ziccardi M.H., Munson L. The stress response to environmental change in captive cheetahs (Acinonyx jubatus) J. Zoo Wildl. Med. 2004;35:8–14. doi: 10.1638/02-084. [DOI] [PubMed] [Google Scholar]
- Whitehouse-Tedd K.M., Lefebvre S.L., Janssens G.P. Dietary factors associated with faecal consistency and other indicators of gastrointestinal health in the captive cheetah (Acinonyx jubatus) PLoS One. 2015;10:e0120903. doi: 10.1371/journal.pone.0120903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielebnowski N. Reassessing the Relationship between Juvenile Mortality and Genetic Monomorphism in Captive Cheetahs. Zoo Biology. 1996;15:353–369. [Google Scholar]
- Wielebnowski N. Behavioral differences as predictors of breeding status in captive cheetahs. Zoo Biol. 1999;18:335–349. [Google Scholar]
- Witte C.L., Lamberski N., Rideout B.A., Fields V., Shields Teare C., Barrie M., Haefele H., Junge R., Murray S., Hungerford L.L. Development of a case definition for clinical feline herpesvirus infection in cheetahs (Acinonyx jubatus) housed in zoos. J. Zoo Wildl. Med. 2013;44:634–644. doi: 10.1638/2012-0183R.1. [DOI] [PubMed] [Google Scholar]
- Zhang B., Une Y., Fu X., Yan J., Ge F., Yao J., Sawashita J., Mori M., Tomozawa H., Kametani F., Higuchi K. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proc. Natl. Acad. Sci. USA. 2008;105:7263–7268. doi: 10.1073/pnas.0800367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Une Y., Ge F., Fu X., Qian J., Zhang P., Sawashita J., Higuchi K., Mori M. Characterization of the cheetah serum amyloid A1 gene: critical role and functional polymorphism of a cis-acting element. J. Hered. 2008;99:355–363. doi: 10.1093/jhered/esn015. [DOI] [PubMed] [Google Scholar]
- Zordan M., Deem S.L., Sanchez C.R. Focal palatine erosion in captive and free-living cheetahs (Acinonyx jubatus) and other felid species. Zoo Biol. 2012;31:181–188. doi: 10.1002/zoo.20392. [DOI] [PubMed] [Google Scholar]


