Abstract
A significant number of biosafety level 2 (BSL-2) laboratories have been established in many countries for studies of various types of pathogenic agents and other infectious biological materials. The harmonized management of biological risks in such diverse laboratories thus appears as a real challenge. Zhejiang Province in China has taken the initiative to establish a comprehensively integrated laboratory biosafety management system called SINS (Standardization, Informatization, Normalization and Systematization). The SINS model system has been introduced and adopted in 1,721 BSL-2 laboratories in Zhejiang Province, and thus lead to an increase in the number of biosafety committees from 20% to more than 95% from 2007 to 2018, and the number of biosafety laboratory managers who knows biosafety-related laws and regulations increase from 52.7% to 83.7% from 2009 to 2017. Such achievements indicate that the successful implementation of SINS model has increased the effective control of biological risks in BSL-2 laboratories of the Zhejiang Province. SINS model and its main effects on leading the improvement of laboratory biosafety management was presented in this review. The SINS model helps to strengthen laboratory biosafety and thus effectively reduces occurrence of biosafety-related incidences. This model can potentially be used by other regions or countries where harmonized biosafety management system is still under-developing.
Keywords: BSL-2 laboratory, Biosafety, Management, Pathogenic agents, SINS model
Highlights
-
•
Zhejiang Province in China took the lead in establishing a comprehensive integrated management system of laboratory biosafety called SINS.
-
•
The SINS model contains Standardization,Informatization, Normalization and Systematization.
-
•
The SINS model can combine the technical with management to strengthen the laboratory biosafety management, and effectively avoid the occurrence of bio-risk.
-
•
SINS model presented in this paper is aimed to cover all aspects in the biosafety management in BSL2 laboratories, consisting of such elements as policy development, administrative supervision, systematic training, documentation and information management.
-
•
SINS model constructed on good experiences in other provinces, serves as a tool for analyzing, checking, and evaluating laboratory management in China.
1. Introduction
Emerging and reemerging infectious diseases caused by pathogenic microorganism such as SARS and MERS Coronavirus, H7N9 Influenza virus, Ebola virus and more recently the soft tick Bunya virus (STBV), have been causing disastrous harm to public health and resulting considerable economic losing world-wide [[1], [2], [3], [4], [5]]. Pathogenic microorganisms are classified into four categories according to China's regulations on the laboratory biosafety management of pathogenic microorganisms [6]. The first type refers to those microorganisms which cause very serious disease in humans or animals, and has not been declared eliminated in China. The second type refers to the microorganisms which cause serious disease in humans or animals, and can be easily transmitted directly or indirectly from human to human, from animal to human, or between animals. Both types of microorganism are collectively referred as “high pathogenic agents”, since they are more susceptible to human and animal; thus easily cause significant impact to health of human and animals. Diagnostic and experimental activities on “high pathogenicity agents” should be carried out in Biosafety level 2 (BSL-2) laboratories or higher level biosafety laboratories. There have been numbers of such certified BSL-2 laboratories in China for conducting activities of the “high pathogenicity agents”. For example, the preliminary screening of HIV is actually carried out in many diagnostic BSL-2 laboratories.
Proper regulation and management of BSL-2 laboratories are very important for their features of handling various “high pathogenicity agents”. Such activities are usually carried out with sophisticated work procedures and equipment for environmental and work personnel's protection, like biosafety cabinets [7,8]. Occasionally, dissemination of pathogenic agents may occur and cause direct or indirect harm to personnel exposed, or even in large scale may exerts social and economic negative impacts. Proper management of BSL-2 laboratory activities appears as challenge to government agencies and related institutes [[9], [10], [11], [12], [13], [14]].
The management of BSL-2 laboratories generally speaking is complicated involving many fields like medicine, biology, agriculture, industry and many other sectors. Cross traditional management model and habits made the management even more difficult. In order to solve these problems, and to strengthen, harmonize and control of BSL-2 laboratory management, Zhejiang Province had launched in 2007 a laboratory management system called SINS (Standardization, Informatization, Normalization and Systematization).
Zhejiang Province is located on the southeast coast of China and west to the Pacific Ocean. It has subtropical monsoon climate and an area of 101,800 km2 with a population of more than 55 million peoples. As of August 20, 2019, Zhejiang Province has 2 BSL-3, 1,721 BSL-2, and more than 500 BSL-1 laboratories. Significant efforts have been implemented in recent years to strengthen the management and safety of all those laboratories. Establishment of SINS (Figure 1 ) counts as part of these efforts and SINS has been applied gradually for the development of a harmonized management approach of these BSL-2 laboratories [15].
Figure 1.
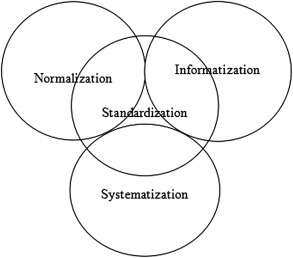
SINS Model for Management of BSL-2 Laboratories.
SINS model presented in this article aims to cover all aspects of biosafety management in BSL-2 laboratories. It includes policy development, administrative supervision, systematic training, information collection and documentation. SINS model constructed by incorporating good experiences from other provinces serves as a useful tool for analyzing, checking, and evaluating biosafety laboratory management in China. It has been practicing for more than 10 years in Zhejiang Province and maybe can be possibly used in other provinces in order to strengthen the management of biosafety in China.
2. Presentation and practice of the SINS model
2.1. Establishment of advanced models and standards (standardization)
2.1.1. Technical specifications
In 2007, Zhejiang Province took the initiative to develop and formulate the Technical Specification for the BSL-2 Laboratories in the provincial scope to standardize all BSL-2 laboratories. More than ten laws and regulations and standard-documents covering laboratory biosafety both in China and abroad were effective integrated. The technical specification focuses on the standardization of the BSL-2 laboratory design, construction and management of BSL-2 laboratories. The specification consists of forty-six clauses in fifteen chapters, including management system, risk assessment, standard operating procedures, facilities and materials, biological agents, biological sample administration, incident response, disinfection and sterilization.
The technical specification presents four main characteristics. First of all, it is user-friendly. This technical specification sorts out straight forward the requirements applicable to BSL-2 laboratories in Zhejiang Province. The technical specification facilitates the task of institutions and laboratory managers, as well as the health administration departments at all levels. Second, it highlights the importance of standardized management and clarifies the specific duties and tasks of all the stakeholders: the laboratory management departments, the laboratory managers and laboratory staff. Third, it is highly practical. The specification has made clear a number of requirements for routine standard operating procedures, for the management of biological agents, toxins and biological samples, for disinfection and sterilization, which should lead to a rapid and better mastery by the laboratory staff. Finally, it features the concept of “prevention first”. The document sets the requirements for job training for anyone enrolled into a BSL-2 laboratory and the requirements for strengthen the access of personnel, with great emphasis being put on personal protection, incident response, and biosafety signage [16].
The specification, adopted by 1,721 BSL-2 laboratories across Zhejiang Province, has achieved remarkable results and has greatly improved the standardized management of BSL-2 laboratories. Based on the number of BSL-2 laboratories recorded in the Zhejiang Province and the data from questionnaires filled by more than 1,000 BSL-2 laboratories in 2007 and 2018, the biosafety committees in the province increased from approx. 20% to more than 95% over the eleven year period. The organization management system has been popularized from sporadic laboratories to full coverage of all the BSL-2 laboratories. The study also reveals that the leaders' awareness and knowledge at all levels has also increased over this period of time. The laboratory construction and management have also evolved onto to a more standardized model.
2.1.2. Laboratory evaluation and standard BSL-2 laboratories
Despite a large number of BSL-2 laboratories in Zhejiang Province, the construction and management initially were not uniformly standardized, and many laboratory leaders were not even knowing about the standards for laboratory design and construction. In order to help develop standard laboratory guidance, another document, “Administrative measures for the BSL-2 laboratory of Zhejiang Province” was published in 2009. This document sets-up the criterions for evaluation of standard laboratories, together with the evaluation of on-site examinations. The evaluation indicators cover the hardware, software, personnel management and operation on-site testing. Based on the “Technical Specifications for the BSL-2 laboratory of Zhejiang Province”, the document aims at highlighting the normative nature of BSL-2 laboratory construction and management.
Up to now, 16 BSL-2 laboratories have been recognized as “standard BSL-2 laboratories” based on this evaluation. Such standard laboratories serve as role models for other laboratories. Through training courses, guidance and demonstration offered by standard laboratories, other laboratory leaders may obtain a better understanding for the design and operation of the BSL-2 laboratory, which facilitates a harmonized approach throughout the province.
2.1.3. Standardized training
Standard training models are presented as supplement components of SINS model standardization, focusing on the standardized training methods and training contents [17]. Training model in SINS has been classified into four forms: teachers' training, newcomers' training, continuing education and self-training. Also, six components of the training are unified across the province: the program, the contents, the trainers' qualification, the tests, the scoring and the certification. The training contents cover the legal requirements for pathogenic microbiology laboratories — “List of pathogenic microorganisms transmitted in humans” [18], and the core aspects such as safe handling practices and biosafety protection. They also highlight standards, contributing to strengthen the promotion and implementation of national industry standards such as GB19489-2008 [19] and WS233-2017 [20].
Nearly 20,000 laboratory personals have been trained since 2007. Test results showed a significant improvement in the awareness and knowledge of laboratory managers: assessed increase from only 52.7% in 2009 to 83.7% in 2017 [21,22]. Standardized training thus leads to a significant improvement in the past ten years.
2.2. Development of a computerized documentation system (Informatization)
2.2.1. Documentation management
According to the relevant laws and regulations, the biosafety information management system of pathogenic microorganism laboratory in Zhejiang Province was first established in 2007. The main framework of the system is shown in Figure 2 . Filing information includes lists of pathogenic agents, tables describing equipment configuration, list of relevant personnel, organization of affiliated units, and documents on the biosafety management system and so on. The information needs to be regularly updated.
Figure 2.
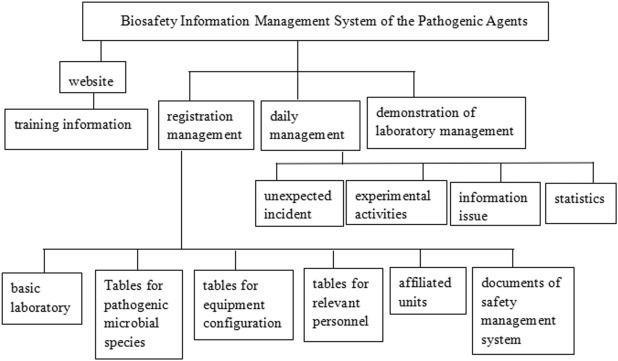
Biosafety Information Management System.
The above-described registration of biological laboratories enables to provide an over-all review on laboratory biosafety management to related authorities in Zhejiang Province. From 2016 to Aug. 2019, the number of BSL-2 laboratories recorded in the Zhejiang Province has increased by 53.44%. According to a regional database, most recorded BSL-2 laboratories are located in Hangzhou (457, as 26.55%), followed by Ningbo (218, as 12.67%). Zhoushan has the least number of BSL-2 laboratories recorded (48, as 2.79%) (Table 1 ). Among all of the registered BSL-2 laboratories 1,102 are affiliated to medical institution (64.03%), 380 are at the Centers for Disease Control and Prevention (CDC) (22.08%), and 13 are within colleges and universities (0.76%) (Table 2 ).
Table 1.
Distribution of BSL-2 laboratories in Zhejiang Province.
| District | Number of BSL-2 laboratories | Proportion (%) |
|---|---|---|
| Hangzhou | 457 | 26.55 |
| Ningbo | 218 | 12.67 |
| Wenzhou | 176 | 10.23 |
| Huzhou | 80 | 4.65 |
| Jiaxing | 135 | 7.84 |
| Shaoxing | 146 | 8.48 |
| Zhoushan | 48 | 2.79 |
| Jinhua | 144 | 8.37 |
| Quzhou | 59 | 3.43 |
| Taizhou | 178 | 10.34 |
| Lishui | 80 | 4.65 |
| Total | 1,721 | 100.00 |
Table 2.
Affiliation distribution of BSL-2 laboratories in different Institutes.
| Laboratory type | Number of laboratories | Proportion (%) |
|---|---|---|
| Medical institution | 1,102 | 64.03 |
| Centers for Disease Control and Prevention (CDC) | 380 | 22.08 |
| Colleges and universities | 13 | 0.76 |
| Other research institutes | 7 | 0.41 |
| Third-party inspection agencies | 76 | 4.42 |
| Private enterprises | 103 | 5.98 |
| Import-export control and quarantine facilities | 22 | 1.28 |
| Other organizations | 18 | 1.05 |
| Total | 1,721 | 100.00 |
As indicated above, the vast majority of BSL-2 laboratories are affiliated facilities of institutes that carry out significant multi-task activities involving many kinds of laboratory experiments, use of sophisticated equipment, and large flow of people. If BSL-2 laboratory biosafety is not managed improperly, it could lead to harmful incidents to public health, and thus may possibly result in huge social panic and economic losses. Therefore, it is critical important to have restrict and efficient daily management and supervision for those medical institutions.
2.2.2. Daily management and monitoring system
Routine on-going biological activities and changes in those routine activities are also need to be documented. Informatization of daily management covers risk assessment, inspection, audit reports, incident inquiries and records, and statistical analysis. High quality of biosafety management depends on daily over all monitoring of laboratory activities; timely identifying potential safety hazards; and following-up the implementation of risk mitigation measures. Information documentation on daily management provides a valuable foundation for technical update for improvement of experimental methods and scientific specifications, and for the biosafety supervision managed by related authorities.
A monitoring and early warning system was established in Zhejiang Province in 2010 by the provincial CDC, which made Zhejiang the first provinces to have such a system in China. At the same time, a database recording reported pathogenic microorganisms every year in the Zhejiang Province was established for the purpose of monitoring and registration of those exiting and newly discovered microorganisms. The system and database was also developed into a model that guides data registration and management, and can be used in for diagnosis, research and laboratory development. This system is able to provide early warning in case of emergence or re-emergence of “high pathogenic agents”. The informatization of recording and monitoring system supports the auxiliary function of the laboratories in early warning. It completes the informatization of all the biosafety aspects related to the management of laboratories in the province. It features simplicity, rapidity, and efficiency of information monitoring in the province and provides technical support for managing public health and reducing laboratory risks.
2.3. Normalization of the management regime, management system and incident handling (normalization)
2.3.1. Normalization of management regime
To standardize laboratory biosafety management, Zhejiang Province has promulgated Measures for pathogenic microbiology laboratory biosafety management of Zhejiang Province (draft). These measures highlight the following characteristics. First, four leading principles were established. They are “prevention first”, “legal compliance”, “clear responsibilities” and “reasonable management of incidents”. Second, the measures clarify the management responsibilities of the main stakeholders, focusing on those legal representative, biosafety management committee and of the laboratory managers. Third, the experimental activities, the transportation of hazardous biological materials, bacterial species preservation, and biological waste management were more clarified and defined. Four, the provisions for facilities and equipment, preparedness of incidents and safety precautions were specified. The management measures made the management of biosafety laboratories more normalization in our province.
2.3.2. Normalization of management system
Document Preparation Manual of BSL-2 Laboratory Management System has been compiled to solve the problems of no scientific, no systematic and no integrity management of the laboratories at district and county level. This manual gives instructions on how to establish a bio-risk management system and provides a model and case for its documentation. The manual aims at a strong system with wide application and easy operation. It has potential for a broad application. The strength of the system is reflected in the eleventh chapters. These include the organization structure, the system documentation elements and the security manuals. The program package provides 30 templates and 11 examples, which facilitates the implementation of such a documentation system in an organization. The release of this manual resulted in a reduction of documentation problems and significantly improved the quality of system documentation in Zhejiang Province.
2.3.3. Normalization of incident handling procedures
Despite all the efforts made in preventing them, such incidents and accidents like spills, misuse of infectious materials, exposure of laboratory staff or accidental release of biological agents may occur. While the occurrence of incidences cannot be totally prevented, their handling should definitely avoid negative consequences [23]. In order to enhance the capabilities in handling incidents. Zhejiang Province introduced Handbook for Emergency of Laboratory Incident [24]. After giving an overview of the characteristics and consequences for laboratory workers and environment of the laboratory incidents both in China and abroad, the handbook provides techniques and practical information for handling 36 types of incidents. It is intended to offer strong and useful guidance for improved and standardized emergency response procedures.
Incidents and accidents such as accidental spills of biological agents, the unusual exposure of infectious materials for laboratory staff or accidental release of biological agents occur from time to time despite every effort have been made in preventing these events. Therefore, correctly handling of those incidents and accidents are critically important in order to avoid farther negative consequences [23]. A Handbook for Emergency of Laboratory Incident has been published in People's medical publishing house since 2016 [24]. This handbook overall reviews the necessary knowledge to laboratory stuff on how to handle these laboratory incidents. Also techniques and procedures are provided for handling 36 types of incidents. The handbook has been used as an official guidance in laboratory for handling incidents and accidents.
2.4. Development of systematic administrative management (systematization)
2.4.1. Systematic management
Zhejiang Province issued a notice in 2016 to practically strengthen laboratory biosafety management with 13 departments. The notice aims at supporting the main responsibility of supervision, improving safety management from awareness, deploying supervision and inspection, and strengthening biosafety from various aspects of management. The system works in a systematic way at three levels: provincial, municipal and county, which helps to standardize, systematize and unify the biosafety management across the province. The notice serves as a model to other provinces.
2.4.2. Supervision and inspection system
The supervision of biosafety management system in Zhejiang Province is based on the unified standards and the territorial and hierarchical responsibilities. Responsibilities of implementation and supervision eventually fall on whoever holds the pertinent position in the hierarchy structure of the territorial health authority. Three-level supervision at provincial, municipal and county has been established in Zhejiang Province for an effective control and reduction of biological risks. Essential biosafety management such as laboratory design and construction, organizational structure and laboratory biosafety measures are reported to different management levels for supervision and inspection. Laboratory self-survey and health departmental supervision are regular approaches to identify potential bio-risk problems.
As part of the overall supervision, experts were invited in September 2018 to inspect and audit 151 BSL-2 laboratories in 44 institutions located in 11 cities of Zhejiang Province. Corrections were made towards a total of 205 issues identified. By the end of October 2018, self-surveys were conducted in more than 5,700 laboratories, and on-site inspections had been organized and carried out in more than 2,200 laboratories. Only in 2018, a survey carried by the Health Commission of Zhejiang Province identified 1,721 violations in 1,358 BSL-2 laboratories through self-inspection reports. The problems stay mainly with laboratory environment, facilities and equipment and daily operation. Through corrective actions, those involved laboratories eliminated the problems and considerably increased their control on bio-risks. The supervision and inspection processes significantly decrease laboratory bio-risks. Since then, not even a single laboratory biosafety incident has occurred in the Zhejiang Province.
3. Discussion and conclusion
Through implementation of the SINS model in Zhejiang Province, standardization and supervision for laboratory biosafety management have improved significantly. First of all, the unified construction and management, as well as the standardized guidance for BSL-2 laboratory allowed a progressive improvement of BSL-2 laboratory infrastructure and management. Then, the normalization of the management schemes has also improved the way BSL2 laboratories are managed in various institutions. Third, the computerized documenting and reporting system facilitates the oversight and general management of different level of authorities. It helps to realize “run once at most” in laboratory biosafety management, simplifies the process, increases efficiency, and strengthens the capability of remote monitoring. Fourth, the systematic management leads to realization of a grid management system of laboratory biosafety, effectively ensure that all management measures are properly implemented and that the biosafety of the laboratory is secured.
By implementing the SINS model which including self-inspection process supervised at the municipal and county levels and the oversight and control by the provincial health authorities, Zhejiang Province has developed a multilayer supervision mechanism that contributes to a more effective control and reduction of biological risks in the entire province.
The SINS management model has been initiated and practiced for more than 10 years since 2007 in Zhejiang Province. Over this period of time, this model has been proved to be effective as management guidance for laboratory biosafety. It has been promoted and widely applied during major campaigns in Zhejiang Province. Now this model has been recognized as a mature laboratory biosafety management model.
China did not establish laboratory biosafety management until 2003 [25]. Even now many BSL-2 laboratories still face the difficulties in biosafety management. SINS management model has been gradually established and improved over the past 12 years based on the hand-on management experience accumulated. We believe that the SINS model can not only lead to effectively enhancement on laboratory biosafety management at provincial level, but also can be further promoted to national level or even international level. It can be used as reference model for laboratory biosafety management in other countries where biological risk management capabilities still need to be improved. It is suggested that the SINS model for laboratory biosafety management should be popularized and applied to ensure the safety of laboratory workers and environments.
Acknowledgements
The authors would like to express our thanks to the Zhejiang Provincial Centers for Disease Control and Prevention and Health Commission of Zhejiang Province, China. We also thank Philippe Stroot PhD for his contribution to this Paper. This study was part of the Project of Health and Medical Technology of Zhejiang Province funded by the Health Commission of Zhejiang Province (2020KY536).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
X. Qiu collected and analyzed the data, and drafted the manuscript. H. Gu designed the study and prepared and revised the manuscript. Jingqing Weng drafted standards. C. Yan analyzed the data. Z. Jiang collected and screened the data.
References
- 1.Cunha C.B., Opal S.M. Middle East respiratory syndrome (MERS): a new zoonotic viral pneumonia. Virulence. 2014;5:650–654. doi: 10.4161/viru.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao L., Chen E., Chen Z., Gong Z. From SARS to H7N9: the mechanism of responding to emerging communicable diseases has made great progress in China. Biosci. Trends. 2013;7:290–293. [PubMed] [Google Scholar]
- 3.Gong Z., Lv H., Ding H., Han J., Sun J., Chai C., Cai J., Yu Z., Chen E. Epidemiology of the avian influenza A (H7N9) outbreak in Zhejiang Province, China. BMC Infect. Dis. 2014;14:244. doi: 10.1186/1471-2334-14-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sell T.K., McGinty E.E., Pollack K., Smith K.C., Burke T.A., Rutkow L. US state-level policy responses to the Ebola outbreak, 2014–2015. J. Public. Health. Manag. Pract. 2017;23:11–19. doi: 10.1097/PHH.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 5.Yu X.J., Liang M.F., Zhang S.Y., Liu Y., Li J.D., Sun Y.L. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.State Council of the People's Republic of China . State Council of the People's Republic of China; Beijing: 2004. Regulations on Biosafety Management of Pathogenic Microbiology Laboratory. [Google Scholar]
- 7.Munson E., Bowles E.J., Dern R., Beck E., Podzorski R.P., Bateman A.C., Block T.K., Kropp J.L., Radke T., Siebers K., Simmons B., Smith M.A., Spray-Larson F., Warshauer D.M. Laboratory focus on improving the culture of biosafety: statewide risk assessment of clinical laboratories that process specimens for microbiologic analysis. J. Clin. Microbiol. 2018;56 doi: 10.1128/JCM.01569-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Duc J.W., Anderson K., Bloom M.E., Estep J.E., Feldmann H., Geisbert J.B., Geisbert T.W., Hensley L., Holbrook M., Jahrling P.B., Ksiazek T.G., Korch G., Patterson J., Skvorak J.P., Weingartl H. Framework for leadership and training of biosafety level 4 laboratory workers. Emerg. Infect. Dis. 2008;14:1685–1688. doi: 10.3201/eid1411.080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huigang L., Cui H., Haixia M., Zhiming Y. High-level biosafety laboratory and biosafety. Bull. Chin. Acad. Sci. 2016;31:452–456. [Google Scholar]
- 10.Zhang H., Wang L., Zhang S.Z. Biosafety issues and current status of construction and development of pathogenic microorganisms. Journal of Medical Pest Control. 2014;9:1062. [Google Scholar]
- 11.Wei F., Yuan Z.M., Chen Z.S. Thoughts and suggestions on standardization management system of biosafety laboratories in China. Bull. Chin. Acad. Sci. 2014;3:309–314. [Google Scholar]
- 12.Wang X.L., Yang Y.S., Shen S.S., Li Z.L. Strengthening the biosafety construction of biological laboratory in colleges and universities. Res. Explor. Lab. 2013;3:243–245. [Google Scholar]
- 13.Li W.Y., Li H., Kuang Y. Construction and thinking of biosafety system in pathogenic biology laboratory. Exp. Sci. Technol. 2013;1:155–157. [Google Scholar]
- 14.Li W.G., Li Y.C., Lu G.J. Essential qualities of laboratory biosafety and personnel. Contemporary Animal Husbandry. 2012;3:27–29. [Google Scholar]
- 15.Ackermann-Gaumann R., Siegrist D., Zust R., Signer J., Lenz N., Engler O. Standardized focus assay protocol for biosafety level four viruses. J. Virol. Methods. 2019;264:51–54. doi: 10.1016/j.jviromet.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Gu H., Zhu W., Weng J.Q. Study on technical specifications of biosafety level-2 laboratory. Chin. J. Health Lab. Technol. 2011;6:1557–1558. [Google Scholar]
- 17.Ndolo D.O., Wach M., Rudelsheim P., Craig W. A curriculum-based approach to teaching biosafety through eLearning. Front. Bioeng. Biotechnol. 2018;6:42. doi: 10.3389/fbioe.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health of the People's Republic of China . Ministry of Health of the People's Republic of China; Beijing: 2006. List of Pathogenic Microorganisms Transmitted From Humans. [Google Scholar]
- 19.China International Standardization Administration Committee . China Standard Press; Beijing: 2008. General Requirements for Laboratory Biosafety (GB19489-2008) [Google Scholar]
- 20.Health and Family Planning Commission of the people Republic of China . 2017. General Biosafety Standard for Causative Bacteria Laboratories (WS 233-2017). Beijing. [Google Scholar]
- 21.Gu H., Jiang Z.G., Cai G.F., Weng J.Q., Sun J.Z., Huang X.T. Analysis of knowledge of biosafety knowledge among medical staff in Zhejiang Province. Chin. J. Health Lab. Technol. 2016;23:3492–3496. [Google Scholar]
- 22.Gu H., Chen S.H., Chen H.P., Gao X.P., Sun J.Z., Wang F. Survey on awareness rate of biosafety knowledge among laboratory workers and manag ers in Zhejiang Province. Chin. Prev. Med. 2009;8:732–734. [Google Scholar]
- 23.Artika M., NisaMa’Roef C. Laboratory biosafety for handling emerging viruses. Asian Pac. J. Trop. Biomed. 2017;7:483–490. doi: 10.1016/j.apjtb.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu H., Weng J.Q. People's Medical Publishing House; Beijing: 2016. Laboratory Incident Emergency Handbook. [Google Scholar]
- 25.Lu B., Li J.J., Cheng H.L., Huang P.T. Current status of construction and Management of Biosafety Laboratories in China. Res. Explor. Lab. 2012;1:192–196. [Google Scholar]


