Abstract
We examine whether investor mood, driven by World Health Organization (WHO) alerts and media news on dangerous infectious diseases, is priced in pharmaceutical companies' stocks in the United States. We argue that disease-related news (DRNs) should not trigger rational trading. We find that DRNs have a positive and significant sentiment effect among investors (on Wall Street). The effect is stronger (weaker) for small (large) companies, who are less (more) likely to engage in the development of new vaccines. A potential negative investor climate (on Main Street) – induced by disease-related fear – does not alter the positive sentiment effect.
Keywords: WHO alerts, Investor sentiment, Pharmaceutical industry, Trading strategies
Highlights
-
•
We examine whether investor mood, driven by World Health Organization alerts on infectious diseases, is priced in pharmaceutical stocks in the U.S.
-
•
Disease-related fear has a negative and significant effect on returns on pharmaceutical stocks.
-
•
Disease-related news (DRN) has a positive and significant effect on returns on pharmaceutical stocks.
-
•
The effect of DRN persists over several days following a WHO alert on infectious diseases.
-
•
The effect of DRN is stronger for smaller companies and is weaker for larger companies.
-
•
Our findings give rise to profitable trading strategies.
1. Introduction
A relatively large number of studies show that investor sentiment may drive agents' investment decisions (De Long et al., 1990, Cen and Liyan-Yang, 2013, Kaplanski and Levy, 2015). In this respect, the behavioral finance literature provides solid evidence supporting the existence of a significant relation between stock returns and investor sentiment (Loughran and Schultz, 2004, Cao and Wei, 2005, Baker and Wurgler, 2006, Kaplanski and Levy, 2010a, Kaplanski and Levy, 2010b, Curatola et al., 2016). Investor sentiment is typically defined as a belief about future cash flows and investment risks that is not justified by the facts at hand (Baker and Wurgler, 2007, p. 129). Certain events may induce either a positive or a negative sentiment that strongly affects investors' investment decisions and, thus, the corresponding stock market prices.
Early studies observe, for example, that sunshine, which is well known to be a driver of peoples' mood, tends to comove positively with daily stock returns (see Saunders, 1993, Hirshleifer and Shumway, 2003). Yuan et al. (2006) find that lunar phases may affect stock market returns. More recent studies find that international sporting games – and in particular soccer games – heavily affect investor sentiment, and in turn, stock market returns (Edmans et al., 2007, Kaplanski and Levy, 2010a). Kaplanski and Levy (2010b) show that major aviation disasters tend to generate a negative sentiment within two days after the event. Horváth and Huizinga (2015) examine the impact of the announcement of the creation of the European Financial Stability Facility on bank share prices.
Key in this research is the possibility – hitherto unaccounted for in the literature – that large events of devastating nature to the economy and society can be regarded as good news to some interest groups, such as stock market investors. Differently from the existing work, we rely on dangerous infectious diseases (i.e., SARS, Influenza A(H1N1), Polio, and Ebola), which are perceived by the general public (Main Street) as large negative developments, but can generate sector-specific positive investor sentiment (Wall Street). Specifically, our main contribution consists of employing a novel mood variable (disease-related news or DRNs), based on the World Health Organization's (WHO's) alerts and media news related to dangerous infectious diseases, to examine the effect of investor sentiment on pharmaceutical stock prices. Our principal hypothesis is that fear of dangerous infectious diseases will be negatively associated with investor sentiment, whereas DRNs will positively affect investment in pharmaceutical stocks. This hypothesis builds on the argument that while dangerous infectious diseases spread fear and generate negative sentiment, investors unrealistically anticipate an increase in the cash flows of pharmaceutical companies due to selling medicines aimed at fighting the disease.
Thus, investor sentiment about the performance of pharmaceutical companies may be one key element that drives financial investment decisions. This notwithstanding, there is a dearth of research into pharmaceutical companies' stock market performance (Himmelmann and Schiereck, 2012, Theodossiou and Theodossiou, 2014).
To the best of our knowledge, the relation between pharmaceutical stock returns and investor sentiment – driven by DRNs – has not been investigated, with the exception of Huberman and Regev (2001). Huberman and Regev (2001) use a case study to investigate the effect of investor enthusiasm to a major breakthrough in cancer research. Interestingly, the stock price of EntreMed, a biopharmaceutical company, responded stronger to the breakthrough five months later, when it was reported in the popular media, than when it was originally announced in Nature. Nevertheless, the authors neither (i) generalize their research findings to the whole pharmaceutical industry, nor (ii) focus on the investor sentiment effect on stock prices and returns, nor (iii) design global disease-news-induced trading strategies.
The main contribution of this study is to evaluate the balance between two contrasting effects of outbreaks of dangerous diseases. On the one hand, we recognize the possibility that some infectious diseases spread fear among the general public and stock market investors, which triggers a negative (fear-induced) sentiment in pharmaceutical stock prices. On the other hand, an outbreak of an infectious disease is expected to have a positive sector-specific sentiment effect on pharmaceutical stock prices. Methodologically, accounting for the two competing (negative and positive) effects manifests in a unique framework that alleviates the possibility of spurious correlations. In this study, we address the following questions that have not been examined in the context of the pharmaceutical stock market. First, does investors' fear gauge, which is provoked by DRNs, lead to a decrease in pharmaceutical stock market returns? Second, does an outbreak of a dangerous infectious disease trigger a positive sector-specific investor sentiment effect on pharmaceutical stock prices? Third, does investor sentiment (optimism or pessimism) about the future performance of pharmaceutical companies persist over time? Fourth, do the stock prices of large pharmaceutical stocks respond to DRNs differently than the stock prices of small pharmaceutical stocks?
To address these issues, we use the prices of 102 pharmaceutical firms' stocks traded in the U.S. stock market to construct four different investment portfolios. In addition, as a robustness check, we consider the S&P 500 Information Technology Index. Our empirical strategy draws on two commonly used—event-study and regression-based—methodologies to evaluate the investor sentiment effect on stock prices of pharmaceutical firms following DRNs.
We identify a significantly positive and persistent investor sentiment in the stock returns of pharmaceutical companies following DRNs. This may be generated by positive beliefs about R&D investments in the aftermath of disease outbreaks. The persistence of investor sentiment may be reflected in information salience as well (Palomino et al., 2009). Furthermore, we construct a fear gauge index by employing Chicago Board Options Exchange's (CBOE's) Volatility Index (VIX) as a proxy for investor fear. The index always exerts a negative and significant effect on the returns of pharmaceutical companies' stocks. We find that sentiment tends to exert a stronger effect on small firms than on large firms. This finding is along the lines of Qiu and Welch (2004), who observe that, under certain conditions, small firm returns become a proxy for investor sentiment (Baker and Wurgler, 2006, Edmans et al., 2007). Our results are supported by a battery of robustness checks.
Overall, our findings give rise to profitable trading strategies where an investor takes a long position in a portfolio of pharmaceutical stocks and a short one in the VIX. These strategies lead to a positive and significant performance.
The remainder of this paper is organized as follows. In Section 2, we provide the background and motivation to the study. In Section 3, we describe our mood variable DRNs and report descriptive statistics. In Section 4, we outline the methodology and formulate our main hypotheses. In Section 5, we discuss the estimation results. In Section 6, we design hypothetical trading strategies. Finally, in Section 7, we provide some concluding remarks and discuss the practical usefulness of our results.
2. Motivation and background
In this section, we motivate our analysis by reviewing studies on economic effects of dangerous infectious diseases and by investigating media coverage after a disease outbreak. We also discuss the recent developments in the U.S. pharmaceutical industry and the institutional investors' attention to pharmaceutical sector.
2.1. Investor sentiment and media coverage: the case of infectious diseases
In the asset pricing literature, a variety of mood variables have been considered. For instance, weather conditions (Saunders, 1993, Hirshleifer and Shumway, 2003), hours of daylight in fall and winter (Kamstra et al., 2003), international soccer results (Edmans et al., 2007, Kaplanski and Levy, 2010a, Curatola et al., 2016), and negative newspaper articles (Tetlock, 2007). Notably, investor sentiment is found to have a significant impact on stock market returns. Mehra and Sah (2002) relate in a theoretical framework the effect of feelings on investors' decision making in financial markets. Nofsinger (2005) finds that interpersonal communication, leading to “social mood,” translates into emotions such as optimism, pessimism, happiness, or anxiety. A general finding is that fear leads to negative asset returns, while positive emotions increase investors' willingness to take risks.
In this study, we hypothesize that increased media coverage of dangerous infectious diseases has a positive and relatively persistent effect on pharmaceutical stocks. Despite the overall negative sentiment in the population due to fear of being infected, public and political demand for containing an infectious disease can lead to additional income channels for pharmaceutical companies. Typical reactions to an infectious illness include higher R&D investment (partially subsidized by the government), vaccine mass orders, or a general increase in demand for preventive measures (e.g., medicine, disinfection agents, and surgical masks). We focus on four major infectious diseases that were regarded as a Public Health Emergency of International Concern (PHEIC) by the WHO. The outbreak of Severe Acute Respiratory Syndrome (SARS) in 2003, Influenza A (H1N1) in 2009, and Polio and Ebola in 2014 were all more or less intensively covered by media.
The role of mass media in communication of potential risks has been subject to intense debate. It is argued that low-probability, high-consequence events, such as potential health risks associated with outbreaks of rare diseases, are overemphasized in media-generated news waves. This unbalanced reporting leads to a disjunction between actual and people's perceived risk (Vasterman et al., 2005, Mairal, 2011, Young et al., 2013). For instance, a representative survey conducted by Blendon et al. (2004) indicates that 69% of respondents living in Ontario, 57% in Canada excluding Ontario, and 32% in the U.S. were concerned about contracting SARS, whereas, educative and informative communication efforts in Singapore resulted in a much lower overall anxiety level. Quah and Hin-Peng (2004) report that only 14% of respondents viewed SARS as a personal risk in May 2003. Public overreaction and panic can also lead to adverse economic effects. In particular, sectors such as tourism or retail sales fall due to individuals' preventive measures, such as reduction in traveling to the affected areas or avoidance of public places. In the case of SARS, Hanna and Yiping (2004) estimate the total cost at about 0.5% of GDP in China, while Keogh-Brown and Smith (2008) assess the global economic impact between 30 billion USD and 100 billion USD. Using a general equilibrium model for the U.K., Smith et al. (2009) estimate costs related to Influenza A (H1N1) between 0.5% and 1.0% of U.K.'s GDP. However, the total economic burden is difficult to quantify since both direct health care costs as well as indirect costs of work absenteeism and loss of productivity have to be taken into account.
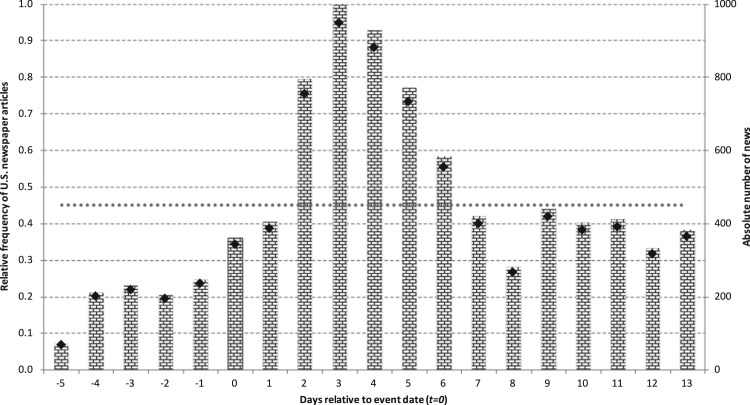
In the spirit of Kaplanski and Levy (2010b), we search for media articles related to dangerous infectious diseases in order to (i) gain better understanding of the scale and timing of the information salience of such diseases and (ii) to evaluate the importance of the information salience as a potential source of investor sentiment in the pharmaceutical industry. Fig. 1 illustrates the normalized number of media news informing the general public about the disease outbreaks mentioned above of the four. It is evident from Fig. 1 that the frequency of the related news notably increases on the announcement day (t=0), that is, when the official WHO PHEIC statement is made. The news coverage intensifies and peaks on the third day after the official PHEIC announcement. In addition, the absolute number of news announcements remains above the average up to six days after the announcement. This is not surprising since PHEIC statements are followed by other official/institutional news, which is used to build our event-day mood variable in the next section. Specifically, in two out of the four cases, an event (i.e., DRNs) was taking place on any of the six days following a PHEIC announcement. These observations provide support for the existence of a relatively strong relation between official news and media coverage and therefore between DRNs and investor sentiment. Moreover, they suggest that the sentiment effect lasts for several days. This evidence provides support for the persistent sentiment hypothesis formulated in Section 4.
Fig. 1.
Media coverage around WHO PHEIC alerts.
Notes: This figure depicts the normalized number of distinct, disease-related newspaper articles published in the U.S. around the event days. The event dates (t=0) are considered to be the official WHO PHEIC statements. The number of articles relies on all four disease announcements and is normalized relative to the its peak value over the 19-day period. The dotted line represents the average relative value across the 19-day period. Black diamonds illustrate the absolute number of disease-related newspaper articles. Data are obtained using the LexisNexis database for global news and business information.
2.2. The pharmaceutical industry
The pharmaceutical industry is at the heart of the U.S. economy. Although public and private spending on pharmaceuticals in the U.S. collectively contributed just over 2% (around 1000 USD) to the country's GDP per capita in 2011 (OECD, 2013),1 this share was the largest among the OECD countries. In 2009, the overall U.S. health care expenditures accounted for 18% of the country's GDP, but it is estimated to contribute 37% in 2050.2 Furthermore, biopharmaceutical companies in the U.S. account for the largest share of all U.S. companies' R&D expenditures, which represents nearly 20% of all domestic R&D (PhRMA, 2015).3 It is one of the fastest growing industries. Indeed, both revenues and R&D spending by the U.S. biopharmaceutical industry tripled from 1996 to 2008 (Lazonick and Tulum, 2011). Further, Offit (2005) documents an increase in the market concentration of vaccine producers in the U.S. over past decades. Specifically, there are currently only four FDA-approved U.S.-based publicly traded vaccine producers and distributors.4 The reasons for companies undertaking or quitting vaccine production are multifold. On the one hand, research, development, testing, and manufacturing of vaccines are costly; on the other hand, the market for selling vaccines is smaller than the market for pharmaceutical drugs, to name just few reasons. However, high entry barriers for the development of a new vaccine bears potential for monopoly. Additionally, there is no manufacturing of generic vaccines, as vaccine production is substantially more complicated and technologically advanced than drug production.5 The result is an increase in vaccine shortages, especially for flu vaccines (Hinman et al., 2006). The emergence of a dangerous infectious disease can thus be regarded as a potentially new market for vaccine producers, along with subsidized R&D. Therefore, it is surprising that research focusing on the effects of investor sentiment on the stock prices of pharmaceutical companies has been underwhelming.
2.3. The U.S. stock market and investor attention to pharma-stocks
In this study, we focus on the U.S. stock market. There are two main reasons to select the U.S. stock market for this study: (1) it is one of the most closely followed markets in the world and, as such, it is very efficient with respect to new information and it is also the most liquid market (Kaplanski and Levy, 2010a); (2) the U.S. stock market is one of the leading stock markets in the world and accounts for about 40% of the global market (Hou et al., 2011).6 Our study is further motivated by the growing role of pharmaceutical stocks within the industry of financial services in the United States. Perceptive Life Sciences Offshore Fund (859 million USD), Traxis Sivik Global Healthcare Offshore (44 million USD), and Visium Balanced Offshore Fund CL2 (3697 million USD) are examples of the sector-specific equity funds investing exclusively in biopharmaceutical stocks.7 There is also a large number of funds with significant exposure to the stocks of (main) individual pharmaceutical companies. Indeed, Table 1 indicates that, as of second quarter of 2014, institutional investors held significant positions in the largest pharmaceutical firms' stocks, including Pfizer, Johnson & Johnson, Merck & Company, AstraZeneca, Amgen, and Gilead Sciences Inc.
Table 1.
Institutional investors' positions in (main) pharmaceutical stocks.
| Pharmaceutical company | Fund (% of pharmaceutical stocks held) |
|---|---|
| Pfizer | Jana Partners (0.05%); Fairfax Financial Holdings (0.48%); Orbimed Advisors (1.42%); Eton Park Capital Management (1.53%); Boyar Asset Management (3.94%); York Capital Management Global Advisors (1.35%); Farallon Capital Management (0.43%); Muhlenkamp and Co (0.21%) |
| Johnson & Johnson | Berkshire Hathaway (0.03%); Fairfax Financial Holdings (0.61%); Perry Corp (0.99%); Orbimed Advisors (0.59%); Boyar Asset Management (2.14%) |
| GlaxoSmithKline | Chou Associates Management (0.14%) |
| Merck & Co. | Fairfax Financial Holdings (0.05%); Orbimed Advisors (1.72%); Healthcor Management (1.69%); Vertex One Asset Management (0.67%); Sarissa Capital (7.87%) |
| Abbott Laboratories | Southeastern Asset Management (2.98%) |
| AstraZeneca | Chou Associates Management (0.28%); Third Point (0.91%); Corvex Management (1.09%); Duquesne Family Office (1.11%); Highfields Capital Management (0.76%); York Capital Management Global Advisors (1.20%) |
| Amgen | Third Point (0.65%); Orbimed Advisors (3.12%); Pennant Capital Management (0.50%); Highline Capital Management (7.06%); Sarissa Capital (1.81%) |
| Eli Lilly and Company | Highfields Capital Management (2.13%) |
| Bristol-Myers Squibb | Orbimed Advisors (4.09%); Boyar Asset Management (2.12%); Muhlenkamp and Co (1.82%) |
| Gilead Sciences | Baker Bros. Advisors (0.26%); Palo Alto Investors (0.95%); Orbimed Advisors (3.34%); Pennant Capital Management (3.07%); Argonaut Capital Management (1.25%); Healthcor Management (2.69%); Parnassus Investments (2.44%); Muhlenkamp and Co (2.12%) |
| Vertex Pharmaceuticals | Orbimed Advisors (0.59%); Healthcor Management (3.88%) |
| Novo Nordisk | Markel Corp (1.42%) |
Source: Form 13F (SEC) – Reports filed by institutional investment managers as of Q2 2014.
3. Data
In this section, we briefly describe our DRNs collection procedure and the construction of the pharmaceutical portfolios.
3.1. Announcements of dangerous infectious diseases
The data cover the entire history of dangerous infectious diseases that were considered as PHEICs by the WHO – a 12-year period from March 2003 to December 2014. The infectious diseases period incorporates 146 DRNs, which we consider to be our event days.8 We categorize the events into the following categories: WHO Statement, WHO Disease Outbreak News, Approval, Government Order, Government Order Cancel, Research Funding, and Statement. Events that are considered to be a WHO Statement9 or WHO Disease Outbreak News10 are obtained from the official website of WHO. All the other events are obtained through a rigorous online search.
WHO Statements are official statements communicated to the public with regard to any new and substantial information related to a certain disease. For instance, on 8 August 2014 the “Statement on the 1st meeting of the IHR Emergency Committee on the 2014 Ebola outbreak in West Africa” informed on the current state of the Ebola outbreak. Additionally, the emergency committee stated that conditions for a PHEIC had been met and provided advice on how to address the Ebola outbreak in the affected countries. Typically, the popular media uses such WHO Statements to communicate the news to the public.
WHO Disease Outbreak News, on the other hand, are to some extent regular updates on the current situation and include, for instance, news about the first cross-border transmissions of a disease. In the case of the SARS outbreak in 2003, there were periods of daily updates, while news on the 2009 Influenza A (H1N1) outbreak were covered on a weekly basis. We recognize that all regularly spaced updates may be anticipated by stock market investors and may be priced in prior to the actual update. For this reason, our sample of announcements comprises only those updates that documented first-time cross-border transmissions. This strategy helps ensure the independence of subsequent announcements, insofar as cross-border transmissions of infectious diseases occur unpredictably. In the case of SARS, we observe a rapid spread across countries within the first two months.11 In total, 29 countries were affected by the disease. In the case of Influenza A (H1N1), the official list contains more than 214 countries and overseas territories or communities as of 1 August 2010. The virus spreads quickly and our list includes a sizable number of first cross-border transmission dates (around 50). For polio, we abstain from identifying first outbreak dates as there are only 10 event dates in total. Considering Ebola, we count 10 cross-border transmissions in total. However, the disease was not under control at the time this paper was written.
In addition to WHO Statements and WHO Disease Outbreak News, we include release dates of official statements provided by government ministries and agencies, as well as individual publicly traded companies. Newly developed vaccines are subject to governmental approval and a positive feedback might have a substantial impact on the share price of the vaccine producing company, as well as on its competitors/followers. We use official press release dates provided by the website of the U.S. Department of Health and Human Services (HHS) and label them Approval. This category is only relevant for Influenza A (H1N1). For SARS and Ebola, vaccines are still in development, while potent polio vaccines have existed for decades. Government Order and Government Order Cancel refer to government purchases of vaccine and subsequent cancellations of orders. Again, these dates refer to the Influenza A (H1N1) outbreak only. In the case of the SARS outbreak, companies were granted research funding to develop a potent vaccine. We label the official dates of funding announcements as Research Funding. The category Statement subsumes different, potentially influential, statements provided by government officials and companies referring to the current situation with regard to a disease. All DRNs are classified and summarized in Table A1 in the Appendix.
3.2. Descriptive statistics
The stock market data for end-of-trading-day prices and market values are retrieved from Thomson Reuters Datastream. The cross-section includes 102 pharmaceutical companies that are listed either on the New York Stock Exchange (NYSE) or National Association of Securities Dealers Automated Quotations (NASDAQ). The pharmaceutical companies are listed in Table A2 in the Appendix.
In addition, we retrieve the S&P 500 Information Technology Index. For non-U.S. based companies, we use the data on American Depository Receipts (ADRs). This gives rise to a 12-year period with 3097 trading days from January 2003 to November 2014. To test for the impact of DRNs on pharmaceutical stock returns, we employ the rates of return on a variety of portfolios comprising pharmaceutical stocks. Specifically, we construct four benchmark pharmaceutical portfolios. The first is an equally weighted portfolio (EW). The EW portfolio draws on the literature on the optimality of portfolios of naïve investors (De Miguel et al., 2009). The second is a value-weighted portfolio of all pharmaceutical stocks with time-varying market-value based weights (VW). The third portfolio is a constant-value-weighted portfolio accounting for the 10 largest pharmaceutical stocks (TOP). The fourth is a constant-value-weighted portfolio of the 10 smallest pharmaceutical stocks (BOTTOM). The VW portfolio emphasizes the role of large companies as opposed to small companies. The TOP portfolio illustrates a real-world situation where investors are constrained by cardinality constraints. The BOTTOM portfolio addresses our Hypothesis 4 stated in the next section. Finally, the inclusion of the S&P 500 Information Technology Index (S&P 500 IT), a standardized and tradable diversified portfolio of stocks, seeks to project our hypotheses on sectors other than the pharmaceutical. In doing so, we show that the DRNs positive sentiment spreads to other sectors and confirm that the breadth of sentiment is not confined only to the pharmaceutical industry.
We compute continuously compounded day-to-day percentage returns on the aforementioned portfolios. The descriptive statistics are presented in Table 2 .
Table 2.
Descriptive statistics.
| Statistic | EW | VW | TOP | BOTTOM | S&P 500 IT |
|---|---|---|---|---|---|
| Mean (%) | 0.0077 | 0.0549 | 0.0159 | −0.0125 | 0.0366 |
| Median (%) | 0.0405 | 0.0586 | 0.0209 | 0.0000 | 0.0559 |
| Maximum (%) | 10.5582 | 10.1711 | 10.3673 | 8.2638 | 11.4610 |
| Minimum (%) | −7.1160 | −6.5238 | −7.7975 | −11.2926 | −9.6701 |
| Std. Dev. (%) | 1.2198 | 1.1391 | 1.0262 | 1.6259 | 1.3702 |
| Skewness | −0.3194 | 0.0210 | −0.1041 | −0.1746 | −0.0439 |
| Excess Kurt. | 4.5993 | 4.6354 | 9.0512 | 2.6647 | 6.3379 |
| Jarque–Bera | 2782.39 | 2772.99 | 10577.2 | 931.996 | 5184.43 |
| (0.0000) | (0.0000) | (0.0000) | (0.0000) | (0.0000) | |
| Ljung–Box Q5 | 29.581 | 8.2820 | 24.890 | 12.497 | 21.378 |
| (0.0000) | (0.1414) | (0.0001) | (0.0286) | (0.0007) | |
| Ljung–Box | 1036.22(0.0000) | 813.167(0.0000) | 1277.68(0.0000) | 54.547(0.0000) | 901.965(0.0000) |
| Observations | 3097 | 3097 | 3097 | 3097 | 3097 |
Notes: This table reports the descriptive statistics of continuously compounded day-to-day percentage returns on the five investment portfolios. EW denotes returns on an equally weighted portfolio of pharmaceutical stocks. VW denotes returns on a (time-varying) value-weighted portfolio of pharmaceutical stocks. TOP denotes returns on a constant-value-weighted portfolio of 10 largest pharmaceutical firms. BOTTOM denotes returns on a constant-value-weighted portfolio of 10 smallest pharmaceutical stocks. S&P 500 IT denotes returns on the S&P 500 Information Technology Index. Q5 denotes the Ljung–Box test statistic for the fifth-order cumulative autocorrelation of stock returns, and denotes the Ljung–Box test statistic for the fifth-order cumulative autocorrelation of returns squared. The table also provides the p-values for the significance tests of the Jarque–Bera and Ljung–Box statistics (in parentheses). We use daily data for the period 01/01/2003–11/13/2014 (a total of 3097 observations).
Over the sample period, the mean return on the VW portfolio of pharmaceutical stocks with time-varying weights, 0.0549%, was considerably greater than the mean of the remaining portfolios. The median follows a similar pattern with one notable exception, where the difference between the mean and the median (and hence the ensuing asymmetries in the probability density function) is largest for the EW portfolio. The range of variation between the maximum and the minimum returns is greatest for the S&P 500 IT portfolio (11.46% and −9.67%, respectively) and lowest for the VW portfolio (10.17% and −6.52%, respectively). The BOTTOM portfolio has the highest idiosyncratic risk, as measured by the standard deviation (1.63%), whereas the TOP portfolio is the least risky to invest (1.03%). Moreover, portfolio returns are negatively skewed, with an exception of the VW portfolio. The negative skewness implies that large negative returns are more likely than large positive returns. Furthermore, returns on the five portfolios are leptokurtic, where the coefficient of excess kurtosis is greater than zero. A high value of excess kurtosis contributes to the observed non-normality of returns for the five portfolios, as measured by the Jarque–Bera test statistic. The Ljung–Box test statistic provides evidence of serial correlation in (squared) returns. Therefore, our regression-based methodology in Section 4.3 is designed so as to account for heteroscedasticity.
4. Hypotheses and methodology
4.1. Testable hypotheses
In this section, we outline the testable hypotheses for pharmaceutical stock returns around DRNs. DRNs may give rise to two conflicting sentiment effects: the DRNs positive effect and the fear effect. In this respect, Kaplanski and Levy (2012) document a “negative and significant war sentiment effect” during the 1973 Arab–Israeli War in Israel, and a positive and significant “holiday sentiment effect.” Firstly, DRNs may lead to anxiety, bad moods, and pessimism among the general public and stock market investors, associated with a panic effect generated by overemphasized media coverage of dangerous infectious diseases. In this scenario, anxiety and fear spread across different stock market sectors and may instigate a drop in the pharmaceutical stock prices. In Hypothesis 1, we posit that the investor's fear gauge, provoked by DRNs, may lead to a decrease in the rate of return of pharmaceutical stocks.
Hypothesis 1
DRNs may lead to bad moods among investors, which may negatively affect the portfolio returns of pharmaceutical stocks.
Secondly, dangerous infectious diseases may be perceived by investors as a profitable investment opportunity. They unreasonably anticipate that a disease will trigger an increase in R&D expenditure by pharmaceutical companies. This development generates a positive sentiment and hence raises demand for pharmaceutical stocks. As a result, portfolio valuation increases. Thus, in Hypothesis 2, we posit a positive relation between DRNs and returns on pharmaceutical stocks.
Hypothesis 2
DRNs have a positive effect on the portfolio returns of pharmaceutical stocks.
Our third hypothesis predicts that investors initially underreact to an announcement of a dangerous infectious disease but they overreact thereafter. Further, the degree of overreaction depends on the announcement's relative salience as measured by media coverage (Palomino et al., 2009). Klibanoff et al. (1998) report that the larger the information salience of an announcement, the faster the contents are incorporated into closed-end country funds' prices. Within the pharmaceutical industry, considerable media coverage can cause significant changes in stock prices through continuous overreporting, even though no genuinely new information becomes available to the market (Huberman and Regev, 2001). Thus, motivated by the literature and the observed inflow of information around PHEIC alerts (see Fig. 1), we posit in Hypothesis 3 that a positive sentiment by stock market investors can remain at an elevated level after the event.
Hypothesis 3
DRNs have a persistent effect on the portfolio returns of pharmaceutical stocks. Such persistence is consistent with an inflow of disease-related information in the stock market.
Our fourth hypothesis builds on the notion that investor sentiment should affect small cap stocks stronger relative to large cap stocks (Brown and Cliff, 2005). Therefore, we expect that on and after the event day, investor sentiment will be potentially stronger for small pharmaceutical firms than for large firms. This hypothesis is supported by recent studies suggesting that the stocks of small firms are mainly held by local investors who tend to be largely influenced by specific events (Edmans et al., 2007). Furthermore, even though large pharmaceutical companies act as forerunners of R&D activities, it may be less costly for small pharmaceutical companies to act as followers in the development of new drugs.12 However, vaccine production requires more costly and sophisticated technologies than drug production (Hinman et al., 2006). The costs of developing effective new vaccines place a constraint on small pharmaceutical firms. As a result, they are likely to develop new vaccines less frequently than large firms. Thus, we posit in Hypothesis 4 that investor sentiment, driven by DRNs, will have a larger effect on the prices of small pharmaceutical stocks in comparison with large stocks.
Hypothesis 4
DRNs have a greater effect on stock returns of small companies relative to large companies.
Two commonly used—the event-study and regression-based—methodologies are used to test Hypothesis 1, Hypothesis 2, Hypothesis 3, Hypothesis 4.
4.2. Event study methodology
In the spirit of traditional event studies, we begin our analysis by computing cumulative abnormal returns (CARs) around DRNs. The abnormal returns are defined as the difference between the observed rate of return of a pharmaceutical portfolio and its ex-post expected rate of return over the whole length of the event window. The expected rate of return is estimated based on the Fama–French three-factor model.13 We choose an event window length to cover 14 days prior and 14 days after DRNs. The estimation window for the model is 252 days long.
In our sample, we observe temporal clustering of DRNs. This means that if all DRNs were taken into account, the CARs would suffer from overlapping event windows. For this reason, our CAR calculations include only a subset of all available DRNs. The DRNs are selected according to the following two criteria. In the first selection procedure, Last event, we choose the DRNs only if it is not followed by other DRNs within 28 days after its occurrence. For First event, on the other hand, we select the DRNs in a chronological order. We start with the first DRNs in the sample, ignore all DRNs showing up in the proximate 28 days, take the next DRNs in succession, ignore the following 28 days, and so on. This iteration proceeds until the whole sample is exhausted.
For illustration, assume there are five DRNs taking place at dates τ 0, τ 1, τ 2, τ 3, and τ 4, where τ 1, τ 2, and τ 3 are temporally clustered. Then, for Last event, we use DRNs for CAR calculation occurring on days τ 0, τ 3, and τ 4; for First event, we choose τ 0, τ 1, and τ 4. Panels A and B in Fig. 2 illustrate these two simple examples.
Fig. 2.
Cumulative abnormal returns of pharmaceutical stocks.
Notes: This figure depicts the average cumulative residuals around the event date (t=0) for pharmaceutical portfolios EW, VW, TOP, and BOTTOM. The residual on day t is calculated as the difference between observed rate of return and the ex-post expected rate of return on day t. The three-factor model is , where rt is the pharmaceutical portfolio return and , smbt and hmlt are Fama–French factors. The estimation window is 252 days. The solid (dashed) line represents the Last (First) event approach when estimating the CARs. This ensures that event windows are non-overlapping. Panels A and B illustrate the two different estimation procedures graphically. The Last (First) event approach includes event days τ0, τ3 and τ4 (τ0, τ1 and τ4) in the estimation procedure. The events occurred during a 12-year period (March 2003–December 2014) which includes 33 event days with non-overlapping event windows.
4.3. Regression-based methodology
We follow existing empirical studies (Kamstra et al., 2003, Edmans et al., 2007, Kaplanski and Levy, 2010a, Kaplanski and Levy, 2010b, Curatola et al., 2016) to evaluate the impact of DRNs on pharmaceutical stock returns, and to identify the negative fear effect. We thus implement the following regression model:
| (1) |
where is the daily rate of return on a pharmaceutical stock portfolio p at time t, β 0 is the regression intercept, and are lagged dependent variables. D 1, D 2, D 3, and D 4 are dummy variables for Monday, Tuesday, Wednesday, and Thursday, respectively, and D TAX t is a dummy variable for the first five days of the taxation year. Further, E t represents the DRNs effect variable, FI t denotes a fear index, and is an error term. In line with existing studies, the VIX is used to proxy the investment fear index also known as “investor fear gauge” (Whaley, 2009).14 Specifically, in the spirit of Bloom (2009), our fear indicator is a dummy variable that takes on value 1 when the U.S. stock market volatility exceeds by more than 1.65 standard deviations the Hodrick–Prescott detrended mean of the VIX, and 0 otherwise.15
The coefficient β 4 captures the contemporaneous effect of DRNs on the portfolio rate of return. It should be noted that most DRNs (85%) are published by the WHO, which is headquartered in Geneva, Switzerland. Due to different time zones, we assume that the U.S. stock market reacts on the day of DRNs publication. Further, previous days' rates of return, , variables are embedded in our main regression to account for possible serial correlation. We choose five lagged returns to be sure that all serial correlations have been accounted for. The dummy variables for the days of the week are employed to account for the so-called “Monday effect.”
To account for a possible positive sentiment effect on the days following DRNs, or differently, for a possible reversal effect, we run also the following regression:
| (2) |
5. Results
5.1. Event study methodology
Fig. 2 depicts the CARs around the event date. The solid (dashed) line depicts the Last event (First event) approach. The Last event approach gives rise to positive abnormal returns following the event day for the EW and BOTTOM portfolios. The increase prior to the event can be attributed to the sensitivity of the Last event approach to the contiguous (preceding) events in the overlapping window. The First event approach points to an increase in returns of the EW and BOTTOM portfolios on the event day. This increase is rather persistent on the days following the event, thus, confirming that those DRNs that were discarded in the CAR calculation had a positive impact on pharmaceutical stock returns. The results are less supportive for the VW and TOP portfolios. The event study results are weaker than the regression results reported in the next section due to the fact that only 33 out of 146 DRNs were employed in the CAR analysis. In general, EW Last/First (VW, TOP, and BOTTOM) experienced in 27%/30.7% (34.6%/42.3%, 38.4%/42.3%, and 53.8%/50%) of all event cases a significant CAR at the 10% level. The CAR analysis provides evidence supporting the persistent (positive) sentiment effect hypothesis (Hypothesis 3). One could anticipate that the SMB factor in the three-factor Fama–French model already captures some of the effect of investment sentiment, biasing the estimate of CARs. Indeed, if investors holding smaller cap stocks become more optimistic, then the SMB factor increases, and the CAR decreases. We also compute the CARs by employing the excess return on the market in a one-factor model.16 The unreported results suggest more pronounced CARs for the BOTTOM portfolio. Overall, our event study analysis reveals a positive and persistent effect of DRNs on the stock returns of pharmaceutical companies.
5.2. Regression analysis
Results examining the effect of the fear gauge and DRNs on the pharmaceutical stock market returns are reported in Table 3 . Panel A documents our main findings that result from incorporating all events in the regression analysis. For robustness purposes, we also rerun our model on a subsample of DRNs by employing WHO-related announcements only (see Panel B). Consistently with Hypothesis 1, a negative and significant effect of the fear gauge index is obtained. This effect is robust across the five portfolios. The estimated coefficient β 5 ranges from −0.1378 (TOP portfolio) to −0.2056 (VW portfolio). Thus, the effect of investor fear gauge is strongest for diversified portfolios of stocks and weakest for large firms. Further, the DRNs variable effect is always positive and generally significant at the 5% level, vindicating Hypothesis 2. The coefficient estimate ranges from 0.1781 (VW) to 0.3883 (S&P 500 IT). Thus, in the wake of DRNs, stock returns increase by roughly 18–39 bps. This is an interesting finding, insofar as disease outbreaks, such as Ebola, may not actually spread in the United States. However, investor decisions can be guided by sentiment. Paradoxically, while dangerous infectious diseases may impose non-negligible costs on the economy and may potentially lead to the depletion of resources, DRNs can produce an optimistic view and a positive sentiment effect among U.S. investors. They unreasonably anticipate that pharmaceutical companies whose stocks or ADRs are listed on the NYSE or NASDAQ will invest in R&D and, potentially, raise their future cash flows.
Table 3.
Regression results (DRNs and fear effects).
| Portf. | Const. | Rt−1 | Rt−2 | Rt−3 | Rt−4 | Rt−5 | Mon. | Tues. | Wed. | Thurs. | DTAX | Et | FIt | Logl. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panel A: All DRNs + fear effects | ||||||||||||||
| EW | 0.1304⁎⁎⁎ | 0.0650⁎⁎ | 0.0222 | 0.0243 | 0.0194 | −0.0129 | −0.1093 | 0.0098 | −0.0544 | −0.0354 | 0.4168⁎⁎⁎ | 0.3861⁎⁎⁎ | −0.1819⁎⁎⁎ | −4977.15 |
| (0.0032) | (0.0151) | (0.4916) | (0.3863) | (0.4605) | (0.6770) | (0.1037) | (0.8746) | (0.3915) | (0.5939) | (0.0009) | (0.0004) | (0.0000) | ||
| VW | 0.1134⁎⁎ | −0.0346 | −0.0487 | 0.0049 | −0.0047 | −0.0022 | 0.0386 | 0.1777⁎⁎⁎ | 0.0433 | 0.0359 | 0.2843⁎⁎ | 0.1781⁎ | −0.2056⁎⁎⁎ | −4774.72 |
| (0.0165) | (0.1894) | (0.1313) | (0.8509) | (0.8568) | (0.9387) | (0.5338) | (0.0040) | (0.4748) | (0.5798) | (0.0284) | (0.0742) | (0.0000) | ||
| TOP | 0.0361 | −0.0393 | −0.0777⁎ | 0.0248 | 0.0061 | −0.0110 | 0.0531 | 0.1437⁎⁎⁎ | 0.0373 | 0.0249 | 0.1972⁎ | 0.2274⁎⁎⁎ | −0.1378⁎⁎⁎ | −4448.79 |
| (0.3625) | (0.2301) | (0.0633) | (0.4355) | (0.8453) | (0.7664) | (0.3433) | (0.0090) | (0.4877) | (0.6678) | (0.0893) | (0.0063) | (0.0000) | ||
| BOTTOM | 0.2192⁎⁎⁎ | −0.0309 | 0.0181 | −0.0096 | 0.0465⁎⁎ | 0.0105 | −0.3420⁎⁎⁎ | −0.2324⁎⁎⁎ | −0.0475 | −0.1129 | 0.5659⁎⁎⁎ | 0.3877⁎⁎ | −0.1842⁎⁎⁎ | −5872.04 |
| (0.0011) | (0.1472) | (0.3684) | (0.6147) | (0.0218) | (0.5691) | (0.0001) | (0.0088) | (0.5946) | (0.2241) | (0.0025) | (0.0235) | (0.0012) | ||
| S&P 500 IT | 0.0203 | −0.0837⁎⁎⁎ | −0.0350 | 0.0105 | −0.0056 | −0.0175 | 0.1016 | 0.2086⁎⁎⁎ | 0.1201 | 0.1027 | 0.3400⁎ | 0.3883⁎⁎⁎ | −0.1792⁎⁎⁎ | −5342.82 |
| (0.6953) | (0.0027) | (0.2696) | (0.7092) | (0.8526) | (0.6115) | (0.1827) | (0.0057) | (0.1057) | (0.1695) | (0.0976) | (0.0007) | (0.0000) | ||
| Panel B: WHO Disease Outbreak News + WHO Statements + fear effects | ||||||||||||||
| EW | 0.1426⁎⁎⁎ | 0.0650⁎⁎ | 0.0224 | 0.0252 | 0.0187 | −0.0132 | −0.1289⁎ | 0.0013 | −0.0631 | −0.0396 | 0.4184⁎⁎⁎ | 0.3968⁎⁎⁎ | −0.1811⁎⁎⁎ | −4978.71 |
| (0.0013) | (0.0151) | (0.4868) | (0.3676) | (0.4796) | (0.6712) | (0.0552) | (0.9834) | (0.3212) | (0.5514) | (0.0009) | (0.0012) | (0.0000) | ||
| VW | 0.1184⁎⁎ | −0.0350 | −0.0494 | 0.0048 | −0.0062 | −0.0022 | 0.0263 | 0.1754⁎⁎⁎ | 0.0393 | 0.0349 | 0.2884⁎⁎ | 0.2720⁎⁎ | −0.2089⁎⁎⁎ | −4773.51 |
| (0.0124) | (0.1841) | (0.1254) | (0.8535) | (0.8141) | (0.9400) | (0.6724) | (0.0045) | (0.5167) | (0.5897) | (0.0260) | (0.0111) | (0.0000) | ||
| TOP | 0.0431 | −0.0399 | −0.0781⁎ | 0.0247 | 0.0048 | −0.0106 | 0.0400 | 0.1393⁎⁎ | 0.0322 | 0.0228 | 0.1998⁎ | 0.2723⁎⁎⁎ | −0.1390⁎⁎⁎ | −4448.62 |
| (0.2764) | (0.2236) | (0.0618) | (0.4371) | (0.8786) | (0.7744) | (0.4748) | (0.0113) | (0.5499) | (0.6946) | (0.0844) | (0.0068) | (0.0000) | ||
| BOTTOM | 0.2335⁎⁎⁎ | −0.0314 | 0.0191 | −0.0087 | 0.0472⁎⁎ | 0.0112 | −0.3544⁎⁎⁎ | −0.2450⁎⁎⁎ | −0.0564 | −0.1190 | 0.5605⁎⁎⁎ | 0.2043 | −0.1760⁎⁎⁎ | −5875.19 |
| (0.0005) | (0.1414) | (0.3422) | (0.6487) | (0.0198) | (0.5440) | (0.0001) | (0.0057) | (0.5263) | (0.1999) | (0.0026) | (0.2936) | (0.0020) | ||
| S&P 500 IT | 0.0328 | −0.0828⁎⁎⁎ | −0.0346 | 0.0110 | −0.0071 | −0.0169 | 0.0830 | 0.1994⁎⁎⁎ | 0.1112 | 0.0979 | 0.3406⁎ | 0.3716⁎⁎ | −0.1773⁎⁎⁎ | −5344.64 |
| (0.5269) | (0.0030) | (0.2753) | (0.6966) | (0.8142) | (0.6244) | (0.2769) | (0.0083) | (0.1348) | (0.1906) | (0.0962) | (0.0110) | (0.0000) | ||
Notes: This table reports the results of Eq. (1). Results of regressions of continuously compounded day-to-day percentage returns on an equally weighted portfolio of pharmaceutical stocks (EW), value-weighted portfolio (VW), constant-value-weighted portfolio of 10 largest pharmaceutical stocks (TOP), a constant-value-weighted portfolio of 10 smallest pharmaceutical stocks (BOTTOM), and S&P 500 Information Technology Index (S&P 500 IT) are reported. VW is computed using time-varying weights, whereas TOP and BOTTOM are computed using constant weights, based on the initial market capitalization. Et represents the DRNs effect variable. DRNs (i.e., event days) are reported in Table A1. In Panel A, Et is composed of all DRNs. In Panel B, Et is composed of WHO Disease Outbreak News and WHO Statements only. The log-likelihood value of the estimated model is shown in the last column. The p-values are in parentheses. The Eicker–White standard error estimates are robust to heteroscedasticity. We use daily data for the period 01/01/2003–11/13/2014 (a total of 3097 observations).
Significance at the 10% level.
Significance at the 5% level.
Significance at the 1% level.
However, investors overlook the fact that the patent system imposes constraints on investment and productivity growth in the pharmaceutical industry (Boldrin and Levine, 2013). At the same time, they underestimate the probability that vaccines and medicines are not necessarily effective (Shortridge, 2004). The results unambiguously indicate that investors should buy stocks when an infectious disease outbreak is announced. Moreover, investment in a portfolio dominated by large cap stocks (such as TOP or VW) when a disease outbreak is announced yields a lower return than investment in a portfolio of small cap stocks (such as BOTTOM), ceteris paribus.17 This finding supports Hypothesis 4, wherein small cap stocks respond stronger to investor sentiment than large cap stocks. This result agrees with Brown and Cliff (2005) and Edmans et al. (2007).
Table 4 further shows that the investor fear gauge has the smallest (largest) effect (in absolute value) for the TOP (VW) portfolio (−0.1481 and −0.2163, respectively). The investor fear gauge reflects investor pessimism regarding portfolio investment, which has a negative effect on stock prices and returns of pharmaceutical companies. This result confirms Hypothesis 1, in which we posit a negative relation between investor fear gauge and returns on pharmaceutical stocks. It is qualitatively similar to the results reported by Kaplanski and Levy (2012), wherein the investor fear gauge provoked by the 1973 Arab–Israeli War is responsible for a negative effect on the stock market return of the Tel Aviv Stock Exchange. Table 4 also indicates that the investor sentiment effect is positive and persistent. We use six lags of DRNs to capture the persistence. The number of lags is dictated by Fig. 1, which indicates that the number of media articles remains above average six days after PHEIC event. We thus expect that the positive sentiment effect will last for six days due to a continuous flow of information in the stock market. We test the combined effect of the contemporaneous and lagged DRNs on pharmaceutical stock returns. To this end, we test for the significance of the coefficient sum , where I=6. Entries related to this coefficient sum highlight that the DRNs positive sentiment effect is stronger among small pharmaceutical firms (0.8169) and is weaker for the VW portfolio (0.4860). In all cases, the coefficient is significant at the 1% level. The estimated lagged effects are consistent with Kaplanski and Levy (2010b), who argue that the announcement typically receives headlines several days after the event. The estimation results for the five portfolios show a positive and significant effect (at the 5% level) for at least six trading days after DRNs are released. Thus, our results also support Hypothesis 3, wherein positive investor sentiment remains significant several days after the DRNs. This result agrees with Huberman and Regev (2001), who underscore the importance of the information salience of a breakthrough in biopharmaceutical research. The contemporaneous and lagged effects provide unequivocal evidence that DRNs have a positive and significant (either contemporaneous or lagged) effect on pharmaceutical stock prices.
Table 4.
Regression results (DRNs contemporaneous and lagged effects and fear effects).
| Portf. | Const. | Rt−1 | Rt−2 | Rt−3 | Rt−4 | Rt−5 | Mon. | Tues. | Wed. | Thurs. | DTAX | FIt | LogL. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panel A: All DRNs + fear effects | ||||||||||||||
| EW | 0.1235⁎⁎⁎ | 0.0616⁎⁎ | 0.0196 | 0.0229 | 0.0163 | −0.0166 | −0.1171⁎ | −0.0005 | −0.0505 | −0.0327 | 0.4392⁎⁎⁎ | 0.7597⁎⁎⁎ | −0.1959⁎⁎⁎ | −4971.54 |
| (0.0055) | (0.0214) | (0.5431) | (0.4153) | (0.5375) | (0.5958) | (0.0820) | (0.9941) | (0.4272) | (0.6217) | (0.0005) | (0.0000) | (0.0000) | ||
| VW | 0.1099⁎⁎ | −0.0363 | −0.0490 | 0.0040 | −0.0071 | −0.0045 | 0.0281 | 0.1635⁎⁎⁎ | 0.0417 | 0.0405 | 0.3009⁎⁎ | 0.4860⁎⁎⁎ | −0.2163⁎⁎⁎ | −4767.39 |
| (0.0195) | (0.1668) | (0.1293) | (0.8769) | (0.7851) | (0.8784) | (0.6514) | (0.0085) | (0.4940) | (0.5319) | (0.0202) | (0.0011) | (0.0000) | ||
| TOP | 0.0333 | −0.0419 | −0.0795⁎ | 0.0227 | 0.0034 | −0.0134 | 0.0442 | 0.1316⁎⁎ | 0.0351 | 0.0253 | 0.2134⁎ | 0.5171⁎⁎⁎ | −0.1481⁎⁎⁎ | −4443.67 |
| (0.4030) | (0.2020) | (0.0580) | (0.4767) | (0.9143) | (0.7182) | (0.4309) | (0.0173) | (0.5157) | (0.6630) | (0.0658) | (0.0004) | (0.0000) | ||
| BOTTOM | 0.2182⁎⁎⁎ | −0.0335 | 0.0159 | −0.0124 | 0.0454⁎⁎ | 0.0091 | −0.3508⁎⁎⁎ | −0.2471⁎⁎⁎ | −0.0638 | −0.1262 | 0.5928⁎⁎⁎ | 0.8169⁎⁎⁎ | −0.1993⁎⁎⁎ | −5867.43 |
| (0.0012) | (0.1180) | (0.4290) | (0.5175) | (0.0248) | (0.6236) | (0.0001) | (0.0054) | (0.4761) | (0.1757) | (0.0015) | (0.0018) | (0.0005) | ||
| S&P 500 IT | 0.0160 | −0.0852⁎⁎⁎ | −0.0360 | 0.0096 | −0.0070 | −0.0188 | 0.0950 | 0.2034⁎⁎⁎ | 0.1237⁎ | 0.1041 | 0.3527⁎ | 0.6166⁎⁎⁎ | −0.1876⁎⁎⁎ | −5340.99 |
| (0.7577) | (0.0023) | (0.2570) | (0.7338) | (0.8165) | (0.5869) | (0.2140) | (0.0072) | (0.0979) | (0.1652) | (0.0862) | (0.0009) | (0.0000) | ||
| Panel B: WHO Disease Outbreak News + WHO Statements + fear effects | ||||||||||||||
| EW | 0.1370⁎⁎⁎ | 0.0621⁎⁎ | 0.0209 | 0.0233 | 0.0163 | −0.0169 | −0.1283⁎ | −0.0052 | −0.0550 | −0.0348 | 0.4348⁎⁎⁎ | 0.8091⁎⁎⁎ | −0.1978⁎⁎⁎ | −4973.93 |
| (0.0022) | (0.0205) | (0.5174) | (0.4055) | (0.5365) | (0.5883) | (0.0601) | (0.9344) | (0.3936) | (0.6014) | (0.0006) | (0.0001) | (0.0000) | ||
| VW | 0.1144⁎⁎ | −0.0363 | −0.0503 | 0.0032 | −0.0073 | −0.0044 | 0.0266 | 0.1727⁎⁎⁎ | 0.0369 | 0.0459 | 0.2989⁎⁎ | 0.5685⁎⁎⁎ | −0.2208⁎⁎⁎ | −4769.27 |
| (0.0161) | (0.1682) | (0.1191) | (0.9024) | (0.7797) | (0.8792) | (0.6713) | (0.0059) | (0.5455) | (0.4803) | (0.0210) | (0.0007) | (0.0000) | ||
| TOP | 0.0380 | −0.0414 | −0.0800⁎ | 0.0220 | 0.0029 | −0.0136 | 0.0436 | 0.1389⁎⁎ | 0.0304 | 0.0313 | 0.2111⁎ | 0.5780⁎⁎⁎ | −0.1511⁎⁎⁎ | −4444.44 |
| (0.3418) | (0.2077) | (0.0564) | (0.4902) | (0.9255) | (0.7146) | (0.4406) | (0.0130) | (0.5760) | (0.5924) | (0.0683) | (0.0009) | (0.0000) | ||
| BOTTOM | 0.2305⁎⁎⁎ | −0.0331 | 0.0153 | −0.0113 | 0.0447⁎⁎ | 0.0084 | −0.3466⁎⁎⁎ | −0.2614⁎⁎⁎ | −0.0512 | −0.1401 | 0.5886⁎⁎⁎ | 0.8869⁎⁎⁎ | −0.2025⁎⁎⁎ | −5868.26 |
| (0.0007) | (0.1204) | (0.4486) | (0.5544) | (0.0279) | (0.6506) | (0.0001) | (0.0035) | (0.5693) | (0.1356) | (0.0015) | (0.0025) | (0.0004) | ||
| S&P 500 IT | 0.0300 | −0.0839⁎⁎⁎ | −0.0345 | 0.0101 | −0.0089 | −0.0182 | 0.0815 | 0.1968⁎⁎ | 0.1177 | 0.1035 | 0.3447⁎ | 0.4854⁎⁎ | −0.1816⁎⁎⁎ | −5341.46 |
| (0.5660) | (0.0027) | (0.2777) | (0.7206) | (0.7701) | (0.5994) | (0.2898) | (0.0104) | (0.1194) | (0.1709) | (0.0926) | (0.0382) | (0.0000) | ||
Notes: This table reports the results of Eq. (2). Results of regressions of continuously compounded day-to-day percentage returns on an equally weighted portfolio of pharmaceutical stocks (EW), value-weighted portfolio (VW), constant-value-weighted portfolio of 10 largest pharmaceutical stocks (TOP), a constant-value-weighted portfolio of 10 smallest pharmaceutical stocks (BOTTOM), and S&P 500 Information Technology Index (S&P 500 IT) are reported. VW is computed using time-varying weights, whereas TOP and BOTTOM are computed using constant weights, based on the initial market capitalization. Et−i () represents the DRNs contemporaneous and lagged effect variables. measures the accumulated effect of the lagged DRNs. DRNs (i.e., event days) are reported in Table A1. In Panel A, Et is composed of all DRNs. In Panel B, Et is composed of WHO Disease Outbreak News and WHO Statements only. The log-likelihood value of the estimated model is shown in the last column. The p-values are in parentheses. The Eicker–White standard error estimates are robust to heteroscedasticity. We use daily data for the period 01/01/2003–11/13/2014 (a total of 3097 observations).
Significance at the 10% level.
Significance at the 5% level.
Significance at the 1% level.
Fear gauge and DRNs can not only provoke changes in the pharmaceutical stock prices but also in the S&P 500 IT, an industry unrelated to dangerous infectious diseases, thus lending further support to the investment sentiment effect. Specifically, our results indicate that fear gauge (DRNs) always has a negative (positive) and significant effect on the stock returns of the S&P 500 IT. This result shows that the investor sentiment effect can be widespread across various industries and lead to profitable trading strategies.
We validate our research findings with a number of robustness checks. First, we examine the extent to which investor sentiment can spread to other sectors of the economy and even to other countries. In this regard, the presence of investor sentiment is identified in the S&P 500 Industrials Index, an industry seemingly unrelated to dangerous infectious diseases. The impact of fear gauge and DRNs was also tested on regions that were directly affected by the four dangerous infectious diseases. In particular, the estimation results show a negative (positive) and significant effect of fear gauge (DRNs) on returns on the MSCI Emerging Markets stock market index. Second, to account for the presence of conditional heteroscedasticity in daily stock returns, our regression methodology is extended to include a battery of GARCH specifications. As in the benchmark methodology, the results show a negative (positive) and significant effect of fear gauge (DRNs) on pharmaceutical stock returns, endorsing the main findings. Third, as a complementary exercise, we vary the number of lags in Eq. (2). The estimation results broadly support Hypothesis 3. Fourth, we perform all our regressions excluding the control variables (i.e., with the DRNs and fear gauge variables only). Again, the main findings can be upheld. The general conclusion that can be drawn from these robustness checks is that investor sentiment, triggered by dangerous infectious diseases, is neither confined to the pharmaceutical industry nor is model-specific. By contrast, the presence of investor sentiment is robust to the type of activity, the geographical scope, and the various methodological underpinnings.18
6. Exploiting sentiment and fear effects
Our empirical evidence asserts that an investor should be willing to exploit both sentiment and fear effects in response to DRNs by building leverage positions in pharmaceutical stocks. For this purpose, we consider a zero-cost strategy. On event days that coincide with high-volatility periods, as measured by the fear index, the zero-cost spread portfolio takes a 100% long position in one of the four above mentioned pharmaceutical portfolios and a 100% short position in the VIX. We assume that the investment horizon of the spread portfolio varies from one day (1D) to three days (3D). To eliminate timing-inconsistencies, the trade is initiated at the closing price of the event day. This procedure ensures that no trade precedes DRNs publication on any given event day.19 Table 5 reports the strategies' average performance. In Panel A, the results are for all DRNs, while Panel B reports only the WHO-related subsample. The 1D strategy does not yield a significant performance for all four pharmaceutical portfolios. Differently, a holding period of two or three days is remarkably profitable. Such profitability is higher in the case of the EW and BOTTOM portfolios.
Table 5.
Trading strategies.
| Investment horizon | EW | VW | TOP | BOTTOM |
|---|---|---|---|---|
| Panel A: All DRNs | ||||
| 1D | 0.518 | 0.3755 | 0.4208 | 0.3941 |
| (0.5208) | (0.5085) | (0.5036) | (0.5226) | |
| 2D | 1.6302⁎⁎ | 1.2548⁎⁎ | 1.2544⁎⁎ | 1.4261⁎⁎ |
| (0.7754) | (0.7551) | (0.7386) | (0.7592) | |
| 3D | 1.913⁎⁎ | 1.433⁎⁎ | 1.4542⁎⁎ | 1.9172⁎⁎⁎ |
| (0.8539) | (0.8076) | (0.8006) | (0.8063) | |
| Panel B: WHO Disease Outbreak News + WHO Statements | ||||
| 1D | 0.4991 | 0.3831 | 0.4181 | 0.424 |
| (0.5725) | (0.5618) | (0.5555) | (0.5877) | |
| 2D | 1.6275⁎⁎ | 1.2644⁎ | 1.2491⁎ | 1.3749⁎ |
| (0.8505) | (0.8368) | (0.8174) | (0.8572) | |
| 3D | 2.0802⁎⁎ | 1.6035⁎⁎ | 1.6335⁎⁎ | 2.1461⁎⁎ |
| (0.9783) | (0.9301) | (0.9224) | (0.9296) | |
Notes: This table reports the average performance (in %) of a spread portfolio that is invested 100% long in a pharmaceutical portfolio and 100% short in the VIX. Long and short positions are taken only during high volatility states of the world (as indicated by the fear index) and using closing prices of the DRNs day. The investment horizon of the portfolio varies between one (1D) and three days (3D). In Panel A, the trading strategy is based on all DRNs. In Panel B, the trading strategy is based on WHO Disease Outbreak News and WHO Statements only. The trading period runs from January 2003 to November 2014 (3097 trading days). Standard errors are reported in parentheses.
Significance of a one-tailed t-test at the 10% level.
Significance of a one-tailed t-test at the 5% level.
Significance of a one-tailed t-test at the 1% level.
The longer term profitability of overweighting pharmaceutical stocks and underweighting the VIX is illustrated in Fig. 3 . Different from the zero-cost spread portfolio, whose profitability is summarized in Table 5, we now assume that an investor holds a benchmark portfolio that is fully exposed to the stock market. That is, during tranquil times, 100% of an investor's wealth is invested in the market portfolio, which is proxied by the S&P 500. On event days that coincide with high volatility periods, the investor reallocates funds by going 150% long in a pharmaceutical portfolio and 50% short in the VIX and holds the position between one (1D) and three (3D) days. The depicted outperformance is calculated as the difference between the cumulative performance of a trading strategy and the market portfolio. A visual inspection suggests a similar performance between the VW and TOP portfolios, while EW and BOTTOM are the more profitable strategies for all three investment horizons (except during the period 2009–2014 in Panel A).
Fig. 3.
Outperformance of DRNs and fear effect strategies.
Notes: This figure depicts the outperformance of portfolios that follow DRNs-based trading strategies. The strategies initiate a 150% long position in a pharmaceutical portfolio (using closing prices of the DRNs day) and a 50% short position in the VIX on those event dates that coincide with high-volatility states of the world as indicated by the fear index. During tranquil times (i.e., absence of DRNs), 100% of an investor's wealth is invested in the market portfolio, which is proxied by the S&P 500. On event days that coincide with high volatility periods, the investor reallocates funds by going 150% long in a pharmaceutical portfolio and 50% short in the VIX and holds the position one day (Panel A: 1D), two days (Panel B: 2D) or three days (Panel C: 3D). The outperformance is calculated as the difference between the cumulative performance of the four trading portfolios and a long-only position in the market portfolio. Assuming an initial investment of 1 USD, the application of the trading strategies 1D–3D to EW (VW, TOP, and BOTTOM) yields on average between 0.48 and 2.05 USD (0.22–0.67 USD, 0.24–0.66 USD, and 0.29–2.02 USD) more than a long-only market exposure. The trading period runs from January 2003 to November 2014 (3097 trading days).
For robustness purposes, we ignore the fear effects and consider a simple buy-and-hold strategy that initiates a long position in a pharmaceutical portfolio at the closing price of an event day and holds it for 14 days. The unreported results confirm that this strategy would also have yielded a positive outperformance over the period examined. The EW (VW, TOP, and BOTTOM) portfolio has on average a 1.6% (0.7%, 0.3%, and 1.7%) higher performance than the S&P 500 during a 14-day period.
Overall, we find that investment strategies exploiting sentiment and fear effect related to DRNs lead to higher profitability. Once again, this result is most pronounced for the BOTTOM portfolio, confirming that investor sentiment tends to have a greater effect on small cap stocks.
7. Conclusion
Motivated by the abundance of recent behavioral finance studies showing that particular events (e.g., St. Patrick's Day, the 1973 Arab–Israeli War, Rosh Hashanah, international sporting games) may have a strong effect on investors' moods, we investigate whether DRNs have a positive and significant sentiment effect on investors interested in U.S. pharmaceutical companies. This research builds on the notion that vaccine production for dangerous infectious diseases is concentrated in a few large pharmaceutical companies. Indeed, the number of vaccine producers in the U.S. decreased dramatically over the last four decades (Masignani et al., 2003).
Rational investors design trading strategies that are based on expectations of future cash flows of these companies. We argue that rational trading should not occur for two reasons. First, in the wake of DRNs, uncertainty surrounding distant cash flows of vaccine developers may be too high. Second, the resources of smaller pharmaceutical companies may be too limited to engage in large-scale R&D. For instance, typically at least 850 million USD is needed to develop and license a new pharmaceutical product (Masignani et al., 2003). As a result, pharmaceutical stock prices should not respond to DRNs. Thus, DRNs can only lead to higher stock prices and returns of other pharmaceutical companies through altering investor sentiment about their future performance and leading to irrational trading (Kaplanski and Levy, 2010b). The potential negative sentiment effect induced by fear and anxiety due to DRNs is also tested, allowing us to account for two conflicting sentiment effects.
We find that during the period from 2003 to 2014 DRNs exert a positive and significant effect on pharmaceutical firms' stock returns. This effect is relatively stronger for a portfolio of small cap stocks. We also find that the DRNs effect lasts for several days. Consistent with the widely held view that a DRNs can also generate panic, fear, and anxiety among investors, we observe that an ad hoc fear gauge index appears to exert a negative and significant effect on the returns of pharmaceutical companies' stocks. Again, the effect is stronger for small relative to large cap stocks. In this research, we show that large events of devastating nature to the economy can be considered as good news to some interest groups, such as stock market traders.
Overall, we show that investor optimism and pessimism induced by DRNs can significantly influence portfolio investment decisions in the pharmaceutical industry. Our findings are of utmost importance and practical usefulness for institutional and individual investors, portfolio managers, financial analysts, and pharmaceutical firms. Indeed, we identify a range of exploitable investment opportunities. To this end, we design a number of trading strategies that involve a portfolio of pharmaceutical stocks and the VIX. The results of the exercise unambiguously show that trading strategies yield positive and significant returns. The outcome of this exercise is also useful for portfolio managers who provide advice for investors. Financial analysts can provide a comprehensive analysis of investment opportunities in the pharmaceutical sector. Furthermore, by issuing and selling new stocks to investors, pharmaceutical firms can benefit from increasing market valuation and, hence, from lower cost of funding for R&D spending when an infectious disease outbreak occurs and accelerates.
Acknowledgments
We would like to thank Michela Altieri, Giuliano Curatola, Alessandro Gioffré, Longarela Iñaki Rodríguez, Marie Lalanne, Robin Lumsdaine, Christian Schlag, and Ansgar Wohlschlegel, along with seminar participants at the 2015 Frankfurt-Muenster Asset Pricing Workshop, the 11th BMRC-DEMS Conference on Macro and Financial Economics/Econometrics, 2015 PBS Research Conference, the XXIII Spanish Finance Forum, Graduate School of Social Sciences (Hiroshima University), the 2015 SFM Conference, ISM University of Management and Economics (Vilnius), Nagasaki University, South Western University of Finance and Economics (Chengdu), University of Malaga, and the 2016 Portsmouth-Fordham Conference on Banking and Finance. We would also like to thank Amit Goyal and one anonymous referee for their insightful comments that helped improve our paper. We gratefully acknowledge research support from the Research Center SAFE, funded by the State of Hessen initiative for research LOEWE. Some of the work on this paper was completed while Kizys was visiting the Research Center SAFE and while Donadelli and Riedel were visiting Portsmouth Business School.
Footnotes
Source: OECD Health Statistics (2013).
Source: The Statistics Portal (Statista), 2015 (available at www.statista.com/statistics/215163/us-health-expenditure-as-percentage-of-gdp-forecast).
Source: The Pharmaceutical Research and Manufacturers of America (PhRMA) (available at www.phrma.org/economic-impact).
FDA's “Complete List of Vaccines Licensed for Immunization and Distribution in the U.S.” includes Emergent Biosolutions, Johnson & Johnson, Merck, and Pfizer (available at www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm).
A generic drug is defined by the WHO as “a pharmaceutical product, usually intended to be interchangeable with an innovator product, that is manufactured without a license from the innovator company and marketed after the expiry date of the patent or other exclusive rights.” Available at www.who.int/trade/glossary/story034/en.
The average volume of stocks traded (as % of GDP) in the U.S. over the last decade is above 200% (Source: World Development Indicators).
Source: HSBC Hedge Weekly n. 28 - Investment Fund Performance Review, July 2014.
WHO announcements and disease-based news released on weekend days are assumed to have an effect on Monday.
Occasionally there were false alarms. Some countries were temporarily included and subsequently removed from the list of affected countries (if a SARS outbreak could not be confirmed in retrospect). We also include such false alarm dates in our analysis since the public is likely to take the information as a fact without questioning its reliability at the time of publication.
Despite the well-documented first-mover advantage in the pharmaceutical industry, “under the current law, the chemical formula and the efficacy of the cure as established by clinical trials are made available for competitors essentially for free” (Boldrin and Levine, 2013, p. 13). When patents expire, low-cost generics are immediately introduced into the market.
The daily data for the factors SMB, HML, and the excess return on the market were obtained from Kenneth French's website (http://mba.tuck.dartmouth.edu/pages/faculty/ken.french).
We consider the VIX an adequate proxy as the correlation between the 252-day rolling standard deviation of the S&P 500 Index (S&P 500) and the pharma portfolios is rather high (average correlations are reported in parentheses): EW (0.79), VW (0.72), TOP (0.82), and BOTTOM (0.78).
The fear effect FIt overlaps with the sentiment effect generated by DRNs in 119 out of 146 cases.
In the one-factor model, EW Last/First (VW, TOP, and BOTTOM) experienced in 42.3%/42.3% (46.1%/50%, 46.2%/50%, and 53.9%/50%) of all event cases a significant CAR at the 10% level.
Either due to lower expenses related to sub-contracting (Assid et al., 2015) or less costly imitation of larger pharmaceutical companies (Boldrin and Levine, 2013), small pharmaceutical stocks can potentially generate higher returns than large stocks.
All the additional checks are not reported but are available from the authors upon request.
A more precise approach would involve the exact publication time of the news in a given country and consider the time zone difference between the U.S. and the respective country. Unfortunately, most DRNs do not have a time stamp. Nevertheless, our trading strategy is consistent in timing for those DRNs that are accompanied with an exact publication time.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.finmar.2016.12.003.
Contributor Information
Michael Donadelli, Email: michael.donadelli@gmail.com.
Renatas Kizys, Email: renatas.kizys@port.ac.uk.
Max Riedel, Email: max.riedel@web.de.
Appendix A.
Table A1.
Event days.
| Date | Disease | Topic | News |
|---|---|---|---|
| 12/03/03a | SARS | WHO Statement | PHEIC |
| 17/03/03 | SARS | WHO Statement, WHO Disease Outbreak News | WHO issues emergency travel advisory (15/03/03), Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update (16/03/03) |
| 18/03/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) multi-country outbreak – update 3 |
| 19/03/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) multi-country outbreak – update 4 |
| 20/03/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) multi-country outbreak – update 5 |
| 21/03/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) multi-country outbreak – update 6 |
| 01/04/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 16 |
| 03/04/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 20 |
| 07/04/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 22 (05/04/03) |
| 10/04/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 26 |
| 14/04/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 29 |
| 15/04/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 30 |
| 16/04/03 | SARS | WHO Statement | Coronavirus never before seen in humans is the cause of SARS |
| 17/04/03 | SARS | WHO Disease Outbreak News, Statement | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 32, Pasteur Institute signs deal with Glaxo to find SARS vaccine |
| 21/04/03 | SARS | Research Funding, WHO Disease Outbreak News | F.D.A. approval of nasal vaccine for flu expected (19/04/03), Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 35 (21/04/03) |
| 24/04/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – Multi-country outbreak Update 38 |
| 28/04/03 | SARS | Research Funding, Statement | GenVec Wins Government SARS Vaccine Contract, Roche aims to launch Sars test by end July |
| 29/04/03 | SARS | WHO Statement, WHO Disease Outbreak News | WHO welcomes ASEAN unity against SARS, Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 42 |
| 30/04/03 | SARS | Statement | Glaxo accelerates work on possible SARS vaccine |
| 01/05/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS) – multi-country outbreak – update 44 |
| 06/05/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS)-multi-country outbreak – update 48 |
| 09/05/03 | SARS | WHO Disease Outbreak News | Severe Acute Respiratory Syndrome (SARS)-multi-country outbreak – update 51 |
| 23/05/03 | SARS | WHO Statement | World Health Organization changes Hong Kong, Guangdong travel recommendations |
| 02/06/03 | SARS | WHO Disease Outbreak News | Update 71 – status of diagnostic tests, training course in China |
| 26/06/03 | SARS | Statement | Pfizer sees progress against SARS |
| 07/07/03 | SARS | WHO Statement | SARS outbreak contained worldwide (05/07/03) |
| 20/08/03 | SARS | WHO Statement | Joint WHO, Food and Agriculture Organization and Chinese government mission on SARS animal reservoir and possible transmission to Humans |
| 21/08/03 | SARS | WHO Statement | Joint mission on SARS animal reservoir and necessary next steps |
| 26/09/03 | SARS | WHO Statement | Inadequate plumbing systems likely contributed to SARS transmission |
| 29/09/03 | SARS | Research Funding | Baxter/Aventis receive 18 million USD SARS funding |
| 05/11/03 | SARS | WHO Statement | Global search for SARS vaccine gains momentum |
| 17/12/03 | SARS | WHO Statement | SARS case in laboratory worker in Taiwan, China |
| 13/09/04 | SARS | Research Funding | AlphaVax receives 4.8 million USD SARS vaccine grant |
| 01/10/04 | SARS | Research Funding | ID Biomedical announces NIH grant to develop nasally delivered SARS vaccine |
| 04/02/05 | SARS | Research Funding | Novavax receives SARS vaccine funding from NIH |
| 24/04/09 | H1N1 | WHO Statement | Influenza-like illness in the United States and Mexico |
| 27/04/09a | H1N1 | WHO Statement, WHO Disease Outbreak News, Statement | PHEIC, Swine influenza – update 3, Mexico granted 205 million USD swine flu loan from World Bank |
| 28/04/09 | H1N1 | WHO Disease Outbreak News | Swine influenza – update 4 |
| 29/04/09 | H1N1 | WHO Statement, WHO Disease Outbreak News | Influenza A(H1N1), Influenza A(H1N1) – update 5 |
| 30/04/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 6 |
| 01/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 8.1 |
| 04/05/09 | H1N1 | WHO Statement, WHO Disease Outbreak News, WHO Disease Outbreak News, WHO Disease Outbreak News | Joint FAO/WHO/OIE/WTO Statement on Influenza A (H1N1) and the safety of pork (02/05/09), Influenza A (H1N1) – update 10 (02/05/09), Influenza A (H1N1) – update 12 (03/05/09), Influenza A (H1N1) – update 14 |
| 06/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 18 |
| 07/05/09 | H1N1 | WHO Statement, WHO Disease Outbreak News | WHO statement on pork and pork consumption, Influenza A(H1N1) – update 20 |
| 08/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 22 |
| 11/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 25 |
| 13/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 27 |
| 15/05/09 | H1N1 | Gvt Order | British government orders 90 million dosages of swine flu vaccine from Baxter, Glaxo |
| 18/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 32 |
| 20/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 34 |
| 22/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 36 |
| 25/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 38 |
| 27/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 39 |
| 28/05/09 | H1N1 | Gvt Order | Australia orders 10 million H1N1 vaccines |
| 29/05/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 41 |
| 01/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 42 |
| 03/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 43 |
| 08/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 45 |
| 10/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 46 |
| 11/06/09 | H1N1 | WHO Statement | World now at the start of 2009 influenza pandemic |
| 15/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 49 |
| 17/06/09 | H1N1 | WHO Statement, WHO Disease Outbreak News | WHO welcomes Sanofi-Aventis's donation of vaccine, Influenza A(H1N1) – update 50 |
| 19/06/09 | H1N1 | WHO Disease Outbreak News, Statement | Influenza A(H1N1) – update 51, Secretary amended an earlier declaration, providing immunity and enabling compensation (contingent upon appropriations) for the use of the antiviral drugs Tamiflu and Relenza for treatment of illnesses caused by H1N1 pandemic flu |
| 22/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 52 |
| 24/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 53 |
| 25/06/09 | H1N1 | Statement | HHS Secretary Kathleen Sebelius issued a declaration under the PREP Act for the use of H1N1 pandemic vaccines that are currently under development, thereby providing immunity and enabling the compensation program, contingent upon appropriations |
| 26/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 54 |
| 29/06/09 | H1N1 | WHO Disease Outbreak News | Influenza A(H1N1) – update 55 |
| 01/07/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 56 |
| 03/07/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 57 |
| 06/07/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 58 |
| 08/07/09 | H1N1 | WHO Statement | Viruses resistant to oseltamivir (Tamiflu) identified |
| 13/07/09 | H1N1 | WHO Statement | WHO recommendations on pandemic (H1N1) 2009 vaccines |
| 15/07/09 | H1N1 | Gvt Order | France orders 94 mln doses of flu vaccine; GlaxoSmithKline, Sanofi, Novartis |
| 23/07/09 | H1N1 | Gvt Order | U.S. purchases 195 million doses of H1N1 Vaccine; CSL Limited, AstraZeneca, Novartis, Sanofi, GlaxoSmithKline |
| 24/07/09 | H1N1 | WHO Statement | Preliminary information important for understanding the evolving situation |
| 27/07/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 59 |
| 31/07/09 | H1N1 | WHO Statement | Pandemic influenza in pregnant women |
| 04/08/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 60 |
| 06/08/09 | H1N1 | WHO Statement | Safety of pandemic vaccines |
| 12/08/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 61 |
| 13/08/09 | H1N1 | Gvt Order | Australia orders 21 million doses of H1N1 vaccine; Roche, Sanofi, GlaxoSmithKline, Novartis, Baxter, CSL Limited, Solvay |
| 21/08/09 | H1N1 | WHO Statement, WHO Disease Outbreak News | Recommended use of antivirals, Pandemic (H1N1) 2009 – update 62 (revised 21 August 2009) |
| 28/08/09 | H1N1 | WHO Statement, WHO Disease Outbreak News | Preparing for the second wave: lessons from current outbreaks, Pandemic (H1N1) 2009 – update 63 |
| 04/09/09 | H1N1 | Gvt Order, WHO Disease Outbreak News | Sinovac obtains initial order of H1N1 vaccine from Chinese Central Government, Pandemic (H1N1) 2009 – update 64 |
| 11/09/09 | H1N1 | WHO Statement, WHO Disease Outbreak News | Measures in school settings, Pandemic (H1N1) 2009 – update 65 |
| 15/09/09 | H1N1 | Approval | Four companies are licensed to produce the 2009 H1N1 influenza vaccine for the U.S.; CSL Limited, AstraZeneca, Novartis, Sanofi |
| 18/09/09 | H1N1 | WHO Statement, WHO Disease Outbreak News | Pandemic vaccine donations for the developing world, Pandemic (H1N1) 2009 – update 66 |
| 24/09/09 | H1N1 | WHO Statement | Pandemic influenza vaccines: current status |
| 30/09/09 | H1N1 | Gvt Order | Sinovac Biotech receives second H1N1 vaccine order from Chinese Central Government |
| 09/10/09 | H1N1 | Statement, WHO Disease Outbreak News | NIH prepares to launch 2009 H1N1 influenza vaccine trial in people with asthma, Pandemic (H1N1) 2009 – update 69 |
| 16/10/09 | H1N1 | WHO Statement | Clinical features of severe cases of pandemic influenza |
| 23/10/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 71 |
| 30/10/09 | H1N1 | WHO Statement | Experts advise WHO on pandemic vaccine policies and strategies |
| 05/11/09 | H1N1 | WHO Statement | Infection of farmed animals with the pandemic virus |
| 06/11/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 73 |
| 10/11/09 | H1N1 | WHO Statement, Approval | Agreement for donation of pandemic H1N1 vaccine signed; GlaxoSmithKline, CSL Limited swine flu vaccine now approved for infants |
| 13/11/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 74 |
| 19/11/09 | H1N1 | WHO Statement | Safety of pandemic vaccines |
| 20/11/09 | H1N1 | WHO Statement | Public health significance of virus mutation detected in Norway |
| 27/11/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 76 |
| 02/12/09 | H1N1 | WHO Statement | Oseltamivir resistance in immunocompromised hospital patients |
| 03/12/09 | H1N1 | WHO Statement | WHO use of advisory bodies in responding to the influenza pandemic |
| 11/12/09 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 78 |
| 22/12/09 | H1N1 | WHO Statement | Comparing deaths from pandemic and seasonal influenza |
| 07/01/10 | H1N1 | Gvt Order Cancel | German states cancel swine flu vaccine orders |
| 11/01/10 | H1N1 | Statement | U.S. scales back H1N1 vaccine, cuts CSL order in half |
| 22/01/10 | H1N1 | WHO Statement, WHO Disease Outbreak News | Statement of the World Health Organization on allegations of conflict of interest and ‘fake’ pandemic, Pandemic (H1N1) 2009 – update 84 |
| 05/02/10 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 86 |
| 12/02/10 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 87 |
| 26/02/10 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 89 |
| 06/04/10 | H1N1 | Gvt Order Cancel | U.K. Government cuts H1N1 vaccine order by two-thirds |
| 16/04/10 | H1N1 | WHO Disease Outbreak News | Pandemic (H1N1) 2009 – update 96 |
| 10/08/10 | H1N1 | WHO Statement | H1N1 in post-pandemic period |
| 22/05/12 | SARS | Research Funding | Baylor College of Medicine receives over 6 million USD from NIH to develop SARS vaccine |
| 05/08/13 | Polio | Statement | Japan steps up to help stop polio outbreak in Somalia |
| 13/11/13 | Polio | WHO Statement | WHO update on polio outbreak in Middle East |
| 17/03/14 | Polio | WHO Disease Outbreak News | Poliovirus in Cameroon – update |
| 21/03/14 | Polio | WHO Disease Outbreak News | Polio outbreak in the Middle East – update |
| 24/03/14 | Ebola | WHO Disease Outbreak News | Ebola virus disease in Guinea (23/03/14) |
| 31/03/14 | Polio | Statement, WHO Disease Outbreak News | Iraqi Ministry of Health declared a polio outbreak (30/03/14), Ebola virus disease in Liberia (30/03/14) |
| 07/04/14 | Polio | WHO Disease Outbreak News, Statement | Ebola virus disease, West Africa – update (05/04/14), first mass vaccination campaigns start since polio found in Iraq (06/04/14) |
| 17/04/14 | Polio | WHO Disease Outbreak News | Update on polio in central Africa – polio confirmed in Equatorial Guinea, linked to outbreak in Cameroon |
| 05/05/14a | Polio | WHO Statement | PHEIC |
| 22/05/14 | Polio | WHO Disease Outbreak News | Wild poliovirus in the Horn of Africa |
| 23/06/14 | Polio | WHO Disease Outbreak News | Detection of poliovirus in sewage, Brazil |
| 25/06/14 | Polio | WHO Disease Outbreak News | Update on polio in central Africa |
| 17/07/14 | Polio | WHO Disease Outbreak News | Update on polio in Equatorial Guinea |
| 18/07/14 | H1N1 | Statement | Janssen Pharmaceuticals pays 30 million USD to develop Cambridge-based Vertex Pharmaceuticals flu drug |
| 31/07/14 | Ebola | WHO Disease Outbreak News | Ebola virus disease, West Africa – update |
| 06/08/14 | Ebola | WHO Statement | WHO to convene ethical review of experimental treatment for Ebola |
| 08/08/14a | Ebola | WHO Statement | PHEIC |
| 11/08/14 | Polio | Statement | Mass polio vaccination campaign supported by WHO and UNICEF kicks off in Iraq |
| 12/08/14 | Ebola | WHO Statement | Ethical considerations for use of unregistered interventions for Ebola virus disease (EVD) |
| 18/08/14 | Ebola | WHO Statement | Statement on travel and transport in relation to Ebola virus disease outbreak |
| 27/08/14 | Ebola | WHO Disease Outbreak News | Ebola virus disease – Democratic Republic of Congo |
| 28/08/14 | Ebola | WHO Statement | WHO issues roadmap to scale up international response to the Ebola outbreak in west Africa |
| 01/09/14 | Ebola | WHO Disease Outbreak News | Ebola virus disease update – Senegal (30/08/14) |
| 05/09/14 | Ebola | WHO Statement | Statement on the WHO consultation on potential Ebola therapies and vaccines |
| 08/09/14 | Polio | WHO Disease Outbreak News | Poliovirus in Cameroon – update (06/09/14) |
| 16/09/14 | Ebola | WHO Statement | WHO welcomes the extensive Ebola support from the United States of America |
| 01/10/14 | Ebola | WHO Disease Outbreak News | Ebola virus disease – united States of America |
| 09/10/14 | Ebola | WHO Disease Outbreak News | Ebola virus disease – Spain |
| 28/10/14 | Ebola | WHO Statement | WHO welcomes Swissmedic approval of Ebola vaccine trial at Lausanne University Hospital |
| 31/10/14 | Ebola | WHO Disease Outbreak News | Ebola virus disease – Mali |
| 06/11/14 | Ebola | WHO Statement | WHO welcomes strong commitment from Australia to beating Ebola |
Note: Disease-related news (event days).
Indicates Public Health Emergency of International Concern (PHEIC) events.
Table A2.
Pharmaceutical companies.
| No. | Name | MCap | Cap weight | Cum sum 1 | Cum sum 2 |
|---|---|---|---|---|---|
| 1 | Pfizer | 188,593.19 | 15.5172 | 15.5172 | 100.0000 |
| 2 | Johnson & Johnson | 180,892.81 | 14.8836 | 30.4008 | 84.4828 |
| 3 | GlaxoSmithKline | 124,710.26 | 10.2610 | 40.6617 | 69.5992 |
| 4 | Merck & Co. | 114,423.03 | 9.4146 | 50.0763 | 59.3383 |
| 5 | Abbott Laboratories | 73,520.95 | 6.0492 | 56.1255 | 49.9237 |
| 6 | AstraZeneca | 71,562.09 | 5.8880 | 62.0135 | 43.8745 |
| 7 | Amgen | 68,315.08 | 5.6209 | 67.6344 | 37.9865 |
| 8 | Eli Lilly and Company | 62,379.55 | 5.1325 | 72.7669 | 32.3656 |
| 9 | Bristol-Myers Squibb | 61,626.74 | 5.0706 | 77.8374 | 27.2331 |
| 10 | Gilead Sciences | 35,021.13 | 2.8815 | 80.7189 | 22.1626 |
| 11 | Novo Nordisk | 34,284.67 | 2.8209 | 83.5398 | 19.2811 |
| 12 | Teva Pharmaceutical Industries | 28,821.62 | 2.3714 | 85.9112 | 16.4602 |
| 13 | Biogen | 20,895.21 | 1.7192 | 87.6304 | 14.0888 |
| 14 | Allergan | 17,904.00 | 1.4731 | 89.1035 | 12.3696 |
| 15 | Genzyme | 14,939.50 | 1.2292 | 90.3327 | 10.8965 |
| 16 | Forest Laboratories | 13,182.62 | 1.0846 | 91.4174 | 9.6673 |
| 17 | Valeant Pharmaceuticals International | 9007.84 | 0.7412 | 92.1585 | 8.5826 |
| 18 | Actavis | 7885.84 | 0.6488 | 92.8074 | 7.8415 |
| 19 | Elan Corporation | 7077.87 | 0.5824 | 93.3897 | 7.1926 |
| 20 | Mylan | 6572.24 | 0.5408 | 93.9305 | 6.6103 |
| 21 | Alexion Pharmaceuticals | 6338.23 | 0.5215 | 94.4520 | 6.0695 |
| 22 | Sigma-Aldrich | 5935.03 | 0.4883 | 94.9403 | 5.5480 |
| 23 | Vertex Pharmaceuticals | 5909.63 | 0.4862 | 95.4265 | 5.0597 |
| 24 | Regeneron Pharmaceuticals | 5319.34 | 0.4377 | 95.8642 | 4.5735 |
| 25 | Perrigo | 4580.84 | 0.3769 | 96.2411 | 4.1358 |
| 26 | Cephalon | 4198.43 | 0.3454 | 96.5866 | 3.7589 |
| 27 | Amylin Pharmaceuticals | 2882.00 | 0.2371 | 96.8237 | 3.4134 |
| 28 | IDEXX Laboratories | 2858.65 | 0.2352 | 97.0589 | 3.1763 |
| 29 | OSI Pharmaceuticals | 2295.20 | 0.1888 | 97.2477 | 2.9411 |
| 30 | Onyx Pharmaceuticals | 2212.30 | 0.1820 | 97.4298 | 2.7523 |
| 31 | NBTY | 2154.64 | 0.1773 | 97.6070 | 2.5702 |
| 32 | Bio-Techne | 2134.91 | 0.1757 | 97.7827 | 2.3930 |
| 33 | PDL BioPharma | 1658.89 | 0.1365 | 97.9192 | 2.2173 |
| 34 | Cubist Pharmaceuticals | 1521.30 | 0.1252 | 98.0444 | 2.0808 |
| 35 | Pharmacyclics | 1377.55 | 0.1133 | 98.1577 | 1.9556 |
| 36 | Immucor | 1200.89 | 0.0988 | 98.2565 | 1.8423 |
| 37 | Ionis Pharmaceuticals | 1113.69 | 0.0916 | 98.3482 | 1.7435 |
| 38 | Par Pharmaceutical | 1078.85 | 0.0888 | 98.4369 | 1.6518 |
| 39 | Nektar Therapeutics | 1074.37 | 0.0884 | 98.5253 | 1.5631 |
| 40 | Chattem | 1023.82 | 0.0842 | 98.6096 | 1.4747 |
| 41 | ViroPharma | 945.58 | 0.0778 | 98.6874 | 1.3904 |
| 42 | Martek Biosciences Corporation | 906.69 | 0.0746 | 98.7620 | 1.3126 |
| 43 | Questcor Pharmaceuticals. | 857.44 | 0.0705 | 98.8325 | 1.2380 |
| 44 | QLT | 850.32 | 0.0700 | 98.9025 | 1.1675 |
| 45 | NPS Pharmaceuticals | 793.55 | 0.0653 | 98.9678 | 1.0975 |
| 46 | Enzon Pharmaceuticals | 699.04 | 0.0575 | 99.0253 | 1.0322 |
| 47 | Meridian Bioscience | 583.74 | 0.0480 | 99.0733 | 0.9747 |
| 48 | USANA Health Sciences | 524.64 | 0.0432 | 99.1165 | 0.9267 |
| 49 | ImmunoGen | 523.70 | 0.0431 | 99.1596 | 0.8835 |
| 50 | Cambrex | 518.72 | 0.0427 | 99.2022 | 0.8404 |
| 51 | Vivus | 485.42 | 0.0399 | 99.2422 | 0.7978 |
| 52 | Neogen Corporation | 472.80 | 0.0389 | 99.2811 | 0.7578 |
| 53 | Quidel Corporation | 396.04 | 0.0326 | 99.3137 | 0.7189 |
| 54 | Geron | 392.13 | 0.0323 | 99.3459 | 0.6863 |
| 55 | CTI BioPharma | 354.98 | 0.0292 | 99.3751 | 0.6541 |
| 56 | AMAG Pharmaceuticals | 354.75 | 0.0292 | 99.4043 | 0.6249 |
| 57 | Amarin Corporation | 353.94 | 0.0291 | 99.4335 | 0.5957 |
| 58 | Celldex Therapeutics | 339.25 | 0.0279 | 99.4614 | 0.5665 |
| 59 | Astex Pharmaceuticals | 328.90 | 0.0271 | 99.4884 | 0.5386 |
| 60 | OraSure Technologies | 323.29 | 0.0266 | 99.5150 | 0.5116 |
| 61 | Xoma Corporation | 312.45 | 0.0257 | 99.5407 | 0.4850 |
| 62 | Progenics Pharmaceuticals | 311.14 | 0.0256 | 99.5663 | 0.4593 |
| 63 | Sarepta Therapeutics | 270.25 | 0.0222 | 99.5886 | 0.4337 |
| 64 | Cerus Corporation | 269.78 | 0.0222 | 99.6108 | 0.4114 |
| 65 | Novavax | 261.06 | 0.0215 | 99.6323 | 0.3892 |
| 66 | Flamel Technologies | 252.94 | 0.0208 | 99.6531 | 0.3677 |
| 67 | Hi-Tech Pharmaceuticals | 230.69 | 0.0190 | 99.6720 | 0.3469 |
| 68 | BioCryst Pharmaceuticals | 227.57 | 0.0187 | 99.6908 | 0.3280 |
| 69 | Depomed | 218.50 | 0.0180 | 99.7087 | 0.3092 |
| 70 | ArQule | 211.10 | 0.0174 | 99.7261 | 0.2913 |
| 71 | SciClone Pharmaceuticals | 192.37 | 0.0158 | 99.7419 | 0.2739 |
| 72 | Vical | 191.09 | 0.0157 | 99.7577 | 0.2581 |
| 73 | Peregrine Pharmaceuticals | 191.09 | 0.0157 | 99.7734 | 0.2423 |
| 74 | ARCA Biopharma | 177.98 | 0.0146 | 99.7880 | 0.2266 |
| 75 | Oncothyreon | 163.07 | 0.0134 | 99.8015 | 0.2120 |
| 76 | Trinity Biotech | 161.77 | 0.0133 | 99.8148 | 0.1985 |
| 77 | Discovery Laboratories | 160.32 | 0.0132 | 99.8280 | 0.1852 |
| 78 | Repligen | 156.18 | 0.0129 | 99.8408 | 0.1720 |
| 79 | Anika Therapeutics | 131.30 | 0.0108 | 99.8516 | 0.1592 |
| 80 | SIGA Technologies | 130.37 | 0.0107 | 99.8623 | 0.1484 |
| 81 | DUSA Pharmaceuticals | 124.59 | 0.0103 | 99.8726 | 0.1377 |
| 82 | CASI Pharmaceuticals | 121.12 | 0.0100 | 99.8826 | 0.1274 |
| 83 | Theragenics Corporation | 117.74 | 0.0097 | 99.8922 | 0.1174 |
| 84 | Repros Therapeutics | 101.24 | 0.0083 | 99.9006 | 0.1078 |
| 85 | La Jolla Pharmaceutical Company | 100.30 | 0.0083 | 99.9088 | 0.0994 |
| 86 | OncoGenex Pharmaceuticals | 99.50 | 0.0082 | 99.9170 | 0.0912 |
| 87 | Hemispherx Biopharma | 99.27 | 0.0082 | 99.9252 | 0.0830 |
| 88 | rEVO Biologics | 96.88 | 0.0080 | 99.9332 | 0.0748 |
| 89 | Poniard Pharmaceuticals | 90.93 | 0.0075 | 99.9406 | 0.0668 |
| 90 | CytRx | 84.84 | 0.0070 | 99.9476 | 0.0594 |
| 91 | Vericel Corporation | 84.66 | 0.0070 | 99.9546 | 0.0524 |
| 92 | Harbor Diversified | 74.89 | 0.0062 | 99.9607 | 0.0454 |
| 93 | AmpliPhi Biosciences | 74.32 | 0.0061 | 99.9669 | 0.0393 |
| 94 | Palatin Technologies | 66.26 | 0.0055 | 99.9723 | 0.0331 |
| 95 | Mateon Therapeutics | 64.49 | 0.0053 | 99.9776 | 0.0277 |
| 96 | Heska Corporation | 56.34 | 0.0046 | 99.9823 | 0.0224 |
| 97 | United-Guardian | 54.06 | 0.0044 | 99.9867 | 0.0177 |
| 98 | ProPhase Labs | 52.64 | 0.0043 | 99.9910 | 0.0133 |
| 99 | Teligent | 39.72 | 0.0033 | 99.9943 | 0.0090 |
| 100 | Natural Alternatives International | 39.02 | 0.0032 | 99.9975 | 0.0057 |
| 101 | Cyanotech Corporation | 19.08 | 0.0016 | 99.9991 | 0.0025 |
| 102 | ImmuCell Corporation | 11.16 | 0.0009 | 100.0000 | 0.0009 |
Note: List of pharmaceutical companies sorted by market capitalization (MCap). MCap is the sample average during the analyzed period in millions of USD. Market capitalization shares for each company are reported in column Cap weight. Cum sum 1 accumulates capitalization. The last column shows capitalization shares in reverse order, i.e., the overall capitalization attributed to the remaining firms, once the largest firms are accounted for.
Appendix B. Supplementary data
References
- Assid M., Gharbi A., Dhouib K. Joint production and subcontracting planning of unreliable multi-facility multi-product production systems. Omega. 2015;50:54–69. [Google Scholar]
- Baker M., Wurgler J. Investor sentiment and the cross-section of stock returns. J. Financ. 2006;61:1645–1680. [Google Scholar]
- Baker M., Wurgler J. Investor sentiment in the stock market. J. Econ. Perspect. 2007;21:129–151. [Google Scholar]
- Blendon R., Benson J.M., Des-Roches C.M., Raleigh E., Taylor-Clark K. The public's response to Severe Acute Respiratory Syndrome in Toronto and the United States. Clin. Infect. Dis. 2004;38:925–931. doi: 10.1086/382355. [DOI] [PubMed] [Google Scholar]
- Bloom N. The impact of uncertainty shocks. Econometrica. 2009;77:623–685. [Google Scholar]
- Boldrin M., Levine D.K. The case against patents. J. Econ. Perspect. 2013;27:3–22. [Google Scholar]
- Brown G.W., Cliff M.T. Investor sentiment and asset valuation. J. Bus. 2005;78:405–440. [Google Scholar]
- Cao M., Wei J. Stock market returns: a note on temperature anomaly. J. Bank. Financ. 2005;29:1559–1573. [Google Scholar]
- Cen L., Liyan-Yang H. Investor sentiment, disagreement, and the breadth return relationship. Manag. Sci. 2013;59:1076–1091. [Google Scholar]
- Curatola G., Donadelli M., Kizys R., Riedel M. Investor sentiment and sectoral stock returns: evidence from world cup games. Financ. Res. Lett. 2016;17:267–274. [Google Scholar]
- De Long J.B., Shleifer A., Summers L.H., Waldmann R.J. Noise trader risk in financial markets. J. Polit. Econ. 1990;98:703–738. [Google Scholar]
- De Miguel V., Garlappi L., Uppal R. Optimal versus naive diversification: how inefficient is the 1/N portfolio strategy? Rev. Financ. Stud. 2009;22:1915–1953. [Google Scholar]
- Edmans A., García D., Norli Ø. Sports sentiment and stock returns. J. Financ. 2007;62:1967–1998. [Google Scholar]
- Hanna D., Yiping H. The impact of SARS on Asian Economies. Asian Econ. Pap. 2004;3:102–112. [Google Scholar]
- Himmelmann A., Schiereck D. Drug approval decisions: a note on stock liquidity effects. J. Empir. Financ. 2012;19:640–652. [Google Scholar]
- Hinman A.R., Orenstein W.A., Santoli J.M., Rodewald L.E., Cochi S.L. Vaccine shortages: history, impact, and prospects for the future. Ann. Rev. Publ. Health. 2006;27:235–259. doi: 10.1146/annurev.publhealth.27.021405.102248. [DOI] [PubMed] [Google Scholar]
- Hirshleifer D., Shumway T. Good day sunshine: stock returns and the weather. J. Financ. 2003;58:1009–1032. [Google Scholar]
- Horváth B., Huizinga H. Does the European Financial Stability Facility bail out sovereigns or banks? An event study. J. Money Credit Bank. 2015;47:177–206. [Google Scholar]
- Hou K., Karolyi G.A., Kho B.-C. What factors drive global stock returns? Rev. Financ. Stud. 2011;24:2527–2574. [Google Scholar]
- Huberman G., Regev T. Contagious speculation and a cure for cancer: a non-event that made stock prices soar. J. Financ. 2001;56:387–396. [Google Scholar]
- Kamstra M.J., Kramer L.A., Levi M.D. Winter blues: a SAD stock market cycle. Am. Econ. Rev. 2003;93:324–343. [Google Scholar]
- Kaplanski G., Levy H. Exploitable predictable irrationality: the FIFA World Cup effect on the U.S. stock market. J. Financ. Quant. Anal. 2010;45:535–553. [Google Scholar]
- Kaplanski G., Levy H. Sentiment and stock prices: the case of aviation distastes. J. Financ. Econ. 2010;95:174–201. [Google Scholar]
- Kaplanski G., Levy H. The holiday and Yom Kippur War sentiment effects: the Tel Aviv Stock Exchange (TASE) Quant. Financ. 2012;12:1283–1298. [Google Scholar]
- Kaplanski G., Levy H. Do happy people make optimistic investors? J. Financ. Quant. Anal. 2015;50:145–168. [Google Scholar]
- Keogh-Brown M.R., Smith R.D. The economic impact of SARS: how does the reality match the predictions? Health Policy. 2008;88:110–120. doi: 10.1016/j.healthpol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanoff P., Lamont O., Wizman T. Investor reaction to salient news in closed-end country funds. J. Financ. 1998;53:673–699. [Google Scholar]
- Lazonick W., Tulum O. US biopharmaceutical finance and the sustainability of the biotech business model. Res. Policy. 2011;40:1170–1187. [Google Scholar]
- Loughran T., Schultz P. Weather, stock returns, and the impact of localized trading behavior. J. Financ. Quant. Anal. 2004;39:343–364. [Google Scholar]
- Mairal G. The history and the narrative of risk in the media. Health Risk Soc. 2011;13:65–79. [Google Scholar]
- Masignani M., Lattanzi M., Rappuoli R. The value of vaccines. Vaccine. 2003;21(Suppl. 2):S110–S113. doi: 10.1016/s0264-410x(03)00209-3. [DOI] [PubMed] [Google Scholar]
- Mehra R., Sah R. Mood fluctuations, projection bias, and volatility of equity prices. J. Econ. Dyn. Control. 2002;26:869–887. [Google Scholar]
- Nofsinger J.R. Social mood and financial economics. J. Behav. Financ. 2005;6:144–160. [Google Scholar]
- Offit P.A. Why are pharmaceutical companies gradually abandoning vaccines? Health Affairs. 2005;24:622–630. doi: 10.1377/hlthaff.24.3.622. [DOI] [PubMed] [Google Scholar]
- Palomino F., Renneboog L., Zhang C. Information salience, investor sentiment, and stock returns: the case of British soccer betting. J. Corp. Financ. 2009;15:368–387. [Google Scholar]
- Qiu, L., Welch, I., 2004. Investor Sentiment Measures. NBER Working Paper No. 10794.
- Quah S., Hin-Peng L. Crisis prevention and management during SARS outbreak, Singapore. Emerg. Infect. Dis. 2004;10:364–368. doi: 10.3201/eid1002.030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders E. Stock prices and Wall Street weather. J. Financ. 1993;83:1337–1345. [Google Scholar]
- Shortridge R.T. Market valuation of successful versus non-successful R&D efforts in the pharmaceutical industry. J. Bus. Financ. Account. 2004;31:1301–1325. [Google Scholar]
- Smith R.D., Keogh-Brown M.R., Barnett T., Tait J. The economy-wide impact of pandemic influenza on the UK: a computable general equilibrium modelling experiment. Br. Med. J. 2009:1–7. doi: 10.1136/bmj.b4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlock P.C. Giving content to investor sentiment: the role of media in the stock market. J. Financ. 2007;62:1139–1168. [Google Scholar]
- Theodossiou A., Theodossiou P. Stock return outliers and beta estimation: the case of U.S. pharmaceutical companies. J. Int. Financ. Mark. Inst. Money. 2014;30:153–171. [Google Scholar]
- Vasterman P., Yzermans J.C., Dirkzwager A.J. The role of the media and media hypes in the aftermath of disasters. Epidemiol. Rev. 2005;27:107–114. doi: 10.1093/epirev/mxi002. [DOI] [PubMed] [Google Scholar]
- Whaley R. Understanding the VIX. J. Portf. Manag. 2009;35:98–105. [Google Scholar]
- Young M.E., King N., Harper S., Humphreys K.R. The influence of popular media on perceptions of personal and population risk in possible disease outbreaks. Health Risk Soc. 2013;15:103–114. [Google Scholar]
- Yuan K., Zheng L., Zhu Q. Are investors moonstruck? Lunar phases and stock returns. J. Empir. Financ. 2006;13:1–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.