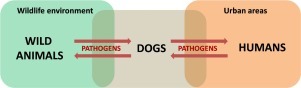
Graphical abstract
Keywords: Dogs, Spillover, Infectious diseases, Zoonoses, Public health
Highlights
-
•
The presence of dogs in protected/vulnerable areas affects wildlife in different ways.
-
•
Domestic dogs transit between wild environments and urban areas.
-
•
Dogs can transmit various pathogens to wild animals.
-
•
Wild animals can transmit different pathogens to domestic dogs.
-
•
Dogs can act as spillover bridges, transferring pathogens from wild animals to humans.
Abstract
The presence of domestic/free-ranging dogs in Brazilian protected areas and native vegetation fragments is an important problem, mainly because these animals pose a threat to wild species that live in such areas. In addition, dogs constantly circulate between wildlife environments and urban regions, acting as “bridges” in spillover events. Dogs are traditionally recognized as vectors of zoonoses, which are correct, but their roles as facilitating agents for the “jump” of pathogens from wild animals to humans (and vice versa) are sparsely debated. In this context, this work briefly describes the different roles of dogs in the dynamics and ecology of infectious diseases, using the Brazilian scenario as a study model.
The presence of domestic/free-ranging dogs in Brazilian protected areas is an important problem that affects wildlife, including various endangered species (Lessa et al., 2016). Also, dogs can be considered the most abundant carnivore in some areas of the Brazilian Atlantic Forest (Srbek-Araujo and Chiarello, 2008, Paschoal et al., 2016, Ribeiro et al., 2019). Dogs threaten wild animals due to predation, competition for territory, and disturbance (chasing or harassment) (Young et al., 2011, Silva-Rodríguez and Sieving, 2012, Lessa et al., 2016, Doherty et al., 2017). In addition, the risk of pathogen transmission from dogs to other animals, mainly mammals, is also an important problem. Dogs can act as pathogen reservoirs and can transmit Leishmania spp. (leishmaniasis), Leptospira interrogans (leptospirosis), Toxoplasma gondii (toxoplasmosis), Neospora caninum (neosporosis), Dirofilaria immitis (dirofilariasis/heartworm disease), Brucella canis (brucellosis), Sarcoptes scabiei (scabies), Echinococcus spp. (echinococcosis), Rickettsia rickettsii (Brazilian spotted fever), different canine viruses (e.g., distemper virus, adenovirus, coronavirus, herpesvirus, parvovirus), rabies virus, among other pathogens, to both humans and wildlife (Craig et al., 1992, Fiorello et al., 2006, Fiorello et al., 2017, Dantas-Torres, 2007, Labruna et al., 2007, Pinter et al., 2008, Piranda et al., 2008, Yabsley et al., 2008, Moraes-Filho et al., 2009, Brunetti et al., 2011, Ogrzewalska et al., 2012, Furtado et al., 2013, Millán et al., 2013, Parrish et al., 2015, Basano et al., 2016, Campos et al., 2016, Curi et al., 2016, Doherty et al., 2017, Lessa et al., 2016, Faccini-Martínez et al., 2017). Importantly, dogs are amplifier hosts of different pathogens (Dantas-Torres, 2007, Piranda et al., 2011, Szabó et al., 2013), which means that they can develop infection at sufficient levels to infect other species, such as vectors, increasing the disease transmission (Kilpatrick and Altizer, 2010, Labruna et al., 2011, Piranda et al., 2011).
Recently, Kossel et al. (2018) evaluated the occurrence of domestic dogs in the Tijuca National Park (city of Rio de Janeiro, southeastern Brazil). This study demonstrated that dogs were widely present throughout the entire park. Also, the absence of dog pups in the park area indicated that dogs were probably derived from regions around the park, suggesting a steady flow of these animals between the park and urban areas. These results, together with other fundamental studies (Galetti and Sazima, 2006, Srbek-Araujo and Chiarello, 2008, Lacerda et al., 2009, Torres and Prado, 2010, Curi et al., 2016, Lessa et al., 2016, Paschoal et al., 2016, Fernandez et al., 2017, Rosa and Souza, 2017, Vieira et al., 2017, Paschoal et al., 2018), clearly confirm that Brazilian forest areas (mainly those close to urban centers) are strongly influenced by the activity of domestic/free-ranging dogs.
In a study addressing the factors involved in the invasion of dogs in forest remnants, Ribeiro et al. (2019) evaluated 12 landscapes (2830 ha each) of the Brazilian Atlantic Forest. The number of detected dogs ranged from 5 to 27 per landscape, with an estimated number of up to 46 dogs per landscape. Overall, the authors found a high abundance of dogs in the studied forest. According to the same study, high human population density, density of dogs, and landscape disturbances are important factors involved in the ecological problems caused by dogs in Brazil, which invade even forest remnants far from their homes (Ribeiro et al., 2019).
Dogs circulate in forest fragments of protected and non-protected areas, being registered in the most different environments (Doherty et al., 2017), such as agroforests (Frigeri et al., 2014, Santos et al., 2017), caatinga (Bezerra et al., 2014, Lessa et al., 2016), and the Amazon region (Basano et al., 2016). In Brazil, 53% of native vegetation is located on private properties (Soares-Filho et al., 2014). Therefore, it is important to consider that the ecological problems caused by dogs are more critical in protected regions, but they also occur in wildlife environments outside these areas. Furthermore, such problems are probably more frequent in non-protected areas due to the extension of these territories and greater interaction with human agglomerates.
The consequences of dogs-associated disturbances are more complex and worrisome than just the direct impact of dogs on wild species living in the invaded areas. From an ecological perspective, the circulation of dogs between wildlife environments (considering protected and non-protected areas) and urban regions make the dogs facilitators of the movement of zoonotic diseases between wild animals and humans.
The interaction of domestic dogs with wildlife is a driver for disease emergence/re-emergence in dogs, humans, and in native animals (Lessa et al., 2016, Fiorello et al., 2017). The transmission of a particular pathogen from domestic dogs to wild animals will depend on various factors, including the susceptibility of the wild species, the pathogen circulation among dogs, and the contact of dogs with the wildlife (Fiorello et al., 2006). Predation of dog pups or debilitated/sick dogs by wild animals also facilitates the transmission of pathogens from dogs to wild animals (Millán et al., 2013).
Importantly, the movement of pathogens can also occur in the opposite direction, from wild animals to dogs (Otranto et al., 2015a, Otranto et al., 2015b). As a consequence, dogs can potentially act as drivers for spillover events, the “jump of pathogens” from wild animals to other animal species, including humans. This event is also called “host jump”, and describes the process by which a particular pathogen infects a new previously unaffected (and usually taxonomically distant) host species (Stukenbrock and McDonald, 2008). Humans and our most classical companion animals (cats and dogs) share more than 60 parasitic zoonoses (Macpherson, 2005), which shows that many microorganisms capable to infect dogs can also infect humans. Indeed, different studies have shown that dogs are pathogen reservoirs or amplifiers hosts with an important role in the transmission risk of some zoonotic diseases to humans, evidencing that dogs can act as “bridges” for the transmission of pathogens between wild animals and human (Craig et al., 1992, Dantas-Torres, 2007, Pinter et al., 2008, Salb et al., 2008, Brunetti et al., 2011, Piranda et al., 2011, Ramírez et al., 2013, Szabó et al., 2013, Campos et al., 2016, Fiorello et al., 2017).
As mentioned above, some pathogens detected in dogs may have the ability to infect humans as well, even considering they were originated from wild animals. However, spillover is a very complex event, with pathogens having to overcome a number of ecological, physical and molecular barriers, including reservoir distribution and density, pathogen prevalence, infection intensity, pathogen release from reservoir host, pathogen survival and spread, human exposure, structural barriers, host immune response, among others (Plowright et al., 2017). Genetic factors from host species and pathogens also influence the spillover events (Ellwanger et al., 2018). In the case of viruses, to consider dogs as drivers/bridges for the movement of pathogens between wild animals and humans, the pathogen in question needs to infect the hosts using generalist receptors, present in the wild species source of the pathogen, in dogs, and in humans. The cellular machinery used for viral replication also needs to be present in all three hosts (Woolhouse et al., 2001, Woolhouse and Gowtage-Sequeria, 2005, Plowright et al., 2017, Barclay, 2019). Although these demands require complex and seemingly unlikely events, rabies virus and bat influenza viruses can infect different animal species, being good examples of generalist pathogens (Woolhouse et al., 2001, Barclay, 2019, Karakus et al., 2019).
In brief, host plasticity (pathogens with a diverse host range: generalist pathogens) is a fundamental aspect for the occurrence of a zoonotic spillover (Cleaveland et al., 2001, Woolhouse et al., 2001, Woolhouse and Gowtage-Sequeria, 2005, Elena et al., 2009, Johnson et al., 2015). Although most spillover events do not result in outbreaks, they can cause important isolated cases of human infections. Moreover, when the spillover happens, it creates a chance for the emergence of a new disease in humans and should be considered a concern of the public health agents and translated into prevention actions.
Dogs can also transfer pathogen vectors from wild animals to humans, such as ticks and fleas, carriers of various bacterial and viral pathogens that can infect both wild animals and humans (Parola and Raoult, 2001, Labruna et al., 2007, Bitam et al., 2010, Chomel, 2011, Szabó et al., 2013, Campos et al., 2016, Mansfield et al., 2017, Vogel et al., 2018). We speculate that the role of dogs as “vectors of vectors” can be a problem even harder to handle than the direct participation of dogs in spillover events.
It is also important to remember that humans can transmit pathogens to other animal species, a phenomenon known as reverse-zoonosis or zooanthroponosis (Messenger et al., 2014, Nelson and Vincent, 2015). Although this process is poorly studied, it can have a major impact on wildlife and the role of dogs as mediators of reverse-zoonosis should be evaluated in greater detail (Messenger et al., 2014).
The various roles of dogs in the transmission of pathogens between different species were summarized in Fig. 1 . Looking at this scenario from a different perspective, the participation of dogs in the transmission of infectious diseases creates a valuable opportunity when it is not possible, in practice, to control the circulation of dogs in protected or vulnerable areas. In such cases, dogs can be used as sentinels for infectious disease surveillance and even the discovery of new pathogens with potential of infecting humans (Labruna et al., 2007, Pinter et al., 2008, Campos et al., 2016, Bowser and Anderson, 2018, Ellwanger et al., 2019). Currently, there are a number of genetic-based methods which could be employed to achieve these goals (Ellwanger et al., 2017, Gardy and Loman, 2018). We acknowledge that the use of sentinel dogs to quantify the risk of zoonotic diseases in humans can be difficult, although the monitoring of dog populations already in contact with wildlife environments (such as the previously mentioned animals found in the Tijuca National Park) could, alternatively, be used to assess trends and patterns involved in microbial epidemiology of zoonotic pathogens, and ultimately to detect emerging infectious diseases (Bowser and Anderson, 2018). However, we emphasize that the use of dogs as sentinels must be considered only when it is not possible to remove these animals from protected areas. When this can be done, the use of native animal species as sentinels to study the dynamics of infectious diseases (or for surveillance purposes) is the best option.
Fig. 1.
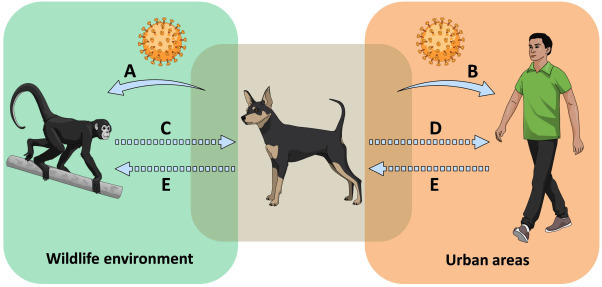
Potential roles of dogs in the transmission of pathogens. Dogs transit between wildlife environments and urban areas, interacting with wild animals and humans. Dogs can transmit pathogens to wild animals (A) and to humans (B, zoonotic diseases). Transmission of pathogens from wild animals to dogs (C) and the subsequent transmission of the pathogen to humans (D) is less likely because the pathogen would need to cross a number of barriers. However, considering the existence of multi-host pathogens, the role of dogs as “spillover bridge” is possible and should not be overlooked. Moreover, dogs can transport pathogen vectors (e.g., ticks) from wild animals to humans, facilitating human infection with pathogens derived from wild animals. Finally, the role of dogs as mediators of reverse-zoonosis (E, pathogen transmission from humans to wild animals) is little studied and should be evaluated in greater detail. See text for references.
This figure was created using Mind the Graph illustrations (available at www.mindthegraph.com).
An eventual low potential for transmission of a particular pathogen can be compensated by the high number of infected-dogs generally present in urban areas (Mackenstedt et al., 2015). Based on data collected in São Paulo State, we can assume that the dog/human ratio in Brazil is very high (Alves et al., 2005). Moreover, considering all the ecological and health problems associated with the large number of dogs in Brazil, some actions need to be put into practice more intensively. Firstly, understanding the extent of dogs’ impacts on wildlife will help to structure public policies and action plans to mitigate such impacts (Young et al., 2011). Next, effective policies of dog population control need to be implemented in Brazil. Dog control plans must involve residents and users of areas surrounding and in the vicinity of parks, protected or vulnerable areas, park managers, professionals responsible for public health promotion and control of zoonoses, politicians, and representatives of animal protection organizations. These plans have to consider prevention, control, and eradication strategies (Galetti and Sazima, 2006, Lessa et al., 2016). More specifically, the following actions are required: evaluate the impacts of dogs–wildlife interactions; promote campaigns explaining to the public and dogs’ owners the negative impacts of dogs on wildlife, indicating how to avoid these impacts; exclude domestic dogs from critical wildlife habitats and protected/vulnerable areas; monitor and control the circulation of dogs in the vicinity of critical and protected areas (buffer zones and transition areas); vaccination of domestic animals; sterilization of dogs; when hunting is necessary for subsistence, hunters need to be advised not to include ill dogs in hunting activities; keep dogs healthy and in hygienic conditions; remove garbage from vulnerable areas; promote actions focused on responsible dog-ownership (Macpherson, 2005, Fiorello et al., 2006, Lacerda et al., 2009, Young et al., 2011, Furtado et al., 2013, Soto and Palomares, 2015, Ellwanger and Chies, 2018). In other words, the One Health perspective must be applied, in which animal, human, and environmental factors are considered together aiming the control of infectious diseases (Cunningham et al., 2017, Destoumieux-Garzón et al., 2018).
In conclusion, it is clear that the circulation of dogs between wildlife environments and urban areas facilitate the transmission of infectious diseases, besides damaging native species. However, the set of problems caused by the poor management of the dog populations in Brazil is still a neglected issue. The contact between humans and dogs is historical (Macpherson, 2005), but needs to be carefully controlled in order to balance the issues and the benefits arising from this relationship.
Funding
JHE received a doctoral scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). JABC receives a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
Conflicts of interest
No conflicts of interest to declare.
References
- Alves M.C.G.P., de Matos M.R., Reichmann M.L., Dominguez M.H. Dimensionamento da população de cães e gatos do interior do Estado de São Paulo. Rev. Saúde Pública. 2005;39:891–897. doi: 10.1590/S0034-89102005000600004. [DOI] [PubMed] [Google Scholar]
- Barclay W.S. Receptor for bat influenza virus uncovers potential risk to humans. Nature. 2019;567:35–36. doi: 10.1038/d41586-019-00580-5. [DOI] [PubMed] [Google Scholar]
- Basano S.A., Tarso P., Soares H.S., Costa A.P., Marcili A., Labruna M.B., Dias R.A., Camargo L.M.A., Gennari S.M. Toxoplasma gondii, Neospora caninum and Leishmania amazonensis antibodies in domestic dogs in the western Brazilian Amazon region. Braz. J. Vet. Res. Anim. Sci. 2016;53:1–9. doi: 10.11606/issn.1678-4456.bjvras.2016.103903. [DOI] [Google Scholar]
- Bezerra C.M., Cavalcanti L.P.G., Souza R.C.M., Barbosa S.E., Xavier S.C.C., Jansen A.M., Ramalho R.D., Diotaiut L. Domestic, peridomestic and wild hosts in the transmission of Trypanosoma cruzi in the Caatinga area colonised by Triatoma brasiliensis. Mem. Inst. Oswaldo Cruz. 2014;109:887–898. doi: 10.1590/0074-0276140048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitam I., Dittmar K., Parola P., Whiting M.F., Raoult D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010;14:e667–e676. doi: 10.1016/j.ijid.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Bowser N.H., Anderson N.E. Dogs (Canis familiaris) as sentinels for human infectious disease and application to canadian populations: a systematic review. Vet. Sci. 2018;5:83. doi: 10.3390/vetsci5040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti E., Garcia H.H., Junghanss T., International C.E., Workshop in Lima, Peru, 2009 Cystic echinococcosis: chronic, complex, and still neglected. PLoS Negl. Trop. Dis. 2011;5:e1146. doi: 10.1371/journal.pntd.0001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos S.D.E., da Cunha N.C., Almosny N.R.P. Brazilian spotted fever with an approach in veterinary medicine and one health perspective. Vet. Med. Int. 2016 doi: 10.1155/2016/2430945. 2430945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel B. Tick-borne infections in dogs-an emerging infectious threat. Vet. Parasitol. 2011;179:294–301. doi: 10.1016/j.vetpar.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Cleaveland S., Laurenson M.K., Taylor L.H. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P.S., Deshan L., MacPherson C.N., Dazhong S., Reynolds D., Barnish G., Gottstein B., Zhirong W. A large focus of alveolar echinococcosis in central China. Lancet. 1992;340:826–831. doi: 10.1016/0140-6736(92)92693-A. [DOI] [PubMed] [Google Scholar]
- Cunningham A.A., Daszak P., Wood J.L.N. One Health, emerging infectious diseases and wildlife: two decades of progress? Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0167. 20160167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curi N.H.A., Massara R.L., de Oliveira Paschoal A.M., Soriano-Araújo A., Lobato Z.I., Demétrio G.R., Chiarello A.G., Passamani M. Prevalence and risk factors for viral exposure in rural dogs around protected areas of the Atlantic forest. BMC Vet. Res. 2016;12:21. doi: 10.1186/s12917-016-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 2007;149:139–146. doi: 10.1016/j.vetpar.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Destoumieux-Garzón D., Mavingui P., Boetsch G., Boissier J., Darriet F., Duboz P., Fritsch C., Giraudoux P., Le Roux F., Morand S., Paillard C., Pontier D., Sueur C., Voituron Y. The One Health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018;5:14. doi: 10.3389/fvets.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T.S., Dickman C.R., Glen A.S., Newsome T.M., Nimmo D.G., Ritchie E.G., Vanak A.T., Wirsing A.J. The global impacts of domestic dogs on threatened vertebrates. Biol. Conserv. 2017;210:56–59. doi: 10.1016/j.biocon.2017.04.007. [DOI] [Google Scholar]
- Elena S.F., Agudelo-Romero P., Lalić J. The evolution of viruses in multi-host fitness landscapes. Open Virol. J. 2009;3:1–6. doi: 10.2174/1874357900903010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger J.H., Chies J.A.B. Zoonotic spillover and emerging viral diseases – time to intensify zoonoses surveillance in Brazil. Braz. J. Infect. Dis. 2018;22:76–78. doi: 10.1016/j.bjid.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger J.H., Kaminski V.L., Chies J.A.B. How to detect new viral outbreaks or epidemics? We need to survey the circulation of viruses in humans and other animals using fast, sensible, cheap, and broad-spectrum methodologies. Braz. J. Infect. Dis. 2017;21:211–212. doi: 10.1016/j.bjid.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger J.H., Kaminski V.L., Chies J.A.B. Emerging infectious disease prevention: where should we invest our resources and efforts? J. Infect. Public Health. 2019;12:313–316. doi: 10.1016/j.jiph.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Zambra F.M.B., Guimarães R.L., Chies J.A.B. MicroRNA-related polymorphisms in infectious diseases – tiny changes with a huge impact on viral infections and potential clinical applications. Front. Immunol. 2018;9:1316. doi: 10.3389/fimmu.2018.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccini-Martínez Á.A., Ramírez-Hernández A., Barreto C., Forero-Becerra E., Millán D., Valbuena E., Sánchez-Alfonso A.C., Imbacuán-Pantoja W.O., Cortés-Vecino J.A., Polo-Terán L.J., Yaya-Lancheros N., Jácome J., Palomar A.M., Santibáñez S., Portillo A., Oteo J.A., Hidalgo M. Epidemiology of spotted fever group rickettsioses and acute undifferentiated febrile illness in Villeta, Colombia. Am. J. Trop. Med. Hyg. 2017;97:782–788. doi: 10.4269/ajtmh.16-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F.A.S., Rheingantz L.M., Genes L., Kenup C.F., Galliez M., Cezimbra T., Cid B., Macedo L., Araujo B.B.A., Moraes B.S., Monjeau A., Pires A.S. Rewilding the Atlantic forest: restoring the fauna and ecological interactions of a protected area. Perspect. Ecol. Conserv. 2017;15:308–314. doi: 10.1016/j.pecon.2017.09.004. [DOI] [Google Scholar]
- Fiorello C.V., Noss A.J., Deem S.L. Demography, hunting ecology, and pathogen exposure of domestic dogs in the Isoso of Bolivia. Conserv. Biol. 2006;20:762–771. doi: 10.1111/j.1523-1739.2006.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorello C.V., Straub M.H., Schwartz L.M., Liu J., Campbell A., Kownacki A.K., Foley J.E. Multiple-host pathogens in domestic hunting dogs in Nicaragua's Bosawás Biosphere Reserve. Acta Trop. 2017;167:183–190. doi: 10.1016/j.actatropica.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Frigeri E., Cassano C.R., Pardini R. Domestic dog invasion in an agroforestry mosaic in southern Bahia, Brazil. Trop. Conserv. Sci. 2014;7:508–528. doi: 10.1177/194008291400700310. [DOI] [Google Scholar]
- Furtado M.M., de Ramos Filho J.D., Scheffer K.C., Coelho C.J., Cruz P.S., Ikuta C.Y., Jácomo A.T.A., Porfírio G.E.O., Silveira L., Sollmann R., Tôrres N.M., Ferreira Neto J.S. Serosurvey for selected viral infections in free-ranging jaguars (Panthera onca) and domestic carnivores in Brazilian Cerrado, Pantanal, and Amazon. J. Wildl. Dis. 2013;49:510–521. doi: 10.7589/2012-02-056. [DOI] [PubMed] [Google Scholar]
- Galetti M., Sazima I. Impact of feral dogs in an urban Atlantic forest fragment in southeastern Brazil. Nat. Conserv. 2006;4:146–151. [Google Scholar]
- Gardy J.L., Loman N.J. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat. Rev. Genet. 2018;19:9–20. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.K., Hitchens P.L., Evans T.S., Goldstein T., Thomas K., Clements A., Joly D.O., Wolfe N.D., Daszak P., Karesh W.B., Mazet J.K. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci. Rep. 2015;5:14830. doi: 10.1038/srep14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakus U., Thamamongood T., Ciminski K., Ran W., Günther S.C., Pohl M.O., Eletto D., Jeney C., Hoffmann D., Reiche S., Schinköthe J., Ulrich R., Wiener J., Hayes M.G.B., Chang M.W., Hunziker A., Yángüez E., Aydillo T., Krammer F., Oderbolz J., Meier M., Oxenius A., Halenius A., Zimmer G., Benner C., Hale B.G., García-Sastre A., Beer M., Schwemmle M., Stertz S. MHC class II proteins mediate cross-species entry of bat influenza viruses. Nature. 2019;567:109–112. doi: 10.1038/s41586-019-0955-3. [DOI] [PubMed] [Google Scholar]
- Kilpatrick A.M., Altizer S. Disease ecology. Nature Education Knowledge. 2010;3:55. Available at: https://www.nature.com/scitable/knowledge/library/disease-ecology-15947677 (accessed 28.05.2019) [Google Scholar]
- Kossel K.V., Kenup C.F., Kreischer C., Fernandez F.A.S., Pires A.S. Who let the dogs out? Occurrence, population size and daily activity of domestic dogs in an urban Atlantic Forest reserve. Perspect. Ecol. Conserv. 2018;16:228–233. doi: 10.1016/j.pecon.2018.09.001. [DOI] [Google Scholar]
- Labruna M.B., Horta M.C., Aguiar D.M., Cavalcante G.T., Pinter A., Gennari S.M., Camargo L.M.A. Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro municipality, western Amazon, Brazil. Vector Borne Zoonotic Dis. 2007;7:249–255. doi: 10.1089/vbz.2006.0621. [DOI] [PubMed] [Google Scholar]
- Labruna M.B., Ogrzewalska M., Soares J.F., Martins T.F., Soares H.S., Moraes-Filho J., Nieri-Bastos F.A., Almeida A.P., Pinter A. Experimental infection of Amblyomma aureolatum ticks with Rickettsia rickettsii. Emerg. Infect. Dis. 2011;17:829–834. doi: 10.3201/eid1705.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda A.C.R., Tomas W.M., Marinho-Filho J. Domestic dogs as an edge effect in the Brasília National Park, Brazil: interactions with native mammals. Anim. Conserv. 2009;12:477–487. doi: 10.1111/j.1469-1795.2009.00277.x. [DOI] [Google Scholar]
- Lessa I., Guimarães T.C.S., Bergallo H.G., Cunha A., Vieira E.M. Domestic dogs in protected areas: a threat to Brazilian mammals? Nat. Conserv. 2016;14:46–56. doi: 10.1016/j.ncon.2016.05.001. [DOI] [Google Scholar]
- Mackenstedt U., Jenkins D., Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson C.N.L. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005;35:1319–1331. doi: 10.1016/j.ijpara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Mansfield K.L., Jizhou L., Phipps L.P., Johnson N. Emerging tick-borne viruses in the twenty-first century. Front. Cell. Infect. Microbiol. 2017;7:298. doi: 10.3389/fcimb.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger A.M., Barnes A.N., Gray G.C. Reverse zoonotic disease transmission (zooanthroponosis): a systematic review of seldom-documented human biological threats to animals. PLoS One. 2014;9:e89055. doi: 10.1371/journal.pone.0089055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J., Chirife A.D., Kalema-Zikusoka G., Cabezón O., Muro J., Marco I., Cliquet F., León-Vizcaíno L., Wasniewski M., Almería S., Mugisha L. Serosurvey of dogs for human, livestock, and wildlife pathogens, Uganda. Emerg. Infect. Dis. 2013;19:680–682. doi: 10.3201/eid1904.121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes-Filho J., Pinter A., Pacheco R.C., Gutmann T.B., Barbosa S.O., Gonzáles M.A.R.M., Muraro M.A., Cecílio S.R.M., Labruna M.B. New epidemiological data on Brazilian spotted fever in an endemic area of the state of São Paulo, Brazil. Vector Borne Zoonotic Dis. 2009;9:73–78. doi: 10.1089/vbz.2007.0227. [DOI] [PubMed] [Google Scholar]
- Nelson M.I., Vincent A.L. Reverse zoonosis of influenza to swine: new perspectives on the human–animal interface. Trends Microbiol. 2015;23:142–153. doi: 10.1016/j.tim.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrzewalska M., Saraiva D.G., Moraes-Filho J., Martins T.F., Costa F.B., Pinter A., Labruna M.B. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of São Paulo, Brazil. Parasitology. 2012;139:1283–1300. doi: 10.1017/S0031182012000546. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Pfeffer M., Dantas-Torres F., Brianti E., Deplazes P., Genchi C., Guberti V., Capelli G. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: Protozoa and tick-borne agents. Vet. Parasitol. 2015;213:12–23. doi: 10.1016/j.vetpar.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Dantas-Torres F., Brianti E., Pfeffer M., Genchi C., Guberti V., Capelli G., Deplazes P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet. Parasitol. 2015;213:24–37. doi: 10.1016/j.vetpar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- Parrish C.R., Murcia P.R., Holmes E.C. Influenza virus reservoirs and intermediate hosts: dogs, horses, and new possibilities for influenza virus exposure of humans. J. Virol. 2015;89:2990–2994. doi: 10.1128/JVI.03146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschoal A.M.O., Massara R.L., Bailey L.L., Doherty P.F., Jr., Santos P.M., Paglia A.P., Hirsch A., Chiarello A.G. Anthropogenic disturbances drive domestic dog use of atlantic forest protected areas. Trop. Conserv. Sci. 2018;11:1–14. doi: 10.1177/1940082918789833. [DOI] [Google Scholar]
- Paschoal A.M.O., Massara R.L., Bailey L.L., Kendall W.L., Doherty P.F., Jr., Hirsch A., Chiarello A.G., Paglia A.P. Use of Atlantic Forest protected areas by free-ranging dogs: estimating abundance and persistence of use. Ecosphere. 2016;7:e01480. doi: 10.1002/ecs2.1480. [DOI] [Google Scholar]
- Pinter A., Horta M.C., Pacheco R.C., Moraes-Filho J., Labruna M.B. Serosurvey of Rickettsia spp. in dogs and humans from an endemic area for Brazilian spotted fever in the State of São Paulo, Brazil. Cad. Saude Publica. 2008;24:247–252. doi: 10.1590/S0102-311X2008000200003. [DOI] [PubMed] [Google Scholar]
- Piranda E.M., Faccini J.L., Pinter A., Pacheco R.C., Cançado P.H., Labruna M.B. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis. 2011;11:29–36. doi: 10.1089/vbz.2009.0250. [DOI] [PubMed] [Google Scholar]
- Piranda E.M., Faccini J.L., Pinter A., Saito T.B., Pacheco R.C., Hagiwara M.K., Labruna M.B. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: clinical and laboratory findings. Mem. Inst. Oswaldo Cruz. 2008;103:696–701. doi: 10.1590/S0074-02762008000700012. [DOI] [PubMed] [Google Scholar]
- Plowright R.K., Parrish C.R., McCallum H., Hudson P.J., Ko A.I., Graham A.L., Lloyd-Smith J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J.D., Turriago B., Tapia-Calle G., Guhl F. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet. Parasitol. 2013;196:216–219. doi: 10.1016/j.vetpar.2012.12.054. [DOI] [PubMed] [Google Scholar]
- Ribeiro F.S., Nichols E., Morato R.G., Metzger J.P., Pardini R. Disturbance or propagule pressure? Unravelling the drivers and mapping the intensity of invasion of free-ranging dogs across the Atlantic forest hotspot. Divers. Distrib. 2019;25:191–204. doi: 10.1111/ddi.12845. [DOI] [Google Scholar]
- Rosa C.A., Souza A.C. Large and medium-sized mammals of Nova Baden State Park, Minas Gerais, Brazil. Check List. 2017;13:2141. doi: 10.15560/13.3.2141. [DOI] [Google Scholar]
- Salb A.L., Barkema H.W., Elkin B.T., Thompson R.C., Whiteside D.P., Black S.R., Dubey J.P., Kutz S.J. Dogs as sources and sentinels of parasites in humans and wildlife, northern Canada. Emerg. Infect. Dis. 2008;14:60–63. doi: 10.3201/eid1401.071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C.L.A., Silva A.P., Santos S.B., Pardini R., Cassano C.R. Dog invasion in agroforests: the importance of households, roads and dog population size in the surroundings. Perspect. Ecol. Conserv. 2017;15:221–226. doi: 10.1016/j.pecon.2017.08.001. [DOI] [Google Scholar]
- Silva-Rodríguez E.A., Sieving K.E. Domestic dogs shape the landscape-scale distribution of a threatened forest ungulate. Biol. Conserv. 2012;150:103–110. doi: 10.1016/j.biocon.2012.03.008. [DOI] [Google Scholar]
- Soares-Filho B., Rajão R., Macedo M., Carneiro A., Costa W., Coe M., Rodrigues H., Alencar A. Cracking Brazil's forest code. Science. 2014;344:363–364. doi: 10.1126/science.1246663. [DOI] [PubMed] [Google Scholar]
- Soto C.A., Palomares F. Human-related factors regulate the presence of domestic dogs in protected areas. Oryx. 2015;49:254–260. doi: 10.1017/S0030605313000604. [DOI] [Google Scholar]
- Srbek-Araujo A.C., Chiarello A.G. Domestic dogs in Atlantic forest preserves of south-eastern Brazil: a camera-trapping study on patterns of entrance and site occupancy rates. Braz. J. Biol. 2008;68:771–779. doi: 10.1590/S1519-69842008000400011. [DOI] [PubMed] [Google Scholar]
- Stukenbrock E.H., McDonald B.A. The origins of plant pathogens in agro-ecosystems. Annu. Rev. Phytopathol. 2008;46:75–100. doi: 10.1146/annurev.phyto.010708.154114. [DOI] [PubMed] [Google Scholar]
- Szabó M.P.J., Pinter A., Labruna M.B. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front. Cell. Infect. Microbiol. 2013;3:27. doi: 10.3389/fcimb.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres P.C., Prado P.I. Domestic dogs in a fragmented landscape in the Brazilian Atlantic Forest: abundance, habitat use and caring by owners. Braz. J. Biol. 2010;70:987–994. doi: 10.1590/S1519-69842010000500010. [DOI] [PubMed] [Google Scholar]
- Vieira F.V., Hoffmann D.J., Fabri C.U.F., Bresciani K.D.S., Gameiro R., Flores E.F., Cardoso T.C. Circulation of canine parvovirus among dogs living in human–wildlife interface in the Atlantic forest biome, Brazil. Heliyon. 2017;3:e00491. doi: 10.1016/j.heliyon.2017.e00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Foley J., Fiorello C.V. Rickettsia africae and novel rickettsial strain in Amblyomma spp. ticks, Nicaragua, 2013. Emerg. Infect. Dis. 2018;24:385–387. doi: 10.3201/eid2402.161901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M.E.J., Taylor L.H., Haydon D.T. Population biology of multihost pathogens. Science. 2001;292:1109–1112. doi: 10.1126/science.1059026. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley M.J., McKibben J., Macpherson C.N., Cattan P.F., Cherry N.A., Hegarty B.C., Breitschwerdt E.B., O’Connor T., Chandrashekar R., Paterson T., Perea M.L., Ball G., Friesen S., Goedde J., Henderson B., Sylvester W. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Vet. Parasitol. 2008;151:279–285. doi: 10.1016/j.vetpar.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Young J.K., Olson K.A., Reading R.P., Amgalanbaatar S., Berger J. Is wildlife going to the dogs? Impacts of feral and free-roaming dogs on wildlife populations. Bioscience. 2011;61:125–132. doi: 10.1525/bio.2011.61.2.7. [DOI] [Google Scholar]


