Abstract
Pneumonia is one of the leading causes of morbidity, hospitalization, and mortality in both industrialized and developing countries. In particular, pulmonary infections acquired in the community, and pneumonias arising in the hospital setting, represent a major medical and economic problem and thus a continuous challenge to health care. For the radiologist, it is important to understand that community-acquired pneumonia (CAP) and nosocomial pneumonia (NP) share a number of characteristics, but should, in many respects be regarded as separate entities. CAP and NP arise in different populations, host different spectra of causative pathogens, and pose different challenges to both the clinician and the radiologist. CAP is generally seen in outpatients, is most frequently caused by Streptococcus pneumoniae, Mycoplasma pneumoniae, Haemophilus influenzae, and Chlamydia, and its radiologic diagnosis is relatively straightforward. NP, in contrast, develops in the hospital setting, is commonly caused by gram-negative bacteria, and may generate substantial problems for the radiologist. Overall, both for CAP and NP, imaging is an integral component of the diagnosis, important for classification and differential diagnosis, and helpful for follow-up.
Keywords: Pneumonia, Community-acquired pneumonia, Nosocomial pneumonia, Pulmonary infections, Radiologic diagnosis
Introduction
Pulmonary infections are among the most frequent causes of morbidity and mortality throughout the world. In the non-immunocompromised population, pneumonia is one of the two major infectious diseases. It is the most prevalent community-acquired infection (CAP) and the second most common nosocomial infectious disorder. Nosocomial pneumonia (NP) is associated with the highest mortality rate of nosocomial infections that contribute causally to death [1]. Many infections occur in individuals with concomitant intrapulmonary or extrathoracic disease, but may also affect otherwise healthy persons. Despite advances in diagnosis and treatment, pneumonia remains one of the leading causes of death, and mortality is particularly high in immunocompromised patients, in children, and in the elderly population [2].
Radiography plays an important role in the detection and management of patients with pneumonia. Among all diagnostic tests, chest radiography has a pivotal position in confirming or excluding the diagnosis of pneumonia. Furthermore, it allows narrowing of the differential diagnosis, helps to direct additional diagnostic measures, and serves as an ideal tool for follow-up examinations. Nevertheless, its diagnostic yield can be substantially enriched by the integration of radiologic findings with epidemiologic facts, histopathologic fundamentals, and clinical features of the individual patient.
In this article we review the most important theoretical, clinical, and radiological principles regarding CAP and NP. Specific attention is paid to the role of imaging in diagnosis and management of disease, and on how radiologic methods can be used optimally in dealing with CAP and NP.
CAP and NP: the basics
Community-acquired pneumonia
CAP is defined as pneumonia acquired in the community setting, i.e., in the environment outside hospitals [3]. Some controversy exists regarding this definition, specifically for pneumonia in nursing home residents, which is classified as CAP in most countries, whereas it is not in the UK. Also, the categorization of CAP in mildly immuno-compromized outpatients with disorders, such as diabetes, alcoholism, or renal insufficiency, is debated. Nevertheless, most guidelines define these pneumonias as CAPs.
CAP is a major health care problem. This is documented by the fact that there are 2–4 million cases per year in the US, and between 450,000 and 1 million patients are hospitalized [4]. CAP affects mainly the young (15–35 of 1000 children per year) and the elderly (30–40 of 1000 persons over 60 years of age) [5, 6]. Mortality in CAP is considerable, averaging 14% in a meta-analysis of 33,148 patients, and ranging from 5% in an unselected outpatient population to 37% in those patients who need intensive care treatment [7].
In CAP, the infection is usually transmitted from person-to-person via small droplets of nasal or oral secretions laden with microorganisms such as bacteria or viruses. The spectrum of causative pathogens includes gram-positive bacteria such as Streptococcus pneumoniae (Pneumococcus) and Staphylococcus aureus, gram-negative bacteria like Haemophilus influenzae, atypical bacterial organisms including Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophilia, and viral agents, mainly the influenza viruses and adenoviruses (Table 1) [3, 4, 6]. Recently, a string of corona viruses has been identified as the most likely cause of the severe acute respiratory syndrome (SARS) epidemic [8, 9]. Nevertheless, a recent study suggests that nearly half the cases of ambulatory CAP are due to atypical bacteria [10]. The term “atypical pneumonia” was originally applied to pneumonias with atypical clinical, laboratory, and/or radiographic features, and was primarily used to describe atypical bacterial, viral, protozoal, and fungal infections. Currently, this term is reserved for pneumonias of bacterial origin, in which the organism is difficult to isolate. In CAP and NP, as already mentioned, atypical pneumonias are predominantly caused by Mycoplasma pneumoniae, Chlamydia, and Legionella pneumophila.
Table 1.
Community-acquired pneumonia: spectrum of common pathogens. (Modified from [3])
| Patient previously well and/or <65 years old | Co-morbid Illness and/or >65 years old | |
|---|---|---|
| Organisms | Streptococcus pneumoniae | Streptococcus pneumoniae |
| Mycoplasma pneumoniae | Haemophilus influenzae | |
| Chlamydia pneumoniae | Oral anaerobes | |
| Haemophilus influenzae | Gram-negative rods | |
| Viruses | Staphylococcus aureus | |
| Legionella species |
For practical purposes it is important to emphasize that CAP is almost never caused by protozoa and fungi. These groups of organisms are exclusively seen in immuno-compromized individuals, who are either neutropenic (fungi) or have impaired cellular or humoral immune function (protozoa).
It appears from the literature that the list of etiologic agents implicated in CAP varies according to patient-related, seasonal, geographic, and diagnostic factors (Table 1). For example, the health and socio-economic status of a given patient has a certain impact on the selection of organisms potentially causing CAP. Otherwise healthy people are most likely to contract Mycoplasma pneumonia or a mild form of Pneumococcus pneumonia. In contrast, debilitated patients, alcoholics, and chronically ill persons more often present with severe pneumococcal pneumonia, or infections caused by Haemophilus influenzae, Staphylococcus aureus, gram-negative bacilli, or tuberculosis [11]. Legionella species and Chlamydia infections seem to be more common in patients with some forms of mild immunologic compromization. Patients with poor oral hygiene and individuals suffering from occasional loss of consciousness (epilepsy, alcoholism) may acquire anaerobic pulmonary infections. Also, in these patients, Mycobacterium tuberculosis infections are more prevalent in comparison with healthy persons without risk factors. Although not commonly listed among the causative organisms of CAP, tuberculosis should be considered as a relatively common cause of infection in outpatients and inpatients, especially when individuals are immuno-compromized. (Tuberculosis is not covered in detail in this article, since it is covered elsewhere in this series.)
The recent outbreak of SARS demonstrates that seasonal and geographic factors play an important role in the diagnosis and management of CAP, whereas Asian states, such as China, Hong Kong, and Singapore, as well as North American cities, were substantially affected by the epidemic, virtually no cases were registered in Europe [9]. The seasonal outbreak of viral epidemics has not only been observed in southern China (where SARS is suspected to have started) but is a well-known phenomenon in the rest of the world.
In CAP, patients usually present with fever, cough, dyspnea, sputum production, and pleuritic chest pain [4]. Because these symptoms are non-specific (most people who have fever and cough do not have pneumonia), radiographic methods and specifically the chest X-ray are play an exquisite role in diagnosing CAP. The radiographic identification of a pulmonary infiltrate is, in the appropriate clinical setting, indicative of pneumonia [12]. A patient who has fever and cough, and does not have radiologic proof of pneumonia, cannot be considered to have pneumonia. It is important to emphasize that in CAP proof of disease is not based on the identification or cultivation of a specific organism. This is because non-invasive tests, such as sputum cultures, are ineffective and correctly identify the offending organism in only 10-50% of cases [13]. On the other hand, invasive procedures (bronchoscopy, lavage, biopsy) are rarely used in patients with CAP. As a consequence, the radiologic diagnosis constitutes an important basis for the diagnosis and (often empiric) treatment of CAP.
Nosocomial pneumonia
NP is defined as lower respiratory tract infection that is neither present nor incubating at the time of admission to the hospital [17]. It is diagnosed when a patient develops fever and leukocytosis, when pathogenic organisms are isolated from tracheobronchial secretions, and when a new infiltrate appears on the chest radiograph at least 72 h after a patient has been hospitalized [15]. NP is the most common hospital-acquired infection with an incidence ranging from 0.5 to 5 cases per 100 admissions [16]. In the subgroup of ventilated patients in an intensive care setting, however, as many as 7–41% may develop NP. Reported mortality rates range from 20% in multihospital studies to 50% or higher in single referral centers and university hospitals. Apparently, prognosis associated with gram-negative pneumonias is considerably worse than with gram-positive or viral agents.
There are several factors contributing to the development of NP [18]. Firstly, host factors, including immune status, underlying disorders, and age. Secondly, a high number of hospitalized patients receive antacids and histamine2-blockers, resulting in a rise of the gastric pH and an increase in gastric gram-negative bacterial counts. This gastric reservoir for gram-negative organisms frequently serves as a seed point for pharyngeal colonization, and nasogastric tubes, which are present in many severely ill patients, may provide a pathway for the bacteria to migrate. Gram-negative bacterial overgrowth of the oropharyngeal epithelium may then occur particularly when the normally adherent gram-positive flora is lost [17]. Thirdly, tracheal intubation and mechanical ventilation may allow the pharyngeal germs direct access to the lungs. Fourthly, in supine patients and patients with decreased sensorium, direct aspiration of gastrointestinal contents into the lungs triggers the development of NP. Fifthly, microbial contamination of inserted drainage tubes, lines, and catheters is an important pathogenetic factor for the development of NP. Also, NP may result from bacteremia originating from right-sided endocarditis or septic pelvic thrombophlebitis. Finally, hospital personnel and patients with active infections may serve as sources of infections with direct person-to-person transmission of the microorganisms. Whatever the source of infection, the inappropriate use of broad-spectrum and prophylactic antibiotics is an additional and important factor leading to an increased susceptibility to hospital-acquired pneumonias.
In NP, the spectrum of causative organisms differs from that in CAP and is dominated by gram-negative bacilli such as Pseudomonas aeruginosa, Klebsiella species, Enterobactericeae species, Escherichia coli, Serratia marrescens and Proteus species. Gram-positive cocci (Streptococcus pneumoniae, Staphylococcus aureus), atypical bacteria (Legionella species), and viruses, including the respiratory syncytial virus, complement the list of pathogens (Table 2) [14].
Table 2.
Nosocomial pneumonia (NP; excluding patients with immunosuppression): spectrum of common pathogens. MRSA methicillin-resistant Staphylococcus aureus. (Modified from [14])
| Early onset mild to moderate NP; enteric gram-negative bacilli | Onset, any time, mild to moderate NP; enteric gram-negative bacilli | Late-onset severe NP; enteric gram-negative bacilli |
|---|---|---|
| Enterobactericeae species | Enterobactericeae species | Enterobactericeae species |
| Escherichia coli | Escherichia coli | Escherichia coli |
| Klebsiella species | Klebsiella species | Klebsiella species |
| Proteus species | Proteus species | Proteus species |
| Serratia marrescens | Serratia marcescens | Serratia marcescens |
| Haemophilus influenzae | Haemophilus influenzae | Haemophilus influenzae |
| Staphylococcus aureus | Staphylococcus aureus | Staphylococcus aureus |
| Streptococcus pneumoniae | Streptococcus Pneumoniae | Streptococcus Pneumoniae |
| Plus | Plus | |
| Anaerobesa | Pseudomonas aeruginosa | |
| Staphylococcus aureus b | Acinetobacter species | |
| Legionella speciesc | Consider MRSA | |
| Pseudomonas aeruginosa d |
aRecent abdominal surgery, witnessed aspiration
bComa, head trauma, diabetes mellitus, and renal failure
cHigh-dose steroids
dProlonged ICU stay, steroids, antibiotics, and structural lung disease
For the clinician, it can be difficult to diagnose NP. This is mainly because hospitalized patients with pneumonia do not always present with the characteristic symptoms of new fever, cough, sputum production, and elevated leukocyte count. If present, these symptoms may not necessarily be caused by pneumonia, but by a variety of other disorders [19]. Furthermore, the causative pathogen is frequently not readily identified: cultures from sputum and unprotected lavage fluid are of limited value because of the problems in distinguishing contamination from true infection. In addition, pulmonary disease in a hospital environment is frequently produced by more than one agent. These are just a few reasons why the identification of pulmonary infection, the use of various methods to identify the causative microorganism, the methodology to obtain a specimen, and the value of isolation of potential pathogens are matters of constant discussion in the clinical diagnosis of NP. At present, it seems that protected catheter brushing is the best possible method to identify an organism potentially responsible for pneumonia in inpatients.
Role of radiology revisited
Radiology has a pivotal position in the diagnosis and management of CAP and NP. The role of imaging is not limited to the detection or exclusion of pneumonia but involves the radiologist in the etiologic work-up, in the establishment of a differential diagnosis, in the follow-up procedure and, if necessary, in planning additional diagnostic measures. Chest radiography is the initial imaging method to evaluate patients with suspected pneumonia because of its reasonable accuracy, its wide availability, and its low cost and low radiation burden. It is used to confirm or exclude pneumonia, narrow the differential diagnosis, evaluate complications, and monitor disease at follow-up. Computed tomograpy is reserved for unclear cases, particularly when chest radiograms are normal in patients with a high level of clinical suspicion of pneumonia, and in immuno-compromized patients, in whom early diagnosis of pneumonia may influence a patient’s fate. Also, CT is mandatory in cases of persistent or recurrent infiltrates. Magnetic resonance currently plays a very limited role in the diagnosis and management of pneumonia.
Diagnosing pneumonia
Community-acquired pneumonia is most often seen in the offices of general practitioners, private radiologists, and in the outpatient department or the emergency room of hospitals. In patients with CAP, the primary role of the radiologist is to detect or to exclude pneumonia, and the chest radiogram is both sensitive and specific in this task [20]. The diagnosis of pneumonia is based on several diagnostic criteria; however, in all existing definitions of pneumonia, the presence of a pulmonary abnormality, compatible with pneumonia, is an indispensable prerequisite [16]. The American Thoracic Society guidelines recommend that a chest radiograph should be obtained whenever pneumonia is suspected [14].
Although some variation exists regarding the time frame between the onset of clinical symptoms and the development of a radiographically visible abnormality, the vast majority of infiltrates appears within the time period of 12 h (Fig. 1) [21]. This time frame allows detection or exclusion in most cases of CAP, where patients are generally seen by the radiologist within a few days following initial clinical presentation. Moreover, the majority of patients suspected to have CAP are otherwise healthy individuals without abnormalities of the lungs that would render the identification of an infiltrate difficult; thus, one should think that the detection or exclusion of outpatient pneumonia is, in most cases, relatively straightforward. However, several studies have demonstrated that the interobserver agreement in the diagnosis of CAP is only fair to good for experienced radiologists, and poor to fair for inexperienced radiologists and residents, respectively [22, 23].
Fig. 1a,b.
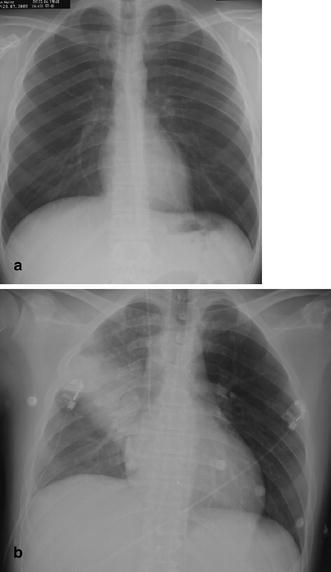
Rapid development of pneumonia in a 39-year-old man with a hematologic disorder. a When the patient developed fever, the first chest radiogram was taken 5 h after the onset of symptoms. b Follow-up 20 h after the onset of symptoms demonstrates an extensive alveolar infiltrate in the right upper lung representing lobar pneumonia due to Staphylococcus aureus infection
Likewise, caution must be exercised in patients with nosocomial infections, i.e., in patients who develop pulmonary infections in a hospital setting. These patients may be seen in the radiology department within a matter of hours after the onset of clinical symptoms, a time period in which a visible radiographic abnormality may not have developed. Moreover, in immuno-compromized patients, the appearance of a detectable radiographic abnormality may be delayed, particularly when an individual is neutropenic [24, 25]. Zornoza et al. [24] investigated a series of 175 consecutive patients with gram-negative pneumonia who were neutropenic following anti-neoplastic chemotherapy. In these patients, 70 episodes of pneumonia were initially diagnosed clinically, in the absence of radiographically detectable disease. In 27 of 70 episodes, an infiltrate was subsequently found on follow-up chest radiography. In 25 of 57 patients with no radiographically detectable infiltrates, the diagnosis of pneumonia was established at autopsy [24].
The radiographic appearance of a visible pneumonic infiltrate may be delayed not only in neutropenic patients but also in patients with functional defects of granulocytes due to diabetes, alcoholism, and uremia (Fig. 2). Some controversy exists in the literature regarding the influence of a patient’s state of hydration on the development of pneumonia [26, 27]. From a practical point of view, the radiologist must be aware that in the above-mentioned group of patients, pneumonia may exist without the typical appearance of a pulmonary infiltrate on the chest radiograph. Computed tomography and especially thin-section CT can be helpful in these patients since it is more sensitive in the detection of subtle abnormalities and may show findings suggestive of pneumonia up to 5 days earlier than chest radiographs (Fig. 3) [28]. Consequently, CT can aid in the early institution of therapy and thus help to lower mortality rates.
Fig. 2.
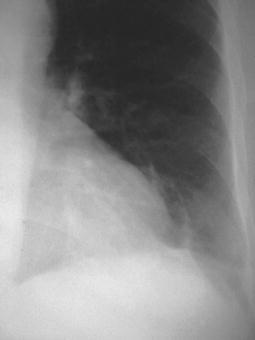
A 54-year-old diabetic inpatient with fever, non-productive cough, and elevated white cell count. The chest radiograph shows a faint segmental density in the left lower lobe representing delayed and incomplete development of left lower lobe pneumonia. At bronchoscopy, E. coli was identified as the causative organism
Fig. 3a,b.
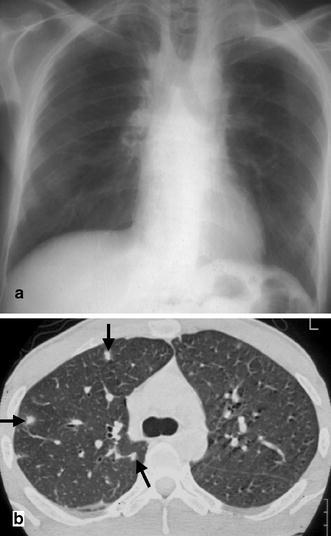
A 38-year-old patient with relapsing Hodgkin’s disease. In the neutropenic phase following chemotherapy the patient developed fever. a The chest radiograph demonstrates paramediastinal fibrosis as a consequence of the initial radiotherapy but reveals no signs of infection. b In contrast, the CT scan in the same patient 2 days later shows three small focal lesions with a halo phenomenon (arrows). Based on the CT findings, the patient was diagnosed to suffer from invasive aspergillosis and was treated successfully
Particularly in multimorbid hospitalized patients, the identification of a pulmonary infiltrate and the diagnosis of pneumonia may be hampered by pre-existing or concomitant pulmonary disorders (Fig. 4), or limited by processes that have a radiological appearance similar to that of pneumonia; these include atelectasis, edema, aspiration, hemorrhage, infarct, idiopathic interstitial pneumonias, pulmonary involvement in collagen-vascular disorders adult respiratory distress syndrome (ARDS), and pleural effusion. All of the above-mentioned abnormalities may mimic pneumonia or obscure or alter the otherwise characteristic radiographic appearance of an infiltrate [29].
Fig. 4.
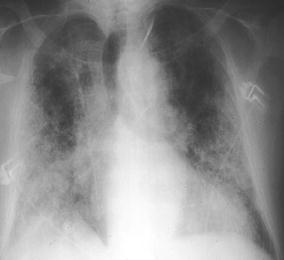
A 62-year-old inpatient with previous smoking history and proven idiopathic pulmonary fibrosis. Clinical symptoms included cough, fever, dyspnea, and malaise: CRP was 26 mg/dl and white cell count was 23.6 g/l. Chest radiography does not allow identification of a pneumonic infiltrate, although the patient was diagnosed as suffering from Pseudomonas aeruginosa infection (using protected catheter brushing)
NP may be particularly difficult to diagnose in intensive care unit (ICU) patients requiring mechanical ventilation, and the identification of pneumonia in patients with ARDS remains an unsolved problem (Fig. 5). Wunderink et al. compared the pre-mortem chest X-ray findings with pulmonary autopsy studies in ventilated patients with NP. No radiographic sign had a diagnostic efficiency greater than 68%. The only abnormality that correctly predicted 60% of pneumonias was the presence of an air bronchogram [30]. The most specific sign, although uncommon, was an air-space process abutting a fissure. Overall, the specificity of chest radiography in establishing the diagnosis of pneumonia ranges from 27 to 35% in comparison with autopsy or protected specimen brush culture [30, 31, 32]. Winer-Muram et al. [33] analyzed 40 intensive care patients with clinical signs and symptoms of pulmonary infection and new pulmonary abnormalities that were detected on chest radiography. In these patients, fiberoptic bronchoscopy with protected specimen brushing and bronchoalveolar lavage was performed and the findings were correlated with those of chest radiography. For the diagnosis of pneumonia, chest radiography provided an overall accuracy of 52%, and, when ARDS coexisted with pneumonia, of 42%. Interestingly, the use of clinical information does not ameliorate the diagnostic accuracy of chest radiography in the ICU setting. In Winer-Muram’s study, a further drop in accuracy resulted when the radiologist was given clinical information [33]. These unfavorable results reflect false-negative and false-positive results, the latter originating from misinterpretation of non-pneumonic opacities and from clinical bias.
Fig. 5.
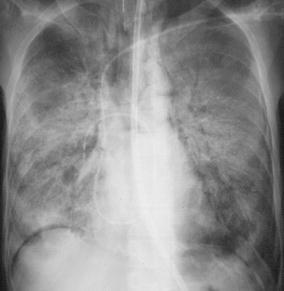
A 28-year-old patient with adult respiratory distress syndrome (ARDS) and sepsis. Supine portable chest radiogram displays extensive consolidation in both lungs, characteristic of ARDS, but does not allow either identification of pneumonic abnormalities or differentiation between ARDS and pneumonia. Protected catheter brushing revealed Pseudomonas aeruginosa infection
The same workgroup also determined the diagnostic accuracy of CT of 31 patients with ARDS receiving mechanical ventilation [34]. The CT scans obtained in 16 patients were interpreted as having no pneumonia present, and results of bronchoscopy revealed that 13 of these 16 patients had no pulmonary infection. Findings in this study have demonstrated that CT features are less useful in accurate identification of patients who have pneumonia but showed a fair diagnostic accuracy for ventilator-associated pneumonia in patients with ARDS owing primarily to identification of patients without pneumonia [34].
Finally, it is important to emphasize that the quality of radiology reports plays a critical role in the management of patients with suspected pneumonia. Webber-Chapman et al. demonstrated that report clarity, i.e., the lack of ambiguity in a report, reflects several factors or a combination of these [35]. In their study, three independent variables were associated with unambiguous reports including an interpretation of whether findings supported the diagnosis of pneumonia (in reports with pneumonia-related observations), short sentences, and the redundancy of pneumonia-related observations. In contrast, uncertainty modifiers and use of only descriptive terms introduced ambiguity in the reports.
Narrowing of the differential diagnosis
A second and very important task for the radiologist is to aid the clinician in the narrowing of the etiologic differential diagnosis. This relates to the notion that it is frequently impossible for the clinician to identify the causative organism of a pneumonic infiltrate. Reviewing the clinical literature on this topic, it becomes clear that with the full battery of microbiologic tests, the causative organisms can be identified in only 30–70% of cases. Moreover, sputum tests, which are commonly used to diagnose outpatient pneumonia, are frequently contaminated by upper respiratory tract colonization. This often results in the incorrect identification of organisms by sputum cultures in a high percentage of patients with CAP [36, 37]. Finally, the use of invasive procedures to identify the potential causative agent is frequently limited in nosocomial infections, especially in patients who are immuno-compromized and suffer from coagulation disorders.
In this situation, radiology may help. Narrowing of the etiologic differential diagnosis is indeed possible using pattern recognition, and with the integration of epidemiologic, clinical, laboratory, and radiographic information. Pattern recognition is based on the categorization of the radiographic features of pneumonia (such as form, shape, and density) into different morphologic “patterns” and the correlation of these patterns with the histopathologic changes caused by microbial agents. Practically, radiological pattern recognition allows the identification of different groups of potentially underlying organisms. This may be of value particularly in the etiologic diagnosis of CAP, where bacteria and viruses account for more than 95% of all microbial agents. Levy et al. analyzed the value of initial noninvasive bacteriologic and radiologic investigations in 420 patients with CAP [20]. They demonstrated that (focal segmental or lobar) alveolar infiltrates were caused by bacterial agents in over 90% of cases, whereas the majority of diffuse interstitial or mixed abnormalities could be attributed to viral, atypical bacterial, or tuberculous infections (Figs. 6, 7).
Fig. 6.
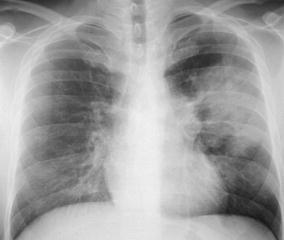
Acute air-space pneumonia in a male outpatient with symptoms suggestive of pneumonia. A chest radiogram shows a large area of consolidation with relatively well-defined borders and a central discrete air bronchogram in the left lung. This acute air-space pneumonia was caused by Streptococcus pneumoniae
Fig. 7.
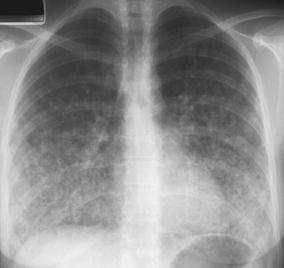
A 27-year-old woman with high-grade fever, dyspnea, and maculo-papular rash. Varicella zoster pneumonia in this patient is characterized by diffuse bilateral patchy and confluent nodular densities, a common pattern in pulmonary VZV infection
Notably, pattern recognition can hardly ever be carried further into the specific diagnosis of a single causative agent. For example, differentiation of the radiographic patterns of typical bacterial pneumonia (caused, for example, by Haemophilus influenzae, Streptoccocus pneumoniae, Staphylococcus aureus, and aerobic gram-negative bacilli) and atypical bacterial pneumonia (caused by Mycoplasma pneumoniae and Chlamydia species) is generally not possible. In a prospective study of 359 adults with CAP, Fang et al. compared the radiographic, clinical, and laboratory features of “typical” bacterial pneumonia with the findings of patients with atypical bacteria pneumonia and found no parameters that could reliably differentiate these groups [38]. Granados et al. prospectively compared the clinical and radiologic features of CAP caused by Legionella pneumophila to patients with pneumococcal infections. The authors concluded that Legionella, clinically as well as radiologically, may look like a typical bacterial pneumonia [39].
From a practical point of view, pattern recognition can aid in the differentiation of bacterial and viral pneumonias (which account for almost all community-acquired and nosocomial pneumonias). This may have implications on the therapeutic management of individual patients. Once the suspicion is raised that a patient suffers from a bacterial pneumonia, pattern recognition is of limited importance, since most recent therapeutic guidelines now recommend combining antibiotic regimens covering both typical and atypical bacteria.
Radiographic patterns of pneumonia
Localized air-space disease
In acute air space pneumonia (synonymously termed “lobar pneumonia”), the microorganism causes damage to the terminal air spaces (alveoli). As a result, edema pours into the alveoli, spreads rapidly through the terminal airways and pores of Kohn, and may involve large portions of the lung parenchyma, or an entire segment or lobe. Erythrocytes (which may already be present in early phases of the insult), leukocytes, and macrophages subsequently invade the involved parenchyma. Finally, fluid, cellular infiltration, and subsequent fibrin accumulation lead to consolidation of the lung parenchyma (Fig. 8) [1, 40].
Fig. 8.
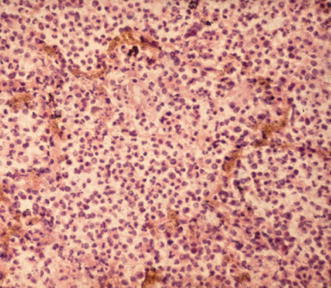
Pathohistologic correlate of acute air-space pneumonia (hematoxylin–eosin stain). An abundance of neutrophils fill the alveolar spaces and are accompanied by a few macrophages and fibrin accumulation
In patients with CAP and NP, acute air-space disease is most commonly caused by gram-positive bacteria such as Streptococcus pneumoniae, gram-negative bacilli, and atypical bacteria such as Mycoplasma pneumoniae and Legionella pneumophila [1, 20, 39, 41, 42, 43]. Occasionally, however, it may also be seen in viral infections [44]. Here, consolidation is not uncommon in adeno-, hanta-, and coronavirus (SARS) pneumonia [44, 45, 46]. Localized air-space disease is also a well-known feature of fungal and protozoal pneumonias, but these pneumonias almost never occur in CAP and NP, unless patients are immuno-compromized.
Acute air-space pneumonia (lobar pneumonia) is characterized by a mostly homogeneous consolidation of lung parenchyma, is relatively sharply demarcated, and does not typically respect segmental boundaries (Fig. 6). Initially, lobar pneumonia may appear in the periphery of the lungs, and may then spread toward the medulla and/or the hilar region, resulting in a segmental, lobar, or non-segmental infiltrate (Fig. 9) [41, 47, 48]. In the latter case, occasionally, a “cloud-like” appearance or a rounded lesion is seen, particularly in streptococcal and Legionella pneumonia (Fig. 6) [41, 42]. In acute air-space pneumonia, an air bronchogram is very common (Fig. 9). The loss of the silhouette of an adjacent mediastinal structure or a hemidiaphragm is another important diagnostic sign (Fig. 10). Infiltrates are either unilateral or bilateral. Cavitation is rare in infections with atypical bacteria but typical in Staphylococcus aureus and gram-negative pneumonias, as well as in infections with mycobacteria and anaerobes (Fig. 10) [41]. The lung volume is usually normal in gram-positive pneumonias and often increased in gram-negative pneumonias (KIebsiella species) [49]. At CT, acute lobar pneumonias in patients with CAP most commonly show air-space consolidation with segmental and non-segmental lesions, the latter with a round or cloud-like shape (Fig. 11), and involvement of the middle and outer zones of the lung [50, 51]. Occasionally, areas of ground-glass attenuation may abut air-space disease (Fig. 11) [50].
Fig. 9.
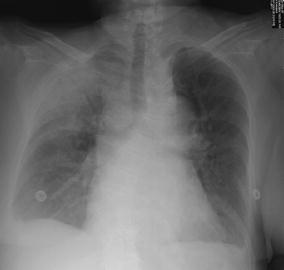
Typical upper lobe pneumonia in a 63-year-old outpatient. Note that acute air-space disease may involve predominantly the periphery of the lungs while the perihilar area is relatively spared. The aerobronchogram is indicative of an air-space process
Fig. 10.
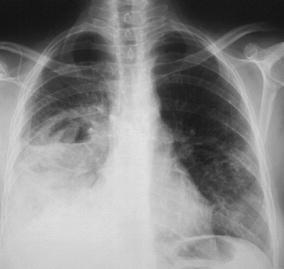
Typical radiographic characteristics of acute lobar pneumonia in a 53-year-old inpatient. The chest radiogram displays an extensive, dense alveolar infiltrate in the right mid- and lower lung fields with loss of the silhouettes of the lower right heart border and right hemidiaphragm. These features suggest involvement of the right lower lobe and middle lobe by the pneumonic infiltrate. In addition, an air–fluid level indicates cavitation. Note that the infection has spread transbronchially to the left lung. The patient was diagnosed to have E. coli pneumonia
Fig. 11.
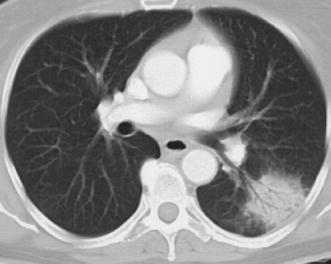
Computed tomography signs of acute lobar pneumonia due to Streptococcus pneumoniae. At CT, the air-space consolidation in the left lower lobe displays a round shape and an air bronchogram, and is surrounded by a halo of ground-glass attenuation (which is occasionally seen in acute air-space pneumonia)
Lobular pneumonia
Staphylococcus aureus and Pseudomonas aeruginosa are the two organisms most commonly causing the radiographic pattern defined as lobular or bronchopneumonia. This form of pneumonia differs from lobar pneumonia in that the causative organism directs its attack against the peripheral airways and damages particularly the walls of terminal and respiratory bronchioles, resulting in a necrotizing bronchiolitis and bronchitis. Subsequently, inflammatory aggregates involve the adjacent lung parenchyma, triggering in the typically patchy appearance of disease (Fig. 12). Over time, and with progression, dense consolidation of the involved parenchyma may develop [21, 48, 52]. Lobular pneumonia can also be caused by Haemophilus influenzae, Mycoplasma pneumoniae, Mycobacterium tuberculosis, and viruses [53]. These organisms also tend to affect small airways and the surrounding lung parenchyma, leading to patchy centrilobular infiltrates.
Fig. 12a,b.
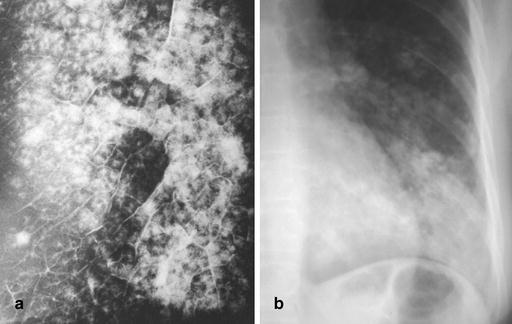
Radiographic appearance of lobular (broncho) pneumonia. a Specimen radiogram of lobular (broncho) pneumonia. This radiogram, obtained from a post-mortem examination of a patient with Staphylococcus aureus pneumonia, demonstrates the centrilobular origin of this particular form of pneumonia. As disease spreads from the walls of terminal and respiratory bronchioles, large portions of secondary lobules are involved, and confluent disease may cause frank lobar consolidation. [By courtesy of Saunders, Philadelphia. Potchen J, Grainger R, Greene R (eds) Pulmonary radiology.] b Radiographic appearance of lobular (broncho) pneumonia caused by Staphylococcus aureus and shows patchy and partly confluent densities in the left lower lobe. This pattern is distinctly different from the one seen in acute lobar (air-space) pneumonia
The radiographic appearance of early bronchopneumonia is patchy with air-space nodules (centrilobular lesions with poorly defined margins measuring 4–10 mm in diameter), lobular consolidation, and confluent focal areas of consolidation (Fig. 12) [54, 55]. In the later phase of disease frank lobar consolidation may be seen [54]. The disease is typically segmental (involving one or more segments) or lobar in distribution. In more than half of the cases of Staphylococcus aureus infection, the infiltrates are bilateral. Cavitation is rare in early bronchopneumonia but common in late-phase consolidation.
At CT, centrilobular lesions and focal areas of circumscribed air-space disease (air-space nodules) are the most common findings in early bronchopneumonia (Fig. 13) [51, 53, 55]. In Mycoplasma pneumoniae, centrilobular lesions, focal and patchy acinar lesions, areas of ground-glass attenuation and segmental infiltrates may be isolated findings, or may coexist in a complex pattern (Fig. 14) [51, 53]. In these cases pneumonia has the tendency to affect both medulla and cortex of the lung [51]. As Reittner et al. pointed out, areas of ground-glass attenuation in a lobular distribution are a distinct feature of Mycoplasma infection, and are not seen in any other form of pneumonia (Fig. 14) [53].
Fig. 13.
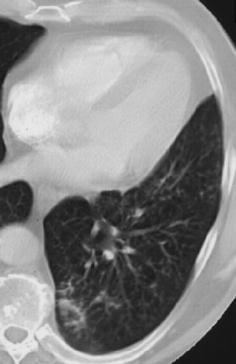
Computed tomography findings in early bronchopneumonia. The CT section through the left lower lobe in a patient with Haemophilus influenzae pneumonia demonstrates small nodular and patchy acinar lesions with a tendency towards confluence in the posterior basal segment of the left lower lobe
Fig. 14a–c.
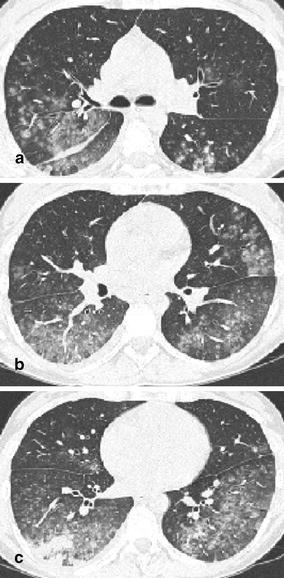
Computed tomography features of Mycoplasma pneumonia. The CT scans in a 31-year-old woman with fever, incessant cough, malaise, and fatigue demonstrates excessive areas of ground-glass attenuation in all pulmonary lobes. Certain ground-glass densities show a centrilobular location (a), whereas others have formed widespread ground-glass infiltrates (b,c). Areas of ground-glass attenuation in a lobular distribution are a distinct feature of Mycoplasma pneumonia
Interstitial pneumonia
Throughout many years, the term “interstitial pneumonia” (diffuse interstitial or mixed alveolar–interstitial) was a widely accepted classification for diffuse and mostly bilateral pneumonic abnormalities. This terminology, however, is somewhat misleading, since in most cases of “interstitial” pneumonias, we find interstitial changes and alveolar disease side by side. Initially, the inhaled microbial agent (mostly viruses and protozoa) damages the ciliated epithelial cells and bronchial mucous gland cells. Subsequently, edema and mononuclear (lymphocytic) cellular infiltration lead to widening of alveolar septa, and later, to the involvement of interlobular septa (Fig. 15). This stage represents the “simple” viral pneumonia [48, 52]. It may be complicated by foci of inflammation characterized by leukocytic infiltration and focal necrosis, occasionally of bacterial origin, particularly in the later stages. In both instances, a cascade of pathophysiologic events may lead to capillary leakage with accumulation of hemorrhagic interstitial and/or alveolar edema [48, 52]. Polymorphonuclear cellular infiltration and hyaline membrane formation are other distinct morphologic features of this process which, in cases of severe damage, can resemble diffuse alveolar damage and/or ARDS, histopathologically as well as radiographically [48, 52].
Fig. 15.
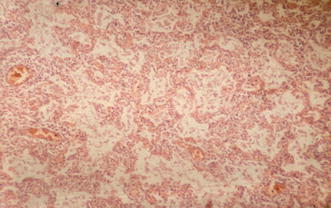
Histopathologic correlate of interstitial pneumonia. This histopathologic specimen (hematoxylin–eosin stain) demonstrates moderate lymphocytic infiltrates surrounding a blood vessel and extending into the neighboring alveolar walls. The presence of macrophages and pneumocytes suggest the beginning of acute alveolar damage
Diffuse interstitial pneumonias are most commonly caused by viruses and protozoa, and are rarely seen in bacterial pneumonias. Therefore, this form of pneumonia is rare in patients with CAP; however, if identified in normally healthy outpatients, it is most commonly caused by a viral agent such as the respiratory syncytial virus (RSV).
Radiographically, interstitial diffuse lung disease may encompass a wide variety of findings. These include reticular, reticulonodular, nodular, patchy, and alveolar densities, and areas of increased background (ground-glass) density (Figs. 7, 16, 17) [41, 42, 44, 56, 57, 58, 59]. Frequently, interstitial diffuse lung disease presents as a mixed interstitial–alveolar disease, sometimes even in a pure alveolar abnormality. Two or more of these findings may co-exist. In the vast majority of cases, the disease is bilateral and diffusely distributed. In herpes simplex virus 1 pneumonia, chest radiograms demonstrate mostly bilateral patchy subsegmental, segmental and lobar ground-glass densities and consolidation [44, 58]. In RSV pneumonia, the radiographic abnormalities are characterized by discrete interstitial perihilar linear abnormalities. In Herpes varicellae (Varicella zoster) pneumonia, diffusely distributed, well-defined patchy or nodular densities are the characteristic feature (Fig. 7) [57]. In organ transplant recipients a symmetric, diffuse bilateral linear, or discrete to marked nodular pattern, potentially combined with patchy alveolar densities, is most commonly caused by CMV infection [41, 44]. Pleural effusions are uncommon. The CT findings include bilateral and diffusely distributed areas of ground-glass densities, centrilobular patchy lesions, reticular or nodular abnormalities, and mixed reticular-alveolar infiltrates (Fig. 17) [44].
Fig. 16.
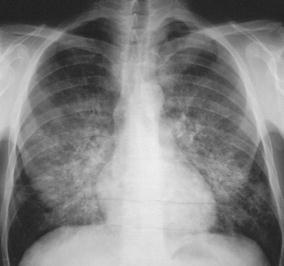
Interstitial pneumonia. In a 36-year-old HIV-positive patient with Pneumocystis carinii pneumonia the disease is characteristized by reticular, reticulo-nodular, and alveolar patchy densities predominately located in the central and basal portions of the lungs. The pattern may occasionally be confused with hydrostatic lung edema
Fig. 17a,b.
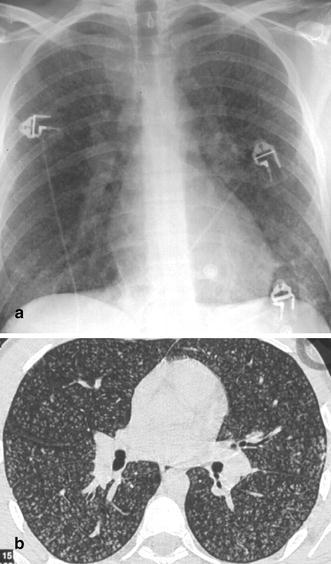
Acute viral broncholitis and pneumonia in a 19-year-old recruit. a At chest radiography, the viral infection is characterized by a myriad of tiny nodules scattered throughout the lung parenchyma bilaterally. b Thin-section CT reveals unimorphous centrilobular densities demarcating the peripheral terminal and respiratory bronchioles
Nodular lesions
Macronodular lesions are the result of infection with bacteria, such as Nocardia asteroides, by mycobacteria, fungi, and septic emboli (Fig. 18) [41, 42, 60, 61, 62, 63, 64, 65]. In outpatients, mycobacterial infections are the most common cause of macronodular lesions (Fig. 19). In the nosocomial setting, nodular abnormalities are more frequently seen in immuno-compromized hosts, and due to infection with bacteria and fungi. Nocardia asteroides causes single or multiple nodular infiltrates with or without cavitation [62]. Aspergillus fumigatus (in invasive pulmonary aspergillosis), Mucor mycosis, and Cryptococcus neoformans can present with single or multiple nodular infiltrates, which often quickly progress to wedge-shaped areas of consolidation [61, 64, 65]. Cavitation is common and occurs both in bacterial and fungal lesion; in the latter, the so-called air-crescent sign points towards invasive pulmonary aspergillosis. A halo phenomenon, i.e., a rim of ground-glass attenuation around a focal nodular opacity, may be seen on CT scans of different inflammatory lesions. In the appropriate clinical setting, however, it is suggestive of invasive pulmonary aspergillosis [64, 65].
Fig. 18.
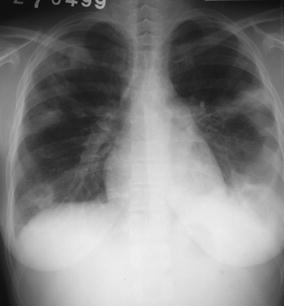
Nodular lesions in a young female drug addict with endocarditis. The chest radiogram demonstrates peripheral densities in both lungs showing a nodular aspect in a right upper and lower lobes. The peripheral location, nodular aspect, and central cavitation is characteristic of septic emboli in this patient setting. Note the enlargement of the cardiac silhouette and the left pleural effusion
Fig. 19.
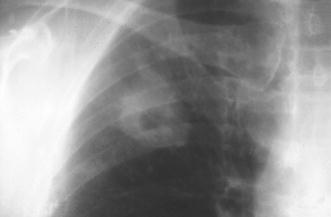
A 58-year-old patient 2 years after renal transplant surgery. The isolated, centrally cavitating macronodular lesion in the right upper lobe is caused by Mycobacterium avium intracellulare infection
Diffusely and randomly distributed well-defined micronodules (miliary disease) are most commonly associated with the hematogenous spread of tuberculous disease (Fig. 20) [66]. In contrast, bronchogenic spread of TB results in larger ill-defined nodules (Fig. 20). These “air-space” nodules may also be seen in infectious broncholitis from viruses and Mycoplasma pneumoniae [53]. Viral pneumonia in herpes or Varicella zoster infection tends to present with larger nodules ranging from 5 to 7 mm in diameter and displaying hazy borders (Fig. 7) [57, 58]. In patients with granulocytopenia, small and well-defined nodules are frequently caused by Candida infection (Fig. 21). Chest radiograms commonly do not show the full extent and morphologic details of pulmonary micronodules, and CT is frequently required to more precisely characterize the disease [57].
Fig. 20a,b.
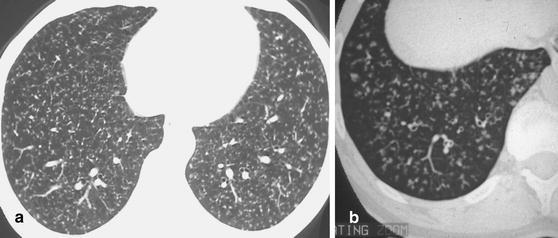
Patterns of micronodular disease caused by tuberculous infection. a Miliary pattern with small, well-defined interstitial nodules scattered randomly throughout the lung parenchyma. b Micronodular disease originating from tuberculous infection of the small airways. Note that the tree-in-bud phenomena, seen in the lung periphery, are characteristic of infectious small airways disease
Fig. 21a,b.
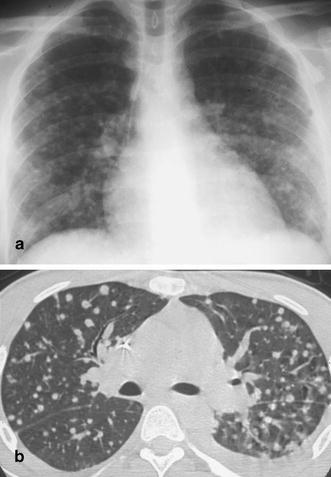
Micronodular disease caused by Candida infection. The a chest radiogram and b CT scan demonstrate well-defined nodules scattered throughout the parenchyma of both lungs. In patients with long-standing granulocytopenia and symptoms of infection, this pattern is indicative of Candida infection with small Candida abscesses. Occasionally, these abscesses are also seen in the liver and the spleen
Specific CT patterns
In lobar, lobular, and diffuse interstitial pneumonias, the patterns at CT patterns generally correspond to those seen at chest radiography. These patterns have been described in the foregoing sections. In addition, distinct findings, signs, and patterns of pneumonia and lower respiratory tract infection are revealed with the use of CT. One of the most important CT features of infection of the small airways is the tree-in-bud sign (Fig. 20b) [67, 68]. This sign is suggestive of infectious bronchiolitis and is not uncommonly seen in patients suspected of having community-acquired infection. In this patient group, it is etiologically most frequently due to infections with viruses and Mycoplasma pneumoniae. In addition, bronchogenic spread of post-primary tuberculosis is a prominent cause of this specific sign [67, 68]. The tree-in-bud phenomenon reflects inflammatory changes in the small peripheral bronchioli with secretions in the lumen of the airways, wall thickening, and peribronchiolar inflammation [67, 68]; thus, with respect to the pathophysiologic and histologic changes, some overlap exists with the initial phase of lobular pneumonia. Indeed, it may be difficult to distinguish between advanced infectious bronchiolitis caused by Mycoplasma pneumoniae, and early lobular pneumonia triggered by the same microorganism. As pointed out by Reittner et al. [53], the thin-section CT findings in Mycoplasma pneumoniae include centrilobular nodules, often in a lobular distribution, and thickening of the peribrochovasular and interlobar septal interstitium. These findings are often difficult to identify on chest radiographs but can usually be recognized on CT scans [53].
Overall, the recognition of radiographic patterns provides the opportunity to aid the clinician in the etiologic diagnosis of pulmonary infections. In principle, pattern recognition may help classify the large groups of organisms (i.e., bacilli, viruses, fungi); however, there are limitations to this approach. Firstly, patterns overlap. Secondly, a single organism may produce different patterns in different patients. The prototype for such chameleon-like behavior is Mycoplasma pneumoniae. This atypical organism has the potential to cause acute air-space disease, bronchopneumonia, bilateral and diffuse disease, centrilobular lesions, ground-glass densities, and tree-in-bud phenomena. The same holds true for the corona virus which was identified as causing SARS. The same organism, in this case a virus, triggered radiologic changes with a variety of patterns including acute air-space disease, ground-glass lesions, and bilateral diffuse abnormalities (Fig. 22). Thirdly, radiographic patterns change with the immunologic status of the patient. This has been demonstrated for mycobacteria or Aspergillus fumigatus, which may cause three different patterns depending upon the immunologic situation of an individual [69, 70]. Finally, patterns may be altered by pre- or co-existing lung disease. Pattern recognition should only be attempted on an extensive knowledge of the radiographic features of pulmonary infections, and with the integration of clinical information.
Fig. 22a–d.
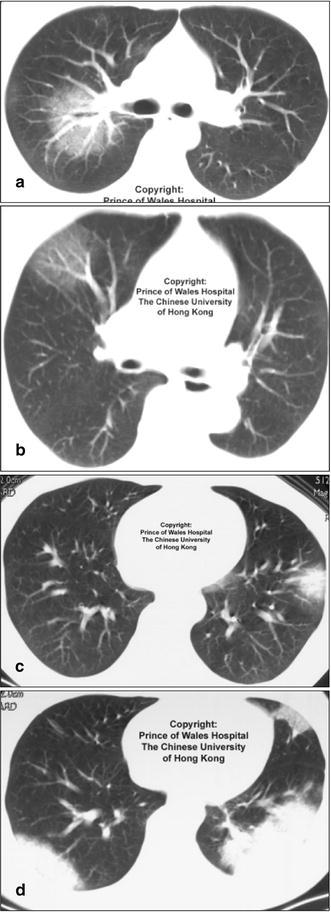
Different patterns of disease caused by the same organism in severe acute respiratory syndrome (SARS). Images from a patient with proven SARS. (From the Princess of Wales Hospital, The Chinese University of Hong Kong) demonstrate different patterns of disease including a,b ground-glass densities, c focal acute air-space disease with halo phenomenon, and d bilateral areas of consolidation. The SARS has been shown to cause several different radiographic patterns at chest radiography and CT
Non-infectious disorders
Not surprisingly, several disorders may mimic pneumonia, clinically as well as radiologically. Focal alveolar densities (as seen in lobar pneumonia) are a common feature in pulmonary infarction, atypical focal edema, hemorrhage, atelectasis, bronchiolitis obliterans with organizing pneumonitis (BOOP), and malignancies including bronchogenic carcinoma (with postobstructive pneumonitis) and lymphoma (Figs. 23, 24) [15, 42, 71]. In diffuse bilateral disease, eosinophilic pneumonia, edema, diffuse alveolar damage, ARDS, acute damage to the alveolo-capillary unit in systemic lupus (Fig. 25), and idiopathic interstitial pneumonias are only a few entities among many that can look like viral or protozoal pneumonia. From a practical point of view, the list of potential differential diagnoses is mostly short in suspected CAP in otherwise healthy outpatients, but potentially long in hospitalized multimorbid individuals. In such patients, edema especially, ARDS, drug toxicity, atelectasis, and infarcts may render diagnosis of pneumonia difficult if not impossible [33]. For example, basilar atelectasis is common in ICU patients, individuals following cardiac surgery, and patients with pleural effusions [29, 72]. It can easily be misinterpreted as pneumonia.
Fig. 23a,b.
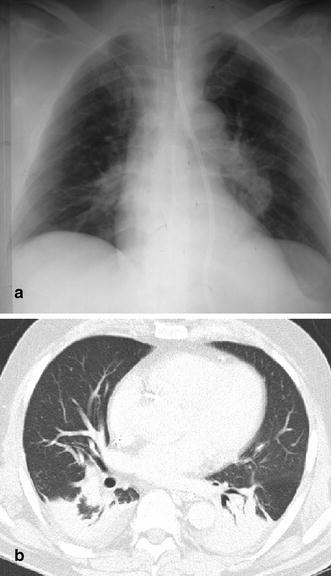
Partial atelectasis of the left lower lobe mimicking pneumonia. a In this 64-year-old stroke patient with arterial hypertension, an alveolar opacity in the medial aspects of the left lower lobe was misdiagnosed as pneumonia. b At CT, this density was identified as partial atelectasis of the left lower lobe
Fig. 24.
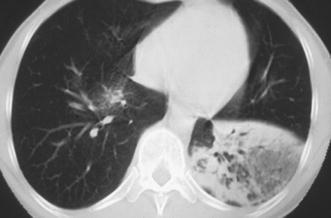
Lobar lymphoma mimicking lobar pneumonia. A 28-year-old outpatient with subfebrile temperatures and a chronic left lower lobe infiltrate, non-responsive to standard antibiotic treatment. The patient was biopsied and a low-grade B-cell lymphoma was identified histopathologically
Fig. 25.
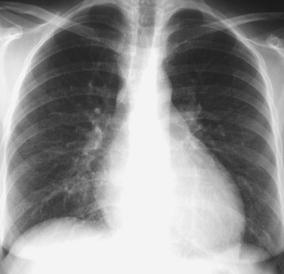
Lupus pneumonitis mimicking pneumonia. A young woman with known systemic lupus erythematosus, presenting with an episode of fever and cough. The chest radiogram demonstrates bilateral diffuse interstitial abnormalities and an increased background density of the lung parenchyma. No causative organism could be identified. The enlargement of the cardiac silhouette, due to a pericardial effusion and the amount of circulating anti-DSDNA antibodies, pointed towards acute damage to the alveolar capillary unit (acute lupus pneumonitis)
Prognosis
Radiology has many roles in the diagnosis and management of community-acquired and nosocomial pulmonary infections. In a meta-analysis and review of the medical literature on prognosis and outcome in patients with CAP, Fine et al. found that imaging may also contribute to an estimate of mortality in a certain case [7]. Multilobar involvement by pneumonia on chest radiographs was found to be an independent predictor of mortality (in addition to several other factors such as co-morbid illnesses, symptoms and signs, and laboratory features) [6, 7, 73].
Planning of additional diagnostic procedures
In patients with CAP, diagnosis and management most frequently rely on chest radiography and do not require further diagnostic tests. In these patients, CT and invasive diagnostic procedures are reserved only for cases in which treatment failure or complications, such as abscess, influence the course of disease. Conversely, in nosocomial or opportunistic infections, cross-sectional imaging techniques and procedures, such as needle or bronchoscopically guided biopsy, are more often required. This is because nosocomial pneumonias are associated with a high mortality rate, and because 25–45% of these patients remain without etiologic diagnosis even when extensive noninvasive diagnostic testing is performed; thus, identification of the causative organism is more intensively pursued, with the use of fiberoptic bronchoscopic lavage, brushing, and/or biopsy. In many institutions, imaging methods, such as CT, are used for the guidance of invasive methods into areas of maximum disease.
The use of transthoracic CT aspiration needle biopsy in the diagnosis of pulmonary infection is controversial. Nevertheless, when noninvasive techniques, such as sputum examination and cultures, are non-diagnostic, a choice must be made between empiric therapy and an invasive test. While the majority of patients are treated empirically, the nature and course of pneumonias in nosocomial infections and in immunosuppressed individuals frequently dictates a more aggressive approach. In such cases, transthoracic needle biopsy may help to identify the causative organism [74, 75]. Sanchez-Nieto et al. reviewed a series of 441 transthoracic needle aspirations to evaluate the use of the procedure in diagnosis of pulmonary infections [75]. In 67 patients in whom pulmonary infection was suspected, a specific diagnosis was made with needle biopsy in 45 cases. In 46 cases in which infection was ultimately found to be present, aspiration biopsy identified the organism in 35 cases. Overall, clinically useful information was obtained in 81% of aspiration biopsies for pulmonary infection. Since other authors report similar results, needle biopsy should enrich the radiologist’s armamentarium in diagnosing and managing pulmonary infections.
As an alternative imaging method, the interest in MR imaging of the lung parenchyma has been growing. Although imaging quality is limited by low proton density of normal lung and susceptibility artifacts caused by the extensive air–tissue interfaces of the parenchyma, several recent studies report promising results regarding improved lesion conspicuity [76]. Leutner et al. compared T2-weighted turbo spin-echo MR imaging with CT in patients with opportunistic pneumonia. Features such as consolidation, ground-glass hyperintense lesions, nodules, reticular infiltrations, cavities, and cystic disease could be identified on high-quality MR images and showed fair correlation with CT scans [76]. Especially the depiction of necrotizing pulmonary lesions using the “reverse target sign” underlines the potential diagnostic possibilities of MRI.
Patient follow-up
The role of radiography in the follow-up of pulmonary infections is currently under debate. Because of increasing economic restrictions, imaging tests are used less routinely to monitor the resolution of pulmonary infiltrates. Many institutions now do not follow patients radiographically when the clinical course indicates successful treatment. In other institutions, in different health care systems, radiographic documentation of the healing process is required for medico-legal reasons.
If follow-up exams are performed, it is important to consider the time period in which a pneumonic infiltrate may resolve. As a general rule, most pneumonias regress within 10–21 days. After 3 months, two-thirds of patients with CAPs have cleared their lungs [77]; in the rest, however, complete resolution may take up to 6 months, especially in patients with underlying lung disease, such as chronic obstructive pulmonary disease, in immuno-compromized individuals, and in the elderly [77, 78].
For the radiologist it is important to separate patients with a continuous (slow) improvement of disease from those who do not adequately respond to treatment; for the latter, a list of differential diagnoses needs to be established. Firstly, it is not uncommon that patients are not treated effectively and either typical or atypical bacteria are not covered by a single drug strategy; thus, in a recent article by Halm and Teirstein, it is suggested that patients with CAP always receive combination therapy covering typical and atypical bacteria [4]. Secondly, one should consider that the disease, although mimicking infection clinically, is not related to pneumonia but represents noninfectious inflammation such as BOOP (Fig. 26), eosinophilic pneumonia or lupus pneumonitis, acute alveolar sarcoid, or a malignant disorder including bronchoalveolar cell carcinoma, bronchogenic carcinoma with post-obstructive pneumonitis, and lymphoma. It is a well-established fact that all of these disorders may look like pneumonia and that misdiagnosis is not uncommon; therefore, an important role of the radiologist in these patients is to critically judge the course of the disease and to plan further diagnostic procedures, including CT scanning, if needed.
Fig. 26a–c.
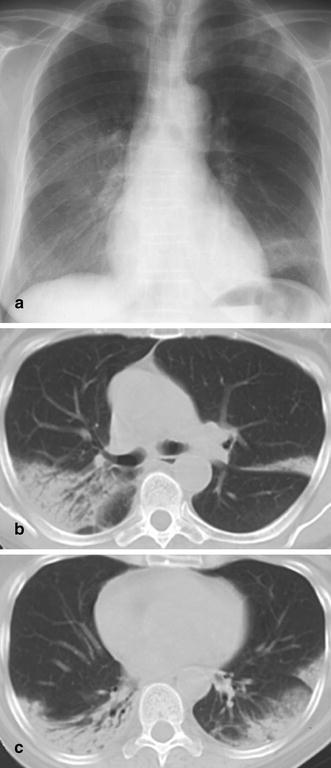
Bronchiolitis obliterans with organizing pneumonia (BOOP) mimicking pneumonia. A 62-year-old woman with a 3 month history of subfebrile temperatures, cough, and failure of response to several antibiotic regimens. a The plain chest radiogram demonstrates “ground-glass” opacities in the right middle and lower lung fields as well as in the left upper lobe and left lower lobe. b,c At CT, alveolar consolidation is seen in the periphery of both upper lobes and both lower lobes. The distinct peripheral location of the abnormalities and the discretely ectatic airways raised the suspicion of BOOP. The diagnosis was confirmed through video-assisted fluoroscopic biopsy
Computed tomography scanning is also the method of choice to evaluate patients with recurrent pulmonary infiltrates. Such recurrent infections usually indicate some sort of underlying problem such as congenital or acquired immunologic disorders, cardiac abnormalities (congestive heart failure), or systemic diseases such as diabetes, chronic alcoholism, and intravenous drug abuse. If occurring in the same anatomical location, they may be the result of underlying structural defects including airway abnormalities such as chronic bronchitis, bronchiectasis, large cavities, and bronchogenic carcinoma (Fig. 27). Here, CT scanning is indicated to search for or exclude an underlying disorder and to plan further therapeutic measures.
Fig. 27a–c.
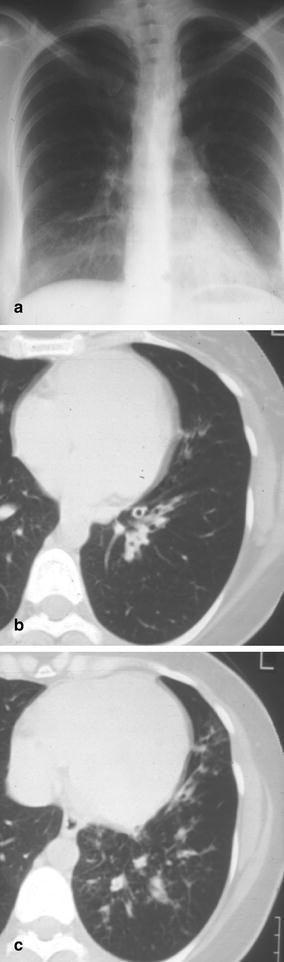
Recurrent left lower lobe pneumonia in a young woman with bronchiectasis. a The left lower lobe pneumonia due to Pseudomonas aeruginosa infection is well documented at chest radiography. b,c After successful treatment, the patient underwent CT scanning which showed dilated airways in the left lower lobe with wall thickening and peribronchial and peribronchiolar inflammation
Conclusion
In patients with suspected pneumonia, the radiologist has an important role in the detection and exclusion of pulmonary infiltrate, in the narrowing of the differential diagnostic spectrum, the planning of further diagnostic procedures, and in follow-up. The chest radiograph is the single most important test to perform the above tasks, and diagnosis and management of pneumonia is impossible without its use. It remains the initial imaging test in any suspected pulmonary infection. Computed tomography is indicated in cases of unclear findings at chest radiography, in patients in whom subtle abnormalities may be due to infectious bronchiolitis, in the detection and evaluation of complication, and in persistent and recurrent pneumonia. The accuracy of imaging is good to excellent in suspected CAP (in otherwise healthy outpatients) and limited to poor in multimorbid, hospitalized individuals.
Footnotes
ECR 2004 - Categorical Course “Infection in the adult today”
References
- 1.Vincent J Am Med Assoc. 1995;274:634. doi: 10.1001/jama.274.8.639. [DOI] [Google Scholar]
- 2.National Monthly Vital Stat Rep. 1993;42:1. [Google Scholar]
- 3.Mandell Chest. 1995;108:35S. doi: 10.1378/chest.108.2_supplement.35s. [DOI] [PubMed] [Google Scholar]
- 4.Halm N Engl J Med. 2002;347:2039. doi: 10.1056/NEJMcp020499. [DOI] [PubMed] [Google Scholar]
- 5.Jokinen Am J Epidemiol. 1993;137:977. doi: 10.1093/oxfordjournals.aje.a116770. [DOI] [PubMed] [Google Scholar]
- 6.Marrie Semin Respir Infect. 1990;5:260. [PubMed] [Google Scholar]
- 7.Fine J Am Med Assoc. 1996;10:275. [Google Scholar]
- 8.Poutanen N Engl J Med. 2003;348:1. doi: 10.1056/NEJMoa030634. [DOI] [Google Scholar]
- 9.Drazen N Engl J Med. 2003;348:e6. doi: 10.1056/NEJMe030062. [DOI] [PubMed] [Google Scholar]
- 10.Marrie Am J Med. 1996;101:508. doi: 10.1016/S0002-9343(96)00255-0. [DOI] [PubMed] [Google Scholar]
- 11.American Am Rev Respir Dis. 1993;148:1418. doi: 10.1164/ajrccm/148.5.1418. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett N Engl J Med. 1995;333:1618. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- 13.Bowton J Thorac Imaging. 1991;3:1. doi: 10.1097/00005382-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 14.American Am J Respir Crit Care Med. 1995;153:1711. [Google Scholar]
- 15.Lipchik Radiol Clin North Am. 1996;34:47. [PubMed] [Google Scholar]
- 16.Torres Am Rev Resp Dis. 1990;142:523. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 17.Johanson Ann Intern Med. 1972;77:701. doi: 10.7326/0003-4819-77-5-701. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous MMWR. 1997;46:1. [Google Scholar]
- 19.Chastre Am J Med. 1988;85:499. doi: 10.1016/s0002-9343(88)80085-8. [DOI] [PubMed] [Google Scholar]
- 20.Levy Chest. 1988;92:43. [Google Scholar]
- 21.Spencer H (1985) Pathology of the lung, 4th edn. Pergamon Press, Oxford, UK
- 22.Albaum Chest. 1996;110:343. doi: 10.1378/chest.110.2.343. [DOI] [PubMed] [Google Scholar]
- 23.Melbye Acta Radiol. 1992;33:79. [PubMed] [Google Scholar]
- 24.Zornoza Am J Roentgenol. 1976;127:989. doi: 10.2214/ajr.127.6.989. [DOI] [PubMed] [Google Scholar]
- 25.Donowitz Arch Intern Med. 1991;151:701. doi: 10.1001/archinte.151.4.701. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell Am Rev Respir Dis. 1975;112:651. doi: 10.1164/arrd.1975.112.5.651. [DOI] [PubMed] [Google Scholar]
- 27.Cooligan Am Rev Respir Dis. 1980;121:122. [Google Scholar]
- 28.Heussel AJR. 1997;169:1374. [Google Scholar]
- 29.Goodman LR (1992) Acute inflammatory disease. In: Goodman LR, Putnam CE (eds) Critical care imaging. Saunders, Philadelphia, pp 83–88
- 30.Wunderink Chest. 1992;101:458. doi: 10.1378/chest.101.2.458. [DOI] [PubMed] [Google Scholar]
- 31.Fagon Am Rev Respir Dis. 1988;138:110. doi: 10.1164/ajrccm/138.1.110. [DOI] [PubMed] [Google Scholar]
- 32.Lefcoe Chest. 1994;105:885. doi: 10.1378/chest.105.3.885. [DOI] [PubMed] [Google Scholar]
- 33.Winer-Muram Radiology. 1993;188:479. doi: 10.1148/radiology.188.2.8327701. [DOI] [PubMed] [Google Scholar]
- 34.Winer-Muram Radiology. 1998;208:189. doi: 10.1148/radiology.208.1.9646813. [DOI] [PubMed] [Google Scholar]
- 35.Webber-Chapman Acad Radiol. 2001;8:57. doi: 10.1016/S1076-6332(03)80744-4. [DOI] [PubMed] [Google Scholar]
- 36.Bartlett Conn Med. 1970;34:347. [PubMed] [Google Scholar]
- 37.Woodhead Lancet. 1987;21:671. doi: 10.1016/S0140-6736(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 38.Fang Medicine. 1990;69:307. [Google Scholar]
- 39.Granados Eur Respir J. 1989;2:130. [PubMed] [Google Scholar]
- 40.Grant L (1974) The sticking and emigration of white blood cells in inflammation. In: Zeifach BW, Grant L, McCluskey RT (eds) The inflammatory response, vol 2, 2nd edn. Academic Press, New York, pp 233–239
- 41.Woodring JH (1997) Pulmonary bacterial and viral infections. In: Freundlich IM, Bragg DG (eds) A radiologic approach to diseases of the chest. Williams and Wilkins, Baltimore, p 436
- 42.Franquet Eur Respir J. 2001;18:196. doi: 10.1183/09031936.01.00213501. [DOI] [PubMed] [Google Scholar]
- 43.Shah J Thorac Imaging. 2002;17:53. doi: 10.1097/00005382-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Kim Radiographics. 2002;22:137. [Google Scholar]
- 45.Wong Radiology. 2003;228:401. [Google Scholar]
- 46.Wong Radiology. 2003;228:395. [Google Scholar]
- 47.Genereux Semin Roentgenol. 1980;15:9. doi: 10.1016/0037-198x(80)90035-8. [DOI] [PubMed] [Google Scholar]
- 48.Fraser RS, Müller NL, Colman N, Pare PD (1999) General features of pulmonary infection. In: Fraser and Pare’s diagnosis of the chest. Saunders, Philadelphia, pp 697–733
- 49.Barnes Aust NZ J Med. 1988;18:130. [Google Scholar]
- 50.Reittner Eur Radiol. 2003;13:515. doi: 10.1007/s00330-002-1490-3. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka J Comput Assist Tomogr. 1996;20:600. doi: 10.1097/00004728-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 52.Heitzman ER (1993) Radiologic diagnosis of acute pneumonia in adults: a contemporary review emphasizing radiologic–pathologic correlations. In: Potchen J, Grainger R, Greene R (eds) Pulmonary radiology. Saunders, Philadelphia, pp 210–226
- 53.Reittner AJR. 2000;174:37. [Google Scholar]
- 54.Macfarlane Thorax. 1996;51:539. doi: 10.1136/thx.51.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itho Am J Roentgenol. 1978;130:223. doi: 10.2214/ajr.130.2.223. [DOI] [PubMed] [Google Scholar]
- 56.Rosmus J Can Assoc Radiol. 1968;19:74. [PubMed] [Google Scholar]
- 57.Kim AJR. 1999;172:113. doi: 10.2214/ajr.172.1.9888749. [DOI] [PubMed] [Google Scholar]
- 58.Aquino J Comput Assist Tomogr. 1998;22:795. doi: 10.1097/00004728-199809000-00024. [DOI] [PubMed] [Google Scholar]
- 59.Umans Eur Radiol. 2001;11:990. doi: 10.1007/s003300000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruckner J Thorac Imaging. 1990;5:28. doi: 10.1097/00005382-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Herold Radiology. 1989;173:717. doi: 10.1148/radiology.173.3.2813776. [DOI] [PubMed] [Google Scholar]
- 62.Raby Radiology. 1990;174:713. doi: 10.1148/radiology.174.3.2406779. [DOI] [PubMed] [Google Scholar]
- 63.Huang AJR. 1989;153:41. [Google Scholar]
- 64.Kuhlman Radiology. 1985;157:611. doi: 10.1148/radiology.157.3.3864189. [DOI] [PubMed] [Google Scholar]
- 65.Herold CJ, Mostbeck G, Kramer J, Schwarzinger I, Wrba F, Haller J, Tscholakoff D. Invasive pulmonary aspergillosis: radiologic and magnetic resonance tomographic characteristics. RöFo. 1990;153:569–574. doi: 10.1055/s-2008-1033440. [DOI] [PubMed] [Google Scholar]
- 66.Kwong Chest. 1996;110:339. doi: 10.1378/chest.110.2.339. [DOI] [PubMed] [Google Scholar]
- 67.Eisenhuber Radiology. 2002;222:771. doi: 10.1148/radiol.2223991980. [DOI] [PubMed] [Google Scholar]
- 68.Aquino J Comput Assist Tomogr. 1996;20:594. doi: 10.1097/00004728-199607000-00018. [DOI] [PubMed] [Google Scholar]
- 69.Ikezoe AJR. 1992;159:1175. doi: 10.2214/ajr.159.6.1442377. [DOI] [PubMed] [Google Scholar]
- 70.Gefter Radiology. 1981;140:313. doi: 10.1148/radiology.140.2.7255704. [DOI] [PubMed] [Google Scholar]
- 71.Strain Crit Care Med. 1985;13:534. doi: 10.1097/00003246-198507000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Shevland Br J Radiol. 1983;56:531. doi: 10.1259/0007-1285-56-668-531. [DOI] [PubMed] [Google Scholar]
- 73.Daley J Am Med Assoc. 1988;260:3617. doi: 10.1001/jama.260.24.3617. [DOI] [Google Scholar]
- 74.Conces AJR. 1989;152:3. [Google Scholar]
- 75.Sanchez-Nieto Am J Resp Crit Care Med. 1998;157:371. doi: 10.1164/ajrccm.157.2.97-02039. [DOI] [PubMed] [Google Scholar]
- 76.Leutner AJR. 2000;175:391. [Google Scholar]
- 77.Mittl Am J Respir Crit Care Med. 1994;149:630. doi: 10.1164/ajrccm.149.3.8118630. [DOI] [PubMed] [Google Scholar]
- 78.Israel Med Clin North Am. 1956;40:853. doi: 10.1016/s0025-7125(16)34506-0. [DOI] [PubMed] [Google Scholar]


