Abstract
In this paper, a susceptible-vaccinated-exposed-infectious-recovered (SVEIR) epidemic model for an infectious disease that spreads in the host population through horizontal transmission is investigated, assuming that the horizontal transmission is governed by an unspecified function . The role that temporary immunity (vaccinated-induced) and treatment of infected people play in the spread of disease, is incorporated in the model. The basic reproduction number is found, under certain conditions on the incidence rate and treatment function. It is shown that the model exhibits two equilibria, namely, the disease-free equilibrium and the endemic equilibrium. By constructing a suitable Lyapunov function, it is observed that the global asymptotic stability of the disease-free equilibrium depends on as well as on the treatment rate. If , then the endemic equilibrium is globally asymptotically stable with the help of the Li and Muldowney geometric approach applied to four dimensional systems. Numerical simulations are also presented to illustrate our main results.
Keywords: Epidemic model, Reproduction number, Lyapunov function, Geometric approach, Global stability, Susceptible–Vaccinated–Exposed–Infectious–Recovered
Introduction
Mathematical modeling enjoys popularity in both preventing and controlling infectious diseases such as severe acute respiratory syndrome (SARS) [1], human immunodeficiency virus infection/acquired immune deficiency syndrome (HIV/AIDS) [2], H5N1 (avian flu) [3] and H1N1 (swine flu)[4]. In recent years, a lot of efforts have been made to develop realistic diseases and further study the asymptotic behavior of such epidemic models [5]. In the field of studying epidemic model behavior, one of the most important parts is to analyze steady states together with their stability [6]. In general, there are two distinct techniques named Lyapunov’s direct method and Li–Muldowney’s geometric approach to give sufficient conditions of global stability for the equilibrium states (see, for example, [7–14]). We would like to mention some related work concerned with the existence of positive solutions for the discrete fractional boundary value problem [15], the sensitivity analysis for optimal control problems governed by nonlinear evolution inclusions [16] and the nonexistence of global in time solution of the mixed problem for the nonlinear evolution equation with memory generalizing the Voigt–Kelvin rheological model [17].
It is well known that the rate of incidence plays the main part in modeling infectious diseases. The rise and fall of epidemics can be influenced by some factors, such as density of population and life style [18, 19]. Many researchers have adopted different nonlinear incidence rates in their works. For more details, we refer the reader to [8–14, 20–34] and the references therein. When it comes to control of a disease, it is generally known that the spread of many diseases can be prevented by vaccinating. When massive vaccination is impossible, the second stage of defensive mechanism could be medical treatment. Individuals need to bear in mind that the treatment is an indispensable way to take precautions for some diseases (for instance, measles, phthisis and influenza). In recent years, many treatment functions have been introduced by several authors to study some epidemic models under different conditions (see, for instance, [12, 14, 27, 31, 35–38]).
Recently, Dénes and Röst [27] investigated the following SI model:
| 1.1 |
where a population of constant size (assumed to be equal to 1) is divided into three compartments: susceptible (denoted by S), infected (denoted by I) and recovered (denoted by R). The transmission of the infection is governed by the incidence rate and μ is birth rate as well as the death rate of the susceptible class. The nonlinear function denotes the sum of the death rate and the recovery rate for the infected individuals satisfying and for . Using a Dulac function approach, which aims at eliminating the existence of the periodic solution and proving the global stability by the Poincaré-Bendixson theorem (see [39], p. 54), they obtained the global stability of the disease-free equilibrium and the endemic equilibrium for system (1.1).
Very recently, Upadhyay et al.[12] considered the following e-epidemic model:
| 1.2 |
with initial conditions: , , , and . All the parameters in model (1.2) are positive and are defined as follows: S, E, I, R and V represent the number of susceptible nodes, exposed nodes, infectious nodes, recovered nodes and vaccinated nodes at time t, respectively; A is the recruitment rate of new nodes, c is the half saturation constant for susceptible nodes S, α is the contact rate or the rate of transfer of virus from an infectious node to the susceptible node, η is rate at which the vaccinated nodes lose their immunity and join the susceptible class, β is the maximal treatment capacity of a network, is the natural crashing rate of nodes all classes, a is the half saturation constant for an infected node I, μ is the vaccination rate coefficient, is the virus induced crashing rate and , are the state transition rates. Using a Lyapunov function and a geometric approach, they obtained the global stability of virus-free equilibrium and endemic equilibrium for system (1.2).
As pointed out by Liu and Yang [11], due to the high similarity between computer virus and biological virus, it is acceptable to establish dynamical models describing biological virus among a population by modifying an e-epidemic model. Thus, it is interesting and important to extend model (1.2) to study the biological virus in the infectious disease.
Inspired by these research results above, in this paper, we consider the following system with five compartments:
| 1.3 |
where , , , , are the number of susceptible population, exposed population, infective population, recovered population, vaccinated population, respectively. The two-variable function represents incidence rate and the nonlinear function denotes the removal rate of infective individuals because of the treatment of infective. The initial conditions for system (1.3) are as follows:
| 1.4 |
Clearly, denotes the total number of high-risk human population at time t.
The model parameters of system (1.3) are described as follows:
- A:
the rate at which new individuals (including newborns and immigrants) enter the susceptible population,
- :
natural death rate of population all classes,
- η:
the rate at which the vaccinated population lose their immunity and join the susceptible class,
- μ:
vaccination rate coefficient,
- :
the rate at which exposed population become infective,
- :
natural recovery rate of infective population,
- :
disease-related death rate of infective population.
Model (1.3) involves certain assumptions which consist of the following:
-
(i)
The new individuals enter the population with a constant rate and all the new individuals are susceptible.
-
(ii)
Susceptible individuals move to exposed class by adequate contact with infective individuals and after some time (i.e., latency period), they become infectious and move to infectious class.
-
(iii)
The infectious individuals are assumed to leave the infectious class as a result of natural death and disease-related death as well as recovery of infected individuals.
-
(iv)
After recovery the individuals become immunized and hence they are no longer susceptible to it.
-
(v)
It is assumed that a fraction of susceptible individuals get vaccinated and join the vaccinated class. A part of vaccinated individuals may lose their immunity and rejoin the susceptible class.
It is easy to see that system (1.3) includes (1.1) and (1.2) as special cases and so model (1.3) provides a uniform setting for the computer virus and biological virus studies. Following the classical assumptions [27, 40], it is reasonable to suppose that the transmission of the infection is governed by an incidence rate in model (1.3). Moreover, as pointed out by Wang [31], the recovery rate is naturally dependent on the number of infected individuals provided the health care resources are constrained and so it is natural to use the nonlinear function as the treatment function in model (1.3).
The main purpose of this paper is to derive the expression for the basic reproduction number and further show the global stability of disease-free as well as endemic equilibria by the aid of Lyapunov function and Li–Muldowney geometric approach applied to four dimensional systems. This paper is organized as follows. In Sect. 2, some elementary assumptions on the functions f and g will be given, and the basic reproduction number is provided. Also the equilibrium points are discussed. The global stability of disease-free equilibrium and endemic equilibrium are analyzed in Sects. 3 and 4, respectively. All our important analytical findings are numerically verified with the help of Mathlab in Sect. 5. Finally, a brief conclusion is given in Sect. 6.
Basic reproduction number and equilibrium
To define the basic reproduction number and indicate the existence of equilibrium, we give some hypotheses.
- is differentiable such that
- for all ;
- for all ;
- for all and ;
- for all ;
- for all .
is differentiable such that , and for .
holds for all , where for .
Remark 2.1
- It is easy to check that the classes of satisfying (H1) include incidence rates such as
for and . - It is straightforward to show that the classes of satisfying (H2) include removal rates such as
for , , . By hypothesis (H2), we know that is a monotone decreasing function on .
- The assumption (H3) is equivalent to the following inequality:
which can be found in [27]. - By the assumptions, it is easy to find that system (1.3) always has a disease-free equilibrium point , where
We shall assume that (H1), (H2) and (H3) hold in the rest of this paper.
Now we define the basic reproduction number for model (1.3) as follows:
where
In order to find the positive equilibria of model (1.3), set
| 2.1 |
It follows that and .
Substituting the above equalities into the second equation in (2.1), one has
Let
| 2.2 |
Then it is easy to see that the positive equilibrium points of system (2.1) are given by zeros of F in the interval .
We denote for convenience. Then it is easy to see
Therefore, we conclude that has at least one root named Ĩ in the interval . That is, and so
Since , we know that and so
If , then system (2.1) has a positive equilibrium point , where
In the following, we show that is the unique positive equilibrium point of system (2.1). For any positive equilibrium point , by (2.2) and hypotheses (H1) and (H2), we have
| 2.3 |
Since and hypotheses (H1) and (H2) hold, we have
| 2.4 |
Since and g is differentiable on , there exists such that . By using hypotheses (H2) and (H3), one has
| 2.5 |
Thus, it follows from (2.3), (2.4) and (2.5) that .
Suppose that there exists another positive equilibrium point . Then due to the property of continuous function. This is a contradiction. Therefore, system (2.1) has a unique endemic equilibrium when . It can be stated as follows.
Theorem 2.1
System (1.3) has a disease-free equilibrium as follows:
which exists for all parameter values. For , the endemic equilibrium admits the unique positive equilibrium point for system (1.3).
Remark 2.2
From the proof of the existence of endemic equilibrium , it is not difficult to arrive at such a conclusion that the nonlinear treatment function has an upper bound , which is reasonable for limited medical resources in our daily life.
Proposition 2.1
The set
is a positively invariant and attracting region for the disease transmission model given by system (1.3) with initial conditions (1.4).
Proof
Summing up the five equations in system (1.3) and denoting
we get
i.e.,
Now integrating both sides of the above inequality and using the theory of differential inequality [41], we obtain
Clearly, as . If , then . Thus, the set Ω is positive-invariant, that is, all initial solutions belong to Ω remain in Ω for all . □
Global stability of the disease-free equilibrium by means of Lyapunov function
In this section, we investigate the global stability of the disease-free equilibrium for system (1.3).
Theorem 3.1
If , then the disease-free equilibrium of system (1.3) is globally asymptotically stable in the feasible region Ω. If , then is unstable.
Proof
The Jacobian matrix of system (1.3) at is
Obviously, is an eigenvalue of . The other eigenvalues of are determined by the equations
and
respectively. If , then one eigenvalue is positive. Thus, is unstable.
When , to prove the disease-free equilibrium is globally asymptotically stable, we consider the Lyapunov function . The derivative of along system (1.3) satisfies
Furthermore, iff . Thus, the largest compact invariant set in , when , is the singleton . By the LaSalle invariance principle theorem ([42], p. 30), the disease-free equilibrium is globally asymptotically stable if . This completes the proof. □
Global stability of the endemic equilibrium by means of geometric approach
In this section, we analyze the stability of the endemic equilibrium . First, we show the local stability of the endemic equilibrium of system (1.3) around the endemic equilibrium .
Theorem 4.1
If , then the endemic equilibrium exists and is locally asymptotically stable if the following conditions hold:
-
(i)
;
-
(ii)
;
-
(iii)
and . Here all the parameters , , , , are defined in (4.1). The values of h and z equal to and , respectively.
Proof
The Jacobian matrix of system (1.3) at is given by
where
| 4.1 |
Clearly, one of the roots of is negative, i.e. . The remaining roots can be determined from the following equation:
where
Using assumptions (i) and (ii), by a direct calculation, we have for . It follows from the Routh–Hurwitz criteria [43] that all the eigenvalues associated to have negative real parts iff for and .
Now,
if (iii) holds. This ends the proof. □
To find the global stability of system (1.3), it is necessary to reduce system (1.3) first. Since recovered class R does not have any effect on the dynamics of S, V, E and I class, we shall investigate the following system:
| 4.2 |
The solutions of (4.2) corresponding to nonnegative initial values remain nonnegative for all time. Moreover, we observe that the total population size of (4.2) denoted by satisfies , so that we can study the model in the region:
Here we follow the approach used in [8] for a SVEIR model of SARS epidemic spread.
Let us consider the following autonomous dynamical system:
| 4.3 |
where , which is an open set, simply connected and . Suppose that is an equilibrium point of (4.3), i.e. . Therefore, is said to be globally stable in D if it is locally stable and all trajectories in D converge to .
Let be a matrix-valued function of order that is on D. We also consider the matrix A which is defined as
where the matrix is
and the matrix M is the second additive compound matrix of the Jacobian matrix J. Further the Lozinskiĭ measure μ̄ of A with respect to a vector norm can be defined in as follows:
We will apply the following theorem according to [44].
Lemma 4.1
If is a compact absorbing subset in the interior of D, and there exist and a Lozinskiĭ measure for all , then every omega limit point of system (4.2) in the interior of D is an equilibrium in .
Theorem 2.1 states that implies the existence and uniqueness of the endemic equilibrium . Further, we know that the disease-free equilibrium is unstable when . The instability of , together with , which implies the uniform persistence of the state variables (see [45]). Thus, there exists a constant such that any solution with in the orbit of system (4.2) satisfies
The uniform persistence of system (4.2), incorporating the boundedness of Θ, suggests that the compact absorbing set in the interior of Θ; see [46]. Hence, Lemma 4.1 may be applied, with .
According to [47], the Lozinskiĭ measure in Lemma 4.1 can be evaluated as:
where is the right-hand derivative. The endemic equilibrium is locally asymptotically stable, provided . Hence, to get the global asymptotic stability, according to Lemma 4.1, the trick of the proof is to find a norm such that for all x in the interior of Θ.
Starting with the Jacobian matrix J of (4.2),
the second additive compound matrix is given by
Hence, the second additive compound matrix of J is given as follows:
where
Now we consider the following matrix:
| 4.4 |
Then we obtain the matrix , where is the derivative of Q in the direction of the vector field f. More accurately, we have
Hence, in view of the fact that
we obtain
where
As in [8], we consider the following norm on :
| 4.5 |
where , with components , , and is defined as:
and is defined as
In the next, we will use the following inequalities:
and
Furthermore, we assume that
| 4.6 |
We will use the inequalities mentioned above to get the estimates on .
Theorem 4.2
For , system (4.2) admits an unique endemic equilibrium which is globally asymptotically stable in the interior of Θ, provided that inequality (4.6) is satisfied and that
| 4.7 |
for some positive constant ω, where
Proof
The basic idea of the proof is to obtain the estimate of the right derivate of the norm (4.5). For this purpose, we need to discuss sixteen cases according to the different orthants and the definition of the norm (4.5) within each orthant.
Case 1:
| 4.8 |
This shows that
By using and , it follows from (4.8) that
Case 2:
| 4.9 |
Thus, we have
Using and , in view of (4.9), one has
The discussion for the other fourteen cases are similar to the ones discussed in [7] and so we omit it here. Thus, we can get the following estimate:
where
Now the global stability follows from Lemma 4.1. □
Remark 4.1
As pointed out by Buonomo and Lacitignola [7], in some real situations, different choices of the matrix Q and of the vector norm may lead to better sufficient conditions than those we presented here, in the sense that the assumptions on the parameters may be weakened. Thus, it is worth to note that sufficient conditions (4.6) and (4.7) in Theorem 4.2 are derived from the application of the method and numerical simulations suggest that they may be not necessary (see Example 5.1).
Numerical simulations
The aim of this section is to give a numerical example to illustrate our main results.
Example 5.1
Consider the system
| 5.1 |
which is a particular case of system (1.3) by letting and , where m, n, γ, a are positive and . The other parameters in (5.1) have the same biological meanings as in model (1.3).
We first consider the case when
by using the parameter values given in Table 1. Using these parameter values, for different initial conditions the dynamics of model (5.1) is presented in Figs. 1–5. It shows that system (5.1) has a disease-free equilibrium and it is globally asymptotically stable. This numerical verification supports the result stated in Theorem 3.1.
Figure 2.

Time series plot of the exposed population for with various initial conditions, parameter values are given in Table 1
Figure 3.
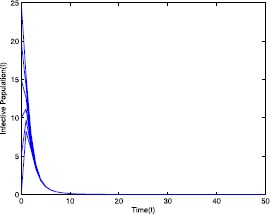
Time series plot of the infective population for with various initial conditions, parameter values are given in Table 1
Figure 4.

Time series plot of the recovered population for with various initial conditions, parameter values are given in Table 1
Table 1.
| Parameter | Values |
|---|---|
| A | 2 |
| 0.2 | |
| m | 0.2 |
| n | 2 |
| η | 0.2 |
| μ | 0.4 |
| 0.8 | |
| 0.5 | |
| 0.55 | |
| γ | 0.3 |
| a | 2 |
Figure 1.

Time series plot of the susceptible population for with various initial conditions, parameter values are given in Table 1
Figure 5.

Time series plot of the vaccinated population for with various initial conditions, parameter values are given in Table 1
Next, we consider the case when using the parameter values given in Table 2. Using these parameter values, for different initial conditions the dynamics of model (5.1) is presented in Figs. 6–10. It shows that system (5.1) has an endemic equilibrium and it is globally asymptotically stable with different initial values, which supports our analytical results stated in Theorem 4.2.
Figure 7.
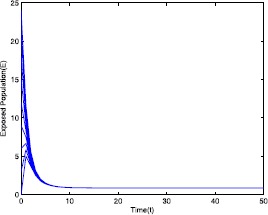
Time series plot of the exposed population for with various initial conditions, parameter values are given in Table 2
Figure 8.

Time series plot of the infective population for with various initial conditions, parameter values are given in Table 2
Figure 9.
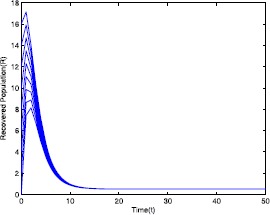
Time series plot of the recovered population for with various initial conditions, parameter values are given in Table 2
Table 2.
| Parameter | Values |
|---|---|
| A | 6 |
| 0.5 | |
| m | 0.8 |
| n | 2 |
| η | 0.2 |
| μ | 0.4 |
| 0.8 | |
| 0.5 | |
| 0.55 | |
| γ | 0.3 |
| a | 2 |
Figure 6.
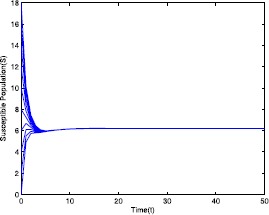
Time series plot of the susceptible population for with various initial conditions, parameter values are given in Table 2
Figure 10.
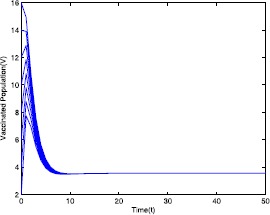
Time series plot of the vaccinated population for with various initial conditions, parameter values are given in Table 2
Conclusions
In this paper, we have considered an SVEIR epidemic model with general nonlinear incidence rate. In model (1.3), we have divided the total population into five compartments, namely susceptible, exposed, infective, recovered, vaccinated population and investigated the dynamical behavior of this model. Here, we have found that
is a basic reproduction number of system (1.3), which helps us to determine the dynamical behavior of the system. We have showed that system (1.3) to be globally asymptotically stable at disease-free equilibrium when
When , the endemic equilibrium stable both locally and globally has been derived and analyzed under some conditions. The important mathematical findings for the dynamical behavior of model (1.3) have also numerically been verified for a special case of model (1.3). We would like to point out that the model considered in this paper is not a case study and so it is difficult to choose parameter values from quantitative estimation. We have used hypothetical sets of parameters to verify our analytical results. It is worth to mention that the results presented in this paper improve and extend some related results in [9, 10, 12, 13].
Finally, we remark that there are quite a few spaces to deserve further investigation. For example, we can continue the research in this line considering the vaccination rate μ in our model (1.3) as a continuous function, and, later, a discontinuous function. On the other hand, as is well known, epidemiological models which incorporate the control strategies can be useful to both control the spread of disease and minimize the intervention costs. For our model, it is natural to consider vaccination rate coefficient as a control to reduce the disease burden. Thus, it is important and interesting to prove the existence of optimal control, characterize the optimal control, prove the uniqueness of optimal control, compute the optimal control numerically and investigate how the optimal control depends on various parameters in the models. We will devote to these questions our future work.
Acknowledgments
Acknowledgements
We would like to thank the editors and referees for their valuable comments and suggestions to improve our paper.
Availability of data and materials
Not applicable.
Abbreviations
- (SVEIR)
Susceptible–Vaccinated–Exposed–Infectious–Recovered
Authors’ contributions
All authors contributed equally to this work. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (11371015, 11471230, 11671282) and the Natural Science Foundation of Sichuan Provincial Education Department (Grant Nos. 18ZB0581, 14ZB0142) and the Meritocracy Research Funds of China West Normal University (17YC373).
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nan-jing Huang, Email: nanjinghuang@hotmail.com.
Cong Zhang, Email: congmike@163.com.
References
- 1. Naheed, A., Singh, M., Lucy, D.: Numerical study of SARS epidemic model with the inclusion of diffusion in the system. Appl. Math. Comput. 229, 480–498 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Billarda, L., Dayananda, P.W.A.: A multi-stage compartmental model for HIV-infected individuals: I waiting time approach. Math. Biosci. 249, 92–101 (2014) [DOI] [PubMed] [Google Scholar]
- 3. Upadhyay, R.K., Kumari, N., Rao, V.S.H.: Modeling the spread of bird flu and predicting outbreak diversity. Nonlinear Anal., Real World Appl. 9, 1638–1648 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pongsumpun, P., Tang, I.M.: Dynamics of a new strain of the H1N1 influenza a virus incorporating the effects of repetitive contacts. Comput. Math. Methods Med. 2014, Article ID 487974 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou, X., Cui, J.: Analysis of stability and bifurcation for an SEIV epidemic model with vaccination and nonlinear incidence rate. Nonlinear Dyn. 63, 639–653 (2011) [Google Scholar]
- 6. Kyrychko, Y., Blyuss, K.: Global properties of a delayed SIR model with temporary immunity and nonlinear incidence rate. Nonlinear Anal., Real World Appl. 6, 495–507 (2005) [Google Scholar]
- 7. Buonomo, B., Lacitignola, D.: Global stability for a four dimensional epidemic model. Note Mat. 30, 81–93 (2010) [Google Scholar]
- 8. Gumel, A.B., McCluskey, C.C., Watmough, J.: An SVEIR model for assessing potential impact of imperfect anti-SARS vaccine. Math. Biosci. Eng. 3, 485–512 (2006) [DOI] [PubMed] [Google Scholar]
- 9. Khan, M.A., Badshah, Q., Islam, S., et al.: Global dynamics of SEIRS epidemic model with non-linear generalized incidences and preventive vaccination. Adv. Differ. Equ. 2015, 88 (2015). 10.1186/s13662-015-0429-3 [Google Scholar]
- 10. Khan, M.A., Khan, Y., Khan, S., et al.: Global stability and vaccination of an SEIVR epidemic model with saturated incidence rate. Int. J. Biomath. 9, 59–83 (2016). 10.1142/s1793524516500686 [Google Scholar]
- 11. Liu, X., Yang, L.: Stability analysis of an SEIQV epidemic model with saturated incidence rate. Nonlinear Anal., Real World Appl. 13, 2671–2679 (2012) [Google Scholar]
- 12. Upadhyay, R.K., Kumari, S., Misra, A.K.: Modeling the virus dynamics in computer network with SVEIR model and nonlinear incident rate. J. Appl. Math. Comput. 54, 485–509 (2017) [Google Scholar]
- 13. Yang, Y., Zhang, C.M., Jiang, X.Y.: Global stability of an SEIQV epidemic model with general incidence rate. Int. J. Biomath. 8, 103–115 (2015). 10.1142/S1793524515500205 [Google Scholar]
- 14. Zhang, X., Liu, X.N.: Backward bifunction of an epidemic model with saturated treatment. J. Math. Anal. Appl. 348, 433–443 (2008) [Google Scholar]
- 15. Chidouh, A., Torres, D.: Existence of positive solutions to a discrete fractional boundary value problem and corresponding Lyapunov-type inequalities. Opusc. Math. 38, 31–40 (2018) [Google Scholar]
- 16. Papageorgiou, N.S., Radulescu, V.D., Repovs, D.D.: Sensitivity analysis for optimal control problems governed by nonlinear evolution inclusions. Adv. Nonlinear Anal. 6, 199–235 (2017) [Google Scholar]
- 17. Pukach, P., Il’kiv, V., Nytrebych, Z., Vovk, M.: On nonexistence of global in time solution for a mixed problem for a nonlinear evolution equation with memory generalizing the Voigt–Kelvin rheological model. Opusc. Math. 37, 735–753 (2017) [Google Scholar]
- 18. Xu, R., Ma, Z.: Global stability of a delayed SEIRS epidemic model with saturation incidence rate. Nonlinear Dyn. 61, 229–239 (2010) [Google Scholar]
- 19. Alexander, M., Moghadas, S.: Bifurcation analysis of an SIRS epidemic model with generalized incidence. SIAM J. Appl. Math. 65, 1794–1816 (2005) [Google Scholar]
- 20. Bianca, C., Pennisi, M., Motta, S., et al.: Immune system network and cancer vaccine. AIP Conf. Proc. 1389, 945–948 (2011). 10.1063/1.3637764 [Google Scholar]
- 21. Bianca, C., Pappalardo, F., Motta, S., et al.: Persistence analysis in a Kolmogorov-type model for cancer-immune system competition. AIP Conf. Proc. 1558, 1797–1800 (2013). 10.1063/1.4825874 [Google Scholar]
- 22. Cai, L.M., Li, X.Z.: Analysis of a SEIV epidemic model with a nonlinear incidence rate. Appl. Math. Model. 33, 2919–2926 (2009) [Google Scholar]
- 23. Capasso, V., Serio, G.: A generalization of the Kermack-McKendrick deterministic epidemic model. Math. Biosci. 42, 43–61 (1978) [Google Scholar]
- 24. Capasso, V.: Global solution for a diffusive nonlinear deterministic epidemic model. SIAM J. Appl. Math. 35, 274–284 (1978) [Google Scholar]
- 25. Capasso, V.: Mathematical Structures of Epidemic Systems. Lecture Notes in Biomathematics, vol. 97. Springer, Berlin (1993) [Google Scholar]
- 26. Ghergu, M., Radulescu, V.D.: Nonlinear PDEs, Mathematical Models in Biology, Chemistry and Population Genetics. Springer Monographs in Mathematics. Springer, Heidelberg (2012) [Google Scholar]
- 27. Dénes, A., Röst, G.: Global stability for SIR and SIRS models with nonlinear incidence and removal terms via dulac functions. Discrete Contin. Dyn. Syst., Ser. B 21, 1101–1117 (2016) [Google Scholar]
- 28. Hu, Z.X., Bi, P., Ma, W.B., et al.: Bifurcations of an SIRS epidemic model with nonlinear incidence rate. Discrete Contin. Dyn. Syst., Ser. B 15, 93–112 (2012) [Google Scholar]
- 29. Liu, W., Levin, S.A., Iwasa, Y.: Influence of nonlinear incidence rates upon the behavior of SIRS epidemiological models. J. Math. Biol. 23, 187–204 (1986) [DOI] [PubMed] [Google Scholar]
- 30. Ruan, S.G., Wang, W.D.: Dynamical behavior of an epidemic model with a nonlinear incidence rate. J. Differ. Equ. 188, 135–163 (2003) [Google Scholar]
- 31. Wang, W.D.: Backward bifurcation of an epidemic model with treatment. Math. Biosci. 201, 58–71 (2006) [DOI] [PubMed] [Google Scholar]
- 32. Wang, F., Zhang, Y., Wang, C., et al.: Stability analysis of a SEIQV epidemic model for rapid spreading worms. Comput. Secur. 29, 410–418 (2010) [Google Scholar]
- 33. Xiao, D., Ruan, S.G.: Global analysis of an epidemic model with nonmonotone incidence rate. Math. Biosci. 208, 419–429 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang, Y., Xiao, D.: Influence of latent period and nonlinear incidence rate on the dynamics of SIRS epidemidogical models. Discrete Contin. Dyn. Syst., Ser. B 13, 195–211 (2010) [Google Scholar]
- 35. Gao, D.P., Huang, N.J.: A note on global stability for a tuberculosis model. Appl. Math. Lett. 73, 163–168 (2017) [Google Scholar]
- 36. Gao, D.P., Huang, N.J.: Optimal control analysis of a tuberculosis model. Appl. Math. Model. 58, 47–64 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laarabi, H., Abta, A., Hattaf, K.: Optimal control of a delayed SIRS epidemic model with vaccination and treatment. Acta Biotheor. 63, 87–97 (2015) [DOI] [PubMed] [Google Scholar]
- 38. Wang, W.D., Ruan, S.G.: Bifurcation in an epidemic model with constant removal rate of the infectives. J. Math. Anal. Appl. 291, 775–793 (2004) [Google Scholar]
- 39. Hale, J.K.: Ordinary Differential Equations, 2nd edn. Krieger, Melbourne (1980) [Google Scholar]
- 40. Korobeinikov, A.: Lyapunov functions and global stability for SIR and SIRS epidemiological models with nonlinear transmission. Bull. Math. Biol. 30, 615–626 (2006) [DOI] [PubMed] [Google Scholar]
- 41. Brikhoff, G., Rota, G.C.: Ordinary Differential Equations. Ginn, Boston (1982) [Google Scholar]
- 42. LaSalle, J.P.: The Stability of Dynamical Systems. Regional Conference Series in Applied Mathematics. SIAM, Philadephia (1976) [Google Scholar]
- 43. Kot, M.: Elements of Mathematical Ecology. Cambridge University Press, Cambridge (2001) [Google Scholar]
- 44. Li, M.Y., Muldowney, J.S.: On R.A. Smith’s autonomous convergence theorem. Rocky Mt. J. Math. 25, 365–378 (1995) [Google Scholar]
- 45. Freedman, H.I., Ruan, S.G., Tang, M.X.: Uniform persistence and flows near a closed positively invariant set. J. Differ. Equ. 6, 583–600 (1994) [Google Scholar]
- 46. Hutson, V., Schmitt, K.: Permanence and the dynamics of biological systems. Math. Biosci. 111, 1–71 (1992) [DOI] [PubMed] [Google Scholar]
- 47. Martin, R.H.: Logarithmic norms and projections applied to linear differential systems. J. Math. Anal. Appl. 45, 432–454 (1974) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


