Abstract
Purpose
To provide current knowledge on respiratory virus-induced heterologous immunity (HI) with a focus on humoral and cellular cross-reactivity. Adaptive heterologous immune responses have broad implications on infection, autoimmunity, allergy and transplant immunology. A better understanding of the mechanisms involved might ultimately open up possibilities for disease prevention, for example by vaccination.
Methods
A structured literature search was performed using Medline and PubMed to provide an overview of the current knowledge on respiratory-virus induced adaptive HI.
Results
In HI the immune response towards one antigen results in an alteration of the immune response towards a second antigen. We provide an overview of respiratory virus-induced HI, including viruses such as respiratory syncytial virus (RSV), rhinovirus (RV), coronavirus (CoV) and influenza virus (IV). We discuss T cell receptor (TCR) and humoral cross-reactivity as mechanisms of HI involving those respiratory viruses. Topics covered include HI between respiratory viruses as well as between respiratory viruses and other pathogens. Newly developed vaccines, which have the potential to provide protection against multiple virus strains are also discussed. Furthermore, respiratory viruses have been implicated in the development of autoimmune diseases, such as narcolepsy, Guillain-Barré syndrome, type 1 diabetes or myocarditis. Finally, we discuss the role of respiratory viruses in asthma and the hygiene hypothesis, and review our recent findings on HI between IV and allergens, which leads to protection from experimental asthma.
Conclusion
Respiratory-virus induced HI may have protective but also detrimental effects on the host. Respiratory viral infections contribute to asthma or autoimmune disease development, but on the other hand, a lack of microbial encounter is associated with an increasing number of allergic as well as autoimmune diseases. Future research might help identify the elements which determine a protective or detrimental outcome in HI-based mechanisms.
Keywords: respiratory virus, cross-reactivity, adaptive immunity, autoimmunity, asthma
Introduction
Respiratory viruses, such as respiratory syncytial virus (RSV), rhinovirus (RV) and influenza virus (IV) frequently cause upper (URTI) and lower respiratory tract infections (LRTI). Such infections include the common cold, pneumonia, bronchitis and bronchiolitis.
Direct and indirect costs associated with viral respiratory tract infections other than IV add up to $40 billion annually in the USA [1]. The annual burden due to IV epidemics is estimated to be around $87 billion in the USA [2]. Seasonal IV epidemics affect about 1 billion of the global population and cause up to half a million deaths every year (WHO). A viral aetiology is found in ~70 % [3] of all common cold cases, while RV alone accounts for ~50 % [3]. Furthermore, RV was detected in 9 % of patients hospitalized for severe community-acquired pneumonia, i.e. more often than IV (6 %) or Streptococcus pneumoniae (5 %) in the same study [4].
Especially children, adults > 65 years of age and the chronically ill are at high risk of developing severe disease upon LRTI. Acute LRTI are one of the major causes of childhood mortality worldwide [5]. RSV and IV are among the main pathogens causing acute LRTI in children under 5 years with at least 53 million cases of acute- and 4,4 million cases of severe acute LRTI annually [6, 7].
Viral RTI, especially RV infection, frequently cause chronic obstructive pulmonary disease (COPD) [8] and asthma [9] exacerbations. Respiratory viruses have also been implicated in the development and persistence of asthma [9, 10] as well as the initiation of autoimmune disease [11]. Despite the large impact on society, treatment of these viral infections is mostly supportive.
We discuss respiratory virus-induced adaptive heterologous immune mechanisms in infections, autoimmunity and asthma. Specifically, we describe published data in the involved virus strains, implicated T/B cell epitopes and final outcome among others. A better understanding of heterologous immunity (HI) potentially leads to new therapeutic or preventive strategies for a range of immunologically mediated disorders.
Heterologous Immunity
HI is the altered immune response towards an antigen as a result of a preceding encounter with an unrelated antigen. Thus, immune memory is a central requirement for HI. Therefore, heterologous immune responses have exclusively been linked to the adaptive immune system. However, in recent years, innate immune memory has been described [12] and some vaccines have been associated with substantial innate heterologous effects [13]. Heterologous innate immune stimulation is a way to alter adaptive immune responses towards an antigen. This involves the induction of tolerance, Th polarization, substitution, breaking of tolerance or enhancement of adaptive immune cell responses, while maintaining antigen specificity (see Fig. 3) [14].
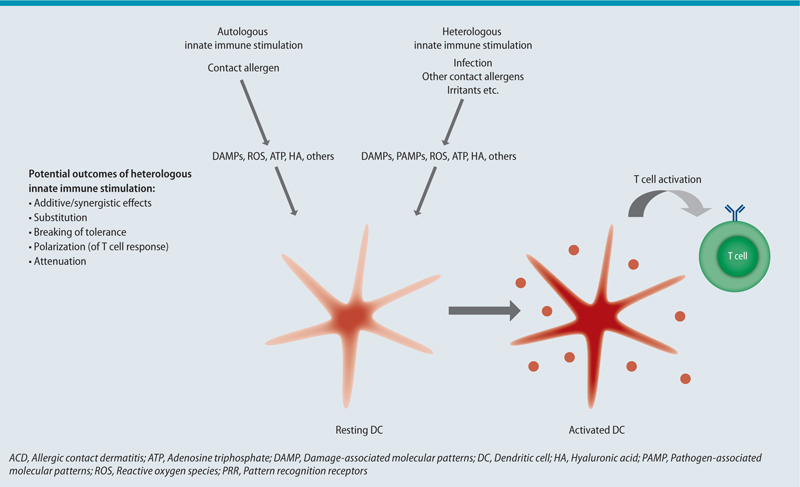
Both, B and T cells have been shown to mediate heterologous effects. Antibodies have been shown to protect from heterologous virus challenge [15]. On the other hand, antibodies induced by viral infection contribute to autoimmune disease [11] and possibly play a role in alloreactivity [16]. Evidence suggests that T cell receptor (TCR) cross-reactivity is common between respiratory viruses [17, 18, 19, 20], but it has also been shown between unrelated viruses [21, 22, 23] and even between viruses and other microbial species [23]. Cross-reactive T cells were shown to protect from heterologous virus challenge [18, 20]. Furthermore, pathogen-derived mimics of a tumor-associated antigen are able to enhance the T cell response towards the tumor antigen [24]. Therefore, pathogen-derived epitopes might be used in a tumor vaccine. HI also has detrimental effects on the host. For example, pre-existing T memory (Tm) cells can restrict the priming of protective naïve T cells to heterologous antigen [25]. Furthermore, pre-existing Tm cells can narrow the primary T cell response by shifting towards proliferation of high affinity clones only [26]. A narrowed T cell response may lead to escape variants and has been shown to be associated with severe disease progression [27, 28]. Furthermore, virus-mediated TCR cross-reactivity has also been shown to involve allo- [16] as well as autoantigens [11, 29]. Cross-reactive CD8+ T cells contributed to transplant rejection in many [16], although not all cases [30].
Unspecific activation of Tm cells has also been associated with HI in some settings. Different mechanisms have been suggested for unspecific T cell activation, e.g. IL-15 [31], IL-12 and IL-18 [32], type I interferons (IFN) [33] and type II IFN [34] signalling (Fig. 1). Bystander activated Tm cells can contribute to early pathogen control [32, 35]. Tissue resident memory (Trm) cells have an important role in pathogen clearance in the lungs. Since Trm stay at the site of infection after pathogen clearance, they provide rapid protection upon homologous virus challenge in mice [36] and humans [37]. Lung Trm were shown to protect from heterosubtypic IV challenge in mice [38, 39]. Of note, HI may alter the immunodominance, induce changes in Th polarisation or result in loss of specific Tm cells [28]. In addition, heterologous immune responses are not necessarily reciprocal [40].
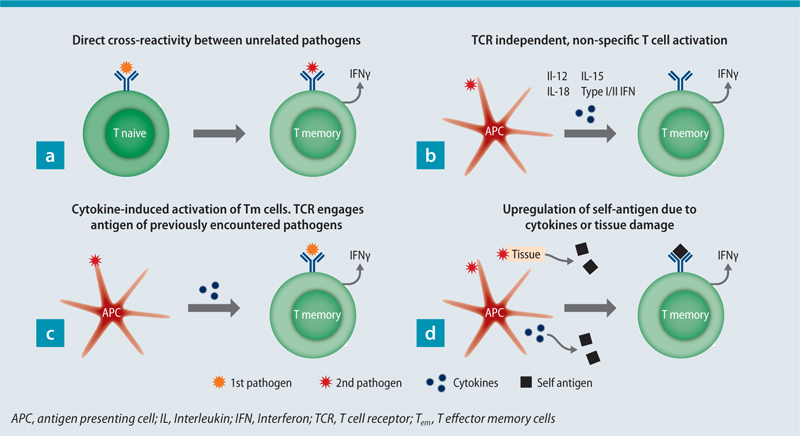
Cellular/humoral cross-reactivity
T cells are equipped with TCRs, with whom they sense their cognate antigen. Major histocompatibility complex (MHC) molecules present peptide antigen to T cells in the form of peptide-MHC (pMHC) complexes. MHC I molecules present peptides 8 to 14 mers of length [41]. MHC class II molecules are able to present even longer peptides. The estimated number of divergent TCRs in the human native T cell pool is < 108 [42], whereas the number of potential foreign peptides presented by MHC molecules is suggested to be > 1015 [41]. Taken together, broad TCR cross-reactivity is inevitable for sufficient immune protection [41, 43]. This theory is further supported by the finding that one TCR is able to recognize > 1 million different peptides presented by one MHC molecule [44]. Cross-reactivity is common between peptides with a high degree of sequence homology [23, 45, 46, 47], but also peptides with little homology are able to elicit cross-reactive immune responses [29, 48, 49, 50, 51]. Moreover, TCR cross-reactivity is restricted to peptides of the same length, when presented via MHC class I [52]. Cross-recognition between seemingly non-related peptides might occur due to hotspot binding, where the peptide-TCR interaction is focused on a hotspot, while tolerating substitutions in other positions [53].
B and plasma cells contribute to host protection by producing antibodies, which can neutralize pathogens and/or toxins. The recognition of antigen occurs at the binding cleft of the antibody, which is located in the fragment antigen binding (Fab) domain. The binding cleft contains multiple paratopes, which recognize B cell epitopes on antigens [54]. Therefore, all antibodies are potentially polyspecific [54], which might be necessary to provide sufficient immune protection against the majority of pathogens. B cell epitopes constitute of 15 amino acids on average [55] and most of them are, in contrast to Tscell epitopes, conformational or discontinuous epitopes [56]. In addition, hotspot recognition is also likely in antibody-antigen interaction [56].
Heterologous immunity between respiratory viruses
Coronaviruses (CoV)
Middle East respiratory syndrome (MERS)-CoV and severe acute respiratory syndrome (SARS)-CoV caused recurrent epidemics, which were associated with a high mortality. CD4+ and CD8+ T cells as well as antibodies have all been suggested to have protective effects against SARS-CoV infection [57]. Humoral cross-reactivity between SARS- and MERS-CoV was absent in several studies [58]. But recently, Tai et al [59] showed that immunization of mice with recombinant receptor binding-domain (rRBD) of the spike (S) protein from different MERS-CoV strains induced broadly neutralizing antibodies against up to 17 human and camel MERS-CoVs. Intranasal vaccination with a viral vaccine vector, which encodes a conserved SARS-CoV CD4+ T cell epitope protected mice from homologous and heterologous challenge with MERS-CoV. Protection was dependent on cross-reactive CD4+ T cells, producing IFNγ [60].
Influenza virus (IV)
CD4+ and CD8+ T cells generated in a preceding IV infection or vaccination are able to provide protection against heterosubtypic IV infection in humans [18, 20] or mice [17]. T cell cross-strain protection is due to recognition of conserved IV proteins. Seasonal IV vaccines generate strain-specific neutralizing antibodies against HA and NA, but fail to induce a significant cross-reactive response. Therefore, a major goal is to develop IV vaccines, which induce a cross-reactive T cell and/or antibody response.
One target might be the immunodominant human leukocyte antigen(HLA)-A2-M158 epitope, which is conserved over strains for many years, although mutations were detected [47]. Valkenburg et al [47] showed that M158-specific CD8+ T cells also recognized three naturally occurring M158-peptide variants. In addition, M158-specific Tem cells from unexposed adults lysed IV A H1N1 2009 pandemic (A(H1N1)pdm09) infected cells ex vivo [61]. Therefore, the M158-epitope is a potential target for a broadly IV protective vaccine.
Prime-boost vaccination with the licenced live attenuated influenza vaccine (LAIV) conferred enhanced protection against heterosubtypic IV A challenge compared to FluZone or control. Protection was dependent on CD4+/CD8+ T cells, which also protected against heterosubtypic challenge [62]. In addition, the 2014–2015 and 2015–2016 seasons LAIV vaccine induced lung CD4+ CD44+ CD62Llo CD69+ Trm cells in C57BL/6 mice [39]. Mice were protected against heterosubtypic challenge for up to 45 weeks [39]. LAIV vaccination was also shown to boost pre-existing cross-reactive T cells in 50 % of vaccinated children [63].
Vaccination with self-amplifying mRNA (SAM®) (GlaxoSmithKline, London, UK) in lipid nanoparticles, encoding for conserved internal IV A proteins (nucleoprotein [NP] and/or matrix protein 1 [M1]), induced proliferation of NP- and M1-specific CD4+ Th1 cells as well as NP147–155-specific CD8+ T cells in mice. All vaccinated mice survived heterosubtypic IV A challenge [64]. Evidence suggests that innate immune stimulation leads to a broader adaptive immune response [64]. A Toll-like receptor 2 (TLR2)-agonist together with a split IV vaccine, but not vaccine alone, protected mice against homologous and heterologous virus challenge. Heterologous effects were dependent on CD8+ T cells specific for NP147–155 [65].
The HA consists of the highly variable globular head domain, which is the main target of the antibody response, and the stalk/stem domain. The stalk domain is highly conserved among two groups in IV A [66]. Anti-stalk antibodies occur in lower titers and less frequent than anti-head antibodies and are infrequently induced by inactivated IV vaccines [66, 67]. An inactivated H5N1 vaccine showed on average a fourfold anti-stalk antibody increase in humans after the first immunization [67]. Different approaches for a stalk vaccine are under investigation and hold promise for a universal IV vaccine [68].
Computationally optimized broadly reactive antigen (COBRA) vaccines of the HA head domain have the potential to generate broadly protective antibodies. Seasonal and pandemic-derived H1N1 COBRA HAs with the broadest HAI activity were inoculated into mice, using virus-like particles (VLP). Vaccination induced broadly-reactive antibodies and protected mice from A(H1N1)pdm09 challenge [69].
Another approach to overcome strain-specific immunity are vaccines containing the highly conserved extracellular domain of the IV matrix protein 2 (M2e). Many different VLPs are used to enhance the otherwise low immunogenicity of M2e [70]. Different M2e-based vaccines induced anti-M2e antibodies [38, 70], but also CD4+ or CD8+ T cells [71, 72], which were protective against heterologous virus challenges in mice. Furthermore, M2e-VLP-induced lung CD8+ Trm cells, which mediated long lived (> 4 months) heterologous protection in mice [38]. Different M2e-vaccines [70] and an anti-M2e monoclonal antibody (mAb) [73] were safe in human trials, but immunity can still be improved.
Respiratory syncytial virus (RSV)
In response to RSV infection, the anti-fusion (F) protein and anti-attachment glycoprotein (G) are the main antibodies produced [74, 75]. CD8+ T cells contribute to RSV clearance in murine models [74] and lung CD8+ Trm have protective effects in human RSV challenge [37]. No vaccine is currently available against RSV, although many approaches for a broadly protective vaccine have been discussed [74]. Vaccination of mice with a recombinant fusion protein, containing a conserved region of the G protein131–230 of RSV-A and RSV-B strains, resulted in IgA and IgG antibodies specific for both RSV-A and RSV-B G proteins. This vaccination protected mice from challenge with RSV-A or RSV-B [76]. The calf animal model is closer to RSV infection in humans. Taylor et al. [77] vaccinated calves with viral vectors expressing sequences of the F, N and M2-1 proteins of human RSV (HRSV). The vaccination induced neutralizing antibodies as well as CD4+ IFNγ+ T cells. Calves were protected from heterologous bovine RSV (BRSV) challenge, possibly because of cross-reactivity, since HRSV and BRSV have a high degree of sequence homology. Cross-reactivity of human antibodies has also been detected between two epitopes of the G protein of RSV-A and RSV-B. Such human IgG antibodies showed neutralizing effects against both viruses in HEp-2 cell culture [75]. Furthermore, human mAbs, cross-neutralizing RSV and human metapneumovirus (HMPV), have been identified [15, 78]. One of these mAbs also reacted to two other paramyxoviruses [15], while protective effects upon infection with the aforementioned viruses in murine models have been described [15, 78].
Rhinovirus (RV)
Infection with RV generates serotype-specific antibodies, which can prevent infection with the same serotype. Since there are over 160 distinct RV strains characterized to date [9], reinfection with other strains is common. Viral capsid proteins (VP) of RV contain sequences [79] and T cell epitopes [80], which are conserved across strains. Therefore, humoral or cellular cross-reactivity might provide cross-strain protection against heterologous RV infection.
Immunization with RV-A16-derived VP0 and a Th1 promoting adjuvant protected mice from heterologous RV-A1B challenge [9, 79]. CD4+ Th1 cells were preferentially expanded. Lung T cells from immunized and RV-A1B-infected mice showed increased IFNγ production compared to control, upon stimulation with RV-A16 VP0 and heterologous RV14 and RV-A1B-VP0 peptides. Immunization also enhanced neutralizing antibodies in heterologous RV challenge. Cross-reactive IgG1 VP1-specific antibodies, especially between RV-A and -C, have been detected in humans [81]. Limitations might arise from the fact that some antibodies bind nonprotective epitopes, which might lead to immune escape of RV [82].
Seronegative, healthy humans have CD4+ and CD8+ T cells against RV-A39 epitopes [19]. Co culture of DCs, RV-A39 and T cells resulted in proliferation of CD4+ and CD8+ T cells and enhanced IFNγ production. Muehling et al. [80] showed that pre-existing CD4+ Tm cells, specific to conserved epitopes of the VP region, proliferate upon RV-A16 challenge in seronegative donors. CD4+ Tm cells mainly showed a Th1 or T follicular helper phenotype. Furthermore, RV-A16 VP2162–181-specific T cells also recognized the VP2169–188 epitope of RV-A39. The results suggest that Tm cells specific for conserved RV regions may mediate heterologous protection. Conserved sequences might be used in a peptide vaccine, which could be especially useful in asthmatics or COPD patients.
Heterologous immunity between respiratory and other viruses
Epstein-Barr virus (EBV)
EBV is the causative pathogen of infectious mononucleosis (IM), the disease severity of which varies substantially. Children usually show mild to no symptoms, whereas adolescents and adults often present with more severe symptoms. Reactivation of IV-M158-specific CD8+ T cells, which are cross-reactive to the EBV BamHI M fragment leftward open reading frame 1280–288 (BMLF1280) epitope were shown to contribute to lymphoproliferation in IM ([48]; Tab. 1). In addition, frequency of IV-M158 and M158-EBV BMLF1280 tetramer+ CD8+ T cells correlated with IM disease severity [83]. This was associated with different TCR repertoire usage and enhanced IFNγ production. Others found bystander activation, but no expansion of IV-specific CD8+ T cells in IM [84]. BMLF1280-specific CD8+ T cells of human donors were shown to recognize up to two IV-derived and two EBV-derived epitopes [49]. Private TCR repertoire usage might explain differences in the number of peptides recognized by BMLF1280-specific CD8+ T cells between donors [49]. Recent data suggest that T cell cross-reactivity between IV-M158, and BMLF1280 and BamHI R fragment leftward open reading frame 1109–117 protects some adults from primary EBV infection [85]. Seronegative status was associated with usage of a private oligoclonal TCR repertoire and higher frequency of CD103+ IV-M1-specific T cells. The authors speculate that cross-reactive Trm might prevent primary EBV infection of B cells in the tonsils.
| Allele | Respiratory virus | Respiratory virus epitope | Sequence | Other pathogen | Other epitope | Sequence | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| HLA-A2 | IV | M158 | GILGFVFTL | EBV | BMLF1280–288 | GLCTLVAML | detrimental | [48, 83] |
| beneficial | [85] | |||||||
| M158 | GILGFVFTL | EBV | BRLF109–117 | YVLDHLIVV | beneficial | [85] | ||
| NP85–94 | KLGEFYNQMM | EBV | BMLF1280–288 | GLCTLVAML | — | [49] | ||
| M158 | GILGFVFTL | HCV | NS31073–1081 | CINGVCWTV | beneficial | [86] | ||
| NA231–239 | CVNGSCFTV | HCV | NS31073–1081 | CINGVCWTV | detrimental | [27] | ||
| M158 | GILGFVFTL | HIV-1 | P17 GAG77–85 | SLYNTVATL | — | [21, 51] | ||
| HA398–410 | SVIEKMNTQFTAV | T. vaginalis | Hypothetical protein118–130 | KMIEKMNTQTEVR | — | [23] | ||
| SVIEKMNTQFTAV | F. magna | Hypothetical protein131–143 | EKVEKMNTQYTAT | — | [23] | |||
| HLA-A2 | CoV | NS252–60 | TMLDIQPED | HPV 16 | E711–19/20 | YMLDLQPET(T) | — | [46] |
| C57Bl/6 Human | Ad5 | — | — | HCV | Various | — | beneficial | [90] |
Ad5, Adenovirus serotype 5; BMLF1, BamHI M fragment leftward open reading frame 1; BRLF1, BamHI R fragment leftward open reading frame 1 (both from EBV-derived immediate-early lytic protein); CoV, Coronavirus, E7, Transforming protein E7; F. magna; Finegoldia magna; EBV, Epstein-Barr-Virus; HA, Hemagglutinin; HCV, Hepatitis C Virus; HIV, Human Immunodeficiency Virus; HLA, human leukocyte antigen; HPV 16, Human Papillomavirus type 16; IV, Influenza Virus; M1, Matrix protein 1; NA, Neuraminidase; NP, Nucleoprotein; NS2, Nonstructural protein 2; NS3, Nonstructural protein 3; p17 GAG, group-specific antigen(gag)derived Matrix Protein (p17); T. vaginalis, Trichomonas vaginalis
Hepatitis C virus (HCV)
Acute HCV infection is variable in its symptoms, ranging from asymptomatic to severe disease. The HLA-A2 restricted nonstructural protein 31073–1081 (NS31073) epitope of HCV is a target for CD8+ T cells in HCV infection. NS31073-specific T cells were detected in the blood of HCV positive donors, but also in HCV seronegative (HCV-SN) donors [22, 86]. Further analysis showed first that NS31073-specific T cells are cross-reactive to the IV-derived NA231–239 epitope and second that IV infection induced HCV specific T cells [22]. Another study found the cross-reactivity between those epitopes to be weak and recognition of the NA231–239 epitope was dependent on preceding HCV infection [87]. NS31073-reactive T cells were shown to be cross-reactive to cytomegalovirus-(CMV), Epstein-Barr virus(EBV)-derived and the IV M158 epitopes in vitro [86]. Therefore, NS31073-reactive T cells might originate from infection with one of these viruses. Pre-existing cellular immunity towards the NS31073 epitope can either result in an enhanced immunity, as shown in evaluation of a HCV peptide vaccine trial [86], or have detrimental effects, as shown by Urbani et al. ([27]; Tab. 1). The latter found that patients with severe HCV liver disease used a private TCR repertoire, with T cells cross-reactive to NA231–239 and NS31073 epitopes. In those patients the CD8+ T cell response was narrowly focused on the NS31073 epitope [27].
Adenoviruses (Ad) are known for their potential as viral vectors in vaccination against infection [88] and have also been utilized for gene therapy [89]. Inoculation of Ad serotype 5 (Ad5) into mice induced robust humoral and cellular immunity against multiple HCV peptides in vitro and resulted in enhanced virus clearance [90]. Moreover, HCV-SN donors with pre-existing Ad immunity showed cross-reactive humoral and cellular immunity towards HCV peptides [90]. Further studies are needed to determine the possible use of Ad in the development of a vaccine for HCV. Limitations may arise from pre-existing Ad immunity, which possibly leads to lack of response to vaccination.
Human immunodeficiency virus (HIV)
T cell cross-reactivity was detected for the HLA-A2 restricted IV-M158 and the HIV-1 p17 GAG77–85 epitopes in vitro, among both HIV seropositive and seronegative donors ([21]; Tab. 1). Cross-reactivity was weak in some seronegative donors, which suggests that a strong T cell response to the IV-M158 is necessary to induce HIV-1 reactive T cells. A larger cohort study with 175 HIV seropositive HLA-A2+ subjects confirmed HIV-1 and IV cross-reactivity. T cells of HIV+ individuals frequently targeted the p17 GAG77–85 and the IV-M158 epitopes in vitro [51]. About 40 % showed T cells specific for both epitopes in vitro [51]. No effect of IV and HIV cross-reactive T cells on the course of HIV infection could be detected.
Adenoviral vectors are used to form an HIV vaccine. To avoid formation of strain specific antibodies, rare adenovirus strains are utilized. Unfortunately, also pre-existing cellular immunity against adenoviral vectors can impede successful vaccination. Frahm et al. [91] showed that pre-existing Ad5-specific CD4+ T cells led to decreased numbers of CD4+ HIV-specific T cells and to a narrowed CD8+ T cell response upon Ad5-based HIV vaccination in humans. In addition, extensive T cell cross-reactivity between adenovirus strains was shown. Furthermore, CD4+ HIV-cross-reactive Tm cells have been detected in unexposed adults [23], which further complicates prediction of anti-HIV immunity.
Human papilloma viruses (HPV)
High risk HPVs, such as type 16, 18 and others are the main risk factor for multiple genital cancers. Nilges et al. [46] described cross-reactivity between HLA-A2-binding epitopes E711–19/20 of HPV type 16 and the NS252-60-derived epitope of human CoV OC43 (Tab. 1). HPV E7-reactive CD8+ T cells were found in patients with cervical cancer and even more often in healthy blood donors. E711–19/20-reactive T cells in healthy donors were possibly formed in CoV infection. Whether T cell cross-reactivity here has negative effects on antitumor immunity or might support tumor clearance remains to be determined.
Heterologous immunity between respiratory viruses and pathogens other than viruses
Pre-existing HA391–410-specific CD4+ Tm cells showed expansion after seasonal IV vaccination. These Tm cells expanded after stimulation with the Trichomonas vaginalis (T. vaginalis)-derived hypothetical protein118–130- and the Finegoldia magna (F. magna)-derived hypothetical protein131–143 peptide in vitro (Tab. 1). HA391–410-specific CD4+ T cells from one donor recognized both peptides, whereas in the other donor the T cells only recognized the F. magna peptide. Furthermore, the two peptides stimulated different IV-reactive T cell clones with distinct affinity [23]. These findings might be a result of first, differential shaping of HI based on encounter with diverse pathogens and second the fact that HI is not necessarily reciprocal.
The oral live-attenuated salmonella typhi Ty21a strain vaccine induced both an increase of Ty21a-reactive and influenza-reactive T cells in the duodenal mucosa of healthy adults [92]. Homing markers were upregulated in Ty21a-reactive and influenza-reactive T cells. More studies are needed to better determine the mechanism behind the increase of influenza-specific T cells in the duodenal mucosa.
Autoimmunity
Acute disseminated encephalomyelitis (ADEM)
ADEM is preceded by either infection in up to 77 % of cases [93] or vaccination in 5–10 % of cases [94]. Episodes of infection or vaccine related ADEM may also occur in the same patient [95]. HMPV [96], parainfluenza [97] and IV infection [98] or IV vaccination [94] preceding ADEM, have all been reported. Influenza infection has been shown to trigger [99] or exacerbate [100] disease in experimental autoimmune encephalomyelitis (EAE) models, which might be a useful to study ADEM [101].
In patients affected by ADEM, myelin basic protein (MBP)-reactive T cells [102] as well as different neuronal antibodies, including anti-myelin oligodendrocyte protein (MOG) have been detected [103]. Generation of these autoreactive T cells and antibodies is probably due to molecular mimicry. TCR cross-reactivity between MBP/MOG-derived and respiratory virus-derived epitopes has been shown for coronavirus [104], adenovirus [29] and influenza A virus HA epitopes ([2950]; Tab. 2). Anti-MOG antibodies, which are frequently found in ADEM [103], might have a pathogenic role, since they induce demyelinating disease in EAE animal models [105].
| Disorder | Respiratory viruses (association) | Immune cells involved | Pathogen-derived protein | Pathogen-derived epitope sequence | Host-derived epitope | Host-derived epitope sequence | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|
| ADEM | Ad, HMPV, HPIV, IV infection / vaccination | T cells | IV HA | YRNLVWFIKKNTRYP | MBP85-99 | ENPVVHFFKNIVTPR | — | [29] |
| Ad 12 ORF | DFEVVTFLKDVLPE | ENPVVHFFKNIVTPR | ||||||
| IV HA306–318 | YVKQNTLKLA | MOG | VLIKNTLRSL | — | [50] | |||
| YVKQNTLKLA | SAANNNIKLL | |||||||
| CD | Ad | T/B cells |
Ad 54kDa E1b384–395 |
LRRGMFRPSQCN | A-gliadin206–217 | LGQGSFRPSQQN | — | [45] |
| GBS | IV infection / vaccination | B cells | IV HA | — | GM1 | — | — | [108] |
| Myocarditis | Ad, IV, RSV, CV | T cells | CV | — | MYHC-α334–352 | DSAFDVLSFTAEEKAGVYK | — | [129] |
| B cells | CV | — | Collagen IV, actin fibronectin, others | — | Multiple homologies between CV and Col IV | [130] | ||
| Narcolepsy | IV infection / vaccination | B cells | IV A NP111–121 | YDKEEIRRIWR | HCRTr234–45 | YDDEEFLRYLWR | [111] | |
| [GM3; TRIB2] | ||||||||
| Neuropsychiatric | IV | B cells | IV HA | Various | AGP | Various | Potential cross reactivity, based on sequence alignment | [119] |
| IV | NMDA A2 | [120] | ||||||
| [122] | ||||||||
| SARS-CoV, IV | B cells | Various | Various | ACTH | Various | |||
| SS | CV | B cells | CV A21/A13 2B protein |
MVTSTITEKLLKNLVKI MVTSVLTEKLLKNLIKI |
Ro60 kDa216–232 | KALSVETEKLLKYLEAV | [134] | |
| T1DM | CV | B cells | IV HA (mAbs) | — | Pancreatic α-cells | — | Tissue staining with mAbs | [140] |
ACTH, Adrenocorticotropic hormone; ADEM, Acute disseminated encephalomyelitis; Ad, Adenovirus; AGP, Axon guidance proteins; CD, Celiac disease; Col IV, Collagen IV; CoV, Coronavirus; CV, Coxsackie virus; GBS, Guillain-Barré Syndrome; GM1, Monosialotetrahexosylganglioside; GM3, Monosialodihexosylganglioside; HA, Hemagglutinin; HCRTr2, Hypocretin Receptor 2; IV, Influenza virus; HPIV, Parainfluenza virus; mAbs, monoclonal antibodies; MBP, Myelin Basic Protein; MOG, Myelin Oligodendrocyte Glycoprotein; MYHC-α, cardiac myosin heavy chain-α; NA, Neuraminidase; NMDA, A2 N-methyl-D-aspartate receptor A2 subunit; ORF, Open reading frame; RSV, Respiratory syncytial virus; SS, Sjogren Syndrome; TRIB2, Tribbles homolog 2; T1DM, Type 1 diabetes mellitus
Guillain-Barré syndrome (GBS)
About 60 % of all GBS cases are thought to be infection-related [106], most frequently gastrointestinal or respiratory tract infections including influenza [98]. Molecular mimicry of antibodies against pathogen-derived and self-antigens seem to play a major role in the initiation of GBS [106] and this is best described for Campylobacter jejuni.
A recent meta-analysis found a slight, but significant increase in the relative risk of influenza vaccine-associated GBS among 39 studies published between 1981 and 2014 [107]. Others found no such increase in disease risk [106]. The link between influenza infection and subsequent development of GBS is better established [106].
The mechanisms of influenza- and influenza-vaccine-induced GBS largely remain unknown. A first clue might be the findings of Nachamkin et al. [108], who showed that the A/NJ/1976 (H1N1) vaccine as well as trivalent vaccines from 1992–1993 and 2004–2005 seasons induced anti-HA and also anti-GM1 antibodies in mice after immunization. In addition, the 2004–2005 vaccine contains glycolipid-like structures, as shown by positive anti-GM1 immunostaining [108]. Anti-GM-1 antibodies showed a low, but detectable hemagglutination inhibition activity.
Narcolepsy
Narcolepsy was associated with the IV A(H1N1)pdm09 vaccine Pandemrix® (GlaxoSmithKline, London, UK) [109] and also with A(H1N1)pdm09 infection [110]. Recently, Ahmed et al. [111], showed that the Pandemrix® vaccine, in some HLA-DQB1*06:02-positive individuals, induced IV A NP111–121 antibodies, which were cross-reactive to the hypocretin receptor 234–45 (Tab. 2). Although hypocretin receptor 2 autoantibodies were detected in 85 % of patients with Pandemrix®-associated narcolepsy [111], the exact mechanism of the antibody-induced narcolepsy remains to be determined.
Other autoantibodies with a potential link to narcolepsy are anti-monosialodihexosylganglioside (GM3) [112] — and anti-Tribbles homolog 2 (TRIB2) [113] antibodies. Anti-GM3 antibodies were detected more frequently in patients with Pandemrix®-associated narcolepsy than in vaccinated healthy controls [112], whereas no such correlation was evident for anti-TRIB2 antibodies after Pandemrix® vaccination [114]. Nonetheless, anti-TRIB2 antibody titers were found to be increased in narcolepsy patients, compared to controls [113]. Furthermore, transfer of pooled anti-TRIB2 positive IgG samples from the blood of narcolepsy patients into mice resulted in narcolepsy-like symptoms and orexin-neuron loss [115].
Other neurologic/neuropsychiatric disorders
Anti-N-methyl-D-aspartate receptor (NMDAR) antibodies were detected in patients with herpes simplex encephalitis [116], although results are inconsistent [117]. These findings suggest that infections are a possible trigger for psychiatric diseases.
Maternal infection, including influenza, has been suggested to play a role in the development of psychiatric disorders in the child [118]. Lucchese et al. identified influenza epitope mimics in multiple neuronal proteins ([119, 120]; Tab. 2). Cross-reactivity might lead to neuropsychiatric disorders, although experimental verification is needed.
Other autoantibodies, which may play a role in neuropsychiatric disorders, such as anorexia nervosa, chronic fatigue syndrome or major depression, are anti-adrenocorticotropin (ACTH) antibodies [121], which may cause ACTH deficiency. Wheatland proposed that SARS-CoV infection can induce pathogen-specific antibodies, which are cross-reactive to ACTH ([122]; Tab. 2).
Celiac disease (CD)
Gastrointestinal infections and to a lesser extent also respiratory infections in early life increased risk of developing CD [123]. Ad may contribute to CD development. A sequence mimic of the A-gliadin protein206–217 has been identified in the 54 kilodalton (kDa) E1b protein of Ad 12384–395 [45]. Rat antiserum generated against the E1b384–395 epitope cross-reacted with A-gliadin as well as a synthetic A-gliadin211–217 peptide ([45]; Table 2). CD patient serum antibodies were also shown to react to a synthetic A gliadin212–217 peptide [124]. Furthermore, T cell cross-reactivity to a synthetic peptide resembling the A-gliadin/E1b sequence have been detected in CD patients [125]. These results were inconsistent in follow-up studies [126].
Myocarditis
Infectious myocarditis is caused by different pathogens, including respiratory viruses such as Ad, IV, RSV and CV [98, 127]. Viral and immune mechanisms contribute to disease onset and persistence in myocarditis [127]. Massilamany et al. [128] showed that immunization of A/J mice with peptide mimics of cardiac myosin heavy chain (MYHC)-α334–352 induced cross-reactive T cells and led to the development of myocarditis. Additionally, CV B3 infection led to the generation of such MYHC-α334–352-reactive CD4+ T cells and associated myocarditis in A/J mice ([129]; Tab. 2). Different antibodies, including those against cardiac myosin and actin, are associated with myocarditis [127]. CV mimics sequences of actin, myosin, collagen and laminin [130]. Moreover, anti-CV antibodies were shown to bind to actin, collagen IV and fibronectin [130].
Sjögren’s syndrome (SS)
Viral infections, including CV have been suggested to play a role in the development of SS [131]. In SS, antibodies and/or T cells to different autoantigens, frequently Ro (SSA) and La (SSB) are present [132, 133]. Sequence homologies between the 2B protein of CV A21/A13 and the Ro60 kDa antigen may induce cross-reactive autoantibodies. Stathopoulou et al. [134] showed that serum of SS patients recognized synthetic peptides from the homologous regions of both proteins more frequently than serum of systemic lupus erythematosus (SLE) patients or controls. Cross-reactivity was confirmed in inhibition assays, using both synthetic peptides ([134]; Tab. 2). Mimics of Ro60 kDa T cell epitopes have been identified in various bacteria from the human skin, oral cavity, intestine and vaginal flora [135]. Peptide mimics were able to stimulate Ro60 kDa-reactive T cells [135].
Type 1 diabetes mellitus (T1DM)
Development of T1DM has been linked to different viral infections, especially enterovirus infection. Also respiratory viral infections, including IV may be associated to T1DM [136]. One possible mechanism, contributing to autoimmunity in T1DM is molecular mimicry [44, 137]. CMV or rotavirus infection may induce cross-reactive T cells to pancreatic autoantigens [138, 139], whereas for coxsackie virus (CV) such findings are inconsistent [137].
Recently, Qi et al. [140] stained pancreatic tissue with monoclonal antibodies specific for different influenza HA epitopes. Two distinct antibodies were cross-reactive to human pancreatic β-cells, but not β-cells (Tab. 2). As shown before in mice, after almost complete diphtheria toxin-induced β-cell loss, pancreatic ?-cells are able to differentiate into insulin producing cells [141]. If pancreatic α cells are the progenitors to β-cells, influenza-induced antibodies against α-cell antigens eventually result in the onset of diabetes.
Asthma/allergy
About 300 million people are currently affected by asthma worldwide [142], while the prevalence might rise to 1 billion in 2050 [143]. Characteristics of asthma are chronic airway inflammation, airway hyperreactivity, over production of mucus and remodelling of airways, which becomes relevant particularly in chronic disease.
One major risk factor for the development of asthma are recurrent wheezing episodes early in life, which are caused by viruses in 62–98 % [144, 145] of cases. RSV-or RV-induced wheezing in children < 3 years, with at least one asthmatic parent, was associated with an increased risk for asthma at 6 years of age [146]. Recently, Lukkarinen et al. [145] followed up children with a severe wheezing episode for 7 years. They identified RV-induced wheezing, sensitization and eczema as risk factors for the development of atopic asthma, whereas non-atopic asthma risk factors included first wheezing at < 12 months of age caused by viruses other than RV/RSV and parental smoking. Early onset asthma can resolve spontaneously, but recurrent infections with respiratory viruses over time makes spontaneous resolution less likely [10]. Therefore, viral respiratory tract infections also contribute to the persistence of asthma.
Most asthma exacerbations are also caused by respiratory viral infection, such as RV, RSV, IV, CoV, HMPV, parainfluenza virus and adenovirus [144]. RV is the pathogen detected most frequently in all age groups, whereas RSV affects mostly preschool children and IV is most prevalent in adults [144].
On the other hand, the prevailing concept to explain the rising prevalence of allergic and autoimmune diseases in industrialized countries is the hygiene hypothesis [147]. According to the latter, less frequent exposure to pathogens in early life is associated with the development of allergies [148]. Protective effects of bacteria or bacterial products on asthma development have been well characterized [148], but also viruses [149] as well as respiratory viruses, including IV [150], were shown to protect mice from asthma. Correlates of protection are induction of T1 immune responses, e.g. by stimulation of innate immune receptors, such as Toll-like receptors [148, 151]. Viral infections were shown to protect from asthma by induction of an natural killer T (NKT) cell subset [150] or monocytes with a regulatory phenotype [149].
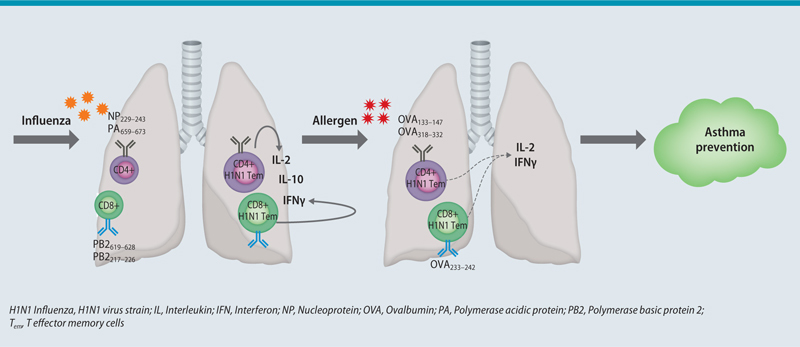
Our group further examined the role of respiratory viral infection on asthma protection in a murine model. In agreement to earlier reports [150, 152], we found that IV A infection of Balb/c mice confers protection against ovalbumin (OVA)-induced, but also house dust mite (HDM)-induced asthma. Protection was dependent on CD4+ and CD8+ Tem cells, which were cross-reactive to IV A- and OVA-derived peptides, as predicted by bioinformatics analysis. Upon ex vivo restimulation with the predicted influenza A- or OVA-derived peptides, lung T cells showed increased production of IL-2 and IFNγ. Furthermore, peptide immunization with the predicted virus-derived peptides also provided asthma protection through Tem cells. This is possibly due to the production of IFNγ by virus-specific T cells upon allergen challenge, as an augmented IFN? response can protect from experimental asthma [152]. Thus, we provide evidence for Tem-mediated HI between viruses and allergens as a protective mechanism against allergic asthma ([153]; Fig. 2).
Conclusions and Outlook
HI involving respiratory viruses may have various protective, but also detrimental effects on the host. Because of differences in the private TCR repertoire, the clinical outcome of cross-reactivity between the same epitopes may be detrimental in one and beneficial in another person, as seen for example between IV and EBV [48, 85]. IV vaccination has been associated with autoimmune diseases in a few cases [94, 107, 109]. Nevertheless, an association between autoimmune disease and respiratory viral infection has been more extensively discussed. Different approaches for broadly protective vaccines are currently under investigation. Some vaccines were shown to induce lung Trm cells, the role of which in heterologous protection from respiratory tract infections is yet to be determined in humans.
Our group showed that HI between respiratory viruses and allergens protects from experimental asthma [153], thus expanding the hygiene hypothesis. Further studies are needed to determine whether HI is a broadly applicable concept between other respiratory viruses and environmental allergens. Moreover, it will be interesting to see whether any of the currently licenced or future vaccines has the potential to induce heterologous protection from viral infection as well as asthma. Recently, gammaherpesvirus infection was shown to induce regulatory monocytes, which prevented experimental asthma in mice [149]. Therefore, heterologous innate immune stimulation with tolerogenic or T1 promoting adjuvants [14] might be utilised to induce allergen tolerance (Fig. 3).
Appendix
Glossary
Acute disseminated encephalomyelitis (ADEM): ADEM is a rare autoimmune disease affecting the central nervous system (CNS), with an incidence of 0.6–0.8/100,000 people/year [94]. Especially young children suffer from ADEM, but adults may also be affected. ADEM is an autoimmune mediated, demyelinating disease of the central nervous system (CNS) with a usually monophasic course. Clinically, a vast array of neurological symptoms is possible, from varying focal deficits to encephalopathy (confusion, reduced consciousness, irritability).
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis: Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis belongs to the heterogeneous group of autoimmune epilepsies, which mainly occur as paraneoplastic syndromes [154]. Antibodies directed against cancer antigens are thought to cross-react with neuronal antigens.
Celiac disease (CD): Prevalence of CD in the European population is approximately 1 % [155]. Genetically susceptible individuals with a genetic background of HLA-DQ2 and/or HLA-DQ8, usually develop symptoms at childhood, although disease onset may occur later in life. Different infections are thought to promote or prevent CD development [156].
Computationally optimized broadly reactive antigen (COBRA) HA: The HA amino acid compositions from many isolated IV A strains is analysed. The aim is to define a consensus sequence for every amino acid in the HA protein.
Guillain-Barré syndrome (GBS): GBS is a rare neurological disease with an incidence of 0.4–4/100,000 people per year [106]. Classical GBS, also called acute inflammatory demyelinating polyneuropathy (AIDP), is caused by an autoimmune demyelination of peripheral nerves, which leads to subacute ascending paralysis with muscle weakness and sensory deficits in the limbs. Severe cases can present with respiratory failure or autonomic instability. Axonal forms of GBS, namely AMAN and AMSAN are associated with anti-GM1 and/or anti GD1a antibodies, while in Miller Fisher syndrome and to a lesser extent also in Bickerstaff brainstem encephalitis, anti-GQ1b antibodies are found. No antibody specific for AIDP has been detected yet.
Heterologous innate immune stimulation: The “original” or homologous pathogen/antigen often induces an adaptive immune response. Heterologous pattern recognition receptor (PRR) ligands stem from other sources than the original antigen and mostly do not induce adaptive immune responses. Heterologous PRR stimulation alters the immune response towards the homologous antigen. PRR ligands include various substances, such as vaccine adjuvants, other pathogens or commensal bacteria and endogenous ligands (e.g. hyaluronic acid) [14].
Heterosubtypic immunity: Immunity towards one virus also provides heterologous immunity against a substrain of the first virus. The term heterosubtypic immunity is mostly used when referred to IV A infection.
Heterologous immunity (HI): The immune response towards one antigen alters the immune response towards a subsequent encounter with an unrelated antigen. This involves allo-, auto- or allergen-derived antigens as well as pathogen-derived antigens. Heterologous antigen encounter may have protective or detrimental effects on the host.
Myocarditis: The initial phase of the disease is thought to be mediated by direct myocardial damage through distinct agents (e.g. infection, toxins, drugs), which is followed by an immune mediated phase. Ongoing infection and/or autoimmune disease leads to chronic myocarditis [127]. Myocarditis can result in dilated cardiomyopathy or sudden cardiac death [127].
Narcolepsy: Narcolepsy is characterized by daytime sleepiness, cataplexy and sleep attacks and affects about 30 per 100,000 people [109]. Loss of hypocretin (orexin)-producing neurons in the hypothalamus is characteristic for type 1 narcolepsy, but not for type 2 narcolepsy. Disease onset is typically between 10 and 30 years of age [109]. About 98% of patients with narcolepsy and cataplexy are HLA-DQB1*06:02 positive, which suggests a role for T cells in disease pathogenesis [109].
Pandemrix®: Pandemrix® is a monovalent A(H1N1)pdm09 vaccine. It was broadly used in Europe during the 2009 swine flu pandemic. Pandemrix contained much higher doses of NP than other A(H1N1)pdm09 vaccines [111].
Paratope: The antigen binding region of an antibody contains multiple paratopes, which recognize their epitope on a given antigen.
Private TCR repertoire: The public TCR repertoire consists of T cell clones, which are identical for all individuals, whereas T cell clones, which are unique for an individual form the private TCR repertoire. The private TCR repertoire leads to variability in immune recognition and cross-reactivity phenomena. For example, the recognition of the same epitopes by different T cells may result in detrimental or beneficial disease outcomes in the respective hosts.
Sjögren’s syndrome (SS): SS is characterized by lymphocyte infiltration of salivary glands (SGL). Decreased SGL function causes xerostomia and xerophthalmia.
T cell receptor (TCR) cross-reactivity: The ability of the TCR to recognize more than one antigen is referred to as TCR cross-reactivity.
Type 1 diabetes mellitus (T1DM): T1DM is characterized by autoimmune mediated loss of insulin-producing β cells in the pancreas, while glucagon-producing α cells and somatostatin producing δ cells are spared. Disease is thought to be T cell mediated, which means that autoreactive T cells attack pancreatic β cells.
Acknowledgments
The work was supported by the German Research Foundation, SFB 1021, Project C04 and the German Center for Lung Research (DZL)
Abbreviations
- ACTH
Adrenocorticotropin
- Ad
Adenoviruses
- ADEM
Acute disseminated encephalomyelitis
- APC
Antigen presenting cell
- BRSV
Bovine RSV
- CD
Celiac disease
- CMV
Cytomegalovirus
- COBRA
Computationally optimized broadly reactive antigen
- COPD
Chronic obstructive pulmonary disease
- CoV
Coronavirus
- CV
Coxsackie virus
- EAE
Experimental autoimmune encephalomyelitis
- _
Epstein-Barr virus
- F
Fusion protein
- Fab
Fragment antigen binding
- G
Attachment glycoprotein
- GBS
Guillain-Barré syndrome
- GM3
Monosialodihexosylganglioside
- HCV
Hepatitis C virus
- HCV-SN
HCV seronegative
- HDM
House dust mite
- HI
Heterologous immunity
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- HMPV
Human metapneumovirus
- HPV
Human papilloma viruses
- IFN
Interferon
- IL
Interleukin
- IM
Infectious mononucleosis
- IV
Influenza virus
- kDa
Kilodalton
- LAIV
Live attenuated influenza vaccine
- LRTI
Lower respiratory tract infections
- mAb
Monoclonal antibody
- MBP
Myelin basic protein
- MERS
Middle East respiratory syndrome
- MHC
Major histocompatibility complex
- MOG
Myelin oligodendrocyte protein
- MYHC
Myosin heavy chain
- NKT
Natural killer T cell
- NMDAR
Anti-N-methyl-D-aspartate receptor
- OVA
Ovalbumin
- pMHC
peptide-MHC
- rRBD
Recombinant receptor binding-domain
- RSV
Respiratory syncytial virus
- RTI
Respiratory tract infections
- RV
Rhinovirus
- S
Spike protein
- SARS
Severe acute respiratory syndrome
- SLE
Systemic lupus erythematosus
- SS
Sjögren’s syndrome
- T1DM
Type 1 diabetes mellitus
- TCR
T cell receptor
- Tem
T effector memory cells
- TLR2
Toll-like receptor 2
- Tm
T memory
- TRIB2
Tribbles homolog 2
- Trm
Tissue resident memory
- URTI
Upper respiratory tract infections
- VP
Viral capsid proteins
Footnotes
Cite this as
Pusch E, Renz H, Skevaki C. Respiratory virus-induced heterologous immunity — Part of the problem or part of the solution? Allergo J Int 2018,27:79–96 10.1007/s40629-018-0056-0
Conflict of interest
C. Skevaki has received grants from the German Research Foundation, the German Center for Lung Research, Hycor and Mead Johnson Nutritional and consultancy fees by Hycor and Bencard. H. Renz has received a grant from the German Research Foundation and the German Center for Lung Research and payment for lectures from Allergopharma, Novartis, Thermo Fisher, Danone, Mead Johnson Nutritional, and Bencard and has received payment for research and development projects from Hycor, Mead Johnson, and Beckman Coulter. E. Pusch declares no relevant conflicts of interest.
References
- 1.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The Economic Burden of Non-Influenza-Related Viral Respiratory Tract Infection in the United States. Arch Intern Med. 2003;163:487. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Molinari N-A, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, et al. Viruses and Bacteria in the Etiology of the Common Cold. J Clin Microbiol. 1998;36:539–42. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373:415–27. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: An updated systematic analysis. The Lancet. 2015;385:430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 6.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. The Lancet. 2011;378:1917–30. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 7.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedzicha JA, Seemungal TAR. COPD exacerbations: Defining their cause and prevention. The Lancet. 2007;370:786–96. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140:895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nature Medicine. 2012;18:726–35. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 11.Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol. 2012;42:102–11. doi: 10.1007/s12016-011-8294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–61. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Goodridge HS, Ahmed SS, Curtis N, Kollmann TR, Levy O, Netea MG, et al. Harnessing the beneficial heterologous effects of vaccination. Nature Reviews Immunology. 2016;16:392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin SF. Adaptation in the innate immune system and heterologous innate immunity. Cell Mol. Life Sci. 2014;71:4115–30. doi: 10.1007/s00018-014-1676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501:439–43. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 16.D’Orsogna L, van den Heuvel H, van Kooten C, Heidt S, Claas FHJ. Infectious pathogens may trigger specific allo-HLA reactivity via multiple mechanisms. Immunogenetics. 2017;69:631–41. doi: 10.1007/s00251-017-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H, Topham DJ. Multiple Distinct Forms of CD8+ T Cell Cross-Reactivity and Specificities Revealed after 2009 H1N1 Influenza A Virus Infection in Mice. PLoS ONE. 2012;7:1–11. doi: 10.1371/journal.pone.0046166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nature Medicine. 2013;19:1305–12. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 19.Steinke JW, Liu L, Turner RB, Braciale TJ, Borish L. Immune Surveillance by Rhinovirus-Specific Circulating CD4+ and CD8+ T Lymphocytes. PLoS ONE. 2015;10:e0115271. doi: 10.1371/journal.pone.0115271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nature Medicine. 2012;18:276–82. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 21.Acierno PM, Newton DA, Brown EA, Maes L, Baatz JE, Gattoni-Celli S, et al. Cross-reactivity between HLA-A2-restricted FLU-M1:58-66 and HIV p17 GAG:77-85 epitopes in HIV-infected and uninfected individuals. Journal of Translational Medicine. 2003;1:3. doi: 10.1186/1479-5876-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and Influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392–400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su L, Kidd B, Han A, Kotzin J, Davis M. Virus-Specific CD4+ Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity. 2013;38:373–83. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vujanovic L, Shi J, Kirkwood JM, Storkus WJ, Butterfield LH. Molecular mimicry of MAGE-A6 and Mycoplasma penetrans HF-2 epitopes in the induction of antitumor CD8+ T-cell responses. Oncoimmunology. 2014;3:e954501. doi: 10.4161/21624011.2014.954501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson LR, Weizman O-E, Rapp M, Way SS, Sun JC. Epitope-Specific Vaccination Limits Clonal Expansion of Heterologous Naive T Cells during Viral Challenge. Cell Rep. 2016;17:636–44. doi: 10.1016/j.celrep.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberle SG, Hanna-El-Daher L, Chennupati V, Enouz S, Scherer S, Prlic M, Zehn D. A Minimum Epitope Overlap between Infections Strongly Narrows the Emerging T Cell Repertoire. Cell Rep. 2016;17:627–35. doi: 10.1016/j.celrep.2016.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbani S, Amadei B, Fisicaro P, Pilli M, Missale G, Bertoletti A, Ferrari C. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med. 2005;201:675–80. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–66. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heutinck KM, Yong SL, Tonneijck L, van den Heuvel H, van der Weerd NC, van der Pant KAMI, et al. Virus-Specific CD8(+) T Cells Cross-Reactive to Donor-Alloantigen Are Transiently Present in the Circulation of Kidney Transplant Recipients Infected With CMV and/or EBV. Am J Transplant. 2016;16:1480–91. doi: 10.1111/ajt.13618. [DOI] [PubMed] [Google Scholar]
- 31.Younes S-A, Freeman ML, Mudd JC, Shive CL, Reynaldi A, Panigrahi S, et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J Clin Invest. 2016;126:2745–56. doi: 10.1172/JCI85996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander Activation of CD8+ T Cells Contributes to the Rapid Production of IFN- in Response to Bacterial Pathogens. The Journal of Immunology. 2001;166:1097–105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 33.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamath AT, Sheasby CE, Tough DF. Dendritic Cells and NK Cells Stimulate Bystander T Cell Activation in Response to TLR Agonists through Secretion of IFN- and IFN- The Journal of Immunology. 2005;174:767–76. doi: 10.4049/jimmunol.174.2.767. [DOI] [PubMed] [Google Scholar]
- 35.Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, et al. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 2013;3:701–8. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol. 2015;195:203–9. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y-N, Lee Y-T, Kim M-C, Gewirtz AT, Kang S-M. A Novel Vaccination Strategy Mediating the Induction of Lung-Resident Memory CD8 T Cells Confers Heterosubtypic Immunity against Future Pandemic Influenza Virus. The Journal of Immunology. 2016;196:2637–45. doi: 10.4049/jimmunol.1501637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zens KD, Chen JK, Farber DL. JCI Insight. 2016. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Che JW, Selin LK, Welsh RM. Evaluation of non-reciprocal heterologous immunity between unrelated viruses. Virology. 2015;482:89–97. doi: 10.1016/j.virol.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sewell AK. Why must T cells be cross-reactive? Nature reviews. Immunology. 2012;12:669–77. doi: 10.1038/nri3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arstila TP. A Direct Estimate of the Human T Cell Receptor Diversity. Science. 1999;286:958–61. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 43.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunology Today. 1998;19:395–404. doi: 10.1016/S0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 44.Wooldridge L, Ekeruche-Makinde J, van den Berg HA, Skowera A, Miles JJ, Tan MP, et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem. 2012;287:1168–77. doi: 10.1074/jbc.M111.289488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kagnoff MF, Austin RK, Hubert JJ, Bernardin JE, Kasarda DD. J Exp Med. 1984. Possible role for a human adenovirus in the pathogenesis of celiac disease; pp. 1544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilges K, Höhn H, Pilch H, Neukirch C, Freitag K, Talbot PJ, Maeurer MJ. Human papillomavirus type 16 E7 peptide-directed CD8+ T cells from patients with cervical cancer are cross-reactive with the coronavirus NS2 protein. J Virol. 2003;77:5464–74. doi: 10.1128/JVI.77.9.5464-5474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valkenburg SA, Josephs TM, Clemens EB, Grant EJ, Nguyen THO, Wang GC, et al. Molecular basis for universal HLA-A*0201-restricted CD8+ T-cell immunity against influenza viruses. Proceedings of the National Academy of Sciences. 2016;113:4440–5. doi: 10.1073/pnas.1603106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, et al. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. Journal of Clinical Investigation. 2005;115:3602–12. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim S-K, Naumov YN, et al. CD8 T Cell Cross-Reactivity Networks Mediate Heterologous Immunity in Human EBV and Murine Vaccinia Virus Infections. The Journal of Immunology. 2010;184:2825–38. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markovic-Plese S, Hemmer B, Zhao Y, Simon R, Pinilla C, Martin R. High level of cross-reactivity in influenza virus hemagglutinin-specific CD4+ T-cell response: Implications for the initiation of autoimmune response in multiple sclerosis. J Neuroimmunol. 2005;169:31–8. doi: 10.1016/j.jneuroim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Huckelhoven AG, Etschel JK, Bergmann S, Zitzelsberger K, Mueller-Schmucker SM, Harrer EG, Harrer T. Cross-Reactivity Between Influenza Matrix- and HIV-1 P17-Specific CTL-A Large Cohort Study. J Acquir Immune Defic Syndr. 2015;69:528–35. doi: 10.1097/QAI.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 52.Ekeruche-Makinde J, Miles JJ, van den Berg HA, Skowera A, Cole DK, Dolton G, et al. Peptide length determines the outcome of TCR/peptide-MHCI engagement. Blood. 2013;121:1112–23. doi: 10.1182/blood-2012-06-437202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams JJ, Narayanan S, Birnbaum ME, Sidhu SS, Blevins SJ, Gee MH, et al. Structural interplay between germline interactions and adaptive recognition determines the bandwidth of TCR-peptide-MHC cross-reactivity. Nat Immunol. 2016;17:87–94. doi: 10.1038/ni.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Regenmortel MHV. Specificity, polyspecificity, and heterospecificity of antibody-antigen recognition. J Mol Recognit. 2014;27:627–39. doi: 10.1002/jmr.2394. [DOI] [PubMed] [Google Scholar]
- 55.Kringelum JV, Nielsen M, Padkjær SB, Lund O. Structural analysis of B-cell epitopes in antibody:protein complexes. Mol Immunol. 2013;53:24–34. doi: 10.1016/j.molimm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sela-Culang I, Kunik V, Ofran Y. Front Immunol. 2013. The structural basis of antibody-antigen recognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol. 2014;88:11034–44. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu WJ, Zhao M, Liu K, Xu K, Wong G, Tan W, Gao GF. T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai W, Wang Y, Fett CA, Zhao G, Li F, Perlman S, et al. J Virol. 2017. Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao J, Zhao J, Mangalam AK, Channappanavar R, Fett C, Meyerholz DK, et al. Airway Memory CD4(+) T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity. 2016;44:1379–91. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, et al. Cytotoxic T Lymphocytes Established by Seasonal Human Influenza Cross-React against 2009 Pandemic H1N1 Influenza Virus. J Virol. 2010;84:6527–35. doi: 10.1128/JVI.00519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Arévalo MT, Chen Y, Chen S, Zeng M. T-cell-mediated cross-strain protective immunity elicited by prime-boost vaccination with a live attenuated influenza vaccine. International journal of Infectious diseases. 2014;27:37–43. doi: 10.1016/j.ijid.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohn KGI, Zhou F, Brokstad KA, Sridhar S, Cox RJ. Boosting of Cross-Reactive and Protection-Associated T Cells in Children After Live Attenuated Influenza Vaccination. J Infect Dis. 2017;215:1527–35. doi: 10.1093/infdis/jix165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magini D, Giovani C, Mangiavacchi S, Maccari S, Cecchi R, Ulmer JB, et al. Self-Amplifying mRNA Vaccines Expressing Multiple Conserved Influenza Antigens Confer Protection against Homologous and Heterosubtypic Viral Challenge. PLoS ONE. 2016;11:e0161193. doi: 10.1371/journal.pone.0161193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chua BY, Wong CY, Mifsud EJ, Edenborough KM, Sekiya T, Tan ACL, et al. Inactivated influenza vaccine that provides rapid, innate-immune- system-mediated protection and subsequent long-term adaptive immunity. mBio. 2015;6:1–11. doi: 10.1128/mBio.01024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–30. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellebedy AH, Krammer F, Li G-M, Miller MS, Chiu C, Wrammert J, et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A. 2014;111:13133–8. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krammer F. Novel universal influenza virus vaccine approaches. Curr Opin Virol. 2016;17:95–103. doi: 10.1016/j.coviro.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter DM, Darby CA, Lefoley BC, Crevar CJ, Alefantis T, Oomen R, et al. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J Virol. 2016;90:4720–34. doi: 10.1128/JVI.03152-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng L, Cho KJ, Fiers W, Saelens X. M2e-Based Universal Influenza A Vaccines. Vaccines (Basel) 2015;3:105–36. doi: 10.3390/vaccines3010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eliasson DG, Omokanye A, Schön K, Wenzel UA, Bernasconi V, Bemark M, et al. Mucosal Immunology. 2017. M2e-tetramer-specific memory CD4 T cells are broadly protective against influenza infection. [DOI] [PubMed] [Google Scholar]
- 72.Schotsaert M, Ysenbaert T, Smet A, Schepens B, Vanderschaeghe D, Stegalkina S, et al. Nature Scientific Reports. 2016. Long-Lasting Cross-Protection Against Influenza A by Neuraminidase and M2e-based immunization strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramos EL, Mitcham JL, Koller TD, Bonavia A, Usner DW, Balaratnam G, et al. Efficacy and safety of treatment with an anti-m2e monoclonal antibody in experimental human influenza. J Infect Dis. 2015;211:1038–44. doi: 10.1093/infdis/jiu539. [DOI] [PubMed] [Google Scholar]
- 74.Graham BS. Vaccines against respiratory syncytial virus: The time has finally come. Vaccine. 2016;34:3535–41. doi: 10.1016/j.vaccine.2016.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cortjens B, Yasuda E, Yu X, Wagner K, Claassen YB, Bakker AQ, et al. J Virol. 2017. Broadly Reactive Anti-Respiratory Syncytial Virus G Antibodies from Exposed Individuals Effectively Inhibit Infection of Primary Airway Epithelial Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J-Y, Chang J. Universal vaccine against respiratory syncytial virus A and B subtypes. PLoS ONE. 2017;12:e0175384. doi: 10.1371/journal.pone.0175384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor G, Thom M, Capone S, Pierantoni A, Guzman E, Herbert R, et al. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci Transl Med. 2015;7:300ra127. doi: 10.1126/scitranslmed.aac5757. [DOI] [PubMed] [Google Scholar]
- 78.Schuster JE, Cox RG, Hastings AK, Boyd KL, Wadia J, Chen Z, et al. A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus. J Infect Dis. 2015;211:216–25. doi: 10.1093/infdis/jiu307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glanville N, Mclean GR, Guy B, Lecouturier V, Berry C, Girerd Y, et al. Cross-Serotype Immunity Induced by Immunization with a Conserved Rhinovirus Capsid Protein. PLoS Pathogens. 2013;9:e1003669. doi: 10.1371/journal.ppat.1003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muehling LM, Mai DT, Kwok WW, Heymann PW, Pomes A, Woodfolk JA. Circulating Memory CD4+ T Cells Target Conserved Epitopes of Rhinovirus Capsid Proteins and Respond Rapidly to Experimental Infection in Humans. J Immunol. 2016;197:3214–24. doi: 10.4049/jimmunol.1600663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iwasaki J, Smith W-A, Stone SR, Thomas WR, Hales BJ. Species-specific and cross-reactive IgG1 antibody binding to viral capsid protein 1 (VP1) antigens of human rhinovirus species A, B and C. PLoS ONE. 2013;8:e70552. doi: 10.1371/journal.pone.0070552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niespodziana K, Napora K, Cabauatan C, Focke-Tejkl M, Keller W, Niederberger V, et al. Misdirected antibody responses against an N-terminal epitope on human rhinovirus VP1 as explanation for recurrent RV infections. FASEB J. 2012;26:1001–8. doi: 10.1096/fj.11-193557. [DOI] [PubMed] [Google Scholar]
- 83.Aslan N, Watkin LB, Gil A, Mishra R, Clark FG, Welsh RM, et al. mBio. 2017. Severity of Acute Infectious Mononucleosis Correlates with Cross-Reactive Influenza CD8 T-Cell Receptor Repertoires. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Odumade OA, Knight JA, Schmeling DO, Masopust D, Balfour HH, JR, Hogquist KA. Primary Epstein-Barr virus infection does not erode preexisting CD8(+) T cell memory in humans. J Exp Med. 2012;209:471–8. doi: 10.1084/jem.20112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watkin LB, Mishra R, Gil A, Aslan N, Ghersi D, Luzuriaga K, Selin LK. Unique influenza A cross-reactive memory CD8 T-cell receptor repertoire has a potential to protect against EBV seroconversion. Journal of Allergy and Clinical Immunology. 2017;140:1206–10. doi: 10.1016/j.jaci.2017.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S, Bakshi RK, Suneetha PV, Fytili P, Antunes DA, Vieira GF, et al. Frequency, Private Specificity, and Cross-Reactivity of Preexisting Hepatitis C Virus (HCV)-Specific CD8+ T Cells in HCV-Seronegative Individuals: Implications for Vaccine Responses. J Virol. 2015;89:8304–17. doi: 10.1128/JVI.00539-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kasprowicz V, Ward SM, Turner A, Grammatikos A, Nolan BE, Lewis-ximenez L, et al. Defining the directionality and quality of influenza virus - specific CD8 + T cell cross-reactivity in individuals infected with hepatitis C virus. Journal of Clinical Investigation. 2008;118:1143–53. doi: 10.1172/JCI33082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ertl HC. Viral vectors as vaccine carriers. Curr Opin Virol. 2016;17:1–8. doi: 10.1016/j.coviro.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 89.Kotterman MA, Chalberg TW, Schaffer DV. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 90.Singh S, Vedi S, Samrat SK, Li W, Kumar R, Agrawal B. Heterologous immunity between adenoviruses and hepatitis C virus: A new paradigm in HCV immunity and vaccines. PLoS ONE. 2016;11:1–23. doi: 10.1371/journal.pone.0146404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122:359–67. doi: 10.1172/JCI60202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pennington SH, Thompson AL, Wright AKA, Ferreira DM, Jambo KC, Wright AD, et al. Oral Typhoid Vaccination With Live-Attenuated Salmonella Typhi Strain Ty21a Generates Ty21a-Responsive and Heterologous Influenza Virus-Responsive CD4 + and CD8 + T Cells at the Human Intestinal Mucosa. Journal of Infectious Diseases. 2016;213:1809–19. doi: 10.1093/infdis/jiw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tenembaum S, Chitnis T, Ness J, Hahn JS. Acute disseminated encephalomyelitis. Neurology 2007:23–3 [DOI] [PubMed]
- 94.Karussis D P P T s o p-v i C d syndromes} Autoimmun Rev. 2014;13:215–24. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Ravaglia S, Ceroni M, Moglia A, Todeschini A, Marchioni E. Post-infectious and post-vaccinal acute disseminated encephalomyelitis occurring in the same patients. J Neurol. 2004;251:1147–50. doi: 10.1007/s00415-004-0498-9. [DOI] [PubMed] [Google Scholar]
- 96.Athauda D, Andrews TC, Holmes PA, Howard RS. Multiphasic acute disseminated encephalomyelitis (ADEM) following influenza type A (swine specific H1N1) J Neurol. 2012;259:775–8. doi: 10.1007/s00415-011-6258-8. [DOI] [PubMed] [Google Scholar]
- 97.Au WY, Lie AKW, Cheung RTF, Cheng PW, Ooi CGC, Yujenc K-Y, Kwong Y-L. Acute disseminated encephalomyelitis after para-influenza infection post bone marrow transplantation. Leuk Lymphoma. 2002;43:455–7. doi: 10.1080/10428190290006350. [DOI] [PubMed] [Google Scholar]
- 98.Sellers SA, Hagan RS, Hayden FG, Fischer WA. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11:372–93. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blackmore S, Hernandez J, Juda M, Ryder E, Freund GG, Johnson RW, Steelman AJ. Influenza infection triggers disease in a genetic model of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2017;114:E6107–E6116. doi: 10.1073/pnas.1620415114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Q, Liu Y, Lu A, Ni K, Xiang Z, Wen K, Tu W. Influenza virus infection exacerbates experimental autoimmune encephalomyelitis disease by promoting type I T cells infiltration into central nervous system. J Autoimmun. 2017;77:1–10. doi: 10.1016/j.jaut.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 101.Sriram S, Steiner I. Experimental allergic encephalomyelitis: A misleading model of multiple sclerosis. Ann Neurol. 2005;58:939–45. doi: 10.1002/ana.20743. [DOI] [PubMed] [Google Scholar]
- 102.Pohl-Koppe A, Burchett SK, Thiele EA, Hafler DA. Myelin basic protein reactive Th2 T cells are found in acute disseminated encephalomyelitis. J Neuroimmunol. 1998;91:19–27. doi: 10.1016/S0165-5728(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 103.Hennes E-M, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89:900–8. doi: 10.1212/WNL.0000000000004312. [DOI] [PubMed] [Google Scholar]
- 104.Boucher A, Desforges M, Duquette P, Talbot PJ. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin Immunol. 2007;123:258–67. doi: 10.1016/j.clim.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peschl P, Bradl M, Höftberger R, Berger T, Reindl M. Myelin Oligodendrocyte Glycoprotein: Deciphering a Target in Inflammatory Demyelinating Diseases. Front Immunol. 2017;8:529. doi: 10.3389/fimmu.2017.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lehmann HC, Hartung H-P, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infectious Diseases. 2010;10:643–51. doi: 10.1016/S1473-3099(10)70140-7. [DOI] [PubMed] [Google Scholar]
- 107.Martín Arias LH, Sanz R, Sáinz M, Treceño C, Carvajal A. Guillain-Barré syndrome and influenza vaccines: A meta-analysis. Vaccine. 2015;33:3773–8. doi: 10.1016/j.vaccine.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 108.Nachamkin I, Shadomy SV, Moran AP, Cox N, Fitzgerald C, Ung H, et al. Anti-ganglioside antibody induction by swine (A/NJ/1976/H1N1) and other influenza vaccines: insights into vaccine-associated Guillain-Barré syndrome. J Infect Dis. 2008;198:226–33. doi: 10.1086/589624. [DOI] [PubMed] [Google Scholar]
- 109.Partinen M, Kornum BR, Plazzi G, Jennum P, Julkunen I, Vaarala O. Narcolepsy as an autoimmune disease: The role of H1N1 infection and vaccination. The Lancet Neurology. 2014;13:600–13. doi: 10.1016/S1474-4422(14)70075-4. [DOI] [PubMed] [Google Scholar]
- 110.Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 111.Ahmed SS, Volkmuth W, Duca J, Corti L, Pallaoro M, Pezzicoli A, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7:294ra105. doi: 10.1126/scitranslmed.aab2354. [DOI] [PubMed] [Google Scholar]
- 112.Saariaho A-H, Vuorela A, Freitag TL, Pizza F, Plazzi G, Partinen M, et al. Autoantibodies against ganglioside GM3 are associated with narcolepsy-cataplexy developing after Pandemrix vaccination against 2009 pandemic H1N1 type influenza virus. J Autoimmun. 2015;63:68–75. doi: 10.1016/j.jaut.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 113.Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, Dauvilliers Y, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–9. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lind A, Ramelius A, Olsson T, Arnheim-Dahlström L, Lamb F, Khademi M, et al. A/H1N1 antibodies and TRIB2 autoantibodies in narcolepsy patients diagnosed in conjunction with the Pandemrix vaccination campaign in Sweden 2009-2010. J Autoimmun. 2014;50:99–106. doi: 10.1016/j.jaut.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 115.Katzav A, Arango MT, Kivity S, Tanaka S, Givaty G, Agmon-Levin N, et al. Passive transfer of narcolepsy: anti-TRIB2 autoantibody positive patient IgG causes hypothalamic orexin neuron loss and sleep attacks in mice. J Autoimmun. 2013;45:24–30. doi: 10.1016/j.jaut.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 116.Prüss H, Finke C, Höltje M, Hofmann J, Klingbeil C, Probst C, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72:902–11. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berger B, Pytlik M, Hottenrott T, Stich O. Absent anti-N-methyl-D-aspartate receptor NR1a antibodies in herpes simplex virus encephalitis and varicella zoster virus infections. Int J Neurosci. 2017;127:109–17. doi: 10.3109/00207454.2016.1147447. [DOI] [PubMed] [Google Scholar]
- 118.Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272–6. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucchese G, Capone G, Kanduc D. Peptide sharing between influenza A H1N1 hemagglutinin and human axon guidance proteins. Schizophr Bull. 2014;40:362–75. doi: 10.1093/schbul/sbs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lucchese G. Understanding Neuropsychiatric Diseases, Analyzing the Peptide Sharing between Infectious Agents and the Language-Associated NMDA 2A Protein. Front Psychiatry. 2016;7:60. doi: 10.3389/fpsyt.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wheatland R. Chronic ACTH autoantibodies are a significant pathological factor in the disruption of the hypothalamic-pituitary-adrenal axis in chronic fatigue syndrome, anorexia nervosa and major depression. Med Hypotheses. 2005;65:287–95. doi: 10.1016/j.mehy.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 122.Wheatland R. Molecular mimicry of ACTH in SARS - implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 2004;63:855–62. doi: 10.1016/j.mehy.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beyerlein A, Donnachie E, Ziegler A-G. Am J Epidemiol. 2017. Infections in early Life and development of celiac disease. [DOI] [PubMed] [Google Scholar]
- 124.kagnoff MF, paterson YJ, kumar PJ, kasarda DD, carbone FR, unsworth DJ, austin RK. Evidence for the role of a human intestinal adenovirus in the pathogenesis of coeliac disease. Gut. 1987;28:995–1001. doi: 10.1136/gut.28.8.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mantzaris GJ, Karagiannis JA, Priddle JD, Jewell DP. Cellular hypersensitivity to a synthetic dodecapeptide derived from human adenovirus 12 which resembles a sequence of A-gliadin in patients with coeliac disease. Gut. 1990;31:668–73. doi: 10.1136/gut.31.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kupfer SS, Jabri B. Pathophysiology of celiac disease. Gastrointest Endosc Clin N Am. 2012;22:639–60. doi: 10.1016/j.giec.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 128.Massilamany C, Gangaplara A, Steffen D, Reddy J. Identification of novel mimicry epitopes for cardiac myosin heavy chain-? that induce autoimmune myocarditis in A/J mice. Cell Immunol. 2011;271:438–49. doi: 10.1016/j.cellimm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 129.Gangaplara A, Massilamany C, Brown DM, Delhon G, Pattnaik AK, Chapman N, et al. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-α-reactive CD4 T cells in A/J mice. Clin Immunol. 2012;144:237–49. doi: 10.1016/j.clim.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 130.Root-Bernstein R. Rethinking Molecular Mimicry in Rheumatic Heart Disease and Autoimmune Myocarditis: Laminin, Collagen IV, CAR, and B1AR as Initial Targets of Disease. Front Pediatr. 2014;2:85. doi: 10.3389/fped.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Igoe A, Scofield RH. Autoimmunity and infection in Sjögren’s syndrome. Curr Opin Rheumatol. 2013;25:480–7. doi: 10.1097/BOR.0b013e32836200d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tong L, Koh V, Thong B-H. Review of autoantigens in Sjögren’s syndrome: An update. J Inflamm Res. 2017;10:97–105. doi: 10.2147/JIR.S137024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh N, Cohen PL. The T cell in Sjogren’s syndrome: Force majeure, not spectateur. J Autoimmun. 2012;39:229–33. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stathopoulou EA, Routsias JG, Stea EA, Moutsopoulos HM, Tzioufas AG. Cross-reaction between antibodies to the major epitope of Ro60 kD autoantigen and a homologous peptide of Coxsackie virus 2B protein. Clin Exp Immunol. 2005;141:148–54. doi: 10.1111/j.1365-2249.2005.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szymula A, Rosenthal J, Szczerba BM, Bagavant H, Fu SM, Deshmukh US. T cell epitope mimicry between Sjögren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol. 2014;152:1–9. doi: 10.1016/j.clim.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lönnrot M, Lynch KF, Elding Larsson H, Lernmark Å, Rewers MJ, Törn C, et al. Diabetologia. 2017. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: The TEDDY study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus—why the β cells? Nature Reviews Endocrinology. 2016;12:263–73. doi: 10.1038/nrendo.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hiemstra HS, Schloot NC, van Rood JJ, Willemen SJM, Franken KLMC, de Vries RRP, et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 2001;98:3988–91. doi: 10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Honeyman MC, Stone NL, Falk BA, Nepom G, Harrison LC. Evidence for molecular mimicry between human T cell epitopes in rotavirus and pancreatic islet autoantigens. J Immunol. 2010;184:2204–10. doi: 10.4049/jimmunol.0900709. [DOI] [PubMed] [Google Scholar]
- 140.Qi Z, Hu H, Wang Z, Wang G, Li Y, Zhao X, et al. J Diabetes Investig. 2017. Antibodies against H1N1 influenza virus cross-react with ?-cells of pancreatic islets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–54. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 143.Lötvall J, Pawankar R, Wallace DV, Akdis CA, Rosenwasser LJ, Weber RW, et al. We Call for iCAALL: International Collaboration in Asthma, Allergy and Immunology. The World Allergy Organization Journal. 2012;5:39–40. doi: 10.1097/WOX.0b013e3182504245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations—a GA2 LEN-DARE systematic review. Allergy. 2011;66:458–68. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lukkarinen M, Koistinen A, Turunen R, Lehtinen P, Vuorinen T, Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140:988–95. doi: 10.1016/j.jaci.2016.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Garn H, Renz H. Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology. 2007;212:441–52. doi: 10.1016/j.imbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 149.Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C, et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol. 2017;18:1310–20. doi: 10.1038/ni.3857. [DOI] [PubMed] [Google Scholar]
- 150.Chang Y-J, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206:2869–77. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wohlleben G, Muller J, Tatsch U, Hambrecht C, Herz U, Renz H, et al. Influenza A Virus Infection Inhibits the Efficient Recruitment of Th2 Cells into the Airways and the Development of Airway Eosinophilia. J Immunol. 2003;170:4601–11. doi: 10.4049/jimmunol.170.9.4601. [DOI] [PubMed] [Google Scholar]
- 153.Skevaki C, Hudemann C, Matrosovich M, Möbs C, Paul S, Wachtendorf A, et al. J Allergy Clin Immunol. 2017. Influenza-derived peptides cross-react with allergens and provide asthma protection. [DOI] [PubMed] [Google Scholar]
- 154.Bien CG, Bauer J. Autoimmune epilepsies. Neurotherapeutics. 2014;11:311–8. doi: 10.1007/s13311-014-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–95. doi: 10.3109/07853890.2010.505931. [DOI] [PubMed] [Google Scholar]
- 156.Lerner A, Arleevskaya M, Schmiedl A, Matthias T. Microbes and Viruses Are Bugging the Gut in Celiac Disease. Are They Friends or Foes? Front Microbiol. 2017;8:1392. doi: 10.3389/fmicb.2017.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]


