- I.
- II.
- III.
- IV.
I. General Remarks
Widely divergent views regarding vaccines and their use have appeared recently in the human, veterinary, and lay literature (Carmichael, 1983; Dodds, 1991; Holmes, 1996; Pitcairn, 1995; Priest, 1996; Smith, 1995; Starita-Mehan, 1997; Tizzard, 1990; Yarnall, 1995). Strong opinions have been voiced by many individuals and an increasingly wide public desires to know “the facts” that underlie vaccine use and the basis of immunization regimens. Impassioned, sometimes uninformed, concerns have been expressed regarding vaccine efficacy and safety, the need for certain vaccines, the frequency with which vaccines are given, the need for annual vaccination—indeed, whether vaccines should be used at all! The questions are not new, for they have been raised since Mithridates VI, an ancient Greek king of Pontus (first century b.c.), attempted to protect himself against poisoning by repeatedly taking small amounts of noxious substances, in honey (theriaca)—a practice not dissimilar to certain contemporary holistic rituals. Since the introduction of variolation from the Near East in the early eighteenth century and the use of cowpox virus against Variola, there has been public concern about the safety and efficacy of vaccines. The concept of “safety” has changed with time, for reactions that were common when the risks of a serious disease were great are no longer acceptable. Although often exaggerated by individual passions, or groups who champion their own doctrines, several of today's concerns seem justified, especially when recognized problems with vaccines are not addressed in the light of existing knowledge or experience.
Rapidly changing attitudes toward pets, their value, and their health care have provoked vigorous and widespread discussion of the use of vaccines in small animal practice (Priest, 1996; Schultz, 1995; Smith, 1995; Tizzard, 1990). This is illustrated by the full-day session devoted to vaccination practices at the July 1996 annual meeting of the AVMA at Cornell's Feline Practitioner's Seminar and at a recent symposium, European Symposium on Pet Vaccinology, held in France in September 1996. In an ideal world, real or presumed problems with companion animal vaccines would be addressed quickly and responsibly by industry, government regulatory officials, and the veterinary profession as soon as they are identified. Unfortunately, problems often are neglected or avoided. This circumstance contributes to confusion and the creation of myths, which often are enhanced by differing views of “experts” who have sometimes formed their own conclusions with limited data or biased sampling designs.
Misfortunes with vaccines are well documented in the literature (Appel and Gillespie, 1972; Carmichael, 1983, 1997; Martin, 1985; Rikula et al., 1995; Tizzard, 1990; Wilbur et al., 1994; Wilson et al., 1986). They have often become elevated to catastrophes, however, especially by those who advocate a radical philosophy but ignore the benefits provided by vaccines. Concerns have sometimes led to the senseless conclusion that all vaccines are dangerous and are a direct or indirect cause of chronic illness (“vaccinosis”). “Vaccinoses” are claimed to range from “devastated immune systems, laziness, bowel disease, bloat, stained teeth, ulcers, chronic gastroenteritis, autoimmune hemolytic anaemia, and seizures,” to list but a few conditions that have been cited (Duval and Giger, 1996; Pitcairn, 1995; Priest, 1996; Starita-Mehan, 1997; Yarnall, 1995). However, there are truths between the passion of some and indifference of others. Advocates of holistic vaccination practices, as inane as they may seem, may actually be doing a service to pet fanciers by bringing issues to the fore which have largely been ignored. Unfortunately, legitimate safety or efficacy problems have sometimes been disregarded until major misfortunes occurred. Nevertheless, those who experienced the rampant distemper outbreaks prior to the mid-1960s are amazed by the arguments presented by some critics, especially holistic believers. Within 2–3 years of the advent of efficacious distemper vaccines, the disease practically disappeared in vaccinated populations, but it has reappeared whenever vaccination had diminished. Undeniable progress has been made in the suppression of canine distemper and infectious hepatitis and, more recently, in controlling the canine parvovirus pandemic in a remarkably short period of time. However, the recent outbreaks of distemper in Scandinavia, and this year's epizootic in Alaska and northern Quebec, illustrate what may occur when distemper vaccine efficacy, or vaccine use, diminishes. Veterinarians and the public have become more sophisticated—and litigious; they want to know the facts about treatments they use. Unfortunately, many essential facts regarding vaccines are lacking and myths continue to flourish.
Questions commonly asked by dog owners/breeders and veterinarians are usually complex: Are all vaccines available for dogs necessary? Are vaccines safe in very young pups? How effective are they in preventing disease? Do both live and inactivated vaccines produce a sterilizing immunity so as to interrupt transmission? How soon does immunity occur after vaccination and how long does it endure? Why do vaccines continue to be developed against diseases that are still poorly understood? Are too many agents packaged as multicomponent vaccines, and what are the consequences? It has been well established that the immune system can respond normally to several different antigens—an issue that seem to persist; however, some combined vaccines that had inadequate field trial data prior to release have given rise to serious consequences in regard to safety. Unfortunately, answers to the questions above often reflect individual experiences, vested interests, or a disinclination to state that true answers are not known.
It has been estimated that more than 50% of office visits to veterinarians are associated with vaccination. Several vaccines for dogs (and cats) have been licensed that have poor or questionable efficacy; yet they continue to be produced and promoted, for example, Leptospira bacterins, some canine coronavirus (CCV) vaccines and, in the recent past, several canine parvovirus type 2 (CPV-2) vaccines.
New or “improved” vaccines are introduced almost yearly, yet even perfunctory examination reveals a sparse amount of data that often overstates claims for a particular product. On the other hand, questions posed by veterinarians, dog owners, or by those who oppose vaccination on philosophical grounds often defy factual answers because of the paucity of published results. Questions are often based on the perception that valid data are available. Also, many individuals do not accept the reality that vaccination, as other medical practices, sustain some risk. To a large extent, problems in standardizing veterinary vaccines resist solution because of the complexity inherent in the number of different vaccines and viral strains available for pet animals, most of which are poorly characterized.
I share the belief that expectations for vaccines are at a turning point. In this article I outline some personal views and experiences, note unsettled problems, and point out the difficulties in resolving some of the commonly asked questions. Notwithstanding, I am aware that my remarks will have little impact unless veterinarians concerned with dog vaccines show the same concerns as those raised by the Feline Practitioner's Association and act to gain a better understanding of vaccines, how they work, a realistic appreciation of the problems that can occur, and how they might be remedied.
II. Veterinary Vaccines
Most veterinary vaccines continue to be developed empirically. With the technology now available, new vaccines will doubtless continue to be developed, including subunit vaccines, vectored recombinant vaccines, deletion mutants, nucleic acid (plasmid DNA) vaccines and, perhaps, even “recombinant nosodes” (sic). When made available, however, their merits should be evaluated against presently used products, not merely for the sake of novelty. Some recombinant vaccines, for example, vaccinia-vectored rabies for wildlife, a recently licensed canary pox-vectored distemper vaccine, and a Lyme disease vaccine, have shown merit in their utility, safety, or, in some cases, superior efficacy.
With few exceptions, modified live virus (MLV) vaccines are the most common products used worldwide (Appel, 1987; Carmichael, 1997). Most vaccines comprise virus strains which were selected as spontaneous mutants that emerged from the native viral populations during repeated passage in cell cultures or other laboratory hosts. But, the majority of vaccines consist of viral populations that contain multiple mutations and few canine vaccinal strains have been biologically cloned so as to suppress the generation of nonimmunizing mutants during laboratory passage to vaccine.
Mutants that grow in the intended host, yet are replication restricted in critical tissues, constitute vaccines with different degrees of loss of natural virulence (“attenuated virus”) Nonimmunizing mutants also may emerge during laboratory passage. Such variants may fail to grow in the natural host, yet proliferate luxuriantly in tissue cultures or chick embryos. Because “attenuation” means reduction, not absolute loss of the capacity to produce disease, safety problems may not be revealed until extensive field tests have been conducted; unfortunately, this has occurred after a product has been licensed and marketed. A conspicuous example of such failure was the large number of dogs that died or suffered serious illness following the introduction of a live canine coronavirus vaccine in 1983 (Martin, 1985; Wilson et al., 1986). Also a vaccine judged harmless for one species may provoke illness in another one (Appel, 1987; Carmichael, 1997; Tizzard, 1990). Because of the uncertainty of absolute safety with certain vaccines, for example, distemper vaccinal strains propagated in canine cell cultures, live viral vaccines are not recommended for most wildlife species, pregnant animals, unweaned pups, or pups that are ill. Yet, breeders and some veterinarians continue to vaccinate pregnant dams, pups as early as 2 weeks of age, or use vaccines for pet species where safety information in limited (e.g., ferrets).
Efficacy problems persist with certain “primary” vaccines, such as some canine parvovirus vaccines and certain canine distemper products (Appel and Gillespie, 1972; Carmichael, 1983, 1989, 1997; Schultz, 1995, 1996). However, the recent improvements in several canine vaccines, especially parvovirus vaccines that previously had poor or marginal efficacy, have been greatly improved, and they now appear to provoke good immune responses. Whether the improvements will be sustained depends in large measure on the care taken by vaccine producers in selecting and conserving their seed stock.
III. Comments on Selected Vaccines
A. Canine Distemper
Virtually all licensed canine distemper (CD) vaccines consist of living attenuated viral strains (Appel, 1987; Appel and Gillespie, 1972; Carmichael, 1997). The majority are produced from the egg-adapted or avian cell culture-adapted Onderstepoort strain or the “Rockborn strain,” which is propagated in canine cell cultures (Rockborn et al., 1965). The Rockborn strain is produced legitimately only by the European company authorized by Professor Gunnar Rockborn (G. Rockborn, personal communication, 1996). That virus had undergone ≤56 passages in cell cultures, whereas virus in several U.S. vaccines is used at lower passage levels. Thus, the designation “Rockborn CD vaccine strain” has been misrepresented by several authors in the past, including myself. Certain products also are claimed to contain the attenuated “Snyder Hill” strain, also grown in canine cell cultures, or a ferret-origin strain cultivated in avian cell cultures. It is difficult to determine the origin of those viral strains in a CD vaccine because some strains have been given novel designations by manufacturers and there are few genetic markers. Regardless of the viral strain employed, attenuated CD vaccines have proved highly effective when administered to dogs lacking maternal immunity, but they are variably effective in dogs with low levels of maternal antibodies.
As noted earlier, a recognized problem with certain CD vaccines, especially those propagated in canine cell cultures, is the variable occurrence of postvaccinal (PV) encephalitis, but actual risks are unknown (Appel, 1978, 1987; Appel and Gillespie, 1972; Carmichael, 1983). Some CD vaccines are virulent for several zoo or wildlife species, some of which are now considered pets (e.g., ferrets, skunks, raccoons). Also, reversion to virulence of the attenuated Rockborn CD strain was demonstrated after serial passage in dogs, or in dog lung macrophages (Appel, 1978). The canine cell-adapted vaccines are not recommended for pups less than 6 weeks of age, or for wildlife species, because of the greater risk of postvaccinal encephalitis. Field experience has demonstrated enhanced virulence of CD vaccines produced in canine cell cultures when administered in combination with certain other viruses. The most conspicuous have been canine adenovirus type 1 (CAV-1, ICH) and live CCV vaccines (Carmichael, 1983; Martin, 1985; Wilson et al., 1986). Notwithstanding the risks noted earlier, the Rockborn strain (at passage level ~55–60) has been used as our laboratory's principal experimental vaccine for >40 years. The vaccine is adjusted to ~103 TCD50/dose, since the minimal immunizing dose (MID) of the “Rockborn strain” is ~20 TCD50. In such instances, no cases of postvaccinal encephalitis have been observed in field use. However, in laboratory experiments, when the vaccine dose was more than 10.5.5 TCD50/ml, or when vaccine was given together with live CAV-1, encephalitis was a frequent occurrence about 10–12 days postvaccination. Field reports indicated that the frequency of postvaccinal CD encephalitis diminished greatly after the substitution of CAV-2 for CAV-1 in combined vaccines. This was one reason for advocating the Substitution of CAV-2 for CAV-1 in canine vaccines, in addition to the marked, but not total, reduction in postvaccinal “blue eyes” that occurred frequently after vaccination with CAV-1. Manufacturers who utilize canine cell-grown CDV should, therefore, determine optimal safe doses. My personal view is that each vaccine should have the MID indicated on the package insert; this seems important with both CD and CPV-2 vaccines, but for different reasons (see below). If CD vaccinal titers were kept low (≤103.0/dose), the excellent immunity provided by CD vaccines grown in canine cells would probably be attended by a more acceptable risk of PV reactions.
Duration of immunity data for most commercial distemper vaccines are limited. In one study at the Baker Institute (L. E. Carmichael, unpublished results, 1980), nine beagles were vaccinated with the Rockborn CD strain and maintained in strict isolation. All dogs had high levels of neutralizing antibodies >6 years later. Also, we have recently confirmed 6.5-year immunity (SN titers ≥1:80) in male dogs that were vaccinated with a commercial (multiple) vaccine and kept as breeding stock in a kennel that maintains strict isolation. Nevertheless, the rates of immunity following vaccination differ between CD vaccines (Appel, 1987; Appel and Gillespie, 1972; Carmichael, 1977; Rikula et al., 1995). As with other canine vaccines, maternal antibodies interfere with immunization. Recently, substantial differences were reported in the ability of CD vaccines to immunize pups with similar levels of maternal antibodies at 6–7 weeks of age (Schultz, 1996).
Early studies on duration of antibody persistence at levels that were estimated to ensure immunity (neutralizing antibody titers 2:1:100) waned within 1 year in 33% of dogs vaccinated with the chick embryo-adapted “Lederle low passage” CD strain; 2 years later another 33% had antibody titers < 1:100 (Baker et al., 1962, 1962). Those limited data appear to be the basis for the common practice of annual revaccination. Whether an SN titer of 1:100, by the tests done then, is required for protection is uncertain, for it has been stated that SN titers of 1:20 are protective (Appel and Gillespie, 1972; Gorham, 1966). Neutralizing antibodies to the low egg passage Onderstepoort strain also have been reported to last from 3 to 6 years in almost 90% of dogs kept in isolation (Prydie, 1966). Since distemper vaccine efficacy has generally improved in recent years, it now seems reasonable, without being radical, to discontinue recommending annual vaccination after the first year of life, and to limit vaccinations to 3- to 5-year intervals. Notwithstanding, most veterinarians and dog breeders will likely continue annual vaccinations for pecuniary, or other, reasons.
1. Comments
-
•
Minimum immunizing doses for each canine vaccinal strain should be determined. Egg-adapted CD strains appear to vary somewhat in efficacy, while canine-cell adapted strains vary in their capacity to provoke PV encephalitis. The “Rockborn-type” strains should probably contain about 500 MIDs, unless safety has been ensured.
-
•
Duration of immunity data are needed. Some vaccines, especially those propagated in the chick embryo or Vero cells, appear to provoke shorter durations of immunity than do other vaccines. However, data are scant. Such data are essential to the formulation of rational recommendations.
-
•
Safety of canine-cell grown (“Rockborn-type” including “Snyder Hill” strains) should be more rigorously studied, especially if used in combination with other agents.
-
•
There is a need for an effective nonliving CD vaccine especially for wildlife species. Promising experiments with a recombinant (canarypox) distemper product that protected dogs against challenge with virulent distemper virus suggests the possibility for success of such vaccines (Taylor et al., 1994). One recombinant CD product has recently been licensed in the United States, but unequivocal recommendation should be withheld until field studies have demonstrated its efficacy and duration of immunity.
B. Canine Parvovirus Type 2
Several vaccines have been developed for CPV-2 infection, but immune response data on most CPV-2, or CPV-2a, -b, strains are limited, except for brief periods (2–3 weeks) following vaccination. Immunity to CPV-2 is believed to be antibody mediated and hemagglutination-inhibiting (HI) titers ≥1:80 are considered protective (Carmichael, 1983, 1994, 1997; Carmichael et al., 1983; Pollock and Carmichael, 1990). However, serologic tests are not standardized and comparison of antibody titers from different laboratories is not too meaningful (Luff et al., 1987).
Inactivated and MLV vaccines are available in most countries for immunization of dogs. Although inactivated vaccines for CPV-2 provide only limited protection against infection, dogs may be exempt from disease for several months (Carmichael, 1983; Pollock and Carmichael, 1990). Like distemper, reports of the actual duration of immunity to inactivated CPV-2 vaccines are very limited. It is not known whether immunologic memory provides immunity beyond the period when antibody has declined below detectable levels; it also is not known whether all killed vaccines perform in a similar manner since the magnitude of the antibody responses is related to the amount of viral antigen administered. Because inactivated vaccines do not interrupt transmission of virulent virus, except for belief periods of time (~2–3 months), they are not recommended where large numbers of dogs are raised, that is, breeding kennels, pet shops, and animal shelters or where dogs are at high risk of exposure, such as at shows or field trials. It should be obvious that inactivated vaccines should not be followed by MLV vaccines, or the reverse, because antibodies engendered by the killed vaccine will neutralize the live virus; in the latter instance, the killed vaccine would be wasted if the MLV vaccine had immunized.
Efficacious modified-live CPV-2 vaccines have been highly successful in preventing parvovirus infection when administered to seronegative pups, or to dogs with very low antibody titers. They normally engender rapid and enduring immunity, and it is probable that immunity persists for several years. HI antibody titers > 1:320 persisted for periods as long as 6 years in 13 dogs vaccinated with one strain (Cornell LP strain 780916). In recent tests, 5 male dogs that had received a commercial product (combined vaccine), and were maintained in a commercial specific pathogen-free colony, had titers > 1:320 more than 6.5 years later. Similar studies with other CPV-2 vaccines have not been published, but tests done in our laboratory in 1987–1990 revealed that serum HI antibody titers in dogs that had received certain commercial vaccines had declined to ≤l:10 within 2–2.5 years. Thus, differences have been observed between vaccines, but several of the ones tested earlier have now been replaced by “new generation” products.
As with CD, a principal cause of vaccination failures in pups is maternal antibody interference, which has been amply exploited by biologics producers in promoting “new vaccines” that claim to immunize pups earlier than do competing products. The reality is that live virus vaccines differ in their capacity to evade low levels of antibodies and no vaccine has been shown to immunize pups at the time when they have maternal antibody levels that prevent infection with virulent virus. The concept of the “critical period” (or “window of vulnerability”) was developed to describe that period of time when pups become susceptible to infection with virulent virus, but respond unpredictably to vaccines (Carmichael, 1989; Pollock and Carmichael, 1990). The critical period has been shown to range from 2–5 weeks, but it is briefer with some vaccines than with others; that is some vaccines may immunize pups earlier than to others, regardless of age (Carmichael, 1989, 1997; Hoskins et al., 1995; Schultz, 1995). Failures to respond to efficacious vaccines relate to prevaccination antibody titers, but not age.
No modified live CPV-2 vaccine has been reported to cause adverse reactions, and the myth of “immunosuppression” by virulent CPV-2, or vaccine virus, has been discredited (Brunner and Swango, 1985; Phillips and Schultz, 1987). Indeed, a recent study in Japan indicated that modified live CPV-2 vaccines enhance cellular immune responses; when vaccine was given to dogs prior to surgery, it prevented the postsurgical immunosuppression attending the use of halothane anaesthesia (Taura et al., 1995).
Despite the general benefit derived from CPV-2 vaccines, consistent efficacy has been a recurring problem. Several commercial (MLV) vaccines that were studied in our laboratory, and found effective at the time they were launched, later had poor efficacy. This is likely due to genetic heterogeneity of the seed stock. Such occurrences have prompted new products, including vaccines prepared from isolates that represent variants (CPV-2a,-b) of the original CPV-2. However, it is evident that vaccines prepared from the more recent CPV-2 types have no discernible advantage over efficacious vaccines prepared from the original isolates.
The USDA's “master seed principle” does not appear to function well with CPV-2 vaccines. The principle may be sound, but it hasn't always worked in practice. Reasons are not documented for most vaccine strains, but mutant viruses that fail to provoke immunity often predominate after several passages in cell cultures (Fig. 1 ). Vaccines that we have examined, with two exceptions, consisted of mixed viral populations. Manufacturers should, therefore, prepare seed virus from biologically cloned stock, selecting those clones shown to immunize and which are stable during subsequent cell culture passage from seed stock to vaccine.
Fig. 1.
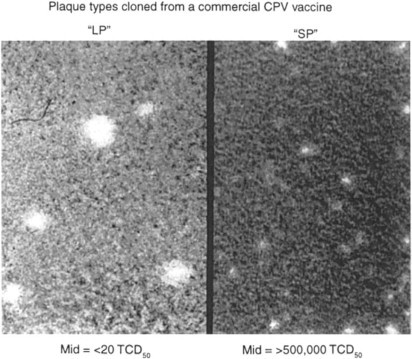
Plaque variants and immunogenicity of clones from a CPV-2 vaccine (1989). The original vaccinal virus population was predominantly “small plaque” (SP), with approximately 2% “large plaques” (LP). The vaccine immunized pups at > 10.5.5 TCD50 virus, but not with 102 TCD50. Selected “SP” variants failed to provoke HI antibody responses in pups, even at doses > 106. In contrast, the “LP” variants produced strong HI antibody responses (1:5120–1:10,240) within 10 days of vaccination.
The term high titered vaccine has been promoted in advertising, but the term has very little meaning if the minimal immunizing dose is not revealed. A few years ago, we tested two widely used commercial vaccines that had viral infectivity titers of >105.5/dose, yet they provoked only low antibody responses in SPF dogs. Those products have been supplanted by “new generation vaccines” and it appears that, during the past 2 years, most CPV-2 vaccines have improved substantially. Most CPV-2 cases/outbreaks now are reported in unvaccinated dogs, breeding kennels or animal shelters. Animals vaccinated with ineffective vaccines, pups that had interfering levels of maternal antibodies at the time of vaccination, and puppies in contaminated kennels are at greatest risk of infection, especially where stringent hygiene is not practiced.
Claims that a vaccine will “immunize pups more efficiently at a particular age” are misleading. Failures to respond to good vaccines are related to prevaccinal antibody levels, not age. Also, we have never observed the failure of a susceptible dog to respond to efficacious CPV-2 or CD vaccines, regardless of breed (e.g., Rottweilers). Studies in our laboratory of nearly 1000 field sera from breeding-age dogs indicated that approximately 25% would not be expected to respond until after 12 weeks of age. On the other hand, studies on vaccine response-versus-age have reported a higher success rate at 12 weeks of age in pups from kennels where the dams’ antibody titers were probably low as a result of vaccination rather than infection (Hoskins et al., 1995; Larson and Schultz, 1996; Schultz, 1995). Control of CPV-2 during the initial 3 months of a pup's life should be based on stringent management and prudent vaccination—pups should be isolated as much as possible and kept in a sanitary environment. The availability of dependable good vaccines is essential.
The foregoing remarks notwithstanding, the general success of vaccines in controlling canine parvovirus infections has been remarkable and the improved vaccine efficacy during the past 2 years inspires confidence that parvoviral infections will continue to be uncommon in vaccinated dogs reared in hygienic environments. Inactivated, MLV, and heterotypic (feline parvovirus) vaccines are currently available, but homologous MLV vaccines are recommended for most dogs because they interrupt virulent CPV-2 transmission. Although all MLV vaccines have not been the same with regard to their efficacy there have been no documented safety problems with any parvovirus vaccine in the 15 years since introduction. Note also that attempts to “boost” low antibody titer (i.e., HI titers ≥1:40) are ineffectual.
1. Comments
-
•
Vaccinal seed stock strains should be biologically cloned to provide more uniform and stable viral populations in order to ensure more constant efficacy.
-
•
It would seem beneficial to consider “primary” vaccines for pups less than 3 months of age that contain only CD and CPV-2 components. Multiple vaccines are suggested at 12 weeks of age, unless pups are at high risk for respiratory infections (e.g., animal shelters, pet shops, etc.).
-
•
Because efficacious CPV-2 and distemper vaccines have been shown to provide immunity for at least 5 years, revaccination at 3–5 years, after the first year, seems a conservative strategy.
-
•
Parvovirus vaccines are exceptionally safe. Dogs that develop signs and symptoms of parvovirus infection within 5 days of vaccination should be considered as infected with virulent virus prior to, or at the time of, vaccination. This is still a common occurrence where parvovirus is more likely to be present in the environment (e.g., “puppy mills,” pet shops, dog shows, animal shelters, veterinary clinics).
C. Canine Coronavirus
Canine infections caused by CCV, a virus that may infect both cats and dogs, occur as sporadic cases or kennel outbreaks of mild to explosive (Appel, 1987; Binn et al., 1975; Pastoret, 1984; Pollock and Carmichael, 1990; Tennant et al., 1993). Although CCV is frequently observed by electron microscopy (EM) in the feces of both normal and diarrheic dogs, the true role that CCV plays in canine enteric illness, or the need for vaccines, has yet to be agreed on; however, millions of doses have been sold. Disease associated with CCV is usually attended by low mortality, but occasional deaths occur in young pups. Dogs under stress of intensive training or crowding and those who shelter additional enteric pathogens seem to be at greater risk of illness.
The biology of CCV, and its close relatives in cats and pigs, is still unclear. Serologic cross-reactions have been demonstrated between CCV, feline infectious peritonitis/feline enteric coronavirus (FECV), and transmissible gastroenteritis of swine, but cross-protection has been reported only between CCV and FECV (Coyne and May, 1995). Most reports on CCV have been case reports or epizootiologic studies, where CCV particles in diarrheic feces have ranged from <1% of normal stools to about 75% prevalence in rescue kennels (Rimmelzwaan, 1990; Tennant et al., 1993; Vieler and Herbst, 1995). A controlled study in the Netherlands detected CCV by ELISA tests in 7% of normal stools and in 11% of diarrheic stools (Rimmelzwaan, 1990). Cases are rarely reported since they are usually mild, with the exception of infrequent outbreaks with fatal cases, usually in young pups.
Laboratory studies have confirmed that mixed infections by CCV and CPV-2 result in more severe disease than that caused by either virus alone (Appel, 1988), an argument commonly used to justify the use of CCV vaccines. However, it has not been reported that vaccination of dogs with CCV vaccine prevents the severe manifestations of concurrent, or closely spaced, infections with both viruses. One study of possible benefit by an inactivated CCV vaccine to prevent the serious consequences attending infection by CPV-2 and CCV failed to demonstrate protection (M. Appel, unpublished results, 1985). Also, dual infections now appear to be rare in vaccinated dogs as the result of the extensive use of CPV-2 vaccines.
Both inactivated and live CCV vaccines are available (Carmichael, 1997; Coyne and may, 1995; Edwards et al., 1985; Fulker et al., 1995). The history of CCV vaccines is convoluted and not without misfortune. The first licensed modified live CCV vaccine was rescinded shortly after its introduction in 1983 because of severe adverse reactions with lesions that resembled those of FIP (Martin, 1985). Those reactions occurred in an estimated 5% of vaccinated pups, generally ones <12 weeks of age. An inactivated CCV vaccine that had been licensed also was withdrawn from the market shortly after it was issued because of inadequate efficacy. In addition, a second licensed modified live CCV product which was combined with a canine cell-grown CD vaccine was withdrawn because of a high frequency of post vaccinal CD encephalitis. That vaccine has since been reformulated to exclude the distemper component, which appears to have contributed to the problem. Interestingly, the latter CCV vaccine strain had been licensed for use in California, where it had been marketed for more than 10 years in combination with CPV-2 and distemper vaccine, and the manufacturer affirmed that there had been no adverse reactions. The most recently licensed CCV vaccine comprises a killed FECV product, but information on that vaccine, as well as with most others, is limited mainly to promotional information.
The status of CCV infection is controversial since authenticated cases or outbreaks are seldom reported. Notwithstanding, in January 1997 we made several isolations of a CCV from an outbreak of mild enteric disease in a kennel in New Jersey that breeds and trains dogs for the blind. Of interest was the finding that the recent isolates differed from previous isolates in their failure to grow in feline cell cultures and its lack of affinity for the aminopeptidase-N cell receptor, typical of other coronaviruses from cats, dogs, pigs, and humans which were studied (Tresnan et al., 1996; D. Tresnan, personal communication, 1997).
It would seem, therefore, that the development and distribution of CCV vaccines was mainly the result of marketing decisions, not clearly demonstrated need. On the other hand, factual information on CCV disease is limited. Presently, there seems inadequate medical justification for recommending the use of coronavirus vaccines in dogs until further research results are available. A lesson from the experiences with CCV vaccines is that veterinarians should be cautious when administering new products, especially when little data are available other than that required for product licensing.
IV. Summary
The most important canine viral infections are distemper and CPV-2. Problems of variable CD vaccine safety and efficacy persist, but CD vaccines have greatly reduced the prevalence of disease and cases in vaccinated dogs are now rare. Canine hepatitis (ICH, CAV-1 infection) also has been controlled well by vaccines for more than 35 years and it is now rare; the sporadic cases seen in the 1990s have usually occurred in unvaccinated dogs. CAV-2 vaccines should, therefore, continue to be given since they have proved to be safe and effective, and prevent hepatitis as well as adenoviral tracheobronchitis. Failure to vaccinate would likely result in increase in cases of ICH, a serious disease, but never as significant as distemper and CPV infection.
“Are we vaccinating too often?” The question is complex, but the dominant opinion is “yes” (Smith, 1995). The question cannot be responded to unequivocally, however, since manufacturers employ different strains that vary in their immunizing capacity and, probably, duration of immunity. This question was frequent with distemper in the 1960s. At that time, many veterinarians tested batches of the vaccine they used by providing pre- and postvaccinal sera to competent diagnostic laboratories. That practice appeared to benefit veterinarians and dogs, as well as the quality of vaccines.
Unfortunately, many owners and some veterinarians seem to hold the view that infectious diseases such as parvovirus infection can be controlled by frequent vaccination alone. The common practice of dog breeders of vaccinating their animals several times each year is senseless.
Revaccination for distemper and parvovirus infection is suggested at 1 year of age, but recommendations regarding the frequency of most vaccinations given after that time are unclear. Since most distemper and CPV-2 vaccines probably provide immunity that endures several years, vaccination at 3- to 5-year intervals, after the first year, seems a reasonable practice until more data on duration of immunity become available.
“Are too many kinds of vaccines being promoted for dogs?” Distemper and parvovirus vaccines are essential; canine adenovirus vaccines are recommended since the few cases brought to our attention in recent years have been in unvaccinated dogs. Vaccination against respiratory infections is recommended for most dogs, especially those in kennels, or if they are to be boarded. Need has not been clearly established for coronavirus vaccines; Lyme disease vaccines (see below) are useful in preventing illness in areas where the disease exists, but are unnecessary elsewhere since dogs respond rapidly to appropriate antibiotics; current Leptospira bacterins are without benefit since they contain serovars that fail to protect in most areas (noted below).
Lyme disease (LD) was not considered here, but newer recombinant (OspA) vaccines are now available that appear to be safe and effective for at least 1 year and they have not caused vaccine-induced postvaccinal lameness, which has been documented with certain whole-cell Lyme disease bacterins. Lyme disease vaccines should be restricted to dogs in, or entering, endemic areas where infested ticks reside. More than 85% of LD cases occur in the mid-Atlantic and Northeastern States, about 10% in six Midwestern states (Michigan, Minnesota, and Wisconsin), and a smaller percentage in restricted areas of northern California and the Pacific Northwest.
Leptospirosis also was not discussed here, but vaccines are commonly reported as a cause of anaphylaxis and current vaccines do not contain the serovars prevalent in most regions. The vast majority of cases diagnosed at the New York State Diagnostic Lab at Cornell are grippotyphosa and pomona serovars and there have been no recent cases caused by canicola or icterohemorrhagiae serovars. Because leptospirosis is an important disease of dogs, there is an urgent need for more research and the development of safer vaccines that contain the prevalent serovars. In Mexico, dogs may be infected with several serovars and some canine vaccines contain 8–10 serovars.
The conditio sine qua non is the availability of consistently good vaccines. Without standardization of vaccines, it seems difficult to formulate general vaccine recommendations. Effort should be directed to improving and standardizing the important vaccines in current use, not the development of new products, unless need is demonstrated.
The public is becoming increasingly aware of vaccine problems, perhaps even more so than the benefits of vaccination. The reality that all vaccines carry some risk is not fully perceived by many owners and veterinarians. Alternative veterinary medicine is now a growing reality; such practices are being taught in some veterinary colleges and questions pertaining to vaccine safety and efficacy will continue to vex veterinarians, vaccinologists, and vaccine producers. They will have to be addressed. There is a need for better appreciation of the risk of adverse reactions (Duval and Giger, 1996).
Finally, the issues that have been discussed, or recommendations that might be made, will have little influence unless biologics manufacturers and regulatory officials exercise greater responsibility in controlling vaccine quality. This could be encouraged by the appointment of a committee of unbiased experts to review vaccines for each disease and provide recommendations based on available evidence. This view has been discussed at meetings on several occasions during the past 30 years, but it has been largely neglected because of considerations that involve industry interests, indifferent or overburdened government authorities, and the trust by veterinarians and dog owners in advertising. Vaccines and vaccination guidelines for physicians are supervised by the American Academy of Pediatric's Committee on Infectious Diseases and the Advisory Committee on Immunization Practices who advise the medical profession and regulatory authorities (Holmes, 1996). Until the veterinary profession insists on a responsible advisory council, concerns and questions regarding vaccines will continue to be met by conflicting opinions and open the door to “Nosodes” and “Thuja”—whose benefits seem to be understood only by those who use and profit from them.
References
- Appel M. Reversion to virulence of attenuated canine distemper virus in vivo and in vitro. J. Gen. Virol. 1978;41:385–390. [Google Scholar]
- Appel M. In: Horzinek M.C., editor. Vol 1. Elsevier; Amsterdam: 1987. pp. 29–160. (Virus Infections of Vertebrates). [Google Scholar]
- Appel M.J.G. Does canine coronavirus augment the effects of subsequent parvovirus infection? Vet. Med. 1988;83:360–366. [Google Scholar]
- Appel M.J.G., Gillespie J.H. Canine distemper virus. Virol. Monogr. 1972;11:27–48. [Google Scholar]
- Baker J.A., Robson D.S., Hildreth B., Pakkala B. Breed response to distemper vaccination. Proc. Anim. Care Panel. 1962;12:157–162. [PubMed] [Google Scholar]
- Binn L.N., Lazar E.C., Keenan, et al. Recovery and characterization of a coronavirus from military dogs with diarrhea. Proc. 78th Annu. Meet., U.S. Anim. Health Assoc.; Roanoke, VA, 1974 ; 1975. pp. 359–366. [PubMed] [Google Scholar]
- Brunner C.J., Swango L.J. Canine parvovirus infection: Effects on the immune system and factors that predispose to severe disease. Compend. Contin. Educ. Vet. Pract. 1985;7:979–988. [Google Scholar]
- Carmichael L.E. Immunization strategies in puppies—why failures? Compend. Contin. Educ. Pract. Vet. 1983;5:1043–1051. [Google Scholar]
- Carmichael L.E. Canine parvovirus immunization: “Myths and realities.”. Am. Kennel Club Gaz. 1989;Decembers:94–102. [Google Scholar]
- Carmichael L.E. Canine parvovirus type-2: An evolving pathogen of dogs. Ann. Med. Vét. 1994;138:459–464. [Google Scholar]
- Carmichael L.E. In: Veterinary Vaccinology. Pastoret P.-P., Balncou J., Vannier P., Vereschueren C., editors. Elsevier; Amsterdam: 1997. Vaccines for dogs; pp. 327–331. [Google Scholar]
- Carmichael L.E., Pollock R.H.V., Joubert J.C. A modified live canine parvovirus strain with novel plaque characteristics. II: Immune response. Cornell Vet. 1983;71:13–29. [PubMed] [Google Scholar]
- Coyne M.J., May S.W. Considerations in using a canine coronavirus vaccine. Top. Vet. Med. 1995;6:32–34. [Google Scholar]
- Dodds W.J. Vaccine safety and efficacy. Kennel Hotline. 1991;8:2–4. [Google Scholar]
- Duval D., Giger U. Vaccine-associated immune-mediated hemolytic anaemia in the dog. J. Vet. Intern. Med. 1996;10:290–295. doi: 10.1111/j.1939-1676.1996.tb02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards B.G., Fulker R.H., Acree W.M. Evaluating a canine coronavirus vaccine through antigen extinction and challenge studies. Vet. Med. 1985;80:28–33. [Google Scholar]
- Fulker R., Wasmoen T., Atchison H.-J., Acree W. In: Corona- and Related Viruses. Talbot P.J., Levy G.A., editors. Plenum; New York: 1995. Efficacy of an inactivated vaccine against clinical disease caused by canine coronavirus. [DOI] [PubMed] [Google Scholar]
- Gorham J.R. Duration of vaccination immunity and the influence on subsequent prophylaxis. J. Am. Vet. Med. Assoc. 1966;149:699–704. [PubMed] [Google Scholar]
- Holmes S.J. Review of recommendations of the Advisory Committee on Immunization Practices. Centers for Disease Control and Prevention. J. Infect. Dis. 1996;174:S342–S344. doi: 10.1093/infdis/174.supplement_3.s342. [DOI] [PubMed] [Google Scholar]
- Hoskins J.D., Taylor H.W., Gourley K.R. Challenge trial of a new attenuated canine parvovirus vaccine. Proc. Annu. Vet. Med. Forum Am. Coll. Vet. Intern. Med. 1995;Vol. 13:1012. [Google Scholar]
- Larson L.J., Schultz R.D. High-titer canine parvovirus vaccine: Serologic response and challenge-of-immunity study. Vet. Med. 1996;March:1–5. [Google Scholar]
- Luff P.R., Wood G.W., Thornton P.H. Canine parvovirus serology: Collaborative assay. Vet. Rec. 1987;120:270–273. doi: 10.1136/vr.120.12.270. [DOI] [PubMed] [Google Scholar]
- Martin M.L. Canine coronavirus enteritis and a recent outbreak following modified live virus vaccination. Compend. Contin. Educ. Pract. Vet. 1985;7:1012–1017. [Google Scholar]
- Pastoret P.-P. Les infections digestives d'origin viral chez le chien. Ann. Med. Vet. 1984;128:473–483. [Google Scholar]
- Phillips T.R., Schultz R.D. Failure of vaccine or virulent strains of canine parvovirus to induce immunosuppressive effects on the immune system of the dog. Viral Immunol. 1987;1:135–144. doi: 10.1089/vim.1987.1.135. [DOI] [PubMed] [Google Scholar]
- Pitcairn R.H. 2nd ed. Rodale Press; Emmaus, PA: 1995. (Dr. Pitcairn's Complete Guide to Natural Health for Dogs & Cats). [Google Scholar]
- Pollock R.H.V., Carmichael L.E. In: Infectious Diseases of the Dog and Cat. Greene C.E., editor. Saunders; Philadelphia: 1990. Enteric viruses; pp. 226–283. [Google Scholar]
- Priest S.A. Holistic remedies are getting a shot in the arm. Homeopathic nosodes are being examined by some vets as an option to yearly vaccines. Dog World. 1996;January:24–30. [Google Scholar]
- Prydie J. Persistence of antibodies following vaccination against canine distemper and effect of revaccination. Vet. Rec. 1966;78:486–488. doi: 10.1136/vr.78.14.486. [DOI] [PubMed] [Google Scholar]
- Rikula U., Sihvonen L., Voipio H.M., et al. Serum antibody response to canine distemper virus vaccines in beagle dogs. Front. Lab. Anim. Sci. 1995:199. [Google Scholar]
- Rimmelzwaan G. State University of Utrecht; The Netherlands: 1990. Application of enzyme-linked immunosorbant assay systems for the serology and antigen detection in parvovirus, coronavirus and rotavirus infections in dogs in The Netherlands. Canine Parvovirus Infection: Novel Approaches to Diagnosis and Immune Prophylaxis; pp. 39–56. (Thesis). [Google Scholar]
- Rockborn G., Lannek N., Norby E. Comparison between the immunizing effect in dogs and ferrets of living distemper vaccines attenuated in dog tissue culture and embryonated eggs. Res. Vet. Sci. 1965;6:423. [PubMed] [Google Scholar]
- Schultz R.D. Proc. Int. Gastroenter. Symp. North Am. Vet. Conf. 1995. Emerging issue: Vaccines strategies for canine viral enteritis; pp. 19–24. [Google Scholar]
- Schultz R.D. Intervet; Millsboro, DE: 1996. Canine distemper: Comparison of the leading multi-component commercial vaccines; pp. 1–2. (Infect. Dis. Bull.). [Google Scholar]
- Smith C.A. Current concepts: Are we vaccinating too much? J. Am. Vet. Med. Assoc. 1995;207:421–425. [PubMed] [Google Scholar]
- Starita-Mehan D. The Dangers of Vaccinations, and the Advantages of Nosodes for Disease Prevention. Nosode Vaccination. County Way Veterinary Care, Boring, OR. 1997 [Google Scholar]
- Taura Y., Ishi K., Nagami M., et al. Changes in lymphoproliferation and DTH responses after vaccination immediately before surgery in puppies. J. Vet. Med. Sci. 1995;57:899–904. doi: 10.1292/jvms.57.899. [DOI] [PubMed] [Google Scholar]
- Taylor J., Tartaglia J., Riviere, et al. Applications of canarypox (ALVAC) vectors in human and veterinary vaccines. Dev. Biol. Stand. 1994;82:131–135. [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Jones R.C., et al. Studies on the epizootiology of canine coronavirus. Vet. Rec. 1993;132:7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]
- Tizzard I. Risks associated with the use of live vaccines. J. Am. Vet. Med. Assoc. 1990;196:1851–1858. [PubMed] [Google Scholar]
- Tresnan D.B., Levis R., Holmes K.V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine and human coronavirus in serogrup I. J. Virol. 1996:8669–8674. doi: 10.1128/jvi.70.12.8669-8674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieler E., Herbst W. electron microscopic determination of viruses in feces of dogs with diarrhea. Tieraerztl. Prax. 1995;23:66–69. [PubMed] [Google Scholar]
- Wilbur L.A., et al. Abortion and death in pregnant bitches associated with a canine vaccine contaminated with bluetongue virus. J. Am. Vet. Med. Assoc. 1994;204:1762–1765. [PubMed] [Google Scholar]
- Wilson R.B., Holladay J.A., Cave J.A. A neurologic syndrome associated with use of a canine coronavirus-parvovirus vaccine in dogs. Compend. Contin. Educ. Pract. Vet. 1986;8:117–124. [Google Scholar]
- Yarnall C. Tuttle; Rutland, VT: 1995. (Cat Care Naturally). [Google Scholar]
Uncited References
- Baker J.A., Robson D.S., Carmichael L.E., Gillespie J.H., Hildreth B. Control procedures for infectious diseases of dogs. Proc. Anim. Care Panel. 1961;11:234–244. [Google Scholar]
- Schultz R.D. Current and future canine and feline vaccination programs. Vet. Med. 1998;93(3):233–254. Editor's note: See also two very recent publications on this subject. [Google Scholar]
- Schultz R.D. Vaccine immunity challenges for the 21st century. Suppl. Compend. Contin. Educ. Pract. Vet. 1998;20(5B):5–18. [Google Scholar]


