Significance
The transition from simple, microscopic forms to the abundance of complex animal life that exists today is recorded within soft-bodied fossils of the Ediacara Biota (571 to 539 Ma). Perhaps most critically is the first appearance of bilaterians—animals with two openings and a through-gut—during this interval. Current understanding of the fossil record limits definitive evidence for Ediacaran bilaterians to trace fossils and enigmatic body fossils. Here, we describe the fossil Ikaria wariootia, one of the oldest bilaterians identified from South Australia. This organism is consistent with predictions based on modern animal phylogenetics that the last ancestor of all bilaterians was simple and small and represents a rare link between the Ediacaran and the subsequent record of animal life.
Keywords: bilaterian, Ediacaran, Ediacara Biota, phylogenetics, trace fossil
Abstract
Analysis of modern animals and Ediacaran trace fossils predicts that the oldest bilaterians were simple and small. Such organisms would be difficult to recognize in the fossil record, but should have been part of the Ediacara Biota, the earliest preserved macroscopic, complex animal communities. Here, we describe Ikaria wariootia gen. et sp. nov. from the Ediacara Member, South Australia, a small, simple organism with anterior/posterior differentiation. We find that the size and morphology of Ikaria match predictions for the progenitor of the trace fossil Helminthoidichnites—indicative of mobility and sediment displacement. In the Ediacara Member, Helminthoidichnites occurs stratigraphically below classic Ediacara body fossils. Together, these suggest that Ikaria represents one of the oldest total group bilaterians identified from South Australia, with little deviation from the characters predicted for their last common ancestor. Further, these trace fossils persist into the Phanerozoic, providing a critical link between Ediacaran and Cambrian animals.
The first macroscopic animal fossils are recognized within the soft-bodied Ediacara Biota (1, 2). Among these are candidate poriferans (3), cnidarians (4), and ctenophores (5). Rare Ediacaran taxa have been interpreted as putative bilaterians, namely, Kimberella (6, 7). However, small furrowed trace fossils are generally accepted as definitive evidence for total group bilaterians in the Ediacaran (8–10). The size and morphology of these trace fossils suggest that they were produced by millimeter-scale organisms that would be difficult to recognize in the fossil record (11).
Helminthoidichnites are horizontal trace fossils found in Ediacaran and Phanerozoic deposits globally (12, 13). Helminthoidichnites is a curvilinear burrow that can be preserved on both bed tops as well as bottoms and occurs most commonly on the base of thin (submillimeter to millimeter scale) discontinuous sand bodies, or shims (8, 14). The preservation of Helminthoidichnites in negative relief flanked by positive levees on bed bottoms indicates that the progenitor moved under thin sand bodies following deposition and burial, displacing sediment (8, 9, 11, 14). Observed relationships between intersecting Helminthoidichnites indicates the ability of the progenitor to move vertically, albeit on millimeter scales (11). Rare Helminthoidichnites penetrating body fossils of macroscopic taxa may represent the oldest evidence of scavenging (11).
In modern environments, Helminthoidichnites-type structures can be produced by a variety of bilaterians (9, 11). A likely progenitor for Ediacaran Helminthoidichnites has yet to be identified, although it has been suggested that these were produced by simple “worm-like animals” (9). Critically, based on the nature of sediment displacement by a horizontally burrowing organism, it would have been small, with a maximum diameter less than that observed for Helminthoidichnites. Such behavior necessitates anterior–posterior differentiation, as well as a coelom, consistent with bilaterian-grade tissue organization (8, 9, 11, 15).
Helminthoidichnites are preserved abundantly within the Ediacara Member, Rawnsley Quartzite in the Flinders Ranges and surrounding regions of South Australia (16). The Ediacara Member consists of shallow marine sandstone event beds 50 to 500 m below a basal Cambrian disconformity (17). At the National Heritage Nilpena site, the excavation and reconstruction of 37-m-scale fossiliferous bed surfaces reveals in situ communities of the Ediacara Biota (18). At Nilpena, and sections within the Flinders Ranges, Helminthoidichnites occurs more than 100 m below the first appearance of Kimberella (19, 20). There are currently no radiometric dates to constrain the absolute age of the Ediacara Member; however, significant overlap of taxa with well-established deposits from the White Sea region of Russia indicates that these are likely between 560 and 551 million years old (21–24). A similar pattern of leveed, horizontal trace fossils (although in this case assigned to the ichnogenus Archaeonassa) occurring stratigraphically below classic White Sea assemblage body fossils in Russia (9, 23) may corroborate the early appearance of trace fossils in South Australia.
Results
Here, we report the discovery of the new genus, new species Ikaria wariootia, the interpreted progenitor of Helminthoidichnites. We have identified 108 Ikaria on a single bed surface (1T-A) and 19 from float at multiple localities, preserved in negative hyporelief on the base of sandstone beds (Fig. 1). Ikaria is found in fine-grained sandstones in two facies representing deposition in relatively shallow marine environments between fair-weather and storm-wave base (14, 17, 25).
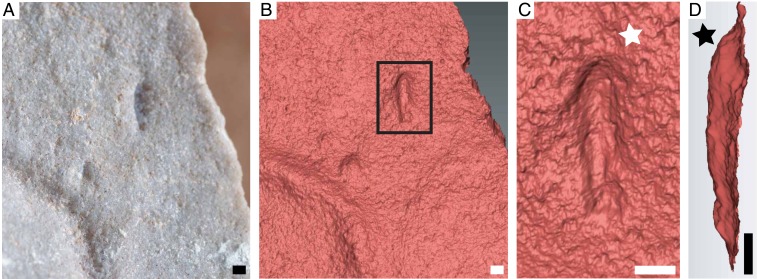
Fig. 1.
Type specimen of I. wariootia from Nilpena, including (A) photograph; and (B–D) 3D laser scans. Notice distinct bilateral symmetry (wider end identified by white star in C and deeper end by black star in D). P57685. (Scale bars, 1 mm.)
Systematic Description
Ikaria wariootia gen. et sp. nov.
Etymology.
The generic name is after the word “Ikara,” which is the Adnyamathanha name for Wilpena Pound, and means “meeting place” in the Adnyamathanha language. Ikara is the major landmark in view from Nilpena, and the fossil has been named to acknowledge the original custodians of the land; species are named for Warioota Creek, which runs from the Flinders Ranges to Nilpena Station.
Holotype.
Paratype.
P57686 (Fig. 2A; South Australia Museum).
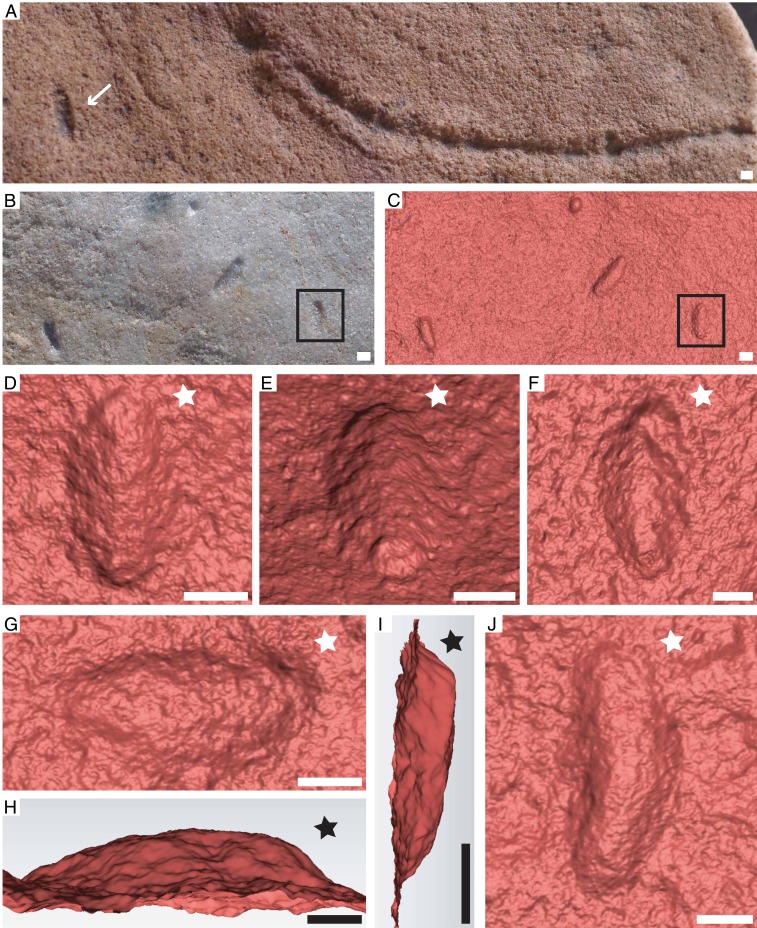
Fig. 2.
Photographs (A and B) and 3D laser scans (C–J) of I. wariootia. (A) Specimen (white arrow) associated with Helminthoidchnites. (B–E) Associated specimens; black boxes in B and C are the same specimen shown in close up in negative hyporelief (D) and inverted (E). (F and J) Bent specimens. (G and H) N bedding plane (G) and profile (H) of the same specimen. (I) Profile demonstrating variable relief. Notice correlation between broader, wider end (white stars) in the bedding-pane view and more significant relief end (black stars) in the profile. (A) P57686. (B–E) 1T-A 001 to 003. (F) 1T-A 004. (G and H) 1T-A 005. (I) 1T-A 006. (J) 1T-A 007. (Scale bars, 1 mm.)
Field Paratypes.
1T-A bed 001 to 007 (Fig. 2 B–J; Nilpena).
Horizon and Locality.
Ediacara Member, Rawnsley Quartzite at the National Heritage Nilpena field site and Bathtub Creek.
Diagnosis.
Irregular millimeter-scale ovoid preserved in negative hyporelief. The major axis length averages 2.3 times the minor axis. There is distinct asymmetry along the major axis with one end wider and more broadly curved (white star in Figs. 1C and 2 D–F, G, and J). In profile, the broader end is preserved in more significant negative relief and with a steeper curvature (black star in Figs. 1D and 2 H and I). Rare specimens are bent about the long axis (Fig. 2 F and J) and/or exhibit potential evidence of modularity, with two to five body divisions (Fig. 2 D and E).
Description.
I. wariootia are well-defined elongate ovals, fusiform in shape (Figs. 1 and 2). Three-dimensional (3D) laser scans demonstrate clear anterior/posterior differentiation, with one end distinctly smaller and more tightly curved. Length of the major axis ranges from 1.9 to 6.7 mm and the minor axis from 1.1 to 2.4 mm. Preserved depth ranges from 0.6 to 1.6 mm. There is a consistent linear relationship between total length and total width (SI Appendix, Fig. S1A). The relationships between total length and depth (SI Appendix, Fig. S1B) as well as width and depth (SI Appendix, Fig. S2) are irregular. Depth is always less than width, suggesting that fossils of Ikaria are compressed. This confirms previous interpretations that the preserved depth of specimens from the Ediacara Member is strongly influenced by taphonomic processes (e.g., ref. 26).
While the morphology of Ikaria is very simple, it is consistent across specimens and is unambiguously distinct from other structures. The consistent shape and length-to-width ratio are not what is observed for rip-up clasts of organic mats, which are irregular (14). Although mat rip-ups are found within the Ediacara Member, they do not occur in the same lithologies and facies as Ikaria (14, 25), which represent deposition in a lower-energy environment. Furthermore, rip-up clasts have a different biostratinomic and diagenetic history than Ikaria and all other body fossils (14). The outer margin of Ikaria is sharp, and they are preserved with considerable relief, distinct from the surrounding matrix and organic mat textures (Fig. 2). This is consistent with other nonsessile taxa from the Ediacara Member (27), suggesting that Ikaria represents the body fossil of a free-living organism.
Ikaria can be easily differentiated from other taxa preserved on the same bed surface and of similar size and scale (SI Appendix, Fig. S3). Thus, it is unlikely a juvenile form of a previously described taxon. The lack of larger specimens with comparable morphology suggests that maximum size is ∼7 mm. The recognition of other taxa on the same surface preserved at the same scale and with similarly well-defined outer margins distinct from the organic mat corroborates the biologic, body-fossil origin of Ikaria.
Specimens of Ikaria are found in association with Helminthoidichnites, albeit rarely (Fig. 2A). The range of Ikaria widths plots entirely within those measured for Helminthoidichnites with the maximum size of body fossils not exceeding that of trace fossils (SI Appendix, Fig. S4). Further, the Anderson–Darling test indicates that size-frequency distributions are not significantly different (P value 0.448). This, combined with clear anterior–posterior differentiation, suggests that Ikaria is the only known contemporaneous body fossil with the suite of characters predicted for the progenitor of Helminthoidichnites.
Discussion
In general, it is rare to have trace fossils and the organisms that produced them preserved together, particularly with respect to mobile metazoans. This can be attributed to both the different preservational pathways between body and trace fossils and the ability of the animal to move away from the area where it left evidence of activity (28, 29). In certain cases, the morphological characteristics of body fossils from the same deposits can be used to reliably determine the progenitors of particular trace fossils (e.g., ref. 30).
Body fossils in the Ediacara Member, including Ikaria, are well preserved on the bottoms of centimeter-scale sandstone beds with early mineralization of overlying sand casting the tops of these organisms following burial (8, 14, 16). Although counterparts are identified in rare cases on bed tops, these are poorly preserved and at a resolution that is unlikely to produce identifiable features at the same scale as Ikaria. In contrast, Helminthoidichnites is found on both bed tops and bottoms, but most commonly on the base of millimeter-thick shims, where well preserved body fossils are rare (8, 14). Negative hyporelief preservation indicates that Helminthoidichnites formed after the deposition of overlying sand, with the organism that produced it capable of moving into and out of thin layers of sand (11). This predicts that we should only find Helminthoidichnites and its progenitor on the same bed bottom in rare instances when it died while burrowing underneath thin sand bodies. Given the simple morphology and preservation of both body and trace fossil in negative relief, even if Ikaria was preserved at the end of a trail, it is unlikely that it would be possible to confidently identify as distinct from that trace. We interpret the surprising discovery of Helminthoidichnites with nearby Ikaria (Fig. 2A) as the result of vertical movement from the bedding plane in the region between the end of its trace fossil and its final resting place. While this scenario was likely exceedingly rare, it may represent the only situation in which it would be possible to distinguish associated body and trace fossils and further corroborates interpretations of Ikaria as the progenitor of Helminthoidichnites.
We propose that Ikaria is the trace maker of Helminthoidichnites and potentially the oldest, definitive bilaterian, at least as represented in the fossil record of South Australia. Kimberella, the only other taxon from the Ediacara Member that is consistently reconstructed as a bilaterian, occurs significantly higher stratigraphically than the earliest appearance of Helminthoidichnites (6, 7, 19, 20). Similarities between taxonomic assemblages have been consistently cited as evidence that White Sea assemblage fossils from the Ediacara Member, including Kimberella, are conservatively 560 to 551 Ma (21–24). The stratigraphic position of Helminthoidichnites suggests that the first appearance of Ikaria was likely within this age range or possibly earlier. Burrows initially interpreted to be from much older Ediacaran rocks in Uruguay have uncertain age constraints (31, 32). Trace fossils from Brazil, representing the activity of meiofaunal bilaterians, occur 30 to 40 m above a tuff dated at 555 Ma and in close association with Cloudina, indicating that they are likely younger than Ikaria (10, 24). A recently described segmented bilaterian from South China, associated with trace fossils, is interpreted to be younger, larger, and more complex than Ikaria (33).
The ability to move and produce recognizable trace fossils is not unique to bilaterians. Complex body and trace fossils from older Ediacaran deposits were probably produced by muscular eumetazoans interpreted to be cnidarians (34). Dickinsonia, and similar Ediacara Biota fossils, likely do not represent crown-group bilaterians, but were mobile and left trace fossils (35, 36). Modern protists generate simple burrows, but are typically smaller than Ikaria (37, 38). Laboratory experiments demonstrate that mobile foraminifera form burrows in clay and silt; however, they do not produce burrows in fine-grained or coarser sand (37). Large testate amoeba in deep-sea environments are associated with horizontal trails similar to those observed in the Ediacaran, but these are surficial and represent movement by rolling (38). Flatworms are mobile, but do not burrow below the sediment–water interface and rarely leave trace fossils (39). Among these examples, expression on bed bottoms with furrows is unique to Helminthoidichnites and suggests mobility associated with significant displacement of medium sand grains. This is consistent with reconstructions of Ikaria containing musculature and a coelom (15, 40). Combined with the relative size of body and trace fossils, these characteristics are unique to bilaterians.
Polarity of relief and curvature characterize anterior/posterior differentiation in Ikaria (Fig. 3), supported by directed movement in trace fossils. Preservation of v-shaped transverse ridges within Helminthoidichnites suggests peristaltic mobility (ref. 11; Fig. 2A). Ikaria morphology implies a potentially modular body construction, which would have aided in muscular organization required for peristalsis (40). Sediment displacement and scavenging reveal that Ikaria likely had a coelom, mouth, anus, and through-gut (11, 15, 40), although these are unlikely to be reproduced in the fossil record. Preferential preservation of Helminthoidichnites under thin sand bodies indicates that Ikaria sought out these environments, possibly due to increased oxygen availability (11, 14, 20). Ultimately, as the depth of overlying sand increases, oxygenated environments give way to sulfidic, inhospitable settings due to decomposition of organic matter, supported by the restriction of Helminthoidichnites to beds <15 mm thick (11, 14, 20). Ikaria was likely able to detect organic matter buried in well-oxygenated environments as well as potentially toxic conditions, suggesting rudimentary sensory abilities. Combined, these features suggest that, despite the simple morphology that can be directly observed in fossil specimens of Ikaria, this organism was remarkably complex, compared with contemporaneous Ediacara Biota taxa.
Fig. 3.
Reconstruction of Ikaria in life position forming a Helminthoidichnites-type trail.
Molecular phylogenetic analysis of modern metazoans demonstrates that developmental programing is highly conserved between disparate groups. Initially, this led to hypotheses that the last common ancestor (LCA) of bilaterians (animals with two openings and a through-gut) was relatively complex, containing many of the features common to a variety of such groups, including eyes, segmentation, appendages, and a heart (41–43). Expansion of this analysis to nonbilaterian animals and their closest single-celled ancestors instead indicates that components of these conserved developmental pathways have deep ancestry (see ref. 44 for discussion). Combined with recent evidence for a sister-group relationship between Xenacoelamorpha and Bilateria, this suggests that the bilaterian LCA was a simple, small, mobile organism with anterior/posterior differentiation and limited sensory abilities (44–49). Remarkably, these predictions agree closely with the characters identified here for Ikaria.
Recognition of the totality of traits in Ikaria is reliant on both body and associated trace fossils. Given the simple morphology of Ikaria, it is unlikely that we would be able to confidently assign it to the Bilateria, or even Metazoa, without this relationship. This is consistent with hypotheses that the apparent gap between molecular clock predictions for the early divergence of bilaterians and their later appearance in the fossil record is the result of their predicted simple morphology (44, 49). Thus, similar prephylum, total group bilaterians may be found elsewhere in the Precambrian fossil record; Ikaria provides a search image for the future identification of such forms.
The stratigraphic position of Helminthoidichnites suggests that Ikaria is the oldest total group bilaterian from the fossil record of South Australia. Ikaria represents a rare example in early animal evolution where phylogenetic predictions correspond directly with the fossil record. Further, the global distribution and recognition of Helminthoidichnites in Cambrian strata (12, 13) is distinct from the overwhelming majority of the Ediacara Biota. While Ikaria is not necessarily responsible for the production of all examples of Helminthoidichnites, it is likely that Ikaria and/or related taxa are rare fossil animals that existed across the Ediacaran–Cambrian boundary.
Materials and Methods
Fossil specimens from the National Heritage fossil site at Nilpena remain in the field due to occurrence on large (square-meter to square-decameter scale) bedding planes (18). These specimens are identified by bed and field numbers (e.g., 1T-A 001). Float specimens from Nilpena are collected and housed at the South Australia Museum in Adelaide and identified by P numbers.
Specimens of Ikaria and Helminthoidichnites were documented through digital photography, using a Pentax K-50 digital single-lens reflex, and latex molds. Helminthoidichnites width was measured by using digital calipers directly on fossil specimens. Detailed morphological investigation was made possible by 3D laser scans, collected by using the HDI Compact C506 3D laser scanner. The accuracy of this scan system is reported to 12 μm. Scans were processed by using the FlexScan3D software. Measurements were conducted on 3D scans by using the FlexScan3D software. Screenshots of these scans are presented in Figs. 1 and 2.
We used the Anderson–Darling test to statistically compare the size frequency distributions of Helminthoidichnites and Ikaria using the freely available PAST software (https://folk.uio.no/ohammer/past/). For this analysis, we compared the average widths of 606 individual Helminthoidichnites with the maximum widths of 112 Ikaria from Nilpena (Dataset S1). This analysis produced a statistically significant P value (>0.05) of 0.448, indicating that we cannot reject the null hypothesis that the two samples are taken from populations with equal distributions.
Data Availability Statement.
All data discussed in this paper are available in Dataset S1.
Supplementary Material
Acknowledgments
This work was supported by NASA Exobiology Program Grant NNX14AJ86G (to M.L.D.) and NASA Earth and Space Science Fellowship Program Grant NNXPLANET17F-0124 (to S.D.E.). S.D.E. and M.L.D. were supported by the NASA Astrobiology Institute under Cooperative Agreement NNA15BB03A, issued through the Science Mission Directorate. We thank R. and J. Fargher for access to the National Heritage Nilpena Ediacara fossil site on their property, acknowledging that this land lies within the Adnyamathanha Traditional Lands. Fieldwork was facilitated by M. A. Binnie, M. Droser, R. Droser, M. Dzaugis, M. E. Dzaugis, P. Dzaugis, M. Ellis, C. Hall, E. Hughes, C. Peddie, J. Perry, D. Rice, R. Surprenant, and L. Tarhan. We thank D. Erwin and J. Irving for helpful discussion regarding this manuscript. S. Wasif created Fig. 3.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001045117/-/DCSupplemental.
References
- 1.Xiao S., Laflamme M., On the eve of animal radiation: Phylogeny, ecology and evolution of the Ediacara biota. Trends Ecol. Evol. (Amst.) 24, 31–40 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Erwin D. H., et al. , The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science 334, 1091–1097 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Clites E. C., Droser M. L., Gehling J. G., The advent of hard-part structural support among the Ediacara biota: Ediacaran harbinger of a Cambrian mode of body construction. Geology 40, 307–310 (2012). [Google Scholar]
- 4.Droser M. L., Gehling J. G., Synchronous aggregate growth in an abundant new Ediacaran tubular organism. Science 319, 1660–1662 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Zhu M., Gehling J. G., Xiao S., Zhao Y., Droser M. L., Eight-armed Ediacara fossil preserved in contrasting taphonomic windows from China and Australia. Geology 36, 867–870 (2008). [Google Scholar]
- 6.Ivantsov Y., Trace fossils of Precambrian metazoans “Vendobionta” and “Mollusks”. Stratigr. Geol. Correl. 21, 252–264 (2013). [Google Scholar]
- 7.Gehling J. G., Runnegar B. N., Droser M. L., Scratch traces of large Ediacara bilaterian animals. J. Paleo. 88, 284–298 (2014). [Google Scholar]
- 8.Jensen S., The Proterozoic and earliest Cambrian trace fossil record; patterns, problems and perspectives. Integr. Comp. Biol. 43, 219–228 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Buatois L. A., Mángano M. G., “Ediacaran ecosystems and the dawn of animals” in The Trace-Fossil Record of Major Evolutionary Events, Buatois L. A., Mángano M. G., Eds. (Springer, Berlin, Germany, 2016), vol. 1, pp. 27–72. [Google Scholar]
- 10.Parry L. A., et al. , Ichnological evidence for meiofaunal bilaterians from the terminal Ediacaran and earliest Cambrian of Brazil. Nat. Ecol. Evol. 1, 1455–1464 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Gehling J. G., Droser M. L., Ediacaran scavenging as a prelude to predation. Emerg. Top. Life Sci. 2, 213–222 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Buatois L. A., Narbonne G. M., Mángano M. G., Carmona N. B., Myrow P., Ediacaran matground ecology persisted into the earliest Cambrian. Nat. Commun. 5, 3544 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Wood R., et al. , Integrated records of environmental change and evolution challenge the Cambrian Explosion. Nat. Ecol. Evol. 3, 528–538 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Tarhan L. G., Droser M. L., Gehling J. G., Dzaugis M. P., Microbial mat sandwiches and other anactualistic sedimentary features of the Ediacara member (Rawnsley Quartzite, South Australia): Implications for interpretation of the Ediacaran sedimentary record. Palaios 32, 181–194 (2017). [Google Scholar]
- 15.Budd G. E., Jensen S., The origin of the animals and a ‘Savannah’ hypothesis for early bilaterian evolution. Biol. Rev. Camb. Philos. Soc. 92, 446–473 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Jensen S., Droser M. L., Gehling J. G., “A critical look at the Ediacaran trace fossil record” in Neoproterozoic Geobiology and Paleobiology, Xiao S., Kaufman A. J., Eds. (Springer, Berlin, Germany, 2006), pp. 116–159. [Google Scholar]
- 17.Gehling J. G., Environmental interpretation and a sequence stratigraphic framework for the terminal proterozoic Ediacara member within the Rawnsley Quartzite, South Australia. Precambrian Res. 100, 65–95 (2000). [Google Scholar]
- 18.Droser M. L., et al. , Piecing together the puzzle of the Ediacara biota: Excavation and reconstruction at the Ediacara national Heritage site Nilpena (South Australia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 513, 132–145 (2019). [Google Scholar]
- 19.Gehling J. G., Droser M. L., Ediacaran stratigraphy and the biota of the Adelaide Geosyncline, South Australia. Episodes 35, 236–246 (2012). [Google Scholar]
- 20.Gehling J. G., García-Bellido D. C., Droser M. L., Trahan L. G., Runnegar B., The Ediacaran-Cambrian transition: Sedimentary facies versus extinction. Estud. Geol. 75, e099 (2019). [Google Scholar]
- 21.Martin M. W., et al. , Age of Neoproterozoic bilatarian body and trace fossils, White Sea, Russia: Implications for metazoan evolution. Science 288, 841–845 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Waggoner B., The Ediacaran biotas in space and time. Integr. Comp. Biol. 43, 104–113 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Grazhdankin D. V., Patterns of evolution of the Ediacaran soft-bodied biota. J. Paleo. 88, 269–283 (2014). [Google Scholar]
- 24.Boag T. H., Darroch S. A., Laflamme M., Ediacaran distributions in space and time: Testing assemblage concepts of earliest macroscopic body fossils. Paleobiology 42, 574–594 (2016). [Google Scholar]
- 25.Gehling J. G., Droser M. L., How well do fossil assemblages of the Ediacara Biota tell time? Geology 41, 447–450 (2013). [Google Scholar]
- 26.Evans S. D., Droser M. L., Gehling J. G., Highly regulated growth and development of the Ediacara macrofossil Dickinsonia costata. PLoS One 12, e0176874 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Droser M. L., Evans S. D., Dzaugis P. W., Hughes E. B., Gehling J. G., Attenborites janeae: A new enigmatic organism from the Ediacara member (Rawnsley Quartzite), South Australia. Aust. J. Earth Sci., 10.1080/08120099.2018.1495668 (2018). [DOI] [Google Scholar]
- 28.Bromley R. G., Ed., Trace Fossils: Biology Taphonomy and Applications (Taylor and Francis, Abingdon, UK, 1996). [Google Scholar]
- 29.Seilacher A., Ed., Trace Fossil Analysis (Springer, Berlin, Germany, 2007). [Google Scholar]
- 30.Gibb S., Pemberton S. G., Chatterton B. D. E., Arthropod trace fossils of the Upper Lower Cambrian Gog Group, Southern Rocky Mountains of Canada. Ichnos 24, 91–123 (2016). [Google Scholar]
- 31.Pecoits E., et al. , Bilaterian burrows and grazing behavior at >585 million years ago. Science 336, 1693–1696 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Gaucher C., Poiré D. G., Bossi J., Bettucci L. S., Beri Á., Comment on “Bilaterian burrows and grazing behavior at >585 million years ago”. Science 339, 906 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Chen Z., Zhou C., Yuan X., Xiao S., Death march of a segmented and trilobate Bilaterian elucidates early animal evolution. Nature 573, 412–415 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Liu A. G., McIlroy D., Matthews J. J., Brasier M. D., Confirming the metazoan character of a 565 Ma trace-fossil assemblage from Mistaken Point, Newfoundland. Palaios 29, 420–430 (2014). [Google Scholar]
- 35.Sperling E. A., Vinther J., A placozoan affinity for Dickinsonia and the evolution of late Proterozoic metazoan feeding modes. Evol. Dev. 12, 201–209 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Evans S. D., Gehling J. G., Droser M. L., Slime travelers: Early evidence of animal mobility and feeding in an organic mat world. Geobiology 17, 490–509 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Serevin K. P., Culver S. J., Blanpied C., Burrows and trails produced by Quinqueloculina impressa Reuss, a benthic foraminifer, in fine-grained sediment. Sedimentology 29, 897–901 (1982). [Google Scholar]
- 38.Matz M. V., Frank T. M., Marshall N. J., Widder E. A., Johnsen S., Giant deep-sea protist produces Bilaterian-like traces. Curr. Biol. 18, 1849–1854 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Valentine J. W., Erwin D. H., Jablonski D., Developmental evolution of metazoan bodyplans: The fossil evidence. Dev. Biol. 173, 373–381 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Clark R. B., Locomotion and the phylogeny of the Metazoa. Ital. J. Zool. 48, 11–28 (1981). [Google Scholar]
- 41.Carroll S. B., Grenier J. K., Weatherbee S. D., DNA to Diversity: Molecular Genetics and the Evolution of Animal Design (Wiley, New York, NY, 2001). [Google Scholar]
- 42.Knoll A. H., Carroll S. B., Early animal evolution: Emerging views from comparative biology and geology. Science 284, 2129–2137 (1999). [DOI] [PubMed] [Google Scholar]
- 43.De Robertis E. M., The molecular ancestry of segmentation mechanisms. Proc. Natl. Acad. Sci. U.S.A. 105, 16411–16412 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erwin D. H., Early metazoan life: Divergence, environment and ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20150036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erwin D. H., Davidson E. H., The last common bilaterian ancestor. Development 129, 3021–3032 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Struck T. H., et al. , Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of spiralia. Mol. Biol. Evol. 31, 1833–1849 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Hartenstein V., Stollewerk A., The evolution of early neurogenesis. Dev. Cell 32, 390–407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon J. T., et al. , Xenacoelomorpha is the sister group to Nephrozoa. Nature 530, 89–93 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Cunningham J. A., Liu A. G., Bengtson S., Donoghue P. C. J., The origin of animals: Can molecular clocks and the fossil record be reconciled? BioEssays 39, 1–12 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in this paper are available in Dataset S1.