Abstract
This chapter discusses the infections caused by DNA viruses and also RNA viruses. The chapter focuses on the detection, diagnosis, risk assessment, and decision-making regarding viral infections. Several infections caused by DNA viruses are parvoviruses, rat cytomegalovirus, poxviruses, adenovirus, and papovavirus. Several RNA viruses and infections caused by these viruses are coronaviruses, paramyxoviruses, rotavirus and reovirus, and picornaviruses. Monitoring for viral infections should cover at least three venues: animals in established breeding and experimental colonies, animals held in entry quarantine, and animal tissues and products destined for in vivo use. For established colonies, monitoring should be tailored to local conditions. Effective monitoring should encompass sampling on a pre-arranged schedule, which can be intensified if evidence or suspicion of viral infection emerges. Because viral infections of rats can spread insidiously, early detection and epidemiologic “staging” should employ a detection matrix that includes clinical observation, appropriate sampling, and sensitive and specific diagnostic testing.
I. INTRODUCTION
The previous edition of this chapter began with an observation that is still largely true:“The number of viruses naturally infectious for rats is small (Table 12-1 ), and most cause inapparent infections which usually are detected by serological monitoring.” This statement was not meant either then or now to imply that viral infections of rats are trivial. Their prevalence, and their capacity or potential for disruption of research, belies their modest number. A national survey (Jacoby and Lindsey, 1998) found that two virus families in particular—parvoviruses and coronaviruses—remain widely distributed in the United States: with a recent prevalence of more than 30% among rat colonies at 72 major biomedical research centers. Viruses of laboratory rats also are highly infectious and, at least for parvoviruses, can persist in animals and in the environment, thereby extending their impact on research.
TABLE 12-1.
Viruses of Laboratory Rats
| Virus | Signs | Duration | Transmission | Lesions | |
|---|---|---|---|---|---|
| DNA | Adenovirus | None | Presumed acute | Feces | Intranuclear inclusions in entcrocytes |
| Papovavirus |
Euthymie: none Athymie: wasting |
Unknown | Urine, saliva |
Euthymie: Intranuclear inclusions in lung Athymie: Intranuclear inclusions in salivary gland, respiratory tract, kidney |
|
| Poxvirus | None through dermal pox and deaths | Unknown | Skin contact, respiratory aerosol | Seven eases: Pox lesions in skin; necrotizing, rhinotracheitis and pleuropneumonia | |
| Rat cytomegalovirus | None | Persistent | Saliva | Intranuclear inclusions and cytomegaly in salivary glands; variable sialoadenitis | |
| Rat parvovirus | None | Persistent | Presumed urine, possibly feces | None | |
| Rat virus/H-1 virus | Usually none Dams: rarely abortion, fetal resorption Infants: rarely icterus, diarrhea, ataxia AH ayes: rarely sudden death |
Acute alter exposureas adults; Persistent after pre- or perinatal exposure, and in athymic rats | Presumed urine, possibly feces, intrauterine | Necrosis, hemorrhage in liver. CNS. lymphoid tissue; intranuclear inclusions, fetal deaths | |
| RNA | Coronavirus | Photophobia, lucri malion, sneezing, cervical swelling | Acute in immunocompetent ratsPersistent in athymie rats | Respiratory aerosol, saliva, potentially tears; potentially urine in athymie rats | Necrotizing, rhinitis, sialodacryoadenitis, keratoconjunctivitis; interstitial pneumonia |
| Hantavirus | None | Persistent | Bite wounds, skin, urine | None, mild mullisystemic inflammation, insulitis | |
| Rat respiratory virus (presumed hanta-like virus) | None | Unknown | Presumed respiratory aerosol | Interstitial pneumonia | |
| Picornavirus | None | Unknown | Presumed feces | None | |
| Pneumonia virus of mice | None | Acute | Respiratory aerosol | Rhinitis, pulmonary perivasculitis, interstitial pneumonia | |
| Rotavirus |
Adults: none Infants: diarrhea |
Acute | Feces | Fluid and gas in bowels, enterocytic syncytia and necrosis, occasional intracytoplasmic inclusions | |
| Sendai virus | None, respiratory distress | Acute | Respiratory aerosol | Rhinitis, bronchiolitis, pneumonia | |
| DNA | Adenovirus | Small intestine (based on pathology) | Small intestine | Not determined | None documented |
| Papovavirus | Salivary glands, lung, kidney (based on pathology) | Lung, salivary gland, kidney | Not determined | None documented | |
| Poxvirus | Skin, lung | Skin, lung | Euthanasia, chlorine dioxide disinfection | Clinical disease, potential zoonotic hazard | |
| Rat cytomegalovirus | Salivary glands, lacrimal glands | Salivary glands, lacrimal glands | Cesarean rederivation, embryo transfer | None documented, but potentially disruptive to studies of salivary or lacrimal glands | |
| Rat parvovirus | Mesenteric lymph nodes | Small intestine, mesenteric lymph nodes—both for ISHa | Quarantine, cesarean rederivation, embryo transfer, chlorine dioxide disinfection | Potential to disrupt biological responses that depend on cell proliferation in vivo and in vitro | |
| Rat virus/H-1 virus | Liver, brain, spleen, lymph nodes, mesenteric vessels | Liver, brain, spleen, lymph nodes, mesenteric vessels—and for ISH or IHCb | As for rat parvovirus | As for rat parvovirus; poor breeding performance, sudden death | |
| RNA | Coronavirus | Submandibular salivary glands, Hardcrian glands, nasal washes, lung | Salivary glands, lacrimal glands, nasal turbinates, lung, eye | Quarantine, cessation of breeding, chlorine dioxide disinfection | Disrupted respiratory studies, anesthetic risks, disrupted ophthalmology studies, poor reproductive performance, reduced food intake, retarded growth |
| Hantavirus | Lung, vessels, kidney | Lung, vessels, kidney | Euthanasia, chlorine dioxide disinfection | Zoonotic hazard | |
| Rat respiratory virus (presumed hantalike virus) | Not determined | Lung | Noi determined | None documented | |
| Picornavirus | Small intestine | Small intestine | As for rat Coronavirus | None documented | |
| Pneumonia virus of mice | Lung | Lung | As for rat coronavirus | None documented | |
| Rotavirus | Small intestine | Small intestine | As for rat coronavirus | Clinical disease, retarded growth | |
| Sendai virus | Lung | Lung | As for rat coronavirus | Clinical disease, poor reproductive performance, disrupted respiratory studies, disrupted immune responses |
Notes: Signs, duration, and lesions listed are typical for immunocompetent rats, except as noted. Immunodeficient rats can be expected to have infection with increased severity and duration.
Acute infection: Termination of infection correlates with onset of immunity, which usually occurs within 10 14 day after exposure.
Persistent infection: Infection persists for varying periods after onset of immunity.
Transmission: Transmission by direct contact should be assumed in addition to routes listed.
Major lesions: Represents acute infection, especially prior to the onset of immunity.
a = in situ hybridization.
b = immunohistocheniistry.
Laboratory rats also are susceptible to less prevalent viruses. These include Sendai virus, pneumonia virus of mice, rat cytomegalovirus, rat rotavirus, and hantaviruses. Finally, poxviruses, papovaviruses, and picornaviruses can induce natural infections in rats, but are rarely found. In this edition, we have reduced or omitted discussion of agents that were reported transiently in older literature or were misconstrued as viral because of incomplete diagnostic assessment. These include rat salivary gland virus, MHG virus, Novy virus, and virus-like pneumotropic agents such as “gray lung virus” and wild rat pneumonia agent.
The major sections of this chapter are divided between DNA viruses (Section II) and RNA viruses (Section III). However, please note that Section IV provides a general discussion of detection, diagnosis, risk assessment, and decision-making regarding viral infections.
II. INFECTION CAUSED BY DNA VIRUSES
A. Parvoviruses
The Parvoviridae are small (18–30 nm), nonenveloped, single-stranded, negative-sense DNA viruses with a genome of approximately 5 kb. Productive replication requires cellular factors that are expressed only during cell differentiation and division (Tattersall and Cotmore, 1986; Cotmore and Tattersall, 1987). They account for the predilection of parvoviruses for mitotically active cells and the pathogenicity of several serotypes. Infection begins with binding of virions to cell receptors, internalization by endocytosis, and transport to the nucleus where they replicate and from which they are released during apoptic or lytic cell death. Recent evidence from in vivo studies also suggests that productive replication may not lead irrevocably to cell lysis (Jacoby, et al., 2000) a possibility that requires confirmation. In this context, nonproductive infection (abortive, cryptic, or restrictive) has been demonstrated in vitro for the murine parvovirus, minute virus of mice (MVM) but not for the parvoviruses of rats (Tattersall and Cotmore, 1986; Cotmore and Tattersall, 1987; Jacoby and Ball-Goodrich, 1995; Jacoby et al., 1996).
Current knowledge, based partially on extrapolation from studies of parvoviruses of mice, indicate that parvoviruses of rats replicate autonomously; that is, they do not require a helper virus. They encode two nonstructural regulatory proteins, NS1 and NS2, which are conserved and account for immunologic cross-reactivity among serotypes (Cross and Parker, 1972). They also encode two capsid proteins, VP1 and VP2, which are serotype specific. VP2 is the major capsid protein, although its sequence is contained within the VP1 coding region. A third capsid protein, VP3, results from the proteolytic cleavage of VP2 but is present in only minute quantities in mature virions.
Three established serotypes of parvoviruses infect laboratory rats. The prototype agent is rat virus (RV), which was isolated from a transplantable neoplasm of rats by Kilham and Olivier (Kilham and Olivier, 1959). Therefore, it also has been called Kilham rat virus (KRV). An antigenically distinct virus (H-1 virus) was isolated shortly afterward by Toolan from a human tumor cell line that had been passaged through rats (Toolan et al., 1960). A third serotype, rat parvovirus (RPV), was isolated recently from naturally infected rats (Ball-Goodrich, et al., 1998). A similar agent has been identified by Japanese workers who initially referred to it as “rat orphan parvoviruscenter (Ueno et al., 1996). Most recently, three strains of a putative fourth serotype have been identified by Wan and co-workers (Wan et al, 2002), which they have named, collectively, minute virus of rats. The genomic and amino acid sequences for these isolates are closely related to KRV and H-1 virus but are significantly different from RPV. Because parvovirus infections remain prevalent among laboratory rats and have the potential to distort rat-based research results, efforts to detect, eliminate, and exclude them should receive justifiably high priority.
Many early descriptions of parvoviruses of rats were based on experimentally induced infections (Kilham, 1960; Kilham, 1961; Kilham and Margolis, 1964; Kilham and Margolis, 1966; Kilham and Margolis, 1969), often induced by parenteral inoculation. This reflected interest in using these agents for developing rodent models of virusinduced birth defects. Contemporary studies have emphasized inoculation by natural (oronasal) routes at various ages in both immunocompetent and immunodeficient rats to resemble more closely exposure during natural outbreaks (Jacoby et al., 1987, 1988, 1991; Gaertner et al., 1989, 1993, 1995, 1996).
Agents. The three documented serogroups of parvoviruses infective for rats (RV, H-1 virus, and RPV) each contain multiple isolates. Because they are small, nonenveloped viruses, they are highly resistant to environmental inactivation (Yang et. al., 1995), a characteristic that complicates control and elimination. Early work with RV and H-1 virus showed that they retain infectivity after exposure to lipid solvents and are stable over a pH range of 1 to 12 (Siegl, 1976). They also are highly heat resistant. For example, RV can remain infectious after exposure to 80°C for up to 2 hours and for up to 60 days at 40°C. Rodent parvoviruses are resistant to ultrasonication, treatment with RNAse, DNAse, papain, and trypsin—properties which, however, also facilitate preparation of purified virus from infected cell cultures. RV and H-1 agglutinate guinea pig erythrocytes and aggregate, to variable degrees, erythrocytes of other species (Tattersall and Cotmore, 1986). This property was useful for serotyping viral isolates by hemagglutination inhibition (HAI), a method that has been superseded by more modern serodiagnostic tests, discussed below. There is no evidence that strains within a given serogroup are antigenically distinguishable, even though they may differ in nucleic acid sequence, replication kinetics, and pathogenicity. For example, RV-UMass and RV-Y belong to the same serogroup, but RV-UMass appears to replicate faster in vitro and to be more pathogenic in vivo (Ball-Goodrich et al., 2001).
RV and H-1 virus grow well in primary rat embryo cells and C6 rat glial cells. RV also replicates in continuous cell lines such as 324K (human embryonic kidney) and BHK21 (hamster kidney). H-1 virus also can be propagated in rat nephroma cells (Tattersall and Cotmore, 1986; Paturzo et al., 1987) whereas RPV replicates well in 324K cells (Ball-Goodrich, et al., 1998). Productively infected cells usually develop cytopathic effects (CPE), beginning with intranuclear inclusions and proceeding rapidly to cytolysis. The kinetics and severity of CPE depend on virus strain and dose and the level of confluence of the cell substrate. High concentrations of input virus can induce intranuclear antigen indicative of replication within 12 hours and CPE within several days, whereas low concentrations may not induce measurable effects for a week or more. Cultures that contain actively dividing cells are more susceptible to viral infection and replication than confluent cultures.
Clinical signs. Natural parvoviral infections in rats are usually asymptomatic and first detected by seroconversion or suspected from distorted research results. The prevalence of clinical signs depends on virus strain and dose, host age, and route of exposure. Host genotype is not known to affect susceptibility to infection or disease. In the context of these variables, RV and H-1 serotypes are demonstrably pathogenic after experimental inoculation, whereas RPV is not. Clinical signs of RV or H-1 virus infection are most readily elicited in pregnant or infant rats. Resistance to clinical illness appears to develop by 1 week postpartum (Gaertner et al., 1996). The onset of resistance has been attributed to a decline in mitotic activity among target tissues and postnatal development of immune competence.
Prenatal infection with pathogenic serotypes can cause fetal deaths resulting in partial or complete loss of litters in dams that appear otherwise clinically normal (Kilham and Ferm, 1964; Kilham and Margolis, 1966; Kilham and Margolis, 1969; Jacoby et al., 1988; Gaertner et al., 1996). It is unclear whether intrauterine mortality reduces a dam's subsequent reproductive performance. Infection in late pregnancy or within several days after parturition can cause severe or fatal disease in infants, particularly due to hemorrhage and necrosis in the liver and central nervous system (Kilham and Margolis, 1966; Cole et al., 1970; Margolis and Kilham, 1970). Signs in affected infants can include ataxia, icterus, and diarrhea, or sudden death (Figs. 12-1 and 12-2 ). Rats that survive acute disease may have locomotion deficits or may die later from chronic active hepatitis and progressive hepatic fibrosis. Although clinical signs are rare in rats exposed to virus beyond 1 week after birth, fatal hemorrhagic lesions have been reported in weanlings and immunosuppressed adults (El Dadah et al., 1967; Coleman et al., 1983). They also have been elicited in infected rats with induced increases in hepatic mitotic activity (Ruffolo et al., 1966).
Fig. 12-1.

Acute rat virus infection. Ataxia (splayed feet) from RV-induced cerebellar hypoplasia.
Fig. 12-2.
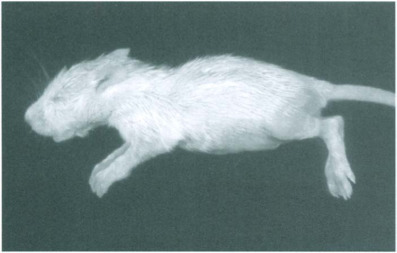
Acute rat virus infection. Diarrhea in a rat pup with severe hepatic necrosis.
Epidemiology. The relative prevalence of the three known serotypes has been complicated by the use of generic antigens for serologic testing. This raises the possibility that RPV has contributed to seroconversions attributed historically to RV and/or H-1 virus. The recent availability of serotype-specific antigens (see below) should make differentiation of parvoviral infections more precise.
Rattus norvegicus is the only known natural host for RV, H-1 virus, and RPV. Based largely on studies with RV, infections should be considered highly contagious, ratto-rat transmission occurring primarily by contact with infectious animals per se or particulate contaminants, such as animal bedding (Jacoby et al., 1988; Yang et al., 1995). Both RV and RPV (and presumably H-1 virus) infect renal tubular epithelium (Figs. 12-3 and 12-4 ) which facilitates excretion in urine. There is some evidence that RV can infect intestinal mucosa and lead to fecal excretion (Lipton et al., 1973), but intestine does not appear to be a prominent target tissue. Intestinal infection is prominent with RPV, although affecting predominantly the lamina propria Fig. 12-5 ). Therefore, its contribution to fecal excretion of virus is unresolved. Natural postnatal exposure to parvoviruses of rats appears due to inhalation and, or ingestion of virus or virus-contaminated fomites. Prenatal transmission is secondary to maternal infection. However, it appears to require exposure of dams to large doses of virus and/or virulent strains (of RV or H-1 virus) (Kilham and Margolis, 1966; Jacoby et al., 1988; Gaertner et al., 1996; Kajiwara et al., 1996). This requirement helps to explain why prenatal infection is uncommon during natural outbreaks in breeding colonies.
Fig. 12-3.
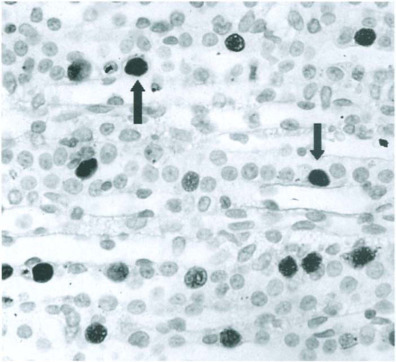
Acute rat virus infection. Intranuclear rat virus antigen (arrows) in renal tubular epithelial cells detected by immunoperoxidase staining.
(Modified from Jacoby et al., 1987 with permission from Archives of Virology.)
Fig. 12-4.

Acute rat parvovirus infection. Viral DNA in renal tubular epithelium detected by in situ hybridization.
Fig. 12-5.

Acute rat parvovirus infection. Viral DNA in an intestinal villus detected by in situ hybridization (arrow).
The risk of transmission is prolonged by persistent infection in rats (Jacoby et al., 1991) and protracted environmental contamination. These properties have been clearly established for RV but should be assumed for all parvoviruses of rats. Susceptibility to persistent RV infection depends heavily on age and immunologic status. Infection in adult immunocompetent rats is usually eliminated within 4 weeks, whereas pre- or perinatal infection can lead to persistent infection lasting at least 6 months, despite the onset of antiviral humoral immunity (Gaertner et al., 1989, 1991, 1995; Jacoby et al., 1991). Persistently infected rats also can excrete virus for up to 3 months, and episodic excretion over longer periods cannot be ruled out (Jacoby et al., 1988). Passive immunization with RV immune serum protects rats from acute and persistent infection (Gaertner et al., 1991). Similarly, maternally acquired immunity appears to protect rats born to persistently infected dams (Jacoby et al., 1988). However, passive immunity is most effective if it is established prior to infection. Antibody administered even 1 day after exposure to virus provides only partial protection against infection. Thus, the potential for persistent infection develops soon after virus reaches its cellular targets. Immunodeficient (athymic) rats, unsurprisingly, are susceptible to persistent RV infection at any age (Gaertner et al., 1989). Administration of RV-immune serum to infected athymic adults suppresses but does not eliminate virus, which can reemerge after immunity decays (Gaertner et al., 1991). The environmental stability of RV has been demonstrated by Yang and co-workers, who found that virus remained infectious on plastic surfaces for at least 5 weeks (Yang et al., 1995).
Pathology and pathogenesis. Pioneering studies by Kilham, Margolis, Toolan, Cole, Nathanson, and others indicated that the predilection of RV and H-1 virus for mitotically active cells was responsible for pathogenic infections in fetal and infant rats, especially in the liver, central nervous system, and lymphopoietic tissues (Toolan et al., 1960; Kilham and Ferm, 1964; Kilham and Margolis, 1966; Margolis et al., 1968; Cole et al., 1970; Novotny and Hetrick, 1970). Virus infection in these tissues has been documented more recently by immunohistochemistry and in situ hybridization (Jacoby et al., 1987; Gaertner et al., 1993), indicating that oronasal exposure leads to viremic dissemination. Lesions caused by pathogenic strains are often widespread but most readily detected in liver, which is both susceptible to infection and mitotically active during infancy. Viral replication causes intranuclear inclusions Fig. 12-6 ), followed rapidly by degenerative changes, including ballooning degeneration, intensified cytoplasmic eosinophilia, dissociation of histoarchitecture, nuclear pyknosis and karyorrhexis, and cell lysis. Associated lesions may include focal hemorrhage, bile stasis, and formation of blood-filled spaces resembling peliosis hepatis. These changes are reflected by gross lesions that may include icterus and yellow-red mottling of the liver accompanied by distorted lobular contours caused by necrosis. Attempts at repair result in increased mitotic activity, which may include the formation of polyploid giant cells. Persistent infection can incite chronic active hepatitis. Portal triads in affected livers may sustain chronic inflammation with infiltration by mononuclear cells, fibrosis, nodular hyperplasia, and biliary hyperplasia Fig. 12-7 ). Corresponding gross lesions can include fibrosis and nodular distortion of one or more lobes.
Fig. 12-6.
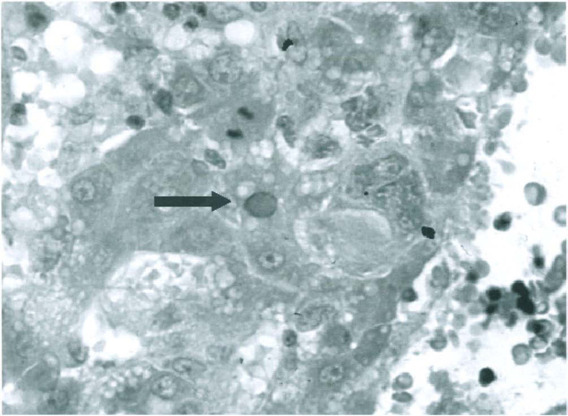
Acute rat virus infection. Hepatocellular necrosis, hemorrhage, and an intranuclear hepatocytic inclusion (arrow).
Fig. 12-7.
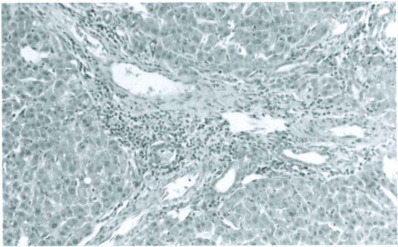
Persistent rat virus infection. Chronic active hepatitis, fibrosis and biliary hyperplasia.
Necrosis and hemorrhage can occur virtually anywhere in the central nervous system but often affect the cerebellum. Segmental or pancerebellar destruction of the external germinal layer Fig. 12-8 ) leads to granuloprival cerebellar hypoplasia Fig. 12-9 ). Hemorrhage may be significant in the cerebellum or other sites and may result in infarction and malacia. Lymphoreticular lesions also are typified by necrosis, which can affect thymus, lymph nodes, and spleen. Intrauterine uterine infection often results in necrosis of fetuses, placentas, and degrees of fetal resorption. Corresponding gross lesions may vary from a reduction to a total loss of viable fetuses and include segmental reddish-black discoloration of the uterus and accumulation of necrotic debris at placentation sites.
Fig. 12-8.

Acute rat virus infection. Necrosis of the external germinal layer of the cerebellum.
(Modified from Jacoby et al., 1987 with permission from Archives of Virology.)
Fig. 12-9.
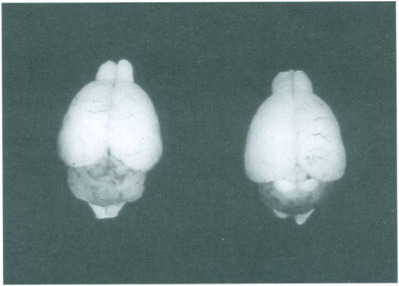
Acute rat virus infection. Hypoplastic cerebellum (right) compared with normal cerebellum (left). Note hemorrhage in affected cerebellum.
The prevalence of hemorrhagic lesions illustrates the importance of vasculotropism in pathogenic parvovirus infections. Hemorrhage during acute infection appears to result from viral-induced endothelial injury (Cole et al., 1970). Inclusion bodies, viral DNA, and viral antigens have been demonstrated in endothelium Fig. 12-10 ) (Margolis and Kilham, 1970; Jacoby et al., 1987; Gaertner et al., 1993; Jacoby et al., 2000). Infection may cause cells to swell or lyse, thereby disrupting vascular integrity. Baringer and Nathanson (1972) found that platelet-fibrin aggregates attached preferentially to RV-infected cells and suggested that endothelial infection activated the clotting mechanism, resulting in infarction and hemorrhage. Additionally, RV infection of megakaryocytes with resulting thrombocytopenia can occur during acute infection (Margolis and Kilham, 1972). Endothelial infection may also exacerbate viremia and facilitate fetal infection through involvement of placental and fetal vasculature.
Fig. 12-10.

Acute rat virus infection. Rat virus antigen in swollen hepatic endothelial cell (open arrow) and hepatocyte (solid arrow) detected by immunoperoxidase staining.
(Modified from Jacoby et al., 1987 with permission from Archives of Virology.)
RV infects vascular and intestinal smooth muscle cells (SMC), which appear to be major sites of persistent infection Fig. 12-11 ) (Jacoby et al., 2000). Persistent vascular SMC infection may be accompanied by perivascular mononuclear cell infiltrates, which most often are found in kidney and liver. Viral DNA can be demonstrated in adjacent vessels by in situ hybridization Fig. 12-12 ) (Jacoby et al., 2000). The mechanisms of viral persistence are unknown, but recent studies suggest that SMC sequester non-replicating RV. RV replication also may continue among mitotically quiescent SMC (an observation that requires confirmation) (Jacoby et al., 2000). Persistent infection of SMC does not cause prominent necrosis, but may provoke focal myolysis. Focal SMC infection also could exacerbate mitotic activity in attempts at repair and may provide additional targets for viral infection.
Fig. 12-11.
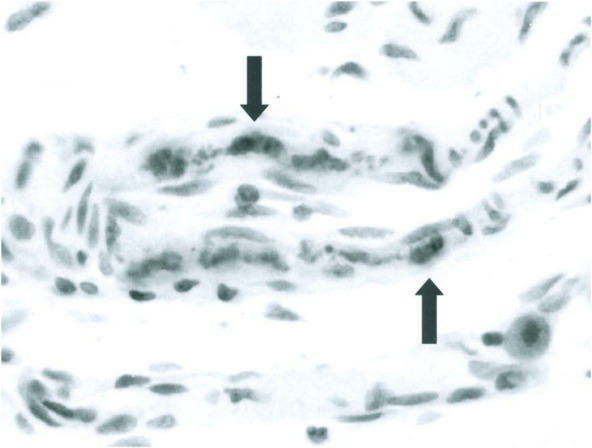
Persistent rat virus infection. Viral DNA detected by in situ hybridization in nuclei of arteriolar smooth muscle cells (arrows).
(Modified from Jacoby et al., 2000 with permission of Journal of Virology.)
Fig. 12-12.
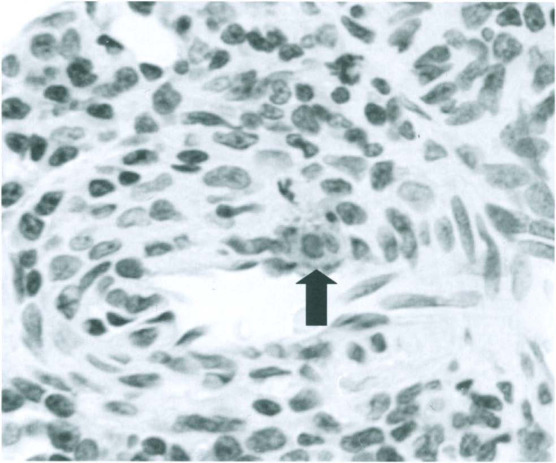
Persistent rat virus infection. DNA detected by in situ hybridization in the nucleus of a smooth muscle cell of a renal arteriole (arrow). Note adjacent accumulations of mononuclear cells.
(Modified from Jacoby et al., 2000 with permission of Journal of Virology.)
The role of host immunity in parvoviral infection is only partially understood. Although pre-existing humoral immunity can prevent infection, anti-viral antibody reduces but does not eliminate infection (Robey et al., 1968; Gaertner et al., 1991, 1995). The increased susceptibility of athymic rats confirms an important role for T lymphocytes in eliminating infection (Gaertner et al., 1989, 1991), whereas mononuclear cell infiltrates that develop during convalescent or persistent infection suggest at least an ancillary role for cell-mediated immunity.
In contrast to RV and H-1 virus, RPV is non-pathogenic even in infant rats. However, it does express similar tissue tropisms. In particular, it infects vascular endothelium and renal tubular epithelium Fig. 12-4) as well as regional lymph nodes Fig. 12-13 ) (Ball-Goodrich et al., 1998). However, unlike RV and H-1 virus, it also infects the lamina propria of intestinal mucosa Fig. 12-5), a tropism comparable to that of MVM and mouse parvovirus (MPV). Therefore, mechanisms of productive RPV replication and excretion may differ from those of the cytolytic parvoviruses. Further, there is no evidence that RPV is transmitted in utero. The duration of RPV infection and factors that may influence duration have not been studied adequately. Virus has been detected after seroconversion in both infant and adult rats for as long as 8 weeks (Ueno et al., 1997; Ball-Goodrich et al., 1998; Ueno et al., 1998), a finding consistent with persistent infection.
Fig. 12-13.
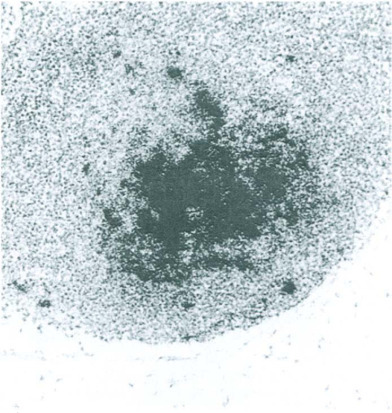
Acute rat parvovirus infection. Viral DNA detected by in situ hybridization in the germinal center of a mesenteric lymph node.
Diagnosis. The diagnosis of parvovirus infection depends on the complementary use of several methods. Clinical signs can be dramatic, but occur rarely. Standard pathologic examination can be useful, especially to detect inclusions and/or lesions compatible with pathogenic infection. However, diagnostic sensitivity and specificity is best achieved with tissue sections by immunostaining or in situ hybridization. Fixation of tissues in freshly prepared paraformaldehyde-lysine-periodate for not more than 16 hours preserves cytoarchitecture and minimizes denaturation of parvoviral antigens and DNA (Jacoby et al., 1987; Gaertner et al., 1993). Therefore, this method should be used prior to paraffin embedding to permit maximum flexibility in primary and follow-up examination of tissues or cells if parvovirus infection is suspected.
Because infections are frequently asymptomatic, serology is essential for primary detection. Enzyme-linked immunosorbent assays (ELISAs) or immunofluorescence assays (IFAs) using virions or infected cells as generic antigens can be used to detect all known serotypes. Alternatively an ELISA using rNS-1 from MPV can detect infection caused by parvoviruses of rats because NS-1 is highly conserved among rodent parvoviruses (Riley et al., 1996). Follow-up testing can employ HAI to directly distinguish RV and H-1 virus infection. Additionally, sera positive by ELISA and/or IFA and negative by HAI would suggest RPV infection. However, the sensitivity of the generic assays is higher than that of HAI, so testing of sera with low concentrations of antibody could produce a false-negative HAI result. Neutralization serology also can be used to distinguish among serotypes, as all replicate in at least one established cell line. This option is comparatively expensive, time consuming, and technically more demanding, making it best suited to use in reference laboratories. The most promising emerging strategy for specific, sensitive cost-effective serologic detection employs recombinant, serotype-specific (capsid) ELISA antigens. They have been produced for RV and RPV as recombinant VP-2 (Ball-Goodrich et al., 2002). Addition of a corresponding H-1 antigen should be straightforward.
Although serologic testing indicates historical or contemporary exposure to virus, immunostaining and molecular diagnostics may be required to obtain evidence for active infection. Antisera against rat parvoviruses are available and can be applied to snap-frozen or aldehyde-fixed tissues. Serotype-specific antibodies are not yet generally available, and immunostaining results from experimental studies indicate that antibodies to NS-1 proteins are more sensitive than those against VP2 proteins. Thus, immunostaining alone does not currently provide the means to confirm serotype specificity. In situ hybridization can employ strand-specific probes to detect not only virus genome, but also replicative forms and mRNA indicative of active infection.
The rat antibody production (RAP) test, adapted from a method developed for detection of mouse viruses, can detect viral antigens in clinical specimens. Test tissues are homogenized and inoculated into non-immune pathogen-free rats to elicit antibodies against suspected viral agents. Testing requires that live rats be held in quarantine for several weeks to allow antibodies to develop, making it relatively laborious and expensive. Although, in practical terms, it also requires that infectious virus be present in the test inoculum, it does not inherently rule out seroconversions elicited by viral antigen that may be present in non-infectious samples.
Four polymerase chain reaction (PCR) assays are currently available to detect parvoviruses of rats. They are very sensitive and improved primers make them increasingly specific. One assay amplifies a region of the NS-1 gene, thereby detecting all serotypes, but not distinguishing among them. The other three amplify VP region sequences for RV, H-1 virus, or RPV, making them serotype-specific (Besselsen et al. 1995a,b; Yagami et al., 1995). They can be used to test tissues, excreta, or cells (e.g., tumor lines) for viral DNA.
Virus isolation can be accomplished by inoculation of the cell lines listed above or by explant culture, which is an amplification technique especially conducive to detecting small quantities of infectious virus, such as those occurring during persistent infection (Paturzo et al., 1987). Briefly, it entails culture of multiple small fragments of suspect tissue for about 3 weeks. Cultures are harvested and aliquots are inoculated into indicator cells, which are examined for viral antigen and/or CPE.
Differential diagnosis. Pathogenic parvoviral infections should be differentiated from chemical toxicity, neoplasia, trauma, genetically determined developmental abnormalities, or rotavirus infection. Reproductive failures or reduced litter size also can occur during Sendai virus or coronavirus infection or environmental insults.
Control and prevention. Control and prevention must take into account the ability of parvoviruses to persist in rats and in the environment, and their capacity for both pre-and postnatal transmission. Initial assessment of an outbreak should begin with quarantine of seropositive rooms, extensive serologic testing in each room, in adjacent or regional facilities, and sites to which potentially affected rats or rat tissues may have been transported, including research laboratories. Cell lines, transplantable tumors, and other biological products, which may be either a source of or exposed to infection, should be tested rapidly by molecular diagnostics, or the RAP method and contaminated items should be discarded. Strategies to eliminate infection depend on multiple factors, including the value and immunologic status of infected or exposed animals and the ability to replace them from specific pathogen-free sources. Selective culling can be attempted if animals are housed in isolation or individually ventilated cages, but one must consider risks posed by persistent infection and environmental contamination. Attempts to minimize further transmission should include use of barrier caging and change stations, limiting personnel traffic, barrier garb, and strict adherence to room order entry and sanitation during cage changing, including heat sterilization of soiled equipment and supplies. Directional air flow should be tested and affected rooms placed under negative pressure.
Rederivation can be accomplished by embryo transfer or cesarean section under stringently aseptic conditions. However, offspring should be tested for prenatal infection by contact transmission (which may include testing of foster dams) or pathologic examination of strategically chosen animals (e.g., at least one per litter). It is important to remember that the prenatal transfer of maternal antibody may render derived progeny at least transiently seropositive, yet protected. Therefore, they should be tested to ensure that antibody titers decay completely by 3 months after birth. A reversal of decreasing titer would suggest active infection.
Because pre-existing humoral immunity protects rats from infection, it can be exploited to help rederive breeding colonies. For example, progeny weaned from seropositive dams can be segregated from virus-free rats until they lose maternally derived immunity. However their dams should remain segregated if they will be used for further matings or be discarded as suspects for persistent infection. The use of contact sentinels to confirm the absence of infection in rederived rats is an optional step. This immunologically based strategy may take 3- to 4 months to complete, but research “down time” can be minimized by re-initiation of breeding among rederived rats while the decay of maternal immunity proceeds. Incremental serologic surveillance of facilities housing or previously housing infected rats is wise until confidence in elimination of infection is secure. Refer to Sections III A. and IV for additional information about the prevention and control of viral agents in rats.
Previously infected rooms should be evacuated and thoroughly disinfected, including physical removal of any debris or fomites from floors, walls, and ceilings. Detergent washes should be followed by disinfection with an oxidizing agent, such as chlorine dioxide, and a drying period of 2- to 3 days (Saknimit et al., 1988). Additional checks on the thoroughness of decontamination may include placement of sentinel rats and/or swabbing of surfaces for PCR analysis.
Interference with research. Although infection with pathogenic strains can cause illness and death, the more likely effects of parvovirus infection will be disruption of biological responses that rely on cell proliferation. RV is lymphocytotropic and can induce functional distortions in immune function. These include: interference with T cell responses to transplantable neoplasms (Campbell et al., 1977), provocation of autoimmune diabetes in diabetes-resistant rat strains by disruption of Th1-like T lymphocyte responses (Guberski et al., 1991; Brown et al., 1993; Ellerman et al., 1996), and diminished proliferative and cytolytic responses to alloantigens in mixed lymphocyte cultures (McKisic et al., 1995). Further, parvoviruses of rats are oncotropic, raising the possibility for disruption of tumor kinetics (Bergs, 1969). Finally, contamination of cell cultures or other biological products must be considered (Nicklas et al., 1993). It can lead to cytopathic effects or inadvertent transmission to rats if such products are used in vivo.
B. Rat Cytomegalovirus
Rat cytomegalovirus (RCMV) is the only known herpesvirus of rats. It has physical, chemical, and biological characteristics typical of the Herpesviridae, including the capacity for persistent infection of salivary glands (Priscott and Tyrell, 1982; Bruggeman et al., 1983; Bruggeman et al., 1985). Virus can be propagated in primary rat embryo fibroblasts, rat kidney cells, and hamster kidney cells. Infection of immunocompetent rats is asymptomatic. Experimental infection of rats immunosuppressed by whole body irradiation induced systemic disease with high mortality, but no studies of infection in naturally immunodeficient rats have been reported. RCMV appears to be infectious only for rats. There is no published evidence for natural cross-infection of rats with cytomegaloviruses of other species or for infection of other species with RCMV. Although infection has been detected in wild rats, the prevalence of RCMV in contemporary rat colonies is believed to be very low. However, epidemiologic data are inadequate to confirm this perception because serologic surveillance is performed sporadically. Nevertheless, prevention of infection in vivaria should focus on exclusion of wild rats, especially from support areas such as those used for supply and equipment storage.
Although acute infection may be generalized, persistent infection occurs in the salivary glands and may affect the lacrimal glands (Lyon et al., 1959). Virus can be excreted in saliva for at least several months. Typical lesions include cytomegaly with formation of intranuclear inclusions, primarily in ductal epithelium Fig. 12-14 ), accompanied by mild, non-suppurative interstitial inflammation. Infection can be detected from typical histopathologic changes, which are most apparent during the first few weeks of infection. An ELISA is available for serologic testing (Bruggeman et al., 1983). Virus can be isolated by inoculation of or cultivation with rat embryo fibroblasts (Rabson et al., 1969).
Fig. 12-14.
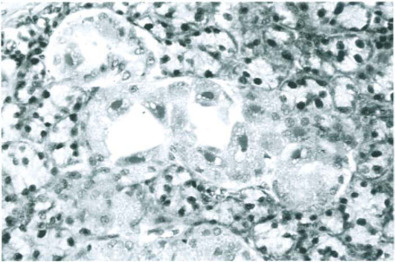
Rat cytomegalovirus infection. Intranuclear inclusions in acinar epithelial cells of a submandibular salivary gland accompanied by mild interstitial inflammation.
Effects on research. Reported effects on research include altered macrophage function, transient suppression of humoral immunity, transfer of infection during organ transplant studies, and exacerbation of collagen-induced arthritis (Bruggeman et al., 1985). Latent infection can be activated by immunosuppression. Conversely, RCMV replication can be augmented by allogeneic immune responses (Tamura et al., 1999). No significant effects on research have been reported from natural infections.
C. Poxviruses
An agent called Turkemia rodent poxvirus, distinct from ectromelia virus, has been reported in rats from Eastern Europe and the former Soviet Union (Iftimovici et al., 1976; Marrenikova and Shelukhina, 1976; Krikun, 1977; Marenikova et al., 1978; Kraft et al., 1982). Although infection can be asymptomatic, if clinical signs occur they resemble mousepox including the development of dermal lesions, tail amputation, and high mortality. Rats also develop lesions of the upper and lower respiratory tract. The latter can include interstitial pneumonia, pulmonary edema and hemorrhage, and pleural effusions.
D. Adenovirus
There is serologic and histologic evidence that rats are susceptible to infection with one or more adenoviruses antigenically related to mouse adenovirus-2 (Ward and Young, 1976). However, the causative agent(s) has not been isolated. Infection, to the best of available knowledge, is asymptomatic. Histologic changes are minimal and resemble those found in adenoviral infections of mice, with the sporadic development of intranuclear inclusions in enterocytes of the small intestine.
E. Papovavirus
A polyoma virus antigenically distinct from polyoma virus of mice has been detected in rats. It was found initially in athymic rats that developed a wasting disease accompanied, in a minority of animals, by pneumonia and sialoadenitis (Ward et al., 1984). Intranuclear inclusions consistent with papovavirus infection occurred in parotid salivary glands, and viral antigen was detected in salivary glands, respiratory airway mucosa, and kidney. Immunocompetent rats did not develop disease. More recent studies in athymic rats have detected intranuclear inclusions in alveolar lining cells and associated with interstitial pneumonia (Percy and Barthold, 2001).
III. INFECTION AND DISEASE CAUSED BY RNA VIRUSES
A. Coronaviruses
Agents. The Coronaviridae are enveloped, pleomorphic single-stranded RNA viruses with club-like projections (peplomers) radially arranged on their capsids. They replicate in the cytoplasm and are released by budding through the endoplasmic reticulum (Compton et al., 1993). They are widespread among mammals and birds, including laboratory rats and mice, but individual viruses are largely species-specific. Classification of the coronaviruses of rats has been somewhat confused by the context in which the two prototype strains were initially identified. In 1964, Hartley and co-workers detected antibodies in rat sera to a common coronavirus of mice, mouse hepatitis virus (MHV), which suggested that rats were susceptible to an antigenically related agent (Hartley et al., 1964). Parker and associates subsequently isolated a coronavirus from lungs of clinically normal rats which became widely known as Parker's rat coronavirus (Parker et al., 1970). Within several years, a second coronavirus was isolated by Bhatt and co-workers from rats with sialodacryoadenitis and was named sialodacryoadenitis virus (Bhatt et al., 1972). Subsequent studies showed that RCV and SDAV were similar antigenically and physicochemically, and that both induced sialodacryoadenitis and inflammation of the respiratory tract. Therefore, RCV and SDAV should be perceived as different strains of a coronavirus indigenous to rats and called, most inclusively, RCV (Percy and Williams, 1990).
Replication of single-stranded RNA viruses is more error-prone than that of DNA viruses and leads to emergence of divergent strains during natural infections. Thus, additional RCV isolates have been reported since the initial isolations by Parker and Bhatt. These include Japanese isolates—CARS (Kojima et al., 1980; Maru and Sato, 1982)— and U.S. isolates RCV-BCMM, RCV-W (Compton et al., 1999a), and RCV-NJ (Compton et al., 1999b).
RCV has a non-segmented, positive-sense RNA genome (Compton et al., 1993). It is sensitive to lipid solvents and relatively stable at acid pH. It can be stored at −60° C for at least 7 years, but loses infectivity rapidly if stored at −20° C (Bhatt et al., 1972; Jacoby et al., 1979). It replicates in vitro in primary rat kidney (PRK) cells in which most virus strains form multinucleate syncytia typical of coronaviral CPE (Bhatt et al., 1972). Virus can be detected antigenically in infected cells within 12 hours and CPE can develop within 24 hours. Established cell lines such as BHK-21, VERO, Hep-2, and NCTC 1469 resist RCV infection, but mouse L2 fibroblasts and sublines, as well as rat LBC cells and intestinal RCV-9 cells, also can be infected (Hirano et al., 1986; Percy et al., 1989; Percy et al., 1990b; Gaertner et al., 1996; Ohsawa et al., 1996). L2 cell sublines and LBC cells also have been used for plaque assays and plaque purification (Gaertner et al., 1993c).
RCV antigens appear to be similar among all isolates, although some also have a hemagglutinating surface protein (Gaertner et al., 1996a; Gagneten et al., 1996). Because RCV is antigenically related to MHV (Barker et al., 1994), MHV antigens are routinely used in serologic testing to detect RCV infection (Peters and Collins, 1981; Smith and Winograd, 1986; Percy et al., 1991).
Clinical signs. Clinical signs may vary with RCV strain, but their overall expression is characteristic of coronavirus infection. Mild infection can be clinically silent and revealed first by seroconversion of sentinel or index rats, but clinical signs are common in many outbreaks. They can occur in non-immune rats of any age, usually last about a week, and occur in various combinations and with varying severity (Jacoby et al., 1975; Bhatt and Jacoby, 1985). Early signs, especially in sucklings, often include squinting, photophobia, and lacrimation Fig. 12-15 ). Older rats frequently develop audible sneezing and palpable enlargement of cervical salivary glands. Closer examination may also reveal serous nasal discharge and pawing at the external nares resulting in wet forefeet. Red-tinged discharges containing porphyrin (chromodacryorrhea) may stain periorbital and perinasal skin Fig. 12-16 ). Severe ocular inflammation is expressed initially as keratoconjunctivitis, which may resolve quickly or become chronic. Chronic ocular inflammation can lead to corneal opacities and ulcers, pannus, hypopyon, hyphema, and megaioglobus (Lai et al., 1976) (Figs. 12.17 and 12.18 ). Ancillary effects attributable to the discomfort of infection include transient anorexia and weight loss (Sato et al., 2001) and disruption of estrus (Utsumi et al., 1978, 1980). Additionally, birth rates and fertility in breeding colonies have been observed to markedly decline during acute epizootics. Although morbidity may be high, RCV infection rarely causes mortality. Most rats recover, with the most frequent long term sequelae associated with ocular disease.
Fig. 12-15.

Rat coronavirus infection. Photophobia and lacrimation due to keratoconjunctivitis and secondary to RCV infection of the lacrimal glands.
Fig. 12-16.

Rat coronavirus infection. Periocular porphyrin staining (chromodacryorrhea).
Fig. 12-17.

Rat coronavirus infection. Opaque cornea indicating keratitis.
Fig. 12-18.

Rat coronavirus infection. Postinfectious hyphema and megaloglobus.
Epidemiology. RCV spreads rapidly among rats housed in “open” cages and among rooms in conventional facilities. Transmission appears to be primarily by direct contact with infected rats or by aerosol. However, limited tests have shown that RCV can remain infectious for at least 2 days when dried on plastic surfaces (Gaertner et al., 1993a). This suggests that fomite transmission also may occur. Experimental studies have demonstrated the ease of horizontal transmission of RCV (LaRegina et. al., 1992), but there is no evidence for intrauterine transmission. Morbidity frequently reaches 100% among conventionally housed rats.
Colony infections often occur in either of two patterns: explosive epizootics among non-immune animals or endemic infection in rooms where young rats are protected transiently by maternal antibody. Non-immune, immunocompetent rats excrete virus in oronasal and lacrimal discharges for about 1 week (Bhatt and Jacoby, 1985). The onset of antiviral immunity terminates infection and is most easily detected by seroconversion. Immunity offers protection against recurrence of clinical disease, but protection is relatively short-lived (< 6 months) and does not preclude transient re-infection, virus shedding, and a resultant increase in antibody titer (Percy et al., 1990a; Weir et al., 1990a; Percy et al., 1991; Percy and Scott, 1991). Re-infection of seropositive animals implies exposure to antigenically variant strains. This risk is inherent to coronavirus infections because of spontaneous mutations leading to the emergence of variant strains, especially during endemic infection. Infection of immunodeficient (e.g., athymic) rats is persistent (Weir et al., 1990b; Hajjar et al., 1991). Such rats can sustain chronic necrosis and inflammation in the salivary and lacrimal glands and the respiratory tract, as described below. Additionally, they can excrete virus for prolonged periods because their capacity for immune elimination of infection is impaired. Apart from oronasal and lacrimal excretion, immunodeficient rats can develop urinary tract infection, which makes transmission by contaminated urine likely. In contrast to the prolonged course of RCV infection in athymic rats, Hanna and co-workers (Hanna et al., 1984) found that immunosuppression with prednisolone had a negligible effect on the course of infection, and treatment with cyclophosphamide resulted in only a modest prolongation.
Although there is no firm evidence for genetic resistance and susceptibility to RCV infection (Percy et al., 1984), recent studies suggested that Lewis rats developed more severe clinical signs than other rat strains tested (Liang et al., 1995). There also is evidence that RCV strains may vary in virulence, but corresponding antigenic differences detected by plaque neutralization are not useful to predict the severity of clinical disease (Compton et al., 1999b).
Rats appear to be the sole hosts for naturally occurring infection, although there is experimental evidence that mice develop transient infection and interstitial pneumonia after inoculation with virus or contact exposure to infected rats (Bhatt et al., 1977; Barthold et al., 1990; La Regina et al., 1992). Mice experimentally inoculated with large doses of RCV can transmit infection to mice in close contact; however, RCV infection does not appear to spread mouse-to-mouse under natural conditions.
A national survey found that RCV infection was prevalent among institutional rat colonies in the United States (Jacoby and Lindsey, 1998) and similar results were reported from France (Zenner and Regnault, 2000). Commercial breeders have made significant strides in eliminating infection. Nevertheless, a recent break in preventive procedures at a commercial vendor resulted in widespread contamination of client colonies. This episode underscores the highly contagious character of RCV infection and the need for effective vigilance.
Pathology and pathogenesis. The lesions of RCV reflect acute inflammation of the salivary and lacrimal glands, lymphoid tissue, respiratory tract, and eye (Jacoby et al., 1975; Bhatt and Jacoby, 1977). They occur in various combinations and with varying severity. The submandibular and parotid salivary glands and adjacent cervical connective tissue are often edematous and the glands proper may be pale yellow to white, with red spots caused by vascular congestion Fig. 12-19 ). The cervical lymph nodes also may be enlarged, edematous and contain multiple red foci. The exorbital and infraorbital lacrimal glands also may be pale and enlarged, whereas the Harderian lacrimal gland located on the dorso-lateral and posterior aspects of the eye globe may be flecked with yellow-gray foci. Reddish-brown mottling of the Harderian gland, however, indicative of porphyrin pigment, is a normal finding. The thymus may be small (as a result of stress atrophy) and the lungs may contain small grey foci. The external nares and eyes may reveal serous exudate and staining of adjacent skin by porphyrin pigment. Acute ocular inflammation includes corneal opacities and swollen conjunctiva, whereas advanced ocular disease may, as noted above, include pannus, hypopyon, hyphema, and megaloglobus (Lai et al., 1976).
Fig. 12-19.

Rat coronavirus infection. Pale, enlarged salivary glands and interstitial inflammatory edema.
Histologic lesions of RCV infection develop initially in the nasopharynx. They include necrosis of respiratory epithelium Fig. 12-20 ) and submucosal glands with inflammatory edema of the lamina propria. Similar changes also affect the vomeronasal organ. The nasal meatus often contains neutrophils, cell debris, and mucus (Jacoby et al., 1975; Bhatt and Jacoby, 1977; Bihun and Percy, 1995). Mild, nonsuppurative tracheitis with focal necrosis of respiratory epithelium also may occur and hyperplastic peribronchial lymphoid nodules may develop. Pulmonary lesions are less frequent and are characterized by focal, interstitial pneumonia presenting as infiltration of alveolar septae by mononuclear cells and neutrophils Fig. 12-21 ). More severe lesions may be accompanied by alveolar exudates containing exfoliated pneumocytes, macrophages, and edema fluid. Although such lesions are typically asymptomatic, Parker and colleagues demonstrated that RCV can induce lethal interstitial pneumonia in neonatal rats (Parker et al., 1970).
Fig. 12-20.

Rat coronavirus infection. Necrosis of respiratory epithelium and inflammation of nasal mucosa.
Fig. 12-21.
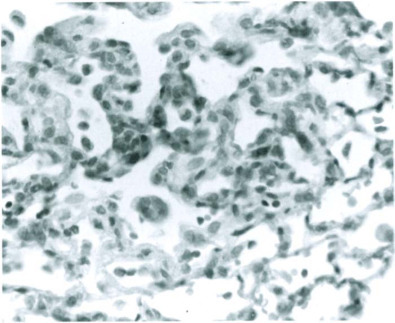
Rat Coronavirus infection. Interstitial pneumonia.
Salivary gland lesions occur in serous or mixed parenchyma and are, therefore, most easily visualized in the submandibular and parotid salivary glands, although smaller serous glands lining the oral cavity also are susceptible. Mucous salivary glands are not affected. Lesions begin as necrosis of ductular epithelium Fig. 12-22 ), which progresses rapidly to diffuse acinar necrosis. Moderate to severe interstitial inflammatory edema also develops within glandular parenchyma and in periglandular connective tissues. The combined effects of necrosis and inflammation often lead to regional or panacinar effacement of affected glands. Lacrimal gland lesions develop in a similar pattern Fig. 12-23 ).
Fig. 12-22.

Rat coronavirus infection. Necrosis of a salivary duct during early stages of infection.
(Modified from Jacoby et al., 1975 with permission from Veterinary Pathology.)
Fig. 12-23.

Rat coronavirus infection. Necrosis in a Harderian gland.
Histologic lesions in immunodeficient (athymic) rats are characterized by chronic active inflammation in the respiratory tract, salivary glands Fig. 12-24 ), and lacrimal glands. They represent chronic tissue damage caused by persistent infection concomitant with ineffective attempts by the host to eliminate virus and effect tissue repair. As noted above, viral antigen has been detected in the urinary tract of athymic rats, indicating that defective immunity leads to altered tissue tropism and the likelihood of prolonged urinary excretion Fig. 12-25 ) (Weir et al., 1990b).
Fig. 12-24.

Rat coronavirus infection in an athymic (rnu) rat. Chronic, active sialoadenitis with fibrosis in a submandibular salivary gland.
(From Weir et al. 1990b, with permission from the American Association for Laboratory Animal Science.)
Fig. 12-25.

Rat Coronavirus infection in an athymic (rnu) rat. Viral antigen in epithelium of urinary bladder mucosa detected by immunoperoxidase staining.
Immunohistochemistry has shown that the foregoing lesions, irrespective of immune status, result from initial infection of the respiratory tract, which extends to the salivary and lacrimal glands. It is not clear how this transition occurs. No viremic phase has been detected during experimental infections, but detection of viral antigen in salivary excretory ducts suggests that retrograde infection from the pharynx may occur. Retrograde infection of nasolacrimal ducts also could account for lacrimal gland infection.
Cervical lymph nodes often sustain focal necrosis and inflammatory edema succeeded by hyperplasia as immunity develops. Mild thymic necrosis with widening of interlobular septae is viewed as a non-specific response to the stress of infection.
Tissue repair in immunocompetent rats commences 5 to 7 days after infection begins. Reconstitution of respiratory mucosa and alveolar parenchyma is rapid and uneventful, although transient squamous metaplasia may occur in respiratory epithelium. Healing in salivary and lacrimal glands is characterized by prominent squamous metaplasia of ductular epithelium, including the tubuloalveolar epithelium of the Harderian gland, and proliferation of hyperchromatic regenerating acinar cells (Fig. 12.26 ). Restoration of cytoarchitecture is substantially complete in 4 to 6 weeks, facilitated by survival of basement membranes, which provide an effective framework for parenchymal regeneration. Residual lesions include focal lymphocytic infiltrates and mild fibrosis.
Fig. 12-26.

Rat coronavirus infection. Squamous metaplasia in a submandibular salivary gland.
Keratoconjunctivitis does not result from direct RCV infection of the eye. It is attributed to impeded tear production by compromised lacrimal glands resulting in keratitis sicca. Changes are characterized by focal or diffuse interstitial keratitis with superficial corneal ulceration and associated conjunctivitis. These lesions may resolve within 4 to 6 weeks without further complication or progress to permanent scarring or more severe sequelae in a small number of rats. Severe outcomes include transmural corneal ulceration, hypopyon, hyphema, synechia, and glaucoma with lenticular and retinal degeneration. Keratitis also may facilitate secondary bacterial infection and increase the severity of ocular lesions.
Diagnosis. Clinical signs, serology, lesions, immunohistochemistry, and virus isolation can all aid the diagnosis of RCV infection. However, the severity and prevalence of clinical signs can vary enough to caution against using them as the sole basis for diagnosis. Gross and microscopic lesions, particularly necrosis and inflammation of the salivary and lacrimal glands, are virtually pathognomonic for RCV infection, as they are not duplicated in other known diseases of rats. The course of infection and tissue repair is sufficiently prolonged (4–6 weeks) to facilitate detection of acute or reparative stages, provided the number of rats sampled is adequate. Because acute lesions develop within 5 days of exposure to virus and are often reflected in clinical signs, it is best to select animals for pathologic study that have active clinical signs or have been in recent contact with clinically affected animals. Immunohistochemistry to detect RCV antigens in aldehyde-fixed, paraffin-embedded tissues is useful to visualize virus in tissues collected during acute infection, as virus is eliminated rapidly with the onset of host immunity.
Serology is used routinely to detect or confirm infection. However, it generally takes 7 to 10 days after initial exposure to virus before an individual rat becomes seropositive. The preferred serologic tests are an immunofluorescent assay (IFA) using virus-infected cells or an ELISA (Peters and Collins, 1981; Smith and Winograd, 1986; Machii et al., 1988; Percy et al., 1991), both of which use MHV as antigen to exploit its cross-reactivity with RCV. Virus isolation is rarely used or needed but can be accomplished by inoculation of test specimens, such as clarified homogenates of cervical salivary gland into PRK cells or L2 or LBC cell lines (Bhatt et al., 1972; Hirano et al., 1986; Hirano, 1990; Gaertner et al., 1996a; Hirano et al., 1995). Molecular probes for RCV have been developed and can be used to detect infection in situ or in tissue extracts. Reverse-transcriptase PCR techniques also have been used to identify cages housing infected rats rapidly-and prior to seroconversion (Compton et al., 1999b).
Differential diagnosis. Salivary gland lesions are virtually pathognomonic for RCV infection. Rat cytomegalovirus (II.B.) can cause mild, asymptomatic lesions of the salivary glands, but corresponding lesions are characterized by enlargement of ductal epithelial cells that may contain herpetic, intranuclear inclusions. Porphyrintinged naso-ocular discharges can occur when rats are subject to other stressors, or during other infectious diseases of the respiratory tract such as mycoplasmosis. Irritants such as ammonia can cause photophobia and lacrimation. Respiratory tract lesions due to RCV should be differentiated from those caused by Sendai virusa recently recognized and putative hantavirus-like respiratory virus of rats (Simmons and Riley, 2002), pneumonia virus of mice, or pathogenic bacteria such as Mycoplasma pulmonis. One cannot exclude, in this regard, the potential for RCV to facilitate or exacerbate secondary bacterial infection (Michaels and Myerowitz, 1983). Bacterial conjunctivitis can occur in rats, but RCV must be ruled out as an underlying cause.
Control and prevention. Effective use of barrier equipment and procedures is essential to control and prevent RCV infection, because it spreads rapidly and can remain endemic in any colony where non-immune rats are regularly added. However, rapid spread and limited duration also can be exploited toward control; that is, it is reasonable to expect that, in a given colony room, all rats should be infected and develop immunity within 3 to 5 weeks. This latter interval may be longer among rats housed in static isolation caging or in individually ventilated caging. Thus, strict quarantine of infected rooms is essential for at least 5 weeks, and preferably 6 to 8 weeks. Additionally, quarantined rooms should be kept under negative air pressure with respect to shared corridors and should be serviced last. Personnel entering barrier or quarantined animal rooms should wear protective garb and should not enter rooms housing uninfected rats without thorough personal sanitation steps, as fomite transmission of RCV can occur (La Regina et al., 1992). Cages should be serviced in HEPA-filtered stations and soiled equipment should be sent for sanitation by a predetermined and secure route, to prevent cross-infection. Finally, serologic testing should be intensified in adjacent colonies to determine if quarantine has been effective in containing the spread of infection. The quarantine period can be reduced by purposefully exposing all rats to infection, which will accelerate the development of immunity, provided that it will not interfere with research objectives. Production should cease in breeding colonies for about 6 weeks. After this interval, seropositive rats can be moved to a different room to resume breeding, because they should no longer be contagious (Brammer et al., 1993). Here, as well, purposeful exposure of breeding stock will accelerate colony-wide immunity, which also will lead to protection of normally susceptible litters by maternally acquired immunity. The foregoing strategies assume that attempts will be made to salvage infected animals. Under certain conditions, such as those applicable during pharmacologic testing, even transient infection and RCV seropositivity may not be tolerable and may require euthanasia of entire colonies.
Because RCV is relatively labile, routine disinfection will inactivate virus. Sanitation of caging and equipment should include thorough washing at 83°C, but need not require autoclaving. Similarly, room surfaces can be sanitized with standard disinfectants, such as chlorine dioxide, followed by enforced vacancy for 2 to 3 days (Saknimit et al., 1988).
Prevention of infection depends on effective surveillance as outlined in Chapter 16. Because infected rats are thought to be the major source of contamination, procurement from vendors with sound barrier housing and serologic monitoring programs is essential. This includes demonstrated attention to separation of functions, such as surgical manipulations, within vendor facilities. An effective quarantine program for biological materials for use in rats and for rats arriving from non-commercial vendors is also essential (Nicklas et al., 1993; Shek and Gaertner, 2002). Irrespective of source, it is prudent to decontaminate the external surfaces of shipping containers before unpacking animals.
Interference with research. RCV infection may hamper studies involving the respiratory system, salivary glands, lacrimal glands, the immune system, and the eye. This includes the distortion of physiologic responses or the interpretation of histologic changes. Furthermore, acutely infected rats may be at increased risk for death during inhalation anesthesia, as inflammatory exudate in the airways and enlarged salivary glands may impede normal respiration. Eye research can be compromised by the ocular manifestations of infection, a problem especially relevant to long-term toxicologic studies. Published reports include other diverse effects upon research, including altered feeding behavior (Sato et al., 2001), inhibition of phagocytosis and interleukin-1 production by pulmonary macrophages (Boschert et al., 1988), enhancement of nasal colonization with Haemophilus influenzae type b (Michaels and Myerowitz, 1983), depletion of salivary gland epidermal growth factor (Percy et al., 1988), and graft-versushost disease in rats with transplanted bone marrow (Rossie et al., 1988). Finally, it is worth remembering that pneumotropic viruses can facilitate co-infection by opportunistic bacteria, as illustrated in the following description of Sendai virus infection. Thus, bacterial pneumonias of rats should be assessed for underlying coronaviral involvement, especially during active RCV infection.
B. Paramyxoviruses (Sendai Virus, Pneumonia Virus of Mice)
1. Sendai Virus
Sendai virus infection, with resultant necrosis and inflammation in the respiratory tract, was a major impediment to rodent-based research for many years. Its prevalence has abated owing to improved housing, husbandry, and serologic surveillance. Mice were the primary targets of Sendai virus infection. There are, however, several reports of natural outbreaks in rats. Because Sendai virus also is infectious for hamsters and guinea pigs, infection would place multiple species at risk.
Agent. Sendai virus is an enveloped, pleomorphic, spherical, or filamentous virus of the family Paramyxoviridae. It has a single-stranded RNA genome that encodes six proteins including a surface HN glycoprotein with hemagglutinin and neuraminidase moieties that are responsible for adsorption to host cells, and a surface F glycoprotein that facilitates cell fusion important to virus entry, expressed cytologically as syncytia formation (Brownstein, 1986). Sendai virus is also known as parainfluenza virus 1 and can be serologically differentiated from strains 2, 3 and 4. It grows well in embryonated eggs and in multiple established cell lines, including BHK-21 cells. Other biological and biochemical characteristics of Sendai virus have been described elsewhere (Brownstein, 1986).
Clinical signs. Infection in rats, in contrast to mice, is primarily asymptomatic. However, clinical signs have been observed during natural (Makino et al., 1972) and experimental (Castleman, 1983; Giddens et al., 1987) infection. These include dyspnea, anorexia, and ruffled pelage. Indirect clinical effects have been attributed to the general stress of infection and include fetal resorption, reduced litter size, and retarded growth (Coid and Wardman, 1971).
Epidemiology. Rats of all ages are susceptible to infection, but it is acute and self-limiting in immunocompetent animals. Elimination of virus coincides with the onset of antiviral immunity at about 1 week after initial exposure. Because infection is highly contagious and transmitted by aerosol, rapid spread is common. This effect has been documented by Makino and co-workers (et al., 1972) who observed infection in a colony of 500 breeding rats with subsequent outbreaks re-occurring at 8- to 10-month intervals as newly susceptible rats emerged from the breeding program. Signs of respiratory disease and retarded growth among suckling rats occurred during each episode. Endemic infection is punctuated by fresh clinical outbreaks, typical for acute viral infections in which recovery leads to transient maternally derived immunity followed by renewed susceptibility. Antibody responses to virus remain high for at least several months after acute infection, but decline to low or undetectable levels within 9 months. In these contexts, the epidemiologic pattern and risks from Sendai virus infection in rats resemble those described for RCV infection. However, there is no specific evidence regarding long-term risks for re-infection with Sendai virus. Infection has not been described in immunodeficient rats, but one should assume, as for immunodeficient mice, that it would persist in individual animals and that the risks for transmission would be high. There is no evidence for prenatal infection.
Pathology and pathogenesis. Patterns of infection and lesions are similar in infant, weanling, and young adult rats, but viral clearance is slower in infants (Castleman, 1983; Castleman et al., 1987; Giddens et al., 1987). Thus, virus can be detected beyond the first week of infection, whereas older rats generally clear virus by 1 week. This difference is reflected in a slower onset of humoral anti-Sendai virus immunity in infant rats. Infection causes rhinitis with subsequent development of bronchiolitis and pneumonia. Grossly, affected portions of lung are dark red and consolidated. Otitis media may develop in some rats. Upper respiratory lesions begin within 4 days of exposure to virus, but viral antigen can be detected in respiratory epithelium within 1- to 2 days. Early lesions are characterized by epithelial necrosis with mucosal infiltration by neutrophils and lymphoid cells, all of which increase in severity during the ensuing week. Bronchiolitis and pneumonia may begin as early as 2 days after exposure to virus and feature necrosis and inflammation Fig. 12-27 ). Bronchiolar epithelium undergoes necrosis and erosion, which may be accompanied by epithelial hyperplasia. Viral replication occurs in type I and type II pneumocytes and in macrophages, resulting in necrosis and interstitial infiltration by neutrophils, macrophages, and lymphocytes. Pneumonic lesions are most severe at 5 to 8 days, depending on the age of affected rats, with lesions peaking earlier in weanling or young adult rats than in infant rats. Resolution of pneumonia in immunocompetent rats begins within 2 weeks after exposure and is largely complete within 3 weeks. Residual lesions include perivascular and peribronchial accumulations of mononuclear cells, which may last up to several weeks. Some rats may develop interstitial fibrosis.
Fig. 12-27.
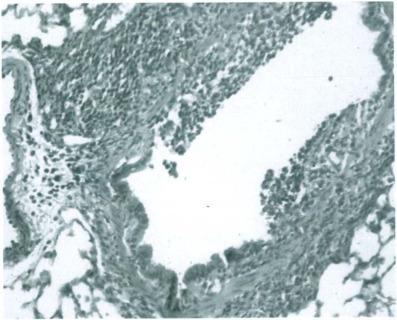
Sendai virus infection. Bronchopneumonia with necrosis of bronchial epithelium.
Diagnosis. Because clinical signs occur sporadically, they are not a reliable indicator of infection. However, Sendai virus infection should be considered if unexpected morbidity or deaths occur during procedures that impact the respiratory tract, or if unexplained distortions occur in immune responses. Serologic testing, accompanied or followed by pathologic assessment, is the primary means for detecting infection. Sensitive IFA and ELISA tests are available for detection of antiviral antibodies (Smith, 1983; Wan et al., 1995). Histopathologic examination of the upper and lower respiratory tracts should be performed in clinically affected animals, on those that have recently seroconverted, or even in asymptomatic rats that have been exposed to affected animals. Immunohistochemistry to detect viral antigen can be performed on aldehyde-fixed, paraffin-embedded tissues harvested during acute infection. Respiratory epithelial cells and alveolar pneumocytes should be examined for intracytoplasmic viral antigen. Virus can be isolated by inoculation of nasobronchial washes or clarified lung homogenates into embryonated eggs, or preferably, BHK-21 cells. Cultures should be checked for viral antigen by 1 week after inoculation, whether or not CPE has developed.
Differential diagnosis. Sendai virus infections must be differentiated from those caused by RCV, PVM, and rat respiratory virus (RRV) (putative hanta-like virus). RCV pneumonia is primarily interstitial and infection also causes sialodacryoadenitis. RRV also appears to target the alveolar parenchyma, so rhinotracheitis and bronchitis are not characteristic features. PVM causes pulmonary vasculitis and interstitial pneumonia. Sendai virus pneumonia can be confused with early stages of murine respiratory mycoplasmosis, which it also can exacerbate. However, the latter often progress to chronic respiratory disease with bronchiectasis and bronchiolectasis. Other bacterial pneumonias also can be considered in the differential diagnosis and are described in the chapter on bacterial disease by Weisbroth, Kohn, and Boot. Because Sendai virus infection has been associated with fetal resorption and retarded growth, it must be differentiated from parvovirus infection.
Control and prevention. Strategies for control and prevention are equivalent to those described for RCV infection. Because mice, rats, hamsters, and guinea pigs are susceptible to Sendai virus, precautions should be taken to prevent cross-infection. The essential components of containment are quarantine of infected rooms, strict sanitary measures, and tightly controlled room servicing procedures and schedules. Because Sendai virus is highly labile in the environment, clean up and disinfection should follow recommendations described for RCV. Transport of rats to laboratories should be discouraged. As with RCV, the primary source of infection is likely to be infected animals, so strict control of animal entry, including vendor surveillance and quarantine and testing of animals from non-commercial sources is essential.
Interference with research. Because Sendai virus infects the respiratory tract, experimental procedures ranging from anesthesia to inhalation toxicology can be affected. This includes the distortion of physiologic responses or the interpretation of histologic changes. There also is evidence that systemic immune responses can be transiently impaired (Garlinghouse and van Hoosier, 1978). Finally, Sendai virus should be considered a potential copathogen or aggravating factor in other respiratory infections of rats, as demonstrated by its aforementioned relationship with murine mycoplasmosis (Schoeb et al., 1985).
2. Pneumonia Virus of Mice
Agent. Pneumonia virus of mice (PVM) is a member of the genus Pneumovirus in the family Paramyxoviridae. It is most widely known as a cause of rhinotracheitis and interstitial pneumonia in mice, but also is naturally infectious for rats.
Clinical signs. Infection in rats is asymptomatic and thus detected primarily by serology, for which sensitive ELISA and IFA tests are available (Descoteaux et al., 1981; London, et al., 1983; Smith, 1983). PVM infection is mildly pathogenic, so diagnosis can be supplemented by pathologic assessment during acute infection.
Epidemiology. Rats, mice, hamsters, gerbils, and possibly guinea pigs and rabbits are susceptible to infection. A recent survey of major biomedical research centers reported that approximately 20% of rat colonies had some evidence of PVM infection (Jacoby and Lindsey, 1998).
Pathology. The following picture has emerged from anecdotal observations and a brief report of experimental infection (Votsberger et al., 1982; Brownstein, 1985). Although rhinotracheitis may occur, the most prominent lesions are perivascular mononuclear cell infiltrates, nonsuppurative interstitial pneumonia and hyperplasia of bronchial-associated lymphoid tissue, all of which may last up to several weeks. The vascular orientation is a point of differentiation from pneumonia caused by Sendai virus or rat coronavirus.
Control and prevention. Prevention, control, and elimination follow principles described above for rat coronaviruses.
Interference with research. The risks to research pertain to PVM's potential capacity as a co-pathogen and to transmission to other susceptible species, notably mice. It is worth noting, in these contexts, that PVM can exacerbate pneumocystosis in immunocompromised (SCID) mice (Bray et al., 1993). It is not known if immunodeficient rats are susceptible to this effect.
C. Hantaviruses and Rat Respiratory (Hanta-like) Virus
Agents. Hantaviruses are tri-segmented, negative-sense, single-stranded RNA viruses of the genus Hantavirus in the family Bunyaviridae (Nichol, 2001). There are more than 20 known strains, each of which is closely associated with a rodent or insectivorous reservoir host (Schmaljohn and Hjelle, 1997; Simmons and Riley, 2002). Several hantavirus strains, including Seoul virus, are indigenous to Rattus norvegicus. Black Creek Canal virus, a strain that is highly virulent for humans, has been isolated from cotton rats (Sigmodon hispidus) and implies that extreme caution should be employed if wild-caught cotton rats are used for research. In fact virtually all hantaviruses are known or have the potential to be zoonotic for man with varying pathogenicity ranging from asymptomatic to lethal infection. A newly recognized agent, named rat respiratory virus (RRV), has been detected in laboratory rats with interstitial pneumonia (Simmons and Riley, 2002). Serologic tests suggest that it is related to the hantaviruses.
Clinical signs. Rodent hantavirus infections are typically asymptomatic but persistent, a condition attributed to co-evolution of hantaviruses with their respective hosts.
Epidemiology. Rat-to-rat transmission occurs through direct contact and bite wounds, although indirect contact by fomites contaminated with excreta or secreta is possible. Recent in situ hybridization studies have detected persistent infection in kidney, exocrine pancreas, salivary gland, and skin—sites conducive to secretion/excretion of infectious virus (Compton et al., 2003). Further, one must consider the possibility for transmission by contaminated transplantable rat tumors. Hantavirus infection should be considered persistent in individual rats despite antiviral immunity (Meyer and Schmaljohn, 2000). The mechanism(s) of persistence is not well-understood, but prolongs risks for transmission. The distribution of rat-associated infection is virtually worldwide and has included zoonotic infections among laboratory animal workers (Desmyter et al., 1983; Lloyd et al., 1984; Lloyd and Jones, 1986; LeDuc, 1987; Tsai, 1987). As noted above, human infection has a variety of clinical outcomes ranging from asymptomatic infection to lethal hemorrhagic fevers (Hart and Bennett, 1999). The prevalence of virulent and/or zoonotic hantaviruses in laboratory rats is extremely low. Early assessments of RRV infection indicate a prevalence of approximately 8% among laboratory rats and more than 20% among laboratory mice (Simmons and Riley, 2002).
Pathology. Most studies of hantavirus pathology in rats have utilized experimental infection with Asian isolates such as Seoul virus (Lee et al., 1986; Compton et al., 2003). Acute hantavirus infection has a widespread tissue distribution and a predilection for endothelial cells, as well as glandular and renal epithelium. Infection does not cause significant tissue damage, but can provoke mononuclear cell infiltrations in affected tissues, including lung, salivary gland, pancreas, and kidney, especially during persistent infection. Pancreatic lesions may include insulitis with at least transient elevations in blood glucose (Compton et al., 2003). As noted above, RRV infection appears to cause interstitial pneumonia.
Diagnosis. Diagnosis relies primarily on detection of specific anti-hantavirus antibodies by IFA, with an ELISA serving primarily as a back-up test (Lee et al., 1999). Immunostaining of tissue sections or molecular techniques (in situ hybridization or RT-PCR) also can be used; however, primer sets are not readily available for all strains. Further, RT-PCR is complicated by heterogeneity inherent to hantavirus genomes. Hantaviruses can be propagated in cell culture, with Vero-6 cells being the preferred substrate, but replicate slowly and with low yield.
Attempts at virus isolation should be reserved for only the most experienced and well-equipped laboratories, because of technical and safety concerns.
Differential diagnosis. Interstitial pneumonia, which may be caused be RRV, must be differentiated from other causes of similar lesions including rat coronavirus, Sendai virus, and PVM.
Control and prevention. Serologic monitoring is the key to prevent entry and spread of hantavirus infection. Infected or exposed rats should be placed under strict quarantine, and attempts to speciate the causative agent by a qualified laboratory should be undertaken as a means to assess zoonotic risk. Thus, the Asian hantaviruses present a clear zoonotic hazard, whereas there is currently no evidence for zoonotic transmission of RRV. Confirmation of a zoonotic strain should provoke depopulation of affected and exposed animals and thorough decontamination of facilities, equipment, and supplies. Personnel working in affected or potentially affected areas should don full protective gear, including ventilated hoods. If the potential for infection has been identified, the safest action is to notify and engage institutional safety officers. It also is prudent to assess rat-derived biological materials obtained from countries where hantavirus infection is endemic.
Effects on research. Zoonotic threats from virulent strains are the major impediment to research. As noted above, infection of rats with Seoul virus can cause insulitis and hyperglycemia. There is no evidence for or against the possibility that interstitial pneumonia associated with RRV alters pulmonary function or susceptibility to opportunistic respiratory infections.
D. Rotavirus and Reovirus
A rotavirus has been incriminated as the cause of infectious diarrhea of infant rats (IDIR), a non-lethal condition that resembles rotavirus-caused epidemic diarrhea of infant mice (EDIM) (Vonderfecht et al., 1984; Vonderfecht, 1986). The causative agent is an antigenically distinct group B rotavirus that may be of human origin. Infected suckling rats develop clinical signs within a day or two after initial exposure. These include diarrhea for up to 1 week accompanied by cracking and bleeding of the perineal region, dry, flaky skin, and transient growth retardation. Although rats of all ages are susceptible to infection, resistance to clinical disease develops at about 2 weeks of age. Gross lesions are limited to the gastrointestinal tract. The stomach usually contains milk. The ileum and colon may be distended with yellow-green fluid and gas. Histologic lesions are most prominent in the ileum and are characterized by enterocytic syncytia, which may contain intracytoplasmic inclusions, enterocytic necrosis, and attenuation of villi. Viral antigen and rotaviral particles may be found in affected enterocytes. Diagnosis rests on characteristic clinical and pathologic changes accompanied by detection of rotaviral antigens and/or particles. Serologic assays have not been developed. Differential diagnoses should include clinically apparent infection by pathogenic enterotropic bacteria. The significance of IDIR pertains primarily to transient growth retardation and the possibility that it originated as a human infection.
Margolis and Kilham have induced prenatal reovirus 3 infection in fetal rats (Margolis and Kilham, 1973) and there is anecdotal evidence that rats can develop antibodies to reoviruses, but there are no documented reports of natural reovirus infection. Because reovirus antigens are fairly widespread in nature, it is decidedly unclear whether such seroconversions are due to active infection or exposure to inert reovirus antigens from sources such as plant matter.
E. Picornaviruses
Two types of picornavirus infection have been linked to rats. Theiler's mouse encephalomyelitis virus (TMEV) is a member of the cardiovirus genus of the family Picornaviridae, the natural host for which is the mouse (Jacoby et al., 2002). Anecdotal observations indicate that rats can develop antibodies to TMEV, but no associated disease or lesions have been found. In historical perspective, a TMEV-like agent called MHG virus was isolated from rats and infected intestine, lung, and brain after experimental inoculation. A second type of cardiovirus genetically distinct from TMEV has been isolated from rats and designated rat cardiovirus (Ohsawa et al., 2003). It does not appear to be pathogenic after experimental inoculation.
IV. COMMENTS ON DETECTION, DIAGNOSIS, RISK-ASSESSMENT AND DECISION-MAKING
Contemporary biomedical research should use rats free of adventitious viruses with the potential to cause illness or distort research results. Validation is based primarily on the absence of antibodies to common viruses. Broad agreement among experts in the field regarding antigen panels, sampling protocols, and serologic methods has not yet materialized. Therefore, definitions of health status vary and must be established for each set of housing and husbandry conditions. This step is essential to protect the integrity of individual colonies, to reduce risks for spread of infection during transport or exchange of rats among laboratories, and to document the virologic status of rats in research grant applications and in scientific reports.
A. Detection and Diagnosis
Monitoring for viral infections should cover at least three venues: animals in established breeding and experimental colonies, animals held in entry quarantine, and animal tissues and products destined for in vivo use. For established colonies, monitoring should be tailored to local conditions. Effective monitoring should encompass sampling on a pre-arranged schedule, which can be intensified if evidence or suspicion of viral infection emerges. Although sampling should utilize index animals, such as retired breeders, whenever possible, the most widely accepted approach to assess contemporary conditions in a colony is serologic monitoring of strategically placed and exposed sentinels. Sentinels must be immunocompetent, virus-antibody free, and protected from exposure to infectious agents during shipment. The number of sentinels used should reflect assessment of risks to a colony, as illustrated in Chapter 16. Cages of sentinels should be placed, by rack and room, to facilitate controlled exposure to potential sources of infection. One popular configuration for rats housed in conventional wire-top caging is to place sentinels on the lowest shelf of a rack to maximize natural exposure to bedding expelled from overlying cages. Additionally, samples of soiled bedding collected during routine cage changing (e.g., weekly) can be placed in sentinel cages to increase the sensitivity of detection. This strategy also helps to compensate for the use of isolation and individually ventilated caging, which limits airborne transmission of viral agents and by doing so, also may inhibit early detection of infection. However, limited durability of excreted virus in soiled bedding and the potential that small amounts of virus could be diluted to nonimmunogenic levels in pooled bedding samples also must be considered. Targeted selection of soiled bedding samples also can be helpful to trace infection to a sector of a suspect rack or shelf. For this procedure to work satisfactorily, cage positions should not be changed during the testing interval.
Sampling intervals for sentinel rats generally range from 30 to 90 days, but, minimally, must be long enough to complete desired exposure and allow opportunity for seroconversion, which typically occurs by 2 weeks after an immunogenic exposure. Sentinel animals can be sampled repeatedly if they remain seronegative, but should be replaced if they develop antibodies. As a general rule, sentinels, regardless of serologic status, should be replaced every 6 to 12 months to permit incremental assessment, such as histopathology.
Molecular techniques can be used selectively to confirm serologic results. For example, PCR testing of excreta, soiled bedding, equipment, or surfaces can be used in lieu of virus isolation to help determine if infection initially detected by serology is currently active. Additionally, there is preliminary evidence from studies with mouse coronavirus that PCR sampling of exhaust air manifolds can provide a rapid, “realtime” overview of infection among individually ventilated cage racks (SR Compton, personal communication); however, it remains to be seen whether this technology is applicable to viruses of rats.
Sampling of quarantined arrivals, which is likely to increase with the wider use and exchange of genetically altered rats, should be based on the same sampling principles outlined above. Depending on source, age, gender, animal number and behavioral considerations, sentinel exposures can use soiled bedding or direct placement with index animals. Because cohorts of imported rats are generally small, sentinel exposure for 30 days should be adequate to elicit potential seroconversion. Sampling of animal products destined for in vivo use, such as transplantable tumors or blood products, is essential for a sound monitoring program. It also is important, in this regard, to counsel investigators about the hazards of introducing untested biological products into rats. Aliquots can be tested by inoculation of susceptible cell lines or by molecular methods. The aforementioned RAP test, although apparently sensitive, tends to be costly, time consuming, and likely to be replaced by molecular methods.
Because viral infections of rats can spread insidiously, early detection and epidemiologic “staging” should employ a detection matrix that includes clinical observation, appropriate sampling, and sensitive and specific diagnostic testing. The value of serology to detect infection and determine prevalence and incidence is obvious. As noted in previous sections of this chapter, sensitive and specific tests are available for the most common viruses of laboratory rats. We re-emphasize, however, that the number of test animals should be large enough and the duration of exposure long enough to maximize the likelihood of detecting infection in a room or colony. Further, the clinician/pathologist should have high confidence that the testing laboratory has the testing conditions and expertise to produce incontrovertible results.
Clinical signs are helpful if they are comparatively specific, such as the cervical and ocular manifestations of coronavirus infection. However, non-specific signs, such as inappetence, altered reproduction, problematic anesthesia, distorted immunity, also may signal underlying viral infection.
Exposed rats, and especially those with clinical morbidity, should be subjected to pathologic examination, beginning with a thorough necropsy and including proper preservation of tissues for histopathology, collection of serum for antibody testing, and selection of tissues for potential molecular and/or virologic analysis. Tissue selection and preservation should be guided by a differential diagnosis emerging from clinical and gross pathology examinations complemented by a sound working knowledge of viral infections of rats. Such knowledge should account for the possibility that signs and gross lesions will be absent. Table 12-1 summarizes current recommendations for tissue collection, preservation, and examination. As noted earlier, aldehyde fixation followed by embedding in paraffin wax is generally suitable for routine histopathology. However, we recommend fixation for approximately 16 hours in freshly prepared paraformaldehyde-lysine-periodate (McClean and Nakane, 1974) if immunohistochemistry and/or in situ hybridization are contemplated, because it provides improved stabilization of viral antigens and nucleic acids. We also recommend freezing selected tissues at −60° C. These samples can be used for virus isolation, molecular testing, or antibody production testing, as initial results warrant. Careful attention to minimize contamination during collection of such samples is critical, especially if the option for PCR testing is to be preserved. It also is prudent to complement examinations for potential viral infection with other microbiological testing to assess the possibility for dual infections or emergence of opportunistic bacterial infections secondary to viral infection.
B. Risk-Assessment and Decision-Making
If evidence of viral infection is obtained, the decision to take corrective action should be based on several key questions: Is the infection a threat to animal or human health? Is the infection a threat to research at the affected or collaborating sites? What are the options for control and elimination? Are the options epidemiologically effective, minimally disruptive to research, and fiscally sound? What are the options to prevent reinfection? These questions are best addressed through a thorough understanding of the biology and pathobiology of the offending agent and the functional and structural characteristics of the affected vivarium. Characteristics such as mode of spread, environmental stability, duration, and virulence in immunocompetent compared with immunodeficient hosts and known effects on research can lead to well-established actions for containment and elimination that have been illustrated for specific agents in this chapter. Further, working knowledge of viral infections can facilitate extrapolation of what is known to what may be conjectural due to lack of specific data. For example, the predilection of parvoviruses for mitotically active cells should be a generic factor in considering the potential impact of parvovirus infection on research. However, decision-making is likely to become more problematic as the use of genetically altered rats increases. Rats with either targeted or serendipitous alterations in resistance to infection may have correspondingly altered responses to viral infections. This potential is illustrated by cryptic immunodeficiencies in laboratory mice that can occur as unintended sequelae of genetic manipulation. Therefore, it will be important to consider phenotype as integral to risk assessment, and to broaden clinical, epidemiologic, and pathologic conception of the “textbook” spectra of infection.
REFERENCES
- Ball-Goodrich L.J., Johnson E.A., Jacoby R.O. Divergent replication kinetics in two phenotypically different parvoviruses of rats. J. Gen. Virol. 2001;82:537–546. doi: 10.1099/0022-1317-82-3-537. [DOI] [PubMed] [Google Scholar]
- Ball-Goodrich L.J., Leland S.E., Johnson E.A., Paturzo F.X., Jacoby R.O. Rat parvovirus type 1: the prototype for a new rodent parvovirus serogroup. J. Virol. 1998;72:3289–3299. doi: 10.1128/jvi.72.4.3289-3299.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball-Goodrich L.J., Paturzo F.X., Johnson E.A., Steger K., Jacoby R.O. Immune responses to the major capsid proteins during parvovirus infection of rats. J. Virol. 2002;76:10044–10049. doi: 10.1128/JVI.76.19.10044-10049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baringer J.R., Nathanson N. Parvovirus hemorrhagic encephalopathy of rats: electron microscopic observations of the vascular lesions. Lab. Invest. 1972;27:514–522. [PubMed] [Google Scholar]
- Barker M.G., Percy D.H., Hovland D.J., MacInnes J.I. Preliminary characterization of the structural proteins of the coronaviruses, sialodacryoadenitis virus and Parker's rat coronavirus. Can. J. Vet. Res. 1994;58:99–103. [PMC free article] [PubMed] [Google Scholar]
- Barthold S.W., deSouza M.S., Smith A.L. Susceptibility of laboratory mine to intranasal and contact infection with corona-viruses of other species. Lab. Anim. Sci. 1990;40:481–485. [PubMed] [Google Scholar]
- Bergs V.V. Rat virus-mediated suppression of leukemia induction by Moloney virus in rats. Cancer Res. 1969;29:1669–1672. [PubMed] [Google Scholar]
- Besselsen D.G., Besch-Williford C.L., Pintel D.J., Franklin C.L., Hook R.R., Jr., Riley L.K. Detection of newly recognized rodent parvoviruses by PCR. J. Clin. Microbiol. 1995;33:2859–2863. doi: 10.1128/jcm.33.11.2859-2863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselsen D.G., Besch-Williford C.L., Pintel D.J., Franklin C.L., Hook R.R., Jr., Riley L.K. Detection of H-1 parvovirus and Kilham rat virus by PCR. J. Clin. Microbiol. 1995;33:1699–1703. doi: 10.1128/jcm.33.7.1699-1703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P.N., Jacoby R.O. Epizootiological observations of natural and experimental infection with sialodacryoadenitis virus in rats. Lab. Anim. Sci. 1985;35:129–134. [PubMed] [Google Scholar]
- Bhatt P.N., Jacoby R.O. Experimental infection of adult axenic rats with Parker's rat coronavirus. Arch. Virol. 1977;54:345–352. doi: 10.1007/BF01314779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P.N., Jacoby R.O., Jonas A.M. Respiratory infection in mice with sialodacryoadenitis virus, a coronavirus of rats. Infect. Immun. 1977;18:823–827. doi: 10.1128/iai.18.3.823-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P.N., Percy D.H., Jonas A.M. Characterization of the virus of sialodacryoadenitis of rats: a member of the coronvarivus group. J. Infect. Dis. 1972;126:123–130. doi: 10.1093/infdis/126.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihun C.G., Percy D.H. Morphologic changes in the nasal cavity associated with sialodacryoadenitis virus intection in the Wistar rat. Vet Pathol. 1995;32:1–10. doi: 10.1177/030098589503200101. [DOI] [PubMed] [Google Scholar]
- Boschert K.R., Schoeb T.R., Chandler D.B., Dillehay D.L. Inhibition of phagocytosis and interleukin-1 production in, pulmonary macrophages from rats with sialodacryoadenitis virus infection. J. Leukoc. Biol. 1988;44:87–92. doi: 10.1002/jlb.44.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammer D.W., Dysko R.C., Spilman S.C., Oskar P.A. Elimination of sialodacryoadenitis virus from a rat production colony by using seropositive breeding animals. Lab. Anim. Sci. 1993;43:633–634. [PubMed] [Google Scholar]
- Bray M.V., Barthold S.W., Sidman C.L., Roths J., Smith A.L. Exacerbation of Pneumocystis carinii pneumonia in immu-nodeficient (SCID) mice by concurrent infection with a pneumovirus. Infect. Immun. 1993;61:158–1588. doi: 10.1128/iai.61.4.1586-1588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.W., Welsh R.M., Like A.A. Infection of peripancreatic lymph nodes but not islets precedes Kilham rat virus-induced diabetes in BB/Wor rats. J. Virol. 1993;67:5873–5878. doi: 10.1128/jvi.67.10.5873-5878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein D.G. Pneumonia virus of mice infection, lung, mouse and rat. In: Jones T.C., Mohr U., Hunt R.D., editors. Monographs on Pathology of Laboratory Animals: Respiratory System. Springer-Verlag; New York: 1985. pp. 206–210. [Google Scholar]
- Brownstein D.G. Viral and Mycoplasmal Infections of Rodents: Effects on Biomedical Research. Academic Press; Orlando, FL: 1986. Sendai virus. [Google Scholar]
- Bruggeman C.A., Debie W.M., Grauls G., Majoor G., van Boven C.P. Infection of laboratory rats with a new cytomegalo-like virus. Arch. Virol. 1983;76:189–199. doi: 10.1007/BF01311103. [DOI] [PubMed] [Google Scholar]
- Bruggeman C.A., Meijer H., Bosman F., van Boven C.P.A. Biology of rat cytomegalovirus infection. Intervirol. 1985;24:1–9. doi: 10.1159/000149612. [DOI] [PubMed] [Google Scholar]
- Campbell D.A., Staal S.P., Manders E.K., Bonnard G.D., Oldham R.K., Staal S.P., Manders E.K., Bonnard G.D., Oldham R.K., Salzman R.K., Herberman R.B. Inhibition of in vitro lymphoproliferative responses by in vivo passaged rat 13762 mammary adenocarcinoma cells. II. Evidence that Kilham rat virus is responsible for the inhibitory effect. Cell. Immunol. 1977;33:378–391. doi: 10.1016/0008-8749(77)90166-6. [DOI] [PubMed] [Google Scholar]
- Castleman W.L. Respiratory tract lesions in weanling outbred rats infected with Sendai virus. Am. J. Vet. Res. 1983;44:1024–1031. [PubMed] [Google Scholar]
- Castleman W.L., Brudnage-Anguish L.J., Kreitzer L., Neuenschwander S.B. Pathogenesis of bronchiolitis and pneumonia induced in neonatal and weanling rats by parainfluenza (Sendai) virus. Am. J. Pathol. 1987;129:277–296. [PMC free article] [PubMed] [Google Scholar]
- Cole G.A., Nathanson N., Rivet H. Viral hemorrhagic encephalopathy of rats. II. Pathogenesis of central nervous system lesions. Am. J. Epidemiol. 1970;91:339–350. doi: 10.1093/oxfordjournals.aje.a121144. [DOI] [PubMed] [Google Scholar]
- Coid R., Wardman G. The effect of parainfluenza type 1 (Sendai) virus infection on early pregnancy in the rat. J. Reprod. Fertil. 1971;29:39–43. doi: 10.1530/jrf.0.0240039. [DOI] [PubMed] [Google Scholar]
- Coleman G.L., Jacoby R.O., Bhatt P.N., Jonas A.M. Naturally occurring lethal parvovirus infection of juvenile and young adult rats. Vet. Pathol. 1983;20:49–56. doi: 10.1177/030098588302000105. [DOI] [PubMed] [Google Scholar]
- Compton S.R., Barthold S.W., Smith A.L. The cellular and molecular pathogenesis of coronaviruses. Lab. Anim. Sci. 1993;43:15–28. [PubMed] [Google Scholar]
- Compton S.R., Jacoby R.O., Paturzo F.X., Smith A.L. 2003. Persistent Seoul virus infection in Lewis rats. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton S.R., Smith A.L., Gaertner D.J. Comparison of the pathogenicity in rats of rat coronaviruses of different neutralization groups. Lab. Anim. Sci. 1999;49:514–518. [PubMed] [Google Scholar]
- Compton S.R., Vivas-Gonzales B.E., Macy J.D. Reverse transcriptase chain reaction-based diagnosis and molecular characterization of a new rat coronavirus strain. Lab. Anim. Sci. 1999;49:506–513. [PubMed] [Google Scholar]
- Cotmore S.F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv. Virus. Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Cross S.S., Parker J.C. Some antigenic relationships of the murine parvoviruses: minute virus of mice, rat virus, H-1 virus. Proc. Soc. Exp. Biol. Med. 1972;139:105–108. doi: 10.3181/00379727-139-36088. [DOI] [PubMed] [Google Scholar]
- Descoteaux J.P., Payment P. Comparison of hemagglu-tination inhibition, serum neutralization and ELISA PVM tests for the detection of antibodies to pneumonia virus of mice in rat sera. J. Virol. Meth. 1981;3:83–87. doi: 10.1016/0166-0934(81)90004-5. [DOI] [PubMed] [Google Scholar]
- Desmyter J., LeDuc J.W., Johnson K.M., Brasseur F., Deckers C., van Ypersele de Strihou Laboratory rat associated outbreak of hemorrhagic fever with renal syndrome due to Hantaan-like virus in Belgium. Lancet. 1983;2:1445–1448. doi: 10.1016/s0140-6736(83)90797-3. [DOI] [PubMed] [Google Scholar]
- El Dadah A.H., Nathanson N., Smith K.O., Squire R.A., Santos G.W., Melby E.C. Viral hemorrhagic encephalopathy in rats. Science. 1967;156:392–394. doi: 10.1126/science.156.3773.392. [DOI] [PubMed] [Google Scholar]
- Ellerman K.E., Richards C.A., Guberski D.L., Shek W.R., Like A.A. Kilham rat virus triggers, T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes. 1996;45:557–562. doi: 10.2337/diab.45.5.557. [DOI] [PubMed] [Google Scholar]
- Gaertner D.J., Compton S.R., Winograd D.F. Environmental stability of rat coronaviruses (RCVs) Lab. Anim. Sci. 1993;43:403–404. [PubMed] [Google Scholar]
- Gaertner D.J., Compton S.R., Winograd D.F., Smith A.L. Growth characteristics and protein profiles of prototype and wild-type rat coronavirus isolates grown in a cloned subline of mouse fibroblasts (L2p.176 cells) Virus Res. 1996;41:55–68. doi: 10.1016/0168-1702(95)01274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner D.J., Jacoby R.O., Johnson E.A., Paturzo F.X., Smith A.L. Persistent rat virus infection in juvenile athymic rats and its modulation by antiserum. Lab. Anim. Sci. 1995;45:249–253. [PubMed] [Google Scholar]
- Gaertner D.J., Jacoby R.O., Johnson E.A., Paturzo F.X., Smith A.L., Brandsma J.L. Characterization of acute rat parvovirus infection by in situ hybridization. Virus Res. 1993;28:1–18. doi: 10.1016/0168-1702(93)90085-2. [DOI] [PubMed] [Google Scholar]
- Gaertner D.J., Jacoby R.O., Paturzo F.X., Johnson E.A., Brandsma J.L., Smith A.L. Modulation of lethal and persistent rat parvovirus infection by antibody. Arch. Virol. 1991;118:1–9. doi: 10.1007/BF01311299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner D.J., Jacoby R.O., Smith A.L., Ardito R.B., Paturzo F.X. Persistence of rat virus in athymic rats. Arch. Virol. 1989;105:259–268. doi: 10.1007/BF01311362. [DOI] [PubMed] [Google Scholar]
- Gaertner D.G., Smith A.L., Jacoby R.O. Efficient induction of persistent and prenatal infection with a parvovirus of rats. Virus Res. 1996;44:67–78. doi: 10.1016/0168-1702(96)01351-2. [DOI] [PubMed] [Google Scholar]
- Gaetner D.J., Winograd D.F., Compton S.R., Patruzo F.X., Smith A.L. Development and optimization of plaque assays for rat coronaviruses. J. Virol Meth. 1993;43:53–64. doi: 10.1016/0166-0934(93)90089-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneten S., Scanga C.A., Dveksler G.S., Beauchemin N., Percy D.H., Holmes K.V. Attachment glycoproteins and receptor specificity of rat coronaviruses. Lab. Anim. Sci. 1996;46:159–166. [PubMed] [Google Scholar]
- Garlinghouse L.E., van Hoosier G.L., Jr. Studies on adjuvant-induced arthritis, tumor transplantability, and serologic response to bovine serum albumin in Sendai virus-infected rats. Am. J. Vet. Res. 1978;39:297–300. [PubMed] [Google Scholar]
- Giddens W.E., van Hoosier G.L., Garlinghouse L.E. Experimental Sendai virus infection in laboratory rats. II. Pathology and histochemistry. Lab. Anim. Sci. 1978;37:442–448. [PubMed] [Google Scholar]
- Guberski D.L., Thomas V.A., Shek W.R., Like A.A., Handler E.S., Rossini A.A., Wallace J.E., Welsh R.M. Induction of type I diabetes by Kilham rat virus in diabetes-resistant BB Wor rats. Science. 1991;254:1010–1013. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- Hajjar A.M., DiGiacomo R.F., Carpenter J.K., Bingel S.A., Moazed T.C. Chronic sialodacryoadenitis virus (SDAV) infection in athymic rats. Lab. Anim. Sci. 1991;41:22–25. [PubMed] [Google Scholar]
- Hanna P.E., Percy D.H., Paturzo F.X., Bhatt P.N. Sialodacryoadenitis in the rat: effects of immunosuppression on the course of the disease. Am. J. Vet. Res. 1984;45:2077–2083. [PubMed] [Google Scholar]
- Hart C.A., Bennett M. Hantavirus infections: epidemiology and pathogenesis. Microbe Infect. 1999;1:1229–1237. doi: 10.1016/s1286-4579(99)00238-5. [DOI] [PubMed] [Google Scholar]
- Hartley J.W., Rowe W.P., Bloom J.J., Turner H.C. Antibodies to mouse hepatitis virusin human sera. Proc. Soc. Exp. Biol. Med. 1964;115:414–418. doi: 10.3181/00379727-115-28928. [DOI] [PubMed] [Google Scholar]
- Hirano N., Suzuki Y., Ono K., Murakami T., Fujiwara K. Growth of sialodacryoadenitis virus in LBC cell culture. Nippon Juigaku Zasshi. 1986;48:193–195. doi: 10.1292/jvms1939.48.193. [DOI] [PubMed] [Google Scholar]
- Hirano N. Plaque assay and propagation in rat cell line LBC cells of rat coronavirus and 5 strains of sialodacryoadenitis virus. Zentralbl. Veterinarmed. 1990;37:91–96. doi: 10.1111/j.1439-0450.1990.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Hirano N., Ono K., Nomura R., Tawara T. Isolation and characterization of sialodacryoadenitis virus (coronavirus) from rats by established cell line LBC. Zentralbl. Veterinarmed. 1995;42:147–154. doi: 10.1111/j.1439-0450.1995.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Iftimovici R., Iacobescu V., Mutui A., Puca D. Enzootic withectromelia symptomatology in Sprague-Dawley rats. Rev. Roum. Med. Virol. 1976;27:65–66. [PubMed] [Google Scholar]
- Jacoby R.O., Ball-Goodrich L.B. Parvovirus infections of mice and rats. Sem. Virol. 1995;6:329–333. [Google Scholar]
- Jacoby R.O., Ball-Goodrich L.J., Besselsen D.G., McKisic M.D., Riley L.K., Smith A.L. Rodent parvovirus infections. Lab. Anim. Sci. 1996;46:370–380. [PubMed] [Google Scholar]
- Jacoby R.O., Bhatt P.N., Gaertner D.J., Smith A.L., Johnson E.A. The pathogenesis of rat virus infection in infant and juvenile rats after oronasal inoculation. Arch. Virol. 1987;95:251–270. doi: 10.1007/BF01310784. [DOI] [PubMed] [Google Scholar]
- Jacoby R.O., Bhatt P.N., Jonas A.M. Pathogenesis of sialodacryoadenitis in gnotobiotic rats. Vet. Pathol. 1975;12:196–209. doi: 10.1177/030098587501200305. [DOI] [PubMed] [Google Scholar]
- Jacoby R.O., Bhatt P.N., Jonas A.M. Viral diseases. In: Baker H., Lindsey J.R., Weisbroth S.H., editors. First edition. Volume 1. Academic Press; San Diego: 1979. pp. 271–306. (The Laboratory Rat). [Google Scholar]
- Jacoby R.O., Fox J.G., Davisson M. Biology and diseases of mice. In: Fox J.G., Anderson L.C., Loew F.M., Quimby F.W., editors. Laboratory Animal Medicine. Second edition. Academic Press; San Diego: 2002. pp. 35–120. [Google Scholar]
- Jacoby R.O., Gaertner D.J., Bhatt P.N., Paturzo F.X., Smith A.L. Transmission of experimentally-induced rat virus infection. Lab. Anim. Sci. 1988;38:11–14. [PubMed] [Google Scholar]
- Jacoby R.O., Johnson E.A., Paturzo F.X., Ball-Goodrich L.J. Persistent rat virus infection in smooth muscle of euthymic and athymic rats. J. Virol. 2000;74:11841–11848. doi: 10.1128/jvi.74.24.11841-11848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby R.O., Johnson E.A., Paturzo F.X., Gaertner D.J., Brandsma J.L., Smith A.L. Persistent rat parvovirus infection in individually housed rats. Arch. Virol. 1991;117:193–205. doi: 10.1007/BF01310765. [DOI] [PubMed] [Google Scholar]
- Jacoby R.O., Lindsey J.R. Risks of infection among laboratory rats and mice at major biomedical research institutions. ILAR J. 1998;39:266–271. doi: 10.1093/ilar.39.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara N., Yutaka U., Takahashi A., Sugiyama F., Yagami K. Vertical transmission to embryo and fetus in maternal infection with rat virus (RV) Exp. Anim. 1996;45:239–244. doi: 10.1538/expanim.45.239. [DOI] [PubMed] [Google Scholar]
- Kilham L., Olivier L. A latent virus of rats isolated in tissue culture. Virology. 1959;7:428–437. doi: 10.1016/0042-6822(59)90071-6. [DOI] [PubMed] [Google Scholar]
- Kilham L. Mongolism associated with rat virus (RV) infection in hamsters. Virology. 1960;13:141–143. doi: 10.1016/0042-6822(61)90043-5. [DOI] [PubMed] [Google Scholar]
- Kilham L. Rat virus (RV) infections in hamsters. Proc. Soc. Exp. Biol. Med. 1961;106:825–829. doi: 10.3181/00379727-106-26489. [DOI] [PubMed] [Google Scholar]
- Kilham L., Ferm V.H. Rat virus (RV) infections of pregnant, fetal and newborn rats. Proc. Soc. Exp. Biol. Med. 1964;117:874–879. doi: 10.3181/00379727-117-29723. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Cerebellar ataxia in hamsters inoculated with rat virus. Science. 1964;143:1047–1048. doi: 10.1126/science.143.3610.1047. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Spontaneous hepatitis and cerebellar hypoplasia in suckling rats due to congenital infection with rat virus. Am. J. Pathol. 1966;49:457–475. [PMC free article] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Transplacental infection of rats and hamsters induced by oral and parenteral inoculations of H-1 and rat viruses (RV) Teratology. 1969;2:111–123. doi: 10.1002/tera.1420020206. [DOI] [PubMed] [Google Scholar]
- Kojima A., Fujimani F., Doi K., Yasoshima A., Okaniwa A. Isolation and properties of sialodacryoadenitis virus of rats. Jikken Dobutsu. 1980;29:409–418. doi: 10.1538/expanim1978.29.4_409. [DOI] [PubMed] [Google Scholar]
- Kraft L.M., D'Amelio E.D., D'Amelio F.E. Morphological evidence for natural poxvirus infection in rats. Lab. Anim. Sci. 1982;32:648–654. [PubMed] [Google Scholar]
- Krikun V.A. Pox in rats: isolation and identification of poxvirus. Vopr. Virusol. 1977;22:371–373. [PubMed] [Google Scholar]
- Lai Y.L., Jacoby R.O., Bhatt P.N., Jonas A.M. Keratoconjunctivitis associated with sialodacryoadenitis in rats. Invest. Ophthalmol. 1976;15:538–541. [PubMed] [Google Scholar]
- La Regina M., Woods L., Klender P., Gaertner D.J., Paturzo F.X. Transmission of sialodacryoadenitis virus (SDAV) from infected rats to rats and mice through handling, close contact, and soiled bedding. Lab. Anim. Sci. 1992;42:344–346. [PubMed] [Google Scholar]
- LeDuc J.W. Epidemiology of Hantaan and related viruses. Lab. Anim. Sci. 1987;37:413–418. [PubMed] [Google Scholar]
- Lee H.W., Callisher C.H., Schmaljohn C., editors. Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. WHO Collaborating Center for Virus Reference and Research (Hantaviruses) Asian Institute for Life Sciences; Seoul. Korea: 1999. [Google Scholar]
- Lee H.W., French G.R., Lee P.W., Baek L.J., Tsuchiya K., Foulke R.S. Observations on natural and laboratory infections with the etiologic agent of Korean hemorrhagic fever. Am. J. Trop. Med. Hyg. 1981;30:477–482. doi: 10.4269/ajtmh.1981.30.477. [DOI] [PubMed] [Google Scholar]
- Lee P.W., Yanagihara R., Gibbs C.J., Jr., Gadjusek D.C. Pathogenesis of experimental Hantaan virus infection in laboratory rats. Arch. Virol. 1986;88:57–66. doi: 10.1007/BF01310890. [DOI] [PubMed] [Google Scholar]
- Liang S.C., Schoeb T.R., Davis J.K., Simecka J.W., Cassell G.H., Lindsey J.R. Comparison severity of respiratory lesions of sialodacryoadenitis virus and Sendai virus infections in LEW and F344 rats. Vet. Pathol. 1995;32:661–667. doi: 10.1177/030098589503200607. [DOI] [PubMed] [Google Scholar]
- Lipton H.G., Nathanson N., Hodous J. Enteric transmission of parvoviruses: pathogenesis of rat virus infection in adult rats. Am. J. Epidemiol. 1973;6:443–446. doi: 10.1093/oxfordjournals.aje.a121476. [DOI] [PubMed] [Google Scholar]
- Lloyd G., Bowen E.T., Jones N., Pendry A. HFRS outbreak associated with laboratory rats in UK. Lancet. 1984;1:1175–1176. doi: 10.1016/s0140-6736(84)91413-2. [DOI] [PubMed] [Google Scholar]
- Lloyd G., Jones N. Infection of laboratory workers with hantavirus acquired from immunocytomas propagated in laboratory rats. J. Infect. 1986;12:117–125. doi: 10.1016/s0163-4453(86)93533-4. [DOI] [PubMed] [Google Scholar]
- London B.A., Kern J., Gehle W.D. Detection of murine virus antibody by ELISA. Lab. Anim. 1983;12:40–47. [Google Scholar]
- Lyon H.W., Christian J.J., Mitler C.W. Cytomegalic inclusion disease of lacrimal glands in male laboratory rats. Proc. Soc. Exp. Biol. Med. 1959;101:164–166. doi: 10.3181/00379727-101-24868. [DOI] [PubMed] [Google Scholar]
- Machii K., Iwai H., Otsuka Y., Ueda K., Hirano N. Reactivities of 4 murine coronavirus antigens with immunized or naturally infected rat sera by enzyme linked immunosorbent assay. Jikken Dobutsu. 1988;37:251–255. doi: 10.1538/expanim1978.37.3_251. [DOI] [PubMed] [Google Scholar]
- Makino S., Seko S., Nakao H., Midazuki K. An epizootic of Sendai virus infection in a rat colony. Exp. Anim. 1972;22:275–280. doi: 10.1538/expanim1957.22.4_275. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L. Parvovirus infections, vascular endothelium and hemorrhagic encephalogpathy. Lab. Invest. 1970;22:478–488. [PubMed] [Google Scholar]
- Margolis G., Kilham L. Pathogenesis of intrauterine infection in rats due to reovirus type 3. II. Pathologic and fluorescent antibody studies. Lab. Invest. 1973;28:605–613. [PubMed] [Google Scholar]
- Margolis G., Kilham L. Rat virus infection of megakaryocytes: a factor in hemorrhagic encephalopathy. Exp. Molec. Pathol. 1972;16:326–340. doi: 10.1016/0014-4800(72)90008-1. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L., Ruffulo P.R. Rat virus disease, an experimental model of neonatal hepatitis. Exp. Molec. Pathol. 1968;8:1–20. doi: 10.1016/0014-4800(68)90002-6. [DOI] [PubMed] [Google Scholar]
- Marrenikova S.S., Shelukhina E.M. White rats as a source of pox infection in carnivora of the family Felidae. Acta Virol. 1976;2:422. [PubMed] [Google Scholar]
- Marrenikova S.S., Shalukhina E.M., Fimina V.A. Pox infection in white rats. Lab. Anim. 1978;12:33–36. doi: 10.1258/002367778780953288. [DOI] [PubMed] [Google Scholar]
- Maru M., Sato K. Characterization of a coronavirus isolated from rats with sialodacryoadenitis. Arch. Virol. 1982;73:33–43. doi: 10.1007/BF01341725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean I.W., Nakane P.K. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J. Histochem. Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- McKisic M.D., Paturzo F.X., Gaertner D.J., Jacoby R.O., Smith A.L. A nonlethal rat parvovirus infection suppresses rat T lymphocyte effector functions. J. Immunol. 1995;155:3979–3986. [PubMed] [Google Scholar]
- Meyer B.J., Schmaljohn C.S. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 2000;8:61–67. doi: 10.1016/s0966-842x(99)01658-3. [DOI] [PubMed] [Google Scholar]
- Michaels R.H., Myerowitz R.L. Viral enhancement of nasal colonization with Haemophilus influenzae type b in the infant rat. J. Pediatr. Res. 1983;17:472–473. doi: 10.1203/00006450-198306000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol S.N. Bunyaviruses. In: Knipe D., Howley P., editors. Field's Virology. Lippincott Williams & Wilkins; Philadelphia, Pa.: 2001. pp. 1603–1633. [Google Scholar]
- Nicklas W., Kraft V., Meyer B. Contamination of transplantable tumors, cell lines, and monoclonal antibodies with rodent viruses. Lab. Anim. Sci. 1993;43:296–300. [PubMed] [Google Scholar]
- Novotny J.F., Hetrick F.M. Pathogenesis and transmission of Kilham rat virus infection in rats. Infect. Immun. 1970;2:298–303. doi: 10.1128/iai.2.3.298-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K., Watanabe Y., Takakura A., Itoh T., Sato H. Replication of rat coronaviruses in intestinal cell line, RCN-9, derived from F344 rats. J. Exp. Anim. 1996;45:389–393. doi: 10.1538/expanim.45.389. [DOI] [PubMed] [Google Scholar]
- Ohsawa K., Watanabe Y., Miyata H., Sato H. Genetic analysis of a Theiler-like virus isolated from rats. Comp. Med. 2003;53:191–196. [PubMed] [Google Scholar]
- Parker J.C., Cross S.S., Rowe W.P. Rat coronavirus (RCV): a prevalent, naturally occurring pneumotropic virus of rats. Arch. Gesamte Virusforsch. 1970;31:293–302. doi: 10.1007/BF01253764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturzo F.X., Jacoby R.O., Bhatt P.N., Smith A.L., Gaertner D.G., Ardito R.B. Persistence of rat virus in seropositive rats as detected by explant culture. Arch. Virol. 1987;95:137–142. doi: 10.1007/BF01311341. [DOI] [PubMed] [Google Scholar]
- Percy D.H., Barthold S.W. Second edition. Iowa State University Press; Ames, Iowa: 2001. p. 109. (Pathology of Laboratory Rodents and Rabbits). [Google Scholar]
- Percy D.H., Bond S., MacInnes J. Replication of sialodacryoadenitis virus in mouse L-2 cells. Arch. Virol. 1989;104:323–333. doi: 10.1007/BF01315553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy D.H., Bond S.J., Paturzo F.X., Bhatt P.N. Duration of protection from reinfection following exposure to sialodacryoadenitis virus in Wistar rats. Lab. Anim. Sci. 1990;40:144–149. [PubMed] [Google Scholar]
- Percy D.H., Hanna P.E., Paturzo F.X., Bhatt P.N. Comparison of strain susceptibility to experimental sialodacryoadenitis in rats. Lab. Anim. Sci. 1984;34:255–260. [PubMed] [Google Scholar]
- Percy D.H., Haves M.A., Kocal T.E., Wojcinski Z.W. Depletion of salivary gland epidermal growth factor by sialodacryoadenitis virus infection in the Wistar rat. Vet. Pathol. 1988;25:183–192. doi: 10.1177/030098588802500301. [DOI] [PubMed] [Google Scholar]
- Percy D.H., Scott R.A. Coronavirus infection in the laboratory rat: immunization trials using attenuated virus replicated in L-2 cells. Can. J. Vet. Res. 1991;55:60–66. [PMC free article] [PubMed] [Google Scholar]
- Percy D.H., Williams K.L. Experimental Parker's rat coronavirus infection in Wistar rats. Lab. Anim. Sci. 1990;40:603–607. [PubMed] [Google Scholar]
- Percy D.H., Williams K.L., Bond S.J., MacInnes J.L. Characteristics of Parker's rat coronavirus (PRC) replicated in L-2 cells. Arch. Virol. 1990;112:195–202. doi: 10.1007/BF01323164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy D.H., Williams K.L., Paturzo F.X. A comparison of the sensitivity and specificity of sialodacryoadenitis virus, Parker's rat coronavirus, and mouse hepatitis virus-infected cells as a source of antigen for the detection of antibody to rat coronaviruses. Arch. Virol. 1991;119:175–180. doi: 10.1007/BF01310668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R.L., Collins M.J. Use of mouse hepatitis virus antigen in an enzyme-linked immunosorbent assay for rat coronaviruses. Lab. Anim. Sci. 1981;31:472–475. [PubMed] [Google Scholar]
- Priscott P.K., Tyrell D.A.J. The isolation and partial characterization of a cytomegalovirus from the brown rat Rattus norvegicus. Arch. Virol. 1982;73:145–150. doi: 10.1007/BF01314723. [DOI] [PubMed] [Google Scholar]
- Rabson A.S., Edgcomb J.H., Legallais F.Y., Tyrell S.A. Isolation and growth of rat cytomegalovirus in vitro. Proc. Soc. Exp. Biol. Med. 1969;131:923–927. doi: 10.3181/00379727-131-34010. [DOI] [PubMed] [Google Scholar]
- Riley L.K., Knowles R., Purdy G., Salome N., Pintel D., Hook R.R., Jr., Franklin C.L., Besch-Williford C.L. Expression of recombinant parvovirus NS1 protein by a baculovirus and application to serologic testing of rodents. J. Clin. Microbiol. 1996;34:440–444. doi: 10.1128/jcm.34.2.440-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey R.E., Woodman D.R., Hetrick F.M. Studies on the natural infection of rats with the Kilham rat virus. Am. J. Epidemiol. 1968;88:139–143. doi: 10.1093/oxfordjournals.aje.a120863. [DOI] [PubMed] [Google Scholar]
- Rossie K.M., Sheridan J.F., Barthold S.W., Tutschka P.J. Graft-versus-host disease and sialodacryoadenitis viral infection in bone marrow transplanted rats. Transplantation. 1988;45:1012–1016. doi: 10.1097/00007890-198806000-00003. [DOI] [PubMed] [Google Scholar]
- Ruffolo P.R., Margolis G., Kilham L. The induction of hepatitis by partial hepatectomy in resistant adult rats injected with H-1 virus. Am. J. Pathol. 1966;49:795–824. [PMC free article] [PubMed] [Google Scholar]
- Saknimit M., Inatsuki I., Sugiyama Y., Yagami K. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Jikken Dobutsu. 1988;37:341–345. doi: 10.1538/expanim1978.37.3_341. [DOI] [PubMed] [Google Scholar]
- Sato T., Mequid M.M., Quinn R.H., Zhang L., Chen C. Feeding behavior during sialodacryoadenitis viral infection in rats. Physiol. Behav. 2001;72:721–726. doi: 10.1016/S0031-9384(01)00420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C., Hjelle B. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeb T.R., Kervin K.C., Lindsey J.R. Exacerbation of murine respiratory mycoplasmosis in gnotobiotic F344/N rats by Sendai virus infection. Vet. Pathol. 1985;22:272–282. doi: 10.1177/030098588502200310. [DOI] [PubMed] [Google Scholar]
- Shek W.R., Gaertner D.J. Microbiological quality control for laboratory rodents and lagomorphs. In: Fox J.G., Andersen L.C., Loew F.W., Quimby F.W., editors. “Laboratory Animal Medicine” Second Edition. Academic Press; San Diego: 2002. pp. 365–393. [Google Scholar]
- Siegl G. The parvoviruses. In: Gard S., Hallauer G., editors. Vol. 15. Springer-Verlag; Berlin: 1976. (Virology Monographs). [Google Scholar]
- Simmons J.H., Riley L.K. Hantaviruses: an overview. Comp. Med. 2002;52:97–110. [PubMed] [Google Scholar]
- Singh S.B., Lang C.M. Enzyme-linked immunosorbent assay in the diagnosis of Kilham rat virus infection in rats. Lab. Anim. Sci. 1984;18:364–370. doi: 10.1258/002367784780865289. [DOI] [PubMed] [Google Scholar]
- Smith A.L. An immunofluorescence test for detection of serum antibody to rodent coronaviruses. Lab. Anim. Sci. 1983;33:157–160. [PubMed] [Google Scholar]
- Smith A.L., Winograd D.F. Two enzyme immunoassays for the detection of antibody to rodent coronaviruses. Virol. Methods. 1986;14:335–343. doi: 10.1016/0166-0934(86)90035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K., Ohsawa, K., Koji, T., Watanabe, Y., Katamine, S., Sato, H. and Ayabe, H. Allogeneic immune responses augment rat cytomegalovirus replication in rats. Transplant. Proc.31, 1376–1377. [DOI] [PubMed]
- Tattersall P., Cotmore S.F. The rodent parvoviruses. In: Bhatt P.N., Jacoby R.O., Morse H.C., New A.E., editors. Viral and Mycoplasmal Infections of Rodents: Effects on Biomedical Research. Academic Press; Orlando, FL: 1986. pp. 305–348. [Google Scholar]
- Toolan H.W., Dalldorf G., Barclay M., Chandra S., Moore A.E. An unidentified filterable agent isolated from transplanted human tumors. Proc. Nat. Acad. Sci. U.S.A. 1960;46:1256–1259. doi: 10.1073/pnas.46.9.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y., Iwama M., Ohshima T., Sugiyama Y., Takakura A., Itoh T., Sugiyama K. Prevalence of “orphan” parvovirus infections in rats and mice. Exp. Anim. 1998;47:207–210. doi: 10.1538/expanim.47.207. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Sugiyama F., Sugiyama Y., Ohsawa K., Sato H., Yagami K. Epidemiological characterization of newly recognized rat parvovirus. “rat orphan parvovirus”. J. Vet. Med. Sci. 1997;59:265–269. doi: 10.1292/jvms.59.265. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Sugiyama F., Yagami K. Detection and in vivo transmission of rat orphan parvovirus (ROPV) Lab. Anim. 1996;30:114–119. doi: 10.1258/002367796780865763. [DOI] [PubMed] [Google Scholar]
- Utsumi K., Ishikawa T., Maeda T., Shimizu S., Tatsumi H., Fujiwars K. Infectious sialodacryoadenitis and rat breeding. Lab. Anim. 1980;14:303–307. doi: 10.1258/002367780781071229. [DOI] [PubMed] [Google Scholar]
- Utsumi K., Maeda T., Tatsumi H., Fujiwara K. Some clinical and epizootiological observations of infectious sialoadenitis in rats. Jikken Dobutsu. 1978;27:283–287. doi: 10.1538/expanim1978.27.3_283. [DOI] [PubMed] [Google Scholar]
- Vonderfecht S.L. Infectious diarrhea of infant rats (IDIR) induced by an antigenically distinct rotavirus. In: Bhatt P.N., Jacoby R.O., Morse H.C., New A.E., editors. Viral and Mycoplasmal Infections of Rodents: Effects on Biomedical Research. Academic Press; Orlando, Fl.: 1986. pp. 245–252. [Google Scholar]
- Vonderfecht S.L., Huber A.C., Eiden J., Mader L.C., Yolken R.H. Infectious diarrhea of infant rats produced by a rotavirus-like agent. J. Virol. 1984;52:94–98. doi: 10.1128/jvi.52.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votsberger L.M., Stromberg P.C., Rice J.M. Histological and serological response of B6C3F1 mice and F344 rats to experimental pneumonia virus of mice infection. Lab. Anim. Sci. 1982;32:419. [Google Scholar]
- Wan C.H., Riley M.I., Hook R.R., Jr., Franklin C.L., Besch-Williford C.L., Riley L.K. Expression of Sendai virus nucleocapsid protein in a baculovirus expression system and application to diagnostic assays for Sendai virus. J. Clin. Microbiol. 1995;33:2007–2011. doi: 10.1128/jcm.33.8.2007-2011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C.H., Soderlund-Venermo M., Pintel D.J., Riley L.K. Molecular characterization of three newly recognized rat parvoviruses. J. Gen. Virol. 2002;83:2075–2083. doi: 10.1099/0022-1317-83-8-2075. [DOI] [PubMed] [Google Scholar]
- Ward J.M., Lock A., Collins M.J., Gonda M.A., Reynolds C.W. Papovaviral sialoadenitis in athymic nude rats. Lab. Anim. 1984;18:84–89. doi: 10.1258/002367784780864884. [DOI] [PubMed] [Google Scholar]
- Ward J.M., Young D.M. Latent adenoviral infection of rats: intranuclear inclusions induced by treatment with a cancer chemotherapeutic agent. J. Am. Vet. Med. Assoc. 1976;169:952–953. [PubMed] [Google Scholar]
- Weir E.C., Jacoby R.O., Paturzo F.X., Johnson E.A. Infection of SDAV-immune rats with SDAV and rat coronavirus. Lab. Anim. Sci. 1990;40:363–366. [PubMed] [Google Scholar]
- Weir E.C., Jacoby R.O., Paturzo F.X., Johnson E.A., Ardito R.B. Persistence of sialodacryoadenitis virus in athymic rats. Lab. Anim. Sci. 1990;40:138–143. [PubMed] [Google Scholar]
- Yagami K., Goto Y., Ishida J., Ueno Y., Kajiwara N., Sugiyama F. Polymerase chain reaction for detection of rodent parvoviral contamination in cell lines and transplantable tumors. Lab. Anim. Sci. 1995;45:326–328. [PubMed] [Google Scholar]
- Yang F-C., Paturzo F.X., Jacoby R.O. Environmental stability and transmission of rat virus. Lab. Anim. Sci. 1995;45:140–144. [PubMed] [Google Scholar]
- Zenner L., Regnault J.P. Ten-year long monitoring of laboratory mouse and rat colonies in French facilities: a retrospective study. Lab. Anim. 2000;34:76–83. doi: 10.1258/002367700780577957. [DOI] [PubMed] [Google Scholar]


