Significance
Zika virus (ZIKV) infection during pregnancy can cause fetal abnormalities. Vaccines against ZIKV are under development, but because of potential safety concerns due to disease-enhancing antibodies, and the time required by active immunization to induce protective antibodies, there is a need to explore alternative strategies. Recombinant monoclonal antibodies can be modified to prevent enhancement of infection, and thus could be an efficacious and safe alternative to vaccines to confer rapid protection. We show that prophylactic administration of two engineered antibodies, Z004 and Z021, to pregnant macaques partially protects against fetal neurologic damage and limits vertical transmission of ZIKV.
Keywords: Fc domain modifications, Zika virus, macaque pregnancy model, antibody-dependent enhancement, congenital Zika syndrome
Abstract
Human infection by Zika virus (ZIKV) during pregnancy can lead to vertical transmission and fetal aberrations, including microcephaly. Prophylactic administration of antibodies can diminish or prevent ZIKV infection in animal models, but whether passive immunization can protect nonhuman primates and their fetuses during pregnancy has not been determined. Z004 and Z021 are neutralizing monoclonal antibodies to domain III of the envelope (EDIII) of ZIKV. Together the two antibodies protect nonpregnant macaques against infection even after Fc modifications to prevent antibody-dependent enhancement (ADE) in vitro and extend their half-lives. Here we report on prophylactic coadministration of the Fc-modified antibodies to pregnant rhesus macaques challenged three times with ZIKV during first and second trimester. The two antibodies did not entirely eliminate maternal viremia but limited vertical transmission, protecting the fetus from neurologic damage. Thus, maternal passive immunization with two antibodies to EDIII can shield primate fetuses from the harmful effects of ZIKV.
ZIKV is a mosquito-borne flavivirus that in humans is often asymptomatic but can manifest as febrile illness and rarely as neurologic disease (1, 2). Although ZIKV was discovered in 1947, its devastating effects on fetal health were only recognized during the 2015 outbreak in the Americas (3). ZIKV infection during pregnancy can result in a range of birth defects that predominantly involve the central nervous system, including microcephaly (4–6). The rate of adverse outcomes varies but can surpass 40% in some regions (7–10). Similar consequences are observed in macaques, where experimental infection during gestation can lead to fetal neuropathology and demise (11–13).
Although the path to approval is challenging due to the waning epidemic, several ZIKV vaccine candidates have entered clinical trials (14, 15). In preclinical settings, vaccine efficacy was demonstrated in mice and macaques (16, 17), including mouse and macaque pregnancy models (18–21). One of the potential issues with vaccination is that active immunization regimens require time to induce protective immunity and therefore vaccination after conception may be ineffective in preventing fetal disease in the setting of an active outbreak. In addition, there is concern about vaccine-mediated enhancement because features of maternal antibodies that enhance ZIKV infection in vitro are associated with an increased risk of human microcephaly and other neurologic defects in humans and macaques (22). Thus, there is a need to develop strategies to limit maternal and fetal pathology that are rapid, effective, and safe.
Results
The combination of human IgG1 monoclonal antibodies Z004 and Z021 significantly reduces plasma viral loads and prevents emergence of viral escape mutations in nonpregnant rhesus macaques challenged with a superphysiologic dose of ZIKV i.v. (105 plaque-forming units [PFU]) (23). Similar results were obtained when the antibodies were modified to prevent antibody-dependent enhancement (ADE) in vitro by mutations that abrogate Fc-γ receptor engagement (GRLR; refs. 23 and 24).
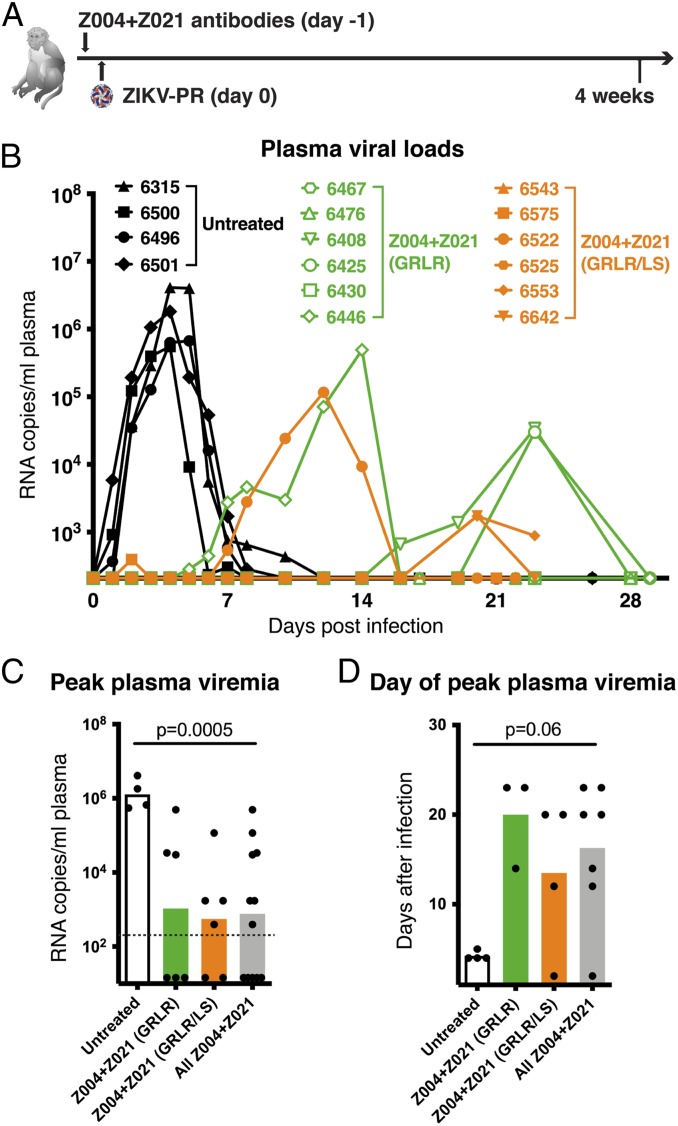
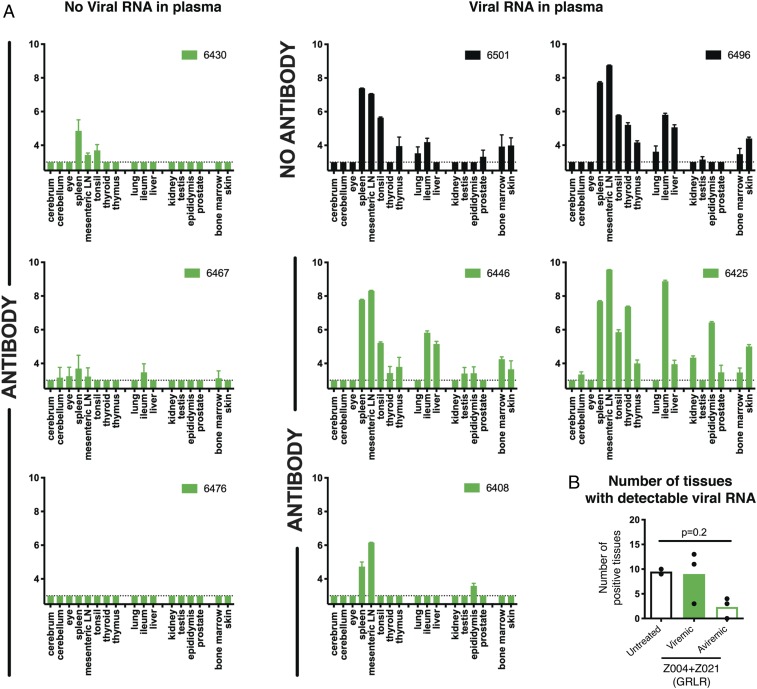
To evaluate the protective effect of these antibodies against what is considered to be a more physiologic dose and route of administration, we administered Z004GRLR + Z021GRLR to macaques 24 h before s.c. inoculation with 103 PFU of ZIKV (Fig. 1A) (25, 26). In untreated controls, plasma viremia peaked on days 4 to 5 after infection, reaching between 105 and 107 RNA copies/mL (n = 4; black in Fig. 1B). In contrast, plasma viremia was undetectable in three out of six antibody-treated macaques, and it was lower and delayed in the remaining three animals (green in Fig. 1 B–D). Since ZIKV can be detected in macaque tissues even when absent from plasma (13, 27), we measured viral RNA in a panel of 17 tissues (Fig. 2). Viral RNA was evident in multiple tissues in control animals as well as in the three animals that showed low and delayed viremia after infection (Fig. 2 A, Right). In contrast, viral RNA was low or undetectable in tissues from macaques with no detectable plasma viremia (Fig. 2 A, Left and Fig. 2B). Thus, prophylactic administration of Z004GRLR + Z021GRLR prevents or reduces viral accumulation in plasma and tissues upon low-dose ZIKV challenge.
Fig. 1.
Administration of Z004GRLR/LS + Z021GRLR/LS antibodies reduces magnitude and duration of ZIKV viremia in nonpregnant rhesus macaques. (A) Schematic of the experiment. Macaques were administered Fc-modified human monoclonal antibodies Z004 and Z021 1 d prior to s.c. challenge with 103 PFU of Puerto Rican Zika virus (ZIKV-PR). (B) Antibody treatment alters plasma viral loads. Macaques received either the GRLR version of the antibodies (green), the GRLR/LS version (orange), or were left untreated (black). Shown are plasma ZIKV RNA levels over time as determined by qRT-PCR. The x axis is set at the limit of detection (2.3 log RNA copies/mL). (C) Peak plasma viral RNA levels are decreased in antibody-treated macaques (based on B). In gray is the peak viral load of both Z004GRLR + Z021GRLR and Z004GRLR/LS + Z021GRLR/LS groups combined. The dotted line represents the limit of detection of the assay and samples with undetectable ZIKV were arbitrarily assigned a value equal to half of the limit of detection. (D) Peak plasma viremia is delayed in antibody-treated macaques (based on B). Bars in C and D represent the mean; the P values were determined with the Mann–Whitney U test.
Fig. 2.
Zika viral RNA levels in tissues are decreased in antibody-treated male macaques. (A) Viral RNA was measured in a panel of tissues obtained at time of killing (days 26 to 29 postinfection). Values represent log10 of viral RNA copies per gram of tissue, as determined by qRT-PCR. Data are represented as mean ± SD of triplicate measurements. In green are macaques treated with Z004GRLR + Z021GRLR and in black are untreated controls. Antibody-treated macaques are arranged according to whether they had detectable plasma viremia at any time point after ZIKV inoculation. (B) Shown for each macaque is the number of tissues with detectable viral RNA. Bars represent the mean; the P values were determined with the Mann–Whitney U test.
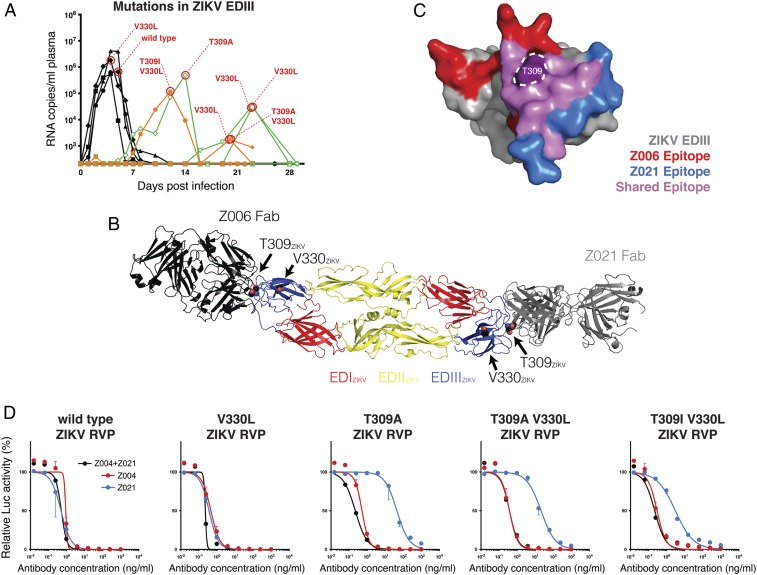
The half-life of Z004 and Z021 can be extended by altering the antibody’s Fc domain (LS mutation; refs. 28 and 29) without altering ZIKV neutralizing activity in vitro or in vivo (23). To determine whether Z004GRLR/LS + Z021GRLR/LS remains efficacious in macaques, we administered the combination before low-dose s.c. challenge with ZIKV. Z004GRLR/LS + Z021GRLR/LS showed extended half-lives and their levels in plasma remained more than 4 logs above the in vitro inhibitory concentration throughout the monitoring period (SI Appendix, Fig. S1). The treatment prevented plasma viremia in two of six macaques; one macaque had a single episode of low-level viremia (less than 103 viral RNA copies/mL on day 2 postinfection), and the remaining three had lower and delayed viral loads (orange in Fig. 1 B–D) compared to untreated controls. The macaques that developed plasma viremia after administration of Z004GRLR + Z021GRLR or Z004GRLR/LS + Z021GRLR/LS had viruses with mutations in the envelope domain III (EDIII) region: T309A, T309A/V330L, T309I/V330L, and V330L (Fig. 3 A–C). To determine whether these substitutions confer resistance to the antibodies, we produced reporter virus particles (RVPs; ref. 30) with these mutations and evaluated them for sensitivity to the two antibodies (Fig. 3D). Both antibodies retained potency against RVPs containing the V330L mutation, which was also found in untreated control animals (Fig. 3A). This mutation is located outside the epitope of either antibody (Fig. 3B). Although T309 is in the epitope of both antibodies (Fig. 3 B and C), its mutations affected the potency of Z021 alone. Since T309 mutations did not alter ZIKV sensitivity to the combination of antibodies in vitro, they are unlikely to represent virus escape (Fig. 3D). Consistent with these findings, the mutations involved residues other than those altered in the viruses emerging from macaques treated with the individual antibodies (E393 and K394 with Z004, and T335 with Z021; ref. 23 and SI Appendix, Fig. S2). We conclude that Z004GRLR/LS + Z021GRLR/LS prevents emergence of viral escape variants found with monotherapy (ref. 23 and SI Appendix, Fig. S2) and are as efficacious as their GRLR counterparts against ZIKV in macaques.
Fig. 3.
Mutations in Zika virus emerging in plasma from antibody-treated nonpregnant macaques remain sensitive to Z004 and Z021. (A) Summary of the amino acid changing mutations in the EDIII of ZIKV. The virus EDIII region recognized by Z004 and Z021 was amplified and sequenced at peak plasma viremia. Shown is a graphic summary of the identified mutations. Note that V330L was also detected in one untreated macaque. We were unable to amplify the EDIII from one sample with RNA copies/mL below 103. (B) The mutations are mapped on the structure of the sE dimer of ZIKV (PDB ID: 5JHM). The structures of the Z004-related antibody Z006 (PDB ID:5VIG) and of Z021 (PDB ID:6DFI) in complex with the EDIII of ZIKV are structurally aligned to the sE dimer to show the location of residues T309 and V330 relative to the binding sites of the antibodies. The ZIKV EDIII from the Z006-EDIII and Z021-EDIII structures are omitted for clarity. (C) The epitopes of ZIKV EDIII recognized by the Z004-related antibody Z006 (in red), by Z021 (in blue), and by both antibodies (in purple) are shown. Residue T309 is highlighted. (D) Z004, Z021, and the two antibodies together (Z004 + Z021) neutralize RVPs corresponding to ZIKV wild-type sequence or ZIKV mutated at the indicated residues. For Z004 + Z021, each of the antibodies is added at the indicated concentration (e.g., 10 ng/mL contains 10 ng/mL of Z004 plus 10 ng/mL of Z021). Data are represented as mean ± SD of triplicates and a representative of two experiments is plotted. Values are relative to isotype control.
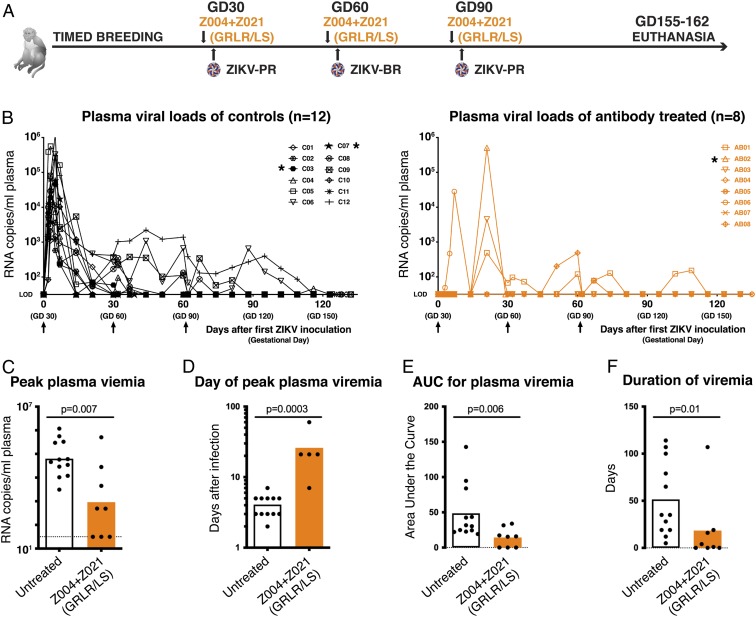
ZIKV infection of pregnant macaques is associated with fetal demise and neuropathology (11–13). To evaluate the effect of anti-ZIKV antibodies on fetal development and health in pregnant primates, we performed timed breeding experiments and challenged the dams three times with 103 PFU of ZIKV s.c. on gestational days (GDs) GD30, GD60, and GD90, which correspond to first and second trimester (normal gestation of rhesus macaques is ∼165 d) (21). Macaques in the treatment group received i.v. infusions of Z004GRLR/LS + Z021GRLR/LS antibodies 24 h before each virus challenge (Fig. 4A). Both dam and fetus were closely monitored; animals were killed near the end of gestation for detailed tissue analysis (SI Appendix, Tables S1 and S2). During this whole observation period, human IgG plasma levels remained 3 logs or higher than the in vitro inhibitory concentration (Materials and Methods and SI Appendix, Fig. S3). Untreated macaques developed plasma viremia that peaked between days 2 and 5 after the first ZIKV inoculation (Fig. 4 B, Left and ref. 21). In contrast, out of the eight antibody-treated dams, three had no detectable viremia, while four other animals had delayed peak viremia on days 7 (n = 1) or 21 (n = 3) after the first inoculation, and a fifth developed peak viremia on GD90, 30 days after the second ZIKV inoculation (Fig. 4 B, Right). In addition, viremia was significantly lower and of shorter duration in antibody-treated dams (Fig. 4 B–F). Fetal death (i.e., no heartbeat on ultrasound) was detected in two control animals (C03 and C07) on GD35 and GD60 (i.e., 5 and 30 d after the first ZIKV inoculation), with detectable viral RNA in placental and fetal tissues (21). Fetal death was observed on GD51 in one of the antibody-treated macaques (AB02). This occurred 1 wk after undergoing routine amniocentesis and was coincident with a sudden spike of maternal viremia (>105 RNA copies/mL; Fig. 4B) and ZIKV in the amniotic fluid (104 RNA copies/mL) and placental-amniotic tissues (>104 RNA copies/g tissue). In the antibody-treated pregnant dams, mutations in the EDIII region of the emerging viruses were similar to the previously observed mutations that did not alter sensitivity to the antibodies in combination and therefore are unlikely to represent escape (Fig. 3 and SI Appendix, Fig. S4). We conclude that the Z004GRLR/LS + Z021GRLR/LS combination prevents or significantly reduces maternal viral loads during pregnancy.
Fig. 4.
Administration of Z004GRLR/LS + Z021GRLR/LS prevents or reduces ZIKV viral loads in pregnant macaques. (A) Schematic of the experiment. Pregnant macaques were administered Z004GRLR/LS + Z021GRLR/LS antibodies 1 d prior to s.c. challenge with 103 PFU of ZIKV on each of GD30, GD60, and GD90. PR is Puerto Rican and BR is Brazilian ZIKV. Blood samples were obtained throughout the pregnancy. (B) Antibody treatment alters plasma viral loads. Macaques were either untreated (Left) or treated with Z004GRLR/LS + Z021GRLR/LS (Right). Shown are plasma ZIKV RNA levels over time. LOD, limit of detection. Asterisks indicate animals with early fetal death. (C) Peak plasma viral RNA levels are decreased in antibody-treated macaques (based on B). The dotted line represents the LOD of the assay where samples with undetectable ZIKV were arbitrarily assigned a value equal to the LOD. (D) Peak plasma viremia is delayed in treated animals. (E) Overall plasma viral loads are decreased in treated macaques. (F) Duration of viremia is reduced in animals that received antibodies. Bars in C–F represent the mean; the P values were determined with the Mann–Whitney U test.
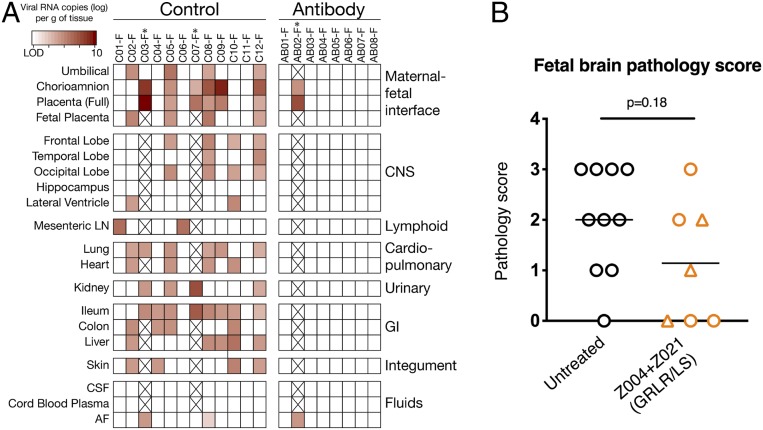
Amniocentesis was performed regularly to monitor potential ZIKV transmission to the placental-fetal compartment. Six control animals (50%) had detectable ZIKV RNA in at least one amniotic fluid sample. In contrast, except for antibody-treated animal AB02 at time of fetal death, none of the other antibody-treated animals had detectable vRNA in amniotic fluid samples (SI Appendix, Fig. S5). Since vertical transmission of ZIKV can cause congenital Zika syndrome (CZS), we examined the fetuses in the pregnancies that reached term. Fetal body, placenta, brain, and other fetal organ weights were indistinguishable between controls and treatment groups (SI Appendix, Fig. S6). In 9 of the 10 control untreated fetuses that made it to the end of gestation, ZIKV RNA was detected in at least 1 of the 17 examined tissues. These include the maternal–fetal interface (MFI), lymphoid, cardiopulmonary, urinary, gastrointestinal, and central nervous systems, and the skin (average of 6 ZIKV RNA positive tissues per fetus; Fig. 5A). In contrast, viral RNA was undetectable in the same fetal tissues from the seven antibody-treated animals that reached end of gestation (Fig. 5A). Moreover, fetuses that received antibodies had less brain pathology overall, as only 1 out of 7 fetuses (14%) had a brain pathology score of moderate (score of 3) or above, in contrast to 4/10 (40%) of the control fetuses (ref. 21, Fig. 5B, and SI Appendix, Fig. S7 and Table S3). Though its dam had no detectable viremia, it is possible that the moderate brain pathology score in one antibody-treated fetus (AB08-F) reflects earlier infection that was cleared by the time the fetus was harvested. Pathology scores of placenta and amnion were not significantly different between groups (SI Appendix, Fig. S7 and Table S3). Finally, there was no correlation between maternal viremia and pathology scores; while aviremic animal AB05 had normal fetus and placenta, macaque AB07 was also aviremic but its fetus and placenta showed mild to moderate pathology scores, respectively; in contrast, the fetus of animal AB06, which had higher than 104 RNA copies/mL at peak maternal viremia, had normal brain findings but moderate placental pathology (Fig. 5B and SI Appendix, Fig. S7). We conclude that maternal administration of Z004GRGR/LS + Z021GRLR/LS limits vertical transmission and fetal brain damage by ZIKV.
Fig. 5.
Protection of macaque fetuses by Z004GRLR/LS + Z021GRLR/LS. (A) With exception of AB02-F (i.e., the fetus of dam AB02), ZIKV RNA is undetectable in placental and fetal tissues from antibody-treated dams. Viral RNA was measured in a panel of 17 fetal tissues and three fluids harvested at pregnancy termination. LN, lymph node; CSF, cerebrospinal fluid; AF, amniotic fluid; GI, gastrointestinal; and CNS, central nervous system. * indicates the two control animals and one antibody-treated animal with early fetal death. (B) Lower fetal brain pathology in animals receiving Z004GRLR/LS + Z021GRLR/LS. Triangles indicate fetuses whose dams had no detectable plasma viremia. Horizontal lines represent mean values and the P value was determined with the Mann–Whitney U test. Pregnancies that terminated prematurely (GD60 or earlier) were omitted from this panel. Pathology scores of fetal tissues are summarized in SI Appendix, Fig. S7.
Discussion
Recombinant antibodies represent an alternative to vaccines for at-risk populations. Passive immunization reduces or prevents ZIKV infection in nonpregnant rodents and macaques (23, 25, 26, 31, 32). In addition, antibodies diminish ZIKV viral burden in the placenta and fetal organs, and also reduce fetal demise in mouse pregnancy models (33–35). Some of these ZIKV mouse pregnancy models reproduce features of the human disease, including brain pathology (4–6). However, mice are not natural hosts for ZIKV, and the development of pathology typically requires an impaired immune system. As opposed to immunocompetent rodents, macaques can be infected readily with ZIKV and their pregnancies are anatomically and physiologically far more similar to humans (36). Whether antibodies can prevent fetal disease in macaques had not been determined. Our experiments show that the combination of two ZIKV neutralizing human monoclonal antibodies is sufficient to thwart or significantly reduce maternal viremia, prevent virus accumulation in fetal tissues, and reduce fetal pathology following repeated ZIKV challenge in macaques. The results have many similarities, but also some differences, with results obtained using the ZIKV DNA vaccine VRC5283 that was tested in the same animal model of congenital ZIKV disease (21).
VRC5283 is a DNA vaccine that produces subviral particles with premembrane, membrane, and envelope proteins, is immunogenic in a phase 1 clinical trial, and is currently being evaluated in a phase 2/2b (17, 37, 38). In the macaque pregnancy experiment with similar design to the present study, two doses of vaccine prior to ZIKV inoculation significantly reduced maternal viremia when animals were challenged between 4 d to 1 y after the second immunization (21). Viremia was undetectable in 5 of 13 animals (38%), a rate of protection comparable to the current antibody treatment study (37.5%; n = 8). In addition, in the ZIKV DNA vaccine study, the presence or absence of detectable viremia in vaccinated dams correlated with the titer of neutralizing antibody responses at the time of the first ZIKV challenge (i.e., animals with antibody titers above a threshold had no detectable viremia). For vaccinated animals with detectable viremia, the reduced viremia was always early (peak viremia ≤5 d after ZIKV inoculation), and short in duration (≤14 d after the first ZIKV challenge), consistent with the observed rapid augmentation of antiviral antibody and T cell-mediated immune responses in vaccinated animals after challenge (21).
In the passive immunization studies with Z004 and Z021, antibodies failed to prevent viremia when nonpregnant macaques were inoculated i.v. with a high dose of virus (105 PFU; ref. 23). In contrast, we achieved an overall 40% protection from detectable viremia in 20 animals infected with a more moderate dose of 103 PFU of ZIKV administered s.c. (5 protected out of 12 nonpregnant macaques and 3 protected out of 8 pregnant dams). This dose is still higher than the typical dose received from a mosquito bite, which in laboratory experiments was ∼30 PFU for both the ZIKV isolates used in this study (39). This protection was observed irrespective of Fc domain mutations that modify Fc receptor binding. Neutralizing antibody levels on day of challenge were similar between antibody-treated animals with or without detectable viremia and were ≥3 logs higher than the in vitro inhibitory concentration. However, animals showing low levels and even no ZIKV in plasma had detectable viral RNA in tissues. This indicates that in some animals the virus was able to spread despite high levels of the neutralizing antibodies in serum. These results contrast with earlier reports of sterilizing protection by antibodies under similar virus challenge conditions (s.c. inoculation with 103 PFU). However, sampling was less frequent and over a shorter time period in those studies and therefore transient or delayed viremia may have gone undetected (25, 26). The delay in viremia in the current study, despite high levels of the two neutralizing antibodies in the circulation, suggests that following moderate- to high-dose ZIKV inoculation, virus can still reach tissues and initiate replication, either because in some tissues antibodies did not penetrate sufficiently, or because certain cell types became infected via mechanisms (e.g., via different cell receptors) that are insufficiently captured in the standard in vitro neutralization assays. The delayed peak viremia (7 to 60 d postinoculation) in the pregnant dams likely reflects burst of virus replication in such reservoirs, which then triggered the development of active immune responses. The observation of early fetal losses in two control fetuses and one antibody-treated dam, with high levels of virus in fetal or placental tissues, and rapid disappearance of plasma viremia following fetectomy, indicates the fetal-placental compartment as a possible reservoir that is shielded from antiviral immune responses. The observation of delayed viremia also in antibody-treated nonpregnant macaques suggests the existence of additional reservoirs, of which the identification will improve our knowledge of ZIKV pathogenesis and our ability to develop intervention strategies.
For the unimmunized control pregnancy arm, we previously reported a positive association between fetal pathology scores and prolonged maternal viremia (21). In contrast, although overall pathology scores were reduced compared to the control arm, some fetuses in the active or passive immunization arms had demonstrable fetal or placental pathology despite having no detectable viral RNA in tissues or circulation. While small group sizes are an inherent limiting factor, several other factors may contribute to this finding. As mentioned earlier, the absence of viral RNA at time of killing does not exclude the possibility of earlier ZIKV replication followed by viral clearance. In addition, independent of ZIKV, the frequent amniocentesis procedures may also be a confounding factor that can directly affect amnioplacental pathology scores and ultimately fetal brain development (reviewed in ref. 40). Finally, although representative specimens were sampled to our best ability, pathological lesions and viral RNA may be localized temporally and spatially, rather than evenly distributed.
Despite these study limitations, and despite differences in the kinetics of viremia, early versus delayed viremia in vaccinated versus antibody-treated pregnant dams, the reduced maternal viremia and associated improved fetal outcomes were similar in the two studies (21). Thus, the VRC5283 vaccine and the Z004GRLR/LS + Z021GRLR/LS antibodies are comparably efficacious vis-à-vis fetal protection, with each strategy having their intrinsic advantages and limitations in terms of speed of induction, potential for enhancement, and durability of protective immunity.
Dengue is a flavivirus closely related to ZIKV. Dengue virus reinfection can lead to antibody-enhanced disease by a mechanism that is associated with serologic cross-reactivity between different dengue serotypes and prior dengue infection has been associated with increased risk of Zika microcephaly (41–44). Antibodies to dengue also cross-react with ZIKV and experiments in mice and with human placental explants have shown that ZIKV infection can be enhanced by antibodies in these systems (45–48). Conversely, antibodies to ZIKV that are maternally acquired cause more severe disease in mice infected with dengue (49). Finally, features of the antibodies that increase ZIKV infection in in vitro ADE assays correlate with an enhanced risk of Zika microcephaly in humans and brain pathology in macaque (22). Thus, the potential for enhancement is a safety concern for ZIKV vaccine development that clinical trials need to address. Since monoclonal antibodies that are Fc modified to prevent ADE can shield against ZIKV infection and fetal damage, they represent a potentially safe and efficacious alternative intervention strategy in the face of a ZIKV outbreak.
Materials and Methods
Cell Lines.
Human embryonic kidney (HEK) 293-6E cells were cultured at 37 °C in 8% CO2, shaking at 120 rpm. The other cell lines were cultured at 37 °C in 5% CO2, without shaking: human hepatocyte (Huh)-7.5 cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 1% nonessential amino acids (NEAAs) and 5% fetal bovine serum (FBS); human Lenti-X 293T cells were grown in DMEM supplemented with 10% FBS.
Antibodies.
The human IgG1 antibodies Z004 and Z021 and their Fc variants (GRLR and GRLR/LS) were previously described (23, 30). Z004GRLR and Z021GRLR were prepared by transient transfection of mammalian HEK 293-6E cells with equal amounts of Ig heavy and light chain expression vectors. The supernatant was harvested after 7 d and the antibodies were purified using Protein G Sepharose 4 Fast Flow. Lipopolysaccharide (LPS) was removed with Triton X-114 and the antibodies concentrated in phosphate-buffered saline (PBS). Z004GRLR/LS (TDI-Y-001) and Z021GRLR/LS (TDI-Y-002) were produced by WuXi Biologics and provided by the Tri-Institutional Therapeutics Discovery Institute (TDI). In TDI-Y-002, the Z021 heavy chain variable sequence was modified (C50V).
Macaque Infection Experiments.
Animals and care.
All rhesus macaques (Macaca mulatta) in the study were of Indian origin and were born and raised in the conventional (i.e., not specific pathogen free) breeding colony at the California National Primate Research Center (CNPRC) (SI Appendix, Table S4). While none of the animals were positive for type D retrovirus, simian immunodeficiency virus (SIV), or simian lymphocyte tropic virus type 1, two animals in the pregnancy control group (C02 and C07) were seropositive for West Nile virus due to prior outdoor housing. All animals in the pregnancy experiment had prior pregnancies (range 2 to 10).
The CNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal care was performed in compliance with the 2011 Guide for the Care and Use of Laboratory Animals provided by the Institute for Laboratory Animal Research. Macaques were housed indoor in stainless steel cages (Lab Product, Inc.) whose sizing was scaled to the size of each animal, as per national standards, and were exposed to a 12-h light/dark cycle, 64 to 84° F, and 30 to 70% room humidity. Animals had free access to water and received commercial chow (high protein diet, Ralston Purina Co.) and fresh produce supplements. The study was approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Experiments with nonpregnant macaques.
All 18 rhesus macaques (M. mulatta) were healthy juvenile males and females (∼2 to 4 y of age). When necessary for inoculations or sample collections, macaques were immobilized with 10 mg/kg ketamine hydrochloride (Parke-Davis) injected intramuscularly after overnight fasting. Z004 and Z021 antibodies were administered by i.v. route to macaques at doses of 15 mg/kg body weight 24 h before s.c. inoculation with Puerto Rican ZIKV (ZIKV-PR; 103 PFU, strain PRVABC-59; GenBank KU501215). For the experiments in SI Appendix, Fig. S2, two macaques were administered antibody Z021 (with wild-type Fc) and infected i.v. with high dose of Brazilian ZIKV (ZIKV-BR; 105 PFU, strain SPH2015; GenBank KU321639.1) similar to previously reported (23). Macaques were evaluated at least daily for clinical signs of disease, including poor appetence, stool quality, dehydration, diarrhea, and inactivity. None of the animals that received the antibodies developed any clinical signs, suggesting a good safety profile. Animals were sedated for antibody administration (minus 24 h), at time 0 (virus inoculation), daily for 7 to 8 d, and then every few days for sample collection. Ethylenediaminetetraacetic acid (EDTA) anti-coagulated blood samples were collected using venipuncture and plasma was isolated. At the end of the study, animals were killed for tissue collection. The samples were processed, and the viral RNA was measured from plasma and tissues as described below for pregnant macaques.
Experiments with pregnant macaques.
Time-mated breeding and pregnancy selection.
For time-mated breeding, the female macaques were monitored for reproductive cycle and at the time of optimal receptiveness, temporarily housed with breeding males to induce pregnancy. Gestational ages were determined from the menstrual cycle of the dam and the fetus length at initial ultrasound compared to growth data in the CNPRC rhesus macaque colony. Fetal health and viability were rechecked via ultrasound immediately before the first ZIKV inoculation (∼GD30) and regularly thereafter.
Antibody treatment and virus inoculations.
Z004 and Z021 antibodies were administered to macaques by i.v. route at doses of 15 mg/kg body weight each by 24 h before each virus inoculation. Each pregnant animal was inoculated with virus three times. Whereas the normal gestation of rhesus macaques is 165 d, inoculations occurred at approximately GD30, 60, and 90, corresponding to first and second trimester of human gestation. The GD30 and GD90 inoculations were done with a 2015 Puerto Rico isolate (PRVABC-59; GenBank KU501215), while the GD60 inoculation was done with a 2015 Brazil isolate (strain Zika virus/H.sapiens-tc/BRA/2015/Brazil_SPH2015; GenBank KU321639.1), the same strain as tested earlier in pregnant and nonpregnant animals (11, 27). The use of two strains was intended to mimic an endemic area where different variants may circulate. Aliquots of both virus stocks were kept frozen in liquid nitrogen, and a new vial was thawed shortly before each inoculation. For each inoculation, the inoculum was adjusted to 1,000 PFU in 0.5 mL of RPMI-1640 medium, then kept on wet ice and injected s.c. to simulate the route of mosquito feeding. This dose is higher than the typical dose received from a mosquito bite, which in laboratory vector competence experiments with Californian Aedes aegypti was ∼30 PFU for both the ZIKV isolates used in this study (39).
Sample collection and clinical observations and monitoring.
Macaques were evaluated twice daily for clinical signs of disease, including poor appetence, stool quality, dehydration, diarrhea, and inactivity. When necessary, macaques were immobilized with ketamine hydrochloride (Parke-Davis) at 10 mg/kg and injected intramuscularly after overnight fasting. Animals in both the ZIKV-treated and placebo cohorts were sedated at days 0 (time of first virus inoculation; approximately GD30), 2, 3, 5, 7, 14, 21, 30 (second ZIKV inoculation at GD60), 32, 37, 44, 51, 60 (third ZIKV inoculation; GD90), 62, 67, and then weekly until time of killing between GD155 and GD162, for sample collection and ultrasound monitoring of fetal health. The antibody-treated animals had additional time points of sedation for blood collection and IV antibody administration 1 d before each ZIKV inoculation. EDTA anti-coagulated blood was collected using venipuncture at every time point for complete blood counts (with differential count), and a separate aliquot of blood was centrifuged for 10 min at 800 × g to separate plasma from cells. The plasma was spun an additional 10 min at 800 × g to further remove cells, and aliquots were immediately frozen at −80 °C.
Ultrasound-guided amniocentesis was conducted starting at day 14 after inoculation (GD44), and then at all time points listed above with exception of days 32 and 62 after initial infection. The amniocentesis was conducted using sterile techniques by inserting a 22-gauge, 1.5-inch spinal needle into the amniotic sac. The fetal heart rate was obtained before and after amniocentesis. The area of umbilical entry through the amniotic sac was always avoided to prevent damage to the umbilical arteries and vein. Whenever possible, placental tissue was avoided during the collection of amniotic fluid. Amniotic fluid was spun to remove cellular debris, and the supernatant was aliquoted and immediately cryopreserved at −80 °C for viral RNA assays.
Necropsy and tissue collection.
All necropsies were performed by a board-certified anatomic pathologist and a pathology technician. For animals that had early fetal loss, the procedures described below were performed to the best extent possible based on fetal size. Hysterotomy was performed by a veterinary surgeon on the pregnant macaques under inhalation anesthesia. After collection of amniotic fluid and cord blood, the fetus was killed with an overdose of sodium pentobarbital (≥120 mg/kg). Fetal and organ weights were measured with a scale, and crown–rump length, biparietal diameter, head height, head length, and femur length, were derived from caliper measurements. A detailed tissue dissection was performed. Except for some animals that had early fetal loss and were maintained several weeks after removal of the fetus, all other mothers were killed shortly after their fetus with an overdose of sodium pentobarbital for tissue collection.
Each tissue was grossly evaluated in situ, and then excised, with further dissection with separate forceps and razor blades for each tissue to minimize risks for cross-contamination. Tissues were collected for viral analyses in RNAlater (according to manufacturer’s instructions); extra available samples were snap frozen and stored at −70 °C. Tissues were also preserved in 10% neutral-buffered formalin and routinely paraffin embedded, and slides were created and routinely stained with hematoxylin and eosin (H&E).
Isolation and quantitation of viral RNA from fluids and tissues for determination of infection status.
ZIKV RNA was isolated from samples and measured in triplicate by qRT-PCR according to methods described previously (27), modified to increase the initial volume of sample tested from 140 to 300 µL to increase sensitivity. According to the volume available, the limit of detection for plasma and amniotic fluid ranged from 1.5 to 2.3 log10 viral RNA copies per milliliter of fluid. RNAlater preserved tissues were homogenized to a liquid state with glass beads (Fisher Scientific) or a 5-mm steel ball (Qiagen). For tissues, the limit of detection (LOD) varied depending on the weight of tissue sampled with a mean of 3.5 log10 RNA copies/g of tissue, determined according to the calculations described in ref. 50.
For maternal plasma, amniotic fluid, fetal cord blood, and fetal cerebrospinal fluid, all samples from both control and vaccine group animals that could be collected were tested. For the maternal tissue samples, as maternal infection was not the main focus of this study, a limited selection of lymphoid tissues most likely to have viral RNA based on prior studies was tested (11, 27), with addition of uterus due to proximity to the placenta and fetus. For the analysis of fetal tissue samples for viral RNA, we started our analysis with the control fetuses and tested 26 fetal and MFI tissues, consisting of tissues most likely to contain viral RNA based on our prior study (11). Next we generated a heatmap and selected a list of 17 tissues consisting of those most likely to be infected and diagnosed the most infections in the control fetuses, with addition of brain regions most likely to have histological lesions in the current study (such as hippocampus), or found to be infected in human fetuses affected by CZS (51). The criteria to define infection were used systematically for both control and antibody-treated group fetuses: a fetus was considered ZIKV infected if at least one tissue had a consistent qRT-PCR signal (3/3 replicates positive for viral RNA); this situation applied to 11 of the 12 control fetuses. In a rare case, when a fetus only had a sample with inconsistent qRT-PCR signal (1 out of 3 replicates positive) while all other tissues were negative, retesting was performed, generally on a different aliquot. If the retest result was negative (3/3 replicates), the sample was considered not to contain detectable ZIKV RNA; if this occurred for all tissues from a fetus, the fetus was considered not infected. One control fetus (C07-F) and one antibody-group fetus (AB05-F) met these criteria and were therefore considered uninfected.
Histopathology.
Sections of fixed and embedded fetal tissues were stained with H&E and evaluated by a board-certified anatomic pathologist. Scoring of critical fetal tissues was done according to criteria outlined in SI Appendix, Table S3.
Virus Sequencing.
For the detection of virus mutations at the EDIII region, ZIKV RNA was extracted at peak plasma viremia and Qiagen One-Step RT-PCR was performed using either of two primer sets targeting sequences surrounding the ZIKV EDIII region, as previously detailed (23). Due to low levels of viremia in some samples, reliable EDIII sequence information was not always obtained in samples with amounts below 103 RNA copies/mL plasma. Mutations reported are based on changes in the majority nucleotide in sequencing chromatograms; concordant sequences in both directions were used to identify mutations.
Reporter Virus Particles.
Luciferase encoding RVPs bearing the wild-type or mutant ZIKV E proteins of interest were produced by cotransfection of Lenti-X-293T cells with plasmid pWNVII-Rep-REN-IB (WNV replicon expression construct; ref. 52) in combination with plasmids expressing flavivirus CprME (1 μg of pWNVII-Rep-REN-IB and 3 μg of the CprME plasmid) as previously detailed (30).
The plasmids for expression of wild-type ZIKV CprME with E protein sequence corresponding to Puerto Rican ZIKV (strain PRVABC59) that was used in this study was generated from plasmid pZIKV/HPF/CprM*E* (30) by replacing a BspHI/SacII fragment with sequences corresponding to the PRVABC59 strain that were generated by PCR using oligos RU-O-24379 and RU-O-24380- and PRVABC59-derived cDNA as template. The resulting plasmid was designated pZIKV/HPF/CprM*PRVABC59E*. Assembly PCR-based site-directed mutagenesis was then used to introduce in pZIKV/HPF/CprM*PRVABC59E* the V330L, T309A, V330L/T309A, and V330L/T309I mutations that resulted in plasmids pZIKV/HPF/CprM*PRVABC59E* (V330L), pZIKV/HPF/CprM*PRVABC59E* (T309A), pZIKV/HPF/CprM*PRVABC59E* (V330L/T309A), and pZIKV/HPF/CprM*PRVABC59E* (V330L/T309I). Similarly, the ZIKV RVPs encoding for the T335 mutations (SI Appendix, Fig. S2) were generated by modifying plasmid pZIKV/HPF/CprM*E* (30) to introduce the T335A and T335I modifications resulting in pZIKV/HPF/CprM*E* (T335A) and pZIKV/HPF/CprM*E* (T335I), respectively. The primers used for assembly PCR and mutagenesis are listed in SI Appendix, Table S5. All PCR-derived plasmid regions were verified by sequencing.
In Vitro Neutralization Assay.
RVP neutralization of luciferase-encoding RVPs by antibodies was performed with Huh-7.5 cells in 96-well plates, in triplicate, as previously detailed (30), and the luciferase activity was read using a FLUOstar Omega luminometer (BMG LabTech). The neutralization capacity of the antibodies against wild-type or mutant RVPs was determined by the percentage of luciferase activity obtained relative to RVPs incubated with isotype control antibody 10-1074 (53). Representatives of at least two independent experiments are shown.
Measurements of Human IgG in Macaque Plasma.
ELISA was used for the detection of human IgG as previously detailed (30). Briefly, the biotinylated anti-human IgG capture antibody was added to neutravidin-coated 96-well plates. Upon washes, the plates were blocked with 2% bovine serum albumin (BSA), blotted, and then serial dilutions of the macaque plasma were added to the wells (5 steps of 1:4 dilutions in PBS-T, starting with 1:10). Each plate included serial dilutions of the standard, in duplicates (Z004 IgG, 11 steps of 1:3 dilutions in PBS-T, starting with 10 μg/mL). Plates were incubated for 1 h at room temperature and washed prior to adding the detection reagent anti-human IgG-horseradish peroxidase (HRP). After washes, the reaction was developed with 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) substrate (Life Technologies).
Measurements of Human IgG in Mouse Serum.
ELISA was used for the detection of human IgG as previously detailed (54). Briefly, FcRn/FcγR-humanized mice were injected i.v. with 100 μg of Z004 or Z021 Fc variants. High-binding 96-well microtiter plates (Nunc) were coated overnight at 4 °C with neutravidin. All sequential steps were performed at room temperature. Plates were blocked for 1 h with PBS/2% BSA and incubated with biotinylated goat anti-human IgG antibodies for 1 h (5 μg/mL; catalog 109-066-170, Jackson ImmunoResearch). Serum samples were serially diluted and incubated for 1 h, followed by incubation with HRP-conjugated anti-human IgG. After washes, the reaction was developed with the 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase substrate system (KPL) and stopped with 2 M phosphoric acid. Αbsorbance at 450 nm was immediately recorded using a SpectraMax Plus spectrophotometer (Molecular Devices), background absorbance from negative control samples was subtracted, and duplicate wells were averaged.
Statistical Analyses.
Statistical analyses were performed with Prism 8 (GraphPad).
Data Availability.
All data are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank Kai-Hui Yao and Daniel Yost at Rockefeller University, as well as A. Gibbons, M. Allen, V. Bakula, M. Christensen, I. Cazares, W. von Morgenland, the veterinary staff, pathology staff, and the staffs of Colony Management and Research Services and the Clinical Laboratory at the California National Primate Center for expert assistance. We also thank Mohsan Saeed (Boston University) for construction of plasmid pZIKV/HPF/CprM*PRVABC59E. This work was supported by awards from NIH, U19AI111825, P01AI138938, and UL1TR001866 (to D.F.R.), R01AI037526, UM1AI100663, U19AI111825, P01AI138938, and UL1TR001866 (to M.C.N.); funding from Lyda Hill Philanthropies (to M.C.N.); grants R01AI124690 and U19AI057229 (CCHI pilot project); The Rockefeller University Development Office, and anonymous donors (to C.M.R. and M.R.M.); the Office of Research Infrastructure Programs/OD (grant P51OD011107); start-up funds from the Pathology, Microbiology, and Immunology Department (to L.L.C.); and grant R21AI129479-S (to K.K.A.V.R.). Support was also provided by the Robertson Therapeutic Development Fund (to D.F.R. and M.C.N.) and a scholarship from the Studienstiftung des Deutschen Volkes (to T.K.). S.R.E. is supported by NIH National Research Service Award Fellowship F30AI147579 and NIH National Institute of General Medical Sciences Training grant T32-GM008042 through the University of California, Los Angeles-Caltech Medical Scientist Training Program. M.C.N. is a Howard Hughes Medical Institute Investigator.
Footnotes
Competing interest statement: The Rockefeller University, D.F.R., and M.C.N. have filed a patent application for antibodies Z004 and Z021.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000414117/-/DCSupplemental.
References
- 1.Gould E. A., Solomon T., Pathogenic flaviviruses. Lancet 371, 500–509 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Weaver S. C., Reisen W. K., Present and future arboviral threats. Antiviral Res. 85, 328–345 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen L. R., Jamieson D. J., Honein M. A., Zika virus. N. Engl. J. Med. 375, 294–295 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Del Campo M., et al. ; Zika Embryopathy Task Force-Brazilian Society of Medical Genetics ZETF-SBGM , The phenotypic spectrum of congenital Zika syndrome. Am. J. Med. Genet. A. 173, 841–857 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Pierson T. C., Diamond M. S., The emergence of Zika virus and its new clinical syndromes. Nature 560, 573–581 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Musso D., Ko A. I., Baud D., Zika virus infection–After the pandemic. N. Engl. J. Med. 381, 1444–1457 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Brasil P., et al. , Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 375, 2321–2334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleber de Oliveira W., et al. , Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy–Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 65, 242–247 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Hoen B., et al. , Pregnancy outcomes after ZIKV infection in French Territories in the Americas. N. Engl. J. Med. 378, 985–994 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Nielsen-Saines K., et al. , Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 25, 1213–1217 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffey L. L., et al. , Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat. Commun. 9, 2414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley D. M., et al. , Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat. Med. 24, 1104–1107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinot A. J., et al. , Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell 173, 1111–1122.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan C., Xie X., Shi P. Y., Zika virus vaccine: Progress and challenges. Cell Host Microbe 24, 12–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond M. S., Ledgerwood J. E., Pierson T. C., Zika virus vaccine development: Progress in the face of new challenges. Annu. Rev. Med. 70, 121–135 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Larocca R. A., et al. , Vaccine protection against Zika virus from Brazil. Nature 536, 474–478 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowd K. A., et al. , Rapid development of a DNA vaccine for Zika virus. Science 354, 237–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richner J. M., et al. , Vaccine mediated protection against Zika virus-induced congenital disease. Cell 170, 273–283.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X., et al. , A single-dose live-attenuated Zika virus vaccine with controlled infection rounds that protects against vertical transmission. Cell Host Microbe 24, 487–499.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan C., et al. , A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 8, 676 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Rompay K. K. A., et al. , DNA vaccination before conception protects Zika virus-exposed pregnant macaques against prolonged viremia and improves fetal outcomes. Sci. Transl. Med. 11, eaay2736 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbiani D. F., et al. , Risk of Zika microcephaly correlates with features of maternal antibodies. J. Exp. Med. 216, 2302–2315 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keeffe J. R., et al. , A combination of two human monoclonal antibodies prevents Zika virus escape mutations in non-human primates. Cell Rep. 25, 1385–1394.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton H. M., et al. , Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 68, 8049–8057 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Abbink P., et al. , Therapeutic and protective efficacy of a dengue antibody against Zika infection in rhesus monkeys. Nat. Med. 24, 721–723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnani D. M., et al. , Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci. Transl. Med. 9, eaan8184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffey L. L., et al. , Zika virus tissue and blood compartmentalization in acute infection of rhesus macaques. PLoS One 12, e0171148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalevsky J., et al. , Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 28, 157–159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautam R., et al. , A single injection of crystallizable fragment domain-modified antibodies elicits durable protection from SHIV infection. Nat. Med. 24, 610–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbiani D. F., et al. , Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 169, 597–609.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stettler K., et al. , Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353, 823–826 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Abbink P., et al. , Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353, 1129–1132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caine E. A., Jagger B. W., Diamond M. S., Animal models of Zika virus infection during pregnancy. Viruses 10, E598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapparapu G., et al. , Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540, 443–447 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez E., et al. , Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat. Immunol. 18, 1261–1269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stouffer R. L., Woodruff T. K., Nonhuman primates: A vital model for basic and applied research on female reproduction, prenatal development, and women’s health. ILAR J. 58, 281–294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaudinski M. R., et al. ; VRC 319 ; VRC 320 study teams , Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 391, 552–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett A. D. T., Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. NPJ Vaccines 3, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Main B. J., et al. , Vector competence of Aedes aegypti, Culex tarsalis, and Culex quinquefasciatus from California for Zika virus. PLoS Negl. Trop. Dis. 12, e0006524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shallie P. D., Naicker T., The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. Int. J. Dev. Neurosci. 73, 41–49 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Carvalho M. S., Freitas L. P., Cruz O. G., Brasil P., Bastos L. S., Association of past dengue fever epidemics with the risk of Zika microcephaly at the population level in Brazil. Sci. Rep. 10, 1752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halstead S. B., Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 60, 421–467 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Katzelnick L. C., et al. , Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salje H., et al. , Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 557, 719–723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bardina S. V., et al. , Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356, 175–180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmerman M. G., et al. , Cross-reactive dengue virus antibodies augment Zika virus infection of human placental macrophages. Cell Host Microbe 24, 731–742.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown J. A., et al. , Dengue virus immunity increases Zika virus-induced damage during pregnancy. Immunity 50, 751–762.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathore A. P. S., Saron W. A. A., Lim T., Jahan N., John A. L. St., Maternal immunity and antibodies to dengue virus promote infection and Zika virus–induced microcephaly in fetuses. Science Adv. 5, eaav3208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fowler A. M., et al. , Maternally acquired Zika antibodies enhance dengue disease severity in mice. Cell Host Microbe 24, 743–750.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forootan A., et al. , Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol. Detect. Quantif. 12, 1–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azevedo R. S. S., et al. , Zika virus epidemic in Brazil. II. Post-mortem analyses of neonates with microcephaly, stillbirths, and miscarriage. J. Clin. Med. 7, E496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierson T. C., et al. , A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology 346, 53–65 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Mouquet H., et al. , Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U.S.A. 109, E3268–E3277 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weitzenfeld P., Bournazos S., Ravetch J. V., Antibodies targeting sialyl Lewis A mediate tumor clearance through distinct effector pathways. J. Clin. Invest. 129, 3952–3962 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in SI Appendix.