Introduction
The subject matter of this article concerns the structure and function of a prototypical animal cell in its role as a host for viruses (Fig. 1 ). It is now clearly evident from the information acquired during the recent past, particularly from data developed by molecular biology techniques, that viruses in the form of quasi-autonomous macromolecular complexes are propagated by expression of genomes which originated from their hosts. The viral genes have undergone alteration to a varying degree during evolutionary selection so as to give the virus parasite an advantage in metabolic efficiency. Detailed knowledge about cell organization reveals the existence of a great variety of relationships connecting cellular components of direct relevance to every stage of the infectious process with individual virus types. Owing to the brevity of this treatise it has been necessary to omit information about cell organelles and structures that have only a minor or peripheral relationship to the virus life cycle.
Figure 1.
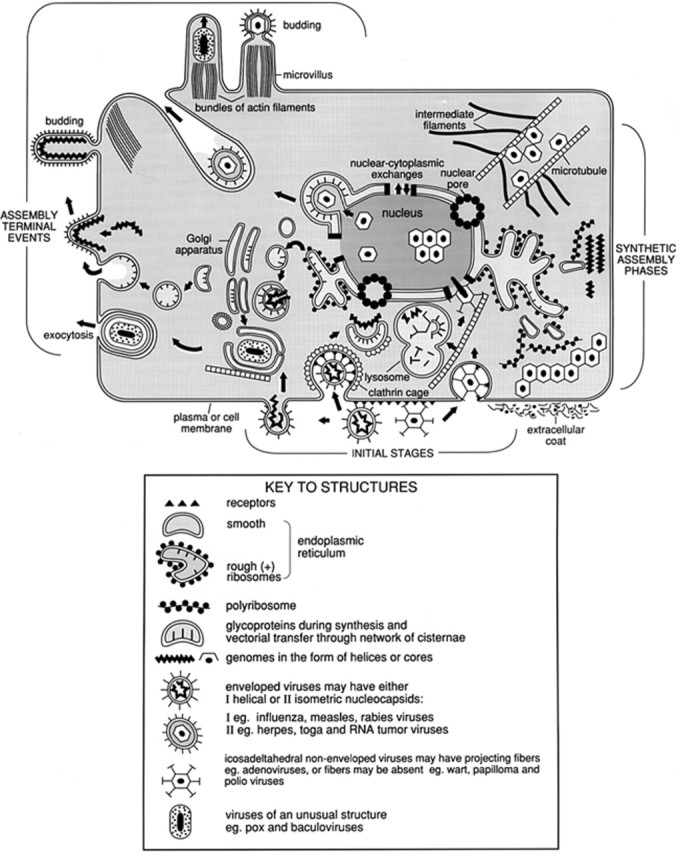
Schematic representation of a prototypical animal cell. The illustration emphasizes cellular organization as it relates to various phases of the virus replication cycle.
The Surface
Tissue organization and cell polarity
When discussing cellular structure and function with respect to virology, one must realize that within the animal host, initiation and spread of virus infections frequently follows encounters at epithelial and endothelial surfaces such as those in skin, lung, gut and vascular lining. These surface sheets are assemblies of polarized cells that possess apical and basolateral membranes exposed to the fluid milieu. The free movement of particulates from one side of these sheets to the other is, however restricted by lateral cell-to-cell contacts termed tight junctions. This manner of organization at cellular surfaces determines the routes of virus ingress, egress and dissemination within the animal. Data derived from infected animals and explants of cultured epithelia demonstrate that specific viral receptors may be confined to either the apical or basolateral surfaces, depending on the virus type, although dual receptors at opposite sides of polarized cells have been uncovered. Cell polarization also influences the membrane sites at which envelopment and assembly of viruses can occur. This may be due primarily to mechanisms for sorting and vectorial transfer of nascent glycoproteins destined for incorporation into the virus progeny within specialized membrane components. The interrelationship between molecular sorting and vectorial transfer is clearly exemplified by differences between influenza virus, which is assembled on the apical side, vesicular stomatitis virus, which buds preferentially on the basolateral face, and the coronaviruses, which use for assembly membranes of intracellular vesicles neighboring the Golgi apparatus. The direction of vectorial movement through the cellular membrane network is actually inherent in specific amino acid sequences of polypeptides and glycoproteins, whether they are encoded by cells or viruses.
Extracellular coats
Ubiquitous glycoprotein and glycolipid molecules, which are integral to the organization of the plasma membrane bilayer, are usually decorated by extracellular branched polysaccharide chains. A surface meshwork of such chains forms the glycocalyx. Carbohydrate residues of the glycocalyx provide initial attachment sites for ligands on the virus inoculum, exemplified by adsorption of influenza hemagglutinin to N-acetylneuraminic (or sialic) acid of the cell receptor. At the terminal formative stages of enveloped viruses, regions of cell surface contiguous to those of the emerging particles become desialylated by viral neuraminidases, thereby ensuring that individual virions are released efficiently from the glycocalyx. Other types of carbohydrates such as the elongated mucopolysaccharides that coat cells of the respiratory epithelium represent another type of barrier which must be dispersed by enzyme on the virus before the inoculum can reach the cell receptor to establish a stable link. Other types of extensive coats also exist, termed extracellular matrix, with various properties adapted to specialized functions of the tissue where they occur. The substances present in the extracellular matrix include hydrated gel-like glycosaminoglycans linked to proteins forming proteoglycans. Proteoglycans can be permeated by a meshwork of fibrous proteins, exemplified by collagen and materials such as fibronectin and laminin, which promote cell adhesion to supporting layers. The extracellular matrix is involved in kidney diseases such as certain forms of glomerulonephritis which may develop during chronic persistent infections when massive quantities of virus–antibody immune complexes filtrated out of the circulation are deposited in the matrix. This disease process occurs among human carriers chronically infected with hepatitis B virus and other agents.
The cell or plasma membrane
The cell membrane, interchangeably termed plasma membrane, forms a semipermeable barrier enclosing the contents of the cell. Like all cellular membranes it is organized into a bilayer of amphipathic phospholipids and glycolipids, transversed by a great variety of transmembrane polypeptides and glycoprotein molecules. The carbohydrates of the glycoproteins, present as multiple branches of oligosaccharides, are frequently attached to the externally protruding N-termini of the polypeptide. Organization into bilayers favors a high degree of membrane flexibility during a constant state of flux and the lateral mobility of molecules inserted within the bilayer. The dynamic state of membranes allows for their rapid deformability into invaginations from the surface leading to formation of vesicles. Evaginations may result in the formation of transient elongations and more permanent processes such as microvilli. Membrane dynamics also provide mechanisms for membrane-to-membrane fusion or, conversely, separation between neighboring membrane segments, phenomena that occur frequently throughout the cell and do not compromise the bilayer continuity. These properties of membranes make the plasma membrane a site for the formation of autonomous endocytic vesicles, and coalescence with shuttle vesicles, which carry materials for exocytosis. Sealing between membranes can be promoted by the influence of fusogenic proteins. The constant traffic away from and towards the surface facilitates continuous circulation and turnover of membrane components not only at the surface but throughout the cell. Sideways mobility of molecules within the plane of the lipid bilayer provides a mechanism for establishing intermolecular contacts and conformational changes related to metabolic activities. Specificity of interactions within the membrane bilayer between proteins and glycoproteins can create heteromeric or multimeric molecular assemblies, functioning themselves as receptors or via connections to the receptors. Such groupings of molecules are commonly involved with immune response phenomena, with formation of toxins or transmitter-gated channels of the type present in interneural and neuromuscular junctions. Rapid physiological responses can occur at the surface initiated via receptors recognizing extracellular signals, whether they are of the autocrine variety like lectins and growth factors, of an endocrine type such as insulin or in the category of second messengers represented by the cyclic nucleotides. The rapid transfer of externally initiated signals towards intracellular response foci can be mediated through connections provided by elements of the cytoskeleton, or by membrane-to-membrane coupling or through vesicular shuttles trafficking between membrane-enclosed compartments and the cell exterior.
Homeostasis of the cell with respect to cell volume, ion concentration and pH is maintained by means of proteinaceous ion pumps present in the plasma membrane. Such pumps facilitate internalization of Na+ and Cl− and removal of excess H+ or HCO3− derived from cell metabolism. Other membrane proteins catalyze transmembrane transport of metabolites or, inadvertently, the uptake of chemotherapeutic drugs. Overall the cell membrane can be conceptualized as a mosaic of domains, with each type of domain fulfilling a specialized function in the life of a cell. Regions of dense coat along the inner cytoplasmic face of the plasma membrane are due to formation of cages or basket-like scaffolds organized out of triskeletal macromolecular assemblies of the protein clathrin. Clathrin cages also enclose endocytic and other specialized vesicles shuttling to and from the surface. Other types of specialized coated vesicles, surrounded by proteins other than clathrin, can also be formed and participate in trafficking with the plasma membrane.
As one of its primary functions, which also has a bearing on viruses, the plasma membrane selectively concentrates and internalizes extracellular material. Sequestration of particulates inside endocytic vesicles is termed endocytosis, and uptake of fluid-phase components is pinocytosis. Endocytosis becomes highly efficient and selective due to the sequential binding via a zippering-like process termed receptor-mediated endocytosis of ligands. The ligands present on the particulate destined for engulfment become bound to multiple receptors mobilized by lateral diffusion along the plasma membrane. Receptor-mediated endocytosis decrees the precise configuration of envelopment in endosomes. The internal milieu of newly formed endosomes is close to neutrality, but during their inward movement away from the surface the fluid contents of endosomes become acidified by accumulation of H+ pumped out of the cytosol. The presence of a repertoire of acidic hydrolases, acquired via transfer vesicles from the Golgi apparatus, converts the late endosomes into prelysosomes, the latter predestined to coalesce with mature lysosomes. The lysosomes containing acidic enzymes able to hydrolyze lipids, nucleic acids and proteins function as the primary sites for intracellular digestion to dispose of damaged organelles or any undesirable materials acquired accidentally by engulfment. The strategies that viruses employ to utilize the properties of the plasma membrane during initial and terminal stages of their life cycle clearly manifest coevolutionary origin with that of the host. A stable attachment is established when a virus ligand makes contact with host cell receptor during the initial collisions at the surface. The presence of multiple ligands on the virus surface and an abundance of receptors, perhaps 104–106 on each host cell, increases the probability that a productive encounter will occur. The display of certain receptors on specific differentiated cell types and/or along specialized cell surfaces can be the determinant regulating tissue or organ tropisms for different virus types. Among notable examples are CD4 molecules on human T cells which function as receptors for the human immunodeficiency virus (HIV), β-adrenergic receptors on neurons in mouse brain which can bind reovirus type 3, receptors for the neurotransmitter acetylcholine at neuromuscular junctions where attachment of rabies virus is evident and the asialoglycosphingolipids in gut epithelium which function as receptors for rotaviruses. Receptors for polio-, rhino- and cardiovirus groups are macromolecules that are functional in uninfected cells related to a super family of glycoproteins with the basic configuration of immunoglobulin. As already mentioned, dual receptors for viruses such as herpes simplex can occur on distinctive surfaces on polarized epithelial cells, or alternative receptors may be present on different host cells, as is evident with attachment of HIV to either its principal T cell CD4 receptors or to galactocerebroside receptors on certain glial cells in the nervous system.
The entry of virus inoculum particles to effect an intracellular release or uncoating of their genomes depends on fusogenic activity carried by the virus which is used to disrupt temporarily membrane integrity. Viral fusogens are rapidly activated at the surface by specific host proteases. Fusion of this type, exemplified by paramyxoviruses, can be activated at neutral pH, bringing about a comingling between viral and cell membranes at the surface so as to create an entrance for the virus nucleocapsid into the cytosol. Access of encapsidated virus genomes into the cytosol, facilitated by fusion of viral envelopes with the cell membrane, can be regulated by interactions with coreceptors. With HIV they are chemokine receptors CCR5 in the case of macrophage tropic and CXCR for T lymphocyte tropic virus strains.
Other fusogens are activated under acidic conditions inside endosomes where virus membrane fusion creates an escape passage, observed during penetration of influenza, rhabdo-, toga-, adeno- and other virus groups. Sequestration inside endosomes rapidly destabilizes the icosahedral picornavirus coats effecting release of the RNA genome, which then passes across the membrane into the cytosol. Other types of icosahedral agents such as the adenoviruses can rupture endosome membranes, escaping thereby into the cytosol without apparent loss of integrity (Fig. 2 ). From the above examples it should be evident that viruses are able to initiate infections successfully because they circumvent the usual transfer and hydrolysis of engulfed particulates in lysosomes. Under circumstances that abolish or block the activity of viral fusogens, as happens on denaturation at elevated temperature or as a result of steric hindrance after attachment of specific antibodies, the engulfed inoculum virus is, in fact, transferred into lysosomes where it is destroyed.
Figure 2.
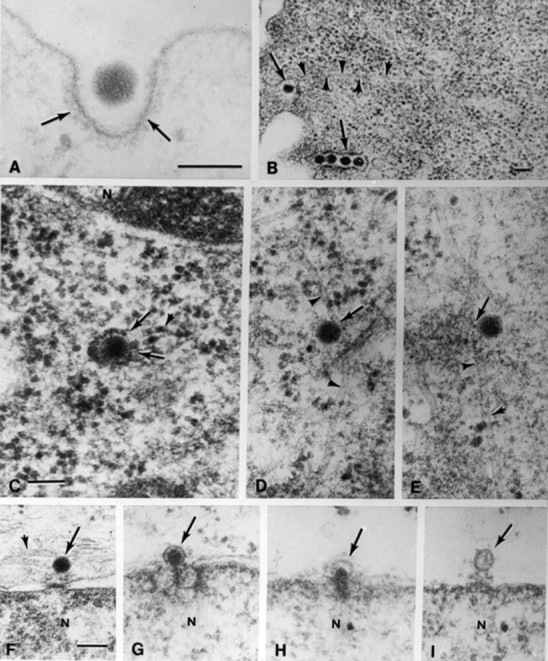
Intracellular events during the penetration and uncoating of inoculum adenoviruses. (A, B) Engulfment of virions at the plasma membrane is indicated by arrows. In (B) the microtubule, delineated by arrowheads, appears to be in contact with the plasma membrane. In (C) arrows indicate where the endosome membrane has been ruptured. (D, E) Arrows point to contacts between virions free in the cytosol and microtubules. (F–I) Progressive stages of uncoating at the nuclear pore complexes indicated by arrows. Following injection of dense, fibrous DNA into the nuclear compartment (N), illustrated in (G) and (H) the empty capsids in (I) remain behind. Magnifications (A) ×140000; (B) ×40000; (C–E) ×80000; (F–I) ×65000. (Reproduced from (A) Chardonnet Y and Dales S (1970) Virology 40: 462; (B–I) Dales S and Chardonnet Y (1973) Virology 56: 465.
Membrane network
The intracellular network of membrane-enclosed cisternae, vesicles and membranous organelles occupies about one-half of the volume of the cytoplasmic or cytosol compartment. Numerous membranous organelles that reside in the cytosol, among them the mitochondria, which are involved in energy metabolism, and microbodies, which participate in detoxification reactions, are omitted from Fig. 1 and are not further discussed because they have only a peripheral role in virus infections. The predominant structures enclosed by membranes are the endoplasmic reticulum (ER) and Golgi apparatus, which are transiently interconnected with each other and the plasma membrane by transport vesicles. A specialized continuum of the ER are the cisternae enclosed by the nuclear envelopes surrounding the nucleus. This network of lacunae functions as a conduit for an orderly flow of materials between intracellular compartments and the exterior.
Some regions of the ER which are devoid of ribosomes are termed smooth ER (SER) whereas other regions encrusted with ribosomes on the membrane facing the cytosol are designated as the rough ER (RER). Synthesis of proteins destined for cisternae or membrane-enclosed organelles or for transfer to the cell surface is initiated on RER when ribosomes already engaged with messenger or mRNA in a translation complex become bound to receptors on the SER. The growing polypeptide chain is orientated from the ribosome across the membrane into the lumen of ER cisternae. The vectorial insertion of the nascent polypeptide is due to recognition of signal sequences of hydrophobic amino acids. The destination to which polypeptides synthesized in RER are dispatched is also determined by specific amino acid sequences. Some nascent molecules enter into organelles such as the lysosomes and mitochondria, others are destined to become plasma membrane components, and yet others are scheduled for removal from the cell by exocytosis. Many proteins moving through the ER are modified by addition of carbohydrate chains in a complex sequence of reactions involving enzymes bound to the luminal side of the membrane, termed glycosyltransferases. Following the formation of intermediate mannose-rich oligosaccharide chains linked to a dolichol lipid carrier, the carbohydrate becomes attached to asparagine residues at specific signal sites on the polypeptide forming an N-linkage. In addition to a role in producing proteins and glycoproteins, the other functions of the ER include synthesis of phospholipids and glycolipids required for membrane addition and turnover throughout the cell.
The ER provides a surface for enzymes such as those that catalyze the detoxification of drugs prominent in the liver, and as an attachment surface for ion pumps such as those for sequestering Ca2+ in the sarcoplasmic reticulum of muscle. Carrier vesicles engaged in transfer of materials between various cisternae are also generated from the ER. The Golgi apparatus, which is organized into aggregates or stacks of flat interconnected cisternae, is the compartment in which completion of synthesis, sorting and distribution of glycoproteins towards various membrane compartments takes place. The groups of Golgi cisternae facing towards the cell interior, termed cis, are involved in dispatching molecules to centrally positioned organelles, whereas trans cisternae, oriented towards the surface, are associated with products transferred to the lysosomes or to the plasma membrane by carrier vesicles, some enclosed within clathrin cages. Additional glycosyltransferase enzymes in the Golgi are used to modify the N-linked oligosaccharides, produced in the ER, by deletion and terminal addition of carbohydrate. These enzymes also synthesize exclusively in the Golgi oligosaccharides attached by the -O- linkage to serine residues of the polypeptide. Certain virus groups such as the coronaviruses, which possess the O-linked carbohydrate of an envelope glycoprotein, are assembled not at the cell surface but on membranes of Golgi vesicles. The majority of enveloped virus types is organized into particles by budding from the plasma membrane (Fig. 3 ). Therefore, the cell surface becomes a region for organizing the glycoprotein molecules that constitute the peplomers or spikes on the virus envelope after they have been delivered to the plasma membrane in transfer vesicles from the Golgi cisternae. Once they are in place, peplomers become foci for mobilizing the nucleocapsid and inner membrane protein(s), then budding takes place. Some virus-induced glycoproteins, which are not a constituent per se of virus partlcles, are nevertheless inserted into cell membranes, thus altering their properties. For example, the cisternae may be formed into rigid channels through which particles of herpes simplex virus can pass to the cell surface, or, as in the case of poxviruses, membrane modifications promote recognition between surfaces on the virus and Golgi vesicles which then surround the virus particle and fuse with each other to form a continuous double-membrane cisterna. The wrapped virus is moved towards the surface where exocytosis occurs as a result of fusion between the outer wrapping and plasma membranes (Fig. 4 ).
Figure 3.
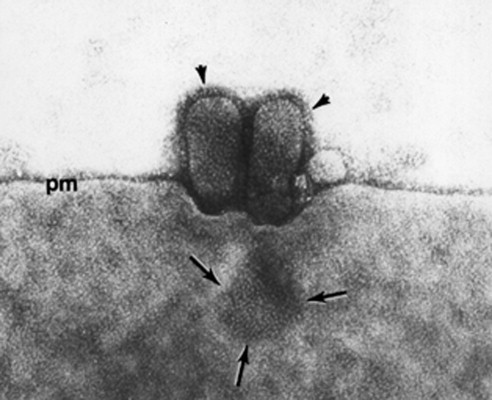
Emergence of influenza virus by budding from the plasma membrane (pm). In the electron micrograph of an infected primate cell arrowheads indicate presence of peplomers on the virions’ surface. Arrows point towards a grouping of peplomers where a presumptive virion is assembling; magnification ×165000. (Reproduced from Choppin PW (1963) Virology 21: 278.)
Figure 4.
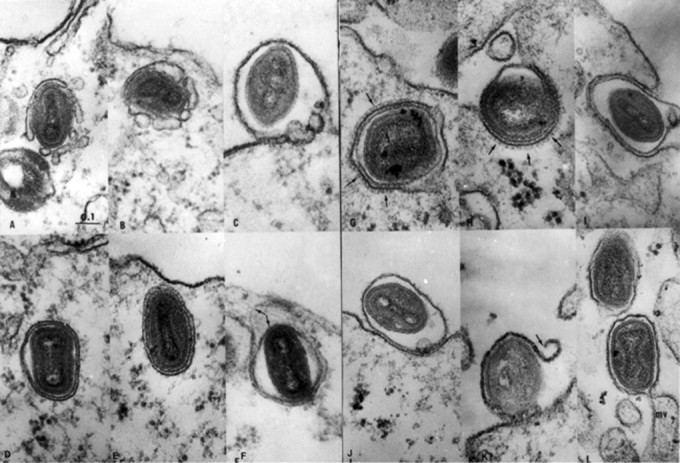
Electron micrographs illustrating a reconstructed sequence of the envelopment, migration to the surface and release through the plasma membrane of progeny vaccinia virus. (A, B) Envelopment in vesicles that failed to fuse into a continuous cisterna. (C) Two vesicles interposed between the plasma membrane and the inner cisterna membrane surrounding a discharged virion. (D, E) Migrating virions enclosed by double membrane cisternae. In (F) arrows indicate a separation of the two cisterna membranes. (G, H) Arrows indicate the presence of dense coats on some wrapping membranes. (I, J) Release of virions wrapped by the inner cisterna membrane, which has been ruptured in (K). (L) Virion in the process of discharge through a microvillus (mv), the alternative method of egress. Magnification ×61000. (Reproduced from Ichihashi Y et al (1971) Virology 46: 507.)
The Cytosol
The cytosolic compartment occupies the cytoplasm exclusive of the membrane network and membrane-enclosed organelles. Proteins formed in the cytosol, termed ‘free’, are translated from mRNA on polyribosomal complexes that do not become attached to the ER. The ‘free’ proteins, whether they remain in the cytosol or enter into the nuclear compartment, are components of enzyme assemblies, which may be attached to membranes on the cytosolic side. They also form organelles such as the ribosomes, fibrous filaments of the cytoskeleton and nucleosome proteins of the chromatin. It is notable that the polyribosomes engaged in synthesis of large precursor polyproteins of viruses such as polio are attached to SER vesicles at one end. Virus-specified proteins synthesized in the cytosol accumulate into pools of material out of which individual icosahedral virions can be assembled. The proteins are sometimes sufficiently abundant for formation of higher order crystalline aggregates out of assembled virus particles (Fig. 5 ).
Figure 5.

An area of the cytosol in a human cell infected 7 h previously with poliovirus type 1. A prominent crystalline aggregate of virions occupies the center of the electron micrograph. Magnification ×83000. (Reproduced from Dales S et al (1965) Virology 26: 379.)
The cytoskeleton represents an array of three principal types of fibrous materials, actin microfilaments arranged in bundles, intermediate filaments of several types and cylindrical microtubules. These three elements are interconnected with each other, and can make contacts via a large repertoire of associated proteins with membranes around cell organelles, the nucleus and at the cell surface. Actin filament bundles, prominent in the peripheral cortex region of the cytosol and inside microvilli, are involved in surface mobility during formation of pseudopodia and participate in other changes in cell form. The filaments which have a directional polarity are continually disassembled and reassembled from actin protomers. Circumstantial evidence connects actin filaments with virus infections during initial stages of penetration, migration of the progeny through microvilli and assembly by budding at the cell surface. The intermediate filaments contain four chemically distinguishable but related fibrous proteins. In all cells, they form a net of nuclear lamins situated next to the nuclear envelopes. The organ or tissue in which a cell occurs determines the composition of the intermediate filament(s). Intermediate filaments in cells of epithelia, connective tissue, muscle and the nervous system contain respectively cytokeratins, vimentin, desmin, glial fibrillary acidic protein and neurofilament protein. Contact between intermediate filaments and other components most probably plays a role in mechanical support, as, for example, in neuronal and astrocytic processes and intercellular contacts where adhesion foci, such as the desmosomes, are present. Intermediate filaments have been implicated in the assembly of some picorna- and reoviruses by their presence among the progeny aggregates of virus. Microtubules, the thickest of the cytoskeletal elements, contain α and/or β tubulins organized into cylindrical walls of helical protofilaments. The presence of microtubules imparts the shape and polarity to cells and provides a mechanism for orderly segregation of daughters during cell division. The microtubules provide surfaces along which movement of vesicles and other membrane-enclosed organelles can be directed. Among the notable functions are: (1) fast axonal transport along groups of microtubules which connect the region of synthesis in the cell body with synaptic termini, (2) involvement in the beating of cilia or flagella and (3) as pathways for the rapid and reversible movement of granules in pigment epithelial cells. The energy for effecting translocations along the microtubules is obtained from the hydrolysis of ATP.
Microtubules are utilized by viruses during infection in various ways. They provide attachment sites for inoculum adeno- and herpes simplex viruses and for conveying them vectorially towards the nuclear pore complexes where uncoating and injection of the DNA occur (Fig. 2). The virions may be propelled along the microtubules by the action of a molecular motor, kinesin. Microtubules and connected intermediate filaments become loci for assembly of reovirus type 3 progeny, as is evident in fibroblasts and axons of neurons.
With another virus type there exists an interaction between neuronal microtubules and the N nucleocapsid protein of coronavirus JHM (Fig. 6 ). This relationship occurs perhaps due to molecular mimicry, as revealed by sequence relatedness of N with cellular microtubule-associated proteins tau and MAP-4 (Fig. 7 ). This mimicry draws attention to evolution of viruses which, as intracellular parasites, exploit the cell’s structure and function properties for self propagation.
Figure 6.
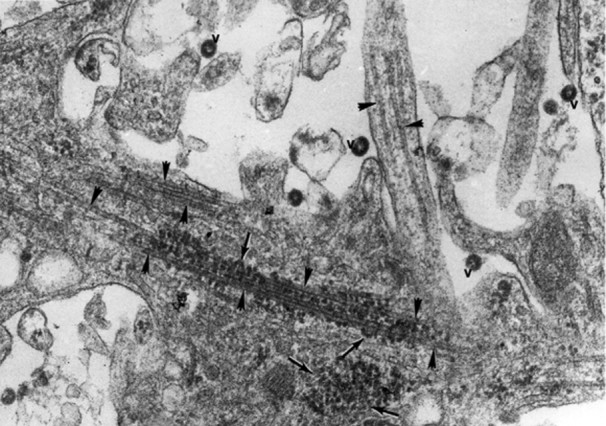
Association between the nucleocapsids of coronavirus JHM (arrows) and bundles of microtubules (arrowheads) within neurites of an infected hippocampal neuron of a mouse. Extracellular virions are marked ‘v’. Magnification ×115000. (Reproduced from Kalicharran K and Dales S (1996) Trends Microbiol. 4: 264.)
Figure 7.

Sequence relatedness between the nucleocapsid protein N of coronavirus JHM and the microtubule-associated proteins tau and MAP-4. (A) The tandemly repeated sequence involved in binding to microtubules is matched with a sequence of N. (B) Shows homologies at the serine- and proline-enriched segments. In the optimal alignment amino acid identity is shown by connecting lines and relatedness, depending on closeness, by one or two dots. (Reproduced from Kalicharran K and Dales S (1996) Trends Microbiol. 4: 264.)
The Nucleus
Maintained as an autonomous compartment during the interphase of the cell cycle, the nucleus is where synthesis occurs relevant to duplication of the DNA and gene expression in the form of transcription into messenger, ribosomal, transfer and other RNA species. The genomic DNA of the cell and associated proteins, including such basic proteins as the histones, form the chromatin, which consists of a multitude of primary units, the nucleosomes. Nucleosomes are organized into hierarchical orders to form the ultimate units of structure, the chromosomes. At interphase, during the most active synthetic phase, the chromatin is dispersed maximally. Chromatin compaction into cytologically identifiable chromosomes commences upon initiation of cell division. In the chromatin, individual genes can be expressed transcriptionally as units. DNA synthesis and transcription each require specific polymerase enzymes and numerous additional cofactors. These factors, some acting cooperatively and others competing with the polymerases, can bring about relaxation of the double-stranded DNA template, promote the copying of the forward and retrograde DNA strands and act in transcription of individual genes. Since the DNA is organized into interrupted stretches of informational sequences (exons) separated by intervening noncoding sequences (introns), a functional RNA template for translation into protein is formed from the primary RNA transcript by removal or splicing out of the introns, usually catalyzed by specific protein complexes termed spliceosomes. Further enzymatic modifications of the RNA template bring about an addition at the 5′ end of specific nucleotide sequences termed ‘caps’ and at the 3′ end stretches of polyadenylic acid. The above metabolic activities within the cell nucleus are diverted for their own use by viruses with a nuclear phase of their life cycles. Members of the papovavirus group, which possess circular DNA genomes organized as nucleosomes, can subvert DNA and RNA polymerases as well as other cellular factors to promote viral gene expression by splicing and terminal modifications of the mRNA in the manner of the host. To initiate their replication, papovaviruses require the host DNA to be in a synthetic phase of the cell cycle. The viral genome expression is favored over that of the host because a sequence at the origin of viral DNA replication can bind avidly the virus-induced T antigen protein which, in turn, has a high binding affinity for host polymerase. Other DNA viruses, including those of the herpes- and adenovirus groups, also initiate their replication by utilizing, to a varying degree, host polymerases. Replication of influenza viruses exemplifies another highly specific, albeit limited, requirement for the cell’s metabolism. These agents possess fragmented RNA genomes of the minus sense which are transcribed into mRNAs by an RNA polymerase carried within the inoculum particles. However, to become functional, the influenza mRNAs must acquire their 5′ terminal caps from among those synthesized inside the nucleus under the aegis of host enzymes. Thus, association of influenza viruses with the nucleus is best explained in terms of transcapping of the viral mRNAs.
Nuclear–Cytoplasmic Exchanges
The mRNA, transfer RNA and ribosomal RNAs of large and small subunits are products of the nucleus which become components of a cytoplasmic translation complex. The individual ribosomal subunits are assembled in the nucleolus, a specialized site of the nucleus, out of ribosomal RNA and numerous types of ribosomal polypeptides, the latter synthesized in the cytoplasm. Compartmentalization of the above synthetic events highlights the necessity for a continual and efficient transfer of transcription products into the cytoplasm and the reverse movement of proteins made in the cytoplasm into the nucleus. Passive transfer of smaller proteins of less than 20 kDa occurs rapidly, within 2 min of synthesis, and that of larger proteins of about 40 kDa takes place more slowly, after perhaps 30 min. Proteins of even greater molecular mass can enter the nucleus only by means of active transport. Such proteins are first bound to specific receptors on the cytoplasmic side of nuclear pores, and are then able to enter into the nucleus because they possess specific signal sequences for import through the pores, as is evident in the case of 90 kDa T antigen of papovavirus. Another function of nuclear–cytoplasmic exchanges in virus infections includes uncoating by injection of adenovirus and baculovirus DNA through nuclear pores (Fig. 2). Cytological evidence for the presence inside nuclei of large numbers of assembled adeno- and herpesvirus particles, sometimes grouped into crystalline arrays, demonstrates that large quantities of viral proteins can enter the nucleus. This type of evidence is supported by biochemical studies which reveal very rapid and efficient intranuclear sequestration of nascent viral polypeptides.
Concluding Remarks
This very brief account of cellular structure and function emphasizes throughout the contribution of the host towards the infectious process. The dependence and close intimacy between viruses and their host cells demonstrates adaptation through evolution by the parasite to take every advantage for self-propagation. The examples used to describe cell organization and metabolism in the context of virology should provide the reader with hints about the use that has been made of viruses as incisive probes of the living cell itself.
See also:
PATHOGENESIS | Animal Viruses; PATHOGENESIS | Plant Viruses; REPLICATION OF VIRUSES; VIRAL RECEPTORS; VIRUS–HOST CELL INTERACTIONS.


