Abstract
Three experiments were conducted to investigate the effects of dietary crude protein (CP) level and N-carbamylglutamate (NCG) supplementation on apparent total tract digestibility (ATTD) and ileal digestibility of nutrients and digestive enzyme activity of jejunum in growing pigs. In experiment 1, 10 Duroc × Landrace × Yorkshire barrows (initial BW: 48.7 kg) were allotted to a three-period switchback design with five experimental diets and two replicate pigs per diet in each period. Diets were categorized as high CP (HP, 18% CP), moderate low CP (MLP, 15% CP), very low CP (VLP, 12% CP), and MLP and VLP with 0.1% NCG supplementation. Feces and urine were collected from day 6 to day 11 after a 5-d adaptation period. The DE, ME, and ATTD of GE, OM, CP, NDF, ADF, and P decreased (P < 0.01) with a reduction of dietary CP, but no effect of dietary treatments on pig daily N retention was detected. The NCG supplementation increased (P < 0.01) DE and ATTD of ADF of the VLP diet. In experiment 2, 10 jejunal-cannulated Duroc × Landrace × Yorkshire barrows (initial BW: 44.5 kg) were fed five diets for three periods as experiment 1. Jejunal fluid was collected on days 6 and 8 after a 5-d adaptation period. The digestive enzymes activity was not affected by dietary CP level, except for α-amylase, for which there was a decrease (P < 0.01) in pigs fed VLP diets compared to HP and MLP diets. In experiment 3, 12 ileal-cannulated Duroc × Landrace × Yorkshire barrows (initial BW: 46.7 kg) were allotted to a three-period switchback design with six diets and two replicate pigs per diet in each period. The six experimental diets consisted of five experimental diets as experiment 1 and one N-free diet. Ileal digesta was collected from day 6 to day 8 after a 5-d adaptation period. Results indicated that apparent ileal digestibility (AID) of CP and P and ileal digestibility of Arg, His, Ile, Leu, Phe, and all dispensable AA, except Pro, decreased (P < 0.01) in pigs fed VLP diet compared to HP and MLP diets, but AID of GE, OM, EE, NDF, and ADF were not affected. The supplementation of NCG in the VLP diet increased (P < 0.01) the AID of CP and ileal digestibility of Arg, His, Leu, Phe, Val, Ser, and Tyr. In conclusion, reducing dietary CP level decreased nutrient digestibility, but improved the efficiency of dietary N utilization and reduced N emission. Moderate reduction of dietary CP level had a minimal effect on nutrient digestibility and digestive enzyme activity. Additionally, NCG supplementation plays a beneficial effect on nutrient digestion only if the dietary CP level is extremely lowered.
Keywords: amino acid, crude protein, digestibility, enzyme activity, growing pig
Introduction
Reducing dietary crude protein (CP) level is an effective way to decrease N excretion in pig production (Jha and Berrocoso, 2016). There is growing interest in low-CP (LP) diets to improve the efficiency of protein resources (Wang et al., 2018). Previous studies have extensively investigated the effects of LP AA balanced diets on growth performance, N utilization, and gut health in growing pigs (Kerr et al., 2003; Nyachoti et al., 2006; Galassi et al., 2010; Gloaguen et al., 2014). However, little is known about the impact of LP diets on nutrient digestion in pigs. An adequate understanding of nutrient digestion for the regulation of protein and AA intake in pigs is crucial for formulating effective LP diets. Specifically, digestive enzymes in the gastrointestinal tract (GIT) are required to break down dietary macronutrients into a form ready for digestion and absorption (He et al., 2016). The chemical essence of the digestive enzyme is protein; theoretically, an inadequate supply of dietary CP may affect enzyme synthesis and activity, resulting in attenuated digestion efficiency (Zhao et al., 2007).
N-carbamylglutamate (NCG), an activator of endogenous Arg synthesis, has been reported to increase mTOR signaling activity and improve protein synthesis in skeletal muscle (Frank et al., 2007; Wu et al., 2010). In growing pigs, 0.1% NCG supplementation in LP diets can improve longissimus dorsi muscle cross-section area and decrease fat deposition (Ye et al., 2017; Wang et al., 2019), primarily through improving the endogenous synthesis of the Arg and the Arg family of AA (Zeng et al., 2015). Arginine has been shown to stimulate muscle protein synthesis of pigs through inducing the phosphorylation of mTOR (Yao et al., 2008). Additionally, decreased concentrations of serum urea N in diets supplemented with NCG indicate higher rates of net protein synthesis (Yang et al., 2013). Therefore, NCG supplementation may improve the efficiency of dietary N utilization, resulting in beneficial effects on N digestibility. However, the effects of NCG supplementation on nutrient digestion in growing pigs have not been determined. One hypothesis was that an LP diet supplemented with NCG may support increased nutrient digestibility and improved dietary N utilization efficiency when fed to growing pigs.
Therefore, the objective of this study was to determine the effect of an LP diet, administered without or with NCG supplementation, on the apparent total tract digestibility (ATTD) and ileal digestibility of nutrients, N balance, and digestive enzyme activity in growing pigs.
Materials and Methods
The protocol for the experiment was reviewed and approved by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China). The study was conducted in the FengNing Swine Research Unit of China Agricultural University (Academician Workstation in Chengdejiuyun Agricultural & Livestock Co., Ltd, Chengde, Hebei, China).
Animals, experimental design, and sample collection
Three experiments were conducted simultaneously in this study.
Experiment 1
Experiment 1 was conducted to determine the effects of reducing dietary CP on the ATTD of nutrients and N balance in growing pigs. Ten crossbred barrows (initial BW: 48.7 ± 3.6 kg; Duroc × Landrace × Yorkshire) were allotted to a three-period switch-back design with five experimental diets and two replicate pigs per diet in each period, resulting in six replicate pigs per diet. No pig received the same diet more than once during the experiment.
The experiment lasted 11 d in each period. The initial 5 d was considered an adaptation period to the diet, whereas feces and urine were collected from 0800 h on day 6 to 0800 h on day 11. The amount of feed refusals and spillage were collected daily before feeding to be dried, weighed, and recorded. Feces samples were stored at −20 °C immediately after collection. Urine was collected in a bucket containing 50 mL of 6 mol/L HCl. The bucket was covered by gauze to prevent solids from contaminating the urine. Each day, the volume of collected urine was measured, and 10% of the daily urine collection was transferred to a screw-capped bottle and stored at −20 °C. At the end of the experiment, feces and urine were thawed, pooled within pigs and period, and homogenized. All fecal samples were dried at 65 °C in a forced air oven for 72 h, weighed, and subsampled. Before analysis, fecal subsamples were finely ground through a 1-mm screen.
Experiment 2
Experiment 2 was conducted to determine the effects of reducing dietary CP on the digestive enzyme activity of jejunum in growing pigs. Ten crossbred barrows (initial BW: 44.5 ± 3.3 kg; Duroc × Landrace × Yorkshire) were surgically equipped with a T-cannula in the upper jejunum 170 cm distal to the pylorus and allotted to a three-period switchback design with five experimental diets and two replicate pigs per diet in each period, resulting in six replicate pigs per diet. The diets were the same as in experiment 1. No pig received the same diet more than once during the experiment.
Each period included 5 d of acclimatization and 3 d of jejunal digesta collection. To minimize the influence of environmental temperature on the digestive enzyme activity of digesta collected by jejunal cannula, a sampling bottle was used as described in detail by Zhao et al. (2007). Briefly, this bottle was placed into a larger bottle, and the section between the two bottles was filled with water and placed at 4 °C for at least 3 h before sampling. Jejunal digesta was collected for 1 h after each feeding on days 6 and 8 of each period. Jejunal fluid was recovered by centrifuging jejunal digesta samples for 10 min at 3000 × g and 4 °C according to the procedure described by Zhao et al. (2007). The supernatant was transferred to a 2-mL centrifuge tube and stored at −20 °C until subjected to digestive enzyme activity assay.
Experiment 3
Experiment 3 was conducted to determine the effects of reducing dietary CP on the ileal digestibility of dietary nutrients in growing pigs. Twelve crossbred barrows (initial BW: 46.7 ± 3.8 kg; Duroc × Landrace × Yorkshire) were surgically equipped with a T-cannula in the distal ileum and allotted to a three-period switchback design with six diets and two replicate pigs per diet in each period, resulting in six replicate pigs per diet. No pig received the same diet more than once during the experiment.
The six experimental diets consisted of the five experimental diets used in experiment 1 plus an N-free diet. Chromic oxide (0.3%) was supplemented as an indigestible marker in all diets to determine nutrient digestibility using the indicator method (Li et al., 2015).
Each experimental period included 5 d of acclimatization and 3 d of ileal digesta collection. Ileal digesta was collected into a plastic bag attached to the cannula barrel from 0800 to 1800 h. Bags were replaced as soon as they filled with digesta, or at least once every 30 min, and were frozen at −20 °C upon collection to prevent bacterial degradation of AA in the digesta. At the end of the experiment, collected ileal digesta samples were thawed and pooled within the experimental period and pig, and a subsample was collected for chemical analysis. Ileal digesta samples were freeze-dried and ground to pass through a 0.5-mm screen before analysis.
Diets and feeding
The five experimental diets consisted of decreasing CP content: a high-CP (HP, 18% CP) diet, a moderate LP (MLP, 15% CP) diet, a very LP (VLP, 12% CP) diet, and MLP or VLP diet supplemented with 0.1% NCG (MLP + NCG or VLP + NCG). All diets were formulated to contain similar concentrations of standardized ileal digestible (SIDI) Lys, Met + Cys, Thr, and Trp, and NE as the recommendations of NRC (2012). The content of SIDI Val in the HP diet was considerably more than needed for growing pigs, and L-Val was supplemented in LP diets to meet the requirements for 50.0 kg growing pigs of NRC (2012). The same batches of corn, soybean meal, rapeseed meal, and wheat bran were used to prepare all diets (Table 1). The N-free diet in experiment 3 was used to determine endogenous losses of N.
Table 1.
Ingredient composition of experimental diets (%, as-fed basis)
| Item | HP | MLP | VLP | N-free diet |
|---|---|---|---|---|
| Corn | 71.42 | 75.68 | 79.67 | – |
| Soybean meal | 18.95 | 9.17 | – | – |
| Rapeseed meal | 3.10 | 4.00 | 4.24 | – |
| Wheat bran | 3.70 | 7.67 | 11.95 | – |
| Cornstarch | – | – | – | 73.50 |
| Sucrose | – | – | – | 15.00 |
| Soybean oil | – | – | – | 3.00 |
| Cellulose acetate | – | – | – | 4.00 |
| l-Lys·HCl | 0.28 | 0.53 | 0.78 | – |
| dl-Met | 0.01 | 0.08 | 0.15 | – |
| l-Thr | 0.05 | 0.16 | 0.27 | – |
| l-Trp | – | 0.04 | 0.08 | – |
| l-Val | – | 0.12 | 0.25 | – |
| Dicalcium phosphate | 0.59 | 0.58 | 0.57 | 2.50 |
| Limestone | 1.10 | 1.17 | 1.24 | 0.50 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Potassium carbonate | – | – | – | 0.30 |
| Chromic oxide1 | – | – | – | 0.30 |
| Magnesium oxide | – | – | – | 0.10 |
| Vitamin and mineral premix2 | 0.50 | 0.50 | 0.50 | 0.50 |
| Calculated composition3 | ||||
| CP | 18.00 | 15.00 | 12.00 | – |
| NE4, kcal/kg | 2480 | 2480 | 2480 | – |
| SIDI5 Lys | 0.85 | 0.85 | 0.85 | – |
| SIDI Met + Cys | 0.48 | 0.48 | 0.48 | – |
| SIDI Thr | 0.52 | 0.52 | 0.52 | – |
| SIDI Trp | 0.15 | 0.15 | 0.15 | – |
| SIDI Val | 0.64 | 0.60 | 0.60 | – |
1All the experimental diets were supplemented with 0.30% chromic oxide for experiment 3.
2Provided the following quantities of vitamins and micro-minerals per kilogram of complete diet: vitamin A as retinyl acetate, 8500 IU; vitamin D3 as cholecalciferol, 850 IU; vitamin E as dl-alpha-tocopheryl acetate, 55 IU; vitamin K3 as menadione nicotinamide bisulfite, 4 mg; vitamin B12, 50 μg; riboflavin, 10 mg; pantothenic acid as dl-calcium pantothenate, 20 mg; niacin, 30 mg; choline chloride, 600 mg; folacin, 2 mg; vitamin B1 as thiamine mononitrate, 1 mg; vitamin B6 as pyridoxine hydrochloride, 3 mg; biotin, 4 mg; Mn as MnO, 25 mg; Fe as FeSO4·H2O, 100 mg; Zn as ZnSO4, 100 mg; Cu as CuSO4·5H2O, 50 mg; I as KI, 0.5 mg; Se as Na2SeO3, 0.15 mg.
3Calculated from the analyzed composition of ingredients and adjusted for 88.0% DM.
4Values for NE content of ingredients are calculated from analyzed chemical composition of ingredients (Noblet et al., 1994).
5Values for SIDI AA of ingredients are calculated from the analyzed AA contents and SID AA of the ingredients (NRC, 2012).
Pigs were housed individually in stainless-steel metabolism (1.5 m × 0.7 m × 0.8 m) crates equipped with a water nipple and a feeding trough and placed in an environmentally controlled room (25 ± 2 °C). All pigs were fed at 3.4 times the maintenance energy requirement daily (i.e., 197 kcal of ME/kg BW0.60; NRC, 2012) and had free access to water throughout the experiment. In each experiment, the daily feed allotments were divided into two equal meals at 0800 and 1600 h. Pig weights were recorded at the beginning and conclusion of the experiment.
Chemical analysis
Diets, ileal digesta, and fecal samples were analyzed for DM (Method 930.15; AOAC International, 2007), ash (Method 942.05; AOAC International, 2007), CP (Method 990.03; AOAC International, 2007), starch (Method 948.02; AOAC International, 2007), and P (Method 975.03; AOAC, 2007). Gross energy was analyzed using an isoperibol bomb calorimeter (Model 6400, Parr Instruments, Moline, IL) using benzoic acid as the internal standard. The ether extract was determined using the petroleum ether extraction method with an automated analyzer (Ankom XT15 Extractor; Ankom Technology, Macedon, NY). The NDF and ADF contents in the samples were determined using a fiber analyzer (Model A220 fiber analyzer; Ankom Technology, Macedon, NY) and filter bags following a modification of the procedure of van Soest et al. (1991). The concentration of NDF was analyzed using heat-stable α-amylase and sodium sulfite without correction for insoluble ash. Urine was analyzed for CP using the Kjeldahl method.
Diets and ileal digesta were also analyzed for chromium and AA concentrations. Chromium concentration was estimated using a polarized Zeeman Atomic Absorption Spectrometer (Hitachi Z2000, Tokyo, Japan) after nitric acid–perchloric acid wet ash sample preparation (Li et al., 2015). Within the exception of tryptophan, AA contents were determined using an Amino Acid Analyzer (Hitachi L-8900, Tokyo, Japan). The samples were hydrolyzed with 6 mol/L HCl at 110 °C for 24 h before analysis. The Met and Cys were analyzed after cold performic acid oxidation overnight and hydrolyzed with 7.5 mol/L HCl at 110 °C for 24 h. Tryptophan was determined after hydrolysis with LiOH at 110 °C for 22 h using High-Performance Liquid Chromatography (Agilent 1200 Series, Santa Clara, CA).
The digestive enzyme activity of jejunum was measured by spectrophotometry. The α-amylase activity was determined with soluble starch as a substrate using the method described by Dahlqvist (1962). Trypsin and chymotrypsin activity was determined with Nα-p-toluolsulfonyl-l-arginine methyl ester hydrochloride and N-benzoyl-l-tyrosine ethyl ester as substrate, respectively, using the method described by Wirnt (1974a, 1974b). The activity of lipase (catalog number A054-1-1), lactase (catalog number A082-1-1), sucrase (catalog number A082-2-1), and maltase (catalog number A082-3-1) was determined using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) according to the manufacturer’s protocols. All digestive enzyme activities were expressed in U per microgram of total protein (U/mg protein) of the jejunal fluid, and the concentration of total protein was determined with a BCA Protein Assay Kit (Pierce Thermo Fisher Scientific, Thermo Scientific, catalog number 23227).
Calculation and statistical analysis
The ATTD (%) of nutrients was calculated for each diet according to the following equation (She et al., 2018):
where Nutrin and Nutrout are the nutrient intake in diet DM and output in feces DM, respectively.
The values for DE and ME were calculated by subtracting the GE analyzed in feces or feces and urine from the GE in the diets, respectively (NRC, 2012).
The retention (%) of N was calculated using the following equation:
where Nr is the retention (%) of N, Ni is the intake (g) of N, and Nf and Nu are the fecal and urinary loss (g) of N, respectively.
The apparent ileal digestibility (AID) (%) of nutrients, standardized ileal digestibility (SID) (%) of AA, and basal endogenous of AA (IAAend, g/kg of DMI) were calculated using the following equation (Stein et al., 2007):
where Crd and Crid are the chromium concentration in diet and ileal digesta DM, respectively; Nid and Nd are the nutrient concentration in ileal digesta and diet DM, respectively; AAd is the AA concentration of the diet.
All statistical analyses were performed using the SAS program (SAS 9.4 software; SAS Inst. Inc., Cary, NC). Outliers were identified and eliminated as values that deviated from the treatment mean by more than three times the interquartile range. Data were analyzed using PROC MIXED in a randomized complete design with a pig as the experimental unit for all analyses. The statistical model included diet as the fixed effects and pig and period as random effects. Treatment means were calculated using the LSMEANS statement, and differences among means were separated using the PDIFF option with Tukey’s adjustment. Statistical significance and tendency were defined at P < 0.05 and 0.05 ≤ P < 0.10, respectively.
Results
Nutrient composition of diets
The concentrations of nutrients in all diets were close to expected values (Tables 1 and 2). The analyzed concentration of CP in the HP diet was 0.9 percentage units less than expected, and that in the MLP and VLP diets were 0.9 and 2.4 percentage units higher than expected, respectively, but it was assumed that this small difference did not affect the results. Additionally, as expected, the N and AA contents in the N-free diet were observed in only small amounts.
Table 2.
Analyzed nutrient composition of experimental diets (%, as-fed basis)
| Item | HP | MLP | VLP | N-free diet |
|---|---|---|---|---|
| DM | 89.32 | 89.41 | 89.47 | 90.43 |
| GE, kcal/kg | 4013 | 3986 | 3932 | 3763 |
| CP | 17.83 | 15.14 | 12.29 | – |
| EE | 3.09 | 3.07 | 3.01 | 1.78 |
| OM1 | 95.72 | 95.90 | 9 | 96.56 |
| Starch | 39.60 | 44.08 | 48.89 | 65.14 |
| NDF | 13.59 | 13.98 | 13.22 | 4.94 |
| ADF | 5.02 | 4.85 | 4.79 | 1.69 |
| P | 0.50 | 0.51 | 0.51 | 0.43 |
| Indispensable AA | ||||
| Arg | 1.09 | 0.88 | 0.60 | – |
| His | 0.51 | 0.44 | 0.34 | – |
| Ile | 0.77 | 0.56 | 0.39 | – |
| Leu | 1.61 | 1.45 | 1.12 | – |
| Lys | 1.14 | 1.13 | 1.12 | – |
| Met | 0.31 | 0.33 | 0.36 | – |
| Phe | 0.87 | 0.67 | 0.49 | – |
| Thr | 0.69 | 0.70 | 0.67 | – |
| Trp | 0.16 | 0.18 | 0.17 | – |
| Val | 0.90 | 0.81 | 0.80 | – |
| Dispensable AA | ||||
| Ala | 0.98 | 0.82 | 0.70 | – |
| Asp | 1.58 | 1.24 | 0.70 | – |
| Cys | 0.26 | 0.22 | 0.18 | – |
| Glu | 2.94 | 2.54 | 1.93 | – |
| Gly | 0.71 | 0.61 | 0.45 | – |
| Pro | 1.45 | 1.34 | 1.10 | – |
| Ser | 0.77 | 0.63 | 0.45 | – |
| Tyr | 0.59 | 0.45 | 0.36 | – |
1OM = (DM – ash)/DM × 100%, DM basis.
ATTD of nutrients in diets
The DE and ME content, as well as the ATTD of GE, OM, CP, EE, NDF, ADF, and P, differed among the dietary treatments (P < 0.01; Table 3). Regardless of NCG supplementation groups, the nutrient digestibility decreased (P < 0.05) with the reduction of the dietary CP content. Supplementation of NCG in the VLP diet increased (P < 0.05) the DE and ATTD of ADF compared with the VLP diet, but no effects of NCG supplementation on the ATTD of these parameters in the MLP diet were detected.
Table 3.
Effects of reducing dietary CP content and NCG supplementation on ATTD of nutrients when fed to growing pigs1 (%, experiment 1)
| MLP | VLP | ||||||
|---|---|---|---|---|---|---|---|
| Item | HP | −NCG | +NCG | −NCG | +NCG | SEM | P-value |
| DE, kcal/kg | 3544a | 3411b | 3398b | 3261d | 3327c | 20 | <0.01 |
| ME, kcal/kg | 3442a | 3303b | 3276b | 3163c | 3223bc | 22 | <0.01 |
| ATTD | |||||||
| GE | 88.31a | 85.58b | 85.52b | 82.93c | 84.67bc | 0.49 | <0.01 |
| OM | 90.66a | 88.10b | 88.22b | 85.98c | 87.59bc | 0.42 | <0.01 |
| CP | 88.84a | 86.52ab | 86.48ab | 82.16c | 84.51bc | 0.80 | <0.01 |
| EE | 54.06a | 52.41a | 51.59a | 46.23b | 50.73ab | 1.48 | 0.01 |
| NDF | 67.86a | 60.82ab | 61.05ab | 48.06c | 54.90bc | 2.18 | <0.01 |
| ADF | 73.09a | 59.81b | 63.34b | 47.62c | 56.20b | 1.79 | <0.01 |
| P | 48.68a | 45.03ab | 45.30ab | 41.39b | 44.88ab | 0.98 | <0.01 |
1Data are means of six observations per treatment.
a–cMeans in a row with different superscripts are different (P < 0.05).
Nitrogen balance
Nitrogen intake, urinary N, and total N losses were greater (P < 0.01) in pigs fed the HP diet compared with pigs fed the other diets (Table 4). However, no differences among diets in fecal excretion of N were observed. Collectively, there was no effect of dietary treatment on pig daily N retention. Therefore, the N retention to intake ratio was greater (P < 0.01) in pigs fed the MLP and VLP diets compared with pigs fed the HP diet with no difference observed between MLP and VLP diets. Unfortunately, NCG supplementation in MLP and VLP diets did not affect N retention.
Table 4.
Effects of reducing dietary CP content and NCG supplementation on N balance when fed to growing pigs1 (g/d, experiment 1)
| MLP | VLP | ||||||
|---|---|---|---|---|---|---|---|
| Item | HP | −NCG | +NCG | −NCG | +NCG | SEM | P-value |
| N intake | 55.78a | 49.41b | 49.44b | 40.29c | 40.35c | 0.78 | <0.01 |
| Fecal N losses | 6.23 | 6.66 | 6.68 | 7.19 | 6.25 | 0.37 | 0.36 |
| Urinary N losses | 22.88a | 16.04b | 14.71b | 9.54c | 9.65c | 0.64 | <0.01 |
| Total N losses | 29.11a | 22.70b | 21.39b | 16.73c | 15.90c | 0.60 | <0.01 |
| N retention | 26.67 | 26.71 | 28.05 | 23.56 | 24.45 | 1.10 | 0.05 |
| N retention/intake, % | 47.66b | 54.03ab | 56.71a | 58.42a | 60.41a | 1.61 | <0.01 |
1Data are means of six observations per treatment.
a–cMeans in a row with different superscripts are different (P < 0.05).
Digestive enzyme activities of the jejunum
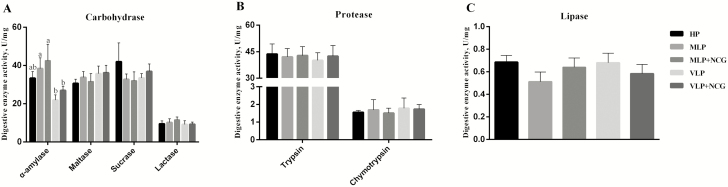
The α-amylase activity of jejunum from pigs fed VLP diet was lower (P < 0.01) than pigs fed other diets (Figure 1A), but there were no differences in the activities of trypsin, chymotrypsin, lipase, maltase, sucrase, and lactase among dietary treatments (Figure 1).
Figure 1.
Effects of reducing dietary CP content and NCG supplementation on carbohydrase (A), protease (B), and lipase (C) activity of jejunum when fed to growing pigs (experiment 2). Values are presented as means ± SEM, n = 6. a,bMeans without common letters differ at P < 0.05.
AID of nutrients in diets
There were no differences among diets in the AID of GE, OM, EE, NDF, and ADF (Table 5). The AID of CP decreased (P < 0.01) with CP reduction, but pigs fed the VLP + NCG diet had greater (P < 0.05) AID of CP than pigs fed the VLP diet. Pigs fed the HP diet had greater (P < 0.01) AID of P than pigs fed the MLP and VLP diets, but no differences were observed between the MLP and VLP diets.
Table 5.
Effects of reducing dietary CP content and NCG supplementation on AID of nutrients when fed to growing pigs1 (%, experiement 3)
| MLP | VLP | ||||||
|---|---|---|---|---|---|---|---|
| Item | HP | −NCG | +NCG | −NCG | +NCG | SEM | P-value |
| GE | 73.77 | 71.87 | 71.56 | 71.89 | 71.63 | 0.85 | 0.35 |
| OM | 75.89 | 74.45 | 74.34 | 75.01 | 74.97 | 0.75 | 0.61 |
| CP | 76.24a | 74.43ab | 73.92ab | 69.36c | 71.91b | 0.92 | <0.01 |
| EE | 61.67 | 60.63 | 60.21 | 59.24 | 61.91 | 2.36 | 0.93 |
| NDF | 33.44 | 27.59 | 28.34 | 30.44 | 29.14 | 2.67 | 0.57 |
| ADF | 20.95 | 18.71 | 19.92 | 20.27 | 22.06 | 2.68 | 0.93 |
| P | 43.09a | 37.91b | 38.81b | 36.55b | 38.86ab | 1.03 | <0.01 |
1Data are means of six observations per treatment.
a–cMeans in a row with different superscripts are different (P < 0.05).
AID and SID of AA in diets
For indispensable AA, no difference in AID (Table 6) and SID (Table 7) of Lys, Met, Thr, and Trp was observed among dietary treatments. Regardless of NCG supplementation groups, pigs fed the HP and MLP diets had greater (P < 0.01) AID and SID of Arg, His, Ile, Leu, and Phe than pigs fed the VLP diet, but there was no difference between the HP and MLP diets. With NCG supplementation, pigs fed the VLP + NCG diet showed greater (P < 0.01) AID and SID of Arg, His, Leu, Phe, and Val than pigs fed the VLP diet. For most dispensable AA, pigs fed the VLP diet had lower (P < 0.01) AID and SID of AA than pigs fed the HP and MLP diets, except for Pro, regardless of NCG supplementation groups. Supplementation of NCG in VLP diets improved (P < 0.01) AID and SID of Ser and Tyr. Overall, the AID and SID of all AA did not differ between the HP and MLP diets but were higher (P < 0.01) than the values obtained with the VLP diet.
Table 6.
Effects of reducing dietary CP content and NCG supplementation on AID of AA when fed to growing pigs1 (%, experiment 3)
| MLP | VLP | ||||||
|---|---|---|---|---|---|---|---|
| Item | HP | −NCG | +NCG | −NCG | +NCG | SEM | P-value |
| Indispensable AA | |||||||
| Arg | 86.45a | 83.50a | 83.20a | 74.19c | 79.29b | 1.00 | <0.01 |
| His | 82.48a | 82.47a | 81.38a | 73.09b | 79.24a | 1.13 | <0.01 |
| Ile | 81.26a | 78.31a | 77.55a | 65.10b | 68.69b | 1.31 | <0.01 |
| Leu | 82.75a | 82.20a | 81.83a | 75.78c | 79.02b | 0.89 | <0.01 |
| Lys | 83.99 | 83.56 | 83.54 | 82.97 | 84.05 | 0.59 | 0.71 |
| Met | 84.96 | 84.67 | 84.47 | 84.92 | 85.45 | 0.62 | 0.84 |
| Phe | 81.61a | 81.02a | 78.95ab | 69.67c | 75.40b | 0.97 | <0.01 |
| Thr | 75.52 | 75.98 | 75.59 | 73.70 | 75.63 | 0.69 | 0.18 |
| Trp | 72.94 | 74.21 | 72.93 | 72.13 | 74.21 | 0.86 | 0.38 |
| Val | 77.92ab | 76.65b | 76.91ab | 76.00b | 78.66a | 0.59 | 0.03 |
| Mean | 80.99a | 80.25ab | 79.64ab | 74.75c | 77.96b | 0.64 | <0.01 |
| Dispensable AA | |||||||
| Ala | 77.32a | 76.17a | 75.54ab | 68.73b | 70.95bc | 1.12 | <0.01 |
| Asp | 79.40a | 75.97a | 75.32a | 62.84b | 66.69b | 1.10 | <0.01 |
| Cys | 73.94a | 71.52a | 70.75a | 63.77b | 66.51ab | 1.80 | <0.01 |
| Glu | 83.89a | 83.64a | 83.88a | 77.31b | 80.28ab | 0.88 | <0.01 |
| Gly | 69.08a | 69.06a | 65.85ab | 55.60c | 60.04bc | 1.56 | <0.01 |
| Pro | 80.33 | 79.55 | 79.15 | 76.22 | 77.41 | 1.19 | 0.13 |
| Ser | 77.22a | 76.53a | 75.16a | 65.44b | 73.96a | 1.07 | <0.01 |
| Tyr | 79.95a | 78.78ab | 78.89ab | 67.80c | 73.87b | 1.37 | <0.01 |
| Mean | 77.64a | 76.40a | 75.54a | 67.22c | 71.21b | 0.88 | <0.01 |
| All AA | 79.50a | 78.54a | 77.82ab | 71.40c | 74.96b | 0.70 | <0.01 |
1Data are least squares means of six observations per treatment.
a–cMeans in a row with different superscripts are different (P < 0.05).
Table 7.
Effects of reducing dietary CP content and NCG supplementation on SID of nutrients when fed to growing pigs1 (%, experiment 3)
| MLP | VLP | ||||||
|---|---|---|---|---|---|---|---|
| Item | HP | −NCG | +NCG | −NCG | +NCG | SEM | P-value |
| Indispensable AA | |||||||
| Arg | 90.35a | 87.98ab | 87.74ab | 81.34c | 85.85b | 1.00 | <0.01 |
| His | 85.81a | 85.89a | 84.92a | 77.76b | 83.81a | 1.13 | <0.01 |
| Ile | 85.50a | 83.40a | 82.71ab | 73.25c | 77.30bc | 1.31 | <0.01 |
| Leu | 85.78a | 85.36a | 84.99a | 79.92b | 83.25a | 0.89 | <0.01 |
| Lys | 87.76 | 87.20 | 87.19 | 86.81 | 87.89 | 0.59 | 0.69 |
| Met | 87.25 | 86.86 | 86.65 | 86.93 | 87.44 | 0.62 | 0.90 |
| Phe | 85.24a | 84.98a | 83.06a | 75.65b | 81.32a | 0.97 | <0.01 |
| Thr | 82.57 | 82.48 | 82.05 | 80.96 | 82.88 | 0.69 | 0.35 |
| Trp | 80.44 | 80.75 | 79.48 | 79.32 | 81.40 | 0.86 | 0.41 |
| Val | 82.67ab | 81.52ab | 81.78ab | 80.94b | 83.60a | 0.59 | 0.03 |
| Mean | 85.34a | 84.64a | 84.06a | 80.29b | 83.47a | 0.64 | <0.01 |
| Dispensable AA | |||||||
| Ala | 82.65a | 81.44a | 81.01a | 75.43b | 78.30ab | 1.12 | <0.01 |
| Asp | 83.93a | 81.49a | 80.98a | 72.50b | 76.32b | 1.10 | <0.01 |
| Cys | 78.72a | 76.96ab | 76.19ab | 70.39b | 73.19ab | 1.80 | 0.02 |
| Glu | 86.83a | 86.79a | 87.06a | 81.70b | 84.58ab | 0.88 | <0.01 |
| Gly | 77.90a | 78.36a | 75.63a | 68.45b | 73.28ab | 1.56 | <0.01 |
| Pro | 92.31 | 91.50 | 91.04 | 90.50 | 92.09 | 1.19 | 0.81 |
| Ser | 83.38a | 82.76a | 81.64a | 75.15b | 82.38a | 1.07 | <0.01 |
| Tyr | 84.72a | 83.77a | 83.92a | 75.96b | 81.24a | 1.37 | <0.01 |
| Mean | 83.80a | 82.88a | 82.18a | 76.26b | 80.17a | 0.88 | <0.01 |
| All AA | 84.65a | 83.86a | 83.22a | 78.50b | 82.00a | 0.70 | <0.01 |
1Data are means of six observations per treatment.
a–cMeans in a row with different superscripts are different (P < 0.05).
Discussion
A previous study by our group found a lower gain to feed ratio during the growing phase when pigs were fed LP diets compared with HP diets under commercial conditions, possibly due to lower ATTD of nutrients in LP diets (Wang et al., 2019). However, the effect of reduced CP level on nutrient digestion in different intestinal segments has not been specifically studied in growing pigs.
It is generally assumed that diet CP content does not influence nutrient digestion in pigs and hence not considered in diet formulations. However, in the present study, there was a decrease in DE, ME, and ATTD of GE with decreasing dietary CP level for growing pigs formulated with the same dietary NE content. The DE or ME system used in preparing LP diets may underestimate the true available energy for pigs, resulting in increased fat deposition compared to control diets with similar dietary DE or ME content (Noblet et al., 1994). As an effective solution, the NE system has been confirmed to be suitable for preparing LP diets because NE is the only system in which energy requirements of pigs and available energy of feed ingredients can be expressed on the same basis (Le Bellego et al., 2001). Theoretically, similar dietary NE content in LP and control diets means lower DE and ME content in LP diets, for DE or ME system overestimates the available energy of protein and simultaneously underestimates starch energy availability (Wang et al., 2018). Decreased energy digestibility was also observed by Bikker et al. (2006) in weanling piglets, Lei et al. (2019) in growing pigs, and O’Connell et al. (2006) in finishing pigs when reducing the dietary CP level, although LP diets contained the same SIDI Lys, Met, Thr, and Trp as control diets. However, Qiu et al. (2018) reported no difference in the ATTD of energy when dietary CP was reduced from 18% to 14% in growing pigs. The discrepancy in the response of energy digestibility in diets with different CP levels among studies may be due to different types and amounts of crystalline AA supplemented in diets. In the study of Qiu et al. (2018), all indispensable AA were supplemented to ensure the experimental diets provided similar SIDI AA, but this study was just supplemented l-Lys, l-Thr, dl-Met, l-Trp, and l-Val in LP diets to meet the requirements for pigs, and other indispensable AA would be insufficient and affect nutrients digestion. Additionally, the ATTD of CP was reduced a total of 6% from HP to VLP diets. However, N retention per day remained similar among dietary treatments, which was attributed to a 7% to 8% reduction in total N excretion for every 10 g/kg reduction in dietary CP concentration (Wang et al., 2018). The present data also showed a decrease in the ATTD of OM, EE, NDF, ADF, and P with a declining dietary CP level. One plausible explanation for this response might be the higher content of rapeseed meal in LP diets compared to the HP diet. The digestibility of nutrients in rapeseed meal is less than that in corn or soybean meal, which provides a possible explanation for the reduced ATTD of nutrients in LP diets (Li et al., 2017). Additionally, the digestive or fermentative capability at different sites of GIT might be affected by the CP level (Portune et al., 2016).
Dietary nutrient digestibility is positively associated with the activity of digestive enzymes (Guzmán-Pino et al., 2014). Several studies of pigs have suggested that activity of the predominate digestive enzymes in the jejunum including carbohydrase (α-amylase, maltase, sucrase, and lactase), protease (trypsin and chymotrypsin), and lipase are reflective of the digestive capacity of the small intestine (Zhao et al., 2007; Tian et al., 2016). Yue and Qiao (2008) demonstrated that reducing dietary CP changed gut morphology and decreased the disaccharidase activities of the proximal jejunum in weaned piglets, resulting in negative effects on the growth rate. However, no difference was observed in growing and finishing pigs (He et al., 2016). In the current study, the digestive enzyme activity did not vary with different CP levels, except α-amylase. This phenomenon indicates that the digestive capacity of endogenous enzymes can adapt to a wide range in dietary CP level in growing pigs (He et al., 2016). Unexpectedly, the α-amylase was lower in pigs fed the VLP diet compared to pigs fed the HP and MLP diets, even though the VLP diet had higher starch concentration to stimulate the secretion of α-amylase, speculate there may have been insufficient AA supply for endogenous synthesis of α-amylase in VLP diet fed pigs. This speculation is consistent with the results of He et al. (2016) who indicated that the mRNA level for α-amylase was higher in pigs fed the 13% or 16% CP diet than in pigs fed the 10% CP diet. To some extent, the reduced α-amylase activity may explain the lower digestibility of nutrients in the VLP diet. These results suggest that reducing dietary CP content by 3 percentage units can maintain the activity of digestive enzymes for pigs. However, a further reduction by 6 percentage units and supplemented only with the first five indispensable AA, the AA or N supply would be insufficient for the de novo synthesis of endogenous enzymes and extremely impaired the digestive capacities (Bikker et al., 2006).
We investigated the effects of dietary CP level on AID of nutrients. In the present study, reducing CP content decreased the AID of P, which is consistent with the findings of Xue et al. (2017), who reported that there was a limiting effect of decreased CP levels on ileal P digestion in growing pigs. The decreased ileal P digestion caused by reducing CP content might be that the limited AA intake impaired the synthesis or gene expression of NaPi-IIb, the main transporter in the P digestion system (Xue et al., 2016). As expected, the change in AID of P with dietary CP level was consistent with the change in ATTD of P because the majority of dietary P is digested and absorbed in the stomach and small intestine in growing pigs (Liu et al., 2018). For CP digestion, the AID of CP can be maintained when dietary CP is reduced by 3 percentage units. However, when dietary CP is reduced by 6 percentage units, the AID of CP decreased even though the activity of protease was not impaired, which is consistent with the variation in AID or SID of all AA. These results suggested that a 6 percentage unit reduction of dietary CP, supplemented with the first five indispensable AA, led to an imbalance of AA, resulting in an insufficient supply of N and poor digestive capacity of the intestine (O’Connell et al., 2006; Adebiyi et al., 2015). This effect may contribute to the decreased CP and AA digestibility in the VLP diet. Additionally, dietary CP level did not affect AID of GE, OM, EE, NDF, and ADF in the current study, which implied that the difference in the ATTD of nutrients mainly varied in the large intestine. Two factors likely played a role in the alterations in digestibility presumed to have occurred in the large intestine. First, the bacterial fermentation capabilities might be impaired by the LP diets (Lei et al., 2019), and second, more endogenous losses of nutrients in the large intestine of LP diet fed pigs compared to HP diet fed pigs (O’Connell et al., 2006). Future research is warranted to test this supposition.
In the current study, we observed that 0.1% NCG supplementation in VLP diet greatly improved DE, ATTD of ADF, it may be due to the improved fermentative capability of microbiome in the large intestine, for no difference on AID of energy and carbohydrate was observed, but the effect of NCG supplementation on microbiome need further research. The higher AID of CP and ileal digestibility of most AA observed in VLP supplemented with NCG attributed to the increased endogenous AA synthesis and muscle protein accretion in pigs (Zeng et al., 2015; Ye et al., 2017). Additionally, Wu et al. (2010) reported that NCG supplementation increased the relative weight of the small intestine and intestinal morphology of pigs, which is beneficial for intestinal digestion. However, in this study we have found that NCG supplementation in the LP diet had limited effects on nutrient digestibility, it might be the content of AA, especially Arg and Arg family of AA in LP diet was sufficient to meet the requirement of pigs. Unfortunately, no effect of NCG supplementation on the activity of digestive enzymes was observed in the current study is likely because the digestive tract of growing pigs is well developed and not easily affected by NCG supplementation (Kiarie et al., 2016).
In conclusion, reducing dietary CP level by 6 percentage units decreased ATTD of nutrients, α-amylase activity, AID of CP and P, ileal digestibility of most AA, except for Lys, Thr, Met, Trp, Val, and Pro, but improved the efficiency of dietary N utilization and reduced the N output in the urine. Moderate reduction of dietary CP level within 3 percentage units decreased ATTD of nutrients and AID of P, but had no effects on ileal digestibility of other nutrients, implied the fermentative capability of microbiome in large intestine may be responsible for the differences in ATTD of nutrients. Additionally, NCG supplementation only in the VLP diet had beneficial effects on nutrient digestion. Taken together, the effects of dietary CP level on ATTD of nutrients should be considered in preparing the dietary formula.
Acknowledgments
This study was financially supported by Beijing Advanced Innovation Center for Food Nutrition and Human Health of China Agricultural University, Special Funds for Basic Scientific Research Operating Expenses of China Agricultural University (2019TC139), and Beijing Swine Innovation Team of Modern Agriculture Industry Technological System, Financial Projects of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (1619007).
Glossary
Abbreviations
- AID
apparent ileal digestibility
- ATTD
apparent total tract digestibility
- CP
crude protein
- GIT
gastrointestinal tract
- HP
high crude protein
- LP
low crude protein
- MLP
moderate low crude protein
- NCG
N-carbamylglutamate
- SID
standardized ileal digestibility
- VLP
very low crude protein
Conflict of interest statement
All authors have no conflicts of interest.
Literature Cited
- Adebiyi A. O., Ragland D., Adeola O., and Olukosi O. A.. . 2015. Apparent or standardized ileal digestibility of amino acids of diets containing different protein feedstuffs fed at two crude protein levels for growing pigs. Asian-Australas. J. Anim. Sci. 28:1327–1334. doi: 10.5713/ajas.14.0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC International. 2007. Official methods of analysis. 18th ed.Hortwitz W. and Latimer G. W. Jr, editors. Gaithersburg (MD): AOAC International. [Google Scholar]
- Bikker P., Dirkzwager A., Fledderus J., Trevisi P., le Huërou-Luron I., Lallès J. P., and Awati A.. . 2006. The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. J. Anim. Sci. 84:3337–3345. doi: 10.2527/jas.2006-076 [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. 1962. A method for the determination of amylase in intestinal content. Scand. J. Clin. Lab. Invest. 14:145–151. doi: 10.3109/00365516209079686 [DOI] [PubMed] [Google Scholar]
- Frank J. W., Escobar J., Nguyen H. V., Jobgen S. C., Jobgen W. S., Davis T. A., and Wu G.. . 2007. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J. Nutr. 137:315–319. doi: 10.1093/jn/137.2.315 [DOI] [PubMed] [Google Scholar]
- Galassi G., Colombini S., Malagutti L., Crovetto G. M., and Rapetti L.. . 2010. Effects of high fibre and low protein diets on performance, digestibility, nitrogen excretion and ammonia emission in the heavy pig. Anim. Feed Sci. Technol. 161:140–148. doi: 10.1016/j.anifeedsci.2010.08.009 [DOI] [Google Scholar]
- Gloaguen M., Le Floc’h N., Corrent E., Primot Y., and van Milgen J.. . 2014. The use of free amino acids allows formulating very low crude protein diets for piglets. J. Anim. Sci. 92:637–644. doi: 10.2527/jas.2013-6514 [DOI] [PubMed] [Google Scholar]
- Guzmán-Pino S. A., Solà-Oriol D., Figueroa J., and Pérez J. F.. . 2014. Influence of the protein status of piglets on their ability to select and prefer protein sources. Physiol. Behav. 129:43–49. doi: 10.1016/j.physbeh.2014.02.029 [DOI] [PubMed] [Google Scholar]
- He L., Wu L., Xu Z., Li T., Yao K., Cui Z., Yin Y., and Wu G.. . 2016. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids 48:21–30. doi: 10.1007/s00726-015-2059-1 [DOI] [PubMed] [Google Scholar]
- Jha R., and Berrocoso J. F. D.. . 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. Technol. 212:18–26. doi: 10.1016/j.anifeedsci.2015.12.002 [DOI] [Google Scholar]
- Kerr B. J., Yen J. T., Nienaber J. A., and Easter R. A.. . 2003. Influences of dietary protein level, amino acid supplementation and environmental temperature on performance, body composition, organ weights and total heat production of growing pigs. J. Anim. Sci. 81:1998–2007. doi: 10.2527/2003.8181998x [DOI] [PubMed] [Google Scholar]
- Kiarie E., Walsh M. C., and Nyachoti C. M.. . 2016. Performance, digestive function, and mucosal responses to selected feed additives for pigs. J. Anim. Sci. 94(suppl 3):169–180. doi: 10.2527/jas.2015-9835 [DOI] [Google Scholar]
- Le Bellego L., van Milgen J., Dubois S., and Noblet J.. . 2001. Energy utilization of low-protein diets in growing pigs. J. Anim. Sci. 79:1259–1271. doi: 10.2527/2001.7951259x [DOI] [PubMed] [Google Scholar]
- Lei X. J., Lee S. I., and Kim I. H.. . 2019. Effects of different levels of dietary protein with or without plant extract YGF251 on growth performance, nutrient digestibility, blood profiles, fecal microbial shedding, and fecal gas emission in growing pigs. Anim. Sci. J. 90:547–553. doi: 10.1111/asj.13162 [DOI] [PubMed] [Google Scholar]
- Li Z., Li Y., Lyu Z., Liu H., Zhao J., Noblet J., Wang F., Lai C., and Li D.. . 2017. Net energy of corn, soybean meal and rapeseed meal in growing pigs. J. Anim. Sci. Biotechnol. 8:44. doi: 10.1186/s40104-017-0169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. L., Wu F., Chen Y. F., Wang J. R., Guo P. P., Li Z. C., Liu L., and Lai C. H.. . 2015. Determination of the energy content and amino acid digestibility of double-low apeseed cakes fed to growing pigs. Anim. Feed Sci. Technol. 210:243–253. doi: 10.1016/j.anifeedsci.2015.10.012 [DOI] [Google Scholar]
- Liu J. B., Xue P. C., Cao S. C., Liu J., Chen L., and Zhang H. F.. . 2018. Effects of dietary phosphorus concentration and body weight on postileal phosphorus digestion in pigs. Anim. Feed Sci. Technol. 242:86–94. doi: 10.1016/j.anifeedsci.2018.06.003 [DOI] [Google Scholar]
- National Research Council 2012. Nutrient requirements of swine. 11th ed.Washington (DC): National Academy of Sciences. [Google Scholar]
- Noblet J., Fortune H., Shi X. S., and Dubois S.. . 1994. Prediction of net energy value of feeds for growing pigs. J. Anim. Sci. 72:344–354. doi: 10.2527/1994.722344x. [DOI] [PubMed] [Google Scholar]
- Nyachoti C. M., Omogbenigun F. O., Rademacher M., and Blank G.. . 2006. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 84:125–134. doi: 10.2527/2006.841125x [DOI] [PubMed] [Google Scholar]
- O’Connell J. M., Callan J. J., and O’Doherty J. V.. . 2006. The effect of dietary crude protein level, cereal type and exogenous enzyme supplementation on nutrient digestibility, nitrogen excretion, faecal volatile fatty acid concentration and ammonia emissions from pigs. Anim. Feed Sci. Technol. 127:73–88. doi: 10.1016/j.anifeedsci.2005.09.002 [DOI] [Google Scholar]
- Portune K. J., Beaumont M., Davila A. M., Tomé D., Blachier F., and Sanz Y.. . 2016. Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. Trends Food Sci. Technol. 57:213–232. doi: 10.1016/j.jpgs.2016.08.011 [DOI] [Google Scholar]
- Qiu K., Zhang X., Jiao N., Xu D., Huang C., Wang Y., and Yin J.. . 2018. Dietary protein level affects nutrient digestibility and ileal microbiota structure in growing pigs. Anim. Sci. J. 89:537–546. doi: 10.1111/asj.12946 [DOI] [PubMed] [Google Scholar]
- She Y., Sparks J. C., and Stein H. H.. . 2018. Effects of increasing concentrations of an Escherichia coli phytase on the apparent ileal digestibility of amino acids and the apparent total tract digestibility of energy and nutrients in corn-soybean meal diets fed to growing pigs. J. Anim. Sci. 96:2804–2816. doi: 10.1093/jas/sky152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H. H., Sève B., Fuller M. F., Moughan P. J., and de Lange C. F.. . 2007. Invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J. Anim. Sci. 85:172–180. doi: 10.2527/jas.2005-742 [DOI] [PubMed] [Google Scholar]
- Tian Z. M., Ma X. Y., Yang X. F., Fan Q. L., Xiong Y. X., Qiu Y. Q., Wang L., Wen X. L., and Jiang Z. Y.. . 2016. Influence of low protein diets on gene expression of digestive enzymes and hormone secretion in the gastrointestinal tract of young weaned piglets. J. Zhejiang Univ. Sci. B. 17:742–751. doi: 10.1631/jzus.B1600229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Wang Y. M., Yu H. T., Zhou J. Y., Zeng X. F., Wang G., Cai S., Huang S., Zhu Z. P., Tan J. J., Johnston L. J., . et al. 2019. Effects of feeding growing-finishing pigs with low crude protein diets on growth performance, carcass characteristics, meat quality and nutrient digestibility in different areas of China. Anim. Feed Sci. Technol. 256:114256. doi: 10.1016/j.anifeedsci.2019.114256 [DOI] [Google Scholar]
- Wang Y., Zhou J., Wang G., Cai S., Zeng X., and Qiao S.. . 2018. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 9:60. doi: 10.1186/s40104-018-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirnt R. 1974a. Chymotrypsin, measurements with n-benzoyll-tyrosin ethyl ester as substrate. In: Ulrich Bergmeyer ed. Methods of enzymatic analysis. 2nd ed.Weinheim (Germany): Verlag Chemie; p. 1009–1012. [Google Scholar]
- Wirnt R. 1974b. Trypsin, measurement with nα-p-toluenesulfonyl-l-arginine methyl ester as substrate. In: Ulrich Bergmeyer ed. Methods of enzymatic analysis. 3rd ed.Weinheim (Germany): Verlag Chemie; p. 1021–1024. [Google Scholar]
- Wu X., Ruan Z., Gao Y., Yin Y., Zhou X., Wang L., Geng M., Hou Y., and Wu G.. . 2010. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 39:831–839. doi: 10.1007/s00726-010-0538-y [DOI] [PubMed] [Google Scholar]
- Xue P. C., Ajuwon K. M., and Adeola O.. . 2016. Phosphorus and nitrogen utilization responses of broiler chickens to dietary crude protein and phosphorus levels. Poult. Sci. 95:2615–2623. doi: 10.3382/ps/pew156 [DOI] [PubMed] [Google Scholar]
- Xue P. C., Ragland D., and Adeola O.. . 2017. Influence of dietary crude protein and phosphorus on ileal digestion of phosphorus and amino acids in growing pigs. J. Anim. Sci. 95:2071–2079. doi: 10.2527/jas.2016.1293 [DOI] [PubMed] [Google Scholar]
- Yang H. S., Fu D. Z., Kong X. F., Wang W. C., Yang X. J., Nyachoti C. M., and Yin Y. L.. . 2013. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J. Anim. Sci. 91:2740–2748. doi: 10.2527/jas.2012-5795 [DOI] [PubMed] [Google Scholar]
- Yao K., Yin Y. L., Chu W., Liu Z., Deng D., Li T., Huang R., Zhang J., Tan B., Wang W., . et al. 2008. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 138:867–872. doi: 10.1093/jn/138.5.867 [DOI] [PubMed] [Google Scholar]
- Ye C., Zeng X., Zhu J., Liu Y., Ye Q., Qiao S., and Zeng X.. . 2017. Dietary N-carbamylglutamate supplementation in a reduced protein diet affects carcass traits and the profile of muscle amino acids and fatty acids in finishing pigs. J. Agric. Food Chem. 65:5751–5758. doi: 10.1021/acs.jafc.7b02301 [DOI] [PubMed] [Google Scholar]
- Yue L. Y., and Qiao S. Y.. . 2008. Effects of low-protein diets supplemented with crystalline amino acids on performance and intestinal development in piglets over the first 2 weeks after weaning. Livest. Sci. 115:144–152. doi: 10.1016/j.livsci.2007.06.018 [DOI] [Google Scholar]
- Zeng X., Huang Z., Zhang F., Mao X., Zhang S., and Qiao S.. . 2015. Oral administration of N-carbamylglutamate might improve growth performance and intestinal function of suckling piglets. Livest. Sci. 181:242–248. doi: 10.1016/j.livsci.2015.09.004 [DOI] [Google Scholar]
- Zhao F., Hou S. S., Zhang H. F., and Zhang Z. Y.. . 2007. Effects of dietary metabolizable energy and crude protein content on the activities of digestive enzymes in jejunal fluid of Peking ducks. Poult. Sci. 86:1690–1695. doi: 10.1093/ps/86.8.1690 [DOI] [PubMed] [Google Scholar]