Abstract
A 120-d feeding trial was conducted to determine the effect of alfalfa (Medicago sativa) feeding on growth and chemical composition, fatty acid content, and nutritional and lipid indices of the meat of grass carp (Ctenopharyngodon idella). Two experimental diets were used: alfalfa pellet (AP) diet and artificial grain diet (GD). Final weight, feed conversion rate, and protein efficiency ratio were significantly greater in the GD group (P < 0.05). However, no differences in the length and condition factor were observed. The composition of the meat differed between treatments. The protein content was significantly greater in the AP group (P < 0.05), while the lipid and cholesterol contents were significantly greater in the GD group (P < 0.05). A greater proportion of saturated, n-6 polyunsaturated, and n-6 highly unsaturated fatty acids was obtained in the GD group. The AP group accumulated a greater concentration of eicosapentaenoic (EPA), docosapentaenoic (DPA), and docosahexaenoic (DHA) acids (P < 0.05). The fatty acid composition of the meat determined a significant decrease in the thrombogenicity index and saturation index (S/P) in the AP group (P < 0.05). The Elongase index was greater in the GD group (P < 0.05). In contrast, the AP group had a greater index of Δ9 Desaturase and Δ5 + Δ6 Desaturase for n-3 and n-6 fatty acids (P < 0.05). These results suggest that alfalfa feeding decreases the growth of C. idella but improves the quality of meat by increasing the protein, EPA, and DHA contents. It also reduces cholesterol content and improves nutritional indices.
Keywords: fish meat, grass carp, meat nutritional value, nutrition, productive response
Introduction
Aquaculture is currently seeking economically viable and environmentally sustainable alternatives to replace fishmeal in fish diets (Gatlin et al., 2007; FAO, 2016). One alternative is the culture of herbivore species such as grass carp (Ctenopharyngodon idella), which is capable of feeding on superior plants and is one of the most widely produced carps (China Fishery Statistical Yearbook, 2013).
In culture conditions, C. idella feeds on grain-based diets. However, it may also feed on forage, such as alfalfa (Medicago sativa), which is low-cost and environmentally sustainable. Alfalfa is produced worldwide and has great nutritional value, resulting from its protein content and balance between alpha-linolenic (ALA; C18:3 n-3) and linoleic (LA; C18:2 n-6) fatty acids (García et al., 2016).
Both fatty acids are important and essential for C. idella (Li et al., 2015). These can be elongated and desaturated to fatty acids of 20 or more carbon atoms (highly unsaturated fatty acids: HUFAs). LA is elongated to arachidonic acid (ARA; C20:4 n-6), whereas ALA is elongated to eicosapentaenoic (EPA; C20:5 n-3), docosapentaenoic (DPA; C22:5 n-3), and docosahexaenoic (DHA; C22:6 n-3) acids (Du et al., 2006), the three of which play an important role in the health and growth of fish (Sargent, 2002). Since the enzymes responsible for elongation and desaturation of ALA and LA compete with each other (Brenner, 1999), an adequate ALA/LA ratio could favor the production of EPA, DPA, and DHA (Li et al., 2015). Therefore, in addition to meet the requirements of C. idella, these fatty acids could accumulate in greater concentration in the meat.
The content of EPA, DPA, and DHA is one of the attributes for which fish meat is highly recommended and appreciated for human consumption. In this regard, clinical evidence was reported on the benefits that these compounds offer to human health. Some of these benefits are the development of cognitive ability during the first years of life (Richards et al., 2009; Valenzuela et al., 2011), and a decrease in the incidence of neurodegenerative and heart diseases, diabetes risk, and obesity (Simopoulos, 2008; Bazan et al., 2011; Sioen et al., 2017;Saini and Keum, 2018). Therefore, an improvement in the lipid quality of C. idella meat could be obtained from alfalfa feeding. Besides, this feeding strategy may have a positive impact on consumer perception. It is known that meat from pasture-fed animals is associated with better quality indices and greater nutritional composition (Nilzén et al., 2001; Descalzo et al., 2005; Forrester-Anderson et al., 2006; Ponte et al., 2008). In recent years, consumers have indicated a preference for fish meat naturally enriched with omega 3, vitamins, and antioxidants (Ramalho Ribeiro et al., 2019; Risius et al., 2019). Therefore, alfalfa feeding may improve the nutritional quality of C. idella meat while fulfilling the sustainable criteria of meat production.
The aim of this study was to evaluate the impact of alfalfa (M. sativa) feeding on the growth, chemical and fatty acid composition, and metabolic and lipid nutritional indices of C. idella meat. We hypothesized that grass carp fed alfalfa pellets (AP) can improve the nutritional value of meat for human consumption.
Material and Methods
This research–including all fish handling, sampling, and slaughter procedures–was approved by the Committee of Ethical Use of Animals from Facultad de Ciencias Agrarias, Universidad Nacional de Lomas de Zamora, Lomas de Zamora, Buenos Aires province, Argentina (Protocol number 23060/17), and by the National Institute of Agricultural Technology (INTA).
Experimental diets
Two contrasting diets were used (Table 1): 1) an artificial diet based on grains (GD) and 2) a diet based on AP (M. sativa). The GD is a specific diet for the species developed by the American Soybean Association (ASA) (Cremer et al., 2003), formulated with 50% soy meal, 20% sunflower meal, 16.8% corn husk, 8% corn gluten, 1% soybean oil, and 4.2% vitamin and mineral mixes. The vitamin mix was constituted by thiamine (50 mg/kg diet), riboflavin (50 mg/kg diet), vitamin A (9 mg/kg diet), vitamin E (400 mg/kg diet), vitamin D3 (6 mg/kg diet), pyridoxine HCl (40 mg/kg diet), cyanocobalamin (0.1 mg/kg diet), biotin (6 mg/kg diet), calcium pantothenate (100 mg/kg diet), folic acid (15 mg/kg diet), niacin (200 mg/kg diet), and inositol (2,000 mg/kg diet). The mineral mix was constituted by Ca(H2PO4)2 (9.8 mg/kg diet), calcium lactate (37.9 mg/kg diet), NaCl (2.6 mg/kg diet), K2SO4 (13.1 mg/kg diet), KCl (5.3 mg/kg diet), FeSO4 (0.9 mg/kg diet), ferric citrate (3.1 mg/kg diet), MgSO4 (3.5 mg/kg diet), ZnSO4 (0.4 mg/kg diet), 5H2O.CuSO4 (0.02 mg/kg diet), CoCl2 (0.03 mg/kg diet), and KI (0.02 mg/kg diet). Cellulose was used as a carrier. The animals were fed for a total period of 120 d.
Table 1.
Proximate composition and fatty acid composition of experimental diets
| AP | GD | |
|---|---|---|
| Composition, g kg−1 | ||
| Crude protein | 202.8 ± 0.8 | 175.3 ± 2.0 |
| Crude lipid | 22.0 ± 1.3 | 65.0 ± 1.0 |
| Carbohydrate | 123.2 ± 12.1 | 557.1 ± 11.1 |
| Total fiber | 416.3 ± 10.4 | 47.0 ± 3.0 |
| Moisture | 97.5 ± 1.3 | 111.6 ± 2.0 |
| Ash | 138.2 ± 15.7 | 44.0 ± 1.0 |
| Available energy, kcal kg−1 | 2,400 | 3,100 |
| Fatty acid profile, mg | ||
| C10:0 | 61.9 ± 5.8 | – |
| C12:0 | 10.1 ± 0.9 | – |
| C14:0 | 7.1 ± 0.9 | 8.8 ± 0.11 |
| C15:0 | 5.1 ± 0.2 | – |
| C16:0 | 12.1 ± 0.7 | 726.1 ± 0.6 |
| C17:0 | – | 8.9 ± 0.8 |
| C18:0 | 37.2 ± 2.1 | 331.4 ± 3.3 |
| C20:0 | 16.1 ± 1.2 | 2.1 ± 0.2 |
| C22:0 | 57.6 ± 2.1 | 28.6 ± 1.6 |
| C24:0 | 14.5 ± 0.1 | – |
| Total SAT | 404.3 ± 11 | 1,105.2 ± 4.9 |
| C16:1 n-7 | 12.1 ± 0.7 | – |
| C16:1 n-9 | 5.3 ± 0.6 | 6.4 ± 0.0 |
| C18:1 n-7 | 6.2 ± 0.7 | 75.1 ± 5.7 |
| C18:1 n-9 | 26.9 ± 1.8 | 1,415.4 ± 7.4 |
| Total MUFAs | 50.5 ± 2.3 | 1,498.6 ± 1.8 |
| C18:2 n-6 | 111.1 ± 5.5 | 3,376.6 ± 12.3 |
| C18:3 n-6 | – | 33.3 ± 0.2 |
| C18:3 n-3 | 117.8 ± 2.7 | 369.6 ± 1.7 |
| Total C18 PUFA | 228.8 ± 8.2 | 3,779.6 ± 10.4 |
| C18:3/18:2 | 1.1 ± 0.0 | 0.1 ± 0.0 |
1Values are means ± standard deviation (n = 4).
Animals
Approximately, 400 juvenile grass carps (C. idella), initial weight 13.42 ± 1.13 g and initial length 10.43 ± 0.55 cm, were obtained from the local fish farm (Oberá, Misiones, Argentina). The assay consisted of two treatments with four replicates each. Replicates were reared in individual tanks with 50 animals. The acclimatization period lasted 10 d. During the feeding period, each tank received individual aeration. Once a week, half the volume of water in the tanks was changed. Water temperature was 23 ± 0.7 °C, dissolved oxygen > 5.0 mg/L, pH 7.8 ± 0.3, NH4+-N < 0.5 mg/L, and NO2–N < 0.05 mg/L. The fish tanks had a physical-biological filtering system to maintain the physicochemical characteristics of the water. The fish were fed to satiation twice a day (9:00 a.m. and 5:00 p.m.) according to the corresponding diets. Food residues were removed from the water after each feeding to determine the feed intake per day.
Sampling procedure
At 120 d of the trial, 12 fish from each treatment (3 fish from each of the four replicates) were slaughtered to determine the chemical and fatty acid composition and the lipid nutritional indices of the meat. Prior to slaughter, the animals were fasted for 24 h. Then, they were desensitized by immersion in water at 0 °C, weighed, and measured. Finally, they were slaughtered with a cervical puncture. To produce the bleeding, a cut was made in the caudal region.
Biometrical parameters
A total of 16 fish from each group (4 fish per tank) were used to determine biometric parameters. The determinations were conducted 24, 48, 72, 96, and 120 d after the beginning of the treatments. The weight gain (WG), specific growth rate, feed conversion rate (FCR), and other indices were then calculated using the following formulas:
Biochemical analysis
Diets
The chemical composition of the diets was performed according to the methods of the Association of Official Analytical Chemists (AOAC, 2000). All determinations were made in triplicate. To determine the moisture content, the samples were placed in a drying oven (60 °C) for 36 h. An aliquot of 2 g was incinerated at 550 °C for 24 h to determine the ash content. Crude protein was analyzed by the Kjeldahl method and estimated as N × 6.25 after sulfuric acid (98% m/m) digestion. An automatic Foss Tecator 2200 Kjeltec model was used. The Soxhlet hot extraction method was used to determine the total lipid content (Soxtec System HT 1043 Extraction unit, Tecator USA).
The fatty acid content was determined from 3 g samples of the dry matter. Lipids were extracted using a chloroform–methanol mixture (2:1), according to the method of Folch et al. (1957). Fatty acid methyl esters (FAMEs) were transmethylated with 4% HCl acid in anhydrous methanol, according to the method of Pariza et al. (2001). Methyl nonadecanoate (C19:0) at 1 mg/mL was added as the internal standard.
Analysis of FAME in hexane was performed on gas-liquid chromatography (Varian CP3800, Walnut Creek, CA, USA) fitted with a flame ionization detector. The concentration of fatty acids was expressed in mg/100 g dry matter.
Muscle
The proximate composition analysis of the muscle (three fish per tank) was determined according to AOAC (2000) methods. The moisture was determined by oven drying at 105 °C for 24 h, and the ash content was determined using a muffle furnace at 550 °C for 24 h. Crude protein was analyzed by the Kjeldahl method and estimated as N × 6.25 after sulfuric acid (98% m/m) digestion. An automatic Foss Tecator 2200 Kjeltec model was used. The concentration of lipids was obtained by taking 5 g of muscle using the technique of Folch et al. (1957). The cholesterol level was determined using a cholesterol assay kit (Colestat enzymatic kit, Argentina).
Fatty acid analysis of muscle and lipid nutritional indices
To determine the fatty acid composition, samples of 5 g of muscle (three fish per tank) were taken for lipid extraction using the technique of Folch et al. (1957). FAMEs were transmethylated with 4% HCl acid in anhydrous methanol, according to the method of Pariza et al. (2001). Analysis of FAME in hexane was performed on a gas-liquid chromatography (Varian CP3800, Walnut Creek, CA, USA) fitted with a flame ionization detector. Analytical results were expressed as percentages of total fatty acids. From the lipid content of the meat, the concentration of fatty acids (mg 100 g−1 of meat) was calculated.
Fatty acid means were used to calculate atherogenicity (AI), thrombogenicity (TI), hypocholesterolemic (h), hypercholesterolemic (H), peroxidability (PI), and saturation (S/P) indices using the following formulas, according to Mancini et al. (2017b):
Chromatographic conditions
The FAME profile in diets and muscle was determined by split injection (1:100) into a CP-Sil 88 fused silica capillary column (100 m × 0.25 mm i.d., 0.20 μm film thickness, Varian CP7489) using a gradient temperature program. The column oven was held at 45 °C for 4 min, then increased from 45 to 165 °C at 13 °C/min and held for 35 min, and finally, from 165 to 215 °C at 4 °C/min and held for 30 min. The total run time was 90 min. The carrier gas was helium, at a constant flow of 1.0 mL/min. Temperature of injector and detector was 250 °C. Fatty acids were identified by comparing relative retention times with the individual fatty acid standard (PUFA-2 Animal Source; Grain Fatty acid Methyl Ester Mix [Sigma-Aldrich, USA]) and GLC 481B (Nu Chek Prep. Inc. Elysian, MN, USA).
Estimation of metabolic indices in muscle
Some indices were calculated to estimate the desaturase and elongase activity of muscle tissue. The activity of Δ9 desaturase was calculated from the relationship between the percentages of product and precursor (Okada et al., 2005):
The elongase and thioesterase indices were calculated according to Zhang et al. (2007) as follows:
The determination of Δ5 and Δ6 desaturase activity index was done by the following equations (Sirri et al., 2010):
Statistical analyses
All data were expressed as the means ± standard deviation (SD) for n replicates. The effects of each diet were compared by a one-way analysis of variance followed by Tukey’s post hoc test (P < 0.05). The variation of the biometrical parameters in response to the treatments was studied at different times (24, 48, 72, 96, and 120 d) using repeated measures ANOVA. All analyses were conducted using the XLSTAT Version 2018 (France).
Results
Diet composition
During the trial period, the fish accepted the diets. Voracity, typical of this species, was observed in food consumption. No signs of pathology were visualized in the fish. In addition, the physicochemical parameters of the water were maintained in the optimal ranges.
Table 1 shows the chemical composition and fatty acid concentration of both experimental diets. The AP diet had a significantly (P < 0.05) greater protein content than the GD diet and approximately nine times more fiber. In contrast, the GD had three times more total lipids. This indicated that the GD and AP diets had a carbohydrate:lipid (CHO:L) ratio of 8.57 and 5.6, respectively. Despite the lower content of nonstructural carbohydrates, the AP diet provided more structural carbohydrates (fiber) than the GD diet.
Both diets were contrasting in the composition of fatty acids. The greater content of saturated fatty acid (SFA) in GD was given mainly by palmitic (C16:0) and stearic (C18:0) acids. In addition, the monounsaturated fatty acids (MUFAs) content was 30 times greater in GD. The GD diet also had a greater concentration of LA (C18:2 n-6) and ALA (C18:3 n-3). Conversely, the AP diet had a greater C18:3/C18:2 ratio.
Biometrical parameters
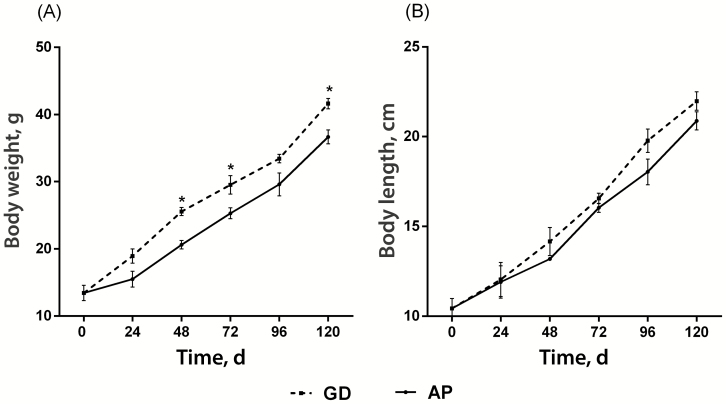
In both treatments, the fish had similar growth parameters (Table 2), but at 48, 72, and 120 d they had a differential response to diets (P < 0.05). Although GD fish were heavier than AP fish, both groups increased their body weight and length (Figure 1) over time, and no differences were observed in the condition factor (CF).
Table 2.
Growth, feed utilization, and biometrical parameters recorded from grass carp (C. idella) fed either with AP or with GD diet, at different times of the growth trial1
| 24 d | 48 d | 72 d | 96 d | 120 d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AP2 | GD | AP | GD | AP | GD | AP | GD | AP | GD | |
| FM, g | 15.4 ± 1.2a | 18.9 ± 1.1a | 20.6 ± 0.6b | 25.5 ± 0.6ª | 25.3 ± 0.8b | 29.5 ± 1.4ª | 29.6 ± 1.7a | 33.4 ± 0.6a | 36.6 ± 1.0b | 41.6 ± 0.5a |
| WG, % | 15.5 ± 0.5b | 41.1 ± 0.9a | 53.7 ± 1.1b | 90.4 ± 2.6a | 88.5 ± 2.0b | 120.4 ± 1.2a | 120.6 ± 2.2b | 149.0 ± 4.6a | 173.1 ± 3.1b | 210.1 ± 5.7a |
| FCR | 13.2 ± 0.8a | 11.2 ± 0.5b | 14.7 ± 0.3a | 15.9 ± 0.9a | 26.2 ± 0.8a | 25.5 ± 0.6a | 35.9 ± 1.4b | 45.7 ± 0.3a | 51.9 ± 1.1b | 56.2 ± 0.5a |
| PER | 0.4 ± 0.0b | 1.6 ± 0.0a | 0.7 ± 0.1b | 1.4 ± 0.1a | 0.5 ± 0.1a | 0.6 ± 0.0a | 0.4 ± 0.1b | 0.6 ± 0.1a | 0.5 ± 0.0b | 1.1 ± 0.1a |
| SR, % | 100 | 99 | 99 | 99 | 96 | 99 | 96 | 99 | 96 | 96 |
| CF | 0.9 ± 0.0b | 1.1 ± 0.2a | 0.9 ± 0.0a | 0.9 ± 0.1a | 0.6 ± 0.0a | 0.6 ± 0.0a | 0.5 ± 0.0a | 0.4 ± 0.0a | 0.4 ± 0.0a | 0.4 ± 0.0a |
1Values are means ± standard deviation, n = 16 per group.
2FM, final mass; SR, survival ratio.
a,bAdjusted means without a common letter differ statistically from each other (Tukey test, P < 0.05).
Figure 1.
Body weight and body length of C. idella juveniles at 24, 48, 72, 96, and 120 d of the growth trial. AP diet treatment; GD treatment. *denotes statistical differences (P < 0.05) between treatments for each time of the trial.
By contrast, significant differences (P < 0.05) were found in the FCR. The AP group had a greater FCR at all times analyzed, except at 72 d. This indicates that the AP group required more feed intake to gain weight probably due to lower efficiency in the use of dietary proteins from AP, as observed in the protein efficiency ratio (PER).
Biochemical composition of muscle
The chemical composition of the meat was determined at slaughter time (120 d) (Table 3). Meat from AP and GD had different contents of proteins, lipids, and cholesterol (P < 0.05). Crude protein and lipid content in meat reflected the protein and lipid composition of the diets (Table 1). Although the AP group reached lower final weight than the GD group (36.6 vs. 41.6 g), the protein concentration in the meat of alfalfa-fed animals was greater. Furthermore, the lipid and cholesterol contents were lower (approximately twice as low) in the AP than the GD group. Despite differences in the lipid content, the moisture content in meat was similar in both groups.
Table 3.
Chemical composition and cholesterol content determined at the slaughter time on the meat of C. idella juveniles fed either with AP or with GD diet1
| AP | GD | |
|---|---|---|
| Crude protein, % | 16.03 ± 0.26a | 14.98 ± 0.34b |
| Crude lipid, % | 2.45 ± 0.28b | 4.35 ± 0.35a |
| Cholesterol, mg | 13.72 ± 0.77b | 22.89 ± 0.94ª |
| Moisture, % | 80.27 ± 0.71a | 79.43 ± 0.15a |
| Ash, % | 1.25 ± 0.47a | 1.24 ± 0.32a |
1Values are means ± standard deviation, (n = 12 per group).
a,bDifferent superscript letters in columns denote statistical differences (P < 0.05).
The diets produced changes in the fatty acid profile of C. idella meat in both experimental groups (Table 4). In the GD group, SATs were greater than in AP meat, especially for myristic (C14:0), palmitic (C16:0), and stearic (C18:0) acids, which were the most abundant. This is important because they are known as H and atherogenic. The oleic acid was the most abundant MUFA in both treatments.
Table 4.
Fatty acid composition of meat of C. idella fed either with AP or with GD diet1
| Fatty acid, % | AP | GD | Significance |
|---|---|---|---|
| C8.0 | 0.34 ± 0.06ª | 0.14 ± 0.00b | 0.0007 |
| C10:0 | 0.19 ± 0.04ª | 0.05 ± 0.01b | 0.0005 |
| C12:0 | 0.12 ± 0.01ª | 0.04 ± 0.00b | <0.0001 |
| C14:0 | 1.36 ± 0.14b | 1.69 ± 0.05b | 0.0094 |
| C15:0 | 0.77 ± 0.04ª | 0.29 ± 0.08b | <0.0001 |
| C16:0 | 13.69 ± 0.26b | 15.62 ± 0.20a | <0.0001 |
| C17:0 | 0.43 ± 0.03ª | 0.11 ± 0.02b | <0.0001 |
| C18:0 | 2.68 ± 0.33b | 4.56 ± 0.15a | <0.0001 |
| C20:0 | 0.41 ± 0.09 | 0.48 ± 0.11 | NS |
| C22:0 | 1.49 ± 0.18 | 2.10 ± 0.62 | NS |
| Total SFA | 21.47 ± 0.50b | 25.09 ± 0.66a | 0.0001 |
| C14:1 cis 9 | 0.18 ± 0.02ª | 0.11 ± 0.07b | 0.0359 |
| C16:1 trans 9 | 0.40 ± 0.06ª | 0.15 ± 0.04b | 0.0019 |
| C16:1 cis 7 | 0.53 ± 0.08 | 0.46 ± 0.05 | NS 2 |
| C16:1 cis 9 | 4.55 ± 0.42b | 5.71 ± 0.01ª | 0.0120 |
| C17:1 cis 10 | 0.18 ± 0.03ª | 0.12 ± 1.23b | 0.0033 |
| C18:1 n-9 | 21.29 ± 0.66b | 25.91 ± 0.31ª | 0.0006 |
| C18:1 cis 11 n-7 | 2.70 ± 0.18 | 2.86 ± 0.01 | NS |
| C22:1 n-9 | 0.30 ± 0.09ª | 0.09 ± 1.51b | 0.029 |
| Total MUFA | 30.13 ± 0.86b | 35.35 ± 0.07a | 0.0009 |
| C18:2 n-6 | 17.88 ± 0.26b | 29.66 ± 0.24ª | <0.0001 |
| C18:3 n-6 | 0.57 ± 0.03ª | 0.24 ± 0.09b | 0.0025 |
| C18:3 n-3 | 3.52 ± 0.10ª | 2.50 ± 0.37b | <0.0001 |
| Total C18 PUFA | 21.97 ± 0.22b | 32.40 ± 0.48ª | <0.0001 |
| C20:4 n-6 | 4.51 ± 0.37 | 5.09 ± 0.38 | NS |
| C20:5 n-3 | 0.38 ± 0.04ª | 0.12 ± 0.03b | <0.0001 |
| C22:4 n-6 | 2.14 ± 0.14b | 2.57 ± 0.02ª | 0.0009 |
| C22:5 n-3 | 0.40 ± 0.06ª | 0.19 ± 0.02b | 0.0003 |
| C22:6 n-3 | 3.58 ± 0.32ª | 1.02 ± 0.04b | <0.0001 |
| Total HUFA | 11.01 ± 0.25a | 8.99 ± 0.33b | 0.0001 |
| n-3/n-6 | 0.31 ± 0.02a | 0.10 ± 0.01b | <0.0001 |
1Values are means ± standard deviation, (n = 12 per group).
2 NS, non significant.
a,bDifferent superscript letters in columns denote statistical differences (P < 0.05)..
The C18 polyunsaturated fatty acid (C18PUFA) differed between groups (P < 0.05). Accordingly, the GD group had a greater LA and a lower ALA proportion than the AP group. This determined a C18:3/C18:2 ratio of 0.08 in the GD group and 1.22 in the AP group. The difference in this relationship was also reflected in the content of HUFAs. Since no HUFAs were detected in the diets, their presence in the meat reflected the metabolic capacity of C. idella to elongate and desaturate LA and ALA.
The AP group had a greater proportion of all HUFAs n-3: EPA (C20: 5 n-3), DPA (C22: 5 n-3), and DHA (C22: 6 n-3). By contrast, GD had a greater proportion of n-6 PUFAs. Differences in the proportions of n-3 and n-6 fatty acids between groups determined a different n-3/n-6 ratio. In this regard, although the n-3/n-6 ratio was low due to the high proportion of total n-6 in both groups, the n-3/n-6 ratio was better in the AP than in the GD group, in terms of the nutritional benefits of the meat (Ruxton et al., 2004, 2005).
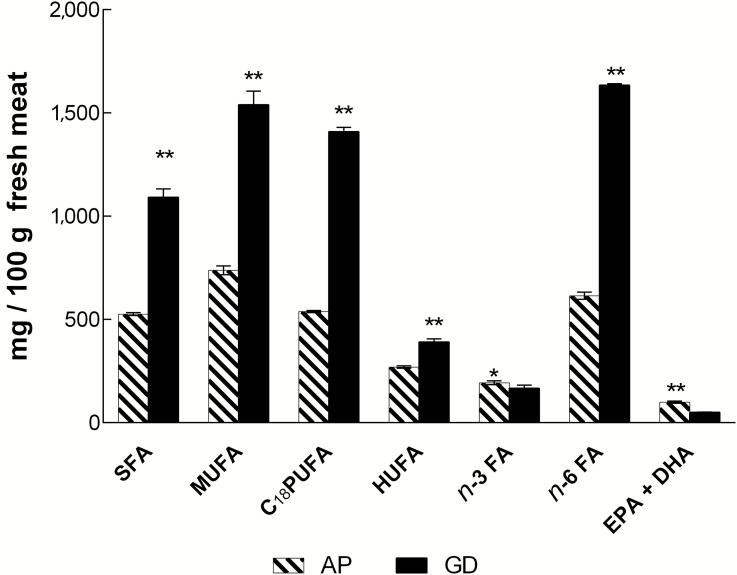
The proportions observed in the fatty acids of fish meat produced differences in the concentration of the main fatty acids of interest to human health (Figure 2). The GD group fish doubled the concentration of SFA and MUFA in their meat, compared to the AP group (1,091 vs. 525.9 mg and 1,540.6 mg vs. 738.2 mg/100 g meat, respectively). Furthermore, the GD had three times as much C18 PUFA and n-6 totals (1,409.4 vs. 538.2 mg and 1,364.1 mg vs. 614.6 mg/100 g meat, respectively). However, the AP group outperformed the GD group in the total n-3 content (193.13 vs. 166.3 mg/100 g meat, respectively) and doubled the EPA + DHA content (96.95 vs. 49.67 mg/100 g meat).
Figure 2.
Concentration of fatty acid groups of nutritional interest in juvenile C. idella meat, expressed in mg/100 g fresh meat. AP diet treatment; GD treatment; SFA, total saturated fatty acid; MUFA, total monounsaturated fatty acid; C18 PUFA, total polyunsaturated fatty acid; HUFA, total highly polyunsaturated fatty acid; n-3 FA, total n-3 series fatty acid; n-6 FA, total n-6 series fatty acid. *Significance P < 0.05; **Significance P < 0.01.
Nutritional and metabolic lipid indices of the meat
Differences in fatty acid content in C. idella meat, in response to diets, produced differences in metabolic and nutritional lipid indices (Table 5) between the GD and AP groups. The AP group had lower TI, H, and S/P. However, it presented greater PI than the GD group, probably due to its greater content of PUFAs and HUFAs.
Table 5.
Lipid, nutritional, and metabolic indices determined at the slaughter time on the meat of C. idella juveniles fed either with AP or with GD diet1
| AP | GD | Significance | |
|---|---|---|---|
| AI | 0.26 ± 0.01 | 0.25 ± 0.01 | NS 2 |
| TI | 0.34 ± 0.01b | 0.46 ± 0.01ª | <0.0001 |
| h | 49.35 ± 0.67b | 62.03 ± 1.03ª | <0.0001 |
| H | 15.05 ± 0.31b | 17.32 ± 0.21ª | <0.0001 |
| h/H | 3.28 ± 0.10b | 3.58 ± 0.09ª | 0.0051 |
| PI | 79.55 ± 0.96ª | 74.63 ± 0.92b | 0.0003 |
| S/P | 1.54 ± 0.04b | 1.65 ± 0.05ª | 0.0109 |
| Elongase index | 0.20 ± 0.01b | 0.29 ± 0.02ª | 0.0001 |
| Thioesterase index | 10.28 ± 0.65ª | 9.22 ± 0.05b | 0.0176 |
| Δ 9 Desaturase (C16) | 24.92 ± 2.06 | 26.76 ± 1.93 | NS |
| Δ 9 Desaturase (C18) | 88.82 ± 0.85ª | 85.01 ± 1.48b | 0.0043 |
| Δ 5+ Δ 6 Desaturase (n-6) | 26.74 ± 0.79ª | 20.78 ± 0.57b | <0.0001 |
| Δ 5+ Δ 6 Desaturase (n-3) | 55.25 ± 0.48a | 35.75 ± 0.90b | <0.0001 |
1Values are means ± standard deviation, (n = 12 per group).
2 NS, non significant.
a,bDifferent superscript letters in columns denote statistical differences (P < 0.05).
No significant differences were observed in the Δ9 Desaturase (C16) index in response to the experimental diets. By contrast, the elongase index was greater (0.09 times) in the GD group. This may be due to the high content of SFA in meat (Table 4). In addition, the AP group had significant differences in the rest of the lipid indices (P < 0.05). The thioesterase index was greater in AP than in GD (10.28% vs. 9.22%, respectively). Also, greater Δ5 − Δ6 (n-3) and Δ5 – Δ6 (n-6) desaturases indices were observed in AP. However, the differences determined for the AP group were greater for n-3 fatty acids (19.5%) than for n-6 fatty acids (5.96%). Thus, the high proportion of n-6 fatty acids in the meat of the GD group was not reflected in a high Δ5 − Δ6 (n-6) desaturase index as expected.
Discussion
It is known that the growth and quality of fish meat are affected by a set of factors, the diet being prominent among them (Gutierrez et al., 2014; Gisbert et al., 2016). In this regard, feed sources and their nutrient content are considered the primary contributors (Zhao et al., 2018). In the present trial, the AP group achieved less growth than the GD group. Similar results for forage-based diets were reported in C. idella by Camargo et al. (2006), Nekoubin and Sudagar (2012), and Zhao et al. (2018), among others. These works attributed the lower growth to the low protein and carbohydrate contents in forage-based diets. However, many forage crops such as alfalfa (M. sativa) and annual ray-grass (Lolium multiflorum) can reach great protein contents (Palladino et al., 2009; Garcia et al., 2016), comparable to those of grains. The AP diet had greater protein content than the GD diet (Table 1); therefore, the lower growth observed in the AP group may have been due to the lower carbohydrate and lipid content of the diet and its high fiber content. Previous observations had a negative correlation between increased fiber content in the diet and WG in C. idella (Yu et al., 2014; Cheng et al., 2016). Thus, the greater fiber content of the AP diet, compared with the GD diet (413.6 vs. 47 g kg−1), may explain the lower digestibility of the diet, observed from the greater FCR in the AP group. In this regard, Gao et al. (2011) and Guo et al. (2015) reported a decrease in growth in C. idella fingerlings with diets whose fiber content was 165 and 173 g kg−1, respectively. The low digestibility of the AP diet may have caused the difference in the PER in the fish of this experimental group.
Biometrical indices other than weight may also be used as indicators of growth. The CF expresses the relationship between length (cm) and weight (g) (Jobling, 1994). In this regard, no differences were observed between both experimental groups for the CF. The CF observed was similar to those reported by Du et al. (2008) when supplementing juveniles of C. idella with 1% artificial diet. Therefore, since the fish showed no differences in total length between treatments, the difference observed in total weight may be ascribed to the high lipid deposition in the meat.
As previously mentioned, the diet, in addition to growth, affects the composition of meat (Joo et al., 2013). The processes of muscle growth and meat quality are governed by multiple molecular mechanisms (Salem et al., 2013). These, additionally, depend largely on the nutrient content of the diet (Cheng et al., 2014). In this study, it was observed that the composition of fish meat was related to the composition of the diet. Despite the low relationship reported between the protein content of the diet and the protein content of meat for C. idella (Guo et al., 2015), an increase in the protein content of meat was observed in the AP group. This increase may have stemmed from the greater protein content of the AP diet. It might also be attributed to the lower lipid content in the meat of this experimental group, resulting from lower amounts of lipids in the AP diet.
The greater lipid content of the GD diet produced an increase in meat lipids from this group of fish. Numerous studies reported a similar response from C. idella to different lipid contents in the diet (Du et al., 2006; Gao et al., 2011; Guo et al., 2015; Jiang et al., 2015; Li et al., 2017; Tian et al., 2019). This increase is linked to the great lipid and carbohydrate content of grain-based diets. However, Tian et al. (2019) found a high accumulation of lipids in C idella-fed bean pods (Vicia faba) (0.75% lipids), in comparison to that found in a group fed a grain diet.
Given its relationship with the lipid content of meat, cholesterol concentration differed between treatments. The lowest concentration observed in the meat of the AP group can be attributed to the lower lipid content, since the cholesterol concentration per gram of lipids did not differ between treatments (5.43 ± 0.3 mg cholesterol/g lipid). In agreement with these results, Guo et al. (2015) reported that cholesterol concentrations in C. idella meat varied according to the content of lipids in the diet, but observed no difference in the concentration expressed in mg cholesterol/g lipid. Regardless of the relative cholesterol content, the fish fed alfalfa had a lower total amount of cholesterol. This is of nutritional importance given the recommendations of official health agencies to reduce the consumption of meat with high cholesterol content (Saini and Keum, 2018).
Along with the cholesterol content, fatty acid composition influences the quality of the meat. The meat of the AP group had a lower proportion of SATs, especially myristic (C14:0) and palmitic (C16:0) acids. This is important for consumers because both acids are H (Blank et al., 2002; Russo, 2009; Mancini et al., 2017; Saini and Keum, 2018; Kumar et al., 2019). The difference in the proportion of the acids mentioned reflected their content in the diets. Therefore, the lower concentration of C14:0 in the diet may have resulted in the lower index of the elongases observed in the AP group, as these elongate the C14:0 to C16:0.
As reported by Du et al. (2006, 2008) and Lei et al. (2015), the ability of C. idella to elongate and desaturate fatty acids of 18 carbon atoms was also observed in the present work. Differences in the fatty acid profile of the diets produced differences in the profile of the meat. The meat of the AP group had a greater content of ALA (C18: 3 n-3) while the meat of the GD group had a greater content of LA (C18: 2 n-6); it is known that the growth and quality of fish meat are affected by a set of factors, among which the diet stands out. Besides, based on previous findings, synthesis of ARA (C20:4 n-6) was achieved by sequential desaturation and elongation of LA, whereas the synthesis of n-3 HUFAs (EPA, DPA, and DHA) was achieved by sequential desaturation and elongation of ALA with desaturase and elongase (Δ5 – Δ6) for the bio-conversion (Luo et al., 2012). The AP diet had lower LA content than the GD diet. This determined a greater ALA/LA ratio in the diet (1.1 vs. 0.1). The obtained relation could have led to the greater activity of Δ5 − Δ6 (n-3) desaturase, which favored the biosynthesis of the HUFAs: EPA, DPA, and DHA in the meat of AP. This way, the AP group had a greater endowment of HUFAs (n-3), which is beneficial to human health (Saini and Keum, 2018; Hernando et al., 2019). Accordingly, the obtained concentration of EPA + DHA covers half of the daily consumption requirements of EPA and DHA recommended by official health agencies (FAO, 2012; Sioen et al., 2017).
The amount of and differences in the fatty acids in fish meat in response to the diet determined a difference in lipid nutritional indices. TI shows the tendency to clot formation in blood vessels. The decrease in this index, coupled with the decrease in the saturation index, occurred in response to the enrichment of meat with PUFAs and HUFAs n-3 from alfalfa (AP) feed. Although there is no report in fish meat, similar results were obtained in the meat of other species, such as rabbit (Mancini et al., 2017; Dalle Zotte et al., 2018), pork (Mancini et al., 2017), beef (Selani et al., 2016), and broiler (Kumar et al., 2019). This way, it can be inferred from the low TI that the meat of the AP group may contribute to reducing the risk of numerous cardiovascular diseases (König et al., 2005; Nantapo et al., 2015).
We concluded that feeding C. idella (grass carp) AP (M. sativa) diet is a sustainable alternative in providing meat with attributes of nutritional interest to consumers, although it produces a decrease in the growth rate of fish. Lower lipid and cholesterol contents, coupled with an increase in the number of proteins, EPA, DPA, DHA acids, and a lower TI index, were obtained from the proposed feeding system. Accordingly, not only does this nutritional composition improve the quality of fish meat, but also it fulfills the indications of various official food and human health agencies as well.
Acknowledgment
This work was supported by the Universidad Nacional de Lomas de Zamora (IV LOMASCYT 94), Universidad de Morón (2017–2019: DC/17-06/15-003), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2017-0238). We acknowledge and thank universities, institutes, and Consejo Nacional de Investigaciones Científicas y Tecnicas (CONICET).
Glossary
Abbreviations
- AI
atherogenicity
- ALA
alpha-linolenic acid
- AP
alfalfa pellet
- CF
condition factor
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- FAMEs
fatty acid methyl esters
- FCR
feed conversion rate
- GD
grain diet
- H
hypercholesterolemic
- h
hypocholesterolemic
- HUFA
highly unsaturated fatty acids
- LA
linoleic acid
- MUFA
monounsaturated fatty acids
- PER
protein efficiency ratio
- PI
peroxidability
- PUFA
polyunsaturated fatty acid
- TI
thrombogenicity
- WG
weight gain
Conflict of interest statement
The authors declare that they have no conflict of interest.
Literature Cited
- AOAC , 2000. Official methods of analysis. Washington (DC): Association of Official Analytical Chemists. [Google Scholar]
- Bazan N. G., Molina M. F. and Gordon W. C... 2011. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 31:321–3 51. doi: 10.1146/annurev.nutr.012809.104635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C., Neumann M. A., Makrides M., and Gibson R. A... 2002. Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J. Lipid Res. 43:1537–1543. doi: 10.1194/jlr.m200152-jlr200 [DOI] [PubMed] [Google Scholar]
- Brenner R. R. 1999. Factors influencing fatty acid chain elongation and desaturation. In: Vergroesen A. J. and Crawford M., editors. The role of fat in human nutrition. London: Academic Press; p. 46–79. [Google Scholar]
- Camargo J. B. J., Neto J. R., Emanuelli T., Lazzari R., Costa M. L., Eliseu M., de Lima R. L., Scherer R., Augusti P. R. and Pedron F... 2006. Crescimento de alevins de carpa capim (C. idella) alimentados com ração e forragens cultivadas 12:5.doi: 10.1590/S0103-84782008000200031 [DOI] [Google Scholar]
- Cheng J. H., Sun D. W., Han Z. and Zeng X. A... 2014. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: a review. Compr. Rev. Food Sci. Food Saf. 13:52–61.doi: 10.1111/1541-4337.12043 [DOI] [PubMed] [Google Scholar]
- Cheng H. H., Xie C. X., Li D. P., Xiao Y. H., Tian X. and Chen J... 2016. The study of muscular nutritional components and fish quality of grass carp (Ctenopharyngodon idellus) in ecological model of cultivating grass carp with grass. Fish. China 40:1050–1059. doi: 10.11964/jfc.20150709964 [DOI] [Google Scholar]
- China Fishery Statistical Yearbook. 2013. Fishery Bureau, Ministry of Agriculture. Beijing: China Agriculture Press (in Chinese). [Google Scholar]
- Cremer M. C., Zhang J. and Zhou E... 2003. Mejoramiento de carpa herbívora y tilapia con alimentos basados en soja, en China. ASA. International Aquafeed, 6(3):24–30. [Google Scholar]
- Dalle Zotte A., Cullere M., Martins C., Alves S. P., Freire J. P. B., Falcão-e-Cunha L. and Bessa R. J. B... 2018. Incorporation of Black Soldier Fly (Hermetia illucens L.) larvae fat or extruded linseed in diets of growing rabbits and their effects on meat quality traits including detailed fatty acid composition. Meat Sci. 146:50–58. doi: 10.1016/j.meatsci.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Descalzo A. M., Insani E. M., Biolatto A., Sancho A. M., García P. T., Pensel N. A. and Josifovich J. A... 2005. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 70:35–44. doi: 10.1016/j.meatsci.2004.11.018 [DOI] [PubMed] [Google Scholar]
- Du Z. Y., Clouet P., Huang L. M., Degrace P., Zheng J. G., He I. G., Tian L. X. and Liu Y. J... 2008. Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): mechanism related to hepatic fatty acid oxidation. Aquac. Nutr. 14:77–92. doi: 10.1111/j.1365-2095.2007.00507.x [DOI] [Google Scholar]
- Du Z. Y., Liu Y. J., Tian L. X., Wang J. T., Wang Y. and Liang G. Y... 2006. Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 11:39–146. doi: 10.1079/BJN20061733 [DOI] [Google Scholar]
- FAO 2012. El estado mundial de la pesca y la acuicultura .Vol. 231 Roma: FAO; p. 28–39. [Google Scholar]
- FAO 2016. El estado mundial de la pesca y la acuicultura 2016. Contribución a la seguridad alimentaria y la nutrición para todos . Roma: FAO; p. 224. [Google Scholar]
- Folch J., Lees M., and Sloane-Stanley G. H. S... 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- Forrester-Anderson I. T., McNitt J., Way R., and Way M... 2006. Fatty acid content of pasture-reared fryer rabbit meat. J. Food Compos. Anal. 19:715–719. doi: 10.1016/j.jfca.2006.02.011 [DOI] [Google Scholar]
- Gao W., Liu Y. J., Tian L. X., Mai K. S., Liang G. Y., Yang H. J., Huai M. Y. and Luo W. J... 2011. Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngodon idella). Aquac. Nutr. 16:327–333. doi: 10.1111/j.1365-2095.2009.00668.x [DOI] [Google Scholar]
- Garcia P. T., Pordomingo A., Perez C. D., Rios M. D., Sancho A. M., Volpi Lagreca G. and Casal J. J... 2016. Influence of cultivar and cutting date on the fatty acid composition of forage crops for grazing beef production in Argentina. Grass Forage Sci. 71:235–244. doi: 10.1111/gfs.12167 [DOI] [Google Scholar]
- Gatlin D. M., Barrows F. T. and Brown P... 2007. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquacult. Res. 38:551–579. doi: 10.1111/j.1365-2109.2007.01704.x [DOI] [Google Scholar]
- Gisbert E., Mozanzadeh M. T., Kotzamanis Y. and Estévez A... 2016. Weaning wild flathead grey mullet (Mugil cephalus) fry with diets with different levels of fish meal substitution. Aquaculture 462:92–100. doi: 10.1016/j.aquaculture.2016.04.035 [DOI] [Google Scholar]
- Guo X., Liang X. F., Fang L., Yuan X., Zhou Y., Zhang J., and Li B... 2015. Effects of dietary non-protein energy source levels on growth performance, body composition and lipid metabolism in herbivorous grass carp (Ctenopharyngodon idella Val.). Aquac. Res. 46: 1197–1208. doi: 10.1111/are.12275 [DOI] [Google Scholar]
- Gutierrez P. T., Almeida F. L., Carani F. R., Vechetti I. J., Padovanic C. R. and Salomão R. A. S... 2014. Rearing temperature induces changes in muscle growth and gene expression in juvenile pacu (Piaractus mesopotamicus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 169:31–37. doi: 10.1016/j.cbpb.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Hernando S., Requejo C., Herran E., Ruiz-Ortega J. A., Morera-Herreras T., Lafuente J. V., Ugedo L., Gainza E., Pedraz J. L., Igartua M.,. et al. 2019. Beneficial effects of n-3 polyunsaturated fatty acids administration in a partial lesion model of Parkinson’s disease: The role of glia and NRf2 regulation. Neurobiol. Dis. 121:252–262. doi: 10.1016/j.nbd.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Jiang J., Shi D., Zhou X. Q., Feng L., Liu Y., Jiang W. D., Wu P., Tang L., Wang Y. and Zhao Y... 2015. Effects of lysine and methionine supplementation on growth, body composition and digestive function of grass carp (Ctenopharyngodon idella) fed plant protein diets using high-level canola meal. Aquac. Nutr. 22: 1126–1133. doi: 10.1111/anu.12339 [DOI] [Google Scholar]
- Jobling M. 1994. The compensatory growth response of the Atlantic cod: effects of nutritional history. Aquac. Int. 2:75–90. doi: 10.1007/BF00128802 [DOI] [Google Scholar]
- Joo S. T., Kim G. D., Hwang Y. H. and Ryu Y. C... 2013. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 95:828–836. doi: 10.1016/j.meatsci.2013.04.044 [DOI] [PubMed] [Google Scholar]
- König A., Bouzan C., Cohen J. T., Connor W. E., Kris-Etherton P. M., Gray G. M., Lawrence R. S., Savitz D. A., and Teutsch S. M... 2005. A quantitative analysis of fish consumption and coronary heart disease mortality. Am. J. Prev. Med. 29:335–346. doi: 10.1016/j.amepre.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Kumar F., Praveen K. T. Mir N. A., Pramod K. T. Dev K., Bera I., Biswas A. K., Sharma D., Mandal A. B., and Deo C... 2019. Role of flaxseed meal feeding for different durations in the lipid deposition and meat quality in broiler chickens. J. Am. Oil Chem. Soc. 96:261–271. doi:doi: 10.1002/aocs.12190 [DOI] [Google Scholar]
- Lei C. X., Tian J. J., Ji H., Chen L. Q., and Du Z. Y... 2015. Dietary alpha-linolenic acid affects lipid metabolism and tissue fatty acid profile and induces apoptosis in intraperitoneal adipose tissue of juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 21:1–15. doi: 10.1111/anu.12377 [DOI] [Google Scholar]
- Li C., Liu P., Ji H., Huang J., and Zhang W... 2015. Dietary n-3 highly unsaturated fatty acids affect the biological and serum biochemical parameters, tissue fatty acid profile, antioxidation status and expression of lipid-metabolism-related genes in grass carp, Ctenopharyngodon idellus. Aquac. Nutr. 21:373–383. doi: 10.1111/anu.12169 [DOI] [Google Scholar]
- Li X., Zhou L., Mo H., Pan Q., and Gan L... 2017. Interaction effects of dietary lipid and lysine on growth feed utilization and body composition of juvenile grass carp (Ctenopharyngodon idella). Aquac. Int. 25:1591–1606. doi: 10.1007/s10499-017-0132-3 [DOI] [Google Scholar]
- Luo Z., Tan X. Y., Li X. D., and Yin G. J... 2012. Effect of dietary arachidonic acid levels on growth performance, hepatic fatty acid profile, intermediary metabolism and antioxidant responses for juvenile Synechogobius hasta. Aquac. Nutr. 18:340–348. doi: 10.1111/j.1365-2095.2011.00906.x [DOI] [Google Scholar]
- Mancini S., Paci G., Fratini F., Torracca B., Nuvoloni R., Dal Bosco A., Roscini V., and Preziuso G... 2017a. Improving pork burgers quality using Zingiber officinale Roscoe powder (ginger). Meat Sci. 129:161–168. doi: 10.1016/j.meatsci.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Mancini S., Preziuso G., Dal Bosco A., Roscini V., Parisi G., and Paci G... 2017b. Modifications of fatty acids profile, lipid peroxidation and antioxidant capacity in raw and cooked rabbit burgers added with ginger. Meat Sci. 133:151–158. doi: 10.1016/j.meatsci.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Nantapo C. W. T., Muchenje V., Nkukwana T. T., Hugo A., Descalzo A. M., Grigioni G., and Hoffman L. C... 2015. Socio-economic dynamics and innovative technologies affecting health-related lipid content in diets: implications on global food and nutrition security. Food Res. Int. 76:896–905. doi: 10.1016/j.foodres.2015.05.033 [DOI] [Google Scholar]
- Nekoubin H., and Sudagar M... 2012. Effect of formulate and plant diets on growth performance and survival rate of juvenile grass carp (Ctenopharyngodon idella). World J. Fish Mar. Sci. 4(4):386–389. doi: 10.5829/idosi.wjfms.2012.04.04.62143 [DOI] [Google Scholar]
- Nilzén V., Babol J., Dutta P. C., Lundeheim N., Enfält A.-C., and Lundström K. 2001. Free range rearing of pigs with access to pasture grazing — effect on fatty acid composition and lipid oxidation products. Meat Sci. 58:267–275. doi: 10.1016/S0309-1740(00)00164-9 [DOI] [PubMed] [Google Scholar]
- Okada T., Furuhashi N., Kuromori Y., Miyashita M., Iwata F., and Harada K... 2005. Plasma palmitoleic acid content and obesity in children. Am. J. Clin. Nutr. 82:747–750. doi: 10.1093/ajcn/82.4.747 [DOI] [PubMed] [Google Scholar]
- Palladino R. A., O’Donovan M., Kennedy E., Murphy J. J., Boland T. M., and Kenny D. A... 2009. Fatty acid composition and nutritive value of twelve cultivars of perennial ryegrass. Grass Forage Sci. 64:219–226. doi: 10.1111/j.1365-2494.2009.00683.x [DOI] [Google Scholar]
- Pariza M. W., Park Y., and Cook M. E... 2001. The biologically active isomers of conjugated linoleic acid. Prog. Lipid Res. 40:283–298. doi: 10.1016/s0163-7827(01)00008-x [DOI] [PubMed] [Google Scholar]
- Ponte P. I. P., Alves S. P., Bessa R. J. B., Ferreira L. M. A., Gama L. T., Brás J. L. A., Fontes C. M. G. A., and Prates. J. A. M.. 2008. Influence of pasture intake on the fatty acid composition, and cholesterol, tocopherols, and tocotrienols content in meat from free-range broilers. Poult. Sci. 87:80–88. doi: 10.3382/ps.2007-00148 [DOI] [PubMed] [Google Scholar]
- Ramalho Ribeiro A., Altintzoglou T., Mendes J., Nunes M.L., Dinis M.T., and Dias J... 2019. Farmed fish as a functional food: perception of fish fortification and the influence of origin – insights from Portugal. Aquaculture 501:22–31. doi: 10.1016/j.aquaculture.2018.11.002 [DOI] [Google Scholar]
- Richards M. P., Pettitt P. B., Stiner M. C., and Trinkaus E... 2009. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc. Natl. Acad. Sci. U.S.A.. 98:6528–6532. doi: 10.1073/pnas.111155298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risius A., Hamm U., and Janssen M... 2019. Target groups for fish from aquaculture: Consumer segmentation based on sustainability attributes and country of origin. Aquaculture 499:341–347. doi:doi: 10.1016/j.aquaculture.2018.09.044 [DOI] [Google Scholar]
- Russo L. G. 2009. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 77:937–946. doi: 10.1016/j.bcp.2008.10.020 [DOI] [PubMed] [Google Scholar]
- Ruxton C. H. S., Calder P. C., Reed S. C., and Simpson M. J. A... 2005. The impact of long-chain n-3 polyunsaturated fatty acids on human health. Nutr. Res. Rev. 18:113–129. doi: 10.1079/NRR200497 [DOI] [PubMed] [Google Scholar]
- Ruxton C. H., Reed S. C., Simpson M. J., and Millington K. J... 2004. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J. Hum. Nutr. Diet. 17:449–459. doi: 10.1111/j.1365-277X.2004.00552.x [DOI] [PubMed] [Google Scholar]
- Saini R. K., and Keum Y. S... 2018. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance – a review. Life Sci. 203:255–267. doi: 10.1016/j.lfs.2018.04.049 [DOI] [PubMed] [Google Scholar]
- Salem M., Manor M. L., Aussanasuwannakul A., Kenney P. B., Weber G. M., and Yao J... 2013. Effect of sexual maturation on muscle gene expression of rainbow trout: RNA-Seq approach. Physiol. Rep. 1:e00120. doi: 10.1002/phy2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent J. R., Tocher D. R., and Bell J. G... 2002. The lipids. In: Halver J. E., and Hardy R. W., editors. Fish nutrition, 3rd ed.San Diego (CA): Academic Press, Elsevier; p. 181–257. [Google Scholar]
- Selani M. M., Shirado G. A. N., Margiotta G. B., Rasera M. L., Marabesi A. C., Piedade S. M. S., Contreras-Castillo C. J., Canniatti-Brazaca S.G.. 2016. Pineapple by-product and canola oil as partial fat replacers in low-fat beef burger: Effects on oxidative stability, cholesterol content and fatty acid profile. Meat. Sci. 115:9–15. doi: 10.1016/j.meatsci.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Simopoulos A. P. 2008. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 233(6):674–688. doi: 10.3181/0711-MR-311 [DOI] [PubMed] [Google Scholar]
- Sioen I., van Lieshout L., Eilander A., Fleith M., Lohner S., Szommer A., Petisca C., Eussen S., Forsyth S., Calder P. C.,. et al. 2017. Systematic review on n-3 and n-6 polyunsaturated fatty acid intake in European countries in light of the current recommendations – focus on specific population groups. Ann. Nutr. Metab. 70:39–50. doi: 10.1159/000456723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri F., Castellini C., Roncarati A. and Meluzzi A... 2010. Effect of feeding and genotype on lipid profile of organic chicken meat. Eur. J. Lipid Sci. Tech. 112:994–1002. doi: 10.1002/ejlt.200900204 [DOI] [Google Scholar]
- Tian J. J., Ji H., Wang Y. F., Xie J., Wang G. J., Li Z. F., Yu E. M., Yu D. G., Zhang K., and Gong W. B... 2019. Lipid accumulation in grass carp (Ctenopharyngodon idellus) fed faba beans (Vicia faba L.). Fish Physiol. Biochem. 45:631–642. doi: 10.1007/s10695-018-0589-7 [DOI] [PubMed] [Google Scholar]
- Valenzuela R., Tapia G., Gonzalez M., and Valenzuela A... 2011Omega-3 fattyacids (EPA and DHA) and its applications in diverse clinical situations. Rev. Chil. Nutr. 38:356–367. doi: 10.4067/S0717-75182011000300011 [DOI] [Google Scholar]
- Yu E. M., Liu B. H, Wang G. J., Yu D. G., Xie J., and Xia Y... 2014Molecular cloning of type I collagen cDNA and nutritional regulation of type I collagen mRNA expression in grass carp. J. Anim. Physiol. Anim. Nutr. 98:755–765. doi: 10.1111/jpn.12132 [DOI] [PubMed] [Google Scholar]
- Zhang S., Knight T. J., Stalder K. J., Goodwin R.N., Lonergan S. M., Beitz D. C... 2007. Effects of breed, sex, and halothane genotype on fatty acid composition of pork longissimus muscle1. J. Anim. Sci. 85:583–591. doi:doi: 10.2527/jas.2006-239 [DOI] [PubMed] [Google Scholar]
- Zhao H., Xia J., Zhang X., He X., Li L., Tang R., Chi W., and Li D... 2018. Diet affects muscle quality and growth traits of grass carp (Ctenopharyngodon idellus): a comparison between grass and artificial feed. Front. Physiol. 9:1–13. doi: 10.3389/fphys.2018.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]