Publisher Summary
Naked mole rats are mouse-sized rodents that have become an important animal model in biomedical research. They play a unique mammalian role in behavioral and ecophysiological research of life underground. This chapter studies the general physiology, anatomy of organ systems, husbandry, and uses in research of the naked mole rats. Naked mole rats belong to the order Rodentia in that they have two incisor teeth on the upper and lower arcade that continuously grow. The skin is loose, wrinkled, and brownish pink in color. The body is for the most part absent of hairs with the exception of tactile hairs that are regularly arranged throughout the body and which are particularly prominent around the face and to a lesser extent on the tail. They are typically housed at 28–30°C, and at 50–60% relative humidity. Because naked mole rats are social and have cooperative behaviors, the study of their conduct has more applicability to people. The chapter describes the models of experimental research on the naked mole rat such as the model of reproductive suppression, model of somatosensory processing, model of bone elongation, and model of aging.
Keywords: naked mole rat, diseases, uses in research, anatomy, reproduction, physiology, health, Heterocephalus glaber, Batherygidae
This chapter describes the use of the naked mole rat in the research laboratory setting giving details on its anatomy, natural habitat, reproductive behavior, and physiology followed by a detailed description of its organ systems. This is followed by sections on aging, husbandry of the naked mole rat, colony health, and finally the main models used in research.
Naked mole rats are mouse-sized rodents that in recent years have become an important animal model in biomedical research in addition to their unique mammalian role in behavioral and ecophysiological research of life underground.
History
Naked mole rats (Heterocephalus glaber) were first described by Ruppell in 1842 and for many years his taxonomic description was refuted and they were considered to be either neonates of a much larger species or mammals that had lost their fur as a consequence of disease. Subsequent collections and descriptions were reported in the 1890s through 1957 and are summarized by Brett (1986). Most of those studies focused upon the unusual anatomy of this species and morphological adaptations to life underground. Jennifer Jarvis began studying these animals in the late 1960s and was the first person to bring these hairless rodents into the laboratory. She observed that these animals were highly social and appeared to work together to excavate tunnels and find food. She also noted that she seldom found pregnant animals in the wild and even in captivity had little success with the formation and maintenance of breeding colonies. Subsequently, a group of biologists primarily working with Jarvis (Brett, Buffenstein, Clarke, Faulkes) began studying and reporting on their unique biology. In the mid 1970s, Alexander, an evolutionary biologist whose research focused on the evolution of eusociality (a colonial lifestyle with a strict division of labor culminating in the presence of a single breeding female), described the ideal hypothetical eusocial mammal. At that time no eusocial mammals had yet been identified and only the wasps, ants, and bees were known to be eusocial. He described his hypothetical eusocial mammal as strictly subterranean, living in hard soils and cooperatively foraging on large underground tubers. A colleague and friend of Jarvis, TL Vaughan, told Alexander that he had aptly described the naked mole rat, rodents that Vaughan had seen in the office of Jarvis. Alexander, his doctoral student Sherman, and Jarvis thereafter began an extensive collaborative study on eusociality and behavior in the naked mole rat.
Biologists Bennett, Clarke, Faulkes, O’Riain, and Sherman have contributed greatly to the understanding of naked mole rat behavior. Buffenstein studied with Jarvis and accompanied her on her trip to Kenya in 1980 to assess if naked mole rats in the wild also live in colonies with only one breeding female. Jarvis published this seminal finding in Science in 1981 (Jarvis, 1981). Her team published extensively on ecophysiological responses which allow naked mole rats to survive and thrive in their dark, dank environment in the arid and semiarid regions of northeast Africa. These responses include mineral metabolism in the absence of sunlight (Buffenstein et al., 1994), kidney function (Urison and Buffenstein, 1994), thermoregulation (Buffenstein and Yahav, 1991, Withers and Jarvis, 1980), gastrointestinal function (Yahav and Buffenstein, 1991), microecological aspects of the naked mole rat (Bennett and Jarvis, 1995) , and finally the biological characteristics that contribute to the exceptional longevity of this, the longest-lived rodent known. Naked mole rats have been documented to live longer than 30 years in the lab (Buffenstein et al., 2008). More recently, neurophysiologists (Catania, Comer, Crish, Goldman, and Park) have analyzed the nervous system of these animals and have identified many unique neurological features that allow these animals to thrive in their environment. One of the interesting aspects of the naked mole rat nervous system is their lack of ability to sense chemical pain (capsaicin and acid) and inflammatory pain (Park et al., 2008), although they respond normally to acute pinch and heat (Kanue and Hole, 1990, Kanui et al., 1993, Park et al., 2008, Towett et al., 1993).
Taxonomy
Naked mole rats belong to the order Rodentia in that they have two incisor teeth on the upper and lower arcade that continuously grow. They are in the family Bathyergidae which includes six extant genera (Kock et al., 2006), and includes solitary (Bathyergus; Georychus; Heliophobius), and social taxa (Cryptomys, Fukomys and Heterocephalus). The Suborder is difficult to classify since the criteria for classification are the origination of the masseter muscle and the degree to which the muscle passes through the infraorbital foramen. The Bathyergidae have a small infraorbital foramen and the masseter muscle does not enter into it. Nonetheless, the lateral angle of the jaw relative to the plane of the incisor teeth appears more hystricomorph-like than Myomorph or Sciuromorph. In addition, the Bathyergidae share several Hystricognath features which include similar fetal membranes and sacculus rethralis, a fused malleus and incus, an anterior opening in the pterygoid foss, multiserial incisor enamel, the loss of the internal carotid artery, and albumin immunology (Honeycutt et al., 1991). Taxonomists thus classify the Bathyergidae in the suborder Hystricognathi. This suborder consists of three super families (the Phiomorpha (e.g. mole rats, rock rats, and cane rats), Caviomorpha (e.g., guinea pigs), and Hystricidae (e.g. porcupines). Interestingly, most hystricognath rodents are found in the western hemisphere, and there are no close relationships between members of the Bathyergidae family and other hystricomorph families.
Molecular genetic data suggest that the naked mole rat is a sister lineage to the group comprising all other genera in the family. Heterocephalus forms a monotypic genus with no morphological variation nor variation in chromosome number (n = 60). Earliest fossil records of this species were found in East Africa and date back to the early Miocene ~26 million years ago.
General Description
Good descriptions are available in the literature (Jarvis et al., 1991, Lacey and Sherman, 1991). Naked mole rats are cylindrical-shaped with short limbs and a dorsally arched back over lumbar and sacral regions (Hamilton, 1928) (Figure 45.1 ). The head is cone-shaped and blunted anteriorly in the area of a horseshoe-shaped region that surrounds the nares. Procumbent incisor teeth extend well outside the mouth and are characteristic of the Bathyergidae. Naked mole rats have either two or three molar teeth. The eyes are tiny with thickened eyelids and minute eyelashes. The auditory meatus is raised slightly and pinnae are absent. The masseter muscles are prominent.
Figure 45.1.

Photograph of typical naked mole rats. Their elongate tubular shape allows them to move through tunnels. Teeth are exterior to the mouth, and the ears and the eyes are tiny with thickened eyelids and minute eyelashes. The auditory meatus is raised slightly and pinnae are absent. The masseter muscles are prominent.
The skin is loose, wrinkled, and brownish pink in color. The body is for the most part absent of hairs with the exception of tactile hairs that are regularly arranged throughout the body and which are particularly prominent around the face and to a lesser extent on the tail. The outer edges of the hind feet are fringed with stiff hairs that increase the surface area of the foot. There are five toes on each foot. The tail is about half the length of the body.
Naked mole rats are sexually monomorphic; the external genitalia of males and females appear very similar. Males have internal testes and most females have unperforated vaginas. Males and females have similar-looking external genitalia, ano–genital distance, perineal muscles, perineal motoneurons, brain, and spinal cord (Holmes et al., 2007, Lacey and Sherman, 1991, Peroulakis et al., 2002, Seney et al., 2009) as well as femoral bone morphology and microarchitecture and strength (Pinto et al., 2009). However, these traits differ among breeders and subordinates. There is no sex difference in adult body weight.
Animals raised in captivity have body weights that vary with age, food availability, colony composition, and social and reproductive status (Jarvis, 1985, Jarvis et al., 1991, Lacey and Sherman, 1991, O’Riain and Jarvis, 1998, O’Riain et al., 2000). Most mature animals range from 25–50 grams in captivity but can get as large as 110 grams. This large range in adult body mass is uncommon among small mammals (Jarvis, 1991).
Naked mole rats are one of the slowest-growing mammals and reach stable body mass by the end of the first year of age. Individuals born in the first few litters of a newly established breeding female grow more rapidly than those of later litters (O’Riain and Jarvis, 1998). By 2 years of age all non-breeding animals have stopped growing. Should a dominant animal die or be removed from the colony many individuals will have a growth surge, and this is most pronounced if the breeding female dies.
Any animal within the colony is capable of becoming a breeder and individuals as young as 6 months or as old as 24 years have changed their social status. Should a female change her social status in the colony and become a breeder; she is capable of significant vertebral growth regardless of her age. Indeed a female that became a breeder for the first time at 24 years of age still underwent a prolonged period of lumbar vertebral growth.
Natural Habitat
The members of the Bathyergidae family are all African rodents and are found in distinct geographic locations from the southern-most tip of the continent to about 10°N of the equator. Bathyergids are found in several distinct climatic zones, at different altitudes and in a wide variety of soil types. Naked mole rats are found in the arid and semi-arid regions of eastern equatorial Africa, commonly found in Kenya, Ethiopia, and Somalia. Their habitat gets irregular rain, but it is generally hot and dry with little seasonal or daily variation in climatic conditions, followed by intense short periods of high rainfall. The soil in which they burrow varies from sandy to hard clay soils. The only evidence of the presence of naked mole rats below the surface is small volcano-like mounds of soil excavated by the mole rats during burrow extensions.
Naked mole rats live in an extensive maze of underground tunnels and chambers. Depending upon the size of the colony these may extend more than 1 mile (1.6 km) in length and may reach depths of up to 2 meters. There are multiple layers of interconnected tunnels and chambers. Some tunnels are more permanent (highways) while others near the periphery and surface are opened and closed depending on the needs of the colony. The more superficial tunnels are used for gathering food from tuberous plants, and may be used during behavioral thermoregulaion by animals basking in warmer regions of the burrow system. These near-surface tunnels also serve as a path to eliminate soil from new burrows. The deepest tunnels generally open into football-sized nest chambers in which most of the mole rats will huddle together, often resting with many animals piled up on top of each other. The nest area is lined with tufts of grass roots, skins of tubers and bulbs, and other soft detritus encountered in the burrow system. Often near the nests are deep, blind-ending tunnels that are presumed to be bolt holes and which may possibly serve as drainage systems during periods of heavy rainfall. Interconnecting tunnels that run at a slant are thought to play an important role in ventilation of the colony. Adjacent to the nest chambers in the deeper confines of the burrow system is a blind-ending toilet chamber in which naked mole rats generally urinate and defecate. When these become full, the chamber is plugged with soil and a new toilet chamber excavated. Similarly food hoards or pantry-like chambers are located near the nest; here small bulbs, corms, and roots that have been located during foraging are carried back to the nest and stored or shared among the colony.
Mole rats maintain their burrows all year round, but predominantly extend their burrow system shortly after rains occur when the dry sun-baked soils are moistened and no longer hard and impenetrable.
A sealed subterranean burrow complex protects the inhabitants from predation and also protects against climatic extremes. While surface temperatures above ground may become unbearably hot, the thermal conductivity properties of soil result in attenuated fluctuations in burrow temperatures such that burrow temperatures in the warm confines of equatorial Africa vary by less than 1°C seasonally (Bennett et al., 1988). While superficial burrows may heat up during the day and remain considerably warmer than deeper burrows, burrows at depths greater than 10 cm are thermally stable.
The gaseous atmosphere in the burrow system, and in particular in the deep nests, is extremely challenging (Bennett and Faulkes, 2000). The burrows are usually sealed and gas exchange through soil is limited. This coupled with so many organisms respirating (naked mole rats, commensals such as insects and lizards and microfauna) result in an hypoxic (10–15% oxygen) and hypercapnic (~5% CO2) atmosphere almost saturated with water, even when soils are dry. Air movement through the burrow is extremely limited and primarily facilitated by animals in tight burrows acting like pistons pushing air in front of them as they move through tunnels. Respiration in such atmospheres, especially in animals that are metabolically active or resting in large communal groups may be especially problematic. Furthermore the impact of high levels of ammonia or methane in nests and latrines could exacerbate respiration and acid–base balance.
Behavior
Naked mole rats are eusocial, strictly subterranean mammals in the wild. The term eusocial describes species that live in colonies of overlapping litters in which one or a few individuals produce all the offspring and the rest serve as functionally sterile helpers in colony maintenance, rearing juveniles and protecting the colony (Michener, 1969). The only other mammal that meets these criteria of eusociality is the naked mole rat’s close cousin the Damaraland mole rat (Fukomys damarensis). A typical naked mole rat colony has only one breeding female (queen) and a few (1–4) breeding males. The remainder of the colony is loosely categorized by body size. The larger animals tend to be the first captured in field studies and are thought to be the defenders of the colony. The smaller animals function as food gatherers and burrow diggers. With the exception of the “dispersomorphs,” animals that occasionally leave their natal colony, presumably to outbreed and form new colonies, naked mole rats only come onto the surface if flooded out of a burrow system or if their burrow system has been breached.
Despite their evolution in a formidable dark, dank environment, naked mole rats thrive in a laboratory setting and display many of the social behaviors that make them unique and of considerable interest for biological and biomedical research. In the wild, colonies of naked mole rats may vary from a few animals to an average of 70 animals to as many as 295 or more animals (Brett, 1991). In an institutional setting, colonies may be formed from breeding pairs and despite being housed in much smaller burrow systems may reach comparable sizes to wild colonies. Average colony size in captivity is ~50, with maximum colony size exceeding 120 individuals. Naked mole rats are extremely xenophobic and readily attack and kill conspecifices from foreign colonies. Members of a colony may roll in the excreta within the toilet to confer a colony scent. This enables colony members to identify each other as members of the group or strangers.
Naked mole rats are strictly herbivorous, feeding on roots, bulbs, corms, and tubers which they locate by excavating tunnels until they stumble across a large tuber or a patch of small bulbs. This is an energetically costly and blind process. NMRs dig/forage in working groups. Typically a lead member digs and passes the soil back to other members who move the soil to other areas of the tunnel system or to the surface. This effort has been described as a chain of diggers. Occasionally members will move the soil to the surface and deposit it in volcano-like structures. This is the only time tunnels are open to predation.
Mole rats generally carry back to the nest small foods (roots, bulbs) they encounter and bites of the large tubers they find. The large tubers may weigh as much as 50 kg and tend to be “farmed” in that animals will gnaw at parts of the tuber, carefully removing the often poisonous outer layers and then plug the regions they have consumed with soil, thereby enabling the tuber to regenerate. Such large tubers may sustain the colony during the dry season when burrow excavations are energetically prohibitive (Lovegrove and Wissel, 1988). Given the high cost associated with random foraging in regions where food is patchily distributed, a single animal may easily starve to death before foods are found. By working in foraging groups; animals are able to search for food in multiple areas at once and the colony increases its chances of finding a patch of food or a tuber that is large enough to sustain the entire colony. New food sources are communicated to the group through an odor trail (Judd and Sherman, 1996) and cheep-like vocalizations.
Naked mole rats have an extensive vocal communicate repertoire. Pepper et al. (1991) described 18 different vocalizations that include alarm calls, food recruitment calls, mating calls, toilet-assembly calls, and vocalizations specific to pups. These vocalizations range from 65 Hz to 12.8 kHz at 60 dB (Hefner and Hefner, 1993), but the majority of noises are low frequency (1–9 kHz) (Judd and Sherman, 1996). Lower-frequency noises propagate better underground and NMRs take advantage of this. The soft chirp is characteristic of individual members of the group and may be important in identifying individual members (Yosida et al., 2007). There are also several distinct alarm calls employed during cooperative predator avoidance and the defense of the colony.
Colony defense is an important behavior in the colony. If a major predator or member of a foreign colony is encountered in the tunnels, naked mole rats produce a pronounced alarm call and an obnoxious odor, and defenders are recruited in mass to attack the offender. Indeed even snakes may be killed by the wall of teeth they encounter, should they not capture the first mole rat they come upon.
Young pups in the colony employ many different kinds of begging behavior and emit various vocalizations to induce an appropriate response from the breeders and older siblings. One of the most common types of these behaviors is the begging for feces from adults. Adults respond by voiding feces which are directly consumed from the anus.
Reproductive Behavior
Captive colonies of naked mole rats normally contain one breeder female, and she can retain this position for many years (Jarvis, 1991). The breeding females may produce more than 1100 offspring during their breeding reign and litter size tends to increase sometime after the breeders become established. On rare occasions there may be an additional female breeding within the colony. The level of dominant female aggression appears to influence whether this occurs and in most cases the offspring of the second breeder in the colony seldom survive.
Reproductive suppression occurs by the queen’s dominance-related behavioral aggressiveness and requires direct contact between the queen and subordinates (Faulkes and Abbott, 1997, Margulis et al., 1995). The direct contact takes the form of pushing and shoving. Once dominance is established subordinates readily display subordinate behaviors, they allow the breeders to eat first and they generally tend to be walked over when they encounter a breeder in the tunnel. Aggression in a colony occurs mainly between the queen and other challenging females. Often challengers attack and kill the breeding female when she is at her most gravid or during parturition. If the dominant female dies, succession appears to be rapid, but it may take months to years for colonies to stabilize. Succession appears to come from animals that tend to be larger. This is usually the only time when fighting within a colony occurs. Typically, when the queen identifies a colony member to attack the subordinate animal submits and may even be killed.
Captive colonies of naked mole rats have several breeding males. In contrast to females, there is no evidence of breeding competition in males (Clarke and Faulkes, 1999). Both dominant breeding males and females have higher levels of sex steroid hormones than the non-reproductive colony members.
Lordosis and mounting behavior are commonly observed in the queen and breeding male respectively during the periovulatory period as is seen in other rodent species (Goldman et al., 2006). The female is restless during estrus and initiates courtship behavior by thrusting her rear end into the face of the male and lordosing. Mating occurs several times between a 2–24-hour period and often occurs in the tunnels. Ano–genital nuzzling in breeders is also observed and occurs at all phases of the estrus cycle. In this behavior the queen and a breeding male lie side-by-side in the naso–anal position. This latter behavior is not dependent on androgen status in the male, and is most commonly observed in the nest. Naso–genital stimulation of the breeding males is thought to play an important role in elevating and maintaining active hypothalamic/pituitary/gonadal axes compared to non-breeders. The latter have low levels of testosterone and extremely low levels of luteinizing hormone and furthermore are unresponsive to administration of exogenous gonadotropin releasing hormone (Faulkes et al., 1991).
Spermatogenesis occurs in both breeding and non-breeding males. Non-breeding males tend to be sterile, producing fewer and predominantly non-motile sperm (Endo et al., 2002, Faulkes et al., 1994). Similarly subordinate females for all intensive purposes appear to be sterile. Endocrine and anatomical studies reveal that these females show no signs of ovarian cyclicity or ovulation and that their ovaries are in a functionally quiescent state. This state may change should the subordinate female be isolated from the breeding female of her colony.
Since usually only one female breeds in a naked mole rat colony, the majority of animals (males and females) spend their entire lives in a suspended sterile “prepubescent” reproductive state. These subordinates spend their entire lives maintaining the colony and its burrow systems and generally helping raise the young.
A breeding female breeds continuously throughout the year and may have as many as four litters per year. The average litter size is 12, but may be as small as one pup and as large as 29. In captivity, the gestation period ranges from 64–74 days and there is a 6–11-day post partum estrus, such that the inter-birth interval is approximately 80 days. The first sign of pregnancy is a marked increase in body temperature of the breeding female (Urison and Buffenstein, 1994) and during this time the breeding female spends considerable time basking under heat lamps or in the warmer confines of the burrow. The female becomes obviously pregnant at about 40 days gestation, when her weight begins to increase significantly. Similarly in the last trimester of pregnancy the very gravid queen spends more time in the nest or basking under heat lamps, and other females and males in the colony show signs of nipple enlargement and some females even show perforate vaginas. By the end of pregnancy a female may more than double her non-pregnant body mass. Both pregnancy and lactation are energetically costly processes, increasing metabolic rate1.5–3-fold (Urison and Buffenstein, 1995). The young weigh about 1.0–2.5 g. The queen nurses the pups for 6 weeks, although they start eating solids and begging for and eating feces from older siblings at 14 days. Fecal consumption provides the appropriate microfaunal innoculum necessary to digest fiber.
Naked mole rats have the highest inbreeding coefficient known for wild mammals (Faulkes et al., 1997, Honeycutt et al., 1991) with a mean coefficient of relatedness among colony members estimated at 0.81 (Reeve et al., 1990) and there is considerable evidence that most new colonies form by fission. There is, however, strong evidence that there is an outbreeding dispersomorph caste with a morphologically, physiologically, and behaviorally distinct phenotype (O’Riain et al., 1996). This disperser caste may occasionally promote outbreeding in naked mole rats, especially in those colonies that are not successfully reproducing. These dispersers are unwilling to engage in colony maintenance, are laden with fat, especially in the neck and pelvic regions, exhibit elevated levels of luteinizing hormone, and solicit mating behaviors with non-colony members and as such are readily accepted by foreign colonies. In captivity, these animals are often seen standing on their hind limbs, trying to escape from the colony and it seems that if there is a colony escapee it is usually a member of this caste.
General Physiology
Naked mole rats have low metabolic rates, approximately 70% of that of similar-sized rodents (Buffenstein and Yahav, 1991). Outside the confines of their warm, humid equatorial burrows, naked mole rats cannot regulate their body temperature over a broad range of environmental temperatures (12–37°C) and are thus considered poikilotherms (Buffenstein and Yahov, 1991, Hislop and Buffenstein, 1994). Indeed both their metabolic and body temperature profiles at temperatures below 28°C, closely track that expected for a similar-sized lizard. At temperatures above 29°C, they display a typical mammalian endothermic pattern with the highest metabolic rate at the lowest temperature and a decline in metabolic rate, with the lowest rate at temperatures between 31–34°C; temperatures considered the apparent themoneutral zone (McNab, 1966). The relative increase in metabolic rate from 29–32°C is similar to that observed in mice housed within their thermoneutral zone and in a refrigerator at 4°C. Hence, given their lack of insulatory pelage, naked mole rats have a much narrower window in which they can thermoregulate relative to other mammals. Indeed it is only at subzero temperatures that mice show the similar hypothermic “reptilian” response of a decline in metabolism and body temperature as conditions become colder. At temperatures above 29°C, naked mole rats employ non-shivering thermogenesis (Hislop and Buffenstein, 1994) initiated by catecholaminergic innervation of interscapular brown fat (Daly et al., 1997). Brown fat is also found in the neck, inguinal, axillary, and perirenal areas. This can generate heat and may maintain a constant body temperature if the animals are housed in large groups in a humid environment that prevents evaporative cooling. Naked mole rats naturally have low thyroid hormone levels, in keeping with the warm temperatures they encounter in burrows or under institutional housing conditions. However, they show increased thyroid activity if housed in the cold for prolonged periods, thus conforming to general mammalian cold acclimation trends (Buffenstein et al., 2001).
Although naked mole rats do not enter a daily or seasonal torpor, like many small mammals do, metabolic rate decreases following prolonged food restriction and may be a mechanism to cope with sporadic and restricted food supplies (Goldman et al., 1999).
Circadian locomotor activity was not observed in general for animals monitored while they were in a colony setting (Riccio and Goldman, 2000a). However, nocturnal rhythms were noted in four males in the colony that were thought to be dispersomorphs. For individually housed naked mole rats, 65% showed robust circadian rhythms in locomotor activity, and three of four naked mole rats showed circadian rhythms of body temperature and metabolic rate (Riccio and Goldman, 2000b).
Anatomy and Physiology of Organ Systems
Although good descriptions (Hill et al., 1955) of naked mole rat anatomy have been published, very little is known about the basic physiology of naked mole rats. The following are some salient features known about the systems biology of the naked mole rat relative to above ground dwellers.
Digestive System
A unique identifying feature of naked mole rats is the large incisor teeth that are external to the mouth. Having the teeth external enables the animals to dig without the earth coming inside the mouth. Naked mole rats have either two or three molar teeth. The mandibles connect at their anterior extent by a flexible mandibular symphysis, allowing the lower incisor teeth to move independently and provide a greater range of movements and dexterity than is typical of most mammals (Catania and Remple, 2002). The incisor teeth appear to be greatly involved in somatosensory perception (Henry et al., 2006). The parotid, submandibular, and sublingual salivary glands are all well developed (Hill et al., 1955). The esophagus of the naked mole rat has keratinized stratified squamous epithelium and smooth muscle surrounding it. The stomach is monogastric. The length of the small intestine is relatively short, and leads into a very large cecum (Rechkemmer et al., 1988). The intestinal mucosa does not have distinct subdivisions based on duodenal, jejunal, or ileal villar length. The cecum accounts for 8% of the body weight (Buffenstein and Yahav, 1991). Despite the fact that members of the Bathyergidae are strictly herbivorous and eat a diet high in fiber, they nevertheless exhibit the highest digestive efficiency of all mammals (Bennett and Jarvis, 1995). This is primarily due to comparatively long retention times in the gut and highly efficient microbial fermentation processes in the cecum (Buffenstein and Yahav, 1991, Porter, 1957) coupled with coprophagy.
The cecum has a mixed microbial population of ciliate and holotrich protozoa, biflagellates, and fungi as well as an assortment of rod- and cone-shaped bacteria (Buffenstein et al., 1994, Porter, 1957) (Figure 45.2 ). These abundant microorganisms within the hindgut, provide naked mole rats with high-energy compounds in the form of volatile fatty acids (such as acetic and butyric acid) that are rapidly absorbed into the bloodstream and provide the host with more than 60% of their basal energy requirements (Parra, 1978). To further maximize energy extracted from the diet, NMRs practice coprophagy which provides a good source of proteins in the form of digested microbes.
Figure 45.2.
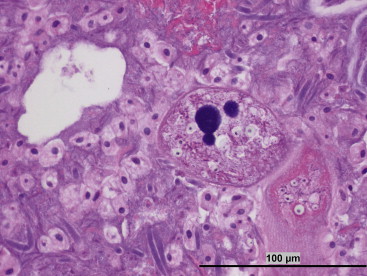
Cecal contents (H&E): The hindgut fermentation of the naked mole rat in health is associated with protozoa and bacteria. This high-power photomicrograph demonstrates several large protozoal ciliates, likely Balantidium spp., as well as several smaller flagellates. Also noted are large fusiform organisms.
Fermentation processes in the gut of the naked mole rat function most efficiently at their low body temperature of 32°C. Surprisingly, because fermentation processes are generally considered to be endothermic, cecal temperature in the mole rat is cooler than core temperature (Yahav and Buffenstein, 1992). The scientific reason for this phenomenon has not yet been explained.
The liver has four principal lobes and a number of minor ones, the main lobes being separated by deep fissures extending in most cases completely throughout the hepatic substance, and a gall bladder is absent (Hill et al., 1955).
Respiratory System
The anatomy and physiology of the respiratory system in subterranean animals is of great interest because their environment can have high levels of carbon dioxide (7–8%) and low levels of oxygen (6%) (Arieli, 1979, Contreras and McNab, 1990, Kennerly, 1964, Nevo, 1999). Naked mole rats have three turbinates (maxillo, ethmo, and sphenoidal), three lung lobes on the right and left sides, and a caudate lobe on the right side (Hill et al., 1955). Histologically, they have a preponderant double pulmonary capillary arrangement, incomplete differentiation of alveolar pneumocytes, and extension of the cuboidal epithelium to the immediate vicinity of the alveoli (Maina et al., 1992, Maina et al., 2001) (Figure 45.3 ). Observations between field animals and animals maintained in the lab were similar, suggesting the naked mole rat is intrinsically intractable to environmental shifts in its habitat. Consistent with their overall reduced metabolism, the respiratory rate of naked mole rats is about half the respiratory rate of mice (Blass et al., 2009).
Figure 45.3.
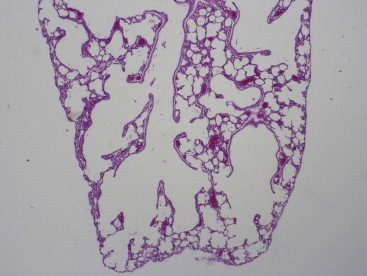
Lung (H&E) section of diaphragmatic portion of the naked mole rat lung. In contrast to rat and mouse lungs, the terminal bronchiole is ectatic; and alveolar ducts are short or non-existent, allowing alveolar sacs to branch almost directly from the terminal bronchioles. Terminal bronchioles are lined by cuboidal epithelium, the alveoli by flattened type I pneumocytes. Thus the most distal alveolar spaces abut the pleura and connect with the terminal bronchioles. The lungs thus have an “emphysematous” appearance at low magnification. The relation of this design to the low oxygen needs of the naked mole rat is not understood.
Circulatory System
Subterranean rodents have increased capillary densities in skeletal, cardia, and pulmonary tissues, as well as increased fractional volumes of mitochondria in tissue (Arieli and Ar, 1981, Widmer et al., 1997). Also, elevated myoglobin levels have been report in other subterranean species (Ar et al., 1977). In addition, lower resting heart rate and greater heart mass have been reported in ground squirrels and chipmunks (Burlington et al., 1970, Jones and Wang, 1976). In the naked mole rat, the left precaval vein is retained and is larger than the right precaval vein (Hill et al., 1955). However, little else is known about the cardiovascular system of this species. The left ventricular free wall and interventricular septum are thicker in relation to the right ventricular free wall than seen in rats and mice.
Hematopoietic System
Naked mole rats have the typical variety of leukocytes (Johansen et al., 1976) as seen under light microscopy, but there are no studies that define cells by markers or immunological function. Erythrocytes are similar to those of other mammals. The spleen is in the typical rodent location and is more slender than typical rodent spleens. Extramedullary hematopoiesis is noted in the spleen of young and pregnant animals.
The hemoglobin molecule has higher affinity for oxygen compared to most other mammalian species, and this is viewed as an adaptation for living under chronic low-oxygen conditions in subterranean burrows (Boggs et al., 1984). Naked mole rats exhibit levels similar to those in other mammals for 2,3-diphosphoglycerate in red blood cells (Johansen et al., 1976).
Integumentary System
The skin of the naked mole rat has many features which are well-suited to its warm, subterranean niche. These include the lack of an insulating layer, and a loosely folded morphological arrangement, both contributing to poikilothermic responses to changing temperatures (Daly and Buffenstein, 1998). The presence of pigment-containing cells in the dermis, rather than the epidermis, which is typical of most mammals, also reflects an environment devoid of sunlight. The lack of fur is compensated by a thicker epidermal layer and a marked reduction in sweat glands. In contrast to having fur composed of numerous short hairs, the naked mole rat has an array of sparsely distributed long vibrissa-like hairs (Crish et al., 2003, Daly and Buffenstein, 1998). The follicles of these body hairs are exceptionally large and well-innervated, similar to guard hair of furred species (Park et al., 2003) (Figure 45.4 ). The role of these sensory hairs is discussed below under somatosensory perception. The arrectores pilorum muscles are lacking, and the sebaceous glands are degenerate (Hill et al., 1955).
Figure 45.4.
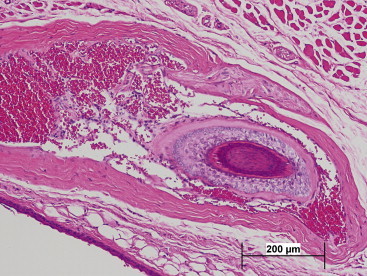
Photomicrograph of sensory hair (tactile, sinus hair, vibrissa-like hair) (H&E): The most important structure to the naked mole rat sensorium, the tactile hair is found distributed throughout the body surface, with concentration at the face, tail, and feet. These coarse long hairs have follicles that contain prominent vascular sinusoids and nerve endings. Striated muscle can be seen at the upper quadrant.
Urinary System
Water balance of naked mole rats is dependent on utilizing the water from food sources only, since pools of water are not normally available in a subterranean environment and even in captivity naked mole rats meet all their water requirements through the consumption of foods. The kidneys of the naked mole rat are unipapillate and unlike other arid-dwelling organisms are capable of only a moderate concentrating of urine (1500 mOsm) (Urison and Buffenstein, 1994). Given the high humidity in their burrows in the wild, this may be adequate for both pulmonary and cutaneous evaporative water loss. However, if high humidity is not maintained under captive conditions, evaporative water loss reaches the highest levels recorded for homeotherms (Buffenstein and Yahav, 1991). Not surprisingly, when water stressed under captive conditions, given their low metabolic rates, metabolic water production is too low to counteract high rates of insensible evaporative water loss and the animals cannot maintain plasma osmolality or body mass.
Reproductive System
Both males and females are able to breed at 6 months of age, should the opportunity arise. The external genitalia show no dramatic difference between the sexes. The males have abdominal testes and there may be a slightly greater ano–genital distance in non-breeding males and females. The only accessory sex gland is the vestibular gland. Non-breeding females have a vaginal closure membrane that is slightly dark in color. Breeding males are generally larger, thinner, and the penis is slightly more raised. Breeding females (queens) are large, elongate (Jarvis, 1991, O’Riain et al., 2000), with a swollen and raised genital area, patent vagina, and large mammae (Sherman, 1999). The uterus is bicornuate, there are no fornices, and the ovarian bursa is absent. The ovary is covered by a greatly expanded infundibulum of the uterine tube that has no fimbriae (Hill et al., 1955). The non-breeding males have hyperplastic testicular interstitial cells, reduced numbers of seminiferous tubules, and a simple epididymus without a tail for capacitation of sperm.
Reproductive suppression of non-breeding female naked mole rats is profound in that the majority of females remain in a pre-pubertal state for their entire lives. Concentrations of urinary and plasma progesterone are consistently low in subordinate naked mole rat females (Faulkes et al., 1990a) yet the pituitaries of subordinate females are responsive to GnRH (Faulkes et al., 1990b). This suggests that the primary site of reproductive hormone inhibition is at the level of the brain (Holmes et al., 2009). Non-breeding males generally have lower levels of urinary testosterone compared to breeding males, but the difference is not as large as in the females. The rapid rise in urinary progesterone in subordinate females following removal from the colony is probably not an indication of reproductive capacity in that breeding rarely occurs in less than 5–6 months (Holmes et al., 2009).
Other anatomical features associated with reproduction that are sexually monomorphic in naked mole rats include Onuf’s nucleus of the spinal cord, perineal muscles, and brain regions (Holmes et al., 2009).
The induction of ovulation has not been determined in naked mole rats, but the Damaraland mole rats are capable of spontaneous ovulation and the act of coitus may advance the onset of ovulation (Snyman et al., 2006).
Average number of mammary glands in males and females averages 11.6 ± 1.6 (Sherman et al., 1999). Neither total numbers of mammae, nor fluctuation asymmetries in mammary numbers differ significantly between males and females, or between breeders and non-breeders. Also, there is no relationship between litter sizes and numbers of mammae.
Endocrine System
Naked mole rats have an unusual endocrine profile relative to above-ground rodents, although several subterranean-dwelling rodents show similar profiles. As mentioned above, naked mole rats have low levels of sex steroids; however they also have low levels of other steroid hormones.
Living in the dark, it is not surprising that naked mole rats are naturally deficient in the “sunshine hormone”, vitamin D. Levels of 25-hydroxyvitamin D are undetectable and the principal active metabolite is low (Buffenstein et al., 1994). Surprisingly, even when exposed to sunlight for prolonged periods, hormone levels remain low and the enzyme responsible for synthesis of the active metabolite (1-alpha hydroxylase) is rapidly down-regulated. Despite these low levels, naked mole rats are able to tightly regulate serum calcium levels and adequately maintain mineral homeostasis, using passive gastrointestinal absorption and vitamin-D-independent mechanisms to retain calcium, magnesium, and phosphorus within the body (Skinner et al., 1991).
Thyroid hormones and thyroid-stimulating hormones are also maintained at low levels for mammals and may contribute to the low basal metabolic rate of this species (Buffenstein et al., 2001). Thyroid morphology is similar to that of most mammals and the thyroid responds positively to cold stress such that thyroid follicular cell height and free throxine levels were significantly greater in cold-acclimated individuals.
Insulin levels in naked mole rats cannot be detected using standard rodent antibody-based assays, suggesting that naked mole rats either have extremely low levels of insulin or have an insulin molecule with a different protein composition to that of other rodents. Although we have not as yet attempted to sequence naked mole rat insulin, there is considerable evidence that the insulin structure in the hystricognaths (e.g. guinea pigs) is substantially different to that of laboratory mice. Unlike laboratory mice, and in a similar morpholothe, insulin-producing cells are distributed in a mantle around the periphery of the islet, rather than in the more usual location of the central core. Similarly, glucagon-producing cells are not located in the mantle, as previously reported in most vertebrates. Indeed, only horses show a similar pancreatic arrangement. Like most mammals, a greater number of insulin-producing cells than glucagon-producing cells are present, and somatostatin-producing and pancreatic polypeptide-producing cells are considerably more scarce and randomly scattered within the islet (Kramer and Buffenstein, 2004) (Figure 45.5 ).
Figure 45.5.
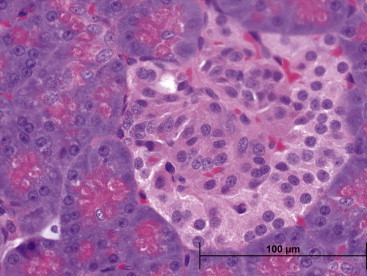
Photomicrograph of pancreatic islet cells (H&E); The islet distribution of beta and alpha cells which produce insulin and glucagon respectively have a distinct zonal arrangement with the mantle cells of beta cells framing a centrally located mass of glucagon producing cells. Human islets are not organized in this manner, but cells of all types are rather randomly distributed within the islet.
Naked mole rats have low fasting blood glucose levels, and show an impaired glucose tolerance test, maintaining elevated glucose levels for more than 2 hours. When given a physiologically determined dose of human insulin, blood glucose rapidly drops to extremely low levels (20 mg/dl) and remains there for several hours. The exquisite sensitivity to human insulin coupled with the abnormal glucose tolerance test and undetectable levels of insulin suggest that naked mole rats naturally only produce small quantities of this hormone and that this is usually sufficient for their needs, given their predominant reliance on volatile fatty acids produced during fermentation for their energy requirements. As such, they are unable to regulate blood glucose when given a supraphysiological dose of sugar.
Little is known about the hormones of the adrenal gland. The right adrenal is medial to the right kidney and the left adrenal is ventro-medial to the left kidney. There are no published data on the adrenal cortical axis in naked mole rats. Mole rats have very low levels of corticosterone compared to mice and rats, but predominantly secrete cortisol. It is not known if these two hormones have identical functions or have differences in their regulatory mechanisms.
Apart from the hormones described above, little if anything is known about other hormone secretions and functions and considerable research is needed to glean a better understanding of the endocrine function of this species.
Nervous System
The naked mole rat brain has the normal anatomy of a typical rodent brain (Xiao et al., 2006). What makes the naked mole rat different is the relative amount of cortex devoted to the different sensory inputs. The somatosensory system occupies one-third of the sensory cortex and appears to be a more important sensory input (Henry et al., 2006).
Unlike the majority of vertebrate species, subordinate and breeding naked mole rats lacked vassopressin innervation of the lateral septum and vassopressin-immunoreactive cells in the nucleus of stria terminalis (Rosen et al., 2007). Instead, the dorsomedial septum contained dense vassopressin-immunoreactive innervation, which did not vary with sex or breeding status. Immunoreactive oxytocin fibers were found in the periaqueductal gray, locus coeruleus, parabrachail nucleus, nucleus of the solitary tract, and nucleus ambiguous (Rosen et al., 2008). The high levels in the nucleus accumbens and preoptic area are associated with maternal behavior in other animals (Francis et al., 2000). Breeders, regardless of sex, had more cells than subordinates in the ventromedial nucleus of the hypothalamus and a larger volume of the bed nucleus of the stria terminalis, paraventricular nucleus, and medial amygdale (Holmes et al., 2007). Non-breeding males and females did not differ on any measure.
Special Senses
Somatosensation
The sensation of touch is an important sense in the naked mole rat. The area of the sensory cortex devoted to the sensation of touch is much larger in comparison to other mammals (Catania and Remple, 2002, Henry et al., 2006). The queen suppresses reproductive activity via touch by pushing and shoving (Faulkes and Abbott, 1997). Like most mammals, naked mole rats have well-developed facial vibrissae (whiskers). However, they also have a system of vibrissa-like hairs that are regularly spaced in rows over the body, and naked mole rats have a robust and accurate orientating response when these hairs are stimulated (Crish et al., 2003). A large number of vibrissa-like hairs are also present on the tail, consistent with the naked mole rat moving as quickly in reverse as in the forward direction.
Naked mole rats also naturally lack neuropeptides and behaviors associated with signaling of chemical irritants on the skin and nasal cavity (LaVinka et al., 2009, Park et al., 2008) which will be discussed in the Models section.
Olfaction and Gustation
Naked mole rats demonstrate prominent scent-marking behaviors and members of the colony are frequently seen rolling in the toilet areas of housing systems. Yet, reproductive suppression does not appear to be influenced by hormones or pheromones present in the urine from the breeding animals (Smith et al., 1997).
In most vertebrates, chemical (pheromonal) communication involves the vomeronasal organ, a structure within the nasal cavity. For rodents in general, the vomeronasal organ is proportionally large (relative to body size) compared to other mammals (Smith et al., 2005). Yet, the vomeronasal organ in naked mole rats is remarkably small, about ten-fold smaller than vomoeronasal organs in social rodents such as the meadow vole and mouse. Also, the naked mole rat is the only known mammal to show no post-natal growth of the vomeronasal organ (Smith et al., 2007). This supports the notion that pheromones and chemical sense do not play a major role in sexual suppression in naked mole rats. With regards to traditional odor sensation involving the olfactory system (apart from the vomeronasal system), naked mole rats perform as well as mice in distinguishing odors in a behavioral laboratory test (LaVinka et al., 2009).
Auditory
Naked mole rats do not have pinnae (important for sound localization), and have narrow external ear canals. The majority of subterranean rodents have ear canals filled with cerumen (Burda et al., 1990). Definitive anatomical descriptions of the auditory system are not available for the naked mole rat with one exception (Hefner and Hefner, 1993). They described the brainstem auditory nuclei as being relatively small but present. Generally though, middle ears are vestigial and the inner ear resembles the guinea pig anatomically more than murid rodents in subterranean rodents (Begal et al., 2007).
In contrast to other rodents, the naked mole rat has hearing and communication calls that are primarily in the low-frequency range (Pepper et al., 1991). This is probably because low-frequency sound propagates well through soil. However, naked mole rats perform poorly in tasks based on sound localization ability (Hefner and Hefner, 1993). This may reflect that these tests are not appropriately designed to match the unusual (tubular) environment and behavior of this species.
Vision
Subterranean rodents live in a light-free environment and as a consequence have very small eyes and reduced or absent visual capacities. The naked mole rat is not an exception. They have a rod-dominated retina and have cones that are S-opsin dominated which is similar to other Bathyergidae and unlike any other mammalin species (Peichl et al., 2004). The eye contains 11-cis retinal, the chromophore of visual pigment. The optic nerve cross-sectional area is 10% of the gerbil and had an ERG response typical of other mammals but extremely attenuated and with much slower kinetics (Hetling et al., 2005). They display a variable degree of retinal detachment which may be associated with the less than gelatinous nature of the vitreous humor (Hetling et al., 2005, Nikitina et al., 2004). The superior collicullus and lateral geniculate nucleus are very small in the naked mole rat (Crish et al., 2006). Collectively these findings show that the retinogeniculocortical system in the naked mole rat is considerably smaller than that of rodents that rely heavily on their visual system, but is nevertheless less regressed than that of the extensively studied blind mole rat; this may facilitate limited responses to visual stimuli (Xiao et al., 2006). Indeed all these findings suggest the naked mole rat eye functions for amorphous light detection that may serve for circadian function and/or for the simple assessment of whether or not the burrow has been breached and requires repair.
Magnetoreception
Magnetoreception is magnetic compass orientation. It has been reported in the African Ansell’s mole rat (Burda et al., 1990; Marhold et al., 1997), the blind mole rat Spalax ehrenbergi (Middle Eastern mole rat unrelated to the Bathyergidae; Burda et al., 1991), the Siberian hamster (Deutschlander et al., 2003), and the laboratory mouse (Muheim et al., 2006). There are no reports in the naked mole rat.
Aging
Aging, the progressive decline in functional morphology that over time leads to increased mortality risk is markedly abrogated in the naked mole rat. Naked mole rats are the longest-lived rodents known, living approximately 30 years in captivity; 5.3 times longer than predicted on the basis of body size and nine times longer than similar-sized laboratory mice. In captivity both breeders and non-breeders have similar maximum lifespan, whereas in the wild the more protected breeders live considerably longer (17 years; S. Braude, personal communication) than working, predation-vulnerable subordinates (4 years). Not only do naked mole rats have an extraordinarily long lifespan but they show sustained good health for at least 85% of their maximum longevity and show no age-associated acceleration in mortality risk. From the age of 2 to 20 years naked mole rats maintain lean muscle mass (O’Connor et al., 2002), bone health (Pinto et al., 2009), mitochondrial mass, and enzyme activities (Ungvari et al., 2008). Furthermore they sustain their metabolic rate (O’Connor et al., 2002), level of gastrointestinal absorption (Yang and Buffenstein, 2002), and cardiovascular youthfulness (Csiszar et al., 2007) in addition to their level of activity and reproductive potential well into their third decade (Buffenstein, 2008).
Naked mole rats support the evolutionary theory of aging which posits that species that have evolved in areas of low extrinsic mortality such as thermally buffered, sealed burrows that are protected from predation will evolve mechanisms better-suited for extended tissue maintenance and concomitant longevity; whereas those species living in a dangerous environment fraught with challenging climatic conditions and/or high predation risk will evolve life history traits that facilitate early reproduction and shorter lifespans. Naked mole rats also support the environmentally selected lifespan theory which posits that animals that live where resource availability is unpredictable will exhibit extended longevity. The life history of naked mole rats provides some support for this theory: location of food is a random, blind, and energetically costly process and food resources are patchily distributed. The low metabolic rate of NMRs may help them survive periods of limited resources. Extended longevity, like lower extrinsic mortality, is correlated with group-living, such that cave-roosting bats, social insects, and humans all have long lifespans. The cellular, molecular, and biochemical mechanisms facilitating this extended longevity, good health, and delayed aging are however poorly understood.
Naked mole rats do not support the telomere theory of aging. Telomere length and maintenance will govern the number of times a cell may divide and thereby replace damaged cells. Naked mole rats, like humans, have relatively short telomeres compared with mice and lack telomerase and as such cannot maintain telomeres indefinitely. These will shorten with each replication passage and eventually become too short for further replication events.
Similarly, the naked mole rat only provides equivocal support for the most widely accepted theory of aging, the oxidative stress theory of aging that posits that the characteristic traits of aging are due to accrued damage by unchecked reactive oxygen species (ROS), generated during aerobic metabolism. Naked mole rats produce similar amounts of ROS to those produced by shorter-lived rats and mice (Lambert et al., 2007), do not have a stellar antioxidant defense (Andziak et al., 2006), and accrue more oxidative damage even at a young age than do shorter-lived species (Andziak et al., 2006). However, accrued damage appears to be maintained at steady-state levels throughout their long lives (Andziak and Buffenstein, 2006), suggesting that these animals have a higher tolerance threshold for damage prior to inducing mechanisms to remove and repair accrued oxidation products.
Currently, the mechanisms facilitating prolonged good health and lifespan remain elusive and new studies are focusing on mechanisms that may facilitate protein and genomic stability even in the face of cellular endogenous and environmental insults.
Husbandry
Naked mole rats are popular zoological attractions (Petry, 2003) and are also studied at numerous research institutions (Artwohl et al., 2002). They are typically housed at 28–30°C, and humidity levels at 50–60% relative humidity. If housing temperatures are below 25°C, mole rats will stop breeding (Woodley, 2002). The only way to obtain this environment is to curtail the ventilation into the room significantly. The benefit of turning on the light–dark cycle has not been proven since colony-housed animals have not demonstrated a circadian rhythm. As in most facilities that house breeding rodents, noise should be minimized. Wall-mounted shelves can be used or racks that do not rock and have locking wheels can be used. Some facilities put the rack wheels in sand to reduce vibrations in the floor extending into the rack.
Various bedding material can be provided. All current types of mouse bedding have been used with naked mole rats. Each of these kinds of bedding has different pros and cons. For example, wood shavings from pine and ash may have chemicals or odors that may be irritating, causing scratching of the skin. Unless it is of a fine high quality it can also cause splinters in the feet. The advantage of wood shavings is that the shavings are less dusty and tend not to stick to skin. Vermiculite and corn cob bedding are particularly harsh. Some types of paper bedding produce fine particles of dust that may block noses and irritate eyes of naked mole rats. A highly absorbent material should be placed in the toilet chamber and paper towel, tissues, and corn husks should be given in addition for nesting. Naked mole rats like to move the bedding material and typically gather materials into the nesting chamber. Occasionally naked mole rats will make a ramp with bedding material that can be used to escape from their enclosure.
No water is generally provided when housing naked mole rats in captivity. Fresh food should thus always be available. Food items such as sweet potato can be soaked in water prior to offering the food. The high humidity in the room will diminish insentient water loss. The staple diet for naked mole rats is sweet potato. Some institutions soak the tuber in 10% bleach or weak acid (lemon/vinegar) for a period of time since fecal organisms can regularly be cultured from them. Sweet potatoes are difficult to disinfect because of their porous nature. Additional fresh foods include apple, grapes, bananas, oranges, melons, squash, cucumbers, lettuce, and peppers. These can be supplemented with frozen beans and corn. Mole rats are particularly fond of melons, however, these water-rich foods lead to frequent urination and the need to clean cages more frequently. A vitamin supplement such as Pronutro (Wadeville, South Africa) is frequently offered.
In a laboratory setting, caging is typically made from caging materials that are readily sanitized in a cage washer. Housing systems consist of single and multiple cages that can be connected via 2-inch-diameter pipe. Sanitizing complex caging that utilizes connecting pipes is more labor-intensive since pipes should be periodically scrubbed. Enrichment for naked mole rats includes offering nesting materials for making a nest, increased cage complexity (Figure 45.6 ) that would include multiple pipe connections or soils in which to burrow. One strategy is to provide un-husked corn. The animals eat the corn and make a nest out of the husk material. Naked mole rats are relentless chewers and constantly gnaw at the corners of chambers. Blocking tubes with whole corn or yams will stimulate burrowing behavior in the tubes and may possibly reduce repairs to the housing structure. That said, care must also be taken to check for holes and loose tubes or lids to prevent escapes from occurring.
Figure 45.6.

Example of a complex caging system used to house colonies of naked mole rats. Cages are connected by 2-inch vinyl tubes which allow the naked mole rats’ functional separations of toilet and nesting areas.
Cages can be spot cleaned daily by removing soiled food and spoiled substrates, particularly in the toilet chamber. The entire system can be cleaned every 2–4 weeks with scent-free mild soaps.
Adult naked mole rats can be easily marked using felt-tipped pens which may last up to 10 days. They do not seem to lick the markings off themselves. Toe clipping and transponders can also be used (Braude and Ciszek, 1998). Subcutaneous implanted transponders however can migrate to different areas of the body and often fall out. Tattoos have also worked in naked mole rats.
Colony Health
Neonatal and weanling mortality is common in naked mole rats. Facilities should severely limit traffic and curtail noises in rooms with neonates. Naked mole rats respond to noises and vibrations and can bury or carry neonates as part of their care-taking behavior. Sometimes new litters die simply because the mother does not take notice of the neonates.
Problems associated with the birthing process include dystocia and endometritis. Naked mole rats can have very large litters and uterine fatigue can occur. Oxytocin given at mouse dosages helps this condition. Queens with endometritis typically die a few days after birth. Opportunistic bacteria can be isolated from the uterus.
The queen’s aggression is part of the eusocial life of naked mole rats. Aggression is heightened when other females are trying to usurp the queen’s control. Fight wounds can be observed when this occurs and are one of the most commonly observed clinical problems. These often result in staphylococcal infections. Wounds should be treated with organic iodines.
Naked mole rats have various non-infectious conditions that occur on occasion. Bloating is not an uncommon sign. It is often associated with weaning and can be associated with ingestion of less-complex carbohydrate food sources. Usually this sign will abate with time, but occasionally the bloat is debilitating, leading to dehydration and can cause death. Clinical evaluation and treatment are necessary in these cases.
A metastatic calcification condition has been observed (Margulis, personnal communication (Figure 45.7, Figure 45.8 ). The cause for the condition was probably dietary-related since a higher level of rodent chow was used and naked mole rats typically eat diets low in calcium. Some calcium deposits may be dystrophic since they can be seen in areas of inflammation such as around fight wounds and foot lesions. The foot lesions would be considered painful. The calcium deposits are made of a pasty white material that appears like an abscess, but are often sterile and radio-opaque. Similarly London Zoo reported nephrocalcinosis and mineral deposition in soft tissues in animals fed a calcium-rich diet supplemented with vitamin D.
Figure 45.7.
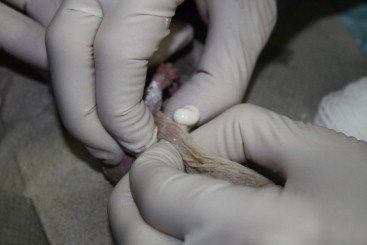
Metastatic calcification deposit being expressed. This condition should be considered for any mass in a naked mole rat that appears like an abscess.
Figure 45.8.
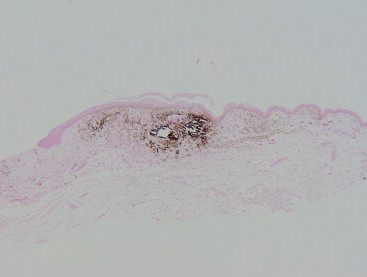
Photomicrograph (Von Kossa). A focus of cutaneous mineralization which is stained dark.
Few pathogens have been reported in naked mole rats, which is noteworthy considering the preponderance of naked mole rat colonies in zoos and conventional institutional rodent facilities. Zoos are environments that have less control over the spread of pathogens. Naked mole rats seem to be extremely resilient to pathogens and other toxic insults. Perhaps naked mole rats are less susceptible to murine pathogens because of their lower resting body temperature (33°C), or perhaps because naked mole rats are typically housed at higher room temperatures than other rodents, necrosis sets in rapidly when they die such that pathogens and cause of death cannot be easily identified. As such, incidences of pathogenic outbreaks may not be reported. Surveys of zoo pathologists however support the premise that naked mole rats are extremely resilient and resistant to infectious agents.
Ross-Gillespie et al. (2007) described an acute coronavirus epizootic that caused acute diarrhea, dehydration, and severe enteric hemorrhaging that killed 161 of the 365 animals (44.1%). Age (younger animals more susceptible), sex (males were more susceptible), and the degree of inbreeding (inbreeding increased susceptibility) influenced mortality. The source of the coronavirus was not identified.
An acute death syndrome has been noted at multiple institutions and zoos. It is characterized by multiple deaths, sometimes in multiple species (naked mole rats and Damaraland mole rats) in animals that are in good body condition. Sometimes diarrhea is noted. Available tests for clostridial enterotoxin and mycotoxins were negative. Edematous ileum, cecum, and colon were noted histologically. Since naked mole rats have a complex microflora in this region of the intestine, consideration of dysbiosis as described in rabbits or a pathogen that requires special culture conditions should be made for the cause. Naked mole rats occasionally get localized abscesses where opportunistic pathogens such as Pasteurella, Pseudomonas or Staphylococcus sp. are isolated. Helicobacter sp. have not been identified in NMRs.
Small colonies are particularly vulnerable to dehydration and cracked skin in response to low humidity. Give small colonies mini-chambers to increase humidity. If skin is dry add moist cloths and/or supplement nesting with fresh corn husks, lettuce, or fresh (washed) grass. In the past we have rubbed Vaseline intensive care or hypoallergic moisturizer creams onto skin with considerable success. Recently however, a small proportion (up to 10% of a colony) of animals has gone into shock and although we were able to resuscitate them, we have replaced this moisturizing spa treatment with a rub of olive oil. Ring tail has also been observed in naked mole rats (Figure 45.9 ).
Figure 45.9.

Ringtail in a naked mole rat.
The immune function of naked mole rats has not been described. Consideration for susceptibilities to experimental infections should be considered. Artwohl et al. (2009) reported 100% mortality in naked mole rats given a recombinant herpes simplex virus type 1 that was avirulent to mice. The virus was administered via scarification of the foot.
Neoplasia has not been reported in naked mole rats (Buffenstein, 2008).
It is possible but not substantiated adequately by pathology that old naked mole rats may suffer from cardiovascular insults such as aneurysms and possible strokes.
A couple of considerations when treating naked mole rats are that they take a long time to recover from anesthesia, and the reintroduction of animals back into the colony can cause a xenophobic response. Gaseous anesthesia is preferred and dosages of analgesics are much lower than dog dosages. In addition, the greater amount of time the naked mole rat is removed from the colony, the greater the chance of queen aggression on the animal. Gloves should be cleaned before removing the animal, minimal disinfectants should be used that would impart a foreign scent, and post-procedural monitoring should be performed to be the sure the animal is not killed after reintroduction. One tactic is to roll the animal in the toilet area before placing it back in the colony. Animals that are mobbed by colony members after reintroduction should be removed again. Some shoving by the queen is acceptable and usually does not last long.
Use in Research
Model of Social Cooperation
Because naked mole rats are social and have cooperative behaviors, the study of their behavior has more applicability to people. Functional castes within a colony have been identified (Bennett and Faulkes, 2000, Sherman et al., 1992), and some means of communication. No wonder they are popular attractions at zoos and in the biology class.
Model of Reproductive Suppression
Naked mole rats are one of only two species of mammals identified as being eusocial. The advantage of studying this species in comparison to the Damaraland mole rats (the other eusocial mammal) is that they are much smaller and colonies reach much higher numbers of animals. The basic mechanisms involved in reproductive suppression have not been elucidated, but there appears to be some psychological aspect to it (Faulkes and Abbott, 1997).
Model of Somatosensory Processing
The sensation of touch is an important sense in the naked mole rat since they inhabit an environment that is devoid of light. The area of the sensory cortex devoted to the sensation of touch is larger in comparison to other mammals (Catania and Remple, 2002, Henry et al., 2006). Like most mammals, naked mole rats have well-developed facial vibrissae (whiskers). However they also have a system of vibrissa-like hairs that are regularly spaced in rows over the body, and naked mole rats display a robust and accurate orientating response when these hairs are stimulated (Crish et al., 2003). The highly organized pattern of the body hairs and the decisive orienting responses to their touch suggest that the hairs convey somatosensory-based spatial information.
Since naked mole rats have no fur, the vibrissa-like body hairs are easily accessed for precise stimulation by investigators. The superior colliculus, a midbrain center involved in directing orientation movements to somatosensory, auditory, and visual stimuli, provides an excellent area for studying the neural basis of orientation to somatosensory stimuli in the absence of vision (which is dominant in the superior colliculi of more visually guided animals) (Crish et al., 2006). Taken together, the tractable number of body hairs (~50), their ease of access for stimulation, and the lack of visual inputs to the superior colliculus make the somatosensory system of the naked mole rat an excellent system for studying somatosensory processing as it relates to orientating behavior.
Model of Pain Insensitivity
Naked mole rats show a unique and remarkable lack of cutaneous pain-related behaviors to two potent chemical irritants, capsaicin (from chili peppers) and acid (Park et al., 2008), and a lack of trigeminal pain-related behaviors to capsaicin and ammonia (LaVinka et al., 2009). Furthermore, when exposed to inflammatory insults or known mediators (e.g. Freunds’ adjuvant), naked mole rats do not display sensitization to heat on the inflamed area (thermal hyperalgesia) as do other mammals. In contrast, naked mole rats do display normal (mouse-like) pain behaviors to mechanical stimuli (pinch) and heat.
Naked mole rats display several physiological and anatomical features that explain their insensitivity to noxious chemical and inflammatory stimuli. Most notably, nerve fibers in the skin that normally respond to acid in other animals are completely insensitive to acid in naked mole rats (Park et al., 2008). Also, while nerves in the skin do respond to capsaicin, those nerves lack the neurotransmitters that would usually be released onto spinal cord cells to signal pain (Park et al., 2003). The neurotransmitters that are missing are substance P (SP) and calcitonin gene related peptide (CGRP).
Why do naked mole rats lack pain from chemical irritants? As mentioned earlier, naked mole rats have a very unusual combination of ecological and social characteristics. They are fully subterranean, extremely social, and they live in colonies with many individuals. In other words, naked mole rats live in large numbers in very tight quarters and in very poorly ventilated spaces where metabolic activity increases carbon dioxide (CO2) and ammonia to extremely high levels. In other mammals, exposure to chronically high levels of CO2 and ammonia induces pain, especially in the eyes and nose. It appears that the insensitivity to chemical irritants in naked mole rats is an adaptation to living in an otherwise painful environment.
Model of Cutaneous Substance P (SP) and Calcitonin Gene Related Peptide (CGRP) Knockout
In addition to functioning in certain aspects of pain processing, SP and CGRP also play an important role in regulating blood flow, for example in thermoregulation and digestion. The absence of these neuropeptides in naked mole rats may be related to the finding that naked mole rats are the only known mammalian poikilotherms, and they may not require cutaneous vasodilation to regulate body temperature. Cutaneous vasodilation in other mammals is mediated by nerve cells that release SP and CGRP onto small arteries in the skin to increase regional blood flow and heat transfer. Of interest, naked mole rats have SP/CGRP innervation to mesenteric arteries, which presumably subserves regulation of blood flow related to digestion (Park et al., 2003).
SP and CGRP have been implicated in a direct effector role in tissue maintenance. Absence of this peptidergic innervation raises questions about the capacity of the skin of naked mole rats to respond to insult and could explain the extreme susceptibility to herpes simplex I administered cutaneously (Artwohl et al., 2009).
Model of Delayed Puberty and Prolonged Hypogonadism
Hypogonadism, be it induced by delayed puberty, exercise-induced ammenorhea, or testicular malfunction, impacts upon bone quality, resulting in impaired bone strength with reduced bone mass and area as well as changes in trabecular architecture (Chevalley et al., 2009). Suboptimal skeletal development during puberty affects long-term bone strength, leading to an increase in fracture risk later in life (Yingling et al., 2008). Current animal models to study delayed puberty and hypogonadism have several drawbacks. Ovariectomy models often manifest with high GnRH, since it is inhibition by sex steroids and inhibition is reduced, whereas GnRH antagonists affect not only the entire hypothalamic–pituitary–gonadal axis, but also have a tendency to increase body weight, serum IGF-1 levels, and complete suppression of the onset of puberty is dose-dependent (Roth et al., 2000; Yingling and Taylor, 2008). While these studies have yielded considerable information in this regard, the short-term nature of these studies as well as the extended duration of bone remodeling and turnover, limits these studies in what they can tell us about “catch-up” growth or chronic hypogonadism and begs the question of whether or not there is a natural animal model for long-term hypogonadic effects on bone.
One such novel animal model may be the naked mole rat. They are naturally suppressed from entering puberty and do not show differences in other hypothalamic pituitary hormones or serum IGF-1 levels, a clear advantage to other models. Further, “puberty” can be initiated within 7 days once isolated from the dominant female and levels of the sex steroid hormones in these recently isolated NMRs reach concentrations comparable to those of their long-term breeding counterparts (Clarke and Faulkes, 1997, Clarke and Faulkes, 1998). This activation of gonadal activity and fertility is extremely rapid, especially when considering that non-breeders may have been reproductively suppressed for many years, and even 24-year-old females are capable of exhibiting rapid changes from reproductive suppression to ovarian cycling. Further studies in which hormone status may be experimentally manipulated in young individuals both prior to and after attainment of adult mass may shed more light as to whether or not bone morphology and mechanical properties are sustained by sex steroid hormone-independent mechanisms. Elucidating the mechanisms facilitating sustained bone structure and strength in hypogonadic subordinates may yield pivotal insights into potential mechanisms and therapies for sustaining bone in delayed puberty and exercise-induced hypogonadism.
Model of Bone Elongation
Naked mole rat queens have significantly longer lumbar vertebrae for their body size compared with all other colony members (Jarvis et al., 1991, O’Riain et al., 2000). This finding parallels eusocial insects in that the dominant breeder is physically different. The length of the spine is proportional to the number of pregnancies experienced and suggests that hormones related to pregnancy may have a role in bone growth (Henry et al., 2007). Queens no longer experienced net gain in lumbar spine length after eight pregnancies, but did have some transient elongation during pregnancy after the eighth pregnancy (Dengler-Crish and Catania, 2009).
Model of Aging
Few animal models used in aging studies show slow rates of aging with affiliated prolonged good health-span and lifespan. Rather, traditional aging models are chosen primarily because such animals have poor defenses against aging and are short-lived. For example the most common mammal used in aging is the C57Bl/6 mouse that only lives 50% as long as predicted by body size. Use of short-lived species enables rapid evaluation of whether experimental manipulation of environmental (e.g., caloric restriction (CR)) and/or genetic factors alter lifespan (Miller and Nadon, 2000). Although we recognize the many advances in understanding aging processes in these short-lived models, we do not know if their lifespan extension mechanisms are pertinent to long-lived mammals, such as humans, that naturally age slowly. Long-lived species may possess certain traits germane to retarding aging that enable them to attain their impressive longevity (Austad, 2009). Thus, a complementary approach for studying mechanisms of aging, that we as humans would like to use in translational research, would be to exploit the untapped resource of natural variation in longevity and focus on long-lived successfully aging species such as the naked mole rat.
Similarly, since naked mole rats do not show any signs of spontaneous neoplasia during aging and also exhibit negligible declines in cardiovascular function, bone quality, and cognitive function; this unusually long-lived and healthy species may also prove to be useful models in many age-associated diseases. As such this species may reveal novel, potentially causal, mechanisms naturally involved in abrogated aging and age-associated diseases. Such knowledge will enable therapies aimed at activating mechanisms to delay human aging, prevent degenerative disease, and extend quality of life in old age.
References
- Andziak B., O’Connor T.P., Buffenstein R. Antioxidants do not explain the disparate longevity between mice and the lonest-living rodent, the naked mole rat. Mechan. Aging Dev. 2005;126:1206–1212. doi: 10.1016/j.mad.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Andziak B., O’Connor T.P., Qi W., DeWaal E.M., Pierce A., Chaudhuri A.R. High oxidative damage levels in the longest-living rodent, the naked mole rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Ar A., Arieli R., Shkolnik A. Blood-gas properties and function in the fossorial mole rat under normal and hypoxic hypercapnic atmosphere conditions. Resp. Physiol. 1977;30:201–218. doi: 10.1016/0034-5687(77)90031-7. [DOI] [PubMed] [Google Scholar]
- Arieli R. The atmospheric environment of fossorial mole rat (Spalax ehrenbergi): effect of season, soil texture, rain, temperature and activity. Comp. Biochem. Physiol. A. 1979;63:569–575. [Google Scholar]
- Arieli R., Ar A. Blood capillary density in heart and skeletal muscles of the fossorial mole rat. Physiol. Zool. 1981;54:22–27. [Google Scholar]
- Artwohl J., Hill T., Comer C., Park T. Naked mole rats: unique opportunities and husbandry challenges. Lab. Anim. 2002;31(5):32–36. doi: 10.1038/5000156. [DOI] [PubMed] [Google Scholar]
- Artwohl J., Ball-Kell S., Valyi-Nahy T., Wilson S.P., Lu Y., Park T.J. Extreme susceptibility of African naked mole rats (Heterocephalus glaber) to experimental infection with Herpes Simplex virus Type 1. J. Am. Assoc. Lab. Anim. Sci. 2009;59(1):83–90. [PMC free article] [PubMed] [Google Scholar]
- Austad S.N. Comparative biology of aging. J. Gerontol. 2009;64A:199–201. doi: 10.1093/gerona/gln060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begal S., Lange S., Schleich C.E., Burda H. Acoustics, audition and auditory system. In: Lacey W.A., Patton J.L., Cameron G.N., editors. Subterranean Rodents, News from Underground. Springer-Verlag; Berlin, GE: 2007. pp. 97–112. [Google Scholar]
- Bennett N.C., Jarvis J.U.M., Davies K.C. Daily and seasonal temperatures in the burrows of Africn rodent moles. S. Afr. J. Zool. 1988;23:189–195. [Google Scholar]
- Bennett N.C., Jarvis J.U.M. Coefficients of digestibility and nutritional values of geophytes and tubers eaten by southern African mole rats. (Rodentia: Bathyergidae) J. Zool. 1995;231:179–186. [Google Scholar]
- Bennett N.C., Faulkes C.G. African Mole Rats: Ecology and Eusociality. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]
- Blass, G.R.C., Smith, E., Lewin, G.R., Minshall, R.D., Park, T.J., 2009. Resistance to CO2-induced pulmonary edema in naked mole rats and free-tailed bats. Society for Neuroscience Abstracts 196.18.
- Boggs D.E., Kilgore D.L., Birchard G.F. Respiratory physiology of burrowing mammals and birds. Comp. Biochem. Physiol. 1984;77A:1–7. [Google Scholar]
- Braude S., Ciszek D. Survival of naked mole rats marked by implantable transponders and toe-clipping. J. Mammal. 1998;79(1):360–363. [Google Scholar]
- Brett, R.A., 1986. The ecology and behavior of the naked mole rat Heterocephalus glaber (Ruppell) (Rodentia: Bethyergidae), Ph.D. dissertation, University of London, United Kingdom, 391 pp.
- Brett R.A. The ecology of naked mole rat colonies: burrowing, food, and limiting factors. In: Sherman P.W., Jarvis J.U.M., Alexander R.D., editors. The Biology of the Naked Mole Rat. Princeton University Press; Princeton, NJ: 1991. pp. 137–184. [Google Scholar]
- Buffenstein R., Yahov S. Is the naked mole rat Heterocephalus glaber an endothermic yet poikilothermic mammal? J. Therm. Biol. 1991;16:227–232. [Google Scholar]
- Buffenstein R., Yahav S. The effect of diet on microfaunal population and function in the caecum of a subterranean naked mole rat, Heterocephalus glaber. Brit. J. Nutri. 1991;65:249–258. doi: 10.1079/bjn19910084. [DOI] [PubMed] [Google Scholar]
- Buffenstein R., Jarvis J.U.M., Opperman L.A., Cavaleros M., Ross F.P., Pettifor J.M. Subterranean mole rats naturally have an impoverished calciol status, yet synthesize calciol metabolites and calbindins. Eur. J. Endocrin. 1994;130:402–409. doi: 10.1530/eje.0.1300402. [DOI] [PubMed] [Google Scholar]
- Buffenstein R., Woodley R., Thomadakis C., Daly T.J.M., Gray D.A. Cold-induced changes in thyroid function in a poikilothermic mammal, the naked mole rat. Am. J. Physiol. J. Regul. Int. Comp. Physiol. 2001;280:R149–R155. doi: 10.1152/ajpregu.2001.280.1.R149. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole rat: insights from a successfully aging species. J. Comp. Physiol. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- Burda H., Marhold S., Westenberger T., Wiltschko R., Wiltschko W. Magnetic compass orientation in the subterranean rodent (Cryptomys hottentotus Bathyergidae. Experientia. 1990;46:528–530. doi: 10.1007/BF01954256. [DOI] [PubMed] [Google Scholar]
- Burda H., Bruns V., Hickman G.C. The ear in subterranean Insectivora and Rodentia in comparison with ground-dwelling representatives. I. Sound conducting system of the middle ear. J. Morphol. 1990;214:49–61. doi: 10.1002/jmor.1052140104. [DOI] [PubMed] [Google Scholar]
- Burda H., Beiles A., Marhold S., Simpson S., Nervo E., Wiltschko W. Magnetic orientation in subterranean mole rats of the superspecies Spalax ehrenbergi: experiments, patterns, and memory. Isr. J. Zool. 1991;37:182–183. [Google Scholar]
- Burlington R.F., Whitten B.K., Sidel C.M., Posiviata M.A., Salkovits I.A. Effect of hypoxia on glycolysis in perfused hearts from rats and ground squirrels (Citelus lateralis) Comp. Biochem. Physiol. 1970;35:403–414. [Google Scholar]
- Catania K.C., Remple M.S. Somatosensory cortex dominated by the representation of teeth in the naked mole rat brain. Proc. Natl. Acad. Sci. U.S.A. 2002;99(8):5692–5697. doi: 10.1073/pnas.072097999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalley T., Bonjour J.P., Ferrari S., Rizzoli R. The influence of pubertal timing on bone mass acquisition: a predetermined trajectory detectable five years before menarche. J. Clin. Endocrinol. Metab. 2009;94(9):3424–3431. doi: 10.1210/jc.2009-0241. [DOI] [PubMed] [Google Scholar]
- Clarke F.M., Faulkes C.G. Dominance and queen succession in captive colonies of the eusocial naked mole rat, Heterocephalus glaber. Proc. R. Soc. Lond. B. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke F.M., Faulkes C.G. Hormonal and behavioral correlates of male dominance and reproductive status in captive colonies of the naked mole rat, Heterocephalus glaber. Proc. R. Soc. Lond. 1998;265:1391–1399. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke F.M., Faulkes C.G. Kin discrimination and female mate choice in the naked mole rat Heterocephalus glaber. Proc. R. Soc. Lond. B. 1999;266:1995–2002. doi: 10.1098/rspb.1999.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I.C., McNab B.K. Thermoregulation and energetics in subterranean mammals. In: Nevo E., Reig O.A., editors. Evolution of Subterranean Mammals at the Organismal and Molecular Levels. Wiley-Liss; New York: 1990. pp. 231–250. [Google Scholar]
- Crish S.D., Rice F.L., Park T.J., Comer C.M. Somatosensory organization and behavior in naked mole rats I: vibrissa-like body hairs comprise a sensory array that mediates orientation to tactile stimuli. Brain Behav. Evol. 2003;62:141–151. doi: 10.1159/000072723. [DOI] [PubMed] [Google Scholar]
- Crish S.D., Dengler-Crish D.M., Catania K.D. Central visual system of the naked mole rat (Heterocephalus glaber) Anat. Rec. 2006;288A:205–212. doi: 10.1002/ar.a.20288. [DOI] [PubMed] [Google Scholar]
- Crish S.D., Dengler-Crish D.M., Comer C.M. Population coding strategies and involvement of the superior colliculus in the tactile orienting behavior of naked mole rats. Neuroscience. 2006;139:1461–1466. doi: 10.1016/j.neuroscience.2005.11.073. [DOI] [PubMed] [Google Scholar]
- Csiszar A., Labinskyy N., Orosz Z., Xiangmin Z., Buffenstein R. Vascular aging in the longest-living rodent, the naked mole rat. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- Daly T.J.M., Williams L.A., Buffenstein R. Catecholaminergic innervation of interscapular brown adipose tissue in the naked mole rat (Heterocephalus glaber) J. Anat. 1997;190:321–326. doi: 10.1046/j.1469-7580.1997.19030321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly T.J.M., Buffenstein R. Skin morphology and its role in thermoregulation in mole rats, Heterocephalus glaber and Cryptomys hottentotus. Anatomy. 1998;193:495–502. doi: 10.1046/j.1469-7580.1998.19340495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler-Crish C.M., Catania K.C. Cessation of reproduction-related spine elongation after multiple breeding cycles in female naked mole rats. Anat. Rec. 2009;292:131–137. doi: 10.1002/ar.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschlander M.E., Freake M.J., Borland S.C., Phillips J.B., Anderson L.E., Wilson B.W. Learned magnetic compass orientation by the Siberian hamster, Phodopus sungorus. Anim. Behav. 2003;65:779–786. [Google Scholar]
- Endo H., Okanoya K., Park T.J., Sasaki M., Tanemura K., Kimura J. Localization of the cytochrome p450 side-chain cleavage enzyme in the inactive testis of the naked mole rat. Zool. Sci. 2002;19:673–678. doi: 10.2108/zsj.19.673. [DOI] [PubMed] [Google Scholar]
- Faulkes C.G., Abbot D.H., Jarvis J.U.M. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole rats, Heterocephalus glaber. J. Reprod. Fertil. 1990;88:559–568. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- Faulkes C.G., Abbott D.H., Jarvis J.U.M., Sherriff F.E. LH responses of female naked mole rat, Heterocephalus glaber, to single and multiple doses of exogenous GnRH. J. Reprod. Fertil. 1990;89:317–323. doi: 10.1530/jrf.0.0890317. [DOI] [PubMed] [Google Scholar]
- Faulkes C.G., Abbott D.H. Social control of reproduction in breeding and non-breeding naked mole rats (Heterocephalus glaber) J. Reprod. Fert. 1991;93:427–435. doi: 10.1530/jrf.0.0930427. [DOI] [PubMed] [Google Scholar]
- Faulkes C.G., Trowell S.N., Jarvis J.U.M., Bennett N.C. Investigation of sperm numbers and motility in reproductively active and socially suppressed males of two eusocial African mole rats, the naked mole rat (Heterocephalus glaber), and the Damaraland mole rat (Cryptomys damarensis) J. Reprod. Fertil. 1994;100:411–416. doi: 10.1530/jrf.0.1000411. [DOI] [PubMed] [Google Scholar]
- Faulkes C.G., Abbott D.H. Proximate mechanisms regulating a reproductive dictatorship: a single-dominant female controls male and female reproduction in colonies of naked mole rats. In: Solomon N.G., French J.A., editors. Cooperative Breeding in Mammales. Cambridge University Press; Cambridge, UK: 1997. pp. 302–334. [Google Scholar]
- Faulkes C.G., Abbott D.H., O’Brien H.P., Lau L., Roy M., Wayne R.K. Micro- and macro-geographical genetic structure of colonies of naked mole rats Heterocephalus glaber. Mol. Ecol. 1997;6:615–628. doi: 10.1046/j.1365-294x.1997.00227.x. [DOI] [PubMed] [Google Scholar]
- Francis D.D., Champagne F.C., Meaney M.J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Goldman B.D., Goldman S.L., Lanz T., Magaurin A., Maurice A. Factors influencing metabolic rate in naked mole rats (Heterocephalus glaber) Physiol. Behav. 1999;66(3):447–459. doi: 10.1016/s0031-9384(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Goldman S.L., Forger N.G., Goldman B.D. Influence of gonadal sex hormones on behavioral components of the reproductive hierarchy in naked mole rats. Horm. Behav. 2006;50:77–84. doi: 10.1016/j.yhbeh.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Hamilton W.J. Heterocephalus the remarkable African burrowing rodent. Brooklyn Mus. Sci. Bull. 1928;3:173–184. [Google Scholar]
- Hefner R.S., Hefner H.E. Degenerate hearing and sound localization in naked mole rats (Heterocephalus glaber), with an overview of central auditory structures. J. Comp. Neurol. 1993;331:418–433. doi: 10.1002/cne.903310311. [DOI] [PubMed] [Google Scholar]
- Henry E.C., Remple M.S., O’Riain J., Catania K.C. Organization of somatosensory cortical areas in the naked mole rat (Heterocephalus glaber) J. Comp. Neurol. 2006;495:434–452. doi: 10.1002/cne.20883. [DOI] [PubMed] [Google Scholar]
- Henry E.C., Dengler-Crish C.M., Catania K.C. Growing out of a caste – reproduction and the making of the queen mole rat. J. Exp. Biol. 2007;210:261–268. doi: 10.1242/jeb.02631. [DOI] [PubMed] [Google Scholar]
- Hetling J.R., Baig-Silva M.S., Comer C.M., Pardue M.T., Samaan D.Y., Qtaishat N.M. Features of visual function in the naked mole rat Heterocephalus glaber. J. Comp. Physiol. 2005;191:317–330. doi: 10.1007/s00359-004-0584-6. [DOI] [PubMed] [Google Scholar]
- Hill W.C.O., Porter A., Bloom R.T., Seago J., Southwick M.D. Field and laboratory studies of the nake mole rat, Heterocephalus glaber. Zool. Soc. Lond. 1955;128:45–514. [Google Scholar]
- Hislop M.S., Buffenstein R. Noradrenaline induces non-shivering thermogenesis in both the naked mole rat (Heterocephalus glaber) and the Damara mole rat (Crystomys damarensis) despite very different modes of thermoregulation. J. Thermal Biol. 1994;19:25–32. [Google Scholar]
- Holmes M.M., Rosen G.J., Jordan C.L., de Vries G.J., Goldman B.D., Forger N.G. Social control of brain morphology in a eusocial mammal. PNAS. 2007;104(25):10548–10552. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M.M., Goldman B.D., Goldman S.L., Seney M.L., Forger N.G. Neuroendocrinology and sexual differentiation in eusocial mammals. Front. Neuroendocrin. 2009;30:519–533. doi: 10.1016/j.yfrne.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt R.L., Nelson K., Schlitter D.A., Sherman P.W. Systematics and evolution of the family Bathyergidae. In: Sherman P.W., Jarvis J.U.M., Alexander R.D., editors. The Biology of the Naked Mole Rat. Princeton University Press; Princeton, NJ: 1991. pp. 45–65. [Google Scholar]
- Honeycutt R.L., Nelson K., Schlitter D.A., Sherman P.W. Genetic variation within and among populations of the naked mole rat: evidence from nuclear and mitochondrial genomes. In: Sherman P.W., Jarvis J.U.M., Alexander R.D., editors. The Biology of the Naked Mole Rat. Princeton University Press; Princeton, NJ: 1991. pp. 195–208. [Google Scholar]
- Jarvis J.U.M. Eusociality in a mammal: cooperative breeding in nake mole rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- Jarvis J.U.M. Ecological studies on Heterocephalus glaber, the naked mole rat, in Kenya. Nat. Geog. Soc. Res. Rep. 1985;20:429–437. [Google Scholar]
- Jarvis J.U.M., O’Riain J.J., McDaid E. Growth and factors affecting of size in naked mole rats. In: Sherman P.W., Jarvis J.U.M., Alexander R.D., editors. The Biology of the Naked Mole Rat. Princeton University Press; Princeton, NJ: 1991. pp. 358–383. [Google Scholar]
- Jarvis J.U.M. Reproduction of naked mole rats. In: Sherman P.W., Jarvis J.U.M., Alexander R.D., editors. The Biology of the Naked Mole Rat. Princeton University Press; Princeton, NJ: 1991. pp. 384–425. [Google Scholar]
- Johansen K., Lykkeboe G., Weber R.E., Maloiy G.M.O. Blood respiratory properties in the naked mole rat Heterocephalus glaber, a mammal of low body temperature. Respir. Physiol. 1976;28:303–314. doi: 10.1016/0034-5687(76)90025-6. [DOI] [PubMed] [Google Scholar]
- Jones D.L., Wang L.C.H. Adaptive cardiovascular modifications in the west chipmunds, gunes Eutamias. J. Comp. Physiol. 1976;112:307–315. [Google Scholar]
- Judd T.M., Sherman P.W. Naked mole rats recruit colony mates to food sources. Anim. Behav. 1996;52:957–969. [Google Scholar]
- Kanue T.I., Hole K. Morphine induces aggression but no analgesia in the naked mole rat (Heterocephalus glaber) Comp. Biochem. Physiol. 1990;96C:131–133. doi: 10.1016/0742-8413(90)90057-g. [DOI] [PubMed] [Google Scholar]
- Kanui T.I., Karim F., Towett P.K. The formalin test in the naked mole rat (Heterocephallus glaber): analgesic effects of morphine, nefopamand, paracetamol. Brain Res. 1993;600:123–126. doi: 10.1016/0006-8993(93)90409-g. [DOI] [PubMed] [Google Scholar]
- Kennerly T.E. Microenvironmental conditions of the pocket gopher burrow. Texas J. Sci. 1964;16:395–441. [Google Scholar]
- Kock D., Ingram C.M., Frabotta L.J., Honeycutt R.L., Burda H. On the nomenclature of Bathyergidae and Fukomys n. gen. (Mammalia: Rodentia) Zootaxa. 2006;1142:51–58. [Google Scholar]
- Kramer B., Buffenstein R. The pancreas of the naked mole rat (Heterocephalus glaber): an ultrastructural and immunocytochemical study of the endocrine component of thermoneutral and cold acclimated animals. Gen. Comp. Endocrin. 2004;139:206–214. doi: 10.1016/j.ygcen.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Lacey E.A., Sherman P.W. Social organization of naked mole rat colonies: evidence for divisions of labor. In: Sherman P.W., Jarvis J.U.M., Alexander R.D., editors. The Biology of the Naked Mole Rat. Cambridge University Press; United Kingdom: 1991. pp. 275–336. [Google Scholar]
- Lambert A.J., Boysen H.M., Buckinghom J.A., Yang T., Podlutsky A., Austad S.N. Low rates of hydrogen peroxide production by isolated heart mitochondria associated with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- LaVinka P.C., Brand A., Landau V.J., Wirtshafter D., Park T.J. Extreme tolerance to ammonia fumes in African naked mole rats animals that naturally lack neuropeptides from trigeminal chemosensory nerve fibers. J. Comp. Physiol. 2009;195:419–427. doi: 10.1007/s00359-009-0420-0. [DOI] [PubMed] [Google Scholar]
- Lovegrove B.G., Wissel C. Sociality in mole rats: metabolic scaling and the role of risk sensitivity. Oecologia (Berl.) 1988;74:600–606. doi: 10.1007/BF00380059. [DOI] [PubMed] [Google Scholar]
- Maina J.N., Maloiy G.M.O., Makanya A.N. Morphology and morphometry of the lungs of two East African mole rats, Tachyoryctes slendens and Heterocephalus glaber (Mammalia, Rodentia) Zoomorphology. 1992;112:167–171. [Google Scholar]
- Maina J.N., Gebreegziabher Y., Woodley R., Buffenstein R. Effects of change in environmental temperature and natural shifts in carbon dioxide and oxygen concentrations on the lungs of captive naked mole rats (Heterocephalus glaber): a morphological and morphometric study. J. Zool. Lond. 2001;253:371–382. [Google Scholar]
- Margulis S.W., Saltzman W., Abbott D.H. Behavioral and hormonal changes in female naked mole rats (Heterocephalus glaber) following removal of the breeding female from a colony. Horm. Behav. 1995;29:227–247. doi: 10.1006/hbeh.1995.1017. [DOI] [PubMed] [Google Scholar]
- Marhold S., Wiltschko W., Burda H. A magnetic polarity comjpass for direction finding in a subterranean mammal. Naturwiss. 1997;84:421–423. [Google Scholar]
- McNab B.K. Metabolism of fossorial rodents. Ecology. 1966;47:712–733. [Google Scholar]
- Michener C.D. Comparative social behavior of bees. Annu. Rev. Entomol. 1969;14:299–342. [Google Scholar]
- Muheim R., Edgar N.M., Sloan K.A., Phillips J.B. Magnetic compass orientation in C57BL/6 mice. Learn. Behav. 2006;34:366–373. doi: 10.3758/bf03193201. [DOI] [PubMed] [Google Scholar]
- Miller R.A., Nadon N.L. Principles of animal use for gerontological research. Gerontol. A Biol. Sci. Med. Sci. 2000;55(3):B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo E. Mosiac Evolution of Subterranean Mammals: Regression, Progression, and Global Convergence. Oxford University Press; Oxford: 1999. [Google Scholar]
- Nikitina N.V., Maughan-Brown B., O’Riain J.J., Kidson S.H. Postnatal development of the eye in the naked mole rat (Heterocephalus glaber) Anat. Rec. 2004;277a:317–337. doi: 10.1002/ar.a.20025. [DOI] [PubMed] [Google Scholar]
- O’Connor T.P., Lee A., Jarvis J.U.M., Buffenstein R. Prolonged longevity in naked mole rats: age-related changes in the metabolism, body composition and gastrointestinal function. Comp. Biochem. Physiol. A. 2002;133:835–842. doi: 10.1016/s1095-6433(02)00198-8. [DOI] [PubMed] [Google Scholar]
- O’Riain M.J., Jarvis J.U.M., Faulkes C.G. A dispersive morph in the naked mole rat. Nature. 1996;380:619–621. doi: 10.1038/380619a0. [DOI] [PubMed] [Google Scholar]
- O’Riain M.J., Jarvis U.M. The dynamics of growth in naked mole rats: the effects of litter order and changes in social structure. J. Zool. Lond. 1998;246:49–60. [Google Scholar]
- O’Riain M.J., Jarvis J.U.M., Alexander R., Buffenstein R., Peeters C. Morphological castes in a vertebrate. Proc. Nat. Acad. Sci. 2000;97:13194–13197. doi: 10.1073/pnas.97.24.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T.J., Comer C., Carol A., Lu Y., Hong H.S., Rice F.L. Somatosensory organization and behavior in naked mole rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. J. Comp. Neurol. 2003;465:104–120. doi: 10.1002/cne.10824. [DOI] [PubMed] [Google Scholar]
- Park T.J., Lu Y., Juttner R., Smith E.S.S., Hu J., Brand A. Selective inflammatory pain insensitivity of the African maked mole rat (Heterocephalus glaber) PLOS Biol. 2008;6(1):156–170. doi: 10.1371/journal.pbio.0060013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra R. Comparison of foregut and hindgut fermentation in herbivores. In: Montgomery B.B., editor. The Ecology of Arboreal Folivores. Smithsonian Institute Press; Washington, D.C: 1978. pp. 205–229. [Google Scholar]
- Peichl L., Nemec P., Burda H. Unusual cone and rod properties in subterranean African mole rats (Rodentia, Bethyergidae) Eur. J. Neurosci. 2004;19:1545–1558. doi: 10.1111/j.1460-9568.2004.03263.x. [DOI] [PubMed] [Google Scholar]
- Pepper J.W., Braude S.H., Lacey E.A., Sherman P.W. Vocalizations of the naked mole rat. In: Sherman P.W., Jarvis J.U.M., Alexander R.D., editors. The Biology of the Naked Mole Rat. Princeton University Press; Princeton, NJ: 1991. pp. 241–274. [Google Scholar]
- Peroulakis M.W., Goldman B., Forger N.G. Perineal muscles and motoneurons are sexually monomorphic in the naked mole rat (Heterocephalus glaber) J. Neurobiol. 2002;51(1):33–42. doi: 10.1002/neu.10039. [DOI] [PubMed] [Google Scholar]
- Petry H. Zur artgerechten Haltung von afrikanischen Nacktmullen (Heterocephalus glaber) J. Anim. Physiol. A. Anim. Nutr. 2003;87:421–432. doi: 10.1046/j.0931-2439.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Pinto M., Jepsen K.J., Terranova C.J., Buffenstein R. Lack of sexual dimorphism in femora of the eusocial and hypogonadic naked mole rat: a novel animal model for the study of delayed puberty on the skeletal system. Bone. 2009 doi: 10.1016/j.bone.2009.08.060. Sep 15 (Epub. ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. Entozoa and endophyta of the naked mole rat. Proc. Zool. Soc. Lond. 1957;128:515–527. [Google Scholar]
- Rechkemmer G., Ronnau K., Engelhardt W.V. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp. Biochem. Physiol. 1988;90A:563–568. doi: 10.1016/0300-9629(88)90668-8. [DOI] [PubMed] [Google Scholar]
- Reeve H.K., Westneat D.F., Noon W.A., Sherman P.W., Aquadro C.F. DNA ‘fingerprinting’ reveals high levels of inbreeding in colonies of the eusocial naked mole rat. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2496–2500.N. doi: 10.1073/pnas.87.7.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A.P., Goldman B.D. Circadian rhythms locomotor activity in naked mole rats (Heterocephalus glaber) Physiol. Behav. 2000;71:1–13. doi: 10.1016/s0031-9384(00)00281-x. [DOI] [PubMed] [Google Scholar]
- Riccio A.P., Goldman B.D. Circadian rhythms of body temperature and metabolic rate in naked mole rats. Physiol. Behav. 2000;71:15–22. doi: 10.1016/s0031-9384(00)00280-8. [DOI] [PubMed] [Google Scholar]
- Rosen G.J., De Cries G.J., Goldman S.L., Goldman B.D., Forger N.G. Distribution of vasopressin the brain of the eusocial naked mole rat. J. Comp. Neurol. 2007;500:1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- Rosen G.J., De Vries G.J., Goldman S.L., Goldman B.D., Forger N.G. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155:809–817. doi: 10.1016/j.neuroscience.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Gillespie A., O’Riain M.J., Keller L.F. Viral epizootic reveals inbreeding depression in a habitually inbreeding mammal. Evolution. 2007;61(9):2268–2273. doi: 10.1111/j.1558-5646.2007.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C., Leonhardt S., Seidel C., Luft H., Wuttke W., Jarry H. Comparative analysis of different puberty inhibiting mechanisms of two GnRH agonists and the GnRH antagonist cetrorelix using a female rat model. Pediatr. Res. 2000;48(4):468–474. doi: 10.1203/00006450-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Seney M.L., Kelly D.A., Goldman B.D., Sumbera R., Forger N.G. Social structure predicts genital morphology in African mole rats. PLoS One. 2009;4(10):e7477. doi: 10.1371/journal.pone.0007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P.W., Jarvis J.U.M., Bruade S.H. Naked mole rats. Sci. Am. 1992;267:42–48. [Google Scholar]
- Sherman P.W., Braude S., Jarvis J.U.M. Litter sizes and mammary numbers of naked mole rats: breaking the one-half rule. J. Mammal. 1999;80(3):720–733. [Google Scholar]
- Skinner D.C., Moodley G.P., Buffenstein R. Is vitamin D3 essential for mineral metabolism in the damara mole rat (Cryptomys damarensis)? Gen. Comp. Endocrinol. 1991;81:500–505. doi: 10.1016/0016-6480(91)90178-9. [DOI] [PubMed] [Google Scholar]
- Smith T.E., Faulkes C.G., Abbott D.H. Combined olfactory contact with the parent colony and direct contact with nonbreeding animals does not maintain suppression of ovulation in female naked mole rats (Heterocephalus glaber) Horm. Behav. 1997;31:277–288. doi: 10.1006/hbeh.1997.1384. [DOI] [PubMed] [Google Scholar]
- Smith T.D., Bhatnagar K.P., Burrows A.M., Shimp K.I., Dennis J.C., Smith M.A. The vomeronasal organ of greater bushbabies (Otolemur spp.): species, sex, and age differences. J. Neurocytol. 2005;34:135–147. doi: 10.1007/s11068-005-5053-9. [DOI] [PubMed] [Google Scholar]
- Smith T.D., Bhathagar K.P., Dennis J.C., Morrison E.E., Park T.J. Growth-deficient vomeronasal organs in the naked mole rat (Heterocephalus glaber) Brain Res. 2007;1132:78–83. doi: 10.1016/j.brainres.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Snyman P.C., Jackson C.R., Bennett N.C. Do dispersing non-reproductive female Damaraland mole rats (Cryptomys damarensis (Rodentia: Bethyergidae) exhibit spontaneous or induced ovulation? Horm. Behav. 2006;31:277–288. doi: 10.1016/j.physbeh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Towett P.K., Kanue T.I., Titus I. Effects of pethidine, acetylsalicylic acid, and indomethacin on pain and behavior in the mole rat. Pharmacol. Biochem. Behav. 1993;45:153–159. doi: 10.1016/0091-3057(93)90099-f. [DOI] [PubMed] [Google Scholar]
- Ungvari Z., Buffenstein R., Austad S.N., Podlutsky A., Kaley G., Csiszar A. Oxidative stress in vascular senescence: lesions from successfully aging species. Front Biosci. 2008;13:5056–5070. doi: 10.2741/3064. [DOI] [PubMed] [Google Scholar]
- Urison N.T., Buffenstein R. Kidney concentrating ability of a subterranean xeric rodent, the naked mole rat (Heterocephalus glaber) J. Comp. Physiol. B. 1994;163:676–681. doi: 10.1007/BF00369519. [DOI] [PubMed] [Google Scholar]
- Urison N.T., Buffenstein R. Metabolic and body temperature changes during pregnancy and lactation in the naked mole rat (Heterocephalus glaber) Physiol. Zool. 1995;68:402–420. [Google Scholar]
- Widmer H.R., Hoppeler H., Nevo E., Taylor C.R., Weibel E.R. Working underground: respiratory adaptations in the blind mole rat. Proc. Nat. Acad. Sci. U.S.A. 1997;94:2062–2067. doi: 10.1073/pnas.94.5.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers P.C., Jarvis J.U.M. The effect of huddling on thermoregulation and oxygen consumption for the naked mole rat. Comp. Biochem. Physiol. A, Comp. Physiol. 1980;66:215–219. [Google Scholar]
- Xiao J., Levitt J.B., Buffenstein R. A steriotaxic atlas of the brain of the naked mole rat (Heterocephalus glaber) Neuroscience. 2006;141(3):1415–1435. doi: 10.1016/j.neuroscience.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Yahav S., Buffenstein R. The effect of temperature on caecal fermentation processes in a poikilothermic mammal, Heterocephalus glaber. J. Comp. Physiol. 1991;16(6):345–349. doi: 10.1007/BF00357526. [DOI] [PubMed] [Google Scholar]
- Yahav S., Buffenstein R. Caecal function provides the energy of fermentation without liberating heat in the poikilothermic mammal, Heterocephalus glaber. J. Comp. Physiol. B. 1992;162:216–218. doi: 10.1007/BF00357526. [DOI] [PubMed] [Google Scholar]
- Yang T., Buffenstein R. Disparate age effects on gastrointestinal enzymes in naked mole rats. Integr. Comp. Biol. 2002;42:1340–1341. [Google Scholar]
- Yingling V.R., Taylor G. Delayed pubertal development by hypothalamic suppression causes in increase in periosteal modeling but a reduction in bone strength in growing female rats. Bone. 2008;42(6):1137–1143. doi: 10.1016/j.bone.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosida S., Kobayasi K.I., Ikebuchi M., Ozaki R., Okanoya K. Antiphonal vocalization of a subterranean rodent, the naked mole rat (Heterocephalus glaber) Ethology. 2007;113:703–710. [Google Scholar]


