Abstract
Mucosal immune responses within the middle ear and eustachian tube generally provide an effective and efficient response to the presence of microbial pathogens, with approximately 80% of clinically recognizable middle ear infections resolved within 7 days. Particularly for young children aged less than 3 years of age, the proximity and direct connection of the middle ear, via the eustachian tube, to the nasopharynx provide increased risk of commensal bacteria and upper respiratory tract viruses infecting the middle ear. Mucosal immunological defense in the middle ear and eustachian tube utilizes a number of mechanisms, including physicochemical barriers of mucus and the mucosal epithelial cells and innate immune responses such as inflammation, cellular infiltration, effusion, and antimicrobial protein secretions, in addition to adaptive host immune responses. Recent advances in otopathogen recognition via microbial pattern recognition receptors and elucidation of complex signaling cascades have improved understanding of the coordination and regulation of the middle ear mucosal response. These advances support vaccine development aiming to reduce the risk of otitis media in children.
Keywords: Adaptive immunity, Cytokine, Eustachian tube, Innate immunity, Intracellular signaling, Middle ear, Otitis media, Otopathogen, Vaccine
Introduction
Mucosal immunological defense in the middle ear utilizes a number of mechanisms, including physicochemical barriers of mucus and the mucosal epithelial cells and innate immune responses such as inflammation, cellular infiltration, effusion, and antimicrobial protein and peptide secretions, in addition to adaptive host immune responses.
Immunology of the tubotympanum has been studied primarily during infection states. Development of mucosal immune responses to antigens continues through infancy and early childhood (Chapter 11) (Cripps et al., 1987, Ogra, 2010), at a time when eustachian tube morphology increases the child’s risk of otopathogen access to the middle ear and development of otitis media (OM) (Lim, 1976, Bluestone and Doyle, 1988). Furthermore, infants and children experiencing gastroesophageal reflux may have increased risk of OM due to ongoing upregulation of mucosal responses by the presence of pepsin within the eustachian tube and middle ear. Pepsin is reported to stimulate middle ear inflammation and increase the risk of chronic otitis media with effusion (COME) (Miura et al., 2012). Infancy and early childhood are the periods of highest incidence of OM, with the peak incidence of acute otitis media (AOM) occurring in the first 6 months of life and the recurrence of OM being common up to 7 years of age (Ogra, 2010).
This chapter describes the morphology of immunological tissue within the tubotympanum, the characteristics of local immune responses to common otopathogens, and regulation of the mucosal immune responses in the middle ear. Throughout this chapter, discussion is informed by the immune responses observed during OM from animal models and human clinical studies (for review see Murphy et al. (2013)).
Mucosal Immune Characteristics of the Tubotympanum
Anatomically, the tubotympanum comprises the middle ear and the eustachian tube. The middle ear is a unique cavity and is normally considered a “sterile” environment (Kurono and Mogi, 1996) despite its proximity to the nasopharynx, a region of extensive antigen exposure in healthy individuals (Lim et al., 2000). The middle ear is lined with a simple mucosal membrane with typically few immunocytes present (Lim, 1979, Ichimiya et al., 1990), and the eustachian tube forms a direct conduit between the middle ear and nasopharynx, increasing the opportunity for microbial and allergenic antigen exposure.
Mucosal Tissue Organization of the Middle Ear
Structurally, the human middle ear cavity may be considered anatomically partitioned at the level of the tympanic membrane into an attic or epitympanic region above the membrane, then descending through the mesotympanic region adjacent to the tympanic membrane to the hypotympanic or anterior inferior region of the middle ear cavity (Ars et al., 2012, Bluestone and Doyle, 1988, Lim, 1979). See Figure 1 .
Figure 1.
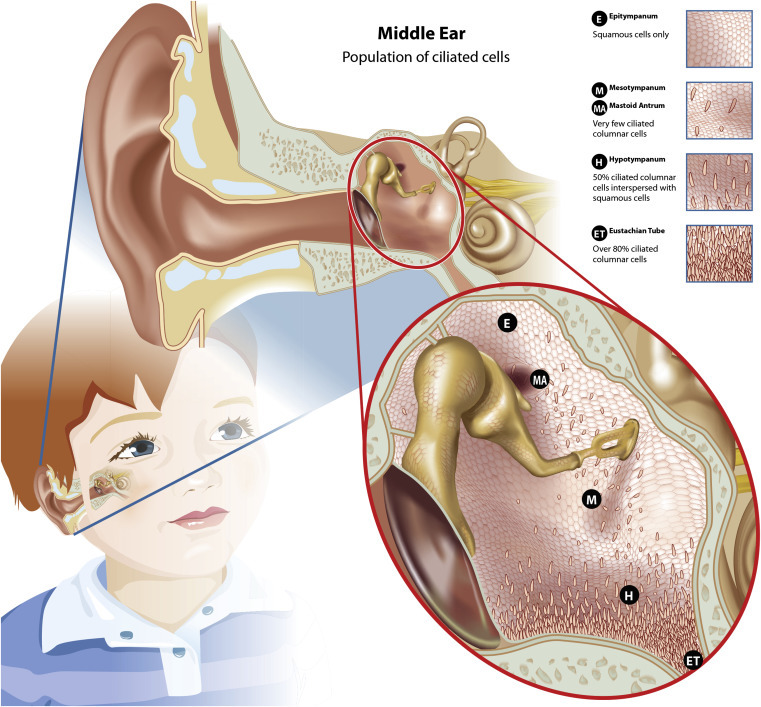
Diagrammatic representation of the middle ear showing the distribution of the ciliated cells within the epitympanum, mesotympanum, and hypotympanum, which form the mucociliary transport pathways from the middle ear to the eustachian tube.
Adapted from Lim (1979) and Lim et al. (2000).
The middle ear cavity is lined with a thin mucosal membrane that covers all structures, such as the ossicles, and is continuous with the mucosal membrane in the mastoid antrum, eustachian tube, and nasopharynx (Lim, 1976, Lim, 1979). The mucosal epithelium within the epitympanic region is composed primarily of squamous epithelia with islands of ciliated columnar cells. These islands form a functional mucociliary pathway from the mastoid antrum in the epitympanum, through the mesotympanum, toward the eustachian tube. Similarly, the squamous epithelium of the mesotympanum has two paths of ciliated columnar cells, one continuing the path from the epitympanum and mastoid antrum and the other connecting the tympanic membrane through the hypotympanum to the eustachian tube.
In the healthy middle ear, the mucosal surface of the mesotympanum is composed equally of squamous and ciliated columnar cells. The number of ciliated columnar epithelial cells in the mucosal lining progressively increases toward the eustachian tube to constitute about 80% of the cells adjacent to the eustachian tube entrance. These histomorphological changes evidence the progressive transformation from flat, nonsecretory squamous epithelium to respiratory epithelium that is pseudostratified, ciliated columnar cells just proximal to the eustachian tube entrance (Bluestone and Doyle, 1988, Lim, 1976, Lim, 1979, Ars et al., 2012). The changing cellular architecture of the middle ear cavity facilitates function of an organized mucociliary transportation system from primarily the epitympanum and hypotympanum to the eustachian tube, as illustrated in Figure 1.
Within the eustachian tube, respiratory epithelial cells are tightly interconnected and utilize occludin and various members of the claudin family at tight junctions and E-cadherin at adherin junctions to optimize physical resilience of the membrane (Tsukita et al., 2008, Yonemura, 2011). These epithelial cells are ciliated and, in addition to goblet cells, also secrete mucus (Lim, 1979). Within the middle ear and eustachian tube, secretory glands secrete two types of mucus: mucoid and serous (Lim, 1979). Serous fluid is less viscous and is considered the origin of the periciliary fluid that aids mucociliary clearance of these regions. (For review, see Lim (1979).) Mucoid secretion also provides lubrication of the epithelial cell boundary of the mucosal membrane (Ueno and Lim, 1991) and together, the glandular and mucosal epithelial cells in these regions are the front line for secretion of molecules of innate immunity (Lim, 1976, Lim, 1979, Lim et al., 2000). For example, in children, mucoid effusions from chronically infected middle ears contain higher concentrations of lysozyme, an antimicrobial enzyme (Liu et al., 1975) that was localized to middle ear epithelial goblet cells (Lim et al., 1976) and has been demonstrated to reduce susceptibility to OM in the murine model (Shimada et al., 2008).
Efficiency of middle ear mucociliary clearance in infants and young children is impaired by the shorter, wider, more horizontal morphology of the eustachian tube, which increases the opportunity for antigen exposure or pathogen colonization while reducing the opportunity for mucosal immune responses, compared to the adult morphology (Bluestone and Doyle, 1988). To assist mucociliary clearance of the middle ear, the epithelium of the epitympanum, although primarily squamous, is well vascularized and performs a gas exchange role with the mastoid and middle ear cavities. Intermittent opening of the eustachian tube, during swallowing and yawning, in conjunction with the mastoid antrum gas pressurization, improves clearance of secretions from the middle ear into the nasopharynx (Ars et al., 2012, Lim, 1976, Bluestone and Doyle, 1988, Marom et al., 2012, Lim, 1979).
Overall, healthy eustachian tube functions include protection from microbial colonization by remaining predominantly closed, ventilating to regulate air pressure, and using gas-assisted mucociliary activity to improve clearance of secretions from the middle ear and eustachian tube. Obstruction of the eustachian tube by inflammation or impaired mucociliary clearance contributes to increased susceptibility to middle ear infection (Mygind and Pedersen, 1983). Ultimately, the relative sterility of the middle ear is maintained by continuous mucociliary clearance of the middle ear cavity in combination with host innate and adaptive immune responses (Lim et al., 2000).
Immunocompetent Cells in the Middle Ear Mucosa
Healthy middle ear mucosa is characterized by a simple, thin membrane with typically few immunocompetent cells, as observed in both animal models (Jecker et al., 2001, Jecker et al., 1996, Ichimiya et al., 1990) and the human middle ear (Suenaga et al., 2001). Mast cells and macrophages are the most commonly observed immunocompetent cell types in non-inflamed tissue, with mast cells predominant in healthy middle ears. Mast cells are preferentially distributed in the well-vascularized mucosa or ciliated mucosal epithelial layer of the tubotympanum rather than within the subepithelial layer (Palva et al., 1991, Watanabe et al., 1991). Immunocompetent cell numbers progressively fall as the healthy middle ear is approached from the nasopharynx via the eustachian tube (Widemar et al., 1986, Watanabe et al., 1991). Electron microscopic examination of the human tympanic membrane reported non-epithelial cells within the basal epidermis (Yamanaka, 1987) that have been subsequently identified as dendritic cells (DC) (Freijd et al., 1984). The presence of these antigen presenting cells (APC) within the normal tympanic membrane, albeit sourced from cadaveric specimens, suggests a mechanism for local initiation of a primary immune response. DC and upregulation of cytokine secretion are recognized as elements of the local immunological response of the tympanic membrane to chronic OM and perforation (Corscadden et al., 2013).
Antigen sampling via mucosa-associated lymphoid tissue (MALT) is not typically observed in the healthy middle ear in either animal models or healthy human ears. A single report of post mortem tissue suggests that MALT is present in the eustachian tube and middle ear of children; however, this study did not examine the clinical history of the subjects and OM was determined by the presence of effusion or cellular infiltration at post mortem (Matsune et al., 1996). In the rat, the frequency of MALT observed in the eustachian tube reduced as the middle ear was approached and suggests an effector rather than inductive immune role for the middle ear epithelium.
Otopathogens
Viral Otopathogens
Immune responses within the middle ear and eustachian tube are frequently stimulated during upper respiratory tract infections (URI), with innate inflammatory responses to infection contributing to the development of OM in some children (Mandel et al., 2008). Viral infection of the upper respiratory tract mucosae, reported in prospective human studies, often precedes and predisposes the middle ear cavity to development of OM (Mandel et al., 2008). During one 12-month prospective study, 97% of children aged 6 months to 3 years experienced one or more URI per year, with approximately 10% of children less than 3 years of age experiencing 10 or more infections. AOM was identified in 61% of children experiencing a URI while otitis media with effusion (OME) was observed in 25% of children, with child age being the strongest predictor (Chonmaitree et al., 2008). It is not uncommon for children to experience six to eight URI per year (Heikkinen and Jarvinen, 2003), and approximately 70% of children with a diagnosis of OM have symptoms of a URI. Furthermore, approximately 77% of these children have a virus detected in their nasal secretions (Mandel et al., 2008, Winther et al., 2007). Adenovirus, respiratory syncytial virus (RSV), influenza A and influenza B virus, parainfluenza virus, coronavirus, enterovirus, and rhinoviruses are commonly associated with OM (Heikkinen and Chonmaitree, 2003, Alper et al., 2009).
The number and frequency of viral URI experienced by young children increases the risk and opportunity for ascension via the eustachian tube to infect the middle ear. Although viruses are a commonly detected microbe within the middle ear fluid (MEF) from children with OM, there is recent debate as to whether viral presence equates to OM pathogenesis (Chonmaitree et al., 2012). Viruses have been detected in 70% of MEF samples from children with AOM and viral detection alone was observed in only 4% of cases, with 66% of children exhibiting concurrent viral and bacterial infections. Only 26% of the AOM cases exhibited bacterial infection alone (Ruohola et al., 2006); however, viral infection of the upper airways does have a critical role in the pathogenesis of bacterial OM. A number of studies using combined viral and bacterial otopathogens in the chinchilla model have been recently reviewed (Bakaletz, 2010) and confirm that a highly specific interrelationship exists between a respiratory tract viral infection and the bacteria associated with OM as recognized in children (Heikkinen, 2000, Chonmaitree et al., 2008). This relationship varies depending upon the otopathogens involved; for example, in Moraxella catarrhalis-induced OM, more than one viral or bacterial co-pathogen may be necessary for OM establishment. Viral/bacterial partnership (interrelationship) facilitates the transfer of nasopharyngeal commensal bacteria into the middle ear via the eustachian tube and increases the risk and severity of bacterial OM infection (Garcia-Rodriguez and Fresnadillo Martinez, 2002). A review on the bacterial and viral interactions on the development of AOM has been recently published (Marom et al., 2012).
Clinically, concurrent viral and bacterial infection of the middle ear results in significant worsening of the clinical course of OM in animal models (Giebink et al., 1980) and children compared to virus or bacterial infection alone. For children, increased detection of both virus and bacteria within the MEF was observed in cases of antibacterial failure after 2–4 days of antibiotic administration (Chonmaitree et al., 1990). Viral presence within the middle ear can also reduce antibiotic penetration from the serum into MEF (Canafax et al., 1998).
Investigation of the immunological responses of the middle ear and their immunoregulation has utilized both acute and chronic OM pathogenesis models using a variety of animal models including, mice, rats, chinchillas, gerbils, monkeys, and guinea pigs and a range of differing modes of pathogen introduction. A recently published modified methodology (Stol et al., 2009) that permits noninvasive, pressurized administration of otopathogens may provide further insight into OM, particularly otopathogen ascension via the eustachian tube from the nasopharynx.
Immunological responses of the middle ear to viral infection may increase the risk of OM development in a number of ways, including enhancing bacterial adherence to epithelial cells (Avadhanula et al., 2006), which is recognized to optimize establishment of a superinfection (Sanford et al., 1978). Upper respiratory tract viruses also stimulate increased expression of eukaryotic receptors such as intercellular adhesion molecule 1, carcinoembryonic antigen-related cell adhesion molecule 1, and platelet-activating factor receptor on the mucosal or host cell surface (Griffiths et al., 2007). Bacteria use these surface receptors as adherence sites and to alter rates of mucus secretion. Viral infection also stimulates innate mucosal immune responses, including inflammation and mucosal membrane thickening and increased mucus secretion by goblet cells, leading to reduced middle ear pressurization (Giebink et al., 1987). These local immune responses in the middle ear and eustachian tube have been clearly associated with enhanced susceptibility to OM in the chinchilla model and have been recently reviewed (Bakaletz, 2010).
Middle ear inflammation after viral exposure can increase the rate of mucus secretion (Chung et al., 1993) while altering fluid viscosity through upregulation of gel-like mucin secretion. Mucosal epithelial cell synthesis of antimicrobial peptides within the mucus can be impaired by viral infection (Mcgillivary et al., 2007), and changes to fluid and ion transport within and through the epithelial cell membrane increases the formation of an edematous mucosal lining and effusion to the middle ear (Macarthur et al., 2011a). Furthermore, reduction of the number and activity of epithelial cell cilia within the eustachian tube by influenza A virus has been demonstrated to reduce the opportunity for neutralizing or clearing otopathogens from the middle ear in the chinchilla model (Park et al., 1993).
Clearly, viral infection results in interruption or dysregulation of normal inflammatory responses of the mucosa within the eustachian tube and middle ear and permits transit of commensal nasopharyngeal bacteria to the middle ear. Subsequent inability to open the eustachian tube provides minimal opportunity for mucociliary clearance to the nasopharynx and enhances mucosal tissue damage through generation of pressure differential and local superinfection within the middle ear.
Bacterial Otopathogens
Streptococcus pneumoniae, non-typeable Haemophilus influenzae (NTHi), and M. catarrhalis are normal commensal flora and the most common bacterial microbes detected in MEF from children with AOM (Ruohola et al., 2006). Group A Streptococcus is also reported as an otopathogen (Segal et al., 2005) and Alloiococcus otiditis is reported as a significant otopathogen in some population groups (Ashhurst-Smith et al., 2012). Mucosal immune responses within the middle ear and eustachian tube may be overwhelmed by the extent of early bacterial colonization and result in unresolved inflammation within the middle ear. For example, the nasopharyngeal carriage rate for NTHi is higher in children with OME compared to healthy children. Children who experience dense bacterial colonization of the nasopharynx early in life are at significantly increased risk of early OM development and more severe disease, including tympanic membrane perforation, and are at very high risk of suppurative complications of OM (Leach et al., 1994, Smith-Vaughan et al., 2006). Effective mucosal immune responses within the middle ear may reduce the impact of bacterial load and/or virulence of the otopathogen. For example, single gene deletion of slrA (streptococcal lipoprotein rotamase A) or in combination with ppmA gene (putative proteinase maturation protein A) significantly reduced bacterial load and virulence in the nasopharynx and middle ear in a murine experimental AOM model (Stol et al., 2009). The additive effects of deletion of these genes on bacterial colonization and virulence provides further challenge for vaccine development.
The effectiveness of the endogenous mucosal immune responses to AOM, for the children of developed countries at “low risk” of severe OM (i.e., low rates of suppurative complications of OM) is indicated by the high rate of self-resolving OM episodes (80%) (Glasziou et al., 2004), in turn implying the success of the host mucosal immune responses. For healthy children, middle ear effusion normally resolves within 7 days in 40% of cases, and in 75–90% of cases resolution occurs within 4 weeks (Mandel et al., 2008).
Failure of the middle ear infection to self-resolve can result in ongoing persistence of bacterial OM, as either COME or recurrent AOM. Persistent bacterial infection of the adenoids has been the focus of recent microbial studies that demonstrated that S. pneumoniae, NTHi, and M. catarrhalis can invade and survive within adenoidal cells (Forsgren et al., 1994). Furthermore, intracellular localization of bacteria within the middle ear mucosal cells has now been confirmed and the bacterial species subsequently identified (Coates et al., 2008, Thornton et al., 2011). Multi-species bacterial biofilms containing these species have been visualized on the middle ear mucosa and characterized using confocal microscopy. The presence of multiple bacterial species, within the same middle ear mucosal samples, could provide additional protection to each microbe, protecting them from host innate and acquired immune defenses (Armbruster et al., 2010). Biofilm formation is known to significantly increase bacterial protection from antibiotic treatment (Slinger et al., 2006) and the host immune system responses.
Biofilm formation has also been observed in both the nasopharynx and middle ear in the chinchilla model after initial intranasal inoculation with influenza A followed by S. pneumoniae a week later (Hoa et al., 2009). Inoculation with a variety of bacterial species, in combination with prior viral infection, increases the incidence and severity of OM (in a murine model) (Krishnamurthy et al., 2009). Biofilm formation occurs rapidly and the presence of bacteria, not successfully cleared by local or systemic immune responses or antibiotic therapy, poses a challenge for vaccine efficacy. The presence of multiple otopathogens within biofilms also increases the rate of horizontal gene transfer between microbes (Madsen et al., 2012), which may further increase the challenge for the host mucosal immune response and targeting of successful vaccine development for OM.
Importantly, bacterial OM, including biofilm formation, is most frequently the result of infection by commensal bacteria. Commensal microbes are well adapted to minimize recognition by the host’s immune system through molecular mimicry or modulation of host cell innate immune regulation. Recent evidence shows that existing biofilms can be eradicated, in vitro, using an antibody directed against PilA, suggesting that it is possible to develop vaccine strategies that may both prevent and treat OM (Novotny et al., 2012) through activation of the host immune system.
Immunoregulation in the Middle Ear
Mucosal responses to bacterial and viral colonization within the eustachian tube and middle ear include a rapid inflammatory response that effectively seals the eustachian tube. The resultant reduced pressurization of the middle ear causes extravasation of fluid into the middle ear chamber. Together, trapped antigens and otopathogens activate recruitment of immunocompetent cells and then stimulate local antibody secretion, providing evidence that the middle ear is an active effector site.
Over the longer term, mucosal immune responses within the middle ear, as demonstrated by OM, induce pathogenic changes within the tubotympanum mucosa. These changes include increased numbers of mucous glands, goblet cells, and lymphoid follicles containing germinal centers. The thickened mucosa also exhibits increased mucus secretion arising from the mucosal hyperplasia within the eustachian tube (Matsune et al., 1996). Within the middle ear, persistent or COME induces increased epithelial thickness and converts the flattened epithelial cells to secretory ciliated pseudostratified columnar cells with significant basal cell hyperplasia (Ars et al., 2012, Tos, 1980).
Pathogen Recognition
Innate host defenses recognize invading pathogens by activation of one or multiple microbial pattern recognition receptors (PRRs) located in the extracellular, membranous, and cytoplasmic compartments of the middle ear epithelium (Leichtle et al., 2011, Lee and Kim, 2007). PRRs aid the host in discriminating “self” from “non-self” and these receptors may be located at the cell surface, such as transforming growth factor β (TGF-β), or traversing the cell membrane as transmembranous proteins, such as Toll-like receptors (TLR) and C-type lectin receptors. Receptors located within the cytosol include nucleotide-binding oligomerization domain (NOD)-like receptors NOD-1 and NOD-2 and retinoic acid-inducible gene (RIG)-1-like receptors (Lee and Kim, 2007). Furthermore, bacterial and other non-self DNA may also be detected by PRRs such as TLR9, located on endosomes within the cytoplasm of the epithelial cell, or other cytosolic PRRs such as DNA-dependent activator of IFN regulatory factor (DAI), RNA polymerase III (Pol-III), and absent in melanoma 2 (AIM2) receptors (Leichtle et al., 2009).
Regardless of their cellular or subcellular location within the middle ear or eustachian tube epithelia, PRRs recognize conserved molecular signatures produced by the pathogens, defined as pathogen-associated molecular patterns (PAMPs), which, in turn, facilitate upregulation of the host immune response. Each pathogen may produce a variety of PAMP molecules including proteins, lipoproteins, lipids, and nucleic acids and thus may activate multiple signaling pathways. A recent study reported upregulated expression of genes involved with non-self DNA sensing via the TLR9, DAI, Pol-III, and AIM2 receptors in the middle ear mucosa of mice following NTHi infection and confirmed their role in bacterial DNA recognition of NTHi induction of OM in this model (Leichtle et al., 2012a). These multiple parallel pathways for DNA signaling during the early innate immune responses to pathogens within the middle ear indicates potential synergistic and/or redundant activation mechanisms to the same pathogen via stimulation via a number of PRRs. Furthermore, as evidenced by microarray analyses, NTHi and S. pneumoniae can activate upregulation of the TGF-β signaling pathway overall, despite individual genes being up- or downregulated at the time of study (Lee et al., 2011).
Intracellular Signaling in the Middle Ear
Ultimately, PAMP activation of PRRs induces complex signaling cascades that regulate the local innate immune response within the tissue and can then stimulate both local and systemic adaptive immune responses. Immunoregulatory mucosal immune responses may include alteration of the secretion and timing of various cytokines, interferons, and chemokines from cells located within and recruited to the mucosal epithelium. Cross-talk between these molecules may optimize activation of innate immune responses such as inflammation, but also facilitates attenuation of signaling to minimize the risk of local tissue damage. Evidence of the complexity of TLR-induced signaling in response to NTHi-induced OM in the mouse is explored in a 2011 review (Leichtle et al., 2011). Middle ear immune responses to bacterial infection are mediated through a variety of receptors and pathways, including TLR-dependent and TLR-independent pathways (NOD-like receptors, RIG-1 receptors, and other cytosolic PRRs), with TLR-dependent pathways the best described.
Increasingly, OM is recognized as a polymicrobial disease and PRRs such as TLR2 and TLR4 can respond to multiple otopathogens, including NTHi and S. pneumoniae, to activate innate immune responses, including inflammation of the middle ear mucosa (Trune and Zheng, 2009, Kweon et al., 2006). For example, multiple otopathogens activate multiple inflammatory inducers through simultaneous activation of nuclear factor kappa beta (NF-κB). This synergistic activation of inflammation inducers occurs through multiple intracellular signaling pathways (Kweon et al., 2006) and is indicative of involvement of multiple PRRs in middle ear mucosal responses to infection by common otopathogens.
TLRs are among the best-characterized PRRs within the upper respiratory tract and middle ear, and are located within a variety of cell types including macrophages, mast cells, and DC, as well as the mucosal epithelial cells. TLR receptors in the middle ear mucosa can respond to a number of NTHi PAMP ligands, including peptidoglycans or peptidoglycan-associated proteins such as outer membrane protein P6 and lipooligosaccharide (LOS). These ligands activate both TLR2 and TLR4 receptors (Chen et al., 2004, Shuto et al., 2001, Demaria et al., 1996) and are components of bacterial cell membranes, leading to interest in their use as prospective vaccine targets.
A significant body of research has been undertaken using a variety of animal models to explore middle ear immune responses and signaling pathways in NTHi-induced OM. In brief, NTHi activates both TLR2 and TLR4 receptors that recruit and activate TLR adaptor molecules, myeloid differentiation primary response gene 88 (MyD88), and cytoplasmic Toll/interleukin-1 receptor (TIR) domain-containing adaptor-inducing IFN-β (TRIF), respectively. NTHi stimulation of NOD-2 receptors in the cytoplasm can also activate the MyD88-dependent pathway used by all the TLRs (except TLR3), resulting in proinflammatory cytokine production via activation of NF-κB and/or mitogen-activated protein kinase (MAPK) and/or Jun N-terminal protein kinase (JNK). In contrast, TLR4 activation of the TRIF pathway stimulates type 1 interferon and tumor necrosis factor (TNF) production through IFN regulatory factor 3 and acts to delay expression of other inflammatory cytokines. Recently, host cell recognition of M. catarrhalis has also been reported to use multiple TLR receptors, including TLR2, TLR4, and TLR9, in addition to the membrane-bound CD14-TLR4 complex. Activation of these receptors results in subsequent activation of both MyD88- and TRIF-dependent pathways within the host innate immune responses. MAPK activation leads to upregulation of TNFα secretion and, together with IL-6, these proinflammatory cytokines are necessary to reduce bacterial loads in TLR4 mutant C3H/HeJ mice (Hassan et al., 2012). Different otopathogens may upregulate host inflammatory responses via similar PRRs and signaling pathways, although extensive investigation of NTHi infection demonstrates that a number of different signaling pathways may cooperatively activate host immune responses, as illustrated in Figure 2 . NTHi infection also activates the TGF-β signaling pathway through transmembrane receptors and stimulates phosphorylation of Mothers against decapentaplegic homolog 2 (SMAD2/3) protein, which relocates the SMAD/2/3/4 complex to the nucleus. The SMAD/2/3/4 complex, together with transcription factor, triggers expression of genes involved with extracellular matrix formation, which is an important regulator of tissue remodeling within the middle ear during OM (Lee et al., 2011). Viral otopathogens are detected via a variety of PRRs, for example, single-stranded RNA of influenzavirus stimulates TLR7 (mouse) and TLR8 (human) PRRs within the endosome. Furthermore, genomic RNA can be detected in the cytoplasm by RIG-1, which signals, via IRF3/7 and NF-κB transcription factors, upregulation of inflammatory cytokines and type 1 interferons (Takeuchi and Akira, 2010). NACHT domain, leucine-rich-repeat and PYD-containing protein 3 (NLRP3) inflammasomes have a role in detection of the M2 protein of influenzavirus and then activate caspase-1, facilitating production of proinflammatory cytokines IL-1β, IL-18, and IL-33 (Ichinohe et al., 2010).
Figure 2.
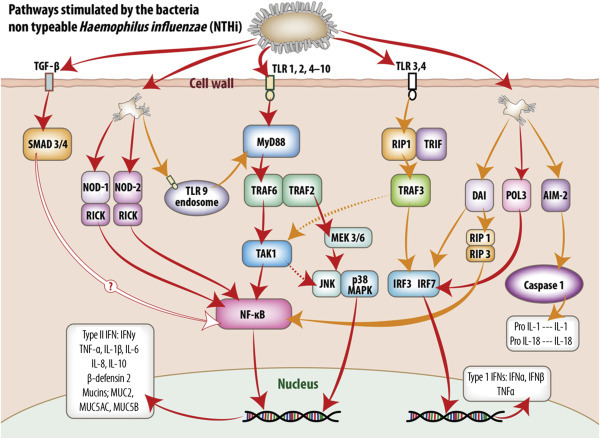
Intracellular signaling pathways utilized by middle ear mucosa in response to stimulation by non-typeable Haemophilus influenzae (NTHi).
Pattern recognition receptors (PRR) detect conserved molecular signatures of invading microbes and facilitate activation of synthesis and secretion of downstream cytokine cascades. This figure schematically represents the signaling pathways activated by NTHi both at the cell membrane and within the cell cytoplasm of the middle ear mucosal cells. The information portrayed was extracted from published reports of experiments utilizing middle ear cell lines and middle ear epithelium and mucosa from animal models and human biopsy material. Transmembrane TLR receptors 1, 2, and 4–10 predominate at the cell surface and their activation results in translocation of NF-κB to the nucleus and upregulation of type II interferons and a range of proinflammatory and antimicrobial molecules. TLR3 utilizes an MYD88-independent pathway via TRIF and IRF3 to upregulate type I interferons. In addition, stimulation of TLR3 and 4 may also activate a delayed response (dotted lines) through TAK1, then the JNK p38MAPK pathway or NF-κB to stimulate type II interferons and proinflammatory molecule production. TGF-β activates SMAD 3 and SMAD 4, ultimately upregulating NF-κB; however, the mechanism for this pathway, although assumed similar to that exhibited by respiratory epithelia, has not been confirmed for middle ear mucosa as indicated. PRR within the cell cytoplasm, such as NOD-1 and NOD-2 and TLR9 receptors on the endosomes, can also upregulate NF-κB stimulation of type II interferons and proinflammatory and antimicrobial molecules via MyD88-independent and -dependent pathways, respectively. DAI may activate both NF-κB and NF-κB-independent stimulation of type II and type 1 interferons, respectively. POL3 via IRF7 stimulates the MyD88-independent pathway utilized by TLR3, while AIM2, via caspase 1, stimulates formation of IL1 and IL18 formation within the cytoplasm.
The effects of NTHi-induced OM, particularly through activation of TLR signaling pathways, has provided considerable detail of the molecules involved in the signaling cascade within the middle ear mucosal cells. Only recently, however, has the relative importance of the individual molecules in activation of innate immune responses been described in mice, using dysfunctional or gene knockouts for key molecules of innate immunity. These molecules included TLR2, TLR4, MyD88, and TNFα, and the study confirmed their key role in normal immunoregulation of the middle ear innate immune responses including inflammation, mucosal hyperplasia, and cellular recruitment to the middle ear (Leichtle et al., 2011, Leichtle et al., 2009). (For review see Leichtle et al., 2012b.)
It is anticipated that the complexity of signaling cascades resulting from activation of TLRs may be underestimated due to the involvement of common signaling pathways. Furthermore, the timing and importance of individual TLR activations, within the response profile, may also differ. For example, within murine middle ear epithelia, TLR2 is the predominant PRR expressed in response to NTHi (Lee et al., 2008), although TLR4 expression is also upregulated (Leichtle et al., 2009, Lim et al., 2008). However, early TLR4 expression is essential for optimizing the TLR2 upregulation and is necessary for rapid inflammation and clearance of pathogens from the middle ear. Higher levels of TLR2 and TLR4 receptor expression have been observed within the healthy rat middle ear mucosa, compared to the levels observed within either the eustachian tube or nasopharyngeal mucosa (Song et al., 2009).
The role of signaling molecules in innate responses to pathogens within the middle ear mucosa is further demonstrated by a reduced rate of recovery from NTHi-induced OM in response to deficiencies in TLRs and their downstream signaling molecules, MyD88 and TRIF. Furthermore, MyD88-deficient mice exhibit a prolonged recovery from NTHi-induced OM compared to TRIF-deficient mice, which may indicate a predominant role for MyD88 in recovery from NTHi infection of the middle ear in this species (Hernandez et al., 2008).
Subsequent activation of NF-κB further down the signaling cascade mediates expression of a variety of molecules in the middle ear mucosa, including inflammatory-related cytokines such as IL-1α, IL-1β, IL-6, IL-10, TNFα, chemokines CCL3 (macrophage inflammatory protein-1a or MIP1a) and CXCL2 (macrophage inflammatory protein-2 or MIP2), and antimicrobials such as β-defensin 2 and mucin genes (Macarthur et al., 2011b, Macarthur et al., 2011a). In the rat model, NTHi stimulates stronger activation of the TGF-β pathway than is observed for pneumococcus-infected ears and contributes to the formation of granulation tissue in response to infection (Lee et al., 2011). The roles performed by individual inflammatory mediators in OM pathogenesis have been comprehensively reviewed (Juhn et al., 2008).
Investigations using the mouse model have clearly demonstrated that PRR activation results in rapid upregulation of inflammatory cytokine expression in the middle ear, which peaks between 6 and 24 h of original infection. Inflammatory cytokine expression in these mice typically returns toward normal levels within a week. In contrast, the resultant middle ear effusion often lasts considerably longer and genes regulating ion and water movement within middle ear epithelia are mostly downregulated for up to 72 h after infection, with many remaining downregulated a week after infection (Macarthur et al., 2011a). These findings from the mouse model are consistent with the timing of middle ear immune responses to pathogen colonization and OM pathogenesis in humans.
Systemic cytokine profiles in children show that recurrent or chronic OM results in increased serum concentrations of T-helper (Th)-2 cytokines, IL-4 and IL-5, in affected children compared with healthy children. Furthermore, serum IL-6 levels are also increased in children with AOM due to S. pneumoniae (Heikkinen et al., 1998). Attempts to utilize the characteristics of proinflammatory cytokine secretion to assist with clinical differentiation of chronic versus acute OM in children have been reported. For example, middle ear effusate (MEE) samples from chronic OME patients show TNFα, IL-1β, and IL-8 in 77–91%, 67–97%, and 92–100% of samples, respectively (Smirnova et al., 2002). In addition, upregulation of TNFα may also be an important factor in the persistence of OME, whereas IL-8, a PMN (Polymorphonuclear)-related inflammatory cytokine, may impact AOM recurrence or delay recovery (Chonmaitree et al., 1996).
Overall, immune responses in otitis-prone children show evidence of reduced capacity to prevent or regulate bacterial colonization at all stages of the immune response, including gene variation in TNFA-238G, TNFA-376G, TNFA-863A, IL6-174G, and IL10-1082A (Emonts et al., 2007). Otitis-prone children exhibit reduced expression of TLR9 and other PRRs, including NOD-1, RIG-1 mRNA in their MEE (Kim et al., 2010), and lower levels of IL-1β, IL-6, and TNFα in nasopharyngeal secretions (Lindberg et al., 1994). Finally, otitis-prone children also demonstrate reduced proliferation of lymphocytes in adenoidal tissue after inoculation with the NTHi outer membrane protein P6 (Kodama et al., 1999), fewer CD4+ T and total T cells in adenoidal tissue, and reduced levels of total serum IgG2 (Freijd et al., 1985). Together, these reports suggest that dysfunction in both innate and adaptive immune responses within the middle ear may increase host susceptibility to recurrent OM.
Local Immune Response in Middle Ear Mucosa
Cellular Response
Innate immune responses within the middle ear mucosa occur rapidly and nonspecifically to facilitate dual objectives, otopathogen eradication and stimulation of adaptive immune defenses for the prevention of subsequent infection by the same pathogens.
Although immunocompetent cells are rarely observed in healthy middle ear mucosa, experimental induction of OM in the rat results in a rapid, large increase in granulocytes with moderate increases in T and B lymphocytes, primarily Th (CD4+) and IgG+ and IgM+ B lymphocytes, as well as NK cells (Jecker et al., 2001). MEE, adenoid, and peripheral blood samples from children experiencing OME with effusion exhibit a predominance of CD4+ memory cells and naïve CD8+ cells (Skotnicka et al., 2005).
Proliferation of lymphocytes within the adenoids results in homing to the middle ear; however, inflammation also stimulates increased local proliferation of lymphocytes within the middle ear mucosa itself in a number of animal models, including mouse and rat (Takahashi et al., 1992, Jecker et al., 2001, Ichimiya et al., 1990). Surprisingly, the number of proliferating B and T lymphocytes observed in rat middle ear mucosa is higher than previously observed in normal lymphatic organs such as the spleen, with dendritic cell, macrophage, and natural killer cell proliferation even higher. In contrast, leukocyte proliferation and recruitment within eustachian tube mucosa is less than observed for the middle ear in a rat AOM model (Jecker et al., 2001) and may provide further evidence of the specific localization of the immunocompetent cell proliferation to the middle ear mucosa.
Cellular proliferation is another characteristic feature of experimentally induced OM and has been observed in a variety of animal models. Mucosal epithelial cell hyperplasia and edema is stimulated by inflammatory cytokine release and the resultant increased mucosal thickness reduces the opportunity for pathogen invasion of the epithelial cells through the middle ear mucosa.
The extent of mucosal hyperplasia, in response to otopathogens such as NTHi, is regulated by release of local proinflammatory cytokines, including TNFα, as demonstrated using TNFα knockout mice. These mice exhibit middle ear mucosal thickening, increased leukocyte infiltration, increased macrophage rather than neutrophil recruitment, and persistence of leukocytes within the middle ear mucosae, characterizing OM infection (Ebmeyer et al., 2011). Several intracellular signaling pathways are involved in the stimulation of mucosal hyperplasia during OM—three primary groups of distinctly regulated MAPK cascades, including extracellular signal-regulated kinase 1/2 (ERK1/ERK2), p38 MAPK, and JNK (Furukawa et al., 2007), although MAPK-independent pathways may also be involved.
Mast Cells
Mast cell presence and degranulation also contribute to the rapid, nonspecific inflammatory responses by mucosal epithelia to bacterial antigens and result in phagocytosis of bacteria and release of proinflammatory cytokines (Mekori, 2004, Dawicki and Marshall, 2007). More generally, there is evidence to suggest that mast cells may also modulate responses by the adaptive immune system (Galli et al., 2005, Shelburne et al., 2009). Although predominantly examined in vitro, mast cell secretion of biologically active molecules such as histamine, TNFα, and IL-6 may influence the migration, maturation, or differentiation of DC, T cells, and B cells, respectively. (For review, see Galli et al., 2005, Galli et al., 2011.)
In some animal models, such as in the murine middle ear, mast cells are not essential for early response to bacterial infection; however, their presence optimizes the rapidity and magnitude of the response. These roles have been demonstrated using NTHi inoculation in W/Wv mice, which lack mast cells, and it was estimated that up to half the strength of early mucosal inflammatory response to NTHi results from mast cell degranulation (Ebmeyer et al., 2005, Pajor et al., 2011). Importantly, mast cell-independent factors including proinflammatory cytokines TNFα, IL-1β, IL-6, and IL-8 may dominate responses in the longer term (Smirnova et al., 2002). Reconstitution of the mast cell population in these knockout mice restored the mucosal response, and together these findings support the mast cell role as a cellular sentinel in the normal innate response in the middle ear mucosa. In humans, the presence of increased density of tryptase-positive mast cells within granulation tissue in the middle ear mucosa of patients with chronic OM is associated with poorer postoperative healing and increased risk of OM recurrence (Pajor et al., 2011).
Mucus and Fluid Flow
Bacterial antigens also rapidly stimulate the eustachian tube and middle ear to secrete mucus. The secreted mucus predominantly contains mucin (MUC), a diverse family of highly glycosylated proteins (Kerschner, 2007, Linden et al., 2008). In general, MUC1, MUC4, and MUC16 appear to facilitate localized adherence to the cell membrane while MUC2, MUC5AC, MUC5B, and MUC19 help to form a movable mucus layer (Linden et al., 2008). The movable mucus layer then permits mucus protection of epithelial cells from pathogens by provision of a mechanism for removal, via cilia action, through the eustachian tube to the nasopharynx (Evans and Koo, 2009).
Mucin transcription is frequently upregulated in response to common otopathogens such as S. pneumoniae and NTHi via the TLR2/4-MyD88-TAK1 signaling cascade. Furthermore, these otopathogens can act synergistically to upregulate MUC5AC transcription by activation of the mitogen-activated protein kinase kinase 3/6 (MKK3/6 p38) and ERK MAPK pathways (Shen et al., 2008). Human middle ear epithelium expresses many of the known mucin genes, including MUC1, MUC2, MUC3, MUC4, MUC5AC, MUC5B, MUC7, MUC8, MUC9, MUC11, MUC13, MUC15, MUC16, MUC18, MUC19, and MUC20 (Kerschner, 2007). Human middle ear epithelial (HMEEC) cell lines have provided considerable evidence of identification and regulation of intracellular signaling pathways in response to S. pneumoniae and NTHi infection and indicate that predominantly MUC5AC is the mucin upregulated (Shen et al., 2008, Komatsu et al., 2008, Lim et al., 2009, Lee et al., 2012). In contrast, evidence from COM patients suggests that MUC5B upregulation was predominant over MUC5AC and that MUC2, MUC19, and MUC7 were not detected in the MEE (Preciado et al., 2010). Recent cell-culture evidence suggests that MUC1 mucin upregulation in A549 airway epithelial cells may help control inflammation during infection with NTHi through suppression of TLR2 signaling and reduced IL-8 production (Kyo et al., 2012). These findings suggest a potential anti-inflammatory role for MUC1 mucin in OM pathogenesis.
Mucus production and its viscosity within the middle ear are also influenced by water flow through and between the subepithelial, epithelial, and apical or luminal compartments of the mucosal epithelium. Aquaporins are a family of integral membrane proteins that act as specialized channels to facilitate water passage through cell membranes in a wide variety of organisms including bacteria, animals, and plants. Aquaporins 1, 4, and 5 are expressed in the rat eustachian tube and middle ear epithelium and are localized within the subepithelial fibroblasts, basolateral membranes of the ciliated epithelial cells, and apical surface of the serous glands (Kang et al., 2007), respectively. Murine expression of aquaporins within the eustachian tube showed similar localization of aquaporin proteins 1, 4, and 5 within the mucosal epithelia, with aquaporin 3 (Takahashi et al., 2009) also localized to the basal membrane of mucosal epithelial cells. Heat-killed NTHi injected into the middle ear resulted in downregulation of the majority of middle ear aquaporin genes in the murine model (Macarthur et al., 2011a); thus NTHi infection within the middle ear may contribute to effusion by this mechanism.
Fluid regulation and ion transport also contribute to water movement through mucosal membranes and may contribute to the transudation of fluid into the middle ear cavity during AOM. Recent studies have reported rapid upregulation of eight key inflammatory cytokines, IL-1α, IL-1β, IL-6, IL-10, MIP-1α, MIP-2, KC (proinflammatory chemokine), and TNFα, within 6–24 h after injection of heat-killed NTHi into the murine middle ear, whereas vascular endothelial growth factor (VEGF) and MAPK8 expression were downregulated (Macarthur et al., 2011b, Macarthur et al., 2011a). In contrast, most of the 24 inner ear ion homeostasis genes examined were downregulated during the inflammation, particularly aquaporin and Na+, K+-ATPase genes, which supports the early transudation of fluid into the middle ear. While the proinflammatory cytokine gene expression returned to normal over the following week, ion homeostasis genes remained downregulated, perhaps in response to the fluid within the middle ear (Macarthur et al., 2011b), which might help to explain why middle ear effusion persists after the infection is cleared.
Fluid movement over mucosal epithelial surfaces is improved by the action of surfactant, which reduces surface tension in liquid–air interfaces such as the respiratory tract and tubotympanum. Lung surfactant protein A (SP-A) and lung surfactant protein D (SP-D) are two large hydrophilic proteins of the collectin family that act to enhance phagocytosis of gram-negative bacteria. Both are expressed within the eustachian tube and middle ear epithelium. SP-A and SP-D expression has been localized to the microvillar epithelial cells in the pig eustachian tube (Paananen et al., 2001) and may contribute to the prevention of bacterial ascension to the middle ear. Furthermore, surfactant apoprotein has also been detected in human middle ear effusions (Yamanaka et al., 1991) and children susceptible to recurrent OM show differences in specific SP haplotypes (Ramet et al., 2001).
Antimicrobial Proteins and Peptides
Overlaying the mucosal epithelial cells, mucus also provides an antimicrobial barrier containing molecules such as lysozyme, lactoferrin, and β-defensins, which act independently and together to inhibit bacterial colonization and stimulate adaptive immune responses (Hellstrom et al., 1997, Underwood and Bakaletz, 2011). The importance of antimicrobial peptides within the middle ear mucosa to reduce middle ear colonization and invasion has been demonstrated in the chinchilla model. In this model, inoculation with an NTHi mutant, NTHi sapA, that exhibits an increased sensitivity to an antimicrobial protein, recombinant chinchilla β-defensin 1 (cBD-1), a human-defensin 3 homolog, resulted in OM disease attenuation and reduced bacterial survival (Mason et al., 2005). Antimicrobial proteins perform both bactericidal and nonbactericidal actions to maintain normal middle ear function. For example, small interfering RNA knockdown of short palate, lung, and nasal epithelium clone 1 (SPLUNC1) in the chinchilla did not alter NTHi survival in the middle ear but demonstrated that expression of SPLUNC1 peptide was essential for maintenance of normal mucociliary clearance and middle ear pressurization (Mcgillivary and Bakaletz, 2010).
Lysozyme
Lysozyme is a potent antibacterial molecule produced by PMNs and mononuclear inflammatory cells that acts by disruption of bacterial cell membranes to reduce the opportunity for bacterial colonization and invasion of middle ear mucosae. Lysozyme rapidly accumulates within the MEE, within 6 h of experimental NTHi infection. Accumulation of lysozyme occurs approximately 6 h before inflammatory cell influx in the guinea pig model (Kawana, 1995). Furthermore, MEE concentrations of lysozyme are higher in children with mucoid OM compared to children experiencing serous OM (Harada et al., 1990, Liu et al., 1975), and lysozyme secretion was localized to middle ear goblet cells in children (Lim et al., 1976). In contrast, lysozyme was localized to the serous glands and epithelial cells and middle ear mucosa in the chinchilla (Hanamure and Lim, 1986). Lysozyme knockout mice (M−/−) are more susceptible to pathogen colonization of the middle ear and develop a more severe middle ear inflammatory response after exposure to S. pneumoniae 6B infection (Shimada et al., 2008), thus supporting the antibacterial role of lysozyme within the middle ear. Furthermore, human lysozyme can act synergistically with β-defensin 2 to reduce S. pneumoniae 6B viability by directly killing the invading pathogen (Lee et al., 2004). Clearly, antimicrobial proteins such as lysozyme may act both independently and together to prevent bacterial colonization and maintain normal function of the middle ear.
Defensins
Defensins are endogenous broad-spectrum antimicrobial peptides considered to be antecedents of the immune response. These peptides are active against a wide variety of bacterial, viral, and fungal pathogens and are an evolutionarily conserved characteristic of the innate immune system (Lehrer and Ganz, 1999). HMEEC can secrete lysozyme and a range of human β defensin (hBD) molecules, including hBD1, hBD2, and hBD3. Pathogen testing confirms hBD1 has bactericidal activity to M. catarrhalis while hBD2 is bactericidal for all three predominant bacterial otopathogens, NTHi, S. pneumoniae, and M. catarrhalis (Lee et al., 2004). Within the human middle ear, expression of hBD2 and hBD3, but not hBD1, are upregulated in human middle ear cholesteatoma epithelium (Song et al., 2007, Park et al., 2003). Both hBD1 and hBD2 act primarily against gram-negative bacteria, but hBD2 may also target gram-positive bacteria and hBD3 appears to provide a broad-spectrum killing of multiple pathogenic microbes (Harder et al., 2001).
Similarly, in the murine model, expression of murine β-defensin (mBD) molecules mBD-2, mBD-3, and mBD-4 is increased; however, mBD-1 and mouse α-defensin expression were unchanged in response to injection of endotoxin into the middle ear (Jin Shin et al., 2006). Invading pathogens such as RSV can also suppress cBD-1, a hBD 3 ortholog; upregulation; and peptide secretion, thus resulting in enhanced otopathogen colonization of the middle ear in the chinchilla model (Mcgillivary et al., 2009). Furthermore, subsequent intranasal administration of exogenous recombinant cBD-1 reduced the bacterial load of NTHi recovered from airway mucosae (Mcgillivary et al., 2009). The relevance of bacterial load is also evidenced from human middle ear cell lines where NTHi induced IL-1α secretion and NTHi components synergistically upregulate epithelial cell secretion of β-defensin (Moon et al., 2006).
Overall, viral suppression of expression of cBD-1, and secretion of its antimicrobial peptide, can increase bacterial colonization in the middle ear and nasopharynx, a key event in failure of pathogen clearance by the middle ear and OM pathogenesis.
Complement
Complement is also a significant component of early mucosal defense of the middle ear and deficiency in complement proteins has long been associated with impaired opsonization in children experiencing OM (Stenfors and Raisanen, 1992).
The importance of the role of complement in NTHi-induced OM was investigated experimentally by complement depletion induced using cobra venom factor. Complement depletion restored the virulence of two otherwise avirulent NTHi strains (NTHi siaβ mutants) that exhibit reduced capacity to sialylate lipopolysaccharide in a murine OM model (Figueira et al., 2007).
Mannose binding lectin (MBL) can also facilitate opsonization of bacterial pathogens through specific oligosaccharide binding activation of the complement pathway; however, significant reduction in serum MBL was not observed in a prospective study of children experiencing recurrent OME (Straetemans et al., 2005).
Further evidence of the role of complement proteins in reducing the risk of OM development is demonstrated by increased binding of complement C3 protein (C3). Streptococcus pneumoniae 6A strains that exhibited either low or high C3 binding were examined using a murine model and increased deposition of C3 protein resulted in improved pathogen clearance from the middle ear, through facilitated opsonization of invading otopathogens. Thus, ultimately, the opportunity for bacterial adherence to the mucous epithelial cell layer was reduced, subsequently reducing the risk of OM development (Sabharwal et al., 2009).
Cytokines
The middle ear and eustachian tube possess an effective innate immune system. Innate immune responses are rapidly induced in response to otopathogens in a nonspecific manner through stimulation of a variety of cell signaling pathways, including local production of inflammatory cytokines and type I interferons (Takeuchi and Akira, 2010). Investigation of the expression of inflammatory cytokine gene expression in the middle ear has been investigated using a number of animal models, including the AOM murine model induced by trans-tympanic injection of S. pneumoniae. In this model, AOM mice demonstrated upregulation of IL-1α, IL-1β, IL-2, TNFα, and IL-6, with IL-1α, IL-1β predominant 24 h after administration of the pathogen (Macarthur et al., 2011b). Examination of chronic OM, using a spontaneously induced OM murine model, the C3H/HEJ mouse (defective TLR4 thus mounts an ineffective immune response to gram-negative bacteria), showed further increase of the upregulation of IL-1α, IL-1β, and TNFα but also demonstrated cytokine genes related to tissue remodeling (TNFα, FGF, BMP) and angiogenesis (VEGF) (Macarthur et al., 2011b). Together, these studies emphasize the intricate control and progressive modification of cytokine gene expression during both the initial and chronic inflammatory responses to otopathogens in the middle ear and their potential role in chronic OM pathogenesis. These responses reduce the opportunity for bacterial adherence to the mucosa and activate adaptive immune responses to upregulate acquired immune responses to then further reduce pathogen invasion within the middle ear mucosa.
Acquired Immune Response and the Middle Ear
The middle ear mucosa is proximal to the adenoids and palatine tonsils, which are key components of the regional MALT, or more specifically, the nasal associated lymphoid tissue (NALT) in association with other small structures in the Waldeyer ring in humans. NALT is considered a key immune induction site of the upper airways, where antigens can be directly sampled via microfold (M) cells in the lymphoid follicle-associated epithelium for processing and presentation by APC such as DC to naïve lymphocytes. In addition, within the middle ear mucosa, antigens may be sampled by intra- and subepithelial DC, which then migrate through the draining lymphatic vessels to regional cervical lymph nodes.
Antigen sampling and key immune induction sites may vary between species, and recent focus on improved immune responses to vaccination via inhalation have frequently used murine models. (For review, see Sabirov and Metzger, 2008b, Sabirov and Metzger, 2008a.) Selective surgical removal of specific tissues comprising the NALT in mice clearly demonstrated that for this species, the cervical lymph nodes performed a greater role in induction of the immune response than did NALT (Sabirov and Metzger, 2008b).
Recently, respiratory M cells or isolated individual antigen sampling cells were identified in the murine airways. These isolated cells function equivalently to M cells within MALT but are located locally within mucosal tissue such as nasal cavity epithelium. CD11C+ DC migrate under these cells to receive antigen after intranasal infection. These M cell-like cells may provide a NALT-independent induction pathway for generation of antigen-specific mucosal immune responses (Kim et al., 2011). Furthermore, if present in the middle ear, these cells may result in the induction of a specific antibody response within the middle ear, particularly in patients who have chronic OM where germinal centers can be identified in the middle ear and eustachian tube epithelium (Matsune et al., 1996).
Secretory Immunoglobulin A in the Middle Ear
Acquired immunological defense in the upper respiratory tract, including the middle ear, in brief, is dependent on the formation of secretory immunoglobulin A (S-IgA), which can minimize bacterial adherence and improve bacterial clearance via opsonization and phagocytosis. During the mucosal immune response, B-cell stimulation of S-IgA secretion occurs initially in the induction sites such as MALT, with S-IgA subsequently secreted at effector sites, which often include but are not limited to the site of pathogen colonization.
IgA is regarded as a hallmark for local immune responses at mucosal surfaces (Cerutti et al., 2011) and early evidence of the middle ear acting as an effector site of the mucosal immune system was the presence of S-IgA in the MEE at a greater concentration than IgG and IgM during antigen-induced inflammation (Ogra et al., 1974). Antigen-specific IgA-producing cells have been identified in the inflamed middle ear; however, other immunoglobulin isotypes are also present. In a study of the anti-NTHi P6 antibody profiles in MEE from children who were prone to OM, but undergoing an acute or subacute infection episode due to NTHi, it was demonstrated that 92% of the MEEs were positive for IgG anti-P6 antibody, 78% for IgA, 70% for IgM, and 45% for SIgA. While the level of IgG, IgA, and IgM anti-P6 antibodies correlated well with those observed in serum, SIgA levels did not (Yamanaka and Faden, 1993). Interestingly, the MEF from patients with AOM has been reported to show higher IgA concentrations compared to blood samples from patients with OME, while IgM and IgG concentrations are less in MEF compared to serum (Sloyer et al., 1976, Yamanaka et al., 1987). Another report indicates that IgG and IgA concentrations within the serum and MEF concentrations are reduced in patients with recurrent OME compared to control patients (Yeo et al., 2008). No significant differences have been reported in serum IgA, IgA2, IgG, and IgG2 between children 3–10 years of age, regardless of whether they have OME or not (Drake-Lee et al., 2003). To our knowledge, no studies have reported more comprehensive IgA subclass profiles in MEEs or serum within otitis-prone children. Furthermore, a recent report indicates a nonsignificant increase in MEF IgA levels against the pneumococcal IgA-1 protease protein in S. pneumoniae-negative/COME-positive children, which may indicate a role in improving the binding of IgA to improve bacterial clearance. This study also reported evidence of a positive correlation between antigen-specific serum and MEE IgG levels for both M. catarrhalis and S. pneumoniae antibodies, which suggests that the antibody response in the middle ear is derived from both the systemic circulation and local mucosal tissue in the middle ear (Verhaegh et al., 2012).
Another study reported that the IgG:IgA ratio in MEF resembled that observed in serum but that small amounts of SIgA were also detected (Kaur et al., 2012).
It is likely that the relationship between the mucosal and systemic responses may change as the infection of the middle ear progresses, as suggested by Takada et al. (1998), whereby antibodies to M. catarrhalis outer membrane proteins detected in the early phase of middle ear infection may be primarily derived from blood and a more local antibody response, accompanied by increasing levels of SIgA, occurs as the infection progresses. If this is the case, it may explain why children who have a generally lower systemic IgG response to otopathogens are prone to OM (Freijd et al., 1985, Takada et al., 1998, Veenhoven et al., 2003, Kaur et al., 2011) and that once colonization and infection occurs, it would prove increasingly difficult for the local immune response stimulated to clear the middle ear of microbes. Furthermore, the formation of biofilms and/or intracellular colonization of the middle ear epithelia may then reduce the opportunity for the successful clearance of microbes from the middle ear by a subsequent local immune response.
Unfortunately, it is likely that pathogenesis of OM is more complex than suggested above, since antigen-specific serum IgG or IgA antibody responses following vaccination with a pneumococcal conjugate vaccine are not impaired in otitis-prone children (Menon et al., 2012). Furthermore, children experiencing recurrent AOM can produce adequate IgG, IgG1, and IgG2 responses against pneumococcal polysaccharides induced by either vaccination or natural exposure (Corscadden et al., 2013). Unfortunately, children with low levels of nasopharyngeal SIgA-specific anti-P6 antibodies are unable to reduce the density of NTHi colonization, irrespective of the load of initial NTHi colonization (Harabuchi et al., 1994), and in these children, the frequency of OM was directly related to the frequency of NTHi colonization. Subsequent studies, however, do support a relationship between the concentration of specific antibody responses following natural exposure to common otopathogens in serum and MEF in children who are prone to recurrent AOM (Berman et al., 1992, Sharma et al., 2011, Straetemans et al., 2005). Indeed, rapid inactivation and clearance of pathogenic bacteria are observed in children where elevated specific serum antibodies, particularly IgG, are identified (Freijd et al., 1985); however, recurrent OM remains a key clinical indicator for identifying children with potential immunodeficiency (Wilson and Hogan, 2008).
Cellular Recruitment and Local Proliferation in the Middle Ear
The concept that local immune responses within the middle ear and eustachian tube, in response to AOM, are the result of a combination of local proliferation of immunocompetent cells as well as being the destination of immunocompetent cells from surrounding NALT has been illustrated by a number of animal models, including rat (Jecker et al., 1996), mouse (Leichtle et al., 2011), and chinchilla (Bakaletz et al., 1989). In these models, the relative paucity of immunocompetent cells within the middle ear mucosa was significantly increased within 24 h of otopathogen administration to the middle ear. In response to immune stimulation, lymphocytes have been reported to enter the middle ear mucosa from a variety of lymphatic sources as determined using chromium-51-labeled lymphocytes in guinea pig (Ryan et al., 1990). A subsequent study examined lymphocytes in the MEE of children experiencing either OME or adenoid hypertrophy and reported that the adenoid may be a primary source for T lymphocytes that can home to middle ear mucosa during immune response to antigenic challenge and that the majority of these cells were CD45RO+, that is, expressed the memory phenotype (Mattila et al., 2000). Furthermore, the hypothesis that local proliferation of lymphocytes occurs within the middle ear mucosa (Takahashi et al., 1992) has been examined during AOM in the rat model using the DNA precursor bromodeoxyuridine to label proliferating leukocytes. Within the middle ear mucosa, the local proliferation rate ranged between 2% and 9% within immunocyte subsets (Jecker et al., 2001).
Lymphocyte subpopulations identified in MEE samples from children with chronic OME are reported to consist predominantly of T cells (CD3+) and primarily T-helper (CD4+) cells with fewer CD8+ cytotoxic T cells (Skotnicka et al., 2005). MEE samples from these children show that the CD4+ lymphocytes are primarily memory cells (CD4+CD45RO+), whereas for CD8+ cells, there is a predominance of naïve T cells (CD8+CD45RA+). Middle ear effusions from these children were also reported to show a higher percentage of CD4+ and CD8+ cells and a higher CD4+/CD8+ ratio compared to that observed within their peripheral blood. Differences between lymphocyte profiles between MEE and blood samples further suggest that the lymphocyte profile is locally regulated, yet these patients exhibited the same phase of mucosal response occurring in both ears at the same time (Skotnicka et al., 2005).
T-cell Regulation and Implication for the Middle Ear
The middle ear mucosa is the primary target for adenoid-derived lymphocytes. Hypertrophic adenoids from children with chronic OME are reported to show increased percentages of CD4+ and CD8+ T lymphocytes with increased expression of CD127 and CD4+ T lymphocytes expressing CD132, both of which indicate increased IL-7R expression. IL-7 secretion facilitates timely proliferation and activation of T and B cells to support the development of an effective immune response within the middle ear (Żelazowska-Rutkowska et al., 2012). An earlier study reported that for children with COME, percentages of CD4+ and CD8+ T cells within the adenoid are decreased (Wysocka et al., 2001).
Another study reports that within the adenoid there is an increased proportion of CD69+ Foxp3high CD25high Treg cells with potent immunosuppressive function. These cells were observed in higher numbers in children who were culture positive for S. pneumoniae in their nasopharynx, which may be suggestive of adenoidal Treg cells having a role in delayed clearance or persistence of pneumococcal carriage (Zhang et al., 2011). Furthermore, it has been reported that the proportion of Foxp3+CD4+ CD25+T/CD4+ T cells in the blood of adults with COME was significantly higher than that observed in acute OME, but also higher than that in normal volunteers, which may indicate a role of Treg immunosuppression in the persistence of middle ear infection (Zhao et al., 2009). Alternatively, Treg cells during chronic middle ear effusion may perform a significant role in the homeostatic control of inflammation in tissues frequently exposed to pathogens, not simply by suppression of T cell production in total but by increasing the opportunity for T cells that recognize self-antigens to escape selection in the thymus and thus contribute actively to chronic inflammatory processes.
Immune responses to common otopathogens within the middle ear must be tightly regulated given that mucosal surfaces are delicate, often one cell thick. Active regulation of innate immune response is therefore vital to minimize immune-generated tissue destruction and damage. It has been reported that within the middle ear mucosa, antigen-specific Treg cells can improve suppression of immune-mediated OM by inhibition of IgG and IgM plasma cell precursors while stimulating IgA-forming plasma cells (Ueyama et al., 1988). Thus, mucosal immunization may significantly reduce induction of immune-mediated damage in the middle ear through reduced risk of reduction of IgG-induced mucosal inflammation and increased antigen-specific S-IgA secretion. Further studies to explore the role of Treg cells in the middle ear are required. However, given observations in the adenoid, Treg cell immunity may be important in the control of inflammatory processes in middle ear infection.
Mucosal Immunization to Prevent Middle Ear Infection
The four predominant otopathogens are S. pneumoniae, NTHi, M. catarrhalis, and RSV, and vaccine strategies to prevent OM should initially be targeted toward these microbes. For S. pneumoniae, the vaccine must include the major serotypes responsible for disease. There are a number of pneumococcal vaccines, developed primarily for prevention of invasive pneumococcal disease, currently available (Cripps and Otczyk, 2006, Cripps et al., 2005, Cripps and Kyd, 2003, Schuerman et al., 2009). One of these vaccines, a polysaccharide-conjugated formulation, uses a protein from NTHi (protein D) as the main carrier protein. Conjugate vaccine efficacy against S. pneumoniae-induced AOM for vaccine serotypes is in the order of 55%. A prototype of the conjugate vaccine containing protein D as the polysaccharide carrier demonstrated an efficacy against AOM of 35.3% due to NTHi, as well as a 57.6% efficacy against serotypes included in the vaccine (Prymula et al., 2006). For OM resulting from S. pneumoniae non-vaccine serotypes, little protection has been observed (Schuerman et al., 2009, Prymula et al., 2006). Studies using animal models and the mucosal immunization route have demonstrated that immunization was able to significantly enhance clearance of each bacterium from the middle ear when subsequently challenged with live bacteria (Cripps and Kyd, 2007). Inclusion of multiple serotypes in an experimental LOS-based vaccine against M. catarrhalis elicited humoral and cellular immune protection against most strains within a mouse model (Ren et al., 2011). For humans, the disappointing outcomes of current polysaccharide conjugate vaccines on AOM, despite good systemic antibody responses, may be several: serotype replacement following vaccination by non-vaccine serotypes or other bacterial otopathogens; different causative S. pneumoniae serotypes from those that cause invasive disease; suboptimal antibody responses following immunization to some serotypes; and the lack of induction of mucosal immune responses in the middle ear following systemic immunization (Cripps and Otczyk, 2006). A greater understanding of immune mechanisms in the middle ear will facilitate the development of vaccination strategies for OM, although it is without doubt that a polymicrobial vaccine will be required.
Conclusion
The global impact of OM on both individuals and the health care system has generated considerable research interest in mucosal immunological response of the middle ear. Ascension of commensal bacteria from the nasopharynx via the eustachian tube to the middle ear initiates innate responses within the middle ear mucosa that, through a complex series of signaling cascades, result in either clearance or failure to clear the pathogen from the middle ear. Secretion of SIgA by the middle ear mucosa may provide improved immunoprotection by prevention of pathogen colonization of the middle ear. Although the majority of childhood middle ear infections resolve uneventfully in most children, very young children are at greater risk of pathogen colonization despite upregulation of innate and acquired immune responses by the middle ear. Mucosal vaccination, targeted to the most common otopathogens, may act via enhanced SIgA response within the middle ear mucosa to prevent development of acute and chronic OM in young children. Finally, the role of regulatory T cells in controlling the middle ear immune response to pathogens requires further investigation. Specifically, the role of regulatory T cells as potential moderators of middle ear inflammatory responses post infection may further reduce the risk of development of chronic or recurrent OM.
Dedication
This chapter is dedicated to our late colleague and dear friend Professor Goro Mogi.
Acknowledgments
The authors gratefully acknowledge Susan Stone for her assistance with the illustrations and Dr Diana Otczyk for reviewing the manuscript.
References
- Alper C.M., Winther B., Mandel E.M., Hendley J.O., Doyle W.J. Rate of concurrent otitis media in upper respiratory tract infections with specific viruses. Arch. Otolaryngol. Head Neck Surg. 2009;135:17–21. doi: 10.1001/archotol.135.1.17. [DOI] [PubMed] [Google Scholar]
- Armbruster C.E., Hong W., Pang B., Weimer K.E.D., Juneau R.A., Turner J., Swords W.E. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio. 2010;1 doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ars B., Dirckx J., Ars-Piret N., Buytaert J. Insights in the physiology of the human mastoid: message to the surgeon. J. Int. Adv. Otol. 2012;8:296–310. [Google Scholar]
- Ashhurst-Smith C., Hall S.T., Stuart J., Burns C.J., Liet E., Walker P.J., Dorrington R., Eisenberg R., Robilliard M., Blackwell C.C. Alloiococcus otitidis: an emerging pathogen in otitis media. J. Infect. 2012;64:233–235. doi: 10.1016/j.jinf.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Avadhanula V., Rodriguez C.A., Devincenzo J.P., Wang Y., Webby R.J., Ulett G.C., Adderson E.E. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J. Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz L.O. Immunopathogenesis of polymicrobial otitis media. J. Leukoc. Biol. 2010;87:213–222. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz L.O., Tallan B.M., Andrezejewski W.J., Demaria T.F., Lim D.J. Immunological responsiveness of chinchillas to outer membrane and isolated fimbrial proteins of nontypeable Haemophilus influenzae. Infect. Immun. 1989;57:3226–3229. doi: 10.1128/iai.57.10.3226-3229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S., Lee B., Nuss R., Roark R., Giclas P.C. Immunoglobulin G, total and subclass, in children with or without recurrent otitis media. J. Pediatr. 1992;121:249–251. doi: 10.1016/s0022-3476(05)81197-7. [DOI] [PubMed] [Google Scholar]
- Bluestone C.D., Doyle W.J. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J. Allergy Clin. Immunol. 1988;81:997–1003. doi: 10.1016/0091-6749(88)90168-6. [DOI] [PubMed] [Google Scholar]
- Canafax D.M., Yuan Z., Chonmaitree T., Deka K., Russlie H.Q., Giebink G.S. Amoxicillin middle ear fluid penetration and pharmacokinetics in children with acute otitis media. Pediatr. Infect. Dis. J. 1998;17:149–156. doi: 10.1097/00006454-199802000-00014. [DOI] [PubMed] [Google Scholar]
- Cerutti A., Cols M., Gentile M., Cassis L., Barra C.M., He B., Puga I., Chen K. Regulation of mucosal IgA responses: lessons from primary immunodeficiencies. Ann. N.Y. Acad. Sci. 2011;1238:132–144. doi: 10.1111/j.1749-6632.2011.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Lim J.H., Jono H., Gu X.-X., Kim Y.S., Basbaum C.B., Murphy T.F., Li J.-D. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2–TAK1-dependent p38 MAPK-AP1 and IKKβ-IκBα-NF-κB signaling pathways. Biochem. Biophys. Res. Commun. 2004;324:1087–1094. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Owen M.J., Howie V.M. Respiratory viruses interfere with bacteriologic response to antibiotic in children with acute otitis media. J. Infect. Dis. 1990;162:546–549. doi: 10.1093/infdis/162.2.546. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Patel J.A., Sim T., Garofalo R., Uchida T., Howie V.M., Owen M.J. Role of leukotriene B4 and interleukin-8 in acute bacterial and viral otitis media. Ann. Otol. Rhinol. Laryngol. 1996;105:968–974. doi: 10.1177/000348949610501207. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Revai K., Grady J.J., Clos A., Patel J.A., Nair S., Fan J., Henrickson K.J., Chonmaitree T., Revai K., Grady J.J., Clos A., Patel J.A., Nair S., Fan J., Henrickson K.J. Viral upper respiratory tract infection and otitis media complication in young children. Clin. Infect. Dis. 2008;46:815–823. doi: 10.1086/528685. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonmaitree T., Ruohola A., Hendley J.O. Presence of viral nucleic acids in the middle ear: acute otitis media pathogen or bystander? Pediatr. Infect. Dis. J. 2012;31:325–330. doi: 10.1097/INF.0b013e318241afe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.H., Griffith S.R., Park K.H., Lim D.J., Demaria T.F. Cytological and histological changes in the middle ear after inoculation of influenza A virus. Acta Otolaryngol. 1993;113:81–87. doi: 10.3109/00016489309135771. [DOI] [PubMed] [Google Scholar]
- Coates H., Thornton R., Langlands J., Filion P., Keil A.D., Vijayasekaran S., Richmond P. The role of chronic infection in children with otitis media with effusion: evidence for intracellular persistence of bacteria. Otolaryngol. Head Neck Surg. 2008;138:778–781. doi: 10.1016/j.otohns.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Corscadden K.J., Kirkham L.A., Thornton R.B., Vijayasekaran S., Coates H.L., Richmond P.C., Wiertsema S.P. High pneumococcal serotype specific IgG, IgG1 and IgG2 levels in serum and the middle ear of children with recurrent acute otitis media receiving ventilation tubes. Vaccine. 2013;31:1393–1399. doi: 10.1016/j.vaccine.2012.12.078. [DOI] [PubMed] [Google Scholar]
- Cripps A.W., Clancy R.L., Gleeson M., Hensley M.J., Dobson A.J., Firman D.W., Wlodarcyk J., Pang G.T. Mucosal immunocompetence in man – the first five years. In: Mestecky J., Mcghee J.R., Bienenstock J., Orga P.L., editors. Recent Advances in Mucosal Immunology, Part B. Plenum Publishing Corporation; 1987. [PubMed] [Google Scholar]
- Cripps A.W., Kyd J. Bacterial otitis media: current vaccine development strategies. Immunol. Cell Biol. 2003;81:46–51. doi: 10.1046/j.0818-9641.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- Cripps A.W., Kyd J.M. Comparison of mucosal and parenteral immunisation in two animal models of pneumococcal infection: otitis media and acute pneumonia. Vaccine. 2007;25:2471–2477. doi: 10.1016/j.vaccine.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Cripps A.W., Otczyk D.C. Prospects for a vaccine against otitis media. Expert Rev. Vaccines. 2006;5:517–534. doi: 10.1586/14760584.5.4.517. [DOI] [PubMed] [Google Scholar]
- Cripps A.W., Otczyk D.C., Kyd J.M. Bacterial otitis media: a vaccine preventable disease? Vaccine. 2005;23:2304–2310. doi: 10.1016/j.vaccine.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Dawicki W., Marshall J.S. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Demaria T.F., Urwin D.M., Leake E.R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect. Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake-Lee A.B., Hughes R.G., Dunn C. Serum IgA and IgG functional antibodies and their subclasses to Streptococcus pneumoniae capsular antigen found in two aged-matched cohorts of children with and without otitis media with effusion. Clin. Otolaryngol. Allied Sci. 2003;28:335–340. doi: 10.1046/j.1365-2273.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- Ebmeyer J., Furukawa M., Pak K., Ebmeyer U., Sudhoff H., Broide D., Ryan A.F., Wasserman S. Role of mast cells in otitis media. J. Allergy Clin. Immunol. 2005;116:1129–1135. doi: 10.1016/j.jaci.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Ebmeyer J., Leichtle A., Hernandez M., Ebmeyer U., Husseman J., Pak K., Sudhoff H., Broide D., Wasserman S.I., Ryan A.F. TNFA deletion alters apoptosis as well as caspase 3 and 4 expression during otitis media. BMC Immunol. 2011;12:12. doi: 10.1186/1471-2172-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonts M., Veenhoven R.H., Wiertsema S.P., Houwing-Duistermaat J.J., Walraven V., De Groot R., Hermans P.W., Sanders E.A., Emonts M., Veenhoven R.H., Wiertsema S.P., Houwing-Duistermaat J.J., Walraven V., De Groot R., Hermans P.W.M., Sanders E.A.M. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007;120:814–823. doi: 10.1542/peds.2007-0524. [DOI] [PubMed] [Google Scholar]
- Evans C.M., Koo J.S. Airway mucus: the good, the bad, the sticky. Pharmacol. Ther. 2009;121:332–348. doi: 10.1016/j.pharmthera.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira M.A., Ram S., Goldstein R., Hood D.W., Moxon E.R., Pelton S.I. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect. Immun. 2007;75:325–333. doi: 10.1128/IAI.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren J., Samuelson A., Ahlin A., Jonasson J., Rynnel-Dagoo B., Lindberg A. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect. Immun. 1994;62:673–679. doi: 10.1128/iai.62.2.673-679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freijd A., Hammarstrom L., Persson M.A., Smith C.I. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin. Exp. Immunol. 1984;56:233–238. [PMC free article] [PubMed] [Google Scholar]
- Freijd A., Oxelius V.-A., Rynnel-Dagöö B. A prospective study demonstrating an association between plasma IgG2 concentrations and susceptibility to otitis media in children. Scand. J. Infect. Dis. 1985;17:115–120. doi: 10.3109/00365548509070430. [DOI] [PubMed] [Google Scholar]
- Furukawa M., Ebmeyer J., Pak K., Austin D.A., Melhus A., Webster N.J., Ryan A.F. Jun N-terminal protein kinase enhances middle ear mucosal proliferation during bacterial otitis media. Infect. Immun. 2007;75:2562–2571. doi: 10.1128/IAI.01656-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S.J., Borregaard N., Wynn T.A. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S.J., Nakae S., Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez J.A., Fresnadillo Martinez M.J. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J. Antimicrob. Chemother. 2002;50:59–73. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- Giebink G.S., Berzins I.K., Marker S.C., Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect. Immun. 1980;30:445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G.S., Ripley M.L., Wright P.F. Eustachian tube histopathology during experimental influenza A virus infection in the chinchilla. Ann. Otol. Rhinol. Laryngol. 1987;96:199–206. doi: 10.1177/000348948709600212. [DOI] [PubMed] [Google Scholar]
- Glasziou P.P., Del Mar C.B., Sanders S.L., Hayem M. Antibiotics for acute otitis media in children. Cochrane Database Syst. Rev. 2004;1(1):1–72. doi: 10.1002/14651858.CD000219.pub2. CD000219. [DOI] [PubMed] [Google Scholar]
- Griffiths N.J., Bradley C.J., Heyderman R.S., Virji M. IFN-γ amplifies NFκB-dependent Neisseria meningitidis invasion of epithelial cells via specific upregulation of CEA-related cell adhesion molecule 1. Cell. Microbiol. 2007;9:2968–2983. doi: 10.1111/j.1462-5822.2007.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamure Y., Lim D.J. Normal distribution of lysozyme- and lactoferrin-secreting cells in the chinchilla tubotympanum. Am. J. Otolaryngol. 1986;7:410–425. doi: 10.1016/s0196-0709(86)80017-5. [DOI] [PubMed] [Google Scholar]
- Harabuchi Y., Faden H., Yamanaka N., Duffy L., Wolf J., Krystofik D. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. J. Infect. Dis. 1994;170:862–866. doi: 10.1093/infdis/170.4.862. [DOI] [PubMed] [Google Scholar]
- Harada T., Juhn S.K., Adams G.L. Lysozyme levels in middle ear effusion and serum in otitis media. Arch. Otolaryngol. Head Neck Surg. 1990;116:54–56. doi: 10.1001/archotol.1990.01870010058017. [DOI] [PubMed] [Google Scholar]
- Harder J., Bartels J., Christophers E., Schroder J.M. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Hassan F., Ren D., Zhang W., Merkel T.J., Gu X.-X. Moraxella catarrhalis activates murine macrophages through multiple toll like receptors and has reduced clearance in lungs from TLR4 mutant mice. PLoS One. 2012;7:e37610. doi: 10.1371/journal.pone.0037610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T. The role of respiratory viruses in otitis media. Vaccine. 2000;19(Suppl. 1):S51–S55. doi: 10.1016/s0264-410x(00)00278-4. [DOI] [PubMed] [Google Scholar]
- Heikkinen T., Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin. Microbiol. Rev. 2003;16:230–241. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T., Ghaffar F., Okorodudu A.O., Chonmaitree T. Serum interleukin-6 in bacterial and nonbacterial acute otitis media. Pediatrics. 1998;102:296–299. doi: 10.1542/peds.102.2.296. [DOI] [PubMed] [Google Scholar]
- Heikkinen T., Jarvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom S., Eriksson P.O., Yoon Y.J., Johansson U. Interactions between the middle ear and the inner ear: bacterial products. Ann. N.Y. Acad. Sci. 1997;830:110–119. doi: 10.1111/j.1749-6632.1997.tb51883.x. [DOI] [PubMed] [Google Scholar]
- Hernandez M., Leichtle A., Pak K., Ebmeyer J., Euteneuer S., Obonyo M., Guiney D., Webster N., Broide D., Ryan A., Wasserman S. Myeloid differentiation primary response gene 88 is required for the resolution of otitis media. J. Infect. Dis. 2008;198:1862–1869. doi: 10.1086/593213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa M., Tomovic S., Nistico L., Hall-Stoodley L., Stoodley P., Sachdeva L., Berk R., Coticchia J.M. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int. J. Pediatr. Otorhinolaryngol. 2009;73:1242–1248. doi: 10.1016/j.ijporl.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Ichimiya I., Kawauchi H., Mogi G. Analysis of immunocompetent cells in the middle ear mucosa. Arch. Otolaryngol. Head Neck Surg. 1990;116:324–330. doi: 10.1001/archotol.1990.01870030088015. [DOI] [PubMed] [Google Scholar]
- Ichinohe T., Pang I.K., Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jecker P., Pabst R., Westermann J. The mucosa of the middle ear and eustachian tube in the young rat: number of granulocytes, macrophages, dendritic cells, NK cells and T and B lymphocytes in healthy animals and during otitis media. Acta Otolaryngol. 1996;116:443–450. doi: 10.3109/00016489609137871. [DOI] [PubMed] [Google Scholar]
- Jecker P., Pabst R., Westermann J. Proliferating macrophages, dendritic cells, natural killer cells, T and B lymphocytes in the middle ear and Eustachian tube mucosa during experimental acute otitis media in the rat. Clin. Exp. Immunol. 2001;126:421–425. doi: 10.1046/j.1365-2249.2001.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Shin D., Gan-Undram S., Jin Kim S., Joon Jun Y., Jung Im G., Hyun Jung H. Expression of beta-defensins in the tubotympanum of experimental otitis media. Acta Otolaryngol. 2006;126:1040–1045. doi: 10.1080/00016480600672626. [DOI] [PubMed] [Google Scholar]
- Juhn S.K., Jung M.-K., Hoffman M.D., Drew B.R., Preciado D.A., Sausen N.J., Jung T.T.K., Kim B.H., Park S.-Y., Lin J., Ondrey F.G., Mains D.R., Huang T. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin. Exp. Otorhinolaryngol. 2008;1:117–138. doi: 10.3342/ceo.2008.1.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.H., Chang K.H., Ohcho S., Lee H.Y., Cha K., Moon S.K., Andalibi A., Lim D.J. Expression of water channel proteins (aquaporins) in the rat Eustachian tube and middle ear mucosa. Acta Otolaryngol. 2007;127:687–692. doi: 10.1080/00016480500452574. [DOI] [PubMed] [Google Scholar]
- Kaur R., Casey J.R., Pichichero M.E. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29:1023–1028. doi: 10.1016/j.vaccine.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Kim T., Casey J.R., Pichichero M.E. Antibody in middle ear fluid of children originates predominantly from sera and nasopharyngeal secretions. Clin. Vaccine Immunol. 2012;19:1593–1596. doi: 10.1128/CVI.05443-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana M. Early inflammatory changes of the Haemophilus influenzae-induced experimental otitis media. Auris Nasus Larynx. 1995;22:80–85. doi: 10.1016/s0385-8146(12)80104-0. [DOI] [PubMed] [Google Scholar]
- Kerschner J.E. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007;117:1666–1676. doi: 10.1097/MLG.0b013e31806db531. [DOI] [PubMed] [Google Scholar]
- Kim D.Y., Sato A., Fukuyama S., Sagara H., Nagatake T., Kong I.G., Goda K., Nochi T., Kunisawa J., Sato S., Yokota Y., Lee C.H., Kiyono H. The airway antigen sampling system: respiratory M cells as an alternative gateway for inhaled antigens. J. Immunol. 2011;186:4253–4262. doi: 10.4049/jimmunol.0903794. [DOI] [PubMed] [Google Scholar]
- Kim M.G., Park D.C., Shim J.S., Jung H., Park M.S., Kim Y.I., Lee J.W., Yeo S.G. TLR-9, NOD-1, NOD-2, RIG-I and immunoglobulins in recurrent otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2010;74:1425–1429. doi: 10.1016/j.ijporl.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Kodama H., Fadden H., Harabuchi Y., Kataura A., Bernstein J.M., Brodsky L. Cellular immune response of adenoidal and tonsillar lymphocytes to the P6 outer membrane protein of non-typeable Haemophilus influenzae and its relation to otitis media. Acta Otolaryngol. 1999;119:377–383. doi: 10.1080/00016489950181422. [DOI] [PubMed] [Google Scholar]
- Komatsu K., Jono H., Lim J.H., Imasato A., Xu H., Kai H., Yan C., Li J.-D. Glucocorticoids inhibit nontypeable Haemophilus influenzae-induced MUC5AC mucin expression via MAPK phosphatase-1-dependent inhibition of p38 MAPK. Biochem. Biophys. Res. Commun. 2008;377:763–768. doi: 10.1016/j.bbrc.2008.10.091. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy A., Mcgrath J., Cripps A.W., Kyd J.M. The incidence of Streptococcus pneumoniae otitis media is affected by the polymicrobial environment particularly Moraxella catarrhalis in a mouse nasal colonisation model. Microbes Infect. 2009;11:545–553. doi: 10.1016/j.micinf.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Kurono Y., Mogi G. Mucosal immunity of the middle ear. In: Kiyono H., Ogra P.L., Mcghee J., editors. Mucosal Vaccines. Academic Press; California, USA: 1996. [Google Scholar]
- Kweon S.-M., Wang B., Rixter D., Lim J.H., Koga T., Ishinaga H., Chen L.-F., Jono H., Xu H., Li J.-D. Synergistic activation of NF-κB by nontypeable H. influenzae and S. pneumoniae is mediated by CK2, IKKβ-IκBα, and p38 MAPK. Biochem. Biophys. Res. Commun. 2006;351:368–375. doi: 10.1016/j.bbrc.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo Y., Kato K., Park Y.S., Gajhate S., Umehara T., Lillehoj E.P., Suzaki H., Kim K.C. Antiinflammatory role of MUC1 mucin during infection with nontypeable Haemophilus influenzae. Am. J. Respir. Cell Mol. Biol. 2012;46:149–156. doi: 10.1165/rcmb.2011-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach A., Boswell J., Asche V., Nienhuys T., Mathews J. Bacterial colonisation of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants. Pediatr. Infect. Dis. J. 1994;13:983–989. doi: 10.1097/00006454-199411000-00009. [DOI] [PubMed] [Google Scholar]
- Lee H.-Y., Takeshita T., Shimada J., Akopyan A., Woo J.-I., Pan H., Moon S., Andalibi A., Park R.-K., Kang S.-H., Kang S.-S., Gellibolian R., Lim D. Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC Infect. Dis. 2008;8:87. doi: 10.1186/1471-2334-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.Y., Andalibi A., Webster P., Moon S.K., Teufert K., Kang S.H., Li J.D., Nagura M., Ganz T., Lim D.J. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect. Dis. 2004;4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Komatsu K., Lee B.C., Lim J.H., Jono H., Xu H., Kai H., Zhang Z.J., Yan C., Li J.-D. Phosphodiesterase 4B mediates extracellular signal-regulated kinase-dependent up-regulation of mucin MUC5AC protein by Streptococcus pneumoniae by inhibiting cAMP-protein kinase A-dependent MKP-1 phosphatase pathway. J. Biol. Chem. 2012;287:22799–22811. doi: 10.1074/jbc.M111.337378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Kim Y. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol. Cells. 2007;23:1. [PubMed] [Google Scholar]
- Lee Y.-W., Chung Y., Juhn S.K.M.D., Kim Y., Lin J. Activation of the transforming growth factor beta pathway in bacterial otitis media. Ann. Otol. Rhinol. Laryngol. 2011;120:204–213. doi: 10.1177/000348941112000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R.I., Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- Leichtle A., Hernandez M., Lee J., Pak K., Webster N.J., Wollenberg B., Wasserman S.I., Ryan A.F. The role of DNA sensing and innate immune receptor TLR9 in otitis media. Innate Immun. 2012;18:3–13. doi: 10.1177/1753425910393539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichtle A., Wasserman S.I., Wasserman S.I., Ryan A.F. Otitis media: immunology and vaccines. Otorinolaringologia. 2012;62:91–100. [Google Scholar]
- Leichtle A., Hernandez M., Pak K., Yamasaki K., Cheng C.-F., Webster N.J., Ryan A.F., Wasserman S.I. TLR4-mediated induction of TLR2 signaling is critical in the pathogenesis and resolution of otitis media. Innate Immun. 2009;15:205–215. doi: 10.1177/1753425909103170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichtle A., Lai Y., Wollenberg B., Wasserman S.I., Ryan A.F. Innate signaling in otitis media: pathogenesis and recovery. Curr. Allergy Asthma Rep. 2011;11:78–84. doi: 10.1007/s11882-010-0158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.J. Functional morphology of the mucosa of the middle ear and Eustachian tube. Ann. Otol. Rhinol. Laryngol. 1976;85:36–43. doi: 10.1177/00034894760850S209. [DOI] [PubMed] [Google Scholar]
- Lim D.J. Normal and pathological mucosa of the middle ear and Eustachian tube. Clin. Otolaryngol. 1979;4:213–234. doi: 10.1111/j.1365-2273.1979.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Lim D.J., Chun Y.M., Lee H.Y., Moon S.K., Chang K.H., Li J.D., Andalibi A. Cell biology of tubotympanum in relation to pathogenesis of otitis media—a review. Vaccine. 2000;19(Suppl. 1):S17–S25. doi: 10.1016/s0264-410x(00)00273-5. [DOI] [PubMed] [Google Scholar]
- Lim D.J., Liu Y.S., Birck H. Secretory lysozyme of the human middle ear mucosa: immunocytochemical localisation. Ann. Otol. Rhinol. Laryngol. 1976;85:50–60. doi: 10.1177/000348947608500109. [DOI] [PubMed] [Google Scholar]
- Lim J.H., Ha U., Sakai A., Woo C.H., Kweon S.M., Xu H., Li J.D. Streptococcus pneumoniae synergizes with nontypeable Haemophilus influenzae to induce inflammation via up-regulating TLR2. BMC Immunol. 2008;29(9):40. doi: 10.1186/1471-2172-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.H., Kim H.J., Komatsu K., Ha U., Huang Y., Jono H., Kweon S.M., Lee J., Xu X., Zhang G.S., Shen H., Kai H., Zhang W., Xu H., Li J.D. Differential regulation of Streptococcus pneumoniae-induced human MUC5AC mucin expression through distinct MAPK pathways. Am. J. Transl. Res. 2009;1:300–311. [PMC free article] [PubMed] [Google Scholar]
- Lindberg K., Rynnel-Dagoo B., Sundqvist K.G. Cytokines in nasopharyngeal secretions; evidence for defective IL-1β production in children with recurrent episodes of acute otitis media. Clin. Exp. Immunol. 1994;97:396–402. doi: 10.1111/j.1365-2249.1994.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden S.K., Sutton P., Karlsson N.G., Korolik V., Mcguckin M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.S., Lim D.J., Lang R.W., Birck H.G. Chronic middle ear effusions. Immunochemical and bacteriological investigations. Arch. Otolaryngol. 1975;101:278–286. doi: 10.1001/archotol.1975.00780340010003. [DOI] [PubMed] [Google Scholar]
- Macarthur C.J., Hausman F., Kempton J.B., Trune D.R. Murine middle ear inflammation and ion homeostasis gene expression. Otol. Neurotol. 2011;32(3):508–515. doi: 10.1097/MAO.0b013e31820e6de4. 510.1097/MAO.1090b1013e31820e31826de31824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur C.J., Pillers D.-A.M., Pang J., Kempton J.B., Trune D.R. Altered expression of middle and inner ear cytokines in mouse otitis media. Laryngoscope. 2011;121:365–371. doi: 10.1002/lary.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J.S., Burmølle M., Hansen L.H., Sørensen S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012;65:183–195. doi: 10.1111/j.1574-695X.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- Mandel E.M., Doyle W.J., Winther B., Alper C.M., Mandel E.M., Doyle W.J., Winther B., Alper C.M. The incidence, prevalence and burden of OM in unselected children aged 1–8 years followed by weekly otoscopy through the “common cold” season. Int. J. Pediatr. Otorhinolaryngol. 2008;72:491–499. doi: 10.1016/j.ijporl.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom T., Nokso-Koivisto J., Chonmaitree T. Viral-bacterial interactions in acute otitis media. Curr. Allergy Asthma Rep. 2012;12:551–558. doi: 10.1007/s11882-012-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason K.M., Munson R.S., Jr., Bakaletz L.O. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect. Immun. 2005;73:599–608. doi: 10.1128/IAI.73.1.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsune S., Takahashi H., Sando I. Mucosa-associated lymphoid tissue in middle ear and Eustachian tube in children. Int. J. Pediatr. Otorhinolaryngol. 1996;34:229–236. doi: 10.1016/0165-5876(95)01265-6. [DOI] [PubMed] [Google Scholar]
- Mattila P.S., Nykänen A., Eloranta M., Tarkkanen J. Adenoids provide a microenvironment for the generation of CD4+, CD45RO+, L-selectin−, CXCR4+, CCR5+ T lymphocytes, a lymphocyte phenotype found in the middle ear effusion. Int. Immunol. 2000;12:1235–1243. doi: 10.1093/intimm/12.9.1235. [DOI] [PubMed] [Google Scholar]
- Mcgillivary G., Bakaletz L.O. The multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airway. PLoS One. 2010;5:e13224. doi: 10.1371/journal.pone.0013224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgillivary G., Mason K.M., Jurcisek J.A., Peeples M.E., Bakaletz L.O. Respiratory syncytial virus-induced dysregulation of expression of a mucosal β-defensin augments colonization of the upper airway by non-typeable Haemophilus influenzae. Cell. Microbiol. 2009;11:1399–1408. doi: 10.1111/j.1462-5822.2009.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgillivary G., Munson R., Bakaletz L. 107th American Society Microbiology General Meeting (Toronto, Ontario, Canada) 2007. Desialylation of an antimicrobial peptide by a causative agent of the polymicrobial disease otitis media is a potential mechanism for immune evasion that also influences colonization by nontypeable Haemophilus influenzae. [Google Scholar]
- Mekori Y.A. The mastocyte: the “other” inflammatory cell in immunopathogenesis. J. Allergy Clin. Immunol. 2004;114:52–57. doi: 10.1016/j.jaci.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Menon V.J., Corscadden K.J., Fuery A., Thornton R.B., Kirkham L.A., Richmond P.C., Wiertsema S.P. Children with otitis media mount a pneumococcal serotype specific serum IgG and IgA response comparable to healthy controls after pneumococcal conjugate vaccination. Vaccine. 2012;30:3136–3144. doi: 10.1016/j.vaccine.2012.01.086. [DOI] [PubMed] [Google Scholar]
- Miura M.S., Mascaro M., Rosenfeld R.M. Association between otitis media and gastroesophageal reflux: a systematic review. Otolaryngol. Head Neck Surg. 2012;146:345–352. doi: 10.1177/0194599811430809. [DOI] [PubMed] [Google Scholar]
- Moon S.K., Lee H.Y., Pan H., Takeshita T., Park R., Cha K., Andalibi A., Lim D.J. Synergistic effect of interleukin 1 alpha on nontypeable Haemophilus influenzae-induced up-regulation of human beta-defensin 2 in middle ear epithelial cells. BMC Infect. Dis. 2006;6:12. doi: 10.1186/1471-2334-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T.F., Chonmaitree T., Barenkamp S., Kyd J., Nokso-Koivisto J., Patel J.A., Heikkinen T., Yamanaka N., Ogra P., Swords W.E., Sih T., Pettigrew M.M. Panel 5: Microbiology and immunology panel. Otolaryngol. Head Neck Surg. 2013;148:E64–E89. doi: 10.1177/0194599812459636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygind N., Pedersen M. Nose, sinus and ear symptoms in 27 patients with primary ciliary dyskinesia. Eur. J. Respir. Dis. Suppl. 1983:96–101. [PubMed] [Google Scholar]
- Novotny L.A., Clements J.D., Bakaletz L.O. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine. 2012;31(34):3417–3426. doi: 10.1016/j.vaccine.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra P.L. Mucosal immune system in neonatal period and early infancy. Pediatr. Health. 2010;4:637–647. [Google Scholar]
- Ogra P.L., Bernstein J.M., Yurchak A.M., Coppola P.R., Tomasi T.B. Characteristics of secretory immune system in human middle ear: implications in otitis media. J. Immunol. 1974;112:488–495. [PubMed] [Google Scholar]
- Paananen R., Sormunen R., Glumoff V., Van Eijk M., Hallman M. Surfactant proteins A and D in Eustachian tube epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L660–L667. doi: 10.1152/ajplung.2001.281.3.L660. [DOI] [PubMed] [Google Scholar]
- Pajor A., Danilewicz M., Jankowski A., Durko T. Participation of mast cells in chronic otitis media. Folia histochemica et cytobiologica/Polish Academy of Sciences. Pol. Histochem. Cytochem. Soc. 2011;49:479–485. doi: 10.5603/fhc.2011.0068. [DOI] [PubMed] [Google Scholar]
- Palva T., Taskinen E., Lehtinen T., Ramsay H., Bjorksten F., Hackman P. Mast cells and histamine in adenoid tissue and middle ear. Acta Otolaryngol. 1991;111:349–353. doi: 10.3109/00016489109137399. [DOI] [PubMed] [Google Scholar]
- Park K., Bakaletz L.O., Coticchia J.M., Lim D.J. Effect of influenza A virus on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann. Otol. Rhinol. Laryngol. 1993;102:551–558. doi: 10.1177/000348949310200711. [DOI] [PubMed] [Google Scholar]
- Park K., Moon S.K., Choung Y.H., Choi H.S. Expression of beta-defensins in human middle ear cholesteatoma. Acta Otolaryngol. 2003;123:236–240. doi: 10.1080/0036554021000028102. [DOI] [PubMed] [Google Scholar]
- Preciado D., Goyal S., Rahimi M., Watson A.M., Brown K.J., Hathout Y., Rose M.C. MUC5B is the predominant mucin glycoprotein in chronic otitis media fluid. Pediatr. Res. 2010;68:231–236. doi: 10.1203/PDR.0b013e3181eb2ecc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prymula R., Peeters P., Chrobok V., Kriz P. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- Ramet M., Lofgren J., Alho O.P., Hallman M. Surfactant protein-A gene locus associated with recurrent otitis media. J. Pediatr. 2001;138:266–268. doi: 10.1067/mpd.2001.110133. [DOI] [PubMed] [Google Scholar]
- Ren D., Xie H., Zhang W., Hassan F., Petralia R.S., Yu S., Lim D.J., Gu X.-X. Intranasal immunization of the combined lipooligosaccharide conjugates protects mice from the challenges with three serotypes of Moraxella catarrhalis. PLoS One. 2011;6:e29553. doi: 10.1371/journal.pone.0029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruohola A., Meurman O., Nikkari S., Skottman T., Salmi A., Waris M., Osterback R., Eerola E., Allander T., Niesters H., Heikkinen T., Ruuskanen O. Microbiology of acute otitis media in children with tympanostomy tubes: prevalences of bacteria and viruses. Clin. Infect. Dis. 2006;43:1417–1422. doi: 10.1086/509332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A.F., Sharp P.A., Harris J.P. Lymphocyte circulation to the middle ear. Acta Otolaryngol. 1990;109:278–287. doi: 10.3109/00016489009107444. [DOI] [PubMed] [Google Scholar]
- Sabharwal V., Ram S., Figueira M., Park I.H., Pelton S.I. Role of complement in host defense against pneumococcal otitis media. Infect. Immun. 2009;77:1121–1127. doi: 10.1128/IAI.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov A., Metzger D.W. Mouse models for the study of mucosal vaccination against otitis media. Vaccine. 2008;26:1501–1524. doi: 10.1016/j.vaccine.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov A., Metzger D.W. Intranasal vaccination of infant mice induces protective immunity in the absence of nasal-associated lymphoid tissue. Vaccine. 2008;26:1566–1576. doi: 10.1016/j.vaccine.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B.A., Shelokov A., Ramsay M.A. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J. Infect. Dis. 1978;137:176–181. doi: 10.1093/infdis/137.2.176. [DOI] [PubMed] [Google Scholar]
- Schuerman L., Borys D., Hoet B., Forsgren A., Prymula R. Prevention of otitis media: now a reality? Vaccine. 2009;27:5748–5754. doi: 10.1016/j.vaccine.2009.07.070. [DOI] [PubMed] [Google Scholar]
- Segal N., Givon-Lavi N., Leibovitz E., Yagupsky P., Leiberman A., Dagan R. Acute otitis media caused by Streptococcus pyogenes in children. Clin. Infect. Dis. 2005;41:35–41. doi: 10.1086/430605. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Casey J.R., Pichichero M.E. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J. Infect. Dis. 2011;204:645–653. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne C.P., Nakano H., St John A.L., Chan C., Mclachlan J.B., Gunn M.D., Staats H.F., Abraham S.N. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Yoshida H., Yan F., Li W., Xu F., Huang H., Jono H., Li J.-D. Synergistic induction of MUC5AC mucin by nontypeable Haemophilus influenzae and Streptococcus pneumoniae. Biochem. Biophys. Res. Commun. 2008;365:795–800. doi: 10.1016/j.bbrc.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Shimada J., Moon S.K., Lee H.Y., Takeshita T., Pan H., Woo J.I., Gellibolian R., Yamanaka N., Lim D.J. Lysozyme M deficiency leads to an increased susceptibility to Streptococcus pneumoniae-induced otitis media. BMC Infect. Dis. 2008;8:134. doi: 10.1186/1471-2334-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto T., Xu H., Wang B., Han J., Kai H., Gu X. Activation of NF-kappa B by nontypeable Haemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha/beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8774–8779. doi: 10.1073/pnas.151236098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotnicka B., Stasiak-Barmuta A., Hassmann-Poznanska E., Kasprzycka E. Lymphocyte subpopulations in middle ear effusions: flow cytometry analysis. Otology Neurotol. 2005;26:567–571. doi: 10.1097/01.mao.0000169050.61630.da. [DOI] [PubMed] [Google Scholar]
- Slinger R., Chan F., Ferris W., Yeung S.-W., St Denis M., Gaboury I., Aaron S.D. Multiple combination antibiotic susceptibility testing of nontypeable Haemophilus influenzae biofilms. Diagn. Microbiol. Infect. Dis. 2006;56:247–253. doi: 10.1016/j.diagmicrobio.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Sloyer J.L., Jr., Howie V.M., Ploussard J.H., Schiffman G., Johnston R.B., Jr. Immune response to acute otitis media: association between middle ear fluid antibody and the clearing of clinical infection. J. Clin. Microbiol. 1976;4:306–308. doi: 10.1128/jcm.4.3.306-308.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova M.G., Kiselev S.L., Gnuchev N.V., Birchall J.P., Pearson J.P. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur. Cytokine Netw. 2002;13:161–172. [PubMed] [Google Scholar]
- Smith-Vaughan H., Byun R., Nadkarni M., Na J., Hunter N., Halpin S., Morris P., Leach A. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 2006;6 doi: 10.1186/1472-6815-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-J., Cho J.G., Woo J.-S., Lee H.-M., Hwang S.-J., Chae S.-W. Differential expression of toll-like receptors 2 and 4 in rat middle ear. Int. J. Pediatr. Otorhinolaryngol. 2009;73:821–824. doi: 10.1016/j.ijporl.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Song J.J., Chae S.W., Woo J.S., Lee H.M., Jung H.H., Hwang S.J. Differential expression of human beta defensin 2 and human beta defensin 3 in human middle ear cholesteatoma. Ann. Otol. Rhinol. Laryngol. 2007;116:235–240. doi: 10.1177/000348940711600312. [DOI] [PubMed] [Google Scholar]
- Stenfors L.E., Raisanen S. In vivo attachment of Streptococcus pneumoniae and Haemophilus influenzae to nasopharyngeal epithelium in children. ORL J. Otorhinolaryngol. Relat. Spec. 1992;54:25–28. doi: 10.1159/000276254. [DOI] [PubMed] [Google Scholar]
- Stol K., Van Selm S., van Den Berg S., Bootsma H.J., Blokx W.A., Graamans K., Tonnaer E.L.G.M., Hermans P.W.M. Development of a non-invasive murine infection model for acute otitis media. Microbiology. 2009;155:4135–4144. doi: 10.1099/mic.0.033175-0. [DOI] [PubMed] [Google Scholar]
- Straetemans M., Wiertsema S., Sanders E.M., Rijkers G., Graamans K., Van Der Baan B., Zielhuis G. Immunological status in the aetiology of recurrent otitis media with effusion: serum immunoglobulin levels, functional mannose-binding lectin and Fc receptor polymorphisms for IgG. J. Clin. Immunol. 2005;25:78–86. doi: 10.1007/s10875-005-0361-8. [DOI] [PubMed] [Google Scholar]
- Suenaga S., Kodama S., Ueyama S., Suzuki M., Mogi G. Mucosal immunity of the middle ear: analysis at the single cell level. Laryngoscope. 2001;111:290–296. doi: 10.1097/00005537-200102000-00019. [DOI] [PubMed] [Google Scholar]
- Takada R., Harabuchi Y., Himi T., Kataura A. Antibodies specific to outer membrane antigens of Moraxella catarrhalis in sera and middle ear effusions from children with otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 1998;46:185–195. doi: 10.1016/s0165-5876(98)00158-x. [DOI] [PubMed] [Google Scholar]
- Takahashi E., Kikuchi T., Katori Y., Kobayashi T. Localization of aquaporins, water channel proteins, in the mouse eustachian tube. Acta Otolaryngol. 2009;129:67–70. doi: 10.1080/00016480902964317. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Kanai N., Watanabe A., Oshima O., Ryan A.F. Lymphocyte subsets in immune-mediated otitis media with effusion. Eur. Arch. Otorhinolaryngol. 1992;249:24–27. doi: 10.1007/BF00175666. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Thornton R., Rigby P., Wiertsema S., Filion P., Langlands J., Coates H., Vijayasekaran S., Keil A., Richmond P. Multi-species bacterial biofilm and intracellular infection in otitis media. BMC Pediatr. 2011;11:94. doi: 10.1186/1471-2431-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tos M. Middle ear epithelia in chronic secretory otitis. Arch Otolaryngol.Head Neck Surg. 1980;106:593–597. doi: 10.1001/archotol.1980.00790340001001. [DOI] [PubMed] [Google Scholar]
- Trune D.R., Zheng Q.Y. Mouse models for human otitis media. Brain Res. 2009;1277:90–103. doi: 10.1016/j.brainres.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Yamazaki Y., Katsuno T., Tamura A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- Ueno K., Lim D.J. Heterogeneity of glycoconjugates in the secretory cells of the chinchilla middle ear and eustachian tubal epithelia: a lectin-gold cytochemical study. J. Histochem. Cytochem. 1991;39:71–80. doi: 10.1177/39.1.1983875. [DOI] [PubMed] [Google Scholar]
- Ueyama S., Kawauchi H., Mogi G. Suppression of immune-mediated otitis media by T-suppressor cells. Arch. Otolaryngol. Head Neck Surg. 1988;114:878–882. doi: 10.1001/archotol.1988.01860200062019. [DOI] [PubMed] [Google Scholar]
- Underwood M., Bakaletz L. Innate immunity and the role of defensins in otitis media. Curr. Allergy Asthma Rep. 2011;11:499–507. doi: 10.1007/s11882-011-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhoven R., Rijkers G., Schilder A., Adelmeijer J., Uiterwaal C., Kuis W., Sanders E. Immunoglobulins in otitis-prone children. Pediatr. Res. 2003;55:159–162. doi: 10.1203/01.PDR.0000099776.66136.39. [DOI] [PubMed] [Google Scholar]
- Verhaegh S.J., Stol K., De Vogel C.P., Riesbeck K., Lafontaine E.R., Murphy T.F., Van Belkum A., Hermans P.W., Hays J.P. Comparative analysis of the humoral immune response to Moraxella catarrhalis and Streptococcus pneumoniae surface antigens in children suffering from recurrent acute otitis media and chronic otitis media with effusion. Clin. Vaccine Immunol. 2012;19:914–918. doi: 10.1128/CVI.05630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Kawauchi H., Fujiyoshi T., Mogi G. Distribution of mast cells in the tubotympanum of guinea pigs. Ann. Otol. Rhinol. Laryngol. 1991;100:407–412. doi: 10.1177/000348949110000511. [DOI] [PubMed] [Google Scholar]
- Widemar L., Hellström S., Stenfors L.-E., Bloom G.D. An overlooked site of tissue mast cells—the human tympanic membrane, implications for middle ear infections. Acta Otolaryngol. 1986;102:391–395. doi: 10.3109/00016488609119422. [DOI] [PubMed] [Google Scholar]
- Wilson N.W., Hogan M.B. Otitis media as a presenting complaint in childhood immunodeficiency diseases. Curr. Allergy Asthma Rep. 2008;8:519–524. doi: 10.1007/s11882-008-0095-6. [DOI] [PubMed] [Google Scholar]
- Winther B., Alper C.M., Mandel E.M., Doyle W.J., Hendley J.O., Winther B., Alper C.M., Mandel E.M., Doyle W.J., Hendley J.O. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119:1069–1075. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- Wysocka J., Hassmann-Poznanska E., Kasprzycka E., Musiatowicz M. Lymphocyte subpopulations in the adenoids analyzed by flow cytometry. Wiadomosci Lek. 2001;54:418–423. [PubMed] [Google Scholar]
- Yamanaka N., Faden H. Low serum IgG antibody levels specific for P6 of nontypeable Haemophilus influenzae in otitis prone children. J. Pediatr. 1993;122:212–218. doi: 10.1016/s0022-3476(06)80115-0. [DOI] [PubMed] [Google Scholar]
- Yamanaka N., Kobayashi K., Kataura A., Kuroki Y., Akino T. Implication of surfactant apoprotein in otitis media with effusion. Ann. Otol. Rhinol. Laryngol. 1991;100:835–840. doi: 10.1177/000348949110001009. [DOI] [PubMed] [Google Scholar]
- Yamanaka N., Somekawa Y., Suzuki T., Kataura A. Immunologic and cytologic studies in otitis media with effusion. Acta Otolaryngol. 1987;104:481–486. doi: 10.3109/00016488709128278. [DOI] [PubMed] [Google Scholar]
- Yeo S.G., Park D.C., Lee S.K., Cha C.I. Relationship between effusion bacteria and concentrations of immunoglobulin in serum and effusion fluid in otitis media with effusion patients. Int. J. Pediat. Otorhin. 2008;72:337–342. doi: 10.1016/j.ijporl.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Yonemura S. Cadherin–actin interactions at adherens junctions. Curr. Opin. Cell Biol. 2011;23:515–522. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Żelazowska-Rutkowska B., Wysocka J., Ratomski K., Kasprzycka E., Skotnicka B. Increased percentage of T cells with the expression of CD127 and CD132 in hypertrophic adenoid in children with otitis media with effusion. Eur. Arch. Otorhinolaryngol. 2012;269:1821–1825. doi: 10.1007/s00405-012-1977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Leong S.C., Mcnamara P.S., Mubarak A., Malley R., Finn A. Characterisation of regulatory T cells in nasal associated lymphoid tissue in children: relationships with pneumococcal colonization. PLoS Pathog. 2011;7:e1002175. doi: 10.1371/journal.ppat.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.Q., Li J., Liu H., Zhang Q.G., Wang Y., Han D.M. Role of interleukin-10 and transforming growth factor beta 1 in otitis media with effusion. Chin. Med. J. 2009;122:2149–2154. [PubMed] [Google Scholar]


