Publisher Summary
Members of the family Paramyxoviridae cause most devastating diseases of animals and humans. In particular, the viruses causing rinderpest, canine distemper, Newcastle disease, measles, and mumps have arguably caused more morbidity and mortality than any other single group of related viruses in history. The impact of these diseases has been dramatically reduced through the use of vaccines in both humans and animals, in combination with depopulation and restrictions on animal movements for diseases such as rinderpest and Newcastle disease. The history of the paramyxoviruses is replete with incorrect reports that complicate their taxonomic classification and confuses assessment of their true ability to cause interspecies infections. The nomenclature of viruses within the family Paramyxoviridae is confusing and fraught with inconsistencies, as individual viruses have variously been named according to their species of origin, geographic sites of discovery, antigenic relationships, or given names related to the diseases that they produce in affected animals or humans. Paramyxovirus virions are pleomorphic, 150–350 nm in diameter. Virions are enveloped, covered with large glycoprotein spikes, and contain a “herringbone-shaped” helically symmetrical nucleocapsid, approximately 1 μm in length and 18 nm (Paramyxovirinae) or 13–14 nm (Pneumovirinae) in diameter. Paramyxoviruses usually cause lytic infection in cell cultures, but adaptation of the virus is usually necessary to achieve high-titer yields of virus.
The family Paramyxoviridae is included in the order Mononegavirales, along with the families Rhabdoviridae, Filoviridae, and Bornaviridae. This order was established to bring together viruses with distant, ancient phylogenetic relationships (Figure 17.1 ) that are also reflected in similarities in their gene order and strategies of gene expression and replication. All these viruses are enveloped and, other than bornaviruses, have prominent envelope glycoprotein spikes. All viruses included in the order have genomes consisting of a single molecule of negative-sense, single-stranded RNA. The features that differentiate the individual families of the order include genome size, nucleocapsid structure, site of genome replication and transcription, manner and extent of messenger RNA (mRNA) processing, virion size and morphology, tissue specificity, host range, and pathogenic potential in their respective hosts (Table 17.1 ).
Figure 17.1.

Phylogenetic tree of members of the order Mononegavirales. The tree was constructed using the sequences of the conserved domain III of the RNA polymerase. aPaV6, avian parainfluenza virus type 6; BDV, Borna disease virus; BEFV, bovine ephemeral fever virus; CDV, canine distemper virus; HeV, Hendra virus; HMPV, human metapneumovirus; HRSV, human respiratory syncytial virus; MARV, Lake Victoria marburgvirus; MeV, measles virus; MuV, mumps virus; NDV, Newcastle disease virus; NiV, Nipah virus; PIV3, parainfluenza virus 3; PVM, pneumonia virus of mice; RABV, rabies virus; SeV, Sendai virus; SV5, simian virus 5; TRTV, turkey rhinotracheitis virus; VSV, vesicular stomatitis virus; ZEBOV, Zaire ebolavirus.
[From Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses (C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, L. A. Ball, eds.), p. 613. Copyright © Elsevier (2005), with permission.]
Table 17.1.
Distinguishing Characteristics of Four Families of the Order Mononegavirales
| Characteristic | Family Paramyxoviridae | Family Rhabdoviridaea | Family Filoviridae | Family Bornaviridae |
|---|---|---|---|---|
| Genome size (kb) | 15–19 | 11–15 | 19 | 9 |
| Virion morphology | Pleomorphic | Bullet-shaped | Filamentous | Spherical |
| Site of replication | Cytoplasm | Cytoplasm | Cytoplasm | Nucleus |
| Mode of transcription | Polar with non-overlapping signals (except pneumoviruses) and stepwise attenuation | Polar with non-overlapping signals and stepwise attenuation | Polar with non-overlapping signals and stepwise attenuation | Complex with mRNA splicing and overlapping start/stop signals |
| Host range | Vertebrates | Vertebrates | Humans, non-human primates and bats | Horses, sheep, cats, birds, (humans?) shrews and possibly other small mammals |
| Pathogenic potential | Mainly respiratory disease | Mild febrile to fatal neurological disease | Hemorrhagic fever | Immune-mediated neurological diseaseProventricular dilation syndrome in birds |
Vertebrate virus members.
Several of the most devastating diseases of animals and humans are caused by members of the family Paramyxoviridae. In particular, the viruses causing rinderpest, canine distemper, Newcastle disease, measles, and mumps have arguably caused more morbidity and mortality than any other single group of related viruses in history. The impact of these diseases has been dramatically reduced through the use of vaccines in both humans and animals, in combination with depopulation and restrictions on animal movements for diseases such as rinderpest and Newcastle disease. Other viruses in this family also cause disease in a wide variety of mammals, birds, and reptiles—including, amongst many examples: respiratory syncytial viruses in cattle, sheep, goats, and wildlife; Sendai virus (murine parainfluenza virus 1) in mice; avian rhinotracheitis virus (metapneumovirus) in turkeys and chickens; phocine morbillivirus in seals; ophidian paramyxoviruses, including Fer-de-Lance virus in snakes. Of recent concern and interest are the paramyxoviruses of the genus Henipavirus that naturally infect various species of bats, but cause high mortality rates in infected humans and animals. As wildlife species come more in contact with humans and domesticated animals through changes in habitat, the opportunities increase for cross-species infections by these and additional, as yet unidentified, paramyxoviruses.
The history of the paramyxoviruses is replete with incorrect reports that complicate their taxonomic classification, and confuses assessment of their true ability to cause interspecies infections. Specifically, interpretation of the results of previous sero-surveys is frequently complicated by the considerable cross-reactivity that occurred as a result of inapparent contamination of the test antigens, as well as the stimulation of heterotypic antibodies after infection of animals with individual viruses. Failure to recognize these limitations led to erroneous conclusions, such as a putative link between parainfluenza virus 3 infection and abortion in cattle and respiratory disease in horses.
Properties of Paramyxoviruses
Classification
The family Paramyxoviridae is subdivided into the subfamilies Paramyxovirinae and Pneumovirinae, the former containing the genera Respirovirus, Avulavirus, Henipavirus, Rubulavirus, and Morbillivirus, and the latter containing the genera Pneumovirus and Metapneumovirus (Table 17.2 ; Figure 17.2 ). The family continues to expand rapidly as new viruses are discovered in wild animal populations, with a growing list of relatively uncharacterized viruses from wild or feral rodents (J paramyxovirus, Nariva virus, and Mossman virus), tree shrews (Tupaia virus), and bats (Menangle virus and Mapuera virus). Several other members of the family have not yet been assigned to the existing genera, including: Fer-de-Lance and a variety of related ophidian paramyxoviruses of reptiles, Salem virus of equines, several viruses of penguins that are distinct from avian paramyxoviruses 1–9, and Atlantic salmon paramyxovirus. The list of members of the family Paramyxoviridae is certain to grow as more wildlife species are analyzed for their respective viruses. Indeed, the family Paramyxoviridae probably will continue to expand, not just with new viruses, but also with new genera.
Table 17.2.
Paramyxoviruses and the Diseases they Cause
| Subfamily/Genus Virus | Animal Species Affected | Disease |
|---|---|---|
| Paramyxovirinae/Respirovirus | ||
| Bovine parainfluenza virus 3 | Cattle, sheep, other mammals | Respiratory disease in cattle and sheep |
| Murine parainfluenza virus 1 (Sendai virus) | Mice, rats, rabbits | Severe respiratory disease in mice (sometimes rats and other laboratory animals) |
| Human parainfluenza viruses 1 and 3 | Humans | Respiratory disease |
| Paramyxovirinae/Rubulavirus | ||
| Avian paramyxovirus 1 (Newcastle disease virus-virulent isolates) | Domestic and wild fowl | Severe generalized disease with central nervous system signs |
| Avian paramyxoviruses 2–9 | Fowl | Respiratory disease |
| Canine parainfluenza virus 5 (SV5) | Dogs | Respiratory disease |
| Porcine rubulavirus (La-Piedad-Michoacan-Mexico virus) | Swine | Encephalitis, reproductive failure, corneal opacities |
| Mumps virus | Humans | Parotitis |
| Human parainfluenza viruses 2, 4a, and 4b | Humans | Respiratory disease |
| Paramyxovirinae/Morbillivirus | ||
| Rinderpest virus | Cattle, wild ruminants | Severe generalized disease |
| Peste des petits ruminants virus | Sheep, goats | Severe generalized disease like rinderpest |
| Canine distemper virus | Dogs and members of families Procyonidae, Mustelidae, Felidae | Severe generalized disease with central nervous system signs |
| Phocine distemper virus | Seals and sea lions | Severe generalized disease with respiratory system signs |
| Dolphin distemper virus | Dolphins | Severe generalized disease with respiratory system signs |
| Porpoise distemper virus | Porpoises | Severe generalized disease with respiratory system signs |
| Bovine morbillivirus (MV-K1) | Cattle | Poorly characterized—significance unknown |
| Measles virus | Humans | Measles, severe systemic disease with respiratory and central nervous system signs |
| Paramyxovirinae/Henipavirus | ||
| Hendra virus | Horses and humans | Acute respiratory distress syndrome in horses and humans |
| Nipah virus | Swine and humans | Acute respiratory distress syndrome in swine and humans |
| Pneumovirinae/Pneumovirus | ||
| Bovine respiratory syncytial virus | Cattle, sheep, goats | Respiratory disease |
| Pneumonia virus of mice | Mice and dogs | Respiratory disease |
| Human respiratory syncytial virus | Humans | Respiratory disease |
| Pneumovirinae/Metapneumovirus | ||
| Turkey rhinotracheitis virus | Turkeys, chickens | Severe respiratory disease in turkeys, swollen head syndrome of chickens |
Figure 17.2.
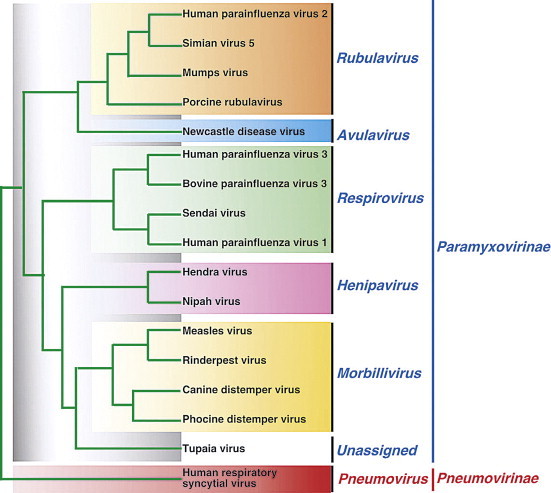
Phylogenetic relationships among the L protein sequences of member viruses of the family Paramyxoviridae
[From Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses (C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, L. A. Ball, eds.), p. 667. Copyright © Elsevier (2005), with permission.]
The nomenclature of viruses within the family Paramyxoviridae is confusing and fraught with inconsistencies, as individual viruses have variously been named according to their species of origin (e.g., porcine rubulavirus, avian paramyxoviruses 2–9), geographic sites of discovery (e.g., Sendai, Hendra, and Newcastle disease viruses), antigenic relationships (e.g., human parainfluenza viruses 1–4), or given names related to the diseases that they produce in affected animals or humans (e.g., canine distemper, rinderpest, measles, and mumps viruses). Indeed, it appears that many members of this family represent related lineages of viruses that are enzootic within one principal host species but periodically cross over to another species (species-jumping), underscoring the continuing potential for cross-species emergence of these viruses as pathogens.
Virion Properties
Paramyxovirus virions are pleomorphic (spherical as well as filamentous forms occur), 150–350 nm in diameter (Figure 17.3 ). Virions are enveloped, covered with large glycoprotein spikes (8–14 nm in length), and contain a “herringbone-shaped” helically symmetrical nucleocapsid, approximately 1 μm in length and 18 nm (Paramyxovirinae) or 13–14 nm (Pneumovirinae) in diameter. The genome consists of a single linear molecule of negative-sense, single-stranded RNA, 13–19 kb in size. The RNA does not contain a 5′ cap and is not polyadenylated at the 3′ end, but does have functional 5′ and 3′ non-coding elements. With the exception of members of the Pneumovirinae, the genomic size follows the “rule of six”—that is, the number of nucleotides is a multiple of six, which appears to be a function of the binding properties of the N protein to the RNA molecule. There are 6–10 genes separated by conserved non-coding sequences that contain termination, polyadenylation, and initiation signals for the transcribed mRNAs; viruses in the genera Respirovirus, Avulavirus, Henipavirus, and Morbillivirus have 6 genes, those in the genus Rubulavirus have 7, the genus Metapneumovirus has 8, and the genus Pneumovirus has 10 (Figure 17.4 ). The genomes of viruses in the subfamily Paramyxovirinae encode 9–12 proteins through the presence of overlapping reading frames within the phosphoprotein (P) locus, whereas those in the subfamily Pneumovirinae encode only 8–10 proteins. Most of the gene products are present in virions either associated with the lipid envelope or complexed with the virion RNA. The virion proteins include three nucleocapsid proteins [an RNA-binding protein (N), a phosphoprotein (P), and a large polymerase protein (L)] and three membrane proteins [an unglycosylated matrix protein (M), and two glycosylated envelope proteins—a fusion protein (F) and an attachment protein, the latter being a hemagglutinin (H), a hemagglutinin–neuraminidase (HN), or a glycoprotein G that has neither hemagglutinating nor neuraminidase activities]. Variably conserved proteins include non-structural proteins (C, NS1, NS2), a cysteine-rich protein (V) that binds zinc, a small integral membrane protein (SH), and transcription factors M2-1 and M2-2.
Figure 17.3.

(Right) Negative contrast electron micrographs of intact simian virus-5 (SV-5) particles (genus Rubulavirus) (Top) and the SV-5 nucleocapsid after detergent lysis of virions (Bottom)(Courtesy of G.P. Leser and R.A. Lamb). The bars represent 100nm. (Left top and bottom) Schematic diagrams of SV-5 particles in cross section (N) (formerly NP): nucleocapsid, P: phosphoprotein, L: large polymerase protein, V: cysteine rich protein that shares its N-terminus with P sequence and for SV-5 is found in virions, M: matrix or membrane protein, F: fusion protein, NH: hemagglutinin-neuraminidase, SH: small hydrophobic protein). Adapted from Kingsbury, D.W. (1990). Paramyxoviridae: the viruses and their replication. In: Virology, 2nd Edn (B.N. Fields and D.M. Knipe, eds). Raven Press, New York, and from Scheid, H. (1987). Animal Virus Structure, (M.V. Nermut, and A.C. Steven, eds). Elsevier, Amsterdam. With permission).
[From Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses (C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, L. A. Ball, eds.), p. 655. Copyright © Elsevier (2005), with permission.]
Figure 17.4.
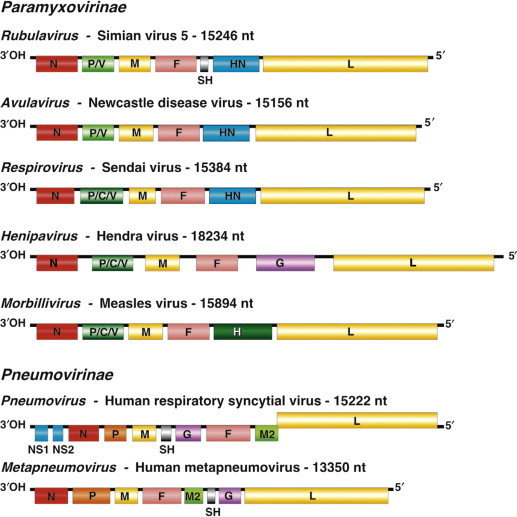
Maps of genomic RNAs (3′ to 5′) of viruses belonging to the seven genera of the family Paramyxoviridae. Each box represents a separately encoded mRNA; multiple distinct ORFs within a single mRNA are indicated by slashes. Numbers indicate nucleotide length of the genomic RNA. Protein letter codes are as in Figure 17.3.
[From Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses (C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, L. A. Ball, eds.), p. 657. Copyright © Elsevier (2005), with permission.]
The envelope spikes of paramyxoviruses are composed of two glycoproteins: the fusion protein (F) and HN (Respirovirus, Avulavirus, Rubulavirus), H (Morbillivirus), or G (Henipavirus, Pneumovirus, Metapneumovirus) (Table 17.3 ). Both envelope proteins have key roles in the pathogenesis of all paramyxovirus infections. One glycoprotein (HN, H, or G) is responsible for cell attachment, whereas the other (F) mediates the fusion of the viral envelope with the plasma membrane of the host cell. Unlike entry of viruses through the endosomal pathway, membrane fusion initiated by the paramyxovirus F protein is not dependent upon a low pH environment. Neutralizing antibodies specific for the attachment glycoprotein (HN, H, or G) inhibit adsorption of virus to cellular receptors, but antibodies specific to F can also neutralize viral infectivity.
Table 17.3.
Functions and Terminology of Virion Proteins in the Family Paramyxoviridae
| Function |
Virion Protein |
||
| Genera Respirovirus and Rubulavirus |
Genus Morbillivirus |
Genus Pneumovirus |
|
| Attachment protein: hemagglutinin, induction of productive immunity | HN | H | Gα |
| Neuraminidase: virion release, destruction of mucin inhibitors | HN | None | None |
| Fusion protein: cell fusion, virus penetration, cell–cell spread, contribution to induction of protective immunity | F | F | F |
| Nucleoprotein: protection of genome RNA | N | N | N |
| Transcriptase: RNA genome transcription | L and P/C/V | L and P/C/V | L and P |
| Matrix protein: virion stability | M | M | M |
| Other | (SH) | – | SH, M2 |
No hemagglutinating activity.
The fusion protein is synthesized as an inactive precursor (F0) that must be activated by proteolytic cleavage by cellular proteases. The cleaved peptides remain in close proximity by virtue of linking disulfide bonds. The specific nature of the cleavage process and the characteristics of the F0 protein differ among viruses in the different genera. However, the paramyxoviruses can be crudely divided into two groups: those with a single basic amino acid at the cleavage site and those with multiple basic amino acids at the cleavage site. The cleavage of F0 is essential for infectivity, and is a key determinant of pathogenicity; for example, virulent strains of avian paramyxovirus 1 (Newcastle disease virus) have multiple basic residues at the cleavage site, which means that the F protein can be cleaved intracellularly by furin, an endopeptidase in the trans-Golgi network (Table 17.4 ). The ubiquitous presence of this enzyme in cells facilitates the production of infectious virus in all cells capable of being infected by Newcastle disease virus. Avirulent forms of the virus have a single basic residue at the cleavage site, and the F0 protein is present in mature virions; these viruses are only activated by extracellular proteases with appropriate substrate specificity or trypsin-like enzymes in epithelial cells of, principally, the respiratory and gastrointestinal tracts. This limited “cleavability” restricts infectivity of the virus to fewer species of birds and significantly reduces the pathogenic potential of these viruses. After cleavage, the newly generated amino-terminal sequence of the F1 protein has a hydrophobic domain, and it is postulated that this is involved directly in fusion, in concert with the attachment protein.
Table 17.4.
Amino Acid Sequences at the F0 Cleavage Site of Strains of Avian Paramyxovirus 1
| Virus Strain | Virulence for Chickens | Cleavage Site Amino Acids 111 to 117 |
|---|---|---|
| Herts 33 | High | -G-R-R-Q-R-R*F- |
| Essex '70 | High | -G-R-R-Q-K-R*F- |
| 135/93 | High | -V-R-R-K-K-R*F- |
| 617/83 | High | -G-G-R-Q-K-R*F- |
| 34/90 | High | -G-K-R-Q-K-R*F- |
| Beaudette C | High | -G-R-R-Q-K-R*F- |
| La Sota | Low | -G-G-R-Q-G-R*L- |
| D26 | Low | -G-G-K-Q-G-R*L- |
| MC110 | Low | -G-E-R-Q-E-R*L- |
| 1154/98 | Low | -G-R-R-Q-G-R*L- |
| Australian isolates | ||
| Peats Ridge | Low | -G-R-R-Q-G-R*L- |
| NSW 12/86 | Low | -G-K-R-Q-G-R*L- |
| Dean Park | High | -G-R-R-Q-R-R*F- |
| Somersby 98 | Low | -G-R-R-Q-R-R*L- |
| PR-32 | ? | -G-R-R-Q-G-R*F- |
| MP-2000 | Low | -G-R-R-Q-K-R*L- |
[From Diseases of Poultry (Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, D. E. Swayne, eds.), 11th ed., p. 69. Copyright © 2003 Wiley-Blackwell, with permission.]
Cleavage point. Basic amino acids are shown in bold. Note that all virulent viruses have phenylalanine (F) at position 117, the F1 N-terminus.
The M or matrix protein is the most abundant protein in the virion. As with other viruses with similar proteins, M interacts with the lipid envelope, the cytoplasmic “tails” of the F- and HN-like proteins, and the ribonucleoprotein. These interactions are consistent with M having a central role in the assembly of mature virions, by providing the structural link between the envelope glycoproteins and the ribonucleoprotein. M proteins are also implicated in controlling the levels of RNA synthesis.
Virus Replication
Paramyxoviruses usually cause lytic infection in cell cultures, but adaptation of the virus (selection for mutants more readily able to replicate in the in-vitro system) is usually necessary to achieve high-titer yields of virus. Formation of syncytia is a characteristic feature of many paramyxovirus infections in non-polarized cell cultures, but less so in polarized cell culture systems; similarly, syncytia are characteristic of some, but certainly not all, paramyxovirus infections in animals (Figure 2.2B). Acidophilic cytoplasmic inclusions composed of ribonucleoprotein structures are characteristic of paramyxovirus infections and, although their replication is entirely cytoplasmic, morbilliviruses also produce characteristic acidophilic intranuclear inclusions that are complexes of nuclear elements and N protein. Hemadsorption is a distinctive feature of paramyxoviruses that encode an HN protein (Figure 2.1D), and of some morbilliviruses, but not of pneumoviruses.
Paramyxoviruses replicate in the cytoplasm of infected cells; virus replication continues in the presence of actinomycin D and in enucleated cells, confirming that there is no requirement for nuclear functions. The virus attachment proteins (HN, H, G), recognize compatible ligands on the surface of host cells. For the rubulaviruses, respiroviruses, and avulaviruses, HN binds to surface molecules containing sialic acid residues—either glycolipids or glycoproteins. The neuraminidase activity of this protein is assumed, by analogy with influenza virus, to assist the virus in release from infected cells by removing the sialic acid residues that could bind virus to an already infected cell. For morbilliviruses, the cell receptor on lymphocytes, macrophages, and dendritic cells is the equivalent to the human CD150 [signaling lymphocyte activation molecule (SLAM)] glycoprotein, which explains the strong tropism of these viruses for these cell types. The receptors for henipaviruses (Hendra and Nipah viruses) are ephrin B2 and B3 cell-surface proteins, with single amino acid differences in the attachment glycoprotein G apparently determining which receptor is preferentially used. The distribution of these receptors may explain in part the pathogenesis of the systemic infections caused by henipaviruses, as these receptors are variably expressed on the surface of endothelial cells and brain stem neurons. The attachment molecules for respiratory syncytial virus (genus Pneumovirus) are ill defined, but may include heparan sulfate.
Following attachment, the processed F protein mediates fusion of the viral envelope with the plasma membrane at physiologic pH. The liberated nucleocapsid must remain intact, with all three of its associated proteins (N, P, and L) being necessary for initial transcription of the genomic viral RNA by the RNA-dependent RNA polymerase [transcriptase (L)]; mRNA synthesis is initiated in the absence of protein synthesis. The polymerase complex initiates RNA synthesis at a single site on the 3′ end of the genomic RNA, and the genome is transcribed progressively into 6–10 discrete mRNAs by a sequential interrupted-synthesis mechanism. This termination–reinitiation process controls the synthesis of mRNA such that the quantity of the individual mRNAs decreases with increasing distance from the 3′ end of the genome. The mRNAs are capped and polyadenylated.
When the concentration of the N protein reaches a critical level, a promoter sequence at the 3′ end of the genomic RNA is transcribed and N protein binds to the nascent RNA chain. This alters the polymerase to ignore the message-termination signals, and a complete positive-sense antigenome strand is made. This antigenome strand complexed with N protein then serves as a template for the production of negative-sense genomic RNA. A second phase of mRNA synthesis then begins from the newly made genomic RNA, thus amplifying dramatically the synthesis of viral proteins.
Whereas most genes encode a single protein, the P gene of the member viruses of the subfamily Paramyxovirinae encodes three to seven P/V/C proteins (Figure 17.4; Table 17.3). Remarkably different strategies for maximizing the coding potential of this gene complex have evolved in the different genera of the subfamily. For example, the gene complex of the member viruses of the genera Morbillivirus, Henipavirus, and Respirovirus encodes 4–7 proteins, the production of which utilizes two distinct transcription mechanisms: (1) internal initiation of translation from different start codons; (2) insertion of non-templated G residues into mRNA to shift the reading frame to that of an otherwise inaccessible open reading frame. Whereas the P protein itself is translated from a faithful mRNA copy of the complete gene, the smaller C protein is read in a different reading frame following initiation of translation from an internal initiation codon. Quite separately, the transcription of the V gene involves the insertion of an extra G nucleotide into its mRNA by polymerase site-specific stuttering (“editing”), which results in the production of a protein that displays N-terminal homology with the P protein, but with a different amino acid sequence downstream of the G insertion. Because the reading frame used to transcribe the V gene is also distinct, all three reading frames are utilized in the transcription of the P/C/V gene complex. In the case of parainfluenza virus 3, a fourth protein, D, is translated by insertion of two non-templated G residues. In the genus Rubulavirus there are additional variations in the transcription of the P/C/V gene complex and the products formed, but in the genus Pneumovirus each of the 10 genes encodes just a single protein, with none of the genomic coding economy and strategies utilized by viruses in the other genera.
The P gene is essential for virus replication but the function(s) of the proteins produced by the alternative transcription/translation of the gene are yet to be clearly defined. The C-terminal of the protein binds to the L protein and the N protein : RNA complex to form a unit that is essential for mRNA transcription. The N-terminal portion of the P protein is also proposed to bind to the newly synthesized N protein to permit synthesis of genomic RNA from the plus-strand template. Protein products of the “P gene” of several paramyxoviruses, including the henipaviruses and morbilliviruses, disrupt innate host defenses (Figure 17.5 ); specifically, mutations affecting these accessory proteins generally do not affect growth of the viruses in cell culture, but, in vivo, the mutants are attenuated. Available data suggest that products of the P gene compromise the interferon response network, possibly through inhibition of the signal transducers and activator of transcription (STAT) proteins, interferon regulatory factor 3 (IRF3), and other interferon response genes. Other activities ascribed to the accessory proteins involve regulation of levels of viral RNA synthesis.
Figure 17.5.
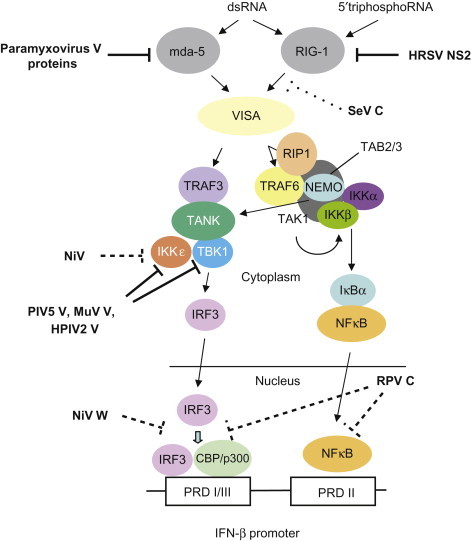
Paramyxovirus accessory proteins target the intracellular viral pattern recognition receptors (PRR). The signaling pathways leading from the RNA helicases mda-5 and RIG-1 to induction of interferon-β (IFN-β) are shown. Paramyxovirus V proteins interact with mda-5 and prevent its activation. Sendai virus (SeV) C protein targets RIG-1, although a specific molecular interaction has yet to be shown, The NS2 (non-structural) protein of human respiratory syncytial virus (HRSV) directly binds to RIG-1 and inhibits its activity. The V proteins of human parainfluenza virus 2 (HPIV2), simian virus 5 (PIV5, formerly SV5), and mumps virus (MuV) interact with and inhibit TBK1 and IKKε, and the V protein of Nipah virus (NiV) inhibits IKKε (although not TBK1). The C protein of rinderpest virus (RPV) and the W protein of NiV have uncharacterized nuclear targets that act downstream of transcription factors. CBP; IKK, inhibitory protein kappa B (IκB) kinase; IRF3, IFN regulatory factor 3; NEMO, NFκB essential modulator; NFκB, nuclear factor κB; PRD; RIP1, receptor-interacting protein 1; TAB2/3; TAK1, transforming growth factor-β-activated kinase 1; TANK, TRAF family member-associated NFκB activator; TBK1, TANK binding kinase-1; TRAF, tumor necrosis factor receptor-associated factor, VISA,
[From S. Goodbourn, R. E. Randall. The regulation of type I interferon production by paramyxoviruses. J. Interferon Cytokine Res. 29, 539–548 (2009), with permission.]
Virion maturation involves: (1) the incorporation of viral glycoproteins into patches on the host-cell plasma membrane; (2) the association of matrix protein (M) and other non-glycosylated proteins with this altered host-cell membrane; (3) the alignment of nucleocapsid beneath the M protein; (4) the formation and release via budding of mature virions (Table 17.5 ).
Table 17.5.
Properties of Members of the Family Paramyxoviridae
| Two subfamilies: Paramyxovirinae, containing the genera Respirovirus, Rubulavirus, Henipavirus, and Morbillivirus, and Pneumovirinae, containing the genera Pneumovirus and Metapneumovirus |
| Virions are enveloped, pleomorphic (spherical and filamentous forms occur), and 150–300 nm in diameter. They are covered with large spikes (8–14 nm in length) |
| Virions contain a “herringbone-shaped” helically symmetrical nucleocapsid, 600–800 nm in length, and 18 nm (genera Respirovirus, Rubulavirus, Morbillivirus) or 13 nm (genera Pneumovirus and Metapneumovirus) in diameter |
| Virion envelope contains two viral glycoproteins and one or two non-glycosylated proteins |
| Genome consists of a single linear molecule of negative sense, single-stranded RNA, 13–19 kb in size, with seven to eight open reading frames encoding 10 to 12 proteins, including NP (or N), P, M, F, L, and HN (or H or G), which are common to all genera |
| Cytoplasmic replication, budding from the plasma membrane |
| Syncytium formation, intracytoplasmic and intranuclear inclusion bodies (genus Morbillivirus) |
Members of the Subfamily Paramyxovirinae, Genus Respirovirus
The genus Respirovirus includes human parainfluenza viruses 1 and 3, bovine parainfluenza virus 3, and Sendai viruses. Counter-intuitively, human parainfluenza viruses 2 and 4 are included in the genus Rubulavirus, despite their antigenic cross-reactivity with the other human parainfluenza viruses. Segregation of these viruses is based on sequence analysis of specific genes (e.g., N protein) and distinctive properties of the viruses in each group. Although species designations are frequently used to identify individual parainfluenza viruses, these viruses do not necessarily respect host species boundaries.
Bovine Parainfluenza Virus 3
Bovine parainfluenza virus 3, although antigenically and genetically related to human parainfluenza virus 3, occupies a distinct branch of the parainfluenza virus 3 group. There is long-standing controversy as to whether bovine parainfluenza virus 3 infection alone causes disease in cattle and other ruminants, independently of its putative role of predisposing to secondary bacterial infections of the respiratory tract. It is the potential role of the virus in initiating so-called shipping fever of cattle, or bovine respiratory disease complex, which has prompted most attention and controversy. Shipping fever occurs in cattle subsequent to transportation or other stressful situations; the term refers to an ill-defined disease syndrome caused by a variety of agents acting in concert or sequentially, culminating in severe bacterial bronchopneumonia that is most commonly caused by Mannheimia haemolytica. The syndrome remains an economically important problem, particularly in feedlots.
Clinical Features and Epidemiology
Bovine parainfluenza virus 3 has a worldwide distribution and can infect many species of ungulates, including cattle, sheep, goats, and wild ruminants, as well as humans and non-human primates. In contrast to human parainfluenza virus 3, bovine parainfluenza virus 3 is both non-pathogenic and poorly transmitted between humans. The most important routes of transmission of bovine parainfluenza virus 3 in susceptible animals are by aerosol and fomites contaminated with nasal discharges, because this virus is exclusively a respiratory tract pathogen that rarely, if ever, becomes systemic. In calves, lambs, and goat kids, infection is generally subclinical, but sometimes may manifest as fever, lacrimation, serous nasal discharge, depression, dyspnea, and coughing. Some animals may develop bronchointerstitial pneumonia that selectively affects the anteroventral portions of the lungs. The uncomplicated respiratory infection caused by bovine parainfluenza virus 3 runs a brief clinical course of 3–4 days that is usually followed by complete and uneventful recovery. However, in stressful circumstances, cattle and sheep may subsequently develop severe bacterial bronchopneumonia—that is, shipping fever. In this case the infection, alone or in concert with other viral infections (e.g., bovine adenovirus, bovine coronavirus, bovine viral diarrhea virus, infectious bovine rhinotracheitis virus, bovine respiratory syncytial virus), predisposes to secondary bacterial infection, especially Mannheimia haemolytica infection. This syndrome is characterized by purulent nasal discharge, cough, rapid respiration, anorexia, fever, general malaise, and substantial mortality from acute fibrinous bronchopneumonia. Poor hygiene, crowding, transport, harsh climatic conditions, and other causes of stress typically initiate this important disease syndrome.
Pathogenesis and Pathology
Under farm conditions, clinical signs of bovine parainfluenza virus 3 infection are often obscured by concurrent infections with other agents. Upon intranasal or intratracheal inoculation of bovine parainfluenza virus 3 alone, calves show only mild fever and serous nasal discharge. Infection results in necrosis and inflammation in small airways in the lungs—specifically bronchiolitis and bronchitis—with accumulation of cellular exudate in the lumen of affected airways. Epithelial cells of the respiratory tract are the primary target cells for bovine parainfluenza virus 3, but type II pneumocytes and alveolar macrophages also are infected, sometimes with the presence of acidophilic intracytoplasmic and/or intranuclear inclusion bodies. Infection of alveolar macrophages and interference with the normal protective mucociliary clearance mechanisms of the lung predispose to bacterial invasion and pneumonia.
Diagnosis
The diagnosis of bovine parainfluenza virus 3 infection is most frequently made by virus isolation or by serology to demonstrate increasing antibody titers. Available serological assays include hemagglutination-inhibition and virus neutralization. The virus is easily isolated in a variety of cells, and virus isolation also provides a mechanism for screening for the other viruses associated with bovine respiratory disease. Nasal swabs or tracheal wash fluids are the samples of choice for virus detection, and the virus can be recovered from the nasal discharges for 7–9 days after infection, by cultivation in bovine cell cultures. The virus also may be identified in nasal discharges or respiratory tissues by immunofluorescence staining, reverse transcriptase-polymerase chain reaction (RT-PCR) tests, or immunohistochemistry. However, because of the extensive variety of agents associated with bovine respiratory disease and the high incidence of subclinical parainfluenza virus 3 infection, mere detection of the virus is not proof of any disease causality. Interpretation of results requires an assessment of the overall clinical condition in the individual animal and the herd.
Immunity, Prevention, and Control
Convalescent animals develop a strong immune response, indicated by the presence of virus-specific antibodies that mediate hemagglutination-inhibition, neuraminidase inhibition, and virus neutralization. These antibodies are predominantly directed against the hemagglutinin–neuraminidase protein. The role of the cellular response in protective immunity has not been thoroughly investigated. Sterile immunity is short lived, as it is with many respiratory pathogens, and animals become susceptible to reinfection after several months. Colostral antibodies prevent clinical disease. Inactivated and live-attenuated virus vaccines for intranasal and parenteral use are available that induce protective antibodies. Typically, combined vaccines are formulated to include various combinations of protective antigens of bovine herpesvirus 1 (infectious bovine rhinotracheitis virus), bovine respiratory syncytial virus, bovine viral diarrhea virus, and Mannheimia haemolytica. These vaccines are readily used to control disease problems associated with bovine parainfluenza virus 3 infection in dairy cattle, but the different management issues confronted in beef production complicate control of multifactorial disease syndromes such as the respiratory disease complex in feedlot cattle. Bovine parainfluenza virus 3 vaccines also have been used for protective immunization of sheep.
Sendai Virus (Murine Parainfluenza Virus 1)
Sendai virus was discovered in 1952, after inoculation of lung material from pneumonic human infants into laboratory mice during attempts to isolate human respiratory viruses. These original studies occurred in Sendai, Japan, thus the designation as Sendai virus. It was subsequently shown that laboratory and feral rodents, rabbits, pigs, and non-human primates also may be infected with Sendai virus, which is closely related to human parainfluenza virus 1. This relationship has fuelled debate as to whether Sendai virus originated from humans or mice. However, although Sendai virus can replicate to an equivalent degree in a variety of animal species, including non-human primates, human parainfluenza virus 1 infects animals with markedly less efficiency.
Clinical Features and Epidemiology
Sendai virus infection of wild and laboratory rodents occurs worldwide. Although previously common in laboratory rodents, the virus has been curiously absent in recent decades. Sendai virus was a scourge of laboratory rodent colonies during the 1950s to 1980s, when it had a somewhat mysterious pattern of seasonal outbreaks in widely separated locations, suggesting exposure to human populations. Sendai virus is among a very few naturally occurring viruses that can cause severe respiratory disease with high mortality in adult mice and, to a much lesser extent, in rats and other laboratory animals.
Sendai virus is highly contagious among rodents. Affected mice exhibit a roughened hair coat, crusting of the eyes, dyspnea, mortality in adult and post-weanling-aged mice, weight loss, and fetal resorption in pregnant animals. There is a remarkable genetic basis of susceptibility to clinical Sendai viral pneumonia among inbred strains of mice, some strains manifesting high mortality, whereas others are subclinically infected. T-cell-deficient animals such as athymic nude and severe combined immunodeficiency mice develop chronic wasting disease, with progressive weight loss and dyspnea. Immunocompetent mice that survive clinical infection recover with no persistence of the virus. Infection of other laboratory rodents and rabbits is generally subclinical or mild.
Pathogenesis and Pathology
The strict respiratory tropism of Sendai virus is related to the processing of the viral fusion (F) protein. A single basic amino acid at the cleavage site of the F protein precludes intracellular processing; rather, an endopeptidase similar to clotting factor Xa that is secreted by Clara cells within the bronchiolar epithelium of rats and mice is responsible for cleavage of the F protein, thereby allowing the virus to replicate and amplify within the respiratory tract. The pathogenesis of Sendai virus infection has been studied extensively, and provides insight into the pathogenesis of other parainfluenza virus infections. Sendai virus is largely non-cytolytic, and selectively infects respiratory epithelium in the nose, trachea, and bronchioles, as well as type II pneumocytes. Disease characterized by necrotizing rhinitis, tracheitis, bronchiolitis, and interstitial pneumonia arises during the “immune” phase of infection, wherein cytotoxic T cells destroy virus-infected cells. Thus clinical disease occurs in fully immunocompetent mice, with variable morbidity and mortality depending on the strain, immunocompetence, and age of the mice. A critical determinant of survival is the extent of immune-mediated destruction of infected type II pneumocytes, as extensive injury to these progenitor stem cells prevents repair. Older and genetically resistant strains of mice tend to develop less severe disease, because virus fails to reach the distal airways before the advent of the immune response. Likewise, when infection is enzootic within a population, young mice with waning maternal antibody are partially resistant. Animals devoid of cellular immunity, such as nude mice, do not develop the pathognomonic immune-mediated necrotizing bronchiolitis, but rather develop a chronic progressive interstitial pneumonia. Laboratory rats, other rodents, and lagomorphs usually develop very mild or subclinical infections.
Diagnosis
Enzyme immunoassays and immunofluorescence assays are most commonly used for the serological diagnosis of Sendai virus infections in laboratory rodent colonies. Antibodies are detected by approximately 7 days after infection, and their presence characteristically coincides with the advent of immune-mediated clinical signs of necrotizing bronchiolitis and pneumonia. The use of sentinel animals is a standard method for surveillance of infection in mouse colonies. The virus can be isolated in numerous cell culture systems (monkey kidney, Vero, and BHK-21 cells with trypsin in the culture medium) and embryonated eggs, and the presence of virus is confirmed by immunofluorescence or immunohistochemical staining of infected monolayers. RT-PCR testing now is standard for rapid testing and confirmation of isolates.
Immunity, Prevention, and Control
Sendai virus does not persist in immunocompetent animals that recover from infection, and antibodies remain detectable throughout life. When infections have been diagnosed, depopulation, disinfection of the premises, and screening of incoming animals are required for control. Infected colonies can be re-established by cesarean re-derivation and foster nursing, by embryo transfer, or by isolating seropositive (recovered) immunocompetent breeding mice, which will subsequently give birth to uninfected (but transiently seropositive) pups. Cesarean or embryo transfer derivation is useful for immunodeficient mice, because virus is restricted to the respiratory tract. Nevertheless, all progeny must be carefully screened to assure successful re-derivation before initiating breeding or reintroduction of animals into uninfected populations.
Parainfluenza Virus 3 in Laboratory Rodents
Guinea pigs are commonly infected asymptomatically with a parainfluenza virus 3 that is closely related to human parainfluenza virus 3. Parainfluenza virus 3 also causes natural infection and transient pulmonary lesions in laboratory rats. Parainfluenza virus 3 infections of laboratory rodents are generally discovered during sero-surveillance for Sendai virus infection, because antibodies to Sendai virus and human parainfluenza virus 3 are cross-reactive.
Members of the Subfamily Paramyxovirinae, Genus Rubulavirus
The genus Rubulavirus includes mumps virus, human parainfluenza viruses 2 and 4, and simian viruses 5 (synonymous with canine parainfluenza virus 5), and 41 that are closely related to human parainfluenza virus 2, but distinguished on the basis of sequence analysis of specific genes (e.g., N protein) and their host range.
Canine Parainfluenza Virus 5 (Simian Virus 5)
Canine parainfluenza virus 5 and simian virus 5 are essentially the same virus. Simian virus 5 was the first virus to be isolated from monkey cell cultures, but it is generally now believed that the dog is the natural primary host of this virus. There are unproven reports that canine parainfluenza virus 5 is zoonotic, but this debate is complicated by the antigenic cross-reactivity between human parainfluenza virus 2 and canine parainfluenza virus 5. Although the two viruses are genetically distinct, their close relationship is further reflected by the fact that the canine virus historically was referred to as parainfluenza virus 2, and it now is proposed that the virus be classified as type 5 parainfluenza virus, specifically canine parainfluenza virus 5. It also has been claimed that other species are naturally infected with this virus, but the validity of these claims is dubious, as they probably reflect either contamination or confusion with infection with closely related viruses such as human parainfluenza virus 3 infection in guinea pigs.
Canine parainfluenza virus 5 causes inapparent infection or mild respiratory disease in dogs, and the virus has been incriminated as an uncommon cause of congenital hydrocephalus also. Serological studies indicate that the infection of dogs occurs worldwide. Canine parainfluenza virus 5 is implicated in the pathogenesis of the acute respiratory disease of canines (kennel cough syndrome), and more serious, chronic respiratory disease may develop when additional microbial or viral agents, poor hygiene, or stress complicate infections. There is an incubation period of 3–10 days after infection, followed by disease that is characterized by the sudden onset of a serous nasal exudate, paroxysmal coughing episodes, and fever, lasting 3–14 days. Virus is shed for 6–8 days after infection and is spread by fomites or short-distance aerosols. Disease is most frequently seen in kennels, animal shelters, or day-care settings, and is more prevalent in younger dogs. The virus causes destruction of the ciliated epithelial cells of the respiratory tract, which predisposes infected dogs to secondary bacterial infections. Coughing can continue long after the virus has been cleared. In severe cases (mostly in malnourished or young dogs) there is also conjunctivitis, tonsillitis, anorexia, and lethargy. Because a number of other infectious agents (canine distemper virus, canine pneumovirus, canine influenza virus, canine adenovirus 2, canine herpesvirus) can induce similar clinical signs, definitive diagnosis depends on virus isolation or nucleic acid detection by RT-PCR from nasal or throat swabs. Serology can also be used to define the presence of canine parainfluenza virus 5. Vaccines are available and are usually used in various combination formulations containing antigens of other canine viral and microbial pathogens. Vaccination can complicate the interpretation of diagnostic test results, specifically RT-PCR and serology.
Porcine Rubulavirus (La-Piedad-Michoacan-Mexico Virus) and mapuera virus
A series of outbreaks of neurological disease, conjunctivitis, and corneal opacity, with moderate to high mortality, occurred among young pigs in commercial pig farms in central Mexico, beginning in 1980. Corneal opacity was the only manifestation of the disease in older non-pregnant animals, hence the common name for the disease, “blue eye.” In pregnant sows there was an increase in abortions, stillbirths, and mummified fetuses. Characteristic histological changes in the brain were non-suppurative encephalomyelitis with perivascular cuffing, neuronal necrosis, and meningitis. A paramyxovirus was isolated from affected pigs and the disease syndrome was reproduced by inoculation of pigs with this virus. Sequence analysis resulted in designation of the causative virus as porcine rubulavirus, because of its similarities to human mumps virus. It is speculated that the virus spread to pigs from a wildlife reservoir, as porcine rubulavirus is genetically similar to a virus (Mapuera virus) that was isolated from a fruit bat in Brazil in 1979. A seropositive bat was detected in the affected region of Mexico, further supporting the speculation on the origin of porcine rubulavirus.
Menangle and Tioman viruses
In 1997, an apparently new paramyxovirus was isolated from mummified and deformed stillborn piglets in Australia. Abnormalities present in the stillborn piglets included arthrogryposis, spinal and craniofacial deformities, and central nervous system malformation. Disease was not evident in postnatal animals. There was a high seroprevalence amongst swine on the affected farm, and on several adjacent ones. Two humans on the property who had experienced undiagnosed febrile illnesses coincidentally with the recognition of the disease in the pigs had serum antibody to the new virus, named Menangle virus. As this outbreak occurred just 3 years after the initial identification of Hendra virus, it was quickly determined that fruit bats were the source of Menangle virus. Another related paramyxovirus (Tioman virus) was isolated in 2001 from pteropodid bats on Tioman Island, Malaysia. This virus can also infect pigs, although it caused only very mild disease. Both of these viruses are genetically distinct from other paramyxoviruses, and they have been tentatively placed in the genus Rubulavirus.
Members of the Subfamily Paramyxovirinae, Genus avulavirus
All viruses in the genus Avulavirus exhibit both hemagglutinin and neuraminidase activity. These viruses are most related to those in the genus Rubulavirus, but there are essential differences in the coding assignments of their respective genomes. The genus includes significant pathogens of birds, in particular Newcastle disease virus.
Newcastle Disease and Other Avian Paramyxovirus Type 1 Viruses
Newcastle disease has become one of the most important diseases of poultry worldwide, negatively affecting trade and poultry production in both developing and developed countries. The disease was first observed in Java, Indonesia, in 1926, and in the same year it spread to England, where it was first recognized in Newcastle-upon-Tyne, hence the name. The disease is one of the most contagious of all viral diseases, spreading rapidly among susceptible birds. Newcastle disease virus is by definition a virulent virus, classified in the genus Avulavirus in the avian paramyxovirus serotype 1 group, but some virus strains in this group are not Newcastle disease virus, as they are either avirulent or of low virulence. The genus Avulavirus also contains other species of low-virulent avian paramyxoviruses, designated as avian paramyxoviruses 2–9. Natural and experimental avian paramyxovirus serotype 1 group virus infections have been described in more than 240 bird species from 27 of the 50 orders of birds, but this virus group has the potential to infect most, if not all, bird species. The signs of the infection vary greatly depending on the species of bird and the strain of virus.
Because of the severe economic consequences of an outbreak of virulent Newcastle disease in commercial poultry, the disease is reportable to the World Organization for Animal Health (Office International des Epizooties: OIE). However, in view of the wide variation in disease caused by avian paramyxovirus serotype 1 strains, very specific criteria were established for defining an outbreak as Newcastle disease. The disease is defined as an infection of birds caused by an avian paramyxovirus serotype 1 virus that meets one of the following criteria for virulence: (1) the virus has an intracerebral pathogenicity index in day-old chickens (Gallus gallus domesticus) of 0.7 or greater, or (2) multiple basic amino acids have been demonstrated in the virus (either directly or deduced) at the C-terminus of the F2 protein and phenylalanine at residue 117, which is the N-terminus of the F1 protein (Table 17.4). The term “multiple basic amino acids” refers to at least three arginine or lysine residues between residues 113 and 116. Failure to demonstrate the characteristic pattern of amino acid residues as described above would require characterization of the isolated virus by an intracerebral pathogenicity index test. As a corollary, Newcastle disease can only be caused by a virulent strain of avian paramyxovirus serotype 1 virus.
Clinical Features and Epidemiology
Chickens, turkeys (Meleagridis gallapavo), pheasants (Phasianus colchicus), guinea fowl (Numida meleagris), Muscovy (Cairina moschata) and domestic (Anas platyrhynchos) ducks, geese (Anser anser), pigeons (Columba livia), and a wide range of captive and free-ranging semi-domestic and free-living birds, including migratory waterfowl, are susceptible to avian paramyxovirus serotype 1 infections, including virulent strains—that is, Newcastle disease virus. Most low-virulent or avirulent avian paramyxovirus serotype 1 strains are maintained in migratory waterfowl and other feral birds, whereas others are maintained in domestic poultry. Newcastle disease virus strains are primarily maintained in and spread between domestic poultry, but cormorants (Phalacrocorax auritus) have been identified as reservoir hosts in North America that were implicated in the spread of the virus to domestic turkeys. Introduction of the Newcastle disease virus into a country has been documented through the smuggling of exotic birds and illegal trade in poultry and poultry products. Recent outbreaks of Newcastle disease in Australia and United Kingdom were the result of specific mutations within the fusion gene, changing an enzootic, avirulent avian paramyxovirus serotype 1 virus to a virulent Newcastle disease virus.
The clinical signs associated with avian paramyxovirus serotype 1 viral infections in chickens are highly variable and dependent on the virus strain, thus virus strains have been grouped into five pathotypes: (1) viscerotropic velogenic; (2) neurotropic velogenic; (3) mesogenic; (4) lentogenic; (5) asymptomatic enteric. The viscerotropic, neurotropic, and mesogenic strains are those that produce moderate to high mortality rates and are associated with officially designated Newcastle disease. Whereas velogenic strains kill virtually 100% of infected fowl, naturally avirulent strains of avian paramyxovirus serotype 1 virus (lentogenic and enteric strains) have even been used as vaccines against Newcastle disease because they induce cross-protective antibodies.
Virus is shed for up to 4 weeks in all secretions and excretions of birds that survive the infection. Transmission occurs by direct contact between birds via inhalation of aerosols and dust particles, or via ingestion of contaminated feed and water, because respiratory secretions and feces contain high concentrations of virus. Mechanical spread between flocks is facilitated by the relative stability of the virus and its wide host range. On rare occasions, vertical transmission has been documented for lentogenic virus strains, and virus-infected chicks have hatched from virus-containing eggs. It remains uncertain as to whether there is vertical transmission of more pathogenic viruses, although, in one experimental study, very low doses of virulent Newcastle disease virus inoculated into eggs resulted in isolation of the virus from a few hatched chicks. Vertical transmission is unclear, or a rare occurrence at best.
Legal trade of caged and aviary birds and poultry and their products has played a key role in the spread of Newcastle disease virus from infected to non-infected countries, but with implementation of stringent quarantine and testing procedures such introductions are now uncommon. However, smuggling of birds and products remains a high risk for spread of virulent Newcastle disease virus, especially with fighting cocks as occurred in Southern California in 2002–2003, and with psittacine birds as occurred in parts of the United States in 1991. Some psittacine species may become persistently infected with virulent Newcastle disease virus and excrete virus intermittently for more than 1 year without showing clinical signs. Virus may also be disseminated by frozen chickens, uncooked kitchen refuse, foodstuffs, bedding, manure, and transport containers. The greatest risk for spread is via human activity, through mechanical transfer of infective material on equipment, supplies, clothing, shoes, and other fomites. Wind-borne transmission and movement by wild birds are much less common modes of transfer.
Respiratory, circulatory, gastrointestinal, and nervous signs are all characteristic of avian paramyxovirus serotype 1 viral infections in chickens; the particular set of clinical manifestations depends on the age and immune status of the host and on the virulence and tropism of the infecting virus strain. The incubation period ranges from 2 to 15 days, with an average of 5–6 days. The velogenic strains may cause high mortality—close to 100%—without clinical signs. Other velogenic strains may cause increased respiration rate, loss of appetite, listlessness, occasionally edema around eyes and head, and typically ending in a few hours with prostration and death. Respiratory signs may be absent to severe, depending on virus strain. Some birds will have neurological signs including muscle tremors, torticollis, paralysis of legs and wings, and opisthotonos. Neurotropic strains produce severe respiratory disease followed, in 1–2 days, by neurological signs and near cessation of egg production. The infection produces 100% morbidity, but only 50% mortality, in adult chickens; mortality is higher in young birds. Mesogenic strains produce respiratory disease, reduced egg production and, uncommonly, neurological signs, and low mortality. Lentogenic strains usually cause no disease unless accompanied by secondary bacterial infections that result in respiratory signs.
The disease in turkeys is similar but usually less severe than that in chickens; there are signs of respiratory and nervous system involvement. Airsacculitis, rather than tracheitis, is the most common lesion. In ducks and geese most infections are inapparent, although a few cases of severe disease have been reported in domestic ducks. Game birds of most species have experienced outbreaks of Newcastle disease. In pigeons, avian paramyxovirus serotype 1 viral infections cause diarrhea and neurological signs, and the pigeon virus produces signs similar to velogenic or neurotropic virus strains in chickens.
Pathogenesis and Pathology
Strains of avian paramyxovirus serotype 1 virus differ widely in virulence, depending on the cleavability and activation of the fusion (F) glycoprotein. The importance of this feature of the viruses is reflected in the criteria set by the OIE for defining a virulent virus. Low-virulent or avirulent virus strains produce precursor F proteins that are cleaved only by a trypsin-like protease that has a restricted tissue distribution, and which is usually present extracellularly or in epithelial cells of only the respiratory and digestive systems. In contrast, in virulent virus strains these precursor F proteins are cleaved intracellularly by furin-like proteases present in cells lining mucous membranes. The relative ease of intracellular cleavage allows virulent viruses to replicate in more cell types, with attendant widespread tissue injury, viremia and systemic disease.
Avian paramyxovirus serotype 1 virus initially replicates in the mucosal epithelium of the upper respiratory and intestinal tracts, which for lentogenic and enteric strains means that disease is limited to these two systems, with airsacculitis being most prominent. For virulent Newcastle disease viruses the virus quickly spreads after infection via the blood to the spleen and bone marrow, producing a secondary viremia that leads to infection of other target organs: lung, intestine, and central nervous system. Respiratory distress and dyspnea result from congestion of the lungs and damage to the respiratory center in the brain. Gross lesions include ecchymotic hemorrhages in the larynx, trachea, esophagus, and throughout the intestine. The most prominent histologic lesions are foci of necrosis in the intestinal mucosa, especially associated with Peyer's patches and cecal tonsil, submucosal lymphoid tissues, and the primary and secondary lymphoid tissues, and generalized vascular congestion in most organs, including the brain.
Virulent velogenic strains cause marked hemorrhage, in particular at the junctions of the esophagus and proventriculus, and proventriculus and gizzard, and in the posterior half of the small intestine. In severe cases, hemorrhages are also present in subcutaneous tissues, muscles, larynx, trachea, esophagus, lungs, airsacs, pericardium, and myocardium. In adult hens, hemorrhages are present in ovarian follicles. In the central nervous system, lesions are those of encephalomyelitis with neuronal necrosis.
Diagnosis
Because clinical signs are relatively non-specific, and because the disease is such a threat, the diagnosis of Newcastle disease must be confirmed by virus isolation, RT-PCR, and serology. The virus may be isolated from spleen, brain, or lungs from dead birds, or tracheal and cloacal swabs from either dead or live birds, by allantoic sac inoculation of 9–10-day-old embryonating eggs. Any hemagglutinating agents detected can be identified by avian paramyxovirus serotype 1 virus-specific hemagglutination-inhibition tests or RT-PCR tests and subsequent sequence analysis. Determination of the virulence of virus isolates is essential. Immunofluorescence staining of tracheal sections or smears is rapid, although somewhat less sensitive. Demonstration of antibody is diagnostic only in unvaccinated flocks; hemagglutination-inhibition is the test of choice because of the rapidity of the test results. Commercial ELISA kits provide a convenient alternative, but most ELISA tests are only applicable for chickens and turkeys. These serological tests can also be used for surveillance of avian paramyxovirus serotype 1 viral infections in countries where the virus is enzootic, or to monitor vaccinal immunity. Knowing the flock vaccination history is critical in interpreting virological and serological results, because live-attenuated vaccines complicate the interpretation of positive test results for RT-PCR and serological assays in vaccinated flocks.
Immunity, Prevention, and Control
Antibody production is rapid after infection, and hemagglutination-inhibiting and virus-neutralizing antibody can be detected within 6–10 days of infection, peaks at 3–4 weeks, and persists for over a year. The level of hemagglutination-inhibiting antibody is an indirect measure of immunity. Neutralizing antibodies are directed against both the HN and F proteins. Maternal antibodies transferred via the egg yolk protect chicks for 3–4 weeks after hatching as they have a half-life of approximately 4.5 days. ImmunoglobulinG (IgG) is confined to the circulation and does not prevent respiratory infection, but it does block viremia; locally produced IgA antibodies play an important role in protection in both the respiratory tract and the intestine, although some IgG is secreted in the respiratory tract and provides some protection.
Because Newcastle disease is a notifiable disease in most developed countries, legislative measures constitute the basis for control. Where the disease is enzootic, control can be achieved by good hygiene combined with immunization, both live-virus vaccines containing naturally occurring lentogenic virus strains and inactivated virus (injectable oil emulsions) being commonly used. These vaccines are effective and safe, even in chicks. Live virus vaccines may be administered via drinking water or by aerosol, eye or nostril droplets, or beak dipping. The inactivated vaccines must be injected. Broiler chickens are vaccinated a minimum of twice, whereas long-lived chickens, such as laying hens, are revaccinated several times throughout their lives, with inactivated vaccines. Protection against disease can be expected approximately a week after vaccination. Birds vaccinated with live virus will excrete the vaccine virus for up to 15 days after vaccination, hence in some countries birds cannot be moved from vaccinated flocks until 21 days after vaccination. Inactivated vaccine, administered subcutaneously, is usually used for pigeons. New-generation vectored vaccines have been developed, and these would preclude the possibility of reversion of apathogenic vaccine strains and would not complicate the interpretation of diagnostic RT-PCR results in vaccinated birds.
Human Disease
Newcastle disease virus can produce a transitory conjunctivitis in humans; the condition occurs primarily in laboratory workers and in members of vaccination teams exposed to large quantities of virus. Before vaccination was widely practiced, infections were reported in workers eviscerating poultry infected with virulent Newcastle disease virus. In developed countries, birds infected with Newcastle disease virus are not processed, but in village poultry and live markets of developing countries, Newcastle disease is common and may not preclude slaughter of infected birds. The disease has not been reported in individuals who raise poultry or consume poultry products.
Other Avian Avulaviruses (Avian Paramyxoviruses 2–9)
Serologically distinct avulaviruses (avian paramyxoviruses 2–9) have been isolated from numerous species of birds, mostly turkeys with respiratory disease or asymptomatic wild waterfowl. However, the pathogenic significance of many of these viruses is uncertain. They commonly have been isolated from passerine and psittacine birds in import quarantine facilities, or from asymptomatic wild waterfowl during surveillance for avian influenza viruses. There are also additional, unclassified viruses that are not included in the avian paramyxoviruses 1–9 groupings.
Members of the Subfamily Paramyxovirinae, Genus Morbillivirus
Members of the genus Morbillivirus all utilize the same replication strategy and all lack neuraminidase activity. They cause severe but very different disease syndromes in their respective hosts.
Rinderpest Virus
Rinderpest is one of the oldest recorded plagues of livestock. It most probably arose in Asia, and was described in the 4th century. Devastating epizootics of rinderpest occurred across Europe in the 18th and 19th centuries, and a massive epizootic spread throughout sub-Saharan Africa in the late 19th century (1887–1897), decimating populations of cattle and certain wildlife. The 1920 outbreak in Europe led to the founding of the Office International des Epizooties (OIE)—the World Organization for Animal Health—that today coordinates animal infectious disease authorities globally to regulate animal diseases and to facilitate science-based international trade. The historical impact of rinderpest was most eloquently summarized in 1992 by Drs Gordon Scott and Alain Provost when they described the disease as “the most dreaded bovine plague known, belongs to a select group of notorious infectious diseases that have changed the course of history. From its homeland around the Caspian Basin rinderpest, century after century, swept west over and around Europe and east over and around Asia with every marauding army causing the disaster, death and devastation that preceded the fall of the Roman Empire, the conquest of Christian Europe by Charlemagne, the French Revolution, the impoverishment of Russia and the colonization of Africa.”
The causative agent, rinderpest virus, was first shown to be a filterable virus in 1902. On the basis of phylogenetic analysis, it has been suggested that rinderpest virus is the archetype morbillivirus, speculated to have given rise to canine distemper and human measles viruses some 5000 to 10,000 years ago. As of 2008, there has been considerable and increasing optimism that rinderpest has been eradicated from domestic livestock worldwide, as a result of an intensive and coordinated global effort that involved active surveillance, animal culling and movement restrictions, and an intense vaccination program. If true, rinderpest will join smallpox as the only viral diseases to have been successfully eradicated.
Clinical Features and Epidemiology
Rinderpest is a highly contagious disease of cattle and other artiodactyls. The host range includes domestic cattle, water buffalo, yak, sheep, and goats. Domestic pigs can develop clinical signs and were regarded as an important virus reservoir in Asia. Among wild animals, wildebeest, waterbuck, warthog, eland, kudu, giraffe, deer, various species of antelope, hippopotami, and African buffalo are all susceptible, although there is a wide spectrum of clinical disease that is most severe in African buffalo, wildebeest, and giraffe and invariably mild or subclinical in several species of antelope and hippopotamus. It may well be that all artiodactyls are susceptible to infection, but not all will exhibit obvious clinical signs. Other species, including rodents, rabbits, and ferrets, are susceptible to experimental infection, but are unlikely to play any significant role in the epidemiology of natural infections.
The clinical features of individual outbreaks of rinderpest reflect the virulence of the infecting strain of virus and the susceptibility of the individual animal host. In its typical manifestation in cattle and other susceptible wild or domestic ruminant species, rinderpest is an acute febrile disease with morbidity in susceptible populations approaching 100% and mortality of perhaps 50% (range 25–90%). Some of the indigenous cattle breeds in Africa are highly susceptible to rinderpest, whereas other breeds experience lower mortality (less than 30%). After an incubation period of 3–5 days, there is a prodromal phase with rapid increase in temperature, decrease in milk production, labored breathing, and cessation of eating. This is followed by congestion of the mucous membranes of the conjunctiva and oral and nasal cavities, and an abundant serous or mucoid oculonasal discharge. Severe cases are characterized by extensive, typically coalescing, erosion and ulceration of the epithelial lining of the entire oral cavity; plaques of caseous necrotic debris overlie foci of epithelial necrosis and inflammation, and affected animals typically drool saliva because of the discomfort associated with swallowing. This is followed by a phase of severe bloody diarrhea and prostration caused by involvement of the gastrointestinal tract. Finally there is a precipitous drop in temperature, at which time affected animals may die from dehydration and shock. Young animals are predisposed to severe disease. Less severe disease is characteristic of infection of susceptible animals with specific virus strains, and inapparent infection invariably occurs within certain host species such as impala and hippopotamus. Disease also is often less severe in sheep and goats. These mild infections are characterized by reduced clinical signs and mucosal injury, little or no diarrhea, and considerably lower mortality.
Once established in a population, rinderpest virus causes a considerably milder disease. The attenuation of rinderpest in enzootic areas probably reflects both selection of less virulent virus strains with the highest potential for transmission, and immunity within populations of susceptible animals. The infection is maintained in enzootic areas in younger animals that become infected as their maternal immunity wanes. Rinderpest virus also can be maintained for long periods through subclinical infections in wildlife, which then can serve as a reservoir for infection of cattle. The virus rapidly can regain its virulence when it spreads from enzootic foci to cause epizootics in susceptible animal populations.
Rinderpest virus is spread in all the secretions and excretions of affected animals, in greatest quantities during the acute febrile stages of the disease. The virus is relatively labile in the environment, thus transmission in enzootic areas predominantly is by direct contact between infected and susceptible animals. Aerosol transmission also can occur, and the virus can be spread by fomites. The virus can persist for several days in infected carcasses. Because infected cattle excrete large amounts of virus during the incubation period before the appearance of clinical signs, acutely infected but asymptomatic animals often introduced rinderpest virus into disease-free areas. The disease was also introduced into new areas by importation of subclinically infected sheep, goats, and possibly other ruminants and wildlife. Subclinically infected swine of any species may act as a source of infection for cattle, but only Asian breeds of swine and warthogs show clinical signs of rinderpest virus infection.
Pathogenesis and Pathology
After nasal entry via infected aerosols, rinderpest virus first replicates within mononuclear leukocytes in the tonsils and mandibular and pharyngeal lymph nodes. Within 2–3 days, virus is transported during leukocyte-associated viremia to lymphoid tissues throughout the body, as well as the epithelium lining the gastrointestinal and respiratory tracts. The virus utilizes the equivalent of human CD150 (signaling lymphocyte activation molecule) as a receptor, which is consistent with the cellular and tissue tropism of rinderpest virus, as this molecule is present on immature thymocytes, activated lymphocytes, macrophages, and dendritic cells. The virus also infects and replicates in endothelial cells and some epithelial cells, presumably through a CD150-independent pathway, causing multifocal necrosis and inflammation in a variety of mucous membranes.
Rinderpest virus infection triggers a rapid innate and acquired immune response, including a vigorous interferon response. However, a viral protein or proteins, most likely the P protein, block the interferon response through inhibition of the phosphorylation and nuclear translocation of STAT proteins (Figure 17.5). Profound lymphopenia occurs in infected animals as a consequence of virus-mediated destruction of lymphocytes in all lymphoid tissues, including the gut-associated lymphoid tissue (Peyer's patches). The immune cells that support replication of rinderpest virus also produce numerous potent immunoregulatory cytokines after appropriate stimulation. The production and release of these cytokines, coupled with severe virus-induced lymphopenia, probably is responsible for the profound but transient immunosuppression that very characteristically occurs in animals infected with rinderpest virus.
The profuse diarrhea that occurs in severely affected animals rapidly leads to dehydration and fatal hypovolemic shock. The lesions present in infected animals reflect the virulence of the infecting virus strain, and in severe, acute cases include: marked dehydration (sunken eyes, for example); disseminated erosions and ulcers throughout the mucosal lining of the oral cavity, esophagus, and forestomachs; diffuse hemorrhage and necrosis of the mucosa of the abomasum; focal congestion and hemorrhage in the mucosa of the intestinal tract, with hemorrhagic necrosis of Peyer's patches. Segmental vascular congestion within the mucosa of the large intestine can produce characteristic “zebra stripes.” Hemorrhage and congestion can also occur in the mucosal lining of the urinary bladder and upper respiratory tract and trachea. Secondary bacterial pneumonias are common because of the transient but severe immunosuppression in infected animals. Histologic lesions include widespread necrosis of lymphocytes and multifocal epithelial necrosis; epithelial syncytia and intracytoplasmic and, less often, intranuclear eosinophilic inclusion bodies are characteristically present in affected tissues.
Diagnosis
In countries where rinderpest was enzootic, clinical diagnosis was usually considered sufficient. Rinderpest historically could be confused with other diseases causing mucosal congestion, erosions or ulcers, such as bovine viral diarrhea, malignant catarrhal fever, and, in the early stages, infectious bovine rhinotracheitis and foot-and-mouth disease. These diagnostic problems have largely been resolved with the development of specific PCR tests for all of these “look-alike” diseases. Quantitative (real-time) RT-PCR assays are now available for rinderpest virus that rapidly can distinguish it from the related peste des petits ruminants virus. Historically, virus isolation was done in a variety of different cell lines, routinely in primary bovine kidney cell cultures. Virus neutralization and, more recently, ELISA have been used to assess the prevalence of rinderpest virus infection in a given region, which has required that only unvaccinated animals be evaluated to assess the success of eradication programs.
Immunity, Prevention, and Control
Cattle that survive rinderpest virus infection have life-long immunity. Neutralizing antibodies appear 6–7 days after the onset of clinical signs, and maximum titers are reached during the 3rd and 4th weeks postinfection. With the advent of molecular typing, three distinct genetic lineages of rinderpest virus were defined, two from Africa and one from Asia. All strains belong to the same serotype, which permitted use of a vaccine that contained a single strain of virus. In recent times, lineage 3 was restricted to Asia, lineage 2 to East and West Africa, and lineage 1 to Ethiopia and Sudan. As of April 2007, there were no reports of rinderpest virus infection in any countries reporting to OIE, which includes all of Asia and Africa. Kenya became the last African country to report a self-declared free status. The basis of this report is that there has been no clinical disease in the past 2 years and that active vaccination has ceased. Surveillance must be maintained in order to insure that any unidentified wildlife reservoir does not re-establish infection in domestic livestock.
In rinderpest-free countries, veterinary public health measures were designed to prevent introduction of the virus. Importation of uncooked meat and meat products from infected countries was forbidden, and zoo animals were quarantined before being transported to such countries. In countries with enzootic rinderpest and where the disease had a high probability of being introduced, attenuated virus vaccines were used. Early rinderpest vaccine strains were produced by virus passage in rabbits (lapinized vaccine), embryonated eggs (avianized vaccine), or goats (caprinized vaccine). In the 1960s an attenuated virus vaccine produced in cell culture (Plowright vaccine or TCRV) was developed and has since been used throughout Africa in the program that now appears to have succeeded in eliminating the disease from the continent. This vaccine is efficacious because it induces life-long immunity and it is inexpensive to produce. In fact, it remains one of the best vaccines available for any animal disease, but it is thermolabile and requires a well-maintained “cold chain”—a difficult practical problem in many areas where rinderpest previously occurred. As the number of infected animals decreased, the use of vaccine was suspended in order to facilitate serological surveillance. This was necessary because the immune response to the vaccine was indistinguishable from the wild-type virus infections. Marker vaccines have been developed, but they have not been used widely, as rinderpest apparently has been eradicated with existing reagents and technology.
Peste des Petits Ruminants Virus
Peste des petits ruminants is a highly contagious, systemic disease of goats and sheep that is very similar to rinderpest and caused by a closely related morbillivirus, peste des petits ruminants virus. The infection was first described in West Africa, but now occurs in sub-Saharan Africa, the Middle East, and the Asian subcontinent, including Nepal, Bangladesh, and Tibet. There are suggestions that this virus has recently moved into areas from which rinderpest virus was previously eradicated. Peste des petits ruminants virus has been grouped into four distinct lineages based on the sequence of the F protein. Regardless of lineage, all strains belong to a single serogroup. Lineages 1 and 2 occur in West Africa; lineage 3 in East Africa, the Middle East, and southern India; lineage 4 extends from the Middle East to Tibet. There is some correlation between virulence and lineage; for example, lineage 1 strains in West Africa are more virulent than lineage 2 strains from the same area.
Transmission of the virus is similar to that of rinderpest, and generally requires close contact with infected animals. Virus is excreted for several days before the onset of significant clinical signs, such that spread of the virus is enhanced with the co-mingling of animals. Wild animals are not believed to play a major role in the spread of virus.
The natural infection occurs in sheep and goats, with goats being more severely affected. Different breeds of goat show different morbidity rates, and young animals are more severely affected. Case fatality rates can be as high as 85% in goats, but rarely above 10% in sheep. Peste des petits ruminants virus is very similar to rinderpest virus, and cattle can be experimentally infected with both viruses; some putative cases of rinderpest may in fact have been peste des petits ruminants virus instead. In goats, a febrile response occurs at 2–8 days after infection. Clinical signs include fever, anorexia, nasal and ocular discharges, necrotic stomatitis and gingivitis, and diarrhea. Bronchopneumonia is a frequent complication. The course of the disease may be peracute, acute, or chronic, depending on strain of virus, age of animals and breed of host. The pathogenesis of the infection is probably similar or identical to that of rinderpest virus, with infection of mononuclear cells with a resulting viremia, leukopenia, and systemic infection, principally involving lymphocytes, macrophages, and the epithelial cells lining the alimentary tract. At necropsy, there are extensive erosions and necrosis in the mucosal lining of the oral cavity, esophagus, abomasum, and small intestine. Regional lymph nodes are enlarged and there typically is an interstitial pneumonia.
Diagnosis of the disease, aside from clinical signs, has shifted from virus isolation to quantitative RT-PCR tests. These tests can distinguish between peste des petits ruminants and rinderpest viruses, which has been critical in the rinderpest eradication program. Virus isolation in primary lamb kidney cells is still used to obtain isolates for molecular characterization and comparison. Virus neutralization tests are used to distinguish between antibodies induced in animals by peste des petits ruminants and rinderpest virus infections. A live-attenuated vaccine based on a lineage 2 virus isolate (Nigeria 75/1) is now the vaccine of choice. Rinderpest vaccine is no longer recommended to prevent peste des petits ruminants, because of the rinderpest eradication program.
Canine Distemper Virus
Canine distemper is a highly contagious acute febrile disease of dogs that has been known since at least 1760. Edward Jenner first described the course and clinical features of the disease in 1809; its viral etiology was demonstrated in 1906 by Carré. It is now comparatively rare in domestic dogs in many developed countries, being well controlled by vaccination. The clinical cases that do occur in developed countries invariably are in non-vaccinated or incompletely vaccinated dogs, especially those entering rescue shelters or adoption centers. The continued presence of canine distemper virus in heavily vaccinated dog populations in many countries might suggest that the virus has an alternative reservoir, perhaps in wildlife. Canine distemper virus has recently emerged as a significant pathogen of the large species in the family Felidae. Beginning in 1994, thousands of African lions died in a succession of epizootics, with free-roaming canids (hyenas, feral dogs) being the most likely source of the virus.
Clinical Features and Epidemiology
The host range of canine distemper virus encompasses all species of the families Canidae (dog, dingo, fox, coyote, jackal, wolf), Procyonidae (raccoon, coatimundi, panda), Mustelidae (weasel, ferret, fishers, mink, skunk, badger, marten, otter), the large members of the family Felidae (lions, leopards, cheetahs, tigers), and the collared peccary (Tayassu tajacu). The highly publicized outbreaks of distemper in lions (Panthera leo) in the Serengeti National Park in Tanzania and cases in the Chinese leopard (Panthera pardus japonensis) and other large cats in zoos, have graphically confirmed the capacity of the virus to invade new hosts. In addition to the large cats, canine distemper virus has also caused high mortality rates in black-footed ferrets (Mustela nigripes), the bat-eared fox (Otocyon megalotis), red pandas (Ailurus fulgus), hyenas (genus Hyaena), African wild dogs (genus Lycaon), raccoons (genus Procyon), palm civets (Paradoxurus hermaphroditus), and Caspian (Pusa caspica) and Baikal (P. sibirica) seals. The threat of this virus to wildlife species can only be expected to increase with relentless human encroachment into traditionally undeveloped areas of the world.
At least seven distinct lineages of canine distemper are recognized worldwide, based on sequence analysis of the H gene: Asia-1, Asia-2, American-1, America-2, Arctic-like, European wildlife, Europe. Additional lineages probably will be identified in the future. The traditional vaccine strains of canine distemper virus—Snyder Hill, Onderstepoort, Lederle—are all included in the America-1 lineage; however, field strains of this lineage are not currently circulating in the canine population in North America, although a lineage America-1 virus recently was identified among raccoons in the United States. The European wildlife-like virus has also been isolated in North America, perhaps as a result of unregulated movement of dogs from Eastern Europe. Despite genetic differences amongst field strains of canine distemper virus, cross-neutralization studies show only minor antigenic differences that are not considered significant enough to warrant changes in the existing vaccines.
Clinical signs of canine distemper virus infection depend upon the strain of the virus, the host age and immune status, and levels of environmental stress. A significant proportion (estimated to be 50%) of infections are subclinical or so mild as not to require veterinary care. Dogs with mild disease exhibit fever, signs of upper respiratory tract infection, and become listless and inappetant. Bilateral serous ocular discharges can become mucopurulent with coughing and labored breathing, signs that are often indistinguishable from those of “kennel cough” (acute respiratory disease of canines). In severe generalized distemper, infected dogs first develop a fever after an incubation period of 3–6 days, but a second febrile response ushers in the more serious phase of the infection that coincides with systemic spread of the virus and accompanying profound leukopenia. Signs occurring at this time include anorexia, inflammation of the upper respiratory tract with serous or mucopurulent nasal discharge, conjunctivitis, and depression. Some dogs show primarily respiratory signs, whereas others develop gastrointestinal signs; respiratory signs reflect inflammation and injury to the upper respiratory tract and large airways, causing a productive cough; bronchitis and interstitial pneumonia follow. Gastrointestinal involvement is manifest by vomiting and watery diarrhea. The duration of disease varies, often depending on complications caused by secondary bacterial infections (Figure 17.6 ).
Figure 17.6.

Sequential pathogenesis of canine distemper. CNS, central nervous system.
[From Infectious Diseases of the Dog and Cat, C. E. Greene, 3rd ed., p. 29. Copyright © Elsevier (2006), with permission.]
Profound central nervous system signs develop in some infected animals. Neurologic manifestations of distemper occur at 1–3 weeks after the onset of acute signs, but may also appear after apparent subclinical infection. There is no way to predict which dogs will develop neurological complications. Seizures (so-called chewing gum fits and epileptic seizures), cerebellar and vestibular signs, paraparesis or tetraparesis with sensory ataxia and myoclonus are common. Neurologic signs, whether acute or chronic, are usually progressive, which leads to a poor prognosis and surviving dogs may have permanent central nervous system sequelae. So-called old dog encephalitis is a rather poorly characterized chronic and slowly progressive neurologic disease caused by canine distemper infection in adult dogs that are not necessarily “old.” Hyperkeratosis of foot pads and the nose occurs in some dogs with neurological disease, as a result of epithelial damage caused by the virus.
Canine distemper virus is shed in all secretions and excretions from the 5th day after infection, which is before the onset of clinical signs, and continues, sometimes for weeks. Transmission is mainly via direct contact, droplet, and aerosol, as the virus is not stable in the environment. Young dogs are more susceptible to the disease than older dogs, with the greatest susceptibility being between 4 and 6 months of age, after puppies have lost their maternal antibody.
Pathogenesis and Pathology
Following aerosol respiratory infection, canine distemper virus first replicates within macrophages in the tissues of the upper respiratory tract, and it then quickly spreads to the tonsils and regional lymph nodes. Canine distemper, like other morbilliviruses, infects cells that express the equivalent of human CD150 (SLAM), which is present on thymocytes, activated lymphocytes, macrophages, and dendritic cells. The tropism of canine distemper virus for these cells explains the immunosuppressive effects of the virus, which probably reflect virus-mediated destruction of immune cells as well as induction of various immunomodulatory cytokines. After multiplication in regional lymph nodes, the virus enters the blood stream, where it circulates within infected B and T cells. Primary viremia is synchronous with the first bout of fever, and virus then is spread to lymphoid tissues throughout the body, including gut-associated lymphoid tissues, and fixed tissue macrophages such as Kupffer cells in the liver. Virions formed in these sites are carried by blood mononuclear cells during secondary viremia that coincides with the second peak of fever. Infection of epithelial cells in the lung, bladder, and skin occurs relatively late in the infection, through a CD150-independent mechanism of attachment that may follow direct interaction with infected lymphocytes. Epithelial cells do not possess CD150 (SLAM), and the receptor that facilitates virus entry into epithelial cells is yet to be defined. Infection of the central nervous system occurs relatively late in the course of infection, and only in dogs that do not develop protective immune responses sufficiently quickly to prevent this spread. Infection of neurons and glial cells also occurs through a CD150-independent mechanism.
Puppies with distemper develop pneumonia, enteritis, conjunctivitis, rhinitis, and tracheitis. The lungs are typically edematous; microscopically, there is bronchointerstitial pneumonia with necrosis of the epithelium lining small airways, and thickening of alveolar walls. Secondary bacterial bronchopneumonia is common as a consequence of both virus-mediated immunosuppression and inhibition of normal pulmonary clearance mechanisms. Lesions in the central nervous system of infected dogs with distemper are variable, depending on duration of infection and the properties of the infecting virus strain; these can include any combination of demyelination, neuronal necrosis, gliosis, and non-suppurative meningoencephalomyelitis. Acidophilic inclusions may be present in the nuclei and cytoplasm of infected astrocytes, as well as in epithelial cells in the lung, stomach, renal pelvis, and urinary bladder (Figure 17.7 ). Canine distemper virus infection of neonates can result in failure of development of the enamel of adult teeth (odontodystrophy), and metaphyseal osteosclerosis in long bones.
Figure 17.7.

Canine distemper. (A) Intranuclear and intracytoplasmic inclusion bodies in the brain of an infected badger. (B) Immunohistochemical staining of canine distemper virus in the brain of a dog.
(Courtesy of R. J. Higgins, University of California, Davis.)
Diagnosis
Clinical diagnosis of canine distemper can be complicated by the use of modified live vaccines. Cases of canine distemper can occur in recently vaccinated puppies, raising the obvious question of whether the signs are caused by the vaccine virus or a field strain. This question is not satisfactorily resolved with standard serological, virus isolation, or antigen detection tests. RT-PCR is now becoming a standard method of testing, but the distinction of field and vaccine viruses also requires very specialized RT-PCR assays that are not routinely available.
Laboratory diagnosis is necessary to exclude other diseases with similar clinical manifestations. Virus isolation can be achieved by co-cultivation of lymphocytes from suspect animals with cell lines expressing the CD150 (SLAM) molecule, which has eliminated the need to use activated mononuclear cells for isolation of field strains of canine distemper virus. After initial isolation, the virus can then be adapted to grow in primary dog lung cells or conventional cell lines, including Madin–Darby canine kidney or Vero. Immunohistochemical or fluorescent antibody staining methods are useful for demonstrating the presence of viral antigen in impression smears of the conjunctiva and skin biopsies (antemortem) or sections of lung, intestine, stomach, kidney, brain, and bladder tissue collected at necropsy (Figure 17.7). RT-PCR tests can be done on conjunctival swabs, blood mononuclear cells, any tissue sample that includes epithelium, and urine. The serological status of dogs can be assessed with virus neutralization assays, ELISA, or indirect fluorescent antibody tests.
Immunity, Prevention, and Control
Cell-mediated immunity is important in the protective immune response against morbillivirus infections in general. In human measles, persons with agammaglobulinemia can overcome the infection, but those with inherited or acquired deficiencies in their cell-mediated immune system are at extreme risk. However, those animals with any detectable neutralizing antibody are immune to reinfection, and immunity following morbillivirus infections is life-long.
Control of canine distemper virus infection is based on adequate diagnosis, quarantine, sanitation, and vaccination. The virus is very fragile, and susceptible to standard disinfectants. Thorough disinfection of premises, however, can be very challenging. Successful immunization of pups with attenuated canine distemper virus vaccines depends on the absence of interfering maternal antibody. The age at which pups can be immunized can be predicted from a nomograph if the serum antibody titer of the mother is known; this service is available in some diagnostic laboratories. Alternatively, pups can be vaccinated with modified live-virus vaccine at 6 weeks of age and then at 2- to 4-week intervals until 16 weeks of age, which is often now the standard practice. For treatment, hyperimmune serum or immune globulin can be used prophylactically immediately after exposure. Antibiotic therapy generally has a beneficial effect by lessening the effect of secondary opportunistic bacterial infections.
Standard modified live vaccines should not be used in species other than canids. Adverse reactions (disease) have occurred in other species, including red pandas and foxes. Inactivated (killed) vaccines previously were used to immunize zoo animals; however, these vaccines were often of marginal efficacy. The availability of a canarypox virus vectored vaccine containing only the H and F proteins of canine distemper virus has resolved this dilemma, as this product provides safe and effective immunization without ever exposing animals to live canine distemper virus. This product currently is used for immunization of endangered species such as giant pandas and black-footed ferrets in zoos in the United States, for instance.
Marine (Phocine and Cetacean) Morbilliviruses
In 1988, a major die-off of harbor seals (Phoca vitulina) occurred in the North, Wadden, and Baltic Seas. Estimates of the number of dead animals ranged from 17,000 to 23,000. Animals initially showed a febrile response, with severe depression. The affected seals exhibited clinical signs similar to those of distemper in dogs, such as serous nasal discharge, conjunctivitis, gastroenteritis, cutaneous lesions, and neurologic signs. Lesions in affected seals included pneumonia, encephalitis, and ophthalmitis. The brains of affected seals had lesions consistent with viral encephalitis, with intracytoplasmic and intranuclear acidophilic inclusions. Pulmonary lesions were consistent with interstitial pneumonia. Lymphocyte depletion and necrosis were prominent in the spleen, bronchial lymph nodes, and Peyer's patches. Recovered seals had neutralizing antibodies to canine distemper virus. A morbillivirus was isolated from the affected seals, and genetic typing places this virus (phocine morbillivirus) in a separate species from canine distemper virus in the morbillivirus genus. A second epizootic occurred in 2002 that resulted in an estimated 30,000 deaths. The exact source of the virus causing these epizootics has not been definitively determined, but evidence suggests that other seals in which the virus is enzootic carried the virus to the affected region during a period of migration. Phocine morbillivirus is present in seal populations throughout the North Atlantic, and perhaps among those in some areas of the Pacific Ocean also.
An epizootic that resulted in the deaths of thousands of striped dolphins in the Mediterranean Sea began in 1990. A morbillivirus was isolated and typing indicated that it was a new species (cetacean morbillivirus virus) in the morbillivirus family, distinct from the previous marine isolate. In 1990, a virus was isolated from a harbor porpoise (Phocoena phocoena) in the Irish Sea showing similar signs to that of the harbor seals infected with phocine morbillivirus. This virus is also considered a cetacean morbillivirus. Retrospective studies on Atlantic bottlenose dolphins (Tursiops truncatus) that died between 1987 and 1988 along the east coast of North America showed evidence of morbillivirus infections. Since their identification, epizootics of disease in marine mammals caused by these viruses have occurred sporadically, and another major die-off of striped dolphin (Stenella coeruleoalba) occurred in the Mediterranean Sea in 2007. Recent sero-surveys indicate that cetacean morbillivirus infections occur in a wide variety of marine mammals in all areas of the world. Factors involved in virus transmission are unknown, as are the animal species that are responsible for maintaining enzootic infections.
Measles Virus
Measles (rubeola) is a disease of humans caused by a morbillivirus that is closely related to the morbilliviruses of animals. Measles virus is naturally infectious for several species of non-human primates, including gorillas, macaques, baboons, African green monkeys, colobus monkeys, squirrel monkeys, and marmosets. Infection is rare in wild populations, but may be common in laboratory animal colonies in association with human exposure. Most laboratory animal facilities are careful to exclude exposure of non-human primates to measles virus by vaccination of personnel (or clinical history of recovered measles virus infection). Clinical disease is relatively mild in most monkeys, with the exception of marmosets (family Callitrichidae) and colobus monkeys (genus Colobus), which may develop high mortality. Lesions include exanthematous rash, conjunctivitis, giant-cell pneumonia, and encephalitis. As in humans infected with measles virus, macaques (genus Macaca) may develop subacute sclerosing panencephalitis months or years after recovery from the acute infection. Marmosets may also develop gastritis and enterocolitis, with disseminated foci of necrosis in several other organs. Diagnosis is facilitated by recognition of characteristic syncytia and both intranuclear and intracytoplasmic inclusion bodies.
Members of the Subfamily Paramyxovirinae, Genus Henipavirus
Zoonotic henipaviruses have caused human deaths in Australia, Malaysia, Singapore, India, and Bangladesh. Pteropus species of fruit bats that are distributed throughout the Indo-Pacific region from Madagascar to the South Pacific islands are the known reservoir host of henipaviruses (Figure 17.8 ).
Figure 17.8.

Locations of human deaths from zoonotic henipaviruses in the Indo-Pacific region.
Hendra Virus
In 1994, an outbreak of severe respiratory disease with high mortality occurred in thoroughbred horses stabled in Brisbane, Queensland, Australia. Two persons at the stable developed a severe influenza-like disease and one died. A new virus (Hendra virus) was isolated from both affected horses and a human, and the syndrome was reproduced experimentally in horses. There have since been sporadic but continuing cases of this devastating disease in both horses and humans, including veterinarians who performed necropsies on affected horses. Serological surveillance confirmed that a similar or identical virus infected four species of fruit bats (flying foxes, suborder Megachiroptera) on the east coast of Australia, and Hendra virus ultimately was isolated from two species of fruit bat. Molecular analyses of the viruses isolated from horses, humans, and bats indicated a close relationship with viruses in the genus Morbillivirus, thus the initial designation of the virus as “equine morbillivirus.” To avoid confusion with possible future isolates and not link the virus to a non-natural host, the virus designation was changed to Hendra virus to reflect the location of the first isolation, and Hendra virus has now been placed in a new genus, Henipavirus, of the subfamily Paramyxovirinae.
Clinical Features and Epidemiology
Hendra virus is maintained by enzootic, asymptomatic infection in certain species of fruit bat. The precise mechanism of virus transmission from bats to non-natural hosts such as horses and humans is uncertain, but probably is through environmental contamination by secretions or excretions from the bats (saliva, feces, urine, placental fluids). The sporadic nature of the outbreaks is probably the result of changes in the feeding behavior of the bats due to changes in food supplies or habitat incursions that facilitate close interaction of horses and bats.
Clinical signs exhibited by horses infected with Hendra virus include any combination of initial anorexia, depression, fever, and increased respiratory and heart rates, followed by respiratory or neurological signs. The clinical course is apparently short, with infected horses dying quickly after the onset of clinical signs. The incubation period in experimentally infected horses was from 6 to 10 days. Cats and guinea pigs, but not rabbits or mice, are susceptible to experimental infection, and cats develop a fatal pneumonia identical to that in horses.
Pathogenesis and Pathology
Affected horses often exhibit severe pulmonary edema, with copious thick, foamy, and hemorrhagic fluid in the airways. Pericardial effusion is also characteristic. Histologically, there is severe interstitial pneumonia, with protein-rich fluid and hemorrhage in the airspaces, dilated lymphatics, vascular thrombosis, and necrosis of the walls of small blood vessels. Vasculitis is limited to small arteries, arterioles, and capillaries, with viral antigen within endothelial cells and the tunica media of affected vessels. Syncytia are present in the endothelium of lung capillaries and arterioles. Cytoplasmic inclusion bodies within these syncytia were shown by electron microscopy to consist of massed viral nucleocapsids.
The finding that ephrin-B2, a transmembrane protein that is abundantly expressed on endothelial cells, is the functional receptor for the henipaviruses potentially explains the distribution of virus in the infected host. Like those of morbilliviruses, the Hendra virus P gene encodes proteins that interfere with interferon induction and signaling. This strategy of selective interference with host innate defenses very likely enhances the severity of the infection.
Diagnosis
The epidemiology, clinical signs and florid lesions of Hendra virus infection in horses are all distinctive, but the macroscopic lesions must be distinguished from those of African horse sickness in particular. Rapid diagnosis can be achieved using RT-PCR tests, and the virus also rapidly can be identified in tissues by immunofluorescence or immunohistochemical staining. Virus isolation can be accomplished in a variety of cell types, but Vero cells are generally preferred. Virus isolation should only be done in high-containment facilities, and any work involving live virus must be undertaken in a Bio Safety Level 4 (BSL-4) facility because of the devastating potential consequences of human exposure. Serological testing can be done by virus neutralization, but ELISA is much preferred because of safety issues pertaining to the requirement to use live virus in the neutralization assay.
Immunity, Prevention, and Control
Horses that survive Hendra virus infection develop very high titers of neutralizing antibodies to the virus, but although vaccines are in development they are not yet commercially available. Hendra virus very clearly is a most dangerous zoonotic pathogen that requires appropriate caution when its presence is suspected, and the availability of adequate biocontainment laboratory facilities for its diagnosis.
Nipah Virus
In 1998–1999, there was an outbreak of acute encephalitis with high mortality in workers handling pigs in Malaysia. The morbidity and mortality in the pigs was not abnormally high, and there was some thought that this was an outbreak of Japanese encephalitis. The mortality rate in the first 265 identified human cases was 40%. A concurrent disease in the pigs was characterized as a febrile respiratory illness, with epistaxis, dyspnea, and coughing in young pigs; some older animals showed neurological signs such as ataxia, paresis, seizures, and muscle tremors. A morbillivirus was isolated from human cases and then from the affected pigs. The virus was antigenically related to Hendra virus, but subsequent sequence analysis identified a new species in the genus Henipavirus, now designated as Nipah virus.
Epidemiological investigations identified the source of the virus as fruit bats, as with Hendra virus. The virus occurs in several species of fruit bat in Southeast Asia, with infections being reported as far west as India. In experimentally infected fruit bats, virus can be detected by virus isolation or by RT-PCR in urine samples from the exposed bats. As with Hendra, the likely track of the infection is from the fruit bats to animals in agricultural facilities in close proximity to the feeding bats. The virus can easily be spread among the exposed pigs through the respiratory route. Workers handling the pigs or pig carcasses also became infected, and there is evidence of human-to-human spread. In Malaysia, the infection in pigs was known as “barking pig syndrome” because of characteristic coughing in the affected pigs. Virus can consistently be isolated from pharyngeal swabs from experimentally infected pigs by day 4 post infection, and the virus spread horizontally to control pigs. Cats can also be infected with Nipah virus and can transmit the virus to contacts.
The pathology of Nipah virus disease in pigs and humans is similar to that caused by Hendra virus. A prominent feature in the human cases was a vasculitis with endothelial cell damage, necrosis, and syncytial giant cells in the affected vessels; immunohistochemical staining confirmed that abundant viral antigen was present in endothelial and smooth muscle cells of the small blood vessels. Severe dysfunction of brain stem neurons occurs in humans with Nipah virus encephalitis, probably as a result of the strong tropism of the attachment G protein of Nipah virus for the ephrin-B3 receptor that is abundantly expressed on these cells. Naturally infected pigs developed tracheitis and bronchointerstitial pneumonia with hyperplasia of the airway epithelium. Sero-surveys indicate that many pigs have subclinical infections.
Nipah virus appears to be a more substantial threat to agriculture and humans than Hendra virus, in part because of the role of swine as amplifying hosts of Nipah virus. Experimental vaccines have been developed that are efficacious, and rapid and sensitive diagnostic tests are available, including RT-PCR assays, and immunofluorescence and immunohistochemical staining assays to detect viral antigen. As with Hendra virus, immunoassays are routinely used for serological diagnosis because of the biosecurity issues with handling live Nipah virus.
Other Henipaviruses
Serological evidence exists of henipavirus infection of West African fruit bats (Eidolon helvum), suggesting that viruses that are identical or related to Nipah and Hendra viruses circulate in other regions of the world but in different bat reservoir hosts. Antibodies to henipaviruses also have been found in species of bats other than Pteropus in Madagascar.
Members of the Subfamily Pneumovirinae, Genus Pneumovirus
Viruses in the subfamily Pneumovirinae are genetically and antigenically distinct from those in the subfamily Paramyxovirinae, and they utilize somewhat different replication strategies. Most pneumoviruses lack both a hemagglutinin and a neuraminidase; rather, they utilize a G protein for cell binding. Viruses in the genus Pneumovirus are further distinguished from those in the genus Metapneumovirus on the basis of their sequence relatedness and differences in their genetic constitution.
Bovine Respiratory Syncytial Virus
Bovine respiratory syncytial virus was first detected in Japan, Belgium, and Switzerland in 1967, and was isolated soon thereafter in England and the United States. It is now known to occur worldwide in all bovine species as well as in sheep, goats, and other ungulates. The virus is related closely to human respiratory syncytial virus, and some monoclonal antibodies developed to detect the human virus also will detect the bovine equivalent. Caprine and ovine strains of respiratory syncytial virus are also recognized, and these perhaps represent, with bovine respiratory syncytial virus, a subgroup of ruminant syncytial viruses rather than different species.
In many settings the bovine virus causes inapparent infections, but in recently weaned calves and young cattle it can cause pneumonia, pulmonary edema, and emphysema. Infection also predisposes to other infections of the respiratory tract.
Clinical Features and Epidemiology
Bovine respiratory syncytial virus infection occurs most often during the winter months when cattle, goats, and sheep are housed in confined conditions. However, there have been substantial outbreaks in cow and calf herds in summer as well. The virus spreads rapidly, probably through aerosols or droplets of respiratory tract excretions. Reinfection of the respiratory tract is not uncommon in calves with antibody. Pre-existing antibody, whether derived passively from maternal transfer or actively by prior infection or vaccination, does not prevent virus replication and excretion, although clinical signs may be lessened. The virus persists in herds, most probably through continuous subclinical reinfections or in putatively inapparent virus carriers.
Inapparent infection of cattle is very common. Disease caused by respiratory syncytial virus infection is particularly important in recently weaned beef calves and young cattle, especially when they are maintained in closely confined conditions. Infection is characterized by a sudden onset of high fever, hyperpnea, abdominal breathing, lethargy, rhinitis, nasal discharge, and cough. Secondary bacterial pneumonia, especially that caused by Mannheimia haemolytica, is common. Outbreaks often occur after a sharp drop in temperature. In general, in outbreaks the morbidity is high but mortality is low, and animals that die are also often persistently infected with bovine viral diarrhea virus.
Pathogenesis and Pathology
In calves infected experimentally, the virus causes destruction of the ciliated epithelium of the airways in the lung, so that pulmonary clearance is compromised, which predisposes to secondary bacterial infections. At necropsy, there is interstitial pneumonia with emphysema that affects all lobes of the lungs. Secondary bacterial bronchopneumonia that affects the anteroventral aspects of the lung is common. Syncytia may be present in the airway epithelium lining the bronchi and bronchioles, as well as in alveolar macrophages and type II pneumocytes.
Protection against reinfection is short lived following natural infection, but the clinical signs in subsequent infections are less severe. Passive antibody is protective, in that the attack rate is less in 1-month-old calves than in older calves with no colostral antibodies. Calves immunized with formalin-inactivated vaccine preparations developed more severe lung injury following challenge infection with bovine respiratory syncytial virus than did control animals, and it has been proposed that the enhanced disease may be a consequence of a predominant T-helper 2 (Th2) cell response, with the preferential release of inflammatory cytokines in the absence of a CD8 T cell response. This abnormal Th2 cell response with eosinophilia can be reproduced by immunization with recombinant vaccines expressing only the G protein of the virus. This protein is one of the most unique among viral proteins as a result of its high degree of O-linked glycosylation, a property that may help evade immune surveillance and compromise efforts to develop an effective vaccine.
Diagnosis
Bovine respiratory syncytial virus infection is not reliably diagnosed by virus isolation, as virus frequently is complexed with antibody in cattle that already have developed an immune response. The virus can be isolated from appropriate samples using a number of bovine cell cultures. The presence of virus reliably can be detected in tracheal wash-derived cells by immunofluorescence staining with virus-specific monoclonal antibodies, and in tissue samples from necropsy cases. RT-PCR tests have also been developed for bovine respiratory syncytial virus, and these assays also have the inherent advantage of not being affected by the presence of neutralizing antibodies, although care must be exercised to consider the possible detection of virus from modified live vaccines. Virus neutralization assays can be used to detect neutralizing antibodies, and paired samples from the index case, in addition to age-matched herd mates, should be tested.
Immunity, Prevention, and Control
Although immunity is incomplete and transient following natural bovine respiratory syncytial virus infection of calves, vaccination remains the usual means of control. Several inactivated and attenuated virus vaccines are in current use. Efficacy data has been difficult to obtain because challenge models for cattle are not robust. There is anecdotal evidence in the United States suggesting that vaccination reduces the occurrence of severe outbreaks of disease associated with bovine respiratory syncytial virus infection; however, efforts are ongoing to develop more efficacious products, including vectored virus vaccines and the like.
Pneumonia Virus of Mice
Pneumonia virus of mice was highly prevalent in mouse colonies before routine surveillance programs. This virus or related viruses also infect(s) rats, cotton rats (genus Sigmondia), hamsters (subfamily Cricetinae), gerbils, guinea pigs (Cavia porcellus), and dogs. It can be a clinically silent infection that is typically detected by sero-surveillance, generally by immunoassay (ELISA). It received its name when pneumonia developed in suckling mice following experimental serial passage, but natural disease occurs only in immunodeficient mice. Seropositive immunocompetent mice recover from infection without evidence of a carrier state. Pneumonia virus of mice is a clinically important infection in immunodeficient mice, such as nude and severe combined immunodeficient mice. Like Sendai virus, pneumonia virus of mice is non-cytolytic and infects respiratory epithelium and type II pneumocytes. However, pneumonia virus of mice virus tends to infect individual cells, rather than the entire respiratory epithelial population, so cellular immune responses do not result in recognizable necrotizing lesions that are typical of Sendai virus infection. T-cell-deficient mice develop progressive interstitial pneumonia that is difficult to differentiate from Sendai virus pneumonia in immunodeficient mice. Pneumonia virus of mice infection of marginally immune deficient mice (numerous types of genetic null mutant animals) may exacerbate pneumonias caused by either Pneumocystis spp. or bacterial infections.
Members of the Subfamily Pneumovirinae, Genus Metapneumovirus
Avian Rhinotracheitis Virus (Metapneumovirus)
Avian metapneumovirus causes a variety of disease syndromes, depending on the bird species and virus type (types A, B, C, and D). The first infections were described in turkeys in South African in 1978. These infections were caused by type A viruses, and termed turkey rhinotracheitis. Later, infections caused by type B virus were described in turkeys in Europe. Infections by type A and B viruses causes upper respiratory disease in chickens, termed swollen head syndrome. Type C virus has been reported only in turkeys in the upper Midwest of the United States and in Muscovy ducks in France; and type D virus has been reported in turkeys in France. The currently preferred designation for avian metapneumovirus infections is “avian rhinotracheitis.” Pheasants and guinea fowl with respiratory disease in the United Kingdom have also been infected with type A avian metapneumovirus.
In young turkeys the disease is characterized by inflammation of the respiratory tract, rales, sneezing, frothy nasal discharge, conjunctivitis, swelling of the infraorbital sinuses, and submandibular edema. Coughing and head shaking are frequently observed in older poults. These signs may be exacerbated by secondary infections. In turkey breeding operations, infections cause a decrease in egg production of up to 70%, and an increased incidence of prolapsed uterus from excessive coughing by affected birds. In hens, respiratory disease is milder than in young poults. Morbidity is often 100%; mortality ranges from 0.4 to 50% and is highest in young poults. Swollen head syndrome is a milder form of the disease that occurs in chickens, typically with co-infection by bacteria such as Escherichia coli. This disease is characterized by swelling of the infraorbital sinuses, torticollis, disorientation, and general depression, sometimes also with respiratory distress. In chickens, morbidity is usually less than 4% and mortality less than 2%.
In turkeys, the respiratory tract disease is characterized histologically by: increased glandular secretion, focal loss of cilia, hyperemia, and mild mononuclear mucosal inflammation within the turbinates during the first 2 days after infection epithelial destruction and intense mucosal inflammation on days 3–5; watery to mucoid exudate in turbinates from days 1–9. Tracheal lesions are generally milder, but in severe cases can include complete deciliation of the mucosal lining of the trachea within 4 days. Cytoplasmic eosinophilic inclusions occur in epithelial cells lining the airways and nasal cavities.
Diagnosis of both the turkey and the chicken disease is based most commonly on detection of specific antibodies on ELISA from non-vaccinated animals with recent history of respiratory disease, or detection of avian metapneumovirus genome by molecular tests such as RT-PCR in acute respiratory disease cases. Virus isolation is difficult, but can be achieved by serial passage in 6- to 7-day-old turkey or chicken embryos or in chicken embryo tracheal organ cultures. RT-PCR assays provide data on the subtypes of virus circulating in a given area. Attenuated virus vaccines and inactivated vaccines are available commercially for three of the four genetic subgroup types of avian metapneumovirus (A, B, and C), and appear to give cross-protection against the various strains.
As a curiosity, the only other species for which a metapneumovirus has been identified as a pathogen is humans.
Unclassified Members of Family Paramyxoviridae
Bottlenose Dolphin (Tursiops truncatus) Parainfluenza virus
A paramyxovirus was isolated from a 19-year-old bottlenose dolphin with fatal bronchointerstitial pneumonia. Other significant findings were multifocal erosive and ulcerative tracheitis and laryngitis. Phylogenetic analyses indicated that the virus was most closely linked to bovine parainfluenza virus 3. A sero-survey confirmed that healthy dolphins from Florida and California had previously been exposed to this virus, suggesting that infections are common in bottlenose dolphins and that the virus may be involved in outbreaks of respiratory disease in marine mammals.
Fer-de-Lance and other Ophidian Paramyxoviruses
An apparently new epizootic disease of snakes was first reported in 1976 from a serpentarium in Switzerland. A paramyxovirus-like agent was isolated (Fer-de-Lance virus). Subsequently, similar viruses have been isolated from snakes, lizards, and turtles, thus Fer-de-Lance virus was the first isolate of what appears to be a whole new genus of reptile paramyxoviruses or ophidian paramyxoviruses. It has been proposed that viruses in this group be included in a new genus, Ferlavirus, with Fer-de-Lance virus being the type species.
Snakes infected with these viruses can develop abnormal posturing, regurgitation, anorexia, mucoid feces, head tremors, terminal convulsions, and high mortality. The lungs of affected snakes were congested, and histologic lesions included proliferative interstitial pneumonia with variable degrees of infiltration of mononuclear cells. Intracytoplasmic inclusions were present within epithelial cells of the airways. In the pancreas of several snakes, there were multifocal areas of necrosis. Immunohistochemical staining confirmed the presence of viral antigen at the luminal surfaces of pulmonary epithelium, and multinucleated cells within the pancreas.
Virus can be isolated using viper heart cells or Vero cells, but at reduced incubation temperatures (25–30°C). The ophidian paramyxoviruses hemagglutinate chicken red blood cells, which permitted the development of a serological test for screening of exposed animals. Virus can be detected by immunohistochemical staining of tissues from affected snakes, and by RT-PCR tests.
Salem Virus
In 1992, an outbreak of febrile illness with limb edema occurred in horses at three race tracks in the Northeastern United States. A syncytium-forming virus was isolated from the blood mononuclear cells of one affected horse, and subsequent sequence analysis identified it as a member of the subfamily Paramyxovirinae that obeyed the “rule of six.” This virus, however, did not segregate with viruses in the existing genera in the subfamily. The virus grows in a wide variety of cell cultures, but lacks either neuraminidase or hemagglutinating activity. Sero-surveys indicated that some 50% of horses in the region were seropositive, and sero-reactivity also was demonstrated with canine and porcine sera, but not that of ruminants. Dogs are susceptible to infection, and virus was isolated from them up to 1 month after infection. The pathogenic significance of Salem virus is uncertain, for both dogs and horses.
Atlantic Salmon Paramyxovirus
Atlantic salmon paramyxovirus has been associated with proliferative gill disease, which has a multifactorial etiology, in post-smolt farmed Atlantic salmon in Scandinavia. Sequence analysis of the entire genome indicates that the virus is most similar to viruses in the genus Respirovirus; however, the Atlantic salmon virus is not yet taxonomically classified, and it may represent a new genus within the subfamily Paramyxovirinae. Similar viruses frequently have been isolated from salmonids from the west coast of North America, but without any associated clinical disease.


