Abstract
This chapter reviews the anatomy and physiology of the rabbit eye from a comparative perspective. The anatomy of the rabbit eye reflects its niche as a diurnal herbivore. The rabbit has both photopic and scotopic vision without the benefit of a tapetum. Orbits are laterally situated; the rabbit is one of the few animals in which the orbital axis coincides with the visual axis. The shape of the orbit is circular, compared to the cone shaped human orbit. The orbital walls are of bone, except inferiorly, where the wall is formed partially by the muscles of mastication. The superior orbital wall is formed by the frontal bone. The supraorbital process of the frontal bone contains three supraorbital foramina, which are unique feature of the rabbit orbit; the foramina are incisures formed into apertures by a cartilaginous sheet. The optic foramina share a common canal anteriorly with only a thin boney plate to divide them, which disappears posteriorly to form one canal opening into the cranium as a single foramen. Entropion in young rabbits can occur as a primary or as a secondary condition arising from infection. Viral-induced eyelid proliferations can result from infection by the rabbit myxoma and papilloma viruses. The papilloma virus is a part of the papovaviridae and is a DNA virus transmitted by arthropod vectors. The cottontail rabbit in the mid-western United States is most frequently affected, although domestic rabbits are susceptible.
I. INTRODUCTION
The laboratory rabbit, for reasons both obvious and subtle, has no close competition with regard to being the most commonly utilized species for experimental ophthalmology; use of rabbits in vision research is somewhat tempered, but lagomorphs are well represented here as well. For the ophthalmic researcher, accessibility, economy of acquisition andmaintenance, general tractability, and relatively large prominent globes have been determining factors rather than demonstrated similarities in physioanatomy.
This chapter reviews the anatomy and physiology of the rabbit eye from a comparative perspective, summarizes documented spontaneous ocular conditions, discusses experimentally induced disease in general terms, and concludes with a summary of observations regarding the rabbit as a model for broad categories of research. Presentation is conceptual and general rather than specific, anticipating that this chapter will be the first step, not the last, for those who would seek to know the rabbit eye. References are selective (we hope representative and pertinent) rather than comprehensive (a cursory literature search yielded several thousand references), with emphasis on the contemporary and the in vivo.
II. ANATOMY AND PHYSIOLOGY OF THE VISUAL SYSTEM
Our review of anatomy and physiology is cumulative from classic texts (Davis, 1929; Prince et al., 1960; Prince, 1964; Cole, 1974; Francois and Neetens, 1974; Tripathi, 1974), more recent papers, and personal observations. The anatomy of the rabbit eye reflects its nicheas a diurnal herbivore. The rabbit has both photopic and scotopic vision without benefit of a tapetum. Orbits are laterally situated; the rabbit is one of the few animals in which the orbital axis coincides with the visual axis. There is an angle of 150° to 175° between the two visual axes witha binocular visual field of 10° to 35° in width. By moving the eyes and tilting the head upward the rabbit can achieve a maximum field of vision of almost 360°. The prominent globes, which extend up to 5 mm beyond the inferior and 12 mm beyond the superior orbital rim, and the large corneascontribute to the phenomenal field of vision. Adult globe size is 18 mm horizontal, 17 mm vertical, and 16 mm anterior–posterior.
A. Circulation of the Orbit and Globe
In the rabbit the internal maxillary artery (a branch of the external carotid) enters the orbit through the anterior sphenoidal foramen and gives rise to the external ophthalmic artery, which anastomoses with the internal ophthalmic artery to supply the extraocular muscles and Harder's gland and with both posterior and anterior ciliary arteries (nasal and temporal) which supply the globe. The internal ophthalmic artery is a branch of the external carotid and enters the orbit through the optic foramen. Venous drainage from the vortex veins is into a rather extensive posterior orbital venous sinus which surrounds the muscle cone and Harder's gland.
B. The Orbit and Extraocular Muscles
The shape of the orbit is circular, compared to the cone-shaped human orbit. The orbital walls are of bone, except inferiorly, where the wall is formed partially by the muscles of mastication. The superior orbital wall is formed by the frontal bone. The supraorbital process of the frontal bonecontains three supraorbital foramina, which are an unique feature of the rabbit orbit; the foramina are incisures formed into apertures by a cartilaginous sheet. The optic foramina share a common canal anteriorly with only a thin boney plate to divide them, which disappears posteriorly to form one canal opening into the cranium as a single foramen.
The rabbit has nine extraocular muscles, one more than is acknowledged in other domestic animals; the additional muscle is the depressor palpebrae inferior. In other mammals a short tendinous extension of the inferior rectus muscle depresses the lower lid; the rabbit has a prominent globe which extends beyond the orbital rim and thus requires an additional muscle for depression. The muscle arises from the zygomatic bone slightly inferior to the level of the nasal canthus and inserts into theanterior portion of the lower lid. The remaining muscles originate from the orbital wall between the optic foramen and the orbitorotundum foramen and insert rather close (2–4 mm) to the limbus. The retractor bulbi muscle surrounds the optic nerve deep to the rectus muscles; its point of origin iscloser to the orbitorotundum foramen, and it inserts well behind the equator in an irregular fashion.
C. Eyelids
The palpebral opening in the rabbit is 10 to 16 mm long. The superior lid is shorter and thicker than the inferior lid, with more numerous, posteriorly directed cilia. On the inferior lid the cilia are longer nasally, shorter temporally, and directed straight ahead to allow maximum protection and vision.
The orbicularis oculi muscle is comparatively large in the rabbit. There are 40 to 50 meibomian glands embedded in the tarsus, the palpebral conjunctiva contains numerous lymphatic nodules and intraepithelial glands, and the caruncle has a broad base 5 mm wide which merges with both eyelids. Rabbits blink 10 to 12 times per hour.
The third eyelid of the rabbit is not noticeably active; in fact, it does not nictitate. It can, however, be retracted by applying pressure to the globe. The direction of movement is upward andtemporally, and it does not move more than two-thirds across the cornea. The fornices shallow significantly both anterior and posterior to the third eyelid.
D. Conjunctiva
The conjunctiva is divided into two continuous parts, namely, the bulbar conjunctiva and thepalpebral conjunctiva; total surface area is about 50% of the human conjunctiva. The palpebral conjunctiva is firmly adherent to the lids and is approximately 40 µm in thickness. In the fornix there are numerous goblet cells and intraepithelial glands scattered between the epithelial cells, and the epithelium is thicker. The lacrimal and auxiliary ducts enter into the fornices. The bulbar conjunctiva is thinner (10–30 µm), with fewer goblet cells which increase in number toward the limbus. A superficial epithelial cell type characterized by large osmiophilic granules is not found in primates. The presence of immunoglobulin A (IgA) staining plasma cells and the tendency for dense inflammatory cell infiltrates to accumulate in the conjunctiva in response to inflammatory disease in adjacent tissues speak to its role in immunoresponsiveness.
E. Lacrimal System
The lacrimal system consists of three glands, the lacrimal puncta and canaliculi, nasolacrimal duct, and nasopuncta. The aqueous tear film is produced by the lacrimal gland, Harder's gland, and the gland of the third eyelid; normal Schirmer tear test values in the rabbit are 5.30 ± 2.96 mm/min (Abrams et al., 1990). The lacrimal gland is large, bilobulated, and pale red in color, occupying the orbit adjacent to the lower rim; it is narrow in form with bulbous enlargements at each canthus. The lacrimal gland is a serous-secreting, compound, tubular gland surrounded by a fibrous connective tissue capsule; the lobes are divided into lobules by loose connective tissue septa containing reticular and collagenous elastic fibers. In the rabbit the lacrimal glandplays a lesser role in lubricating the eye than it does in humans, and the effect of removal of the lacrimal gland is diminished by compensatory secretions from the gland of the third eyelid and Harder's gland. Although transient keratoconjunctivitis sicca may be observed following partial removal of the gland, signs disappear, supposedly owing to the regeneration of the tissue.
Harder's gland is quite large and is attached to the inferior nasal medial wall of theorbit. Dimensions are about 19 by 12 to 15 by 4 to 6 mm. The gland is encapsulated and is almost totally surrounded by the orbital venous sinus. The gland is roughly kidney shaped with two distinct lobes, a pink lobe and a white lobe; the size and dispersion of lipid droplets in the gland probably account for the difference in color. The cells of the gland differ in shape as well. In the pink lobe thecells of the acini are cuboidal and filled with lipid droplets which are larger than those in the white lobe. In the white lobe, the acini are formed by columnar epithelium with smaller lipid droplets. The two lobes appear to have similar function, which includes being an integral part of the secretoryimmune system. There is a single duct which opens onto the inferior part of the bulbar surface of thethird eyelid, to which the gland is attached. The gland of the third eyelid closely resembles Harder's gland in structure and is situated surrounding the shaft of the cartilage of the third eyelid. The biochemistry of isolated rabbit lacrimal acini has been described (Bradley et al., 1992).
The rabbit has only one lacrimal punctum which is located in the inferior eyelid, 3 mm from the medial canthus and 3 mm from the inner lid margin. The proximal portion of the canaliculus is very short, approximately 2 mm long, and assumes a funnel-shaped sac at its transition into the nasolacrimal duct. The lacrimal bone in the rabbit does not have a well-defined lacrimal fossa although it does support the lacrimal sac medially. The nasolacrimal duct courses through the semicircular lacrimalcanal from the orbit to the maxilla. In the maxilla the duct courses medially and rostrally for 5 to 6 mm; at this point the duct changes diameter abruptly to 1 mm and curves rostrally. The duct maintains a 2 mm diameter until it reaches the incisor tooth root, where it spirals slightly and is compressed in a saggital plane between the alveolar bone of the premaxilla and the nasal cartilage. The duct courses rostrally and medially to the nasal vestibule, where it exits at the nasopunctum several millimeters posterior to the mucocutaneous junction of the alar fold. The nasolacrimal duct of the rabbitis difficult to cannulate as resistance is encountered at the proximal maxillary curve and the base of the incisor tooth (Burling et al., 1991). Histologically the rabbit nasolacrimal duct is similar to the human nasolacrimal duct.
F. Cornea
The rabbit cornea is quite prominent, having a horizontal dimension of 15 mm and a vertical dimension of 13.5 to 14 mm. The cornea has a uniform thickness of 407 ± 20 µm (Fig. 1 ). The epithelium is thinner in the rabbit than it is in humans, approximately 30 to 40 µm thick, and consists of one row of elongated columnar basal cells beneath three to five layers of wing and surface cells. Descemet's membrane is continually laid down throughout life and gradually thickens; the membrane is usually 7 or 8 µm thick, but may reach up to 15 µm with age. The endothelial cells are hexagonal in shape, about 20 µm in diameter, with a denisty of 2998 ± 326/mm2 (Salistad and Peiffer, 1981), and unlike those of humans, primates, and cats possess regenerative capabilities (Van Horne et al., 1977). The cornea is innervated by ciliary nerves which pass forward from the ciliary body to the limbus between the sclera and the choroid. Humans have 82 nerve bundles serving the cornea; the rabbit has 65.
Fig. 1.
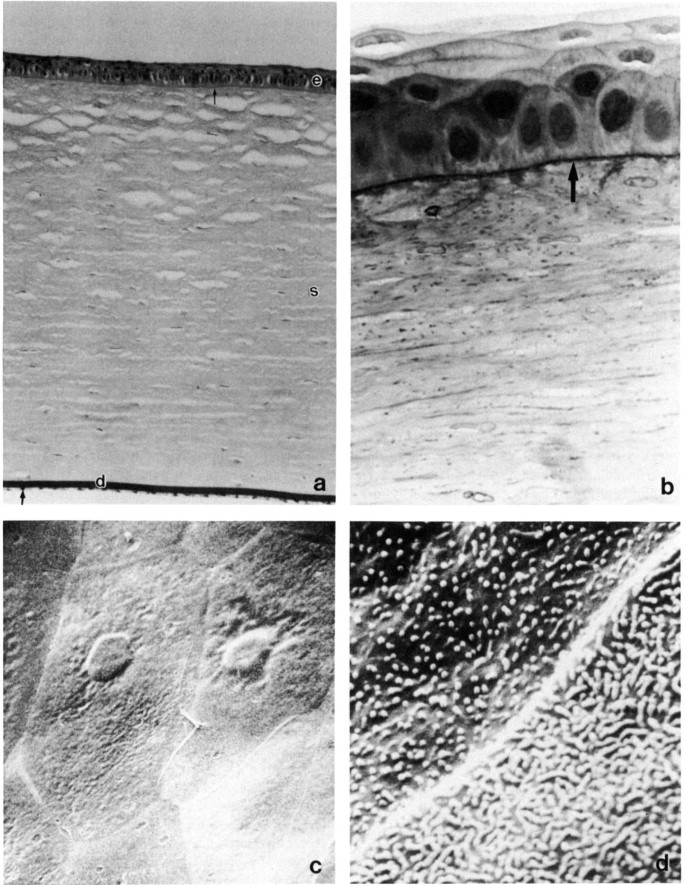
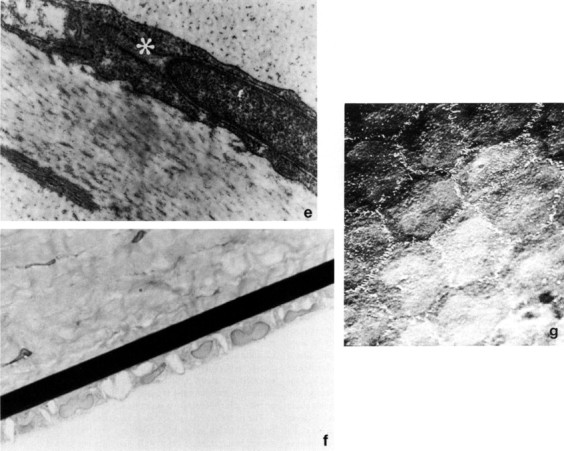
Rabbit cornea. (a) Photomicrograph of cornea with epithelium, basement membrane (small arrow), stroma (s), Descemet's membrane (d), and endothelium (large arrow). Periodic acid–Schiff, × 200. (b) The corneal epithelium is four to five cell layers thick with prominent basement membrane (arrow), irregular basal cell layer, one to two layers of wing cells, andtwo to three layers of surface cells. Toluidine blue, × 1025. (c) Scanning electron microscopy (SEM) of surface endothelium. × 1300. (d) Corneal surface epithelial cell microvilli and microplicae and a linear cell junction. SEM, × 15,000. (e) Corneal stroma comprised of keratocytes (*) and highly organized lamellae of collagen fibers. Transmission electron micrograph, × 31,500. (f) Descemet's membrane lacks distinct inner and outer zones. Toluidine blue, × 1250. (g) Corneal endothelium. Note cellular regularity of monolayer and occasional microvilli. SEM, × 2000.
G. Iridocorneal Angle
Many iris pillars with broad bases pass from the iris root and taper to a fine insertion into the termination of Descemet's membrane and the surrounding sclerocorneal stroma. The broad insertions provide a firm anchorage for the long thin iris and large ciliary process attached to its rear surface, which in turn suspend a comparatively large lens. The pillars are approximately 0.1 to 0.2 mm apart and lead to a prominent ciliary cleft. The trabecular meshwork of the rabbit is relatively rudimentary (Fig. 2 ).
Fig. 2.
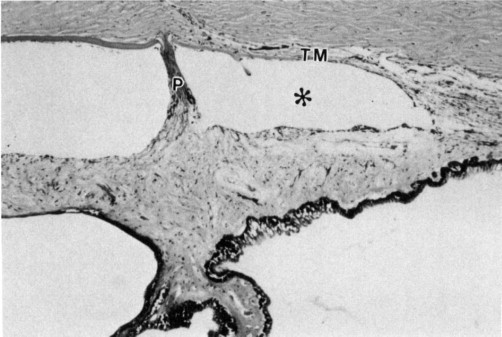
Rabbit iridocorneal angle with a prominent pectinate fiber (P), a prominent ciliary cleft (*), and a rudimentary trabecular meshwork (TM). Hematoxylin and eosin, × 100.
H. Sclera
The rabbit sclera has considerable thickness variation, measuring 0.5 mm adjacent to the limbus and thinning gradually toward the posterior pole, where it is 0.18 mm thick. Along the equator superiorly the average thickness is 0.25 mm and inferiorly 0.2 mm. The ciliary vessels that pass forward in the sclera are found closer to the external surface in rabbits and humans compared to dogs and cats.
I. Uveal Tract
The anterior chamber of the rabbit is quite shallow (3.5 mm). The rabbit pupil is slightly ovoid vertically but is circular in shape when widely dilated; pupillary size varies from 5 to 11 mm. Arising from the ciliary body, the iris has a narrow base of approximately 250 µm, thickening centrally to 270 µm before tapering to 90 µm at the pupillary border. Stromal melanocytes are absent in albinos. The sphincter muscle is well developed and extends to the pupillary margin; the epithelium is nonpigmented in the albino and consists of a posterior layer ofcolumnar cells apposed to a flat cell layer adjacent to the dilator muscle. There is only one main arterial circle, which is halfway between the pupil and the root of the iris; it is formed by four branches of the ciliary arteries, two of which enter temporally and two nasally. Many small vessels branch off the major circle and pass posterior to cross the ora ciliaris retinae and disperse in the choroid. The capillaries near the pupillary margin drain into the radial veins, which drain posteriorly tothe venous system of the choroid.
The ciliary body in the rabbit is poorly developed and flat owing to the scarce muscle fibers, which accounts for the negligible power of accommodation in the rabbit. The ciliary body is 1.5 mmlong from the ora ciliaris retinae to the iris root and is 0.3 mm at its thickest part. The ciliary processes are well developed and differ from those of humans in that they arise from the anterior portion of the ciliary body and merge into the posterior surface of the iris to extend within 1 mm of thepupillary margin (Fig. 3 ). The processes are frequently joined to the iris for much of their length, and not all of the processes join the iris at the same point. All the processes are connected proximally by the “sims” or ciliary web which providesrigidity and vascular anastomosis between the processes. This type of relationship between the ciliary processes and iris is frequently found in mammals with little or no accommodative power; it is theorized that a small amount of accommodative power may be obtained by engorging or decreasing the bloodvolume in the ciliary processes and iris, which changes the diameter of the pupil and position of thelens slightly.
Fig. 3.
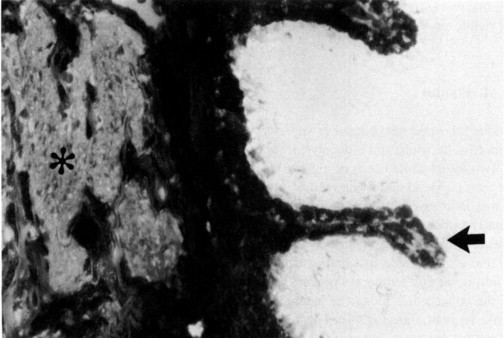
Ciliary processes (arrow) are well developed and inserted anteriorly on the iris. The ciliary musculature (*) is not well developed. Toluidine blue, × 400.
The zonular fibers have a diffuse origin from the ciliary body; they originate as far posterior as the ora ciliaris and from both the ridges and valleys of the ciliary processes. The origin of the vascular supply to the ciliary body is the long posterior ciliary artery, short posterior ciliaryartery, and to some degree the anterior ciliary artery. A circular channel within the ciliary body isformed by two terminal branches of the long posterior ciliary arteries, which form two connecting semicircular channels.
The rabbit choroid is well developed and typically mammalian in structure and without a tapetum. The choroidal thickness varies, being thickest posteriorly and thinning toward the ora ciliaris retinae; it tends to be thicker inferiorly compared to superiorly and is thickest and most heavily pigmented in the region of the visual streak, an area that lies well above the posterior pole of the globe on either side of and below the optic disk.
J. Aqueous Humor
The capillaries within the stroma of the ciliary body have a thin endothelium, 0.15 µm, with fenestrations 200 to 1200 A in size. Fluid flows through the fenestrations and into the stromaof the ciliary body and toward the ciliary epithelium. The ciliary body has an energy-dependent transport mechanism similar to that found in the renal tubules. Sodium and chloride ions are actively pumped into the aqueous and water passively follows. Na+, K+-ATPase has been localized to the inner layer of the nonpigmented ciliary epithelium and may be associated with the “sodium pump.”
The volume of rabbit aqueous is about 300 µl (250 µl anterior chamber, 57 µl posterior chamber). The fractional turnover rate (K 1) is about 0.15, outflow facility (C) between 0.21 and 0.34 ml/min/mm Hg, episcleral venous pressure 9.3 mm Hg, and normal intraocular pressure (IOP) 18–21 mm Hg with a circadian rhythm, being lowest at night and highest during the day.
The concentration of Na+ and K+ in the rabbit aqueous is virtually the same as that found in plasma. The concentrations of bicarbonate and ascorbic acid are much higher than those found in the plasma, and the highest concentrations of both are found in the posterior chamber. Ascorbic acid is actively transported into the posterior chamber, and the higher concentration of bicarbonate is related to the presence of carbonate dehydratase in the ciliary body. Carbonate dehydratase catalyzes the formation of carbonic acid from CO2 and water; the carbonic acid dissociates, and the bicarbonate ions pass into the aqueous.
The concentration of glucose is 10 to 20% lower than that found in the plasma owing to the metabolism of glucose by the lens and cornea. The concentration of lactic acid is the same as in plasma, but most of it is derived from the metabolism of glucose. The concentration of protein is 1% of serum and the ratio of albumin to globulin the same as that found in plasma.
The trabecular plexus, which consists of a large number of anastomosing small intrascleral vessels, lies adjacent to the trabecular meshwork. The aqueous humor enters this plexus and travels tothe perilimbal veins and then, via ciliary veins, enters the orbital venous sinus.
K. Lens
The rabbit lens is larger, is more spherical, and occupies a greater percentage of the globethan in humans, which have considerably larger eyes. The lens is a transparent, avascular, biconvex body. The anterior surface has a curvature radius of 5.3 mm, and the posterior surface has a slightly steeper curvature radius of 5.0 mm. The anterior to posterior dimension is 7 mm, and the equatorial dimension is 9 to 11 mm. There are two linear sutures; the anterior suture is vertically directed, andthe posterior suture is horizontally directed. The capsule is thickened anteriorly and is thickest toward the equator where the zonular fibers insert. At the equator the capsule is 8 to 10 µm thick, and it thins to 4 to 6 µm at the posterior pole. The epithelium is a single layer of cuboidal epithelial cells and averages 17 µm in thickness. The lens fibers form a complicated interlacing and process-interlocking system which is tightly knit yet capable of enough resilience to permit 1.5 D of accommodation. The lens nucleus is not well defined. Removal of the lens leaves the rabbit 10 diopters hypermetropic.
L. Vitreous Body
In the newborn rabbit there is a prominent primary vitreous body with many blood vessels extending from the optic disk to the posterior surface of the lens. After 2 to 3 weeks the vessels disappear, but the hyaloid canal or vascular remnants generally persist.
The rabbit vitreous weighs 1.4 g. The hyalocytes are numerous and are most readily found near the cortex within 30 µm of the surface of the vitreous, where the highest concentration of hyaluronic acid is found. Like the aqueous there is free movement of many substances within the vitreous; half the water in the rabbit vitreous is replaced in 10 to 15 min. The flow in the rabbit vitreousmoves meridionally from the ciliary region toward the posterior pole; one meridional stream is 1 mm from the surface of the retina, while another follows the posterior surface of the lens.
M. Fundus
When viewing the fundus the optic nerve head is superior and nasal to the posterior pole of the globe and is deeply cupped and horizontally shaped. Two broad white bands of myelinated nerve fibers, the medullary rays, extend nasally and temporally from the optic disk; they lose their myelination just short of the equator on either side. Small bundles of myelinated nerve fibers extend from therays, especially inferiorly, and appear as white streaks. The retinal vein and artery divide just before or just after entering the globe into nasal and temporal branches and travel from the center of the cupped disk along the medullary rays. The remaining retina is avascular (Fig. 4 ). The visual streak is an area of the retina that appears to have more sensitivity and is located just inferior to the medullary rays and runs parallel with them; its center is 3mm below the optic disk, it is 3 to 4 mm wide, and the streak is in apposition with the pigment streak of the choroid. Photoreceptors are predominantly rods; however, cones may be found in the visual streak. In nonpigmented rabbits the choroidal vessels are easily seen.
Fig. 4.

Ophthalmoscopic view of the posterior pole of the pigmented rabbit eye. The horizontally ovoid optic disk has a prominent physiological cup, myelinated nerve fibers which form the horizontal medullary rays, and accompanying retinal arterioles and venules.
N. Retina
The rabbit retina varies in thickness; the thickest area is at the visual streak, which is 160 µm thick. Most other areas of the retina are uniformly 120 µm thick, but there is thinning around the ora ciliaris to 90 µm. The rabbit retina is not completely differentiated until6 weeks after birth.
The retinal pigment epithelium (RPE) is devoid of pigment in the albino rabbit. The cells are flat and elongated in saggital section but polygonal in tangential section. The cells are 10 to 15 µm in diameter and 5 to 7 µm thick. There are approximately 40 to 45 receptors per each pigment epithelial cell.
By the second week of life the rabbit photoreceptor layer is sufficiently differentiated to be functional. Most of the receptors in the rabbit retina are rods, but centrally there are atypical cones, found in greatest concentration in the area of the visual streak. The diameter of most of the rod outer segments is 1 µm, and most are 11 µm long; cones have twice the diameter. The receptor layer is thickest at the visual streak (about 40 µm).
Muller cells and the internal and external limiting membranes are rather prominent in the rabbit. Most of the nuclei in the outer nuclear layer are about 4 µm in diameter, and the thickest area of the layer is 30 µm at the visual streak. Most of the retina shows 9 to 10 rows of outer nuclei. The axonal extensions of the photoreceptors are ensheathed in the cytoplasm of the Muller cells to form the outer plexiform layer; the photoreceptor ends dilate to form synaptic expansions, which synapse with the bipolar cells in the inner areas of the layer. The outer plexiform layer has a uniform thickness of 8 to 9 µm.
With regard to the inner nuclear layer of the rabbit retina, the nuclei of the bipolar cellsare 8 to 9 µm in diameter on average, with a nucleolus from 5 to 6 µm, and the cells havea typical dendrite synapsing with the photoreceptor cells. The nuclei of the horizontal, amacrine andMuller cells are also in this layer. A cell that is peculiar to the rabbit is a large (37–38 µm), light-staining cell that sometimes has more than one nucleus. There are usually one three orfour layers of nuclei in the inner nuclear layer, and the layer is thickest (30 µm) in the visual streak and thins toward the ora ciliaris to 18 µm. Above the optic nerve it is 15 µm, and inferior to the medullary rays it is a mere 5 µm.
There is usually one layer of ganglion cells, with the exception of the visual streak, wherethere may be up to 3 to 4 layers. The presence of the largest number of ganglion cells and the concentration of cones in that zone correspond with the general mammalian pattern of retinal structure in areas of highest acuity. An interesting feature of the rabbit retina is the presence of a number of clearly multinucleated cells in the ganglion cell layer (Fig. 5 ).
Fig. 5.
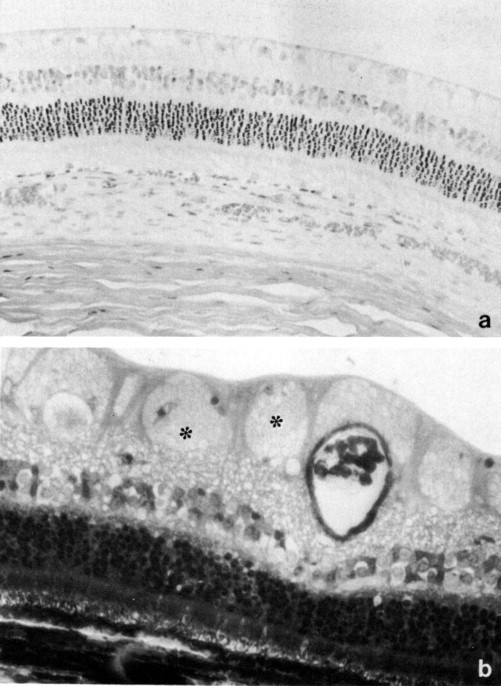
Rabbit retina. (a) Retinal cell layers from a region lacking medullary rays in an albino rabbit. Hematoxylin and eosin, × 250. (b) Retinal section through the medullary rays of a pigmented rabbit. Myelinated axons (*) are present on the retinal surface. Toluidine blue, × 400.
The rabbit retinal vascular pattern is merangiotic, characterized by the presence of blood vessels in a limited part of the retina, with the larger vessels being ophthalmoscopically visible. The retinal circulation is of ciliary origin; one large arteriole and venule rise on either side of theoptic disk and are often accompanied by smaller vessels which penetrate the retina near the optic disk. All the retinal vessels are confined to the area of the myelinated nerve fibers and run collateralto them. This area is about 15 to 18 mm broad and 1 to 2 mm high. The major retinal arterioles and venules can readily be visualized microscopically and have a diameter of 75 and 100 µm, respectively. The large vessels lie on the inner surface of the retina and give off superficial, deep, and peripheral capillaries; the remaining retina is virtually avascular (De Schaepdrijver et al., 1989). Because the rabbit has a merangiotic retina, it is a less than ideal choice for an experimental model to study retinal vascular diseases of humans.
O. Optic Nerve and Visual Pathways
The myelinated optic disk is ovoid, elongated in the horizontal plane, and has a large, deep physiological cup, owing at least in part to the absence of lamina cribrosa (Fig. 6 ). Blood supply is from branches of the ciliary vessels.
Fig. 6.
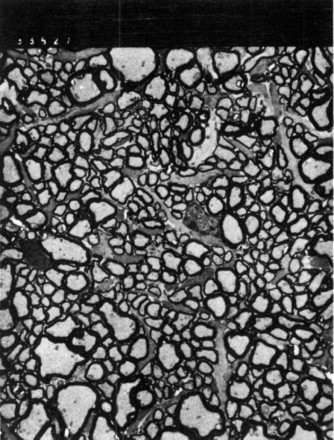
Small myelinated axons predominate in the optic disk. Transmission electron micrograph, × 2100.
The optic nerve is about 1.5 mm in diameter and has an average length of about 12 mm betweenthe optic chiasm and the globe. The average number of nerve fibers is 2,611,000. This is less than inhumans but is accounted for by the different rod and cone ratios and the size of the eye. The optic nerve leaves the globe at an acute ventral angle and passes through the optic foramen. The two optic nerves pass through a common cranial foramina and form the optic chiasm. There is nearly complete decussation or “crossing over” of the optic nerve fibers, with only a few ipsilateral nerve fibers.
The optic tracts pass caudally to the lateral geniculate nucleus; the majority of the axons terminate in the lateral geniculate nucleus as part of the pathway for conscious perception while the rest continue as part of the reflex pathway. The axons which terminate in the lateral geniculate nucleus synapse with ganglion cells whose fibers pass caudally in the optic radiation to the cerebral (visual) cortex, located on the lateral, caudal, and medial aspects of the occipital lobe. The remaining fibers pass over the lateral geniculate nucleus to terminate in the pretectalarea or rostral colliculus. The axons that enter the pretectal region synapse with ganglion cells in the nucleus of Edinger–Westphal (cranial nerve III). Some of the parasympathetic nerve fibers of the oculomotor nerve cross over to the nucleus of the other hemisphere, and the rest of the efferent fibers travel to the ciliary ganglion, via the short ciliary nerve, to terminate in the sphincter muscle. The rostral colliculus receives input from the optic nerve tract, visual cortex, and spinal cord. It influences the spinal cord and the nuclei of the oculomotor nerve, trochlear nerve, and facialnerve. The combination of these pathways function to integrate head, neck, and eye movements in response to visual stimuli.
The ciliary muscle, pupillary dilator muscle, third eyelid, and Muller's muscle all have sympathetic innervation. The sympathetic fibers originate from the hypothalamus and pass down the cervical spinal cord with preganglionic neurons in the first four segments of the thoracic spinal cord. The fibers pass cranially with the vagus nerve and terminate at the cranial cervical ganglion; postganglionic fibers distribute to the various structures.
III. SPONTANEOUS OCULAR DISEASE
Knowledge of spontaneously occurring ophthalmic diseases in the laboratory rabbit is important in the management and husbandry of research colonies. Such knowledge is also necessary to distinguish spontaneous from experimentally induced conditions and to identify potential models for ocular diseases in humans.
A. Eyelid Disorders
Entropion in young rabbits can occur as a primary or as a secondary condition arising from infection. The condition can be surgically corrected with either an everting mattress suture or by blepharoplasty (Fox et al., 1979). Blepharitis can be due to infectious agents including bacteria such as Pasteurella multocida or Staphylococcus aureus, which can infect the eyelids as well as the conjunctiva (Millichamp and Collins, 1986).
Viral-induced eyelid proliferations can result from infection by the rabbit myxoma and papilloma viruses (Ross, 1972). The papilloma virus is part of the papovaviridae and is a DNA virus transmitted by arthropod vectors. The cottontail rabbit in the midwestern United States is most frequently affected, although domestic rabbits are susceptible. Clinically, papillomatosis is characterized by horny warts that are normally found in hairless areas such as eyelids. They may undergo malignant transformation to squamous cell carcinoma.
B. Conjunctival Disorders
Conjunctivitis/dacryocystitis
Serous and purulent conjunctivitis is one of the more common ophthalmic diseases in the rabbit, Pasteurella multocida being the most frequent etiologic agent. Infection typically causes a subacute to chronic conjunctivitis with a mucopurulent discharge; the lacrimal sac may concurrently or singularly beinfected as well (Jones and Carrington, 1988). There are often other associated clinical signs of pansystemic involvement, and rabbits utilized for ophthalmic research are best obtained from and maintained in a Pasteurella-free environment.
Other bacteria, such as Staphylococcus aureus and Haemophilus sp. (Srivastava et al., 1986), as well as environmental factors such as hay dust can also cause conjunctivitis in rabbits (Buckley and Lowman, 1979). Cultures and sensitivities are usually indicated to diagnose this condition definitively.
Viral conjunctivitis is most commonly caused by rabbit myxoma virus. Conjunctivitis and edema of the eyelids are the most consistent ocular manifestations, and animals may develop a mucopurulent blepharoconjunctivitis.
Conjunctival hyperplasia
Rabbits are subject to a unique circumferential hyperplasia of the bulbar conjunctiva that appears to occur as a primary condition(Arnbjev, 1979). The conjunctiva folds on itself as it encroaches onto the cornea without adhering to it. The condition is usually bilateral, and recurrence following excisional conjunctivoplasty and/or cryosurgery is not uncommon (Fig. 7 ).
Fig. 7.
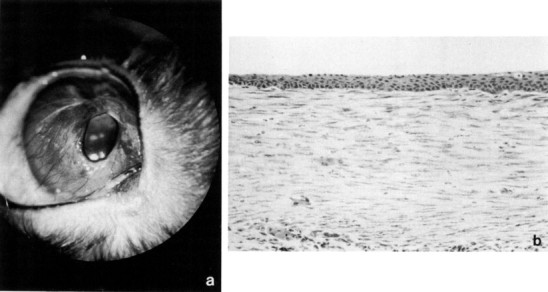
Conjunctival hyperplasia. (a) Circumferential encroachment of folded limbal conjunctiva over the corneal surface. (b) Hyperplastic conjunctiva, which overlies normal cornea (not shown), contains normal conjunctival epithelium and a hyperplastic stroma. Hematoxylin and eosin,× 100.
C. Corneal Disorders
Corneal dystrophy
Corneal dystrophy has been reported in the rabbit in two distinct forms. In the New Zealand White rabbit, two cases of unilateral cornealopacities beginning at the limbus circumferentially and progressing toward the center have been reported (Port and Dodd, 1983). The dystrophy appeared as a smooth, white opacity that was not associated with inflammation. The possibility of trauma, toxicity, or dietary etiologies was excluded. Histologically, the epithelium was a thickened and disorganized layer. The basement membrane and stroma showedno abnormalities.
Spontaneously occurring anterior corneal dystrophy was found in related 6-month-old Dutch Belted rabbits. The animals were either unilaterally or bilaterally affected. The focal, crescent-shaped epithelial and subepithelial opacities involved the central and paracentral cornea. Studies of the breeding colony indicated a familial predisposition. This anterior corneal dystrophy was found to involve epithelium, epithelial basement membrane, and anterior stroma (Moore et al., 1987).
Dietary lipid keratopathy
Lipid keratopathy and other systemic manifestations of high fat diets fed to rabbits have been described (Roscoe and Riccaroli, 1969; Gwin and Gelatt, 1977; Stock et al., 1985), and similar lesions occur in the Wantabe heritable hyperlipidemic (WHHL) rabbit (Garibaldi and Pecquet Goad, 1988). Corneal lipidosis, unlike dystrophies, will induce an inflammatory reaction with corneal neovascularization as wellas macrophage and neutrophil infiltration. Corneal lipid deposition typically occurs in the anterior stroma, basement membrane, and epithelium. The lipid keratopathy usually begins at the limbus either focally or circumferentially and can spread centrally. Dietary-induced lipid infiltration and inflammation have also been reported in the sclera, third eyelid, iris, ciliary body, and choroid.
Other lipid degenerations
Lipid degeneration characterized by the geographic deposition of presumably cholesterol crystals into the subepithelial stroma has been seen in rabbits as a nonspecific change following intraocular surgical procedures.
Viral keratitis
A solitary report exists describing a case of keratitis caused by a viral infection (McLeod and Langlenais, 1988). The rabbit or squirrel fibroma virus was the likely etiology in the rabbit, which was temporarily housed outdoors. The corneal mass was firm, pale yellow, and extended approximately 0.75 cm onto the nasal cornea of one eye.
D. Uveitis
Rabbits experimentally infected with the coronavirus of pleural effusion disease developed anongranulomatous anterior uveitis 3–6 days after exposure that persisted for 2–3 weeks (Fiedelius et al., 1978).
E. Cataracts
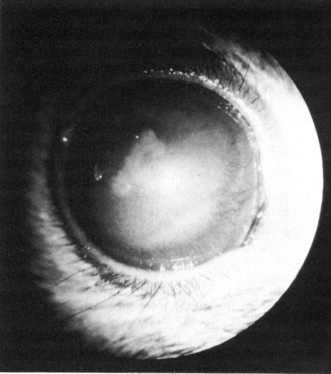
The overall incidence of spontaneous ophthalmic diseases in rabbit populations is low; the prevalence of spontaneously occurring cataracts based on research of rabbit fetuses is 3.6% (Weisse et al., 1974). One study reported 5 of 7 rabbits in one litter to have congenital nuclear and cortical cataracts that did not progress over time. Test matings failed to prove inheritability, and an in utero insult was the likely etiology (Gelatt, 1975). The author has observed sporadic nuclear cataracts, cataracts associated with posterior lenticonus (Fig. 8 ), and cataracts related to the persistence of the primary vitreous and tunica vasculosa lentis.
Fig. 8.
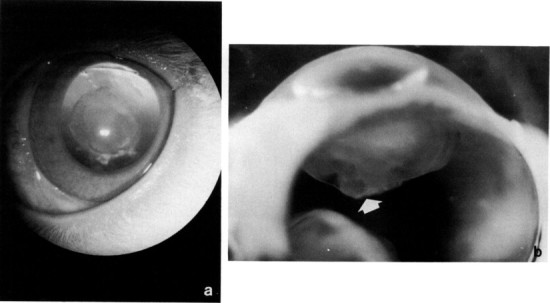
Posterior lenticonus in a young New Zealand White rabbit. (a) The condition was bilateral and appeared as a posterior capsular opacity. (b) Note posterior dimpling of the lens (arrow).
Spontaneous lens capsule rupture
An interesting condition seen not uncommonly in rabbits is a spontaneous rupture of the anterior lens capsule with a resultant zonal granulomatous lens-induced uveitis. The condition is usually unilateral; animals present with cataract, a small pupil fixed by posterior synechiae, a localized bulging forward of adjacent iris (misdiagnosis of an intraocular neoplasm is common), and a mild anterior uveitis. The lens-induced uveitis is characterized by zones of protein-laden macrophages, lymphocytes, and plasma cells, and the defect sealed by iris. Wolfer et al. (1993) have identified Encephalitozoon cuniculi within the lenses and speculate a casual relationship (Fig. 9 ). Lens extraction may be of benefit in preserving visual eyes.
Fig. 9.
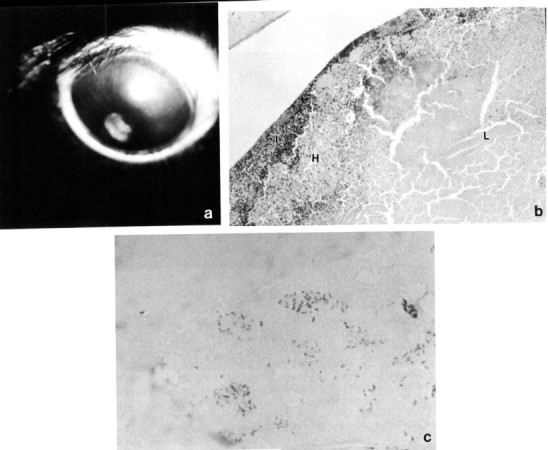
Spontaneous cataract and lens capsule rupture with associated granulomatous lens-induced uveitis. (a) Pupillary constriction, perilimbal corneal vascularization, and corneal edema are evident. (b) Lens cortex (L) is surrounded by acute and chronic inflammatory cells, a zone of histiocytes (H), and adherent infiltrated iris (I). (c) The condition has been associated with Encephalitozoon cuniculi organisms within the lens cortex. Gram stain, × 1000.
Courtesy Dr. J. Wolfer.
F. Glaucoma
Glaucoma occurs as an autosomal recessive trait and as a semilethal condition in the New Zealand rabbit. The disease process has similarities to congenital glaucoma in humans and is associated with goniodysgenesis. Affected animals have impaired aqueous outflow by 3 months of age, and by 6 months clinical changes of megalobulbus, increased corneal diameter, corneal edema and neovascularization, and variable increase in IOP are noted (Fig. 10 ). Morphologically, the uveal tissue inserts anteriorly into the cornea, and there is associated dysplasia or complete absence of trabecular meshwork and ciliary cleft (Tesluk et al., 1982).
Fig. 10.
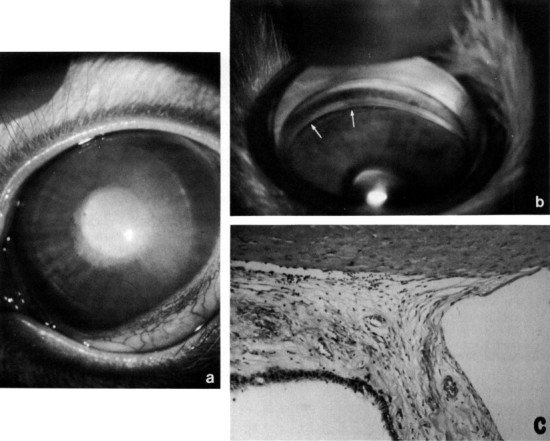
Congenital glaucoma (buphthalmos) in a New Zealand White rabbit. (a) Megaloglobus and diffuse corneal edema. Intraocular pressure elevation is variable. (b) Gonioscopy demonstrates a dysplastic pectinate ligament with only occasional flow holes (arrows). (c) The aqueous outflow pathways are totally dysplastic, without differentiation of pectinate ligament, ciliary cleft, ortrabecular meshwork (compare with Fig. 2). Hematoxylin and eosin, × 200.
G. Retinal Degeneration
An inbred pigmented rabbit strain was described with a retinal degeneration evident ophthalmoscopically as a retinal pigment epitheliopathy: scotopic ERG was affected, and histologically an outer retina degeneration was seen (Reichenbach and Baar, 1985). Although the authors argued for a potential model for retinitis pigmentosa, no heritability data were published, and the morphological evidence was weak.
H. Ocular Neoplasms
Intraocular neoplasms are uncommon; however, metastatic lymphosarcoma may occur. The collection of the author contains a rabbit globe with an adenocarcinoma, presumably of ciliary body epithelium origin, but the possibility of a primary tumor elsewhere with ocular metastases could not be excluded.
IV. EXPERIMENTALLY INDUCED OCULAR DISEASE
A. Infectious and Parasitic Disease
An astonishing array of infectious agents have been applied onto and within the rabbit eye in order to study disease pathogenesis and mechanisms, evaluate therapy, and examine local and systemic responses to infection.
Blepharitis
Rabbits immunized with cell wall antigens of Staphylococcus aureus developed a blepharitis, following topical challenge with viable bacteria, that appeared to be due to a hypersensitivity reaction (Mondino et al., 1987).
Conjunctivitis
Rabbits developed a follicular conjunctivitis 24 hr following topical application of rabbit retrovirus type 70 (Langford et al., 1986).
Keratoconjunctivitis
Preimmunized rabbits challenged with subconjunctival inoculation of Onchocerca volvulus developed conjunctivitis, limbal abscesses, and stromal keratitis; the response was more severe in animals immunized with live versus freeze-killed microfilaria (Duke and Garner, 1975). A model of phlyctenular keratoconjunctivitis was developed by exposing rabbits immunized against Staphylococcus aureus or its cell wall to viable organisms, with subsequent development of phlyctenulae and catarrhal infiltrates (Mondino et al., 1980).
Herpetic keratitis
The rabbit has become the standard experimental model for herpetic keratitis, and a voluminous literature exists dealing with the natural history, pathology, immunology, and treatment of herpes simplex virus (HSV) keratitis. Early studies utilized abrasion of the epithelial surface by one of a variety of methods followed by topical application of HSV; however, abrasion is not required, and simple inoculation and massage through the eyelids routinely cause the disease. A number of significant variables exist, including inoculation technique, virus strain, and breed of rabbit; in general, HSV type 1 (HSV-1) produces a mild to moderate conjunctivitis followed by punctate epithelial erosions by day 4 which coalesce to form a dendritic geographic ulcer by day 7; lesions resolve with minimal scarring and occasional vascularization by days 14–21. Type 2 HSV (HSV-2) tends to have a slightly longer incubation period and more prolonged and severe corneal sequelae. Latency within the trigeminal ganglion and reactivation stimulated by a variety of factors have been documented. Disciform (stromal) keratitis can be reproduced by injection of virus into the corneal stroma.
In general, the rabbit eye is more sensitive to HSV than the human eye, and immune response is an important player in disease pathogenesis; species differences between humans and rabbits in this regard are discussed elsewhere. The rabbit cornea is thus an imperfect paradigm of human HSV keratitis (Merriam, 1984).
Bacterial keratitis
The rabbit cornea, as well as those of other laboratory animal species, is extremely resistant to establishment of experimental infection with some of the more common human ocular pathogens, including Pneumococcus, Streptococcus epidermis, Moraxella lacunata, Nisseria gonococcus, Nocardia asteroides, and Mycobacterium fortuitium; successful rabbit modeling has been achieved with more virulent organisms, such as Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Shigella flexneri, and, with less success, Proteus mirabilis, Pasteurella multocida, Clostridium perfrigens, and Bacteroides fragilis. Studies have involved the application of either an inoculation of organisms or the isolated endo- or exotoxinsby scraping or scratching the corneal surface or by intrastromal injection (Barth, 1989); in most cases, the former situation more closely mimics the human disease.
Fungal keratitis
Francois and Rijsselaere (1974) demonstrated that topical subconjunctival administration of corticosteroids intensified the keratitis resulting from the intrastromal injection of Fusarium or Aspergillus spores. Pretreatment with subconjunctival injection of corticosteroids was required to induce a fungal keratitis in pigmented rabbits injected intralamellarly with actively germinating conidiafrom Fusarium solani; culture-positive ulcers were present at 2 and 3 weeks (Forster and Rebell, 1975). Ellison and Newmark (1973) and Singh et al. (1989) utilized similar models of immunosuppression to induce Aspergillus fumigatus infection; the latter work found the experimental disease to be more severe in albino rabbits. Agrawal et al. (1982) demonstrated an enhanced severity of Curralaria lunata keratitis with pretreatment with penicillin, streptomycin, or cortisone.
Acanthamoeba keratitis
Corneal infection with the ubiquitous protozoan Acanthamoeba is rare in humans and dependent on risk factors that have not yet been characterized. Disease cannot be predictably produced by the intrastromal injection of organisms in rabbit eyes and in vitro studies demonstrated scant binding of parasites to rabbit cornea (Niederkorn et al., 1992).
Herpes virus uveitis
Intravitreal injection of HSV-1 or HSV-2 induced a uveitis in rabbit eyes. Onset of the primary disease was gradual, whereas repeat infection of recovered eyes with either live or inactivated virus resulted in an immediate response, implying that secondary disease is mediated by immunologic mechanisms (Oh, 1984).
Herpetic retinochoroiditis
A rabbit model of neonatal HSV-2 infection was developed by the subcutaneous injection of the virus into newborn rabbits; animals died from systemic infection by day 5. Ocular lesions included retinal folds and necrosis and mild uveitis; HSV-2 was identified in the retina using fluorescent antibody techniques (Oh, 1984).
Borna disease chorioretinitis
Borna disease virus-infected rabbits developed a multifocal chorioretinitis that paralleled the clinical neurological signs. Histopathologically, a perivascular choroidal infiltrate and necrosis of retinal pigment epithelium and photoreceptors was seen. Virus reached the eye via the axons of the optic nerve (Krey et al., 1979).
Toxoplasmosis
Injection of Toxoplasma organisms into the subarachnoidal space of the rabbit eye produced a focal retinochoroiditis, vitritis, and mild anterior uveitis 4–6 days later that persisted for 4–6 weeks; resolution occurred with an atrophic hyperpigmented scar and with persistence of organisms. Recurrences could be induced using intravenous antilymphocytic serum, normal serum, or total body radiation (Nozik and O’Connor, 1970).
Histoplasmosis
Presumed ocular histoplasmosis in humans is a self-limiting disease characterized by minimal inflammation, hemorrhagic maculopathy, peripapillary scarring, and atrophic lesions most commonly seen in the peripheral fundus. Hematogenous infection with measured suspensions of Histoplasma capsulatum will produce a focal choroiditis in rabbit eyes; other described models produce acute anterior segment inflammation, vitreous clouding, or other features not characteristic of the human disease. Intracarotid injection of 25–50,000 G-89 strain organisms caused blepharoconjunctivitis and multifocal choroiditis within 1–6 days; anterior uveitis occurred in 70% of animals within 2–5 days. Development of lesions in contralateral eyes was less predictable and somewhat delayed. Organisms were present in acute lesions; spontaneous resolution with posterior synechiae, chorioretinal scars, and clearing of the organism occurred by the eighth week (O’Connor, 1975).
Bacterial endophthalmitis
Early studies with Staphylococcus aureus or Escherichia coli demonstrated that the anterior chamber of the rabbit resisted infectious agents better than the vitreous, and subsequently Staphylococcus aureus (the organism most frequently isolated from postoperative endophthalmitis in humans) and others (including Pseudomonas aeruginosa and Klebsiella oxytoca) were injected in rabbit vitreous to evaluate efficacy of type, dosage, and route of administration of antibiotics and to compare and contrast medical and surgical (vitrectomy) treatment as well as to study immune responsiveness. Intravitreal injection of the organisms (dependent on virulence and size of inoculum) induces a suppurative response that, untreated, destroyed eyes in as little as 20 hr (Yen-Lowder, 1989). Viable staphylococciwere present up to day 21 but could not be cultured by day 30, correlating with an increase in vitreous levels of IgG (Engstrom et al., 1991).
Fungal endophthalmitis
Ellison (1979) injected Candida albicans into rabbit eyes to study the efficacy of natamycin therapy. Hematogenous endophthalmitis resulted in a high percentage of rabbits injected intravenously with a suspension of Candida albicans; the disease was uni- or bilateral. Locally produced antibodies were demonstrated in aqueous humor (Bessieres et al., 1987).
B. Models of Noninfectious Ocular Inflammatory Disease
1. Scleritis
Sensitization with ovalbumin and Freund's complete adjuvant followed by the limbal injection of ovalbumin resulted in progressive scleral disease 90°–180° from the injectionsite (Hembry et al., 1979).
2. Conjunctivitis
A model of thimerosal antibody-induced immune complex hypersensitivity was created by exposing rabbits previously immunized to thimerosal conjugates via antigen-sensitized contact lenses (Baines et al., 1991). A disease process with similarities to ocular cicatricial pemphigoid occurred in neonatal Dutch Belted rabbits administered subconjunctival or intraperitoneal murine monoclonal antibodies against stratified squamous epithelial basement membrane (Roat et al., 1990).
Allergic conjunctivitis can be mimicked by the topical application of a selective mast cell degranulator, N-methyl-p-methoxyphenethylamine formaldehyde condensation product, which induced an eosinophilic infiltrate (Abelson et al., 1983). Subconjunctival injection of 10–1000 mg of platelet-activating factor induced a conjunctivitis that appeared to be mediated by peptidoleukotrienes (Muller et al., 1990).
3. Lens-Induced Uveitis
Immunization of both albino and pigmented rabbits with heterologous rat lens protein six times at 2-week intervals followed by surgical disruption of the anterior lens capsule induced an uveitis of variable intensity (Uusitalo et al., 1984).
4. Uveitis
Uveitis mechanisms and pharmacology have been studied using a variety of experimental induction methods ranging from paracentesis to neurogenic inflammation by the topical application of neutral formaldehyde (Krootila et al., 1989) to intravitreal injection of endotoxin (Williams and Peterson, 1987; Csukas et al., 1990). Intravitreal injection of bovine serum albumin in presensitized rabbits induced a bilateral uveitis, more severe in the injected eye, that was characterized by an acute elevation of intraocular pressure (IOP) (Uusitalo, 1984; Jamieson et al., 1989). Similar responses were obtained using human serum albumin (Verbey et al., 1988; Hoyng, 1989) or egg albumin (Bonnet et al., 1976). A single intravitreal injection of horse serum likewise caused an uveitis (Pankowska and Boj, 1990).
Uveitis manifested by alterations in vascular permeability resulted from the intravenous injection of bovine γ-globulin (BGG) into immunized animals or BGG–anti-BGG antigen–antibody complexes into normal rabbits. Ciliary processes were notably involved (Howes and McKay, 1975).
Experimental allergic uveitis (EAU) can be induced in rabbits by administering retina (Hempel et al., 1976) or soluble retinal S-antigen (Kalsaw and Wacker, 1986; Rao et al., 1986; Fricke et al., 1990) and complete Freund's adjuvant; 90% of animals develop a chorioretinitis characterized by spontaneous relapses. Immunization with bovine interphotoreceptor retinoid-binding protein (IRBP) will result in a uveoretinitis with macrophagic infiltrate, outer retinal degeneration, and breakdown of the blood–retinal barrier notable by day 18 (Eisenfeld et al., 1987).
5. Optic Neuritis
Sensitization of New Zealand albino rabbits to bovine cerebral white matter or myelin basic protein in Freund's complete adjuvant followed by challenge by intravenous brain-specific basicprotein or indifferent purified tuberculin induced a cell-mediated optic neuritis with demyelination in both groups of animals (Wisniewski and Bloom, 1975).
C. Models of Other Ophthalmic Disease Processes
1. Eyelid Papillomas
A wild strain of Shope papilloma virus was used to induce tumors in rabbits by dermal abrasion followed by both topical application and intradermal injection, in this case to study efficacy of immunotherapy (Smolin et al., 1981).
2. Meibomian Gland Dysfunction
Keratinization of meibomian glands may play a role in meibomian gland dysfunction and associated chronic blepharitis; a similar condition was induced in albino rabbits by the twice-daily topical application of 2% epinephrine over 6–12 months; 56% of eyes developed detectable lesions (Jester et al., 1989).
3. Keratoconjunctivitis Sicca
Partial keratoconjunctivitis sicca (KCS) was induced in rabbits by cauterizing the excretoryduct of the lacrimal gland; total KCS involved surgical removal of the third eyelid and Harderian gland. Both models developed increased tear osmolarity and decreases in conjunctival goblet cell densityand corneal epithelial glycogen and at 44 weeks postoperatively, visualized by Rose Bengal staining (Gilbard et al., 1989).
4. Band Keratopathy
Subepithelial calcification is a common human condition that occurs secondary to chronic anterior segment disease or systemic abnormalities of calcium and phosphate metabolism. The condition can be produced in rabbits by the intravitreal injection of 1 mg of ovalbumin followed in 12–14 days by large doses of intramuscular calciferal (600,000–900,000 units); by corneal freezing, KMnO4 perfusion of the anterior chamber, or corneal endothelial abrasion combined withtreatment with dihydrotachysterol (DHT); in vitamin D-deficient rabbits injected with intravitreal polyethylene sulfonate; and by either CO2 or argon laser treatment (Muirhead and Tomazzoli-Gerosa, 1984). Absence of Bowman's layer in the rabbit warrants consideration in interspecies correlation.
5. Models of Corneal Neovascularization
Corneal neovascularization is a clinical event with potential for both benefit and harm. In addition, the accessibility and visualizability in the lucent cornea provides a convenient model to study angiogenesis in general.
Investigations utilizing the rabbit to study vasoproliferative processes have induced corneal vascularization with a variety of techniques, including trephination (Thoft et al., 1979; Groden et al., 1983), thermal cautery (Ruben, 1981), silver nitrate application (Ausprunk et al., 1978), and suturing (Stock et al., 1985; Cherry and Garner, 1976), as well as the intrastromal injection of a variety of cells, tissues, and other substances (Moore and Sholley, 1985). Blood vessel extension from the limbusoccurs at the rate of 0.2–0.3 mm/day.
6. Models of Bullous Keratopathy
A rabbit model of endothelial dysfunction can be induced by mechanical abrasion or cryodestruction of the corneal endothelium; these techniques induce a transient edema that clears in days to weeks, a tribute to the somewhat unique regenerative capability of rabbit endothelium. Flushing the anterior chamber with 0.05% benzalkonium chloride results in corneal edema within 24 hr with regeneration in only a small percentage of experimental animals over a 2- to 3-month period. Interestingly, intraocular pressure was lowered in experimental eyes (Maurice and Perlman, 1977; Kohchi et al., 1980).
7. Model of Corneal Transplantation
The elegant work by Khodadoust (1968) and Khodadoust and Silverstein (1968) laid the foundation for the use of the rabbit as a model for corneal allografts, host immunomechanisms of graft rejection, and pharmacological manipulation of immunoresponsiveness. Mechanisms of host sensitization appear to be similar across species (Polack, 1972); in rabbits the rate of allograft rejection is low, but the phenomenon can be induced in a high percentage of animals by utilizing techniques to stimulate graft vascularization, including leaving silk sutures in place or focally cauterizing the recipient margin (Mannis and May, 1983). The remarkably regenerative endothelium of rabbits does not make it a good model for the study of endothelial cell responses to penetrating keratoplasty, the cat being preferred.
8. Models of Wound Healing
Much of the basic understanding of the process of healing of traumatic and surgical wounds has its foundation in rabbit studies, from observations on limbal cataract incisions to corneal alterations associated with keratorefractive surgery, including radial keratotomy and excimer laser cornealsculpting. Likewise, studies of corneal epithelial healing and attempts to modulate the same have extensively utilized this species. Epithelial wounds may be created mechanically or chemically; of import and technique-dependent is whether the epithelial basement membrane is removed (Pfister, 1975; Kuwabara et al., 1976; Haik and Zimny, 1971). Experimentally diabetic rabbits are more prone to epithelial basement membrane injury (Hatchell et al., 1982).
Attention has focused on the healing of corneoscleral filtration wounds used to manage glaucoma with the aim of exploring methods, primarily pharmacological, to keep them open and filtering (Gressel et al., 1984; Miller et al., 1985). A comparison of rabbit, dog, and cat sclerotomy and trabeculectomy wounds 1 week after surgery demonstrated a moreprominent myofibroblastic response in pigmented compared to nonpigmented rabbits (Peiffer et al., 1991). Normal healing occurs in about 17 days with a rather prominent inflammatory cell infiltrate (Miller et al., 1985).
9. Glaucoma
Corticosteroids induce elevation of IOP in humans, and numerous studies have attempted to exploit the rabbit as a model for this modest elevation of IOP with both mechanistic and therapeutic aims. Interestingly, variable results have been reported, with rabbit variables (age, sex, and breed), steroid type and dosage, and/or tonometric methodology likely responsible for disparities and inconsistencies.
Most consistent results have been reported utilizing repeated subconjunctival injections of either repository betamethasone (Bonami et al., 1978) or triamcinolone (Bonamiet al., 1978; Hester et al., 1987) twice weekly. Elevation of IOP up to 10 mm Hg occurred, with a lesser and delayed effect occurring in uninjected fellow eyes, suggesting a systemic as well as local effect. Other models of ocular hypertension in rabbits have included water loading (60 ml/kg increased IOP approximately 10 mm Hg for 30–120 min) (Seidenhamel and Dungan, 1974) and the intravenous infusion of 5% glucose (10–12 mm Hg increase of 40min duration with an infusion of 15 ml/kg) (Bonami et al., 1976).
Historically, experimental glaucoma has been produced in rabbits by several methods. Injection of 1% kaolin into the anterior chamber obstructs the outflow channels, and IOP reached 50–70mm Hg within 14 days (Voronina, 1954). Encircling the globe with constricting threads (Skotnicki, 1957) or bands (Flocks et al., 1959) caused high IOP (70–100 mm Hg) which decreased to 35–50 mm Hg within 48 hr; one-third of the eyes in one study developed endophthalmitis (Flocks et al., 1959). Polyethylene tubing threaded into the rabbit iridocorneal angle caused an increase in IOP within 24 hr that remained elevated for 6 months; loss of retinal ganglion cells occurred (Kupfer, 1962; Malik et al., 1970). Intraocular injection of methylcellulose will cause a transient elevation of IOP (Samis, 1962; Kazdan and MacDonald, 1963), whereas injection of cotton fragments induces a more prolonged elevation (deCarvalho, 1962). Injection of 75 units of α-chymotrypsin into the anterior chamber resulted in a chronic moderate elevation of IOP (up to 50 mm Hg) in 50% of both pigmented and nonpigmented rabbit eyes (Best et al., 1975). Subconjunctival injection of sclerosing agents such as phenol causedlong-term elevation of IOP (Malik et al., 1970; Luntz, 1966). Intracameral injection of autologous fixed red blood cells resulted in a chronic, variable, but dramatic elevation of IOP associated with a hemolytic inflammatory process; optic nerve changes were documented in the model (Peiffer et al., 1991) (Fig. 11 ). Perhaps the model that is most desirable utilized an argon laser to occlude the iridocorneal angle inpigmented rabbits; laser parameters included 200–275 50-µm spots, 0.1–0.2 sec, 1.2–1.6 W, and 32.8–78 joules of total energy; an IOP elevation of 28–50 mm Hg occurredin 50% of treated rabbits (Gherezghiher et al., 1986).
Fig. 11.
Experimental glaucoma in Dutch Belted rabbits. Megaloglobus, corneal edema, and episcleral vascular congestion are evident. Intraocular pressure elevations of 5–10 mm Hg were observed for up to 30 days postinjection.
10. Naphthalene Cataracts
Naphthalene-induced cataracts in rats and rabbits are a widely utilized model for oxidative change to the lens and have been studied from the perspectives of both basic and applied research. Oral dosing of pigmented rabbits with naphthalene (1 g/kg) every other day for 4 weeks induced variablecataractogenesis; for the first 2–3 weeks cortical vacuoles and granules developed consistently. Thereafter, one-third progressed to maturity while one-third remained relatively stable. This variability makes the model less than ideal for experimental cataract research (Selzer et al., 1991).
11. Sugar Cataracts
Rabbits will develop sugar cataracts in a fashion similar to that seen in other species. NewZealand rabbits fed a 50% galactose diet developed lens opacities after 5 days (Cheng et al., 1992).
12. Regeneration of Lens
Amphibians can regenerate lens from either corneal or iris epithelium; this ability is largely lost in higher vertebrates. Among mammals, rabbits possess perhaps a relatively unique ability to resynthesize lens following surgical removal; residual lens epithelial cells are the source of the process, which although not uncommon to a lesser extent in other mammals occurs in lagomorphs with particular exuberance. Regeneration occurs slowly (over months), and even though a new lens bow may form, fibers are irregular and the “new” lens is not transparent. Crystalline synthesis occurs, but proteins are somewhat altered from normal rabbit lens (Gwon et al., 1989). While of interest to the basic scientist, lens regeneration may be the bane ofthe researcher who uses the rabbit as a surgical model for cataract extraction and related procedures.
13. Cataracts Associated with Retinal Regeneration
Cataracts associated with spontaneous retinal degenerations have been described in humans, rats, and dogs, and the pathogenesis has been postulated to be due to the release of polyunsaturated lipid peroxidation products from photoreceptor membranes. Intravitreal injection of liposomes preparedfrom phospholipids containing lipid peroxidation products induced posterior subcapsular cataracts in Chinchilla rabbits (Babizhayer and Deyer, 1989).
14. Light-Induced Retinal Degeneration
In 1966 Noell and associates reported that chronic exposure of the rat to moderate-intensitylight resulted in retinal degeneration. A similar phenomenon was demonstrated in Dutch Belted rabbits, with the exposure threshold being considerably higher than that seen in rats (Lawwill, 1973).
15. Other Retinal Degenerations
Rabbit studies have contributed to our knowledge of the retinotoxic effects of intraocular iron; intravitreal placement in both pigmented and albino rabbits resulted in retinal degeneration, with photoreceptors primarily affected (Olsen et al., 1979; Burger and Klintwals, 1974). Retinotoxic effects and the pharmacokinetics of iodate have likewise been studied in rabbits(Regnaut, 1970).
16. Retinal Detachments, Proliferative Vitreoretinopathy, and Retinal Neovascularization
Experimental retinal hemorrhagic detachments in rabbits have been induced to simulate the early stages of human age-related macular degeneration. The technique included transvitreal subretinal injection of fresh autologous blood. Rather rapid irreversible outer retinal degeneration occurred (Glatt and Machemer, 1982).
Tractional retinal detachments were induced in pigmented rabbits simply by penetrating the sclera over the pars plana, extending the wound to 8 mm with scissors, excising prolapsed vitreous, and suturing the sclera with 8-0 silk. Eyes with intraoperative autologous blood injection were likely to develop retinal detachments, whereas those without blood injection did not (Cleary and Ryan, 1979).
The proliferation of preretinal membranes, which is an important component of retinal detachment in humans, has been induced in eyes of pigmented rabbits by the intravitreal injection of autologous retinal pigmented epithelium (Radtke et al., 1981). Retinal neovascularization, a precursor to vitreous hemorrhage and traction, which is a potentially blinding condition associated with diabetes mellitus, retinal vein occlusion, and retinopathy of prematurity, can be induced by the intravitreal injection of fibroblasts and hyaluronidase (Autoszyk et al., 1991).
Dutch rabbits were subjected to the subretinal injection of Hanks’ solution accompanied by either mechanical trauma to the RPE or argon laser photocoagulation to study mechanisms involvedin central serous chorioretinopathy (Negi and Marmor, 1984).
17. Models of Optic Nerve Atrophy and Regeneration
Axonal regeneration has been documented in adult rabbits following mechanical, thermal, or glaucomatous damage to the optic nerve; attempts were not successful in terms of functional reconnection to the brain (Eysel and Peichl, 1985; Schnitzer and Scherer, 1990; Peiffer et al., 1991).
18. Models of Ocular Tissue Responsiveness and Biocompatability
Rabbits have been utilized extensively in safety, irritability, and biocompatability studiesto provide data on how specific chemicals, substances, devices, or other biomaterials interact with the ocular tissues in order to predict tolerability and/or safety in humans. This interspecies extrapolation is predicated on the assumption that mechanisms of inflammation and tissue and immunoresponsiveness are similar; quite the contrary, dramatic species differences have been documented. The relatively labile and less predictable blood–ocular barrier of the rabbit has been mentioned earlier; the rabbit uvea responds to insult with exuberant fibrin exudation. A comparison of response to intravitreal glycosaminoglycans showed the rabbit to have a more severe anterior segment response and a less predictable posterior segment response, compared to the human and monkey (Peiffer, 1991). Thus, both quantitative and qualitative differences in ocular tissue responsiveness make the rabbit less thanan ideal model (Bito, 1984).
The Draize test, utilized for the testing of irritancy of cosmetics, toiletries, agricultural chemicals, occupational and environmental hazards, and certain therapeutic agents, especially ophthalmic formulations, is a technically simple, unsophisticated, semiquantitative method of evaluating the safety of the products as they relate to the external eye (Draize et al., 1944). The Draize test has been controversial both scientifically (rabbit response is exaggerated whencompared to human eyes and quite variable, and the test does not allow for finediscrimination) and ethically; alternatives including Draize modifications (low-volume testing) and in vitro techniques continue to be investigated.
Rabbit eyes have been used to study ocular tissue responses to suture material (Allen et al., 1982), the components of surgical sponges (cotton, collagen, and cellulose) (Peiffer et al., 1983), intraocular lenses (Cook et al., 1986) (Fig. 12 ), and vitreous replacement materials (Peiffer, 1991). Although interspecies differences need to be considered, appreciation of them has allowed for some valid extrapolation.
Fig. 12.
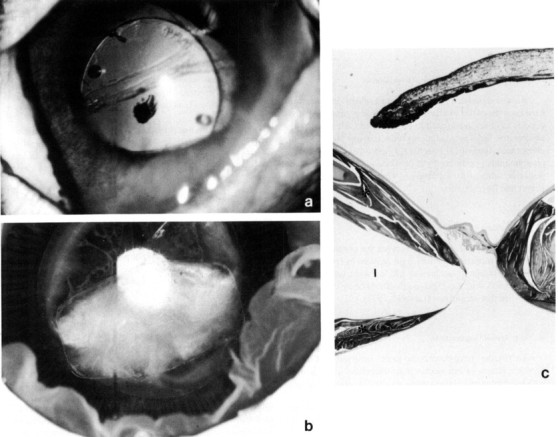
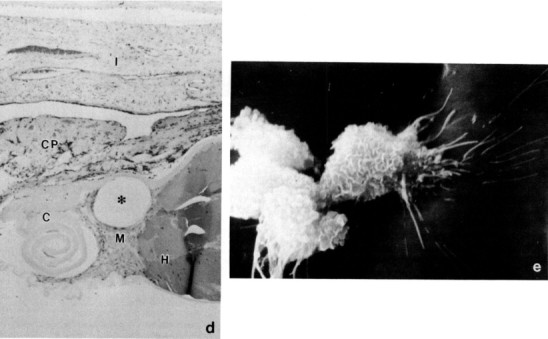
The rabbit as a model for intraocular lens implantation. (a) A polymethylmethacrylate implant (PMMA) with prolene haptics 1 month after implantation in the eye of a Dutch Belted rabbit; note the folds in the anterior lens capsule. (b) Secondary cataract formation (lens fiberregeneration) is seen around a decentered silicone intraocular lens. (c) Marked lens regeneration (*) has occurred around a silicone implant (I, implant cavity). Hematoxylin and eosin, × 4.(d) Epithelial hyperplasia (H) and fibrous metaplasia (M) around a PMMA haptic (*). I, Iris; CP, ciliary process; C, capsule. Hematoxylin and eosin, × 100. (e) Pseudophakic spindle cell precipitates with filopodia are evident on the surface of an intraocular lens removed after 3 months of implantation. SEM, × 200.
V. MODELS IN VISION RESEARCH
Research on the visual process has involved relatively sophisticated investigation of the organization of neurons, their electrophysiological responses, their relationship to one another, and their biochemistry at the level of retina, geniculate, cerebral cortex, and other visually related areas of the brain. Other species, because of relatively unique and/or experimentally desirable variantsof the above, have received more attention from neurophysiologists than has the rabbit.
Papers by van Hof and colleagues provided a qualitative visual psychophysical description of the Dutch Belted rabbit, and this breed has a strong claim to being the standard for vision research. Visual acuity is between 20´ and 40´ of cycle width (Vaney, 1980;van Hof, 1966, 1967; van Hof and Lagers van Haselen, 1973; van Hof and Steele, 1977).
An elegant overview of the “topographical relationships between the anatomy and physiology of the rabbit visual system” by Hughes (1976) summarizes work in this area prior to 1970 and was part of a symposium entitled, “Vision in the Rabbit,” held in Rotterdam in 1970 and published in Documenta Ophthalmogica, Volume 30, in 1971: rather than attempt to summarize these works here I refer the reader to that source.
Of note with regard to work on the retinal neurons is the starburst amacrine cell, which hasa distinctive and regular dendritic geometry with cholinergic input solely to ganglion cells (Famiglietti, 1985). Horizontal cells are larger and more densely populated in the periphery (Reichenbach andWohlarts, 1983); interplexiform cells are present (Oyster and Takahaski, 1977). The development of synapses within the outer and inner plexiform layer has been studied (Dachaux and Miller, 1981a,b).
Retinal ganglion cell morphology, distribution, and function have been investigated, and work on the rabbit eye has contributed to the understanding of the ganglion cell receptive field (Barlowand Levick, 1961; Barlow and Hill, 1963; Barlow et al., 1964; Oyster and Barlow, 1967; Oyster, 1968, Oyster et al., 1972; Vaney et al., 1981; Amthor et al., 1989; Pu and Amthor, 1990). A population of large ganglion cells similar to alpha cells of the cat retina has been described (Peichl et al., 1987).
Connections of the retina to the brain have been studied by a variety of morphological tracer, and degenerative studies; projections to the lateral geniculate, superior colliculus, and accessory optic system have been characterized, and the latter, consisting of the medial terminal nucleus, lateral terminal nucleus, and dorsal terminal nucleus, has been studied rather extensively in the rabbit (Sithi-Amoru, 1976; Hamasaki and Marg, 1962; Oyster et al., 1980; Giolli et al., 1984, 1985; Soodak and Simpson, 1988). Responses are similar to and thus input likely from direction-sensitive retinal ganglion cells. A horseradish peroxidase study showed the rabbit accessory optic system to consist of two fasciculi and lacking a dorsal terminal nucleus (Terubayashi and Fujisawa, 1988). Cortical wiring has also been studied (Giolli and Guthrie, 1971; Giolli et al., 1978; Schmolke and Viebahn, 1986), as have pathways for the pupillary light response in this species (Zuouc and Kiribuchi, 1985) and the innervation of the extraocular muscles (Evinger et al., 1987).
Electrophysiologically, the rabbit ERG b wave can be recorded between postnatal day 11 and 18 (Reuter et al., 1971). The visual evoked response is diurnally cyclical (Bobbert and Brandenburg, 1978).
VI. CONCLUSIONS
Unsleeping eyes, by nature raised to take the horizon in … (Hughes, 1976)
The eighteenth century poet who thus described the rabbit eye alluded to its uniqueness. Rabbits have served us well in our understanding of normal ocular form and function and the pathogenesisand management of ophthalmic disease, but likely not optimally. Species variation in the sensitivity and nature of the blood–ocular barriers has been addressed. Differences between breeds and strains and especially between nonpigmented and pigmented eyes have not been elaborated on but warrant consideration. There are demonstrated differences in drug distribution (Barza et al., 1979) and neural pathways (Oyster et al., 1987) between nonpigmented andpigmented eyes; empirical observations with regard to species-specific tissue responsiveness, hypertensive response to corticosteroids, and numerous other experimental conditions have been made. The albino eye is a reasonable model to study parameters in albinos, but the vast majority of humans (to which most modeling is extrapolated) have melanin in the iris, ciliary, and retinal pigment epithelium as well as the uvea. The continued use of the New Zealand White rabbit as a model for ophthalmic and visual experimentation is difficult to rationalize, and the use of pigmented rabbits will result in better scientific studies.
Footnotes
Supported in part by a Grant from Research to Prevent Blindness and the North Carolina Lions Foundation.
REFERENCES
- Abelson M.B., Udell I.J., Weston J.H. Conjunctival eosinophils in compound 48/80 rabbit model. Arch. Ophthalmoi. 1983;101:631–633. doi: 10.1001/archopht.1983.01040010631021. [DOI] [PubMed] [Google Scholar]
- Abrams K.L., Brooks D.E., Funk R.S., Theran P. Evaluation of the Schirmer tear test in clinically normal rabbits. Am. J. Vet. Res. 1990;51:1912–1913. [PubMed] [Google Scholar]
- Agrawal P.K., Lai B., Shukla P.K., Khan Z.A., Srivastava O.P. Clinical and experimental keratitis due to Currularia lunata (Wakker) Boedyn van aeria (Batista, Lima, and Vasconceles) Ellis. Sabouraudia. 1982;20:215–232. doi: 10.1080/00362178285380331. [DOI] [PubMed] [Google Scholar]
- Allen M.V., Jones E., Snow R., Peiffer R.L. Long-term study of iris sutures in rabbits. Ophthalmoi. Surg. 1982;13:733–736. [PubMed] [Google Scholar]
- Amthor F.R., Takahashi E.S., Oyster C.W. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J. Comp. Neurol. 1989;280:72–96. doi: 10.1002/cne.902800107. [DOI] [PubMed] [Google Scholar]
- Arnbjev J. Pseudoterygium in a pygmy rabbit. Vet. Med. Small Anim. Clin. 1979;79:737–738. [PubMed] [Google Scholar]
- Ausprunk D.H., Falterman K., Folkman J. The sequence of events in the regression of corneal capillaries. Lab. Invest. 1978;38:284–294. [PubMed] [Google Scholar]
- Autoszyk A.N., Gottleib J.L., Casey R.C., Hatchell D.L., Machemer R. An experimental model of preretinal neovascularization in the rabbit. Invest. Ophthalmoi. Visual Sci. 1991;32:46–52. [PubMed] [Google Scholar]
- Babizhayev M.A., Deyer A.I. Lens opacity induced by lipid peroxidation products as a model of cataract associated with retinal disease. Biochim. Biophys. Acta. 1989;1004:124–133. doi: 10.1016/0005-2760(89)90222-1. [DOI] [PubMed] [Google Scholar]
- Baines M.G., Cai F., Backman H.A. Ocular hypersensitivity to thimerosal in rabbits. Invest. Ophthalmoi. Visual Sci. 1991;32:2259–2265. [PubMed] [Google Scholar]
- Barlow H.B., Hill R.M. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- Barlow H.B., Hill R.M., Levick W.R. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J. Physiol. (London) 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H.B., Levick W.R. The mechanism of directionally sensitive units in the rabbit retina. J. Physiol. (London) 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth G. Animal models of bacterial corneal ulcers. In: Tabarra K.E., Cello R.M., editors. “Animal Models of Ocular Diseases”. Thomas; Springfield, Illinois: 1989. pp. 129–135. [Google Scholar]
- Barza M., Kane A., Baum J. Marked differences between pigmented and albino rabbits in the concentration of clindamycin in iris and choroid-retina. J. Infect. Dis. 1979;139:203–208. doi: 10.1093/infdis/139.2.203. [DOI] [PubMed] [Google Scholar]
- Bessieres M.H., Malecaze F., Linas M.D., Recco P., Fleutiaux S., Bec P., Seguela J.P. Local production of specific antibodies in the aqueous humor in experimental Candida endophthalia in rabbits. Ann. Biol. Clin. (Paris) 1987;45:651–656. [PubMed] [Google Scholar]
- Best M., Rabinovitz A.Z., Masket S. Experimental alphachymotrypsin glaucoma. Ann. Ophthalmoi. 1975;7:803–810. [PubMed] [Google Scholar]
- Bito L.Z. Species differences in the response of the eye to irritation and trauma: A hypothesis of divergence in ocular defense mechanisms and the choice of experimental animals for eye research. Exp. Eye Res. 1984;39:807–829. doi: 10.1016/0014-4835(84)90079-4. [DOI] [PubMed] [Google Scholar]
- Bobbert A.C., Brandenburg J. Diurnal changes in the rabbit's visual evoked potential. Int. J. Chrinobiol. 1978;5:307–325. [PubMed] [Google Scholar]
- Bonomi L., Tomazzoli L., Jaria D. An improved model of experimentally induced ocular hypertension in the rabbit. Invest. Ophthalmoi. 1976;15:781–784. [PubMed] [Google Scholar]
- Bonomi L., Perfetti S., Noya E., Belluci R., Tomazzoli L. Experimental corticosteroid ocular hypertension in the rabbit. Arch. Klin. Exp. Ophthalmoi. 1978;209:73–82. doi: 10.1007/BF00407840. [DOI] [PubMed] [Google Scholar]
- Bonnet P., Faure J.P., Le Douarec J.C. Standardization of an experimental immune uveitis in the rabbit for topical testing of drugs. Mod. Probl. Ophthalmoi. 1976;16:285–304. [PubMed] [Google Scholar]
- Bradley M.E., Lambert R.W., Lee L.M., Mircheff A.K. Isolation and subcellular fractionation analysis of acini from rabbit lacrimal glands. Invest. Ophthalmol. Visual Sci. 1992;33:2951–2965. [PubMed] [Google Scholar]
- Buckley P., Lowman D.M.R. Chronic non-infective conjunctivitis in rabbits. Lab. Anim. 1979;13:69–73. doi: 10.1258/002367779780943468. [DOI] [PubMed] [Google Scholar]
- Burger P.L., Klintworth G.K. Experimental retinal degeneration in the rabbit produced by intraocular iron. Lab. Invest. 1974;30:9–19. [PubMed] [Google Scholar]
- Burling K., Murphy C.J., de Silva Curiel J., Koblick P., Bellhorn R.W. Anatomy of the rabbit nasolacrimal duct and its clinical implications. Prog. Vet. Comp. Ophthalmoi. 1991;1:33–40. [Google Scholar]
- Cheng H.M., Kuan W.P., Garrido L., Xiong J., Chang C. High-resolution MR imaging of water diffusion in the rabbit lens. Exp. Eye Res. 1992;54:127–132. doi: 10.1016/0014-4835(92)90076-5. [DOI] [PubMed] [Google Scholar]
- Cherry P.M.H., Garner A. Corneal neovascularization treated with argon laser. Br. J. Ophthalmol. 1976;60:464–472. doi: 10.1136/bjo.60.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P.E., Ryan S.J. Experimental posterior penetrating eye injury in the rabbit. I. Method of production and natural history. Br. J. Ophthalmoi. 1979;63:306–311. doi: 10.1136/bjo.63.5.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.F. Comparative aspects of the intraocular fluids. In: Davson H., Graham L.T., editors. “The Eye”. Academic Press; New York and London: 1974. pp. 71–162. [Google Scholar]
- Cook C.S., Peiffer R.L., Mazzocco T.K. Clinical and pathologic evaluation of a flexible silicone posterior chamber lens design in a rabbit model. J. Cataract Refractive Surg. 1986;12:130–134. doi: 10.1016/s0886-3350(86)80027-x. [DOI] [PubMed] [Google Scholar]
- Csukas S., Paterson C.A., Brown K., Bhattacherjee P. Time course of rabbit ocular inflammatory response and mediator release after intravitreal endotoxin. Invest. Ophthalmol. Visual Sci. 1990;31:382–387. [PubMed] [Google Scholar]
- Dacheux R.E., Miller R.E. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. I. Receptors, horizontal, and bipolar cells. J. Comp. Neurol. 1981;198:307–326. doi: 10.1002/cne.901980209. [DOI] [PubMed] [Google Scholar]
- Dachaux R.E., Miller R.E. An intracellular electrophysiological study of the ontogeny of functional synapse in the rabbit retina. II. Amacrine cells. J. Comp. Neurol. 1981;198:327–334. doi: 10.1002/cne.901980210. [DOI] [PubMed] [Google Scholar]
- Davis E.A. The anatomy and histology of the eye and orbit of the rabbit. Trans. Am. Ophthalmol. Soc. 1929;27:401–441. [PMC free article] [PubMed] [Google Scholar]
- deCarvalho C.A. Histopathology of retina and optic nerve with experimental glaucoma. Arch. Ophthalmol. 1962;67:483–487. doi: 10.1001/archopht.1962.00960020483017. [DOI] [PubMed] [Google Scholar]
- De Schaepdrijver L., Simoens D., Lauwere H., De Geest J.P. Retinal vascular patterns in domestic animals. Res. Vet. Sci. 1989;47:34–42. [PubMed] [Google Scholar]
- Draize J.N., Woodard G., Calvery H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944;82:377–390. [Google Scholar]
- Duke B.O.L., Garner A. Reactions to subconjunctival inoculation of Onchocerca volvulus microfilaria in pre-immunized rabbits. Tropenmed. Parasitoi. 1975;26:435–448. [PubMed] [Google Scholar]
- Eisenfeld A.J., Bunt-Milam A.H., Saari J.C. Uveoretinitis in rabbits following immunization with interphotoreceptor retinoid-binding protein. Exp. Eye Res. 1987;44:425–438. doi: 10.1016/s0014-4835(87)80176-8. [DOI] [PubMed] [Google Scholar]
- Ellison A.C., Newark Effects of subconjunctival primaricias in experimental keratomycosis. Am. J. Ophthalmol. 1973:790–792. doi: 10.1016/0002-9394(73)90883-0. primaricin (II) [DOI] [PubMed] [Google Scholar]
- Ellison A.C. Intravenous effects of primaricin on myotic endophthalmitis. Ann. Ophthalmol. 1979:157–164. (11) [PubMed] [Google Scholar]
- Engstrom R.E., Jr., Mondino B.J., Glasgow B.J., Pitchekian H.H., Adamu S.A. Immune response to Staphylococcus aureus endophthalmitis in a rabbit model. Invest. Ophthalmol. Visual Sci. 1991;32:1523–1533. [PubMed] [Google Scholar]
- Evinger C., Graf W.M., Baker R. Extra and intracellular HRP analysis of the organization of extraocular motoneurons and internuclear neurons in the guinea pig and rabbit. J. Comp. Neurol. 1987;262:429–445. doi: 10.1002/cne.902620307. [DOI] [PubMed] [Google Scholar]
- Eysel U.T., Peichl L. Regenerative capacity of retinal axons in the cat, rabbit, and guinea pig. Exp. Neurol. 1985;88:757–766. doi: 10.1016/0014-4886(85)90086-x. [DOI] [PubMed] [Google Scholar]
- Famiglietti E.V. Starburst amacrine cells: Morphological constancy and systematic variation in the anisotropic field of rabbit retinal neurons. J. Neurosci. 1985;5:562–577. doi: 10.1523/JNEUROSCI.05-02-00562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledelius H., Bruun L., Fennestad K.L., Andersen S.R. Uveitis in rabbits with pleural effusion disease: Clinical and histopathological observations. Acta Ophthalmol. 1978;56:599–606. doi: 10.1111/j.1755-3768.1978.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Flocks M., Tsukahara I., Miller J. Mechanically induced glaucoma in animals. Am. J. Ophthalmol. 1959;48:11–18. doi: 10.1016/0002-9394(59)90236-3. [DOI] [PubMed] [Google Scholar]
- Forster R.K., Rebell G. Animal model of Fusarium solani keratitis. Am. J. Ophthalmol. 1975;79:510–515. doi: 10.1016/0002-9394(75)90629-7. [DOI] [PubMed] [Google Scholar]
- Fox J.G., Shaler M., Beaucage C.M., Smith M. Congenital entropion in a litter of rabbits. Lab. Anim. Sci. 1979;29:509–511. [PubMed] [Google Scholar]
- Francois J., Neetens A. Comparative anatomy of the vascular supply of the eye in vertebrates. In: Davson H., Graham L.T., editors. “The Eye”. Academic Press; New York and London: 1974. pp. 1–70. [Google Scholar]
- Francois J., Rijsselaere M. Corticosteroids and ocular mycoses: Experimental study. Ann. Ophthalmol. 1974:201–217. [PubMed] [Google Scholar]
- Fricke H.J., Schroder K.D., Metzner G., Schroder I., Haroske D., Tilgner S., Sych E.J., Klein S., Jager L. Mitigating effects of dialysable leukocyte extract (DLE) on the experimental allergic uveitis (EAU) of the rabbit. Allergol. Immunopathol. 1990;18:269–275. [PubMed] [Google Scholar]
- Garibaldi B.A., Pecquet Goad M.E. Lipid keratopathy in the Watanabe (WHHL) rabbit. Vet. Pathol. 1988;25:173–174. doi: 10.1177/030098588802500214. [DOI] [PubMed] [Google Scholar]
- Gelatt K.N. Congenital cataract in a litter of rabbits. J. Comp. Neurol. 1975;167:598–599. [PubMed] [Google Scholar]
- Gherezghiher T., March W.E., Nordquist R.E., Ross M.C. Laser induced glaucoma in rabbits. Exp. Eye Res. 1986;43:885–894. doi: 10.1016/0014-4835(86)90067-9. [DOI] [PubMed] [Google Scholar]
- Gilbard J.P., Rossi S.R., Heyda K.G. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalnuol. 1989;96(8):1180–1186. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- Giolli R.A., Guthrie M.D. Organization of the subcortical projections of visual areas I and II in the rabbit. An experimental degenerative study. J. Comp. Neurol. 1971;142:351–376. doi: 10.1002/cne.901420306. [DOI] [PubMed] [Google Scholar]
- Giolli R.A., Towns L.C., Takahashi T.T., Karamanlidis A.N., Williams D.D. An autoradiographic study of the projections of visual cortical area I to the thalamus, pretectum, and superior colliculus of the rabbit. J. Comp. Neurol. 1978;180:743–752. doi: 10.1002/cne.901800406. [DOI] [PubMed] [Google Scholar]
- Giolli R.A., Blanks R.H.I., Torigoe Y. Pretectal and brain stem projections of the medial terminal nucleus of the accessory optic system of the rabbit and rat as studied by anterograde and retrograde neuronal tracing methods. J. Comp. Neurol. 1984;227:228–251. doi: 10.1002/cne.902270208. [DOI] [PubMed] [Google Scholar]
- Giolli R.A., Blanks R.H.I., Torigoe Y., Williams D.D. Projections of medial terminal accessory optic nucleus, ventral tegmental nuclei, and substantia nigra of rabbit and rat as studied by retrograde axonal transport of horseradish peroxidase. J. Comp. Neurol. 1985;232:99–116. doi: 10.1002/cne.902320109. [DOI] [PubMed] [Google Scholar]
- Glatt H., Machemer R. Experimental subretinal hemorrhage in rabbits. Am. J. Ophthalmol. 1982;94:762–773. doi: 10.1016/0002-9394(82)90301-4. [DOI] [PubMed] [Google Scholar]
- Gressel M.G., Parrish R.K., Folberg R. 5-Flurouracil and glaucoma filtering surgery: I. An animal model. Ophthalmology. 1984;911:378–383. doi: 10.1016/s0161-6420(84)34277-4. [DOI] [PubMed] [Google Scholar]
- Groden L.R., Cassel G.H., Laibson P.R. The effect of corneal trephination on neovascularization. Ophthalmic Surg. 1983;14:954–956. [PubMed] [Google Scholar]
- Gwin M., Gelatt K.N. Bilateral ocular lipidosis in a cottontail rabbit fed an all-milk diet. J. Am. Vet. Med. Assoc. 1977;171:887–889. [PubMed] [Google Scholar]
- Gwon A., Enomoto H., Horowitz J., Garner M.H. Induction of de novo synthesis of crystalline lenses in aphakic rabbits. Exp. Eye Res. 1989;49:913–926. doi: 10.1016/s0014-4835(89)80016-8. [DOI] [PubMed] [Google Scholar]
- Haik B.G., Zimny M.L. Scanning electron microscopy of corneal wound healing in the rabbit. Invest. Ophthalmol. 1977;16:787–796. [PubMed] [Google Scholar]
- Hamasaki D., Marg E. Microelectrode study of the accessory optic tract in the rabbit. Am. J. Physiol. 1962;202:480–486. doi: 10.1152/ajplegacy.1962.202.3.480. [DOI] [PubMed] [Google Scholar]
- Hatchell D.L., Pederson H.J., Faculjak M.L. Susceptibility of the corneal epithelial basement membrane to injury in diabetic rabbits. Cornea. 1982;l:227–231. [Google Scholar]
- Hembry R.M., Playfair J., Watson P.G., Dingle J.T. Experimental model for scleritis. Arch. Ophthalmol. 1979;97:1337–1340. doi: 10.1001/archopht.1979.01020020079019. [DOI] [PubMed] [Google Scholar]
- Hempel E., Tilgner S., Meyer W., Schroder K.D. Experimental chorioretinitis in rabbits following injection of autologous retina in Freund's complete adjuvant. Exp. Eye Res. 1976;23:399–401. doi: 10.1016/0014-4835(76)90167-6. [DOI] [PubMed] [Google Scholar]
- Hester D.E., Trites P.N., Peiffer R.L., Petrow V. Steroid-induced ocular hypertension in the rabbit: A model using subconjunctival injections. J. Ocul. Pharmacol. 1987;3:185–189. doi: 10.1089/jop.1987.3.185. [DOI] [PubMed] [Google Scholar]
- Howes E.L., McKay D.G. Circulating immune complexes: Effects on ocular vascular permeability in the rabbit. Arch. Ophthalmol. 1975;93:365–370. doi: 10.1001/archopht.1975.01010020377013. [DOI] [PubMed] [Google Scholar]
- Hoyng E.J. The influence of topical prostaglandin in HSA-induced uveitis in the rabbit. Doc. Ophthalmol. 1989;73:35–41. doi: 10.1007/BF00174125. [DOI] [PubMed] [Google Scholar]
- Hughes A. Topographical relationships between the anatomy and physiology of the rabbit visual system. Doc. Ophthalmol. 1976;30:33–159. doi: 10.1007/BF00142518. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kiribuchi T. Cortical and subcortical pathways for pupillary reactions in rabbits. Jpn. J. Ophthalmol. 1985;29:63–70. [PubMed] [Google Scholar]
- Jamieson L., Meckoll-Brinck D., Keeler N. Characterized and predictable rabbit uveitis model for anti-inflammatory drug screening. J. Pharmacol. Methods. 1989;21:329–338. doi: 10.1016/0160-5402(89)90070-3. [DOI] [PubMed] [Google Scholar]
- Jester J.V., Nicolaides N., Kass-Paloolgyi F., Smith R.E. Mei-bomian gland dysfunction II: The role of keratinization in a rabbit model of MGD. Invest. Ophthalmol. Visual Sci. 1989;30:936–945. [PubMed] [Google Scholar]
- Jones S.M., Carrington S.D. Pasteurella dacryocystitis in rabbits. Vet. Rec. 1988;122:514–515. doi: 10.1136/vr.122.21.514. [DOI] [PubMed] [Google Scholar]
- Kalsow C.M., Wacker W.B. Rabbit ocular and pineal autoimmune response to retinal antigens. Curr. Eye Res. 1986;5:579–586. doi: 10.3109/02713688609015122. [DOI] [PubMed] [Google Scholar]
- Kazdan J.J., MacDonald R.K. Experimental angle block avoiding paracentesis reflex. Am. J. Ophthalmol. 1963;56:836. doi: 10.1016/0002-9394(65)93747-5. [DOI] [PubMed] [Google Scholar]
- Khodadoust A.A. Penetrating corneal transplantation in the rabbit. Am. J. Ophthalmol. 1968;66:899–905. doi: 10.1016/0002-9394(68)90820-9. [DOI] [PubMed] [Google Scholar]
- Khodadoust A.A., Silverstein A.M. Transplantation and rejection of individual cell layers of the cornea. Invest. Ophthalmol. 1968;8:180–195. [PubMed] [Google Scholar]
- Kohchi H., Goto M., Higashino I., Sakimoto T., Kitano S. Bullous keratopathy; endothelial cell changes in experimental corneal edema. Acta Sot: Ophthalmol. Jpn. 1980;84:1244–1254. [PubMed] [Google Scholar]
- Krey H.F., Ludwig H., Boschek C.B. Multifocal retinopathy in Borna disease infected rabbits. Am. J. Ophthalmol. 1979;87:157–164. doi: 10.1016/0002-9394(79)90135-1. [DOI] [PubMed] [Google Scholar]
- Krootila K., Syrjala M., Lehtosalo J.F., Uusitalo H. Platelets and polymorphonuclear leukocytes in experimental ocular inflammation in the rabbit eye. Grafe's Arch. Clin. Exp. Ophthalmol. 1989;227:465–469. doi: 10.1007/BF02172900. [DOI] [PubMed] [Google Scholar]
- Kupfer C. Studies of intraocular pressure II: The histopathology of experimentally increased intraocular pressure in the rabbit. Invest. Ophthalmol. 1962;1:474–479. [PubMed] [Google Scholar]
- Kuwabara T., Perkins D.G., Cogan D.G. Sliding of the epithelium in experimental corneal wounds. Invest. Ophthalmol. 1976;15:4–14. [PubMed] [Google Scholar]
- Langford M.P., Yin M.M., Barber J.C., Heard H.K., Stanton A.J. Conjunctivitis in rabbits caused by enterovirus type 70 (EV70) Invest. Ophthalmol. Visual Sci. 1986;27:915–920. [PubMed] [Google Scholar]
- Lawwill T. Effect of prolonged exposure of rabbit retina to low-intensity light. Invest. Ophthalmol. 1973;12:45–51. [PubMed] [Google Scholar]
- Luntz M.K. Experimental glaucoma in the rabbit. Am. J. Ophthalmol. 1966;61:665–680. doi: 10.1016/0002-9394(66)91202-5. [DOI] [PubMed] [Google Scholar]
- McLeod C.G., Jr., Langlinais P.C. Pox virus keratitis in a rabbit. Vet. Pathol. 1981;18:834–836. doi: 10.1177/030098588101800615. [DOI] [PubMed] [Google Scholar]
- Malik S.R.K., Choudhry S., Gupta A.K. Experimental production of glaucoma in rabbits. Am. J. Ophthalmol. 1970;69:1010–1015. doi: 10.1016/0002-9394(70)91049-4. [DOI] [PubMed] [Google Scholar]
- Mannis M.J., May W.N. Supression of the corneal allograft reaction: An experimental comparison of cyclosporin A and topical steroid. Cornea. 1983;2:95–101. [Google Scholar]
- Maurice D., Perlman M. Permanent destruction of the corneal endothelium in rabbits. Invest. Ophthalmol. Visual Sci. 1977;16:646–649. [PubMed] [Google Scholar]
- Merriam J.C. The rabbit model of herpetic keratitis. In: Tabarra K.F., Cello R.W., editors. “Animal Models of Ocular Diseases”. Thomas; Springfield, Illinois: 1984. pp. 23–37. [Google Scholar]
- Miller M.H., Joseph N.H., Ennis K.W., Grierson I., Hitchings R.A. An animal model of filtration surgery. Trans. Ophthalmol. Surg. U.K. 1985;104:893–897. [PubMed] [Google Scholar]
- Millichamp N.J., Collins B.R. Blepharoconjunctivitis associated with Staphylococcus aureus in a rabbit. J. Am. Vet. Med. Assoc. 1986;189:1154. [PubMed] [Google Scholar]
- Mondino B.J., Kowalski R., Ratajczak H.V., Peters J., Cutler S.B., Brown S.I. Rabbit model of phlyctenulosis and catarrhal infiltrates. Arch. Ophthalmol. 1980;99:891–895. doi: 10.1001/archopht.1981.03930010891022. [DOI] [PubMed] [Google Scholar]
- Mondino B.J., Caster A.I., Dethlefs B. A rabbit model of staphylococcal blepharitis. Arch. Ophthalmol. 1987;105:409–412. doi: 10.1001/archopht.1987.01060030129042. [DOI] [PubMed] [Google Scholar]
- Moore C.P., Dubeilzig K., Glaze M.B. Anterior corneal dystrophy in American Dutch Belted rabbits: Biomicroscopic and histologic findings. Vet. Pathol. 1987;24:28–33. doi: 10.1177/030098588702400106. [DOI] [PubMed] [Google Scholar]
- Moore J.W., Sholley M.M. Comparison of the neovascular effects of stimulated macrophages and neutrophils in autologous rabbit corneas. Am. J. Pathol. 1985;120:87–98. [PMC free article] [PubMed] [Google Scholar]
- Muirhead J.F., Tomazzoli-Gerosa L. Animal models of band keratopathy. In: Tabbara K.F., Cello R.W., editors. “Animal Models of Ocular Disease”. Thomas; Springfield, Illinois: 1984. pp. 221–233. [Google Scholar]
- Muller A., Meynier F., Bonne C. PAF-induced conjunctivitis in the rabbit is mediated by peptido-leukotrienes. J. Ocul Pharmacol. 1990;6:227–232. doi: 10.1089/jop.1990.6.227. [DOI] [PubMed] [Google Scholar]
- Negi A., Marmor M.F. Experimental serous retinal detachment and focal pigment epithelial damage. Arch. Ophthalmol. 1984;102:445–449. doi: 10.1001/archopht.1984.01040030359038. [DOI] [PubMed] [Google Scholar]
- Newton C., Hatchell D.L., Klintworth G.K., Brown C.F. Topical fibronection and corneal epithelial wound healing in the rabbit. Arch. Ophthalmol. 1988;106:1277–1279. doi: 10.1001/archopht.1988.01060140437048. [DOI] [PubMed] [Google Scholar]
- Niederkorn J.Y., Ubelaker J.E., McCulley J.P., Stewart G.L., Meyer D.R., Mellon J.A., Silvany R.E., He Y.G., Pidherney M., Martin J.H. Susceptibility of corneas from various animal species to in vitro bonding and invasion by Acanthomeba castellani. Invest. Ophthalmol. Visual Sci. 1992;33:104–112. [PubMed] [Google Scholar]
- Nozik R.A., O'Connor G.R. Studies on experimental ocular toxoplasmosis in the rabbit. Arch. Ophthalmol. 1970;84:788–791. doi: 10.1001/archopht.1970.00990040790019. [DOI] [PubMed] [Google Scholar]
- O'Connor G.R. Experimental ocular histoplasmosis. Int. Ophthalmol. Clin. 1975;15:93–104. doi: 10.1097/00004397-197501530-00014. [DOI] [PubMed] [Google Scholar]
- Oh J.O. Primary and secondary herpes simplex uveitis in rabbits. Surv. Ophthalmol. 1976;21:178–184. doi: 10.1016/0039-6257(76)90097-7. [DOI] [PubMed] [Google Scholar]
- Oh J.O. Herpes simplex retinochoroiditis in newborn rabbits. In: Tabarra K.F., Cello R.W., editors. “Animal Models of Ocular Diseases”. Thomas; Springfield, Illinois: 1984. pp. 39–51. [Google Scholar]
- Olsen K.J., Ehlers N., Schønheyder F. Studies on the handling of retinotoxic doses of iodate in rabbits. Acta Pharmacol. Toxicol. 1979;44:241–250. doi: 10.1111/j.1600-0773.1979.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Oyster C.W. The analysis of image motion by the rabbit retina. J. Physiol. (London) 1968;199:613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyster C.W., Barlow H.B. Direction selective units in rabbit retina: Distribution of preferred directions. Science. 1967;155:841–842. doi: 10.1126/science.155.3764.841. [DOI] [PubMed] [Google Scholar]
- Oyster C.W., Takahashi E. Interplexiform cells in rabbit retina. Prog. R. Soc. London B. 1977;197:477–484. doi: 10.1098/rspb.1977.0081. [DOI] [PubMed] [Google Scholar]
- Oyster C.W., Takahashi E., Collewijn H. Direction selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res. 1972;12:183–192. doi: 10.1016/0042-6989(72)90110-1. [DOI] [PubMed] [Google Scholar]
- Oyster C.W., Simpson J.I., Takahashi E.S., Soodak R.E. Retinal ganglion cells projecting to the rabbit accessory optic system. J. Comp. Neurol. 1980;190:49–61. doi: 10.1002/cne.901900105. [DOI] [PubMed] [Google Scholar]
- Oyster C.W., Takahashi E.S., Fry K.R., Lam D.M. Ganglion cell density in albino and pigmented rabbit retinas labeled with a ganglion cell specific monoclonal antibody. Brain Res. 1987;425:25–33. doi: 10.1016/0006-8993(87)90479-3. [DOI] [PubMed] [Google Scholar]
- Pankowska B., Boj E. Acute experimental uveitis caused by a single administration of heterologous blood protein to the vitreous body of rabbits. Klin. Oczna. 1990;92:41–43. [PubMed] [Google Scholar]
- Peichl et al. (1987).
- Peiffer R.L. The rabbit as an alternative model for the intraocular testing of viseoelastic substances. Prog. Vet. Comp. Ophthalmol. 1991;1:83–86. [Google Scholar]
- Peiffer R.L., Lipper S., Merritt J.C., Wright W.W., Jones B. Myofibroblasts in the healing of filtering wounds in rabbit, dog, and cat. Glaucoma. 1981;3:277–280. [Google Scholar]
- Peiffer R.L., Safrit H.D., White E., Eifrig D.E. Intraocular response to cotton, collagen, and cellulose in the rabbit. Ophthalmic Surg. 1983;14:582–587. [PubMed] [Google Scholar]
- Peiffer R.L., Petitto J.B., Bouldin T.W., Suits G., Nichols D.N., Walsh T.J. Effects of GM—rul ganglioside on the optic nerve and retina in experimental glaucoma. Glaucoma. 1991;13:58–61. [Google Scholar]
- Pfister R.R. The healing of corneal epithelial abrasions in the rabbit; a scanning electron microscope study. Invest. Ophthalmol. 1975;14:648–661. [PubMed] [Google Scholar]
- Polack F.M. Scanning electron microscopy of corneal graft rejection: Epithelial rejection, endothelial rejection, and formation of posterior graft membranes. Invest. Ophthalmol. 1972;11:1–14. [PubMed] [Google Scholar]
- Port C.D., Dodd D.C. Two cases of corneal epithelial dystrophy in rabbits. Lab. Anim. Sci. 1983;33:587–588. [PubMed] [Google Scholar]
- Prince J.H. “The Rabbit in Eye Research.”. Thomas; Springfield, Illinois: 1964. [Google Scholar]
- Prince J.H., Diesem D.D., Eglitis I., Ruskell G.I. “Anatomy and Histology of the Eye and Orbit in Domestic Animals,”. 1st Ed. Thomas; Springfield, Illinois: 1960. pp. 260–297. [Google Scholar]
- Pu M.L., Amthor F.R. Dendritic morphologies of retinal ganglion cells projecting to the lateral geniculate nucleus in the rabbit. J. Comp. Neurol. 1990;302(3):675–693. doi: 10.1002/cne.903020320. [DOI] [PubMed] [Google Scholar]
- Radtke N.D., Tano Y., Chandler D., Machemer R. Simulation of massive periretinal proliferation by autotransplantation of retinal pigment epithelial cells in rabbits. Am. J. Ophthalmol. 1981;91:76–87. doi: 10.1016/0002-9394(81)90352-4. [DOI] [PubMed] [Google Scholar]
- Rao N.B., Braun C.J., Marak G.E. Ultrastructural analysis of experimental allergic uveitis in rabbits. Ophthalmic Res. 1986;18:15–20. doi: 10.1159/000265408. [DOI] [PubMed] [Google Scholar]
- Regnaut E.R. Vitreous hemorrhage. An experimental study III: Experimental degeneration of the rabbit retina induced by hemoglobin injection into the vitreous. Arch. Ophthalmol. 1970;83:470–474. [PubMed] [Google Scholar]
- Reichenbach A., Baar U. Retinitis-pigmentosa-like tapetoretinal degeneration in a rabbit breed. Doc. Ophthalmol. 1985;60:71–78. doi: 10.1007/BF00164570. [DOI] [PubMed] [Google Scholar]
- Reichenbach A., Wohlarts F. Horizontal cells of the rabbit retina: Some quantitative properties revealed by selective staining. Z. Mikorsk. Anat. Forsch. 1983;97:240–249. [PubMed] [Google Scholar]
- Reuter J.H., Legen C.P.J.J.M.M., van der Mark E., van Hof M.O. The electroretinogram in normal and light-deprived rabbits. Doc. Ophthalmol. 1971;30:349–361. doi: 10.1007/BF00142531. [DOI] [PubMed] [Google Scholar]
- Roat M.I., Alstadt S.P., Betts Carpenter A., Sundar Raj N., Thofx R.A. Antibasement membrane antibody-mediated experimental conjunctivitis. Invest. Ophthalmol. Visual Sci. 1990;31:168–175. [PubMed] [Google Scholar]
- Roscoe H.G., Riccardi B.A. Phospholipid changes in the eye and aorta of cholesterol-fed rabbits. J. Atheroscler. Res. 1969;10:123–130. doi: 10.1016/s0368-1319(69)80091-8. [DOI] [PubMed] [Google Scholar]
- Ross J. Zoological and wildlife review: Myoxomatosis in the rabbit. Br. Vet. J. 1972;128:172–176. doi: 10.1016/s0007-1935(17)37032-x. [DOI] [PubMed] [Google Scholar]
- Ruben M. Corneal vascularization. Int. Ophthalmol. Clin. 1981;21:27–38. [PubMed] [Google Scholar]
- Salistad D.M., Peiffer R.L. Specular microscopic observations of the corneal endothelium in the normal rabbit. Lab. Anim. 1981;15:393–395. doi: 10.1258/002367781780952924. [DOI] [PubMed] [Google Scholar]
- Samis W.D. An experimental method to produce angle block in rabbits and the use of phospholine after angle block. Am. J. Ophthalmol. 1962;54:1089–1091. doi: 10.1016/0002-9394(62)94347-7. [DOI] [PubMed] [Google Scholar]
- Schmolke C., Viebahn C. Dendrite bundles in lamina II/III of the rabbit neocortex. Anat. Embryol. 1986;173:343–348. doi: 10.1007/BF00318917. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Scherer J. Microglial responses in the rabbit retina following transection of the optic nerve. J. Comp. Neurol. 1990;302:779–791. doi: 10.1002/cne.903020410. [DOI] [PubMed] [Google Scholar]
- Seidenhamel R.J., Dungan K.W. Characteristics and pharmacologic utility of an intraocular pressure (IOP) model in unanesthetized rabbits. Invest. Ophthalmol. 1974;13:319–322. [PubMed] [Google Scholar]
- Selzer M., Wegener A., Hockwin O. Regional enzyme profiles in rabbit lenses with early stages of naphthalene cataract. Lens Eye Toxic. Res. 1991;8:415–430. [PubMed] [Google Scholar]
- Singh S.M., Khan R., Sharma S., Chatteriee P.K. Clinical and experimental mycotic corneal ulcer cased by Aspergillus fumigatus and the effect of oral ketaconazole in the treatment. Mycopathologica. 1989;106:133–141. doi: 10.1007/BF00443054. [DOI] [PubMed] [Google Scholar]
- Sitthi-Amoru C. Evidence of an extended representation of the visual field in the superior colliculus of the rabbit eye (Oryctolagus cuniculus) Indian J. Physiol Pharmacol. 1976;20:187–196. [PubMed] [Google Scholar]
- Skotnicki H.C. Experimental glaucoma. Clin. Oczna. 1957;27:27–36. [PubMed] [Google Scholar]
- Smolin G.M., Okumoto M., Friedlaender M., Cyr R. Immunotherapy for rabbit lid papillomas. Ann. Ophthalmol. 1981;13:101–103. [PubMed] [Google Scholar]
- Soodak R.E., Simpson J.I. The accessory optic system of the rabbit. I. Basic visual response properties. J. Neurophysiol. 1988;60:2037–2054. doi: 10.1152/jn.1988.60.6.2037. [DOI] [PubMed] [Google Scholar]
- Srivastava K.K. Characterization of a Haemophilius sp. isolated from a rabbit with conjunctivitis. Lab. Anim. Sci. 1986;36:291–293. [PubMed] [Google Scholar]
- Stock E.L., Mendelsohn A.D., Lo G.G., Ghosh S., O'Grady R.B. Lipid keratopathy in rabbits: An animal model system. Arch. Ophthalmol. 1985;103:726–730. doi: 10.1001/archopht.1985.01050050118030. [DOI] [PubMed] [Google Scholar]
- Terubayashi H., Fujisawa H. The accessory optic system of the rabbit, cat, dog, and monkey: A whole HRP study. Anat. Embryol. 1988;177:285–295. doi: 10.1007/BF00315835. [DOI] [PubMed] [Google Scholar]
- Tesluk G.C., Peiffer R.L., Brown D. A clinical and pathological study of inherited glaucoma in New Zealand White rabbits. Lab. Anim. 1982;16:234–239. doi: 10.1258/002367782780891679. [DOI] [PubMed] [Google Scholar]
- Thoft R.A., Friend J., Murphy H.S. Ocular surface epithelium and vascularization in rabbits. Invest. Ophthalmol. Visual Sci. 1979;18:85–92. [PubMed] [Google Scholar]
- Tripathi R.C. Comparative physiology and anatomy of the outflow pathway. In: Davson H., Graham L.T., editors. “The Eye”. Academic Press; New York and London: 1974. pp. 163–356. [Google Scholar]
- Uusitalo H. An acute ocular inflammatory reaction induced by intravitreal bovine serum albumin in presensitized rabbits: The effect of phentolamine. Acta Ophthalmol. 1984;62:636–642. doi: 10.1111/j.1755-3768.1984.tb03976.x. [DOI] [PubMed] [Google Scholar]
- Uusitalo H., Uusitalo R., Palkama A. Decreased ecto-5-nucleotidase activity in rabbit lymphocytes during experimental lens-induced uveitis. Curr. Eye Res. 1984;3:407–415. doi: 10.3109/02713688408997227. [DOI] [PubMed] [Google Scholar]
- Vaney D.I. The grating acuity of the wild European rabbit. Vision Res. 1980;20:87–89. doi: 10.1016/0042-6989(80)90146-7. [DOI] [PubMed] [Google Scholar]
- Vaney D.I., Levick W.R., Thibos L.W. Rabbit retinal ganglion cells. Receptive field classification and axonal conduction properties. Exp. Brain Res. 1981;44:27–33. doi: 10.1007/BF00238746. [DOI] [PubMed] [Google Scholar]
- van Hof M.W. Discrimination between striated patterns of different orientation in the rabbit. Vision Res. 1966;6:89–94. doi: 10.1016/0042-6989(66)90016-2. [DOI] [PubMed] [Google Scholar]
- van Hof M.W. Visual acuity in the rabbit. Vision Res. 1967;7:749–751. doi: 10.1016/0042-6989(67)90037-5. [DOI] [PubMed] [Google Scholar]
- van Hof M.W. Interocular transfer in the rabbit. Exp. Neurol. 1970;26:103–108. doi: 10.1016/0014-4886(70)90091-9. [DOI] [PubMed] [Google Scholar]
- van Hof M.W., Lagers van Haselen G.C. The retinal fixation area in the rabbit. Exp. Neurol. 1973;41:218–221. doi: 10.1016/0014-4886(73)90192-1. [DOI] [PubMed] [Google Scholar]
- van Hof M.W., Steele R.I. Binocular vision in the rabbit. Physical Behav. 1977;19:121–128. doi: 10.1016/0031-9384(77)90168-8. [DOI] [PubMed] [Google Scholar]
- Van Horn D.L., Sendele D.D., Scideman S., Buco P.J. Regenerative capacity of corneal endothelium in rabbit and cat. Invest. Ophthalmol. 1977;16:597–613. [PubMed] [Google Scholar]
- Verbey N.L., van Haeringen N.J., de Jong P.T. The effects of 3-isobutyl methylxanthine on experimentally provoked uveitis in rabbits. Curr. Eye Res. 1988;7:557–561. doi: 10.3109/02713688809031811. [DOI] [PubMed] [Google Scholar]
- Voronina E.C. Production of experimental glaucoma and its clinical aspects. Biull. Eksp. Biol. Med. 1954;37:30–32. [PubMed] [Google Scholar]
- Weisse I. Spontane congenitale katarakte bei ratte, maus, und kaninehen. Arch. Toxicol. 1974;32:199–207. doi: 10.1007/BF00318434. [DOI] [PubMed] [Google Scholar]
- Williams R.N., Paterson C.B. The influence of topical corticosteroid therapy upon polymorphonuclear leukocyte distribution, vascular integrity, and ascorbate levels in endotoxin induced inflammation of the rabbit eye. Exp. Eye Res. 1987;44:191–198. doi: 10.1016/s0014-4835(87)80003-9. [DOI] [PubMed] [Google Scholar]
- Wisniewski H.M., Bloom B.R. Experimental allergic optic neuritis (EAON) in the rabbit: A new model to study primary demyelinating diseases. J. Neurol. Sci. 1975;24:257–263. doi: 10.1016/0022-510x(75)90237-3. [DOI] [PubMed] [Google Scholar]
- Wolfer J., Grahn B., Wilcock B.P., Percy D. Phacoclastic uveitis in the rabbit. Prog. Vet. Comp. Ophthalmol. 1993;3:92–97. [Google Scholar]
- Yen-Lowder C. Antibiotics in the treatment of experimental bacterial endophthalmitis. In: Tabarra K.F., Cello R.W., editors. “Animal Models of Ocular Diseases”. Thomas; Springfield, Illinois: 1989. pp. 111–120. [Google Scholar]


