Peritonitis in cats is a serious problem that can often prove fatal. Mild or non-specific clinical signs can delay definitive diagnosis and treatment. Therefore awareness of the possibility of peritonitis, careful examination, and appropriate diagnostic tests are necessary for a timely diagnosis. Even with prompt and aggressive treatment the prognosis for survival is still guarded.
Surgical anatomy
The peritoneum is a closed cavity that contains all of the abdominal organs except for the kidneys and the adrenal glands. The parietal peritoneum covers the abdominal wall and diaphragm. The visceral peritoneum covers the abdominal organs (Fig. 26-1 ). The peritoneum consists of a single layer of mesothelial cells covering a basement membrane.1 The peritoneal mesothelial cells have the same embryological origin as vascular endothelial cells2 and produce surfactant that acts as a lubricant.3 The basement membrane is a fibroelastic tissue containing glycosylated proteins, mast cells, macrophages, and lymphocytes and it covers a well-defined network of elastic fibers. The basement membrane is absent in parts of the diaphragm, omentum, and mesentery.1 The absence of the basement membrane in the diaphragm allows large gaps between the mesothelial cells (‘stomata’) to communicate directly with underlying lymphatic channels, facilitating absorption of fluid and particulate matter.
Figure 26-1.
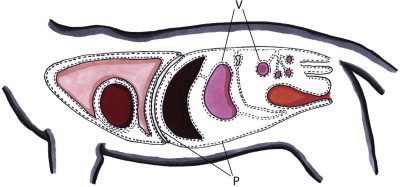
Illustration of the peritoneum in the cat. P, parietal peritoneum; V, visceral peritoneum.
Physiology
The majority of the peritoneum behaves as a semi-permeable membrane allowing bidirectional diffusion of water and solutes. A small amount of serous fluid, formed by the action of Starling forces across the peritoneal capillaries, is normally present in the space between the parietal and visceral peritoneal surfaces. The fluid has a low protein concentration (<3 g/dL protein) and resembles an ultrafiltrate of plasma. In humans, normal peritoneal fluid has antibacterial activity against Gram positive and Gram negative bacteria.4 A normal peritoneal cavity contains approximately 300 cells/mm3, which are largely lymphocytes and macrophages.
Fluid injected into a normal peritoneal cavity is dispersed throughout the entire peritoneal space over a period of between 15 minutes and two hours.5 Contraction of the diaphragm promotes a cranial flow of peritoneal fluid.6 Relaxation of the diaphragm generates a sub-atmospheric pressure that draws fluid and small particles into the large openings between the peritoneal mesothelial cells covering the diaphragm (‘stomata’) that communicate with the underlying lymphatic system. Contraction of the diaphragm closes the stomata and forces fluid into the lymphatic system.1 This lymphatic system transports fluid and other absorbed material into the internal thoracic (or ‘parasternal’) mediastinal lymphatic system and mediastinal and parasternal lymph nodes, then to the thoracic duct system, and eventually to the systemic venous circulation.7 Fluid is normally also absorbed through other sites in the peritoneum, including the omentum.1, 8
Pathophysiology
A physical, chemical, or microbiological injury to the peritoneum activates local defense mechanisms. These defense mechanisms fall into three broad categories (Table 26-1 ).9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Table 26-1.
Local defense mechanisms of the peritoneum
| Defense mechanism | Pathogenesis |
|---|---|
| Removal of bacteria and particulate matter from the peritoneal cavity | Bacteria and small particles are rapidly absorbed by the diaphragm through the stomata |
| Immune cells and systems designed to kill bacteria |
|
| Sequestration of infection locally. |
|
The inflammatory response within the peritoneal cavity and the associated increased peritoneal vascular permeability result in the effusion of a large volume of protein-rich fluid into the peritoneal space. This results in a substantial loss of intravascular volume. In addition, systemic activation of the inflammatory and coagulation cascades causes further loss of fluid and protein and often disregulation of vascular tone and decreased tissue perfusion.19
Systemically, macrophages and other cells in the immune system respond to endotoxin and other bacteria-derived molecules (‘pathogen-associated molecular patterns’ or PAMPS) and endogenous mediators released from dead or dying cells20 through Toll-like receptors to initiate a complex systemic inflammatory response (SIRS). The SIRS response is referred to as ‘sepsis’ when initiated by bacteria. Binding of endotoxin from Gram negative bacteria to the lipopolysaccharide (LPS)-binding protein on macrophages stimulates the release of multiple cytokines.21 The mechanisms by which Gram positive bacteria induce systemic inflammation are less well characterized but several substances they contain, including peptidoglycan, teichoic acid, and their polysaccharide-hyaluronic acid capsules have pro-inflammatory effects, including the production of cytokines (tumor necrosis factor [TNF], interleukin-1 [IL1]) and nitric oxide.22, 23
The macrophage is the main cell initiating the systemic response to bacteria and proteins from damaged cells.19 IL1 and TNFα are rapidly released after macrophage stimulation and promote activation of many of the inflammatory and coagulation cascades. A detailed discussion of cytokines involved in sepsis is beyond the scope of this chapter and the reader is referred elsewhere for a more detailed review.19
The net result of sepsis in peritonitis is a decrease in intravascular volume, decreased cardiac output as a result of decreased preload, abnormal vascular tone, and vascular thrombosis, leading to maldistribution of blood flow, poor tissue perfusion, and organ damage. For more information on shock and SIRS the reader is referred to Chapter 3.
Gastrointestinal tract leakage
The gastrointestinal tract is the most frequent source of peritoneal contamination in cats.24 Usually, only two or three bacterial species are grown in culture, although numerous species of bacteria are found in the gastrointestinal tract. Polymicrobial infections present a significant problem because of bacterial synergism. This appears to be most important in mixed infections with Gram negative and anaerobic bacteria. Anaerobic bacteria alone (such as Bacillus fragilis) seem to cause minimal pathology in experimental models. However, when combined with Gram negative enteric bacteria, the resulting infection is considerably more severe.25 Ingesta, exfoliated cells, blood, gastric mucin, and bile salts are adjuvant substances that worsen peritonitis. Blood is an excellent growth medium for bacteria and hemoglobin interferes with chemotaxis and phagocytosis. Bile salts destroy peritoneal mesothelial cells and inhibit neutrophil function.
Urinary tract leakage
Uroperitoneum occurs when the bladder or peritoneal urethra is damaged by external or iatrogenic trauma. Severe trauma to the kidneys or ureters often damages the peritoneum, allowing urine to leak into the abdominal cavity from the retroperitoneal space. Urine is hyperosmotic and pulls fluid from the vascular space into the peritoneal cavity, resulting in dehydration. Failure to excrete urine also causes acidosis and progressively worsening hyperkalemia. Severe hyperkalemia is truly life threatening. For the effects and treatment of hyperkalemia see Chapters 1 and 3.
Diagnosis and general considerations
The historical and physical examination findings of cats with peritonitis are usually associated with the SIRS syndrome. The historical findings most commonly reported by owners tend to be non-specific. However, a history of trauma or penetrating abdominal wounds may increase the clinician's suspicion of peritonitis. Gastrointestinal signs may be associated with primary gastrointestinal problems (i.e., foreign body) or may solely be due to peritonitis. An icteric animal may suggest primary biliary disease or associated pancreatitis. In both primary (not associated with feline infectious peritonitis [FIP]) and secondary bacterial peritonitis in the cat, common historical findings include lethargy, vomiting, anorexia, and diarrhea.26 Weight loss is another clinical sign reported that appears to be more frequently associated with secondary peritonitis than with primary.26
Physical examination findings
On physical examination, pertinent findings in cats with peritonitis tend to be associated with dehydration and shock, and can include depressed mentation, inadequate hydration, pale mucus membranes, tachypnea, tachycardia, weakness, hyperthermia, and poor nutritional condition.26, 27, 28 Cats with peritonitis and sepsis are often bradycardic and/or hypothermic.26, 27, 28 Abdominal distension, which has been reported to be a common finding in cats with peritoneal effusion, can be identified by owners or on palpation during physical examination.29
In humans, abdominal pain is an early clinical sign of primary peritonitis,30, 31 but it appears not to be a good indicator of peritonitis in cats.24, 27 The overall incidence of abdominal pain elicited in cats with peritonitis ranged from 38–62%.24, 27
Diagnostic procedures
Radiography
Survey abdominal radiographs are often taken to evaluate cats with clinical signs of gastrointestinal, urinary, or non-specific abdominal disease. The presence of free abdominal gas with no history of recent abdominal surgery or penetrating trauma warrants immediate stabilization and surgical exploration (Fig. 26-2 ).32 It can be difficult to definitively diagnose small volumes of free peritoneal gas; close attention should be given to the diaphragmatic crura, as they are often highlighted by gas in the lung cranially and in the peritoneal cavity caudally. Loss of serosal detail and ileus also suggest intraperitoneal disease. Plain abdominal radiography may also diagnose diseases underlying peritonitis, including gastrointestinal foreign bodies, pyometra, cholelithiasis, and abdominal neoplasia.
Figure 26-2.
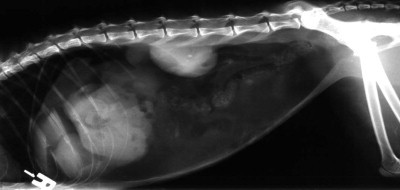
Pneumoperitoneum in a cat secondary to intestinal perforation.
(Courtesy of Dr Adrienne Bentley.)
Ultrasonography
Abdominal ultrasonography (Fig. 26-3 ) is very useful for obtaining samples of free peritoneal fluid. Blind, four quadrant tapping and diagnostic peritoneal lavage have been described for the same purpose. Although these three techniques have not been rigorously compared, ultrasound-guided aspiration has a subjectively higher diagnostic yield than a four quadrant tap and allows fluid to be directly analyzed without the dilutional effect of diagnostic peritoneal lavage. The hair over the abdomen is clipped and prepared with aseptic technique. A fluid accumulation is located with ultrasound and aspirated using a syringe and needle. The fluid's pH, glucose, lactate, and cytology are evaluated (Table 26-2 ).33 Ultrasonography can also evaluate the abdominal organs for masses and abscesses and evaluate the urinary and biliary systems.
Figure 26-3.
A cat with suspected peritonitis undergoing abdominal ultrasound.
Table 26-2.
| Levels | Does the cat have peritonitis? | |
|---|---|---|
| Nucleated cell count | >13,000 cells/µL | 100% sensitivity and specificity* |
| Glucose concentration | <20 mg/dL or more lower than peripheral blood glucose | 86% sensitive, 100% specific* |
| Peripheral venous blood lactate | 1.3–10.6 mmol/L (median = 6.2 mmol/L) | Affected cats |
| 1.2–1.6 mmol/L (median = 1.4 mmol/L) | Unaffected cats | |
| Bile crystals | Presence of intra- and extracellular bile crystals | Bile peritonitis |
| Bilirubin | Two to three times higher than peripheral blood levels | Bile peritonitis |
| Creatinine | Increased levels compared to serum. Mean abdominal fluid creatinine-to-serum creatinine ratio = 2 : 1 (range, 1.1 : 1 to 4.1 : 1) | Uroperitoneum |
| Potassium | Increased levels compared to serum. Mean abdominal fluid K+ to serum K+ ratio was 1.9 : 1 (range, 1.2 : 1 to 2.4 : 1) | Uroperitoneum |
, for diagnosing peritonitis.
Cytological analysis
Cytological analysis has traditionally been used to evaluate peritoneal fluid (Table 26-2). Septic peritoneal effusions generally contain degenerate neutrophils and macrophages. The presence of intracellular bacteria is diagnostic for septic peritonitis.34 A comparison of peritoneal fluid and peripheral blood lactate and glucose concentrations is also useful for confirming the diagnosis of septic peritonitis. Bile peritonitis is diagnosed based on cytological and biochemical analysis of peritoneal fluid. The diagnosis of uroperitoneum is based on a history of either blunt abdominal trauma, recent catheterization, or recent expression of the bladder. In the majority of cats with uroperitoneum, the peritoneal fluid creatinine and potassium levels are higher than those in the serum (see Chapter 3).35
Surgical diseases of the peritoneum
Peritonitis (inflammation or infection of the peritoneum) is the most common disease affecting the peritoneum in the cat. It occurs subsequent to systemic infection or leakage of gastrointestinal tract contents. Peritonitis can be subdivided into primary, secondary, or tertiary peritonitis, reflecting the cause or source of the infection. Neoplasia of the peritoneum is rare, but both primary mesothelioma36, 37, 38 and secondary metastases to the peritoneum have been reported in the cat.
Primary peritonitis
Primary or spontaneous peritonitis is defined as an infection of the peritoneal cavity where no cause for the intraperitoneal bacterial contamination has been identified.30, 31, 39 In primary peritonitis, bacterial contamination of the peritoneal cavity occurs via blood-borne or lymphatic spread or by bacterial translocation from the intestinal tract.30 The true origin of bacterial contamination is difficult or impossible to determine in many instances. Primary peritonitis is more commonly reported in human than veterinary patients and is usually associated with diseases that cause ascites, such as cirrhosis.30, 31, 40, 41 In veterinary medicine, FIP is the most common cause of primary peritonitis.39, 42 FIP is caused by infection with a feline coronavirus and presents in two forms, the granulomatous (dry) form or the effusive (wet) form, which is the form that results in abdominal effusion and peritonitis.43 In a retrospective study on cats with peritonitis, four out of 65 cats had primary peritonitis resulting from the effusive FIP.44 There are numerous reports of primary peritonitis in cats due to other pathogens besides FIP; however, the true source of the infection in most cases is unclear.45, 46, 47, 48, 49
Secondary peritonitis
Secondary peritonitis is the most common form of peritonitis seen in veterinary medicine.31 Bacterial contamination results from penetrating abdominal trauma, bite wounds, contamination from surgery or diagnostic aspiration procedures. Leakage of bacteria from abdominal organs into the peritoneal cavity is another frequent source of contamination in secondary peritonitis.31 While all of the abdominal organs can be potential septic sources of contamination, leakage of contents from the gastrointestinal tract is the most common cause of peritonitis.27, 50, 51, 52, 53 In cats, gastrointestinal leakage causing septic peritonitis is most often the result of neoplasia.27 In one study, neoplasia was the underlying cause of gastrointestinal leakage and subsequent septic peritonitis in 54% of cats.27 Adenocarcinoma and lymphosarcoma were the most common neoplasms identified.27 Other reported causes of gastrointestinal leakage include perforation of the bowel due to a foreign body (Fig. 26-4 ), leakage from a previous surgical site, and perforation of the colon due to megacolon and enteritis.27
Figure 26-4.
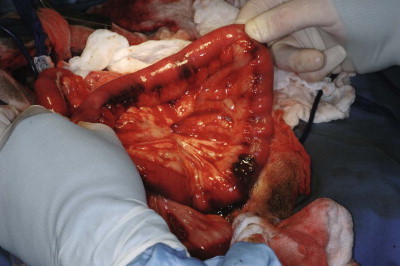
Peritonitis secondary to intestinal perforations from a linear foreign body.
(Courtesy of Dr Karen Tobias.)
Leakage from an enterotomy, gastrotomy, or a resection and anastomosis site is another potential cause of secondary peritonitis.54, 55 Dehiscence occurred in 14% of cases in one study of dogs and cats, although none of the 25 cats had surgical dehiscence and leakage.55 It is difficult to interpret the significance of these findings due to the limited numbers of cases in this study specifically evaluating cats. In a second study, no anastomosis or biopsy site leaked in 70 cats with alimentary lymphosarcoma.56 In dogs, factors suggesting an increase in the risk of postoperative dehiscence include presence of a foreign body or a traumatic injury, preoperative peritonitis, and hypoalbuminemia.54, 55 Perforation of a gastrointestinal ulcer, perhaps associated with carprofen ingestion, has been reported in one cat with peritonitis.57
Secondary peritonitis in cats may occur following bite wounds, gunshot wounds or vehicular trauma.27 Bacteria enter the peritoneal cavity directly from the environment or from trauma resulting in rupture of a viscus. Penetrating wounds warrant emergency surgical exploration to evaluate for possible bowel damage, wound debridement, and lavage.
Septic peritonitis from urinary tract infection is rare and urine is not considered an adjuvant substance in peritonitis.58 In a retrospective study of 51 cases of septic peritonitis in cats, only four cases of peritonitis appeared to have resulted from a urinary tract infection.27 Feline pyometras occasionally rupture and cause secondary peritonitis. In a retrospective study of cats with pyometra, seven out of 183 cats (3.8%) had a uterine rupture, and four of the seven cats died as a result of the septic peritonitis.59
Secondary septic peritonitis can also occur because of biliary leakage resulting from trauma anywhere along the biliary tract, necrotizing cholecystitis, cholelithiasis, or iatrogenic injury from aspiration or surgical manipulation.24, 60, 61, 62, 63 In dogs, it appears that animals with septic bile peritonitis had a decreased survival rate and increased mortality as compared to those with aseptic peritonitis.60, 63
Other potential sources of secondary peritonitis include abscessation of the pancreas, liver, or spleen64 or iatrogenic contamination of the abdominal cavity after abdominal surgery. In 51 cats with secondary peritonitis, three developed septic peritonitis due to septic pancreatitis or a pancreatic abscess, and one due to liver lobe necrosis.27
Tertiary peritonitis
Tertiary or recurrent peritonitis is diagnosed when there is persistent infection of the peritoneal cavity after an effort has been made to treat either primary or secondary peritonitis.65 Tertiary peritonitis can occur even after optimal, standard-of-care treatment and often cases present with systemic sepsis without an obvious focus of infection.66, 67 In humans, the mortality rate is greater than 60%.68 The lack of peritoneal inflammation due to immune paralysis and derangement in the endocrine stress response has been tentatively linked with the occurrence of tertiary peritonitis in people.67 The organisms that are generally found in cases of tertiary peritonitis in humans tend to be from the gastrointestinal tract or lesser pathogens such as Candida spp. or Staphylococcus epidermidis. 30, 68 Tertiary peritonitis has currently not been truly described in cats or dogs.
Mesothelioma
Primary tumors of the peritoneal serosal surfaces are uncommon in cats. They occur within the thoracic or abdominal cavities and are regularly associated with pleural or peritoneal effusions; involvement of the pleura in the cat (see Chapter 44) has been reported more commonly than involvement of the peritoneum. Mesothelial neoplasms can be benign or malignant, and are classified as predominantly epitheloid, mixed (biphasic), or fibrous (spindle cell, fibrosarcomatous).36, 37 Affected cats range in age from 1–17 years and may present with ascites, weakness, and collapse.36 Diagnosis is aided by imaging, fine needle aspiration, and peritoneal fluid analysis.
Surgical findings include infiltration of the omentum, which can form an irregular mass that extends onto the stomach, pancreas, spleen, and proximal intestinal tract.36, 38 The visceral and parietal peritoneal surfaces can be affected and appear thickened with off-white friable material and occasionally firm fibrous plaques.36 Immunohistochemistry may be necessary for confirmation of the suspected diagnosis.36, 37
Preparation for surgery
Cats with peritonitis usually require emergency management and stabilization prior to surgery.
Intravenous fluids
Stabilization of the cat with bacterial peritonitis requires intravenous access, generally with two peripheral catheters or one central catheter. The type of fluid and the rate of resuscitation depend on an assessment of the cat's perfusion, initial laboratory data, and the presence of concurrent respiratory and cardiac diseases. In general, colloids are often used with crystalloids, as endothelial dysfunction and increased vascular permeability are common. Synthetic colloids (Hetastarch, Dextran 70) are administered as a bolus (5 mL/kg) with 20–30 mL/kg of crystalloid. When available, fresh frozen plasma provides albumin and clotting factors. Ongoing resuscitation is determined by the response to therapy, measured by clinical and laboratory parameters, including heart rate, direct or indirect blood pressure, acid/base status, and blood lactate levels. In some cases blood pressure and tissue perfusion do not improve despite apparently adequate intravascular volume expansion. This may occur because of sepsis-associated disruption of vascular tone. In these instances, careful use of a vasopressor (dopamine: 5 µg/kg/minute; norepinephrine: 0.1 µg/kg/minute) can be effective in restoring vascular tone and tissue perfusion.
Antibiosis
Antibiotics are administered perioperatively in all cases of suspected bacterial peritonitis. Samples for bacterial culture and sensitivity testing are taken during surgery. Until these results are obtained, the choice of antibiotics is based on knowledge of the normal bacterial flora of the organ suspected to be the source of the contamination, and antimicrobial pharmacokinetics. Intestinal bacteria are commonly coliforms, enterococci, and anaerobes. Appropriate antibiotics for these bacteria include a combination of ampicillin and either an aminoglycoside or fluoroquinolone. Metronidazole is often added to target anaerobes. Penicillin and cephalosporin antibiotics are ‘time dependent’ as they rely on maintaining serum concentrations above the minimum inhibitory concentration (MIC) by frequent dosing, generally every six hours. Aminoglycosides and fluoroquinolones depend on obtaining the highest possible drug concentration (‘concentration dependent’) by using a single daily dose for their bacteriocidal effect.
Uroperitoneum
In cats with uroperitoneum, rehydration should begin immediately, using warm intravenous fluids. The rate of fluid administration is based on the severity of dehydration. Severely dehydrated animals with no underlying heart disease can be given 40–60 mL/kg over the first hour; subsequent rates of 5–15 mL/kg/hour are used depending on hydration status. Although some clinicians use 0.9% NaCl to avoid administering potassium, it is clear that more balanced electrolyte solutions (lactated Ringer's, Normasol R) allow for a more rapid correction of acid–base status without affecting normalization of serum potassium levels.69, 70 In cases of marked hyperkalemia, calcium gluconate (a functional antagonist of potassium) should be administered slowly, intravenously (0.5 mL/kg). Although it does not lower serum potassium, calcium changes the threshold potential and returns membrane excitability to normal for approximately 20 to 30 minutes. Regular insulin (1 unit/kg) and/or glucose (1–2 g/unit of insulin) can also be administered. Animals are often also severely acidotic. Sodium bicarbonate can be administered (3–9 mEq/kg) to correct acidosis; however, this can paradoxically lower ionized calcium and so worsen the effects of hyperkalemia. Correction of the hyperkalemia and acidosis depends on re-establishing urine flow to allow effective potassium and acid excretion from the body. In animals with uroperitoneum this involves placement of a catheter to drain urine from the peritoneal cavity and ideally placement of a catheter into the bladder via the urethra. In cases where the urethra is completely transected a cystostomy tube is placed surgically or by using interventional radiology techniques.
Surgical management and techniques for peritonitis
The goals of surgery for septic peritonitis are to remove the source of infection, decrease the infectious pathogen load, remove foreign material and other adjuvants, and prevent recurrence or persistence of infection in the peritoneal cavity. Surgery may also include the placement of feeding tubes (i.e., gastrostomy or enterostomy tubes) for postoperative nutritional support (see Chapters 12 and 28).
Celiotomy
A standard ventral midline celiotomy from just caudal to the xiphoid to just cranial to the pubis is recommended to allow for maximum exposure to perform complete exploration of the peritoneal cavity (Fig. 26-5 ) (see Chapter 23). Complete exploration is crucial for determination of the underlying primary cause, correction and elimination of continued contamination, and adequate lavage of the peritoneal cavity. The hepatobiliary, gastrointestinal, and urogenital systems are carefully evaluated to identify the underlying cause of peritonitis. Resection and anastomosis of a gastrointestinal lesion, a cholecystectomy, or ovariohysterectomy to remove a pyometra, may be necessary to treat the primary cause. Debridement of abnormal tissue and removing foreign material and adjuvants such as hemoglobin are also performed during surgery. This will decrease the concentrations of intra-abdominal pathogens, adjuvants, and fibrin, minimizing the risk of persistent infections and clinical deterioration.
Figure 26-5.
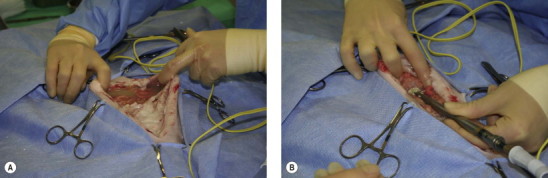
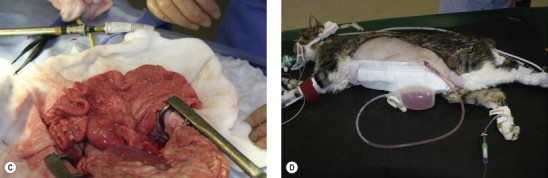
An 8-year-old domestic short-haired cat presented with a chronic history of peritonitis. (A) After celiotomy the abdominal wall is maintained in an elevated position by finger retraction to prevent spillage of peritoneal fluid prior to suction. (B) The fluid is suctioned out using a Poole suction tip. Fibrinous material was present in the fluid. (C) The mesentery and peritoneal surface has a granular appearance with fibrin deposits throughout the abdomen. (D) Multiple biopsies were taken, and following celiotomy closure a closed-suction drain (Jackson–Pratt) was placed. A Nocardia type organism was cultured, thought to be a secondary pathogen; the primary cause was not identified.
(Courtesy of the University of Cambridge.)
Omentalization and serosal patching
Peritonitis is associated with an increased risk of surgical dehiscence and leakage after closure of a viscus.55, 71 Degradation of collagen and the extracellular matrix by the proteolyic enzymes in peritonitis is thought to predispose enterotomies and intestinal anastomoses to dehiscence.72 The omentum plays an important role in the body's defense against injury and infection in the peritoneal cavity. The omentum provides a rich blood supply, has absorptive capabilities, and stimulates angiogenesis.1 The omentum will also form adhesions to help seal off or isolate sources of contamination. Omentalization can be used when the source of the infection cannot be entirely removed, such as prostatic or pancreatic abscesses. The abscess cavity is located by palpation, intraoperative ultrasound, or aspiration. It is opened, drained, and the abscess cavity is gently debrided with a moistened gauze sponge. After thorough local lavage with a warm, balanced electrolyte solution, the omentum is packed loosely into the cavity and the abdominal incision is closed. The immunologic and angiogenic properties of the omentum promote local infection control and healing. The technique has been associated with some success in (six of 12; 50% survival) dogs with pancreatic abscesses.73 Results of a large clinical study of omentalization for abdominal abscesses in cats are lacking.
Omental wrapping involves placing the omentum around the suture line of an enterotomy or anastomosis and has been described for animals with an increased risk of postoperative bowel leakage.55, 74 Serosal patching involves suturing loops of jejunum over the closure site of the viscus. It can be used over suture lines that are at a high risk of dehiscence.71 The technique for making an omental graft is described in Chapter 19.
Peritoneal lavage
The current standard of practice holds that the contaminated peritoneal cavity is thoroughly lavaged after surgery. Whilst the concept of treating the peritoneal cavity in a similar manner to any other contaminated wound seems reasonable, peritoneal lavage has both beneficial and detrimental effects.75 Peritoneal lavage does not necessarily decrease peritoneal bacterial contamination. Once bacteria have colonized and adhered to the peritoneum, they are difficult to remove by lavage.76, 77 Lavage fluid remaining in the peritoneal cavity dilutes complement and other opsonins and suspends bacteria away from neutrophils and macrophages, making phagocytosis more difficult. Lavage, then, should ideally be used to remove blood, purulent material, bile, mucus, and gross intestinal content from the peritoneal cavity but all fluid should be aspirated before the abdominal cavity is closed. The best fluid to use for peritoneal lavage has not been determined. The addition of antiseptics to lavage fluid is not recommended in clinical cases because of toxicity (povidone iodine) or a lack of perceived clinical efficacy in spite of benefits in some experimental models (chlorhexidine). The addition of antibiotics to lavage fluid has no benefit over parenteral antibiotics administered intravenously at the appropriate doses.
Peritoneal drainage
Once the source of the peritonitis has been addressed, access for enteral nutrition should be provided if necessary (see Chapter 11). The surgeon must then decide whether the abdomen should be closed primarily or if some type of local or more general peritoneal drainage is required. Local drainage has largely been recommended for pancreatic abscess or prostatic abscesses in dogs. Localized drainage is used to limit accumulation of a large volume of ongoing infected exudate as this might limit the efficacy of local peritoneal defense mechanisms, prevent localization of the contamination, and speed the systemic absorption of bacteria and endotoxin.75 However, the technique of placing one or more drains into an intra-abdominal abscess has largely been replaced in clinical practice by omentalization (see above).
In generalized peritonitis the surgeon must choose to close the abdomen primarily, close the abdomen after placing drains, or leave the abdomen open. There are no objective criteria and few subjective criteria to guide this decision, and no large scale, randomized clinical trials of these treatment methods in cats with peritonitis.
Primary closure without drainage
A decision to close the peritoneal cavity without drainage is based on control of the source of contamination and adequate decontamination of the peritoneal cavity. The clinician then relies on the body's peritoneal drainage and immune defense systems to resolve residual infection and peritoneal contamination. Fifty-four per cent of dogs with septic peritonitis treated by primary closure survived in one study.78 This form of management is most appropriate when the source of contamination can be controlled definitively and peritonitis is not severe.
Closed-suction drainage
The use of closed-suction drains in generalized peritonitis has been reported in cats and dogs. Some type of suction or vacuum system is necessary for drains to be potentially effective because the peritoneal space within the abdomen is at a sub-atmospheric pressure. Even with experimental insufflation of air, the intraperitoneal pressure never exceeds atmospheric pressure.79 Unless either the intraperitoneal pressure becomes higher than atmospheric or air can enter the peritoneal cavity postoperatively through a vent, drainage from the peritoneal cavity will not occur without the use of a vacuum system. The use of drains within the peritoneal cavity is somewhat controversial because of the discrepancy between reasonable clinical results from their use and experimental studies clearly showing that the omentum and viscera rapidly encase drains inserted into the peritoneal cavity.80 It is therefore not clear if drainage occurs from the peritoneal cavity proper or, more likely, from the area around the encased drain. In an experimental study in dogs, sump-Penrose drains were found encapsulated and isolated from the peritoneal cavity at necropsy performed after 48 hours.5 Despite this isolation, the drains continued to remove radio-opaque contrast material from the peritoneal cavity. In a clinical study of closed-suction silicone drains for septic peritonitis, drains continued to accumulate fluid for up to eight days, seeming to indicate adequate function. However, it is not clear if closed-suction drains are encased to the same extent as sump-Penrose drains and are draining a localized area, or if they retain functional drainage of the peritoneal cavity despite being encased.81 This study reported 70% survival in a cohort of 30 dogs and ten cats with septic peritonitis managed with closed-suction drains. Drains were in place for a mean of 3.6 days with a range of two to eight days. The volume of fluid produced was variable but decreased with time. The drains remained patent until removal, as evidenced by ongoing fluid collection. However, it is unclear what percentage of the total peritoneal fluid volume they drained. No significant complications were reported with clinical use of the closed-suction drains.
Closed-suction drains are relatively inexpensive, easy to place, and seem to be free of significant complications (see Fig. 26-5 and Chapter 11). It should be noted, however, that experimentally, latex drains can significantly impair bowel wound healing. It is not clear if silastic closed-suction drains impair bowel healing in a similar manner. Advantages of closed-suction drainage over open peritoneal drainage include the ability to quantify the volume of effusion and decreased cost and labor.
Commercially available closed-suction drains consist of a fenestrated silicone drain connected to an external reservoir by a non-fenestrated tube (Jackson–Pratt drain, Cardinal Health Australia, Sydney, Australia). The drain is typically positioned near the diaphragm and liver in the most dependent portion of the peritoneal cavity, although a more caudal position may be appropriate, depending on the source of the contamination. The non-fenestrated portion of the drain is exited through a small paramedian incision in the body wall and is secured with a purse-string and finger trap of non-absorbable suture. Compression of the bulb reservoir creates negative pressure within the peritoneal cavity. A bandage is typically applied to cover the exit site of the drain and to provide a means of attaching the bulb reservoir to the patient (see Chapter 4). The contents of the reservoir are easily emptied, typically every 6 hours or when the reservoir is half full.
Open peritoneal drainage
Historically, open peritoneal drainage (Box 26-1 ) has been reserved for the most severe cases of generalized septic peritonitis, which are anecdotally associated with a large volume of effusion postoperatively. Assessment of the severity of peritonitis is largely subjective and based on individual experience.
Box 26-1. Open peritoneal drainage.
Open peritoneal drainage is established through a long abdominal incision, extending from the xiphoid process to the pubis and including the most dependent portion of the abdomen. The falciform fat should be excised according to standard exploratory laparotomy technique (see Chapter 23), but omentectomy is not necessary or desirable. The linea alba is closed with non-absorbable suture in a simple continuous pattern with a gap of 1–3 cm between the edges (Fig. 26-6), depending on the patient's size. The subcutaneous tissues and skin are not closed. A sterile bandage consisting of a non-adherent contact layer, such as petrolatum impregnated gauze, laparotomy sponges, and surgery towels underneath routine bandage material is applied and changed at least daily. If the bandage becomes wet from peritoneal effusion or urine, or if the bandage becomes displaced, the bandage should be replaced as soon as possible. The bandage should be changed in the operating room with the patient sedated or anesthetized. During each bandage change, adhesions at the incision are digitally disrupted and the incision is checked for organ evisceration. The decision to close the peritoneal cavity is based on reassessment of the same factors used in selecting open peritoneal drainage or cytological analysis of the effusion. Closure is performed as a complete laparotomy.
Figure 26-6.
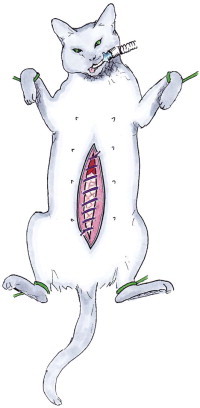
Illustration of closure for open peritoneal drainage in a cat. A simple continuous suture is placed leaving a gap of 1–3 cm between the fascia lata. The skin and subcutaneous tissue is left unsutured. The open abdomen is protected by a sterile bandage.
Clinical studies report a mean duration of open peritoneal drainage of four to five days, with a range of less than one day to up to two weeks.82, 83 Hypoproteinemia and nosocomial infection are reported complications of open peritoneal drainage, although their clinical significance is not well established.
Postoperative care
The postoperative care of the cat with peritonitis is a critical part of the management of the case and will influence its chances of recovery. Particular attention should be paid to the cat's fluid status, electrolyte balance, analgesia, and nutritional balance. The cat should be monitored frequently for complications associated with the procedures performed. The reader is referred to Chapter 3 on postoperative monitoring and management of complications, and Chapter 4 on postoperative nursing for more information.
Perioperative nutrition
Once the primary source of peritonitis has been controlled, the surgeon must consider options for postoperative nutrition (see Chapter 6). In cases of uncomplicated bladder rupture and uroperitoneum the clinician might reasonably expect a cat to eat within one to two days of a successful surgery. However, in cases of generalized bacterial peritonitis, voluntary food intake is unlikely. At the same time, generalized peritonitis is associated with a significant hypermetabolic state, intraperitoneal protein loss, and protein/energy malnutrition. An esophagosotomy, gastrostomy, or jejunostomy tube allows early enteral feeding (see Chapter 12). Enteral feeding maintains mucosal enterocyte health and minimizes bacterial translocation from the gut. In cases where enteral feeding is not tolerated, total parenteral nutrition is considered; ideally, a multiple lumen catheter is placed immediately before or after surgery.
Prognosis for peritonitis
In a study examining 26 cats that were treated surgically for septic peritonitis, 46% of the cats survived to discharge; the remainder of the cats either died or were euthanized due to deterioration of their clinical status.24 Similarly, the survival rate in a study of nine cats was 44% and no differences were detected between cats that had primary or secondary peritonitis.29 In another study, a 30% mortality rate was reported in 23 cats having surgical treatment of septic peritonitis.27 Lastly, when specifically examining primary septic peritonitis in 13 cats where treatment included surgery, the overall mortality rate was 31%.28
References
- 1.Hall JC, Heel KA, Papadimitriou JM, Platell C. The pathobiology of peritonitis. Gastroenterology. 1998;114:185–196. doi: 10.1016/s0016-5085(98)70646-8. [DOI] [PubMed] [Google Scholar]
- 2.Le Gros Clark WE. Oxford University; Oxford, England: 1958. The tissues of the body: an introduction to the study of anatomy. [Google Scholar]
- 3.Beavis J, Harwood JL, Coles GA, Williams JD. Synthesis of phospholipids by human peritoneal mesothelial cells. Perit Dial Int. 1994;14:348–355. [PubMed] [Google Scholar]
- 4.Bercovici B, Michel J, Miller J, Sacks TG. Antimicrobial activity of human peritoneal fluid. Surg Gynecol Obstet. 1975;141:885–887. [PubMed] [Google Scholar]
- 5.Hosgood G, Salisbury SK, Cantwell HD, DeNicola DB. Intraperitoneal circulation and drainage in the dog. Vet Surg. 1989;18:261–268. doi: 10.1111/j.1532-950x.1989.tb01082.x. [DOI] [PubMed] [Google Scholar]
- 6.Last M, Kurtz L, Stein TA, Wise L. Effect of PEEP on the rate of thoracic duct lymph flow and clearance of bacteria from the peritoneal cavity. Am J Surg. 1983;145:126–130. doi: 10.1016/0002-9610(83)90178-2. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Hijleh MF, Habbal OA, Moqattash ST. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat. 1995;186:453–467. [PMC free article] [PubMed] [Google Scholar]
- 8.Flessner MF, Parker RJ, Sieber SM. Peritoneal lymphatic uptake of fibrinogen and erythrocytes in the rat. Am J Physiol. 1983;244:H89–H96. doi: 10.1152/ajpheart.1983.244.1.H89. [DOI] [PubMed] [Google Scholar]
- 9.Dunn DL, Barke RA, Knight NB. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect Immun. 1985;49:257–264. doi: 10.1128/iai.49.2.257-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn DL, Barke RA, Lee JT., Jr Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. VII. Hemoglobin does not inhibit clearance of Escherichia coli from the peritoneal cavity. Surgery. 1983;94:487–493. [PubMed] [Google Scholar]
- 11.Heemken R, Gandawidjaja L, Hau T. Peritonitis: pathophysiology and local defense mechanisms. Hepatogastroenterology. 1997;44:927–936. [PubMed] [Google Scholar]
- 12.Hau T, Ahrenholz DH, Simmons RL. Secondary bacterial peritonitis: The biologic basis of treatment. Curr Probl Surg. 1979;16:1–65. doi: 10.1016/s0011-3840(79)80011-8. [DOI] [PubMed] [Google Scholar]
- 13.Rohrer RJ. Basic immunology for surgeons. In: O'Leary PJ, editor. The Physiologic basis of surgery. 3rd ed. Lippincott Williams and Wilkins; Philadelphia: 2002. pp. 169–177. [Google Scholar]
- 14.Heel KA, Hall JC. Peritoneal defences and peritoneum-associated lymphoid tissue. Br J Surg. 1996;83:1031–1036. doi: 10.1002/bjs.1800830804. [DOI] [PubMed] [Google Scholar]
- 15.Fry DE. Surgical infection. In: O'Leary JP, editor. The Physiologic basis of surgery. Williams & Wilkins; Philadelphia: Lippincott: 2002. pp. 213–234. [Google Scholar]
- 16.Culp WTN, Holt DE. Septic peritonitis. Comp Contin Ed Pract Vet. 2010;32:E1–15. [PubMed] [Google Scholar]
- 17.Vipond MN, Whawell SA, Thompson JN, Dudley HA. Effect of experimental peritonitis and ischemia on peritoneal fibrinolytic activity. Eur J Surg. 1994;160:471–477. [PubMed] [Google Scholar]
- 18.Van Goor H, de Graaf JS, Kooi K. Effect of recombinant tissue plasminogen activator on intra-abdominal abscess formation in rats with generalized peritonitis. J Am Coll Surg. 1994;179:407–411. [PubMed] [Google Scholar]
- 19.Silverstein D, Otto CM. Sepsis. In: Greene CE, editor. Infectious diseases of the dog and cat 4e. Saunders Elsevier; Philadelphia: 2012. pp. 359–369. [Google Scholar]
- 20.Lotze MT, Zeh HJ, Rubartelli A. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans TJ. The role of macrophages in septic shock. Immunology. 1996;195:655–659. doi: 10.1016/S0171-2985(96)80029-5. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 23.Srisskandan S, Cohen J. Gram positive sepsis. Infect Dis Clin North Am. 1999;13:397–412. doi: 10.1016/s0891-5520(05)70082-9. [DOI] [PubMed] [Google Scholar]
- 24.Parsons KJ, Owen LJ, Lee K. A retrospective study of surgically treated cases of septic peritonitis in the cat (2000-2007) J Small Anim Pract. 2009;50:518–524. doi: 10.1111/j.1748-5827.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 25.Onderdonk AB, Bartlett JG, Louie T. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976;13:22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Culp WT, Zeldis TE, Reese MS, Drobatz KJ. Primary bacterial peritonitis in dogs and cats: 24 cases (1990-2006) J Am Vet Med Assoc. 2009;234:906–913. doi: 10.2460/javma.234.7.906. [DOI] [PubMed] [Google Scholar]
- 27.Costello MF, Drobatz KJ, Aronson LR, King LG. Underlying cause, pathophysiologic abnormalities, and response to treatment in cats with septic peritonitis: 51 cases (1990-2001) J Am Vet Med Assoc. 2004;225:897–902. doi: 10.2460/javma.2004.225.897. [DOI] [PubMed] [Google Scholar]
- 28.Ruthrauff CM, Smith J, Glerum L. Primary bacterial septic peritonitis in cats: 13 cases. J Am Anim Hosp Assoc. 2009;45:268–276. doi: 10.5326/0450268. [DOI] [PubMed] [Google Scholar]
- 29.Wright KN, Gompf RE, DeNovo RC., Jr Peritoneal effusion in cats: 65 cases (1981-1997) J Am Vet Med Assoc. 1999;214:375–381. [PubMed] [Google Scholar]
- 30.Johnson CC, Baldessarre J, Levison ME. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin Infect Dis. 1997;24:1035–1047. doi: 10.1086/513658. [DOI] [PubMed] [Google Scholar]
- 31.Laroche M, Harding G. Primary and secondary peritonitis: an update. Eur J Clin Microbiol Infect Dis. 1998;17:542–550. doi: 10.1007/BF01708616. [DOI] [PubMed] [Google Scholar]
- 32.Smelstoys JA, Davis GJ, Learn AE. Outcome of and prognostic indicators for dogs and cats with pneumoperitoneum and no history of penetrating trauma: 54 cases (1988-2002) J Am Vet Med Assoc. 2004;225:251–255. doi: 10.2460/javma.2004.225.251. [DOI] [PubMed] [Google Scholar]
- 33.Bonczynski JJ, Ludwig LL, Barton LJ. Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats. Vet Surg. 2003;32:161–166. doi: 10.1053/jvet.2003.50005. [DOI] [PubMed] [Google Scholar]
- 34.Alleman AR. Abdominal, thoracic, and pericardial effusions. Vet Clin North Am Small Anim Pract. 2003;33:89–118. doi: 10.1016/s0195-5616(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 35.Aumann M, Worth LT, Drobatz KJ. Uroperitoneum in cats: 26 cases (1986-1995) J Am Anim Hosp Assoc. 1998;34:315–324. doi: 10.5326/15473317-34-4-315. [DOI] [PubMed] [Google Scholar]
- 36.Heerkens TM, Smith JD, Fox L, Hostetter JM. Peritoneal fibrosarcomatous mesothelioma in a cat. J Vet Diagn Invest. 2011;23:593–597. doi: 10.1177/1040638711403405. [DOI] [PubMed] [Google Scholar]
- 37.Bacci B, Morandi F, De Meo M, Marcato PS. Ten cases of feline mesothelioma: an immunohistochemical and ultrastructural study. J Comp Pathol. 2006;134:347–354. doi: 10.1016/j.jcpa.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi Y, Usuda H, Ochiai K, Itakura C. Malignant mesothelioma with metastases and mast cell leukaemia in a cat. J Comp Pathol. 1994;111:453–458. doi: 10.1016/s0021-9975(05)80103-3. [DOI] [PubMed] [Google Scholar]
- 39.Kirby BM. Peritoneum and peritoneal cavity. In: Slatter D, editor. Textbook of small animal surgery. WB Saunders; Philadelphia: 2003. pp. 414–445. [Google Scholar]
- 40.Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascities. Hepatology. 2003;37:897–901. doi: 10.1053/jhep.2003.50119. [DOI] [PubMed] [Google Scholar]
- 41.Boixeda D, De Luis DA, Aller R, De Argila CM. Spontaneous bacterial peritonitis. Clinical and microbiological study of 233 episodes. J Clin Gastroenterol. 1996;23:275–279. doi: 10.1097/00004836-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Seim HB. Management of peritonitis. In: Bonagura JD, Kirk RW, editors. Current veterinary therapy xii: small animal practice. WB Saunders; Philadelphia: 1995. pp. 933–937. [Google Scholar]
- 43.Gaskell R, Dawson S. FIP-related disease. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. WB Saunders; Philadelphia: 2000. pp. 137–139. [Google Scholar]
- 44.Wright KN, Gompf RE, DeNovo RC., Jr Peritoneal effusion in cats: 65 cases (1981-1997) J Am Vet Med Assoc. 1999;214:375–381. [PubMed] [Google Scholar]
- 45.Culp WT, Zeldis TE, Reese MS, Drobatz KJ. Primary bacterial peritonitis in dogs and cats: 24 cases (1990-2006) J Am Vet Med Assoc. 2009;234:906–913. doi: 10.2460/javma.234.7.906. [DOI] [PubMed] [Google Scholar]
- 46.Costello MF, Drobatz KJ, Aronson LR, King LG. Underlying cause, pathophysiologic abnormalities, and response to treatment in cats with septic peritonitis: 51 cases (1990-2001) J Am Vet Med Assoc. 2004;225:897–902. doi: 10.2460/javma.2004.225.897. [DOI] [PubMed] [Google Scholar]
- 47.Ruthraff CM, Smith J, Glerum L. Primary bacterial septic peritonitis in cats: 13 cases. J Am Anim Hosp Assoc. 2009;45:268–276. doi: 10.5326/0450268. [DOI] [PubMed] [Google Scholar]
- 48.Ingham B, Brentnall DW. Acute peritonitis in a kitten associated with Salmonella typhimurium infection. J Small Anim Pract. 1972;13:71–74. doi: 10.1111/j.1748-5827.1972.tb06832.x. [DOI] [PubMed] [Google Scholar]
- 49.Dickie CW, Sniff ES. Chlamydia infection associated with peritonitis in a cat. J Am Vet Med Assoc. 1980;176:1256–1259. [PubMed] [Google Scholar]
- 50.Woolfson JM, Dulisch ML. Open abdominal drainage in the treatment of generalized peritonitis in 25 dogs and cats. Vet Surg. 1986;15:27–32. [Google Scholar]
- 51.Greenfield CL, Walshaw R. Open peritoneal drainage for treatment of contaminated peritoneal cavity and septic peritonitis in dogs and cats: 24 cases (1980-1986) J Am Vet Med Assoc. 1987;191:100–105. [PubMed] [Google Scholar]
- 52.Mueller MG, Ludwig LL, Barton LJ. Use of closed-suction drains to treat generalized peritonitis in dogs and cats: 40 cases (1997-1999) J Am Vet Med Assoc. 2001;219:789–794. doi: 10.2460/javma.2001.219.789. [DOI] [PubMed] [Google Scholar]
- 53.Staatz AJ, Monnet E, Seim HB., 3rd Open peritoneal drainage versus primary closure for the treatment of septic peritonitis I dogs and cats: 42 cases (1993-1999) Vet Surg. 2002;31:174–180. doi: 10.1053/jvet.2002.31043. [DOI] [PubMed] [Google Scholar]
- 54.Allen DA, Smeak DD, Schertel ER. Prevalence of small intestinal dehiscence and associated clinical factors: a retrospective study of 121 dogs. J Am Anim Hosp Assoc. 1992;28:70–76. [Google Scholar]
- 55.Ralphs SC, Jessen CR, Lipowitz AJ. Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991-2000) J Am Vet Med Assoc. 2003;223:73–77. doi: 10.2460/javma.2003.223.73. [DOI] [PubMed] [Google Scholar]
- 56.Smith AL, Wilson AP, Hardie RJ. Perioperative complications after full-thickness gastrointestinal surgery in cats with alimentary lymphoma. Vet Surg. 2011;40:849–852. doi: 10.1111/j.1532-950X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 57.Runk A, Kyles AE, Downs MO. Duodenal perforation in a cat following the administration of non-steroidal anti-inflammatory medication. J Am Anim Hosp Assoc. 1999;35:52–55. doi: 10.5326/15473317-35-1-52. [DOI] [PubMed] [Google Scholar]
- 58.Hardie EM. Peritonitis from urogentital conditions. Prob Vet Med. 1989;1:36–49. [PubMed] [Google Scholar]
- 59.Kenney KJ, Matthiesen DT, Brown NO, Bradley RL. Pyometra in cats:183 cases (1979-84) J Am Vet Med Assoc. 1987;191:1130–1132. [PubMed] [Google Scholar]
- 60.Ludwig LL, McLoughlin MA, Graves TK, Crisp MS. Surgical treatment of bile peritonitis in 24 dogs and 2 cats: a retrospective study (1987-1994) Vet Surg. 1997;26:90–98. doi: 10.1111/j.1532-950x.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 61.Kirpensteijn J, Fingland RB, Ulrich T. Cholelithiasis in dogs: 29 cases (1980-90) J Am Vet Med Assoc. 1993;202:1137–1142. [PubMed] [Google Scholar]
- 62.Moores AL, Gregory SP. Duplex gall bladder associated with choledocholithiasis, cholecystitis, gall bladder rupture and septic peritonitis in a cat. J Small Anim Pract. 2007;48:404–409. doi: 10.1111/j.1748-5827.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 63.Mehler SJ, Mayhew PD, Drobatz KJ, Holt DE. Variables associated with outcome in dogs undergoing extrahepatic biliary surgery: 60 cases (1988-2002) Vet Surg. 2004;33:644–649. doi: 10.1111/j.1532-950X.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 64.Sergeeff JS, Armstrong PJ, Bunch SE. Hepatic abscesses in cats: 14 cases (1985-2002) J Vet Intern Med. 2004;18:295–300. doi: 10.1892/0891-6640(2004)18<295:haicc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Malangoni MA. Evaluation and management of tertiary peritonitis. Am Surg. 2000;66:157–161. [PubMed] [Google Scholar]
- 66.Broche F, Tellado JM. Defense mechanisms of the peritoneal cavity. Curr Opin Crit Care. 2001;7:105–116. doi: 10.1097/00075198-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Buijk SE, Bruining HA. Future directions in the management of tertiary peritonitis. Intensive Care Med. 2002;28:1024–1029. doi: 10.1007/s00134-002-1383-6. [DOI] [PubMed] [Google Scholar]
- 68.Nathens AB, Rotstein OD, Marshall JC. Tertiary peritonitis: clinical features of a complex nosocomial infection. World J Surg. 1998;22:158–163. doi: 10.1007/s002689900364. [DOI] [PubMed] [Google Scholar]
- 69.Cunha MG, Freitas GC, Carregaro AB. Renal and cardiorespiratory effects of treatment with lactated Ringer's solution or physiologic saline (0.9% NaCl) solution in cats with experimentally induced urethral obstruction. Am J Vet Res. 2010;71:840–846. doi: 10.2460/ajvr.71.7.840. [DOI] [PubMed] [Google Scholar]
- 70.Drobatz KJ, Cole SG. The influence of crystalloid type on acid-base and electrolyte status of cats with urethral obstruction. J Vet Emerg Crit Care. 2008;18:355–361. [Google Scholar]
- 71.Crowe DT. The serosal patch: Clinical use in 12 animals. Vet Surg. 1984;13:29–38. [Google Scholar]
- 72.Tani T, Tsutamoto Y, Eguchi Y. Protease inhibitor reduces loss of tensile strength in rat anastomosis with peritonitis. J Surg Res. 2000;88:135–141. doi: 10.1006/jsre.1999.5765. [DOI] [PubMed] [Google Scholar]
- 73.Johnson MD, Mann FA. Treatment for pancreatic abscesses via omentalization with abdominal closure versus open peritoneal drainage in dogs: 15 cases (1994-2004) J Am Vet Med Assoc. 2006;228:397–402. doi: 10.2460/javma.228.3.397. [DOI] [PubMed] [Google Scholar]
- 74.McLachlin AD, Denton DW. Omental protection of intestinal anastomoses. Am J Surg. 1973;125:134–140. doi: 10.1016/0002-9610(73)90018-4. [DOI] [PubMed] [Google Scholar]
- 75.Platell C, Papadimitriou JM, Hall JC. The influence of lavage on peritonitis. J Am Coll Surg. 2000;191:672–680. doi: 10.1016/s1072-7515(00)00726-2. [DOI] [PubMed] [Google Scholar]
- 76.Haagen IA, Heezius HC, Verkooyen RP. Adherence of peritonitis-causing staphylococci to human peritoneal mesothelial cell monolayers. J Infect Dis. 1990;161:266–273. doi: 10.1093/infdis/161.2.266. [DOI] [PubMed] [Google Scholar]
- 77.Edmiston CE, Jr, Goheen MP, Kornhall S. Fecal peritonitis: microbial adherence to serosal mesothelium and resistance to peritoneal lavage. World J Surg. 1990;14:176–183. doi: 10.1007/BF01664870. [DOI] [PubMed] [Google Scholar]
- 78.Lanz OI, Ellison GW, Bellah JR. Surgical treatment of septic peritonitis without abdominal drainage in 28 dogs. J Am Anim Hosp Assoc. 2001;37:87–92. doi: 10.5326/15473317-37-1-87. [DOI] [PubMed] [Google Scholar]
- 79.Gold E. The physics of the abdominal cavity and the problem of peritoneal drainage. Am J Surg. 1956;91:415–417. doi: 10.1016/0002-9610(56)90183-0. [DOI] [PubMed] [Google Scholar]
- 80.Yates JL. An experimental study of the local effects of peritoneal drainage. Surg Gynecol Obstet. 1905;1:473–492. [PubMed] [Google Scholar]
- 81.Mueller MG, Ludwig LL, Barton LJ. Use of closed-suction drains to treat generalized peritonitis in dogs and cats: 40 cases (1997-1999) J Am Vet Med Assoc. 2001;219:789–794. doi: 10.2460/javma.2001.219.789. [DOI] [PubMed] [Google Scholar]
- 82.Woolfson JM, Dulisch ML. Open abdominal drainage in the treatment of generalized peritonitis in 25 dogs and cats. Vet Surg. 1986;15:27–32. [Google Scholar]
- 83.Greenfield CL, Walshaw R. Open peritoneal drainage for treatment of contaminated peritoneal cavity and septic peritonitis in dogs and cats: 24 cases (1980-1986) J Am Vet Med Assoc. 1987;191:100–105. [PubMed] [Google Scholar]


