Introduction
Viruses are obligate intracellular parasites that replicate only within living animal, plant, or bacterial cells. Of the 71 taxonomically defined virus families, 24 contain members that infect vertebrates, and these families will be the focus of this overview. Among the smallest vertebrate viruses, the virion consists only of the viral genome and a closely associated protein coat (nucleocapsid), whereas larger viruses possess, in addition to the nucleocapsid, a variety of catalytic, regulatory and structural proteins, and in some cases a host-derived lipid membrane (envelope) containing one or more virus-encoded glycoproteins. In animal cells, virus replication is complete within several hours to at most a few days, and results in the synthesis of 103–105 virions per cell. Virus replication will be discussed in three stages: (1) early events (attachment to susceptible cells, penetration and uncoating), (2) viral biosynthetic events (replication of the viral genome, transcription and translation) and (3) virion assembly. However, because of space limitations, early events and mechanisms of virion assembly will be dealt with briefly so that viral biosynthetic strategies can be considered in greater detail.
Early Events
Attachment
Infection begins with the attachment of a virion, via capsid or envelope proteins, to specific cell-surface macromolecules (viral receptors). Because of the specificity of this interaction, the host range (tropism) of a given virus is determined primarily by the presence of viral receptor molecules on the cell surface. As a group, viruses utilize a variety of proteins, lipids and oligosaccharides as receptors. One class of receptors includes cellular macromolecules involved in ligand binding, endocytosis and cell recognition. For example, the receptors for poliovirus, human rhinovirus (ICAM-1), human immunodeficiency virus type 1 (CD4) and Epstein–Barr virus (CD21) are members of the immunoglobulin superfamily of proteins. In contrast, the receptor for Mahoney leukemia virus is an amino acid transporter and sialic acid-containing glycoproteins serve as receptors for paramyxo- and orthomyxoviruses.
Penetration and uncoating
Following attachment, the virion must enter the cell (penetration) and release its genome (uncoating). The process by which many viruses accomplish this dual task is termed receptor-mediated endocytosis and is the same mechanism used by the cell to import growth factors and other large molecules to which the plasma membrane is not permeable. Virions, bound to their cognate receptors, are transported laterally within the plasma membrane to clathrin-coated pits and enter the cell as the clathrin-coated pit invaginates. Subsequently, this vesicle fuses with an endosome and, within this acidic compartment, uncoating takes place. The acidic pH of the endosome is critical and agents that raise the intraendosomal pH (e.g. NH4Cl, chloroquine, etc.) block virus uncoating. For enveloped viruses, uncoating involves fusion of the viral envelope with the endosomal membrane followed by release of the nucleocapsid into the cytoplasm. In the case of influenza virus, it is thought that low pH changes the conformation of the hemagglutinin (HA) allowing the hydrophobic fusion peptide to interact with target cell membranes. In addition, among negative-stranded viruses, the acidic environment within the endosome promotes release of the matrix (M) protein from the nucleocapsid, a step necessary for subsequent transcription. Nonenveloped viruses also appear to utilize receptor-mediated endocytosis, although here uncoating does not involve membrane–membrane fusion. For example, following attachment of poliovirus to target cells, one of the capsid proteins (VP4) is released exposing hydrophobic residues buried inside the virion. Interaction of these residues with the endosomal membrane may provide a pore through which viral RNA is extruded into the cytoplasm. In the adenovirus system, low endosomal pH induces conformational changes in the capsid which rupture the endosomal membrane at virion–membrane contact points. After its release into the cytoplasm, adenovirus is transported via microtubules to nuclear pores where viral DNA enters the nucleus. In contrast to the above mechanism, several viruses (e.g. paramyxoviruses, herpesviruses and human immunodeficiency virus type 1 [HIV]) do not require an acidic environment for uncoating and enter cells by fusion at the plasma membrane.
Although the presence of viral receptors is a primary determinant for infectivity, not all cells carrying the appropriate receptor are susceptible to infection. In several ‘restrictive’ systems, the synthesis of infectious progeny is blocked at a postattachment step. For example, some mammalian cell lines bind influenza virus and support the synthesis of all viral macromolecules, yet do not generate infectious virions because they lack the protease required to cleave the hemagglutinin precursor (HA0) and generate activated (i.e. fusion-competent) HA1 and HA2. Conversely, some cells that lack the appropriate viral receptor can nonetheless support a productive infection if the viral genome is introduced into the cell by transfection.
Synthesis of Virus-specific Macromolecules
The 24 families of vertebrate viruses, although differing in genomic make-up, virion morphology, and their repertoire of viral-encoded enzymes, can be ordered on the basis of replicative mechanisms. However, before examination of different replication strategies, several common themes need to be addressed.
Viral transcription and genome replication
Viral nucleic acid synthesis is catalyzed by both viral and host enzymes, the relative contribution of which is determined by the type of virus and the specific molecule. Viruses with RNA genomes, except for the retroviruses, synthesize mRNA and replicate their genomes using virus-encoded RNA-dependent RNA polymerases. In contrast, retroviruses synthesize a double-stranded complementary DNA (cDNA) copy of their single-stranded RNA genome using a virion-encoded RNA-dependent DNA polymerase (reverse transcriptase). In subsequent steps, the retroviral cDNA is integrated into the host chromosome and transcribed by host-encoded DNA-dependent RNA polymerase II (pol II) to yield viral messages and genomic RNA. DNA viruses, except for poxviruses, also use host-encoded pol II to transcribe their messages. Poxviruses, because they replicate in the cytoplasm and do not have access to pol II, assemble a novel transcriptase composed of multiple poxvirus-specific (and possibly one or more host-derived) subunits. Most DNA virus families (e.g. Poxviridae, Iridoviridae, Herpesviridae, Adenoviridae) synthesize a virus-encoded DNA-dependent, DNA polymerase. However, two families (i.e. Parvoviridae and Papovaviridae) utilize host DNA polymerase, and the Hepadnaviridae replicate viral DNA through an RNA intermediate using a virus-encoded reverse transcriptase.
Gene regulation
Viruses have evolved a variety of mechanisms to control gene expression and maximize efficiency. In some systems, viral gene expression is divided into temporal phases in which catalytic and regulatory proteins are synthesized early in infection, whereas the synthesis of structural proteins is limited to late times. Alternatively, the expression of viral genes may be controlled by differences in the transcription rate of specific genes (e.g. rhabdoviruses and paramyxoviruses), the translational efficiency of different viral messages (e.g. reovirus) or the replication of transcriptional templates (e.g. influenza virus). Moreover, it is likely that, even within a single virus family, multiple mechanisms regulate gene expression. At the molecular level, gene expression is controlled by both cis- and trans-acting signals. In some cases, the nucleotide sequence of viral messages and transcriptional templates may be the primary factor in determining how efficiently a given sequence is translated or transcribed. For example, the differential synthesis of the various coronavirus mRNAs is thought to be controlled by interaction between trans-acting coronavirus leader RNA and cis-acting sequences located at the beginning of each gene. Furthermore, transcription and genome replication among DNA viruses (and retroviruses) is regulated by the (often) combined action of trans-acting viral- and host-encoded factors with cis-acting viral nucleotide sequences. For example, herpesvirus immediate-early gene transcription requires, aside from pol II, both host- (OTF-1) and virus-encoded (α-TIF) transcription factors. Lastly, in several families (e.g. Orthomyxoviridae, Poxviridae, Herpesviridae and Iridoviridae), there are hints that viral gene expression is also regulated at post-transcriptional and translational levels.
Viral protein synthesis
Viral protein synthesis is completely dependent on the cell’s translational machinery (i.e. ribosomes, tRNAs, initiation factors, etc.). Reflecting that dependence, viral mRNAs, despite some prominent exceptions (e.g. picornaviruses), are similar in overall structure to host messages, i.e. they are capped and methylated at their 5′ terminus and polyadenylated at their 3′ end. Viral mRNAs are monocistronic and are translated as are other eucaryotic transcripts. However, in some systems, viral proteins are synthesized as part of a larger precursor (polyprotein) which is cleaved to generate the final products. This mechanism overcomes the inability of eucaryotic ribosomes to translate polycistronic messages and allows one viral mRNA to code for several proteins. Viruses have also developed several ways to utilize the same nucleotide sequence to encode one or more proteins:
-
1.
HIV-1 and influenza A virus use alternative splicing to generate additional transcripts encoding novel proteins;
-
2.
measles virus and other paramyxoviruses generate a novel P-related protein (V) by RNA editing, a process in which one or more nontemplated nucleotides are added at a site within the 3′ end of some P transcripts;
-
3.
Sendai virus synthesizes five proteins from its P transcript by using alternative translational initiation codons;
-
4.
retroviruses use frameshifting or read-through mechanisms to circumvent a stop codon lying between the capsid and polymerase coding regions of the gag-pol transcript.
Finally, following their translation, viral proteins, like their cellular counterparts, are post-translationally modified (e.g. glycosylated, phosphorylated, etc.) using cellular enzymes.
As infection progresses, viral protein synthesis often supplants cellular translation. In some cases, this simply reflects the increased abundance of viral messages, whereas in others viral messages appear to initiate translation at a higher rate than host messages. Alternatively, virus infection may actively inhibit host translation by (1) proteolytically inactivating or covalently modifying initiation factors required solely or preferentially by cellular messages, (2) selectively degrading host messages, or (3) altering the intracellular ionic environment to favor viral over host translation. Furthermore, because infection can lead to the phosphorylation and functional inactivation of eucaryotic initiation factor 2 (eIF-2), several virus families (Poxviridae, Reoviridae, Orthomyxoviridae, Adenoviridae and Picornaviridae) have evolved mechanisms to block eIF-2 phosphorylation. Virus infection also blocks host cell RNA and DNA synthesis. Although transcriptional shut-off may be the direct result of inactivating specific transcription factors, the inhibition of cellular DNA synthesis is likely due to the earlier inhibition of protein synthesis.
Cytoskeleton
In addition to providing the biochemical components required for replication, the cell also supplies the virus with an intracellular highway to facilitate infection and assembly. There is growing evidence that the transport of infecting virions to the nucleus and the transport of viral proteins into assembly sites takes place along the various fibers of the cellular cytoskeleton.
RNA Viruses
RNA-containing viruses are discussed in the light of four basic transcriptional strategies (Fig. 1 ). These strategies encompass viruses with message-sense (positive-strand viruses) and antisense genomes (negative-strand viruses), viruses that package their replicative form as genome (double-stranded RNA viruses) and viruses that utilize ‘reverse transcription’. Although this approach is conceptually useful, not all viruses within a class conform precisely to the prototypic replication strategy. Despite this caveat, representative examples will be cited to illustrate the replication mechanism.
Figure 1.

Strategies for the production of viral mRNA utilized by 24 families of viruses infecting humans and other animals. (Updated from Baltimore D (1971) Bacteriol. Rev. 35: 235.)
Positive-strand RNA viruses
Positive-strand viruses contain a single-stranded, message-sense RNA genome which is translated immediately after uncoating. To simplify this discussion, poliovirus (family Picornaviridae; genus Enterovirus) will be used as a prototype because it is the most extensively studied positive-strand virus and provides a clear view of this strategy (Fig. 2 ).
Figure 2.
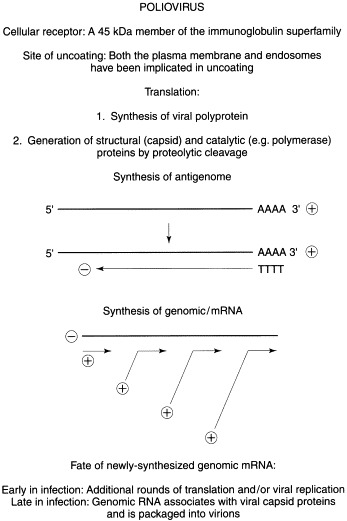
Replication strategy of a representative positive-stranded RNA virus.
Following uncoating, the poliovirus genome is translated to yield an ∼200000 mol. wt polyprotein. Initial cleavage of the polyprotein occurs cotranslationally and is mediated by the autocatalytic activity of a virus-encoded protease, polypeptide 2A. Subsequent cleavages, catalyzed by viral protease 3C, yield structural (capsid) and catalytic (polymerase) proteins. As infection proceeds, translation of capped host mRNAs is blocked due to the virus-induced degradation of the large subunit (eIF-4G) of the mRNA cap recognition factor (eIF-4F). In contrast, viral messages, which are uncapped and possess a highly structured 5′-nontranslated region (5′-NTR), escape shut-off because the 40S ribosomal subunit binds to an internal sequence within the 5′-NTR. The ability of the 40S ribosomal subunit to bind internally, in contrast to the usual scenario in which the 40S ribosome binds at the 5′ end of the message and ‘scans’ until a start codon is encountered, is termed ‘internal ribosome entry’. Thus cap-independent initiation and internal ribosome entry allow poliovirus messages to be selectively translated under conditions where translation of capped messages is progressively compromised.
Following its synthesis, the viral RNA-dependent RNA polymerase catalyzes the synthesis of a full-length negative-sense copy of the genome. Subsequently, the negative-strand serves as template and directs the synthesis of multiple plus-strands. Early in infection, when the concentration of viral structural proteins is low, newly synthesized positive-strands most likely are translated and serve to amplify the synthesis of viral proteins. Later when the concentration of virion precursors is high, newly synthesized plus-strands are encapsidated to generate infectious virus particles. A small virus-encoded protein (VPg) is covalently linked to the 5′ end of all picornavirus RNAs except message. VPg is thought to play a role in RNA synthesis, but it is unclear whether VPg functions as a primer or in some other capacity.
Negative-strand RNA viruses
Because the genome of negative-sense RNA viruses cannot be translated, the first virus-specific biosynthetic event following uncoating is the synthesis of viral mRNA by a virion-associated RNA-dependent RNA polymerase using the viral genome as template. Negative-stranded viruses are divided into two classes: those with unsegmented (monopartite) genomes (i.e. the order Mononegavirales containing the families Paramyxoviridae, Rhabdoviridae and Filoviridae) and those with segmented (multipartite) genomes (i.e. the families Orthomyxoviridae, Bunyaviridae and Arenaviridae). Although each class uses the negative-strand strategy, they possess unique attributes and will be dealt with separately.
The replication of monopartite viruses is discussed using vesicular stomatitis virus (VSV), a rhabdovirus, as the prototype (Fig. 3 ). Immediately after uncoating, the VSV genome is transcribed to yield a short nontranslated ‘leader’ RNA followed, in decreasing molar amounts, by five capped, methylated and polyadenylated viral mRNAs. Transcription occurs within the nucleocapsid, a structure containing the viral genome and multiple copies of three virus-encoded proteins. Aside from the nucleocapsid (N) protein, which tightly encloses the genome and is present in ∼2000 copies/nucleocapsid, two catalytic proteins are also present within the nucleocapsid. The polymerase, polypeptide L (mol. wt ∼200000), is present in about 50 copies per nucleocapsid and catalyzes transcriptional initiation and elongation, as well as capping, methylation and polyadenylation. The phosphoprotein P, present in about 500 molecules per nucleocapsid, plays a variety of roles in RNA synthesis. It binds L to the nucleocapsid, maintains the solubility of free N and may function in chain elongation. The viral transcriptase binds genomic RNA at its 3′ terminus and initiates transcription. At each intergenic junction (with the exception of the leader-N junction), a poly (A) tract is added to the newly synthesized mRNA by repetitive copying (‘stuttering’) of an oligo (U) sequence present at the end of the gene. After synthesis of the poly (A) tract, transcription terminates, releasing newly synthesized mRNA, but maintaining the transcriptase on its template. Re-initiation at the next gene downstream occurs via a conserved start sequence present at the beginning of each gene. However, because re-initiation does not take place every time, downstream genes (i.e. those coding for the envelope and polymerase proteins) are transcribed less frequently than upstream ones encoding the nucleocapsid, phosphoprotein and matrix proteins. Thus viral gene expression is controlled by transcriptional polarity.
Figure 3.

Replication strategy of a representative negative-stranded RNA virus.
Viral genome replication, i.e. the synthesis of a full-length positive-sense copy of the genome and the subsequent generation of progeny negative-strands, is catalyzed by the same polymerase that directs transcription. The switch between the transcriptive and replicative modes appears to be controlled by the concentration of the nucleocapsid protein. When N reaches a critical concentration, it binds newly synthesized RNA within the leader sequence and allows the polymerase to readthrough intergenic regions and synthesize full-size positive-strands (i.e. antigenomes) which will serve as templates for virion RNA synthesis.
Segmented viruses encode their genetic information in multiple molecules of negative-sense RNA. In the case of influenza A virus (Orthomyxoviridae), the genome is composed of eight unique segments of virion RNA. In contrast to most RNA virus families, orthomyxoviruses require a functional cell nucleus for replication. This requirement reflects the fact that the orthomyxovirus polymerase complex can neither initiate transcription de novo nor cap and methylate viral mRNAs. Instead, the complex ‘pirates’ the capped and methylated 5′ terminus from a selected set of newly synthesized host messages and uses these to prime viral transcription. Once initiated, transcription continues until an oligo (U) tract, about 22 nucleotides from the end of virion RNA, signals addition of the 3′ poly (A) tail by repetitive copying. Because of this, viral genomic and mRNAs are not completely complementary, but differ at both their 5′ and 3′ ends. As with unsegmented viruses, the trigger controlling the transition from transcription to replication may be the concentration of nucleocapsid protein.
Other negative-stranded viruses possess additional molecular surprises. Some bunyaviruses (tripartite genome) and all arenaviruses (bipartite genome) possess ‘ambisense’ genomic RNA, in which nonoverlapping subgenomic messages are transcribed from the 3′ ends of both virion RNA and its full-length complement. Furthermore, in what may be the prototype of a new family within the Mononegavirales, Borna disease virus replicates and transcribes its genome within the nucleus and utilizes RNA splicing to generate its messages.
Double-stranded RNA (dsRNA) viruses
Animal viruses with dsRNA genomes are segmented and can be viewed as a variant of the negative-sense strategy in which the virion encapsidates the replicative form of the genome. Genomic dsRNA is transcribed within partially uncoated ribonuclease-resistant viral cores by the virion-associated polymerase to yield viral mRNAs. Early in infection, some progeny plus-strands function as translational templates whereas others associate with nonstructural proteins and form complexes which are transcribed once to yield dsRNA. Newly synthesized dsRNA serves as template for the synthesis of additional viral mRNA which amplifies the replication cycle. Later, as the concentration of core and capsid proteins increases, the dsRNA–protein complex exchanges nonstructural for structural proteins and forms mature virus particles.
RNA viruses that utilize a reverse transcription strategy
Retroviruses replicate their genome and transcribe mRNA using a dsDNA copy of viral genomic RNA as template (Fig. 4 ). This unconventional mechanism, in which single-stranded virion RNA is used as a template for dsDNA synthesis, is catalyzed by a virion-associated, RNA-dependent DNA polymerase (reverse transcriptase). After viral entry, the virion capsid is partially uncoated and a complementary DNA copy of the RNA genome is synthesized using reverse transcriptase. An endonucleolytic activity, integral to the reverse transcriptase, degrades the RNA template and second-strand DNA synthesis begins. Completion of second-strand synthesis results not only in a dsDNA copy of virion RNA, but also generates a unique structure termed the long terminal repeat (LTR). The LTR flanks the viral cDNA and is composed of unique sequences from the 5′ and 3′ ends of the genome and a repeat element common to both ends. Following cDNA synthesis retrovirus DNA is integrated into the host chromosome, and, in this form, is termed the ‘provirus’. Subsequently, the provirus is transcribed by pol II to yield full-length progeny RNA and one or more subgenomic mRNAs. The upstream LTR plays a very important role in retrovirus gene expression because it contains enhancer elements which regulate pol II-catalyzed transcription. (The downstream LTR is not involved in viral gene expression, but may activate host oncogenes and play a role in cell transformation.)
Figure 4.

Replication strategy of a complex retrovirus.
Full-length genome-sized RNA can either be packaged within virions or serve as messenger for the capsid and catalytic viral proteins. Translation of retrovirus genomic RNA yields two classes of polyproteins. The majority (∼95% of the total) encode the capsid, core and matrix proteins and result when translation terminates immediately after the coding region of the nucleocapsid gene. However, a minor population, encoding the aforementioned structural proteins as well as the protease, reverse transcriptase, and integrase results when the stop codon at the end of the capsid/core region is bypassed either by frameshifting or read-through. Envelope glycoproteins are translated from a singly-spliced subgenomic mRNA containing sequences primarily from the 3′ end of the genome, whereas lentiviruses, such as HIV-1, utilize doubly spliced subgenomic mRNAs to direct the synthesis of TAT, REV and several other regulatory proteins.
TAT and REV are the two best-studied of the HIV-1 regulatory proteins. TAT is a trans-acting protein that binds to a sequence present at the 5′ end of all HIV-1 mRNAs and enhances HIV-1 gene expression by relieving a block in transcriptional elongation or by increasing transcriptional initiation. REV mediates the switch between the synthesis of regulatory proteins (i.e. TAT and REV) and the generation of structural and catalytic proteins by binding to cis-acting sequences within viral mRNA and directing the transport into the cytoplasm of unspliced genomic RNA and singly-spliced envelope message.
DNA Viruses
With the exception of parvoviruses and hepadnaviruses, the genomes of which are respectively single-stranded and partially double-stranded, DNA- containing animal viruses possess a dsDNA genome. However, even in these two families, viral mRNA is ultimately transcribed from a dsDNA template using cellular DNA-dependent RNA polymerase (Fig. 1). In place of a detailed discussion of each family, broader issues of viral DNA replication will be discussed. To begin with, DNA viruses differ greatly in their genetic content ranging in size from 5 kbp (Parvoviridae) to greater than 120 kbp (Herpesviridae, Poxviridae and Iridoviridae). Thus the small DNA viruses are about as genetically complex as a typical RNA virus, whereas the larger DNA viruses encode 100 or more proteins. Not unexpectedly, the degree to which virus replication is dependent on cellular functions reflects the genetic complexity of the virus. Thus, parvoviruses and papovaviruses require extensive host involvement to support viral biosynthetic events (including DNA synthesis), whereas other families are progressively more independent.
Among herpes-, pox- and iridoviruses, viral genes are expressed in a coordinated temporal sequence of immediate early, early and late genes. Generally immediate early genes code for proteins required to initiate virus replication, early genes encode catalytic functions (e.g. the viral DNA polymerase), and late genes specify structural proteins. Furthermore, immediate early genes activate early and late gene transcription, whereas specific early and late genes downregulate immediate early and early gene expression respectively. Aside from specific regulatory proteins, full late gene expression also requires viral DNA synthesis, thus inhibitors of viral DNA replication block late gene expression despite the presence of functional immediate early and early activators.
Because DNA polymerase requires a primer with an available 3′-OH to initiate DNA synthesis, all viruses with a linear DNA genome have evolved specialized features that allow them to maintain intact termini during replication. For example, adenoviruses solve the ‘end-problem’ by using a nucleotide-linked terminal protein to initiate DNA replication, herpesviruses replicate through a rolling circle mechanism, and poxviruses and parvoviruses utilize a self-priming mechanism to ensure replication of their termini. In contrast to other DNA viruses, hepadnaviruses possess a circular, partially single-stranded DNA genome that is replicated through an RNA intermediate using virus-encoded reverse transcriptase. Upon entry into the cell the gaps are repaired and dsDNA is transcribed in the nucleus by host polymerase to yield viral mRNAs and pregenomic RNA. The latter is encapsidated and transcribed into complementary DNA using virus-encoded protein P both as the primer and the reverse transcriptase. As with retroviruses, the RNA template is degraded and second-strand DNA synthesis takes place. However, before its completion, the virion is exported from the cell leaving genomic DNA partially single-stranded.
Unlike other DNA viruses, poxviruses replicate solely within the cytoplasm in morphologically distinct viral ‘factories’. Reflecting their metabolic independence from the host cell, poxviruses synthesize unique DNA and RNA polymerases, and their virions contain all the proteins needed to transcribe the earliest class of viral mRNAs. Furthermore, viral transcriptional promoters and termination sequences are unique and are regulated by virus-specific factors.
Iridoviruses, occupying a taxonomic middle-ground between poxviruses and the nuclear DNA viruses, possess several distinctive features. Viral DNA replication takes place in two distinct compartments (genome-length progeny DNA is synthesized in the nucleus, followed by the synthesis of concatemeric DNA in the cytoplasm), whereas virion assembly is confined to cytoplasmic viral ‘assembly sites’. Viral DNA is highly methylated with nearly 25% of cytosine residues present as methylcytosine. Methylation is catalyzed by a virus-encoded enzyme and, as in some bacteriophage systems, appears to function as part of a restriction–modification system. Surprisingly, despite the high content of methylcytosine, host RNA polymerase II has been implicated in at least the early rounds of viral transcription. However, it is not known whether unmodified pol II transcribes viral DNA late in infection or whether viral-encoded proteins modify pol II and alter its specificity.
Virus Assembly
Once sufficient stores of viral nucleic acid and protein have accumulated in the infected cell, nucleocapsid formation and virion assembly begin and continue as long as the cells are metabolically competent. Despite the large number of vertebrate virus families, only three types of nucleocapsids are found: complex, helical and icosahedral (spherical). The nucleocapsids of poxviruses do not conform to the geometric symmetry found among the helical and icosahedral viruses and are considered to be ‘complex’. Little is known about the molecular mechanisms controlling their assembly. Helical nucleocapsids (which, among vertebrate viruses, enclose only RNA genomes) form as viral proteins bind to nascent RNA transcripts and encapsidate them. During virus assembly, helical nucleocapsids migrate to cellular membranes where viral glycoproteins have concentrated. There, through concerted interaction between the nucleocapsid and viral glycoproteins, the nucleocapsid is enveloped by the cellular membrane in a process termed ‘budding’. Host proteins are excluded from the membrane and the resulting envelope contains only virus-encoded glycoproteins. Moreover, because envelopment is not a precise process, dual infections with different strains of the same multipartite virus (e.g. influenza virus A) lead to high-frequency genetic reassortment. Although virion envelopment takes place commonly at the plasma membrane (e.g. among the Paramyxoviridae, Orthomyxoviridae and Rhabdoviridae), intracellular membranes (e.g. those of the Golgi, endoplasmic reticulum and, in the case of DNA viruses, the nucleus) are used by other virus families. In contrast to viruses with helical nucleocapsids, icosahedral nucleocapsids enclose both DNA and RNA genomes. It is thought that nucleocapsids form spontaneously when the concentration of capsid proteins reaches a critical level. In some families, nucleocapsids are not enveloped (i.e. virion equals nucleocapsid), whereas in others nucleocapsids are enveloped as described above. Enveloped icosahedral viruses are released from infected cells by budding, whereas non-enveloped icosahedral virions are liberated by cell lysis.
See also:
Cell Structure and Function in Virus Infections; Virus–Host Cell Interactions.
Further Reading
- Cann A.J. Principles of Molecular Virology. 2nd edn. Academic Press; San Diego: 1997. [Google Scholar]
- Ehrenfeld E. Translational regulation in virus-infected cells. Semin. Virol. 1993;4:199. [Google Scholar]
- Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. 3rd edn. Lippincott–Raven; New York: 1996. [Google Scholar]
- Joklik W.K., Willet H.P., Amos D.B., editors. Zinsser Microbiology. 20th edn. Appleton and Lange; Norwalk, CT: 1992. [Google Scholar]
- Levy J.A., Fraenkel-Conrat H., Owens R.A. Virology. 3rd edn. Prentice-Hall; Englewood Cliffs, NJ: 1994. [Google Scholar]
- Wimmer E. Cellular Receptors for Animal Viruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. [Google Scholar]


