Abstract
Cupressus sempervirens L. (Cupressaceae), known as Mediterranean cypress, is a medicinal and aromatic plant with decorative uses. It is widely distributed throughout the Mediterranean region, including Syria, Turkey, Cyprus, Lebanon, Palestine, and some Greek Islands, as well as the southern coasts of the Caspian Sea in Iran. The plant has a long history of utilization in folk medicine in many countries, mainly for cough and flu treatment, and the research has consistently shown that C. sempervirens has antimicrobial, antiviral, insecticidal, antihyperlipidemic, cytotoxic, antioxidant, antiplatelet, hepatoprotective, and neurobiological activities to varying extents. The mentioned biological activities of the plant are strongly associated with its phytochemical content, which is particularly rich in phenolic constituents and essential oils that could be responsible for most of its antimicrobial, antioxidant, and antiviral activities. This chapter covers botanical information, brief phytochemistry, and pharmacological activities related to C. sempervirens.
Keywords: Cupressus sempervirens, Mediterranean cypress, Biological activity, Phytochemistry, Folk medicine
Introduction
Cupressus sempervirens L., known as Mediterranean cypress, is an ornamental tree and a member of the Cupressaceae family. Among the habitats of the species, northern America, Africa, southeastern Europe, and western Asia can be cited. Using these plants can protect fields from wind damage. Many researchers have reported that the species might demonstrate both fastigiate and horizontal growth habits, and therefore, it has been given subspecific taxonomic ranks. As the fastigiated form has a horticultural origin dating back to early historic or prehistoric times, it is commonly accepted that only the horizontal form precedes human activity.
Cypress species, represented by 25 different taxa in Mediterranean Region, North America, and Asia, are primarily divided into three main groups: Mediterranean cypress, North American cypress, and Asian cypress. Among them, Mediterranean cypress consists of Cupressus sempervirens L., Cupressus atlantica Gaussen, and Cupressus dupreziana A. Camus. Although there are many different subspecies and varieties of this species, the widely accepted varieties by ramification type are branched cypress (Cupressus sempervirens L. var. horizantalis (Mill.) Cord.) and pyramidal (Ehrami) cypress (Cupressus sempervirens L. var. pyramidalis (fastigata = stricta) (Pictures 1 and 2 )).
Picture 1.

Cupressus sempervirens var. horizontalis (by I. Tumen).
Picture 2.

Cupressus sempervirens var. pyramidalis (by M. Karakose).
The Mediterranean cypress naturally exists on the southern coasts of the Caspian Sea in Iran, Syria, Turkey, Cyprus, Lebanon, Palestine, and some Greek Islands (Crete, Rhodes, Samos, Kos, Symi, and Melos). It is one of the ordinary trees in the Mediterranean region and spreads from the Himalayas to China. All cypresses are quite decorative, especially in their early periods, and used in park and garden landscaping as protective, strip, and live fence trees. The Mediterranean cypress can reach up to 20–30 m in height, and its trunk is in a conical shape (Picture 3 ). The most important property distinguishing Mediterranean cypress from other species is the oil glands within long cavities on the backs of its dark green scale leaves [1].
Picture 3.

The fresh cones and leaves of Cupressus sempervirens var. horizontalis (by I. Tumen).
The original native distribution of the species is unknown on account of its long horticultural history. In fact, some researchers attribute the native distribution of the species to Greece (some Aegean islands), Turkey, Crete, north Iran, Lebanon, Syria, and likely Cyprus (which would only be appropriate) (Picture 4 ). It might be endemic to Tunisia and northern Libya in north Africa, and at present, the species is grown or found wild throughout the whole Mediterranean region [2].
Picture 4.

The dry cones and seeds of Cupressus sempervirens var. pyramidalis (by I. Tumen).
Phytochemistry of Cupressus sempervirens
Various classes of phytochemical compounds have been reported in different parts of C. sempervirens, including flavonoid derivatives (rutin, quercetin, quercetin rhamnoside, quercitrin, myricitrin and kaempferol 3-0-rhamnoside, cupressuflavone, amentoflavone, and other biflavonoids) [3,4], diterpenes (neocupressic acid, isocupressic acid, sugiol, communic acid, sandracopimaric acid, imbricatolic acid, acetoxyimbricatolic acid, ferruginol, abita-8,11,13-triene-20-ol) [5], sesquiterpenes (junepediol) [1], catechins and flavonolic oligomers [6], proanthocyanidins [7], essential oils [[8], [9], [10], [11], [12], [13], [14], [15]], phenolic acids (caffeic acid and p-coumaric acid) [4], and fatty acids [16].
Use of Cupressus sempervirens in Folk Medicine
The leaves and cones of C. sempervirens have been used as folk remedies in different parts of the world for antiseptic, antipyretic, anthelminthic, astringent, antirheumatic, antihemorrhoidal, antidiarrhoeic, and vasoconstrictive purposes [[17], [18], [19], [20], [21], [22]].
Biological Activities of Cupressus sempervirens
Depending on the phytochemicals found in C. sempervirens, it has been reported to possess a number of biological activities desired for human health.
Antimicrobial and Antiviral Activity
Early studies revealed that C. sempervirens had strong antimicrobial and antiviral activity [23]. For instance, the proanthocyanidin-rich fraction of C. sempervirens was found to have in vitro antiviral activity against two retroviruses, human immunodeficiency virus and human T-lymphotropic virus III B [24]. The inhibitory activity of the fruit essential oil of C. sempervirens subsp. pyramidalis was examined using severe acute respiratory syndrome coronavirus (SARS-CoV) and Herpes simplex virus type 1 (HSV-1) replication assays, and the oil exerted a mild effect only against SARS-CoV (700 ± 2.3 μg/mL) [11]. C. sempervirens essential oil was identified with marked antiviral activity against HSV-1, having virucidal effects of 68.0% and 53.2% at concentrations of 1:32 and 1:64, respectively [12].
The essential oil of the plant totally inhibited the growth of both antibiotic-susceptible and antibiotic-resistant Helicobacter pylori strains at a concentration of 0.1% (v/v) [25]. In a similar study, an inhibition zone 1.1 cm in diameter was caused by the ethanol extract of C. sempervirens against H. pylori [26]. The remarkable antimicrobial activity of the oil was also demonstrated on the survival and growth of three food pathogens: Escherichia coli, Staphylococcus aureus, and Listeria innocua [27]. The antimicrobial activity of the methanol extract from the male and female leaves and fruits of C. sempervirens var. horizontalis, C. sempervirens var. sempervirens, and C. sempervirens cv. cereifeormis was investigated against Pseudomonas aeruginosa, S. aureus, E. coli, and Candida albicans, using a number of methods, including the disc diffusion, hole plate, cylinder agar diffusion, and agar dilution methods, along with minimum inhibitory concentration (MIC) values determined by the agar dilution method [28]. The extracts exerted antimicrobial activity in varying ranges, but they were most effective against S. aureus. In a similar study [29], the essential oils obtained from the needles and twigs of C. sempervirens were tested against three bacteria (E. coli, Micrococcus luteus, and Bifidobacterium lactis) using the diffusion method. The oils were also tested against seven fungus strains (Aspergillus niger, A. flavus, A. fumigatus, Fusarium solani, F. oxysporum, Penicillium digitatum, and Candida uterus), using flask culture and potato dextrose agar methods, and they were found to have strongest activity against B. lactis. On the other hand, the oils were most active against A. niger in the flask culture method and against C. uterus in the potato dextrose agar method. The leaf essential oil of C. sempervirens was also demonstrated to possess significant antimicrobial and antifungal effects against a wide range of bacteria (Bacillus substilis, S. aureus, E. coli, Pseudomonas aeruginosa) and fungi (A. niger, A. flavus, A. fumigatus, and C. albicans) [30].
Nevertheless, the essential oil of the plant displayed insignificant antifungal activity against Casuarina timber, Aspergillus sp., Penicillium sp., Fusarium sp., and Mucor sp., as tested by the agar disk diffusion method [31]. Consistent with this study, the leaf extract of C. sempervirens, which was determined to be rich in polyphenols expressed as quercetin glycosides (174 μg/mL) and biflavonoids (1460 μg/mL), did not exert any notable antifungal effect against a wide range of isolated strains of 24 yeast species from different countries (Candida albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. zeylanoides, Pichia guilliermondii, Clavispora lusitaniae, Issatchenkia orientalis, Kluyveromyces marxianus, Saccharomyces cerevisiae, Yarrowia lipolytica, Cryptococcus laurentii, Filobasidiella neoformans, Prototheca wickerhamii, and P. zopfii) using an agar diffusion well bioassay [32]. The essential oil of the plant was evaluated another time for its antifungal activity against American type of culture collection strains of five food-spoilage yeasts, including C. albicans, Rhodotorula glutinis, Schizosaccharomyces pombe, S. cerevisiae, and Yarrowia lipolytica, and four fungus strains were notably inhibited by the essential oil with MIC values of 0.06–0.08 mg/mL, while it possessed moderate effect against Y. lypolytica (MIC = 0.23 mg/mL) [33]. The oil was subjected to in vitro assays to establish its antifungal effect toward the human pathogen Aspergillus niger through the inhibition of hyphal growth and spore formation in A. niger [34]. However, it did not show any antifungal effect in these assays.
Antiprotozoal Activity
The antiprotozoal effect of the hydroalcoholic extract of C. sempervirens was found to be mildly active against Leishmania amazonensis [35]. In consistency with this data, the methanol extract of C. sempervirens was ineffective in antiprotozoal assays against the erythrocytic schizonts of Plasmodium falciparum, the intracellular amastigotes of Leishmania infantum, and the Trypanosoma cruzi and free trypomastigotes of T. brucei [36].
Insecticidal Activity
The essential oil obtained from C. sempervirens was reported to exert repellent activity against the codling moth, Cydia pomonella [37]. The flagellate populations of the termite Kalotermes flavicollis were reduced by the essential oil of C. sempervirens on the second (31%) and fourth days (100%). With the hindgut spirochetes of this termite, the essential oil was again effective (46 and 100%) [31]. The repellent and toxic potentials of the essential oil of C. semperviren and its main constituent (cymol) were evaluated against Sitophilus zeamais and Tribolium confusum using impregnation on filter paper discs, as well as coating onto maize grains, and these potentials led to the diminishment of grain weight loss [38]. Moreover, the essential oil had a higher repellent effect than that of cymol. Nevertheless, the cypress essential oil was shown to possess low repellent activity against the mosquito Aedes aegypti [39].
In a study of five Cupressus species (C. arizonica, C. benthamii, C. macrocarpa, C. sempervirens, and C. torulosa), the essential oil from C. benthamii exerted the highest larvicidal activity (LC50 = 37.5 mg/L) against the mosquito Aedes albopictus, while the rest of the essential oils displayed only moderate toxicity against the larvae (LC50 = 47.9–70.6 mg/L) [14]. In addition, C. sempervirens essential oil provided a mild repellent effect against this mosquito species.
Antihyperlipidemic Effect
The cone hydroalcoholic extract of C. sempervirens was tested on two groups of rats, one of which was the control group, for its antihyperlipidemic activity on parameters of serum lipid, muscle and liver enzymes, red and white blood count, platelets, and serum concentrations of uric acid and creatinine [40]. The extract displayed a significant reducing effect on serum cholesterol, whereas it did not cause any changes in triglyceride and high density lipoprotein cholesterol.
Anticancer and Cytotoxic Effect
The cytotoxic effect of the hydroalcoholic fruit extract of C. sempervirens var. horizentalis was tested against three human tumor cell lines by MTT assay. However, the extract did not exhibit any cytotoxic effect in this assay [41]. The cytotoxic effect of C. sempervirens subsp. pyramidalis leaf and cone essential oils was evaluated on several human amelanotic melanoma (C32) and renal adenocarcinoma (ACHN) cells [42]. The leaf oil led to a decrease in ACHN cell viability at 100 μg/mL (53%) and 400 μg/mL (40%), while it displayed cytotoxicity against C32 cells with an IC50 value of 104.90 μg/mL. However, the cone oil was found to be completely inactive in cytotoxicity assays.
In a screening study that tested the plants used in Yemeni traditional medicine on three human cancer cell lines, C. sempervirens was among the most active plant species, having IC50 values below 50 μg/mL [19]. In another study [12], the CHCl3 fraction of C. sempervirens demonstrated a significant cytotoxicity against HeLa cells. The cytotoxicity of the essential oil of the plant was tested on HepG2, Hep3B, A549, MCF-7, and MDA-MB-231 cancer cells, and it showed much less cytotoxicity against these cell lines [42].
Antioxidant Effect
The essential oil of C. sempervirens was evaluated in three antioxidant test systems: 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, β-carotene bleaching test, and luminol-photochemiluminescence (PCL) assay. It showed a mild scavenging effect (below 30%) against DPPH and a marked lipid peroxidation inhibitory effect in the β-carotene bleaching test (around 60%), while it was ineffective in the PCL assay [33].
The antioxidant properties of the dichloromethane, acetone, ethyl acetate, and methanol extracts obtained from the cones and leaves of C. sempervirens var. horizantalis and var. pyramidalis were demonstrated in our study using DPPH and N,N-dimethyl-p-phenylendiamine radical-scavenging activity and metal-chelation capacity with ferric- and phosphomolibdenum-reducing antioxidant power tests, and a variable level of activity according to the methods was observed [43]. In another study on the antioxidant activity of the essential oils obtained from C. sempervirens by supercritical fluid extraction (SFE) with CO2 and hydrodistillation (HD) methods, the essential oil obtained using SFE displayed higher activity in 2,20-azinobis-3-ethylbenzo-thiazoline-6-sulfonate and DPPH radical-scavenging activity tests [15].
The essential oils obtained from the branchlets and fruits of C. sempervirens var. horizontalis were evaluated using a linoleic acid peroxidation test and peroxyl radical-mediated hemolysis of red blood cells (RBC) assays, and RBC hemolysis and lipid peroxidation were observed to be inhibited by both of the oils in a concentration-dependent manner [13].
Antiplatelet and Anticoagulant Activity
The essential oil of C. sempervirens was subjected to in vivo assays in order to determine its antiplatelet activity using clot retraction and platelet aggregation effects in guinea pig and rat plasma [9]. Nonetheless, the oil exerted a deprived effect against arachidonic acid-induced aggregation, as well as in a clot retraction assay at up to 300 μg/mL concentration. In accordance with this study, the authors concluded that the essential oil of the plant was ineffective in a thrombin- and collagen-induced antiplatelet aggregation assay. In contrary, Ulusal et al. [44] reported that the fresh cone water extract of C. sempervirens was shown to have a strong anticoagulant effect in an in vitro assay.
Hepatoprotective Activity
The leaf methanol extract prepared from C. sempervirens was tested against CCl4-induced hepatotoxicity in rats by oral administration [4]. According to the findings, the extract caused a substantial reduction in glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, cholesterol level, and triglycerides, but a significant increase in the total protein level was observed with the extract of C. sempervirens.
Wound Healing and Anti-inflammatory Activity
An earlier study revealed that the proanthocyanidin oligomeric fraction of C. sempervirens was able to block pancreatic elastase via inhibition of esterolytic activity of the enzyme, which is related to wound healing [16].
The probable wound-healing effects of the cone essential oils of C. sempervirens var. horizontalis and var. pyramidalis were examined in linear incision and circular excision experimental wound models, along with their anti-inflammatory activity in an assay involving acetic acid-induced increases in capillary permeability [45]. However, the essential oils were not active in these experiments.
Neurobiological Activity
The possible neuroprotective properties of the dichloromethane, acetone, ethyl acetate, and methanol extracts obtained from the cones and leaves of C. sempervirens var. horizantalis and var. pyramidalis were evaluated by our group through the inhibition of acetyl- (AChE) and butyrylcholinesterase (BChE) enzymes, which are related to Alzheimer’s disease, as well as tyrosinase, an enzyme associated with Parkinson’s disease [43]. The extracts exerted mild to moderate levels of inhibition against cholinesterases and a low level of inhibition against tyrosinase at 200 μg/mL (Figures 1 and 2 ).
Figure 1.
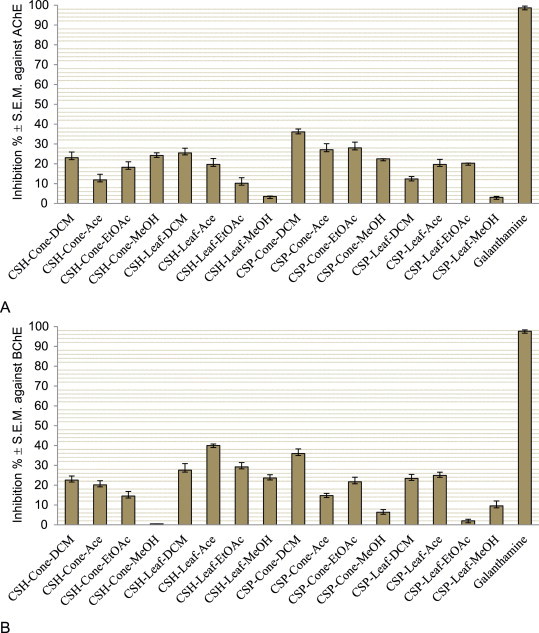
Inhibition of acetylcholinesterase (A) and butyrylcholinesterase (B) (inhibition ± S.E.M.%) by the dichloromethane (DCM), acetone (Ace), ethyl acetate (EtOAc), and methanol (MeOH) extracts of the Cupressus species and the reference (galanthamine) at 200 μg/mL. CSH: Cupressus sempervirens var. horizantalis, CSP: Cupressus sempervirens var. pyramidalis.
Figure 2.
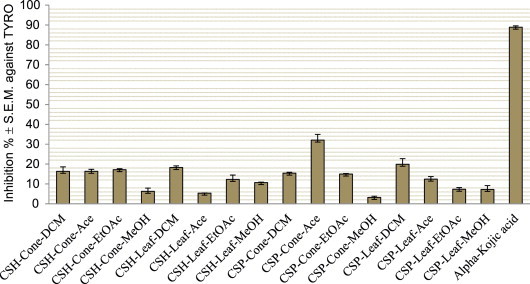
Inhibition of tyrosinase (inhibition ± S.E.M.%) by the dichloromethane (DCM), acetone (Ace), ethyl acetate (EtOAc), and methanol (MeOH) extracts of the Cupressus species and the reference (alpha-kojic acid) at 200 μg/mL. CSH: Cupressus sempervirens var. horizantalis, CSP: Cupressus sempervirens var. pyramidalis.
In a previous study [46], the essential oil of the plant was consistently shown to have moderate level of AChE inhibition, with approximately 70% inhibition at 2.5 mg/mL.
Conclusion
C. sempervirens is an aromatic plant widely used in traditional medicine because it has important biological activities related to human health. However, more research on this species is definitely needed in order to extend its use in health care.
Summary Points
-
•
Cupressus sempervirens L., known as Mediterranean cypress, is a medicinal and aromatic plant.
-
•
The most common varieties are Cupressus sempervirens var. horizantalis and Cupressus sempervirens L. var. pyramidalis.
-
•
Mediterranean cypress has a long history of use in traditional medicine.
-
•
Cypress is rich in polyphenols, terpenes, and essential oils.
-
•
Cypress oil has strong antimicrobial and insecticidal effects.
-
•
Cypress possesses many bioactivities desirable for human health.
Abbreviations
- AChE
acetylcholinesterase
- ACHN
renal adenocarcinoma cell line
- ATCC
American type of culture collection
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- HSV
herpes simplex virus
- IC
inhibitory concentration
- LC
lethal concentration
- MIC
minimum inhibitory concentration
- PCL
luminol-photochemiluminescence
- RBC
red blood cell
- SARS-CoV
severe acute respiratory syndrome coronavirus
- SFE
solid phase extraction
References
- 1.Piovetti L., Francisco C., Pauly G., Benchabane O., Bernard-Dagan C., Diara A. Volatile constituents of Cupressus dupreziana and the sesquiterpenes of Cupressus sempervirens. Phytochemistry. 1981;20:1299–1302. [Google Scholar]
- 2.Vidakovic M. Croatia; Graficki Zavod Hrvatske: 1991. Conifers: morphology and variation. Translated from Croatian by Maja Soljan. [Google Scholar]
- 3.Romani A., Galardi C., Pinelli P., Mulinacci N., Heimler D. HPLC quantification of flavonoids and biflavonoids in Cupressaceae leaves. Chromatographia. 2002;56:469–474. [Google Scholar]
- 4.Ibrahim N.A., El-Seedi H.R., Mohammed M.M.D. Phytochemical investigation and hepatoprotective activity of Cupressus sempervirens L. leaves growing in Egypt. Nat Prod Res. 2007;21:857–866. doi: 10.1080/14786410601132477. [DOI] [PubMed] [Google Scholar]
- 5.Bernard P., Susplugas P., Balansard G., Lallemand M. Rapid separation of diterpenic acids contained in cores of Cypressus sempervirens L. by means of preparative liquid chromatography. Plantes Medicinales et Phytotherapie. 1978;12:137–143. [Google Scholar]
- 6.Jonadet M., Meunier M.T., Villie F., Bastide J., Bastide P. Catechins and flavanolic oligomers from Cupressus sempervirens L. Comparative in vitro elastase inhibitory and in vivo angioprotective activity. Ann Pharm Fr. 1984;42:161–167. [PubMed] [Google Scholar]
- 7.Vennat B., Gross D., Pourrat A., Pourrat H. Polyphenols from Cupressus sempervirens: Qualitative and quantitative assays of proanthocyanidins and flavonoids. Farmaco. 1991;46:685–698. [Google Scholar]
- 8.Kassem F.F., Harraz F.M., El-Sebakhy N.A., De Pooter H.L., Schamp Abou-Schleib N.M.H. Composition of the essential oil of Egyptian Cupressus sempervirens L. cones. Flavour Fragr J. 1991;6:205–207. [Google Scholar]
- 9.Tognolini M., Barocelli E., Ballabeni V., Bruni R., Bianchi A., Chiavarini M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006;78:1419–1432. doi: 10.1016/j.lfs.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Uçar G., Uçar M.B., Fakir H. Composition of volatile foliage isolates from Cupressus sempervirens varieties (var. horizontalis Mill. and pyramidalis Nyman) growing in Turkey. J Essent Oil Res. 2007;19:562–565. [Google Scholar]
- 11.Loizzo M.R., Saab A.M., Tundis R., Statti G.A., Menichini F., Lampronti I. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem Biodivers. 2008;5:461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim N.A., El-Seedi H.R., Mohammed M.M.D. Constituents and biological activity of the chloroform extract and essential oil of Cupressus sempervirens. Chem Nat Comp. 2009;45:309–313. [Google Scholar]
- 13.Asgary S., Naderi G.A., Shams Ardekani M.R., Sahebkar A., Airin A., Aslani S. Chemical analysis and biological activities of Cupressus sempervirens var. horizontalis essential oils. Pharm Biol. 2013;51:137–144. doi: 10.3109/13880209.2012.715168. [DOI] [PubMed] [Google Scholar]
- 14.Giatropoulos A., Pitarokili D., Papaioannou F., Papachristos D.P., Koliopoulos G., Emmanouel N. Essential oil composition, adult repellency and larvicidal activity of eight Cupressaceae species from Greece against Aedes albopictus (Diptera: Culicidae) Parasitol Res. 2013;112:1113–1123. doi: 10.1007/s00436-012-3239-5. [DOI] [PubMed] [Google Scholar]
- 15.Nejia H., Séverine C., Jalloul B., Mehrez R., Stéphane C.J. Extraction of essential oil from Cupressus sempervirens: Comparison of global yields, chemical composition and antioxidant activity obtained by hydrodistillation and supercritical extraction. Nat Prod Res. 2013;27:1795–1799. doi: 10.1080/14786419.2012.755680. [DOI] [PubMed] [Google Scholar]
- 16.Meunier M.T., Villie F., Bastide P. The interaction of Cupressus sempervirens L. proanthocyanidolic oligomers with elastase and elastins. J Pharm Belg. 1994;49:453–461. [PubMed] [Google Scholar]
- 17.Jaradat N.A. Ethnopharmacological survey of natural products in Palestine. An-Najah Univ J Res. 2005;19:13–67. [Google Scholar]
- 18.Tuzlaci E., Emre Bulut G. Turkish folk medicinal plants, part VII: Ezine (Çanakkale) J Pharm Istanbul Univ. 2007;39:39–51. [Google Scholar]
- 19.Mothana R., Gruenert R., Bednarski P.J., Lindequist U. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Pharmazie. 2009;64:260–268. [PubMed] [Google Scholar]
- 20.Benitez G., Gonzalez-Tejero M.R., Molero-Mesa J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J Ethnopharmacol. 2010;129:87–105. doi: 10.1016/j.jep.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Alachkar A., Jaddouh A., Elsheikh M.S., Bilia A.R., Vincieri F.F. Traditional medicine in Syria: folk medicine in Aleppo governorate. Nat Prod Commun. 2011;6:79–84. [PubMed] [Google Scholar]
- 22.Polat R., Satil F. An ethnobotanical survey of medicinal plants in Edremit Gulf (Balikesir—Turkey) J Ethnopharmacol. 2012;139:626–641. doi: 10.1016/j.jep.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Izzo A.A., Di Carlo G., Biscardi D., De Fusco R., Mascolo N., Borrelli F. Biological screening of Italian medicinal plants for antibacterial activity. Phytother Res. 1995;9:281–286. [Google Scholar]
- 24.Amouroux P., Jean D., Lamaison J.-L. Antiviral activity in vitro of Cupressus sempervirens on two human retroviruses HIV and HTLV. Phytother Res. 1998;12:367–368. [Google Scholar]
- 25.Ohno T., Kita M., Yamaoka Y., Imamura S., Yamamoto T., Mitsufuji S. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8:207–215. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 26.Bergonzelli G.E., Donnicola D., Porta N., Corthésy-Theulaz I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob Agents Chemother. 2003;47:3240–3246. doi: 10.1128/AAC.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romeo F.V., De Luca S., Piscopo A., Poiana M. Antimicrobial effect of some essential oils. J Essent Oil Res. 2008;20:373–379. [Google Scholar]
- 28.Afsharzadeh M., Naderinasab M., Najaran Z.T., Barzin M., Emami S.A. In vitro antimicrobial activities of some Iranian conifers. Iranian J Pharm Res. 2013;12:63–74. [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmood Z., Ahmed I., Saeed M.U.Q., Sheikh M.A. Investigation of physico-chemical composition and antimicrobial activity of essential oil extracted from lignin-containing Cupressus sempervirens. BioResources. 2013;8:1625–1633. [Google Scholar]
- 30.Manivannan R., Kumar M.S., Jawahar N., Ganesh E.S., Jubie S. A comparative antimicrobial study on the essential oil of the leaves of various species of Cupressus. Anc Sci Life. 2005;24:131–133. [PMC free article] [PubMed] [Google Scholar]
- 31.Alfazairy A.A.M. Antimicrobial activity of certain essential oils against hindgut symbionts of the drywood termite Kalotermes flavicollis Fabr. and prevalent fungi on termite-infested wood. J Appl Entomol. 2004;128:554–560. [Google Scholar]
- 32.Turehetti B., Pinelli P., Buzzini P., Romani A., Heimler D., Franconi F. In vitro antimycotic activity of some plant extracts towards yeast and yeast-like strains. Phytother Res. 2005;19:44–49. doi: 10.1002/ptr.1622. [DOI] [PubMed] [Google Scholar]
- 33.Sacchetti G., Maietti S., Muzzoli M., Scaglianti M., Manfredini S., Radice M. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–632. [Google Scholar]
- 34.Pawar V.C., Thaker V.S. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses. 2006;49:316–323. doi: 10.1111/j.1439-0507.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 35.García M., Monzote L., Montalvo A.M., Scull R. Screening of medicinal plants against Leishmania amazonensis. Pharm Biol. 2010;48:1053–1058. doi: 10.3109/13880200903485729. [DOI] [PubMed] [Google Scholar]
- 36.Al-Musayeib N.M., Mothana R.A., Matheeussen A., Cos P., Maes L. In vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsular region. BMC Complement Altern Med. 2012;12 doi: 10.1186/1472-6882-12-49. Art. no. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landolt P.J., Hofstetter R.W., Biddick L.L. Plant essential oils as arrestants and repellents for neonate larvae of the codling moth (Lepidoptera: Tortricidae) Environ Entomol. 1999;28:954–960. [Google Scholar]
- 38.Tapondjou A.L., Adler C., Fontem D.A., Bouda H., Reichmuth C. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J Stored Products Res. 2005;41:91–102. [Google Scholar]
- 39.Drapeau J., Fröhler C., Touraud D., Kröckel U., Geier M., Rose A. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flavour Fragr J. 2009;24:160–169. [Google Scholar]
- 40.Karkabounas S., Kiortsis D.N., Zelovitis J., Skafida P., Demetzos C., Malamas M. Effects of Cupressus sempervirens cone extract on lipid parameters in Wistar rats. In Vivo. 2003;17:101–103. [PubMed] [Google Scholar]
- 41.Emami S.A., Sadeghi-Aliabadi H., Saeidi M., Jafarian A. Cytotoxic evaluations of Iranian conifers on cancer cells. Pharm Biol. 2005;43:299–304. doi: 10.1080/13880200590951676. [DOI] [PubMed] [Google Scholar]
- 42.Loizzo M.R., Tundis R., Menichini F., Saab A.M., Statti G.A., Menichini F. Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell Prolif. 2008;41:1002–1012. doi: 10.1111/j.1365-2184.2008.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tumen I., Senol F.S., Orhan I.E. Evaluation of possible in vitro neurobiological effects of two varieties of Cupressus sempervirens (Mediterranean cypress) through their antioxidant and enzyme inhibition actions. Turkish J Biochem. 2012;37:5–13. [Google Scholar]
- 44.Ulusal B.G., Arikan S., Durusoy C. Anticoagulant effect of Cupressus sempervirens. Phytother Res. 2007;21:1116. doi: 10.1002/ptr.2220. [DOI] [PubMed] [Google Scholar]
- 45.Tumen I., Süntar I., Keleş H., Küpeli Akkol E. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evidence-based Complementary and Alternative Medicine. 2012 doi: 10.1155/2012/728281. Art. no. 728281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aazza S., Lyoussi B., Miguel M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules. 2011;16:7672–7790. doi: 10.3390/molecules16097672. [DOI] [PMC free article] [PubMed] [Google Scholar]


