Abstract
This chapter highlights the clinical features of Ebola virus disease and findings on laboratory tests. The steps required to differentiate Ebola virus disease from other endemic infectious diseases are presented. The chapter also describes the precautionary guidelines set up by Center for Disease Control and Prevention in regards to proper usage of personal protective gear and handling of biological specimens of Ebola virus disease patients.
Keywords: Biosafety level, Personal protective equipment, Polymerase chain reaction, Reverse transcriptase polymerase chain reaction, Zmapp
A typical case of Ebola virus disease
A 33-year-old man was working in Liberia as a health-care worker since October 2013. He took his daily prophylaxis against malaria infection. By April 2014, he and his team had established an Ebola treatment unit in Monrovia, Liberia. In July 2014, he woke up feeling feverish and tired. He measured his temperature and found that he was slightly febrile with a temperature of 37.8 °C when measured through a thermometer placed in the mouth (the normal temperature is 37.4 °C). He informed his colleagues of his illness and stayed at his local residence in Liberia. Two rapid malaria tests were negative for identification of malaria infection. With the passage of time, his fever got worse, heightening to a body temperature of 38.6 °C. He also felt nauseated.
Polymerase chain reaction (PCR) tests for Lassa fever, yellow fever, and Ebola virus disease were all negative at the time. As his fever continued, he was treated with intravenous fluids and antibiotics. On day 4 of his illness, tests were repeated for malaria, Lassa fever, yellow fever, and Ebola virus. This time, the test demonstrated that he had Ebola virus disease. It is not uncommon for initial tests to be negative in very early Ebola virus infection because the amount of virus is too small to be detected in the blood.1
On day 6, he developed a petechial (spotty bluish) rash on his arms and chest. These rashes usually represent bleeding from small blood vessels under the skin. His fever spiked to an all-time high of 40 °C. He had severe abdominal pains and diarrhea. He had an episode of bloody stool and was subsequently transfused with a single unit of blood. Later on, his rash has turned into a maculopapular rash (a mix of flat and raised red bumps like lesions).1
On day 7, he was vomiting blood for which he was given another blood transfusion. He also received a unit of convalescent whole blood from an Ebola virus disease survivor. Despite the convalescent whole blood transfusion, his condition continued to worsen. For his fever and muscle pains, he received a gram of generic Tylenol every 6 h. He was given Tang and Gatorade to hydrate himself by mouth because of concerns regarding dehydration in the face of continuing vomiting and diarrhea. He appeared malnourished partly due to the severe anorexia.1
On day 9, a dose of intravenous Zmapp was administered, an experimental antibody-based treatment described in more detail in other chapters. Within 8 h of administration of Zmapp, he reported improvement in his energy level to the point that he was able to walk. The extent of his rash had decreased significantly as well. The professionals caring for him reported improvements in his fever and level of alertness.1
On day 10, he was transferred to Emory University’s Communicable Diseases Unit in Atlanta, Georgia, for treatment. On day 11, he had slight fever with a temperature of 38.8 °C, heart beating fast with 120 beats per minute, and an oxygen saturation of 91%. Oxygen saturation is measured through a monitor taped to one of the fingers and measured the approximate concentration of oxygen in blood by measuring the wavelengths of light passed through arterial blood. The normal value is 95–99%. He was started on 2 L of supplemental oxygen administered via inhalation and resulted in improvement in saturation to 93%. Normally, a resonating sound is heard when lungs are percussed, but there was a dull sound coming from his lungs on percussion, indicating that the low oxygen reading could be a result of inadequate oxygenation of blood because the lungs were not working well. He was severely dehydrated, and low blood pressure and rapid heart rate were recorded every time he stood up. His physical examination showed no obvious bleeding but the persistence of the same petechial rashes. He described pain after a manual pressing of the abdomen during examination, specifically in the right upper quadrant. His liver was not enlarged.1
He had some abnormalities detected on laboratory analysis. His internationalized normal ratio (a test of coagulation parameters) was 1-3, which is elevated and means that blood coagulation was delayed. He was found to have low potassium in serum, commonly seen with diarrhea, which was normalized with oral supplementation. Intravenous and oral fluids were continuously administered to treat dehydration. He received oral protein drinks and a multivitamin pill to improve his nutritional status.1
On days 12 and 15 of his illness, he received additional doses of Zmapp without any side effects. On day 15, his fever disappeared. Between days 14 and 17, the watery diarrhea was resolving, and volume and frequency of his bowel movements were normalizing. On day 17, the diarrhea had completely resolved and he no longer required intravenous fluids.1 Polymerase chain reaction (PCR) tests were performed on his blood and it came back consistently negative. After negative PCR tests, it was decided on day 29 to remove him from isolation unit. He was discharged home the following day.1
The case presented above displayed certain characteristics that are seen in patients suffering from Ebola virus disease. A typical case will occur in a patient with no significant past medical history. The patients may report having been in contact with a stranger suffering from Ebola virus disease or a sick loved one. Since exposure, the patient will usually begin displaying symptoms within 8–12 days, although there is a possibility that the virus infection may not show symptoms for up to 21 days. The disease begins abruptly, patients feeling vague symptoms, a general sense of being unwell, and warm or under-the-weather. Patient can transmit disease to the people around them when these features are present.1
Within a short period of time, typically 1–4 days after initial symptoms, the patient will begin to complain of high fever, chills and shakes, profound weakness, and muscle pain. Some may describe these new occurrences as intense shaking. Weakness is sometimes so severe that the patient cannot even walk to the restroom. The muscle pain can be so severe that the patient does not want to move. Early signs of infection can be evident in the blood as the white blood cell count becomes elevated. White blood cells are elevated because, after being exposed to the infectious agent, the body signals a boost to the defense system. The white count rise is a marker of bodily response to combat the virus.1
Immediately after these symptoms and around 4–8 days after the symptoms initially start, patients can develop diarrhea, vomiting, abdominal pain, red eyes, bleeding, and, rarely, a rash. Patients can lose 20 L of body fluid a day from the severity of the diarrhea and vomiting, causing severe dehydration. The nausea is so bad that the patient can barely take fluids or food by mouth, making replacement of lost fluids and dehydration a challenge. The laboratory measurement of blood urea nitrogen and creatinine provides an estimate of the severity of the dehydration. These substances can be thought of as normal wastes that the body makes. These wastes are delivered to the kidney by the fluid portion of blood. After these wastes reach the kidneys, the kidneys dump these out of the body into the urine.
When there is severe dehydration, as there is in patients with Ebola virus disease, these wastes are not delivered to the kidneys and build up in the blood, hence the reason for their elevation in patients with Ebola virus disease. Normal electrolytes such as sodium, potassium, and chloride tend to be decreased in patients with Ebola virus disease due to enormous loss of fluid when the patients are constantly vomiting and having diarrhea. Potassium is mostly lost with diarrhea, and chloride is mostly lost with vomiting.1
About one in five patients may progress to experience massive bleeding between 6 and 16 days, which can manifest as blood in vomit or blood in diarrhea, making dehydration even more severe. The main blood cell responsible for blood clotting, platelets, start decreasing in the body due to Ebola virus disease. Platelets normally serve to initiate a temporary plug at a site of bleeding. Without platelets, there is a higher chance of bleeding, which explains the bleeding symptoms in Ebola virus disease patients.
At this point in the virus, blood vessels are damaged and a severe drop in blood pressure can lead to multiorgan failure known as hypovolemic shock. This is the point at which the patient is nearing his or her death. Signs of liver failure may be evident in blood tests with accumulation of normally produced liver enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which are normally made inside liver cells. When liver cells are diseased, the cells die and dump AST and ALT into the blood, increasing the concentration of these enzymes. The elevation in Ebola virus disease patients may be either due to the direct attack of virus of these cells or severe dehydration that does not allow for enough blood to be delivered to the liver cells, causing them to die.
Ebola virus infection and spread within the body
A small group of invaders found their way through the walls of the fort. Isolated and too small in numbers to cause any commotion, they moved silently to overwhelm and confiscate the resources of the unsuspecting defending army’s mobile units. Although the invaders were not going to receive any reinforcements, they were multiplying in numbers within the comfort of the confiscated resources at a rate that was unprecedented and out of this world. As the invaders rapidly traveled through the country under the guise of their victims, the defending armies were unable to mount a targeted offense to contain and destroy the invaders. The communications between defending units remained sporadic and ineffective. When the defending armies responded with massive artillery barrage, but without clear targets, the damage inflicted upon their own comrades exceeded the damage suffered by the invaders. The towns were left vulnerable and were quickly overrun by the ever-increasing numbers of invaders; these turned into ghost towns devoid of life and hope. Such was the story of the Ebola virus invaders as they unleashed themselves in the face of a disintegrated and ineffective response by armies of healthy cells.
Ebola virus can enter the body through many pathways: it can be a tear in skin or mucous membranes or it can be transferred from a blood transfusion infected with virus. Once in the body, Ebola virus can infect a large spectrum of cells including circulating white blood cells (that form the inner lining of several organs and blood vessels), fibroblasts and epithelial cells (important components of skin and muscles), and hepatocytes and adrenal cortical cells (secreting cells within the liver and adrenal glands).2
Usually circulating white blood cells, such as macrophages and dendritic cells, are the first cells to be infected irrespective of the point of entry into the human body. These cells become the vehicle of viral transport into various organs but, unfortunately, are not immune to the destructive effect of the virus. Ebola virus replicates rapidly within these cells, leading to their deaths with subsequent releases of large numbers of new viral particles into the extracellular fluid upon cell destruction.3., 4. Regional lymph nodes, which usually function to trap any infectious agent, capture the virus but things get worse when virus continues to replicate in lymph nodes, eventually leading to the destruction of this important defense line.
After that virus continues its rampage by taking down some other warriors of the body, like dendritic cells and macrophages, ultimately spreading to important organs such as liver, spleen, and kidney. Important protective arms of human body such as interferon and lymphocytes are suppressed by Ebola virus, which gives the virus the liberty to replicate and attack any site with any level of severity.4 Studies have shown that the infected dendritic cells fail to mature and cannot produce antibodies. Lack of support signals from dendritic cells cause lymphocytes to undergo self-destruction that makes the body defense system even worse. With the progression of the disease, adrenal cortical cells, hepatocytes, fibroblasts, and many other cell types also become infected, resulting in extensive cell death and tissue necrosis.
The body unfortunately mounts a chaotic defense using expression and secretion of certain chemicals produced from macrophages infected with Ebola virus that are toxic to living cells. Peripheral blood samples have been taken from macaques infected with Ebola virus and these chemicals, along with some other compounds, have been found in an increased amount. Necrotic cells also signal the release of such chemicals. This response kills more of the body’s cells than the virus, resulting in extensive tissue damage and inducing a systemic inflammatory syndrome.3., 4. High concentrations of such compounds in the blood lead to dilation of blood vessels, pooling of blood into various blood vessels, and exudation of fluid into the tissue, causing life-threatening loss of blood pressure and subsequent multiorgan failure as adequate perfusion to such organs is not possible. The condition is also referred to as septic shock to denote the etiological basis of a profound drop in blood pressure. Vomiting is another bodily defense mechanism and leads to loss of body fluids. Due to vomiting, patients are unable to take oral fluids and the psychological stress causes loss of appetite. In addition to the above-mentioned inflammatory substances, these are some other important factors that can contribute to the critical decrease in blood pressure.
The Ebola virus infection is noted for excessive bleeding as blood is unable to clot and thus the term “hemorrhagic fever” is used. Coagulation requires a combination of circulating proteins and adequately functioning blood cells called platelets. Platelets are specialized cells that function to prevent bleeding in the body. Ebola virus infection macrophages also produce some chemicals medically known as cytokines. Cytokines also induce production of tissue factors. Cytokines and tissue factors cause inappropriate and false activation of platelets and other proteins that are involved in blood clotting. This false activation leads to blood clotting that ultimately consumes a lot of platelets and other proteins. Due to depletion of platelets and proteins involved in normal clotting process, the body becomes prone to excessive bleeding and hemorrhages. As the disease progresses, liver injury may also cause a decrease in plasma levels of certain coagulation factors. This whole sequence is called disseminated intravascular coagulation.
Incubation period
In medicine, the time from the moment of exposure until signs and symptoms of the disease appear is called the incubation period. Onset of symptoms typically occurs 8–12 days after exposure to Ebola virus.2., 5. Patients who do not have symptoms of infection but are in their incubation period are not believed to transmit infection. However, there is clear evidence that virus can be communicated to other people from a symptomatic patient; therefore, precautions must be taken against exposure if a person is symptomatic with Ebola virus infection.6 The clinical manifestation of Ebola virus disease starts with nonspecific influenza-like symptoms and progresses into a life-threatening picture with the involvement of multiple bodily functions and organs. The most common signs and symptoms reported from West Africa during the 2014 outbreak included diarrhea, vomiting, fatigue, fever, and anorexia or lack of appetite.2
Clinical manifestations
Ebola and Marburg virus infections have similar clinical manifestations in humans. Marburg virus is considered to be less lethal in terms of case fatality and severity of symptoms. Bleeding is not a common feature of Ebola virus disease, with most patients’ only bleeding during the terminal phase of the infection.
The term “Ebola hemorrhagic fever” was replaced with the term “Ebola virus disease” in modern-day scientific literature. There have been many instances where Ebola virus was confused with another disease that had similar signs and symptoms. Initially, in 1976 when the first Ebola virus disease outbreak occurred, it was thought to be Marburg virus due to the fact that an outbreak had occurred the prior year. In other instances, Ebola virus infection was thought to be either Lassa fever or Yellow fever due to the fact that these diseases have similar symptoms. Back in 1995, during the Kikwit outbreak, many patients were believed to be infected with Shigella, which is a bacterium that can also cause bloody diarrhea.7 Clinical characteristics of Ebola virus disease are discussed below. See Table 9.1 for a comparison of clinical symptoms in Ebola virus disease patients from Donka National Hospital in 2014, Kenema Government Hospital in 2014, and Kikwit General Hospital in 1995.8
Table 9.1.
Demographic and Clinical Characteristics of Hospitalized Patients With Ebola Virus Disease at Donka National Hospital, Conakry, Guinea (March 25–August 5, 2014), Kenema Government Hospital, Sierra Leone (May 25–June 18, 2014), and Kikwit General Hospital, Democratic Republic of Congo (January 6–July 16, 1995)
| Characteristics | Donka National Hospital 2014 | Kenema Government Hospital 20149 | Kikwit General Hospital 199510 |
|---|---|---|---|
| Mean age (years) | 34.0 | NR | 34.7 |
| Men | 63 | 42 | 47 |
| Fever | 66 | 89 | NR |
| Headache | 50 | 80 | 73 |
| Weakness | 79 | 66 | 78 |
| Diarrhea | 43 | 51 | 74 |
| Abdominal pain | 26 | 40 | 56 |
| Vomiting | 50 | 35 | 70 |
| Conjunctivitis | 9 | 31 | 34 |
| Cough | 4 | 20 | NR |
| Rash | 1 | 3 | NR |
| Myalgia and/or arthralgia | 20 | NR | 51 |
| Anorexia | 49 | NR | 73 |
| Dyspnea | 0 | NR | 25 |
| Hiccup | 9 | NR | 14 |
| Any bleeding | 19 | NR | 40–5011 |
| Melena | 6 | NR | 14 |
| Hematemesis | 3 | NR | 13 |
Abbreviations used: NR, not recorded; all values are represented as percentages.
Nonspecific flulike symptoms—Infection initially comes with symptoms consistent with common cold. Patients can have weakness, body aches, runny nose, fever with chills, and headaches. Lower back pain and aches in muscles surrounding the abdomen and back are also commonly seen.12 Like typhoid fever, high-grade fever and decreased heart rate may also be noticed. The throat is inflamed and swollen, and patients complain of feeling a painful lump in the throat. At this stage, the infection cannot be differentiated from any other viral infection that involves the respiratory system including influenza.
Rash—By days 5–7 of the illness, a diffuse red rash may appear on the skin. The rash can cover massive body areas, starting from face and extending down to the trunk with some ulceration of skin as well.7., 12., 13., 14.
Gastrointestinal—Watery diarrhea, nausea, vomiting, and abdominal pain usually develop after many days of initial symptoms. The gastrointestinal symptoms and rash provide the first hints that the infection is more extensive than what would be expected in a respiratory viral illness.14
Hemorrhagic manifestations—Bleeding is not an early feature of the disease, not always present, and, therefore, may not be helpful in the diagnosis of the infection. In the later stages of the infection, excessive bleeding may be evident as ecchymosis/bruising, petechial, oozing from venipuncture sites, and mucosal hemorrhages from nose and gastrointestinal systems. Frank hemorrhages such as bleeding from the nose, rectum, and vagina are seen most commonly in the terminal phase of illness. In the current outbreak, not less than 20% of patients have suffered bleeding, with gastrointestinal tract bleeding being the most common.2., 14.
Myalgia, arthralgia, and fatigue—All viral diseases produce myalgia and fatigue of varying severity. The severe myalgia and fatigue may be secondary to dehydration and uremia seen in acute kidney failure. Poor nutritional status is another contributor to such manifestations. Antigen–antibody complexes are formed during the recovery phase, which may lead to acute arthralgia and other symptoms similar to those seen in autoimmune arthritis.3
Psychological symptoms—Ebola virus disease, like any other untreatable condition, has devastating psychological stress on patients. With its high mortality rate and no proven treatment, patients are more likely to suffer from depressive symptoms. Lack of family support due to the risk of exposure increases the load of mental health problems. Major depression, social withdrawal, loss of appetite, suicidal ideations, and attempts to commit suicide have been reported in patients suffering from Ebola virus disease.15
Miscellaneous findings—Ebola virus disease can present with findings such as difficulty in breathing, pain in the chest, headache, hiccups, confusion, seizures, and swelling in the brain. Redness of eyes and redness of the soft palate are common findings.13 Several of these manifestations are a result of multiorgan failure rather than the direct effect of virus dissemination into muscles or the central nervous system. Spontaneous miscarriages may occur in pregnant women.
Patients demonstrate signs of improvement as early as first 6 days after the onset of symptoms in nonfatal infection.2 Severe symptoms early on in the course of disease with progression to septic shock reflect a bad outcome in terms of survival. In severe Ebola virus disease, death ensues between days 6 and 16 predominantly due to shock.
Laboratory diagnosis of Ebola virus infection
Because of the clinical presentation of Ebola virus disease being similar to several other viral and parasitic infections, diagnosis of Ebola virus infection depends on identifying sources of exposure and confirming virus antigens in serum. Fever in African countries is very frequently due to endemic malaria, typhoid, or Lassa fever and therefore, an unreliable symptom of Ebola virus infection. United States Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommended some guidelines after the outbreak of Ebola virus disease in west part of Africa. Guidelines were provided to screen and manage persons who have been recently exposed to Ebola virus.2., 16., 17., 18., 19., 20. The approach depends on the severity of risk exposure and presence of sign and symptoms consistent with Ebola virus disease.
Because significant amounts of a sample of deoxyribonucleic acid are necessary for molecular and genetic analysis, studies of isolated pieces of deoxyribonucleic acid are nearly impossible without PCR amplification. The Nobel Prize was awarded to Kary B. Mullis, the creator of PCR. PCRs are very significant in a number of laboratory and clinical techniques, including detection of bacteria or viruses (particularly AIDS), DNA fingerprinting, and diagnosis of genetic disorders.
To amplify a segment of DNA using a PCR, DNA is first denatured by heating to separate into two pieces of single-stranded DNA. Then, an enzyme “Taq polymerase” uses the original strand as a template to synthesize two new strands resulting in duplication of original DNA. Two new strands can be created from each of these strands and so on. This cycle of denaturing and synthesizing new DNA is repeated to create billions of exact copies of the original DNA segment. The entire cycling process of PCR is automated, which can be completed in just a few hours. A machine called a thermocycler is designed to alter the temperature of the reaction in alternating manner to allow DNA denaturing and synthesis.
Reverse transcription PCRs (RT-PCRs) take only 3–10 days to detect Ebola virus after the onset of symptoms. The PCR is performed on the serum of suspected individuals. Reverse transcriptase can reverse the isolates and transcribe Ebola virus ribonucleic acid into deoxyribonucleic acid, which is then further amplified by the PCR, generating thousands to millions of copies of a particular deoxyribonucleic acid sequence.
Patients with symptoms of less than 3 days duration may need repeat testing.21 Within the first 3 days, Ebola virus ribonucleic acid concentration may not be significantly high in blood circulation and obtaining a sample for viral detection may produce false-negative results. Enzyme-linked immunosorbent assay can detect viral antigens and specific ribonucleic acid sequences by RT-PCR. Acute infections can be detected by RT-PCR. After the onset of symptoms, viral ribonucleic acid is generally detectable within 3–10 days by RT-PCR.21 Sensitivity of RT-PCR can be affected by genomic variability and changes in ribonucleic acid sequence of Ebola virus.22 Antigen detection may be used for an immediate diagnosis as a confirmatory test.23
The likelihood of exposure to Ebola virus and symptoms and/or basic laboratory tests suggestive of Ebola infection are the factors that determine which individual should be tested for Ebola virus infection.2., 16., 20. Testing for IgG or IgM antibodies to Ebola virus can be used to identify to evaluate for past infection and/or immune response over time. PCR testing is also useful to determine whether a patient has recovered from Ebola infection. According to WHO, individuals without sign and symptoms of Ebola virus disease and two negative PCR tests on whole blood, at least 48 h apart, can be discharged as “infection free”.24
Ancillary laboratory findings
Laboratory testing and phlebotomy should be limited to tests that are required for care.25 Routine blood tests, such as complete blood counts, serum electrolytes, and urea and glucose, allow for quantification of the severity of dehydration and kidney and liver failure. Reduced levels of platelets and white blood cells may provide insights into the severity of blood coagulation impairment and immune suppression. Such tests may allow for early detection and monitoring of such manifestations and determine the response to therapeutic interventions.
Leukopenia—Decrease in number of white blood cells. It is followed by an increase in neutrophil cell count, with an increased percentage of immature cells. In 1967, Marburg outbreak caused many patients to have prominent decrease in the number of white blood cells with counts as low as 1000/μL at the time of clinical presentation.2
Thrombocytopenia—Platelet counts reach a nadir around days 6–8 of illness and are usually stay in the range of 50,000–100,000/μL in contrast to normal counts, which are usually in range of 150,000–350,000/μL.2., 12.
Elevated transaminases—Ebola virus invades hepatocytes (liver cells) and leads to necrosis of these cells. Elevated serum AST rises more than ALT levels. These enzymes are spilled in blood circulation when liver cells die due to viral invasion.9., 24.
Coagulation abnormalities—Fibrin degradation products are elevated, prothrombin and partial thromboplastin times are prolonged, consistent with disseminated intravascular coagulation. These changes are most prominent in severe cases of Ebola virus disease.
Renal abnormalities—Proteins in blood circulation do not filter through kidneys in urine. Due to damage to kidneys, plasma proteins start to seep through kidneys and excrete through urine of the patient. Renal insufficiency occurs with the progression of the disease.
Factors predicting survival or death after Ebola virus infection
Prognosis can be assessed on the basis of clinical and laboratory findings. Certain clinical manifestations such as increased respiratory rate, loss of urine formation, delirium, coma, and shock secondary to impaired oxygen delivery, intravascular volume depletion, and metabolic disturbances are associated with high fatality rate of the disease.26 The clinical course of the disease also provides insights into the chances of recovery as clinical improvement is noted by second week in those who are likely to survive. In such patients, Ebola virus RNA concentrations drop and antibodies to Ebola virus appear during the second week.27., 28.
Viral persistence
The virus can persist for some time in certain bodily fluids, including semen and breast milk. Persistence of virus in breast milk has also been noticed even after peripheral blood is tested negative for the presence of Ebola virus RNA.29 Two children who were breast-fed by Ebola-infected mothers contracted and died of the disease even though the mothers were declared infection-free. Mothers of these babies recovered completely from Ebola infection, but during the course of infection they breastfed the babies.
Studies showed that reverse transcriptase PCR could easily detect viral RNA sequences in the semen of male patients for the period of up to 3 months, and infectious virus was recovered from one patient, 82 days after symptom onset.30 During the 1967 Marburg outbreak, one case caused the transmission of virus through sexual contact because of viral persistence in semen.13., 31.
Convalescence or recovery period
It signifies the later stage of an infectious disease when the patient recovers, but may still be a source of infection even if patient started feeling better. Ebola virus disease has a prolonged convalescence period, which is characterized by extensive sloughing of skin and hair loss most likely due to necrosis of sweat glands.28
CDC guidelines
Risk assessment of Ebola virus disease—Ebola virus disease risk should be evaluated if clinical finding suggestive of Ebola infection is present and there is a possibility of Ebola virus exposure within prior 21 days.2., 16., 20.
Clinical findings—Severe headache, weakness, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage are the symptoms that raise suspicion for Ebola virus disease.
Exposure—The level of exposure varies from high risk to low risk to no known exposure.
Individuals who are at risk for Ebola virus disease include:
-
•
Contact with blood, body fluids, or any human remains of an individual known or suspected to have Ebola virus disease
-
•
Residence in or the recent travel to an area with Ebola virus transmission
-
•
Direct handling of rodents or primates from endemic areas.
As per the recommendations by the CDC, any of the following can be considered close contact:
-
•
Being within the proximity of 3 feet or 1 m to an Ebola virus disease patient
-
•
Being within the room of an Ebola virus disease patient for a prolonged duration without wearing protective equipment
-
•
Contact with the patient while not wearing protection equipment
-
•
Moving through a hospital, walking by a person, or providing care to a patient with Ebola virus disease.
Strict precautionary measures should be taken during the evaluation of patients suspected of Ebola virus infection. Suspected patients should be isolated in a room with a private bathroom, doors should be closed all the time, and health-care teams should wear protective gear to avoid any direct contact or transmission through droplets or body fluids. Gloves, face masks, gowns, and eye protection can be helpful in the prevention of virus transmission. The appropriate staff, the hospital infection control program, and state health departments should also be notified.
Further evaluation
High-Risk Exposure—The CDC recommends testing for Ebola virus infection for all individuals with onset of fever within 21 days of having a high-risk exposure.2., 20. High-risk exposure persons without fever can have the same recommendation if there are other compatible clinical sign and symptoms present and/or laboratory findings are abnormal (i.e., raised transaminases and/or low platelet count, <150,000 cells/μL).
Any of the following includes high-risk exposure24., 25.:
-
•
Mucous membrane or even percutaneous exposure to blood or certain body fluids of a person with Ebola virus disease
-
•
Direct physical contact with skin, blood, or certain body fluids of an Ebola virus disease patient
-
•
Processing blood or certain body fluids from a patient with diagnosed Ebola virus disease without appropriate personal protective equipment or standard biosafety precautions
-
•
Having a direct contact with a dead body (including during funeral rites) without appropriate protective precautions in a country where an outbreak of Ebola virus disease is occurring.
Low-risk exposure—For persons with low-risk exposure who develop fever or other clinical findings consistent with Ebola virus disease, the CDC recommends some additional medical evaluation to check for Ebola virus disease.16., 19. The decision to test the individual is based upon the severity of illness, laboratory findings (e.g., platelet counts), and the likelihood of an alternative diagnosis.
A low-risk exposure includes16., 19.:
-
•
Brief contact with an Ebola-infected patient without exposure to blood or other certain body fluids
-
•
Another close contact with the Ebola virus disease patient in community settings or health-care facility.
Asymptomatic (or symptoms not meeting clinical criteria)—Symptoms and signs of Ebola virus disease should be monitored for certain asymptomatic individuals, as well as for those who do not meet the above clinical criteria. This includes individuals who have:
-
•
Traveled in a country where an outbreak of Ebola virus disease has occurred within the past 21 days
-
•
A high- or low-risk exposure.
Such individuals have to be monitored for 21 days after the last known exposure to a patient (or after leaving the country if no exposure is known) and should immediately report the onset of fever or other symptoms indicative of Ebola virus disease. Whether travel restrictions and/or monitoring by a public health authority are warranted depends upon the risk level of exposure, as described in the CDC recommendations for the monitoring and movement of individuals with Ebola virus exposure.2
WHO and CDC have almost similar recommendations. The WHO considers a person to be at high risk for infection if they have had sexual intercourse with a person recovering from Ebola virus disease or with a sick person.17., 19., 32. PCR testing is also useful to determine whether a patient has recovered from Ebola virus infection. According to the WHO, individuals without signs and symptoms of Ebola virus disease can be discharged if they have two negative PCR tests on whole blood at least 48 h apart.24
Biosafety and biosecurity considerations with Ebola virus disease
Direct interaction through skin abrasions or contact through mucous membranes of the nose and eyes can lead to spread of Ebola virus infection in health-care settings. Another source of spread is through exposure to blood or body fluids of Ebola virus disease-affected persons and through contaminated needles or syringes. According to the CDC guidelines, personal protective equipment can reduce the risk of self-contamination to health-care workers at risk of exposure. The description and levels of personal protective equipment are presented in Table 9.2, Table 9.3 . For effective implementation of these protective guidelines, the role of health-care facilities is essential. To safeguard health-care workers while taking care of Ebola virus disease patients, rigorous safety methods needed to be followed while putting on and taking off personal protective equipment.
Table 9.2.
| Types of Personal Protective Equipment | Designated Use |
|---|---|
| Gloves | Protects health workers’ hands from exposure to infectious agents |
| Respirators and masks | Protects the respiratory tract from airborne infectious agents |
| Goggles | Protects the eyes of the health-care workers to reduce risk of potential splashing or spraying |
| Face shields | Protects face, mouth, nose, and eyes |
| Aprons and gowns | Protects the contamination of skin or clothing of health-care workers |
Table 9.3.
Levels of Personal Protective Equipment in Health-Care Settings35
| Levels of Personal Protective Equipment | Indications | Examples |
|---|---|---|
| Level A | Required for the greatest level of protection to skin, respiratory tract, and eye | Positive pressure, full-face piece self-contained breathing apparatus, disposable protective suit, gloves, and boots |
| Level B | When highest level of respiratory protection is required | Positive pressure, full-face piece self-contained breathing apparatus, inner and outer chemical-resistant gloves, and face shields |
| Level C | Required when the concentration and type of airborne substances is known | Full-face air-purifying respirators, hard hat, escape mask, and inner and outer chemical-resistant gloves |
| Level D | Used when minimum protection is required | Gloves, safety glasses, chemical-resistant, steel-toe boots, or shoes |
Principles of personal protective equipment
According to the CDC guidelines, health-care workers must follow basic principles to ensure efficient use of personal protective equipment, most essential of which is the exposure of skin to virus, which should be avoided at all times. While entering the patient care area, it is essential to put on the personal protective equipment in a correct manner and order. It cannot be readjusted after entering the patient care area because any further manipulation with the protective equipment can result in breach of barriers against virus. A trained observer should monitor this activity to ensure proper compliance.
After handling the body fluids of the infected person, the gloved hands should be disinfected using an alcohol-based hand rub. During patient care activities, if the health-care worker experiences a partial or total breach of their personal protective equipment (through glove separation from sleeves leaving exposed skin, a tear in an outer glove, or a needle stick), then the health-care worker should be moved instantly to the designated area to evaluate the degree of exposure. If indicated during evaluation, the facility’s exposure plan should be implemented emergently. In comparison to putting on protective gear, the removal of used personal protective equipment is a high-risk process that requires an organized approach, a trained observer, and a selected area for removal to warrant protection. This procedure should be slow and vigilant to reduce the probability of self-contamination.
During direct patient care and the personal protective equipment removal process, an additional layer of protection is provided by double gloving method. However, any further addition of layers will cause restraint in movements and lead to an excessively hot environment within the equipment, thus making patient care a challenging assignment. It is strongly suggested by the CDC to train and practice, in case any health-care facility decided to alter and enhance protective procedures with extra personal protective equipment. During the disposal process of used personal protective equipment, human error or omission can be expected. To prevent such occurrences, every step is read aloud to the health-care worker from the procedure worksheet, and each completed step is visually established and documented by a trained observer. The exclusive responsibility of a trained observer during entire put-on and takeoff process is to ensure the safety of the health-care worker and to make sure the facility is not compromised. The trained observer should have all the right knowledge about personal protective equipment recommendations documented in facility’s protocol, including proper donning and doffing procedures, disposal of used personal protective equipment in order to provide right guidance to related health-care worker. In the event of an unintentional breach in procedure, the trained observer should be aware of exposure management plans.
Facilities should have a design that permits for a clear demarcation between clean and possibly contaminated areas. There should also be clearly visible signs to separate these distinct areas and to ensure a one-way movement of care from clean areas (area where personal protective equipment’s donned and unused equipment is stored) to the patient room, and then to the protective equipment removal area where equipment is removed and discarded.36
Handling of biological specimens suspected of containing Ebola virus
While handling specimens from the patients, it is necessary for the laboratory staff to strictly follow safety protocol in order to prevent the transmission of bloodborne pathogens.37 The only possible method to ascertain the etiology of a suspected case will be through a laboratory test, since the early signs of the Ebola virus disease can be vague.38 Unquestionably, Ebola virus is a highly pathogenic agent; therefore, all laboratories should assess their resources and methods along with analyzing the training procedures and skills of their staff so as to conduct a comprehensive biohazard risk assessment. Implementation of correct procedures is necessary to lessen the possibility of the risk.37
Each of the laboratories should design a suitable workflow for processing suspected “Ebola virus-infected” samples to protect health-care providers and laboratory personnel. Specimens should be handled using an “enhanced chain of custody” algorithm. For example, having a designated staff for the shipping and supervision of the specimens within the laboratory is required to make sure that preanalytical, analytical, and postanalytical techniques are correctly tracked to amplify safety to personnel.39 Ebola viruses are categorized as risk group-4 pathogens (see Table 9.4 for levels of pathogens), demanding Laboratory Biosafety Level (BSL)-4-equivalent control for isolation of virus.
Table 9.4.
Categories of Pathogens According to Risk Group40
| Risk Group Pathogens | Definition | Examples |
|---|---|---|
| Risk group 1 | Microorganism causing no or low individual and community risk | Escherichia coli, Bacillus subtilis, Yeast |
| Risk group 2 | Pathogen causing moderate individual risk, low community risk | Hepatitis B and C virus, human immunodeficiency virus (HIV), Salmonella typhi |
| Risk group 3 | Pathogen causing high individual risk, low community risk | Mycobacterium tuberculosis, severe acute respiratory syndrome (SARS) |
| Risk group 4 | Pathogen causing high individual and community risk | Ebola virus |
Only if the virus has been inactivated in a BSL-3 facility (see Table 9.5 for definitions), an on-site molecular analysis can be done. BSL-4 or BSL-3 facility-lacking countries should assure the transportation of the specimens to an established WHO Collaborating Center.22., 38.
Table 9.5.
Grades of Biosafety Level41
| Biosafety Levels (BSL) | Criteria | Examples |
|---|---|---|
| BSL-1 | Agents that pose minimal threat to laboratory workers; does not cause disease in healthy adults | Baculovirus |
| BSL-2 | Agents causing human diseases, thus posing moderate hazard | Equine encephalitis viruses, human immunodeficiency virus (HIV) |
| BSL-3 | Agents that are strictly controlled, can cause lethal disease via aerosol transmission | Yellow fever virus, St. Louis encephalitis virus, West Nile virus |
| BSL-4 | Agents that are extremely dangerous and can pose a high risk of life-threatening disease | Ebola virus, Lassa virus |
Guidelines for the handling of laboratory specimens from suspected or laboratory confirmed Ebola virus disease patients
-
•
Personal protective equipment such as double gloves, impermeable laboratory gown, fluid-resistant gown worn over the laboratory coat or a combination of a sanctioned particulate respirator (e.g., N95 or N100), and eye protection (e.g., goggles/face shields/shroud), or a powdered air-purifying respirator use is strongly suggested, to be put on by the laboratory personnel while handling the samples from suspected Ebola virus disease patients.
-
•
It is necessary to make sure adequate supplies of personal protective equipment and United Nations specimen triple packaging systems is currently available.42
-
•
The samples collected from the suspected Ebola virus disease patients should not be handled in an open area.
-
•
Procedures like pipetting, aspiration, slide preparation having the possibility to create infectious aerosols should be carried out in a biological safety cabinet in a minimum Containment Level-CL2 laboratory.
-
•
The risk of aerosolization is higher with procedures like centrifugation of infectious material and should be carried out using sealed safety cups or sealed rotors that are only opened and unloaded in the biological safety cabinet.
-
•
Use of glass or sharp instruments should be limited wherever necessary and laboratory personnel should be adequately trained in regular practices, including biosafety.
-
•
Before collecting patient samples, label the tubes.
-
•
Requisitions tested need to be stamped as “Ebola virus disease suspect,” and labeling should be done on the exterior of the container.
-
•
Make sure that all the specimens are safely stored and the accessibility should be limited only to authorized staff members.
-
•
Trained staffs skilled in collecting blood should perform phlebotomy.
-
•
During specimen processing, try to avoid unnecessary activities and minimize staff in that area.
-
•
Prior to packaging for shipping to further testing, the surface of the specimen containers should be decontaminated by an effective disinfectant.
-
•
To avoid contamination of the skin and hair, personal protective equipment should be removed in a proper manner. Any contact with the soiled and contaminated items (e.g., gloves, gowns, respirators) should be avoided.
-
•
Proper sterilization is necessary for contaminated clothing and personal protective equipment before discarding, reuse, or removal from the laboratory.
-
•
As soon as the personal protective equipment is removed, wash hands thoroughly.
-
•
The spongy paper towels should be covered with disinfectant and left to soak for 15 min before cleaning up the accidental spills of potentially contaminated material.
-
•
Specimens should be placed in a durable, leak-proof secondary container for transport within a facility. The external surface of a container should be disinfected with 3% sodium hypochlorite.
-
•
Even for shipping within a facility, samples should be placed in a durable, leak-proof secondary container.
-
•
Never use any pneumatic tubes for shipping the samples of suspected Ebola virus disease so as to avoid the risk of breakage or leaks.43
-
•
All specimens should be stamped with a distinct patient ID. It should also be supplemented with a documentation sheet including at least patient’s unique ID, date/time/place of sampling, type of specimen, test requirements, clinical data, along with travel history, and exposure to a suspected or confirmed Ebola virus disease case.44
-
•
With regards to laboratory waste management during handling the specimens infected or contaminated with Ebola virus, the solid waste generated during laboratory testing should be steam sterilized (autoclaving), as the waste treatment process will inactivate the virus.45
-
•
For handling Category A infectious substance like Ebola virus specimen, laboratories should have a Biological Safety Cabinet Certification.45
-
•
If the specimen needs to be tested at the hospital laboratory which is the common laboratory for other hospital and patient-related activities, then the specimen should be double bagged, placed in a biohazard transportation container, and hand-carried to the laboratory37 (Figure 9.1 ).
Figure 9.1.
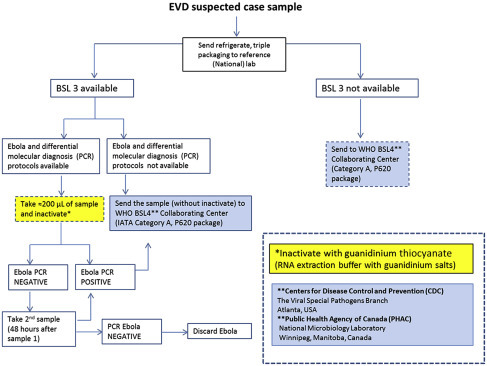
Flowchart for handling of samples derived from suspected Ebola virus disease patients.
World Health Organization (WHO). Algorithm for handling of samples from suspected Ebola virus disease. 2014.
Biosafety recommendations for laboratories conducting diagnostic testing for Ebola virus disease with appropriate BSL-3/BSL-4 facilities
-
•
A virus should only be isolated in a maximum containment BSL-4 laboratory. To prevent accidental or deliberate release of virus isolates, make sure the specimens are properly handled and safely stored.
-
•
According to the detection protocol, the specimens should be inactivated only under BSL-3 conditions.
-
•
Testing procedures like RT-PCR and enzyme-linked immunosorbent assay (ELISA) for noninactivated samples can be done at BSL-3 laboratory.
-
•
For inactivated samples, testing procedures like RT-PCR and ELISA testing can be done at a BSL-2 laboratory.
Biosafety recommendations for laboratories conducting diagnostic testing for Ebola virus disease without appropriate BSL-3/BSL-4 facilities
-
•
Testing procedures like PCR or ELISA for samples should be done in a biosafety cabinet (glove box)-Class III in an isolated laboratory area.
-
•
Once specimens are inactivated, they can be removed from the biosafety cabinet-Class III and the rest of the procedures can be done under BSL-2 conditions.
-
•
All the collected samples can be kept under refrigeration at 2–8 °C up to a week. The biological samples should not be stored under BSL-2 conditions beyond the timeline of necessary shipment.38
Thus, the above-documented guidelines recognize existing precautionary measures and biosafety procedures within laboratories that are satisfactory enough to safeguard the laboratory personnel during most testing procedures.39
References
- 1.Lyon G.M., Mehta A.K., Varkey J.B., Brantly K., Plyler L., McElroy A.K. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014 doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 2.control cfd . 2014. Ebola virus disease information for Clinicians in U.S. Healthcare settings.http://www.cdc.gov/vhf/ebola/hcp/clinician-information-us-healthcare-settings.html [cited 12/17/2014]. Available from: [Google Scholar]
- 3.Mahanty S., Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4(8):487–498. doi: 10.1016/S1473-3099(04)01103-X. [DOI] [PubMed] [Google Scholar]
- 4.Bray M., Geisbert T.W. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol. 2005;37(8):1560–1566. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH . 2014. Specific infectious diseases involving potential health risks for travellers.http://www.who.int/ith/diseases/en/ [cited 12/17/2014]. Available from: [Google Scholar]
- 6.Peters C.J., Jahrling P.B., Khan A.S. Patients infected with high-hazard viruses: scientific basis for infection control. Arch Virol Suppl. 1996;11:141–168. doi: 10.1007/978-3-7091-7482-1_13. [DOI] [PubMed] [Google Scholar]
- 7.Roels T.H., Bloom A.S., Buffington J., Muhungu G.L., MacKenzie W.R., Khan A.S. Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J Infect Dis. 1999;179(Suppl. 1):S92–S97. doi: 10.1086/514286. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi A.I., Chughtai M., Bah E.I., Barry M., Beavogui K., Loua T.O. High survival rates and associated factors among Ebola virus disease patients at Donka National Hospital, Conakry, Guinea. J Vasc Interv Neurol. 2015 [PMC free article] [PubMed] [Google Scholar]
- 9.Schieffelin J.S., Shaffer J.G., Goba A., Gbakie M., Gire S.K., Colubri A. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014 doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan A.S., Tshioko F.K., Heymann D.L., Le Guenno B., Nabeth P., Kerstiens B. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl. 1):S76–S86. doi: 10.1086/514306. [DOI] [PubMed] [Google Scholar]
- 11.Bwaka M.A., Bonnet M.J., Calain P., Colebunders R., De Roo A., Guimard Y. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl. 1):S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 12.Formenty P., Hatz C., Le Guenno B., Stoll A., Rogenmoser P., Widmer A. Human infection due to Ebola virus, subtype Cote d’Ivoire: clinical and biologic presentation. J Infect Dis. 1999;179(Suppl. 1):S48–S53. doi: 10.1086/514285. [DOI] [PubMed] [Google Scholar]
- 13.Martini G.A. Marburg agent disease: in man. Trans R Soc Trop Med Hyg. 1969;63(3):295–302. doi: 10.1016/0035-9203(69)90001-7. [DOI] [PubMed] [Google Scholar]
- 14.Kortepeter M.G., Bausch D.G., Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(Suppl. 3):S810–S816. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 15.Locsin R.C., Matua A.G. The lived experience of waiting-to-know: Ebola at Mbarara, Uganda–hoping for life, anticipating death. J Adv Nurs. 2002;37(2):173–181. doi: 10.1046/j.1365-2648.2002.02069.x. [DOI] [PubMed] [Google Scholar]
- 16.control cfd . 2014. Algorithm for evaluation of the returned traveler.http://www.cdc.gov/vhf/ebola/pdf/ebola-algorithm.pdf [cited 12/17/2014]. Available from: [Google Scholar]
- 17.control cfd . 2014. Travel and transport risk assessment: guidance for public health authorities and the transport sector.http://www.who.int/csr/resources/publications/ebola/travel-guidance/en/ [cited 12/17/2014]. Available from: [Google Scholar]
- 18.Organization WH . 2014. Ebola and Marburg virus disease epidemics: preparedness, alert, control, and evaluation.http://apps.who.int/iris/bitstream/10665/130160/1/WHO_HSE_PED_CED_2014.05_eng.pdf?ua=1 [cited 12/17/2014]. Available from: [Google Scholar]
- 19.Organization WH . 2014. Case definition recommendations for Ebola or Marburg virus diseases.http://who.int/csr/resources/publications/ebola/ebola-case-definition-contact-en.pdf [cited 12/17/2014]. Available from: [Google Scholar]
- 20.control cfd . 2014. Guidance for monitoring and movement of persons with potential Ebola virus exposure.http://www.cdc.gov/vhf/ebola/exposure/monitoring-and-movement-of-persons-with-exposure.html [cited 12/17/2014]. Available from: [PMC free article] [PubMed] [Google Scholar]
- 21.control cfd . 2014. Specimen collection, transport, testing, and submission for patients with suspected infection with Ebola virus disease.http://www.cdc.gov/vhf/ebola/pdf/ebola-lab-guidance.pdf [cited 12/17/2014]. Available from: [Google Scholar]
- 22.Gire S.K., Goba A., Andersen K.G., Sealfon R.S., Park D.J., Kanneh L. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345(6202):1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldmann H. Ebola–a growing threat? N Engl J Med. 2014;371(15):1375–1378. doi: 10.1056/NEJMp1405314. [DOI] [PubMed] [Google Scholar]
- 24.Organization WH . 2014. Laboratory guidance for the diagnosis of Ebola virus disease interim recommendations.http://apps.who.int/iris/bitstream/10665/134009/1/WHO_EVD_GUIDANCE_LAB_14.1_eng.pdf [cited 12/17/2014]. Available from: [Google Scholar]
- 25.control cfd . 2014. Early recognition is critical for infection control.http://www.cdc.gov/vhf/ebola/pdf/could-it-be-ebola.pdf [cited 12/17/2014]. Available from: [Google Scholar]
- 26.Sadek R.F., Khan A.S., Stevens G., Peters C.J., Ksiazek T.G. Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995: determinants of survival. J Infect Dis. 1999;179(Suppl. 1):S24–S27. doi: 10.1086/514311. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez A., Lukwiya M., Bausch D., Mahanty S., Sanchez A.J., Wagoner K.D. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78(19):10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ksiazek T.G., Rollin P.E., Williams A.J., Bressler D.S., Martin M.L., Swanepoel R. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl. 1):S177–S187. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 29.Bausch D.G., Towner J.S., Dowell S.F., Kaducu F., Lukwiya M., Sanchez A. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl. 2):S142–S147. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 30.Rowe A.K., Bertolli J., Khan A.S., Mukunu R., Muyembe-Tamfum J.J., Bressler D. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl. 1):S28–S35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]
- 31.Slenczka W.G. The Marburg virus outbreak of 1967 and subsequent episodes. Curr Top Microbiol Immunol. 1999;235:49–75. doi: 10.1007/978-3-642-59949-1_4. [DOI] [PubMed] [Google Scholar]
- 32.Woo J.H., Park H.S. Successful treatment of severe sympathetically maintained pain following anterior spine surgery. J Korean Neurosurg Soc. 2014;56(1):66–70. doi: 10.3340/jkns.2014.56.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health MDo. Personal protective equipment (PPE) for infection control: Minnesota Department of Health; [cited 2015]. Available from: http://www.health.state.mn.us/divs/idepc/dtopics/infectioncontrol/ppe/.
- 34.Prevention CfDCa. Guidance for the selection and use of personal protective equipment (PPE) in healthcare settings: Centers for Disease Control and Prevention [cited 2015]. Available from: http://www.cdc.gov/HAI/pdfs/ppe/PPEslides6-29-04.pdf.
- 35.Agency USEP. Personal protective equipment: United States Environmental Protection Agency [cited 2015]. Available from: http://www2.epa.gov/emergency-response/personal-protective-equipment.
- 36.Prevention CfDCa . Centers for Disease Control and Prevention; April 5, 2015. Guidance on personal protective equipment to be used by healthcare workers during management of patients with Ebola virus disease in U.S. Hospitals, including procedures for putting on (donning) and removing (doffing)http://www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance.html Available from: [Google Scholar]
- 37.Health NYSDo. Revised NYS/NYC laboratory guidelines for handling specimens from patients with suspected or confirmed Ebola virus disease: New York State Department of Health [cited 2015]. Available from: http://www.health.ny.gov/diseases/communicable/ebola/docs/lab_guidelines.pdf.
- 38.Organization WH . World Health Organization; April 5, 2015. Algorithm for handling of samples from suspected Ebola virus disease.http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=27912&lang=en Available from: [Google Scholar]
- 39.Health BCMo. Draft laboratory biosafety considerations for handling specimens from patients designated as “Suspected Ebola”. British Columbia Ministry of Health. Available from: http://www.health.gov.bc.ca/pho/pdf/bc-vhf-laboratory-biosafety-considerations-aug-19-2014.pdf.
- 40.Association ABS. Risk group classification for infectious agents: American Biological Safety Association. Available from: http://www.absa.org/riskgroups/.
- 41.McLeod V. 2010. Biosafety levels 1, 2, 3 & 4: Lab Manager.http://www.labmanager.com/lab-health-and-safety/2010/12/biosafety-levels-1-2-3-4?fw1pk=2-.VTXnfK2eDRY Available from: [Google Scholar]
- 42.Organization WH . World Health Organization; 2014. Laboratory diagnosis of Ebola virus disease.http://apps.who.int/iris/bitstream/10665/134009/1/WHO_EVD_GUIDANCE_LAB_14.1_eng.pdf Available from: [Google Scholar]
- 43.Canada PHAo . Public Health Agency of Canada; 2014. Interim biosafety guidelines for laboratories handling specimens from patients under investigation for Ebola virus disease.http://www.phac-aspc.gc.ca/id-mi/vhf-fvh/ebola-biosafety-biosecurite-eng.php Available from: [Google Scholar]
- 44.Ebola fever—brief instructions for handling and transport of samples from suspected cases and exposed contacts, including referral for diagnostic confirmation. Quality Assurance Exercises and Networking on the Detection of Highly Infectious Pathogens (QUANDHIP); 2014. http://www.quandhip.info/Quandhip/EN/Home/Ebola_fever_instructions.pdf?__blob=publicationFile [updated September 8, 2014]. 1.5.4. Available from: [Google Scholar]
- 45.Prevention CfDCa . Centers for Disease Control and Prevention; 2014. Guidance from U.S. Laboratories for managing and testing routine clinical specimens when there is a concern about Ebola virus disease.http://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/safe-specimen-management.html Available from: [Google Scholar]


