Abstract
Nature has its own way of protecting mankind from the atrocities of illness and diseases by producing natural products of diverse structures and bioactivities, not only necessary for good health but also to combat diverse life-style and infectious diseases including microbial infections caused by virus, bacteria, fungi, protozoa, and other parasites. The problems of emerging and emerging diseases with drug resistance, latency, and reactivation along with the shorter life span of most synthetic agents or drugs have directed scientists to look into the treasure chests of nature to find out and explore the natural metabolites of plants for the management of diverse diseases including viral diseases. This chapter explains the treasure of “Ethnomedicine” and its journey through time, which reveals the importance of this branch of “natural science” in the current scenario, its potential as “alternative or complementary” medicine, especially against viral diseases with a detailed account of identified antivirals, their isolation and identification as “natural antiviral” to make the readers realize the need to accept “ethnomedicine” as appropriate alternatives to “synthetic chemistry.”
Keywords: Ethnomedicine, tribes, antivirals, plant metabolites, diseases, immunomodulators
3.1. Ethnomedicine: A Boon
A famous quote of Norwegian Artist Edvard Munch “Nature is not all that is visible to the eye… also includes the inner pictures of the soul” speaks many things about the mother “Nature” and her creation. For the past 100 years, mankind have been trying to unravel the secrets of nature, yet continue to be surprised by its revelations day by day. Nature is vast in its resources which not only provide food, clothes, shelter, and antiques but also provide medicine since ages. Out of these resources the greatest gift of nature is her atmosphere with food and medicine, which not only to help mankind to sustain but also to grow, develop, and survive, particularly from the diseases and sufferings. The study of natural resources traditionally used to cure or manage ailments in diverse ethnic culture is collectively termed as “Ethnomedicine” (Chattopadhyay, 2010, Chattopadhyay et al., 2009a).
Scientifically, “Ethnomedicine” is the study of “traditional medicine” of ethnic communities, their knowledge and practices that transmitted orally over centuries, and evolved over millennia of human existence (Chattopadhyay, 2009, Chattopadhyay, 2010). The indigenous people of India till date used their medicaments or “so called medicines” which might be more appropriately defined as the use of plants in the treatment of diseases and should be more accurately termed as “Ethnobotanical medicine” (Fabricant and Farnsworth, 2001). For a considerable period of time, traditional medicine and ethnomedicine were ignored by the clinicians and biomedical practitioners due to a number of factors including the questionable purity, safety, and potency. The raw material to prepare those medicines is not standardized through modern quality control parameters; its chemical profile and their quantification are not known or maintained, and thus their purity is in question. Similarly, their toxicity profile at low doses for long term is unknown, so long-term use in tolerable dosage need to be monitored to rule out the question of long-term toxicity, if any. Although the traditional medicines are used for generations with limited or no major toxic manifestations, which can be considered as “Proof of Concept” in nature’s laboratory. In 21st century the efficacy of any therapeutically useful product should be quantified in terms of modern medicine, so the efficacy of useful traditional and ethnomedicinal plants needs to be validated in modern laboratory by establishing the dosage and the exact mode and mechanism of action in one hand and in vivo efficacy in suitable animal models on the other. Moreover, if possible use of ex vivo model than can mimic the human body for better and global acceptability. With the upsurge research, ethnomedicinally useful plants have become one of the most acceptable resources not only for the pharmaceutical industry to develop therapeutically useful leads, or pharmaceuticals, but also to help in developing supplements or naturaceuticals, and even cosmetics. Reports from WHO-Traditional Medicinal Programme showed that a total of 122 compounds were isolated from 94 plant species, 80% of which were used for the same or related ethnomedical purposes (Fabricant and Farnsworth, 2001). Since these compounds were derived only from 94 species of plants out of an estimated 250,000 flowering plants, one might imagine the abundance of drugs remaining to be identified from plant kingdom.
3.2. Ethnomedicinal Wisdom of Diverse Communities
Ethnomedicine broadly refers to the traditional medical practices concerned with the cultural interpretation of health, diseases, and illness that addresses the healthcare process and healing practices (Krippner and Staples, 2003). It is a vast interdisciplinary science that includes the knowledge about the use of natural pharmaceuticals and the ethnic group from which the same pharmacologically active ingredients belong as well. From Indian Ayurveda to Traditional Chinese Medicine (TCM) of China, from Muti of Africa to Unani medicine of Mughal India, it has been widely practiced in diverse ancient civilizations. The practitioners of traditional medicines follow their traditions, observations, and belief but unaware about the modern theory of treatment. However, their “proof of concept” was based on the end result of using such therapy for generations. The main theme of their treatment was to provide relief to the sufferer, and then find the real cause of the suffering with the belief of “healing from within.” Those traditional practitioners did have a great knowledge about herbalism and ethnobotany as well as about human nature which may not be based on modern anatomy, physiology, biochemistry, and genetics. Species of Hydnocarpus were used by the ancient people of China for the treatment of Leprosy between 3000 and 2730 BC. Finding of opium poppy and castor bean from Egypt tombs revealed the use of phytomedicine in Africa as far back as 1500 BC. The Old Testament also mentions the use of medicinal herb and their cultivation. On the other hand, Ayurveda, the oldest surviving medical system of India about 5000 BC, uses nearly 750 plants like Aconitum, Clitoria, Cosinium, Shorea, and many more. Ayurveda is not only the rational use of medicinal plants but the central tenant of Ayur Bijnan (Science of life) is to maintain the harmony of human body and mind with all the elements of the universe, which can help in the management of life-style or microbial diseases including viral fever, meningitis, genital lesions, Amoebiasis, Leishmaniasis, high blood pressure, asthma, diabetes, and even cancer (Jiang et al., 2013). Like Ayurveda and African Traditional Medicine, ancient Chinese Traditional Medicine (CTM) also relies on harmony or balance between the body and soul in addition to uses of herbs. Traditional Chinese healers use herbs as well as other practices like acupuncture, tai-chi, and ai-gong for the treatment and/or prevention of diseases. Acupuncture involves the stimulation of specific nerve points on the body, while tai-chi and ai-gong involve gentle dance-like body movements with mental focus, breathing, and relaxation. Interestingly, Yoga, practiced by Indus-Sarasvati civilization in Northern India over 5000 years ago, mentioned in the Rig Veda, served as mainstream medical practice to maintain health and longevity. Chinese medicine, particularly CTM, also known to treat several dermatological disorders, coronary heart disease, hypertension, stroke, diabetes, atherosclerosis, etc. (Gong et al., 2017). Other schools of traditional medicines includes traditional Korean medicine, Arabic medicine, Haitian folk medicine, Uyghur traditional medicine, Celtic Medicine, Japanese Kampo medicine, and many more.
3.3. Ethnomedicine in Indian Context
India harbors a rich history of traditional medicine and a great knowledge of medicinal plants. It has been speculated that India’s most known traditional medicine “Ayurveda” existed even at the time of Indus valley civilization, and reached its apex during the Vedic period due to its widespread use and significant development. Ayurveda emphasizes to maintain balance between human body, mind, and the nature. With the use of natural resources, mainly plants and minerals, Ayurveda also focuses on exercise, healthy diet, yoga, and meditation to heal any ailments or disease. One of the main pillars of Ayurveda is the medicinal resources used by ethnic communities (Ethnomedicine) and plant-based treatments using different parts of plants including roots, leaves, fruits, bark, or even seeds. The book Yoga Ratnakara (1700–1800 CE, unknown author) mentioned the use of opium (Papaver somniferum) with minerals as an herbo-mineral formula for diarrhea; while in the book Bhaisajya Ratnavali opium and camphor (Cinnamomum camphora) are used for acute gastroenteritis, and hemp/marijuana/ganja (Cannabis indica) for treating diarrhea. During the British rule the practice of Ayurveda was neglected by the British Indian Government. However, after the independence of India the focus was intensified on Ayurveda and other traditional medical systems. In the past two decades, Ayurveda and other traditional healthcare system such as Yoga, Naturopathy, Unani, Siddha, and Homeopathy (AYUSH) brought under a separate Ministry, and became a part of the Indian National healthcare system with establishment of dedicated colleges and hospitals. Today the AYUSH system of healthcare is running parallel with the mainstream modern medicine in India.
India is inhabited by about 645 indigenous tribes having rich knowledge of wild flora and fauna to manage or cure diseases with other miraculous use. Most of this “traditional wisdom” is undocumented and orally passed over generations. Unfortunately, each time a traditional healer dies without passing their knowledge on to the next generation, the community and the world lose irreplaceable time-tested knowledge about medicinal plants gathered over thousands of years. Indian research policy has turned its attention to validate this untapped knowledge for past few years. However, since 1994 a group of researchers first time initiate a scientific documentation along with validation study to understand why the ancient ethnic communities of Andaman and Nicobar Islands survive, mostly without the help of modern medicine. Five years of rigorous interaction with those communities by establishing personal relationship, the researchers able to unveil some interesting insight of the healthcare culture of Onge, Nicobarese, and Shompen communities through scientific validation of a few useful medicaments which transcribed their traditional “information into modern innovation.” Chattopadhyay et al. have identified and validated a number of useful traditional medicaments of Bay Islands used for the management of infection, inflammatory condition, fever, pain, depression, contraception etc., including the antiviral activity of a useful herb Ophiorrhiza nicobarica collected from Galathia River basin of Great Nicobar Island, and widely used for skin ailments by the local and tribal communities (Chattopadhyay et al., 2006, Chattopadhyay and Bhattacharya, 2008. Later Bag et al., 2013, Bag et al., 2014 validated its potential and efficacy in cutaneously and vaginally infected animals, which lead to the identification of an alkaloid having potent anti-herpes activity at 1.1–1.5 µg/mL, much lower than acyclovir, by blocking the immediate early transcription of Herpes Simplex Virus types 1 (HSV-1) and 2 (HSV-2). While the extract of Achyranthes aspera and its isolated compound Oleanolic acid, used by traditional healers and some tribes of Rajasthan and Gujrat to treat asthma, boils, bronchitis, dysentery, pneumonia, skin diseases, fever, and typhoid (Goyel et al., 2007), was found to inhibit HSV-infection by blocking early stage of virus multiplication (Mukherjee et al., 2013). Similarly the stem bark of Odina wodier, a traditional medicine of Jangalmohal, used for curing ulcer, heart diseases and skin infection is found to prevent HSV-1-infected animals by inhibiting the viral multiplication through modulation of host immunity (Ojha et al., 2013), while the isolated chlorogenic acid regulate COX-2-dependent prostaglandin-E2 and TLR 4 signaling pathway (Ojha et al., 2014). Panda et al. (2017) have screened traditional medicinal plants used by tribes of Similipal Biosphere Reserve, Odisha and reported antiviral activity of few plants against Enterovirus-71.
3.4. Viral Diseases—A Global Health Concern
An inefficient virus kills its host, while a clever virus stays with it.
James Lovelock
Viruses are acellular ultra-microscopic metabolically inert obligate intracellular parasites of cellular hosts (Chattopadhyay et al., 2009b), associated with diverse diseases and are threats to human, wildlife, and livestock (Malosh et al., 2017, Marston et al., 2017, Prkno et al., 2017, Akbari and Elmi, 2017, Vu and Misra, 2018). Many are incurable and have no effective antiviral drugs except a very few (forty) US FDA approved antiviral drugs available for the management of some viral diseases. Complicated life cycle, frequent mutability and unique disease manifestation of each virus are the main reason for the public health concern. Treatment of viral diseases is immensely challenging because of their size, genetic variation, configuration of surface molecules, invasion strategies, mode of transmission, replication and persistent nature with rapid mutability, especially RNA viruses like influenza, severe acute respiratory syndrome (SARS) coronavirus etc., with a very high mutation rate, around 1/genome/replication (Elena and Sanjuan, 2005, Elena et al., 2000). High cost and side effects of present drugs used for treatment are also major contributing factor making antiviral drug development a daunting task. The antiviral drugs Acyclovir against HSV, Zidovudine against human immunodeficiency virus (HIV), Lamivudine against hepatitis B virus (HBV), Ganciclovir against Cytomegalovirus (CMV), Amantadine against influenza, and Pegylated IFN alpha against Hepatitis C virus (HCV) have been proven to be successful at least to some extent. But majority of viruses and viral diseases including Zika, Dengue, Ebola, Marburg, Rabies, Chikungunya, Rotavirus, HIV, etc. do not have any targeted drugs or vaccines till date. The unmet need for successful antivirals can only be fulfilled by thinking out of the box and ancient knowledge of ethnomedicine can be a master stroke against these deadly diseases.
3.5. Ethnomedicine and Virus
Ethnomedicines have been found to be effective as antiviral therapy, due to the following perspectives:
-
•
Plants produce thousands of compounds as secondary metabolites than they require for their survival and propagation (primary metabolites). These secondary metabolites, grouped as phenolics (flavonoids, quinones, coumarins, tannins, and anthocyanins), terpenoids (sterols, saponins, essential oils, and cucurbitacins), alkaloids, proteins, peptides, etc., are species specific and widely varied in structure and bioactivity. These compounds are foul-smelling, toxic and are synthesized mainly as weapons of defense against predators and pathogens.
-
•
Plant extracts are being validated for antiviral activity and use in treatments owing to the fact that many viruses are intractable to the typical antivirals. Also the effective life span of most of the antiviral is limited.
-
•
The problems of antiviral resistance, latency, recurrence, and fastidious spread of new or emerging strains, as in the case of HIV and SARS have compelled the virologists to look for better alternatives in nature, especially for people who have very little or no access to the expensive drugs (Chattopadhyay and Naik, 2007).
Plants have the miraculous treasure of numerous compounds with abilities to cure diseases and make our immunity strong. According to WHO fact sheet 2008, about 80% of population in Asia and Africa depend on plant-derived traditional medicine. Beside Asian countries, South America, Australia, and several countries of the European Union have documented ethnomedicine against various diseases including viral diseases.
The screening of antivirals from medicinal plants of traditional use is based on their wisdom, faith, availability and positive results for generations for curing ailments or diseases. Researchers are exploring those traditional plant-based medicaments to identify new source of antivirals. Most of these plants or plant-derived phytocompounds are reported to have antiinflammatory, antioxidant, antipyretic, antihelminthic, antifungal, antibacterial, and antiviral activities (Chattopadhyay et al., 2009a, Chattopadhyay et al., 2009b). Drug-like activities of plants or plant products, including antiviral activity, have been attributed to the secondary metabolites of plants, mainly alkaloids, flavonoids, saponins, quines, terpenes, lignans, tannins, polysaccharids, steroidal glycoside, thiosulfates, proanthocyanidin, and proteins.
3.5.1. Screening Models for Herbal Antiviral Agents and Their Value in Drug Discovery
-
•
In vitro primary screening
There are different in vitro and in vivo methods used to study the antiviral activities of plant products, but the most commonly used in vitro method for preliminary screening is cytopathic effect reduction (CPE), dye exclusion and plaque reduction assay (PRA) or plaque assay. Another rapid and sensitive in vitro procedure of evaluating antiviral agents is based on Spectrophotometric assessment for viability of virus- and mock-infected cells. Detailed methodologies for studying antiviral activities of plant products are available elsewhere (Chattopadhyay et al., 2009a, Chattopadhyay et al., 2009b, Chattopadhyay et al., 2015).
-
•
High throughput screening
High-capacity antiviral screening assay in 96-well microtiter plates using tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfop henyl)-2H-tetrazolium (MTS) or 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfop henyl)-2H-tetrazolium (MTT) is convenient as well as cost and time saving. Other methods include TaqMan PCR, and microscopic inspection of the cultures.
3.6. Mode of Action of Plant-Derived Antiviral Agents
Catechin, present in the globally most popular beverage green tea leaves, when fermented into theaflavins can neutralize bovine rotavirus and coronavir (Lin et al., 1997). Another common herb Ocimun basilicum or the sweet Basil of India and China has broad spectrum antiviral activity. The aqueous and ethanolic extract along with purified apigenin, linalool, and ursolic acid showed strong activity against HSV-1 (Bag et al., 2012), adenovirus 8 (ADV-8), Coxsackievirus B type-1 (CVB1) (Chattopadhyay and Naik, 2007). While Isoborneol, a monoterpene of essential oils isolated from Egyptian plant Melaleuca alternifolia, exhibited anti-HSV-1 activity by inactivating HSV-1 replication within 30 min of exposure (Armaka et al., 1999). Similarly Vatica cinerea from Vietnam is reported to inhibit HIV-1 replication (Zhang et al., 2003). The plant Cimicifuga racemosa (black cohosh) having antidepressant activity was also reported to have antiviral activity against human retroviruses by inhibiting HIV-1 reverse transcriptase (Sakurai et al., 2004). It is important to note that the viral diseases caused by Picornavirus and Rhinovirus do not have any drugs till date, while the plant-derived compounds chrysoplenol-C, and its glycoside has virucidal effect against both group of viruses (Wei et al., 2004). Scientists have also explored Himalayan flora used in traditional medicine against viral diseases (Amber et al., 2017). Ethnomedicine from India, Pakistan, China, and Nepal has been explored as source of antivirals against Influenza virus, Rhinovirus, Adenovirus, Coronavirus, and Respiratory Syncytial virus (RSV). Different compounds including monoterpenoids, flavonoids, triterpenoids, iridoid glycosides, sesquiterpenes, benzoic acids, and phenolics have strong antiviral potential. Recent emergence of deadly dengue, which is now a public health concern worldwide, has also been shown to be prevented by plant-derived drug. Lupeol isolated from Maytenus gonoclada has shown activity against dengue virus (Silva et al., 2017). Extracts from Carica papaya have now been used for the treatment of dengue in hospitals (Ahmad et al., 2011, Dharmarathna et al., 2013) mainly to prevent the reduction on number of platelet due to platelet aggregation, although the anti-dengue or anti-aggregation of platelet activity of papaya extract has not been scientifically proved till date. In folk medicine, papaya latex is used to cure dyspepsia, external burns and scalds, while its seeds and fruits have excellent antihelminthic and antiamebic activities. However, Chinnappan et al. (2016) have found that the leaf extract of papaya could possess a dengue-specific neutralizing effect on dengue virus-infected plasma that may exert a protective role on platelets. Luteolin, a bioflavonoid isolated from several dietary and medicinal plants, has been shown to have activity against HSV-1 (Ojha et al., 2015), dengue (Peng et al., 2017), Epstein–Barr virus (Wu et al., 2016), Japanese Encephalitis (JE) (Fan et al., 2016), and Chikungunya (Murali et al., 2015). Ginseng, a well-known medicinal herb, has been used in traditional medicine for thousands of years, and was found to be effective for treating influenza (Yoo et al., 2012) and HIV (Park et al., 2014); while in a clinical trial, Ginseng was found to help in curing HBV infection (Choi et al., 2016). Ethnopharmacological use of essential oil extract of three traditional Cretan aromatic plants in Eastern Mediterranean region and Near East claimed to be effective in the prevention and treatment of upper respiratory tract infections of bacterial and viral etiology (Duijker et al., 2015). While an Egyptian plant Nigella sativa extract tested against influenza patients showed better activity in people who cannot be treated with interferon-alpha (Barakat et al., 2013). A great deal of scientific research is being conducted to understand the mechanisms by which plant products exert their antiviral effects. Usually, plant-derived compounds exert antiviral effects through diverse mode and mechanism including (1) inhibition by autophagy, (2) generation of reactive oxygen species (ROS), (3) change in viral gene expression, (4) inhibition of viral entry to host cell including attachment and penetration, (5) inhibition of different steps of replication, (6) inhibition of viral release, as well as modulating the host immune parameters, which are briefly presented in Fig. 3.1 .
Figure 3.1.
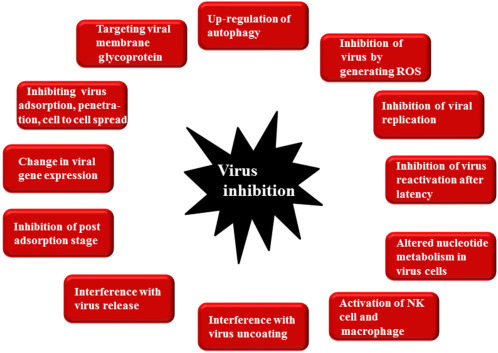
Mode of actions of plant-derived antiviral agents.
3.7. Mechanism of Action of Plant-derived Antiviral Agents
-
•
Viral inhibition by autophagy
Pentagalloylglucose found in many traditional medicinal herbs exhibits several bioactivities including antiinflammatory, anticancer, antioxidant, and antiviral effects. It has been shown to be effective against HSV-1 by induction of autophagosomes that engulfed HSV-1 virion (Pei et al., 2011). Triterpene glycyrrhizic acid, the major compound isolated from Glycyrrhiza glabra, has also been effective against HSV by promoting autophagy (Laconi et al., 2014).
-
•
Inhibition of virus replication
It has been reported that a cranberry extract known as Oximacro and its purified constituent proanthocyanidins can inhibit HSV-1 and HSV-2 replication in vitro and prevent the virus adsorption by targeting the viral envelop glycoprotein gD and gB (Terlizzi et al., 2016). The dichloromethane extract from Angelica archangelica L. fruit is a potential antiviral against HSV-1 by inhibiting viral replication (Rajtar et al., 2017). Fig. 3.1 depicts diverse mechanism of action by which the natural compounds are reported to be effective against different viruses. Resveratrol, a natural component of certain foods, such as grapes, has been shown to limit HSV-1 lesion formation by inhibiting viral replication (Docherty et al., 2005). Pentacyclic triterpenes isolated from birch bark (Betula species) extract of Eurasian and North America can inhibit HSV-1, especially acyclovir-sensitive and acyclovir-resistant clinical isolates of HSV (Heidary Navid et al., 2014). Sulfated polysaccharide from Caesalpinia ferrea can inhibit Poliovirus replication along with virus adsorption, steps after penetration and viral protein synthesis (Lopes et al., 2013). Oxyresveratrol, purified from Thai traditional medicinal plant Artocarpus lakoocha, has been shown to possess therapeutic effects in mice infected with HSV-1, by inhibiting early and late phase of viral DNA replication. While the anti-HSV effects of soybean-derived isoflavonoids were reported to be due to the inhibition of viral DNA replication (Argenta et al., 2018).
-
•
Inhibition of virus by generation of ROS
Oligomeric stilbenoids, polyphenolic phytoalexins from some plants including Cannabis sativa inhibit viral growth by generating ROS (Chen et al., 2012).
-
•
Change in virus gene expression
The n-Docosanol, a behenyl alcohol (22 carbon saturated fatty alcohol) from various plants, used traditionally as an emollient, emulsifier, and thickener in cosmetics, and nutritional supplement, inhibits herpes labialis and immediate early gene expression of the HSV (Treister and Woo, 2010).
-
•
Inhibition of viral entry
The sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata is reported to inhibit the entry of HSV-1 and HIV-2 (Talarico et al., 2004). Mentha suaveolens essential oil and its main component piperitenone oxide exert anti-HSV activity by inhibiting viral adsorption (Civitelli et al., 2014). Moreover, piperitenone oxide from Mentha longifolia could interfere with some redox-sensitive cellular pathways of host cell exploited for viral replication and thus inhibits viral growth and proliferation. While the virucidal effect of peppermint oil, the essential oil of Mentha piperita against HSV can be attributed to the interaction of peppermint oil with viral adsorption at the host cell surface preventing the virion to enter the host cell for establishing infection (Schuhmacher et al., 2003).
-
•
Interference with virus release
Thai medicinal plant Dunbariabella prain interfere with HSV release (Akanitapichat et al., 2006).
3.8. Antiviral Ethno-Pharmacology of Major Classes of Compounds
Plants have always been a major source of various bioactive compounds. Many of them have shown anticancer, antibacterial, antiparasitic activities. Here we will briefly discuss various groups of plant-derived compounds having antiviral activities.
-
•
Alkaloids
Alkaloids are a group of naturally occurring slightly acidic compounds that mostly contain basic nitrogen atoms. More than 10,000 different alkaloids have been discovered in 300 plant families. This group of compounds has been shown to be effective against viruses like hepatitis B (Li et al., 2008), HSV (Ren et al., 2010), dengue (Hishiki et al., 2017), etc.
-
•
Phenolics
Phenols, also called phenolics, are a class of compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group. Phenolic compounds are classified as simple phenols or polyphenols, based on the number of phenol units in the molecule. This group is an excellent candidate for antimicrobial and antiviral research. These compounds was found to be effective against HSV (Likhitwitayawuid et al., 2005), abies virus (Chavez et al., 2006), Influenzavirus (Ha et al., 2016), and many more.
-
•
Coumarins
Coumarins are organic colorless crystalline benzopyrene, naturally found in many plants. In fact its name comes from a French term for the tonka bean coumarou. Coumarins have been proven to be potent against many viruses like HIV (Lin et al., 2011b), HSV (Ghannadi et al., 2014), Dengue and Chikungunya (Gomez-Calderon et al., 2017).
-
•
Flavones, flavonoids, and flavonols
Flavonoids are a class of secondary metabolites of plants and fungus, while flavones are a class of flavonoids and are synthesized in response to microbial infections. Hence, they are broad spectrum antimicrobial agents. Flavonoids represent an important natural source of antiretroviral agents, especially for AIDS therapy (Pasetto et al., 2014).
-
•
Terpenoids and essential oils
Essential oils are the plant-derived phenolic compounds with a C3 side chain and at a lower level of oxidation without oxygen. The oils that are enriched in isoprene structure are called terpenes; but when they contain additional elements like oxygen they become “terpenoids.” These compounds are active against many viruses (Wen et al., 2007, Lu et al., 2015).
-
•
Quinones
Quinones are a class of organic compounds, named after its prototype member 1,4-benzoquinone or cyclohexadienedione, often called as “quinone.” It has been shown to be effective against Dengue (Laurent et al., 2005).
-
•
Tannins
Tannins are a group of polymeric plant phenolics. Studies show that tannins are active against many viruses including enveloped viruses like Influenza virus H3N2 and H5N3, HSV, Vesicular Stomatitis virus, Sendai virus, and Newcastle disease virus; as well as against nonenveloped viruses like Poliovirus, Coxsackievirus, Adenovirus, Rotavirus, Feline calicivirus, etc. (Ueda et al., 2013).
-
•
Lignans
Lignans are polyphenols found in plants. These compounds have also shown potent antiviral action (Cui et al., 2014).
Table 3.1 represents a selected list of ethnomedicinal plants and plant-derived compounds that showed promising antiviral activities against some viruses which are of concern in recent time. Dengue, a flavivirus that causes panic in developing countries including India, has sought attention from government and global research communities to look for new treatment. The use of papaya leaf extract in dengue fever, discussed earlier, while other plant-derived compounds including pentacyclic oxindole (Reis et al., 2008), chebulagic acid (Lin et al., 2011a), punicalagin (Lin et al., 2011b), baicalcin (Zandi et al., 2012), and alpha-mangostin (Tarasuk et al., 2017) reported to have anti-dengue activity. These compounds in general can modulate host cell immune system so that the host cell can fight against the dengue virus as the virus attack immune system, invade platelet, and cause postinfection complicacy including cytokine storm, particularly in the presence of preexisting heterologous antibody due to infection by another serotype of the same virus in same individual. Hepatitis caused by HBV, a common concern for liver dysfunction worldwide, has no effective treatment or vaccine. From ethnomedicinal wisdom, compounds such as oxymatrin (Chen et al., 2016) and cinamic acid (Amano et al., 2017) showed inhibitory effect on HBV by inhibiting viral DNA replication. The herpes caused by HSV-1 and HSV-2 in 90% population, via sexual or close contact from infected to uninfected individual are of great concern due to its silent epidemic nature. In spite of available gold-standard antiviral drug acyclovir, the HSV cannot be eradicated or eliminated from the host nor can prevent latency, rather it inhibits virus replication by blocking thymidine kinase and/or DNA polymerase of the virus, and thereby promote frequent emergence of drug-resistance strains deficient in thymidine kinase which is also a great concern. HSV is used as a standard model for screening of antivirals and its easy laboratory application made it a choice for researchers to study viruses. A vast number of anti-HSV plant extracts and compounds have been cited. But majority of them are from in vitro studies and have been shown to prevent adsorption to cell-to-cell spread. However, latency in HSV is the real twist and we need drugs that can eradicate the virus from brain to prevent its spread. Ursolic acid (Bag et al., 2012), oleanolic acid (Mukherjee et al., 2013), berberine (Song et al., 2014), masilinic acid (Zahmanov et al., 2015), and hermaline (Bag et al., 2013) have been reported to have anti-HSV activity by inhibiting entry, immediate early, early and late replication. Similarly, antiviral plant extracts and compounds have also been reported for Influenzavirus, Poliovirus, JEvirus, and RSV.
Table 3.1.
Selected Ethnomedicines and phytocompounds with antiviral activity
| Plants | Extract or compound | Virus | Mechanism | Reference |
|---|---|---|---|---|
| Peucedanum salinum | Methanol extract | Adenovirus (ADV) type 5 | Inhibit replication | Gao et al. (2011) |
| Radix lithospermi | Naphthaquinones Shikonin | Adenovirus (ADV) type 3 | Inhibit apoptosis and hexon protein expression of virus | Rajtar et al. (2012) |
| Uncaria tomentosa | Pentacyclic oxindoleAlkaloid fraction | DENV-2 | Immunomodulation | Reis et al. (2008) |
| Terminalia chebula Retz | Hydrolyzable tanninChebulagic acid and Punicalagin | DENV, HCV, HCMV, HIV, RSV | Inactivate free virus particles and inhibit virus entry | Lin et al. (2011a) |
|
Flavone Baicalein | DENV-2 | By type I interferon induction and virus adsorption | Zandi et al. (2012) |
| Garcinia mangostana Linn | Xanthones α-mangostin (α-MG) | DENV-2 | Immunomodulation | Tarasuk et al. (2017) |
|
3.4-Dihydroxy benzoic acid, p-OH-phenyl acetic acid, N-argenine | ECV-11, HSV-1, and ADV | Replication | Lazreg Aref et al. (2011) |
|
2-Methyl chromones 8-methoxy-2-methyl-4H-1-benzopyran-4-one | Hepatitis B virus (HBV) | Inhibiting HBV antigens secretion | Zhang et al. (2015) |
| Sophora subprostrata |
|
HBV | Inhibit replication | Yang et al. (2012) |
| Artemisia scoparia |
|
HBV | Inhibit DNA replication | Geng et al. (2015) |
| Sophora flavescens root | Matrine-cytisine alkaloid | HBV | Viral growth | Zhang et al. (2016) |
| Cinnamomum verum bark | Cinnamic acid | HCV | Induction of oxidative stress | Amano et al. (2017) |
| Ficus benjamina (ethanol extract of leaves) | Quercetin 3-O-rutinoside, Kaempferol 3-O-rutinoside and Kaempferol 3-O-robinobioside | HSV-1 | Virus multiplication | Yarmolinsky et al. (2009) |
| Punica granatum (many plants) | Gallic acid, gallic acid gold nanoparticles | HSV | Attachment and penetration | Halder et al. (2018) |
| Sophora subprostrata | Propolis | HSV | Pretreated before infection | Nolkemper et al. (2010) |
|
Pentacyclic triterpeneUrsolic acid | HSV-1, HSV-2 | Inhibiting early stage of multiplication | Bag et al. (2012) |
| A. aspera | Triterpene acidOleanolic acid | HSV-1 and HSV-2 | Modulation of early immunological parameters | Mukherjee et al. (2013) |
| Ophiorrhiza nicobarica Balkr | Indole alkaloid 7-methoxy-1-methyl-4,9-dihydro-3H-pyrido[3,4-b] indole | HSV-2 | Immediate early transcription by blocking recruitment of LSD-1 by HCF-1 | Bag et al. (2013) |
| Caesalpinia ferrea | Sulfated polysaccharide | HSV, poliovirus | Inhibit adsorption, postpenetration, and synthesis of viral proteins | Lopes et al. (2013) |
|
|
HSV |
|
Song et al. (2014) |
| Mentha suaveolens | Essential oilsPiperitenone oxide | HSV | Late phase of replication | Civitelli et al. (2014) |
| Andrographis paniculata | Diterpenoid lactone 3,19-isopropylidenean drographolide | HSV | Inhibit postentry, early gene expression; suppress ICP8 transcription, DNA replication and gD expression | Kongying yoes et al. (2016) |
|
Curcumin | HCMV | Protein expression of Hsp90α | Lv et al. (2015) |
| Angelica archangelica | Xanthotoxin, Bergapten, Imperatorin, Phellopterin, Isoimperatorin (isopentenyloxy moiety at C-8 position) | HSV-1 and Coxsackievirus B3 | Reduce viral titer | Rajtar et al. (2017) |
| Rheum root |
|
HIV |
|
Esposito et al. (2016) |
| Rheum officinale baill | Whole extract | HIV-1 |
|
|
| Schisandra chinensis |
|
HIV-1 | Affect HIV-1 RT | Xu et al. (2015) |
|
Methanol extract of root and bark | HIV | Replication | Tietjen et al. (2016) |
| Cynomorium songaricum Rupr. | Polysaccharide (water soluble) | HIV | Multiplication | Tuvaanjav et al. (2016) |
| Coffea arabica seed | Polyphenol caffeic acid | Influenza A virus | Inhibits multiplication | Utsunomiya et al. (2014) |
|
Allicin and plumbagin | Influenza A (H1N1) | Viral adsorption, nucleoprotein synthesis and polymerase activity | Chavan et al. (2016) |
| Laggera pterodonta | Pterodontic acid | H1 subtype of Influenza A | Block nuclear export of viral RNP complexes | Guan et al. (2017) |
| Forsythia suspense (Thunb.) Vahl fruit | Forsythoside A | Influenza A | Reduction of influenza M1 protein | Law et al. (2017) |
| Dendrobium nobile | Dendrobine | Influenza A | Growth | Li et al. (2017) |
| Rheum palmatum | Chrysophanol and aloe-emodin | Japanese Encephalitis | IFN-γ triggering host innate immune response | Chang et al. (2014) |
| CTM hochu-ekkito (HET) or bu-zhong-Žyi-qi-tang (Chinese) | Mixture of 10 plants | MCMV | Induction of IFN-γ and perforin-mediated cytotoxicity by natural killer (NK) cell activation | Hossain et al. (1999) |
|
Not detected | RSV | Induction of type I interferon-related signaling | Lee et al. (2017) |
HCV, hepatitis C virus; RSV, respiratory syncytial virus; HSV, herpes simplex virus; DENV, dengue virus; HCMV, human cytomegalo virus; MCMV, murine cytomegalo virus; HIV, human immunodeficiency virus; HBV, hepatitis B virus; ADV, adenovirus; JE, Japanese encephalitis virus; ECV, echovirus.
3.9. Challenges for Ethnomedicines as Antivirals
-
•
Many of the viral diseases are incurable and do not have a vaccine yet. Discovery of safe, effective, and inexpensive antivirals from natural source, particularly from ethnomedicinal practices are among the top priorities and challenges in the future.
-
•
Certain natural products show synergistic activities in combination with other natural products or with existing antivirals particularly useful against herpes and retroviral infections. Thus, proper combinatorial techniques to enhance the antiviral activities of these natural products need to be prioritized.
-
•
Recombinant viral vectors mimicking infection and expressing firefly luciferase maker gene are being used in antiviral screening. Therefore, the safety issues involved while screening the antivirals are being met and should be the top most priority in future research as well (Esimone et al., 2005).
-
•
Plants, other than being the sources of natural products, serve as efficient systems for the production of vaccines and pharmaceutical grade peptides/proteins. Several other vaccine antigens have been successfully expressed in plants, after the first subunit vaccine HBsAg was produced in 1992 (Ma Julian et al., 2003; Glenz and Warzecha, 2006).
-
•
Isolation of active ingredient from plants is a challenge and demands continuous up-gradation of technique and laboratory infrastructure through the years, so that those are available at an affordable cost to the public. In China and India, crude extracts are effectively used in healthcare although it is difficult to get these extracts approved by the FDA. In countries where resources are limited, government sponsored explorations will serve as a gateway for merging modern drug discovery with traditional/conventional medicine.
-
•
Many of these natural products prevent the entry of the virus into the host cell or target specific enzymes of the virus, which may be the molecular basis of viral drug resistance. An alternative mechanism of action can be the potential target to tackle the emerging, reemerging and drug-resistant viral infections. Thus, elucidation of mode as well as mechanism of actions of the potential antivirals is equally important in research.
-
•
Herbal medicines are being used individually or in combination, but their effects on the living systems are not scientifically documented. Results of clinical findings of using combination of plants or plant products like coadministration of kava-kava (Piper methysticum) and St. John’s wart (Hypericum perforatum) lead to hepatotoxicity (Musch et al., 2006). This type of information should be available to the healthcare providers practising traditional medicine.
-
•
It is equally important that the experiments on cytotoxicity of traditionally used plant extracts may be conducted as randomized, double-blind, placebo-controlled multicentric clinical trials so that the incorporation of a particular herbal remedy into the healthcare system is safe, besides being effective.
-
•
Several plant species have become extinct, while many phytocompounds mentioned in the classics may have undergone change with time as well as due to anthropogenic and environmental factors. The nature of the natural compound is complex and requires a flexible standardization process from time to time.
-
•
Screening a potential antiviral from millions of plant species is a tedious and time-taking process. A wide variety of computational techniques will help the chemists to virtually screen a huge library to a manageable size.
-
•
Drug discovery based on ethnomedicine follow “reverse pharmacology,” where the already documented traditional plants having healing ability can be further experimented as drug candidates in clinical research. The critical pharmacopoeia tests such as the dissolution time, microbial, pesticide, and heavy metal contamination must be in accordance with the standards globally accepted protocols of antiviral screening (Chattopadhyay et al., 2009b).
3.10. Conclusion
Traditional medicines have been increasingly used by diverse communities in many parts of the world, due to its important role in maintaining good health with increasing awareness and research. According to the WHO, the goal of “health for all” cannot be achieved without the incorporation of herbal medicines in primary healthcare system. Traditional medicine has a long history in disease control and public health management. Today, an increasing number of plants used in traditional system of medicine are reported to have diverse activities in infectious diseases, particularly in viral infections, and thus can be new sources of antivirals. The age-old medicinal system is being revisited to tackle the emerging public health issues. Thus, there is a significant need to enhance drug discovery process with respect to natural products, not just for the next 10 or 20 years, but for the next 20±40 years and beyond, because there are new diseases waiting to discover or invade. Complex structures of phytochemicals, synergistic activity and inadequate validation could not provide the drug which can have the potential to develop as a drug or a drug candidate to inhibit or eradicate the selected viruses alone or in combination with the existing antivirals without much harm to the host. With the advent of new approaches to drug discovery, innovative strategies would be required, that will reveal and contribute the full range of chemical diversity of these valuable natural products to the drug discovery process. It is also important that the development of plants and other natural products for medicinal and other biological purposes should be potentiated on a sustainable and renewable basis in local environment. Thus, pharmaceutical companies should consider developing local resources and decentralizing selected aspects of their research operations globally. Continuous research by using the advanced technology and bowfins can gift us the lead or leads to develop novel antiviral(s) from the treasure of Ethnomedicine.
References
- Ahmad N., Fazal H., Ayaz M., Abbasi B.H., Mohammad I., Fazal L. Dengue fever treatment with Carica papaya leaves extracts. Asian Pac. J. Trop. Biomed. 2011;1:330–333. doi: 10.1016/S2221-1691(11)60055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanitapichat P., Wangmaneerat A., Wilairat P., Bastow K.F. Anti-herpes virus activity of Dunbaria bella Prain. J. Ethnopharmacol. 2006;105:64–68. doi: 10.1016/j.jep.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Akbari M., Elmi R. Herpes simplex virus and human papillomavirus co-infections in hyper immunoglobin E syndrome presenting as a conjunctival mass lesion. Case Rep. Med. 2017;2017:1650841. doi: 10.1155/2017/1650841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano R., Yamashita A., Kasai H., Hori T., Miyasato S., Saito S. Cinnamic acid derivatives inhibit hepatitis C virus replication via the induction of oxidative stress. Antiviral Res. 2017;145:123–130. doi: 10.1016/j.antiviral.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Amber R., Adnan M., Tariq A., Mussarat S. A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. J. Pharm. Pharmacol. 2017;69:109–122. doi: 10.1111/jphp.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenta D.F., Bidone J., Koester L.S., Bassani V.L., Simoes C.M.O., Teixeira H.F. Topical delivery of coumestrol from lipid nanoemulsions thickened with hydroxyethylcellulose for antiherpes treatment. AAPS Pharm. Sci. Tech. 2018;19(1):192–200. doi: 10.1208/s12249-017-0828-8. [DOI] [PubMed] [Google Scholar]
- Armaka M., Papanikolaou E., Sivropoulou A., Arsenakis M. Antiviral properties of Isoborneol, a potent inhibitor of herpes simplex virus type 1. Antiviral Res. 1999;43:79–92. doi: 10.1016/s0166-3542(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Bag P., Chattopadhyay D., Mukherjee H., Ojha D., Mandal N., Sarkar M.C. Anti-herpes virus activities of bioactive fraction and isolated pure constituent of Mallotus peltatus: an ethnomedicine from Andaman Islands. Virol. J. 2012;9:98. doi: 10.1186/1743-422X-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag P., Ojha D., Mukherjee H., Halder U.C., Mondal S., Chandra N.S. An indole alkaloid from a tribal folklore inhibits immediate early event in HSV-2 infected cells with therapeutic efficacy in vaginally infected mice. PLoS One. 2013;8:e77937. doi: 10.1371/journal.pone.0077937. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bag P., Ojha D., Mukherjee H., Halder U.C., Mondal S., Biswas A. A dihydro-pyrido-indole potently inhibits HSV-1 infection by interfering with the viral immediate early transcriptional events. Antiviral Res. 2014;105:126–134. doi: 10.1016/j.antiviral.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Barakat E.M., Wakeel L.M.E.L., Hagag R.S. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J. Gastroenterol. 2013;19:2529–2536. doi: 10.3748/wjg.v19.i16.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.J., Huang S.H., Lin Y.J., Tsou Y.Y., Lin C.W. Antiviral activity of Rheum palmatum methanol extract and Chrysophanol against Japanese encephalitis virus. Arch. Pharm. Res. 2014;37:1117–1123. doi: 10.1007/s12272-013-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D. Ethnomedicinal phytophores in disease management. Internat J. Biomed. Pharmaceut. Sci. 2009;3(Spl1):1–125. ISBN: 9784-903313-35-1. [Google Scholar]
- Chattopadhyay D., editor. Ethnomedicine: a source of complementary therapeutics a review. Research Signpost Trivandrum; 2010. ISBN:978-81-308-0390-6. [Google Scholar]
- Chattopadhyay D., Bhattacharya S.K. Ethnopharmacology: a new search Engine for the development of Antivirals from naturaceuticals. In: Eddouks M., editor. Handbook of Ethnopharmacology. Research Signpost; India: 2008. pp. 129–197. Chapter 5. [Google Scholar]
- Chattopadhyay D., Naik T.N. Antivirals of ethnomedicinal origin: structure–activity relationship and scope. Mini Rev. Med. Chem. 2007;7:275–301. doi: 10.2174/138955707780059844. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Arunachalam G., Mandal A.B., Bhattacharya S.K. Dose dependent therapeutic antiinfectives from Ethnomedicines of Bay Islands. Chemotherapy. 2006;52(3):151–157. doi: 10.1159/000092859. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Mukherjee H., Bag P., Ghosh S., Samanta A., Chakrabarti S. Ethnomedicines in antiviral drug discovery. Int. J. Biomed. Pharmaceut. Sci. 2009;3(1):1–25. [Google Scholar]
- Chattopadhyay D., Sarkar M.C., Chatterjee T., Sharma Dey R., Bag P., Chakraborti S. Recent advancements for the evaluation of anti-viral activities of natural products. Nat. Biotechnol. 2009;25:347–368. doi: 10.1016/j.nbt.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Ojha D., Mondal S., Goswami D. Validation of antiviral potential of herbal ethnomedicine. In: Mukherjee P.K., editor. Evidence-Based Validation of Herbal Medicine. Elsevier Science; USA: 2015. pp. 175–200. Chapter 8. [Google Scholar]
- Chavan R.D., Shinde P., Girkar K., Madage R., Chowdhary A. Assessment of anti-influenza activity and hemagglutination inhibition of Plumbago indica and Allium sativum extracts. Pharmacogn. Res. 2016;8:105–111. doi: 10.4103/0974-8490.172562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez J.H., Leal P.C., Yunes R.A., Nunes R.J., Barardi C.R., Pinto A.R. Evaluation of antiviral activity of phenolic compounds and derivatives against rabies virus. Vet. Microbiol. 2006;116:53–59. doi: 10.1016/j.vetmic.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Chen X., Qiao H., Liu T., Yang Z., Xu L., Xu Y. Inhibition of herpes simplex virus infection by oligomeric stilbenoids through ROS generation. Antiviral Res. 2012;95:30–36. doi: 10.1016/j.antiviral.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Chen J.X., Shen H.H., Niu M., Guo Y.M., Liu X.Q., Han Y.Z. Anti-hepatitis B virus effect of matrine-type alkaloid and involvement of p38 mitogen-activated protein kinase and tumor necrosis factor receptor-associated factor 6. Virus Res. 2016;215:104–113. doi: 10.1016/j.virusres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Chinnappan S., Ramachandrappa V.S., Tamilarasu K., Krishnan U.M., Pillai A.K., Rajendiran S. Inhibition of platelet aggregation by the leaf extract of Carica papaya during dengue infection: an in vitro study. Viral Immunol. 2016;29:164–168. doi: 10.1089/vim.2015.0083. [DOI] [PubMed] [Google Scholar]
- Choi S.H., Yang K.J., Lee D.S. Effects of complementary combination therapy of Korean red ginseng and antiviral agents in chronic hepatitis B. J. Altern. Complement. Med. 2016;22:964–969. doi: 10.1089/acm.2015.0206. [DOI] [PubMed] [Google Scholar]
- Civitelli L., Panella S., Marcocci M.E., De Petris A., Garzoli S., Pepi F. In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine. 2014;21:857–865. doi: 10.1016/j.phymed.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Cui H., Xu B., Wu T., Xu J., Yuan Y., Gu Q. Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein–Barr virus lytic replication. J. Nat. Prod. 2014;77:100. doi: 10.1021/np400757k. [DOI] [PubMed] [Google Scholar]
- Dharmarathna S.L., Wickramasinghe S., Waduge R.N., Rajapakse R.P., Kularatne S.A. Does Carica papaya leaf-extract increase the platelet count? An experimental study in a murine model. Asian Pac. J. Trop. Biomed. 2013;3:720–724. doi: 10.1016/S2221-1691(13)60145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J.J., Fu M.M., Hah J.M., Sweet T.J., Faith S.A., Booth T. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antiviral Res. 2005;67:155–162. doi: 10.1016/j.antiviral.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Duijker G., Bertsias A., Symvoulakis E.K., Moschandreas J., Malliaraki N., Derdas S.P. Reporting effectiveness of an extract of three traditional Cretan herbs on upper respiratory tract infection: results from a double-blind randomized controlled trial. J. Ethnopharmacol. 2015;163:157–166. doi: 10.1016/j.jep.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena S.F., Sanjuan R. Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J. Virol. 2005;79:11555–11558. doi: 10.1128/JVI.79.18.11555-11558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena S.F., Miralles R., Cuevas J.M., Turner P.E., Moya A. The two faces of mutation: extinction and adaptation in RNA viruses. IUBMB Life. 2000;49:5–9. doi: 10.1080/713803585. [DOI] [PubMed] [Google Scholar]
- Esimone C.O., Grunwald T., Wildner O., Nchinda G., Tippler B., Proksch P. In vitro pharmacodynamics evaluation of antiviral medicinal plants using a vector-based assay technique. J. Appl. Microbiol. 2005;99:1346–1355. doi: 10.1111/j.1365-2672.2005.02732.x. [DOI] [PubMed] [Google Scholar]
- Esposito F., Carli I., Del vecchio C., Xu L., Corona A., Grandi N. Sennoside A, derived from the traditional Chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine. 2016;23:1383–1391. doi: 10.1016/j.phymed.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109(Suppl 1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Qian S., Qian P., Li X. Antiviral activity of luteolin against Japanese Encephalitis virus. Virus Res. 2016;220:112–116. doi: 10.1016/j.virusres.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Gao H., Liu L., Qu Z.Y., Wei F.X., Wang S.Q., Chen G. Anti-adenovirus activities of Shikonin, a component of Chinese herbal medicine in vitro. Biol. Pharm. Bull. 2011;34:197–202. doi: 10.1248/bpb.34.197. [DOI] [PubMed] [Google Scholar]
- Geng C.A., Huang X.Y., Chen X.L., Ma Y.B., Rong G.Q., Zhao Y. Three new anti-HBV active constituents from the traditional Chinese herb of Yin-Chen (Artemisia scoparia) J. Ethnopharmacol. 2015;176:109–117. doi: 10.1016/j.jep.2015.10.032. [DOI] [PubMed] [Google Scholar]
- Ghannadi A., Fattahian K., Shokoohinia Y., Behbahani M., Shahnoush A. Anti-viral evaluation of sesquiterpene coumarins from Ferula assa-foetida against HSV-1. Iran. J. Pharm. Res. 2014;13:523–530. [PMC free article] [PubMed] [Google Scholar]
- Glenz K., Warzecha H. New medicinal plants for the production of vaccines. J. für Verbraucherschutz und Lebensmittelsicherheit. 2006;1(Suppl 1):126–130. [Google Scholar]
- Gomez-Calderon C., Mesa-Castro C., Robledo S., Gomez S., Bolivar-Avila S., Diaz-Castillo F. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on Dengue and Chikungunya virus infections. BMC Complement Altern. Med. 2017;17:57. doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P., Li Y., Yao C., Guo H., Hwang H., Liu X. Traditional Chinese medicine on the treatment of coronary heart disease in recent 20 years. J. Altern. Complement. Med. 2017;23:659–666. doi: 10.1089/acm.2016.0420. [DOI] [PubMed] [Google Scholar]
- Goyel B.R., Goyal R.K., Mehta A.A. Phytopharmacology of Achyranthes aspera: A Review. Pharmacognosy Rev. 2007;1(1):143–150. [Google Scholar]
- Guan W., Li J., Chen Q., Jiang Z., Zhang R., Wang X. Pterodontic acid isolated from Laggera pterodonta inhibits viral replication and inflammation induced by influenza A virus. Molecules. 2017:22. doi: 10.3390/molecules22101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T.K., Dao T.T., Nguyen N.H., Kim J., Kim E., Cho T.O. Antiviral phenolics from the leaves of Cleistocalyx operculatus. Fitoterapia. 2016;110:135–141. doi: 10.1016/j.fitote.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Halder A., Das S., Ojha D., Chattopadhyay D., Mukherjee A. Highly monodispersed gold nanoparticles synthesis and inhibition of Herpes Simplex Virus infections. Mater. Sci. Eng. C. 2018;89:413–421. doi: 10.1016/j.msec.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Heidary Navid M., Laszczyk-Lauer M.N., Reichling J., Schnitzler P. Pentacyclic triterpenes in birch bark extract inhibit early step of Herpes Simplex virus type 1 replication. Phytomedicine. 2014;21:1273–1280. doi: 10.1016/j.phymed.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Hishiki T., Kato F., Tajima S., Toume K., Umezaki M., Takasaki T. Hirsutine, an indole alkaloid of Uncaria rhynchophylla, inhibits late step in dengue virus lifecycle. Front. Microbiol. 2017;8:1674. doi: 10.3389/fmicb.2017.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., Takimoto H., Hamano S., Yoshida H., Ninomiya T., Minamishima Y. Protective effects of hochu-ekki-to, a Chinese traditional herbal medicine against murine cytomegalovirus infection. Immunopharmacology. 1999;41:169–181. doi: 10.1016/s0162-3109(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Jiang G., Xiao X., Zeng Y., Nagabhushanam K., Majeed M., Xiao D. Targeting beta-catenin signaling to induce apoptosis in human breast cancer cells by z-guggulsterone and Gugulipid extract of Ayurvedic medicine plant Commiphora mukul. BMC Complement. Altern. Med. 2013;13:203. doi: 10.1186/1472-6882-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongying yoes B., Priengprom T., Pientong C., Aromdee C., Suebsasana S., Ekalaksananan T. 3,19-Isopropylideneandrographolide suppresses early gene expression of drug-resistant and wild type herpes simplex viruses. Antiviral Res. 2016;132:281–286. doi: 10.1016/j.antiviral.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Krippner R., Staples J. Suspected allergy to artemether-lumefantrine treatment of malaria. J. Travel Med. 2003;10:303–305. doi: 10.2310/7060.2003.2744. [DOI] [PubMed] [Google Scholar]
- Laconi S., Madeddu M.A., Pompei R. Autophagy activation and antiviral activity by a licorice triterpene. Phytother. Res. 2014;28:1890–1892. doi: 10.1002/ptr.5189. [DOI] [PubMed] [Google Scholar]
- Laurent D., Baumann F., Benoit A.G., Mortelecqe A., Nitatpattana N., Desvignes I. Structure–activity relationships of dengue antiviral polycyclic quinones. Southeast Asian J. Trop. Med. Public Health. 2005;36:901–905. [PubMed] [Google Scholar]
- Law A.H., Yang C.L., Lau A.S., Chan G.C. Antiviral effect of forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit against influenza A virus through reduction of viral M1 protein. J. Ethnopharmacol. 2017;209:236–247. doi: 10.1016/j.jep.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Lazreg Aref H., Gaaliche B., Fekih A., Mars M., Aouni M., Pierre Chaumon J. In vitro cytotoxic and antiviral activities of Ficus carica latex extracts. Nat. Prod. Res. 2011;25:310–319. doi: 10.1080/14786419.2010.528758. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Chathuranga K., Uddin M.B., Weeratunga P., Kim M.S., Cho W.K. Coptidis rhizoma extract inhibits replication of respiratory syncytial virus in vitro and in vivo by inducing antiviral state. J. Microbiol. 2017;55:488–498. doi: 10.1007/s12275-017-7088-x. [DOI] [PubMed] [Google Scholar]
- Li H.L., Han T., Liu R.H., Zhang C., Chen H.S., Zhang W.D. Alkaloids from Corydalis saxicola and their anti-hepatitis B virus activity. Chem. Biodivers. 2008;5:777–783. doi: 10.1002/cbdv.200890074. [DOI] [PubMed] [Google Scholar]
- Li R., Liu T., Liu M., Chen F., Liu S., Yang J. Anti-influenza A virus activity of dendrobine and its mechanism of action. J. Agric. Food Chem. 2017;65:3665–3674. doi: 10.1021/acs.jafc.7b00276. [DOI] [PubMed] [Google Scholar]
- Likhitwitayawuid K., Sritularak B., Benchanak K., Lipipun V., Mathew J., Schinazi R.F. Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat. Prod. Res. 2005;19:177–182. doi: 10.1080/14786410410001704813. [DOI] [PubMed] [Google Scholar]
- Lin L.T., Chen T.Y., Chung C.Y., Noyce R.S., Grindley T.B., Mccormick C. Hydrolyzable tannins (Chebulagic acid and punicalagin) target viral glycoprotein–glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011;85:4386–4398. doi: 10.1128/JVI.01492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.H., Ke Y.Y., Su C.T., Shiao H.Y., Hsieh H.P., Chao Y.K. Inhibition of HIV-1 Tat-mediated transcription by a coumarin derivative, BPRHIV001, through the Akt pathway. J. Virol. 2011;85:9114–9126. doi: 10.1128/JVI.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.M., Anderson H., Flavin M.T., Pai Y.H., Mata-Greenwood E., Pengsuparp T. In vitro anti-HIV activity of bioflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997;60:884–888. doi: 10.1021/np9700275. [DOI] [PubMed] [Google Scholar]
- Lopes N., Faccin-Galhardi L.C., Espada S.F., Pacheco A.C., Ricardo N.M., Linhares R.E. Sulfated polysaccharide of Caesalpinia ferrea inhibits Herpes Simplex virus and Poliovirus. Int. J. Biol. Macromol. 2013;60:93–99. doi: 10.1016/j.ijbiomac.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Lu H.N., Ma S.G., Liu Y.B., Qu J., Li Y., Xu S. Sesquiterpenes from the roots of Illicium oligandrum. J. Asian Nat. Prod. Res. 2015;17:430–438. doi: 10.1080/10286020.2015.1039524. [DOI] [PubMed] [Google Scholar]
- Lv Y., Gong L., Wang Z., Han F., Liu H., Lu X. Curcumin inhibits human cytomegalovirus by downregulating heat shock protein 90. Mol. Med. Rep. 2015;12:4789–4793. doi: 10.3892/mmr.2015.3983. [DOI] [PubMed] [Google Scholar]
- Ma Julian K.C., Drake Pascal M.W., Christou P. The production of recombinant pharmaceutical proteins in plants. Nature Reviews Genetics. 2003;4:794–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- Malosh R.E., Martin E.T., Ortiz J.R., Monto A.S. The risk of lower respiratory tract infection following influenza virus infection: a systematic and narrative review. Vaccine. 2017;36(1):141–147. doi: 10.1016/j.vaccine.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston B.J., Dokubo E.K., Van Steelandt A., Martel L., Williams D., Hersey S. Ebola response impact on public health programs, West Africa, 2014–2017. Emerg. Infect. Dis. 2017;23 doi: 10.3201/eid2313.170727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee H., Ojha D., Bag P., Chandel H.S., Bhattacharyya S., Chatterjee T.K. Anti-herpes virus activities of Achyranthes aspera: an Indian ethnomedicine, and its triterpene acid. Microbiol. Res. 2013;168(4):238–244. doi: 10.1016/j.micres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Murali K.S., Sivasubramanian S., Vincent S., Murugan S.B., Giridaran B., Dinesh S. Anti-chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac. J. Trop. Med. 2015;8:352–358. doi: 10.1016/S1995-7645(14)60343-6. [DOI] [PubMed] [Google Scholar]
- Musch E., Chrissafidou A., Malek M. Acute hepatitis due to kava-kava and St John's Wort: an immune-mediated mechanism? Dtsch. Med. Wochenschr. 2006;131:1214–1217. doi: 10.1055/s-2006-941754. [DOI] [PubMed] [Google Scholar]
- Nolkemper S., Reichling J., Sensch K.H., Schnitzler P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine. 2010;17:132–138. doi: 10.1016/j.phymed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Ojha D., Mukherjee H., Ghosh S., Bag P., Mondal S., Chandra N. Evaluation of anti-infective potential of a Tribal folklore Odina wodier Roxb against some selected microbes and Herpes Simplex Virus associated with skin infection. J. Appl. Microbiol. 2013;115:1317–1328. doi: 10.1111/jam.12330. [DOI] [PubMed] [Google Scholar]
- Ojha D., Mukherjee H., Mondal S., Jena A., Dwivedi V.P., Mondal K.C. Anti-inflammatory activity of Odina wodier Roxb, an Indian folk remedy, through inhibition of toll-like receptor 4 signaling pathway. PLoS One. 2014;9:e104939. doi: 10.1371/journal.pone.0104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha D., Das R., Sobia P., Dwivedi V., Ghosh S., Samanta A. Pedilanthus tithymaloides inhibits HSV infection by modulating NF-kappaB signaling. PLoS One. 2015;10:e0139338. doi: 10.1371/journal.pone.0139338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S.K., Padhi L., Leyssen P., Liu M., Neyts J., Luyten W. Antimicrobial, antihelminthic, and antiviral activity of plants traditionally used for treating infectious disease in the similipal biosphere reserve, Odisha, India. Front. Pharmacol. 2017;8:658. doi: 10.3389/fphar.2017.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.H., Yum J., Ku K.B., Kim H.M., Kang Y.M., Kim J.C. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J. Ginseng Res. 2014;38:40–46. doi: 10.1016/j.jgr.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasetto S., Pardi V., Murata R.M. Anti-HIV-1 activity of flavonoid Myricetin on HIV-1 infection in a dual-chamber in vitro model. PLoS One. 2014;9:e115323. doi: 10.1371/journal.pone.0115323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Chen Z.P., Ju H.Q., Komatsu M., Ji Y.H., Liu G. Autophagy is involved in anti-viral activity of Pentagalloylglucose (PGG) against Herpes simplex virus type 1 infection in vitro. Biochem. Biophys. Res. Commun. 2011;405:186–191. doi: 10.1016/j.bbrc.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Peng M., Watanabe S., Chan K.W.K., He Q., Zhao Y., Zhang Z. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antiviral Res. 2017;143:176–185. doi: 10.1016/j.antiviral.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Prkno A., Hoffmann D., Goerigk D., Kaiser M., Van Maanen A.C.F., Jeske K. Epidemiological investigations of four cowpox virus outbreaks in Alpaca Herds, Germany. Viruses. 2017;9 doi: 10.3390/v9110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajtar B., Skalicka-Wozniak K., Polz-Dacewicz M., Glowniak K. The influence of extracts from Peucedanum salinum on the replication of adenovirus type 5. Arch. Med. Sci. 2012;8:43–46. doi: 10.5114/aoms.2012.27279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajtar B., Skalicka-Wozniak K., Swiatek L., Stec A., Boguszewska A., Polz-Dacewicz M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem. Toxicol. 2017;109:1026–1031. doi: 10.1016/j.fct.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Reis S.R., Valente L.M., Sampaio A.L., Siani A.C., Gandini M., Azeredo E.L. Immunomodulating and antiviral activities of Uncaria tomentosa on human monocytes infected with Dengue Virus-2. Int. Immunopharmacol. 2008;8:468–476. doi: 10.1016/j.intimp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ren Z., Zhang C.H., Wang L.J., Cui Y.X., Qi R.B., Yang C.R. In vitro anti-viral activity of the total alkaloids from Tripterygium hypoglaucum against herpes simplex virus type 1. Virol. Sin. 2010;25:107–114. doi: 10.1007/s12250-010-3092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai N., Wu J.H., Sashida Y., Mimaki Y., Nikaido T., Koike K. Anti-AIDS agents. Part 57: actein, an anti-HIV principle from the rhizome of Cimicifuga racemosa (black cohosh), and the anti-HIV activity of related saponins. Bioorg. Med. Chem. Lett. 2004;14:1329–1332. doi: 10.1016/j.bmcl.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Schuhmacher A., Reichling J., Schnitzler P. Virucidal effect of peppermint oil on the enveloped viruses Herpes Simplex virus type 1 and type 2 in vitro. Phytomedicine. 2003;10:504–510. doi: 10.1078/094471103322331467. [DOI] [PubMed] [Google Scholar]
- Silva F.C., Rodrigues V.G., Duarte L.P., Lula I.S., Sinisterra R.D., Vieira-Filho S.A. Antidiarrhoeal activity of extracts from Maytenus gonoclada and inhibition of Dengue virus by lupeol. An. Acad. Bras. Cienc. 2017;89:1555–1564. doi: 10.1590/0001-3765201720160046. [DOI] [PubMed] [Google Scholar]
- Song S., Qiu M., Chu Y., Chen D., Wang X., Su A. Downregulation of cellular c-Jun N-terminal protein kinase and NF-kappaB activation by berberine may result in inhibition of herpes simplex virus replication. Antimicrob. Agents Chemother. 2014;58:5068–5078. doi: 10.1128/AAC.02427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico L.B., Zibetti R.G., Faria P.C., Scolaro L.A., Duarte M.E., Noseda M.D. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004;34:63–71. doi: 10.1016/j.ijbiomac.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Tarasuk M., Songprakhon P., Chimma P., Sratongno P., Na-Bangchang K., Yenchitsomanus P.T. Alpha-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 2017;240:180–189. doi: 10.1016/j.virusres.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Terlizzi M.E., Occhipinti A., Luganini A., Maffei M.E., Gribaudo G. Inhibition of herpes simplex type 1 and type 2 infections by Oximacro((R)), a cranberry extract with a high content of A-type proanthocyanidins (PACs-A) Antiviral Res. 2016;132:154–164. doi: 10.1016/j.antiviral.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Tietjen I., Gatonye T., Ngwenya B.N., Namushe A., Simonambanga S., Muzila M. Croton megalobotrys Mull Arg. and Vitex doniana (Sweet): traditional medicinal plants in a three-step treatment regimen that inhibit in vitro replication of HIV-1. J. Ethnopharmacol. 2016;191:331–340. doi: 10.1016/j.jep.2016.06.040. [DOI] [PubMed] [Google Scholar]
- Treister N.S., Woo S.B. Topical n-docosanol for management of recurrent herpes labialis. Expert Opin Pharmacother. 2010;11(5):853–860. doi: 10.1517/14656561003691847. [DOI] [PubMed] [Google Scholar]
- Tuvaanjav S., Shuqin H., Komata M., Ma C., Kanamoto T., Nakashima H. Isolation and antiviral activity of water-soluble Cynomorium songaricum Rupr. Polysaccharides. J. Asian Nat. Prod. Res. 2016;18:159–171. doi: 10.1080/10286020.2015.1082547. [DOI] [PubMed] [Google Scholar]
- Ueda K., Kawabata R., Irie T., Nakai Y., Tohya Y., Sakaguchi T. Inactivation of pathogenic viruses by plant-derived tannins: strong effects of extracts from persimmon (Diospyros kaki) on a broad range of viruses. PLoS One. 2013;8:e55343. doi: 10.1371/journal.pone.0055343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H., Ichinose M., Ikeda K., Uozaki M., Morishita J., Kuwahara T. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014;34:1020–1024. doi: 10.3892/ijmm.2014.1859. [DOI] [PubMed] [Google Scholar]
- Vu L., Misra K. High burden of HIV, syphilis and HSV-2 and factors associated with HIV infection among female sex workers in Tanzania: implications for early treatment of HIV and pre-exposure prophylaxis (PrEP) AIDS Behav. 2018;22(4):1113–1121. doi: 10.1007/s10461-017-1992-2. [DOI] [PubMed] [Google Scholar]
- Wei F., Ma S.C., Ma L.Y., But P.P., Lin R.C., Khan I.A. Antiviral flavonoids from the seeds of Aesculus chinensis. J. Nat. Prod. 2004;67:650–653. doi: 10.1021/np030470h. [DOI] [PubMed] [Google Scholar]
- Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome Coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- Wu C.C., Fang C.Y., Hsu H.Y., Chen Y.J., Chou S.P., Huang S.Y. Luteolin inhibits Epstein–Barr virus lytic reactivation by repressing the promoter activities of immediate-early genes. Antiviral Res. 2016;132:99–110. doi: 10.1016/j.antiviral.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Xu L., Grandi N., Del Vecchio C., Mandas D., Corona A., Piano D. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. J. Microbiol. 2015;53:288–293. doi: 10.1007/s12275-015-4652-0. [DOI] [PubMed] [Google Scholar]
- Yang J.M., Ip S.P., Xian Y., Zhao M., Lin Z.X., Yeung J.H. Impact of the herbal medicine Sophora flavescens on the oral pharmacokinetics of Indinavir in rats: the involvement of CYP3A and P-glycoprotein. PLoS One. 2012;7:e31312. doi: 10.1371/journal.pone.0031312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky L., Zaccai M., Ben-Shabat S., Mills D., Huleihel M. Antiviral activity of ethanol extracts of Ficus binjamina and Lilium candidum in vitro. Nat. Biotechnol. 2009;26:307–313. doi: 10.1016/j.nbt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Yoo D.-G., Kim M.-C., Park M.-K., Park K.-M., Quan F.-S., Song J.-M. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS One. 2012;7(3):1–9. doi: 10.1371/journal.pone.0033678. http://dx.doi.org/10.1371/journal.pone.0033678 e33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahmanov G., Alipieva K., Denev P., Todorov D., Hinkov A., Shishkov S. Flavonoid glycosides profiling in dwarf elder fruits (Sambucus ebulus L.) and evaluation of their antioxidant and anti-Herpes Simplex activities. Ind. Crop. Prod. 2015;63:58–64. [Google Scholar]
- Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M.R., Abubakar S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012;12:214. doi: 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.J., Tan G.T., Hoang V.D., Hung N.V., Cuong N.M., Soejarto D.D. Natural anti-HIV agents. Part IV. Anti-HIV constituents from Vatica cinerea. J. Nat. Prod. 2003;66:263–268. doi: 10.1021/np020379y. [DOI] [PubMed] [Google Scholar]
- Zhang Y.B., Zhan L.Q., Li G.Q., Wang F., Wang Y., Li Y.L. Dimeric matrine-type alkaloids from the roots of Sophora flavescens and their anti-hepatitis B virus activities. J. Org. Chem. 2016;81:6273–6280. doi: 10.1021/acs.joc.6b00804. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Bian Q., Luo P., Sun W. Ethnopharmacological, chemical, and pharmacological aspects of Halenia elliptica: a comprehensive review. Pharmacogn. Rev. 2015;9:114–119. doi: 10.4103/0973-7847.162114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Warzecha H., Mason H.S. Benefits and risks of antibody and vaccine production in transgenic plants. J. Plant Physiol. 2003;160:755–764. doi: 10.1078/0176-1617-01125. [DOI] [PubMed] [Google Scholar]
- Xiang Y.F., Qian C.W., Xing G.W., Hao J., Xia M., Wang Y.F. Anti-herpes simplex virus efficacies of 2-aminobenzamide derivatives as novel HSP90 inhibitors. Bioorg. Med. Chem. Lett. 2012;22:4703–4706. doi: 10.1016/j.bmcl.2012.05.079. [DOI] [PubMed] [Google Scholar]


