Abstract
Although a wide range of diseases have been reported in captive snow leopards, little is known about those affecting the species in the wild. However, the potential threat from diseases to wild snow leopards must not be underestimated as a consequence of lack of health surveillance throughout the inaccessible terrains they occupy. As a felid, the snow leopard is likely to be susceptible to most infectious agents affecting the domestic cat, and here we provide an overview of those with a risk of lethality for free-ranging snow leopards. In contrast to the health of snow leopards themselves, a great deal is known about the diseases affecting their primary prey species. We present these cases and highlight the importance of livestock as the main source of disease spillover to natural prey species. Further studies are required to understand the impact of infectious agents on intra- and interspecific population dynamics of snow leopards and associated prey.
Keywords: infectious disease, wildlife disease, livestock disease, disease spillover
Introduction
This review aims to present the major infectious diseases that may affect free-ranging snow leopards, and those that may impact the abundance of their natural ungulate prey. It is beyond the scope of this review to cite the numerous studies that have documented neoplasia, degenerative diseases, congenital malformations, and infectious diseases (occasionally lethal) in captive snow leopards. In addition it should not be considered as a comprehensive review of infectious diseases; it concentrates only on those with a perceived lethality in nature.
Snow leopards and their ungulate prey inhabit cold arid environments. Because microbial abundance in soil correlates negatively with precipitation (Blankinship et al., 2011), it is predicted that they encounter lower microbial abundance than their counterparts in more mesic, temperate or tropical environments, and may have evolved correspondingly lower immune indices. This circumstance is preoccupying from a conservation standpoint as it may render these species particularly vulnerable to the emergence of pathogens disseminated by fast-spreading populations of domestic species, and to changes in pathogen distribution resulting from climatic changes. The present review shows that at least for snow leopard prey species, disease is already a significant local threat, and may be following an increasing trend, whereas data deficiencies prevent a full evaluation of the disease threat to the snow leopards themselves.
Diseases in free-ranging snow leopards
Causes of Mortality in Snow Leopards
No publications currently provide a comprehensive account of mortality of free-ranging snow leopards. Natural deaths due, for example, to starvation or natural accidents are rarely observed. Human-induced casualties due to poaching, traffic accidents, or poisoning are almost never reported quickly enough for forensic investigations to be performed efficiently. Surveillance of wild populations for infections, based on antemortem testing of blood and feces has been limited. At the time of writing, laboratory investigations of infectious agents circulating in the snow leopard population of the Tost Mountains, in the South-Gobi Province of Mongolia are underway and should provide valuable information with significant sample sizes (Ö. Johansson, personal communication). Incidental reports, confirmed by comprehensive mortality studies carried out in other nondomestic cats (Schmidt-Posthaus et al., 2002) tend to suggest that noninfectious causes are probably responsible for a significant proportion of deaths in free-ranging snow leopards. Hussain Ali, who extensively surveyed the Khunjerab area in northern Pakistan, reports in his diary to have examined 14 dead snow leopards between 2000 and 2008. Two had been poached, four were found dead alongside uneaten carcasses of Siberian ibex (Capra sibirica) and fallen rocks, and presumably died accidentally in the course of chases in steep terrains, three had fallen over cliffs with no dead prey around and possibly as a result of avalanche or rock slide, one was found on the Karakoram Highway and was possibly a road kill or had fallen over a cliff, three (an adult female with her two subadult cubs) could have been poisoned, and one odd case was found dead on top of a juniper tree. Interestingly, of the seven animals that had fallen over cliffs, associated or not to a prey chase, five were young (<2-year-old) animals.
Snow leopards can also be victims of poisons, either intentionally as retaliation to livestock depredation or unintentionally during indiscriminate poisoning campaigns. However, the nature of poisons, extent of use, and impact on snow leopard populations remain largely unstudied. Poaching is usually underestimated because it is rarely reported or missed, such as in the case of alleged starved or “fallen-over-cliff” individuals (Fig. 9.1 ), but it has been reported to cause a significant proportion of the mortality of adult radio-collared felids (e.g., Amur tiger Panthera tigris altaica in Russia; Goodrich et al., 2008). Infectious diseases have almost never been reported or successfully verified by postmortem evaluations in free-ranging snow leopards (Fig. 9.2 ; Batzorig et al., 2011), but similarly to poaching this cause of mortality could easily be underestimated because of the difficulty in detecting and/or investigating cases in the inaccessible terrain they occupy. Infectious diseases may be a normal feature of an otherwise healthy snow leopard population, but effects may be exacerbated by increased stress and occur as a spillover from domestic carnivores or where populations are already compromised by declining numbers.
Figure 9.1.

Determining with accuracy the genuine cause of death of free-ranging snow leopards (P. uncia) could prove a daunting task.
This specimen was found dead at the base of a steep cliff over which local people alleged it had accidentally fallen. However, a thorough necropsy of the animal revealed the presence of bullet fragments in the radius bone (A) (arrow), and an hemorrhagic track through shoulder muscles with additional fragments of a broken up 0.22 caliber rimfire bullet (B), supporting that the fall was consecutive to a gunshot. Wakhan District, Afghanistan, December 2010.
Source: Photo courtesy of S. Ostrowski and Inayat Ali.
Figure 9.2.

A veterinarian saws the skull of a dead radio-collared snow leopard (P. uncia) in an attempt to collect brain tissue for laboratory investigations.
Snow leopards are susceptible to a range of neurotropic infectious agents, such as the viruses responsible of rabies and canine distemper. Tost Mountains, South-Gobi Province, Mongolia, August 2011.
Source: Photo courtesy of T. Lhagvasumberel.
Infectious Diseases
Selected Viral Diseases
A wide variety of viral agents have been found in captive felids, including snow leopards, of which some have severe and sometimes fatal consequences to the host. Clinical disease has been recorded much less frequently in free-ranging wild felids, with a single poorly documented account of rabies infection from 1940 (Heptner and Sludskii, 1992), representing the only clinically significant report of viral disease in a free-ranging snow leopard. However, the true incidence of viral infections in wild snow leopards is hampered by the lack of surveillance in the remote locations they occupy, and the species is likely to be susceptible to a range of pathogens found in other free-ranging felids (Table 9.1 ). Viral infections will only have a negative impact on population viability if they reduce reproductive output either directly or by increasing host mortality that is additive to other causes of death. Contributory risk factors include increased pathogenicity of circulating viruses, the presence of a more abundant reservoir population (domestic or wild), and an increased susceptibility of the host such as in case of coinfection, chronic stress, or decreased genetic variability.
Table 9.1.
Selected Microbial Diseases Potentially Responsible for Morbidity and Mortality in Free-Ranging Snow Leopards
| Disease | Symptoms in felids | Mode of transmission | Perceived lethality | Reference (captive snow leopard) | Reference (free-ranging large nondomestic cats) |
|---|---|---|---|---|---|
| VIRUS | |||||
| Rabies | CNS disease; death | Bite injury; saliva | High | - | Pfukenyi et al., 2009a |
| Canine distemper | CNS disease, pneumonia; death | Ingestion; inhalation | High | Fix et al., 1989 | Seimon et al., 2013 |
| Feline immunodeficiency | Pneumonia; diarrhea; death | Bite injury | Possibly high | Barr et al., 1989b | Roelke et al., 2006c |
| Bluetongue | Lethargy; pneumonia; death | Ingestion; mosquito bite | Possibly high | - | Alexander et al., 1994d |
| Feline panleukopenia | Fever; diarrhea; vomiting; death | Ingestion; transplacentally | Moderate | Fix et al., 1989 | Schmidt-Posthaus et al., 2002 |
| Feline leukemia | Anemia; immunosuppression; death | Bite injury; body fluids | Moderate | - | Meli et al., 2009e |
| Feline papillomavirus | Oral warts; neoplasia | Damaged oral mucosa | Low | Sundberg et al., 2000f | - |
| Feline coronavirus | Enteritis; diarrhea; peritonitis | Ingestion | Low | Kennedy et al., 2002g | Heeney et al., 1990h |
| Feline calicivirus | Upper respiratory tract disease | Inhalation | Low | - | Hofmann-Lehmann et al., 1996i |
| BACTERIA | |||||
| Tuberculosis | Lower respiratory tract disease; emaciation; death | Inhalation | High | Helman et al., 1998 | Michel et al., 2006 |
| Plague | Pneumonia; death | Ingestion; flea bite | High | - | Wild et al., 2006j |
| Anthrax | Sudden death | Ingestion | Moderate | - | Jager et al., 1990 |
| Pseudotuberculosis | Diarrhea; vomiting; lethargy; anorexia; | Ingestion | Moderate | - | Ryser-Degiorgis and Robert, 2006k |
| Tularemia | Lethargy; oral ulcers; enlarged lymph nodes | Ingestion; inhalation; insect bite | Low | - | Girard et al., 2012l |
| PROTOZOA | |||||
| Babesiosis | Fever; anemia; jaundice | Tick bite | Low | - | Munson et al., 2008 |
| Hepatozoonosis | Anemia; emaciation | Tick bite | Low | - | Khoshnegah J. et al., 2012m |
| Toxoplasmosis | CNS disease, pneumonia | Ingestion | Low | Ratcliffe and Worth 1951 | Smith et al., 1995n |
| FUNGI | |||||
| Dermatophytosis | Focal/coalescing skin lesions; alopecia; | Skin contact | Low | - | Rotstein et al., 1999o |
Pfukenyi, D.M., Pawandiwa, D., Makaya, P.V., Ushewokunze-Obatolu, U., 2009. A retrospective study of wildlife rabies in Zimbabwe between 1992 and 2003. Trop. Anim. Health Pro. 41, 565–572.
Barr, M.C., Calle, P.P., Roelke, M.E., Scott, F.W., 1989. Feline immunodeficiency virus infection in nondomestic felids. J. Zoo Wildl. Med. 20, 265–272.
Roelke, M.E., Pecon-Slattery, J., Taylor, S., Citino, S., Brown, E., Packer, C., VandeWoode, S., O’Brien, S.J., 2006. T-Lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J. Wildl. Dis. 42, 234–248.
Alexander, K.A., MacLachlan, N.J., Kat, P.W., House, C., O’Brien, S.J., Lerche, N.W., Sawyer, M., Frank, L.G., Holekamp, K., Smale, L., McNutt, J. W., Laurenson, M.K., Mills, M.G.L., Osburn, B.I., 1994. Evidence of natural bluetongue virus infection among African carnivores. Am. J. Trop. Med. Hyg. 51, 568–576.
Meli, M.L., Cattori, V., Martínez, F., López, G., Vargas, A., Simón, M.A., Zorrilla, I., Muñoz, A., Palomares, F., Lópes-Bao, J.V., Pastor, J., Tandon, R., Willi, B., Hofmann-Lehmann, R., Lutz, H., 2009. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian Lynx (Lynx pardinus). PloS One. 4, e4744.
Sundberg, J.P., Van Ranst, M., Montali, R., Homer, B.L., Miller, W.H., Rowland, P.H., Scott, D.W., England, J.J., Dunstan, R.W., Mikaelian, I., Jenson, A.B., 2000. Feline papillomas and papillomaviruses. Vet. Pathol. 37, 1–10.
Kennedy, M., Citino, S., McNabb, A.H., Moffatt, A.S., Gertz, K., Kania, S., 2002. Detection of feline coronavirus in captive Felidae in the USA. J. Vet. Diag. Invest. 14, 20–522.
Heeney, J.L., Evermann, J.F., McKierman, A.J., Marker-Kraus, L., Roelke, M.E., Bush, M., Wildt, D.E., Meltzer, D.G., Colly, L., Lukas, J., Manton, V.J., Caro, T., O’Brien, S.J., 1990. Prevalence and implications of feline coronavirus infections of captive and free-ranging cheetahs (Acinonyx jubatus). J. Virol. 64, 1964–1972.
Hofmann-Lehmann, R., Fehr, D., Grob, M., Elgizoli, M., Packer, C., Martenson, J.S., O’Brien, S.J., Lutz, H., 1996. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus and immunodeficiency virus, and feline leukemia antigen and the interrelationships of these infections in free-ranging lions in East Africa. Clin. Vacc. Immunol. 3, 554–562.
Wild, M.A., Shenk, T.M., Spraker, T.R., 2006. Plague as a mortality factor in Canada lynx (Lynx canadensis) reintroduced to Colorado. J. Wildl. Dis. 42, 646–650.
Ryser-Degiorgis, M.-P., Robert, N., 2006. Causes of mortality and diseases in free-ranging Eurasian lynx from Switzerland – an update. Proceedings of the Iberian Lynx. Ex situ conservation seminar series. Sevilla and Doñana, Spain, pp. 36–41.
Girard, Y.A., Swift, P., Chomel, B.B., Kasten, R.W., Fleer, K., Foley, J.E., Torres, S.G., Johnson, C.K., 2012. Zoonotic vector-borne bacterial pathogens in California mountain lions (Puma concolor), 1987–2010. Vector-Borne Zoonotic Dis. 12, 913–921.
Khoshnegah, J., Mohri, M., Mirshahi, A., Mousavi, S.J., 2012. Detection of Hepatozoon sp. in a Persian leopard (P. pardus ciscaucasica). J. Wildl. Dis. 48, 776–780.
Smith, K.E., Fischer, J.R., Dubey, J.P., 1995. Toxoplasmosis in a bobcat (Felis rufus). J. Wildl. Dis. 31, 555–557.
Rotstein, D.S., Thomas, R., Helmick, K., Citino, S.B., Taylor, S.K., Dunbar, M.R., 1999. Dermatophyte infections in free-ranging Florida panthers (Felis concolor coryi). J. Zoo Wildl. Med. 30, 281–284.
One viral pathogen of particular importance to populations of other Panthera species is canine distemper virus (CDV). In 1994, populations of lions (P. leo) in the Serengeti National Park declined by two-thirds during an outbreak of CDV, a loss of more than 1,000 animals (Roelke-Parker et al., 1996). More recently, CDV has been identified in Amur tigers in the Russian Far East, where it has contributed to local population declines (Seimon et al., 2013). Snow leopards are susceptible to CDV infection, with two cases recorded in captive animals (Fix et al., 1989, Silinski et al., 2003), although both of these were concurrent with other pathogens. In one case, CDV infection in two leopards was assumed to be a sequel to prior infection with feline panleukopenia virus, whereas in the other immunosuppression related to CDV was thought to have predisposed to an acute infection with Toxoplasma gondii. Coinfections have also been associated with outbreaks of CDV in free-ranging lions, with climatic conditions promoting high tick burdens and Babesia infection contributing to the high mortality recorded during CDV outbreaks in Serengeti in 1994 and Ngorongoro in 2001 (Munson et al., 2008). This may explain the occurrence of so-called “silent” outbreaks among lions in Eastern and Southern Africa, where infection is evident without apparent sickness or mortality (Munson et al., 2008). However, at least some captive and wild outbreaks appear to have been uncomplicated by coinfections (Seimon et al., 2013), and so other factors may contribute to clinical severity, such as strain virulence or additional external stressors.
Classical transmission of CDV is thought to require direct contact between an infected animal and a susceptible host, as it is inactivated by ultraviolet radiation, drying, and moderate temperatures (Green and Appel, 2006). Longer survival at low temperatures raises the possibility of indirect transmission in cold environments (such as viral contamination of carcasses attended by scavengers). However, the most likely source of infection in solitary Panthera species is assumed to be direct transmission when predating infected animals (Gilbert et al., 2014). During the early stages of infection the virus replicates in the respiratory epithelium, leading to respiratory distress signs and, often, purulent oculonasal discharge. The virus spreads systemically by infecting leucocytes, resulting in immunosuppression related to lymphopenia. Infected animals may die at this stage or improve in condition if the immune system is able to overcome the systemic infection. However, in a proportion of animals, the virus infects the central and peripheral nervous system, leading to degenerative neurological signs. For these animals, death is probably inevitable, either from the effects of the disease or as a result of sequelae such as inappropriate behavior. In wild tigers, CDV infections are most evident in these later stages, when neural deficits manifest as aberrant behavior, with a reduced aversion to people and observations of tigers in villages or along roadsides. Whether this is evident in all cases or is an effect of observation bias is unknown. It is unknown whether snow leopards would show similar behavioral signs, but CDV should be considered in any cases where snow leopards present with respiratory infection, ocular and/or nasal discharge, or aberrant behavior, fearlessness, muscle twitching, and/or convulsions.
Populations of large felids are too small and occur at densities that are too low to maintain CDV circulation in the long term. Therefore, infections in free-ranging large felids are the result of spillover from more abundant reservoir hosts and possibly short chains of infection among conspecific contacts. Most terrestrial carnivore species are thought to be susceptible to CDV, and so the exposure of snow leopard populations will depend on the presence of the virus in the wider carnivore community within their habitat. A more abundant susceptible host with high population turnover could act as a reservoir species, or several epidemiologically connected species could act as a reservoir community (Haydon et al., 2002, Viana et al., 2015). In areas that are sparsely settled, the number of domestic dogs may be insufficient to maintain CDV on their own, but the virus could persist if it were to circulate among more abundant carnivores (such as wild canids and/or mustelids), either in concert with or independent of domestic dogs. Modeling has also shown that CDV circulation occurs over wide spatial scales (Almberg et al., 2010), and so the status of CDV in snow leopard habitat could be influenced by transmission in distant locations (such as urban centers) if they are epidemiologically connected to remote carnivore communities.
Selected Bacterial Diseases
Several bacterial agents have the potential to be lethal to free-ranging snow leopards (Table 9.1). However, as in many nondomestic cats, clinical diseases due to bacterial agents are probably most commonly due to ubiquitous bacteria associated with accidental injuries, gingival and dental lesions, and infected wounds (Schmidt-Posthaus et al., 2002). These bacterial infections self-resolve in most cases or remain benign in immunocompetent individuals.
In contrast, mycobacterial infections and particularly tuberculosis due to Mycobacterium bovis have caused significant morbidity and mortality in large free-ranging felids, including lions and leopards (P. pardus; Michel et al., 2006). In wild felids the disease seems to be primarily acquired from feeding on an infected carcass. Assessing the presence of M. bovis in natural prey species and livestock is therefore a crucial indicator of the risk that tuberculosis poses to free-ranging felids. In captivity, snow leopards infected by M. bovis have been found with symptoms of weight loss, persistent cough, and lesions of granulomatous inflammation of the lungs (Helman et al., 1998). This disease should therefore be considered in abnormally thin and emaciated free-ranging snow leopards with clinical signs or lesions of pulmonary disease.
Anthrax, caused by Bacillus anthracis, has been associated with deaths in free-ranging felids in Africa, infected after eating an infected carcass (Jager et al., 1990). The disease has been reported from most states of the snow leopard distribution range. A radio-collared snow leopard found dead in the Gobi desert in April 2011 with marked neck edema, a common sign of anthrax in felids (Jager et al,. 1990), and unclotted bloody discharge from the nostrils, was suspected of anthrax (K. Smimaul, personal communication), although no confirmatory test was carried out and the disease is not known to be endemic in this part of Mongolia (Odontsetseg et al., 2007).
Nondomestic felids are known to succumb to other bacterial infections, usually as incidental hosts (Table 9.1), yet the extent to which snow leopards are susceptible and the occurrence of responsible agents across their range are largely unknown. The exception to this is Yersinia pestis, the agent of plague, which is potentially dangerous to any carnivore it would infect, and occurs in enzootic or epizootic cycles in marmot populations across the snow leopard habitat (Gage and Kosoy, 2005).
Selected Parasitic Infections
Several reports of ectoparasite infestations in free-ranging felids include cases of highly debilitating mange caused by the mite Sarcoptes scabiei in the Eurasian lynx (Lynx lynx) in European countries (Ryser-Degiorgis et al., 2005) and cheetahs (Acinonyx jubatus) in Kenya (Mwanzia et al., 1995). The responsible mites are fairly host-specific, yet most will parasitize humans. Animals affected by these mites can have hair loss and various degrees of encrusting dermatitis affecting more prominently head, feet, and tail. Monitoring of Eurasian lynx populations has however shown that the disease does not constitute a threat to the long-term survival of this species and persists only in areas where it is endemic in coexisting red fox (Vulpes vulpes) populations (Ryser-Degiorgis et al., 2005). The snow leopard is susceptible to sarcoptic mange (Peters and Zwart, 1973), but to date the disease has not been documented with certitude in free-ranging animals, although debilitated individuals with hair loss suggestive of mange have been recorded locally (Pamir Times, 2011). Anecdotal cases of notoedric mange and facial demodicosis, caused by Notoedres cati and Demodex cati, respectively, have been recorded in captive snow leopards and manifested as localized hair loss (Fletcher, 1978, Fletcher, 1980).
Hemoparasites such as piroplasms, including Babesia, Hepatozoon, Trypanosoma and Cytauxzoon spp., appear to be common in free-ranging felids. However, except in a few rare instances (Babesia spp.), most cases of infections in wild felids are subclinical. Hemoparasites associated with clinical disease have yet to be documented in captive or free-ranging snow leopards.
Toxoplasma gondii is a protozoal parasite that infects most species of warm-blooded animals. Felids are the only known definitive hosts for this parasite, and therefore serve as the main reservoir. Cats rarely develop clinical toxoplasmosis, although captive Pallas’ cats (Otocolobus manul) have been reported susceptible to the infection, resulting in high neonatal mortality (Kenny et al., 2002). A mortality case has also been reported in a captive snow leopard (Ratcliffe and Worth, 1951).
Metazoan parasites whether nematodes, cestodes, or trematodes are common in free-ranging felids and do not generally cause clinical disease. Infestations with ascarids such as Toxascaris leonina and Toxocara cati seem common in free-ranging snow leopards (Mozgovoi, 1953, cited in Ganzorig et al., 2003), and all fresh feces from a sample of 8–9 adult animals collected in Afghanistan (5), Pakistan (2), and China (1–2) between 2008 and 2013 had eggs of Toxascaris spp. (SO, personal observation). A case of mortality due to a ruptured aortic aneurysm caused by larvae of Spirocerca lupi was reported in a snow leopard 3 months after being brought into captivity, but authors suggested that the infection was acquired during captivity (Kelly and Penner, 1950).
Diseases in snow leopard natural ungulate prey species
Sarcoptic Mange in Blue Sheep and Other Prey Species
The blue sheep (Pseudois nayaur) is an important prey species for snow leopards (Bagchi and Mishra, 2006). In 2007, an outbreak of sarcoptic mange was reported among blue sheep in extreme northern Pakistan (Fig. 9.3 ), and caused hundreds of fatalities (Dagleish et al., 2007). The disease, first reported by local herders in 1996, occurred throughout the year, affecting both sexes and all age groups and reducing the species’ population over the ensuing decade. Infected animals were in poor condition, presented with severe and extensive skin lesions, especially on the forelegs and chest, and were reluctant to flee when approached (Fig. 9.4 ). Dagleish et al. (2007) suggested that the severity of lesions in blue sheep could have been the result of protein/energy malnutrition, and perhaps also weak immune response due to lack of previous exposure to the ectoparasite. Although the origin of the initial infection was not determined with certainty, the authors believed that the most likely source of infection were infected domestic livestock encroaching into the natural habitat of blue sheep. The gregarious social behavior of the blue sheep may have promoted the dissemination of this novel parasite, which may have been aided by malnutrition due to food competition with livestock (Mishra et al., 2004). The consequences of this mange outbreak appeared to be severe for blue sheep, but potentially also for snow leopards, which rely on this species as an important source of food. The prevalence of mange in blue sheep appeared to have decreased by 2010–2011 (Hussain Ali, personal communication), possibly as a result of host adaptation or selection of more tolerant animals. However, the corollary impacts on the snow leopard population size or dynamics have not been measured. This outbreak raised concerns on the risk posed by sarcoptic mange to other blue sheep populations. Fortunately no similar reports have emerged from elsewhere across the species’ range since 2007.
Figure 9.3.
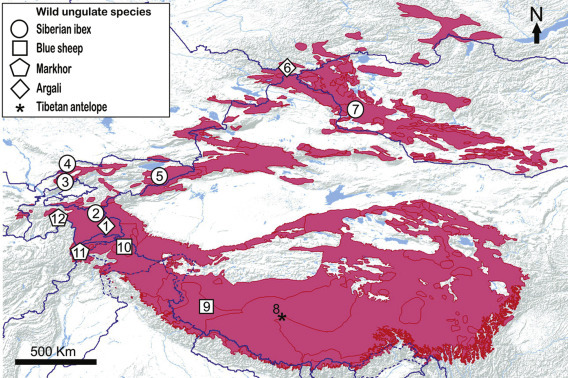
Geographical locations of reported disease outbreaks in natural ungulate prey species plotted over snow leopard distribution range (in pink).
Details of outbreaks are provided hereinafter (disease name/date of outbreak/mountain range/country/reference). 1. Rinderpest and anthrax/1895–1898/Pamir Mountains/Tajikistan/Meklenburtsev (1948). 2. Goats pleuropneumonia disease/1940s/Pamir Mountains/Tajikistan/Heptner, V.G., Nasimovich, A.A., Bannikov, A.G., 1961. Mammals of the Soviet Union. Artiodactyla and Perissodactyla. Vyssahya Shkola, Moscow, USSR (in Russian). 3. Sarcoptic mange/late 1960s/Pamir-Alai Mountains/Uzbekistan/Vyrypaev, V.A., 1973. The status of the Siberian ibex population in the west part of the Chatkal Range (Central Asia). In: Sokolov, V.E. (Ed.) The Rare Mammal Species in the USSR and Their Conservation. Nauka, Moscow, USSR (in Russian). 4. Sarcoptic mange/1968–1971/Pamir-Alai Mountains/Kazakhstan/Fedosenko, A.K., Savinov, E.F., 1983. The Siberian ibex. In: Gvozdev, E.V., Kapitonov, V.I. (Eds.), Mammals of Kazakhstan. Nauka of Kazakh SSSR, Alma-Ata, pp. 92–143 (in Russian). 5. Sarcoptic mange/late 1960s/Tien Shan Mountains/Kyrgyzstan/Yanushevich et al., 1972. cited in Fedosenko, A.K., Blank, D.A., 2001. 6. Tuberculosis/1989/Altai Mountains/Russia/Fedosenko and Blank (2005). 7. Sarcoptic mange/ongoing/Altai Mountains/Mongolia/E. Shiilegdamba personal communication, 2014. 8. Contagious caprine pleuropneumonia/2012/Tibetan Plateau/China/Yu et al. (2013). 9. Peste des petits ruminants/2007–2008/Tibetan Plateau/China/Bao et al. (2011). 10. Sarcoptic mange/1997–2007/Karakoram Mountains/Pakistan/Dalgleish et al., 2007. 11. Foot-and-mouth disease/2011/Hindu Kush Mountains/Pakistan/Shabbir Mir, 2011. Deadly disease kills 12 markhors in Chitral. Express Tribune, April 9, 2011. Available from: http://tribune.com.pk/story/146166/deadly-disease-kills-12-markhors-in-chitral [accessed 06.03.2015.]. 12. Caprine mycoplasmosis/2010/Pamir-Alai Mountains/Tajikistan/Ostrowski et al., 2011.
Figure 9.4.

An adult male blue sheep (Pseudois nayaur) presenting severe and extensive skin lesions on the forelegs and chest due to Sarcoptes scabiei, the ectoparasite responsible of sarcoptic mange.
Debilitated and indifferent to humans, this animal was easily handled by yak herders. Shimshal area, Gilgit-Baltistan Province, Pakistan, July 2000.
Source: Photo courtesy of D. Butz.
Mange has a long history of affecting populations of Siberian ibex, possibly the main prey species for snow leopards range wide. Outbreaks can lead to considerable local mortality, such as in the Aksu-Zhabagly Reserve in Kazakhstan (where ca. 80% of the population was infected in 1968–1971) and in the Chatkal Range of Uzbekistan and in Kyrgyzstan. The disease also seems to be endemic in ibex in Khovd Province of the Mongolian Altai (Fig. 9.3). Clinically sarcoptic mange in ibex is similar to that described in blue sheep, with front legs and thorax being affected first, yet the neck and head also appear to be affected at a later stage. Mortality of infected ibex is particularly high during years of heavy snow (Fedosenko and Blank, 2001). Further studies are required to understand the impact of this infectious agent on intra- and interspecific population dynamics of snow leopards and associated prey.
Mycoplasmosis in Markhor and Other Prey Species
Throughout its range, the markhor (C. falconeri) has to forage in close proximity to domestic goats (Woodford et al., 2004), and is therefore prone to infections of contagious agents transmitted by these animals. In autumn 2010 an outbreak of Mycoplasma capricolum pneumonia killed at least 64 markhor in the southwest of the Hazratishoh Range in Tajikistan (Fig. 9.3; Ostrowski et al., 2011). Several live specimens were observed with clinical signs of labored breathing and the most relevant necropsy findings noted in the field were an abundant serous to mucopurulent nasal discharge; and internally, severe pneumonia associated with a variable level of yellow pleural fluid (Fig. 9.5 ). The clinicopathologic features of the disease resembled contagious caprine pleuropneumonia (CCPP) caused by M. capricolum subsp. capripneumoniae (Frey, 2002), a highly fatal disease-affecting goats in the Middle East, Africa, and Asia. However, a closely related species, M. capricolum subsp. capricolum, associated with respiratory diseases in domestic ruminants was identified, using sensitive molecular techniques, as the most probable causative agent of the fatal pneumonia outbreak in the markhor (Ostrowski et al., 2011). Although the origin of the infection remained unknown, domestic goats, which were occasionally coming in contact with markhor may have been the source of the outbreak. A serological survey carried out 8 months after the outbreak confirmed a CCPP prevalence of 10.1% (95% CI: 6.3–15.2%) in sympatric domestic goats (Peyraud et al., 2014). The susceptibility of ruminants to Mycoplasma infections may be exacerbated by environmental and nutritional factors. The disease appeared in autumn, when livestock and guard dogs force markhor to retreat to suboptimal pastures (Woodford et al., 2004). It was also the end of the dry season, when contact between markhor and livestock increases around dwindling water sources. The consequences of this pneumonia outbreak appeared to be locally severe for markhor but potentially also for the snow leopard, which relies on this species as a source of food. Community guards swiftly implemented control measures, including burning of carcasses and disinfecting contaminated grounds with slaked lime. Shepherds were also asked to avoid using water sources concomitantly to wild ungulates (Stefan Michel, personal communication). As a possible consequence, the markhor population recovered from the outbreak, increasing from an estimated 145 specimens in March 2011 to 236 animals in February 2012 (Michel et al., 2015).
Figure 9.5.

An adult male Heptner’s markhor (C. falconeri heptneri) found dead in the southwest of the Hazratishoh range, Tajikistan.
M. capricolum subsp. capricolum associated with respiratory diseases in domestic ruminants was identified as the most probable causative agent of the fatal pneumonia that killed this markhor, along with at least 63 others, in September 2010.
Source: Photo courtesy of State Veterinary Department of Tajikistan.
The 2010 outbreak highlighted the general risk posed by mycoplasmas to ungulate prey species throughout the snow leopard’s range. Although there have been no similar reports involving markhor populations since 2010, a relative lack of disease surveillance and limited access to sensitive molecular techniques for detection of mycoplasmas in nondomestic ruminants may mask any actual incidence. Primary outbreaks of diseases caused by various Mycoplasma species have had serious consequences for wild Caprinae in Europe. Mortality occasionally occurs due to the disease, and more frequently from associated starvation or falls due to disease-induced behavioral modifications (Giacometti et al., 2002). In Central Asia, the confirmation of CCPP presence in Tajikistan and China in 2009 (Office International des Epizooties, 2009, Chu et al., 2011), followed by a massive outbreak that claimed the lives of ca. 2400 endangered Tibetan antelopes (Pantholops hodgsonii) during September–December 2012 (Fig. 9.3; Yu et al., 2013), raise the specter of an increasing risk of M. capricolum outbreaks in susceptible prey species, which include blue sheep, Siberian ibex, argali (Ovis ammon), markhor, and Himalayan tahr (Hemitragus jemlahicus).
Peste des Petits Ruminants in Blue Sheep and Other Prey Species
Serologic and molecular evidence indicated that peste des petits ruminants (PPR) infection emerged between July and November 2007 in goats and sheep in southwestern Tibet, China (Wang et al., 2009). The disease likely existed for several years in Tibet without being recognized, possibly emerging via cross-border movements of infected livestock from India (Muniraju et al., 2014). The virus has now been reported in all countries bordering southwestern China (i.e., Afghanistan, India, Nepal, Pakistan, and Tajikistan), which encompass a large portion of the snow leopard range. Owing to the wide susceptibility of small ruminants (Furley et al., 1987), it was unsurprising that a fatal outbreak occurred in blue sheep in October 2007 in the same county as the domestic outbreak and then in an adjacent county in January 2008 (Fig. 9.3; Bao et al., 2011). Sick animals presented with ocular and nasal mucopurulent discharge, occasionally associated with diarrhea and lameness. The impact of PPR on this blue sheep population is unknown, but the fact that PPR morbillivirus could be responsible for high morbidity and mortality (> 50%) in wild ungulates (Elzein et al., 2004), and that 19 blue sheep as well as 6 Mongolian gazelles (Procapra gutturosa) were found dead in the course of the Tibetan outbreak suggest that it could have been significant. Several studies have shown that PPR infections are not self-sustaining in wildlife (Elzein et al., 2004). Similarly the closely related rinderpest Morbillivirus was reported in argali in the Pamirs prior to its eradication (Fig. 9.3) (Meklenburtsev, 1948), with infections attributed to spillover contamination while sharing pastures or water sources with infected livestock (Barrett et al., 2006).
Conclusions
The current lack of baseline information on the health of free-ranging snow leopards prohibits an assessment of the potential impact of infectious disease on their populations directly. The remote and inaccessible habitat occupied by the species reduces the chances of detecting disease-related mortality and complicates efforts to transfer diagnostic samples to laboratories for testing. To address these information deficits, researchers should be encouraged to include at least minimal sample collection protocols (appropriate to local circumstances) whenever opportunities arise to handle a snow leopard (e.g., through research projects, conflict situations, or when responding to a debilitated or dead individual). A description of minimal sampling protocols is beyond the scope of this review; therefore researchers are encouraged to seek the advice of a veterinarian with relevant wildlife experience in advance of these situations arising. The population impact of infectious disease should not be dismissed, despite the relatively low rate of contact among conspecifics, and comparatively low densities of other domestic and wild carnivore species occupying snow leopard habitat. Modeling of another wide-ranging solitary felid, the Amur tiger has shown profound impacts on population viability, even when opportunities for disease exposure are infrequent (Gilbert et al., 2014). These effects may be exacerbated by other physiological stressors, such as food availability, climate-related habitat changes, or by the genetic stresses of inbreeding depression. Pathogens with the potential to impact snow leopard populations are likely to be contracted from other carnivore species, therefore whenever possible, introduction of domestic dogs and cats into local carnivore communities is to be discouraged. Ultimately, a population’s ability to withstand the pressures of disease will be maximized by maintaining snow leopards in numerically large subpopulations that are as interconnected as terrain and land use permit.
Snow leopards prey on whatever small and large mammals are available. However, their staple prey, accounting for more than 40% of their diet and without which they cannot survive, are large ungulates, including the blue sheep, Siberian ibex, argali, markhor, and the Himalayan tahr. Monitoring the health condition of these species appears therefore of crucial importance to support snow leopard conservation at local and global scales. In the Asian context of generalized increasing encroachment of livestock into wild habitats, domestic ungulates are the prime target for disease surveillance schemes as they are the most likely source of disease spillover to snow leopard prey. Moreover, livestock can be responsible for upslope range-shift of mountain ungulates into less suitable, stressful foraging habitat, even exceeding in magnitude the worst scenarios of climate-driven effects on their ecology (Mason et al., 2014). Therefore controlling the risk of disease outbreaks in snow leopard prey requires a complex and holistic approach that enforces prevention of disease spillover from livestock to wild ungulates and implements multifaceted controls over livestock numbers and their range use. Limiting other controllable stressors (such as human disturbance) and whenever possible maximizing genetic variability of small fragmented populations through enhanced subpopulation connectivity are also recommended to reduce disease susceptibility (Lafferty and Gerber, 2002). Vaccination of livestock is frequently not available (e.g., sarcoptic mange) or inefficacious (CCPP vaccination in Pakistan; Samiullah, 2013), and when efficiently implemented, may further enhance encroachment of livestock into wild ungulate habitat and nutritional competition as a consequence of increased livestock survival and productivity. Therefore unless implemented in combination, health managers should tend to prioritize measures that limit contact between livestock and wild ungulates rather than prophylactic actions on livestock.
Acknowledgments
We would like to thank the editors and P. Zahler for giving us the opportunity to write this chapter; E. Shiilegdamba, Ö. Johansson, and K. Smimaul for sharing their knowledge on diseases of snow leopard and prey in Mongolia; and Hussain Ali for allowing us to use the information he collected in Khunjerab area, Pakistan. We are also grateful for S. Michel for providing information on disease outbreaks in markhor, and for T. Lhagvasumberel, D. Butz, and the State Veterinary Department of Tajikistan for providing photographs. The writing time for this chapter was generously supported by the Wildlife Health & Health Policy Department of Wildlife Conservation Society.
References
- Almberg E.S., Cross P.C., Smith D.W. Persistence of canine distemper virus in the Greater Yellowstone ecosystem’s carnivore community. Ecol. Appl. 2010;20:2058–2074. doi: 10.1890/09-1225.1. [DOI] [PubMed] [Google Scholar]
- Bagchi S., Mishra C. Living with large carnivores: predation on livestock by the snow leopard (Uncia uncia) J. Zool. 2006;268:217–224. [Google Scholar]
- Bao J., Wang Z., Li L., Wu X., Sang P., Wu G., Ding G., Suo L., Liu C., Wang J., Zhao W., Li J., Qi L. Detection and genetic characterization of peste des petits ruminants virus in free-living bharals (Pseudois nayaur) in Tibet, China. Res. Vet. Sci. 2011;90:238–240. doi: 10.1016/j.rvsc.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Batzorig, B., Tserenchimed, S., Sugir, S., Purevjat, L., Lhagvasumberel, T., 2011. A case of snow leopard mortality in Mongolia. Annual Proceedings of the State Central Veterinary Laboratory; Ulaanbaatar, Mongolia, p. 109–110 (in Mongol).
- Barrett T., Pastoret P.-P., Taylor W.P. Academic Press; London: 2006. Rinderpest and Peste des Petits Ruminants: Virus Plagues of Large and Small Ruminants. [Google Scholar]
- Blankinship J.C., Niklaus P.A., Hungate B.A. A meta-analysis of responses of soil biota to global change. Oecologia. 2011;165:553–565. doi: 10.1007/s00442-011-1909-0. [DOI] [PubMed] [Google Scholar]
- Chu Y., Yan X., Gao P., Zhao P., He Y., Liu J.Z., Lu Z. Molecular detection of a mixed infection of goatpox virus, Orf virus, and Mycoplasma capricolum subsp. capripneumoniae in goats. J. Vet. Diagn. Invest. 2011;23:786–789. doi: 10.1177/1040638711407883. [DOI] [PubMed] [Google Scholar]
- Dagleish M.P., Qurban A., Powell R.K., Butz D., Woodford M.H. Fatal Sarcoptes scabiei infection of blue sheep (Pseudois nayaur) in Pakistan. J. Wildl. Dis. 2007;43:512–517. doi: 10.7589/0090-3558-43.3.512. [DOI] [PubMed] [Google Scholar]
- Elzein E.M.E., Housawi F.M.T., Bashareek Y., Gameel A.A., Al-Afaleq A.I., Anderson E. Severe PPR infection in gazelles kept under semi-free range conditions. J. Vet. Med. B. Infect. Dis. Vet. Public Health. 2004;51:68–71. doi: 10.1111/j.1439-0450.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- Fedosenko A.K., Blank D.A. Capra sibirica. Mamm. Species. 2001;675:1–13. [Google Scholar]
- Fedosenko A.K., Blank D.A. Ovis ammon. Mamm. Species. 2005;773:1–15. [Google Scholar]
- Fix A.S., Riordan D.P., Hill H.T., Gill M.A., Evans E.B. Feline panleukopenia virus and subsequent canine distemper virus infection in two snow leopards (Panthera uncia) J. Zoo Wildl. Med. 1989;20:273–281. [Google Scholar]
- Fletcher K.C. Notoedric mange in a litter of snow leopards. J. Am. Vet. Med. Assoc. 1978;173:1231–1232. [PubMed] [Google Scholar]
- Fletcher K.C. Demodicosis in a group of juvenile snow leopards. J. Am. Vet. Med. Assoc. 1980;177:896–898. [PubMed] [Google Scholar]
- Frey J. Mycoplasmas of animals. In: Razin S., Herrmann R., editors. Molecular Biology and Pathogenicity of Mycoplasmas. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 73–90. [Google Scholar]
- Furley C.W., Taylor W.P., Obi T.U. An outbreak of peste des petits ruminants in a zoological collection. Vet. Rec. 1987;121:443–447. doi: 10.1136/vr.121.19.443. [DOI] [PubMed] [Google Scholar]
- Ganzorig S., Oku Y., Okamoto M., Kamiya M. Specific identification of a taeniid cestode from snow leopard, Uncia uncia, Schreber, 1776 (Felidae) in Mongolia. Mong. J. Biol. Sci. 2003;1:21–25. [Google Scholar]
- Gage K.L., Kosoy M.Y. Natural history of plague: perspectives from more than a century of research. Annu. Rev. Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- Giacometti M., Janovsky M., Jenny H., Nicolet J., Belloy L., Goldschmidt-Clermont E., Frey J. Mycoplasma conjunctivae infection is not maintained in alpine chamois in eastern Switzerland. J. Wildl. Dis. 2002;38:297–304. doi: 10.7589/0090-3558-38.2.297. [DOI] [PubMed] [Google Scholar]
- Gilbert M., Miquelle D.G., Goodrich J.M., Reeve R., Cleaveland S., Matthews L., Joly D.O. Estimating the potential impact of canine distemper virus on the Amur tiger population (Panthera tigris altaica) in Russia. PLoS One. 2014;9(10):e110811. doi: 10.1371/journal.pone.0110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J.M., Kerley L.L., Smirnov E.N., Miquelle D.G., McDonald L., Quigley H.B., Hornocker M.G., McDonald T. Survival rates and causes of mortality of Amur tigers on and near the Sikhote-Alin Biosphere Zapovednik. J. Zool. 2008;276:323–329. [Google Scholar]
- Green C.E., Appel M.J. Canine Distemper. In: Green C.E., editor. Infectious diseases of the dog and cat. third ed. Elsevier; St Louis, MO: 2006. pp. 25–41. [Google Scholar]
- Haydon D.T., Cleaveland S., Taylor L.H., Laurenson M.K. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman R.G., Russel W.C., Jenny A., Miller J., Payeur J. Diagnosis of tuberculosis in two snow leopards using polymerase chain reaction. J. Vet. Diagn. Invest. 1998;10:89–92. doi: 10.1177/104063879801000118. [DOI] [PubMed] [Google Scholar]
- Heptner V.G., Sludskii A.A. Amerind Publishing; New Delhi, India: 1992. Mammals of the Soviet Union, vol. 2. Part 2 (Carnivores: Hyaenas and Cats) [Google Scholar]
- Jager H.G., Booker H.H., Hubschle O.J. Anthrax in cheetahs (Acinonyx jubatus) in Namibia. J. Wildl. Dis. 1990;26:423–424. doi: 10.7589/0090-3558-26.3.423. [DOI] [PubMed] [Google Scholar]
- Kelly A.L., Penner L.R. Spirocerca from the snow leopard. J. Mammal. 1950;31:462. [Google Scholar]
- Kenny D.E., Lappin M.R., Knightly F., Baier J., Brewer M., Getzy D.M. Toxoplasmosis in Pallas’ cats (Otocolobus felis manul) J. Zoo Wildl. Med. 2002;33:131–138. doi: 10.1638/1042-7260(2002)033[0131:TIPCOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Gerber L.R. Good medicine for conservation biology: the intersection of epidemiology and conservation biology. Conserv. Biol. 2002;16:593–604. [Google Scholar]
- Mason T.H.E., Stephens P.A., Appolonio M., Willis S.G. Predicting potential responses to future climate in an alpine ungulate: interspecific interactions exceed climate effects. Glob. Change Biol. 2014;20:3872–3882. doi: 10.1111/gcb.12641. [DOI] [PubMed] [Google Scholar]
- Meklenburtsev, R.N., 1948. Pamir argali Ovis polii polii Blyth. Bulletin of the Moscow Society of Naturalists, Biology Department 53, 65–84 (in Russian).
- Michel A.L., Bengis R.G., Keet D.F., Hofmeyr M., de Klerk L.M., Cross P.C., Jolles A.E., Cooper D., Whyte I.J., Buss P., Godfroid J. Wildlife tuberculosis in South African conservation areas: implications and challenges. Vet. Microbiol. 2006;112:91–100. doi: 10.1016/j.vetmic.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Michel, S., Rosen Michel, T., Saidov, A., Karimov, K., Alidodov, M., Kholmatov, I., 2015. Population status of Heptner’s markhor Capra falconeri heptneri in Tajikistan: challenges for conservation. Oryx 49(3), 506–513.
- Mishra C., Van Wieren S.E., Ketner P., Heitkönig I.M.A., Prins H.H.T. Competition between domestic livestock and wild bharal Pseudois nayaur in the Indian Trans-Himalaya. J. Appl. Ecol. 2004;41:344–354. [Google Scholar]
- Muniraju M., Munir M., Parthiban A.R., Banyard A.C., Bao J., Wang Z., Ayebazibwe C., Ayelet G., El Harrak M., Mahapatra M., Libeau G., Batten C., Parida S. Molecular evolution of peste des petits ruminants virus. Emerg. Infect. Dis. 2014;20:2023–2033. doi: 10.3201/eid2012.140684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson L., Terio K.A., Kock R., Mlengeya T., Roelke M.E., Dubovi E., Summers B., Sinclair A.R.E., Packer C. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS One. 2008;3:e2545. doi: 10.1371/journal.pone.0002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanzia, J.M., Kock, R.A., Wambna, J.M., Kock, N.D., Jarrett, O., 1995. An outbreak of sarcoptic mange in free living cheetah (Acinonyx jubatus) in the Mara region of Kenya. Proceedings AAZV/WDA/AAWV Joint Conference, East Lansing, Michigan, 95–102.
- Office International des Epizooties, 2009. Contagious caprine pleuropneumonia, Tajikistan. May 15. http://web.oie.int/wahis/public.php?page=event_summary&reportid=8610 (accessed 03.11.2014.).
- Odontsetseg N., Tserendorj S., Adiyasuren Z., Uuganbayar D., Mweene M.S. Anthrax in animals and humans in Mongolia. Rev. Sci. Tech. 2007;26:701–710. [PubMed] [Google Scholar]
- Ostrowski S., Thiaucourt F., Amirbekov M., Mahmadshoev A., Manso-Silván L., Dupuy V., Vahobov D., Ziyoev O., Michel S. Fatal outbreak of Mycoplasma capricolum pneumonia in endangered markhor. Emerg. Infect. Dis. 2011;17:2338–2341. doi: 10.3201/eid1712.110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamir Times, 2011. Sick snow leopard captured by villagers in Skardu, dies. Available from http://pamirtimes.net/2011/02/27/sick-snow-leopard-captured-by-villagers-in-skardu-dies/.
- Peyraud A., Poumarat F., Tardy F., Manso-Silván L., Hamroev K., Tilloev T., Amirbekov M., Tounkara K., Bodjo C., Wesonga H., Gacheri Nkando I., Jenberie S., Yami M., Cardinale E., Meenowa D., Reshad Jaumally M., Yaqub T., Shabbir M.Z., Mukhtar N., Halimi M., Ziay G.M., Schauwers W., Noori H., Rajabi A.M., Ostrowski S., Thiaucourt F. An international collaborative study to determine the prevalence of contagious caprine pleuropneumonia by monoclonal antibody-based cELISA. BMC Vet. Res. 2014;10:48. doi: 10.1186/1746-6148-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J.C., Zwart P. Sarcoptesräude bei Schneeleoparden. Erkrankungen der Zootiere. Verhandlungsbericht des XV Symposium uber die Erkrankungen der Zootiere. 1973;15:333–334. [Google Scholar]
- Ratcliffe H.L., Worth C.B. Toxoplasmosis of captive wild birds and mammals. Am. J. Pathol. 1951;27:655–667. [PMC free article] [PubMed] [Google Scholar]
- Roelke-Parker M.E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O’Brien S.J., Pospischil A., Hofmann-Lehmann R., Lutz H., Mwamengele L.M., Mgasa M.N., Machange G.A., Summers B.A., Appel M.J.G. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser-Degiorgis M.-P., Bröjer C., Hård af Segerstad C., Bornstein S., Bignert A., Lutz H., Uggia A., Gavier-Widén D., Tryland M., Mörner T. Assessment of the health status of the free-ranging Eurasian lynx in Sweden. Proceedings of the First Workshop on Lynx Veterinary Aspects, Doñana, November 2005; Ministry of the Environment, Spain; 2005. [Google Scholar]
- Samiullah S. Contagious caprine pleuropneumonia and its current picture in Pakistan: a review. Vet. Med. Czech. 2013;58:389–398. [Google Scholar]
- Schmidt-Posthaus H., Breintenmoser-Wursten C., Posthaus H., Bacciarini L., Breitenmoser U. Pathological investigation of mortality in reintroduced Eurasian lynx (Lynx lynx) populations in Switzerland. J. Wildl. Dis. 2002;38:84–92. doi: 10.7589/0090-3558-38.1.84. [DOI] [PubMed] [Google Scholar]
- Seimon T.A., Miquelle D.G., Chang T.Y., Newton A.L., Korotkova I., Ivanchuk G., Lyubchenko E., Tupikov A., Slabe E., McAloose D. Canine distemper virus: an emerging disease in wild endangered Amur tigers (Panthera tigris altaica) mBio. 2013;4:e00410–e413. doi: 10.1128/mBio.00410-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinski S., Robert N., Walzer C. Canine distemper and toxoplasmosis in a captive snow leopard (Uncia uncia) – a diagnostic dilemma. Verhandlungsbericht des Symposium uber die Erkrankungen der Zootiere. 2003;41:107–111. [Google Scholar]
- Viana M., Cleaveland S., Matthiopoulos J., Halliday J., Packer C., Craft M.E., Hampson K., Czupryna A., Dobson A.P., Dubovi E.J., Ernest E., Fyumagwa R., Hoare R., Hopcraft J.G.C., Horton D.L., Kaare M.D., Kanellos T., Lankester F., Mentzel C., Mlengeya T., Mzimbiri I., Takahashi E., Willett B., Haydon D.T., Lembo T. Dynamics of a morbillivirus at the domestic-wildlife interface: canine distemper virus in domestic dogs and lions. Proc. Natl. Acad. Sci. 2015;112:1464–1469. doi: 10.1073/pnas.1411623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Bao J., Wu X., Liu Y., Li L., Liu C., Suo L., Xie Z., Zhao W., Zhang W., Yang N., Li J., Wang S., Wang J. Peste des petits ruminants virus in Tibet, China. Emerg. Infect. Dis. 2009;15:299–301. doi: 10.3201/eid1502.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford M.H., Frisina M.R., Awan G.A. The Torghar conservation project: management of the livestock, Suleiman markhor (Capra falconeri) and Afghan urial (Ovis orientalis) in the Torghar Hills, Pakistan. Game Wildl. Sci. 2004;21:177–187. [Google Scholar]
- Yu Z., Wang T., Sun H., Xia Z., Zhang K., Chu D., Xu Y., Cheng K., Zheng X., Huang G., Zhao Y., Yang S., Gao Y., Xia X. Contagious caprine pleuropneumonia in endangered Tibetan antelope, China, 2012. Emerg. Infect. Dis. 2013;19:2051–2052. doi: 10.3201/eid1912.130067. [DOI] [PMC free article] [PubMed] [Google Scholar]


