Dreams are nothing but incoherent ideas, occasioned by partial or imperfect sleep.
Benjamin Rush (1746–1813)
EPIDEMIOLOGY
MS is a common cause of neurological disability in middle-aged adults. This disease affects over one million people worldwide and is very common within the USA (Minden et al., 1993; Pugliatti et al., 2002; O'Connor et al., 2002). Age, sex, race, geographical latitude, genetics and environmental exposure are risk factors for developing MS.
The median age of onset of MS is approximately 24 years of age (Minden et al., 1993) with a female to male ratio of two to one (O'Connor et al., 2002). The mean age of death of MS patients is 58 years old, compared to the general national average in the USA of 72 years old (Minden et al., 1993). Within the USA, MS is observed more often in Caucasians compared to African-Americans (Minden et al., 1993). However, the typical clinical course is more severe within African-Americans who are affected (Pugliatti et al., 2002). In regard to geographical patterns, a higher incidence of MS occurs when moving either more north or south of the equator, especially in countries with colder climates (Pugliatti et al., 2002). This latitude effect is also present within the USA as the incidence of MS is highest within the upper Midwest and Northeast compared to the South (Minden et al., 1993).
Family studies have shown that genetic factors contribute to MS (Sadovnick, 2002; Herrera and Ebers, 2003; Kenealy et al., 2003; Willer et al., 2003). The risk for developing MS within dizygotic twins is the same as for other siblings (3–5 per cent). However, the risk for monozygotic twins is greater than 30 per cent (O'Connor et al., 2002; Willer et al., 2003). A greater than 10-fold increased risk of developing MS exists within individuals having certain human leukocyte antigens (HLA) genotypes (O'Connor et al., 2002; Sadovnick, 2002; Herrera and Ebers, 2003; Kenealy et al., 2003). The major predisposing associated haplotype is HLA-DR2 in cellular typing nomenclature (DR15,DQ6 by serology and DRB5*0101-DQA1*0102-DQB1*0602 by sequence-based terminology) (Haegert and Francis, 1993; Hillert, 1994). Even though an association between HLA class II DRB1*15 and DRB1*17 alleles and MS has been seen in individuals of northern European and Mediterranean descent, the presence of these haplotypes does not preclude the development of MS (Sadovnick, 2002; Herrera and Ebers, 2003; Kenealy et al., 2003). Genetic interactions may also occur between certain haplotypes and certain T cell receptors, immunoglobulins and tumor necrosis factors which may lead to an increased chance of developing MS (O'Connor et al., 2002; Alizadeh et al., 2003).
Not only genetic but also one or more exogenous or environmental factors may influence the development of MS (O'Connor et al., 2002). Although twin studies have provided strong evidence for genetic factors in the development of MS, the concordance rate for monozygotic twins has never been demonstrated to be greater than 40 per cent (Compston and Ebers, 1990; Sadovnick, 2002; Willer et al., 2003). Thus, at least 60 per cent of persons with an identical twin who has MS will not develop the disease.
Certain foods, toxins, psychological stress, anesthesia, surgery and physical trauma have been implicated in the development of MS (O'Connor et al., 2002). However, no direct causal relationships have been proven to date. Overall, it is believed that both genetic and environmental factors are responsible for the subsequent development of MS within a susceptible individual (O'Connor et al., 2002). The resulting disability that results from MS leads to a major burden not only to the affected individual but also to the health care system and society (Whetten-Goldstein et al., 1998).
PATHOPHYSIOLOGY
Loss of myelin due to inflammation with limited subsequent remyelination are the two cardinal features of MS (Noseworthy et al., 2000). Breakdown of the blood–brain barrier is typically observed with early inflammatory changes (Smith et al., 1993). Immunocytochemical studies of active MS lesions have shown damage to vascular walls with intramural deposition of complement on smooth muscle components as well as protein-rich leakage (Noseworthy et al., 2000). These abnormalities can occur within hours to days of an acute attack and slowly resolve over subsequent weeks to months (Poser, 1986).
Typical pathological findings seen in MS are plaques containing inflammatory cells scattered throughout the affected parenchyma and arranged in cuffs around blood vessels (Figure 45.1 ). Inflammatory cells that are stimulated during an acute flare include macrophages, lymphocytes, large mononuclear cells and plasma cells (Prineas and Wright, 1978; Tourbah et al., 1993). In MS macrophages are immunologically active and can stimulate T cells leading to the subsequent production of large quantities of cytokines and inflammatory mediators (Hafler and Weiner, 1995; Hohlfeld et al., 1995; Martin and McFarland, 1995; O'Connor et al., 2000). These cytokines can be either pro-or anti-inflammatory in nature (Hafler and Weiner, 1995) with a T cell either stimulatory or inhibitory depending on surrounding environment factors (Hafler and Weiner, 1995; McFarland, 1995; Hohlfeld, 1997).
Figure 45.1.

Circles surround perventricular plaques seen on pathology.
Axonal loss has also shown to be a key factor in the pathogenesis of MS and is responsible for part of the irreversible clinical impairment and disability (O'Connor et al., 2002). Axonal loss could stem from:
-
1
direct autoimmune attack against axons
-
2
a ‘bystander injury’ due to exposure of axons to immune attack following demyelination
-
3
degeneration of axons as a result of chronic demyelination
-
4
primary degeneration of axons, stimulating a secondary inflammatory response (Trapp et al., 1999; Lassmann, 2003).
The importance of these potential associated mechanisms remains to be determined.
ETIOLOGY
The exact etiology of MS remains unknown, but it is believed that this disease most likely results from a viral infection. These organisms can induce disturbances within a previously competent or compromised immune system (Allen and Brankin, 1993; Cook et al., 1995; O'Connor et al., 2002). MS may either be due to infection by viruses that directly induce neurological dysfunction from the onset or result from reactivation later in life. A number of organisms or their nucleic acids have been observed within the CSF or brain parenchyma of patients with MS (Booss and Kim, 1990; O'Connor et al., 2002). These organisms include mycoplasma (Cevassut, 1930), protozoa (Bequignon, 1956), spirochetes (Ichelson, 1957), as well as a number of both DNA and RNA viruses, such as herpes simplex viruses types 1, 2 and 6 (Sanders et al., 1996), rabies virus (Bychkova, 1964), parainfluenza virus type I (ter Meulen et al., 1972), coronavirus (Talbot et al., 1996), canine distemper virus (Rohowsky-Kochan et al., 1995), measles virus (Haase et al., 1981), human immunodeficiency virus type 1 (HIV-1) (Berger et al., 1989) and human T lymphotrophic virus type I (HTLV-I) (Salmi et al., 1983; Greenberg et al., 1989). However, none of the above organisms have been successfully isolated from large groups of clinically definite MS patients. Furthermore, within MS patients infected with these organisms, levels did not correlate with disease activity (O'Connor et al., 2002).
Despite the lack of definitive evidence of a viral source for MS, it is commonly believed that one or more viruses may be responsible for the development of MS within certain genetically susceptible individuals (Allen and Brankin, 1993; Cook et al., 1995). One hypothesis for the mechanism in which viruses cause MS has been proposed which involves both direct and indirect pathways (Clausen, 2003). Viruses could directly damage and lead to functional alterations within oligodendrocytes, resulting in demyelination. Because a single oligodendrocyte can send processes to many neighboring axons, impairment can cause demyelination within a large volume of brain tissue (Silberberg, 1986). Viral invasion may also directly damage endothelial cells within blood vessels and lead to a disruption of the blood–brain barrier. This may subsequently allow for indirect mechanisms in which immunocompetent or activated T lymphocytes can gain entry into the CNS. Demyelination could develop due to the release of cytokines from these activated cells (Poser, 1986; Noseworthy et al., 2000).
Another theory has suggested that viral infection leads to demyelination due to repeated constant exposure of certain antigens to the immune system (Clausen, 2003). An inappropriate immune sensitivity results with antibodies cross-reacting against the oligodendrocyte and/or vascular endothelial cells (Allen and Brankin, 1993; Cook et al., 1995). Alternatively, viral infection might cause a disorder of immunoregulation and secondary demyelination (Salonen et al., 1982). Myelin may not be the primary focus of attack but instead may be an ‘innocent bystander’ with the large-scale destruction that occurs resulting from the interaction of the immune system and reactivated viral infection (Silberberg, 1986; Clausen, 2003).
CLINICAL MANIFESTATIONS
There are no clinical findings that are unique to MS (Kurtzke, 1983; Lisak, 2004). Symptoms and signs that result from MS can include neurological, neuro-ophthalmologic and psychiatric features.
Neurological signs and symptoms
Neurological signs and symptoms of MS are caused primarily by both demyelination and axonal loss that can occur within the cerebral hemispheres, cerebellum, brain stem and spinal cord. Demyelination and destruction of axons produce a variety of somatosensory, motor, cerebellar symptoms and signs (Miller, 1990; Matthews et al., 1991; Kinkel and Rudick, 2002; Lisak, 2004).
Sensory symptoms are the most common clinical manifestations of MS and present as the initial manifestation of the disease in over 70 per cent of MS patients (Kurtzke, 1970; McAlpine, 1972). The anatomic distribution of sensory symptoms often does not correspond to a recognized dermatome, peripheral nerve or homuncular pattern. Some patients may complain of numbness, whereas others are bothered by sensations of tingling, burning or tightness.
Up to 40 per cent of cases of MS will initially present with motor symptoms and signs (Mueller, 1949; Miller, 1990; Matthews et al., 1991; Kinkel and Rudick, 2002; Lisak, 2004). Patients with motor dysfunction may complain of heaviness, stiffness and/or pain in one or more extremities. Typically these symptoms are present in the legs more often than within the arms. Spasticity can also develop and often produces significant discomfort or frank pain (Hooge and Redekop, 1992, 1993).
Cerebellar symptoms and signs can sometimes be seen on initial presentation but typically occur in patients with well-established MS. Cerebellar signs usually manifest as gait ataxia, limb dysmetria and intention tremor (Kurtzke, 1970; McAlpine, 1972). Many patients develop a non-specific impairment of articulation leading to a speech disturbance called ‘scanning speech’.
A Lhermitte's sign, sudden electric-like sensations that radiate down the spine or extremities upon neck flexion, can also be observed. This sign results from a demyelinating lesion in the cervical spinal cord. However, it is not pathognomonic for MS and can be observed within patients with cervical pathology, neck trauma, radiation myelopathy and subacute combined degeneration of the spinal cord (Matthews et al., 1991).
Fatigue can also be seen within MS patients and is usually present in greater than 80 per cent of individuals eventually diagnosed with MS (Tartaglia et al., 2004; Racke and Hawker, 2004; Fisk et al., 1994; Vercoulen et al., 1996; Kinkel and Rudick, 2002; Lisak, 2004). This symptom is particularly bothersome for those with mild to moderate disease. Many patients will complain of a complete lack of motivation. These symptoms can be so severe that they are unable to carry out even the most simple activities of daily living (Miller, 1990; Kinkel and Rudick, 2002). Typically these patients experience fatigue in the setting of motor difficulties (Vercoulen et al., 1996).
Neuro-ophthalmologic manifestations
Optic neuritis (ON) may be the initial presentation of MS (Leibowitz and Alter, 1968; Kuroiwa and Shibasaki, 1973; McDonald and Barnes, 1992). ON usually presents as acute or subacute unilateral eye pain (about 2/3 of the time) accentuated by retro-orbital pain with eye movements. A variable degree of visual loss within the central vision can be observed (Leibowitz and Alter, 1968; Kuroiwa and Shibasaki, 1973). Often the patient with ON will complain of a loss of color vision (red desaturation). Physical examination can reveal a relative afferent pupillary defect. Most often the lesion in ON is retrobulbar with the fundoscopic examination normal during the acute stages (Nordmann et al., 1987). Later, as the disease progresses, the optic disc can become pale as a result of axonal loss and resultant gliosis with temporal pallor present. Typically acute ON will get worse over a time period of several days to 2 weeks, with subsequent visual recovery slowly occurring over 2-4 weeks (Beck et al., 1994). Improvement from ON can continue to progress for up to 1 year after the initial onset of visual symptoms (Beck et al., 1993c).
The Optic Neuritis Treatment Trial (ONTT) was a multicenter trial that attempted to assess the benefit of treatment with corticosteroids on visual recovery in acute optic neuritis (Optic Neuritis Study Group, 1991, 2004; –Beck et al., 1993a; Jacobs et al., 2000; Liu, 2000; CHAMPS Study Group, 2001). The ONTT enrolled 457 patients, aged 18-46 years, with acute unilateral optic neuritis. Patients in the ONTT were randomized to one of three treatment protocols:
-
1
oral prednisone (1 mg/kg/day) for 14 days with a 4-day taper
-
2
intravenous (IV) methylprednisolone sodium succinate (250 mg every 6 h for 3 days) followed by a course of oral prednisone (1 mg/kg/day) for 11 days with a 4-day taper
-
3
oral placebo for 14 days (Beck et al., 1992; Trobe et al., 1996).
The major findings of the ONTT were:
-
1
IV methylprednisolone hastened the recovery of visual function in acute optic neuritis, but had no long-term benefits in visual outcome at either 6 months or at 10 years when compared to placebo or oral prednisone. This medication was most beneficial if given within the first 15 days of presentation
-
2
treatment with oral prednisone alone was associated with an increased rate of recurrent optic neuritis in not only the affected eye but the contralateral eye as well at both 6 months and at 10 years
-
3
monosymptomatic patients in the IV methylpred-nisolone group had a reduced rate for the subsequent development of MS during the first 2 years of follow up, but this benefit was not observed beyond 2 years (Optic Neuritis Study Group, 1991, 2004).
These results suggest that no effective treatment exists for preventing patients who develop acute ON from subsequently developing long-term visual disability (Söderström, 2001; Biousse and Newman, 2002; Foroozan et al., 2002).
The average interval from initial presentation of ON symptoms until subsequent development of MS can vary but typically occurs within 5–7 years (Optic Neuritis Study Group, 1991, 2004). Brain MRI is a powerful technique for predicting subsequent events in MS patients with acute ON (Optic Neuritis Study Group, 1991, 2004; Beck et al., 1993b). The presence of even a single characteristic brain MRI lesion at the time of acute ON is associated with a significantly increased risk (Optic Neuritis Study Group, 2004). More than one lesion on MRI does not substantially increase this risk. Patients with no lesions on MRI and with atypical ON have a very low risk of developing MS.
Internuclear ophthalmoplegia (INO) refers to abnormal horizontal ocular movements with loss or delayed adduction and horizontal nystagmus of the abducting eye due to a lesion in the medial longitudinal fasciculus on the side of diminished adduction (Reulen et al., 1983; Müri and Meienberg, 1985; Gass and Hennerici, 1997). The adduction weakness may be manifest as:
-
1
complete inability to adduct the eye beyond the midline
-
2
a mild limitation of adduction associated with decreased velocity of adduction or
-
3
a mild decrease in the velocity of adducting saccades without any limitation of adduction (Meienberg et al., 1986).
Patients with a unilateral INO can also have a skew deviation with the higher eye on the side of the lesion (Thömke et al., 1992).
Psychiatric manifestations
Neuropsychiatric manifestations may appear at any time of the disease and commonly precede definitive neurological manifestations by months to years (Schiffer and Babigian, 1984; Herndon, 1990; Hutchinson et al., 1993). Overall, cognitive changes can be observed in almost 50 per cent of MS patients (Patti et al., 2003; Benedict et al., 2004). Symptoms tend to progress as the disease worsens (Hutchinson et al., 1993). The most common neuropsychiatric signs and symptoms are disturbances in cognition especially within recent memory, information processing, abstraction and conceptual reasoning (Herndon, 1990; Miller, 1990; Ron and Feinstein, 1992; Benedict et al., 2000; Patti et al., 2003; Lisak, 2004).
Depression can also be a common finding in MS patients with up to 75 per cent experiencing some form of depression during their illness (Schiffer and Babigian, 1984; Kinkel and Rudick, 2002; Patti et al., 2003; Lisak, 2004). The degree of depression can range from mild to severe (Patti et al., 2003; Lisak, 2004) and typically develops after other clinical manifestations. This progression suggests that depression may result within individuals from a greater understanding and knowledge of their disease process rather than due to a direct reaction to the illness. Depression may be quite difficult to identify in some patients, as symptoms can overlap with other disease features, including fatigue, diminished attention and concentration and pain (Kinkel and Rudick, 2002).
GUIDES TO THE DIAGNOSIS OF MS
MS is clinically diagnosed with additional support coming from neuroimaging and paraclinical sources, such as cerebral spinal fluid and evoked potentials. A detailed clinical history requires documentation of at least two discrete episodes within time affecting the CNS (Poser et al., 1983). The McDonald criteria have been developed to assist in the diagnosis of MS. Based on the extent to which the diagnostic criteria are fulfilled for a particular clinical presentation, patients can be categorized as ‘MS’ (diagnostic criteria fulfilled), ‘possible MS’ (criteria not completely met – patient at risk but diagnostic evaluation is equivocal) or ‘not MS’ (criteria fully explored and not met) (McDonald, et al., 2001). The McDonald criteria have successfully been shown to accurately diagnose MS in patients with recent onset of a clinically isolated syndrome. At least two studies have evaluated the sensitivity and specificity of these criteria. One study prospectively evaluated 50 patients at 3 months, 1 year and 3 years of follow up and demonstrated a sensitivity and specificity of 83 per cent (McDonald, et al., 2001). A second study analyzed 139 patients using a retrospective analysis found similar results (Dalton et al., 2004).
The diagnosis of MS is assisted by neuroimaging findings that show evidence of dissemination of lesions within time and space. MRI is particularly sensitive for detecting lesions in the brain stem, cerebellum and spinal cord (Cutler et al., 1986; Ormerod et al., 1986; Grossman and McGowan, 1998; Fazekas et al., 1999; Arnold and Matthews, 2002; Bot et al., 2004) (Figure 45.2 ). Gadolinium (Gad) can be injected and assists in the diagnosis of newly active plaques. Gad enhancement diminishes or disappears after treatment with corticosteroids due to restoration of the blood–brain barrier. Lesions that are more suggestive of MS include those that are 6 mm or more in diameter, ovoid, abutting the lateral ventricles and infratentorial (Arnold and Matthews, 2002). Usually lesions will be observed within the periventricular region, corpus callosum, centrum semiovale and, to a lesser extent, within deeper white matter structures and the basal ganglia. Lesions seen on MRI in MS patients often will fluctuate over time (Grossman and McGowan, 1998; Arnold and Matthews, 2002) with some lesions disappearing, some reappearing, some enlarging, some decreasing and some remaining unchanged (Grossman and McGowan, 1998; Arnold and Matthews, 2002). However, the extent of MRI abnormalities may not correlate with the degree of clinical disability.
Figure 45.2.
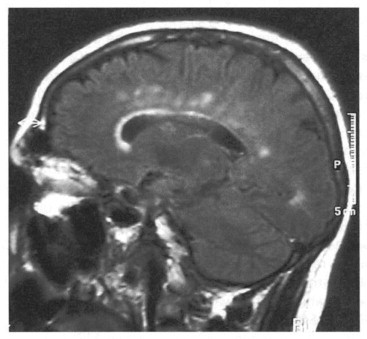
MRI of patient showing multiple demyelinating plaques
Despite these advances in neuroimaging, lesions that are observed may not always be MS (Arnold and Matthews, 2002). Healthy patients over 50 years of age can have similar lesions due to atherosclerotic and hypertensive cerebrovascular disease, CNS vasculitis, migraine, sarcoidosis, venous angiomas and both primary and metastatic brain tumors (Paty, 1990; Boppana and Zagzag, 1993; Arnold and Matthews, 2002). Therefore neuroimaging findings alone cannot be used in the diagnosis of MS.
In addition to demyelinating lesion burden and activity, neuronal and axonal loss are likely to have a crucial role in the development of neurological and visual impairment in MS (Raine and Cross, 1989; Ferguson et al., 1997). Proton MR spectroscopy (MRS) can also provide information on phospholipid metabolism as well as other metabolic components, such as N-acetyl aspartate (NAA) (an exclusively neuronal marker), creatine phosphate (Cr) (a marker of energy metabolism), choline (a cell membrane component), and lactic acid (LA) (Larsson et al., 1991; DeStefano et al., 1995; Kimura et al., 1996; Matthews et al., 1996). Acute MS lesions can have a decrease in NAA and an increase in LA. Chronic MS brains have a reduced amount of NAA as well as a reduced NAA/Cr ratio due to loss of neurons or axons (DeStefano et al., 1995).
Cerebrospinal fluid analysis
Examination of CSF can also assists in the diagnosis of MS (McDonald et al., 2001). Typically glucose and protein levels in the cerebrospinal fluid (CSF) are normal in MS. A few mononuclear cells per cubic millimeter may also be observed. Often a significant elevation of the CSF immunoglobulin level relative to other protein components is present. This immunoglobulin increase is predominantly IgG with an excess primarily in lambda and kappa light chains (Whitaker et al., 1990; Fukazawa et al., 1993). The IgG level may be expressed as a percentage of total protein (normal < 11 per cent), as a percentage of albumin (normal < 27 per cent) or by the use of the IgG index (normal value < 0.66) (Whitaker et al., 1990). CSF IgG production abnormalities, as measured by the IgG index, are found in more than 75 per cent of clinically definite MS patients (Miller et al., 1983; Zeman et al., 1993, 1996; Lowenthal and Karcher, 1994). An elevation in oligoclonal bands can also be observed and is seen in more than 80 per cent of patients with clinically definite MS (Zeman et al., 1993, 1996). More recently, the presence of serum anti-myelin basic protein (MBP) antibodies can predict progression to MS after a clinically isolated event. MBP is one of the major components of myelin and is normally negligible in the CSF During an acute MS flare it rises and falls quickly (Warren and Catz, 2003).
Visually evoked potentials (VEPs)
VEPs are electric potentials or voltages evoked by brief sensory stimuli. These signals are delayed, attenuated or absent within regions of demyelination. An abnormal VEP is seen in 85 per cent of clinically definite MS cases (Brooks and Chiappa, 1982). However, almost any pathophysiologic process that damages the optic nerve can affect VEP results (Asselman et al., 1975; Wilson, 1978). In addition, even though VEPs are considered an ‘objective’ test and are used in the McDonald criteria they may be affected by the patient's age, attention and concentration (Regan, 1972; Regan et al., 1977; Hawkes and Stow, 1981).
TREATMENT
Although some patients with MS have an illness that is slowly progressive from onset (primary progressive MS, or PPMS) and others have an acute, rapidly progressive disease (acute MS), most cases of MS begin with a relapsing-remitting course (RRMS). While not all patients with RRMS develop a progressive course, secondary progressive MS (SPMS) is a long-term sequela of RRMS for many patients (Lublin et al., 1996). While patients with RRMS have clearly defined relapses with a lack of disease progression during inter-relapse intervals, SPMS is characterized by disease progression and worsening of neurologic baseline following an initially relapsing-remitting course. Treatments must therefore be targeted not only toward reducing inflammatory responses and numbers of exacerbations in patients with MS, but ideally should minimize disease progression and axonal loss (Noseworthy et al., 2000; O'Connor, 2002).
Corticosteroids are often used in acute attacks. Treatment usually consists of a short course of IV methylprednisolone, 500 to 1000 mg daily for 3–5 days, with or without a taper. As demonstrated by the ONTT, oral steroid therapy alone should probably not be offered to patients with acute optic neuritis. Patients treated with oral prednisone alone are more likely to suffer recurrent episodes of optic neuritis than those treated with methylprednisolone followed by oral prednisone taper (Optic Neuritis Study Group, 1991, 2004).
Treatment for RRMS consists of starting disease modifying medications, including the interferons or glatiramer acetate. These medications can lead to a decrease in the frequency of relapses and reduce disability in patients with RRMS.
Interferon-beta-1b (Betaseron)
This drug is a cytokine that modulates immune responsiveness, although its precise mechanism of action in MS is unknown. Interferon-beta-1b is administered every other day subcutaneously by self-injection (Arnason, 1993). The efficacy of interferon-beta-1b was demonstrated in a double blind, placebo-controlled trial of patients with RRMS who were randomly assigned to either interferon-beta-1b or placebo (Paty and Li, 1993). After 2 years, the exacerbation rate was significantly lower for patients receiving interferon-beta-1b compared to placebo. The INCOMIN study (independent comparison of interferons) compared interferon-beta-1b (Betaseron) with intramuscular interferon-beta-1a (Avonex) in 188 patients with relapsing-remitting MS and found the former to be more effective on both clinical and MRI outcomes. Over a 2-year period, patients had more relapse free periods as well as developing fewer new MRI lesions on interferon-beta-1b compared to those assigned to interferon-beta-1a (Durelli et al., 2002).
Interferon-beta-1a (Avonex)
Like interferon-beta-1b this drug is a cytokine that modulates immune responsiveness. Interferon-beta-1a is given by weekly intramuscular injections. The efficacy of interferon-beta-1a in patients with RRMS was demonstrated in a randomized, double blind study comparing interferon-beta-1a to placebo (Jacobs et al., 1996). Over a 2-year period, patients treated with interferon-beta-1a had a reduction in the annual exacerbation rate, a decrease in MRI lesion volume burden and less disability compared to patients receiving placebo. In a subsequent study, the Controlled High-Risk Avonex MS Prevention Study (CHAMPS), patients newly diagnosed with MS (those who suffered a first acute clinical demyelinating event and who also had evidence of prior subclinical demyelination on brain MRI) were treated with either Avonex or placebo after initial treatment with corticosteroid therapy. Treatment with Avonex led to a significantly lower probability of developing clinically definitive MS during the subsequent 3 years of follow up compared to patients who were treated with corticosteroids followed by placebo (Jacobs et al., 2000; Galetta, 2001; CHAMPS Study Group, 2002). An extension study of the CHAMPS with all patients on active interferon beta-1a therapy, the Controlled High-Risk Avonex MS Prevention Surveillance (CHAMPIONS) has provided additional data in support of early use of Avonex for the treatment of MS (Kinkel et al., 2003).
Interferon-beta-1a at higher doses (Rebif) has also been shown to be beneficial in RRMS. This medication is given subcutaneously three times a week. The efficacy of interferon-beta-1a was shown through the Prevention of Relapses and Disability by Interferon-beta-1a Subcutaneously in Multiple Sclerosis (PRISMS) study. In this study RRMS patients were given placebo or Rebif three times per week for 2 years. Treatment with Rebif also led to a significant reduction in the MRI lesion burden. Approval for Rebif in the USA arose from results from the EVIDENCE trial in which Rebif was compared directly to Avonex (PRISMS Study Group, 1998). Relapse rate was less frequent and the mean number of active MRI lesions was lower for patients on Rebif compared to those on Avonex.
The main side effects of interferons are flu-like symptoms and depression that tend to diminish with time. Periodic laboratory evaluations including liver enzymes, complete white blood cell counts and thyroid function tests should be performed every 3–6 months. All of the interferons are capable of stimulating the production of neutralizing antibodies that can limit the efficacy of these agents (Sorensen et al., 2003). The risk of antibody formation over 18 months of treatment is highest with Betaseron, intermediate with Rebif and lowest with Avonex (Bertolotto et al., 2003).
Glatiramer acetate (Copaxone)
Glatiramer acetate (Copaxone), a daily subcutaneous injectable synthetic polymer, has been shown to have some positive effects in a small double-blind trial of RRMS (Bornstein et al., 1987). Studies have shown a lower relapse rate and a significant reduction in the number of new T2 lesions compared to placebo. Side effects can include local injection site reactions and transient systemic post-injection reactions such as chest pain, flushing, dyspnea, palpitations and/or anxiety. No laboratory monitoring is necessary and no neutralizing antibodies have been detected.
There are now a number of medications approved by the US Food and Drug Administration for the treatment of RRMS. There are no clear guidelines, nor is there strong evidence for choosing one drug versus another (Galetta et al., 2002). After making a diagnosis of MS the patient should be started on one of the medications listed above. MS patients should be notified that these therapies primarily slow the disease course but are not a cure. These drugs are typically continued indefinitely unless side effects are intolerable or the patient begins to fail in terms of response, after which use of another agent can be considered.
ACKNOWLEDGEMENTS
This chapter was revised from Multiple Sclerosis and Related Demyelinating Diseases by Nancy J. Newman and Laura J. Balcer in Walsh and Hoyt's Clinical Neuro-ophthalmology (2004).
REFERENCES
- Alizadeh M., Babron M.C., Birebent B. Genetic interaction of CTLA-4 with HLA-DR15 in multiple sclerosis patients. Ann Neurol. 2003;54:119–122. doi: 10.1002/ana.10617. [DOI] [PubMed] [Google Scholar]
- Allen I.V., Brankin B. Pathogenesis of multiple sclerosis—the immune diathesis and the role of viruses. J Neuropathol Exp Neurol. 1993;52:95–105. doi: 10.1097/00005072-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Arnason B.G. Interferon beta in multiple sclerosis (editorial; comment) Neurology. 1993;43:641–643. doi: 10.1212/wnl.43.4.641. [DOI] [PubMed] [Google Scholar]
- Arnold D.L., Matthews P.M. MRI in the diagnosis and management of multiple sclerosis. Neurology. 2002;58:S23–S31. doi: 10.1212/wnl.58.8_suppl_4.s23. [DOI] [PubMed] [Google Scholar]
- Asselman P., Chadwick D.W., Marsden C.D. Visual evoked responses in the diagnosis and management of patients suspected of multiple sclerosis. Brain. 1975;98:261–282. doi: 10.1093/brain/98.2.261. [DOI] [PubMed] [Google Scholar]
- Beck R.W., Cleary P.A., Anderson M.M., Jr A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326:581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- Beck R.W., Cleary P.A., Trobe J.D. The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. N Engl J Med. 1993;329:1764–1769. doi: 10.1056/NEJM199312093292403. [DOI] [PubMed] [Google Scholar]
- Beck R.W., Arrington J., Murtagh F.R., The Optic Neuritis Study Group Brain magnetic resonance imaging in acute optic neuritis: Experience of The Optic Neuritis Study Group. Arch Ophthalmol. 1993;50:841–846. doi: 10.1001/archneur.1993.00540080050013. [DOI] [PubMed] [Google Scholar]
- Beck R.W., Cleary P.A., The Optic Neurotis Study Group Optic Neuritis Treatment Trial. One-year follow-up results. Arch Ophthalmol. 1993;111:773–775. doi: 10.1001/archopht.1993.01090060061023. [DOI] [PubMed] [Google Scholar]
- Beck R.W., Cleary P.A., Backlund J.C. The course of visual recovery after optic neuritis: experience of the Optic Neuritis Treatment Trial. Ophthalmology. 1994;101:1771–1778. doi: 10.1016/s0161-6420(94)31103-1. [DOI] [PubMed] [Google Scholar]
- Benedict R.H.B., Weinstock-Guttman B., Fishman I. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol. 2004;61:226–230. doi: 10.1001/archneur.61.2.226. [DOI] [PubMed] [Google Scholar]
- Bequignon R. De l'étiologie de la sclérose en plaques. CR Acad Sci. 1956;242:1380–1382. [PubMed] [Google Scholar]
- Berger J.R., Sheremata W.A., Resnick L. Multiple sclerosis-like illness occurring with human immunodeficiency virus infection. Neurology. 1989;39:324–329. doi: 10.1212/wnl.39.3.324. [DOI] [PubMed] [Google Scholar]
- Bertolotto A., Gilli F., Sala A. Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology. 2003;60:634–639. doi: 10.1212/01.wnl.0000046662.03894.c5. [DOI] [PubMed] [Google Scholar]
- Biousse V., Newman N.J. W. B. Saunders; Philadelphia: 2002. pp. 943–945. (Optic neuritis. In Conn's Current Therapy). [Google Scholar]
- Booss J., Kim J.H. Evidence for a viral etiology of multiple sclerosis. In: Cook S.D., editor. Handbook of Multiple Sclerosis. Marcel Dekker; New York: 1990. pp. 41–62. [Google Scholar]
- Boppana M., Zagzag D. Central nervous system demyelination presenting as a mass lesion: A diagnostic challenge. Ann Neurol. 1993;34:312. [Google Scholar]
- Bornstein M.B., Miller A., Slagle S. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med. 1987;317:408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- Bot J.C.J., Barkhof F., Polman C.H. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology. 2004;62:226–233. doi: 10.1212/wnl.62.2.226. [DOI] [PubMed] [Google Scholar]
- Brooks E.B., Chiappa K.H. A comparison of clinical neuro-ophthalmological findings and pattern shift evoked potentials in multiple sclerosis. In: Courjan J.J., Mauguiere F., Revol M., editors. Clinical Applications of Evoked Potentials in Neurology. Raven Press; New York: 1982. pp. 435–437. [Google Scholar]
- Bychkova E.N. Viruses isolated from patients with encephalomyelitis and multiple sclerosis. Communication I: pathologenic and antigenic properties. Vopr Virusol. 1964;9:173–176. [PubMed] [Google Scholar]
- CHAMPS Study Group Interferon β-1a for optic neuritis patients at high risk for multiple sclerosis. Am J Ophthalmol. 2001;132:463–471. doi: 10.1016/s0002-9394(01)01209-0. [DOI] [PubMed] [Google Scholar]
- CHAMPS Study Group Baseline MRI characteristics of patients at high risk for multiple sclerosis: results from the CHAMPS trial. Multiple Sclerosis. 2002;8:330–338. doi: 10.1191/1352458502ms819oa. [DOI] [PubMed] [Google Scholar]
- Chevassut K. The aetiology of disseminated sclerosis. Lancet. 1930;1:552–560. [Google Scholar]
- Clausen J. Endogenous retroviruses and MS: using ERV's as disease markers. Int MS J. 2003;10:20–21. [PubMed] [Google Scholar]
- Compston A., Ebers G.C. The genetics of multiple sclerosis. In: Cook S.D., editor. Handbook of Multiple Sclerosis. Marcel Dekker; New York: 1990. pp. 25–40. [Google Scholar]
- Cook S.D., Bansil S., Boos J. Total lymphoid irradiation (TLI) and low-dose prednisone (LDP) in progressive multiple sclerosis (PMS) Neurology. 1995;45:A417. [Google Scholar]
- Cutler J.R., Aminoff M.J., Brant-Zawadzki M. Evaluation of patients with multiple sclerosis by evoked potentials and magnetic resonance imaging: A comparative study. Ann Neurol. 1986;20:645–648. doi: 10.1002/ana.410200518. [DOI] [PubMed] [Google Scholar]
- Dalton C.M., Brex P.A., Miszkiel K.A. Application of the new McDonald Criteria to patients with clinically isolated syndromes suggestive of multiple sclerosis. Ann Neurol. 2002;52:47–53. doi: 10.1002/ana.10240. [DOI] [PubMed] [Google Scholar]
- DeStefano N., Matthews P., Antel J. Chemical pathology of acute demyelinating lesions and its correlation with disability. Ann Neurol. 1995;38:901–909. doi: 10.1002/ana.410380610. [DOI] [PubMed] [Google Scholar]
- Durelli L., Verdun E., Barbero P., Independent Comparison of Interferon (INCOMIN) Trial Study Group Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359:1453–1460. doi: 10.1016/s0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- Fazekas F., Barkhof F., Filippi M. The contribution of magnetic resonance imaging to the diagnosis of multiple sclerosis. Neurology. 1998;53:448–456. doi: 10.1212/wnl.53.3.448. [DOI] [PubMed] [Google Scholar]
- Ferguson B., Matyszak M.K., Esiri M.M., Perry V.H. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Fisk J.D., Pontefract A., Ritvo P.G. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- Foroozan R., Buono L.M., Savino P.J. Acute demyelinating optic neuritis. Curr Opin Ophthalmol. 2002;13:375–380. doi: 10.1097/00055735-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Fukazawa T., Moriwaka F., Sugiyama K. Cerebrospinal fluid IgG profiles and multiple sclerosis in Japan. Acta Neurol Scand. 1993;88:178–183. doi: 10.1111/j.1600-0404.1993.tb04213.x. [DOI] [PubMed] [Google Scholar]
- Galetta S.L. The Controlled High-Risk Avonex Multiple Sclerosis Trial (CHAMPS Study) J Neuro-Ophthalmol. 2001;21:292–295. doi: 10.1097/00041327-200112000-00013. [DOI] [PubMed] [Google Scholar]
- Galetta S.L., Markowitz C., Lee A.G. Immunomodulatory agents for the treatment of relapsing multiple sclerosis: a systematic review. Arch Intern Med. 2002;162:2161–2169. doi: 10.1001/archinte.162.19.2161. [DOI] [PubMed] [Google Scholar]
- Gass A., Hennerici M.G. Bilateral internuclear ophthalmoplegia in multiple sclerosis. J Neurol Neurosurg Psychiatr. 1997;63:564. doi: 10.1136/jnnp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S.J., Ehrlich G.D., Abbott M.A. Detection of sequences homologous to retroviral DNA in multiple sclerosis by gene amplification. Proc Natl Acad Sci USA. 1989;86:2878–2882. doi: 10.1073/pnas.86.8.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman R.I., McGowan J.C. Perspectives on multiple sclerosis. Am J Neurol Radiol. 1998;19:1251–1265. [PMC free article] [PubMed] [Google Scholar]
- Haase A.T., Ventrua P., Gibbs C.J., Jr Measles virus nucleotide sequences: Detection by hybridization in situ. Science. 1981;212:672–674. doi: 10.1126/science.7221554. [DOI] [PubMed] [Google Scholar]
- Haegert D.G., Francis G.S. HLA-DQ polymorphisms do not explain HLA class II associations with multiple sclerosis in two Canadian groups. Neurology. 1993;43:1207–1210. doi: 10.1212/wnl.43.6.1207. [DOI] [PubMed] [Google Scholar]
- Hafler D.A., Weiner H.L. Immunologic mechanisms and therapy in multiple sclerosis. Immunol Rev. 1995;144:75–105. doi: 10.1111/j.1600-065x.1995.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Hawkes C.H., Stow B. Pupil size and the pattern evoked visual response. J Neurol Neurosurg Psychiatr. 1981;44:90–91. doi: 10.1136/jnnp.44.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon R.M. Cognitive deficits and emotional dysfunction in multiple sclerosis. Arch Neurol. 1990;47:18. doi: 10.1001/archneur.1990.00530010026013. [DOI] [PubMed] [Google Scholar]
- Herrera B.M., Ebers G.C. Progress in deciphering the genetics of multiple sclerosis. Curr Opin Neurol. 2003;16:253–258. doi: 10.1097/01.wco.0000073924.19076.bb. [DOI] [PubMed] [Google Scholar]
- Hillert J. Human leukocyte antigen studies in multiple sclerosis. Ann Neurol. 1994;36:S15–S17. doi: 10.1002/ana.410360706. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R. Biotechnological agents for the immuno-therapy of multiple sclerosis: Principles, problems and perspectives. Brain. 1997;120:865–916. doi: 10.1093/brain/120.5.865. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Meinl E., Weber F. The role of autoimmune T-lymphocytes in the pathogenesis of multiple sclerosis. Neurology. 1995;45:S33–S38. doi: 10.1212/wnl.45.6_suppl_6.s33. [DOI] [PubMed] [Google Scholar]
- Hooge J.P., Redekop W.K. Multiple sclerosis with very late onset. Neurology. 1992;42:1907–1910. doi: 10.1212/wnl.42.10.1907. [DOI] [PubMed] [Google Scholar]
- Hooge J.P., Redekop W.K. Late-onset MS. Neurology. 1993;43:1629. doi: 10.1212/wnl.43.8.1629. [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Stack J., Buckley P. Bipolar affective disorder prior to the onset of multiple sclerosis. Acta Neurol Scand. 1993;88:388–393. doi: 10.1111/j.1600-0404.1993.tb05365.x. [DOI] [PubMed] [Google Scholar]
- Ichelson R.R. Cultivation of spirochaetes from spinal fluids with multiple sclerosis cases and negative controls. Proc Soc Exp Biol Med. 1957;95:57–58. doi: 10.3181/00379727-95-23117. [DOI] [PubMed] [Google Scholar]
- Jacobs L.D., Cookfair D.L., Rudick R.A. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Jacobs L.D., Beck R.W., Simon J.H. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- Kenealy S.J., Pericak-Vance M.A., Haines J.L. The genetic epidemiology of multiple sclerosis. J Neuroimmunol. 2003;143:7–12. doi: 10.1016/j.jneuroim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Kimura H., Grossman R.I., Lenkinski R.E. Proton MR spectroscopy and magnetization transfer ratio in multiple sclerosis: correlative fidings of active versus irreversible plaque disease. Am J Neurol Radiol. 1996;17:1539–1547. [PMC free article] [PubMed] [Google Scholar]
- Kinkel R.P., Rudick R.A. Multiple sclerosis. In: Rakel R.E., Bope E.T., editors. Conn's Current Therapy. W.B. Saunders; Philadelphia: 2002. pp. 922–937. [Google Scholar]
- Kinkel R.P., the CHAMPIONS Study Group Inttial results of the CHAMPIONS study, an open-label 5-year extension of the CHAMPS study. Presented at the 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); September 17–20, 2003, Milan, Italy; 2003. [Google Scholar]
- Kuroiwa Y., Shibasaki H. Clinical studies of multiple sclerosis in Japan. I. A current appraisal of 83 cases. Neurology. 1973;23:609–617. doi: 10.1212/wnl.23.6.609. [DOI] [PubMed] [Google Scholar]
- Kurtzke J.F. Clinical manifestations of multiple sclerosis. In: Vinken P.J., Bruyn G.W., editors. Vol 9. North-Holland; Amsterdam: 1970. pp. 161–216. (Handbook of Clinical Neurology). [Google Scholar]
- Kurtzke J.F. Epidemiology of multiple sclerosis. In: Hallpike J.F., Adams C.W.M., Toutellotte W.W., editors. Multiple Sclerosis. Williams & Wilkins; Baltimore: 1983. pp. 47–96. [Google Scholar]
- Larsson H.B., Christiansen P., Jensen M. Localized in vivo proton spectroscopy in the brain of patients with multiple sclerosis. Magn Reson Med. 1991;22:23–31. doi: 10.1002/mrm.1910220104. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Axonal injury in multiple sclerosis: renewed interest in axonal destruction in MS (editorial) J Neurol Neurosurg Psychiatr. 2003;74:695–697. doi: 10.1136/jnnp.74.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz U., Alter M. Optic nerve involvement and diplopia as initial manifestations of multiple sclerosis. Acta Neurol Scand. 1968;44:70–80. doi: 10.1111/j.1600-0404.1968.tb07444.x. [DOI] [PubMed] [Google Scholar]
- Lisak R.P. Multiple sclerosis. In: Rakel R.E., Bope E.T., editors. Conn's Current Therapy. W.B. Saunders; Philadelphia: 2004. pp. 973–981. [Google Scholar]
- Liu G.T. Visual loss: optic neuropathies. In: Liu G.T., Volpe N.J., Galetta S., editors. Neuro-Ophthalmology: Diagnosis and Management. W.B. Saunders; Philadelphia: 2000. pp. 103–187. [Google Scholar]
- Lowenthal A., Karcher D. Cerebrospinal fluid in multiple sclerosis. Clin Neurosci. 1994;2:211–214. [PubMed] [Google Scholar]
- Lublin F.D., Reingold S.C., for the National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- McAlpine D. Symptoms and signs. In: McAlpine D., Lumsden C.E., Acheson E., editors. Multiple Sclerosis: A Reappraisal. 2nd edn. Williams & Wilkins; Baltimore: 1972. pp. 132–196. [Google Scholar]
- McDonald W.I., Barnes D. The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J Neurol Neurosurg Psychiatr. 1992;55:747–752. doi: 10.1136/jnnp.55.9.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W.I., Compston A., Edan G. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- McFarland H/F. The multiple sclerosis lesion. Ann Neurol. 1995;37:419–421. doi: 10.1002/ana.410370402. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H.F. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- Matthews P.M., Grancis G., Antel J. Proton magnetic resonance spectroscopy for metabolic characterization of plaques in multiple sclerosis. Neurology. 1991;41:1251–1256. doi: 10.1212/wnl.41.8.1251. [DOI] [PubMed] [Google Scholar]
- Matthews P.M., Pioro E., Narayanan S. Assessment of lesion pathology in multiple sclerosis using quantitative MRI morphometry and magnetic resonance spectroscopy. Brain. 1996;119:715–722. doi: 10.1093/brain/119.3.715. [DOI] [PubMed] [Google Scholar]
- Meienberg O., Müri R., Rabineau P.A. Clinical and oculographic examinations of saccadic eye movements in the diagnosis of multiple sclerosis. Arch Neurol. 1986;43:438–443. doi: 10.1001/archneur.1986.00520050018014. [DOI] [PubMed] [Google Scholar]
- Miller A.E. Clinical features. In: Cook S.D., editor. Handbook of Multiple Sclerosis. Marcel Dekker; New York: 1990. pp. 169–186. [Google Scholar]
- Miller J.R., Burke A.M., Bever C.T. Occurrence of oligoclonal bands in multiple sclerosis and other CNS diseases. Ann Neurol. 1983;13:53–58. doi: 10.1002/ana.410130112. [DOI] [PubMed] [Google Scholar]
- Minden S.L., Marder W.D., Harrold L.N., Dor A. Abt Associates; Cambridge, MA: 1993. Multiple Sclerosis: a statistical portrait. A compendium of data on demographics, disability, and health services utilization in the United States. [Google Scholar]
- Mueller R. Studies on disseminated sclerosis with special reference to symptomatology, course and prognosls. Acta Med Scand. 1949;133(Suppl 222):1–214. [Google Scholar]
- Müri R.M., Meienberg O. The clinical spectrum of internuclear ophthalmoplegia in multiple sclerosis. Arch Neurol. 1985;42:851–855. doi: 10.1001/archneur.1985.04060080029011. [DOI] [PubMed] [Google Scholar]
- Nordmann J.P., Saraux H., Roullet E. Contrast sensitivity in multiple sclerosis: a study in 35 patients with and without optic neuritis. Ophthalmologica. 1987;195:199–204. doi: 10.1159/000309813. [DOI] [PubMed] [Google Scholar]
- Noseworthy J.H., Lucchinetti C., Rodriguez M. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- O'Connor P., The Canadian Multiple Sclerosis Working Group Key issues in the diagnosis and treatment of multiple sclerosis. Neurology. 2002;59:S1–S33. doi: 10.1212/wnl.59.6_suppl_3.s1. on behalf of. [DOI] [PubMed] [Google Scholar]
- Optic Neuritis Study Group The clinical profile of optic neuritis: experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1991;109:1673–1678. doi: 10.1001/archopht.1991.01080120057025. [DOI] [PubMed] [Google Scholar]
- Optic Neuritis Study Group Visual function more than 10 years after optic neuritis: experience of the Optic Neuritis Treatment Trial. Am J Ophthalmol. 2004;137:77–83. doi: 10.1016/s0002-9394(03)00862-6. [DOI] [PubMed] [Google Scholar]
- Ormerod I.E.C., Bronstein A., Rudge P. Magnetic resonance imaging in clinically isolated lesions of the brain stem. J Neurol Neurosurg Psychiatr. 1986;49:737–743. doi: 10.1136/jnnp.49.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti F., Cacopardo M., Palermo F. Health-related quality of life and depression in an Italian sample of multiple sclerosis patients. J Neurol Sci. 2003;211:55–62. doi: 10.1016/s0022-510x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Paty D.W. Neuroimaging in multiple sclerosis. In: Cook S.D., editor. Handbook of Multiple Sclerosis. Marcel Dekker; New York: 1990. pp. 291–316. [Google Scholar]
- Paty D.W., Li D.K. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- Poser C.M. Pathologenesis of multiple sclerosis: A critical reappraisal. Acta Neuropathol. 1986;71:1–10. doi: 10.1007/BF00687954. [DOI] [PubMed] [Google Scholar]
- Poser C.M., Paty D.W., Scheinberg L. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Prineas J.W., wright R.G. Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab Invest. 1978;38:409–421. [PubMed] [Google Scholar]
- PRISMS Study Group Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- Pugliatti M., Sotgiu S., Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:182–191. doi: 10.1016/s0303-8467(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Raine C.S., Cross A.H. Axonal dystrophy as a consequence of long-term demyelination. Lab Invest. 1989;60:714–725. [PubMed] [Google Scholar]
- Regan D. Chapman and Hall, Ltd.; London: 1972. (Evoked Potentials in Psychology, Sensory Physiology and Clinical Medicine). [Google Scholar]
- Regan D., Murray T.J., Silver R. Effect of body temperature on visual evoked potential delay and visual perception in multiple sclerosis. J Neurol Neurosurg Psychiatr. 1977;40:1083–1091. doi: 10.1136/jnnp.40.11.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reulen J.P.H., Sanders E.A.C.M., Hogenuis L.A.H. Eye movement disorders in multiple sclerosis and optic neuritis. Brain. 1983;106:121–140. doi: 10.1093/brain/106.1.121. [DOI] [PubMed] [Google Scholar]
- Rohowsky-Kochan C., Dowling P.C., Cook S.D. Canine distemper virus-specific antibodies in multiple sclerosis. Neurology. 1995;45:1554–1560. doi: 10.1212/wnl.45.8.1554. [DOI] [PubMed] [Google Scholar]
- Ron M.A., Feinstein A. Multiple sclerosis and the mind. J Neurol Neurosurg Psychiatr. 1992;55:1–3. doi: 10.1136/jnnp.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovnick A.D. The genetics of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:199–202. doi: 10.1016/s0303-8467(02)00038-0. [DOI] [PubMed] [Google Scholar]
- Salmi A., Reunanen M., Ilonen J. Intrathecal antibody synthesis to virus antigens in multiple sclerosis. Clin Exp Immunol. 1983;52:241–249. [PMC free article] [PubMed] [Google Scholar]
- Salonen R., Ilonen J., Reunanen M. PPD-, PWM-, and PHA-induced interferon in stable multiple sclerosis: Association with HLD-Dw2 antigen and clinical variables. Ann Neurol. 1982;11:279–284. doi: 10.1002/ana.410110308. [DOI] [PubMed] [Google Scholar]
- Sanders V.J., Waddell A.E., Felisan S.L. Herpes simplex virus in postmortem multiple sclerosis brain tissue. Arch Neurol. 1996;53:125–133. doi: 10.1001/archneur.1996.00550020029012. [DOI] [PubMed] [Google Scholar]
- Schiffer R.B., Babigian H.M. Behavioral disorders in multiple sclerosis, temporal lobe epilepsy, and amyotrophic lateral sclerosis. Arch Neurol. 1984;41:1067–1069. doi: 10.1001/archneur.1984.04050210065016. [DOI] [PubMed] [Google Scholar]
- Silberberg D.H. Pathogenesis of demyelination. In: McDonald W.I., Silberberg D.H., editors. Multiple Sclerosis. Butterworths; London: 1986. pp. 99–111. [Google Scholar]
- Smith M.E., Stone L.A., Albert P.S. Clinical worsening in multiple slerosis is associated with increased frequency and area of gadopentetate dimeglumine-enhancing magnetic resonance imaging lesions. Ann Neurol. 1993;33:480–489. doi: 10.1002/ana.410330511. [DOI] [PubMed] [Google Scholar]
- Söderström M. Optic neuritis and multiple sclerosis. Acta Ophthalmol Scand. 2001;79:223–227. doi: 10.1034/j.1600-0420.2001.790302.x. [DOI] [PubMed] [Google Scholar]
- Sorensen P.S., Ross C., Clemmesen K.M., Danish Multiple Sclerosis Study Group Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet. 2003;362:1184–1191. doi: 10.1016/S0140-6736(03)14541-2. [DOI] [PubMed] [Google Scholar]
- Talbot P.J., Paquette J.S., Ciurli C. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann Neurol. 1996;39:233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M.C., Narayanan S., Francis S.J. The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch Neurol. 2004;61:201–207. doi: 10.1001/archneur.61.2.201. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Koprowski H., Iwasaki Y. Fusion of cultured multiple-sclerosis brain cells with indicator cells: Presence of nucleocapsids and virion with isolation of parainfluenza-type virus. Lancet. 1972;1:1–5. doi: 10.1016/s0140-6736(72)91273-1. [DOI] [PubMed] [Google Scholar]
- Thömke F., Hopf H.C., Breen L.A. Slowed abduction saccades in bilateral internuclear ophthalmoplegia. Neuro-Ophthalmology. 1992;12:241–246. [Google Scholar]
- Tourbah A., Fontaine B., Lyon-Caen O. Immunologie de la sclerose en plaques: Donneés recentes et perspectives therapeutiques. Rev Neurol. 1993;149:373–384. [PubMed] [Google Scholar]
- Trapp B.D., Ransohoff R.M., Fisher E., Rudick R.A. Neurodegeneration in multiple sclerosis: relationship to neurological disability. Neuroscientist. 1999;5:48–57. [Google Scholar]
- Trobe J.D., Beck R.W., Moke P.S. Contrast sensitivity and other vision tests in the Optic Neuritis Treatment Trial. Am J Opthalmol. 1996;121:547–553. doi: 10.1016/s0002-9394(14)75429-7. [DOI] [PubMed] [Google Scholar]
- Vercoulen J.H.M.M., Hommes O.R., Swanink C.M.A. The measurement of fatigue in patients with multiple sclerosis: A multidimensional comprison with patients with chronic fatigue syndrome and healthy subjects. Arch Neurol. 1996;53:642–649. doi: 10.1001/archneur.1996.00550070080014. [DOI] [PubMed] [Google Scholar]
- Warren K.G., Catz I. The relationship between levels of cerebrospinal fluid myelin basic proteien and IgG measurements in patients with multiple sclerosis. Ann Neurol. 1985 doi: 10.1002/ana.410170510. [DOI] [PubMed] [Google Scholar]
- Whetten-Goldstein K., Sloan F.A., Goldstein L.B., Kulas E.D. A comprehensive assessment of the cost of multiple sclerosis in the United States. Multiple Sclerosis. 1998;4:419–425. doi: 10.1177/135245859800400504. [DOI] [PubMed] [Google Scholar]
- Whitaker J.N., Benveniste E.N., Zhou S-r. Cerebrospinal fluid. In: Cook S.D., editor. Handbook of Multiple Sclerosis. Marcel Dekker; New York: 1990. pp. 251–270. [Google Scholar]
- Willer C.J., Dyment D.A., Risch N.J. Twin concordance and sibling recurrence rates in Multiple Sclerosis. Proc Natl Acad Sci USA. 2003;100:12877–12882. doi: 10.1073/pnas.1932604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.B. Visual-evoked response differentiation of ischemic optic neuritis from the optic neuritis of multiple sclerosis. Am J Ophthalmol. 1978;86:530–535. doi: 10.1016/0002-9394(78)90302-1. [DOI] [PubMed] [Google Scholar]
- Zeman A., McLean B., Keir G. The significance of serum oligoclonal bands in neurological diseases. J Neurol Neurosurg Psychiatr. 1993;56:32–35. doi: 10.1136/jnnp.56.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman A., Kidd D., McLean B.N. A study of oligoclonal band negative multiple sclerosis. J Neurol Neurosurg Psychiatr. 1996;60:27–30. doi: 10.1136/jnnp.60.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]


