Abstract
Acute phase proteins (APP) were first identified in the early 1900s as early reactants to infectious disease. They are now understood to be an integral part of the acute phase response (APR) which is the cornerstone of innate immunity. APP have been shown to be valuable biomarkers as increases can occur with inflammation, infection, neoplasia, stress, and trauma. All animals—from fish to mammals—have demonstrable APP, but the type of major APP differs by species. While the primary application of these proteins in a clinical setting is prognostication, studies in animals have demonstrated relevance to diagnosis and detection and monitoring for subclinical disease. APP have been well documented in laboratory, companion, and large animals. With the advent of standardized and automated assays, these biomarkers are available for use in all fields of veterinary medicine as well as basic and clinical research.
Keywords: Acute phase protein, Acute phase response, C-reactive protein, Haptoglobin, Serum amyloid A
The acute phase response or APR is a term given to a collective of reactions to tissue injury resulting from infection, trauma, neoplasia, inflammation, and stress. The response is formulated by a number of different acute phase proteins (APP) that vary in magnitude and type among animal species. These systemic responders act as part of the innate immune defense system with the goal of reestablishing homeostasis and promoting healing. Several publications have documented the role of APP in response to experimental and infectious stimuli in animal models.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Over the past decade, APP have been more widely applied as biomarkers of inflammation in human and veterinary medicine.
I. The Acute Phase Response
The APR is part of broad response of the innate immune or early-defense system. The production of APP is preceded by the perhaps better known local response involving the creation of proinflammatory cytokines such as IL-1, IL-6, and TNF-α. These signal hepatocytes to produce the myriad of APP which then enhance chemotaxis provide other immunomodulatory effects, impart specific bacteriostatic support, and assist in the reduction of oxidative damage (Fig. 1 , Table I ).
Fig. 1.
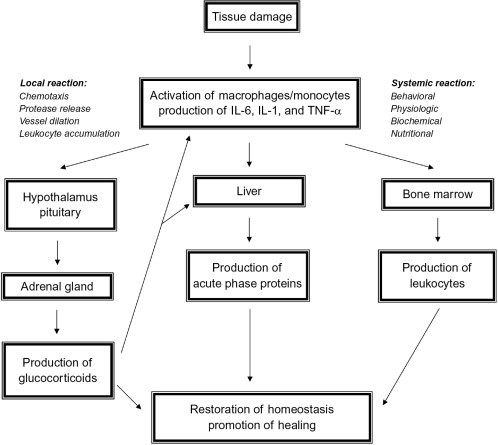
The acute phase response.
Table I.
List of Common Acute Phase Proteins and Their Commonly Used Acronyms and Activities
| Acronym | Acute phase protein | Activities |
|---|---|---|
| AAT | Alpha-1 antitrypsin | Protease inhibition |
| ACT | Alpha-1 antichymotrypsin | Protease inhibition |
| AGP | Alpha-1 acid glycoprotein | Bind drugs and LPS |
| ALB | Albumin | Transport protein |
| A2M | Alpha-2 macroglobulin | Protease inhibition |
| CP | Ceruloplasmin | Transport copper, protect from iron-mediated oxidative injury |
| CRP | C-reactive protein | Enhance opsonization, activate complement, induce cytokines, inhibit chemotaxis |
| FIB | Fibrinogen | Substrate for fibrin, tissue repair |
| HP | Haptoglobin | Bind hemoglobin, bacteriostatic |
| MAP | Pig major acute phase protein | Trypsin inhibition |
| SAA | Serum amyloid A | Chemotaxis, anti-inflammatory activity |
| SAP | Serum amyloid P | Enhance opsonization, activate complement |
| TN | Transferrin | Sequestration of iron |
A. Innate Immunity
The innate immune system serves to prevent infection, eliminate potential pathogens, and initiate the inflammatory response.12 It is often described as compromised of distinct defenses including anatomic barriers (i.e., skin, mucous membranes), physiologic barriers (i.e., low pH and temperature to inhibit bacterial growth), phagocytic barriers (i.e., tissue-based cells that can ingest pathogens), and inflammatory barriers. It is the latter barrier that serves as the backup in the event of the failure of the other arms of the innate immune system and also perhaps is the most recognized given the myriad of symptoms including edema, redness, and fever that are produced with the complex interactions of cytokines, chemokines, and APP.
With the increased knowledge of the inflammatory process, it is recognized that this represents a cascade of very specific and effective immune responses that also initiate several metabolic events.13, 14 The innate immune response is often characterized as nonspecific as it is contrasted to the adaptive immune response which involves antigen-specific responses via T and B cells. While the antigen receptor and its required gene recombination events are a more recent evolutionary acquisition of vertebrates, the innate immune system represents the core from which the adaptive immune system was based. Additionally, these two systems are often examined as mediating separate events. There is a true interface between the APR and adaptive immunity. Yoo and colleagues, with the use of lipopolysaccharide (LPS) treatment of mice, demonstrated that the APR mobilizes antigen presentation by hepatocytes via MHC class I.13 Others have demonstrated that the elimination of toll-like receptors affects both innate and adaptive immune responses.15, 16
B. Induction of the APR
The APR is initiated primarily by tissue macrophages, blood monocytes, and dendritic cells at the site of tissue damage when particular chemical structures in damaged tissue or infectious agents are presented for detection by these first-line defense cells.17 These cells undergo a rapid activation leading to the production of the primary cytokines IL-1, TNF-α, and IL-6 which not only incite the APR but also result in the chemotactic recruitment of additional cells to the affected area to rapidly augment the response. The area of major APP production is the liver; specifically, the induction of APP expression in hepatocytes. APP have also been noted to be produced in other tissues. The production of the major APP serum amyloid A (SAA) has been demonstrated in the gastrointestinal tract, mammary gland, kidneys, and airways.18, 19
The inflammatory stimuli may result in a differential production of cytokines, and the absence of some of the cytokines may abrogate the APR. In a study of LPS-induced inflammation of the mouse lung, the use of neutralizing antibody to IL-6 but not TNF-α resulted in the cessation of the APR.20 In IL-6-deficient mice, the intraperitoneal injection of LPS resulted in an overproduction of TNF-α but still a significant decrease in the production of SAA.21 Similarly, in a mouse model of bacterial pneumonia, SAA increases were completely abrogated in IL-6-deficient mice.22 In wild-type mice, IL-6 levels were increased in both the liver and the lungs of infected mice within 24 h of bacterial instillation.
The apparent conduit between the cytokines and the expression of APP are the transcription factors STAT3 and RelA. It was shown that these factors form a complex after interaction with IL-1 and IL-6 and then induce the transcription of the SAA gene.23 In a mouse model where the levels of STAT3 were significantly reduced, the SAA response was abrogated.24 In vitro studies further demonstrated that the loss of either STAT3 or RelA prevented SAA production in a hepatocyte cell line.22
C. Other Effects of the APR
While specific APP will be discussed in the following sections, it should be noted that the APR is often defined by a myriad of changes not limited to the well-known biomarkers alone (Fig. 1). In addition to the immune system, both the nervous and endocrine systems are also involved and stimulated by the cytokine production resulting in behavioral, physiologic, biochemical, and nutritional changes.25 IL-6 stimulates the corticotrophin-releasing hormone (CRH) synthesis in the hypothalamus which, in turn, modulates ACTH release from the pituitary and glucocorticoid production in the adrenal gland; this is the so called hypothalamic–pituitary–adrenal axis. In IL-6-deficient mice injected with LPS, CRH, and glucocorticoid levels were significantly lower than wild-type controls.26
Other host responses include fever, fatigue, hypoglycemia, anorexia, lipolysis, and muscle catabolism. In the LPS injection model, there is nearly a 10% loss in body weight by day 1.21 Both IL-6 and neural mechanisms have been implicated in this response.21, 27 This is concomitant with a significant decrease in food intake and serum glucose. Fever is thought also to be a response to IL-6 through the production of other mediators including prostaglandins which affect the hypothalamus.28, 29 Decreases in serum iron and zinc concentrations are also often observed with their redistribution to the tissues where they assist in antioxidant activity.25
Leukocytosis is initially mediated through the production of cortisol by the removal of marginal pool cells from the tissues and placement in the blood in what is often called an immediate stress response. The true leukocytosis follows some days after the effects of the proinflammatory cytokine response and its positive effect on the bone marrow, and often involves enhanced levels of both lymphocytes and neutrophils.
Glucocorticoids are known to mediate upregulatory effects on the APR. Experiments have been completed with the application of exogenous dexamethasone into different models of APR; increased APP transcription, IL-6 receptor, and hepatocyte proliferation have been demonstrated.30 Downregulation occurs as the initial stimulus (tissue damage) is addressed. Cytokines can be inhibited by other cytokines and excess cortisol.30, 30, 31 Notably, in addition to their direct roles in addressing the inflammatory tissue and resultant tissue damages, APP also often have anti-inflammatory activity. For example, SAA was found to inhibit myeloperoxidase release, migration, and free oxygen radical production in neutrophils.32
II. Acute Phase Proteins
It is thought that the APR results in changes in more than 200 proteins and is defined as those in which there is a minimum 25% change in concentration.33, 34 APP are categorized as positive or negative. Positive APP increase in concentration and include most of the described proteins. Negative APP decrease in concentration and include albumin and transferrin (in nonavian species). APP are further classified on the basis of the magnitude of their increase.3, 8, 35 Major APP represent those proteins that increase 10- to 1000-fold; most have negligible levels in normal animals. These are often the first responders with rapid increases of large magnitude within 24–48 h of the stimulus. Moderate APP have higher basal levels than major APP and increase 5- to 10-fold with peak levels occurring 3 or more days after the stimulus. Minor APP increase slowly with increases often less than twofold. It should be considered that these classifications are not formalized as such they are dependent on both the type of assay performed and the type of stimulus. In various reviews, there are often discordant classifications of some APP. Of note, the classification of APP by these categories differs by species; this will be further discussed in Section IV. In many cases, the biological activities of the APP are only partially known. Table I provides a list of the most commonly referenced APP.
A. C-Reactive Protein
C-reactive protein (CRP) was the first APP to be identified in the blood of humans and nonhuman primates (NHP) infected with Streptococcus pneumoniae. The “C” fraction (C polysaccharide) of the bacteria was found to react with CRP.36 It is one of the most well-known APP given its use in humans after myocardial infarction.37 As CRP has become more understood, the diagnostic utility has grown to include wellness assessments on clinically normal humans as well as patients with underlying diseases including diabetes and coronary heart disease.14, 38, 39
CRP is composed of five subunits combining to form a pentameric structure.40 It acts as an opsonin binding polysaccharide residues on bacteria, fungi, and parasites to activate complement and phagocytosis.41 CRP has also been described to result in the induction of cytokines and interact with Fc-gamma receptors prompting the hypothesis that CRP is also interactive in the adaptive immune response.42 Serum amyloid P (SAP), a major APP of mice, is considered to have biological functions and structural similarities to CRP.43
CRP is a major APP of the canine species and a canine-specific ELISA is commercially available.44 Recently, a human immunoturbidimetric assay for CRP has been validated for use with canine sera.45 This appears to be manufacturer specific as many other human CRP reagents fail to provide any cross-reactivity.44, 46
B. Serum Amyloid A
SAA is normally found complexed with lipoproteins and different isoforms have been described with numbers varying by species.47 SAA represents one of the most conserved proteins among mammals supporting the premise that it has a basic and essential role in the innate immune system. In species with CRP, SAA often mirrors the magnitude and time course of the response.48 Studies have demonstrated SAA activity in chemotaxis of leukocytes as well as the induction of additional proinflammatory cytokines.49, 50 In addition, SAA has also been described to induce extracellular matrix degrading enzymes which can assist in tissue repair.47
Assays for SAA in animals had been traditionally in the ELISA format. More recently, an immunoturbidimetric assay for human SAA was described with excellent reactivity for equine SAA.51 Similar assays have been described for use with other species.52
C. Haptoglobin
Haptoglobin (HP) is composed of 2α and 2β subunits. There are different subtypes and these may vary with species.8 HP binds free hemoglobin which may be released during various autoimmune, infectious, or inherited diseases.53 This HP–hemoglobin complex is phagocytosed by macrophages via the CD163 receptor. In mouse models, clearance is rapid with a half-life of less than 50 min.53 In HP-deficient mice, high levels of hemoglobin are found to accumulate in the kidney.54 The effective binding to CD163 has been found to stimulate cytokine production, and HP has also been linked to protease activity and immune suppression.55 Interestingly, HP is found both in mammals and in fish but not in chickens and in frogs where another hemoglobin-binding protein has been identified called PIT54.56
Assays for HP in animals are often via spectrophotometry using the natural affinity of HP for hemoglobin.57 Immunoassays for human HP which show cross-reactivity with animal HP are also available but are not widely implemented.58
D. Other APP
Alpha 1-acid glycoprotein (AGP) is a highly glycosylated protein which has the ability to bind drugs including heparin, serotonin, steroids, and histamine.59 This protein has also been implicated in anti-inflammatory responses and has been described to bind LPS.59, 60 Assays for AGP in animals are commonly performed via radial immunodiffusion making this particular APP unenviable for use as a rapid diagnostic test. A feline-specific immunoturbidimetric assay has been described.61
Fibrinogen, like HP, is present in the serum of all vertebrates and has been recognized to be part of the APR for several years. It is a large protein composed of six polypeptide chains and a glycoprotein which is converted by thrombin into fibrin and is essential in the coagulation cascade.62 Many assays are based on heat precipitation methodology.63
Ceruloplasmin (CP) is a glycoprotein which binds most of the free serum copper. In the inflammatory process, it acts as a protectant against damage by free iron which promotes free radical oxidation.64 ELISA-based assays are available for quantitation of this biomarker.
Pig major acute phase protein (Pig-MAP) is one of the main APP in swine. It is a glycoprotein that appears to be unique to this species; it functions as a trypsin inhibitor.65 ELISA methodology is validated for quantitation of this protein.66
Protease inhibitors include MAP as well as alpha 1-antitrypsin, alpha 1-antichymotrypsin, and alpha 2-macroglobulin (A2M).3 Proteases are released during damage as well as from responding neutrophils; these inhibitors are protective from immune-mediated damage.
E. Negative Acute Phase Proteins
Albumin is the major negative APP in all species. As the most abundant protein in the serum, it serves as a source of nutrients and a regulator of osmotic pressure. Decreases can be attributed to protein loss due to kidney or gastrointestinal disease or edema and also due to decreased synthesis related to liver disease or malnutrition.33 In mammals, the iron transporter transferrin (TN) is also a negative APP, although in avian species it has been shown to increase with the APR.67, 68
F. Quantitation of APP
Given the broad range of APP, the differences which occur at the species levels (see Section IV), and the rapid changes in technology over the past 25 years, it is understandable that many of the publications referenced in this review have used a myriad of techniques to assess the APR. At the research level, proteomic profiling has been utilized. With the maturation of immunoassays, immunodiffusion tests, ELISA, and immunoturbidimetric assays have been employed. Where possible, colorimetric assays have also been implemented. Lastly, the APR has been broadly judged by the use of serum protein electrophoresis. This method provides quantitation of albumin and then fractions of APP which are grouped as alpha 1, alpha 2, beta, and gamma globulins based on their migration characteristics.33 Major benefits of this method are not having the need for species-specific reagents and the assessment of the complete APR—albumin and the groups of APP. Protein electrophoresis has been commonly implemented in avian species as an adjunct test to infectious disease assessments.69, 70 A negative to this assay is the limits of detection as fraction sensitivity is at the g/dL level rather than the mg/L level obtained by specific APP assays.71
APP assays have become more available to researchers and veterinarians in recent years. In many cases, labor intensive and costly ELISA-based assays have been replaced by automated assays that have heterologous cross-reactivity with many species. Examples of this type of assay include the Eiken SAA immunoturbidimetric assay (a human assay which reactive with horse and cat but not dog SAA) and the Randox reagents for CRP (a human assay which is reactive with dog CRP).45, 72 These assays afford a commercial source of reagents negating lengthy developmental time. In other cases, species-specific reagents are preferred. These include an immunoturbidimetric assay for feline AGP and a pig-MAP-specific ELISA.61, 66 These needs are based more on the unique APP in particular species which defies the routine use of human or other reagents.
The decision to use a heterologous assay is based on a validation process that ensures not only a low coefficient of variation but also reproducibility. In some cases, a species-specific standard curve can be developed with use with these cross-reactive reagents when such control reagents are commercially available.5, 45 Lacking that, patient-derived controls can be obtained through routine practice and store indefinitely to serve as assessors of different reagent lots. Recently, a four-step validation approach was proposed for these types of assays.5 These steps include assessment of analytical characteristics (i.e., linearity, imprecision), comparison of a small set samples from normal and abnormal animals, evaluation of the assay in an actual clinical setting in which sensitivity and specificity can be judged, and assessment of the clinical and research impact of the test.
Adoption of these types of methods also allows for reproducible results among different laboratories which allows for the first steps toward assay standardization. Within this very review, reference intervals have not been presented due to the diverse types of assays that have been used in the various research studies. This problem was recognized more than 10 years ago at which time a proposal was made to harmonize the types of assays performed among major international laboratories as well as to distribute a reference sample for intralaboratory testing.73 This was formalized into the development of a Concerted Action Group as well as a Colloquium of Acute Phase Proteins where assay information and research findings could be more regularly distributed.74
The positive implementation of APP assays is linked to the development of reference intervals. As discussed above, this is very much method specific but other physiological differences also have a role. CRP levels were examined over a several-day period in normal dogs, and they were found to vary more than 10-fold; no pattern of circadian rhythm was detected.75 Others reported a low biological variability in CRP, HP, AGP, and FIB in dogs76 Age has been demonstrated to influence APP production. In a rat model of LPS stimulation, significantly decreased HP and AGP were observed in aged animals.77 In dogs injected with turpentine oil, significantly higher levels of CRP were found in dogs over 3 months versus 1 month of age.78 Lower resting levels of AGP were observed in these very young dogs versus older dogs prompting the proposal that age should be considered when using and interpretating APP results.79 Similar recommendations were made for newborn dairy calves that gave high levels of APP post-birth followed by a significant decrease through day 21 and eventual stabilization.80
It should also be recognized that APP levels may also be husbandry and strain/breed related as well as reflective of altered physiology or treatment status. Germ-free mice, injected with LPS, were observed to have a lower SAA response.81 Similar differences were observed in dogs housed in clean versus dirty husbandry conditions.82 In addition, SAP low responder and higher responder strains of mice were described; this was not strongly linked to the H-2 complex.83 Differences in pig-MAP levels were also described between Large White and Meishan breeds.84 Pregnancy was also reported to alter CRP levels by nearly 10-fold in dogs.85 Additionally, glucocorticoid treatment and other drugs have described to result in a persistent HP increase.2
III. Clinical Value of APP
The origin of the APP stimulus can be infection, neoplasia, trauma, inflammation/immunologic reaction, and stress (Table II ). In general, APP increases are of high magnitude and can be detected within the first day of stimulus; this rivals other traditional markers of inflammation. In veterinary medicine, applications have been documented in diagnosis, prognosis, detection of subclinical disease, monitoring levels of stress, and viewing the progression of chronic inflammatory processes.
Table II.
Summary of Inflammatory Stimuli That Can Induce an APR
| Stimuli |
|---|
| Experimental—inflammatory agents such as turpentine oil |
| Infection—bacterial, viral, parasitic |
| Surgery and trauma |
| Other trauma including burns |
| Autoimmune diseases |
| Toxins |
| Neoplasia |
| Stress |
A. Comparison to Other Markers of APR
An experimental model of inflammation using the injection of turpentine oil in dogs provides data which demonstrate the differences among the various inflammatory markers (Fig. 2 ).86 Levels of IL-6 and TNF-α were increased within a few hours with maximal response at 12–24 h postinjection. While an early increase (marginal response) in total white blood cells (WBC) was observed, the higher magnitude response was not present until days 4–7. A gradual decrease in serum albumin was present through day 14. The maximal decrease was less than 50% of day 0 values. CRP levels were increased 400-fold by day 2 postinjection and normalized by day 14. Serum AGP was increased approximately twofold on a similar timeline with maximal levels on day 3. Similar results can be found in a study of LPS injection in a model of arthritis in horses.87 An early increase in WBC occurs within 24 h of the stimulus. SAA increases by 24 h with peak values at 48 h and elevated levels through day 5.
Fig. 2.
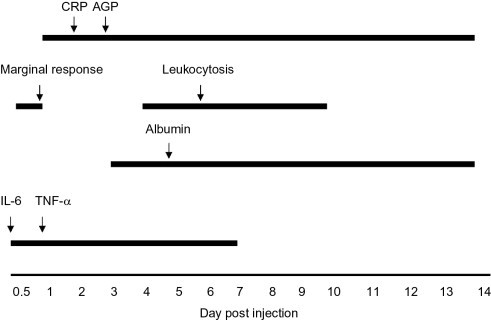
Comparison of expression of acute inflammatory biomarkers after the injection of turpentine oil in a dog. Arrows indicate time of peak expression. Horizontal lines indicate period of expression.
APP are commonly compared to WBC and fibrinogen as they are long-standing hallmarks of inflammatory processes. In a large study of samples from dogs with various inflammatory processes, only a weak correlation (r = 0.44) was observed between CRP levels and WBC counts but no correlation to the presence of band neutrophils which generally indicate bone marrow stimulation.88 In a smaller study, a poor correlation (r = 0.34) was observed between the CRP and the presence of band neutrophils.89 A positive correlation was observed between HP and FIB in dogs with various diseases.90 In a study of horses with various conditions, SAA and FIB were found to be consistently elevated in those animals with bacterial infection, whereas low or normal total WBC and neutrophil counts were observed.91
In our own laboratory, we have compared inflammatory markers in both dogs and horses with various diseases (C. Cray, 2011). The mean total WBC values, while significantly higher than the control group, did not exceed normal reference intervals for this determination. Similarly, there was a mild decrease in the A/G ratio and a mild increase in the fibrinogen between the clinical groups. Changes for all these determinations were less than twofold. Notably, a 70- and 20-fold mean increase was observed for SAA and CRP in horses and dogs, respectively. It is this magnitude of difference which makes the clinical use of APP especially attractive.5 As major APP are normally either not present or present in very low levels, increases are rapid and persistent as other minor and moderate APP are produced during later stages of inflammation.
B. Diagnosis
As APP can be increased by a myriad of different stimuli, it is difficult to state that APP, alone, can lead to a diagnosis (Table II). As discussed above, APP can provide more and earlier information regarding an ongoing inflammatory process over traditional assessments of WBC and fibrinogen. APP can also assist in providing a differential diagnosis. For example, LPS appears to produce the strongest APP response so that animals with high-magnitude APP increases can be suspect of bacterial infection. Thus, elevated APP can provide a basis for an inflammatory etiology which can aid in the development of a diagnosis and early treatment intervention.
SAA was found to differentiate the etiology of colic in horses.92 Those animals with more serious enteritis, colitis, and peritonitis had a 50- or more fold increase in SAA versus those with obstruction, perforation, and ulcers. Similarly, a disorder in cattle called traumatic reticuloperitonitis (related to foreign body ingestion) is difficult to diagnose with just a general examination and often requires radiology and surgery.93 The use of SAA and HP quantitation provided 100% sensitivity and 86% specificity. Lastly, a large study was undertaken to examine cattle with acute and chronic disorders.35 SAA levels were found to be consistently fivefold higher in acutely ill animals versus those with chronic processes and were reported to provide 100% sensitivity in the discrimination between these conditions. The slower forming HP response provided a specificity of 76%. The combined use of APP has been the subject of study, and an APP value index has been proposed for diagnosis and monitoring of clinically ill humans and animals.94
C. Prognosis
Given the high magnitude of response, duration of response, and the short half-life of APP, it is readily understood why APP are favored in prognostication. Studies regarding prognosis have included examination of mortality as well as short-term response to treatment. A large study of the general disorder colic in horses demonstrated a nearly twofold higher proportion of nonsurvivors had marked increases in SAA versus survivors.92 In another study, SAA levels from horses naturally infected with equine influenza virus were observed to be strongly associated with disease severity with eventual normalization of SAA levels with uncomplicated convalescence.95 Similar changes were observed in horses with a positive response to treatment for joint disease.96
In canine medicine, critically ill animals presenting with trauma, sepsis, or pancreatitis demonstrated elevated CRP levels, although no differences were observed between survivors and nonsurvivors.97 Using a single CRP assessment, similar results were observed in dogs diagnosed with sepsis or systemic inflammatory response syndrome.98 As expected, CRP levels decreased with recovery thus predicting survival in 94% of the patients. Acute abdomen syndrome was reported to result in up to 200-fold increases in CRP.99 Nonsurvivors were observed to have significantly higher levels of CRP than survivors.
APP levels have also been used as prognostic indicators in a variety of infections, inflammatory, and neoplastic conditions. In one study, AGP was monitored in dogs with pyometra.100 Nearly fourfold increases in AGP were observed and animals with higher AGP levels had prolonged hospitalization. CRP, SAA, AGP, and HP levels were observed to be elevated in dogs with steroid-responsive meningitis.101 Decreasing levels of APP were reported with response to treatment as well as identifying relapse. CRP was also reported to be a valuable management tool of inflammatory bowel disease, idiopathic arthritis, and autoimmune hemolytic anemia as well as post operative management.102, 103, 104, 105
Prognostic value has also been identified in neoplasia. Dogs with multicentric lymphoma demonstrated higher levels of CRP, and 90% of the animals had normal levels with remission although there was a high variability among the patients.106 In contrast, AGP levels did not serve as a prognostic marker in cats with lymphoma.107 In a mouse model of colorectal cancer with a novel immunotherapy treatment, SAA and SAP levels were higher in nonresponders.108
We have also proposed implementing APP assays as an aid to determine the humane endpoint for different animal models of disease.1 In most studies, the endpoint of an experimental model is often loosely judged on subjective measures and based on the desire of the investigator to obtain endpoint information which may often be clouded by the severe clinical disease in the animal. APP, as a reflection of stress and severe physiologic changes, may have sensitivity in determining an endpoint. Studies are underway to address this proposal.
D. Subclinical Disease
APP have been found to be useful in the detection of subclinical disease. In a large study conducted in cows, clinical signs of inflammatory processes occurred with elevated SAA in 26% of the assessments, whereas normal SAA levels were observed in clinically normal animals in 95% of the assessments.109 These results are similar to that proposing APP as indicators of calf herd health.110 The group with a higher incidence of disease and lower weight gain had significantly higher levels of HP, SAA, and FIB. Increases in HP were also observed before the onset of severe clinical disease in pigs.111 In a similar study, pig-MAP was observed to increase before the onset of postweaning multisystemic wasting syndrome.112
These concepts have led to the implementation of APP quantitation in improving food safety. Toussaint and colleagues suggested the implementation of an APP index that could be used at the slaughterhouse level.113 In another study, HP levels were compared in normal cattle versus culled animals and carcasses removed during slaughterhouse inspection due to gross abnormities.114 Higher HP levels were found in the suspected or confirmed diseased groups. Assays for pig-MAP and HP have also been adapted for use with meat juice of pigs perhaps making this a more viable test panel for the assessment of food safety.115
Our laboratory has studied the application of the herd health concept to the monitoring program of laboratory mice.71 Mice and rats in animal colonies are potentially subject to numerous viral, bacterial, and parasitic infections. Most institutions complete labor intensive and costly screening programs for each pathogen usually by specific serology and PCR detection methods. In our study, we examined mice experimentally infected with Sendai virus and mouse parvovirus and found no significant elevations in SAA, SAP, CRP, and HP. Additionally, APP levels in sentinel mice from colonies with several different endemic infections were also found to be not elevated.
E. Stress
There are several reports of the utility of APP as indicators of stress where these biomarkers rival more traditional assessments of serum cortisol and fecal corticosterone. Most of these studies have involved the use of large animals. Two breeds of cows that differed in nutrition and level of human handling were examined.116 The semi-feral cows that were subject to more nutritional stress demonstrated more than a sixfold increase in SAA. The effects of animal density in housing were also examined in pigs.117 In this study, significant increases in pig-MAP were observed in those animals subjected to a higher stocking rate for two 4-day periods over 26 days. These increases occurred after the second period and were concurrent with the detection of increased antioxidant activity in the serum. Similar changes were reported in pigs subjected to different transport conditions, cows subject to transportation and new housing, and newly weaned calves.118, 119, 120, 121 APP expression was also studied in mice after stress induced by acoustic and immobilization methods.122 CRP levels were increased nearly threefold in animals with acute stress and more than fourfold with chronic exposure.
F. Chronic Inflammation
The goal of the APR is to facilitate tissue repair and the restoration of homeostasis. Under some conditions, however, inflammatory processes are prolonged and can result in further damage. Recent terms have been coined including “metainflammatory” and “parainflammatory” and refer to the observation that chronic elevations of APP have been associated with the onset of different syndromes including atherosclerosis, cardiovascular conditions, obesity, asthma, and diabetes.123, 124 It is suggested that the classic APR results in tissue repair and the metainflammatory APR are initiated with the same mechanisms, but self-tissues are the target rather than pathogens or other inflammatory stimuli in the latter process.
CRP has been associated with cardiovascular disease in humans, and CRP levels are often used to monitor treatment regimens.125 Extravascular inflammation has been proposed as the basis for cardiac damage and the promotion of atherosclerosis, and this may be related to altered HDL particles which occur with increased SAA expression.123 Interestingly, however, a study of a genetic mouse model of atherosclerosis revealed that the recurrent exposure of mice to potent inflammatory agents did not affect the development of disease.126 In contrast, exposure of the same strain of mice to chronic unpredictable stress including temperature changes, light cycle changes, and agitation resulted in the promotion of atherosclerotic lesions as well as an elevated CRP level.127 Obesity has also been proposed as a chronic inflammatory process in animals and humans.123, 128 CRP and HP have been found to be elevated in obese dogs concurrent with insulin resistance and the development of diabetes, and APP have been proposed to be valuable monitors in case management.128 Similarly, HP expression was found to be increased in rats that later developed type 1 diabetes.129 The chronic expression of SAA has been described as the cause of systemic amyloidosis, and this overstimulation has been linked to infectious and autoimmune diseases.130 Amyloidosis has been reported in both humans and animals. The fibril protein AA is derived from the SAA and deposited in tissues as amyloid where it gradually affects tissue function. In early subclinical stages of disease, SAA levels have been reported to be elevated in NHP and genetically predisposed Abyssinian cats.131, 132
IV. APP in Animals
Major, moderate, and minor APP differ by species. This information is presented in Table III and has been drawn from several references.1, 2, 3, 4, 7, 35, 133, 134, 135, 136 Although acute inflammation and APP are common to all species, numerous studies have shown that APP do appear to have differential sensitivity to the types of diseases and disorders which are present in each species. In addition, the unique environment and usage of some species further dictate the usage of APP quantitation. For example, some animals are viewed in a clinical sense as a colony or herd (i.e., laboratory animals, cows, etc.) which create an opportunity for possible novel applications of APP assessment (see Section III.D). Many prototypic studies on animal APP have been conducted using inflammatory agents such as LPS, croton oil, and turpentine oil. These represent the gold standard of the inflammatory process and are included in the following discussion sections as well as studies of diseased animals (Table II).
Table III.
List of APP by Animal Species
| Species | Major APP | Moderate and minor APP |
|---|---|---|
| Cat | AGP, SAA | FIB, HP |
| Chicken | None | AGP, CP, FIB, HP (PIT54), SAA, TN |
| Cow | HP, SAA | AGP, CP, CRP, FIB |
| Dog | CRP, SAA | AGP, CP, FIB, HP |
| Goat | HP, SAA | AGP, FIB |
| Horse | SAA | AGP, CP, FIB, HP |
| Human | CRP, SAA | AGP, FIB, HP |
| Mouse | HP, SAA, SAP | CRP, FIB |
| Nonhuman primate | CRP | A2M, FIB, HP, SAA |
| Pig | HP, MAP, SAA | AGP,CP, CRP, FIB |
| Rabbit | CRP, HP, SAA | AGP, CP, FIB, TN |
| Rat | AGP, A2M | CP, CRP, FIB, HP |
| Sheep | HP, SAA | AGP, CP, CRP, FIB |
A. Rodents and Rabbits
As presented in the previous sections, much of what is known about APP and the APR has been examined in rodent models and transgenic mice. APP have not only been well characterized in these animals, but they also have served as biomarkers in specific animal models of disease. There are several comprehensive reviews on rodent APP.1, 134, 136
The APR of mice has been studied with the injection of the inflammatory agent LPS.48 SAA and CRP levels were found to significantly increase within 24 h; SAP and HP increased by 48 h. This was concomitant with a decrease in serum albumin. Decreasing positive APP levels were observed with the recovery of the animal (i.e., weight gain, clinical appearance) by day 4. Several models of infection have also been examined. Experimental infection with rodent infectious agents Sendai virus and mouse parvovirus was found to not induce an APR; this was also examined in mice naturally infected with several agents which commonly occur in laboratory animal colonies.48 In contrast, experimental infection with malaria was observed to result in increased SAP levels.137 SAP and HP levels were documented in an experimental model of Trypanosoma infection.138 Increases were present early postinfection as well as with the onset of posttreatment encephalopathy.
Significant increases in SAA, SAP, and HP were observed in mice subjected to 20% burn injury; these levels were not completely comparable to that induced with LPS where higher SAA levels were observed.139 Fourfold increases in HP were observed in mice subjected to lethal doses of irradiation.140 SAP was also found to be increased in a mouse model of arthritis.141 Mice injected with a hepatotoxin demonstrated very early transient increases in SAA and SAP, whereas repeated exposure with the same agent resulted in consistent increases at all time points.142 CRP increases were most pronounced within 24 h of mouse exposure to scorpion venom.143
Experimental models of acute inflammation using Freund's adjuvant (CFA) and turpentine oil revealed the major APP of rats.144, 145, 146 CRP and HP increases were observed with CFA injection, but only HP was observed to increase with a single dose of hepatotoxin.144 A marked increase in A2M was observed after intramuscular injection with turpentine oil with maximal levels by day 2 and resolution by day 6.145 With repeated turpentine oil injection, AGP levels also peaked at 2 days.146 Injection of organophosphorus compounds resulted in a twofold increase in HP, sixfold increase in A2M and FIB, and eightfold increase in AGP within 24 h.147 Thirty-fold increases in A2M were also observed in rats inoculated with Staphylococcus aureus.148 An acute septicemia model resulted in elevated CP levels, and the provision of exogenous AGP was observed to decrease the severity of the inflammatory response.149 Trauma related to surgery for castration or oophorohysterectomy induced similar levels.148
Rabbit APP studies have concentrated on experimental inflammatory stimuli. Animals subjected to turpentine oil treatment demonstrated 50-fold increases in CRP by 36 hours with resolution by 96 h.150 CP was also observed to increase. In a similar model, CRP, SAA, HP, and CP were observed to increase with a decrease in albumin.151 TN, a negative APP in most mammals, was also found to increase. Notably, both hepatic and extrahepatic SAA expressions were observed in rabbits injected with turpentine oil, LPS, and casein as well as multiple injections with silver nitrate.152, 153 APP expression has also been studied in other rabbit models. In an arthritis model, CRP was observed to increase immediately after intra-articular injection with normalization 10 days later.141 In our own studies of rabbits with suspected naturally acquired infection with Encephalitozoon cuniculi, we observed up to 30-fold increases in CRP in approximately 50% of the animals with renal, ocular, and neurological signs (C. Cray, 2011).
B. Companion Animals
Small animals including canine and feline species predominate the companion animal category. Both species are readily used in laboratory animal medicine in model development also occupy the major portion of the U.S. veterinary market with ownership of over 70 million cats and dogs.154 Although APP have been well documented in the literature, these assays are not widely used in the United States likely partly due to their poor availability. However, our laboratory has demonstrated a utility for APP quantitation in dogs in a large specialty veterinary practice. In a study of over 200 dogs, a mean increase of 20-fold and 7-fold was observed for CRP and HP, respectively (C. Cray, 2011). These represented animals with varied diseases and disorders including neoplasia, trauma, infection, and autoimmune disorders. Our data are supported by numerous reports of specific diseases and disorders, part of which are reviewed in the following paragraphs and have been previously reviewed by others.2
In a study of the intramuscular injection of turpentine oil in dogs, CRP levels were increased 400-fold by day 2 postinjection and normalization by day 14. CRP increases can certainly be observed in animals with natural infection and disease, but the magnitude of increase is not as common. In a large study of dogs (n = 928) with various diseases, CRP was found to be a major APP with a weak correlation with total WBC counts and a weak negative correlation with albumin.88 Consistently, high levels of CRP were observed in cases with pyometra, polyarthritis, pancreatitis, and panniculitis. Many metabolic diseases were observed not to have significant increases including epilepsy, hypothyroidism, rhinitis, bronchitis, and diabetes. AGP levels were examined in dogs with various diseases.79 Increases were consistently observed in dogs with acute heartworm disease, viral infections including parvovirus and distemper, pyometra, and renal failure.
Nasal disease in dogs can arise from several different etiologies. A recent report demonstrated significant increases in CRP and HP in symptomatic animals.155 Differences among APP were present among the groups with aspergillosis, chronic rhinitis, and neoplasia not only with CRP and HP but also in AGP and SAA levels. Versus other inflammatory stimuli, it is notable that all of these presentations resulted in less than an 80-fold increase in CRP.
Consistent APP increases are observed with infectious diseases in dogs. Two examples are ehrlichiosis and babesiosis which are syndromes related to infection with hematoprotozoans. In an experimental model of infection with Ehrlichia canis, the mean CRP increase was 400-fold with peak levels occurring with evidence of Ehrlichia replication and seroconversion.156 Another study detailed APP levels in naturally infected dogs. Whereas 100-fold increases in CRP were found in all infected dogs, significantly, higher levels of HP, CRP, and SAA were observed in those dogs with the more advanced myelosuppressive infection.157 In animals with naturally occurring babesiosis, CRP and CP levels were significantly increased; maximal levels were linked to disease severity.158 Naturally infected dogs with leishmaniasis, another protozoan, were also studied.159 CRP was increased 30- to 60-fold in infected animals with the highest levels present in symptomatic animals. Significant increases in HP and CP were also observed.
Several studies have been conducted examining APP expression with neoplastic disease. In a studies of dogs with lymphoma, 68% had abnormal levels of CRP.106 Both CRP and HP were increased in dogs with lymphoma, myeloma, and leukemia.160 In dogs with mammary neoplasia, significant increases in CRP, SAA, and HP were observed in only severe metastatic cases or those with ulcerated tumors.161 HP was also reported to be increased in dogs with mammary tumors, but neither HP nor CRP levels were predictive of malignancy.162
Trauma, via surgery, has been documented to increase APP.105, 163 Basic surgeries including tooth extraction and removal of superficial tumors were observed to increase CRP levels two- threefold.163 In dogs with postovariohysterectomy complications (often bacterial infections), 200-fold increases in CRP were observed.105 Whereas animals with uncomplicated surgery demonstrated an early rise and normalization by 10 days postsurgery, elevated levels were observed in those with pyometra for more than 17 days even with appropriate antibiotic treatment. Increased serum AGP levels were also found to be correlative to pyometra.100
Experimental models of trauma show similar changes. In a drug-induced model of acute gastric mucosal injury, significant increases in CRP and SAA were observed on day 1 with normalization by day 4.164 Increases in fibrinogen and HP continued through the terminal endpoint of day 7. In a model of pulmonic stenosis, approximately 30% of the animals demonstrated elevated CRP levels and were those animals that more likely had clinical signs.165 After balloon valvuloplasty, CRP increased twofold. Similar increases were observed in dogs with naturally acquired chronic valvular disease.166
Inflammatory processes in dogs may have an infectious or noninfectious etiology which is the case for canine idiopathic arthritis. CRP levels were markedly increased in all of the study group dogs with values ranging from 3- to 20-fold over healthy controls.104 CRP was also found to be increased nearly 50-fold in dogs with acute pancreatitis, a disease with a high mortality rate.167 In dogs diagnosed with autoimmune hemolytic anemia, 100-fold increases in CRP and 5-fold increases in AGP were reported.103 Steroid-responsive meningitis arteritis represents another autoimmune-based disease in dogs. CRP, SAA, AGP, and HP levels were significantly increased in all study patients.101 More than 100-fold increases in CRP and 1000-fold increases in SAA were observed.
The clinical utility of feline APP has been reviewed in other publications.2, 4 The major APP in cats include SAA and AGP, and mild to moderate HP increases are also observed. In an experimental model of either LPS or turpentine oil injection, significant increases in SAA were observed by 8 h with AGP and HP increased by 24 h postinjection.168 No changes in CRP were observed. In addition, 10-fold increases in SAA and HP and 20-fold increases in AGP were reported in cats hospitalized for various conditions. A larger study examined SAA levels in clinically ill cats and found significant increases in those animals grouped by diagnosis: infection, endocrine disease, and neoplasia.169 The highest levels were observed in the infection group. Others reported significant increases in cats with renal failure and feline lower urinary tract disease.170
Increases in AGP and HP were observed in cats with feline infectious peritonitis (FIP) and feline immunodeficiency virus as well in cats with symptoms similar to FIP.171 A moderate increase in AGP was found to discriminate FIP cats from cats with other inflammatory processes, and a marked increase was supportive of a FIP diagnosis.172 In a study of the efficacy of different diagnostic tests for FIP, AGP was reported to have 100% sensitivity and specificity.173 Other studies have indicated that the assessment of AGP in peritoneal fluid—where a common effusion occurs in acutely ill cats—is correlated with the definitive diagnosis of this infectious disease.61, 171 APP changes were also found in cats infected with feline coronavirus which can progress to FIP disease; the investigators postulated that APP other than SAA, AGP, and HP might have a role in monitoring the progression of disease.174 The APR was also examined in cats naturally infected with Chlamydophila which commonly results in ocular disease. Higher levels of SAA but not AGP were observed in seropositive animals that were actively shedding the organism.
A mean increase of 1.5-fold AGP levels was reported in cats with various malignant tumors.175 No significant differences were observed by tumor type which included carcinoma, sarcoma, and round cell tumors. Increased AGP was also consistent observed in cats with lymphoma.107 In other studies, cats were subjected to surgery to study the APR. In these animals, significant increases in SAA, AGP, and HP were all present by 24 h and persisted until day 4.168 Significant increases in SAA were also reported in cats suffering from various injuries.170 Inconsistent changes in other inflammatory diseases have been reported in cats.2, 4, 170 However, a 160-fold increase in SAA was reported in a clinical case report of pancreatitis.176 SAA was found to decrease with treatment and increase with disease recurrence.
C. Large Animals
APP have been demonstrated to be of value in management of individual cases of disease in large animal species as well as in herd management. These species include horses, cows, pigs, sheep, and goats.
The major APP in horses is SAA.135, 177, 178 Experimental stimuli including the injection of turpentine oil resulted in over 400-fold increases in SAA with the first few days of exposure.178 Twofold increases in CP were also reported.179 Marked increases were also observed in animals with clinical signs including enteritis, pneumonitis, fever, diarrhea, cellulitis, and colic.178 The etiology of the latter disease is multifactorial, and in those animals with a primary inflammatory cause, SAA levels were increased up to 500-fold.92
Experimental and natural bacterial infection has also demonstrated the utility of SAA assessments. In animals experimentally infected with Streptococcus equi in the lung, the levels were observed to increase more than 800-fold with normalization with the decrease in clinical signs.180 Variable concentrations were observed in foals naturally infected with Rhodococcus equi which is a major pathogen in horses.181 SAA was reported to increase with experimental infection with equine herpes virus more than 100-fold.177 SAA increases of 400-fold were observed in horses naturally infected with equine influenza virus; the highest values were observed during the acute phase of infection with the appearance of significant clinical signs.95
APR to surgery has been extensively studied in horses.177, 178, 182 SAA increases were reported to be rapid followed by increases in FIB and HP; levels were found useful in postoperative monitoring. AGP levels were reported to increase by day 2 and normalized in uncomplicated recovery by 28 days.183 A recent study compared the levels of SAA among horses subjected to minimal, intermediate, and extensive surgical trauma.184 SAA and FIB differed by group, whereas total WBC counts did not achieve significance.
Joint disease is a common affliction of horses, and the APR response has been studied in experimental models.87, 185 In a noninfectious arthritis model where inflammation was induced by amphotericin B, SAA increased more than 200-fold with more mild increases in HP.185 The injection of LPS resulted in similar increases in SAA in both the serum and the synovial fluid.87 In animals with naturally occurring joint diseases suspected to have bacterial origin, significant increases in SAA were also observed.96 In grass sickness, a degenerative neurological disease, several APP including AGP, A2M, HP, and CP were observed to be elevated with specific increases often dependent on clinical presentation.186 AGP and HP appeared to be linked to the onset of clinical signs.
Both SAA and HP appear to be important APP in cows.35 After challenge with LPS, more than a 30-fold increase in HP and a 1300-fold increase in SAA were reported by days 2–3.187 A transient decrease in albumin was also observed. Extensive studies have been conducted monitoring APP levels during infection.35 In a model of experimental infection with bovine respiratory syncytial virus (BRSV), strong responses in both HP and SAA were reported.188 In animals naturally infected with BRSV as well as different pathogenic bacteria, similar increases were observed with higher levels of HP when bronchoalveolar fluid was examined.189 APP expression in other infections including bovine herpes virus, foot-and-mouth disease, bovine tropical theileriosis, and bovine diarrhea virus has also been examined.35, 190 After bacterial or viral challenge, mild increases in CP were reported.191 Levels of SAA and HP have also been measured in the serum as well as the milk from cows with mastitis.192 Mild increases in CRP have also been observed in the serum.193 Physiological changes in cows were also observed to affect APP levels. Within the week following calving, significant increases in HP and SAA were observed; this was especially prevalent in cows in their first parturition.194 Lactating cows can commonly suffer from inflammatory disorders relative to the displacement of the abomasum, and some of these animals have secondary or concurrent diseases including hepatic lipidosis.195 Both SAA and HP were observed to be elevated with the displacement, and HP was most associated with the development of lipidosis. In another study involving the implantation of a chamber in the paralumbar fossa during an experimental protocol, mild AGP levels were detected after 4 days.196
In the pig, the APP have been identified as including AGP, CP, HP, CRP, SAA, TN, and pig-MAP.35 The injection of turpentine oil was observed to induce highest early response in CRP and HP with significant increases also in AGP and CP.197 APP levels were also assessed during naturally occurring infections including porcine reproductive and respiratory syndrome virus, Aujeszky's disease virus, porcine circovirus type 2, and Mycoplasma.198 HP levels were the most consistently elevated among the infections. Marked increases in CRP were observed with the circovirus and Mycoplasma infections with moderate increases in SAA and MAP. In an experimental model of bacterial infection, both hepatic and extrahepatic expressions of SAA, CRP, MAP and HP were observed with marked expression of SAA and HP in the serum.199 Animals with trauma (ear/tail bites) as well as arthritis also demonstrated high levels of SAA, CRP, MAP, and HP.198 Experimental surgery was reported to result in transient increases in SAA and HP.200 Lastly, exposure to the mycotoxin deoxynivalenol resulted in significant increases in HP and SAA after 24 h.201 This mycotoxin is produced by the Fusarium spp. of fungus which is a frequent contaminant of livestock foodstuffs.
HP was reported to be a sensitive indicator of infection in sheep.202 On the bases of postmortem diagnoses allowing for the comparison of sheep with bacterial or fungal disease versus noninfectious diseases including metabolic disorders and renal disease, HP was demonstrated to have 85% sensitivity and specificity for infectious processes. In an experimental model using intrathoracic injection of yeast, HP, CP, and FIB levels increased within the first week followed by mild decreases in albumin.203 In another study, after challenge with Corynebacterium pseudotuberculosis, SAA, HP, and AGP levels were significantly increased with a more persistent increase in the latter APP.204 CRP, HP, CP, and FIB were all increased in animals experimentally infected with Mannheimia haemolytica.205 Naturally occurring infection consistently resulted in increased FIB, HP, SAA, and AGP.206 A surgical model resulting in bronchial obstruction was utilized to study the trauma-induced APR.207 Increases were observed in sham animals, but marked increases were especially observed with HP in the obstruction group. CP and FIB increases were also present.
The APP response of goats has not been widely studied. Injection of turpentine oil was reported to result in a marked early response of SAA with significant lagging responses including HP, AGP, FIB as well as a decrease in albumin.208
D. Avian, Exotic, and Wildlife Species
While several research-based studies have documented the presence of APP in various avian, exotic, and wildlife species, APP assays have not be widely adapted for use in these species. This was the subject of a recent paper which demonstrated that several of the standardized assays for CRP, SAA, and HP appear to cross-react with APP from many species, yet samples from some species demonstrated no clinically significant differences which may be a function of the low sample size or the lack of reagent cross-reactivity.209 The information from this and other publications (discussed below) is summarized in Table IV .
Table IV.
List of APP in Avian, Exotic, and Wildlife Species
| Species | APP |
|---|---|
| Avian | AGP, CP, HP (PIT54), SAA, TN |
| Elephant | HP, SAA |
| Fish | A2M, CRP/SAP, HP, SAA, TN |
| Frog | HP (PIT54) |
| Harbor seal | CRP, HP |
| Impala | HP, SAA |
| Manatee | HP, SAA |
| Musk ox | HP, SAA |
| Nonhuman primate | CRP, HP, SAA |
| Sitatunga | HP |
| Turtle | SAA |
As with other species, each wildlife species appears to have its own APP phenotype, that is, major APP. Impala, musk ox, and chimpanzee were found to be reactive on the assay for SAA.209 The chimpanzee was also found to be reactive of the assay for CRP which is consistent with previous studies of NHP.210, 211 Treatment of NHP with recombinant IL-6 was found to result in an increase in A2M.212 HP reactivity was found in the impala, musk ox, sitatunga, and chimpanzee.209 Our laboratory has also found reactivity on the HP colorimetric assay with serum samples from diseased elephants (C. Cray, 2011). HP has been described in clinically healthy camels.213 CP levels have been reported as a marker of pregnancy in the giant panda.214 Considerably, more knowledge will be gained with increased sample size of animals with various diseases and disorders.
Studies of APP in avian species have been dominated by work with chickens.133 TN was identified as a major APP with peak levels obtained 3 days after the injection of croton oil.215 Injection of LPS revealed peak levels of AGP at 2 days after exposure.216, 217 The APR was also studied using infectious agents. Experimental infection with S. aureus resulted in increased TN and SAA, although SAA changes were more consistent and higher.218 Infection with Salmonella enteritidis resulted in a 2.5-fold increase in AGP.219 Escherichia coli inoculation was observed to increase TN, HP, and CP.215, 220 CP, but not HP, increased after inoculation of the parasite Eimeria tenella.220 Challenge with infectious bursal disease virus, a major disease problem in chickens, resulted in 2.5- to 7-fold increases in TN.215 Others reported significant increases in SAA and CP as well as AGP.221, 222 In ongoing studies in our laboratory, we have observed increased SAA, TN, and HP in other avian species undergoing inflammatory processes including parrots and penguins (C. Cray, unpublished observations).218
The evolution of APP has been the focus of several research studies in reptiles and fish. An SAA homolog was recently identified in the soft-shelled turtle.223 Turtles were infected with a Gram-negative bacterium, and SAA levels were found to increase over 1000-fold in the liver by 2 days postinfection. Smaller increases were also observed in the kidney and spleen. Decreases in albumin were also observed supporting a role for this biomarker as a negative APP.
The innate immune response of fish is a subject of an extensive review.224 CRP and SAP like biomarkers have been reported in several species of fish, and both in vivo and in vitro studies have identified SAA. Bacterial infection in trout has been described to result in a 3000-fold increase in SAA.225 Earlier studies documented the diverse inflammatory response to bacteria, virus, and experimental agents.226 Both TN and HP were identified as major APP in another teleost.227 This indicates that TN is a positive APP as observed in avian species. A2M has also been documented in fish.224
APP have also been reported in marine mammals. Harr and colleagues studied the cross-reactivity of SAA, HP, AGP, and CRP assays in serum of manatees.228 CRP and AGP assays were described to not cross-react with the test sera, although, perhaps, this indicates that these are not major APP in this species. SAA was reported to have the highest diagnostic sensitivity for inflammatory disease in this species versus FIB, HP, and total WBC. We have similarly observed SAA to be a valuable prognostic marker in stressed and injured manatees. (C. Cray, 2011). In other studies, specific CRP assays were produced for use with serum samples from harbor seals.229 Animals with pneumonia and other inflammatory processes were found to have as much as a 50-fold increase. HP in harbor seals was characterized in two recent publications.230, 231
V. Concluding Remarks
Dramatic increases in APP occur in response to inflammation and tissue injury, and this process is essential to the development of an innate immune response. The APR is conserved among different species which is a further reflection of its critical function. Although there is marked species variation among the proteins, this has not only resulted in a wealth of publications in different animals but has also facilitated the development of several species-specific and heterologous assays which can be employed to quantitate APP. The implementation of these assays has provided results that have demonstrated clear benefits at the research level with the association of APP with diagnosis, prognosis, the detection of subclinical disease, and applications to food safety. The excitement regarding the applications of APP has been measurable for years.6, 9, 232, 233 At this time, the only impediments are the need for education and acceptance of veterinary practitioners and the more ready access to these methods at the reference laboratory level. APP testing which is available today represents just the start of newly emerging biomarkers for use in animals. As technology improves and research studies ensue, a further understanding of APP and the APR in animals will result in an important step in health monitoring, understanding innate immunity, and potential therapeutic interventions of acute and chronic inflammatory diseases in animals and humans.
References
- 1.Cray C., Zaias J., Altman N.H. Acute phase response in animals: a review. Comp Med. 2009;59:517–526. [PMC free article] [PubMed] [Google Scholar]
- 2.Ceron J.J., Eckersall P.D., Martynez-Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol. 2005;34:85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 3.Murata H., Shimada N., Yoshioka M. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J. 2004;168:28–40. doi: 10.1016/S1090-0233(03)00119-9. [DOI] [PubMed] [Google Scholar]
- 4.Paltrinieri S. The feline acute phase reaction. Vet J. 2008;177:26–35. doi: 10.1016/j.tvjl.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjelgaard-Hansen M., Jacobsen S. Assay validation and diagnostic applications of major acute-phase protein testing in companion animals. Clin Lab Med. 2011;31:51–70. doi: 10.1016/j.cll.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Eckersall P.D. Acute phase proteins: from research laboratory to clinic. Vet Clin Pathol. 2010;39:1–2. doi: 10.1111/j.1939-165X.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 7.Eckersall P.D., Bell R. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J. 2010;185:23–27. doi: 10.1016/j.tvjl.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Eckersall P.D. Proteins, proteomics, and the dysproteinemias. In: Kaneko J.J., Harvey J.W., Bruss M.L., editors. Clinical biochemistry of domestic animals. 6th ed. Academic Press; San Diego, CA: 2008. pp. 117–155. [Google Scholar]
- 9.Eckersall P.D. The time is right for acute phase protein assays. Vet J. 2004;168:3–5. doi: 10.1016/j.tvjl.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Eckersall P.D. Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Rev Med Vet. 2000;151:577–584. [Google Scholar]
- 11.Eckersall P.D. Acute phase proteins as markers of inflammatory lesions. Comp Haematol Int. 1995;5:93–97. [Google Scholar]
- 12.Janeway C.A., Travers P., Walport M., Shlomchik M.J. 5th ed. Garland Publishing; New York, NY: 2001. Immunobiology, vol. 5. (732) [Google Scholar]
- 13.Yoo J.Y., Desiderio S. Innate and acquired immunity intersect in a global view of the acute-phase response. Proc Natl Acad Sci USA. 2003;100:1157–1162. doi: 10.1073/pnas.0336385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 15.Schnare M., Barton G.M., Holt A.C., Takeda K., Akira S., Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 17.Cassatella M.A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 18.Ramadori G., Sipe J.D., Colten H.R. Expression and regulation of the murine serum amyloid A (SAA) gene in extrahepatic sites. J Immunol. 1985;135:3645–3647. [PubMed] [Google Scholar]
- 19.Vreugdenhil A.C., Dentener M.A., Snoek A.M., Greve J.W., Buurman W.A. Lipopolysaccharide binding protein and serum amyloid A secretion by human intestinal epithelial cells during the acute phase response. J Immunol. 1999;163:2792–2798. [PubMed] [Google Scholar]
- 20.Vernooy J.H., Reynaert N., Wolfs T.G., Cloots R.H., Haegens A., de Vries B. Rapid pulmonary expression of acute-phase reactants after local lipopolysaccharide exposure in mice is followed by an interleukin-6 mediated systemic acute-phase response. Exp Lung Res. 2005;31:855–871. doi: 10.1080/01902140600611645. [DOI] [PubMed] [Google Scholar]
- 21.Fattori E., Cappelletti M., Costa P., Sellitto C., Cantoni L., Carelli M. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinton L.J., Jones M.R., Robson B.E., Mizgerd J.P. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect Immun. 2009;77:2417–2426. doi: 10.1128/IAI.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagihara K., Nishikawa T., Sugamata Y., Song J., Isobe T., Taga T. Essential role of STAT3 in cytokine-driven NF-kappaB-mediated serum amyloid A gene expression. Genes Cells. 2005;10:1051–1063. doi: 10.1111/j.1365-2443.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- 24.Alonzi T., Maritano D., Gorgoni B., Rizzuto G., Libert C., Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol Cell Biol. 2001;21:1621–1632. doi: 10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghetti P., Saleri R., Mocchegiani E., Corradi A., Martelli P. Infection, immunity and the neuroendocrine response. Vet Immunol Immunopathol. 2009;130:141–162. doi: 10.1016/j.vetimm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bethin K.E., Vogt S.K., Muglia L.J. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci USA. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarraf P., Frederich R.C., Turner E.M., Ma G., Jaskowiak N.T., Rivet D.J. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akarsu E.S., House R.V., Coceani F. Formation of interleukin-6 in the brain of the febrile cat: relationship to interleukin-1. Brain Res. 1998;803:137–143. doi: 10.1016/s0006-8993(98)00641-6. [DOI] [PubMed] [Google Scholar]
- 29.Coceani F., Akarsu E.S. Prostaglandin E2 in the pathogenesis of fever. An update. Ann N Y Acad Sci. 1998;856:76–82. doi: 10.1111/j.1749-6632.1998.tb08315.x. [DOI] [PubMed] [Google Scholar]
- 30.Yeager M.P., Guyre P.M., Munck A.U. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 31.Loyer P., Ilyin G., Abdel Razzak Z., Banchereau J., Dezier J.F., Campion J.P. Interleukin 4 inhibits the production of some acute-phase proteins by human hepatocytes in primary culture. FEBS Lett. 1993;336:215–220. doi: 10.1016/0014-5793(93)80806-6. [DOI] [PubMed] [Google Scholar]
- 32.Gatt M.E., Urieli-Shoval S., Preciado-Patt L., Fridkin M., Calco S., Azar Y. Effect of serum amyloid A on selected in vitro functions of isolated human neutrophils. J Lab Clin Med. 1998;132:414–420. doi: 10.1016/s0022-2143(98)90112-3. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko J.J. Serum proteins and the dysproteinemias. In: Kaneko J.J., Harvey J.W., Bruss M.L., editors. Clinical biochemistry of domestic animals. 5th ed. Academic Press; San Diego: 1997. pp. 117–138. [Google Scholar]
- 34.Kushner I., Mackiewicz A. Acute phase proteins as disease markers. Dis Markers. 1987;5:1–11. [PubMed] [Google Scholar]
- 35.Petersen H.H., Nielsen J.P., Heegaard P.M. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- 36.Tillett W.S., Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griselli M., Herbert J., Hutchinson W.L., Taylor K.M., Sohail M., Krausz T. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–1740. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenig W., Sund M., Frohlich M., Fischer H.G., Lowel H., Doring A. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 40.Thompson D., Pepys M.B., Wood S.P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7:169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 41.Mold C., Rodic-Polic B., Du Clos T.W. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J Immunol. 2002;168:6375–6381. doi: 10.4049/jimmunol.168.12.6375. [DOI] [PubMed] [Google Scholar]
- 42.Du Clos T.W., Mold C. The role of C-reactive protein in the resolution of bacterial infection. Curr Opin Infect Dis. 2001;14:289–293. doi: 10.1097/00001432-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 43.de Haas C.J. New insights into the role of serum amyloid P component, a novel lipopolysaccharide-binding protein. FEMS Immunol Med Microbiol. 1999;26:197–202. doi: 10.1111/j.1574-695X.1999.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 44.Fransson B.A., Bergstrom A., Wardrop K.J., Hagman R. Assessment of three automated assays for C-reactive protein determination in dogs. Am J Vet Res. 2007;68:1281–1286. doi: 10.2460/ajvr.68.12.1281. [DOI] [PubMed] [Google Scholar]
- 45.Kjelgaard-Hansen M., Jensen A.L., Kristensen A.T. Evaluation of a commercially available human C-reactive protein (CRP) turbidometric immunoassay for determination of canine serum CRP concentration. Vet Clin Pathol. 2003;32:81–87. doi: 10.1111/j.1939-165x.2003.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 46.Klenner S., Bauer N., Moritz A. Evaluation of three automated human immunoturbidimetric assays for the detection of C-reactive protein in dogs. J Vet Diagn Invest. 2010;22:544–552. doi: 10.1177/104063871002200408. [DOI] [PubMed] [Google Scholar]
- 47.Uhlar C.M., Whitehead A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 48.Cray C., Besselsen D.G., Hart J.L., Yoon D., Rodriguez M., Zaias J. Quantitation of acute phase proteins and protein electrophoresis in monitoring the acute inflammatory process in experimentally and naturally infected mice. Comp Med. 2010;60:263–271. [PMC free article] [PubMed] [Google Scholar]
- 49.Patel H., Fellowes R., Coade S., Woo P. Human serum amyloid A has cytokine-like properties. Scand J Immunol. 1998;48:410–418. doi: 10.1046/j.1365-3083.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- 50.Badolato R., Wang J.M., Murphy W.J., Lloyd A.R., Michiel D.F., Bausserman L.L. Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med. 1994;180:203–209. doi: 10.1084/jem.180.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobsen S., Kjelgaard-Hansen M., Hagbard Petersen H., Jensen A.L. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. Vet J. 2006;172:315–319. doi: 10.1016/j.tvjl.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Tamamoto T., Ohno K., Ohmi A., Goto-Koshino Y., Tsujimoto H. Verification of measurement of the feline serum amyloid A (SAA) concentration by human SAA turbidimetric immunoassay and its clinical application. J Vet Med Sci. 2008;70:1247–1252. doi: 10.1292/jvms.70.1247. [DOI] [PubMed] [Google Scholar]
- 53.Levy A.P., Asleh R., Blum S., Levy N.S., Miller-Lotan R., Kalet-Litman S. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12:293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 54.Fagoonee S., Gburek J., Hirsch E., Marro S., Moestrup S.K., Laurberg J.M. Plasma protein haptoglobin modulates renal iron loading. Am J Pathol. 2005;166:973–983. doi: 10.1016/S0002-9440(10)62319-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langlois M.R., Delanghe J.R. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–1600. [PubMed] [Google Scholar]
- 56.Wicher K.B., Fries E. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc Natl Acad Sci USA. 2006;103:4168–4173. doi: 10.1073/pnas.0508723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harvey J.W. Comparison between serum haptoglobin and alpha-2-globulin concentrations in dogs. Vet Clin Pathol. 1986;15:4–5. doi: 10.1111/j.1939-165x.1986.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 58.Tecles F., Subiela S.M., Petrucci G., Panizo C.G., Ceron J.J. Validation of a commercially available human immunoturbidimetric assay for haptoglobin determination in canine serum samples. Vet Res Commun. 2007;31:23–36. doi: 10.1007/s11259-006-3397-y. [DOI] [PubMed] [Google Scholar]
- 59.Fournier T., Medjoubi-N N., Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 60.Moore D.F., Rosenfeld M.R., Gribbon P.M., Winlove C.P., Tsai C.M. Alpha-1-acid (AAG, orosomucoid) glycoprotein: interaction with bacterial lipopolysaccharide and protection from sepsis. Inflammation. 1997;21:69–82. doi: 10.1023/a:1027342909423. [DOI] [PubMed] [Google Scholar]
- 61.Bence L.M., Addie D.D., Eckersall P.D. An immunoturbidimetric assay for rapid quantitative measurement of feline alpha-1-acid glycoprotein in serum and peritoneal fluid. Vet Clin Pathol. 2005;34:335–341. doi: 10.1111/j.1939-165x.2005.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 62.Mosesson M.W. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 63.Millar H.R., Simpson J.G., Stalker A.L. An evaluation of the heat precipitation method for plasma fibrinogen estimation. J Clin Pathol. 1971;24:827–830. doi: 10.1136/jcp.24.9.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roeser H.P., Lee G.R., Nacht S., Cartwright G.E. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970;49:2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lampreave F., Gonzalez-Ramon N., Martinez-Ayensa S., Hernandez M.A., Lorenzo H.K., Garcia-Gil A. Characterization of the acute phase serum protein response in pigs. Electrophoresis. 1994;15:672–676. doi: 10.1002/elps.1150150195. [DOI] [PubMed] [Google Scholar]
- 66.Pineiro M., Lampreave F., Alava M.A. Development and validation of an ELISA for the quantification of pig major acute phase protein (Pig-MAP) Vet Immunol Immunopathol. 2009;127:228–234. doi: 10.1016/j.vetimm.2008.10.318. [DOI] [PubMed] [Google Scholar]
- 67.Gomme P.T., McCann K.B., Bertolini J. Transferrin: structure, function and potential therapeutic actions. Drug Discov Today. 2005;10:267–273. doi: 10.1016/S1359-6446(04)03333-1. [DOI] [PubMed] [Google Scholar]
- 68.Tohjo H., Yadatsu M., Uchida E., Niiyama M., Syuto B., Moritsu Y. Polyacrylamide gel electrophoretic serum protein patterns of acute inflammation induced by intramuscular injection of turpentine in young broiler chickens. J Vet Med Sci. 1996;58:267–268. doi: 10.1292/jvms.58.267. [DOI] [PubMed] [Google Scholar]
- 69.Cray C., Wack A., Arheart K.L. Invalidity of albumin measurement of specimens from clinically ill birds by bromcresol green methodology. J Av Med Surg. 2011;25:14–22. doi: 10.1647/2010-004.1. [DOI] [PubMed] [Google Scholar]
- 70.Cray C., Tatum L. Application of protein electrophoresis in avian diagnostic testing. J Av Med Surg. 1998;12:4–10. [Google Scholar]
- 71.Cray C., Besselsen D.G., Hart J.L., Yoon D., Rodriguez M.R., Zaias J. Application of acute phase protein quantitation and protein electrophoresis in monitoring the acute inflammatory process in experimentally and naturally infected mice. Comp Med. 2010;60(4):263–271. [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobsen S., Kjelgaard-Hansen M., Hagbard Petersen H., Jensen A.L. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. Vet J. 2006;172:315–319. doi: 10.1016/j.tvjl.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 73.Eckersall P.D., Duthie S., Toussaint M.J., Gruys E., Heegaard P., Alava M. Standardization of diagnostic assays for animal acute phase proteins. Adv Vet Med. 1999;41:643–655. doi: 10.1016/s0065-3519(99)80050-0. [DOI] [PubMed] [Google Scholar]
- 74.Skinner J.G. International standardization of acute phase proteins. Vet Clin Pathol. 2001;30:2–7. doi: 10.1111/j.1939-165x.2001.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 75.Otabe K., Sugimoto T., Jinbo T., Honda M., Kitao S., Hayashi S. Physiological levels of C-reactive protein in normal canine sera. Vet Res Commun. 1998;22:77–85. doi: 10.1023/a:1006071211779. [DOI] [PubMed] [Google Scholar]
- 76.Kjelgaard-Hansen M., Mikkelsen L., Kristensen A.T., Jensen A.L. Study on biological variability of five acute-phase reactants in dogs. Comp Clin Pathol. 2003;12:69–74. [Google Scholar]
- 77.Gomez C.R., Acuna-Castillo C., Perez C., Leiva-Salcedo E., Riquelme D.M., Ordenes G. Diminished acute phase response and increased hepatic inflammation of aged rats in response to intraperitoneal injection of lipopolysaccharide. J Gerontol A Biol Sci Med Sci. 2008;63:1299–1306. doi: 10.1093/gerona/63.12.1299. [DOI] [PubMed] [Google Scholar]
- 78.Hayashi S., Jinbo T., Iguchi K., Shimizu M., Shimada T., Nomura M. A comparison of the concentrations of C-reactive protein and alpha1-acid glycoprotein in the serum of young and adult dogs with acute inflammation. Vet Res Commun. 2001;25:117–126. doi: 10.1023/a:1006404902214. [DOI] [PubMed] [Google Scholar]
- 79.Yuki M., Itoh H., Takase K. Serum alpha-1-acid glycoprotein concentration in clinically healthy puppies and adult dogs and in dogs with various diseases. Vet Clin Pathol. 2010;39:65–71. doi: 10.1111/j.1939-165X.2009.00181.x. [DOI] [PubMed] [Google Scholar]
- 80.Orro T., Jacobsen S., Lepage J.P., Niewold T., Alasuutari S., Soveri T. Temporal changes in serum concentrations of acute phase proteins in newborn dairy calves. Vet J. 2008;176(2):182–187. doi: 10.1016/j.tvjl.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 81.Ikeda M., Hamada K., Sumitomo N., Okamoto H., Sakakibara B. Serum amyloid A, cytokines, and corticosterone responses in germfree and conventional mice after lipopolysaccharide injection. Biosci Biotechnol Biochem. 1999;63:1006–1010. doi: 10.1271/bbb.63.1006. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto S., Tagata K., Nagahata H., Ishikawa Y., Morimatsu M., Naiki M. Isolation of canine C-reactive protein and characterization of its properties. Vet Immunol Immunopathol. 1992;30:329–339. doi: 10.1016/0165-2427(92)90103-w. [DOI] [PubMed] [Google Scholar]
- 83.Mortensen R.F., Beisel K., Zeleznik N.J., Le P.T. Acute-phase reactants of mice. II. Strain dependence of serum amyloid P-component (SAP) levels and response to inflammation. J Immunol. 1983;130:885–889. [PubMed] [Google Scholar]
- 84.Clapperton M., Bishop S.C., Pineiro M., Campbell F.M., Glass E.J. The association between plasma levels of acute phase proteins, haptoglobin, alpha-1 acid glycoprotein (AGP), pig-MAP, transthyretin and serum amyloid A (SAA) in Large White and Meishan pigs. Vet Immunol Immunopathol. 2007;119:303–309. doi: 10.1016/j.vetimm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 85.Kuribayashi T., Shimada T., Matsumoto M., Kawato K., Honjyo T., Fukuyama M. Determination of serum C-reactive protein (CRP) in healthy beagle dogs of various ages and pregnant beagle dogs. Exp Anim. 2003;52:387–390. doi: 10.1538/expanim.52.387. [DOI] [PubMed] [Google Scholar]
- 86.Yamashita K., Fujinaga T., Miyamoto T., Hagio M., Izumisawa Y., Kotani T. Canine acute phase response: relationship between serum cytokine activity and acute phase protein in dogs. J Vet Med Sci. 1994;56:487–492. doi: 10.1292/jvms.56.487. [DOI] [PubMed] [Google Scholar]
- 87.Jacobsen S., Niewold T.A., Halling-Thomsen M., Nanni S., Olsen E., Lindegaard C. Serum amyloid A isoforms in serum and synovial fluid in horses with lipopolysaccharide-induced arthritis. Vet Immunol Immunopathol. 2006;110:325–330. doi: 10.1016/j.vetimm.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 88.Nakamura M., Takahashi M., Ohno K., Koshino A., Nakashima K., Setoguchi A. C-reactive protein concentration in dogs with various diseases. J Vet Med Sci. 2008;70:127–131. doi: 10.1292/jvms.70.127. [DOI] [PubMed] [Google Scholar]
- 89.Burton S.A., Honor D.J., Mackenzie A.L., Eckersall P.D., Markham R.J., Horney B.S. C-reactive protein concentration in dogs with inflammatory leukograms. Am J Vet Res. 1994;55:613–618. [PubMed] [Google Scholar]
- 90.Solter P.F., Hoffmann W.E., Hungerford L.L., Siegel J.P., St Denis S.H., Dorner J.L. Haptoglobin and ceruloplasmin as determinants of inflammation in dogs. Am J Vet Res. 1991;52:1738–1742. [PubMed] [Google Scholar]
- 91.Hulten C., Demmers S. Serum amyloid A (SAA) as an aid in the management of infectious disease in the foal: comparison with total leucocyte count, neutrophil count and fibrinogen. Equine Vet J. 2002;34:693–698. doi: 10.2746/042516402776250360. [DOI] [PubMed] [Google Scholar]
- 92.Vandenplas M.L., Moore J.N., Barton M.H., Roussel A.J., Cohen N.D. Concentrations of serum amyloid A and lipopolysaccharide-binding protein in horses with colic. Am J Vet Res. 2005;66:1509–1516. doi: 10.2460/ajvr.2005.66.1509. [DOI] [PubMed] [Google Scholar]
- 93.Nazifi S., Ansari-Lari M., Asadi-Fardaqi J., Rezaei M. The use of receiver operating characteristic (ROC) analysis to assess the diagnostic value of serum amyloid A, haptoglobin and fibrinogen in traumatic reticuloperitonitis in cattle. Vet J. 2009;182:315–319. doi: 10.1016/j.tvjl.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Gruys E., Toussaint M.J., Niewold T.A., Koopmans S.J., van Dijk E., Meloen R.H. Monitoring health by values of acute phase proteins. Acta Histochem. 2006;108:229–232. doi: 10.1016/j.acthis.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 95.Hulten C., Sandgren B., Skioldebrand E., Klingeborn B., Marhaug G., Forsberg M. The acute phase protein serum amyloid A (SAA) as an inflammatory marker in equine influenza virus infection. Acta Vet Scand. 1999;40:323–333. doi: 10.1186/BF03547012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacobsen S., Thomsen M.H., Nanni S. Concentrations of serum amyloid A in serum and synovial fluid from healthy horses and horses with joint disease. Am J Vet Res. 2006;67:1738–1742. doi: 10.2460/ajvr.67.10.1738. [DOI] [PubMed] [Google Scholar]
- 97.Chan D.L., Rozanski E.A., Freeman L.M. Relationship among plasma amino acids, C-reactive protein, illness severity, and outcome in critically ill dogs. J Vet Intern Med. 2009;23:559–563. doi: 10.1111/j.1939-1676.2009.0296.x. [DOI] [PubMed] [Google Scholar]
- 98.Gebhardt C., Hirschberger J., Rau S., Arndt G., Krainer K., Schweigert F.J. Use of C-reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care. 2009;19:450–458. doi: 10.1111/j.1476-4431.2009.00462.x. [DOI] [PubMed] [Google Scholar]
- 99.Galezowski A.M., Snead E.C., Kidney B.A., Jackson M.L. C-reactive protein as a prognostic indicator in dogs with acute abdomen syndrome. J Vet Diagn Invest. 2010;22:395–401. doi: 10.1177/104063871002200308. [DOI] [PubMed] [Google Scholar]
- 100.Hagman R. Serum alpha-1-acid glycoprotein concentrations in 26 dogs with pyometra. Vet Clin Pathol. 2011;40:52–59. doi: 10.1111/j.1939-165X.2011.00294.x. [DOI] [PubMed] [Google Scholar]
- 101.Lowrie M., Penderis J., Eckersall P.D., McLaughlin M., Mellor D., Anderson T.J. The role of acute phase proteins in diagnosis and management of steroid-responsive meningitis arteritis in dogs. Vet J. 2009;182:125–130. doi: 10.1016/j.tvjl.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 102.Jergens A.E., Schreiner C.A., Frank D.E., Niyo Y., Ahrens F.E., Eckersall P.D. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291–297. doi: 10.1111/j.1939-1676.2003.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 103.Mitchell K.D., Kruth S.A., Wood R.D., Jefferson B. Serum acute phase protein concentrations in dogs with autoimmune hemolytic anemia. J Vet Intern Med. 2009;23:585–591. doi: 10.1111/j.1939-1676.2009.0282.x. [DOI] [PubMed] [Google Scholar]
- 104.Ohno K., Yokoyama Y., Nakashima K., Setoguchi A., Fujino Y., Tsujimoto H. C-reactive protein concentration in canine idiopathic polyarthritis. J Vet Med Sci. 2006;68:1275–1279. doi: 10.1292/jvms.68.1275. [DOI] [PubMed] [Google Scholar]
- 105.Dabrowski R., Kostro K., Lisiecka U., Szczubial M., Krakowski L. Usefulness of C-reactive protein, serum amyloid A component, and haptoglobin determinations in bitches with pyometra for monitoring early post-ovariohysterectomy complications. Theriogenology. 2009;72:471–476. doi: 10.1016/j.theriogenology.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 106.Nielsen L., Toft N., Eckersall P.D., Mellor D.J., Morris J.S. Serum C-reactive protein concentration as an indicator of remission status in dogs with multicentric lymphoma. J Vet Intern Med. 2007;21:1231–1236. doi: 10.1892/07-058.1. [DOI] [PubMed] [Google Scholar]
- 107.Correa S.S., Mauldin G.N., Mauldin G.E., Mooney S.C. Serum alpha 1-acid glycoprotein concentration in cats with lymphoma. J Am Anim Hosp Assoc. 2001;37:153–158. doi: 10.5326/15473317-37-2-153. [DOI] [PubMed] [Google Scholar]
- 108.Vafadar-Isfahani B., Laversin S.A., Ahmad M., Ball G., Coveney C., Lemetre C. Serum biomarkers which correlate with failure to respond to immunotherapy and tumor progression in a murine colorectal cancer model. Proteomics Clin Appl. 2010;4:682–696. doi: 10.1002/prca.200900218. [DOI] [PubMed] [Google Scholar]
- 109.Karreman H.J., Wentink G.H., Wensing T. Using serum amyloid A to screen dairy cows for sub-clinical inflammation. Vet Q. 2000;22:175–178. doi: 10.1080/01652176.2000.9695051. [DOI] [PubMed] [Google Scholar]
- 110.Ganheim C., Alenius S., Persson Waller K. Acute phase proteins as indicators of calf herd health. Vet J. 2007;173:645–651. doi: 10.1016/j.tvjl.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harding J.C., Baarsch M.J., Murtaugh M.P. Association of tumour necrosis factor and acute phase reactant changes with post arrival disease in swine. Zentralbl Veterinarmed B. 1997;44:405–413. doi: 10.1111/j.1439-0450.1997.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 112.Grau-Roma L., Heegaard P.M., Hjulsager C.K., Sibila M., Kristensen C.S., Allepuz A. Pig-major acute phase protein and haptoglobin serum concentrations correlate with PCV2 viremia and the clinical course of postweaning multisystemic wasting syndrome. Vet Microbiol. 2009;138:53–61. doi: 10.1016/j.vetmic.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Toussaint M.J., van Ederen A.M., Gruys E. Implication of clinical pathology in assessment of animal health and in animal production and meat inspection. Comp Haematol Int. 1995;5:149–157. [Google Scholar]
- 114.Saini P.K., Riaz M., Webert D.W., Eckersall P.D., Young C.R., Stanker L.H. Development of a simple enzyme immunoassay for blood haptoglobin concentration in cattle and its application in improving food safety. Am J Vet Res. 1998;59:1101–1107. [PubMed] [Google Scholar]
- 115.Pineiro M., Gymnich S., Knura S., Pineiro C., Petersen B. Meat juice: an alternative matrix for assessing animal health by measuring acute phase proteins. Correlations of pig-MAP and haptoglobin concentrations in pig meat juice and plasma. Res Vet Sci. 2009;87:273–276. doi: 10.1016/j.rvsc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Saco Y., Fina M., Gimenez M., Pato R., Piedrafita J., Bassols A. Evaluation of serum cortisol, metabolic parameters, acute phase proteins and faecal corticosterone as indicators of stress in cows. Vet J. 2008;177:439–441. doi: 10.1016/j.tvjl.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 117.Marco-Ramell A., Pato R., Pena R., Saco Y., Manteca X., Ruiz de la Torre J.L. Identification of serum stress biomarkers in pigs housed at different stocking densities. Vet J. 2011 doi: 10.1016/j.tvjl.2011.01.003. doi:10.1016/j.tvjl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 118.Pineiro M., Pineiro C., Carpintero R., Morales J., Campbell F.M., Eckersall P.D. Characterisation of the pig acute phase protein response to road transport. Vet J. 2007;173:669–674. doi: 10.1016/j.tvjl.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Hickey M.C., Drennan M., Earley B. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J Anim Sci. 2003;81:2847–2855. doi: 10.2527/2003.81112847x. [DOI] [PubMed] [Google Scholar]
- 120.Arthington J.D., Eichert S.D., Kunkle W.E., Martin F.G. Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J Anim Sci. 2003;81:1120–1125. doi: 10.2527/2003.8151120x. [DOI] [PubMed] [Google Scholar]
- 121.Lomborg S.R., Nielsen L.R., Heegaard P.M., Jacobsen S. Acute phase proteins in cattle after exposure to complex stress. Vet Res Commun. 2008;32:575–582. doi: 10.1007/s11259-008-9057-7. [DOI] [PubMed] [Google Scholar]
- 122.Depke M., Steil L., Domanska G., Volker U., Schutt C., Kiank C. Altered hepatic mRNA expression of immune response and apoptosis-associated genes after acute and chronic psychological stress in mice. Mol Immunol. 2009;46:3018–3028. doi: 10.1016/j.molimm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 123.Venteclef N., Jakobsson T., Steffensen K.R., Treuter E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol Metab. 2011;22(8):333–343. doi: 10.1016/j.tem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 124.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 125.Correale M., Brunetti N.D., De Gennaro L., Di Biase M. Acute phase proteins in atherosclerosis (acute coronary syndrome) Cardiovasc Hematol Agents Med Chem. 2008;6:272–277. doi: 10.2174/187152508785909537. [DOI] [PubMed] [Google Scholar]
- 126.Ko K.W., Corry D.B., Brayton C.F., Paul A., Chan L. Extravascular inflammation does not increase atherosclerosis in apoE-deficient mice. Biochem Biophys Res Commun. 2009;384:93–99. doi: 10.1016/j.bbrc.2009.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang T., Chen Y., Liu H., Zhou Z., Zhai Y., Yang J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thromb Res. 2010;126:386–392. doi: 10.1016/j.thromres.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 128.German A.J., Ryan V.H., German A.C., Wood I.S., Trayhurn P. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J. 2010;185:4–9. doi: 10.1016/j.tvjl.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 129.Kruger A.J., Yang C., Tam S.W., Hinerfeld D., Evans J.E., Green K.M. Haptoglobin as an early serum biomarker of virus-induced autoimmune type 1 diabetes in biobreeding diabetes resistant and LEW1.WR1 rats. Exp Biol Med (Maywood) 2010;235:1328–1337. doi: 10.1258/ebm.2010.010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Westermark G.T., Westermark P. Serum amyloid A and protein AA: molecular mechanisms of a transmissible amyloidosis. FEBS Lett. 2009;583:2685–2690. doi: 10.1016/j.febslet.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 131.DiBartola S.P., Reiter J.A., Cornacoff J.B., Kociba G.J., Benson M.D. Serum amyloid A protein concentration measured by radial immunodiffusion in Abyssinian and non-Abyssinian cats. Am J Vet Res. 1989;50:1414–1417. [PubMed] [Google Scholar]
- 132.MacGuire J.G., Christe K.L., Yee J.L., Kalman-Bowlus A.L., Lerche N.W. Serologic evaluation of clinical and subclinical secondary hepatic amyloidosis in rhesus macaques (Macaca mulatta) Comp Med. 2009;59:168–173. [PMC free article] [PubMed] [Google Scholar]
- 133.Chamanza R., van Veen L., Tivapasi M.T., Toussaint M.J. Acute phase proteins in the domestic fowl. Worlds Poult Sci J. 1999;55:61–71. [Google Scholar]
- 134.French T. Acute phase proteins. In: Loeb W.F., Quimby F.W., editors. The clinical chemistry of laboratory animals. Pergamon Press; Oxford: 1989. pp. 201–235. [Google Scholar]
- 135.Jacobsen S., Andersen P.H. The acute phase protein serum amyloid A (SAA) as a marker of inflammation in horses. Eq Vet Educ. 2007;19:38–46. [Google Scholar]
- 136.Schreiber G., Tsykin A., Aldred A.R., Thomas T., Fung W.P., Dickson P.W. The acute phase response in the rodent. Ann N Y Acad Sci. 1989;557:61–85. doi: 10.1111/j.1749-6632.1989.tb24000.x. [discussion 85–6] [DOI] [PubMed] [Google Scholar]
- 137.Balmer P., McMonagle F., Alexander J., Phillips R.S. Experimental erythrocytic malaria infection induces elevated levels of serum amyloid P production in mice. Immunol Lett. 2000;72:147–152. doi: 10.1016/s0165-2478(00)00180-2. [DOI] [PubMed] [Google Scholar]
- 138.Eckersall P.D., Gow J.W., McComb C., Bradley B., Rodgers J., Murray M. Cytokines and the acute phase response in post-treatment reactive encephalopathy of Trypanosoma brucei brucei infected mice. Parasitol Int. 2001;50:15–26. doi: 10.1016/s1383-5769(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 139.Duan X., Yarmush D.M., Berthiaume F., Jayaraman A., Yarmush M.L. A mouse serum two-dimensional gel map: application to profiling burn injury and infection. Electrophoresis. 2004;25:3055–3065. doi: 10.1002/elps.200406039. [DOI] [PubMed] [Google Scholar]
- 140.Burger M., Knyszynski A. Haptoglobin and irradiation in mice. Radiat Res. 1971;46:613–620. [PubMed] [Google Scholar]
- 141.Hunneyball I.M., Spowage M., Crossley M.J., Rowe I.F., Baltz M.L. Acute phase protein changes in antigen-induced mono-articular arthritis in rabbits and mice. Clin Exp Immunol. 1986;65:311–318. [PMC free article] [PubMed] [Google Scholar]
- 142.Lockwood J.F., Rutherford M.S., Myers M.J., Schook L.B. Induction of hepatic acute-phase protein transcripts: differential effects of acute and subchronic dimethylnitrosamine exposure in vivo. Toxicol Appl Pharmacol. 1994;125:288–295. doi: 10.1006/taap.1994.1075. [DOI] [PubMed] [Google Scholar]
- 143.Pessini A.C., de Souza A.M., Faccioli L.H., Gregorio Z.M., Arantes E.C. Time course of acute-phase response induced by Tityus serrulatus venom and TsTX-I in mice. Int Immunopharmacol. 2003;3:765–774. doi: 10.1016/S1567-5769(03)00078-X. [DOI] [PubMed] [Google Scholar]
- 144.Giffen P.S., Turton J., Andrews C.M., Barrett P., Clarke C.J., Fung K.W. Markers of experimental acute inflammation in the Wistar Han rat with particular reference to haptoglobin and C-reactive protein. Arch Toxicol. 2003;77:392–402. doi: 10.1007/s00204-003-0458-7. [DOI] [PubMed] [Google Scholar]
- 145.Jinbo T., Sakamoto T., Yamamoto S. Serum alpha2-macroglobulin and cytokine measurements in an acute inflammation model in rats. Lab Anim. 2002;36:153–157. doi: 10.1258/0023677021912433. [DOI] [PubMed] [Google Scholar]
- 146.Honjo T., Kuribayashi T., Matsumoto M., Yamazaki S., Yamamoto S. Kinetics of {alpha}2-macroglobulin and {alpha}1-acid glycoprotein in rats subjected to repeated acute inflammatory stimulation. Lab Anim. 2010;44:150–154. doi: 10.1258/la.2009.009042. [DOI] [PubMed] [Google Scholar]
- 147.Ivanovic-Matic S., Poznanovic G., Grigorov I., Dinic S., Mihailovic M., Grdovic N. The organophosphate-induced acute-phase response is characterized by synthesis of alpha 1-acid glycoprotein that exhibits an immunomodulatory effect. J Appl Toxicol. 2008;28:63–71. doi: 10.1002/jat.1254. [DOI] [PubMed] [Google Scholar]
- 148.Jinbo T., Motoki M., Yamamoto S. Variation of serum alpha2-macroglobulin concentration in healthy rats and rats inoculated with Staphylococcus aureus or subjected to surgery. Comp Med. 2001;51:332–335. [PubMed] [Google Scholar]
- 149.Osikov M.V., Makarov E.V., Krivokhizhina L.V. Effects of alpha1-acid glycoprotein on hemostasis in experimental septic peritonitis. Bull Exp Biol Med. 2007;144:178–180. doi: 10.1007/s10517-007-0283-8. [DOI] [PubMed] [Google Scholar]
- 150.Giclas P.C., Manthei U., Strunk R.C. The acute phase response of C3, C5, ceruloplasmin, and C-reactive protein induced by turpentine pleurisy in the rabbit. Am J Pathol. 1985;120:146–156. [PMC free article] [PubMed] [Google Scholar]
- 151.Mackiewicz A., Ganapathi M.K., Schultz D., Samols D., Reese J., Kushner I. Regulation of rabbit acute phase protein biosynthesis by monokines. Biochem J. 1988;253:851–857. doi: 10.1042/bj2530851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rygg M., Husby G., Marhaug G. Differential expression of rabbit serum amyloid A genes in response to various inflammatory agents. Scand J Immunol. 1993;38:417–422. doi: 10.1111/j.1365-3083.1993.tb02582.x. [DOI] [PubMed] [Google Scholar]
- 153.Ray A., Ray B.K. Persistent expression of serum amyloid A during experimentally induced chronic inflammatory condition in rabbit involves differential activation of SAF, NF-kappa B, and C/EBP transcription factors. J Immunol. 1999;163:2143–2150. [PubMed] [Google Scholar]
- 154.American Veterinary Medical Association . 2007 ed. AVMA; Schaumburg, IL: 2007. U.S. Pet Ownership & Demographics Sourcebook. [Google Scholar]
- 155.Sheahan D., Bell R., Mellanby R.J., Gow A.G., Friend E., Heller J. Acute phase protein concentrations in dogs with nasal disease. Vet Rec. 2010;167:895–899. doi: 10.1136/vr.c5928. [DOI] [PubMed] [Google Scholar]
- 156.Shimada T., Ishida Y., Shimizu M., Nomura M., Kawato K., Iguchi K. Monitoring C-reactive protein in beagle dogs experimentally inoculated with Ehrlichia canis. Vet Res Commun. 2002;26:171–177. doi: 10.1023/a:1015290903332. [DOI] [PubMed] [Google Scholar]
- 157.Mylonakis M.E., Ceron J.J., Leontides L., Siarkou V.I., Martinez S., Tvarijonaviciute A. Serum acute phase proteins as clinical phase indicators and outcome predictors in naturally occurring canine monocytic ehrlichiosis. J Vet Intern Med. 2011;25(4):811–817. doi: 10.1111/j.1939-1676.2011.0728.x. [DOI] [PubMed] [Google Scholar]
- 158.Ulutas B., Bayramli G., Ulutas P.A., Karagenc T. Serum concentration of some acute phase proteins in naturally occurring canine babesiosis: a preliminary study. Vet Clin Pathol. 2005;34:144–147. doi: 10.1111/j.1939-165x.2005.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 159.Martinez-Subiela S., Tecles F., Eckersall P.D., Ceron J.J. Serum concentrations of acute phase proteins in dogs with leishmaniasis. Vet Rec. 2002;150:241–244. doi: 10.1136/vr.150.8.241. [DOI] [PubMed] [Google Scholar]
- 160.Mischke R., Waterston M., Eckersall P.D. Changes in C-reactive protein and haptoglobin in dogs with lymphatic neoplasia. Vet J. 2007;174:188–192. doi: 10.1016/j.tvjl.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 161.Tecles F., Caldin M., Zanella A., Membiela F., Tvarijonaviciute A., Subiela S.M. Serum acute phase protein concentrations in female dogs with mammary tumors. J Vet Diagn Invest. 2009;21:214–219. doi: 10.1177/104063870902100206. [DOI] [PubMed] [Google Scholar]
- 162.Planellas M., Bassols A., Siracusa C., Saco Y., Gimenez M., Pato R. Evaluation of serum haptoglobin and C-reactive protein in dogs with mammary tumors. Vet Clin Pathol. 2009;38:348–352. doi: 10.1111/j.1939-165X.2009.00139.x. [DOI] [PubMed] [Google Scholar]
- 163.Yamamoto S., Shida T., Miyaji S., Santsuka H., Fujise H., Mukawa K. Changes in serum C-reactive protein levels in dogs with various disorders and surgical traumas. Vet Res Commun. 1993;17:85–93. doi: 10.1007/BF01839236. [DOI] [PubMed] [Google Scholar]
- 164.Bayramli G., Ulutas B. Acute phase protein response in dogs with experimentally induced gastric mucosal injury. Vet Clin Pathol. 2008;37:312–316. doi: 10.1111/j.1939-165X.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- 165.Saunders A.B., Smith B.E., Fosgate G.T., Suchodolski J.S., Steiner J.M. Cardiac troponin I and C-reactive protein concentrations in dogs with severe pulmonic stenosis before and after balloon valvuloplasty. J Vet Cardiol. 2009;11:9–16. doi: 10.1016/j.jvc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 166.Rush J.E., Lee N.D., Freeman L.M., Brewer B. C-reactive protein concentration in dogs with chronic valvular disease. J Vet Intern Med. 2006;20:635–639. doi: 10.1892/0891-6640(2006)20[635:cpcidw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 167.Mansfield C.S., James F.E., Robertson I.D. Development of a clinical severity index for dogs with acute pancreatitis. J Am Vet Med Assoc. 2008;233:936–944. doi: 10.2460/javma.233.6.936. [DOI] [PubMed] [Google Scholar]
- 168.Kajikawa T., Furuta A., Onishi T., Tajima T., Sugii S. Changes in concentrations of serum amyloid A protein, alpha 1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Vet Immunol Immunopathol. 1999;68:91–98. doi: 10.1016/s0165-2427(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 169.Hansen A.E., Schaap M.K., Kjelgaard-Hansen M. Evaluation of a commercially available human serum amyloid A (SAA) turbidimetric immunoassay for determination of feline SAA concentration. Vet Res Commun. 2006;30:863–872. doi: 10.1007/s11259-006-3373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sasaki K., Ma Z., Khatlani T.S., Okuda M., Inokuma H., Onishi T. Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. J Vet Med Sci. 2003;65:545–548. doi: 10.1292/jvms.65.545. [DOI] [PubMed] [Google Scholar]
- 171.Duthie S., Eckersall P.D., Addie D.D., Lawrence C.E., Jarrett O. Value of alpha 1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet Rec. 1997;141:299–303. doi: 10.1136/vr.141.12.299. [DOI] [PubMed] [Google Scholar]
- 172.Paltrinieri S., Giordano A., Tranquillo V., Guazzetti S. Critical assessment of the diagnostic value of feline alpha1-acid glycoprotein for feline infectious peritonitis using the likelihood ratios approach. J Vet Diagn Invest. 2007;19:266–272. doi: 10.1177/104063870701900306. [DOI] [PubMed] [Google Scholar]
- 173.Giori L., Giordano A., Giudice C., Grieco V., Paltrinieri S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J Small Anim Pract. 2011;52:152–157. doi: 10.1111/j.1748-5827.2011.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Giordano A., Spagnolo V., Colombo A., Paltrinieri S. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Vet J. 2004;167:38–44. doi: 10.1016/S1090-0233(03)00055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Selting K.A., Ogilvie G.K., Lana S.E., Fettman M.J., Mitchener K.L., Hansen R.A. Serum alhpa 1-acid glycoprotein concentrations in healthy and tumor-bearing cats. J Vet Intern Med. 2000;14:503–506. doi: 10.1892/0891-6640(2000)014<0503:sagcih>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 176.Tamamoto T., Ohno K., Ohmi A., Seki I., Tsujimoto H. Time-course monitoring of serum amyloid A in a cat with pancreatitis. Vet Clin Pathol. 2009;38:83–86. doi: 10.1111/j.1939-165X.2008.00082.x. [DOI] [PubMed] [Google Scholar]
- 177.Pepys M.B., Baltz M.L., Tennent G.A., Kent J., Ousey J., Rossdale P.D. Serum amyloid A protein (SAA) in horses: objective measurement of the acute phase response. Equine Vet J. 1989;21:106–109. doi: 10.1111/j.2042-3306.1989.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 178.Nunokawa Y., Fujinaga T., Taira T., Okumura M., Yamashita K., Tsunoda N. Evaluation of serum amyloid A protein as an acute-phase reactive protein in horses. J Vet Med Sci. 1993;55:1011–1016. doi: 10.1292/jvms.55.1011. [DOI] [PubMed] [Google Scholar]
- 179.Okumura M., Fujinaga T., Yamashita K., Tsunoda N., Mizuno S. Isolation, characterization, and quantitative analysis of ceruloplasmin from horses. Am J Vet Res. 1991;52:1979–1985. [PubMed] [Google Scholar]
- 180.Hobo S., Niwa H., Anzai T. Evaluation of serum amyloid A and surfactant protein D in sera for identification of the clinical condition of horses with bacterial pneumonia. J Vet Med Sci. 2007;69:827–830. doi: 10.1292/jvms.69.827. [DOI] [PubMed] [Google Scholar]
- 181.Cohen N.D., Chaffin M.K., Vandenplas M.L., Edwards R.F., Nevill M., Moore J.N. Study of serum amyloid A concentrations as a means of achieving early diagnosis of Rhodococcus equi pneumonia. Equine Vet J. 2005;37:212–216. doi: 10.2746/0425164054530704. [DOI] [PubMed] [Google Scholar]
- 182.Jacobsen S., Jensen J.C., Frei S., Jensen A.L., Thoefner M.B. Use of serum amyloid A and other acute phase reactants to monitor the inflammatory response after castration in horses: a field study. Equine Vet J. 2005;37:552–556. doi: 10.2746/042516405775314853. [DOI] [PubMed] [Google Scholar]
- 183.Taira T., Fujinaga T., Tamura K., Izumi M., Itoh H., Tsunoda N. Isolation and characterization of alpha 1-acid glycoprotein from horses, and its evaluation as an acute-phase reactive protein in horses. Am J Vet Res. 1992;53:961–965. [PubMed] [Google Scholar]
- 184.Jacobsen S., Nielsen J.V., Kjelgaard-Hansen M., Toelboell T., Fjeldborg J., Halling-Thomsen M. Acute phase response to surgery of varying intensity in horses: a preliminary study. Vet Surg. 2009;38:762–769. doi: 10.1111/j.1532-950X.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 185.Hulten C., Gronlund U., Hirvonen J., Tulamo R.M., Suominen M.M., Marhaug G. Dynamics in serum of the inflammatory markers serum amyloid A (SAA), haptoglobin, fibrinogen and alpha2-globulins during induced noninfectious arthritis in the horse. Equine Vet J. 2002;34:699–704. doi: 10.2746/042516402776250405. [DOI] [PubMed] [Google Scholar]
- 186.Milne E.M., Doxey D.L., Kent J.E., Pemberton A. Acute phase proteins in grass sickness (equine dysautonomia) Res Vet Sci. 1991;50:273–278. doi: 10.1016/0034-5288(91)90123-6. [DOI] [PubMed] [Google Scholar]
- 187.Jacobsen S., Andersen P.H., Toelboell T., Heegaard P.M. Dose dependency and individual variability of the lipopolysaccharide-induced bovine acute phase protein response. J Dairy Sci. 2004;87:3330–3339. doi: 10.3168/jds.S0022-0302(04)73469-4. [DOI] [PubMed] [Google Scholar]
- 188.Heegaard P.M., Godson D.L., Toussaint M.J., Tjornehoj K., Larsen L.E., Viuff B. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet Immunol Immunopathol. 2000;77:151–159. doi: 10.1016/S0165-2427(00)00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Angen O., Thomsen J., Larsen L.E., Larsen J., Kokotovic B., Heegaard P.M. Respiratory disease in calves: microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet Microbiol. 2009;137:165–171. doi: 10.1016/j.vetmic.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nazifi S., Razavi S.M., Esmailnejad Z., Gheisari H. Study on acute phase proteins (haptoglobin, serum amyloid A, fibrinogen, and ceruloplasmin) changes and their diagnostic values in bovine tropical theileriosis. Parasitol Res. 2009;105:41–46. doi: 10.1007/s00436-009-1360-x. [DOI] [PubMed] [Google Scholar]
- 191.Stabel J.R., Spears J.W., Brown T.T., Jr. Effect of copper deficiency on tissue, blood characteristics, and immune function of calves challenged with infectious bovine rhinotracheitis virus and Pasteurella hemolytica. J Anim Sci. 1993;71:1247–1255. doi: 10.2527/1993.7151247x. [DOI] [PubMed] [Google Scholar]
- 192.Eckersall P.D., Young F.J., McComb C., Hogarth C.J., Safi S., Weber A. Acute phase proteins in serum and milk from dairy cows with clinical mastitis. Vet Rec. 2001;148:35–41. doi: 10.1136/vr.148.2.35. [DOI] [PubMed] [Google Scholar]
- 193.Lee W.C., Hsiao H.C., Wu Y.L., Lin J.H., Lee Y.P., Fung H.P. Serum C-reactive protein in dairy herds. Can J Vet Res. 2003;67:102–107. [PMC free article] [PubMed] [Google Scholar]
- 194.Humblet M.F., Guyot H., Boudry B., Mbayahi F., Hanzen C., Rollin F. Relationship between haptoglobin, serum amyloid A, and clinical status in a survey of dairy herds during a 6-month period. Vet Clin Pathol. 2006;35:188–193. doi: 10.1111/j.1939-165x.2006.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 195.Guzelbektes H., Sen I., Ok M., Constable P.D., Boydak M., Coskun A. Serum amyloid A and haptoglobin concentrations and liver fat percentage in lactating dairy cows with abomasal displacement. J Vet Intern Med. 2010;24:213–219. doi: 10.1111/j.1939-1676.2009.0444.x. [DOI] [PubMed] [Google Scholar]
- 196.Walker J.L., Clarke C.R., Lessley B.A., Hague C.M. Effect of Pasteurella haemolytica infection on alpha 1-acid glycoprotein and albumin concentrations in serum and subcutaneous tissue chamber fluid of calves. Res Vet Sci. 1994;56:158–163. doi: 10.1016/0034-5288(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 197.Eckersall P.D., Saini P.K., McComb C. The acute phase response of acid soluble glycoprotein, alpha(1)-acid glycoprotein, ceruloplasmin, haptoglobin and C-reactive protein, in the pig. Vet Immunol Immunopathol. 1996;51:377–385. doi: 10.1016/0165-2427(95)05527-4. [DOI] [PubMed] [Google Scholar]
- 198.Parra M.D., Fuentes P., Tecles F., Martinez-Subiela S., Martinez J.S., Munoz A. Porcine acute phase protein concentrations in different diseases in field conditions. J Vet Med B Infect Dis Vet Public Health. 2006;53:488–493. doi: 10.1111/j.1439-0450.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 199.Skovgaard K., Mortensen S., Boye M., Poulsen K.T., Campbell F.M., Eckersall P.D. Rapid and widely disseminated acute phase protein response after experimental bacterial infection of pigs. Vet Res. 2009;40:23. doi: 10.1051/vetres/2009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jacobson M., Lindberg J.E., Lindberg R., Segerstad C.H., Wallgren P., Fellstrom C. Intestinal cannulation: model for study of the midgut of the pig. Comp Med. 2001;51:163–170. [PubMed] [Google Scholar]
- 201.Mikami O., Kubo M., Murata H., Muneta Y., Nakajima Y., Miyazaki S. The effects of acute exposure to deoxynivalenol on some inflammatory parameters in miniature pigs. J Vet Med Sci. 2011;73:665–671. doi: 10.1292/jvms.10-0461. [DOI] [PubMed] [Google Scholar]
- 202.Skinner J.G., Roberts L. Haptoglobin as an indicator of infection in sheep. Vet Rec. 1994;134:33–36. doi: 10.1136/vr.134.2.33. [DOI] [PubMed] [Google Scholar]
- 203.Pfeffer A., Rogers K.M., O'Keeffe L., Osborn P.J. Acute phase protein response, food intake, liveweight change and lesions following intrathoracic injection of yeast in sheep. Res Vet Sci. 1993;55:360–366. doi: 10.1016/0034-5288(93)90108-r. [DOI] [PubMed] [Google Scholar]
- 204.Eckersall P.D., Lawson F.P., Bence L., Waterston M.M., Lang T.L., Donachie W. Acute phase protein response in an experimental model of ovine caseous lymphadenitis. BMC Vet Res. 2007;3:35. doi: 10.1186/1746-6148-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ulutas P.A., Ozpinar A. Effect of Mannheimia (Pasteurella) haemolytica infection on acute-phase proteins and some mineral levels in colostrum-breast milk-fed or colostrum-breast milk-deprived sheep. Vet Res Commun. 2006;30:485–495. doi: 10.1007/s11259-006-3246-z. [DOI] [PubMed] [Google Scholar]
- 206.Nikunen S., Hartel H., Orro T., Neuvonen E., Tanskanen R., Kivela S.L. Association of bovine respiratory disease with clinical status and acute phase proteins in calves. Comp Immunol Microbiol Infect Dis. 2007;30:143–151. doi: 10.1016/j.cimid.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pfeffer A., Rogers K.M. Acute phase response of sheep: changes in the concentrations of ceruloplasmin, fibrinogen, haptoglobin and the major blood cell types associated with pulmonary damage. Res Vet Sci. 1989;46:118–124. [PubMed] [Google Scholar]
- 208.Gonzalez F.H., Tecles F., Martinez-Subiela S., Tvarijonaviciute A., Soler L., Ceron J.J. Acute phase protein response in goats. J Vet Diagn Invest. 2008;20:580–584. doi: 10.1177/104063870802000507. [DOI] [PubMed] [Google Scholar]
- 209.Bertelsen M.F., Kjelgaard-Hansen M., Grondahl C., Heegaard P.M., Jacobsen S. Identification of acute phase proteins and assays applicable in nondomesticated mammals. J Zoo Wildl Med. 2009;40:199–203. doi: 10.1638/2007-0125.1. [DOI] [PubMed] [Google Scholar]
- 210.Jinbo T., Ami Y., Suzaki Y., Kobune F., Ro S., Naiki M. Concentrations of C-reactive protein in normal monkeys (Macaca irus) and in monkeys inoculated with Bordetella bronchiseptica R-5 and measles virus. Vet Res Commun. 1999;23:265–274. doi: 10.1023/a:1006388602364. [DOI] [PubMed] [Google Scholar]
- 211.De Pablos V., Barcia C., Martinez S., Gomez A., Ros-Bernal F., Zamarro-Parra J. MPTP administration increases plasma levels of acute phase proteins in non-human primates (Macaca fascicularis) Neurosci Lett. 2009;463:37–39. doi: 10.1016/j.neulet.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 212.Myers L.A., Boyce J.T., Robison R.L. The tolerability and pharmacology of interleukin-6 administered in combination with GM-CSF or G-CSF in the rhesus monkey. Toxicology. 1995;101:157–166. doi: 10.1016/0300-483x(95)03081-p. [DOI] [PubMed] [Google Scholar]
- 213.Nazifi S., Saeb M., Ghasemian O., Esmailnezhad Z. Evaluation of serum haptoglobin in clinically healthy Iranian camels (Camelus dromedarius) Comp Clin Pathol. 2006;15:195–197. [Google Scholar]
- 214.Willis E.L., Kersey D.C., Durrant B.S., Kouba A.J. The acute phase protein ceruloplasmin as a non-invasive marker of pseudopregnancy, pregnancy, and pregnancy loss in the giant panda. PLoS One. 2011;6:e21159. doi: 10.1371/journal.pone.0021159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xie H., Newberry L., Clark F.D., Huff W.E., Huff G.R., Balog J.M. Changes in serum ovotransferrin levels in chickens with experimentally induced inflammation and diseases. Avian Dis. 2002;46:122–131. doi: 10.1637/0005-2086(2002)046[0122:CISOLI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 216.Nakamura K., Mitarai Y., Yoshioka M., Koizumi N., Shibahara T., Nakajima Y. Serum levels of interleukin-6, alpha1-acid glycoprotein, and corticosterone in two-week-old chickens inoculated with Escherichia coli lipopolysaccharide. Poult Sci. 1998;77:908–911. doi: 10.1093/ps/77.6.908. [DOI] [PubMed] [Google Scholar]
- 217.Takahashi K., Miyake N., Ohta T., Akiba Y., Tamura K. Changes in plasma alpha 1-acid glycoprotein concentration and selected immune response in broiler chickens injected with Escherichia coli lipopolysaccharide. Br Poult Sci. 1998;39:152–155. doi: 10.1080/00071669889547. [DOI] [PubMed] [Google Scholar]
- 218.Chamanza R., Toussaint M.J., van Ederen A.M., van Veen L., Hulskamp-Koch C., Fabri T.H. Serum amyloid A and transferrin in chicken. A preliminary investigation of using acute-phase variables to assess diseases in chickens. Vet Q. 1999;21:158–162. doi: 10.1080/01652176.1999.9695012. [DOI] [PubMed] [Google Scholar]
- 219.Dunkley C.S., McReynolds J.L., Dunkley K.D., Njongmeta L.N., Berghman L.R., Kubena L.F. Molting in Salmonella enteritidis-challenged laying hens fed alfalfa crumbles. IV. Immune and stress protein response. Poult Sci. 2007;86:2502–2508. doi: 10.3382/ps.2006-00401. [DOI] [PubMed] [Google Scholar]
- 220.Georgieva T.M., Koinarski V.N., Urumova V.S., Marutsov P.D., Christov T.T., Nikolov J. Effects of Escherichia coli infection and Eimeria tenella invasion on blood concentrations of some positive acute phase proteins (haptoglobin (PIT 54), fibrinogen, and ceruloplasmin) in chickens. Rev Med Vet. 2010;161:84–89. [Google Scholar]
- 221.Inoue M., Satoh W., Murakami H. Plasma alpha 1-acid glycoprotein in chickens infected with infectious bursal disease virus. Avian Dis. 1997;41:164–170. [PubMed] [Google Scholar]
- 222.Nazifi S., Dadras H., Hoseinian S.A., Ansari-Lari M., Masoudian M. Measuring acute phase proteins (haptoglobin, ceruloplasmin, serum amyloid A, and fibrinogen) in healthy and infectious bursal disease virus-infected chicks. Comp Clin Pathol. 2010;19:283–286. [Google Scholar]
- 223.Zhou X., Wang L., Feng H., Guo Q., Dai H. Acute phase response in Chinese soft-shelled turtle (Trionyx sinensis) with Aeromonas hydrophila infection. Dev Comp Immunol. 2011;35:441–451. doi: 10.1016/j.dci.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 224.Bayne C.J., Gerwick L. The acute phase response and innate immunity of fish. Dev Comp Immunol. 2001;25:725–743. doi: 10.1016/s0145-305x(01)00033-7. [DOI] [PubMed] [Google Scholar]
- 225.Raida M.K., Buchmann K. Innate immune response in rainbow trout (Oncorhynchus mykiss) against primary and secondary infections with Yersinia ruckeri O1. Dev Comp Immunol. 2009;33:35–45. doi: 10.1016/j.dci.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 226.Gerwick L., Steinhauer R., Lapatra S., Sandell T., Ortuno J., Hajiseyedjavadi N. The acute phase response of rainbow trout (Oncorhynchus mykiss) plasma proteins to viral, bacterial and fungal inflammatory agents. Fish Shellfish Immunol. 2002;12:229–242. doi: 10.1006/fsim.2001.0367. [DOI] [PubMed] [Google Scholar]
- 227.Ahmad R., Khan K.A., Hasnain A., Qayyus S. Distribution of major serum proteins in an air breathing teleost, Channa punctata Bl. (Channidae: Channiformes) Biomed Res. 2007;18:123–128. [Google Scholar]
- 228.Harr K., Harvey J., Bonde R., Murphy D., Lowe M., Menchaca M. Comparison of methods used to diagnose generalized inflammatory disease in manatees (Trichechus manatus latirostris) J Zoo Wildl Med. 2006;37:151–159. doi: 10.1638/05-023.1. [DOI] [PubMed] [Google Scholar]
- 229.Funke C., King D.P., Brotheridge R.M., Adelung D., Stott J.L. Harbor seal (Phoca vitulina) C-reactive protein (C-RP): purification, characterization of specific monoclonal antibodies and development of an immuno-assay to measure serum C-RP concentrations. Vet Immunol Immunopathol. 1997;59:151–162. doi: 10.1016/s0165-2427(97)00059-7. [DOI] [PubMed] [Google Scholar]
- 230.Rosenfeld H., Lassen S., Prange A. Characterization of haptoglobin in the blood plasma of harbor seals (Phoca vitulina) J Proteome Res. 2009;8:2923–2932. doi: 10.1021/pr900035s. [DOI] [PubMed] [Google Scholar]
- 231.Kakuschke A., Erbsloeh H.B., Griesel S., Prange A. Acute phase protein haptoglobin in blood plasma samples of harbour seals (Phoca vitulina) of the Wadden Sea and of the isle Helgoland. Comp Biochem Physiol B Biochem Mol Biol. 2010;155:67–71. doi: 10.1016/j.cbpb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 232.Kent J. Acute phase proteins: their use in veterinary diagnosis. Br Vet J. 1992;148:279–282. doi: 10.1016/0007-1935(92)90081-B. [DOI] [PubMed] [Google Scholar]
- 233.Ceron J.J., Martinez-Subiela S., Ohno K., Caldin M. A seven-point plan for acute phase protein interpretation in companion animals. Vet J. 2008;177:6–7. doi: 10.1016/j.tvjl.2007.12.001. [DOI] [PubMed] [Google Scholar]


