Abstract
Inflammatory demyelinating diseases are a heterogeneous group of disorders, which occur against the background of an acute or chronic inflammatory process. The pathologic hallmark of multiple sclerosis (MS) is the presence of focal demyelinated lesions with partial axonal preservation and reactive astrogliosis. Demyelinated plaques are present in the white as well as gray matter, such as the cerebral or cerebellar cortex and brainstem nuclei. Activity of the disease process is reflected by the presence of lesions with ongoing myelin destruction. Axonal and neuronal destruction in the lesions is a major substrate for permanent neurologic deficit in MS patients. The MS pathology is qualitatively similar in different disease stages, such as relapsing remitting MS or secondary or primary progressive MS, but the prevalence of different lesion types differs quantitatively. Acute MS and Balo's type of concentric sclerosis appear to be variants of classic MS. In contrast, neuromyelitis optica (NMO) and spectrum disorders (NMOSD) are inflammatory diseases with primary injury of astrocytes, mediated by aquaporin-4 antibodies. Finally, we discuss the histopathology of other inflammatory demyelinating diseases such as acute disseminated encephalomyelitis and myelin oligodendrocyte glycoprotein antibody-associated demyelination. Knowledge of the heterogenous immunopathology in demyelinating diseases is important, to understand the clinical presentation and disease course and to find the optimal treatment for an individual patient.
Keywords: multiple sclerosis, neuromyelitis optica, acute disseminated encephalomyelitis, MOG antibodies, aquaporin-4 antibodies, human autoimmune encephalomyelitis
Introduction
Historically the first description of multiple sclerosis (MS) as a neuropathologic entity dates from 1868 by Charcot; subsequently, less prevalent disorders such as neuromyelitis optica (NMO) (Devic, 1894), Marburg type of MS (Marburg, 1906), or concentric sclerosis of Balo (1928) were demonstrated and considered as subtypes of MS. With the availability of immunohistochemical markers it became apparent that there is a considerable pathologic distinction between these disorders and the morphologic patterns were associated with different mechanisms of demyelination (Lucchinetti et al., 2000, Lucchinetti et al., 2002). This observation was further supported by the discovery of autoantibodies that are associated with a specific spectrum of demyelinating diseases. Anti-aquaporin-4 antibodies (AQP4-abs) are associated with NMO and limited forms of the disease, such as isolated optic neuritis or myelitis, which are now subsumed under the term NMO spectrum disorders (NMOSD). Meanwhile, the use of AQP4-abs as biomarker for NMOSD is well established and has major prognostic and therapeutic implications. Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies are found in patients with acute dissemimated encephalomyelitis (ADEM), NMOSD, and, rarely, MS-like clinical presentation (Lennon et al., 2004, Kim et al., 2015, Wingerchuk et al., 2015, Sepulveda et al., 2016), but they may define a unique and specific variant of inflammatory demyelinating diseases. It is quite probable that, even within the classic spectrum of MS, variabilities may represent different disease processes or perhaps different triggering factors and will classify future distinct entities.
Multiple Sclerosis
Clinical and diagnostic features
The clinical diagnosis of MS is based on the demonstration of demyelinating lesions disseminated in time and space. In addition to the neurologic symptoms, the finding of magnetic resonance imaging (MRI) lesions consistent with MS (Barkhof et al., 1997, Tintore et al., 2000), demonstration of oligoclonal bands in cerebrospinal fluid (CSF) (Link and Tibbling, 1977), and/or detection of abnormal visual evoked potentials (delay with a well-preserved wave form) are recommended to provide a correct diagnosis (McDonald et al., 2001). Patients with MS may present a monosymptomatic disease suggestive of MS (clinically isolated syndrome), a classic relapsing remitting course, or a primary or secondary progressive disease. Disease progression is defined as continuous deterioration of neurologic symptoms despite only a few new MRI lesions and a rare incidence of contrast-enhancing lesions. The majority of patients with MS start with a relapsing remitting MS, which later converts into a secondary progressive disease. In primary progressive MS the disease starts with continuous progression from its onset. The pathogenetic mechanism underlying progression is supposed to result at least in part from an ongoing inflammatory reaction behind a closed blood–brain barrier (BBB).
Pathologic features of classic MS
The histopathologic hallmark of MS is the formation of inflammatory demyelinating lesions with variable axonal damage and astrocytic gliosis.
Inflammation
Blood–brain barrier
In the pathogenesis of MS plaques it is currently supposed that autoreactive, myelin-specific lymphocytes are activated outside the central nervous system (CNS), cross the BBB, and form new inflammatory demyelinating lesions (Ciccarelli et al., 2014). The crossing of the BBB is dependent on the interaction of integrins on the surface of lymphocytes with cell adhesion molecules on endothelial cells (Takeshita and Ransohoff, 2012). One of the most important interactions is the binding of the very late antigen-4 integrin on lymphocytes to vascular cell adhesion molecule-1 on brain vascular endothelium (Takeshita and Ransohoff, 2012), which can be therapeutically blocked by the monoclonal antibody natalizumab in relapsing remitting MS (Polman et al., 2006).
The high efficacy of natalizumab treatment is due to its blockade of the migration of many different inflammatory cell types. However, it has been suggested that different inflammatory cell types use different entry pathways, by using distinctly different adhesion molecule interactions or chemokines such as α4integrin, ICAM, ALCAM, CCL5, CXCL10, and CCL2/CCR2 by Th1 +, Th17 + and CD8 + T cells (Engelhardt and Ransohoff, 2005, Cayrol et al., 2008, Ifergan et al., 2011) or CXCL12/CXCR4, CCR7/CCL19/TLR4 by monocytes (Man et al., 2012, Paradis et al., 2016). When these observations are confirmed and validated in different human diseases, including MS, more specific blockade of the migration of those leukocytes, which are most relevant in driving disease and tissue damage, may lead to effective therapies with fewer side-effects, compared to those currently available.
Morphologically, the disturbance of BBB permeability can be visualized with ultrastructural investigation and immunohistochemistry. By ultrastructure, endothelial cells show an increase in pinocytic vesicles in active MS lesions (Brown, 1978) and by immunohistochemistry, a perivascular leakage of serum proteins and, more specifically, an expression of molecular markers for leaky endothelial cells is seen (Kirk et al., 2003, Hochmeister et al., 2006). In MRI studies, the BBB permeability is visualized with contrast enhancement. The dynamic changes from nodular enhancement during early lesion formation to a ring-enhancing lesion in a later stage suggest that BBB leakage initally starts in the inflamed central vein and later mainly occurs at the active margin (Gaitan et al., 2011). It is important to note that contrast enhancement in MRI depicts BBB leakage, but is not a direct marker of inflammation. In particular, in patients with progressive MS, widespread and profound inflammation is seen in the brain, which takes place in part behind a closed or repaired BBB (Hochmeister et al., 2006).
T lymphocytes
Neuropathologic tissue examination recognizes perivascular cell cuffs and meningeal inflammatory infiltrates composed of CD3 + and CD8 + T cells, while CD4 + T cells, CD20-positive B cells, and plasma cells are present in variable and lower numbers (Frischer et al., 2009). Lymphocytic inflammation is associated with profound macrophage infiltration and microglia activation, in particular in lesions with active demyelination or tissue injury. Neutrophils and eosinophils are usually not observed but more characteristic for lesions in NMO. In the parenchyma of MS plaques the inflammatory infiltrates are dominated by CD8 + T cells (Friese and Fugger, 2005) and show a clonal expansion that persists over time (Babbe et al., 2000). In some cases large numbers of cytotoxic T cells were identified in close contact with oligodendrocytes or axons and showed a polarization of granzyme B towards the site of contact (Neumann et al., 2002). For these reasons it has been hypothesized that cytotoxic T cells may in part selectively mediate the inflammatory demyelination in MS and this view is supported by experimental models of brain inflammation induced by oligodendrocyte-reactive CD8 + T cells, which unequivocally show primary demyelination (Saxena et al., 2008). However, it turned out to be very difficult to induce demyelination by passive transfer of CD8 + T cells in animal models (Huseby et al., 2001, Na et al., 2008, Saxena et al., 2008). This was partly explained by an antigen-specific clonal deletion of cytotoxic T cells by oligodendrocytes in the intact CNS (Na et al., 2012), and this effect could be abolished with an immunologic priming of the CNS by an infectious process (Na et al., 2012). An alternative theory suggests that at least a subpopulation of T cells may play a neuroprotective rather than a destructive role (Stadelmann et al., 2005). This is supported also by the detection of HLA-E on endothelial cells and astrocytes in active MS lesions that induce an immunoregulatory phenotype in CD8 + cells (Durrenberger et al., 2012). Interestingly, lower numbers of circulating regulatory T cells (FoxP3 +, CD8 +) in the circulation of relapsing MS patients compared to those in remission were detected and held responsible for the ongoing autoimmune attack in the MS lesion (Boppana et al., 2011), but the numbers of FoxP3 + T cells within MS lesions is very low (Fritzsching et al., 2011).
The CD4 + T cells are present in variable amounts in perivascular and meningeal inflammatory infiltrates, but their number overall is small and clonal expansion is rare in comparison to CD8 + cells (Babbe et al., 2000). It is believed that helper T cells initiate the formation of MS lesions by recruitment of macrophages that subsequently present antigens. The antigen that drives the autoimmune process however is unknown and it is supposed that the antigenic epitope repertoire may increase and change over time (called epitope spreading) (Davies et al., 2005). The theory that CD4 + helper T cells are responsible for the formation of new demyelinating lesions is supported by animal models of experimental autoimmune encephalomyelitis (EAE) that are mainly induced by CD4 + T-cell-driven inflammation (Billiau and Matthys, 2001). However, MS therapies that selectively target CD4 + T-cell response were shown to be ineffective (van Oosten et al., 1997, Segal et al., 2008).
B lymphocytes
B cells are sparse in the parenchyma of MS lesions in comparison to T cells (Frischer et al., 2009). B cells and plasma cells can mainly be found in the perivascular and meningeal inflammatory infiltrates. In patients with pronounced inflammatory pathology, large B-cell aggregates may occur and show features of tertiary lymphoid follicles with germinal center-like structural organization (Serafini et al., 2004, Aloisi and Pujol-Borrell, 2006, Magliozzi et al., 2007). It has been shown that meningeal inflammation correlates with the extent of active demyelination and neurodegeneration in the underlying cortex (see later under demyelinating lesions in the gray matter) (Magliozzi et al., 2010, Howell et al., 2011, Fischer et al., 2013). B cells mature to plasma cells that produce immunoglobulins that are mainly IgG1 and IgG3 isotypes and, less frequently, IgA and IgM isotypes. Lipid-specific IgM in the CSF has been documented in patients with MS and may reflect a negative prognostic marker (Thangarajh et al., 2008). Ig-producing B cells undergo antigen-specific clonal expansion, which is supported by prominent somatic hypermutation of Ig chains within the CSF and brain lesions of MS patients (Obermeier et al., 2011, Beltran et al., 2014). Although the exact role of B lymphocytes in the formation of MS lesions is still unclear, some patients show good response to therapies, which target T- and B-cell infiltration into the CNS (alemtuzumab) (Thompson et al., 2010) or which selectively eliminate circulating B cells (rituximab, ocrelizumab) (Hauser et al., 2008, Hauser et al., 2017, Montalban et al., 2017).
Microglia and macrophages
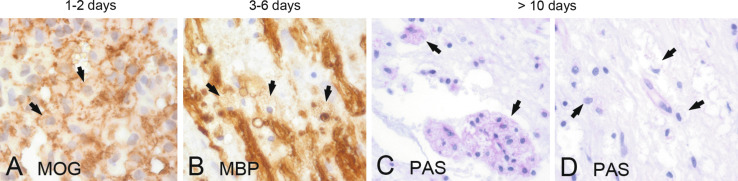
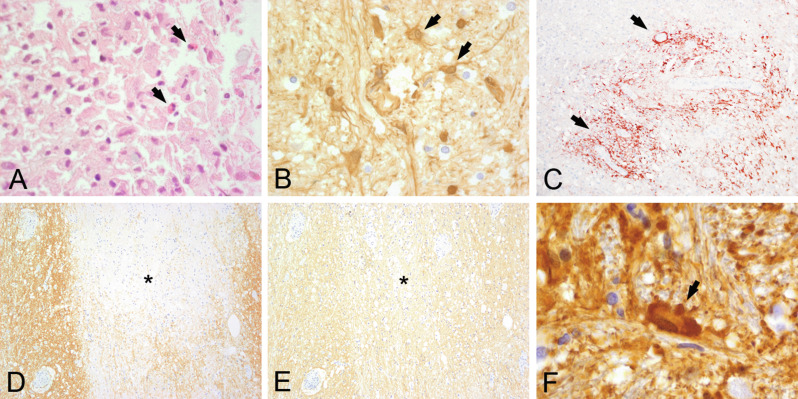
The number of macrophages within MS lesions depends on the stage of activity. Actively demyelinating plaques contain high numbers of lipid-laden macrophages throughout the lesion, chronically active plaques are delineated by a rim of macrophages and activated microglia, while the lesion center is devoid of macrophages, and chronically inactive plaques contain hardly any macrophages (Kuhlmann et al., 2017). The role of microglia and macrophages in the pathogenesis of MS plaques has been extensively studied during the last decades. Originally they were mainly seen as proinflammatory cells exacerbating tissue damage by antigen presentation via major histocompatibility complex (MHC) molecules, production of oxygen and nitric oxide radicals, secretion of proinflammatory cytokines, and phagocytosis. Macrophages were used as parameters in the time staging of MS lesions based on the presence of partly digested myelin components within their cytoplasm (Bruck et al., 1995). In vitro MOG and myelin-associated glycoprotein (MAG) are degraded after 1 day (Fig. 19.1A), while the degradation of proteolipid protein (PLP) and myelin basic protein is much slower following myelin phagocytosis (Fig. 19.1B) (van der Goes et al., 2005).
Fig. 19.1.
Time course of myelin degradation in multiple sclerosis. Minor myelin proteins such as myelin oligodendrocyte glycoprotein (MOG) are degraded within 1–2 days of phagocytosis (A, arrows: MOG-positive myelin debris within macrophages), while major myelin proteins such as myelin basic protein (MBP) may persist for up to 6 days (B, arrows: MBP-positive myelin debris within macrophages). In later stages, macrophages contain periodic acid–Schiff (PAS)-positive residual glycoproteins (C, arrows: PAS-positive macrophages) and lipids (D, arrows: foamy and vacuolated macrophages). × 600.
Immunohistochemical staining for these myelin proteins can give an indication of the time interval between actual myelin destruction and analysis of the tissue (Bruck et al., 1995). In later stages, residual glycoproteins in macrophage lysosomes can be stained with periodic acid–Schiff (Fig. 19.1 C and D). Later it became clear that macrophages may also be involved in remyelination and neuroprotection and their maturation into an anti-inflammatory M2 phenotype occurs after they have ingested myelin (Boven et al., 2006). So far, however, a clear lesion stage-dependent macrophage polarization has not become apparent; most macrophages and microglia showed a phenotype, which is intermediate between M1 and M2 cells (Vogel et al., 2013).
Demyelination
Myelin sheath
Myelin sheaths are formed and maintained by oligodendrocytes (Dhaunchak and Nave, 2007). Myelin assembly and long-term preservation require a number of proteins that are either expressed in the myelin or at the axonal membrane. MOG is localized on the outermost surface of the myelin sheath. The protein belongs to the immunoglobulin superfamily and might serve as cell adhesion molecule, a regulator of microtubule stability, and a mediator of interactions between myelin and the immune system (Johns and Bernard, 1999). Structural myelin proteins such as proteolipid protein and myelin basic protein are necessary for exact membrane-to-membrane spacing, whereas 2’3’-cyclic nucleotide 3’phosphodiesterase (CNP) and MAG are expressed at the periphery of the oligodendrocytes and the latter plays a role in linking the innermost periaxonal loop of oligodendrocytes with the axon (Lappe-Siefke et al., 2003). At the node of Ranvier axoglial proteins like contactin1 and neurofascin155/186 form glioaxonal junctions to keep sodium channels in the nodal region, which is critical for the efficient saltatory conduction of the action potential (Sherman et al., 2005).
Mechanisms of demyelination
Demyelination is the common final phase in the pathology of MS and comprises the stripping of myelin lamellae and removal of myelin fragments by phagocytes. There is evidence that demyelination is driven by different mechanisms involving the adaptive and innate immune system (Lucchinetti et al., 2000). Diffusible oxygen, nitric oxide, and nitrogen species, mainly produced by macrophages and activated microglia, are the key mechanisms for myelin and oligodendrocyte damage during early demyelination (Hill et al., 2004, Haider et al., 2011). In a subgroup of patients (pattern 1) this relatively indiscriminate damage is probably a major mechanism of injury. In about 50% of patients with short and aggressive disease course an additional deposition of immunoglobulins and complement was shown within the lesions, suggesting an antibody-mediated process (pattern 2). First evidence for a direct antibody–antigen interaction was gained in EAE models that were induced by encephalitogenic T cells and modified by monoclonal antibodies against MOG (Schluesener et al., 1987, Linington et al., 1988). Later, autoantibodies to the astrocytic waterchannel AQP4 were discovered and are now sensitive and specific diagnostic markers for NMOSD.
Indirect evidence for antibody-mediated damage was found in patients with IgM antibody deposition on myelin and demyelinated axons that colocalized with complement C3b next to antibody–antigen immuncomplexes in macrophages (Sadaba et al., 2012). In another distinct group of patients, oxidative stress may cause an oligodendrogliopathy that starts in the distal processes of oligodendrocytes with preferential loss of MAG and later results in apoptosis of the oligodendrocyte (pattern 3). Since preferential MAG loss is a prominent feature of early white-matter stroke and some types of viral encephalitis, and HIF1alpha, a marker for hypoxia, is strongly expressed in these lesions, it has been hypothesized that the “dying-back” oligodendrogliopathy may result from a hypoxia-like tissue injury. This may be induced by disturbance of microcirculation or impairment of mitochondrial energy metabolism as a result of reactive oxygen species produced by macrophages (Aboul-Enein et al., 2003, Mahad et al., 2008). Finally, very few cases have been identified with apoptotic oligodendrocytes in the normal-appearing white matter (NAWM), which suggests a primary oligodendrogliopathy in these patients (pattern 4). However, more cases are needed to elucidate the underlying pathogenetic mechanisms.
Neuropathology of MS plaques
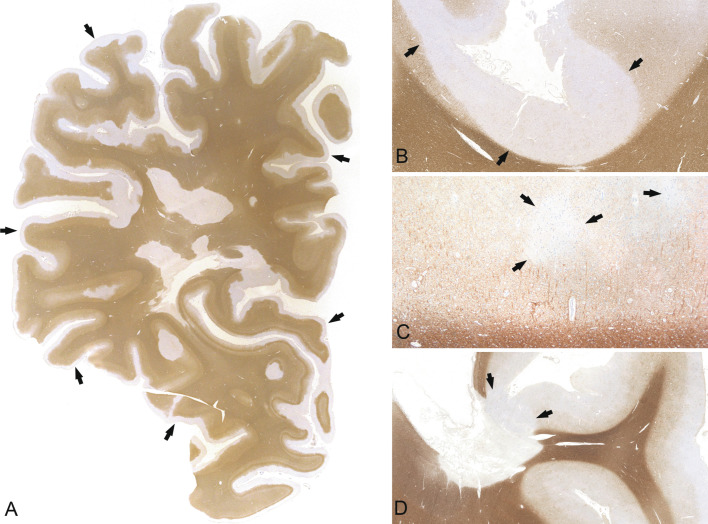
MS lesions can arise at any site in the CNS (Fig. 19.2, Fig. 19.3 ); however, there is a predilection for areas with high venous density, related to the fact that veins are the prime sites for the egress of inflammatory cells through the blood to the brain (Waksman, 1960). In progressive MS, lesions in addition accumulate in watershed areas with low arterial blood supply, such as the periventricular white matter (Haider et al., 2016). This has been attributed to an amplification of oxidative injury and histotoxic hypoxia in areas of reduced arterial perfusion and oxygen supply. The demyelinated lesions are typically well demarcated and centered by an inflamed vein. Finger-like protrusions may occur along inflamed veins and are called Dawson fingers.
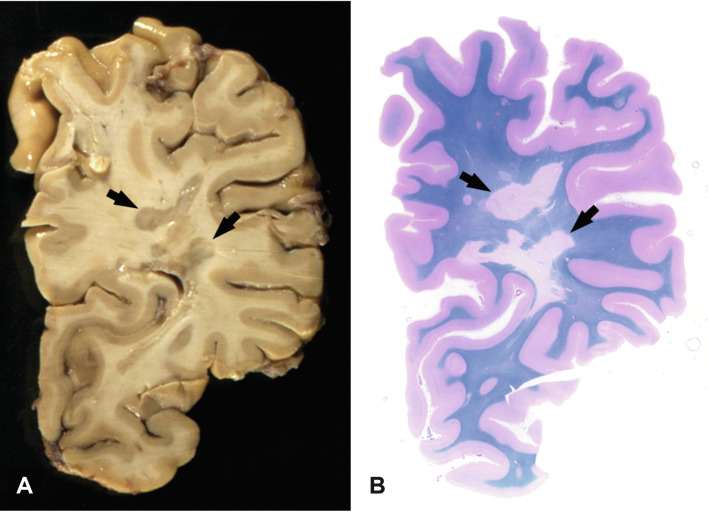
Fig. 19.2.
Macroscopic appearance of multiple sclerosis. Chronic multiple sclerosis in a 61-year-old female with a 33-year history; section through the formalin-fixed brain (A) and the corresponding hemispheric section (B, luxol fast blue) shows multiple well-demarcated demyelinated plaques in the periventricular white matter (arrows).
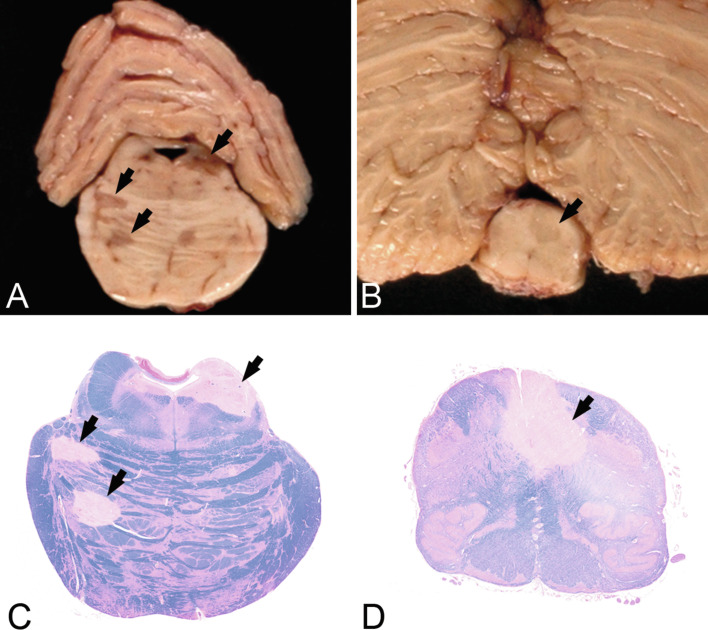
Fig. 19.3.
Macroscopic appearance of multiple sclerosis in the brainstem. Chronic multiple sclerosis lesions (arrows) in the pons (A) and medulla oblongata (B) of a 47-year-old female with an 18-year history. Sections through the formalin-fixed brain (A, B) and corresponding histologic sections (C, D, luxol fast blue) show multiple well-demarcated demyelinated plaques (arrows).
Classic active lesions
Actively demyelinating lesions are mainly found in biopsy specimens or in autopsy tissue of patients who died early after disease onset (Fig. 19.4 ) (Frischer et al., 2015). Active lesions are characterized by numerous macrophages with early myelin degradation products within their cytoplasm and expression of the early macrophage marker MRP14 (Fig. 19.4 A and B). Moreover a considerable number of T cells and a variable number of perivascular and meningeal B cells and plasma cells are visible (Fig. 19.4 C–F). Within the lesions remaining oligodendrocytes show signs of activation with large, round nuclei and broadened cytoplasm, which may reflect early remyelination (Goldschmidt et al., 2009, Hoftberger et al., 2010). When the entire lesion is filled with myelin-containing macrophages, such a lesion is called acute plaque. Chronic active lesions are present, when myelin-containing macrophages are densely packed at the edge of a lesion with an inactive core. Acute axonal damage with amyloid precursor protein-positive spheroids is abundant within active plaques (Kuhlmann et al., 2002). The astrocytes are strongly activated with enlarged cytoplasm and often bizarre or multiple nuclei (so-called Creutzfeldt cells) and form a protoplasmic gliosis. These astrocytic changes are most likely nonspecific and seem to be the consequence of severe inflammation and microglia activation (Sharma et al., 2010). However, activated astrocytes can produce a large number of proinflammatory cytokines and chemokines and therefore these cells may also be involved in the propagation of inflammation and tissue damage (Brosnan and Raine, 2013).
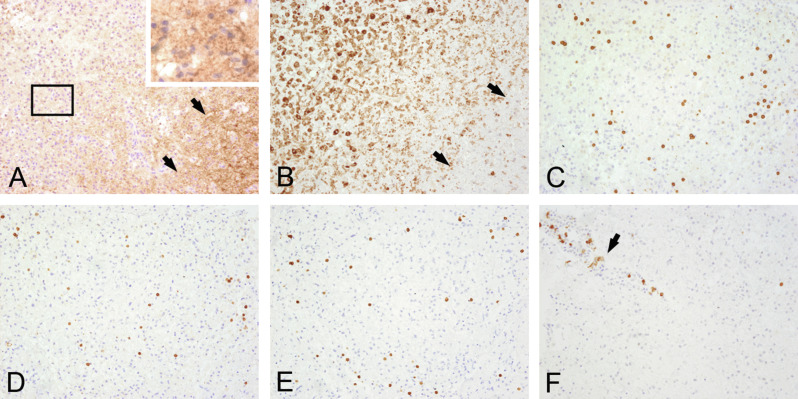
Fig. 19.4.
Actively demyelinating lesion. Serial sections of the edge of an actively demyelinating multiple sclerosis plaque from a white-matter biopsy of a 22-year-old female. Periplaque white matter is seen on the right side of (A) and (B) (arrows). The plaque on the left side shows active demyelination with numerous macrophages containing myelin basic protein-positive myelin debris within their cytoplasm (A, rectangle enlarged upper right). Numerous CD68-positive macrophages (B) are mixed with CD3 + (C), CD4 + (D) and CD8 + parenchymal T cells (E) and perivenous CD79a + B cells/plasma cells (F, arrow). × 200.
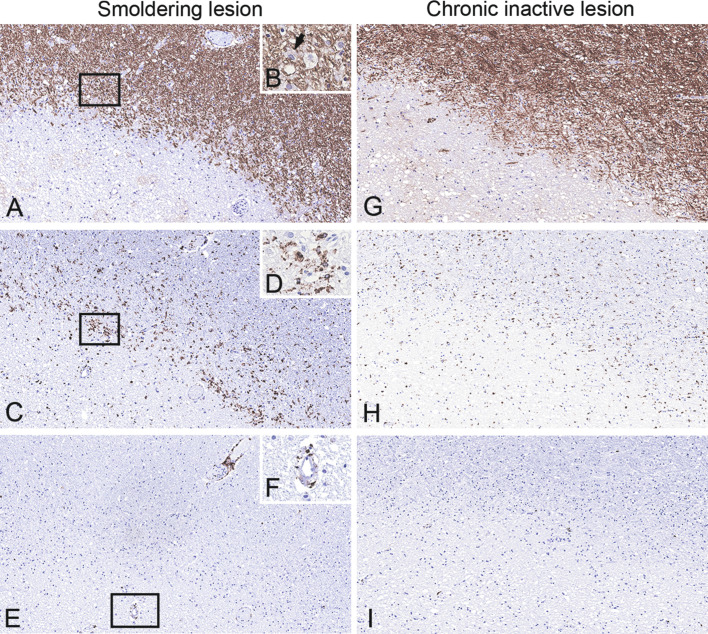
Smoldering (slowly expanding) lesions
These lesions are mainly seen in patients with progressive MS (Frischer et al., 2015) and show a rim of activated microglia at the edge, while the lesion center is inactive (Fig. 19.5 A–D). They contain few macrophages at the edge that are digesting myelin components (Fig. 19.5B) and are accompanied by scattered T cells (Fig. 19.5 E and F), suggesting an ongoing expansion into the surrounding NAWM. Slow expansion of such lesions within a timeframe of 3 years was demonstrated in a recent prospective MRI study (Dal-Bianco et al., 2017). Acute axonal injury is prominent at the edge of these lesions. Moreover, accumulation of iron has been demonstrated in microglia at the lesion edge, resulting in microglial dystrophy, while remyelination is absent (Hametner et al., 2013). The driving force that leads to a continuous expansion of the lesions is still unclear. Since autologous stem cell transplantation failed to terminate the disease progression, it has been hypothesized that a CNS-resident immune response might be involved (Metz et al., 2007).
Fig. 19.5.
Smoldering (slowly expanding) and inactive lesions. Serial sections of the edge of a smoldering demyelinating lesion (A, rectangle enlarged in B; myelin basic protein (MBP)) shows a rim of activated microglia and macrophages at the edge (C, rectangle enlarged in D; CD68). Few macrophages are digesting myelin components (B, arrow: macrophage with MPB-positive degradation products) and are accompanied by scattered CD8 + T cells (E, rectangle enlarged in F), suggesting an ongoing expansion into the surrounding normal-appearing white matter. In contrast, inactive lesions show thin myelin sheaths at the edge, representing remyelination (G), and only contain few microglia/macrophages (H) and T cells (I) at the edge. A, C, F, G–I, × 10; B, D, F, × 400.
Chronic inactive lesions
In long-standing MS, the most common lesion type is the chronic inactive demyelinated lesion. Such lesions are usually hypocellular in the center and may show thin myelin sheaths at the edge, representing remyelination (Fig. 19.5G). Only few macrophages and T cells are found, being present mainly in the perivascular space (Fig. 19.5 H and I). Although myelin is the primary target in MS, there is also pronounced axonal damage, which can lead to an average axonal loss of 20–30% compared to control white matter in newly formed lesions (van Waesberghe et al., 1999, Kornek et al., 2000, Lovas et al., 2000) and of 60–70% in chronic established lesions (Mews et al., 1998, Bjartmar et al., 2000). The astrocytes form a dense fibrillary gliosis.
Demyelinating lesions in the gray matter
In addition to predilection sites in the white matter, the cortex is especially vulnerable to demyelination. Cortical demyelination is more likely to be found in chronic disease stages (Kutzelnigg et al., 2005) and is associated with cognitive deficites and, in some patients, with epilepsy. These lesions can be located subpially or intracortically, or overlap with the white matter (so-called compound plaques) (Kidd et al., 1999), and subpial lesions are disease-specific for MS (Moll et al., 2008, Fischer et al., 2013). The subpial lesions often extend over several gyri and sulci and can cover up to 70% of the cortex in some patients (Fig. 19.6 A and B) (Kutzelnigg et al., 2005), while intracortical lesions are mostly small and inconspicuous (Fig. 19.6C). The compound plaques affect the cortex and the underlying white matter and are mostly more inflammatory and have more microglial and astroglial activation compared to other cortical lesion types (Fig. 19.6D) (Peterson et al., 2001). Subpial cortical lesions are associated with inflammatory infiltrates in the adjacent meninges and are most pronounced in the invaginations of the brain surface, most likely due to the comparatively low CSF flow in these areas (Haider et al., 2016).
Fig. 19.6.
Cortical demyelination. Cortical demyelination located subpially (A, B), intracortically (C), and overlapping with the white matter (so-called compound plaques) (D). A subpial lesion extends over several gyri and sulci and covers almost the entire cortex (A, proteolipid protein, arrows; B, myelin basic protein (MBP), arrows). A small, demyelinated lesion is located intracortically (C, MBP, arrows). A compound plaque is overlapping the cortex and the subcortical white matter (D, MBP, arrows). B, × 1.56; C, × 40; D, × 0.59.
It has been hypothesized that soluble mediators are diffusing into the cortex and induce tissue damage either directly or indirectly through microglia activation and oxidative stress (Howell et al., 2011, Fischer et al., 2013). Not all patients with MS have cortical plaques; however, accumulation of meningeal inflammation with chronicity of the disease, aging of the patient with accumulation of iron in the brain (Lassmann, 2012), and distant lesions within the white or deep gray matter that are in topographic relation to the cortex (Kolasinski et al., 2012) may play a role in vulnerability.
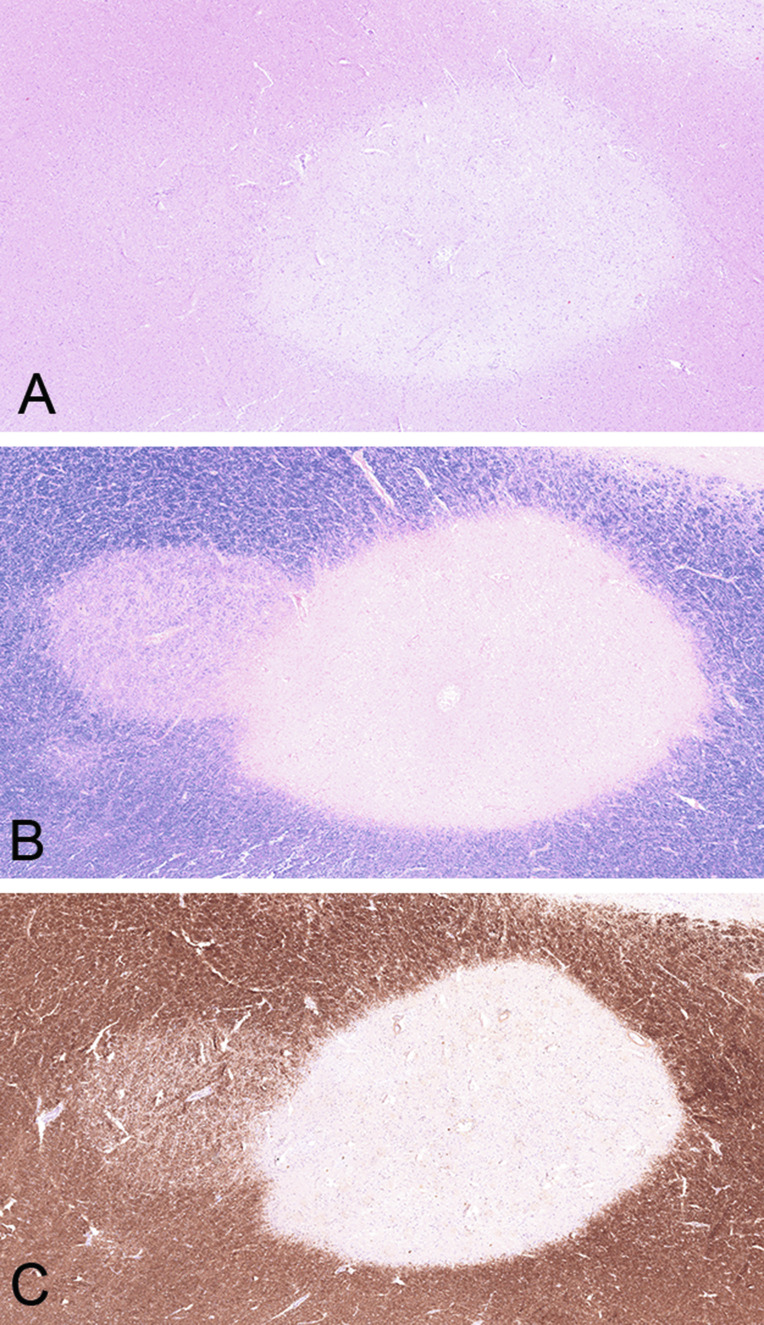
Remyelination
Remyelination of demyelinated lesions occurs spontaneously but is often structurally and functionally incomplete. Remyelination is mediated by oligodendrocyte precursor cells (OPC) that are present in the normal white matter. During remyelination the OPCs are activated and subsequently migrate to the lesion and undergo differentiation into myelin-generating cells. Remyelinated fibers are characterized by thinner myelin sheaths and may be only encountered at the border zone of a lesion or can also fully remyelinate a lesion, which is then called a shadow plaque (Fig. 19.7 A–C). Some shadow plaques may undergo new demyelination, either within or overlapping the previously remyelinated areas (Prineas et al., 1993, Bramow et al., 2010). The extent of remyelination varies considerably between patients and seems to be more prevalent in the early stage of the disease and in lesions with inflammation and macrophage activity (Foote and Blakemore, 2005). Repair capacity is also dependent on the lesion site, with periventricular lesions showing less extensive remyelination (Patrikios et al., 2006). The lack of remyelination in a considerable number of patients has been attributed to a failure of OPC differentiation, which may be influenced by age and hormones (Chang et al., 2002, Chari et al., 2003, Kotter et al., 2011), loss of axons in the lesions (Charles et al., 2002), and the loss of macrophages in the chronic lesion.
Fig. 19.7.
Remyelinating shadow plaque. Overlap of a sharply demarcated chronic inactive plaque centered by a small vein, next to a remyelinated shadow plaque on the left, characterized by thinly myelinated fibers, demonstraded in hematoxylin and eosin (A), luxol fast blue (B), and myelin basic protein (C).
Neurodegeneration
During early and late stages of MS, axons, neurons, and synapses can be damaged, which is commonly termed neurodegeneration (Peterson et al., 2001, Dutta and Trapp, 2011). Axonal loss has been suggested to be a substrate for permanent motor disability in MS (Tallantyre et al., 2010). Acute axonal damage is most pronounced in the active demyelinating lesions and can be visualized with immunohistochemistry for amyloid precursor protein that reflects disturbance of fast axonal transport in dystrophic axons (Ferguson et al., 1997, Trapp et al., 1998, Kuhlmann et al., 2002). The loss of myelin contributes to axonal damgage because of the loss of trophic factors. Moreover, oxidative injury directly damages axons. In addition, antibodies have been identified that target glioaxonal proteins in the juxtaparanodal area, such as anti-neurofascin155 and anti-contactin1 (Mathey et al., 2007), that later turned out to play an important role in a proportion of patients with chronic inflammatory demyelinating polyneuropathy (Ng et al., 2012). In chronic MS lesions the axons are markedly reduced, which is considered to contribute to a failure of remyelination (Kornek et al., 2000).
Neuronal damage may be a consequence of retrograde neurodegeneration when axons are transected and is morphologically associated with chromatolysis in neuronal cell bodies (Haider et al., 2016). In this case, the loss of neurons is in topographic relation to the white-matter lesions and the loss of neurons is most pronounced in the deep layers of the cortex and deep gray-matter nuclei (thalamus and globus pallidus). On the other hand, neuronal damage may be directly mediated by oxidative injury. These neurons are morphologically characterized by dendritic beading and fragmentation and accumulation of oxidized phospholipids in the cytoplasm. This pattern of damage can mainly be found in demyelinating cortical lesions (Haider et al., 2016).
Tumefactive MS
Tumefactive demyelination is a variant of MS that is characterized by lesions with pseudotumoral appearance with a size often larger than 2 cm, cystic changes, or ring enhancement on MRI, and may have a mass effect (Enzinger et al., 2005). The lesions can be solitary or multiple and may occur at any site of the CNS, including the spinal cord. Morphologically, they are typical active demyelinating lesions with inflammation, numerous macrophages, and bizarre reactive gliosis. It is important to know this variant of MS because the lesions are often biopsied or even resected and the differential diagnostic workup may be challenging, especially in very small biopsy specimens (Lucchinetti et al., 2008). For example, the bizarre reactive gliosis in active MS lesions may mimick a glioblastoma or inflammatory infiltrates may raise the possibility of a vasculitis, infectious disease, or even lymphoproliferative disease (Kuhlmann et al., 2008).
Acute MS
Acute MS, also referred to as Marburg´s disease, is an acute monophasic illness that usually follows a rapidly progressive or stepwise deterioriating process and leads to death within 1–6 months of clinical onset (Marburg, 1906). The demyelinating lesions show a similar distribution to that in chronic MS. The plaques appear relatively uniform within the lesion and from one lesion to the other, which correlates with the monophasic disease course. However, even within acute MS, there is often a dissemination in time, as shown by some variability in the lesion stages (Prineas et al., 1989). Acute MS is nowadays extremely rare due to the availability of effective anti-inflammatory treatments and intensive care.
Concentric sclerosis of Balo
Concentric sclerosis of Balo is a variant of MS that is characterized by concentric rings of demyelination that alternate with rings of relatively preserved myelin. The disease often shows an acute onset, progresses steadily, and may even lead to death, but can also show a benign course, with only mild residual deficits. It is supposed that the pathophysiologic mechanism responsible for the formation of concentric lesions is an extensive production of oxidative radicals leading to hypoxia-like tissue injury and dying-back oligodendrogliopathy (Lucchinetti et al., 2000). The zone of periplaque white matter next to the actively demyelinating plaque reacts with the expression of hypoxic preconditioning molecules, such as HIF1alpha and Hsp-70. These molecules have a protective effect and help to preserve a small ring of myelin. In case of aggressive lesion progression, however, the oxidative tissue injury extends beyond the protected tissue ring and leads to a new ring of demyelination (Stadelmann et al., 2005). This may finally end up in the pattern of concentric demyelination and preserved myelin.
Neuromyelitis Optica
Clinical and diagnostic features
NMO is a disease entity distinct from MS, which is characterized by inflammatory demyelination that primarily affects the spinal cord and the optic nerves. The disorder is also termed Devic disease, according to one of the first descriptions that date from 1894. NMO was long considered as a topographically restricted form of MS, although it soon became clear that NMO patients were different from classic MS in terms of disease course, treatment requirements, and lesion morphology, in particular reflected by very prominent perivascular immunoglobulin deposition and complement activation (Lucchinetti et al., 2002). The discovery of autoantibodies to the astrocytic waterchannel AQP4 in a majority of NMO patients (Lennon et al., 2004) was an important step towards the classification of NMO as a distinct entity and this feature is now incorporated into the currently published diagnostic consensus criteria for adult patients with NMO. These criteria are based on core clinical characteristics, AQP4 antibody status, and specific MRI requirements (Table 19.1 ) (Wingerchuk et al., 2015) and allow the inclusion of spatially limited forms (isolated optic neuritis, myelitis) or more extensive forms of the disease (with cerebral, diencephalic, or brainstem lesions) into the spectrum of NMO (so-called NMOSD).
Table 19.1.
Diagnostic criteria of neuromyelitis optica and spectrum disorders (NMOSD) for adult patients (Wingerchuk et al., 2015)
| AQP4 antibody-positive All of the following requirements:
|
| AQP4 antibody-negative or unknown All of the following requirements:
|
AQP-4, aquaporin-4.
Core clinical features: (1) optic neuritis; (2) acute myelitis; (3) area postrema syndrome (hiccups, nausea, and/or vomiting); (4) acute brainstem syndrome; (5) narcolepsy or acute diencephalic clinical syndrome with NMOSD-typical diencephalic MRI lesions; (6) symptomatic cerebral syndrome with NMOSD-typical brain lesions.
Additional magnetic resonance imaging (MRI) requirements for AQP4 antibody-negative NMOSD: (1) optic neuritis with either normal/nonspecific MRI or T2-hyperintense or T1-weighted gadolinium enhancing lesions extending over > 1/2 optic nerve length or involving optic chiasm; (2) acute myelitis with intramedullary MRI lesion extending over three or more vertebral segments (longitudinal extensive transverse myelitis: LETM); (3) area postrema syndrome: dorsal medulla/area postrema lesions in MRI; (4) acute brainstem syndrome: periependymal brainstem lesions in MRI.
Pathologic features of NMOSD
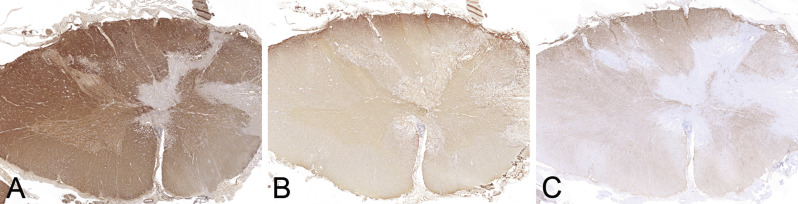
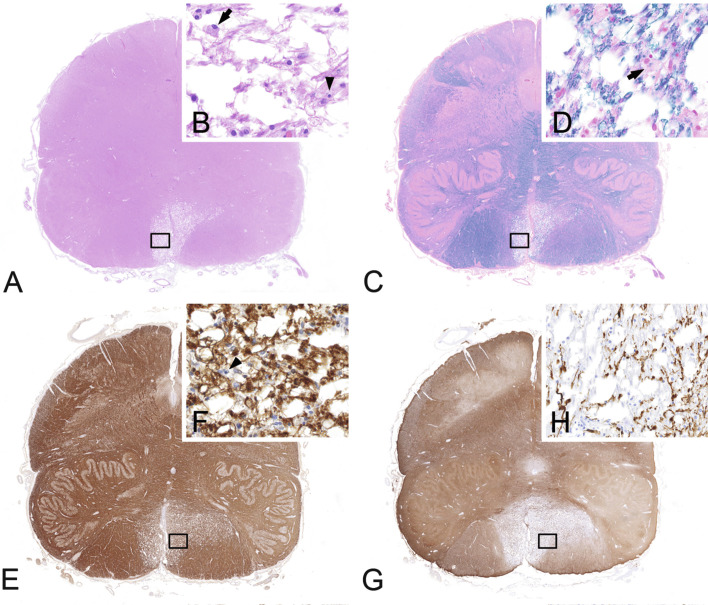
In classic NMO the demyelinating lesions are usually located in the optic nerve and myelon. In the myelon the lesions typically overlap with the gray matter and extend over more than three vertebral segments (Fig. 19.8 A–C). Moreover, lesions may affect the brainstem and exceptionally can cause demyelinating lesions in the brain (Pittock et al., 2006, Wingerchuk et al., 2015). Lesions in NMOSD can present with different morphologic features (Misu et al., 2013).
Fig. 19.8.
Spinal cord lesion in neuromyelitis optica. Destructive demyelinating lesion in the spinal cord (A, myelin basic protein) shows loss of astrocytes (B, aquaporin-4; C, aquaporin-1) and overlaps with the gray matter. × 1.1.
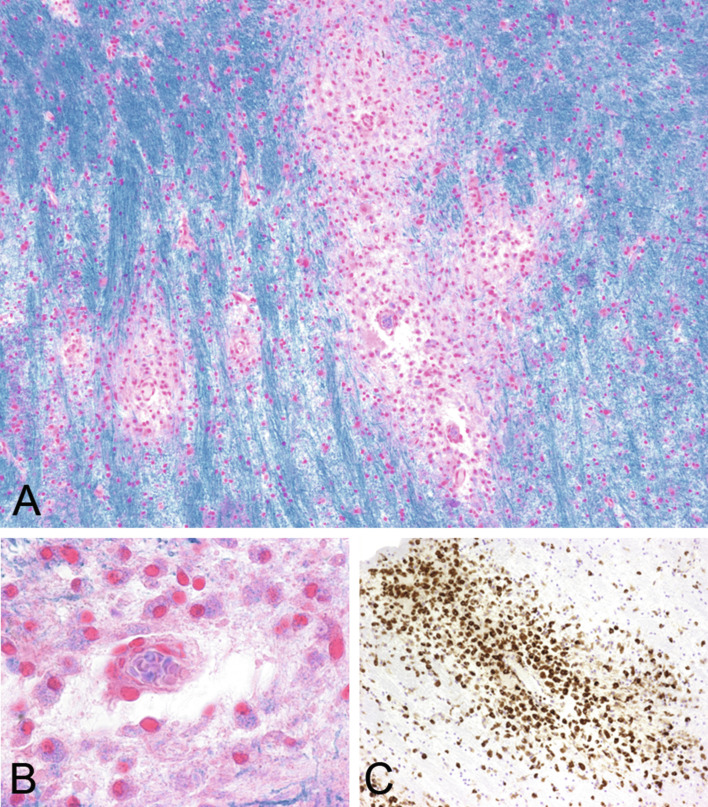
The classic pattern is characterized by demyelination and necrosis with loss of axons and astrocytes, leading to cystic cavitation and loss of AQP4, AQP1, and glial fibrillary acidic protein immunoreactivity. In the active stage, inflammatory infiltrates often contain eosinophilic granulocytes (Fig. 19.9A) next to macrophages and lymphocytes and deposition of IgG (Fig. 19.9B) and IgM as well as activated complement (Fig. 19.9C) (Lucchinetti et al., 2002). In other lesions myelin is only mildly affected and the pathology mainly concentrates on the astrocytes with selective loss of AQP4 (Fig. 19.9 D and E) and/or deposition of complement, loss of perivascular astrocyte processes, and accumulation of bizarre astrocytes with beading and clumping of cell processes (so-called clasmatodendrosis) (Fig. 19.9 F). Some lesions with preserved myelin and loss of AQP4 may show apoptotic oligodendroycytes and expansion of the extracellular space or vacuolation consistent with intramyelinic edema (Fig. 19.10 A–H).
Fig. 19.9.
Active stage of neuromyelitis optica (NMO) lesion. An active NMO lesion shows inflammatory infiltrates with eosinophils (A, hematoxylin and eosin) next to macrophages and lymphocytes, deposition of immunoglobulin G (B, IgG), and C9 neoantigen (C, arrows). Some lesions show selective loss of aquaporin-4 (D, asterisk marks lesion) while aquaporin-1 is well preserved (E) and there is accumulation of bizarre astrocytes with beading and clumping of cell processes (clasmatodendrosis) (F, glial fibrillary acidic protein). A, B, F, × 600; C, × 200; D, E, × 100.
Fig. 19.10.
Vacuolated lesions in neuromyelitis optica (NMO). A vacuolated lesion in NMO is characterized by expansion of the extracellular space and shows some apoptotic nuclei (A, B, hematoxylin and eosin, arrow: apoptotic cell). The myelin is relatively well preserved with only few demyelinating macrophages (C, D, luxol fast blue (LFB); arrow: macrophage with LFB-positive degradation products; E, F, myelin basic protein; arrowhead: apoptotic cell), in contrast, astrocytes are lost (G, H, glial fibrillary acid protein). B, D, F, H: × 250.
Lesions at the floor of the fourth ventricle often show intense inflammation and AQP4 loss, with reactive glial fibrillary acidic protein-positive astrocytes and preserved myelin. Whether these different morphologic patterns represent a different lesion age or reflect variable pathogenetic processes is presently unclear. The loss of AQP4 in active NMO lesions is in contrast to active lesions in MS that are charaterized by upregulation of AQP4 on reactive astrocytes. However, in chronic inactive demyelinated MS plaques, AQP4 may sometimes be absent (Roemer et al., 2007), which may give rise to an erroneous NMO diagnosis.
The deposition of complement and immunoglobulin on perivascular astrocytic processes that show the hightest concentration of AQP4 is consistent with the current concept that humoral autoimmunity to AQP4 mediates the disease and distinguishes it from MS. However, it is still unclear why NMO lesions have a particular predilection for the optic nerves and the myelon.
Acute Disseminated Encephalomyelitis and Related Disorders
Clinical and diagnostic features
ADEM is an acute monophasic inflammatory demyelinating disease of the CNS. Although it can occur at any age, it mainly affects children and young adults (Wingerchuk, 2006). The disease may be preceded by an infectious disease or, less frequently, by vaccination (Wingerchuk, 2003, Karussis and Petrou, 2014), and seasonal accumulation of the disease in winter and spring months has been reported (Dale et al., 2000, Leake et al., 2004). Clinical diagnostic criteria are currently only available for the pediatric age group and include neurologic symptoms and specific MRI features and are summarized in Table 19.2 (Krupp et al., 2013). A subgroup of patients with ADEM has antibodies to a conformational epitope of MOG in serum. These antibodies are particularly prevalent in children and can be used as biomarkers. Relapsing variants of ADEM are multiphasic disseminated encephalomyelitis (MDEM) and ADEM followed by episodes of optic neuritis (ADEMON). Recurrences in MDEM can either affect different brain loci or occur at the same site in each relapse (Cohen et al., 2001) and may reflect either a specific susceptibility or an antigenic modification in the affected brain areas after a local infection. The rate of recurrent variants of ADEM has been reported in up to one-third of patients (Anlar et al., 2003) and the clinical differentiation to MS in these patients is particularly challenging.
Table 19.2.
Diagnostic criteria of acute dissemimated encephalomyelitis (Krupp et al., 2013)
All of the following requirements:
|
Pathologic features of ADEM
The characteristic lesions of ADEM consist of small veins that are surrounded by foamy macrophages with or without T-cell-dominated inflammatory infiltrates (Fig. 19.11A). The adjacent parenchyma shows active demyelination with macrophages that contain luxol fast blue-positive myelin components and neutral lipids (Fig. 19.11 B and C). The demyelination is restricted to the perivascular region and axons are relatively preserved but also show features of acute injury (Young et al., 2010). The vessel walls may sometimes show fibrinous exudates and the adjacent parenchyma may undergo necrosis, indicating an overlap of ADEM and acute hemorrhagic leukoencephalitis. In typical ADEM the demyelinating perivenous lesions appear to be of the same histologic age.
Fig. 19.11.
Acute disseminated encephalomyelitis (ADEM). Lesions in ADEM are characterized by small perivenous demyelinating areas (A, luxol fast blue (LFB)), with numerous macrophages containing LFB-positive degradation products (B, LFB; C, CD68). A, C, × 100; B, × 600.
The lesions can be located throughout the CNS or limited to a single region. Although they are most numerous in white matter, the lesions can also involve the cortex, thalamus, and basal ganglia (Tenembaum et al., 2002). Involvment of spinal cord, brainstem, and cerebellum is also common. Although the occurrence of nonconfluent perivenous lesions is characteristic of ADEM and distinguishes them from the large confluent perivenous lesions of MS (Young et al., 2010), there are occasional patients in whom the clincal history is suggestive of ADEM but pathology overlaps with MS. Whether this represents a different pathogenic process (e.g., anti-MOG antibody-associated ADEM) or patients who have a higher likelihood to convert to MS remains to be clarified.
Immunopathogenesis
ADEM is usually preceded by infectious diseseases or, less frequently, by vaccination. It has been shown that some myelin peptides resemble antigens of viruses such as influenza virus, Epstein–Barr virus, human herpesvirus-6, or coronavirus (Giovannoni et al., 2006). Thus it has been postulated that the infectious agents may stimulate T cells that subsequently attack similar or even identical CNS epitopes. Alternatively, a direct infection of the brain parenchyma exposes CNS antigens to the immune system, affects the BBB, and triggers the autoimmune disease (Steiner and Kennedy, 2015). Finally it has been shown that certain MHC class II alleles are associated with the condition (Woody et al., 1989).
MOG Antibody-Associated Demyelinating Encephalomyelitis
Clinical and diagnostic features
MOG is a minor myelin protein and expressed on the surface of CNS myelin sheaths (Delarasse et al., 2006). MOG belongs to the immunoglobulin superfamily and is believed to play a role as surface receptor or cell adhesion molecule (Martini and Schachner, 1986). Antibodies of the IgG1 subclass against MOG have been found in serum of patients with inflammatory demyelination, including a subgroup of patients with ADEM, ADEMON, seronegative (AQP4-negative) NMOSD, monophasic, or recurrent isolated optic neuritis and transverse myelitis, MDEM, N-methyl-d-aspartate receptor encephalitis overlapping with demyelinating syndromes, and, rarely, MS (Di Pauli et al., 2011, Mader et al., 2011, Probstel et al., 2011, Kitley et al., 2012, Kitley et al., 2014, Rostasy et al., 2012, Rostasy et al., 2013, Huppke et al., 2013, Sato et al., 2014, Titulaer et al., 2014, Hoftberger et al., 2015b, Baumann et al., 2016, Jarius et al., 2016b).
The detection of MOG antibodies in serum and CSF of patients depends on the use of appropriate test methods that conserve the conformation of the protein, such as in live cell-based assays transfected with the full-length human MOG (Mader et al., 2011). MOG antibodies that are tested with these methods serve as sensitive and specific diagnostic markers. MOG antibodies are particularly prevalent among pediatric ADEM patients, where they occur in up to 50%. Children with MOG antibody-positive ADEM show a characteristic MRI pattern with large, hazy, bilateral lesions and wide anatomic involvement, including the myelon with longitudinal extensive transverse myelitis (Baumann et al., 2015). While MOG antibodies are usually transient in patients with ADEM and associated with a monophasic disease course (Probstel et al., 2011), they often persist in patients with optic neuritis and myelitis and may be associated with a relapsing course and severe disability in a substantial number of patients (Jarius et al., 2016b). Demyelinating diseases associated with MOG antibodies are often difficult to categorize and some patients meet the diagnostic criteria of AQP4 antibody-positive NMOSD, while others can be classified as MS. Since it is assumed that all MOG antibody-positive patients have the same underlying immunopathogenesis that requires the same therapy, it has been suggested that it may be useful to subsume them as a distinct disease entity. It is important to note that the therapeutic response in MOG antibody-positive patients is similar to that in NMO, but different from that in MS patients (Jarius et al., 2016b).
Pathologic features of MOG antibody-associated demyelinating encephalomyelitis
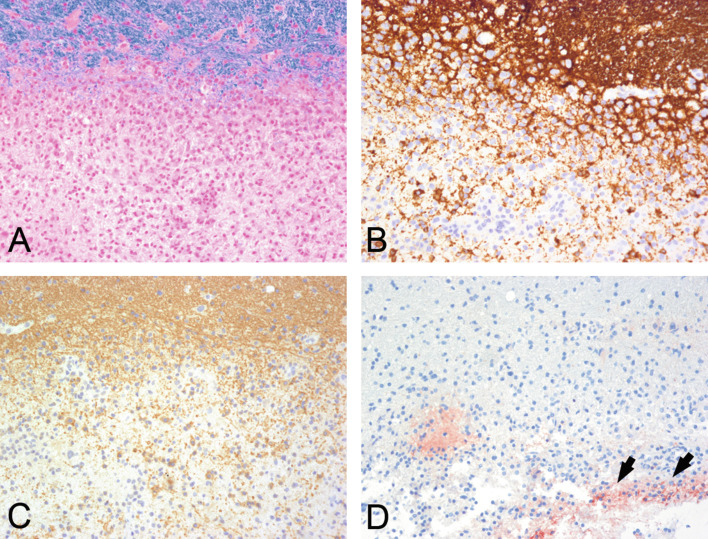
Neuropathology of MOG-antibody associated demyelination has been described in a few patients with relapsing remitting MS, atypical demyelination, clinically isolated syndrome, ADEM, and NMOSD (Table 19.3 ) (Konig et al., 2008, Di Pauli et al., 2015, Spadaro et al., 2015, Jarius et al., 2016a, Wang et al., 2016). Irrespective of the clinical nosology, all cases showed demyelinating features of MS pattern 2, with well-demarcated confluent plaques with loss of myelin (Fig. 19.12A), relative preservation of axons, numerous macrophages, and well-preserved astrocytes. The inflammaotry infiltrates were predominantely composed of perivascular and parenchymal T cells and some perivascular B cells. Lesions were characterized by relatively well-preserved, partly MOG-negative oligodendrocytes, most likely compatible with preoligodendrocytes (Fig. 19.12 B and C) and deposition of terminal complement complex C9neo, indicating a complement-mediated demyelination (Fig. 19.12D). Whether MOG antibodies have a pathogenetic role in disease formation is unclear. Different EAE animal models indicate that MOG antibodies can induce demyelinatin in vitro and in vivo (Linington et al., 1988, Zhou et al., 2006). However, it cannot be excluded that in humans they represent a bystander phenomenon, secondary immune reaction, or even may mediate a beneficial effect (Reindl et al., 2013).
Table 19.3.
Neuropathological reports of myelin oligodendrocyte glycoprotein (MOG) antibody-associated demyelination
| Demyelinating disease | Sex, age (years) | n | Reference | Neuropathology |
|---|---|---|---|---|
| Relapsing remitting MS | F, 49 | 1 | Konig et al., 2008 | MS pattern II; oligodendrocytes preserved (CNPase +; MOG n.d.) |
| Recurrent myelitis with brainstem involvement | F, 66 | 1 | Spadaro et al., 2015 | MS pattern II; oligodendrocytes preserved (CNPase +; MOG–) |
| ADEM/acute MS; seropositive for MOG and AQP4 | M, 71 | 1 | Di Pauli et al., 2011 | MS-pattern II; oligodendrocytes preserved (CNPase +, MOG–) |
| Clinically isolated syndrome | F, 63 | 1 | Jarius et al., 2016a | MS pattern II; oligodendrocytes preserved (CNPase +, MOG +) |
| NMOSD | F, 67 | 1 | Wang et al., 2016 | Pattern classification not done; well-demarcated demyelinating lesion with preserved astrocytes and axons |
| ADEM intrathecal MOG-antibody synthesis |
M, 49 M, 34 |
2 | Körtvelyessy et al., 2017 | Case 1: overlapping features of MS pattern II and III (early MAG loss, apoptotic oligodendrocytes in addition to complement deposition) Case 2: MS pattern III; oligodendrocytes preserved (CNPase +, MOG +) |
ADEM, acute dissemimated encephalomyelitis; AQP4, aquaporin-4; MAG, myelin-associated glycoprotein; MS, multiple sclerosis; NMOSD, neuromyelitis optica and spectrum disorders.
Fig. 19.12.
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated demyelinating encephalomyelitis. A demyelinating lesion associated with MOG antibodies is well demarcated (A, luxol fast blue) and shows relatively well-preserved (B, CNPase; C, MOG) oligodendrocytes and deposition of C9 neoantigen (D, arrows). × 200.
Human Experimental Autoimmune Encephalomyelitis
Human EAE is produced by an unintentional inoculation or vaccination of CNS tissue. Cases have been described in workers of a slaughterhouse, who were repeatedly exposed to an aerosol of brain tissue and developed polyradiculoneuropathy or an ADEM-like condition (Lachance et al., 2010). Moreover, human EAE occurred in association with rabies vaccination using vaccines which contain brain tissue (Semple-type rabies vaccine) (Stuart and Krikorian, 1928, Kulkarni et al., 2004) or after treatment with fresh brain cells or lyophilized brain tissue, which was used in alternative medicine (Jellinger and Seitelberger, 1958). Most patients who survived showed a monophasic, self-limiting disease without evidence of relapse and were reminiscent of clinical and pathologic features of ADEM or inflammatory demyelinating polyneuropathy (Lachance et al., 2010). A rare subset of patients, however, showed a pathologic picture closely resembling acute MS (Uchimura and Shiraki, 1957, Jellinger and Seitelberger, 1958). A detailed immunopathologic analysis of one of these cases revealed close similarities regarding inflammation, demyelination, and neurodegneration to those in acute MS, while essential pathologic features differed from those seen in EAE animal models in primates or rodents (Hoftberger et al., 2015a). However, none of these patients developed chronic disease, when the active sensitization was ceased and the patient survived the acute or subacute disease episode.
References
- Aboul-Enein F., Rauschka H., Kornek B. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- Aloisi F., Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nature reviews. Immunology. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Anlar B., Basaran C., Kose G. Acute disseminated encephalomyelitis in children: outcome and prognosis. Neuropediatrics. 2003;34:194–199. doi: 10.1055/s-2003-42208. [DOI] [PubMed] [Google Scholar]
- Babbe H., Roers A., Waisman A. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balo J. Encephalitis periaxialis concentrica. Arch Neurol Psychiatry. 1928;19:242–264. [Google Scholar]
- Barkhof F., Filippi M., Miller D.H. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120(Pt 11):2059–2069. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- Baumann M., Sahin K., Lechner C. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86:265–272. doi: 10.1136/jnnp-2014-308346. [DOI] [PubMed] [Google Scholar]
- Baumann M., Hennes E.M., Schanda K. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult Scler. 2016;22:1821–1829. doi: 10.1177/1352458516631038. [DOI] [PubMed] [Google Scholar]
- Beltran E., Obermeier B., Moser M. Intrathecal somatic hypermutation of IgM in multiple sclerosis and neuroinflammation. Brain. 2014;137:2703–2714. doi: 10.1093/brain/awu205. [DOI] [PubMed] [Google Scholar]
- Billiau A., Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–860. [PubMed] [Google Scholar]
- Bjartmar C., Kidd G., Mork S. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- Boppana S., Huang H., Ito K. Vol. 78. 2011. Immunologic aspects of multiple sclerosis; pp. 207–220. (The Mount Sinai Journal of Medicine). New York. [DOI] [PubMed] [Google Scholar]
- Boven L.A., Van Meurs M., Van Zwam M. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129:517–526. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- Bramow S., Frischer J.M., Lassmann H. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133:2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- Brosnan C.F., Raine C.S. The astrocyte in multiple sclerosis revisited. Glia. 2013;61:453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- Brown W.J. The capillaries in acute and subacute multiple sclerosis plaques: a morphometric analysis. Neurology. 1978;28:84–92. doi: 10.1212/wnl.28.9_part_2.84. [DOI] [PubMed] [Google Scholar]
- Bruck W., Porada P., Poser S. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Cayrol R., Wosik K., Berard J.L. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Chang A., Tourtellotte W.W., Rudick R. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Charcot J.M. Histologie de le sclerose en plaques. Gazette Hopitaux. 1868;41(557–558):566. [Google Scholar]
- Chari D.M., Crang A.J., Blakemore W.F. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. J Neuropathol Exp Neurol. 2003;62:908–916. doi: 10.1093/jnen/62.9.908. [DOI] [PubMed] [Google Scholar]
- Charles P., Reynolds R., Seilhean D. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain. 2002;125:1972–1979. doi: 10.1093/brain/awf216. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O., Barkhof F., Bodini B. Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. The Lancet. Neurology. 2014;13:807–822. doi: 10.1016/S1474-4422(14)70101-2. [DOI] [PubMed] [Google Scholar]
- Cohen O., Steiner-Birmanns B., Biran I. Recurrence of acute disseminated encephalomyelitis at the previously affected brain site. Arch Neurol. 2001;58:797–801. doi: 10.1001/archneur.58.5.797. [DOI] [PubMed] [Google Scholar]
- Dal-Bianco A., Grabner G., Kronnerwetter C. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25–42. doi: 10.1007/s00401-016-1636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R.C., de Sousa C., Chong W.K. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(Pt 12):2407–2422. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- Davies S., Nicholson T., Laura M. Spread of T lymphocyte immune responses to myelin epitopes with duration of multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:371–377. doi: 10.1093/jnen/64.5.371. [DOI] [PubMed] [Google Scholar]
- Delarasse C., Della Gaspera B., Lu C.W. Complex alternative splicing of the myelin oligodendrocyte glycoprotein gene is unique to human and non-human primates. J Neurochem. 2006;98:1707–1717. doi: 10.1111/j.1471-4159.2006.04053.x. [DOI] [PubMed] [Google Scholar]
- Devic E. Myelite subaigue compliquee de nevrite optique. Le Bulletin Medical (Paris) 1894;8:1033–1034. [Google Scholar]
- Dhaunchak A.S., Nave K.A. A common mechanism of PLP/DM20 misfolding causes cysteine-mediated endoplasmic reticulum retention in oligodendrocytes and Pelizaeus-Merzbacher disease. Proceedings of the National Academy of Sciences of the United States of America; 2007. pp. 17813–17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pauli F., Mader S., Rostasy K. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol. 2011;138:247–254. doi: 10.1016/j.clim.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Di Pauli F., Hoftberger R., Reindl M. Fulminant demyelinating encephalomyelitis: insights from antibody studies and neuropathology. Neurology(R) Neuroimmunology & Neuroinflammation. 2015;2:e175. doi: 10.1212/NXI.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrenberger P.F., Webb L.V., Sim M.J. Increased HLA-E expression in white matter lesions in multiple sclerosis. Immunology. 2012;137:317–325. doi: 10.1111/imm.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R., Trapp B.D. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B., Ransohoff R.M. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Enzinger C., Strasser-Fuchs S., Ropele S. Tumefactive demyelinating lesions: conventional and advanced magnetic resonance imaging. Mult Scler. 2005;11:135–139. doi: 10.1191/1352458505ms1145oa. [DOI] [PubMed] [Google Scholar]
- Ferguson B., Matyszak M.K., Esiri M.M. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Fischer M.T., Wimmer I., Hoftberger R. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain. 2013;136:1799–1815. doi: 10.1093/brain/awt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote A.K., Blakemore W.F. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128:528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- Friese M.A., Fugger L. Autoreactive CD8 + T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- Frischer J.M., Bramow S., Dal-Bianco A. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer J.M., Weigand S.D., Guo Y. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78:710–721. doi: 10.1002/ana.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsching B., Haas J., Konig F. Intracerebral human regulatory T cells: analysis of CD4 + CD25 + FOXP3 + T cells in brain lesions and cerebrospinal fluid of multiple sclerosis patients. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitan M.I., Shea C.D., Evangelou I.E. Evolution of the blood–brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70:22–29. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Cutter G.R., Lunemann J. Infectious causes of multiple sclerosis. The Lancet. Neurology. 2006;5:887–894. doi: 10.1016/S1474-4422(06)70577-4. [DOI] [PubMed] [Google Scholar]
- Goldschmidt T., Antel J., Konig F.B. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- Haider L., Fischer M.T., Frischer J.M. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L., Zrzavy T., Hametner S. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139:807–815. doi: 10.1093/brain/awv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hametner S., Wimmer I., Haider L. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol. 2013;74:848–861. doi: 10.1002/ana.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Bar-Or A., Comi G. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. New England Journal of Medicine. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- Hill K.E., Zollinger L.V., Watt H.E. Inducible nitric oxide synthase in chronic active multiple sclerosis plaques: distribution, cellular expression and association with myelin damage. J Neuroimmunol. 2004;151:171–179. doi: 10.1016/j.jneuroim.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hochmeister S., Grundtner R., Bauer J. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J Neuropathol Exp Neurol. 2006;65:855–865. doi: 10.1097/01.jnen.0000235119.52311.16. [DOI] [PubMed] [Google Scholar]
- Hoftberger R., Fink S., Aboul-Enein F. Tubulin polymerization promoting protein (TPPP/p25) as a marker for oligodendroglial changes in multiple sclerosis. Glia. 2010;58:1847–1857. doi: 10.1002/glia.21054. [DOI] [PubMed] [Google Scholar]
- Hoftberger R., Leisser M., Bauer J. Autoimmune encephalitis in humans: how closely does it reflect multiple sclerosis? Acta Neuropathol Commun. 2015;3:80. doi: 10.1186/s40478-015-0260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftberger R., Sepulveda M., Armangue T. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler. 2015;21:866–874. doi: 10.1177/1352458514555785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell O.W., Reeves C.A., Nicholas R. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134:2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- Huppke P., Rostasy K., Karenfort M. Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult Scler. 2013;19:941–946. doi: 10.1177/1352458512466317. [DOI] [PubMed] [Google Scholar]
- Huseby E.S., Liggitt D., Brabb T. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. Journal of Experimental Medicine. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifergan I., Kebir H., Alvarez J.I. Central nervous system recruitment of effector memory CD8 + T lymphocytes during neuroinflammation is dependent on alpha4 integrin. Brain. 2011;134:3560–3577. doi: 10.1093/brain/awr268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S., Metz I., Konig F.B. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in ‘pattern II multiple sclerosis' and brain biopsy findings in a MOG-IgG-positive case. Mult Scler. 2016;22:1541–1549. doi: 10.1177/1352458515622986. [DOI] [PubMed] [Google Scholar]
- Jarius S., Ruprecht K., Kleiter I. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K., Seitelberger F. Acute fatal demyelinizing encephalitis after repeated injections of dry brain cells. Klin Wochenschr. 1958;36:437–441. doi: 10.1007/BF01478731. [DOI] [PubMed] [Google Scholar]
- Johns T.G., Bernard C.C. The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem. 1999;72:1–9. doi: 10.1046/j.1471-4159.1999.0720001.x. [DOI] [PubMed] [Google Scholar]
- Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Kidd D., Barkhof F., McConnell R. Cortical lesions in multiple sclerosis. Brain. 1999;122(Pt 1):17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- Kim S.M., Woodhall M.R., Kim J.S. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurology(R) Neuroimmunology & Neuroinflammation. 2015;2:e163. doi: 10.1212/NXI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J., Plumb J., Mirakhur M. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. Journal of Pathology. 2003;201:319–327. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- Kitley J., Woodhall M., Waters P. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–1277. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- Kitley J., Waters P., Woodhall M. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71:276–283. doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- Kolasinski J., Stagg C.J., Chance S.A. A combined post-mortem magnetic resonance imaging and quantitative histological study of multiple sclerosis pathology. Brain. 2012;135:2938–2951. doi: 10.1093/brain/aws242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig F.B., Wildemann B., Nessler S. Persistence of immunopathological and radiological traits in multiple sclerosis. Arch Neurol. 2008;65:1527–1532. doi: 10.1001/archneur.65.11.1527. [DOI] [PubMed] [Google Scholar]
- Kornek B., Storch M.K., Weissert R. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. American Journal of Pathology. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körtvelyessy P., Breu M., Pawlitzki M. ADEM-like presentation, anti-MOG antibodies and MS pathology: two case reports. Neurology(R) Neuroimmunology & Neuroinflammation (in press) 2017 doi: 10.1212/NXI.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter M.R., Stadelmann C., Hartung H.P. Enhancing remyelination in disease – can we wrap it up? Brain. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- Krupp L.B., Tardieu M., Amato M.P. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T., Lingfeld G., Bitsch A. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T., Lassmann H., Bruck W. Diagnosis of inflammatory demyelination in biopsy specimens: a practical approach. Acta Neuropathol. 2008;115:275–287. doi: 10.1007/s00401-007-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T., Ludwin S., Prat A. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13–24. doi: 10.1007/s00401-016-1653-y. [DOI] [PubMed] [Google Scholar]
- Kulkarni V., Nadgir D., Tapiawala S. Biphasic demyelination of the nervous system following anti-rabies vaccination. Neurol India. 2004;52:106–108. [PubMed] [Google Scholar]
- Kutzelnigg A., Lucchinetti C.F., Stadelmann C. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Lachance D.H., Lennon V.A., Pittock S.J. An outbreak of neurological autoimmunity with polyradiculoneuropathy in workers exposed to aerosolised porcine neural tissue: a descriptive study. The Lancet. Neurology. 2010;9:55–66. doi: 10.1016/S1474-4422(09)70296-0. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C., Goebbels S., Gravel M. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Cortical lesions in multiple sclerosis: inflammation versus neurodegeneration. Brain. 2012;135:2904–2905. doi: 10.1093/brain/aws260. [DOI] [PubMed] [Google Scholar]
- Leake J.A., Albani S., Kao A.S. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatric Infectious Disease Journal. 2004;23:756–764. doi: 10.1097/01.inf.0000133048.75452.dd. [DOI] [PubMed] [Google Scholar]
- Lennon V.A., Wingerchuk D.M., Kryzer T.J. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- Linington C., Bradl M., Lassmann H. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. American Journal of Pathology. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- Link H., Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977;37:397–401. doi: 10.1080/00365517709091498. [DOI] [PubMed] [Google Scholar]
- Lovas G., Szilagyi N., Majtenyi K. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123(Pt 2):308–317. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Bruck W., Parisi J. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C.F., Mandler R.N., McGavern D. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C.F., Gavrilova R.H., Metz I. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader S., Gredler V., Schanda K. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation. 2011;8:184. doi: 10.1186/1742-2094-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R., Howell O., Vora A. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Magliozzi R., Howell O.W., Reeves C. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68:477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- Mahad D., Ziabreva I., Lassmann H. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008;131:1722–1735. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S., Tucky B., Cotleur A. CXCL12-induced monocyte–endothelial interactions promote lymphocyte transmigration across an in vitro blood–brain barrier. Sci Transl Med. 2012;4:119ra114. doi: 10.1126/scitranslmed.3003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marburg O. Die sogenannte `akute multiple Sklerose’ (Encephalomyelitis periaxialis scleroticans) Jahrbücher für Psychiatrie und Neurologie (Leipzig) 1906:213–311. [Google Scholar]
- Martini R., Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. Journal of Cell Biology. 1986;103:2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey E.K., Derfuss T., Storch M.K. Neurofascin as a novel target for autoantibody-mediated axonal injury. Journal of Experimental Medicine. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W.I., Compston A., Edan G. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Metz I., Lucchinetti C.F., Openshaw H. Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain. 2007;130:1254–1262. doi: 10.1093/brain/awl370. [DOI] [PubMed] [Google Scholar]
- Mews I., Bergmann M., Bunkowski S. Oligodendrocyte and axon pathology in clinically silent multiple sclerosis lesions. Mult Scler. 1998;4:55–62. doi: 10.1177/135245859800400203. [DOI] [PubMed] [Google Scholar]
- Misu T., Hoftberger R., Fujihara K. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 2013;125:815–827. doi: 10.1007/s00401-013-1116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll N.M., Rietsch A.M., Ransohoff A.J. Cortical demyelination in PML and MS: similarities and differences. Neurology. 2008;70:336–343. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- Montalban X., Hauser S.L., Kappos L. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New England Journal of Medicine. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- Na S.Y., Cao Y., Toben C. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131:2353–2365. doi: 10.1093/brain/awn148. [DOI] [PubMed] [Google Scholar]
- Na S.Y., Hermann A., Sanchez-Ruiz M. Oligodendrocytes enforce immune tolerance of the uninfected brain by purging the peripheral repertoire of autoreactive CD8 + T cells. Immunity. 2012;37:134–146. doi: 10.1016/j.immuni.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Neumann H., Medana I.M., Bauer J. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- Ng J.K., Malotka J., Kawakami N. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology. 2012;79:2241–2248. doi: 10.1212/WNL.0b013e31827689ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B., Lovato L., Mentele R. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. J Neuroimmunol. 2011;233:245–248. doi: 10.1016/j.jneuroim.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis A., Bernier S., Dumais N. TLR4 induces CCR7-dependent monocytes transmigration through the blood–brain barrier. J Neuroimmunol. 2016;295–296:12–17. doi: 10.1016/j.jneuroim.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Patrikios P., Stadelmann C., Kutzelnigg A. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Peterson J.W., Bo L., Mork S. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Pittock S.J., Weinshenker B.G., Lucchinetti C.F. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63:964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- Polman C.H., O'Connor P.W., Havrdova E. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. New England Journal of Medicine. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Prineas J.W., Kwon E.E., Goldenberg P.Z. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. [PubMed] [Google Scholar]
- Prineas J.W., Barnard R.O., Revesz T. Multiple sclerosis. Pathology of recurrent lesions. Brain. 1993;116(Pt 3):681–693. doi: 10.1093/brain/116.3.681. [DOI] [PubMed] [Google Scholar]
- Probstel A.K., Dornmair K., Bittner R. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology. 2011;77:580–588. doi: 10.1212/WNL.0b013e318228c0b1. [DOI] [PubMed] [Google Scholar]
- Reindl M., Di Pauli F., Rostasy K. The spectrum of MOG autoantibody-associated demyelinating diseases. Nature Reviews. Neurology. 2013;9:455–461. doi: 10.1038/nrneurol.2013.118. [DOI] [PubMed] [Google Scholar]
- Roemer S.F., Parisi J.E., Lennon V.A. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- Rostasy K., Mader S., Schanda K. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol. 2012;69:752–756. doi: 10.1001/archneurol.2011.2956. [DOI] [PubMed] [Google Scholar]
- Rostasy K., Mader S., Hennes E.M. Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult Scler. 2013;19:1052–1059. doi: 10.1177/1352458512470310. [DOI] [PubMed] [Google Scholar]
- Sadaba M.C., Tzartos J., Paino C. Axonal and oligodendrocyte-localized IgM and IgG deposits in MS lesions. J Neuroimmunol. 2012;247:86–94. doi: 10.1016/j.jneuroim.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Sato D.K., Callegaro D., Lana-Peixoto M.A. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., Bauer J., Scheikl T. Cutting edge: multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. 2008;181:1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- Schluesener H.J., Sobel R.A., Linington C. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987;139:4016–4021. [PubMed] [Google Scholar]
- Segal B.M., Constantinescu C.S., Raychaudhuri A. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. The Lancet. Neurology. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- Sepulveda M., Armangue T., Martinez-Hernandez E. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol. 2016;263:1349–1360. doi: 10.1007/s00415-016-8147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Magliozzi R. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Fischer M.T., Bauer J. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 2010;120:223–236. doi: 10.1007/s00401-010-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D.L., Tait S., Melrose S. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Spadaro M., Gerdes L.A., Mayer M.C. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Annals of Clinical and Translational Neurology. 2015;2:295–301. doi: 10.1002/acn3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C., Ludwin S., Tabira T. Tissue preconditioning may explain concentric lesions in Balo's type of multiple sclerosis. Brain. 2005;128:979–987. doi: 10.1093/brain/awh457. [DOI] [PubMed] [Google Scholar]
- Steiner I., Kennedy P.G. Acute disseminated encephalomyelitis: current knowledge and open questions. J Neurovirol. 2015;21:473–479. doi: 10.1007/s13365-015-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G., Krikorian K.S. The neuro-paralytic accidents of anti-rabies treatment. Ann Trop Med Parasitol. 1928;22:327–377. [Google Scholar]
- Takeshita Y., Ransohoff R.M. Inflammatory cell trafficking across the blood–brain barrier: chemokine regulation and in vitro models. Immunol Rev. 2012;248:228–239. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Bo L., Al-Rawashdeh O. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult Scler. 2010;16:406–411. doi: 10.1177/1352458510364992. [DOI] [PubMed] [Google Scholar]
- Tenembaum S., Chamoles N., Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- Thangarajh M., Gomez-Rial J., Hedstrom A.K. Lipid-specific immunoglobulin M in CSF predicts adverse long-term outcome in multiple sclerosis. Mult Scler. 2008;14:1208–1213. doi: 10.1177/1352458508095729. [DOI] [PubMed] [Google Scholar]
- Thompson S.A., Jones J.L., Cox A.L. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol. 2010;30:99–105. doi: 10.1007/s10875-009-9327-3. [DOI] [PubMed] [Google Scholar]
- Tintore M., Rovira A., Martinez M.J. Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. AJNR. Am J Neuroradiol. 2000;21:702–706. [PMC free article] [PubMed] [Google Scholar]
- Titulaer M.J., Hoftberger R., Iizuka T. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75:411–428. doi: 10.1002/ana.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D., Peterson J., Ransohoff R.M. Axonal transection in the lesions of multiple sclerosis. New England Journal of Medicine. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Uchimura I., Shiraki H. A contribution to the classification and the pathogenesis of demyelinating encephalomyelitis; with special reference to the central nervous system lesions caused by preventive inoculation against rabies. J Neuropathol Exp Neurol. 1957;16:139–203. doi: 10.1097/00005072-195704000-00001. discussion, 203–238. [DOI] [PubMed] [Google Scholar]
- van der Goes A., Boorsma W., Hoekstra K. Determination of the sequential degradation of myelin proteins by macrophages. J Neuroimmunol. 2005;161:12–20. doi: 10.1016/j.jneuroim.2004.12.010. [DOI] [PubMed] [Google Scholar]
- van Oosten B.W., Lai M., Hodgkinson S. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49:351–357. doi: 10.1212/wnl.49.2.351. [DOI] [PubMed] [Google Scholar]
- van Waesberghe J.H., Kamphorst W., De Groot C.J. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- Vogel D.Y., Vereyken E.J., Glim J.E. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman B.H. The distribution of experimental auto-allergic lesions. Its relation to the distribution of small veins. American Journal of Pathology. 1960;37:673–693. [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Jaunmuktane Z., Mummery C. Inflammatory demyelination without astrocyte loss in MOG antibody-positive NMOSD. Neurology. 2016;87:229–231. doi: 10.1212/WNL.0000000000002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk D.M. Postinfectious encephalomyelitis. Curr Neurol Neurosci Rep. 2003;3:256–264. doi: 10.1007/s11910-003-0086-x. [DOI] [PubMed] [Google Scholar]
- Wingerchuk D.M. The clinical course of acute disseminated encephalomyelitis. Neurol Res. 2006;28:341–347. doi: 10.1179/016164106X98251. [DOI] [PubMed] [Google Scholar]
- Wingerchuk D.M., Banwell B., Bennett J.L. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody R.C., Steele R.W., Charlton R.K. Histocompatibility determinants in childhood postinfectious encephalomyelitis. J Child Neurol. 1989;4:204–207. doi: 10.1177/088307388900400311. [DOI] [PubMed] [Google Scholar]
- Young N.P., Weinshenker B.G., Parisi J.E. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010;133:333–348. doi: 10.1093/brain/awp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Srivastava R., Nessler S. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America; 2006. pp. 19057–19062. [DOI] [PMC free article] [PubMed] [Google Scholar]