Abstract
Membrane-anchored serine proteases are a group of extracellular serine proteases tethered directly to plasma membranes, via a C-terminal glycosylphosphatidylinositol linkage (GPI-anchored), a C-terminal transmembrane domain (Type I), or an N-terminal transmembrane domain (Type II). A variety of biochemical, cellular, and in vivo studies have established that these proteases are important pericellular contributors to processes vital for the maintenance of homeostasis, including food digestion, blood pressure regulation, hearing, epithelial permeability, sperm maturation, and iron homeostasis. These enzymes are hijacked by viruses to facilitate infection and propagation, and their misregulation is associated with a wide range of diseases, including cancer malignancy.
Keywords: CAP, Corin, HAT, Hepsin, Matriptase, Membrane-anchored, MSPL, Pericellular proteolysis, Polyserase, Prostasin, Protease, PRSS, Serine protease, Testisin, TTSP
Introduction
Proteases constitute approximately 2% of the human proteome and are critically important for numerous biological processes such as blood coagulation, cell death, tissue morphogenesis, inflammation, and wound healing. Proteases are ubiquitously expressed and can be found secreted into the extracellular environment, anchored to the cell surface, in the cell cytosol or compartmentalized in cellular organelles such as lysosomes. Through cleavage of specific peptide bonds in proteins that are recognized as substrates, proteases mediate many cellular functions including protein degradation, enzymatic activation, and induction of cellular signaling (reviewed in Lopez-Otin and Bond, 2008). Serine proteases are one of the largest families of proteolytic enzymes, constituting over one third of all proteolytic enzymes, and are known to play critical roles in diverse biological functions including blood coagulation, digestion and tissue homeostasis. Serine proteases are defined by the classical histidine, aspartate, and serine amino acid residues which form their catalytic triad, that mediate the process of peptide hydrolysis. Peptide hydrolysis occurs when the nucleophilic serine residue in the enzyme’s active site attacks the carbonyl moiety of the substrate peptide bond, forming an acyl intermediate, and proteolysis follows which also depends upon the histidine and aspartate residues of the enzyme (Hedstrom, 2002, Rawlings and Barrett, 2004). The S1A subfamily of serine proteases are the most widely studied group of serine proteases; the protypical members being trypsin, chymotrypsin and thrombin, which are produced as soluble proteases that are secreted into the extracellular environment. In recent years, a unique sub-group of S1A serine proteases has been identified which are found to be directly anchored to the cell surface. This review focuses on the current knowledge and in vivo functions of this family of membrane-anchored serine proteases. Further information on this family of proteases can be found in the following comprehensive reviews: Antalis et al., 2010, Bugge et al., 2009, Hooper et al., 2001, Netzel-Arnett et al., 2003, Szabo and Bugge, 2011.
Membrane-Anchored Serine Proteases
To date, 20 human and 22 mouse membrane-anchored serine proteases have been identified, which are classified into 3 subclasses based on the manner in which they are anchored to the cell surface (Figure 1 ). These proteases are anchored to the plasma membrane either via a C-terminal glycosylphosphatidylinositol (GPI) linkage (GPI-anchored), a C-terminal transmembrane domain (Type I), or an N-terminal transmembrane domain (Type II). In addition to the extracellular serine protease domain which mediates catalytic activity, the type II transmembrane serine proteases (TTSPs), possess a stem region composed of various combinations of different accessory domains localized adjacent to the cell surface and C-terminal to the serine protease domain (Figure 1; Antalis et al., 2010; Bugge et al., 2009; Netzel-Arnett et al., 2003).
Figure 1.
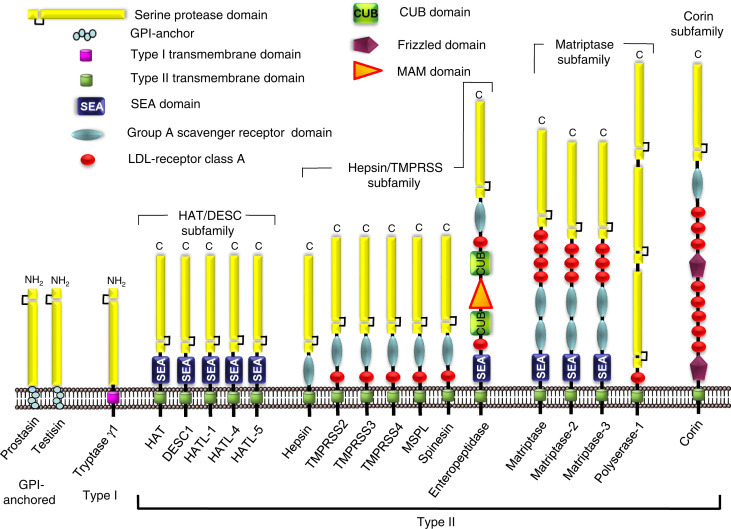
The membrane-anchored serine protease family. The human GPI-anchored serine proteases are prostasin and testisin. Tryptase γ1 is the only known human type I transmembrane serine protease. The type II transmembrane serine proteases (TTSP) may be divided into four subfamilies: (1) the human airway trypsin-like protease/differentially expressed in squamous cell carcinoma (HAT/DESC) subfamily for which the stem regions are all composed of a single SEA-domain, (2) the hepsin/transmembrane protease, serine (TMPRSS) subfamily, each of which have a group A scavenger receptor domain (SRCR) in their stem region, that may be preceded by a single LDL receptor class A-like (LDLRA) domain or in enteropeptidase, an array of SEA, LDLRA, CUB, and MAM domains, (3) the matriptase subfamily, each containing a SEA-domain, two CUB domains, and 3-4 LDLRA domains in their stem region. Polyserase-1 comprises two active, and one catalytically inactive serine protease domains and a stem region containing an LDLRA domain, and (4) the corin subfamily, consisting of a single member, corin, which possesses a complex stem region composed of two frizzled domains, eight LDLRA domains, and one SRCR domain. NH2 indicates amino terminus and C indicates carboxyl terminus.
Adapted from Antalis, T.M., Buzza, M.S., Hodge, K.M., Hooper, J.D., Netzel-Arnett, S., 2010. The cutting edge membrane-anchored serine protease activities in the pericellular microenvironment. Biochemical Journal 428 (3), 325–346 and Bugge, T.H., Antalis, T.M., Wu, Q., 2009. Type II transmembrane serine proteases. Journal of Biological Chemistry 284 (35), 23177–23181.
Catalytic Activity
The catalytic serine protease domains (SPD) are highly homologous, and are 225–230 amino acids in size. Being trypsin-like serine proteases, the membrane-anchored serine proteases all prefer to cleave peptide substrates after the basic amino acids arginine or lysine, and specificity is also influenced by the residues N- and C-terminal to the cleavage site. A comprehensive list of protein and peptide substrates cleaved by the membrane-anchored serine proteases in vitro is found in Antalis et al. (2010). Like other serine proteases, membrane-anchored serine proteases are synthesized as inactive pro-enzymes called zymogens. Proteolytic cleavage of the short N-terminal pro-peptide of the SPD is required to induce a conformationally active protease, with the cleaved pro-domain remaining attached to the protease domain by a disulfide linkage. This activation-inducing cleavage may be mediated by other serine proteases (membrane-anchored or soluble), that recognize the zymogen activation sequence of the protease as a substrate. An example of such a zymogen activation cascade is the activation of the GPI-anchored protease prostasin by the TTSP matriptase in skin (Netzel-Arnett et al., 2006). Once activated, several membrane-anchored-serine proteases can also induce zymogen activation of serine proteases of the digestive, coagulation, and fibrinolytic systems (reviewed in Antalis et al., 2010). In addition, several of the TTSPs are thought to be capable of auto-activation (Oberst et al., 2003b, Velasco et al., 2002). In these cases the amino acid sequence of zymogen activation site of the protease resembles the substrate specificity of the protease, and pro-domain cleavage is thought to occur due to a low level of catalytic activity of the protease zymogen.
Stem Region Accessory Domains
While the functions of the modular domains found in the stem regions of TTSPs are mostly uncharacterized, they are thought to contribute to the cell surface localization, substrate recognition or activation of the protease. Specific mutations in these domains have been described to occur in human diseases (Antalis et al., 2010). The sea urchin sperm protein (SEA)-domain appears to be important for inducing protease activation in matriptase and matriptase-2, and undergoes a spontaneous conformational non-enzymatic cleavage event required for protease activity (Oberst et al., 2003b, Ramsay et al., 2009). In addition, the low density lipoprotein-receptor class A-like (LDLRA) domain of matriptase-2 appears important for cell surface expression (Silvestri et al., 2009), and the frizzled and LDLRA domains of corin are important for macromolecular substrate recognition (Knappe et al., 2004).
Inhibition
After activation, the protease activity of membrane-anchored serine proteases can be regulated by interactions with endogenous protease inhibitors including membrane-anchored Kunitz-type inhibitors which form a reversible inhibitory complex with the protease. The Kunitz-type inhibitors HAI-1/SPINT1 and HAI-2/SPINT2 have been shown to regulate the activity of both matriptase and prostasin in vivo in murine models (Szabo et al., 2007, Szabo et al., 2009a, Szabo et al., 2009b, Szabo et al., 2012). In vitro, the protease activities of several membrane-anchored serine proteases can also be inhibited by various members of the serpin superfamily, which form irreversible covalent complexes with serine proteases (reviewed in Antalis et al., 2010). Inhibitory complexes between matriptase and anti-thrombin III (serpinC1), α1-proteinase inhibitor (serpinA1) and α2-antiplasmin (serpinF2) have been detected in breast milk, suggesting an inhibitory role for these serpins in vivo (Tseng et al., 2008).
While there is still much to learn about the physiological functions of individual members of this family, the development of murine models that are deficient in these proteases, and human diseases where expression or activity is reduced, have provided valuable evidence for the critical importance of many of these proteases in key biological functions as described below, and summarized in Table 1, Table 2 .
Table 1.
Membrane-anchored serine proteases – physiological functions learnt through protease deficiency
| Protease | Function | Implicated substrate | References |
|---|---|---|---|
| GPI-anchored | |||
| Prostasin | Maintenance of epidermal barrier function | Unknown | (Frateschi et al., 2012, Leyvraz et al., 2005, Peters et al., 2014) |
| Regulation of ENaC-mediated fluid clearance in lung and colon epithelium | ENaC-γ subunit | (Frateschi et al., 2012, Malsure et al., 2014, Planes et al., 2010) | |
| Regulation of hepatic insulin sensitivity by cleavage and inactivation of TLR-4 | Toll-like receptor-4 (TLR-4) | (Uchimura et al., 2014) | |
| Testisin | Involved in sperm cell maturation and fertilizing ability | Unknown | (Aimes et al., 2003, Inoue et al., 1998, Netzel-Arnett et al., 2009, Yamashita et al., 2008) |
| Type II | |||
| HAT/DESC subfamily | |||
| HAT | Unknown. Not required for development, long-term health or survival in mice | Unknown | (Bertram et al., 2012, Iwakiri et al., 2004, Takahashi et al., 2001, Yamaoka et al., 1998) |
| HATL-1 | Unknown. Not required for development, long-term health or survival in mice | Unknown | (Faller et al., 2014, Kam et al., 2009, Sales et al., 2011) |
| Hepsin/TMPRSS subfamily | |||
| Hepsin | Important for hearing in mice, where it has a role in cochlear development in inner ear | Unknown | (Faller et al., 2014, Guipponi et al., 2007, Kurachi et al., 1994, Tsuji et al., 1991) |
| Maintenance of liver structural homeostasis in mice | Pro-HGF | (Hsu et al., 2012) | |
| TMPRSS2 | Unknown. Not required for development, long-term health, fertility or survival in mice | Unknown | (Faller et al., 2014, Jacquinet et al., 2001, Kim et al., 2006) |
| TMPRSS3 | Mediates normal hearing in humans. Shown to be critical for cochlear hair cell survival in murine model of deficiency | Unknown | (Fasquelle et al., 2011, Guipponi et al., 2008, Lee et al., 2013, Molina et al., 2013) |
| TMPRSS5 | Mediates normal hearing in humans | Unknown | (Guipponi et al., 2008, Yamaguchi et al., 2002) |
| Enteropeptidase | Critical for digestion of dietary proteins. Initiates a proteolytic cascade that results in the activation of several intestinal proteases | Trypsinogen | (Haworth et al., 1971, Zheng et al., 2009) |
| Matriptase subfamily | |||
| Matriptase | Global role in maintenance of epidermal and epithelial barrier function and homeostasis. | Unknown | (List et al., 2002, List et al., 2009, Yin et al., 2014) |
| Matriptase-2 | Essential regulator of iron homeostasis that prevents iron deficiency anemia | Hemojuvelin | (Du et al., 2008, Folgueras et al., 2008, Truksa et al., 2009) |
| Corin subfamily | |||
| Corin | Regulates systemic salt and water balance to prevent hypertension and cardiac hypertrophy | Pro-ANP, pro-BNP | (Chan et al., 2005, Rame et al., 2009, Wang et al., 2008) |
Table 2.
Membrane-anchored serine proteases – roles in human disease
| Protease | Abnormality | Role in disease | References |
|---|---|---|---|
| Prostasin | Over-expressed in lung epithelium of cystic fibrosis patients | May contribute to pathogenesis of cystic fibrosis by increasing fluid clearance | (Myerburg et al., 2008, Planes et al., 2010) |
| Increased soluble prostasin detect in urine in hypertensive patients | May have a role in development of high blood pressure | (Maekawa et al., 2009, Zhu et al., 2008) | |
| Over-activity in colonic epithelium caused by loss of inhibitor function | Implicated role in fluid secretion in congenital sodium diarrhea | (Faller et al., 2014) | |
| Testisin | Aberrant expression in advanced stage ovarian cancer | May promote tumor growth and metastasis | (Shigemasa et al., 2000, Tang et al., 2005) |
| Lost in male germ cell tumors | Unknown | (Kempkensteffen et al., 2006) | |
| HAT | Increased expression and shedding into airway fluids | Occurs in patients with inflammatory airway diseases such as asthma, function is unknown | (Yasuoka et al., 1997) |
| DESC1 | Lost in head and neck squamous cell carcinoma | Unknown | (Lang and Schuller, 2001, Sedghizadeh et al., 2006) |
| HATL-5 | Significantly decreased in cervical, esophageal, and head and neck carcinomas | Unknown | (Miller et al., 2014) |
| Hepsin | Increased expression in human prostate cancers which correlates with disease severity | Increases prostate cancer progression and metastasis in mouse models | (Wu and Parry, 2007) |
| TMPRSS2 | Frequent gene fusions between the promotor of TMPRSS2 and the ERG protooncogene and related transcription factors in prostate cancers | Androgen responsive elements in TMPRSS2 promotor drive expression of ERG transcription factor to promote prostate cancer progression | (Tomlins et al., 2005, Yu et al., 2010) |
| TMPRSS3 | Point mutations that inhibit TMPRSS3 auto-activation blocking its activity | Causes non-syndromic autosomal recessive deafness | (Lee et al., 2003, Scott et al., 2001, Wattenhofer et al., 2002) |
| TMPRSS4 | Over-expressed in epithelial carcinomas of diverse origins | May have a role in tumor progression | (Choi et al., 2008) |
| TMPRSS5 | Point mutation that inactivates TMPRSS5 | Associated with human deafness | (Guipponi et al., 2008) |
| Enteropeptidase | Intestinal deficiency caused by point mutations | Failure to thrive due to reduced digestive function | (Holzinger et al., 2002) |
| Matriptase | Mutations resulting in an inactive protease | ARIH, a rare human skin disease with ichthyosis and hair follicle defects | (Avrahami et al., 2008, Basel-Vanagaite et al., 2007, Desilets et al., 2008, Lee et al., 2007) |
| Expression downregulated in inflammatory bowel diseases | May contribute to loss of intestinal barrier function and disease pathogenesis | (Kosa et al., 2012, Netzel-Arnett et al., 2012) | |
| Reduced expression in salivary gland epithelium | Causes loss of secretory cell function, contribute to pathogenesis of Sjogren's syndrome | (Yin et al., 2014) | |
| Over-expressed in epithelial carcinomas of diverse origins | Possible role in tumor progression | (List, 2009) | |
| Matriptase-2 | Mutations that affect protease expression and activation | Causal factor in familial iron-refractory iron deficiency anemia | (Finberg et al., 2008, Guillem et al., 2008, Melis et al., 2008) |
| Corin | Polymorphisms that cause reduced protease activity due to decreased zymogen activation | Associated with hypertension and cardiac hypertrophy, worse clinical outcome in patients with heart failure | (Dries et al., 2005, Rame et al., 2007, Rame et al., 2009, Wang et al., 2008) |
| Mutations and reduced expression and in pregnant uterus | May have causal role in the development of pre-eclampsia | (Cui et al., 2012) |
The GPI-Anchored Serine Proteases
The two human GPI–anchored-membrane-anchored serine proteases, prostasin and testisin, are structurally the most simple of this family, being composed solely of an N-terminal SPD, that is linked to the cell surface through a GPI moiety that is added to the C-terminus post-transcriptionally (Chen et al., 2001, Hooper et al., 1999; Figure 1). This lipid anchor is known to compartmentalize these proteases to specialized cholesterol-rich microdomains of the plasma membrane known as lipid rafts (Honda et al., 2002, Verghese et al., 2006). Prostasin, also known as PRSS8 and channel activating protease (CAP)-1, is found ubiquitously expressed in all epithelia (List et al., 2007b). In polarized epithelia such as the gastrointestinal tract and kidney, it specifically localizes to the apical (luminal) membrane (Selzer-Plon et al., 2009, Steensgaard et al., 2010, Verghese et al., 2006). Soluble forms of prostasin have been identified in both human seminal fluid and urine (Koda et al., 2009, Yu et al., 1994), with release from the cell surface shown to be mediated by endogenous phospholipases or by proteolytic shedding (Iwashita et al., 2003, Verghese et al., 2006). Functionally, prostasin was the first identified membrane-anchored serine protease shown to enhance the activity of epithelial sodium channels (ENaC) by cleavage of the ENaCγ subunit to release an inhibitory peptide (Bruns et al., 2007, Carattino et al., 2008). ENaC activity is important for regulating sodium and water flux across polarized epithelium, and in vivo, increased expression or activity of prostasin, and the associated induction of ENaC activation, may be pathologically significant in increased fluid secretion in cystic fibrosis, high blood pressure and congenial sodium diarrhea (Table 2).
In mice, genetic deficiency of prostasin in the skin leads to complete loss of skin barrier function, which appears to be unrelated to defective ENaC activation (Frateschi et al., 2012, Leyvraz et al., 2005, Peters et al., 2014). The identification of prostasin's substrate in the epidermis remains uncertain, however, since genetic deficiency of the type II membrane-anchored serine protease matriptase results in an identical epidermal defect (List et al., 2002), it is thought that these proteases participate in a zymogen activation cascade that mediates epidermal barrier function. This is also supported by in vitro studies showing that expression of either protease is able to induce the activation of the other (Buzza et al., 2013, Netzel-Arnett et al., 2006). Interestingly, one study using murine models suggests that prostasin may mediate skin barrier function by a mechanism that is independent of its catalytic activity (Peters et al., 2014). Using a murine model of prostasin deficiency in the liver, Uchimura et al. found that prostasin regulates hepatic insulin signaling by the cleavage and inactivation of the cell surface toll-like receptor, TLR-4, which suppresses inflammatory signaling (Uchimura et al., 2014).
Testisin (also known as ESP-1 and PRSS21), in contrast to prostasin, exhibits an extremely specific and restricted tissue distribution, being abundantly expressed solely in male germ cells and spermatocytes, with lower expression also identified in microvascular endothelial cells, and eosinophils (Aimes et al., 2003, Inoue et al., 1998, Yamashita et al., 2008). Functionally testisin is important for sperm cell maturation and fertilizing ability in mice (Netzel-Arnett et al., 2009, Yamashita et al., 2008), although the physiological substrate that mediates this activity is unknown. Pathologically, the aberrant testisin expression found in lung cancers and advanced ovarian cancers may contribute to tumour progression (Shigemasa et al., 2000, Tang et al., 2005), and the consequences of its loss in male germ cell tumors remains to be determined.
The Type I Transmembrane Serine Proteases
Tryptase γ1 (also known as PRSS31, transmembrane tryptase and transmembrane protease γ-1) is found only in hematopoietic cells and is stored as the major component of mast cell secretory granules (Caughey et al., 2000, Wong et al., 1999, Wong et al., 2002b). Upon mast cell degranulation, tryptase γ1 is retained on the cell surface by its N-terminal transmembrane domain (Wong et al., 2002a). The physiological function and substrates of tryptase γ1 remain unknown, but it is speculated that it may play a role in pathogen host defense in the human airway, where mast cells are important for protection against bacterial infections (Wong et al., 2002a). In an animal model of exogenous administration to the airway, tryptase γ1 promoted airway hyperresponsiveness and induced the expression of interleukin-13 (IL-13) in bronchiolar lavage fluid (Wong et al., 2002a), which is a key cytokine implicated in the pathogenesis of allergic asthma.
The Type II Transmembrane Serine Proteases
TTSPs are by far the largest group of membrane-anchored serine proteases, with 17 members in humans and 19 members in mice (Bugge et al., 2009, Szabo et al., 2003; Figure 1). All are anchored to the cell surface by a C-terminal transmembrane domain, and have been phylogenetically divided into 4 subfamilies: (1) the human airway trypsin-like (HAT)/differentially expressed in squamous cell carcinoma gene (DESC) subfamily, (2) the hepsin/transmembrane protease, serine (TMPRSS) subfamily, (3) the matriptase subfamily, and (4) the corin subfamily. In comparison to the GPI-anchored and type I serine proteases, which are composed of essentially a SPD and membrane anchor, the TTSPs possess a stem region C-terminal to the protease domain, which is comprised of a variety of modular structural accessory domains (Figure 1), most of which are currently of unknown function, but which are likely to play roles in protease activation, localization, and substrate recognition (Bugge et al., 2009).
The HAT/DESC Subfamily
This subfamily is composed of 5 members in humans; HAT, DESC-1, HAT like-1 (HATL-1), HATL-4 and HATL-5, with mice having two additional members HATL-2 and HATL-3. This subfamily is the simplest of the TTSPs, having just one modular SEA-domain in their stem region. The role of this domain in HAT/DESC protease function has not been determined, but in other TTSPs such as matriptase, this domain undergoes a spontaneous conformation-induced auto-processing event at a conserved glycine residue which is important for protease activation (Macao et al., 2006, Oberst et al., 2003b). As its name implies, HAT protease (also known as TMPRSS11D) is found predominantly expressed in the epithelium of the airways, where it was originally identified as a soluble form found in extracellular lung fluids of asthma patients (Takahashi et al., 2001, Yamaoka et al., 1998, Yasuoka et al., 1997). The normal physiological function of HAT is unknown, and deletion of the gene in mice caused no phenotypical defects in development or long-term health under non-challenged conditions (Sales et al., 2011). Pathologically, HAT is up-regulated in chronic airway diseases (Yamaoka et al., 1998), and has been shown in vitro to increase cell proliferation, and modulate inflammatory processes including increasing mucin production and suppression of fibrin deposition in airway epithelial cultures, suggesting it may play a role in disease suppression (Liu et al., 2013, Matsushima et al., 2006, Yoshinaga et al., 1998). In vitro, HAT has been shown to cleave the urokinase plasminogen activator receptor (uPAR) (Beaufort et al., 2007) and protease activated receptor (PAR)-2 (Iwakiri et al., 2004), although the in vivo relevance of these substrates remains to be demonstrated. Interestingly, HAT may play a role in the propagation and spread of human respiratory viruses. HAT has been shown to cleave and activate the influenza virus hemaglutinin (HA) glycoprotein (Baron et al., 2013, Bertram et al., 2010), and the severe acute respiratory syndrome (SARS) coronavirus spike protein (Bertram et al., 2011), both of which are important for mediating host cell entry, suggesting that inhibition of HAT activity may be a good target for therapeutic intervention in these infections.
Little is known regarding the in vivo function of the other members of this subfamily, which are expressed predominantly in epithelial cells of various organs. DESC-1 (also known as TMPRSS11E) is expressed in the epidermis, prostate, testis, placenta, thymus, and the epithelium of the head and neck (Lang and Schuller, 2001, Sales et al., 2011, Sedghizadeh et al., 2006), where it was originally identified based on it's loss of expression in head and neck squamous cell carcinomas (Sales et al., 2011). HATL-1 (also known as TMPRSS11A) is most highly expressed in the esophagus and trachea (Faller et al., 2014, Kam et al., 2009), and like HAT, the HATL-1 null mouse shows no overt phenotype (Sales et al., 2011). Similarly, this protease may mediate the spread of SARS-corona virus through cleavage of the viral host cell entry spike glycoprotein (Kam et al., 2009). HATL-4 (also known as TMPRSS11F) mRNA is expressed in the esophagus and testis, with lower expression in the cervix, placenta, and trachea (Sales et al., 2011). HATL-5 (also known as TMPRSS11B) displays a relatively restricted tissue expression profile, being expressed in the cervix, esophagus, and oral cavity, with lower expression in the kidney and testis (Miller et al., 2014, Sales et al., 2011). This protease is found expressed in the more differentiated epithelial cells of cervix, esophagus, and trachea (Miller et al., 2014), and is lost in squamous cell carcinomas of these tissues.
The Hepsin/TMPRSS Subfamily
This subfamily is composed of seven members in humans and mice. Hepsin possesses only one additional domain in its stem region, a group A scavenger receptor domain. TMPRSS2, TMPRSS3, TMPRSS4, mosaic serine protease large-form (MSPL), and spinesin have an additional low density lipoprotein receptor class A (LDLA) domain N-terminal to the scavenger domain, while enteropeptidase is much more complex containing multiple other domains types (Figure 1). Hepsin (also known as TMPRSS1), is abundantly expressed in the liver, but is also found at lower levels in other tissues such as the kidney, stomach, prostate, thyroid, and inner ear (Guipponi et al., 2007, Kurachi et al., 1994, Tsuji et al., 1991). Physiological functions for hepsin have been identified in both liver and inner ear using murine models. While hepsin deficient mice do not show any strong defects in development or fertility (Wu et al., 1998), mice lacking hepsin expression have abnormal cochlear structure and are deaf (Guipponi et al., 2007). The physiological substrate cleaved by hepsin to mediate cochlear development has not been determined, but hepsin-deficient mice also show significantly reduced levels of the thyroid hormone thyroxine (Guipponi et al., 2007) which is known to be important for cochlear development. Any relevance of hepsin expression to human hearing is yet to be reported. Recent analysis of liver specific hepsin null mice show its expression is important for maintenance of the structural integrity of the liver, with knock-out livers showing increased hepatocyte size and narrowed liver sinusoids (Hsu et al., 2012). These authors demonstrated that the likely in vivo substrate cleaved by hepsin to regulate liver physiology is pro-hepatocyte growth factor (pro-HGF), a growth factor shown to be a substrate of hepsin in vitro (Kirchhofer et al., 2005). Of importance, treatment of liver specific hepsin null mice with the active form of HGF was able rescue these phenotypic defects (Hsu et al., 2012).
TMPRSS2 (also known as epitheliasin) is expressed in the epithelium of many tissues including the prostate, gastrointestinal tract, breast, lung, kidney, pancreas, ovary, lung, and salivary gland (Faller et al., 2014, Jacquinet et al., 2001). TMPRSS2 is of unknown function and is not required for normal development or health in mice (Kim et al., 2006). Similar to HAT, TMPRSS2 may augment virus entry into airway epithelium increasing virus propagation by cleaving the HA protein of influenza A virus (IAV) (Hatesuer et al., 2013, Sakai et al., 2014), and the SARS coronavirus spike protein (Heurich et al., 2014). Of note, TMPRSS2 deficient mice are highly resistant to experimental IAV virus challenges (H1N1, H3N2, and H7N9 strains), which is associated with reduced HA cleavage in vivo, suggesting TMPRSS2 is a key host protease important for IAV infection and may represent a new therapeutic target in humans (Hatesuer et al., 2013, Sakai et al., 2014). TMPRSS3 (also known as TADG-12), is also expressed in epithelium of a variety of tissues where its function is unknown, however, its expression in the cochlea of the inner ear has been shown to be critically important for hearing in both humans and mice. At least five different point mutations in TMPRSS3 gene that affect protease activity have been linked to autosomal recessive deafness in humans (Guipponi et al., 2008; Lee et al., 2013, Scott et al., 2001, Wattenhofer et al., 2002). Furthermore, mice with TMPRSS3 deficiency are deaf, with reduced cochlear hair cell survival at the onset of hearing (Fasquelle et al., 2011). The identification of the substrate cleaved by TMPRSS3 to mediate hearing is unknown, but expression of hair cell KCNMA1 potassium channels is reduced in mice with mutated TMPRSS3 (Molina et al., 2013), suggesting a role in the acquisition of mature ion channels during cochlear development. Little is known about the biological function of TMPRSS4, also known as CAP-2 for its ability to activate ENaCs in vitro (Andreasen et al., 2006, Passero et al., 2012). However, it is over-expressed in a variety of epithelial carcinomas where it is correlated with disease progression and predicts poor patient survival (reviewed in Choi et al., 2008). In vitro studies suggest TMPRSS4 mediates cancer cell invasion, epithelial–mesenchymal transition, and metastasis (Huang et al., 2014, Kim et al., 2010, Min et al., 2014), where TMPRSS4 proteolytic activation of urokinase plasminogen activator (uPA) may be involved (Min et al., 2014). TMPRSS5 (also known as spinesin), has an unusual expression pattern in comparison to other family members, being highly expressed in the brain and spinal cord (Yamaguchi et al., 2002), where its function is unknown. TMPRSS5 is also expressed in the inner ear, and may be important for human hearing (Guipponi et al., 2008). MSPL (also known as TMPRSS13) is expressed in the lung, placenta, prostate, and pancreas (Kim et al., 2001) where its physiological function is unknown. Similar to HAT and TMPRSS2, it may play a role in influenza virus infection through cleavage of HA (Okumura et al., 2010). Enteropeptidase (commonly known as enterokinase, also known as PRSS7) is expressed in upper small intestine (duodenum and jejunum) on the brush border of enterocytes of the intestinal villus (Hermon-Taylor et al., 1977, Kitamoto et al., 1994, Kitamoto et al., 1995, Yuan et al., 1998). Enteropeptidase has long been known to play a critical role in human digestion by activating a proteolytic cascade that results in the activation of numerous proteases important for human digestion (reviewed in Zheng et al., 2009). Enteropeptidase cleaves and activates pancreatic trypsinogen to trypsin, which in turn activates other digestive zymogens such as chymotrypsinogen, proelastase, procarboxypeptidase, and prolipase in the lumen of the gut (Kunitz, 1939). Mutations that lead to truncated forms of the protease that lack the active site are shown to cause malabsorbtion and failure to thrive in infants (Holzinger et al., 2002).
The Matriptase Subfamily
This TTSP subfamily has four members – matriptase, matriptase-2, matriptase-3, and polyserase-1, which contain various combinations of accessory domains in their stem regions (Figure 1). Matriptase (also known as PRSS14, MT-SP1, CAP3, epithin, ST14) is the most well characterized of this subfamily and is found widely expressed in all epithelia (List et al., 2006, Oberst et al., 2003a). Matriptase has been shown to be critical for epithelial barrier function using mouse models of matriptase deficiency (List et al., 2002, List et al., 2009). In humans, mutations affecting matriptase's enzymatic activity present with a rare form of skin disease (Basel-Vanagaite et al., 2007; ARIH, see Table 2), which is characterized by scaly, itchy skin with increased permeability, similar to the phenotype of matriptase null mice (List et al., 2002, List et al., 2007a). The substrate(s) cleaved by matriptase in the regulation of skin barrier function remain to be elucidated, although matriptase is thought to activate the pro-prostasin zymogen in this tissue (Netzel-Arnett et al., 2006). In the polarized epithelium of the gastrointestinal tract where matriptase localizes to adherens junctions (Buzza et al., 2010), matriptase is also critically important for regulating intestinal epithelial barrier function (Buzza et al., 2010, List et al., 2009), and mice deficient in intestinal matriptase develop chronic inflammation and spontaneous colitis-induced colon adenocarcinoma (Kosa et al., 2012). In murine models, matriptase deficiency increases susceptibility to experimental colitis, and is found to be downregulated in human Crohns disease and ulcerative colitis (Netzel-Arnett et al., 2012). The matriptase substrate that regulates intestinal barrier function is unknown, but matriptase has been reported to induce an intracellular atypical protein kinase C signaling pathway that regulates the composition of the apical tight junctions (Buzza et al., 2010). In the intestine and in contrast to the skin, prostasin appears to act upstream of matriptase and is important for inducing its activation in intestinal epithelium (Buzza et al., 2013). There is also the possibility of a reciprocal zymogen activation cascade between these two proteases in different tissues, suggesting that both matriptase and prostasin zymogens may act as cofactors that can induce the activation of each other, in a mechanism that is independent of catalytic activity (Friis et al., 2013). Matriptase is also lost in the salivary gland of patients with Sjrogen's syndrome which exhibit salivary gland dysfunction and develop autoimmunity. Its loss may be important for disease pathogenesis, since matriptase deficiency in mice induces a primary Sjogren's syndrome-like phenotype with autoimmunity and loss of gland function (Yin et al., 2014).
Matriptase also possesses strong oncogenic activities and in many epithelial cancers, its increased expression or activity (through loss of inhibition) is associated with disease progression (reviewed in List, 2009). Low level over-expression of matriptase in murine skin is sufficient to induce multistage carcinogenesis and invasive squamous cell carcinoma (List et al., 2005). Based on murine models, the mechanism by which matriptase induces squamous cell carcinoma involves cleavage and activation of the matriptase substrate pro-HGF (Lee et al., 2000, Szabo et al., 2011). In vitro studies also identified matriptase as the first membrane-anchored serine protease to activate the G-protein coupled receptor, PAR-2 (Camerer et al., 2010, Takeuchi et al., 2000). In vivo evidence suggests that cleavage of PAR-2, which induces inflammatory signaling pathways, may be an essential component for matriptase induced malignant progression of squamous cell carcinomas in animal models (Sales et al., 2014). Matriptase activity in the skin also has been linked to the initiation of Netherton syndrome, which results from a loss of function mutation in the serine protease inhibitor LEKTI, which is important for regulating the activity of kallikrein proteases in the skin (Sales et al., 2010). Genetic ablation of matriptase in mice deficient in the LEKTI inhibitor reduced inflammation, reduced aberrant protease activity and improved barrier function in the skin, suggesting matriptase is pathologically important in the human disease through kallikrein activation (Sales et al., 2010).
Matriptase-2 (also known as TMPRSS6) is most highly expressed in the liver, but is also found in the kidney and uterine tissue (Hooper et al., 2003, Velasco et al., 2002). Matriptase-2 has a significant function in the regulation of iron metabolism. Humans with point mutations that effect matriptase-2 activity suffer from iron-refractory iron deficient anemia (Finberg et al., 2008, Guillem et al., 2008, Melis et al., 2008), manifested by very low iron levels and severe microcytic anemia. Mice deficient in matriptase-2 expression also show this iron deficient phenotype (Du et al., 2008, Truksa et al., 2009). Matripase-2 is thought to function by suppressing the expression of the liver hormone hepcidin which is known to be an important mediator of iron uptake by liver cells. This suppression occurs indirectly at the mRNA level, and is thought to be mediated by matriptase-2 cleavage of the bone morphogenetic protein (BMP) co-receptor hemojuvelin (Silvestri et al., 2008, Silvestri et al., 2009, Truksa et al., 2009), which has a role in activation of hepcidin transcription. Matriptase-3 (also known as TMPRSS7) is expressed in the brain, skin, salivary gland, and reproductive tissues (Szabo et al., 2005); however, its physiological functions remain to be determined. Polyserase-1 (also known as TMPRSS9 and serase1B) is expressed in skeletal muscle, heart, kidney, liver, placenta, and brain (Cal et al., 2003, Cal et al., 2007). This TTSP is highly unique in that it possesses 3 SPDs but its function is currently unknown.
The Corin Subfamily
Corin (also known as TMPRSS10) is the only member of this subfamily, and is unique in that it possesses two frizzled-like cysteine rich domains in its stem region, along with multiple LDLA domains and a scavenger domain (Figure 1). Corin is highly expressed in cardiomyocytes of the heart, but also in kidney, bone, brain, skin, and pregnant uterus (Hooper et al., 2000, Yan et al., 1999). Corin plays an important role in maintaining heart function and decreasing blood volume and blood pressure, by processing the cardiac hormone pro-atrial natriuretic peptide (pro-ANP) (Wu et al., 2002, Yan et al., 2000). Corin cleaves and activates pro-ANP to mature ANP which promotes natriuresis, diuresis, and vasodilation. In humans, mutations in the corin gene which result in reduced activation and protease activity have been reported in patients with hypertension and cardiac hypertrophy, and are associated with a poorer prognosis in patients with heart failure (Dries et al., 2005, Rame et al., 2007, Rame et al., 2009, Wang et al., 2008). Corin-deficient mice spontaneously develop salt-sensitive hypertension, cardiac hypertrophy and increased body weight, and possess an elevated level of pro-ANP and an absence of active ANP (Chan et al., 2005, Nigrovic et al., 2008). Corin and ANP have also been shown to be important for promoting placental trophoblast invasion and spiral artery remodeling in the pregnant uterus (Cui et al., 2012). Pregnancy in corin- or ANP-deficient mice results in the development of high blood pressure and proteinuria, which are characteristics of pre-eclampsia. Interestingly, in human pre-eclamptic patients, uterine corin expression was found to be reduced, and several corin mutations which cause proteolytic activity were also identified in pre-eclamptic patients, suggesting defective corin may be pathologically important in this disease.
Conclusion
Following the sequencing of the human genome at the turn of the century and the discovery of the family of membrane-anchored serine proteases, these enzymes have emerged to play key roles in many diverse aspects of mammalian physiology. The misregulation of these enzymes is emerging to contribute to the pathology of a variety of diseases. The plasma membrane is a dynamic, fluid microenvironment where cell surface molecules act as cell sensors, initiating signals and relaying information. The extracellular SPDs contained in these molecules likely target specific cellular substrates, growth factors, receptors, and components of the extracellular matrix to modulate irreversible changes in the cellular environment. Clearly these unique enzymes represent potential strategic targets for diagnostic and therapeutic applications for a wide range of diseases.
See also
PROTEIN SYNTHESIS/DEGRADATION: PROTEIN DEGRADATION – PROTEASE CLASSES | ADAMs Regulate Cell–Cell Interactions by Controlling the Function of the EGF-Receptor, TNFα and Notch; PROTEIN SYNTHESIS/DEGRADATION: PROTEIN DEGRADATION – PROTEASE CLASSES | ADAMTS Proteases: Mediators of Physiological and Pathogenic Extracellular Proteolysis; PROTEIN SYNTHESIS/DEGRADATION: PROTEIN DEGRADATION – PROTEASE CLASSES | Matrix Metalloproteinases; PROTEIN SYNTHESIS/DEGRADATION: PROTEIN DEGRADATION – PROTEASE CLASSES | Naturally-Occurring Polypeptide Inhibitors: Cystatins/Stefins, Inhibitors of Apoptosis (IAPs), Serpins, and Tissue Inhibitors of Metalloproteinases (TIMPs). PROTEIN SYNTHESIS/DEGRADATION: PROTEOLYTIC PATHWAYS | Molecular Mechanisms Underlying the Actions of the Complement System; PROTEIN SYNTHESIS/DEGRADATION: PROTEOLYTIC PATHWAYS | Overview of Blood Coagulation and the Pathophysiology of Blood Coagulation Disorders
Biographies

Toni M. Antalis, PhD is a Professor in the Department of Physiology and Associate Director of the Center for Vascular and Inflammatory Diseases at University of Maryland School of Medicine in Baltimore, MD, USA. She obtained her PhD in Biochemistry (1981) from Rice University, Houston, TX, USA. Her research interests are the roles of membrane serine proteases and serine protease inhibitors (serpins) in vascular biology, inflammation and cancer. A particular interest includes how various members of this family are involved in the formation and maintenance of endothelial and epithelial barriers and the impact of their deregulation on disease progression.

Marguerite S. Buzza, PhD is a Research Associate and faculty member of the Department of Physiology and the Center for Vascular and Inflammatory Diseases at University of Maryland School of Medicine in Baltimore, Maryland. She obtained her PhD in Biochemistry and Molecular Biology (2004) from Monash University, IC, Australia. Her graduate studies focused on the biology of cytotoxic serine proteases and their intracellular serine protease inhibitors. Her research interests since joining the University of Maryland (2006) focus on understanding the physiological roles of the membrane anchored serine proteases, matriptase and prostasin, in the regulation of epithelial barrier function in the gastrointestinal tract. She is particularly interested in the consequences of the loss of these proteases in the pathogenesis of inflammatory bowel diseases, and the future development of novel treatment strategies for these diseases.
References
- Aimes R.T., Zijlstra A., Hooper J.D. Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thrombosis and Haemostasis. 2003;89(3):561–572. [PubMed] [Google Scholar]
- Andreasen D., Vuagniaux G., Fowler-Jaeger N., Hummler E., Rossier B.C. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. Journal of the American Society of Nephrology. 2006;17(4):968–976. doi: 10.1681/ASN.2005060637. [DOI] [PubMed] [Google Scholar]
- Antalis T.M., Buzza M.S., Hodge K.M., Hooper J.D., Netzel-Arnett S. The cutting edge: Membrane-anchored serine protease activities in the pericellular microenvironment. Biochemical Journal. 2010;428(3):325–346. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrahami L., Maas S., Pasmanik-Chor M. Autosomal recessive ichthyosis with hypotrichosis syndrome: Further delineation of the phenotype. Clinical Genetics. 2008;74(1):47–53. doi: 10.1111/j.1399-0004.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- Baron J., Tarnow C., Mayoli-Nussle D. Matriptase, HAT, and TMPRSS2 activate the hemagglutinin of H9N2 influenza A viruses. Journal of Virology. 2013;87(3):1811–1820. doi: 10.1128/JVI.02320-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel-Vanagaite L., Attia R., Ishida-Yamamoto A. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. American Journal of Human Genetics. 2007;80(3):467–477. doi: 10.1086/512487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufort N., Leduc D., Eguchi H. The human airway trypsin-like protease modulates the urokinase receptor (uPAR, CD87) structure and functions. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2007;292(5):L1263–L1272. doi: 10.1152/ajplung.00191.2006. [DOI] [PubMed] [Google Scholar]
- Bertram S., Glowacka I., Muller M.A. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. Journal of Virology. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Glowacka I., Steffen I., Kuhl A., Pohlmann S. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Reviews in Medical Virology. 2010;20(5):298–310. doi: 10.1002/rmv.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Heurich A., Lavender H. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7(4):e35876. doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns J.B., Carattino M.D., Sheng S. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. Journal of Biological Chemistry. 2007;282(9):6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- Bugge T.H., Antalis T.M., Wu Q. Type II transmembrane serine proteases. Journal of Biological Chemistry. 2009;284(35):23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzza M.S., Martin E.W., Driesbaugh K.H. Prostasin is required for matriptase activation in intestinal epithelial cells to regulate closure of the paracellular pathway. Journal of Biological Chemistry. 2013;288(15):10328–10337. doi: 10.1074/jbc.M112.443432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzza M.S., Netzel-Arnett S., Shea-Donohue T. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4200–4205. doi: 10.1073/pnas.0903923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cal S., Moncada-Pazos A., Lopez-Otin C. Expanding the complexity of the human degradome: Polyserases and their tandem serine protease domains. Frontiers in Bioscience. 2007;12:4661–4669. doi: 10.2741/2415. [DOI] [PubMed] [Google Scholar]
- Cal S., Quesada V., Garabaya C., Lopez-Otin C. Polyserase-I, a human polyprotease with the ability to generate independent serine protease domains from a single translation product. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9185–9190. doi: 10.1073/pnas.1633392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E., Barker A., Duong D.N. Local protease signaling contributes to neural tube closure in the mouse embryo. Developmental Cell. 2010;18(1):25–38. doi: 10.1016/j.devcel.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattino M.D., Hughey R.P., Kleyman T.R. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. Journal of Biological Chemistry. 2008;283(37):25290–25295. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey G.H., Raymond W.W., Blount J.L. Characterization of human gamma-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. Journal of Immunology. 2000;164(12):6566–6575. doi: 10.4049/jimmunol.164.12.6566. [DOI] [PubMed] [Google Scholar]
- Chan J.C., Knudson O., Wu F. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.M., Skinner M.L., Kauffman S.W. Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. Journal of Biological Chemistry. 2001;276(24):21434–21442. doi: 10.1074/jbc.M011423200. [DOI] [PubMed] [Google Scholar]
- Choi S.Y., Shin H.C., Kim S.Y., Park Y.W. Role of TMPRSS4 during cancer progression. Drug News & Perspectives. 2008;21(8):417–423. doi: 10.1358/dnp.2008.21.8.1272135. [DOI] [PubMed] [Google Scholar]
- Cui Y., Wang W., Dong N. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484(7393):246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilets A., Beliveau F., Vandal G. Mutation G827R in matriptase causing autosomal recessive ichthyosis with hypotrichosis yields an inactive protease. Journal of Biological Chemistry. 2008;283(16):10535–10542. doi: 10.1074/jbc.M707012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries D.L., Victor R.G., Rame J.E. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112(16):2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- Du X., She E., Gelbart T. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller N., Gautschi I., Schild L. Functional analysis of a missense mutation in the serine protease inhibitor SPINT2 associated with congenital sodium diarrhea. PLoS. One. 2014;9(4):e94267. doi: 10.1371/journal.pone.0094267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasquelle L., Scott H.S., Lenoir M. Tmprss3, a transmembrane serine protease deficient in human DFNB8/10 deafness, is critical for cochlear hair cell survival at the onset of hearing. Journal of Biological Chemistry. 2011;286(19):17383–17397. doi: 10.1074/jbc.M110.190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg K.E., Heeney M.M., Campagna D.R. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nature Genetics. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgueras A.R., de Lara F.M., Pendas A.M. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112(6):2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- Frateschi S., Keppner A., Malsure S. Mutations of the serine protease CAP1/Prss8 lead to reduced embryonic viability, skin defects, and decreased ENaC activity. American Journal of Pathology. 2012;181(2):605–615. doi: 10.1016/j.ajpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Friis S., Uzzun S.K., Godiksen S. A matriptase-prostasin reciprocal zymogen activation complex with unique features: Prostasin as a non-enzymatic co-factor for matriptase activation. Journal of Biological Chemistry. 2013;288(26):19028–19039. doi: 10.1074/jbc.M113.469932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem F., Lawson S., Kannengiesser C. Two nonsense mutations in the TMPRSS6 gene in a patient with microcytic anemia and iron deficiency. Blood. 2008;112(5):2089–2091. doi: 10.1182/blood-2008-05-154740. [DOI] [PubMed] [Google Scholar]
- Guipponi M., Antonarakis S.E., Scott H.S. TMPRSS3, a type II transmembrane serine protease mutated in non-syndromic autosomal recessive deafness. Frontiers in Bioscience. 2008;13:1557–1567. doi: 10.2741/2780. [DOI] [PubMed] [Google Scholar]
- Guipponi M., Tan J., Cannon P.Z. Mice deficient for the type II transmembrane serine protease, TMPRSS1/hepsin, exhibit profound hearing loss. American Journal of Pathology. 2007;171(2):608–616. doi: 10.2353/ajpath.2007.070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guipponi M., Toh M.Y., Tan J. An integrated genetic and functional analysis of the role of type II transmembrane serine proteases (TMPRSSs) in hearing loss. Human Mutation. 2008;29(1):130–141. doi: 10.1002/humu.20617. [DOI] [PubMed] [Google Scholar]
- Hatesuer B., Bertram S., Mehnert N. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLOS Pathogens. 2013;9(12):e1003774. doi: 10.1371/journal.ppat.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth J.C., Gourley B., Hadorn B., Sumida C. Malabsorption and growth failure due to intestinal enterokinase deficiency. Journal of Pediatrics. 1971;78(3):481–490. doi: 10.1016/s0022-3476(71)80231-7. [DOI] [PubMed] [Google Scholar]
- Hedstrom L. Serine protease mechanism and specificity. Chemical Reviews. 2002;102(12):4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- Hermon-Taylor J., Perrin J., Grant D.A. Immunofluorescent localisation of enterokinase in human small intestine. Gut. 1977;18(4):259–265. doi: 10.1136/gut.18.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A., Hofmann-Winkler H., Gierer S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. Journal of Virology. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A., Maier E.M., Buck C. Mutations in the proenteropeptidase gene are the molecular cause of congenital enteropeptidase deficiency. American Journal of Human Genetics. 2002;70(1):20–25. doi: 10.1086/338456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A., Yamagata K., Sugiura S., Watanabe K., Baba T. A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. Journal of Biological Chemistry. 2002;277(19):16976–16984. doi: 10.1074/jbc.M112470200. [DOI] [PubMed] [Google Scholar]
- Hooper J.D., Campagnolo L., Goodarzi G. Mouse matriptase-2: Identification, characterization and comparative mRNA expression analysis with mouse hepsin in adult and embryonic tissues. Biochemical Journal. 2003;373(no Pt 3):689–702. doi: 10.1042/BJ20030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper J.D., Clements J.A., Quigley J.P., Antalis T.M. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. Journal of Biological Chemistry. 2001;276(2):857–860. doi: 10.1074/jbc.R000020200. [DOI] [PubMed] [Google Scholar]
- Hooper J.D., Nicol D.L., Dickinson J.L. Testisin, a new human serine proteinase expressed by premeiotic testicular germ cells and lost in testicular germ cell tumors. Cancer Research. 1999;59(13):3199–3205. [PubMed] [Google Scholar]
- Hooper J.D., Scarman A.L., Clarke B.E., Normyle J.F., Antalis T.M. Localization of the mosaic transmembrane serine protease corin to heart myocytes. European Journal of Biochemistry. 2000;267(23):6931–6937. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- Hsu Y.C., Huang H.P., Yu I.S. Serine protease hepsin regulates hepatocyte size and hemodynamic retention of tumor cells by hepatocyte growth factor signaling in mice. Hepatology. 2012;56(5):1913–1923. doi: 10.1002/hep.25773. [DOI] [PubMed] [Google Scholar]
- Huang A., Zhou H., Zhao H. TMPRSS4 correlates with colorectal cancer pathological stage and regulates cell proliferation and self-renewal ability. Cancer Biology & Therapy. 2014;15(3):297–304. doi: 10.4161/cbt.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kanbe N., Kurosawa M., Kido H. Cloning and tissue distribution of a novel serine protease esp-1 from human eosinophils. Biochemical and Biophysical Research Communications. 1998;252(2):307–312. doi: 10.1006/bbrc.1998.9645. [DOI] [PubMed] [Google Scholar]
- Iwakiri K., Ghazizadeh M., Jin E. Human airway trypsin-like protease induces PAR-2-mediated IL-8 release in psoriasis vulgaris. Journal of Investigative Dermatology. 2004;122(4):937–944. doi: 10.1111/j.0022-202X.2004.22415.x. [DOI] [PubMed] [Google Scholar]
- Iwashita K., Kitamura K., Narikiyo T. Inhibition of prostasin secretion by serine protease inhibitors in the kidney. Journal of the American Society of Nephrology. 2003;14(1):11–16. doi: 10.1097/01.asn.0000043900.39397.48. [DOI] [PubMed] [Google Scholar]
- Jacquinet E., Rao N.V., Rao G.V. Cloning and characterization of the cDNA and gene for human epitheliasin. European Journal of Biochemistry. 2001;268(9):2687–2699. doi: 10.1046/j.1432-1327.2001.02165.x. [DOI] [PubMed] [Google Scholar]
- Kam Y.W., Okumura Y., Kido H. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One. 2009;4(11):e7870. doi: 10.1371/journal.pone.0007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempkensteffen C., Christoph F., Weikert S. Epigenetic silencing of the putative tumor suppressor gene testisin in testicular germ cell tumors. Journal of Cancer Research and Clinical Oncology. 2006;132(12):765–770. doi: 10.1007/s00432-006-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.R., Sharmin S., Inoue M., Kido H. Cloning and expression of novel mosaic serine proteases with and without a transmembrane domain from human lung. Biochimica et Biophysica Acta. 2001;1518(1–2):204–209. doi: 10.1016/s0167-4781(01)00184-1. [DOI] [PubMed] [Google Scholar]
- Kim S., Kang H.Y., Nam E.H. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis. 2010;31(4):597–606. doi: 10.1093/carcin/bgq024. [DOI] [PubMed] [Google Scholar]
- Kim T.S., Heinlein C., Hackman R.C., Nelson P.S. Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Molecular and Cellular Biology. 2006;26(3):965–975. doi: 10.1128/MCB.26.3.965-975.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhofer D., Peek M., Lipari M.T. Hepsin activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1B) and HAI-2. FEBS Letters. 2005;579(9):1945–1950. doi: 10.1016/j.febslet.2005.01.085. [DOI] [PubMed] [Google Scholar]
- Kitamoto Y., Veile R.A., Donis-Keller H., Sadler J.E. cDNA sequence and chromosomal localization of human enterokinase, the proteolytic activator of trypsinogen. Biochemistry. 1995;34(14):4562–4568. doi: 10.1021/bi00014a008. [DOI] [PubMed] [Google Scholar]
- Kitamoto Y., Yuan X., Wu Q., McCourt D.W., Sadler J.E. Enterokinase, the initiator of intestinal digestion, is a mosaic protease composed of a distinctive assortment of domains. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(16):7588–7592. doi: 10.1073/pnas.91.16.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe S., Wu F., Madlansacay M.R., Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. Journal of Biological Chemistry. 2004;279(33):34464–34471. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- Koda A., Wakida N., Toriyama K. Urinary prostasin in humans: Relationships among prostasin, aldosterone and epithelial sodium channel activity. Hypertension Research. 2009;32(4):276–281. doi: 10.1038/hr.2009.6. [DOI] [PubMed] [Google Scholar]
- Kosa P., Szabo R., Molinolo A.A., Bugge T.H. Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis. Oncogene. 2012;31(32):3679–3695. doi: 10.1038/onc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitz M. Formation of trypsin from crystalline trypsinogen by means of enterokinase. Journal of General Physiology. 1939;22(4):429–446. doi: 10.1085/jgp.22.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Torres-Rosado A., Tsuji A. Hepsin. Methods in Enzymology. 1994;244:100–114. doi: 10.1016/0076-6879(94)44009-3. [DOI] [PubMed] [Google Scholar]
- Lang J.C., Schuller D.E. Differential expression of a novel serine protease homologue in squamous cell carcinoma of the head and neck. British Journal of Cancer. 2001;84(2):237–243. doi: 10.1054/bjoc.2000.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Baek J.I., Choi J.Y. Genetic analysis of TMPRSS3 gene in the Korean population with autosomal recessive nonsyndromic hearing loss. Gene. 2013;532(2):276–280. doi: 10.1016/j.gene.2013.07.108. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Tseng I.C., Wang Y. Autoactivation of matriptase in vitro: Requirement for biomembrane and LDL receptor domain. American Journal of Physiology – Cell Physiology. 2007;293(1):C95–105. doi: 10.1152/ajpcell.00611.2006. [DOI] [PubMed] [Google Scholar]
- Lee S.L., Dickson R.B., Lin C.Y. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. Journal of Biological Chemistry. 2000;275(47):36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Park D., Kim S.Y., Park W.J. Pathogenic mutations but not polymorphisms in congenital and childhood onset autosomal recessive deafness disrupt the proteolytic activity of TMPRSS3. Journal of Medical Genetics. 2003;40(8):629–631. doi: 10.1136/jmg.40.8.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyvraz C., Charles R.P., Rubera I. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. Journal of Cell Biology. 2005;170(3):487–496. doi: 10.1083/jcb.200501038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List K. Matriptase: A culprit in cancer? Future Oncology. 2009;5(1):97–104. doi: 10.2217/14796694.5.1.97. [DOI] [PubMed] [Google Scholar]
- List K., Currie B., Scharschmidt T.C. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. Journal of Biological Chemistry. 2007;282(50):36714–36723. doi: 10.1074/jbc.M705521200. [DOI] [PubMed] [Google Scholar]
- List K., Haudenschild C.C., Szabo R. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21(23):3765–3779. doi: 10.1038/sj.onc.1205502. [DOI] [PubMed] [Google Scholar]
- List K., Hobson J.P., Molinolo A., Bugge T.H. Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. Journal of Cellular Physiology. 2007;213(1):237–245. doi: 10.1002/jcp.21115. [DOI] [PubMed] [Google Scholar]
- List K., Kosa P., Szabo R. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. American Journal of Pathology. 2009;175(4):1453–1463. doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List K., Szabo R., Molinolo A., Nielsen B.S., Bugge T.H. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. American Journal of Pathology. 2006;168(5):1513–1525. doi: 10.2353/ajpath.2006.051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List K., Szabo R., Molinolo A. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes & Development. 2005;19(16):1934–1950. doi: 10.1101/gad.1300705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li Q., Zhou X., Kolosov V.P., Perelman J.M. Human airway trypsin-like protease induces mucin5AC hypersecretion via a protease-activated receptor 2-mediated pathway in human airway epithelial cells. Archives of Biochemistry and Biophysics. 2013;535(2):234–240. doi: 10.1016/j.abb.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C., Bond J.S. Proteases: Multifunctional enzymes in life and disease. Journal of Biological Chemistry. 2008;283(45):30433–30437. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macao B., Johansson D.G., Hansson G.C., Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nature Structural & Molecular Biology. 2006;13(1):71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- Maekawa A., Kakizoe Y., Miyoshi T. Camostat mesilate inhibits prostasin activity and reduces blood pressure and renal injury in salt-sensitive hypertension. Journal of Hypertension. 2009;27(1):181–189. doi: 10.1097/hjh.0b013e328317a762. [DOI] [PubMed] [Google Scholar]
- Malsure S., Wang Q., Charles R.P. Colon-specific deletion of epithelial sodium channel causes sodium loss and aldosterone resistance. Journal of the American Society of Nephrology. 2014;25:1453–1464. doi: 10.1681/ASN.2013090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima R., Takahashi A., Nakaya Y. Human airway trypsin-like protease stimulates human bronchial fibroblast proliferation in a protease-activated receptor-2-dependent pathway. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2006;290(2):L385–L395. doi: 10.1152/ajplung.00098.2005. [DOI] [PubMed] [Google Scholar]
- Melis M.A., Cau M., Congiu R. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93(10):1473–1479. doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- Miller G.S., Zoratti G.L., Murray A.S. HATL5: A cell surface serine protease differentially expressed in epithelial cancers. PLoS One. 2014;9(2):e87675. doi: 10.1371/journal.pone.0087675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H.J., Lee M.K., Lee J.W., Kim S. TMPRSS4 induces cancer cell invasion through pro-uPA processing. Biochemical and Biophysical Research Communications. 2014;446(1):1–7. doi: 10.1016/j.bbrc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Molina L., Fasquelle L., Nouvian R. Tmprss3 loss of function impairs cochlear inner hair cell Kcnma1 channel membrane expression. Human Molecular Genetics. 2013;22(7):1289–1299. doi: 10.1093/hmg/dds532. [DOI] [PubMed] [Google Scholar]
- Myerburg M.M., McKenna E.E., Luke C.J. Prostasin expression is regulated by airway surface liquid volume and is increased in cystic fibrosis. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2008;294(5):L932–L941. doi: 10.1152/ajplung.00437.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzel-Arnett S., Bugge T.H., Hess R.A. The glycosylphosphatidylinositol-anchored serine protease PRSS21 (testisin) imparts murine epididymal sperm cell maturation and fertilizing ability. Biology of Reproduction. 2009;81(5):921–932. doi: 10.1095/biolreprod.109.076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzel-Arnett S., Buzza M.S., Shea-Donohue T. Matriptase protects against experimental colitis and promotes intestinal barrier recovery. Inflammatory Bowel Diseases. 2012;18(7):1303–1314. doi: 10.1002/ibd.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzel-Arnett S., Currie B.M., Szabo R. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. Journal of Biological Chemistry. 2006;281(44):32941–32945. doi: 10.1074/jbc.C600208200. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S., Hooper J.D., Szabo R. Membrane anchored serine proteases: A rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer and Metastasis Reviews. 2003;22(2–3):237–258. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- Nigrovic P.A., Gray D.H., Jones T. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. American Journal of Pathology. 2008;173(6):1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst M.D., Singh B., Ozdemirli M. Characterization of matriptase expression in normal human tissues. Journal of Histochemistry & Cytochemistry. 2003;51(8):1017–1025. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- Oberst M.D., Williams C.A., Dickson R.B., Johnson M.D., Lin C.Y. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. Journal of Biological Chemistry. 2003;278(29):26773–26779. doi: 10.1074/jbc.M304282200. [DOI] [PubMed] [Google Scholar]
- Okumura Y., Takahashi E., Yano M. Novel type II transmembrane serine proteases, MSPL and TMPRSS13, Proteolytically activate membrane fusion activity of the hemagglutinin of highly pathogenic avian influenza viruses and induce their multicycle replication. Journal of Virology. 2010;84(10):5089–5096. doi: 10.1128/JVI.02605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passero C.J., Mueller G.M., Myerburg M.M. TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the gamma-subunit distal to the furin cleavage site. American Journal of Physiology – Renal Physiology. 2012;302(1):F1–F8. doi: 10.1152/ajprenal.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters D.E., Szabo R., Friis S. The membrane-anchored serine protease prostasin (CAP1/PRSS8) supports epidermal development and postnatal homeostasis independent of its enzymatic activity. Journal of Biological Chemistry. 2014;289:14740–14749. doi: 10.1074/jbc.M113.541318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes C., Randrianarison N.H., Charles R.P. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Molecular Medicine. 2010;2(1):26–37. doi: 10.1002/emmm.200900050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rame J.E., Drazner M.H., Post W. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49(4):857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- Rame J.E., Tam S.W., McNamara D. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in Blacks with systolic heart failure: Results from the genetic risk assessment in heart failure substudy. Circulation: Heart Failure. 2009;2(6):541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay A.J., Quesada V., Sanchez M. Matriptase-2 mutations in iron-refractory iron deficiency anemia patients provide new insights into protease activation mechanisms. Human Molecular Genetics. 2009;18(19):3673–3683. doi: 10.1093/hmg/ddp315. [DOI] [PubMed] [Google Scholar]
- Rawlings N.D., Barrett A.J. Introduction: Serine peptidases and their clans. In: Barrett A.J., Rawlings N.D., Woessner J.F., editors. second ed. vol. 2. Elsevier Ltd; London: 2004. pp. 1417–1439. (Handbook of Proteolytic Enzymes). [Google Scholar]
- Sakai K., Ami Y., Tahara M. The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses. Journal of Virology. 2014;88(10):5608–5616. doi: 10.1128/JVI.03677-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales K.U., Friis S., Konkel J.E. Non-hematopoietic PAR-2 is essential for matriptase-driven pre-malignant progression and potentiation of ras-mediated squamous cell carcinogenesis. Oncogene. 2014 doi: 10.1038/onc.2013.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales K.U., Hobson J.P., Wagenaar-Miller R. Expression and genetic loss of function analysis of the HAT/DESC cluster proteases TMPRSS11A and HAT. PLoS One. 2011;6(8):e23261. doi: 10.1371/journal.pone.0023261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales K.U., Masedunskas A., Bey A.L. Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome. Nature Genetics. 2010;42(8):676–683. doi: 10.1038/ng.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H.S., Kudoh J., Wattenhofer M. Insertion of beta-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nature Genetics. 2001;27(1):59–63. doi: 10.1038/83768. [DOI] [PubMed] [Google Scholar]
- Sedghizadeh P.P., Mallery S.R., Thompson S.J. Expression of the serine protease DESC1 correlates directly with normal keratinocyte differentiation and inversely with head and neck squamous cell carcinoma progression. Head & Neck. 2006;28(5):432–440. doi: 10.1002/hed.20346. [DOI] [PubMed] [Google Scholar]
- Selzer-Plon J., Bornholdt J., Friis S. Expression of prostasin and its inhibitors during colorectal cancer carcinogenesis. BMC Cancer. 2009;9:201. doi: 10.1186/1471-2407-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemasa K., Underwood L.J., Beard J. Overexpression of testisin, a serine protease expressed by testicular germ cells, in epithelial ovarian tumor cells. Journal of the Society for Gynecologic Investigation. 2000;7(6):358–362. [PubMed] [Google Scholar]
- Silvestri L., Guillem F., Pagani A. Molecular mechanisms of the defective hepcidin inhibition in TMPRSS6 mutations associated with iron-refractory iron deficiency anemia. Blood. 2009;113(22):5605–5608. doi: 10.1182/blood-2008-12-195594. [DOI] [PubMed] [Google Scholar]
- Silvestri L., Pagani A., Nai A. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metabolism. 2008;8(6):502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensgaard M., Svenningsen P., Tinning A.R. Apical serine protease activity is necessary for assembly of a high-resistance renal collecting duct epithelium. Acta Physiologica (Oxford) 2010;200(4):347–359. doi: 10.1111/j.1748-1716.2010.02170.x. [DOI] [PubMed] [Google Scholar]
- Szabo R., Bugge T.H. Membrane-anchored serine proteases in vertebrate cell and developmental biology. Annual Review of Cell and Developmental Biology. 2011;27:213–235. doi: 10.1146/annurev-cellbio-092910-154247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R., Hobson J.P., Christoph K. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009;136(15):2653–2663. doi: 10.1242/dev.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R., Kosa P., List K., Bugge T.H. Loss of matriptase suppression underlies spint1 mutation-associated ichthyosis and postnatal lethality. American Journal of Pathology. 2009;174(6):2015–2022. doi: 10.2353/ajpath.2009.090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R., Molinolo A., List K., Bugge T.H. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26(11):1546–1556. doi: 10.1038/sj.onc.1209966. [DOI] [PubMed] [Google Scholar]
- Szabo R., Netzel-Arnett S., Hobson J.P., Antalis T.M., Bugge T.H. Matriptase-3 is a novel phylogenetically preserved membrane-anchored serine protease with broad serpin reactivity. Biochemical Journal. 2005;390(Pt 1):231–242. doi: 10.1042/BJ20050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R., Rasmussen A.L., Moyer A.B. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene. 2011;30(17):2003–2016. doi: 10.1038/onc.2010.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R., Uzzun S.K., Kosa P. Reduced prostasin (CAP1/PRSS8) activity eliminates HAI-1 and HAI-2 deficiency-associated developmental defects by preventing matriptase activation. PLOS Genetics. 2012;8(8):e1002937. doi: 10.1371/journal.pgen.1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R., Wu Q., Dickson R.B. Type II transmembrane serine proteases. Thrombosis and Haemostasis. 2003;90(2):185–193. doi: 10.1160/TH03-02-0071. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Sano T., Yamaoka K. Localization of human airway trypsin-like protease in the airway: An immunohistochemical study. Histochemistry and Cell Biology. 2001;115(3):181–187. doi: 10.1007/s004180000243. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Harris J.L., Huang W. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. Journal of Biological Chemistry. 2000;275(34):26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- Tang T., Kmet M., Corral L. Testisin, a glycosyl-phosphatidylinositol-linked serine protease, promotes malignant transformation in vitro and in vivo. Cancer Research. 2005;65(3):868–878. [PubMed] [Google Scholar]
- Tomlins S.A., Rhodes D.R., Perner S. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- Truksa J., Gelbart T., Peng H. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. British Journal of Haematology. 2009;147(4):571–581. doi: 10.1111/j.1365-2141.2009.07873.x. [DOI] [PubMed] [Google Scholar]
- Tseng I.C., Chou F.P., Su S.F. Purification from human milk of matriptase complexes with secreted serpins: Mechanism for inhibition of matriptase other than HAI-1. American Journal of Physiology – Cell Physiology. 2008;295(2):C423–C431. doi: 10.1152/ajpcell.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Torres-Rosado A., Arai T. Hepsin, a cell membrane-associated protease. Characterization, tissue distribution, and gene localization. Journal of Biological Chemistry. 1991;266(25):16948–16953. [PubMed] [Google Scholar]
- Uchimura K., Hayata M., Mizumoto T. The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nature Communications. 2014;5:3428. doi: 10.1038/ncomms4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G., Cal S., Quesada V., Sanchez L.M., Lopez-Otin C. Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. Journal of Biological Chemistry. 2002;277(40):37637–37646. doi: 10.1074/jbc.M203007200. [DOI] [PubMed] [Google Scholar]
- Verghese G.M., Gutknecht M.F., Caughey G.H. Prostasin regulates epithelial monolayer function: Cell-specific Gpld1-mediated secretion and functional role for GPI anchor. American Journal of Physiology – Cell Physiology. 2006;291(6):C1258–C1270. doi: 10.1152/ajpcell.00637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liao X., Fukuda K. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circulation Research. 2008;103(5):502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenhofer M., Di I., Rabionet V. Mutations in the TMPRSS3 gene are a rare cause of childhood nonsyndromic deafness in Caucasian patients. Journal of Molecular Medicine. 2002;80(2):124–131. doi: 10.1007/s00109-001-0310-6. [DOI] [PubMed] [Google Scholar]
- Wong G.W., Foster P.S., Yasuda S. Biochemical and functional characterization of human transmembrane tryptase (TMT)/tryptase gamma. TMT is an exocytosed mast cell protease that induces airway hyperresponsiveness in vivo via an interleukin-13/interleukin-4 receptor alpha/signal transducer and activator of transcription (STAT) 6-dependent pathway. Journal of Biological Chemistry. 2002;277(44):41906–41915. doi: 10.1074/jbc.M205868200. [DOI] [PubMed] [Google Scholar]
- Wong G.W., Foster P.S., Yasuda S. Biochemical and functional characterization of human transmembrane tryptase (TMT)/tryptase gamma. TMT is an exocytosed mast cell protease that induces airway hyperresponsiveness in vivo via an IL-13/IL-4Ra/STST6-dependent pathway. Journal of Biological Chemistry. 2002;277:41906–41915. doi: 10.1074/jbc.M205868200. [DOI] [PubMed] [Google Scholar]
- Wong G.W., Tang Y., Feyfant E. Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. Journal of Biological Chemistry. 1999;274(43):30784–30793. doi: 10.1074/jbc.274.43.30784. [DOI] [PubMed] [Google Scholar]
- Wu F., Yan W., Pan J., Morser J., Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. Journal of Biological Chemistry. 2002;277(19):16900–16905. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- Wu Q., Parry G. Hepsin and prostate cancer. Frontiers in Bioscience. 2007;12:5052–5059. doi: 10.2741/2447. [DOI] [PubMed] [Google Scholar]
- Wu Q., Yu D., Post J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. Journal of Clinical Investigation. 1998;101(2):321–326. doi: 10.1172/JCI1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Okui A., Yamada T., Nakazato H., Mitsui S. Spinesin/TMPRSS5, a novel transmembrane serine protease, cloned from human spinal cord. Journal of Biological Chemistry. 2002;277(9):6806–6812. doi: 10.1074/jbc.M103645200. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Masuda K., Ogawa H. Cloning and characterization of the cDNA for human airway trypsin-like protease. Journal of Biological Chemistry. 1998;273(19):11895–11901. doi: 10.1074/jbc.273.19.11895. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Honda A., Ogura A. Reduced fertility of mouse epididymal sperm lacking Prss21/Tesp5 is rescued by sperm exposure to uterine microenvironment. Genes to Cells. 2008;13(10):1001–1013. doi: 10.1111/j.1365-2443.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- Yan W., Sheng N., Seto M., Morser J., Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. Journal of Biological Chemistry. 1999;274(21):14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- Yan W., Wu F., Morser J., Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(15):8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka S., Ohnishi T., Kawano S. Purification, characterization, and localization of a novel trypsin- like protease found in the human airway. American Journal of Respiratory Cell and Molecular Biology. 1997;16(3):300–308. doi: 10.1165/ajrcmb.16.3.9070615. [DOI] [PubMed] [Google Scholar]
- Yin H., Kosa P., Liu X. Matriptase deletion initiates a Sjogren's syndrome-like disease in mice. PLoS One. 2014;9(2):e82852. doi: 10.1371/journal.pone.0082852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S., Nakahori Y., Yasuoka S. Fibrinogenolytic activity of a novel trypsin-like enzyme found in human airway. Journal of Medical Investigation. 1998;45(1-4):77–86. [PubMed] [Google Scholar]
- Yu J., Yu J., Mani R.S. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17(5):443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.X., Chao L., Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. Journal of Biological Chemistry. 1994;269(29):18843–18848. [PubMed] [Google Scholar]
- Yuan X., Zheng X., Lu D. Structure of murine enterokinase (enteropeptidase) and expression in small intestine during development. American Journal of Physiology. 1998;274(2 Pt 1):G342–G349. doi: 10.1152/ajpgi.1998.274.2.G342. [DOI] [PubMed] [Google Scholar]
- Zheng X.L., Kitamoto Y., Sadler J.E. Enteropeptidase, a type II transmembrane serine protease. Frontiers in Bioscience (Elite.ed) 2009;1:242–249. doi: 10.2741/E23. [DOI] [PubMed] [Google Scholar]
- Zhu H., Guo D., Li K. Prostasin: A possible candidate gene for human hypertension. American Journal of Hypertension. 2008;21(9):1028–1033. doi: 10.1038/ajh.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]


