Abstract
Galliformes and columbifomes are closely associated with humans and some species have been domesticated for well over 5000 years. Both orders remain diverse, ranging from the common domestic poultry species (e.g., chicken, turkey, and squabs) to the more exotic species found in the wild and in zoological collections. While many species have been benefited from human activities and have increased their ranges, others have declined in numbers and some have become threatened (e.g., Trinidad piping-guan and wood quail) or even extinct (e.g., dodo and passenger pigeon). Nondomestic galliformes and columbiformes are susceptible to many of the same diseases that occur in domestic species, yet predisposition may be different. Furthermore, disease prevalence depends on exposure and potential risk factors. Infectious diseases that tend to be more common under intensive commercial production may not pose as great a risk to exotic and free-living species.
Keywords: bacteria, columbiformes, disease, galliformes, infection, metabolic, parasite, pathology, toxic, virus
Introduction
Galliformes are a diverse order of heavy-bodied, ground-feeding birds with short rounded wings for short-distance flight. The order includes approximately 290 species divided into five families distributed worldwide (Dyke et al., 2003, Payne, 2000): Phasianidae (e.g., chicken, quail, partridges, pheasants, turkeys, peafowl, and grouse), Odontophoridae (New World quails), Numididae (guineafowl), Cracidae (e.g., chachalacas and curassows), and Megapodiidae (e.g., malleefowl, maleo, and brush-turkeys). Galliformes vary in size from the small king quail that weighs 28–40 g to the large North American wild turkey weighing up to 14 kg. Some species have been domesticated and are raised for human consumption of meat and eggs. Grouse, quail, partridges, pheasants, and turkeys are hunted regularly in all parts of the world. The majority of the birds in the group are granivorous to slightly omnivorous.
Columbiformes consists of two families, the Columbidae (pigeons and doves) and Raphidae (Dodos). There are about 310 extant species in the Columbiae family while all species of the Raphidae family are extinct. The terms pigeon and dove are used interchangeably. These birds are common and occur worldwide. Pigeons vary in size and color, from small, sparrow-sized ground doves to large turkey-sized crowned pigeons. Pigeons are kept for various purposes, such as for racing (homing pigeons), show, sports, acrobatics, and pets and as meat (e.g., squab). Some breeds are used in biomedical research.
Pigeons have a fleshy cere, a very large crop that extends on both sides of the neck, vestigial ceca that contains lymphoid tissue, and no gall bladder. Pigeons have an unusual system of veins in the upper part of the neck called the plexus venosus subcutaneous collaris. Extensive hemorrhage and death can occur if a vaccine or treatment is given by injection in this region, especially when it is engorged (e.g., during hot weather, territorial, or sexual displays). Both sexes share in egg incubation and chick care including secretion of crop milk, a thick fluid composed of desquamated epithelial cells, rich in protein, fat, and immunoglobulins that is regurgitated and fed to the young nestlings for about 2 weeks. Production is stimulated by secretion of prolactin in the pituitary gland. During crop milk production, epithelial cells of the crop are hyperemic, hyperplastic, and hypertrophied. Crop milk can be a source of disease transmission, especially when birds are congregated.
Nondomestic galliformes and columbiformes are susceptible to many of the same diseases as that occur in domestic chickens and turkeys. In this chapter, the latter will be used as models for many of the shared, common diseases in domestic and nondomestic species. Specific reference and descriptions of diseases unique to nondomestic species will also be discussed.
Non-infectious diseases
Nutritional
Galliformes and columbiformes are susceptible to a number of nutritional deficiencies; diagnosis for all of these conditions requires evaluation of lesions in combination with feed analysis.
Inadequate intake or lack of absorption of calcium, vitamin D3, or phosphorus causes rickets in young growing birds and osteomalacia in adults (Swayne et al., 2013). Rickets is characterized by enlargement or beading of the junctions of the ribs with sternum and vertebrae (rachitic rosary), bending of the ribs with lateral flattening of the thorax, pathologic bone fractures with callus formation, lateral deviation of the sternum, and spinal deformities are common. Rickets caused by calcium and/or vitamin D3 deficiencies is characterized by a thickened zone of proliferation in the growth plate of bones and enlarged parathyroid glands. Similar growth plate changes in the absence of parathyroid gland enlargement are associated with phosphorus deficiency or calcium excess. With osteomalacia, cortical and trabecular bone are thinner than normal and cortical bone may be porous (osteoporosis).
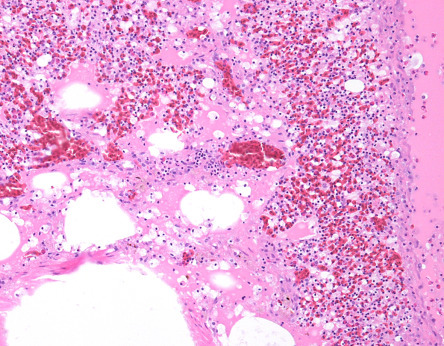
Vitamin E and selenium are antioxidants that protect cell membranes against oxidative damage caused by reactive oxygen species. Vitamin E deficiency (hypovitaminosis E) develops secondary to consumption of diets low in vitamin E and may be accompanied by deficiency in selenium or sulfur-containing amino acids. Accumulation of peroxides and deficiency of antioxidants results in a spectrum of diseases, which includes encephalomalacia, muscular dystrophy, and exudative diathesis (Swayne et al., 2013). Encephalomalacia also known as “crazy chick disease” is a disease of the central nervous system in young gallinaceous birds fed diets low in vitamin E; it is more commonly seen in 2–4 week old birds (Boulianne et al., 2013). Peroxidative damage to capillary walls results in edema, hemorrhage, and malacia of the affected areas. Macroscopically, the cerebellum is swollen and has petechial to diffuse hemorrhage producing a “cherry red” appearance in more severe lesions (Fig. 31.1 ). Microscopically, there is necrosis of the cerebellar folia that affects all layers, capillary thrombosis, edema, and axonal degeneration and hemorrhage in the cerebellar folia and meninges. Birds with muscular dystrophy have white streaks in muscle fibers of the breast, leg, and gizzard. Microscopically, there is degeneration and necrosis of myofibers, myofiber mineralization, satellite cell proliferation, heterophilic cell infiltrates in acute cases with subsequent fibroplasia. Exudative diathesis is characterized by subcutaneous edema and hemorrhage; a green-blue gelatinous appearance commonly seen in the ventral aspect of the thorax and abdomen and less frequently in the mandible and periorbital region (Brugere-Picoux et al., 2015).
Figure 31.1.
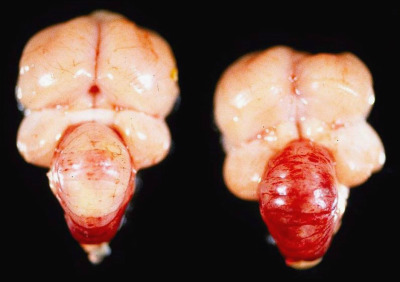
Vitamin E deficiency encephalomalacia in turkey poults.
Swollen and edematous cerebellum with petechiae (left) and diffuse cerebellar hemorrhage (right) with vitamin E deficiency.
Curled-toe paralysis is a disease of young gallinaceous poultry caused by vitamin B2 (riboflavin) deficiency, which is believed to be required for myelin synthesis in peripheral nerves. Deficiency causes a generalized demyelinating polyneuropathy. The sciatic, brachial, cervical, and lumbar nerves and large and medium intramuscular nerves are commonly affected and may be swollen and soft (Cai et al., 2009). The sciatic nerves may be 4–5 times larger than normal (Swayne et al., 2013). Microscopically there is myelin and axonal degeneration, edema, mild lymphocytic infiltrates, gliosis, and hyperplasia of Schwann cells in later stages.
Vitamin A is critical for differentiation of epithelial cells into cuboidal, columnar, or mucous-producing cells. Hypovitaminosis A, causes epithelial metaplasia and hyperkeratosis (Cortes et al., 2006, Swayne et al., 2013). Macroscopically, the mucosa of the tongue, choana, and salivary glands in the oropharynx and esophageal glands are thickened and form “pustule-like” nodules (Fig. 31.2 ) due to hyperkeratosis and distension of glands and ducts with keratin, secretions, and cellular debris. The conjunctiva, bursa of Fabricius, nasal passages, and sinuses may contain caseous exudate, mucous membranes may be dry, corneas may be opaque and dry and plantar surfaces may be thickened and hyperkeratotic.
Figure 31.2.
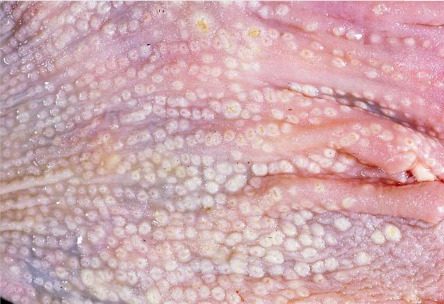
Hypovitaminosis A glandular metaplasia in a chicken.
Distended mucosal glands in the esophagus with vitamin A deficiency.
Choline or manganese deficiency causes perosis or chondrodystrophy, which are diseases of immature gallinaceous birds that result in impaired endochondral bone growth (Swayne et al., 2013). Affected birds are small for their age and have thickened, short, twisted and bent tarsometatarsi, and widened, deformed articular cartilage. In more severe cases, gastrocnemius tendon dislocation occurs. Microscopically, there is a narrow physeal zone of proliferation with disorganized chondrocytes (Fletcher, 2008).
Thyroid hyperplasia (goiter) is considered a common problem in birds and is common in pigeons (Schmidt and Reavill, 2002, Wadsworth and Jones, 1979). The most common causes include dietary deficiency or excess of iodine, consumption of goitrogenic substances (e.g., spinach, cassava, peanuts, soybeans, kale, broccoli, Brussels sprouts, cabbage, canola, cauliflower, mustard greens, radishes, and rapeseed), goitrogenic drugs (e.g., sulfonamides) and a defective negative feedback control by the pituitary.
Metabolic
Gout is a metabolic condition in which white chalky or semifluid-like urates accumulate in soft tissues or joints of various organs in the body. In birds, uric acid is the end-product of protein and purine metabolism (uricotelic) whereas in mammals, urea is the end-product (ureotelic). Gout in birds occurs in acute (visceral) and chronic (articular) forms (Fig. 31.3 ). These two forms differ in age of onset, frequency, sex predilection, gross and microscopic lesions, pathogenesis, and cause/s (Table 31.1 ). A great deal of confusion exists between the two syndromes because urate deposition in joints can occur in both disease forms. For clarity, commonly used terms “visceral gout” and “articular gout” should be avoided and replaced with “acute urate deposition” and “chronic urate deposition”, respectively. Histologically, feathery crystals may be seen within tissues; however, much of the urate deposits are lost when tissues are processed. In chronic cases, granulomatous inflammation is observed.
Figure 31.3.
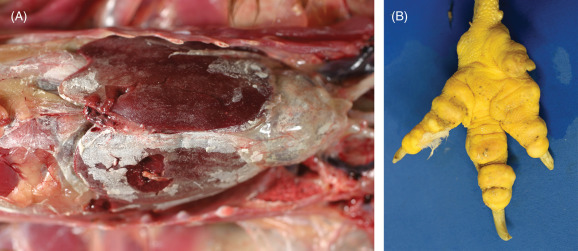
Urate depositions in a chicken.
(A) Acute urate deposition (visceral gout) over viscera. Fine, chalky-white crystalline material is present along the pericardium, across the capsular surface of the liver, and in the soft tissues of the coelom. (B) Chronic urate deposition (articular gout). The toes are enlarged and deformed.
Table 31.1.
Differences Between Acute and Chronic Urate Deposition
| Acute Urate Deposition | Chronic Urate Deposition | |
|---|---|---|
| Frequency | Common | Rare or sporadic |
| Age | Any age | Generally mature birds |
| Renal lesions | Almost always involved, with deposition of white, chalky precipitates. | Normal. |
| Soft tissue lesions | Visceral organs like liver, myocardium, spleen, or serosal surfaces like pleura, pericardium, air sacs, mesentery, etc. are commonly involved. | Soft tissues other than synovium are rarely involved. Occasionally comb, wattles, and trachea are involved. |
| Joint lesions | Soft tissues around the joints may or may not be involved. Surfaces of muscles, synovial sheaths of tendons, and joints are involved in severe cases. | Soft tissues around the joints are always involved, especially feet. Other joints of the legs, wing, spine, and mandible are also commonly involved. |
| Microscopic lesions | Generally no inflammatory reaction in synovium or visceral surfaces. Kidney and viscera have inflammatory reaction around tophus. | Granulomatous inflammation in synovium and other tissues. |
| Pathogenesis | Generally due to renal failure. | Probably due to a metabolic defect in the secretion of urates by the kidney tubules. |
| Causes | Dehydration Nephrotoxicity Neprhotropic infectious agents, vitamin A deficiency Urolithiasis Neoplasia (lymphoma, primary renal tumors) Immune mediated glomerulonephritis |
Genetics High protein diet |
Exertional myopathy is caused by excessive muscle activity during restraint. It commonly affects the wings and legs. Deep pectoral myopathy is an exertional compartment syndrome of the supracoracoid muscle characterized by ischemic necrosis in the absence of other muscular lesions. It occurs in domesticated heavy breeds of chickens and turkeys (Siller, 1985). Deep pectoral myopathy has been reported in zoo white-headed pigeons but not in free-ranging birds (Hill and Miller, 2013). Deep pectoral myopathy can be induced by holding a heavily muscled bird by its legs and allowing it to flap its wings (Siller et al., 1979) or by occlusion of the subclavian artery with muscle stimulation (Wight et al., 1979). Compartmental myopathy produces high levels of lactic acid and myonecrosis. Further complications include lactic acidosis, renal failure, arrhythmias, and death. Initially, the supracoracoid muscle is swollen, pale, and edematous. Later muscle becomes green dry and friable. Histologically, there is necrosis.
Toxic
Galliformes and columbiformes are curious animals that may unintentionally ingest toxic compounds in their environments. Most compounds considered toxic for mammals and other avian groups are likely to be toxic to galliformes and columbiformes (see Supplemental Table e1). As occurs in other avian species, galliformes and columbiformes are more susceptible to inhaled toxins than mammals because of their rapid metabolic rate and highly efficient respiratory system. Good ventilation should be maintained in birds kept in captivity.
Table e1.
Common Toxicities in Galliformes and Columbiformes
| Toxin | Examples | Clinical Effects and Lesions |
|---|---|---|
| Inhaled toxins | ||
| Essential oils | Pine, cedar | Respiratory tract irritation |
| Polytetrafluoro-ethylene | Nonstick cookware, heat lamps | Lung edema and hemorrhage. Histologically, epithelial necrosis of parabronchi and vascular damage |
| Ammonia | Tracheal deciliation, keratoconjunctivitis | |
| Insecticides | Organophosphates, organochlorides | Bronchoconstriction and an increase in bronchial secretions. Hemorrhages in heart and intestinal serosa. No specific histologic changes. |
| Ingested toxins | ||
| Heavy metals | Lead, zinc | Demyelination of peripheral nerves and vascular damage in the cerebellum |
| Mycotoxins | Moldy grains | Mucosal irritation and necrosis, hepatitis, nephritis, secondary infectious due to immunosuppression |
| Anticoagulant rodenticides | Brodifacoum, bromadiolone, chlorophacinone, diphacinone, and warfarin | Hemorrhage, anemia |
| Nonanticoagulant rodenticides | Zinc phosphide | Depression, ruffled feathers, anorexia, diarrhea, muscle spasms, tachypnea. Hydropericardium and ascites, generalized congestion |
| Strychnine* | Fluffed feathers, ataxia, wing droop, salivation, tremors, muscle tenseness, and convulsions, reproductive failure | |
| Sodium chloride | Salt, human foods | Polydipsia, polyuria, neurologic excitement, tremors, pulmonary hypertension, myocardial dilation, ascites |
| Bird baits | 4-aminopyridine (Avitrol) | Neurologic signs. Generalized congestion. Characteristic small pellets usually found in gizzard. |
| Alpha-chloralose | Neurologic signs, but no specific pathology | |
| Ethylene glycol (antifreeze) | Renal tubularepithelial necrosis leading to acute visceral urate deposition. | |
| Thallium | Vacuolar degeneration of hepatocytes and granular and/or hyaline droplet degeneration of renal tubular epithelia. | |
| Insecticides | Organophosphates, organochlorides | Excess salivation, lacrimation, abdominal pain, vomiting, intestinal hypermotility, and diarrhea |
Strychnine-treated baits were the method of choice for controlling pigeon and sparrows by the US Fish and Wildlife Services in 1960s (Hockenyos, 1962. VPC. pp. 271–307 Available from: http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1019&context=vpcone).
Pesticides and anticoagulant rodenticides are known to cause mortality in nontarget species, including wild pigeons, turkeys, and other galliformes (Fleischli et al., 2004, Poppenga et al., 2005, Sarabia et al., 2008). Though more commonly used historically, nonanticoagulant rodenticides (e.g., zinc phosphide or strychnine), can also cause toxicity and should not be ignored. Some species, for example, feral pigeons, may be purposely poisoned if they are considered a nuisance. Presently, there are no nationally registered toxic baits available for use on pigeons in the United States (Woronecki et al., 1992). Common poisons used by private individuals are alpha-Chloralose, 4-aminopyridine (Avitrol), thallium, and ethylene glycol (antifreeze). The first two affect the nervous system. Thallium and antifreeze (ethylene glycol) cause nephrosis and uric acid deposition in many organs (visceral gout).
Heavy metal intoxications, mainly associated with lead and zinc, are some of the most commonly reported and clinically recognized poisons in domestic and free-ranging birds. These metals are included in many man-made products, such as metal tools, coins, batteries, and gun pellets. Clinical signs include weight loss, lethargy, depression, anemia, paralysis, head tremors, convulsions, and death. Microscopically, the most diagnostic lesions are demyelination of peripheral nerves and focal areas of vascular damage in the cerebellum. Heavy metal poisoning should be differentiated from botulism. The primary treatment for lead or zinc poisoning is removal of the toxic metal.
Mycotoxicosis is caused by toxic metabolites of fungi. Hundreds of mycotoxins are known. The most common are aflatoxins and fusariotoxin (e.g., T-2 toxin and deoxynivalenol or DON) (Antonissen et al., 2016, U.S. Geological Survey, 1990). Because galliformes and columbiformes consume primary seed-based diets, they have a high risk of exposure. Susceptibility to mycotoxins varies among species. All mycotoxins cause immunosuppression. Aflatoxins may cause anemia, hemorrhage, hepatic degeneration, and paralysis. Fusariotoxins are associated with feed refusal, extensive necrosis of oral mucosa and in contact skin, and hyperplastic bone marrow.
Inflammatory Non-infectious
Amyloidosis is a well-recognized pathologic disorder in birds (see Chapter 29). Deposition of amyloid protein occurs commonly in the subsinusoidal space in the liver and spleen but can accumulate in other organs. In chickens, turkeys, and pigeons, it is commonly associated with chronic infections including Mycobacterium avium, Mycoplasma spp., and Enterococcus faecalis.
Xanthomas are rare nonneoplastic, white to yellow, nodular masses that occur in the skin, subcutaneous tissues, and internal organs (Kheirandish et al., 2013). Histologically, xanthomas are composed of sheets of large, foamy, and lipoprotein-laden macrophages. Occasionally, cutaneous xanthomas develop over lipomas (Filippich, 2004, Swayne et al., 2013). The majority of xanthomas that are surgically removed do not recur (Reavill, 2004).
Several metabolic diseases that are commonly seen in commercial poultry are occasionally, diagnosed in wild gallinaceous species and pigeons. These include hemorrhagic fatty liver syndrome, aortic rupture, ascites syndrome, dilated cardiomyopathy (round heart disease), tibial dyschondroplasia, and femoral head degeneration. The reader is referred to other publications for information (Boulianne et al., 2013).
Neoplastic
Virus-associated neoplasms tend to be the most common tumors observed in gallinaceous and columbiforms and are summarized in the infectious disease section. The etiopathogenesis of infectious neoplasms is well described for many tumors in domestic chickens (i.e., retrovirus associated lymphoma) and may be similar albeit uninvestigated in nondomestic poultry (Hubbard et al., 1983, Swayne et al., 2013, Wadsworth and Jones, 1981).
Tumors described in gallinaceous birds and pigeons include: oral squamous cell carcinomas in peafowl; fibromas, lipomas, and fibrosarcomas in the skin, skeletal muscle, and visceral tissues of free-ranging pigeon, pheasant, and quail; leiomyomas (often in the mesosalpinx), leiomyosarcomas, rhabdomyomas, and rhabdosarcomas in domestic chickens, quail, and a number of pigeon species; and ovarian or oviductal adenocarcinomas with or without wide metastasis in domestic and nondomestic chickens (Hubbard et al., 1983, Reece, 1992, Swayne et al., 2013).
Infectious diseases
There are a number of important viral infections of domestic galliformes that nondomestic galliformes are presumed susceptible to but that, to date, have not been reported to cause disease (see Supplemental Table e2).
Table e2.
Viral Diseases of Domestic Chickens but of Uncertain Significance in Nondomestic Galliformes and Columbiformes
| Etiology | Disease Name | Gross Lesions | Histologic Lesions | Diagnosis |
|---|---|---|---|---|
| DNA viruses | ||||
| Gyrovirus | Chicken infectious anemia | Pale viscera, fatty and yellowish bone marrow, bursal and thymic atrophy, and hemorrhages | Severe lymphoid depletion of the thymus and bursa of Fabricius. Hypoplasia of erythrocyte and myelocyte precursors in the bone marrow. | Clinical signs, gross and microscopic lesions. Virus detection by PCR and/or isolation. |
| RNA viruses | ||||
| Birnavirus* | Infectious bursal disease (Gumboro disease) | Edema and hemorrhage in bursa of Fabricius with subsequent atrophy. Occasional proventricular and muscle hemorrhage. | Lymphoid depletion, necrosis, interstitial edema, and lymphoid atrophy in the bursa of Fabricius | Gross and microscopic lesions and virus isolation and RT-PCR. |
| Coronavirus* | Infectious bronchitis | Mucosal congestion in the upper respiratory tract. The lungs may be congested and edematous and air sacs are thickened and cloudy. | Deciliation and necrosis of epithelium in the upper respiratory tract. | Virus isolation, RT-PCR, sequencing. |
| Reovirus** | Viral arthritis | Swelling of tibiotarsal-tarsometatarsal joint and occasional tendon rupture. | Edema of tendon sheaths, hypertrophy and hyperplasia of synoviocytes, villous proliferation of synovial membranes, and inflammation. | Virus isolation and identification, direct IF of tendons, PCR, and dot-blot hybridization. |
Note that the non-domestic galliformes are presumed to be susceptible.
OIE, Reportable disease.
Also diagnosed domestic turkeys.
DNA Viruses
The family Adenoviridae is divided into five genera: Mastadenovirus, Aviadenovirus, Atadenovirus, Siadenovirus, and Ichtadenovirus. The majority of adenoviruses in birds are classified as Aviadenoviruses (Harrach et al., 2011).
Quail bronchitis, caused by an Aviadenovirus, is a respiratory disease of bobwhite and Japanese quail (Swayne et al., 2013). Quail under 4 weeks of age are more susceptible. In older birds, infections are usually milder or subclinical (Boulianne et al., 2013). Gross lesions include catarrhal tracheitis, corneal clouding, conjunctivitis, and congestion in the mucosa of the nasal passages and infraorbital sinuses along with pulmonary congestion and consolidation and airsac thickening and opacity. Microscopically, there is lymphoplasmacytic tracheitis and bronchitis with loss of cilia, epithelial cell necrosis, and hyperplasia, and large, basophilic, intranuclear inclusions in intact or sloughed epithelium. Diagnosis is made by evaluating lesions and the presence of characteristic intranuclear inclusions in respiratory epithelium. Virus isolation can also be performed in samples of trachea, lungs, and air sacs for confirmation.
Inclusion body hepatitis (IBH), also caused by an Aviadenovirus, occurs worldwide. It primarily affects chickens, though guinea fowl and turkeys, can also develop disease (Cho, 1976, McFerran et al., 1976). Immunosuppressive viruses, such as chicken anemia virus or infectious bursal disease virus, predispose chickens to IBH. The disease is typically seen in 2–3 week old birds but it has been diagnosed in chickens as young as 4 days and as old as 7 weeks (Boulianne et al., 2013). Gross lesions include an enlarged, friable, and pale liver that in some cases contains multifocal foci of necrosis and hemorrhage. Other lesions include hydropericardium and swollen, pale, mottled, and hemorrhagic kidneys. Microscopically, there is multifocal hepatic necrosis, basophilic to amphophilic intranuclear inclusions in hepatocytes, and inflammation composed of lymphocytes, heterophils, macrophages, and plasma cells. Pancreatic necrosis with intranuclear inclusions has been reported in guinea fowl (Brugere-Picoux et al., 2015). Diagnosis is made by detecting characteristic hepatocellular intranuclear inclusions within lesions (Fig. 31.4 ). Virus isolation, immunofluorescence, and PCR can confirm the diagnosis.
Figure 31.4.
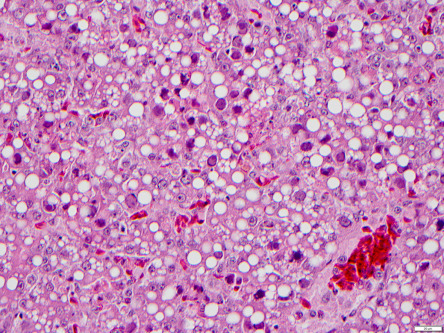
Inclusion body hepatitis in a chicken.
Multifocal hepatic necrosis and basophilic intranuclear inclusions in hepatocytes infected with aviadenovirus.
Pigeon adenovirus occurs in many parts of the world and causes two disease syndromes: classical adenovirus and necrotizing hepatitis (Vereecken et al., 1998). Classical adenovirus occur in young pigeons (<1 year) and causes diarrhea, vomiting, and weight loss with morbidity approaching 100%. This disease is associated with fowl adenoviruses belonging to various serotypes (Marek et al., 2014). Gross lesions include watery intestinal contents and microscopic villous atrophy with basophilic, intranuclear inclusions in enterocytes. Necrotizing hepatitis occurs in pigeons of all ages and results in vomiting, watery yellow diarrhea, and death within 24–48 h. Mortality ranges from 30% to 100%. Grossly, the liver is enlarged and pale yellow. Microscopically, there are multifocal to coalescing, random foci of hepatocyte necrosis with heterophilic inflammation. Karyomegaly may be seen and is due to large, round, and basophilic intranuclear inclusion bodies with occasional margination of chromatin. Adenovirus isolation pigeons is difficult, thus diagnosis is by histopathology and identification of 75–85 nm diameter, icosahedral, and nonenveloped viral particles by transmission electron microscopy.
Hemorrhagic enteritis caused by a Siadenovirus, affects turkeys, Guinea fowl, and chukar partridges (Massi et al., 1995; Shivaprasad, 2008). Lesions are characterized by small intestinal hemorrhage as well as an enlarged, friable, and mottled spleen with reticuloendothelial cell (RE) hyperplasia and basophilic/amphophilic intranuclear inclusions in RE cells. Marble spleen disease, also caused by a Siadenovirus is a disease of pheasants. It affects 3–8 month old birds (Bygrave and Pattison, 1973, Mayeda et al., 1982). Grossly, the spleen is swollen and mottled, and the lungs are congested and edematous. Microscopically, basophilic/amphophilic intranuclear inclusions are present in macrophages and lymphocytes in the spleen and Kupffer cells in the liver (Fitzgerald and Reed, 1989, Fitzgerald et al., 1992). In addition to gross and microscopic lesions, agar gel immunodiffusion or PCR assays can be performed for confirmation.
Pigeon circovirus (PiCV) is a small, nonenveloped, circular, single-stranded DNA virus. It is common in pigeons in the United States of America, Europe, Australia, South Africa, Japan, and China and is probably distributed worldwide. PiCV causes a complex, multifactorial disease primarily in young pigeons (<4 months of age) called young pigeon disease syndrome; adults can be carriers. PiCV can be found in healthy, asymptomatic pigeons (by PCR or ELISA) and has an incidence of 70% or higher (Stenzel et al., 2012, Swayne et al., 2013). Infection causes immunosuppression due to apoptosis of bursal lymphocytes. Sequela includes severe acquired immunosuppression (Abadie et al., 2001) and various secondary infections. Transmission is primarily horizontal but vertical transmission can also occur. Clinical signs vary and often depend on secondary or concurrent infections. Mortality is variable but can approach 100%. Occasionally, abnormal feathers can also be observed. Gross lesions include an enlarged or small bursa of Fabricius with or without luminal, caseous exudate, and splenomegaly. Microscopically, variable degrees of lymphoid necrosis are seen in the bursa: lymphoid depletion in acute disease and cystic follicles in subacute and chronic disease. Lymphoid depletion can also be seen in the spleen, cecal tonsils, and bone marrow. Intranuclear and intracytoplasmic basophilic globular or characteristic botryoid inclusion bodies are present in macrophages and, less commonly, epithelial cells of the bursa of Fabricius (Fig. 31.5 ). Similar inclusions can also be observed in the macrophages of the thymus, spleen, cecal tonsils, lungs, and duodenum. Ultrastructurally, inclusion bodies are composed of 15–20 nm in diameter, nonenveloped, icosahedral viral particles that are either loosely arranged or in a paracrystalline array. Diagnosis is based on a combination of PCR (feces or tissues), ELISA, and identification of characteristic inclusion bodies in the bursa of Fabricius and/or other organs, immunohistochemistry, and electron microscopy.
Figure 31.5.
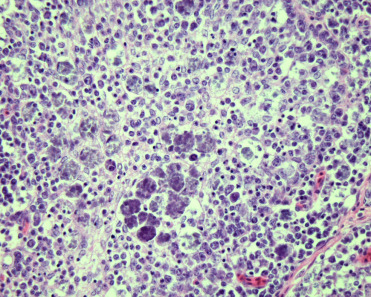
Circoviral infections in the bursa of a pigeon.
Botryoid inclusion bodies present in epithelial cells of the bursa of Fabricius.
The family Herpesviridae is divided into three subfamilies. All avian herpesviruses are in the alphaherpesvirinae subfamily.
Infectious laryngotracheitis (ILT) caused by Gallid herpesvirus 1 (GaHV-1; also known as Avian herpesvirus 1), is an OIE reportable disease. It occurs primarily in chickens, but pheasants, pheasant-chicken crosses, peafowl, and turkeys are susceptible (Crawshaw and Boycott, 1982, Kernohan, 1931, Portz et al., 2008). Virus infects and replicates in respiratory tract epithelium and conjunctiva. Latency is established in the trigeminal ganglia (Bagust, 1986) and viral infection is reactivated in immunosuppressed birds. In mild cases, the larynx, trachea, and primary bronchi contain excess mucus and have hyperemic mucosa with mild petechiae. In more severe lesions, intraluminal accumulation of fibrinonecrotic material and/or abundant hemorrhage (Fig. 31.6 ), which occludes the laryngeal opening and cause suffocation are seen. Affected birds may also have oculonasal discharge, conjunctivitis, and swelling of the infraorbital sinuses. Microscopic lesions in the respiratory tract and conjunctiva consist of epithelial erosion, necrosis, and ulceration with formation of syncytial cells containing eosinophilic, intranuclear inclusion bodies (Fig. 31.7 ). Syncytial cells may be present in tissues or sloughed into lumena and are only evident in the early stages of infection (1–5 days) (Guy et al., 1992). The mucosa and submucosa are edematous, congested, and infiltrated by mononuclear cells and heterophils. In addition to the pathologic changes, diagnosis can be made also by virus isolation, immunofluorescence, and PCR for GaHV-1 on samples of larynx, trachea, and lungs (Tripathy and Garcia, 2008).
Figure 31.6.
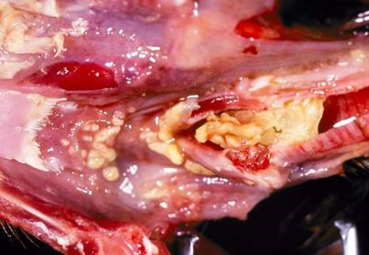
Infectious laryngotracheitis in a chicken.
Accumulation of fibrinonecrotic debris in the larynx of a chicken infected with Gallid Herpesvirus-1.
Figure 31.7.
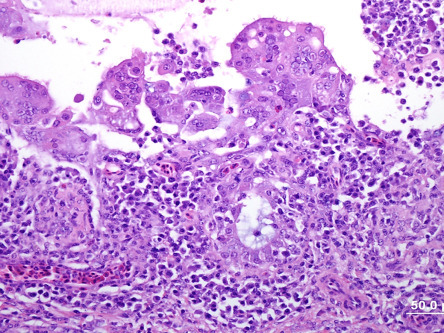
Infectious laryngotracheitis virus (Gallid Herpesvirus-1) in a chicken.
Severe loss of cilia, necrosis of epithelium, and numerous syncytial cells with eosinophilic intranuclear inclusions on the luminal surface.
Gallid herpesvirus 2 (Marek’s disease virus [MDV]), is one of the most common diseases of chickens. Quails, pheasants, and commercial turkeys are also susceptible, and disease in Japanese quail is relatively common (Swayne et al., 2013). The disease is more often seen in unvaccinated and sexually immature chickens that are 2–7 months old, but can occur at any age (Boulianne et al., 2013). Virus replicates in feather follicular epithelium and is shed in feather dander. Marek’s disease pathogenesis has four phases (Boodhoo et al., 2016, Swayne et al., 2013). In the early phase, the inhalation of cell-free virus with feather dander infects the respiratory epithelial cell and local macrophages followed by viremia with cytolytic viral replication in lymphocytes. This is followed by a latency phase in CD4+ T cells and results in systemic viral dissemination. During this phase, cutaneous viral infection, replication, and shedding occur. The third phase of MDV is characterized by reactivation in CD4+ T cells. During this phase, a late cytolytic stage and immunosuppression occurs. The final, proliferative phase is characterized by reactivation of the virus and neoplastic transformation of CD4+ T cells.
Gross lesions include bursal and thymic atrophy; tumor infiltration with enlargement of peripheral nerves (Fig. 31.8 ), white discoloration of the iris with an irregularly shaped pupil, nodular lesions in feather follicles, splenomegaly, hepatomegaly, and the presence of pale white tumors in various organs. Peripheral nerve lesions may be unilateral or bilateral and involve the sciatic, brachial, and vagus nerves and spinal root ganglia. Type A and B lesions are present in peripheral nerves and characterized by an infiltration of neoplastic CD4+ T cells and mixed inflammation with edema, respectively (Burgess et al., 2001, Swayne et al., 2013). Peripheral nerve lesions are rare in quail and turkeys (Paul et al., 1977, Swayne et al., 2013, Witter et al., 1974). Microscopically there is cerebral perivascular lymphocytic cuffing, vasculitis, edema, gliosis, and lymphocytic meningitis. Visceral organs contain pleomorphic and neoplastic T lymphocytes (Fig. 31.9 ). Skin lesions may be inflammatory or neoplastic but usually surround feather follicles. Eosinophilic intranuclear inclusions may be present in cells of the transitional zone and stratum corneum of the feather follicle epidermis (Fletcher, 2008). Lymphoma can be present in the anterior and posterior uvea. Detection of lymphoma in peripheral nerves, brain, and eye by histopathology support a diagnosis of Marek’s disease. As MDV is ubiquitous, viral detection alone is not confirmatory. Diagnosis therefore relies on characteristic lesions combined with detection of Marek’s disease virus antigens (pp38 and meq) in tissues by immunohistochemistry, virus isolation, or PCR of tumor from feather tips, blood, or tissues.
Figure 31.8.
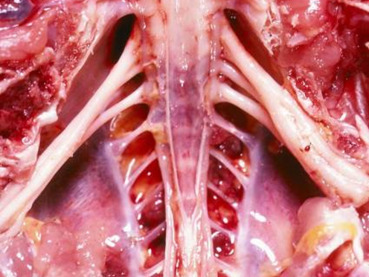
Gallid herpesvirus 2 (Marek’s disease) neuropathy in a chicken.
Enlarged and edematous sciatic nerves in a chicken with Marek’s disease may be due to neoplastic CD4+ T cell infiltrates (Type A) or mixed inflammation and edema (Type B).
Figure 31.9.
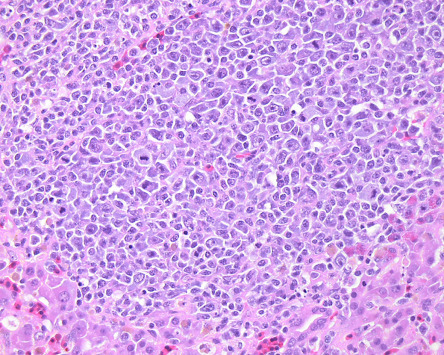
Gallid herpesvirus 2 (Marek’s disease) lymphoma in the liver of a chicken.
Infiltration of pleomorphic and neoplastic CD4+ T lymphocytes due to Marek’s disease.
Pigeon herpesvirus 1 (syn. Columbid herpesvirus 1) causes generalized infection in young pigeons; it’s seen occasionally in adults, who can be asymptomatic carriers. Stress or immunosuppression caused by pigeon circovirus infection predisposes birds to infection and disease (Swayne et al., 2013). Columbid herpesvirus-1 also causes disease in raptors including owls (see Chapter 30) (Gailbreath and Oaks, 2008, Woźniakowski et al., 2013). Virus sources include contaminated environment, food, and water as well as the crop milk, which the adults regurgitate and feed their young. The virus initially infects the mucosa of the conjunctiva, respiratory, and digestive tracts after which the birds develop viremia. Clinical signs include lethargy, depression, excess lacrimation, nasal discharge, diarrhea, neurological signs, and sudden death. Morbidity and mortality are variable in a flock but usually low. Squabs that have maternal immunity are protected against severe disease; they survive but become asymptomatic carriers gel (Marlier and Vindevogel, 2006). Gross lesions include conjunctivitis, fibrinonecrotic exudate in the pharynx, esophagus, and crop and most commonly, hepatomegaly with pale foci of necrosis. The spleen can be enlarged and the pancreas may contain pale, patchy areas of necrosis. Microscopically, there is necrosis of the pharyngeal, esophageal, and ingluvial epithelium with ulceration and inflammation. Epithelial cells subjacent to ulcers often contain eosinophilic intranuclear inclusions. The liver contains multifocal to coalescing areas of coagulative necrosis with mixed inflammatory cell infiltrates. Eosinophilic intranuclear inclusion bodies are present in hepatocytes, pancreatic acinar cells, and splenic mononuclear cells. Syncytia, some with inclusions, may also be present. Other lesions described less frequently include stomatitis, sinusitis, tracheitis, nonsuppurative encephalomyelitis, myocarditis, sialoadenitis, and proventriculitis. Virus isolation, transmission electron microscopy, or PCR for the viral DNA polymerase gene in liver, chicken egg embryos, chorioallantoic membrane, oropharyngeal swab samples, or cell culture are confirmatory when combined with characteristic histologic lesions (Doufour-Zavala et al., 2008).
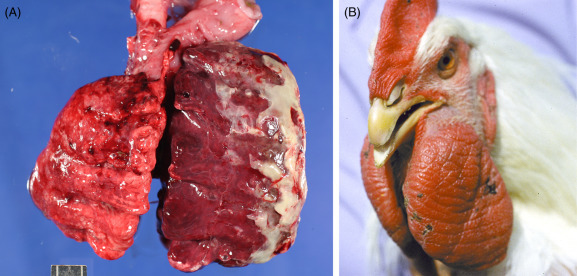
Avian poxvirus infections are caused by Avipoxviruses in the family of Poxviridae. Within the Avipoxvirus genus are numerous species, including fowlpox, pigeonpox, quailpox, and turkeypox. Infections are reported in a wide host range with a degree of host-specificity; morbidity can approach 100% but mortality is low. Avian pox viruses encode a protein similar to epidermal growth factor (EGF) believed to be associated with epithelial cell hyperplasia in infected tissues (Swayne et al., 2013). Gross lesions of cutaneous avian pox consist of proliferative tan nodules primarily on the unfeathered portions of the head (Fig. 31.10 ) and feet. Nodules may become ulcerated, dark brown, and hemorrhagic as a result of localized trauma. The “wet” or diphtheritic form of avian pox is characterized by proliferative and ulcerative lesions with diphtheritic membranes in the mouth, tongue, esophagus, conjunctiva, and upper respiratory tract (Fig. 31.11 ). Sometimes both forms can occur simultaneously. Characteristic microscopic lesions include irregular acanthosis, spongiosis, and ballooning degeneration with brightly eosinophilic, intracytoplasmic inclusions (Bollinger bodies) in epidermal (Fig. 31.12 ), and mucosal epithelial cells. The surface epithelium may be ulcerated and contain secondary bacterial and/or fungal infections. Mononuclear cells are commonly present in the surrounding submucosa or dermis. The microscopic appearance of Bollinger bodies is typically considered as diagnostic, but molecular testing is available. Pox virus infection occurs via ocular, digestive, and respiratory routes by exposure to contaminated environments, food, and water. Mosquito bites aid in the transmission of the virus, which results in breaks in the epidermis that facilitate transmission. The incubation period is between 4 and 10 days and all ages are susceptible. Virus isolation in embryonated chicken egg chorioallantoic membranes, PCR, and electron microscopy (especially in the absence of obvious viral inclusions) (Fig. 31.13 ) support the histologic diagnosis of poxviral infection.
Figure 31.10.
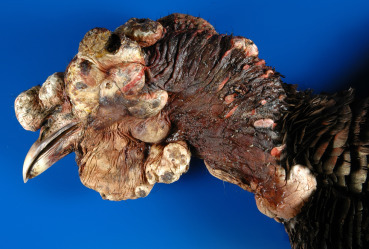
Poxviral dermatitis in a wild turkey.
Multiple proliferative nodules on the head.
Figure 31.11.
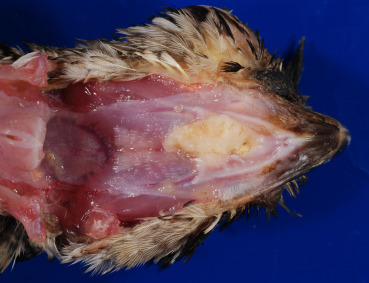
Poxviral proliferative stomatitis in a quail.
Proliferative lesions with diphtheritic membranes within the oral cavity typical of “wet” or diphtheritic form of avian pox.
Figure 31.12.
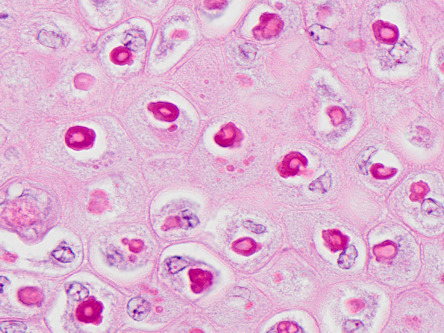
Poxviral epidermal hyperplasia in a chicken.
The epidermis is thickened with ballooning degeneration and eosinophilic intracytoplasmic inclusions.
Figure 31.13.
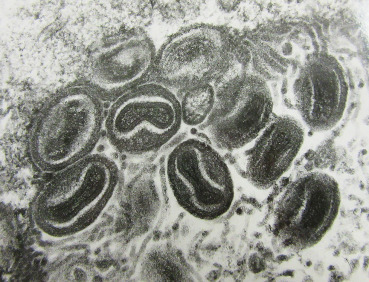
Poxviral particles in the eyelid of a chicken.
Transmission electron micrograph of virus particles with typical large pox virus morphology. TEM.
RNA Viruses
Avian influenza viruses (AIV) are influenza A viruses. They have been detected in more than 105 bird species from different taxa. Wild waterfowl in the orders Anseriformes and Charadriiformes are the natural reservoirs (Stallknecht, 1998). Traditionally, AIVs have been classified by their hemagglutinin (HA) and neuraminidase (NA) surface glycoproteins. Based on the clinical signs and mortality caused experimentally in chickens, AIVs are classified in two pathotypes, low and highly pathogenic AIV (LPAIV and HPAIV, respectively). For domestic species, all AIVs are reportable to the OIE; for wild birds only HPAIVs are reportable (Bonbon et al., 2017). The same AIV may induce a wide range of clinical signs depending on the host species, age, immunity, etc. LPAIV infections may be subclinical or associated with diseases affecting the respiratory, gastrointestinal, urinary, and reproductive tracts. These viruses require trypsin-like enzymes for the cleavage of the HA protein, thus viral replication is usually limited to mucosal epithelial cells of the respiratory and intestinal tracts. Circulation of H5 and H7 subtypes of LPAIV in gallinaceous poultry may result in the acquisition of multiple basic amino acids in the HA protein cleavage site and the emergence of highly pathogenic AIV (HPAIV) (Suarez, 2008).
HPAIV infection causes systemic disease with high mortality in chickens and turkeys. Other gallinaceous poultry species, such as bobwhite quail, ring-necked pheasants, and chukar partridges may survive longer and develop more severe neurological disease than chickens. It has long been thought that pigeons are resistant to HPAI; however, certain strains of HPAIV can cause disease (Jia et al., 2008, Klopfleisch et al., 2006).
Clinical signs of HPAIV infection include depression, paresis, paralysis, and mortality.
Affected birds may have edema and cyanosis of the head and comb, hemorrhage and cyanosis in the skin, edema and hemorrhage in the lungs, hemorrhage in the gastrointestinal mucosa and serosa, and necrotic foci and hemorrhage in visceral organs, such as the pancreas (Stipkovits et al., 2012, Swayne and Pantin-Jackwood, 2008, Swayne et al., 2013). Microscopic lesions of HPAIV infection vary and involve multiorgan necrosis, hemorrhage, and inflammation. Confirmation of disease in suspected cases is through virus isolation, antigen capture immunoassays, and RT-PCR or qRT-PCR in oropharyngeal and cloacal swabs, feces and in samples of affected organs. Rapid diagnosis can be achieved by screening oropharyngeal swabs using a matrix gene for influenza A by qRT-PCR and subsequent testing of positive samples with H5 and H7 subtype-specific qRT-PCR (Spackman et al., 2002). OIE influenza reference laboratories perform the final identification of these viruses.
Newcastle disease virus (NDV), a variant of avian paramyxovirus 1 (APMV-1), causes Newcastle disease (ND). Infection with NDV is OIE notifiable (Bonbon et al., 2017). NDV has been detected in at least 241 bird species (Kaleta, 1988). NDV strains are categorized as velogenic (highly virulent), mesogenic (moderately virulent), and lentogenic (nonpathogenic). Velogenic strains cause severe nervous and respiratory disease and high mortality (up to 100%); mesogenic strains primarily affect egg production, can produce mild respiratory disease and cause low mortality; lentogenic strains usually are asymptomatic in adult birds, but may cause respiratory disease in the young. Chickens are highly susceptible to velogenic NDV. Pathogenesis of ND involves viral replication in mucosal epithelium of the respiratory and intestinal tracts followed by viremia and viral dissemination to multiple visceral organs and brain. Gross lesions include facial edema, ventricular and/or proventricular hemorrhage, necrosis and hemorrhage in the intestinal mucosa, especially at cecal tonsils and Peyer’s patches, and splenomegaly with congestion and mottling (Swayne et al., 2013). Microscopically, affected birds have nonsuppurative encephalitis with gliosis and neuronal degeneration, disseminated vasculitis and thrombosis, and necrosis in lymphoid tissues as well as necrosis and hemorrhage in the mucosa of the conjunctiva, trachea, and gastrointestinal tract. Infections with low virulence NDV can cause mild conjunctivitis, rhinitis, tracheitis, pneumonia, and airsacculitis (Boulianne et al., 2013). Secondary bacterial infections enhance the severity of the respiratory disease caused by low virulence NDV. Virus isolation, RT-PCR, and DNA sequencing of the fusion protein cleavage site on tracheal and cloacal swabs and samples of affected organs support the gross and histological diagnosis; the intracerebral pathogenicity index (ICPI) test is performed to determine the virulence of the virus (OIE, 2012, Swayne et al., 2013).
A coronavirus similar to infectious bronchitis virus of chickens is associated with clinical signs of respiratory disease and pancreatitis in pigeons (Qian et al., 2006). Clinical signs include depression, polydipsia, ocular and nasal discharge, and tracheal rales. Necropsy findings include an enlarged and mottled pancreas, and edema and congestion in the lungs.
Eastern equine encephalitis (EEE) (family Togaviridae; genus Alphavirus); is a zoonotic alphavirus that causes neurological signs and encephalitis in ring-necked pheasants, chukar partridges, and turkeys (Bigler et al., 1976, Swayne et al., 2013). Mortality can be high (up to 80%) in naturally-occurring outbreaks; young birds are most susceptible. Gross lesions have not been observed in pheasants. Chukar partridges may have pale focal areas in the heart and an enlarged, mottled spleen (Ranck et al., 1965, Ranck et al., 1965). Microscopically, affected birds have lymphocytic meningoencephalitis with perivascular cuffing, gliosis, neuronal degeneration, and satellitosis. Myocardial necrosis and lymphocytic myocarditis may also be seen. Lymphoid depletion and necrosis in the bursa of Fabricius and thymus are reported in experimentally infected turkeys (Guy et al., 1993).
Highlands J virus (HJ) is an alphavirus that causes disease in turkeys and chukar partridges. Known distribution is in North and South America (Eleazer and Hill, 1994, Ficken et al., 1993, Wages et al., 1993). Affected birds have a mottled, enlarged spleen, myocardial pallor due to necrotizing myocarditis, intestinal serosal hemorrhage, enteritis with necrosis of gut-associated lymphoid tissue, lymphocytic meningoencephalitis, and lymphoid depletion with lymphoid necrosis in the bursa of Fabricius, spleen, and thymus.
West Nile virus (WNV), a type of flavivirus, is not considered a significant pathogen of galliformes or columbiformes despite recognized low level viremia in infected birds (Allison et al., 2004). Disease, including lymphocytic encephalitis, myocarditis, pancreatitis, ventriculitis, and myositis with bone marrow hyperplasia has been reported in a single wild turkey that was clinically disoriented and failed to flee when approached (Zhang et al., 2006).
Israel turkey meningoencephalitis (ITV) and Bagaza virus (BAGV) have been proposed as single virus species (Jovita Fernández-Pinero et al., 2014). ITV is reported in turkeys (Swayne et al., 2013), and BAGV has been detected in free ranging gallinaceous birds, including partridges and pheasants, and common wood pigeons (Gamino et al., 2012). Disease causes nervous signs and high mortality. Gross lesions vary from none to pallor of the pectoral muscle, pancreas, and myocardium. Injection of encephalic and epicardial vessels may be present. Microscopically, affected birds have nonsuppurative meningoencephalitis and splenitis as well as myocardial necrosis (Gamino et al., 2012, Ianconescu, 1976, Komarov and Kalmar, 1960).
Diagnosis of arboviral diseases is through evaluation of lesions and by performing virus isolation (in level 3 biocontainment facilities), RT-PCR, and immunohistochemistry.
Most enteric viral infections occur in young birds. The clinical signs and lesions induced by different viruses may be similar. Diarrhea and distention of the gastrointestinal tract with gas and liquid are common manifestations. Histologically, there is degeneration of enterocytes, villous blunting, and leukocytic infiltration of the lamina propria. The etiology of many poultry enteric disease syndromes is difficult to elucidate, as many of these viruses cannot be cultured. Astroviruses, reoviruses, rotaviruses, coronaviruses, and parvoviruses, to mention a few, are implicated as causative agents for enteric disease because they have been isolated from or identified in the intestines and intestinal contents of affected poultry (Pantin-Jackwood et al., 2008, Swayne et al., 2013). However, many of these enteric viruses can be also detected in otherwise healthy birds. Current research using viral metagenomics to describe the viral population in the avian gut will hopefully lead to a better understanding of the role viruses play in enteric disease.
Turkey rhinotracheitis (TRT) and swollen head syndrome (SHS) are highly contagious respiratory diseases caused by avian metapneumoviruses (aMPV). TRT is primarily a disease of turkeys, while SHS is primarily a chicken disease. aMPV infections similar to SHS have also been detected in pheasants (Gough et al., 2001, Ogawa et al., 2001, Welchman et al., 2002) and guinea fowl (Litjens et al., 1980). The viruses infect and replicate in epithelial cells of the respiratory tract causing ciliostasis, which predisposes to secondary bacterial infections (Catelli et al., 1998, Jirjis et al., 2004, Jones et al., 1988). Chickens, pheasants, and guinea fowl with SHS may present with swelling of the infraorbital sinuses, periorbital region, eyelid and subcutaneous tissue of the mandible, neck, and wattles due to accumulations of yellow gelatinous to caseous material. Turkeys with TRT have watery to mucoid exudate in the nasal passages and trachea (Jones et al., 1988) and periorbital and infraorbital edema. Fibrinous polyserositis with airsacculitis, pericarditis, perihepatitis, salpingitis, and oophoritis occur in birds with secondary bacterial infections. Microscopically, there is a loss of epithelial cilia in the respiratory tract and the lamina propria is expanded with edema and inflammatory cells. Small eosinophilic intracytoplasmic inclusions may be seen in epithelium of the nasal turbinates and trachea in the acute stage of the disease (Brugere-Picoux et al., 2015). Conjunctivitis, osteomyelitis of cranial bones, and otitis may also occur. Diagnosis is made by evaluating lesions and by virus isolation and/or RT-PCR on upper respiratory tract tissues, swabs, or mucus.
Pigeon Paramyxovirus 1 (PPMV-1) is a variant APMV-1 that is common in pigeon populations worldwide. Pigeons of all ages are susceptible. The APMV-1 variant, affecting pigeons is commonly known as pigeon PMV-1 or PPMV-1. It has a distinct binding pattern with monoclonal antibodies, and by phylogenetic analysis, the majority of PPMV-1 isolates are placed in lineage 4b (VIb) (Aldous et al., 2004). Disease is transmitted most commonly by ingestion of contaminated food and water but also through ocular and respiratory routes. The incubation period is 10–12 days. Lesions are due to viremia and noted in various organs including the brain, inner ear, and viscera. Clinical signs include anorexia, greenish watery diarrhea, lethargy, depression, and most commonly neurological signs, such as ataxia, paralysis of legs and wings, torticollis, and opisthotonus (Fig. 31.14 ). Morbidity can be high, but mortality in a flock is low. Gross lesions include greenish watery intestinal contents, pale tan and enlarged kidneys with increased urates, enlarged and mottled pale tan or red pancreas, and splenomegaly. Microscopically, the most common lesions are lymphoplasmacytic interstitial nephritis, pancreatitis, enteritis and splenitis, nonsuppurative meningoencephalomyelitis, and occasionally otitis interna. PPMV-1 can be diagnosed using the same methods as for any other APMV-1.
Figure 31.14.

Paramyxovirus neurologic signs in a pigeon.
Opisthotonus and torticollis associated with PMV-1 infection.
Avian Paramyxoviruses-2 to -11 are occasionally isolated from wild birds; however, it is unclear if they are responsible for clinical signs and death because experimental infection usually does not induce disease. Serotypes 2, 3, 6, and 7 have been isolated from turkeys. Serotype 7 has been isolated from dead collared doves.
Avian encephalomyelitis (AE) is a neurological disease of chickens, turkeys, quail, and pheasants caused by avian encephalomyelitis virus (genus Tremorvirus). Clinical disease with neurological signs most commonly occurs in 1–3 week old birds. Pathogenesis involves infection of the intestinal epithelium followed by viremia and viral dissemination to other visceral organs, skeletal muscle, and the brain (Swayne et al., 2013). Pale discoloration in the muscular layers of the ventriculus may be the only gross lesion seen in birds with clinical disease or gross lesions may be absent; grayish foci may be seen in the pancreas of pigeons (Toplu and Alcigir, 2004). Cataracts may be observed in birds that survive infection. Microscopically, the brain and spinal cord usually contain encephalomyelitis with lymphocytic perivascular cuffs, gliosis, glial nodules, central chromatolysis of neuronal cell bodies, and neuronal satellitosis. Multifocal lymphocytic infiltration may be present in the myocardium, muscular layer of the gastrointestinal tract, pancreas, skeletal muscle, and peripheral nerves (Brugere-Picoux et al., 2015, Swayne et al., 2013). Diagnosis is based on a combination of clinical signs, disseminated nonpurulent inflammation of the central nervous system, and identification of characteristic central chromatolysis in neuronal cell bodies in the brain stem (Fig. 31.15 ). Virus isolation performed on brain samples confirms the diagnosis. Fluorescent antibody tests can be performed to demonstrate viral antigen in tissues.
Figure 31.15.
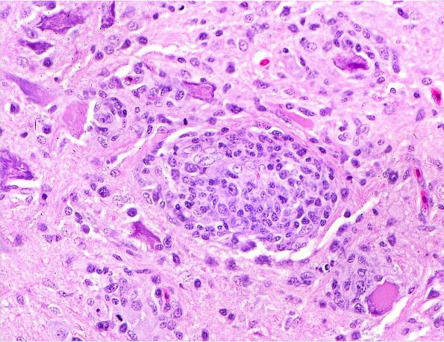
Avian encephalomyocarditis encephalomyelitis in a chicken.
Nonsuppurative encephalomyelitis with glial nodules and central chromatolysis of neuronal cell bodies.
Avian reoviruses are ubiquitous viruses among birds and infection is usually present without disease (an estimated 85%–90% of isolated avian reoviruses are nonpathogenic); one exception being viral arthritis/tenosynovitis in chickens and turkeys (Jones, 2000, Swayne et al., 2013). Reoviruses may be involved in several other disease syndromes, including respiratory, enteric, systemic infections, and the stunting/malabsorption syndrome of poultry. However, reoviruses are not considered the main cause in any of these conditions. Reoviruses have been isolated from tissues or feces of sick pigeons, pheasants, and bobwhite quail; in all these cases other pathogens (e.g., cryptosporidium and Staphylococcus aureus) were also identified in the lesions.
Lymphoid leukosis (LL) is a disease of semimature to mature chickens caused by avian leukosis sarcoma viruses (ALVs). These viruses are classified into 10 subgroups: A, B, C, D, E, F, G, H, I, and J. ALVs may cause a variety of tumors including lymphoid leukosis, soft tissue sarcomas, epithelial cell tumors (primarily renal tumors), and tumors of endothelial cell origin (hemangioma and hemangiosarcoma among others) (Swayne et al., 2013). Lymphoid leukosis is the most common of these neoplastic conditions. Disease is more frequently seen in chickens older than 16 weeks. ALVs infect and replicate in various tissues, but the bursa of Fabricius is the target organ for neoplastic transformation in chickens with LL. ALVs cause insertional activation of c-myc in transformed follicles by placing this gene under the control of viral LTR, which causes maturation arrest and proliferation of bursal stem cells (Neiman et al., 2001, Neiman et al., 2006). Gross lesions of LL consist of splenomegaly, hepatomegaly, and grayish to white soft tumors in various organs but most commonly in liver, spleen and bursa of Fabricius. Microscopically, affected organs have multifocal to coalescing nodules containing a uniform population of large lymphocytes (lymphoblasts) that efface normal tissue architecture and compress adjacent parenchyma. Lymphoma in the bursa of Fabricius has an intrafollicular growth pattern. Diagnosis can be difficult because LL tumors are similar to those caused by Marek’s disease virus and reticuloendotheliosis virus (REV) (Boulianne et al., 2013). Lymphoma with an intrafollicular pattern in the bursa of Fabricius is typical for LL, but bursal lymphoma caused by REV needs to be ruled out. Immunohistochemistry for B-cell and IgM surface markers, virus isolation from peripheral white blood cells and tumor homogenates, and standard or quantitative RT-PCR can be used for confirmation. Because ALVs are ubiquitous, viral detection alone is not confirmatory.
Reticuloendotheliosis, primarily a disease of chickens and turkeys, is caused by REV, a retrovirus in the Gammaretrovirus genus. REV is known to cause lymphoma, acute reticular cell sarcoma (reticuloendotheliosis), and various nonneoplastic lesions in various species of gallinaceous birds. In addition to chickens and turkeys, lymphomas caused by REV occur in quail, pheasants, peafowl, and Attwater’s prairie chickens (Swayne et al., 2013), and have recently been associated with disseminated lymphoma in young and adult breeding pigeons (Zhai et al., 2016). Clinical signs include reduced reproductive performance, poor weight gain, and increased flock mortality. Lesions coupled with virus isolation and PCR positive results from blood and tumor homogenates are diagnostic.
Lymphoproliferative disease (LD), caused by an exogenous retrovirus called lymphoproliferative disease virus (LPDV), occurs in domestic turkeys in Israel and several countries in Europe, and in wild turkeys in North America (Allison et al., 2014, Hayes and Langheinrich, 1992, Thomas et al., 2015). LD is characterized by multifocal to coalescing tan, raised, firm skin nodules composed of pleomorphic lymphoid cells admixed with plasma cells in unfeathered skin on the head or legs (Fig. 31.16 ) (Elsmo et al., 2016) and in visceral organs and tissues. Birds with systemic LD may have hepatomegaly, splenomegaly, and multifocal to coalescing tan, raised nodules or infiltrates in many organs including the liver, kidney, lung, spleen, gastrointestinal tract, and brain; neoplastic lymphocytic infiltrates often also surround blood vessels. While infection with LPDV is common in wild turkeys in the United States, LD lesions are rare. Diagnosis of LD requires histological confirmation of neoplastic infiltrates in the skin or multiple organs. The most sensitive tissue for molecular detection of LPDV is bone marrow, but whole blood, visceral organs, and spleen can also be used (Alger et al., 2015, Thomas et al., 2015). The method of virus transmission is not known.
Figure 31.16.
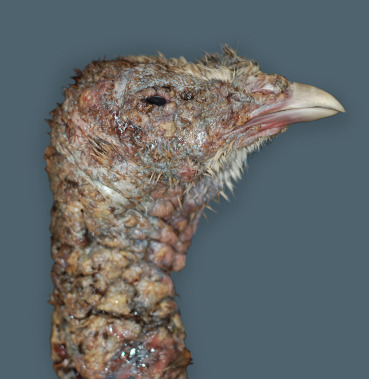
Retroviral lymphoproliferative cutaneous nodules in a turkey.
Nodules in the unfeathered skin on the head are composed of pleomorphic lymphoid cells.
Bacteria
Members of the Enterobacteriaceae family are ubiquitous and normal intestinal flora. Under certain circumstances, they may become pathogenic. They are not part of the normal respiratory or reproductive tract flora. Efforts should be made to avoid contamination during prosection, so that accurate culture results are obtained from these or other sites.
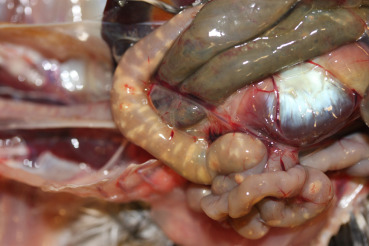
Colibacillosis refers to localized or systemic infection caused by Escherichia coli. It occurs in all types and age groups of poultry and pigeons. E. coli is present in the intestine of birds and excreted in feces. It may be a primary or secondary pathogen. Clinical signs vary from absent, when lesions are mild or localized, to lethargy, anorexia, or peracute death in severely affected birds. Chronically infected birds are often stunted and unthrifty. Colisepticemia or systemic E. coli infection is the most common pathogenic presentation (Fig. 31.17 ). Localized infections include omphalitis in hatchlings, salpingitis in laying hens, cellulitis caused by the secondary infection of skin wounds, synovitis as a sequel to systemic infections, and airsacculitis, pericarditis, and perihepatitis. Chronic granulomatous infections with E. coli are called coligranulomas or Hjarre’s disease. Enteritis caused by pathogenic attaching and effacing E. coli (AEEC) has been reported in gallinaceous birds and pigeons (Brugere-Picoux et al., 2015, Wada et al., 1995).
Figure 31.17.
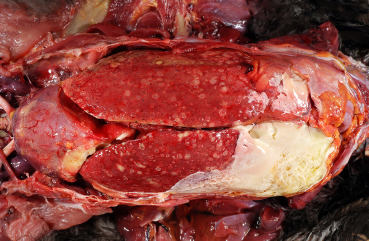
Enterobacterial hepatitis, air saculitis, and pericarditis in a chicken.
Numerous multifocal necrotic foci in the liver, thickening of the pericardium, injection of blood vessels as well as an acute fibrinous coelomitis.
Infections with Salmonella can cause acute and chronic disease. Gallinaceous species are reservoirs of Salmonella. S. enterica subsp. enterica serotypes Pullorum and Gallinarum are responsible for the septicemic diseases known as pullorum disease and fowl typhoid, respectively. These bacteria are nonmotile, generally host specific and reportable to OIE (Bonbon et al., 2017). Clinical signs include anorexia, dehydration, huddling, depression, diarrhea, and death. Mature birds may be asymptomatic or show reduced feed consumption, depression and decreased egg production, fertility, and hatchability. Other lesions may include swelling of the joints, dyspnea, and blindness. Histology is characterized by fibrinoheterophilic inflammation, usually in multiple organs.
The majority of the other serotypes in S. enterica subsp. enterica are motile and nonhost specific, and diseases caused by these bacteria are referred as paratyphoid salmonellosis. Infections are more important as a public health issue, than a disease concern for galliformes. Although all ages are susceptible to infection, clinical signs are usually seen in young and immunosuppressed birds. Birds look depressed and dehydrated, have diarrhea, and increased mortality, mainly in the first 2 weeks of age or at the onset of laying. Adult birds typically do not develop clinical signs. Bacteria are localized to the intestine or gallbladder of carriers and intermittently shed in feces.
In pigeons, one of the most common diseases is wing paralysis caused by S. enterica subsp. enterica serovar Typhimurium var. Copenhagen, a host-adapted pathogen (Rabsch et al., 2002). Clinical signs can be variable ranging from acute death to depression, listlessness, anorexia, greenish diarrhea, corneal opacity, swollen joints, ataxia, opisthotonus, and torticollis and occasionally respiratory signs. Gross lesions include hepatomegaly with green discoloration, enlarged spleens and kidneys in acute cases, and pale yellow dry exudate in the peritoneum, gonads, oviduct, heart, lungs, joints, intestine, brain, and the eye in chronic cases. Microscopically, lesions can vary from hepatocellular necrosis with fibrin exudation and infiltration of heterophils in acute cases to formation of caseous granulomas with infiltration of lymphocytes, macrophages, and multinucleated giant cells in the periphery in chronic cases.
S. enterica subsp. arizonae causes arizonosis in primarily young turkey poults. Infection is egg-transmitted. Disease can be similar to other Salmonella infections, mostly paratyphoid infections (Brugere-Picoux et al., 2015). Clinical signs are nonspecific and include depression, weakness, anorexia, diarrhea, paralysis, torticollis, opisthotonus, and blindness. Gross necropsy findings are nonspecific and include yolk sac retention, peritonitis, cecal cores, enlarged and mottled liver, splenomegaly, cloudy meninges, and cornea.
Diagnosis of salmonellosis is achieved by isolation and identification of the bacteria from internal organs. Isolation of Salmonella sp. from intestine or feces of adult gallinaceous species should be interpreted carefully and in the context of history, clinical signs, and lesions. Gallinaceous birds are the natural host of these bacteria and may carry Salmonella without clinical disease.
As other members of the Enterobacteriaceae family, Yersinia spp. are ubiquitous in the environment and water and can multiply at low temperatures. Yersinia pseudotuberculosis is the most important avian pathogen in this genus causing septicemic disease. Clinical signs depend on disease chronicity with the chronic form being more common (Boulianne et al., 2013). Clinical signs include lethargy, anorexia, loss of weight, diarrhea, dyspnea and dehydration, and sudden death. In chronic infections, signs include weight loss, swollen joints, and paresis. In acute cases, gross changes are associated with swelling of the liver and spleen and bloody to fibrinous exudate into the body cavity. In chronic infections, gross lesions consist of granulomas in organs and skeletal muscle, which resemble avian mycobacteriosis. Microscopically, lesions range from acute coagulative necrosis of hepatocytes, fibrin exudation, and heterophilic infiltration with large numbers of intralesional Gram-negative bacilli to giant cell granulomas. Similar lesions can be present in other organs, including the spleen and kidneys (Fig. 31.18 ).
Figure 31.18.
Yersinia pneumonia in a chicken.
Acute coagulative interstitial pneumonia, with fibrin exudation and heterophilic infiltration due to Yersinia pseudotuberculosis. Numerous intralesional Gram-negative bacilli with fibrin accumulation in the parabronchial lumen.
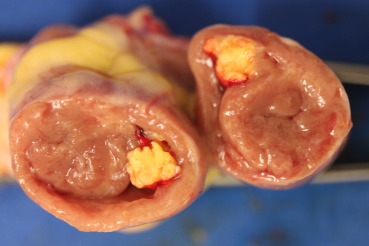
Pasteurella multocida, the causative agent of fowl cholera, occurs frequently as an acute septicemia, with high mortality and morbidity. All birds may be susceptible under appropriate conditions. A chronic, localized form may occur following acute disease (Fig. 31.19 ). Localized infections are found mainly in joints, abscesses of the head (including subcutaneous tissue, sinus, and middle ear), oviduct, and the respiratory tract. Infection between flock mates includes cannibalism of sick and dead birds. Furthermore, many recovered flocks may remain carriers spreading P. multocida to susceptible birds. Carriers may harbor the organism in the choanal cleft contaminating feed, water, and the environment. Cats, dogs, rodents, and pigs have been demonstrated to be reservoirs of P. multocida strains that are virulent for gallinaceous birds.
Figure 31.19.
Pasteurella multocida in a turkey.
(A) Acute unilateral pneumonia with an enlarged, dark red, congested right lung. (B) Swelling of the wattles with chronic P. multocida infection.
Infectious coryza is an acute respiratory disease caused by Avibacterium paragallinarum. Domestic chickens of all ages are susceptible and susceptibility increases with age. Pheasant, guinea fowl, and turkeys have also been reported to develop disease (Morishita, 2015). Transmission is by direct contact, aerosol, and contamination of drinking water. Recovered birds may become carriers. Disease is characterized by upper respiratory clinical signs, including nasal discharge, sneezing, and swelling of the face. There is also a drop in egg production. Lesions include sinusitis, rhinitis, conjunctivitis, tracheitis, bronchitis, and even airsacculitis when other pathogens are involved. Histologically, there is epithelial hyperplasia, edema and infiltration of heterophils, macrophages, and masts cells.
Ornithobacterium rhinotracheale is a Gram-negative bacterium, associated with an acute highly contagious respiratory disease. The bacterium has been isolated from domestic and wild gallinaceous birds, including chicken, chukar partridge, guinea fowl, partridge, pheasant, quail, and turkey. In young birds, the bacterium causes relatively mild respiratory signs. In older birds, especially turkeys, O. rhinotracheale can cause acute pneumonia with high mortality rates (De Rosa et al., 1996, Doufour-Zavala et al., 2008). It may also cause paralysis through arthritis, osteitis, and osteomyelitis, and a drop in egg production and quality (De Rosa et al., 1996, van Empel and Hafez, 1999).
Bordetellosis is a highly contagious upper respiratory tract disease, primarily of young turkeys, quail, and partridges caused by Bordetella avium (Swayne et al., 2013). Disease is characterized by conjunctivitis, sneezing, and coughing, with moist tracheal rales in young birds and dry coughs in older ones. Birds may be dyspneic with open-mouth breathing due to accumulation of mucus in the trachea, and softening and deformation of the tracheal rings. Because B. avium damages the epithelium of the upper respiratory tract, affected birds are susceptible to secondary infections. The severity of disease varies with the strain virulence and the presence of other infectious agents, such as ND, Mycoplasma, Pasteurella, and E. coli.
The genus Clostridium are Gram-positive, spore-forming, catalase-negative, anaerobic bacilli. Many have been recovered from environmental and clinical specimens of wild and domestic avian species. Pathogenicity is mediated through potent toxins and disease may occur as soft tissue infections, intoxications, and toxico-infections. There are four clostridial diseases of importance in gallinaceous birds:
Ulcerative enteritis caused by Clostridium colinum, occurs worldwide in many bird species, including quail, grouse, turkeys, chickens, and partridges (Swayne et al., 2013). It is an acute bacterial infection, primarily of young birds. Lesions are characterized by multiple ulcers with or without hemorrhage throughout the intestinal tract (Fig. 31.20 ) and foci of hepatic necrosis and inflammation. Ulcers can perforate the wall of the intestine causing peritonitis. Birds that recover naturally from the disease develop immunity but remain carriers and shed the bacterium in their feces.
Figure 31.20.
Clostridium enteritis in a quail.
Multiple transmural ulcers throughout the intestinal tract due to Clostridium colinum infection.
Necrotic enteritis is a sporadic, acute, noncontagious disease of the small intestine caused by C. perfringens type A, and to a lesser extent type C (Engström et al., 2003). The novel NetB toxin, not alpha-toxin, as previously believed, is critical in the pathogenesis of necrotic enteritis (Keyburn et al., 2008). Lesions are characterized by severe fibrinonecrotic enteritis with the formation of diphtheric pseudomembrane. Mortality rates are high.
Gangrenous dermatitis is a peracute, fatal disease, caused by C. perfringens or C. septicum. It occurs more frequently in warm and humid environments. It is believed that the bacteria are introduced through cutaneous wounds. Immunosuppression enhances susceptibility to disease. Affected birds are depressed or found dead with darkened and gangrenous skin, with emphysematous or serosanguinous cellulitis.
Botulism, caused by an exotoxin of C. botulinum that produces progressive flaccid paralysis, occurs more often in captive pheasants and occasionally in chickens (Boulianne et al., 2013). It occurs more frequently in warmer months. Of the eight known types of botulism toxins, type C is the most common in gallinaceous birds (Swayne et al., 2013). Birds with type C botulism lack characteristic gross or microscopic lesions. Definitive diagnosis requires detection of toxin in serum, crop, or gastrointestinal contents of moribund birds. C. botulinum can be found in the gut of normal chickens and toxin can be produced after the bird dies. Therefore, finding toxin in tissues of dead birds does not confirm botulism.
Mycoplasma sp. are small (approximately 200 nm), prokaryotic organisms that lack a cell wall phylogenetically related to Gram-positive bacteria (Razin, 1989, Sasaki, 1991). Mycoplasmas tend to be host-specific. Of the many Mycoplasma species that infect birds, only the species capable of causing disease in commercial poultry have received attention by researchers. Mycoplasma spp. can be transmitted vertically or horizontally by contact or by airborne dust or droplets.
Mycoplasma gallisepticum (MG) has been reported in chickens, turkeys, pheasants, partridges, peafowl, guinea fowl, and quail (Morishita, 2015). In turkeys, MG is more prevalent in domestic flocks, but the bacteria are found in wild populations (Luttrell et al., 1991). Infection is often associated with respiratory signs and swelling of the infraorbital sinus. The condition is frequently found as a coinfection with other respiratory pathogens such E. coli, Avibacterium sp., Aspergillus sp., or in ND. Signs of M. synoviae (MS) infection include pale comb, stunting, lameness, and swollen joints. Breast blisters are frequently observed. The infection in the respiratory tract is mostly subclinical. M. meleagridis (MM) and M. iowae (MI) infections affect mainly turkeys, and are responsible for lower egg production and hatchability with minimal or no respiratory signs.
Three species of Mycoplasma; M. columbinum, M. columbarale, and M. columbinasale have been isolated from clinically healthy pigeons and pigeons with respiratory disease (Keymer et al., 1984). Clinical signs can include ocular and respiratory disease; however, most sick birds have concomitant infections and it is difficult to determine whether and which signs are due solely to mycoplasmas. M. columbinum has also been associated with arthritis of the shoulder joint in a juvenile racing pigeon which had a droopy wing and was unable to fly (Hellebuyck et al., 2014).
Histologically, lymphoplasmacytic inflammation of the conjunctiva, sinuses, trachea, and occasionally air sacs and bronchi are the principal lesions of mycoplasmosis. Mycoplasma infection can be diagnosed by isolation on a suitable culture media or by PCR.
Erysipelas is an acute septicemic disease, caused by Erysipelothrix rhusiopatheae. In Galliformes, it occurs primarily in turkeys, occasionally in pheasants and rarely in chickens. The onset of disease can be sudden with birds found dead or dying after a short period of illness. Serosal, cutaneous, and muscular hemorrhage and splenomegaly are characteristic. A chronic form, characterized by polyarthritis or endocarditis, occasionally occurs. Infection is zoonotic.
Staphylococcus aureus, the cause of systemic disease Staphlococcosis, is widespread in the environment and causes a variety conditions, including arthritis, tenosynovitis, osteomyelitis, endocarditis (Fig. 31.21 ), and omphalitis. Skin injuries are a key point of entry, and S. aureus is a common opportunistic infection in ulcerative pododermatitis in many bird species, which include galliformes and columbiformes. The respiratory tract is another important portal of entry.
Figure 31.21.
Staphylococus aureus endocarditis in a chicken.
A large yellow granuloma attached to the right atrio-ventricular valve. The granuloma almost occupies the entire space of the right ventricle
Mycobacterium avium is the causative agent of avian mycobacteriosis. Susceptibility studies suggest that among gallinaceous birds, chickens and partridges are highly susceptible; pheasants, guinea fowl, and domestic turkeys are less susceptible. Epidemiological surveys classify pigeons from moderately susceptible to highly resistant to M. avium (Dhama et al., 2011, Dvorska et al., 2007, U.S. Geological Survey, 1990). In the past, Mycobacteriosis caused by M. genavense has been reported in captive doves (Haridy et al., 2014, Saggese et al., 2008). Contaminated fecal material is the most important means of dissemination of M. avium. Respiratory tract is also a potential source of infection. The bacteria can survive for more than 4 years in the environment, when not under direct sunlight (Dvorska et al., 2007). Infected birds lose weight, their feathers look dull, and birds have a marked leukocytosis due to monocytosis. Abnormal gait or paralysis can occur with joint infection. The most frequent gross lesions are individual or coalescing, granulomas that vary from pinpoint to several centimeters and can be found in many tissues including the liver, spleen, intestine, lung, and bone marrow. Histologically, lesions vary from mild to severe and consist of multifocal granulomatous inflammation or organized granulomas that, in some cases, replace significant portions of parenchymal organs; multifocal caseous necrosis is also seen. Granulomas are characterized by large accumulations of macrophages with abundant cytoplasm surrounded by multinucleated giant cells and fibrosis. Acid fast stains (e.g., Ziehl-Neelsen) highlight large numbers of bacteria within the cytoplasm of macrophages and multinucleated giant cells. These findings are usually sufficient for diagnosis. Other bacterial conditions, such as coligranuloma, Salmonella infections, fowl cholera, etc. must be ruled out as differentials. Mycobacterium can be cultured in suitable media and the species can be identified by PCR and sequencing.
Pseudomonas is an ubiquitous bacterium found in soil, water, and humid environments that can cause respiratory infections, keratitis, omphalitis, or septicemia in young birds.
Riemerella anatipestifer occasionally affects turkeys. See Chapter 30 for a more detailed description.
Chlamydiosis, also called ornithosis, is most commonly caused by Chlamydia psittaci genotype B, but other species of chlamydia, such as C. pecorum, C. trachomatis, and a novel species C. avium have also been detected in a variety of bird species, but their role in causing the disease is not known (Zocevic et al., 2013). Chlamydiosis is a common disease of pigeons of all ages but young birds are more susceptible. Various studies have shown that 19%–96% of pigeons are positive by complement fixation test, 0%–57% by isolation in chickens egg embryos and cell culture systems, and 3%–50% by PCR. Young birds are most susceptible and adults can be carriers. Chlamydiosis, on the other hand, is rarely detected in galliforms (Kaleta and Taday, 2003). Turkeys are the most common species affected (Andersen, 2005). One report from China, demonstrates that C. gallinacean, not C. psittaci, is common in clinically healthy chickens (Guo et al., 2016). The disease characteristics, transmission, and diagnosis are essentially the same as in Psittaciformes (see Chapter 32).
Both Streptococcus sp. and Enterococcus sp. are ubiquitous bacteria found in the intestine of many animals, including galliformes. It is possible that the bacteria enter the blood stream through intestinal lesions. Streptococcus sp. are mainly associated with septicemia, endocarditis, and necrotic hepatitis and splenitis. Streptococcus gallolyticus (previously S. bovis) is an important pathogen in some parts of Europe that causes septicemia and increased mortality as high as 78% in some flocks. The disease is seen more commonly in young pigeons and clinical signs range from acute death to anorexia, inability to fly, lameness, swollen joints, loss of weight, and greenish diarrhea. Grossly, the liver and spleen are enlarged and mottled, kidneys enlarged, and there are occasional pale foci in the heart and consolidation of lungs. Microscopically, there is fibrinoheterophilic inflammation in the liver, spleen, kidneys, heart, lungs, and synovium with occasional involvement of the brain associated with intralesional coccoid bacteria.
Enterococcus cecorum is associated with infections of the skeleton, including spondylitis, femoral head necrosis, and osteomyelitis. E. faecalis has been associated with endocarditis, granulomatous hepatitis, and amyloid arthritis. Clinical signs and lesions are not specific to these bacteria and other bacterial infections need to be ruled out.
Fungi
Aspergillosis is a common infection caused by Aspergillus fumigatus, A. flavus, or A. niger, saprophytic fungi associated with decaying organic matter (see also Chapters 27 and 302730). Birds typically become infected through the inhalation of fungal spores from the environment. The spores germinate in the respiratory tract and incite a strong inflammatory response (Iadrola et al., 1998). The fungi can continue to spread within the respiratory system via production of new spores under aerobic conditions, direct seeding into the coelomic cavity and pneumatized bones can also occur (Converse, 2007, Richard and Thurston, 1983). Systemic spread is via macrophages or because Aspergillus is vasotropic via hematogenous route. Disease, occasionally termed “brooder pneumonia,” tends to be more severe in younger birds (7–10 days after hatching in domestic chickens) in association with concurrent immunosuppression, infections, or stress (O’Mearra and Witter, 1971). In captive settings, disease is commonly associated with poor husbandry, moldy feed or litter, damp bedding, high stocking densities, poor ventilation, or increased species, or breed susceptibility (Atkinson and Brojer, 1998). Aspergillosis causing morbidity and mortality is reported in free-ranging wild turkey (Davidson et al., 1985) and Gambel’s quail (Guillion, 1957).
Gross lesions include mild to severely thickened air sacs and pleura coated with white exudate (mycelia) or green powdery-like (conidia) material (Fig. 31.22 ). Lungs contain a few small, pale yellow nodules ranging from 2 to 5 mm in diameter to larger areas of consolidation. Birds in chronic stages of the disease tend to be in extremely poor body condition and may have underlying or concurrent disease. Plugs of caseonecrotic material may be observed within the lumen of the trachea or syrinx. Ascites is frequently reported in chickens, presumably associated with vascular failure (Julian and Goryo, 1990). Deformities of vertebrae due to osteomyelitis are observed in domestic chickens with chronic infections presumably from hematogenous spread (Bergman et al., 1980). Ocular aspergillosis can be subdivided into two conditions based upon the underlying pathogenesis. Topical exposure results in keratoconjunctivitis with caseous plaques beneath the nictitating membranes (more common in chickens), while hematogenous spread results in posterior opthalmitis or panopthalmitis (more common in turkey poults) (Swayne et al., 2013).
Figure 31.22.
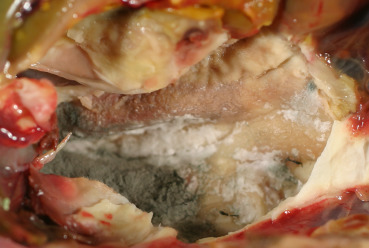
Aspergillus fumigatus air sacculitis in a pigeon.
The right air sac is opaque and thickened. “Bread mold” fungal mat completely overlies the air sac adjacent to the lung of this bird.
Microscopic lesions consist of typical giant cell granulomas with septate, branching hyphae that have 3–6 μm in diameter parallel walls with dichotomous, 45 degree angle, and branching. Fungi stain with both Gomori’s methenamine silver (GMS) and periodic acid-Schiff (PAS) techniques (Chandler et al., 1989). In some instances, large conidiophores (radiating fruiting bodies) are observed at an air interface (Converse, 2007). In acute-subacute cases, inflammatory cells are less organized but organization into granulomas occurs with chronicity as does surrounding fibrosis. In fibrinonecrotic debris, fungal hyphae, and conidiophores are most prevalent at regions of gas exchange (e.g., luminal surface of air sacs). Necrotic lesions associated with hyphae can be found in the brain in some cases, often with a limited inflammatory component. In posterior ocular infections, granulomas are present within the pectin and hyphae can be found in the vitreous humor and the retina.
While the gross and microscopic lesions are almost pathognomonic, infection can be confirmed with culture, molecular techniques (Katz et al., 1996, White et al., 2010), and immunohistochemistry (Carasco et al., 1993). A combination of PCR with galactomannan assays can increase the reliability of molecular results (Musher et al., 2004). In live birds, tracheal washes can be cultured, the granulomas can be visualized and sampled via cytologic preparations or endoscopic methods (Redig, 1998), and an indirect enzyme-linked immunosorbent assay (ELISA) can be used on serum for detection of antibodies and to monitor treatment (Cray et al., 2009). Radiographs can also suggest aspergillosis. As Aspergillus spp. can be ubiquitous in the environment, caution should be used to maintain sterility in sample collection. For cytology, nodules can be digested in 20% potassium hydroxide (KOH) and characteristic conidiophores and hyphae visualized with the assistance of ink dye; fungi can also be seen with Romanowsky or Wright Geimsa stains.
Candidiasis, crop mycosis, or thrush is caused by the ubiquitous and opportunistic yeast Candida albicans. Infection is often associated with concurrent illness or traumatic injury. Found worldwide, this yeast can be isolated from the gastrointestinal tracts of clinically healthy birds. Other species of Candida have also been isolated but are rare (Doufour-Zavala et al., 2008). Candidiasis is one of the most common mycotic diseases in pigeons, especially neonates that are not immunocompetent. Pigeons with concurrent circovirus infections are especially susceptible. Disease also occurs in gallinaceous birds including chickens, turkeys, guinea fowl, pheasants, and quail (Asrani et al., 1993, Moretti et al., 2000, Swayne et al., 2013). Large mortality events have been described in young domestic turkeys and chickens. Younger birds are more susceptible to disease than older birds. In pigeons, transmission of Candida occurs through ingestion of crop milk, contaminated feed and water, and from the environment. Clinical signs include anorexia, diarrhea, depression, regurgitation, crop distension, and increased morbidity and mortality.
Typical lesions of candidiasis include ulcerative and proliferative white plaques, commonly described as “Turkish towel” appearance. Pseudomembranes that can be easily peeled off may be present. Lesions are common in the oral cavity, crop mucosa, and the esophagus. Less commonly, candidiasis may be observed in the proventriculus and ventriculus in association with hemorrhage and ulceration. Rare systemic, dermatologic, and comb infections are reported. Microscopically, lesions include necrosis and epithelial ulceration associated with irregular hyphae and pseudohyphae, fibrin, sloughed epithelial cells, and necrotic debris that are often limited to the stratum corneum. Intraepithelial edema, hyperkeratosis, and secondary bacterial colonization may also be observed. Oval Candida spp. yeast are 3–6 μm. Hyphae are parallel, septate, 3–5 μm in diameter, while pseudohyphae include chains of elongated yeast that resemble hyphae with intermittent constrictions. Candida spp. stains with GMS and PAS and are Gram-positive. Focal periportal necrosis and vascular changes are suspected to be associated with an endotoxin. Candidiasis is diagnosed based on the microscopic appearance of the yeast, hyphae, and pseudohyphae. Infection can be confirmed with culture and molecular methods.
Infection with zygomycete fungi, such as Mucor, Rhizopus, Asidia, Rhizomucor, and Mortierella can present similar to aspergillosis and cause similar lesions. Infections are often secondary to concurrent illness or stress. Hyphae of zygomycete fungi are larger (7–20 μm), aseptate, lack parallel walls, have an irregular appearance with bulging dilations, and branch at right angles (Chandler et al., 1989). Zygomycete hyphae will also stain with GMS and PAS. Diagnosis can be confirmed with fungal culture or molecular methods.
Ochroconosis (or dactylariosis) due to Ochroconus gallopava, causes encephalitis in young domestic turkeys, chickens, and quail. O. gallopava has also been detected in the eye and brain of tinamou chicks (Ossiboff et al., 2015). Mortality events involving a large number of birds are often associated with contaminated litter (Shane et al., 1985, Swayne et al., 2013). Gross lesions described include focally extensive, firm, gray or red foci in the cerebellum or cerebrum as well as rare pulmonary granulomas. Histologic lesions include foci of necrosis associated with numerous heterophils, macrophages, and multinucleated giant cells. The hyphae of Ochroconis spp. are pigmented yellow to light brown, septate, irregularly branched, and 1.2–2.4 μm (Swayne et al., 2013). Diagnosis is based on fungal isolation or PCR.
Megabacteriosis (or macrorhabdosis) is caused by infection with the opportunistic yeast Macrorhabdus ornithogaster that localizes to the proventricular-ventricular junction. Infection has been documented in domestic turkeys, chickens, guinea fowl, quail, and partridge. Gross lesions are consistent with a chronic, debilitating disease of the gastrointestinal tract and include emaciation and thickening of the proventriculus. Histologically, there are lymphoplasmacytic and histocytic infiltrates in the lamina propria of the proventriculus and ventriculus. Organisms are often arranged in parallel bundles within mucus, proventricular crypts, koilin, and rarely, within the mucosa (Swayne et al., 2013). Diagnosis is based on detecting the organism in fecal smears, cytology of gastric mucus, or histology of the proventricular-ventricular junction. Organisms are Gram variable, but will stain with PAS.
Feather abnormalities in wild turkeys are associated with colonization by numerous fungal species including Aspergillus, Curvularia, Cladosporium Dactyella, Exophiala, Helminthosporium, Trichophyton, and Streptomyces. The pathogenesis is unknown. Gross lesions include cracks in the ventral median groove of the rachis with visible mold and broken tail feathers. Histologic lesions include fungal hyphae on the surface of the rachis and within the feather pulp and associated separation of the middle malpighian layer. Diagnosis is based on the microscopic appearance of the fungal hyphae and fungal culture (Davidson et al., 1989).
Metazoa
Most nematodes, that affect galliformes and columbiformes, parasitize the digestive tract with eggs that are shed in droppings. Common parasites, associated lesions and life cycle are summarized in Table 31.2, Table 31.3 (Figure 31.23, Figure 31.24 ).
Table 31.2.
Common Intestinal Parasites
| Parasite | Location in the Host | Clinical Signs/Lesions | Life Cycle |
|---|---|---|---|
| Nematodes | |||
| Capillaria | Crop, small intestine, and ceca | Thickened mucosa, emaciation, hemorrhage/anemia | Direct. May be ingested by earthworms. |
| Dispharynx, Tetrameres, Cyrnea | Proventriculus | Diarrhea, emaciation. Mucosa ulceration, necrosis | Indirect: grasshoppers, cockroaches, sowbugs, and pillbugs are intermediate host |
| Cheilospirura | Gizzard | Damaged gizzard wall | Indirect: grasshoppers, beetle |
|
Ascarids sp. (Fig. 31.23) |
Lumen of small intestine | Weight loss, potential intestinal blockage, and blood loss | Direct: Eggs may be ingested by insects |
| Heterakis | Ceca | Cecal mucosa inflammation and thickening | Direct: Eggs may be ingested by earthworms |
| Cestodes | Small intestine | Mild or unknown, except Raillientina sp. that causes severe lesions and weight loss | Indirect: insect, earthworm, snail |
| Trematode | Small intestine | Mild or unknown | Indirect |
| Protozoa | |||
| Eimeria (Coccidia) | Intestine, region depends on species | Varies with species, from diarrhea to bloody feces and death; from whitish white foci on serosa to hemorrhage and necrosis | Direct |
| Trichomonas | Esophagus, crop | White-yellow plaques (ulcers) | Direct |
| Histomonas | ceca, liver | Necrosis | Indirect: Heterakis gallinarum is the initial intermediate host Direct (?) |
| Cryptosporidium | Small intestine, bursa of Fabricius | Diarrhea, villus atrophy | Direct |
Table 31.3.
Common Non-intestinal Parasites
| Parasite | Location in the Host | Clinical Signs/Lesions | Life Cycle |
|---|---|---|---|
| Nematodes | |||
| Syngamus (Fig. 31.24) | Respiratory system: trachea | Gasping, dyspnea, nodules in trachea | Direct or indirect: earthworm, slugs, snails |
| Oxyspirura | Special senses: eye | Conjunctivitis, found under nictating membrane | Direct or indirect: cockroaches |
| Cestodes | Reproductive tract: oviduct | Weight loss and lower egg production | Indirect: insect, earthworm, snail |
| Protozoa | |||
| Leucocytozoon | Circulatory system | Sudden death, hepatomegaly, splenomegally. Schizogony in tissues. Gametogony in erythrocytes and leukocytes | Indirect: blackflies and culicoid midges |
| Plasmodium | Circulatory system | Schizonts in lung and blood endothelium; merozoites: erythrocytes (displace nucleus to the side) | Indirect: mosquitoes and biting midges |
| Hemoproteus | Circulatory system | Schizonts in lung and blood endothelium; merozoites: erythrocytes (around nucleus) | Indirect: biting flies and midges |
| Cryptosporidium | Respiratory system | Bronchopneumonia | Direct |
Figure 31.23.
Intestinal ascarid infections in a chicken.
Small intestine impaction in a chicken due to Ascaridia.
Figure 31.24.
Syngamus trachea tracheitis in a pheasant.
S. trachea, the gapeworm, are attached to the trachea wall and form granulomas.
Protozoa
Protozoans are a diverse group of unicellular organisms. Most species are free-living but some parasitize animals, including birds. Infections range from asymptomatic to life threatening, depending on the species and strain of the parasite and the resistance of the host.
Eimeria is a genus of intestinal coccidian parasite with a direct life cycle and fecal-oral transmission. Coccidiosis have been reported in numerous galliformes, including chickens, turkeys, quail, grouse, pheasants, chucker, guinea fowl, and peafowl. In general, the different species are host-specific but some species that affect domestic birds may also cause disease in exotic Galliformes (Morishita, 2015). Coccidiosis is also one of the most prevalent parasitic diseases in pigeons (Sokół and Michalczyk, 2009). Parasites may cause inflammation and destruction of intestinal tissue, diarrhea, and often dehydration (Fig. 31.25 ). Disease occurs in young and newly acquired animals. Immunity increases with exposure and older individuals often serve as reservoirs.
Figure 31.25.
Eimeria enteritis in the small intestine of a chicken.
E. necatrix causes inflammation, dilation, hemorrhage, and necrosis of jejunum-ileum. Note the severely swollen jejunum-ileum. Multifocal white foci (necrosis) and hemorrhage are noted in the serosal surface of small intestine.
Trichomonads are flagellated protozoa and most species are commensals of the avian digestive tract. Yet the species Trichomonas gallinae affects the upper digestive tract and causes the condition known as trichomoniasis, canker (pigeons), or frounce (see Chapter 30). Although pigeons are the primary carriers (Sansano-Maestre et al., 2009), T. gallinae also parasitizes chickens, turkeys, and other domestic and wild galliformes. Outbreaks occur more frequently in warmer months. Outbreaks of trichomoniasis in wild birds typically occur in passerines in the family Fringillidae (Robinson et al., 2010) but some mortality events have been reported in free-ranging columbiformes, such as the woodpigeon (Höfle et al., 2004) and the endangered pink pigeon (Swinnerton et al., 2005). Birds become infected by ingesting water or feed contaminated with oral secretions from infected pigeons (e.g., supplemental feeding sites). Young pigeons can become infected from the crop milk is regurgitated and fed by their parents. The protozoa invades the mucosal surface and causes stomatitis, esophagitis, and ingluvitis (Fig. 31.26 ) (Swayne et al., 2013). The affected mucosa becomes necrotic with profuse caseous exudate.
Figure 31.26.
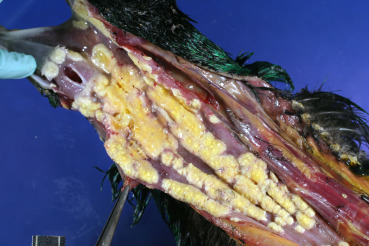
Trichomonas esophagitis in a peafowl.
There is necrosis of the esophagus where the protozoa invade that is overlain by caseous exudate.
Histomonas meleagridis is a well-known and economically important protozoal parasite of gallinaceous birds that causes the disease blackhead. Clinical signs include yellow colored droppings, depression, and death of affected birds. Lesions are characterized by multifocal necrosis of the liver that have a bulls-eye appearance (Fig. 31.27 ) and necrotizing typhlitis with luminal necrotic cecal cores. Domestic chickens may carry this parasite and not demonstrate any clinical signs; however, turkeys, pheasants, and peafowl are highly susceptible. Guinea fowl, chukar, grouse, quail, and partridges can also be infected (Morishita and Schaul, 2007, Swayne et al., 2013). Cases of histomoniasis typically occur in birds that have access to the outdoors and can encounter arthropod intermediate hosts as well as ova within the environment. In captive birds, blackhead is usually passed from bird to bird within the ova of the nematode Heterakis gallinae. Mechanical transmission may occur via earthworms, flies, grasshoppers, sowbugs, and crickets. In a recent survey, blackhead was not evident in any of the wild turkeys surveyed despite identification of Heterakis gallinae in the intestine of several birds (Oates et al., 2005). Histomonas meleagridis is reported periodically in free-ranging wild turkeys (Hurst, 1980), but blackhead has a low prevalence in wild gallinaceous birds (Davidson and Doster, 2003, Davison and Wentworth, 1992).
Figure 31.27.
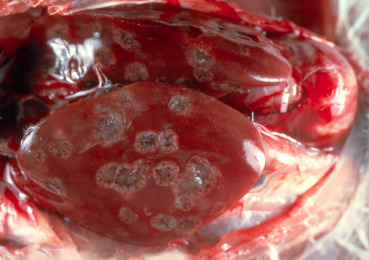
Histomonas meleagridis necrotizing hepatitis in a turkey.
Multiple areas of necrosis in the liver have bullseye appearance.
Hemosporidia, blood-borne, apicomplexan protozoa include parasites of three main genera—Plasmodium (the cause of avian malaria), Hemoproteus, and Leucocytozoon—that are transmitted by mosquitoes. These parasites invade blood and endothelial cells of many domestic and wild species, including galliformes and columbiformes. The life cycle requires two hosts: sporogony in insects, and schizogony (merogony) and gametogony in tissues or blood cells, respectively, of the vertebrate host. Plasmodium insect hosts are sucking mosquitoes (Culicidae), while Hemoproteus uses biting midges (Ceratopogonidae), and louse flies (Hippoboscidae). Leucocytozoon uses blood-sucking blackflies (Simuliidae) as the intermediate host. Avian hosts and their hemosporidian parasites share an evolutionary history in their native geographic distributions and most hemosporidian parasites are not lethal in the wild. Many of the examples of mortality in zoos caused by hemosporidia involve hosts challenged by parasites not found in their native habitat. On the other hand, hemosporidian parasites may act as population modulators because they reduce fitness.
Plasmodium spp. are reported in pheasants and guinea fowl (Morishita, 2015); Hemoproteus spp. are reported in guans, curassows, chachalacas (Bennett et al., 1982, Brugere-Picoux et al., 2015), and free living pigeons and doves (Klei and DeGiusti, 1975, Levine, 1959, Levine, 1980).
Differences in the prevalence, geographic distribution, and host of hemosporidia are associated with the habitat preference of bird hosts. The abundance and feeding habits within habitats of suitable insect vector and innate physiological differences make some avian hosts more susceptible than others. Most of these parasites are not very pathogenic unless parasitic loads are high. Following initial infection and an acute stage, most cases become chronic with occasional relapses when the host is under stress. Blood films stained with Giemsa allow observation of parasites in the erythrocytes. Splenomegaly and hepatomegaly are commonly observed in birds that died from these infections; hemozoin (malarial pigment) may be seen histologically in some infections. PCR may be necessary for confirmation of infection, especially in low level infections, or to speciate the organisms.
Ectoparasites
Lice are highly host-specific. The entire life cycle occurs on live birds and lice cannot live for more than a few days away from the host. Lice are the most commonly reported ectoparasites in wild turkeys and pigeons (Adang et al., 2008, Lane et al., 2006). Transmission is by direct contact. Different common names applied to louse species originate mainly from the preferred site of infestation of the species (Swayne et al., 2013). Lice feed primarily on feathers or scavenged debris from feather surfaces (Fig. 31.28 ); they may also take blood, serum, and skin tissues. Clinical signs are associated with weight loss and restlessness due to constant irritation. Eggs are laid along the feather, and usually at the base of the shaft. Harm to the host is minimal, except in very heavy infections. Bathing, preening, and other grooming behaviors help to eliminate lice.
Figure 31.28.

Chicken body lice (Menacanthus stramineus) on the feathers of a chicken.
These lice are one of the most common parasites found on the body of many gallinaceous birds. This yellowish, chewing lice have a flat body and are about 3 mm long.
Mites are not host specific, although they are more successful on their preferred host (Swayne et al., 2013). They are divided into two groups: (1) mange, which feed on skin cells and cause skin irritation and scabbing, and (2) blood sucking mites. Scaly leg mites (Knemidocoptes, mutans) are the most commonly diagnosed mange in birds with a wide host range. Mites burrow into the skin of the birds’ shanks and feet causing swelling and deformation of scales. In severe infections, loss of digits has been reported (Morishita, 2015). The depluming mite (Knemidocoptes laevis, syn. Neocnemidocoptes gallinae) borrows into the skin at the base of feathers, resulting in severe pruritus and feather pulling. In severe infestations, the mite may cause scaly, thickened, and wrinkled skin, mainly along the neck.
Among the blood sucking mites Dermanyssus gallinae, the red mite, is one of the best known and a common parasite of birds (Brugere-Picoux et al., 2015, Morishita, 2015). It feeds at night and hides during the day. D. gallinae can cause weight loss, anemia, and even death. Another mite that sucks blood and can cause similar serious problems is Qrnithonvssus sylviarum or Northern fowl mite. It visits the host only for feeding and therefore might be missed. In heavy infestations, feathers of the birds appear blackish, especially in the vent region, due to mite droppings.
The main flea in Galliformes is the stick-tight flea, Echidnophaga gallinacean. It infects chickens, pheasants, and quail (Morishita, 2015, Swayne et al., 2013). Females remain embedded in the skin of the host, usually about the head; while the males remain mobile. Adults feed on blood and can cause irritation, anemia, and even death in heavy infections.
Ticks, biting flies, and bedbugs feed periodically on blood. Heavy infestation can lead to severe host anemia, nest desertion, and even death. In addition, they serve as important vectors for other parasites including black flies with Leucocytozoon; biting midges with Hemoproteus; and several genera of mosquitoes with Plasmodium (Swayne et al., 2013). The major concern with these bugs is that they may migrate from birds and infest human houses, when carried in clothing or infected materials.
E-Slides
-
31.e1
Circovirus infection, pigeon, bursa of Fabricius. Lymphoid depletion, mostly in the medulla of bursal follicles. Numerous large basophilic intracytoplasmic inclusion bodies (5-25 um), often in clusters (botryoid), are present in macrophages and lymphocytes. (see Fig. 31.5). eSlide: VM05025
-
31.e2
Infectious laryngotracheitis (ILT), chicken, larynx. The lumen contains large amounts of necrotic cellular debris, heterophils,fibrin and mats of bacterial colonies. The mucosa has diffuse erosion to ulceration of pseudostratified epithelium and rare formation of large, multinucleate syncytia within the mucosa and within sloughed luminal debris. Within syncytia, large, eosinophilic intranuclear inclusion bodies with marginated chromatin are present. (see Figure 31.6, Figure 31.7). eSlide: VM05026
-
31.e3
Infectious laryngotracheitis (ILT), chicken, trachea. Large amount of necrotic cellular debris, inflammatory cells (heterophils and lymphocytes) and fibrin are in the tracheal lumen. There is diffuse deciliation and necrosis and ulceration of mucosal epithelium. Scattered large, multinucleate syncytia are within sloughed luminal debris. Within syncytia, many nuclei contain large, eosinophilic inclusion bodies that marginate chromatin. (see Figure 31.6, Figure 31.7). eSlide: VM05027
-
31.e4
Marek’s disease, chicken, sciatic nerve. Severe, multifocal to diffuse infiltration of pleomorphic and anisokaryotic lymphocytes between the axons of the sciatic nerve. Lymphocytes have discrete borders, small amounts of eosinophilic cytoplasm and central, round nuclei with finely to coarsely stippled chromatin. Surrounding axon sheaths are mildly to moderately dilated. (see Figure 31.8, Figure 31.9). eSlide: VM05028
-
31.e5
Yersinia pseudotuberculosis bronchitis and intestitial pneumonia, chicken, lung. Severe pulmonary congestion and edema. The interparabronchial spaces are expanded by edema and are infiltrated with moderate numbers of heterophils and macrophages. There is also infiltration of inflammatory cells in the airways of parabronchi and air capillaries, occasional necrosis of pneumocytes, and congestion of the capillaries in the parabronchi. (see Fig. 31.18). eSlide: VM04946
-
31.e6
Yersinia pseudotuberculosis interstitial pneumonia, chicken, lung. Gram. Gram stain of lung (H&E: slide SS31.05). Occasional colonies of gram negative bacteria are present within the necrotic areas of the lung. (see Fig. 31.18). eSlide: VM05029
-
31.e7
Histomoniasis (blackhead), chukar, liver. Multifocal coagulative necrosis of hepatocytes with infiltration of a mixed population of inflammatory cells including lymphocytes, plasma cells, macrophages, multinucleated giant cells, and a few heterophils. Within these foci of inflammation there are a large numbers of vacuoles or open spaces of various sizes. Many of these spaces have oval to spherical, faintly staining, eosinophilic protozoa measuring 7 to 15 um in diameter, consistent with Histomonas sp., in their center. (see Fig. 31.27). eSlide: VM05030
Metazoa
Capillaria nematodes infect the crop and esophagus but can also infect the intestine. They cause extensive damage to their hosts because adults feed directly on blood and tissue. Eggs are easily recognized by the prominent opercula at both ends. Some Capillaria species have direct life cycles, while others require intermediate hosts, such as earthworms, beetles, and cockroaches (Levine, 1980, Morishita and Schaul, 2007).
Worms of the proventriculus and gizzard belong to the genera Dispharynx, Tetrameres, and Cheilospirura and all have indirect life cycles (Morishita and Schaul, 2007, Swayne et al., 2013). The intermediate host of D. nasuta is the sowbug. Tetrameres utilize amphipods, grasshoppers, cockroaches, and earthworms as intermediate hosts. Beetles and grasshoppers serve as intermediate hosts to Cheilyspirura. These worms infect a variety of galliformes. All three genera burry in the mucosa causing tissue proliferation and deterioration. Affected birds may appear emaciated.
Ascaridia parasites, or large roundworms, are found in commonly in the intestine of many galliformes. Heavy infestations cause growth retardation, constipation or diarrhea, loss of condition, and sometimes intestinal blockage (Fig. 31.24). The ascarid life cycle is simple with no intermediate host (Levine, 1980). It is also possible that the eggs are ingested by grasshoppers or earthworms and in turn infect an avian host when the invertebrate is eaten. After ingestion, eggs hatch in the proventriculus or upper intestine and larvae cause damage during migration.
Members of the genus Heterakis are parasites mainly of the large intestine (cecum). Heterakis live on intestinal contents and do not migrate during their development, so damage to the host is minimal. But one species, Heterakisgallinae, plays an important role in the transmission of the protozoan parasite Histomonas meliagridis, which causes blackhead in galliformes.
Syngamus trachea or gapeworm has been reported to affect a broad range of captive and wild galliformes and columbiformes (Morishita and Schaul, 2007, Swayne et al., 2013). The parasites are usually encountered in the trachea, and occasionally in the bronchi. The worms attach to the tissue and feed on blood (Fig. 31.25). Clinical signs include gaping, coughing, and pneumonia-like symptoms. The lumen of the trachea may become obstructed, and the bird suffocates. Syngamus eggs are shed with the feces. Syngamus has a direct cycle, although earthworms may serve as intermediate host.
Oxyspiruramansoni, the common eye worm, infects numerous Galliformes, including chicken, turkey, grouse, guinea fowl, peafowl, quail, and prairie chicken (Morishita and Schaul, 2007, Swayne et al., 2013). This parasite is rare in pigeons. Adult worms are found beneath the nictitating membrane of the eye, causing lesions which vary from mild conjunctivitis to severe ophthalmia. The cockroach serves as intermediate host (Morishita and Schaul, 2007, Swayne et al., 2013).
Well over 1000 species of cestode belonging to ten different genera (including Davainea, Raillentina, Metroliasthes, etc.) have been recognized in domestic and exotic Galliformes (Morishita and Schaul, 2007, Swayne et al., 2013). Infection occurs through ingestion of an intermediate host. Most of them are not very pathogenic; however, weight loss has been noted in severely affected birds.
Trematodes (flukes) are small (usually less than 1 cm), flat, leaflike parasites of the gastrointestinal tract. They have an indirect life cycle. Numerous genera of flukes have been reported in domestic and exotic galliformes species and occasionally in pigeons. They are often missed if only fecal flotations are performed. Necropsy may detect specimens as incidental findings. The cause of mortality was associated with heavy infestation of Morishitium in guineafowl (Morishita, 2015).
References
- Abadie J., Nguyen F., Groizeleau C., Amenna N., Fernandez B., Guereaud C., Guigand L., Robart P., Lefebvre B., Wyers M. Pigeon circovirus infection: pathological observations and suggested pathogenesis. Avian Pathol. 2001;30:149–158. doi: 10.1080/03079450124811. [DOI] [PubMed] [Google Scholar]
- Adang K.L., Oniye S.J., Ezealor A.U., Abdu P.A., Ajanusi O.J. Ectoparasites of domestic pigeon (Columba livia domestica, Linnaeus) in Zaria, Nigeria. Res. J. Parasitol. 2008;3:79–84. [Google Scholar]
- Aldous E.W., Fuller C.M., Mynn J.K., Alexander D.J. A molecular epidemiological investigation of isolates of the variant avian paramyxovirus type 1 virus (PPMV-1) responsible for the 1978 to present panzootic in pigeons. Avian Pathol. 2004;33:258–269. doi: 10.1080/0307945042000195768. [DOI] [PubMed] [Google Scholar]
- Alger K., Bunting E., Schuler K., Jagne J., Whipps C.M. Diagnosing lymphoproliferative disease virus in live wild turkeys (Meleagris gallopavo) using whole blood. J. Zoo Wildl. Med. 2015;46:806–814. doi: 10.1638/2015-0037.1. [DOI] [PubMed] [Google Scholar]
- Allison A.B., Keel K.M., Philips J.E., Cartoceti A.N., Munk B.A., Nemeth N.M., Welsh T.I., Thomas J.M., Crum J.M., Lichtenwalner A.B., Fadly A.M., Zavala G., Holmes E.C., Brown J.D. Avian oncogenesis induced by lymphoproliferative disease virus: a neglected or emerging retroviral pathogen? Virology. 2014;450–451:2–12. doi: 10.1016/j.virol.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A.B., Mead D.G., Gibbs S.E.J., Hoffman D.M., Stallknech D.E. West Nile virus viremia in wild rock pigeons. Emerg. Infect. Dis. 2004;10:2252–2255. doi: 10.3201/eid1012.040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A.A. Serotyping of US isolates of Chlamydophila psittaci from domestic and wild birds. J. Vet. Diagn. Invest. 2005;17:479–482. doi: 10.1177/104063870501700514. [DOI] [PubMed] [Google Scholar]
- Antonissen G., Haesendonck R., Devreese M., Broekaert N., Verbrugghe E., Saeger S.D., Audenaert K., Haesebrouck F., Pasmans F., Ducatelle R., Croubels S. The impact of deoxynivalenol on pigeon health: occurrence in feed, toxicokinetics and interaction with salmonellosis. PLoS One. 2016;11:e0168205. doi: 10.1371/journal.pone.0168205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrani R.K., Gupta R.K., Sadana J.R., Pandita A. Experimental candidiasis in Japanese quail: pathological changes. Mycopathologia. 1993;121:83–89. doi: 10.1007/BF01103575. [DOI] [PubMed] [Google Scholar]
- Atkinson, R., Brojer, C., 1998. Unusual presentations of aspergillosis in wild birds. In: Proceedings of the Annual Conference of the Association of Avian Veterinarians, pp. 177–181.
- Bagust T.J. Laryngotracheitis (Gallid-1) herpesvirus infection in the chicken. 4. Latency establishment by wild and vaccine strains of ILT virus. Avian Pathol. 1986;15:581–595. doi: 10.1080/03079458608436317. [DOI] [PubMed] [Google Scholar]
- Bennett G.E., Gabaldan A., Ullva G. Avian haemoproteidae. The haemoproteids of the avian family Cracidae (Galliformes); the quans, curassows, and chachalacas. Can. J. Zool. 1982;60:3105–3112. [Google Scholar]
- Bergman V., Heider G., Vogel K. Mykotische spondylitis als ursache von bewegungsstorungen bei broilern. Montatsh. Veterinarmed. 1980;35:349–351. [Google Scholar]
- Bigler W.J., Lassing E.B., Buff E.E., Prather E.C., Beck E.C., Hoff G.L. Endemic eastern equine encephalomyelitis in Florida: a 20-year analysis, 1955–1974. Am. J. Trop. Med. Hyg. 1976;25:884–890. doi: 10.4269/ajtmh.1976.25.884. [DOI] [PubMed] [Google Scholar]
- Bonbon E., MacDiarmid S.C., Funes G.M. Chapter 1.3. Terrestrial Animal Health Code. 26th ed. World Organisation for Animal Health (OIE); Paris, France: 2017. OIE-Listed diseases, infections and infestations in force in 2017; pp. 5–7. http://www.oie.int/international-standard-setting/terrestrial-code/access-online/. (Accessed 21 January 2018) [Google Scholar]
- Boodhoo N., Gurung A., Sharif S., Behboudi S. Marek’s disease in chickens: a review with focus on immunology. Vet. Res. 2016;47:119. doi: 10.1186/s13567-016-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulianne M., Brash M.L., Fitz-Coy S.H., Fulton R.M., Julian R.J., Jackwood M.W., Ojkic D., Newman L.J., Sander J.E., Shivaprasad H.L., Wallner-Pendleton E., Woolcock P.R. American Association of Avian Pathologists; Jacksonville, FL: 2013. Avian Disease Manual. [Google Scholar]
- Brugere-Picoux J., Vaillacourt J.-P., Shivaprasad H.L., Venne D., Bouzauaia M. Association Francaise pour l’avancement des Sciences (AFAS); Paris, France: 2015. Manual of Poultry Diseases. [Google Scholar]
- Burgess S.C., Basaran B.H., Davison T.F. Resistance to Marek’s disease herpesvirus-induced lymphoma is multiphasic and dependent on host genotype. Vet. Pathol. 2001;38:129–142. doi: 10.1354/vp.38-2-129. [DOI] [PubMed] [Google Scholar]
- Bygrave A.C., Pattison M. Marble spleen disease in pheasants (Phasianus colchicus) Vet. Rec. 1973;92:534–535. doi: 10.1136/vr.92.20.534. [DOI] [PubMed] [Google Scholar]
- Cai Z., Blumbergs P.C., Finnie J.W., Manavis J., Thompson P.D. Selective vulnerability of peripheral nerves in avian riboflavin deficiency demyelinating polyneuropathy. Vet. Pathol. 2009;46:88–96. doi: 10.1354/vp.46-1-88. [DOI] [PubMed] [Google Scholar]
- Carasco L., Bautista M.J., de las Mulas J.M., Jensen H.E. Application of enzyme-immunohistochemistry for the diagnosis of aspergillosis, candidiasis and zygomycosis in three lovebirds. Avian Dis. 1993;37:923–927. [PubMed] [Google Scholar]
- Catelli E., Cook J.K., Chesher J., Orbell S.J., Woods M.A., Baxendale W., Huggins M.B. The use of virus isolation, histopathology and immunoperoxidase techniques to study the dissemination of a chicken isolate of avian pneumovirus in chickens. Avian Pathol. 1998;27:632–640. doi: 10.1080/03079459808419395. [DOI] [PubMed] [Google Scholar]
- Chandler F.W., Kaplan W., Ajello L. Wolfe Medical Publications Ltd; London: 1989. A Colour Atlas and Textbook of the Histopathology of Mycotic Diseases. [Google Scholar]
- Cho B.R. An adenovirus from a turkey pathogenic to both chicks and turkey poults. Avian Dis. 1976;20:714–723. [PubMed] [Google Scholar]
- Converse K.A. Apergillosis. In: Thomas N.J., Hunter D.B., Atkinson C.T., editors. Infectious Diseases of Wild Birds. Blackwell Publishing; Oxford: 2007. pp. 360–374. [Google Scholar]
- Cortes P.L., Tiwary A.K., Puschner B., Crespo R.M., Chin R.P., Bland M., Shivaprasad H.L. Vitamin A deficiency in turkey poults. J. Vet. Diagn. Invest. 2006;18:489–494. doi: 10.1177/104063870601800514. [DOI] [PubMed] [Google Scholar]
- Crawshaw G.J., Boycott B.R. Infectious laryngotracheitis in peafowl and pheasants. Avian Dis. 1982;26:397–401. [PubMed] [Google Scholar]
- Cray C., Watson T., Arheart K.L. Serosurvey and diagnostic application of antibody titers to Aspergillus in avian species. Avian Dis. 2009;53:491–494. doi: 10.1637/8673-030209-Reg.1. [DOI] [PubMed] [Google Scholar]
- Davidson W.R., Doster G.L. Blackhead disease does not really cause black heads. Nat. Wild Turkey Fed. Bul. 2003;25:1–4. [Google Scholar]
- Davidson W.R., Nettles V.F., Couvillion C.E., Howerth E.W. Diseases diagnosed in wild turkeys (Meleagris gallopavo) of the southeastern United States. J. Wildl. Dis. 1985;21:386–390. doi: 10.7589/0090-3558-21.4.386. [DOI] [PubMed] [Google Scholar]
- Davidson W.R., Shotts E.B., Teska J., Moreland D.W. Feather damage due to mycotic infections in wild turkeys. J. Wildl. Dis. 1989;25:534–539. doi: 10.7589/0090-3558-25.4.534. [DOI] [PubMed] [Google Scholar]
- Davison W.R., Wentworth E.J. Population influences: disease and parasites. In: Dickson J.G., editor. The Wild Turkey: Biology and Management. Stackpole Books; Mechanicsburg, PA: 1992. pp. 101–118. [Google Scholar]
- De Rosa M., Droual R., Chin R.P., Shivaprasad H.L., Walker R.L. Ornithobacterium rhinotracheale infection in turkey breeders. Avian Dis. 1996;40:865–874. [PubMed] [Google Scholar]
- Dhama K., Mahendran M., Tiwari R., Singh S.D., Kumar D., Singh S., Sawant P.M. Tuberculosis in Birds: insights into the Mycobacterium avium infections. Vet. Med. Int. 2011;2011:1–14. doi: 10.4061/2011/712369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doufour-Zavala L., Swayne D.E., Pearson J.E., Reed W.M., Jackwood M.W., Woolcock P.R. fifth ed. The American Association of Avian Pathologists; Athens, GA: 2008. Isolation, Identification and Characterization of Avian Pathogens. [Google Scholar]
- Dvorska L., Maltova L., Ayele W.Y., Fischer O.A., Amemori T., Weston R.T., Alvarez J., Beran V., Moravkova M., Pavlik I. Avian tuberculosis in naturally infected captive water birds of the Areideae and Threskiornithidae families studied by srotyping, IS 901 RFLP typing and virulence for poultry. Vet. Microbiol. 2007;119:239–374. doi: 10.1016/j.vetmic.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Dyke G., Gulas B., Crowe T. Suprageneric relationships of galliform birds (Aves, Galliformes): a cladistic analysis of morphological characters. Zool. J. Linnean Soc. 2003;137:227–244. [Google Scholar]
- Eleazer T.H., Hill J.E. Highlands J virus-associated mortality in chukar partridges. J. Vet. Diagn. Invest. 1994;6:98–99. doi: 10.1177/104063879400600118. [DOI] [PubMed] [Google Scholar]
- Elsmo E.E., Allison A.A., Brown J.D. A retrospective study of causes of skin lesions in wild turkeys (Meleagridis gallopavo) in the eastern United States, 1975–2013. J. Wildl. Dis. 2016;52:582–591. doi: 10.7589/2015-05-129. [DOI] [PubMed] [Google Scholar]
- Engström B.E., Fermér C., Lindberg A., Saarinen E., Båverud V., Gunnarsson A. Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet. Microbiol. 2003;94:225–235. doi: 10.1016/s0378-1135(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Ficken M.D., Wages D.P., Guy J.S., Quinn J.A., Emory W.H. High mortality of domestic turkeys associated with Highlands J virus and eastern equine encephalitis virus infections. Avian Dis. 1993;37:585–590. [PubMed] [Google Scholar]
- Filippich L.J. Tumor control in birds. Semin. Avian Exotic Pet Med. 2004;13:25–43. [Google Scholar]
- Fitzgerald S.D., Reed W.M. A review of marble spleen disease of ring-necked pheasants. J. Wildl. Dis. 1989;25:455–461. doi: 10.7589/0090-3558-25.4.455. [DOI] [PubMed] [Google Scholar]
- Fitzgerald S.D., Reed W.M., Burnstein T. Detection of type II avian adenoviral antigen in tissue sections using immunohistochemical staining. Avian Dis. 1992;36:341–347. [PubMed] [Google Scholar]
- Fleischli M.A., Franson J.C., Thomas N.J., Finley D.L., Riley W., Jr. Avian mortality events in the United States caused by anticholinesterase pesticides: a retrospective summary of national wildlife health center records from 1980 to 2000. Arch. Environ. Contamin. Toxicol. 2004;46:542–550. doi: 10.1007/s00244-003-3065-y. [DOI] [PubMed] [Google Scholar]
- Fletcher O. third ed. American Association of Avian Pathologists; Jacksonville, FL: 2008. Avian Histopathology. [Google Scholar]
- Gailbreath K.L., Oaks J.L. Herpesviral inclusion body disease in owls and falcons is caused by the pigeon herpesvirus (Columbid herpesvirus 1) J. Wildl. Dis. 2008;44:427–433. doi: 10.7589/0090-3558-44.2.427. [DOI] [PubMed] [Google Scholar]
- Gamino V., Gutiérrez-Guzmán A.-V., Fernández-de-Mera I.G., Ortíz J.-A., Durán-Martín M., de la Fuente J., Gortázar C., Höfle U. Natural Bagaza virus infection in game birds in southern Spain. Vet. Res. 2012;43:65. doi: 10.1186/1297-9716-43-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough R.E., Drury S.E., Aldous E., Laing P.W. Isolation and identification of avian pneumovirus from pheasants. Vet. Rec. 2001;149:312. [PubMed] [Google Scholar]
- Guillion G.W. Gambel quail disease and parasite investigation in Nevada. Am. Midl. Nat. 1957;40:414. [Google Scholar]
- Guo W., Li J., Kaltenboeck B., Gong J., Fan W., Wang C. Chlamydia gallinacea, not C. psittaci, is the endemic chlamydial species in chicken (Gallus gallus) Sci. Rep. 2016;6:19638. doi: 10.1038/srep19638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J.S., Barnes H.J., Smith L.G. Rapid diagnosis of infectious laryngotracheitis using a monoclonal antibody-based immunoperoxidase procedure. Avian Pathol. 1992;21:77–86. doi: 10.1080/03079459208418820. [DOI] [PubMed] [Google Scholar]
- Guy J.S., Ficken M.D., Barnes H.J., Wages D.P., Smith L.G. Experimental infection of young turkeys with eastern equine encephalitis virus and highlands J virus. Avian Dis. 1993;37:389–395. [PubMed] [Google Scholar]
- Haridy M., Fukuta M., Mori Y., Ito H., Kubo M., Sakai H., Yanai T. Avian Dis.: an outbreak of Mycobacterium genavense infection in a flock of captive diamond doves (Geopelia cuneata) Avian Dis. 2014;58:383–390. doi: 10.1637/10775-011714-Reg.1. [DOI] [PubMed] [Google Scholar]
- Harrach B., Benkő M., Both G.W., Brown M., Davison A.J., Echavarría M., Hess M., Jones M.S., Kajon A., Lehmkuhl H.D., Mautner V., Mittal S.K., Wadell G. Family adenoviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; San Diego: 2011. pp. 125–141. [Google Scholar]
- Hayes L.E., Langheinrich K.A. Reticuloendotheliosis in a wild turkey (Meleagridis gallopavo) from coastal Georgia. J. Wildl. Dis. 1992;25:262–265. doi: 10.7589/0090-3558-28.1.154. [DOI] [PubMed] [Google Scholar]
- Hellebuyck T., Garmyn A., De Cooman L., Boyen F., Pasmans F., Martel A. Mycoplasma columbinum isolated from a racing pigeon (Columba livia) with arthritis. J. Avian Med. Surg. 2014;28:240–241. doi: 10.1647/2013-075. [DOI] [PubMed] [Google Scholar]
- Hill A.G., Miller R. Exercise-induced deep pectoral necrosis in white-headed pigeons (Columba leucomela) J. Zoo Wildl. Med. 2013;44:990–995. doi: 10.1638/2012-0083R1.1. [DOI] [PubMed] [Google Scholar]
- Höfle U., Gortazar C., Ortíz J.A., Knispel B., Kaleta E.F. Outbreak of trichomoniasis in a woodpigeon (Columba palumbus) wintering roost. Eur. J. Wildl. Res. 2004;50:73. [Google Scholar]
- Hubbard G.B., Schmidt R.E., Fletcher K.C. Neoplasia in zoo animals. J. Zoo Anim. Med. 1983;14:33–40. [Google Scholar]
- Hurst G.A. Histomoniasis in wild turkeys in Mississippi. J. Wildl. Dis. 1980;16:357–358. doi: 10.7589/0090-3558-16.3.357. [DOI] [PubMed] [Google Scholar]
- Iadrola P., Lungarella G., Martona P.A., Viglio S., Guglieminetti M., Korzus E., Gorrini M., Cavarra E., Rossi A., Travis J., Luisetti M. Lung injury and degradation of extracellular matrix components by Aspergillus fumigatus serine proteinase. Exp. Lung Res. 1998;24:233–251. doi: 10.3109/01902149809041532. [DOI] [PubMed] [Google Scholar]
- Ianconescu M. Turkey meningo-encephalitis: a general review. Avian Dis. 1976;20:135–138. [PubMed] [Google Scholar]
- Jia B., Shi J., Li Y., Shinya K., Muramoto Y., Zeng X., Tian G., Kawaoka Y., Chen H. Pathogenicity of Chinese H5N1 highly pathogenic avian influenza viruses in pigeons. Arch. Virol. 2008;153:1821–1826. doi: 10.1007/s00705-008-0193-8. [DOI] [PubMed] [Google Scholar]
- Jirjis F.F., Noll S.L., Halvorson D.A., Nagaraja K.V., Martin F., Shaw D.P. Effects of bacterial coinfection on the pathogenesis of avian pneumovirus infection in turkeys. Avian Dis. 2004;48:34–49. doi: 10.1637/7017. [DOI] [PubMed] [Google Scholar]
- Jones R.C. Avian reovirus infections. Rev. Sci. Tech. 2000;19:614–625. doi: 10.20506/rst.19.2.1237. [DOI] [PubMed] [Google Scholar]
- Jones R.C., Williams R.A., Baxter-Jones C., Savage C.E., Wilding G.P. Experimental infection of laying turkeys with rhinotracheitis virus: distribution of virus in the tissues and serological response. Avian Pathol. 1988;17:841–850. doi: 10.1080/03079458808436506. [DOI] [PubMed] [Google Scholar]
- Jovita Fernández-Pinero, Irit Davidson, Maia Elizalde, Jiménez-Clavero M.Á. Bagaza virus and Israel turkey meningoencephalomyelitis virus are a single virus species. J. Gen. Virol. 2014;95:883–887. doi: 10.1099/vir.0.061465-0. [DOI] [PubMed] [Google Scholar]
- Julian J.R., Goryo M. Pulmonary aspergillosis causing right ventricular failure and ascites in meat-type chickens. Avian Pathol. 1990;19:643–654. doi: 10.1080/03079459008418720. [DOI] [PubMed] [Google Scholar]
- Kaleta E.F. Newcastle disease in free-living and pet birds. In: Alexander D.J., editor. Newcastle Disease. Kluwer Academic Publishers; Dordrecht Netherlands, Boston, MA: 1988. pp. 197–246. [Google Scholar]
- Kaleta E.F., Taday E.M. Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathol. 2003;32:435–461. doi: 10.1080/03079450310001593613. [DOI] [PubMed] [Google Scholar]
- Katz M.E., Love S.C.J., Gill H.S., Cheetham B.F. Development of a method for the identification, using the polymerase chain reaction, of Aspergillus fumigatus isolated from ostriches. Aust. Vet. J. 1996;74:50–54. doi: 10.1111/j.1751-0813.1996.tb13735.x. [DOI] [PubMed] [Google Scholar]
- Kernohan G. Infectious laryngotracheitis in pheasants. J. Am. Vet. Med. Assoc. 1931;78:553–555. [Google Scholar]
- Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., Rubbo A.D., Rood J.I., Moore R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;42:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keymer I.F., Leach R.H., Clarke R.A., Bardsley M.E., Mcintyr R.R. Isolation of Mycoplasma spp. from racing pigeons (Columba livia) Avian Pathol. 1984;13:65–74. doi: 10.1080/03079458408418509. [DOI] [PubMed] [Google Scholar]
- Kheirandish R., Azizi S., Azari O. Cutaneous xanthoma in a domestic pigeon: pathologic study (case report) Global Vet. 2013;10:140–143. [Google Scholar]
- Klei T.R., DeGiusti D.L. Seasonal occurrence of Haemoproteus columbae Kruse and its vector Pseudolynchia canariensis Bequaert. J. Wildl. Dis. 1975;11:130–135. doi: 10.7589/0090-3558-11.1.130. [DOI] [PubMed] [Google Scholar]
- Klopfleisch R., Werner O., Mundt E., Harder T., Teifke J.P. Neurotropism of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (Columbia livia f. domestica) Vet. Pathol. 2006;43:463–470. doi: 10.1354/vp.43-4-463. [DOI] [PubMed] [Google Scholar]
- Komarov A., Kalmar E. A hitherto undescribed disease-turkey meningoencephalitis. Vet. Rec. 1960;72:257–261. [Google Scholar]
- Lane R.S., Kucera T.F., Barrett R.H., Mun J., Wu C., Smith V.S. Wild turkey (Meleagris gallopavo) as a host of Ixodid ticks, lice, and Lyme disease spirochetes (Borrelia burgdorferi sensu lato) in California state parks. J. Wildl. Dis. 2006;42:759–771. doi: 10.7589/0090-3558-42.4.759. [DOI] [PubMed] [Google Scholar]
- Levine N.D. second ed. Burgess; Minneapolis, Minnesota: 1980. Nematode Parasites of Domestic Animals and of Man. [Google Scholar]
- Levine N.D.S.K. Check-list of blood parasites of birds of the order columbiformes. J. Wildl. Dis. 1959;1:1. [Google Scholar]
- Litjens J.B., Kleyn van Willigen F.C., Sinke M. A case of swollen head syndrome in a flock of guinea-fowl. Tijdschr. Diergeneeskd. 1980;114:719–720. [PubMed] [Google Scholar]
- Luttrell M.P., Kleven S.H., Davidson W.R. An investigation of the persistence of Mycoplasma gallisepticum in an eastern population of wild turkeys. J. Wildl. Dis. 1991;27:74–80. doi: 10.7589/0090-3558-27.1.74. [DOI] [PubMed] [Google Scholar]
- Marek A., Kaján G.L., Kosiol C., Harrach B., Schlötterer C., Hess M. Complete genome sequences of pigeon adenovirus 1 and duck adenovirus 2 extend the number of species within the genus Aviadenovirus. Virology. 2014;462–463:107–114. doi: 10.1016/j.virol.2014.04.033. [DOI] [PubMed] [Google Scholar]
- Marlier D., Vindevogel H. Viral infections in pigeons. Vet. J. 2006;172:40–51. doi: 10.1016/j.tvjl.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Massi P., Gelmetti D., Sironi G., Dottori M., Lavazza A., Pascucci S. Adenovirus-associated haemorrhagic disease in guinea fowl. Avian Pathol. 1995;24:227–237. doi: 10.1080/03079459508419065. [DOI] [PubMed] [Google Scholar]
- Mayeda B., West G.B., Bickford A.A., Cho B.R. Marble spleen disease in pen-raised pheasants in California; In: Proceedings American Association of Veterinary Laboratory Diagnosticians; 1982. pp. 262–270. [Google Scholar]
- McFerran J.B., Connor T.J., McCracken R.M. Isolation of adenoviruses and reoviruses from avian species other than domestic fowl. Avian Dis. 1976;20:519–524. [PubMed] [Google Scholar]
- Moretti A., Piregili Fioretti D., Boncio L., Pasquali P., Del Rossi E. Isolation of Candida rugosa from turkeys. J. Vet. Med. 2000;47:433–439. doi: 10.1046/j.1439-0450.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- Morishita T.Y. Galliformes. In: Miller R.E., Fowler M.E., editors. Fowler’s Zoo and Wild Animal Medicine. Elsevier; St. Louis, Missouri: 2015. pp. 143–155. [Google Scholar]
- Morishita T.Y., Schaul J.C. Parasites of birds. In: Baker D.G., editor. Flynn’s parasites of laboratory animals. second ed. Blackwell Publishing Professional; Ames, IA: 2007. pp. 217–302. [Google Scholar]
- Musher B., Fredericks D., Leisenring W., Balajee S.A., Smith C., Marr K.A. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalverolar lavage fluid. J. Clin. Microbiol. 2004;42:5517–5522. doi: 10.1128/JCM.42.12.5517-5522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P.E., Kimmel R., Icreverzi A., Elsaesser K., Bowers S.J., Burnside J., Delrow J. Genomic instability during Myc-induced lymphomagenesis in the bursa of Fabricius. Oncogene. 2006;25:6325–6335. doi: 10.1038/sj.onc.1209646. [DOI] [PubMed] [Google Scholar]
- Neiman P.E., Ruddell A., Jasoni C., Loring G., Thomas S.J., Brandvold K.A., Lee R., Burnside J., Delrow J. Analysis of gene expression during myc oncogene-induced lymphomagenesis in the bursa of Fabricius. Proc. Nat. Acad. Sci. USA. 2001;98:6378–6383. doi: 10.1073/pnas.111144898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mearra D.C., Witter J.F. Apergillosis. In: Davis J.N., Anderson R.C., Karstad L., Trainer D.O., editors. Infectious and Parasitic Diseases of Wild Birds. Iowa State University Press; Ames, IA: 1971. pp. 153–162. [Google Scholar]
- Oates D.W., Wallner-Pendleton E.A., Kanev I., Sterner M.C., Cerny H.E. A survey of infectious diseases and parasites in wild turkeys from NEBRASKA. Trans. Nebr. Acad. Sci. 2005;30:25–31. [Google Scholar]
- Ogawa A., Murakami S., Nakane T. Field cases of swollen-head syndrome in pheasants. J. Japan Vet. Med. Assoc. 2001;54:87–91. [Google Scholar]
- OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. seventh ed. OIE; France: 2012. Newcastle disease (Infection with Newcastle disease virus) pp. 555–573. [Google Scholar]
- Ossiboff R.J., Clancy M.M., Terio K.A., Conley K.J., McAloose D. Cerebral and ocular ochroconiasis in 2 elegant crested tinamou (Eudromia elegans) chicks. Vet. Pathol. 2015;52:716–719. doi: 10.1177/0300985814556186. [DOI] [PubMed] [Google Scholar]
- Pantin-Jackwood M.J., Day J.M., Jackwood M.W., Spackman E. Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006. Avian Dis. 2008;52:235–244. doi: 10.1637/8174-111507-Reg.1. [DOI] [PubMed] [Google Scholar]
- Paul P.S., Sautter J.H., Pomeroy B.S. Susceptibility of turkeys to Georgia strain of Marek’s disease virus of chicken origin. Am. J. Vet. Res. 1977;38:1653–1656. [PubMed] [Google Scholar]
- Payne, R., 2000. Birds of the World, Biology 532, Recent Families, Birds of the World. Available from: http://www.ummz.lsa.umich.edu/birds/birddivresources/families.html.
- Poppenga R.H., Ziegler A.F., Habecker P.L., Singletary D.L., Walter M.K., Miller P.G. Zinc phosphide intoxication of wild turkeys (Meleagris gallopavo) J. Wildl. Dis. 2005;41:218–223. doi: 10.7589/0090-3558-41.1.218. [DOI] [PubMed] [Google Scholar]
- Portz C., Beltrao N., Furian T.Q., Junior A.B., Macagnan M., Griebeler J., Lima Rosa C.A., Colodel E.M., Driemeier D., Back A., Barth Schatzmayr O.M., Canal C.W. Natural infection of turkeys by infectious laryngotracheitis virus. Vet. Microbiol. 2008;131:57–64. doi: 10.1016/j.vetmic.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Qian D.H., Zhu G.J., Wu L.Z., Hua G.X. Isolation and characterization of a coronavirus from pigeons with pancreatitis. Am. J. Vet. Res. 2006;67:1575–1579. doi: 10.2460/ajvr.67.9.1575. [DOI] [PubMed] [Google Scholar]
- Rabsch W., Andrews H.L., Kingsley R.A., Prager R., Tschäpe H., Adams L.G., Bäumler A.J. Salmonella enterica serotype Typhimurium and its host-adapted variants. Infect. Immun. 2002;70:2249–2255. doi: 10.1128/IAI.70.5.2249-2255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck F.M., Jr., Gainer J.H., Hanley J.E., Nelson S.L. Natural outbreak of Eastern and Western encephalitis in pen-raised Chukars in Florida. Avian Dis. 1965;9:8–20. [PubMed] [Google Scholar]
- Razin S. Molecular approach to mycoplasma phylogeny. In: Whitcomb R.V., Tully J.G., editors. The Mycoplasmas. Academic Press; San Diego, CA: 1989. pp. 33–69. [Google Scholar]
- Reavill D.R. Tumors of pet birds. Vet. Clin. North Am. Exot. Anim. Pract. 2004;7:537–560. doi: 10.1016/j.cvex.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Redig P.T. Proceedings of the 19th Annual Conference on Avian Medicine and Surgery. Mid-Atlantic States Association States Association of Avian Veterinarians; Lancaster: 1998. Aspergillosis in avians, an update. pp. 172–177. [Google Scholar]
- Reece R.L. Observations on naturally occurring neoplasms in birds in the state of Victoria, Australia. Avian Pathol. 1992;21:3–32. doi: 10.1080/03079459208418815. [DOI] [PubMed] [Google Scholar]
- Richard J.L., Thurston J.R. Rapid hematogenous dissemination of Aspergillus fumigatus and A. flavus spores in turkey poults following aerosol exposure. Avian Dis. 1983;8:1–6. [PubMed] [Google Scholar]
- Robinson R.A., Lawson B., Toms M.P., Peck K.M., Kirkwood J.K., Chantrey J., Clatworthy I.R., Evans A.D., Hughes L.A., Hutchinson O.C., John S.K., Pennycott T.W., Perkins M.W., Rowley P.S., Simpson V.R., Tyler K.M., Cunningham A.A. Emerging infectious disease leads to rapid population declines of common British birds. PLoS One. 2010;5:e12215. doi: 10.1371/journal.pone.0012215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggese M.D., Tizard I., Phalen D.N. Mycobacteriosis in naturally infected ring-neck doves (Streptopelia risoria): investigation of the association between feather colour and susceptibility to infection, disease and lesions type. Avian Pathol. 2008;37:443–450. doi: 10.1080/03079450802210655. [DOI] [PubMed] [Google Scholar]
- Sansano-Maestre J., Garijo-Toledo M.M., Gómez-Muñoz M.T. Prevalence and genotyping of Trichomonas gallinae in pigeons and birds of prey. Avian Pathol. 2009;38:201–207. doi: 10.1080/03079450902912135. [DOI] [PubMed] [Google Scholar]
- Sarabia J., Sánchez-Barbudo I., Siqueira W., Mateo R., Rollán E., Pizarro M. Lesions associated with the plexus venosus subcutaneus collaris of pigeons with chlorophacinone toxicosis. Avian Dis. 2008;52:540–543. doi: 10.1637/8251-020508-Case.1. [DOI] [PubMed] [Google Scholar]
- Sasaki T. Evidence that mycoplasmas, Gram-negative bacteria, and certain Gram-positive bacteria share a similar protein antigen. J. Bacteriol. 1991;173:2398–2400. doi: 10.1128/jb.173.7.2398-2400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.E., Reavill D.R. Thyroid hyperplasia in birds. J. Avian Med. Surg. 2002;16:111–114. [Google Scholar]
- Shane S.M., Marovits J., Snider T.G., III, Harrington K.S. Encephalitis attributed to dactylariosis in Japanese quail chicks (Coturnix coturnix japonica) Avian Dis. 1985;29:822–829. [PubMed] [Google Scholar]
- Shivaprasad H.L. Adenovirus group II-like infection in chukar partridges (Alectoris chukar) Avian Dis. 2008;52:353–356. doi: 10.1637/8032-062007-Case.1. [DOI] [PubMed] [Google Scholar]
- Siller W.G. Deep pectoral myopathy: a penalty of successful selection for muscle growth. Poul. Sci. 1985;64:1591–1595. doi: 10.3382/ps.0641591. [DOI] [PubMed] [Google Scholar]
- Siller W.G., Wight P.A., Martindale L. Exercise-induced deep pectoral myopathy in broiler fowls and turkeys. Vet. Sci. Commun. 1979;2:331–336. [Google Scholar]
- Sokół R., Michalczyk M. Kokcydioza gołębi [Pigeon coccidiosis] Życie Weterynaryjne. 2009;84:407–409. [Google Scholar]
- Spackman E., Senne D.A., Myers T.J., Bulaga L.L., Garber L.P., Perdue M.L., Lohman K., Daum L.T., Suarez D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallknecht D.E. Ecology and epidemiology of avian influenza viruses in wild bird populations: waterfowl, shorebirds, pelicans, cormorants, etc. In: Swayne D.E., Slemons R.D., editors. Proceedings of the Fourth International Symposium on Avian Influenza. US Animal Health Association; Richmond, VA: 1998. pp. 61–69. [Google Scholar]
- Stenzel T.A., Pestka D., Tykałowski B., Śmiałek M., Koncicki A. Epidemiological investigation of selected pigeon viral infections in Poland. Vet. Rec. 2012;171:562–566. doi: 10.1136/vr.100932. [DOI] [PubMed] [Google Scholar]
- Stipkovits L., Glavits R., Palfi V., Beres A., Egyed L., Denes B., Somogyi M., Szathmary S. Pathologic lesions caused by coinfection of Mycoplasma gallisepticum and H3N8 low pathogenic avian influenza virus in chickens. Vet. Pathol. 2012;49:273–283. doi: 10.1177/0300985811415702. [DOI] [PubMed] [Google Scholar]
- Suarez D.L. Avian influenza. In: Swayne D., editor. Influenza A virus. first ed. Blackwell Publishing; Ames, IA: 2008. pp. 3–22. [Google Scholar]
- Swayne D.E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V. thirteenth ed. Wiley-Blackwell; Ames, IA: 2013. Diseases of Poultry. [Google Scholar]
- Swayne D.E., Pantin-Jackwood M. The pathobiology of avian influenza infections in birds and mammals. In: Swayne D.E., editor. Avian Influenza. Blackwell Publishing; Ames, IA: 2008. pp. 87–122. [Google Scholar]
- Swinnerton K.J., Greenwood A.G., Chapman R.E., Jones C.G. The incidence of the parasitic disease trichomoniasis and its treatment in reintroduced and wild pink pigeons Columba mayeri. Ibis. 2005;147:772–782. [Google Scholar]
- Thomas J.M., Allison A.B., Holmes E.C., Phillips J.E., Bunting E.M., Yabsley M.J., Brown J.D. Molecular surveillance for lymphoproliferative disease virus in wild tukeys (Meleagris gallopavo) from the Eastern United States. PLoS One. 2015;10:e0122644. doi: 10.1371/journal.pone.0122644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplu N., Alcigir G. Avian encephalomyelitis in naturally infected pigeons in Turkey. Avian Pathol. 2004;33:381–386. doi: 10.1080/0307945042000220570. [DOI] [PubMed] [Google Scholar]
- Tripathy D.N., Garcia M. Infectious laryngotracheitis. In: Dufour-Zavala L., editor. A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens. fifth ed. OmniPress, Inc; Madison, WI: 2008. pp. 94–98. [Google Scholar]
- U.S.Geological Survey . USGS Biological Resources Division; Washington, D.C: 1990. Field Manual of Wildlife Disease—General Field Procedures and Diseases of Birds. [Google Scholar]
- van Empel P., Hafez H.M. Ornithobacterium rhinotracheale: a review. Avian Pathol. 1999;28:217–227. doi: 10.1080/03079459994704. [DOI] [PubMed] [Google Scholar]
- Vereecken M., de Herdt P., Ducatelle R. Adenovirus infections in pigeons: a review. Avian Pathol. 1998;27:333–338. doi: 10.1080/03079459808419348. [DOI] [PubMed] [Google Scholar]
- Wada Y., Kondo H., Nakazawa M., Kubo M. Natural infection with attaching and effacing Escherichia coli and adenovirus in the intestine of a pigeon with diarrhea. J. Vet. Med. Sci. 1995;57:531–533. doi: 10.1292/jvms.57.531. [DOI] [PubMed] [Google Scholar]
- Wadsworth P.F., Jones D.M. Some abnormalities of the thyroid gland in non-domesticated birds. Avian Pathol. 1979;8:279–284. doi: 10.1080/03079457908418352. [DOI] [PubMed] [Google Scholar]
- Wadsworth P.F., Jones D.M.L.P.S. Some cases of lymphoid leukosis in captive wild birds. Avian Pathol. 1981;10:499–504. doi: 10.1080/03079458108418500. [DOI] [PubMed] [Google Scholar]
- Wages D.P., Ficken M.D., Guy J.S., Cummings T.S., Jennings S.R. Egg-production drop in turkeys associated with alphaviruses: eastern equine encephalitis virus and Highlands J virus. Avian Dis. 1993;37:1163–1166. [PubMed] [Google Scholar]
- Welchman D.B., Bradbury J.M., Cavanagh D., Aebischer N.J. Infectious agents associated with respiratory disease in pheasants. Vet. Rec. 2002;150:658–664. doi: 10.1136/vr.150.21.658. [DOI] [PubMed] [Google Scholar]
- White P.L., Bretagne S., Klingspor L., Melchers W.J., McCulloch E., Schultz B., Finnstrom N., Mengoli C., Barnes R.A., Donnelly J.P., Loeffler J. Aspergillus PCR: one step closer to standardization. J. Clin. Microbiol. 2010;48:1231–1240. doi: 10.1128/JCM.01767-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight P.A.L., Siller W.G., Martindale L., Filshie J.H. The induction by muscle stimulation of a deep pectoral myopathy in the fowl. Avian Pathol. 1979;8:115–121. doi: 10.1080/03079457908418332. [DOI] [PubMed] [Google Scholar]
- Witter R.L., Solomon J.J., Sharma J.M. Response of turkeys to infection with virulent Marek’s disease viruses of turkey and chicken origins. Am. J. Vet. Res. 1974;35:1325–1332. [PubMed] [Google Scholar]
- Woronecki P.P., Dolbeer R.A., Seamans T.W., Lance W.R. Alpha-chloralose efficacy in capturing nuisance waterfowl and pigeons and current status of FDA registration. In: Borrecco J.E., Marsh R.E., editors. Fifteenth Vertebrate Pest Conference. University of California; Davis, CA: 1992. [Google Scholar]
- Woźniakowski G.J., Samorek-Salamonowicz E., Piotr Szymański, Wencel P., Houszka M. Phylogenetic analysis of Columbid herpesvirus-1 in rock pigeons, birds of prey and non-raptorial birds in Poland. BMC Vet. Res. 2013;9:52. doi: 10.1186/1746-6148-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S.L., Chen S.N., Lin T., Wen X.H., Wei W.K., Lv D.H., Chen R.A. Emergence of reticuloendotheliosis virus in pigeons in Guangdong Province, Southern China. Arch. Virol. 2016;61:2007–2011. doi: 10.1007/s00705-016-2870-3. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wilson F., Read R., Pace L., Zhang S. Detection and characterization of naturally acquired West Nile virus infection in a female wild turkey. J. Vet. Diagn. Invest. 2006;18:204–208. doi: 10.1177/104063870601800212. [DOI] [PubMed] [Google Scholar]
- Zocevic A., Vorimore F., Vicari N., Gasparini J., Jacquin L., Sachse K., Magnino S., Laroucau K. A real-time PCR assay for the detection of atypical strains of chlamydiaceae from pigeons. PLoS One. 2013;8:e58741. doi: 10.1371/journal.pone.0058741. [DOI] [PMC free article] [PubMed] [Google Scholar]


