![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Introduction
Initially “that disease” had no name, then we called it atypical pneumonia in China. Later the rest of the world would name it severe acute respiratory syndrome, or SARS … 1
Severe acute respiratory syndrome (SARS) is a newly emerged infectious disease manifested mainly as a severe form of bronchopneumonia that is caused by a novel coronavirus – SARS-CoV.2, 3 The virus was later shown to have jumped host species from horseshoe bat to infect humans via masked palm civets or possibly other mammals sold in live animal markets.4, 5, 6, 7, 8, 9 First having occurred in November 2002 in Guangdong Province of southern China,10, 11 the SARS epidemic that spread to 29 countries in five continents over a few weeks showed its potential to have a pandemic health impact in the absence of precautionary control measures (Fig. 59.1 ).12 Recognition of the SARS epidemic quickly prompted a global response orchestrated by the World Health Organization (WHO) that effectively facilitated the identification of the etiologic agent, the development of diagnostic tests, and the development and evaluation of treatment protocols aiming to reduce morbidity and mortality. With the successful estimation of key epidemiological parameters affecting epidemic dynamics of transmission, appropriate public health interventions were promptly formulated and implemented on a global basis that ultimately brought the epidemic under control; by July 5, 2003, WHO declared the recovery of the last patient and the successful interruption of the chain of SARS transmission in humans.
Figure 59.1.

Cumulative number of severe acute respiratory syndrome (SARS) patients by country in 2003. The star designates a Hong Kong hotel in which a SARS patient from southern China infected 10 guests, whose subsequent international movements spread the virus outside China, causing multicountry outbreaks of SARS in 2003.12
In the following winter in Guangzhou between December 16, 2003 and January 8, 2004, four more community-acquired SARS patients were identified, manifesting only a mild flu-like syndrome with no secondary transmission.13 Information indicated that three of the patients were epidemiologically linked to a restaurant where palm civets were served. The remaining palm civets in the restaurant and in a live animal market that supplied civets to the restaurant were later shown to harbor SARS-CoV that shared near identical nucleotide sequences of the S genes as that detected in specimens collected from some of the patients.7, 8, 9 Following the reemergence of human SARS cases in the winter of 2003–2004, Chinese authorities once again banned trading of live civets in markets, as well as culling all the infected civets in farms, as an attempt to prevent further interspecies transmission to humans. These cases resulted in no grave epidemic impact and provided an opportunity for detailed investigation into an interspecies jump of animal viruses into the human population. It served as a reminder that naturally existing SARS-CoV-like virus may reemerge to cause outbreaks in human populations when given the opportune setting of transmission via interspecies jumping, mutation, and adaptation of the virus to the new host.
The most recent small cluster of two SARS cases that occurred in China in April 2004 originated from a research laboratory. Such an event illustrates another possible source of SARS resurgence in the future.
The Agent
SARS-CoV is a newly identified human pathogen that appears to be phylogenically distinct from the two human pathogenic coronaviruses, 229E and OC43, previously known to cause mostly mild upper respiratory tract infections and more severe influenza-like illness in children and the elderly.14, 15 SARS-CoV has since been classified as antigenic group II coronaviruses that, along with group I, are mainly harbored in bats and mammals, in contrast to group III, that are from birds (for detailed phylogeny, see recent review)16 (Fig. 59.2). Coronaviruses are enveloped, single- and positive-stranded RNA viruses which, by nature, carry a high mutation rate that in essence serves the purposes of genetic diversity, plasticity, and adaptability of the virus to a wide host range.16 The RNA genome, the largest among all RNA viruses, encodes a nonstructural replicase polyprotein and several structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins.17 The S1 subunit of SARS-CoV S protein plays pivotal roles in viral infection and pathogenesis, i.e., it recognizes and binds to host receptors, and the binding brings about subsequent conformational changes in the S2 subunit of the S protein to facilitate fusion between the viral envelope and the host cell membrane.18 Among all structural proteins of SARS-CoV, S protein is the main antigenic component responsible for inducing host immune responses, neutralizing antibodies, and possibly providing protective immunity against viral infection. The S protein has therefore been suggested as an important target for vaccine and antiviral development.
Figure 59.2.

Life cycle of severe acute respiratory syndrome (SARS)-coronavirus (CoV). Cross-species transmission of the 2003 SARS-CoV from the natural reservoir of horseshoe bats to a large-scale human outbreak occurred via several mammalian species sold as exotic food in live animal markets; in the animal market, the virus-infected animal handlers were asymptomatic or exhibited a mild clinical picture. The virus acquired the capacity for efficient human-to-human transmission, and also possibly severe pathogenicity, only after adaptive mutations of the viral genome took place.4, 5, 6, 10, 11, 19, 20, 21, 22
Animal Reservoir
Initiatives to track SARS-CoV back to the animal origin were inspired by the epidemiological information indicating that several food handlers, especially those who handle, kill, or butcher “exotic” animals for food, were among the first index cases of SARS in late 2002 in Guangdong.10, 19 Further serological studies of animal traders from several live animal markets during the time of the SARS outbreak in Guangzhou (Guangdong, China) in 2003 also showed a higher prevalence of immunoglobulin G antibodies against SARS-CoV than vegetable traders,10, 11 and viruses related to human SARS-CoV were subsequently isolated from a number of animals sold in the markets, including Himalaya palm civets.4 However, the palm civets were deemed not to be the natural animal reservoir of SARS-CoV based on the observations that all SARS-CoVs identified from palm civets at that time shared >99.6% nucleic acid sequence identity with one another, implying a recent entry of the virus into the palm civet population in the live animal market. Furthermore, a general absence of antibodies against SARS-CoV among palm civets raised in the farms supplying animals to the markets further corroborates the theory of a wet market-perpetuated transmission of SARS-CoV.4, 20, 21 Subsequently, several SARS-CoV-like viruses, sharing 88–92% nucleotide homology with that of the human SARS-CoV, were detected in species of Chinese horseshoe bats existing in the wild in Hong Kong and southern China.5, 6 These findings culminated in adding SARS-CoV to the long list of viruses that are naturally harbored in a variety of bat species, i.e., rabies, Hendra, Nipha, Ebola, and St Louis encephalitis viruses. Apart from Himalaya palm civets and bats, many other mammalian animal species have also tested positive for SARS-CoV, suggesting a promiscuous nature of SARS-CoV in hosts and the ability for host adaptation (Fig. 59.2) (see reference 22 for detailed review).
Epidemiology
Genetic Evolution
The continuing genetic evolution of SARS-CoV serves as a means for temporal and geographic tracking of viral transmission. Molecular analyses of all available (>100 GenBank deposits) SARS-CoV sequences indicate that most of the genetic diversity of SARS-CoV occurred among the earliest human isolates from Guangdong province, China, and that these human isolates were phylogenically clustered along with the isolates from civets in the live animal markets.4, 19, 23 The rest of the “panglobal isolates” were clustered with viruses epidemiologically linked to the so-called “superspreading event” associated with the Hong Kong hotel transmission by the SARS patient from Guangzhou. The human “panglobal” isolates as a whole all contain a 29-nucleotide deletion (residues 27869 to 27897) that is 246 nucleotides upstream of the start codon of the N gene; this deletion set apart the human isolates from the civet isolates.4, 23 Whether the generation of these “panglobal isolates,” which has clearly demonstrated viral fitness in efficient transmission among human population, is due to the occurrence of critical adaptive mutation(s) of the viral genome to become a human pathogen or as a result of the “superspreading event” occurring by random chance remains to be studied.
Epidemiological Parameters
Retrospective investigation identified possibly 11 index SARS cases in Guangdong Province, China, with the earliest case occurring in mid-November 2002. SARS remained a local disease until February 21, 2003, when a physician infected with SARS-CoV traveled to Hong Kong while still in the early course of illness. Through contact in a Hong Kong hotel, 10 guests were infected. The subsequent movements of these international travelers effectively spread the virus outside China where the patients sought medical care and large-scale nosocomial outbreaks ensued (Fig. 59.1).
The incubation period estimated in cases with well-characterized events of exposure and the onset of illness is between 2 and 10 days, with a mean around 4–5 days.24, 25, 26 The rate of spread of an epidemic largely depends on the basic reproduction number (R 0), defined as the average number of secondary cases infected by one primary case in a susceptible population. Mathematical modeling of the early phase, based on the epidemiological data collected before the implementation of any control measures when transmission was occurring mainly in the hospital setting, estimated the R 0 to be 2.2–3.7.27, 28 The spread of SARS illustrates two important stages of disease transmission in the modern era: the first stage of the long-distance spread via international travelers and the second stage of establishing the chain of local transmission at each local epicenter. The R 0 must be >1 in order for the disease to establish sustainable local transmission. However, a highly heterogeneous potential of transmission from individual index cases is usually observed, i.e., a “superspreading event,” which entails an index case capable of infecting multiple household members and health care workers in both social/family and hospital settings, and these “superspreading events” most often serve to ignite subsequent explosive SARS outbreaks at the local site. The attack rate for SARS-CoV ranges from 10.3% to 60% or 2.4 to 31.3 cases/1000 exposure-hours, depending on the setting and the unit of measurement.25 The overall case-fatality rate was ~15%, but can be as high as 60% in elderly patients with a high virus load.29
Route of Transmission
The virus can be readily detected in the blood and excreta of infective individuals. Transmission occurs mainly via respiratory droplets generated by coughing or sneezing or through contaminated environmental surfaces30 and fomites.24, 25 Under rare and special circumstances, aerosolized human excreta, exemplified by the fecal–oral or fecal–aerosol transmission in the large outbreak in an apartment complex due to a faulty sewage system, may also contribute to transmission.31 The infectivity of SARS patients begins a few days after the onset of disease symptoms, most notably fever (Fig. 59.3 ); the infectivity increases, correlating with increasing virus shedding, during the first 10 days of disease.32, 33 The key epidemiological parameter that renders the control of SARS epidemic conducive to isolation and quarantine strategies is that the infectivity of SARS is preceded by the onset of recognizable clinical symptoms in infected patients (Fig. 59.3).
Figure 59.3.
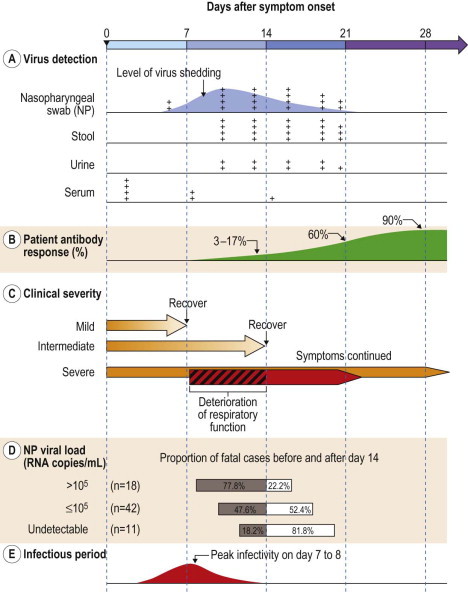
Summary of events corresponding to the clinical course of SARS infection (days after symptom onset). (A) Relative rate of virus detection and (B) antibody response, (C) time of clinical deterioration and recovery in patients of various severity, (D) time of death in relation to virus load, and (E) the approximate infection period.29, 31, 36, 39, 65, 66
The Disease
On Presentation
While SARS is mainly a respiratory disease, the clinical features (Table 59.1 ) on initial presentation are generally indistinguishable from that of influenza infection or atypical pneumonia of other causes (i.e., Mycoplasma, Chlamydia, or Legionella). The most common features at onset of illness include fever, chills, myalgia, malaise, nonproductive cough, headache, and dyspnea.34, 35 Less common symptoms include sputum production, sore throat, rhinorrhea, nausea/vomiting, and diarrhea. During the epidemic period, elderly persons with chronic illness may seek medical care of the underlying disease and have atypical clinical presentation of SARS, i.e., seeming without fever or respiratory symptoms.36 Watery nonbloody diarrhea, along with recurrent fever, was the predominant extrapulmonary symptom during the first week of illness among patients infected in an apartment complex where fecal–aerosol or fecal–oral transmission was thought to have occurred.32
Table 59.1.
| Symptom | Patients with Symptom (%) |
|---|---|
| Fever >38°C | 100 |
| Chills/rigors | 55–73 |
| Nonproductive cough | 29–57 |
| Myalgia | 38–61 |
| Headache | 11–56 |
| Dizziness | 43 |
| Malaise | 35 |
| Coryza | 32 |
| Sputum production | 15–29 |
| Sore throat | 7–23 |
| Diarrheaa | 1–20 |
| Nausea and vomiting | 20 |
| Dyspneaa | 0–10 |
By day 8, diarrhea occurred in 73% of patients, and respiratory symptoms worsened in 45% of patients.27
Progression and Clinical Spectrum
A majority of patients have a mild to intermediate clinical course that resolves with bed rest and nasal oxygen supplement (Fig. 59.3). About 20–36% require intensive care, and 13–26% progress into acute respiratory distress syndrome (ARDS), necessitating invasive ventilatory support.24, 32, 37, 38, 39 The clinical course follows a typical biphasic pattern that presumably is linked to events of an initial phase of viral replication and a second phase of immunopathological response (Fig. 59.3).29, 32 During the initial clinical phase of up to 10 days, the increasing viral load is associated with clinical features of mainly systemic symptoms, including, most notably, fever and myalgia, which generally improve within days among patients having a short mild to intermediate clinical course. In some patients, despite the falling viral load during the second through the third week, immunopathological damage may ensue with persistent or recurrent fever, oxygen desaturation, and radiologic progression of pneumonia.12, 24, 32, 37, 38 For those in whom ARDS (up to 20%) later ensues, pulmonary function begins to show progressive worsening in the second week of illness.
Retrospective analyses of serial chest radiographs in all 1373 SARS patients in Hong Kong40 and of high-resolution computed tomographic scan of 14 patients41 corroborate the progression of radiographic opacities or bilateral fibrotic lung changes during week 2 to be useful prognostic predictors of the ultimate clinical severity. Furthermore, disease severity was intensified by slower and prolonged recovery with complications of pulmonary fibrosis occurring in the third week in some patients.42
Chest Radiological Findings
Up to 25% of SARS patients have normal chest radiographs on presentation.24, 35, 43, 44 While radiographic appearances of SARS, sharing common features with atypical pneumonia of other causes, in general, are characterized by ground-glass opacities and focal consolidations, the abnormalities tend to be localized in the periphery and subpleural regions of the lower zones and are mostly devoid of cavitation, hilar lymphadenopathy, or pleural effusion.24, 42, 44 Progression from unilateral to bilateral involvement is not uncommon in SARS patients42, 44 and sometimes with shifting of radiographic shadows, i.e., resolving opacity in one area while a new opacity occurs in another area of lung, and spontaneous pneumomediastinum (12% in one series).32, 45 High-resolution computed tomography of the thorax is useful in detecting lung opacities early in cases with unremarkable chest radiographs.46
Extrapulmonary Involvement
SARS is a systemic disease, especially in severe cases, in which a wide spectrum of tissue and cell types is directly infected by virus or is affected indirectly. Diarrhea is the most common extrapulmonary manifestation during the early phase, presumably due to viral replication in the gastrointestinal tract.32 Elevated liver transaminases suggest that hepatic dysfunction is common, though virus is not found in liver; dizziness may be related to central nervous virus invasion; the left ventricular ejection fraction may be decreased though the pathogenic mechanism is unclear47; petechiae, myositis, neuromuscular abnormalities, and seizures have also occurred.47, 48 Circulating lymphocytes are widely infected,49 and lymphopenia is very common.50 Orchitis was observed in a series of autopsies of fatal SARS patients, suggesting that reproductive function in male patients who recover from SARS should be monitored.49
Laboratory Findings
Peripheral absolute lymphopenia (reduction in both CD4 and CD8 lymphocytes) is a prominent feature, occurring in 98% of patients. Features of low-grade disseminated intravascular coagulation (thrombocytopenia, prolonged activated partial thromboplastin time, raised D-dimers), and elevated lactate dehydrogenase, alanine transaminases, and creatine kinase are frequent laboratory features of SARS.24, 35, 37, 43
Pathogenesis and Immunity
Tissue tropism of SARS-CoV is stipulated by a specific receptor-facilitated process; angiotensin converting enzyme 2 (ACE2) has been identified as the cellular receptor that binds directly to the viral S protein.51 ACE2 is expressed in alveolar epithelial cells and in surface enterocytes of the small intestine, both of which are the primary target cells of SARS-CoV infection.32 ACE2, which acts as a negative regulator of the local renin–angiotensin system and is down-regulated by viral infection, can protect the lung against external damage in experimental animal models. In addition, the S protein of SARS-CoV can also bind to C-type lectins, i.e., CD209 (also known as dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin, or DC-SIGN) and CD209L, and gain access to cell entry.52 Although SARS-CoV particles and genomic sequence are detected in a large number of circulating lymphocytes, monocytes, and lymphoid tissues during the early phase of infection,49 no virus has been found in dendritic cells. Viremia, with or without cell association, occurs early in the clinical course, thus contributing to the spread of virus to organs other than the site of entry.
The intestinal tract is an important extrapulmonary site of viral replication; specimens taken by colonoscopy or at necropsy reveal evidence of active viral replication within both the small and large intestinal mucosa but with minimal pathological changes,32 and SARS-CoV RNA may be detected by reverse transcriptase polymerase chain reaction (RT-PCR) from gastrointestinal specimens for up to 10 weeks after onset.53 In an autopsy series of 18 patients who died between days 14 and 62, epithelial cells of the digestive tracts of all patients were virally infected but displayed only mild inflammatory changes.49 The most obvious lesion in the digestive tract is depletion of the submucosal lymphoid tissues. The minimal pathology in the gastrointestinal tract contrasts sharply with the diffuse alveolar damage in the lung, while both organs serve as primary sites of viral replication. Thus, the pathogenesis must involve tissue-specific host responses, which are most likely intensified during week 2 of illness when pulmonary function worsens with concomitant decreasing viral load in the airways (Fig. 59.3).32, 54 Clinical studies of cytokines during the acute phase55, 56 suggest that activation of Th1 cell-mediated immunity and an excessive innate inflammatory response, rather than direct damage from uncontrolled virus growth, are responsible for the pathogenic process in severe cases who survive through week 2.32
A protracted clinical course was intensified by slower and prolonged convalescence due to complications of pulmonary fibrosis occurring in week 3 in some patients.42 Results of high-resolution computed tomographic scans in follow-up of SARS patients corroborate this observation, with a strong correlation between bilateral fibrotic lung changes and clinical severity.41
Based on analysis of 18 autopsies on patients who died between days 14 and 62, SARS-CoV infects multiple cell types in several organs other than the epithelial cells of the respiratory tract and the mucosa of the intestine, i.e., the epithelium of the renal distal tubules, showing focal hemorrhage, neurons of the hypothalamus and cortex in all patients, and infiltrating macrophages in other organs.49 Clinical manifestations correlate directly with the sites where viral infection occurs, such as higher viral shedding in the stool associated with diarrhea, and a higher urine viral load associated with abnormal urinalysis, probably due to renal involvement.48, 57, 58 Status epilepticus in two SARS patients whose cerebrospinal fluid and serum samples contained SARS-CoV was most likely due to central nervous system infection.59, 60 Involvement of other organs includes hepatocellular injury in the centrilobular zone in five out of eight patients with no detectable virus, and the testes of seven male patients displayed focal atrophy with no virus detected. Organs with no pathologic change include heart, pancreas, adrenal gland, thyroid gland, and skeletal muscle, though SARS-CoV-laden lymphocytes and monocytes were present in some of these organs. Immune cells and pulmonary epithelium are the main sites of injury.
Viral Load and Mortality
Viral shedding in the nasopharynx, measured by quantitative RT-PCR, peaks on day 10 (Fig. 59.3).32 However, analysis of 265 laboratory-confirmed SARS patients in Taiwan demonstrates that, on any given day of the clinical course, SARS-CoV shedding in the nasopharynx varies widely from individual to individual, ranging from below the detection limit to as high as 108 RNA copies/mL; male patients and elderly patients are more likely to have detectable virus shedding,29 suggesting that individual host differences in viral shedding surpass the variation during the clinical course for each individual.
Higher nasopharyngeal and serum viral loads are associated with oxygen desaturation, mechanical ventilation, and mortality;29, 48, 57 furthermore, a higher nasopharyngeal virus titer is associated with early death occurring within the first 2 weeks of illness (Fig. 59.3).29
Genetic Predisposition
Individual differences are genetically attributed to single base differences (single nucleotide polymorphisms, SNPs) of genes. Several studies have been carried out to search for genetic predisposition to severe clinical outcomes of SARS in the hope of understanding the pathogenesis through the function of the polymorphism gene with predisposing risk. Human leukocyte antigen (HLA)-B*4601 was associated with both predisposition to infection and severity of illness among both Taiwanese and Hong Kong patients.61, 62 HLA-B*0703, which is an allele of very low frequency in ~3% of the general population, is a predisposition allele.62 One SNP of FGL2, of nonsynonymous (G53E) nature, was associated with SARS-CoV shedding in the nasopharynx, as well as with a prolonged clinical course of Taiwanese SARS patients;29, 63 FGL2, an interferon-γ-inducible protein expressed by lymphocytes, macrophages, and endothelium, is a prothrombinase that is reported to contribute to fibrin deposition during viral hepatitis, and its expression is associated with a number of pathological conditions. The SNP in the promoter region of CXCL10/IP-10 and heme oxygenase 1, both of which play roles in modulating inflammation, is associated with the SARS clinical outcome.63 Other SNPs of interleukin (IL)-1α, IL-18 and RelB, all gene products related to innate immunity and interferon pathway, are also associated with nasopharyngeal virus shedding.29 More studies are needed to determine the biological significance of these genetic determinants in relation to susceptibility to infection and to the host–pathogen interaction contributing to the clinical outcome of the infection.
Diagnosis
Virus Detection
Nasopharyngeal and oral pharyngeal mucosa is the site of viral entry, as well as the major site of viral replication for SARS-CoV; after infection, virus may be detected in nasopharyngeal aspirate, stool, serum/plasma, and urine with variable sensitivity during the clinical course (Fig. 59.3). Virus isolation is not routinely carried out for SARS-CoV in the clinical setting as it requires a high containment laboratory for biosafety and biosecurity considerations. Instead, detecting viral nucleic acids based on primers of various segments of the viral genome by conventional RT-PCR or by quantitative RT-PCR is routinely used as the standard diagnostic method. With optimized RNA extraction methods and applying quantitative real-time RT-PCR techniques, the sensitivity for diagnosis of SARS in the first 3 days of disease is as high as 80% when nasopharyngeal aspirate specimens were tested,64 which is a tremendous improvement when compared with the generally low detection rate by conventional RT-PCR during the first week of illness. In a longitudinal follow-up study of 20 SARS patients with detectable nasopharyngeal SARS-CoV virus by RT-PCR, the virus load increased from day 5 to day 10 and decreased by day 15 of illness.32 On day 14 of illness, the sensitivity of detection in urine, nasophargyngeal aspirate, and stool specimens is 42%, 68%, and 97%, respectively.32
Viremia occurs early in the clinical course. Testing of serum by quantitative RT-PCR showed a detection rate of 80% on the first day of hospital admission, dropping to 75% and 42% on days 7 and 14, respectively.65, 66
Serological Diagnosis
Antibody against SARS-CoV may be detected, by either neutralization testing or enzyme-linked immunosorbent assay, by the second week of illness in some 17.4%,39 but usually requires 4 weeks to reach a detection rate above 90%; the antibody level peaks between weeks 5 and 8, then declines thereafter.32, 39 A short survival, i.e., mortality within 2 weeks of onset, was noted among those who developed neutralizing antibody early in the clinical course.39 Thus, the level of neutralizing antibody response correlates with the severity of illness.
Treatment and Prognosis
Supportive Care
Since oxygen desaturation and respiratory failure, associated with ARDS and alveolar destruction, represent the majority of decompensating and terminal events in SARS patients, supportive management of pulmonary function to maintain oxygen saturation is imperative. Nasal oxygen supplementation with strict bed rest to reduce oxygen demand is a general practice, and noninvasive positive-pressure ventilation has been used with success in severely ill cases of SARS patients.67 However, due to the potential risk of viral transmission via positive-pressure mask leakage and flow compensation, causing dispersion of contaminated aerosol, health care workers should adhere to appropriate precautionary practices in wearing personal protective equipment, including mask, gown, and gloves, before noninvasive positive-pressure ventilation is initiated, preferably in a negative-pressure hospital isolation ward or in an isolation room with adequate air exchange.
Antiviral Agents
Treatment of SARS during the outbreak in 2003 was mostly empirical and at times anecdotal owing to the novel nature of the newly emerged SARS and a lack of understanding of the pathogenesis and clinical course. A number of anti-human immunodeficiency virus (HIV) agents were tried. The combination formulation of lopinavir/ritonavir when used in the initial phase was shown in a retrospective multicenter analysis to be associated with a reduction in the overall death rate to 2.3% and intubation rate to 0%, as well as a lower rate of treatment with methylprednisolone when compared with historic controls or with a matched cohort without antiviral treatment.68, 69 The use of ribavirin, on the other hand, appears to be associated with hemolysis and a decrease in hemoglobin, as well as a rise in transaminases.43
Immunomodulators
Intravenous high dose (0.5 g daily) of methylprednisolone was given with the intention of preventing immunopathological lung injury based on the rationale that progression of the pulmonary disease may be mediated by the host inflammatory response during week 2, when progressive worsening of radiological findings and hypoxemia occurs.24, 32, 68 The use of high-dose pulse methylprednisolone during clinical progression was reported to be associated with more favorable clinical improvement.68 Intravenous immunoglobulin 1 g/kg/day for 2 days was routinely used as standard therapy in many patients in Taiwan based on the rationale of possibly suppressing an overly elevated cytokine response, though no control group was available for comparison of its effects.70
In an uncontrolled trial in Toronto, interferon alfacon-1 plus corticosteroids was reported to reduce disease-associated impaired oxygen saturation, to achieve a more rapid resolution of radiographic lung opacities, and to lower levels of creatine kinase.71 It should be noted, however, that in the future event of SARS resurgence, any treatment regimen should undergo clinical trials of randomized placebo-controlled design. New antiviral agents and immunomodulating agents such as hyperimmune globulin and monoclonal antibody have been developed and are awaiting the opportunity for clinical evaluation.
Clinical Prognostic Indicators
Advanced age and presence of comorbidities, notably diabetes mellitus and chronic hepatitis B carriage, are poor prognostic indicators associated with higher SARS mortality;24, 32, 43 pregnancy also carries significant risk for mortality.72 Infections in children appear to be milder than those in adults.73
Low counts of CD4 and CD8 T lymphocytes at presentation are associated with adverse clinical outcome.50 Among the abnormal laboratory findings, elevated lactate dehydrogenase level, hypouricemia, acute renal failure, more extensive pulmonary radiological involvement, and a high neutrophil count on presentation are all poor prognostic indicators.16, 24, 38
Patients having higher nasopharyngeal and serum viral loads are more likely to experience oxygen desaturation, require mechanical ventilation, and die.29, 48, 57 Furthermore, higher nasopharyngeal viral shedding was associated with early death occurring within the first 2 weeks of illness (Fig. 59.3).29 The correlation between high viral loads in specific specimens and the clinical manifestations suggests that viral replication during the initial phase contributes to the clinical manifestations.58
Long-term Prognosis
Abnormalities in the nature of restrictive pulmonary function due to residual lung fibrosis are common.74 Pulmonary function testing of SARS survivors 1 year after recovery showed a reduction in diffusion capacity and a lower exercise endurance capacity than the healthy population.75
Prevention and Control
The global responses have successfully broken the chain of human transmission of the 2003 SARS epidemic that occurred in 29 countries in five continents.12 Now that several winter seasons have elapsed since the 2003 SARS outbreak without the occurrence of any further SARS case, we can exert a much higher level of confidence that the human population is truly free of SARS-CoV transmission even in a covert manner, though potential resurgence remains in the future either via reintroduction of the virus from a natural animal reservoir (Fig. 59.2) or spillage from a research laboratory. SARS is zoonotic in origin, and establishing the capability for early detection in initial human cases is the key to preventing large-scale human transmission. In light of the disease carrying a considerable need for surge capacity of health care services, continued vigilance in determining diagnoses of all cases of atypical or severe respiratory infection in a timely manner, as well as stringent practices to minimize nosocomial infection in general, is imperative.
References
- 1.Ho MS. I think I got “That Disease”: an interview with a medical student who had recovered from SARS in Guangzhou, China. Public Health. 2006;120:6. doi: 10.1016/j.puhe.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.Guan Y, Zheng BJ, He YQ. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 5.Lau SK, Woo PC, Li KS. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Shi Z, Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 7.Kan B, Wang M, Jing H. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J Virol. 2005;79:11892. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song HD, Tu CC, Zhang GW. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc Natl Acad Sci USA. 2005;102:2430. doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Yan M, Xu H. SARS-CoV infection in a restaurant from palm civet. Emerg Infect Dis. 2005;11:1860. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu HF, Wang M, Zhang ZB. [An epidemiologic investigation on infection with severe acute respiratory syndrome coronavirus in wild animals traders in Guangzhou.] Zhonghua Yu Fang Yi Xue Za Zhi. 2004;38:81. [PubMed] [Google Scholar]
- 11.Xu RH, He JF, Evans MR. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS). Geneva: WHO; 2003. http://www.who.int/csr/sars/country/table2003_09_23/en/ Available online at. accessed November 30, 2009.
- 13.Liang G, Chen Q, Xu J. Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg Infect Dis. 2004;10:1774. doi: 10.3201/eid1010.040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vabret A, Mourez T, Gouarin S. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis. 2003;36:985. doi: 10.1086/374222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Sahly HM, Atmar RL, Glezen WP. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo PC, Lau SK, Huang Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med (Maywood) 2009;234:1117. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 17.Marra MA, Jones SJ, Astell CR. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 18.Wong SK, Li W, Moore MJ. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong NS, Zheng BJ, Li YM. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tu C, Crameri G, Kong X. Antibodies to SARS coronavirus in civets. Emerg Infect Dis. 2004;10:2244. doi: 10.3201/eid1012.040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon LL, Chu DK, Chan KH. Identification of a novel coronavirus in bats. J Virol. 2005;79:2001. doi: 10.1128/JVI.79.4.2001-2009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z, Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133:74. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinese SMEC Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 24.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 25.Varia M, Wilson S, Sarwal S. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169:285. [PMC free article] [PubMed] [Google Scholar]
- 26.Leung GM, Hedley AJ, Ho LM. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141:662. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 27.Riley S, Fraser C, Donnelly CA. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 28.Lipsitch M, Cohen T, Cooper B. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen WJ, Yang JY, Lin JH. Nasopharyngeal shedding of severe acute respiratory syndrome-associated coronavirus is associated with genetic polymorphisms. Clin Infect Dis. 2006;42:1561. doi: 10.1086/503843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowell SF, Simmerman JM, Erdman DD. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin Infect Dis. 2004;39:652. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicastri E, Petrosillo N, Puro V. Evidence of airborne transmission of SARS. N Engl J Med. 2004;351:609. author reply 609. [PubMed] [Google Scholar]
- 32.Peiris JS, Chu CM, Cheng VC. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MS Ho, IJ Su. Preparing to prevent severe acute respiratory syndrome and other respiratory infections. Lancet Infect Dis. 2004;4:684. doi: 10.1016/S1473-3099(04)01174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poutanen SM, Low DE, Henry B. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 35.Tsang KW, Ho PL, Ooi GC. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 36.Chow KY, Lee CE, Ling ML. Outbreak of severe acute respiratory syndrome in a tertiary hospital in Singapore, linked to an index patient with atypical presentation: epidemiological study. BMJ. 2004;328:195. doi: 10.1136/bmj.37939.465729.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan JW, Ng CK, Chan YH. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsui PT, Kwok ML, Yuen H. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9:1064. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho MS, Chen WJ, Chen HY. Neutralizing antibody response and SARS severity. Emerg Infect Dis. 2005;11:1730. doi: 10.3201/eid1111.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonio GE, Ooi CG, Wong KT. Radiographic–clinical correlation in severe acute respiratory syndrome: study of 1373 patients in Hong Kong. Radiology. 2005;237:1081. doi: 10.1148/radiol.2373041919. [DOI] [PubMed] [Google Scholar]
- 41.Chiang CH, Shih JF, Su WJ. Eight-month prospective study of 14 patients with hospital-acquired severe acute respiratory syndrome. Mayo Clin Proc. 2004;79:1372. doi: 10.4065/79.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai EK, Deif H, LaMere EA. Severe acute respiratory syndrome: quantitative assessment from chest radiographs with clinical and prognostic correlation. AJR Am J Roentgenol. 2005;184:255. doi: 10.2214/ajr.184.1.01840255. [DOI] [PubMed] [Google Scholar]
- 43.Booth CM, Matukas LM, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 44.Wong KT, Antonio GE, Hui DS. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228:401. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- 45.Chu CM, Leung YY, Hui JY. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23:802. doi: 10.1183/09031936.04.00096404. [DOI] [PubMed] [Google Scholar]
- 46.Wong KT, Antonio GE, Hui DS. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228:395. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 47.Li SS, Cheng CW, Fu CL. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. 2003;108:1798. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 48.Cheng VC, Hung IF, Tang BS. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin Infect Dis. 2004;38:467. doi: 10.1086/382681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu J, Gong E, Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong RS, Wu A, To KF. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Moore MJ, Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang ZY, Huang Y, Ganesh L. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol. 2004;78:5642. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leung WK, To KF, Chan PK. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheung TM, Yam LY, So LK. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845. doi: 10.1378/chest.126.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong CK, Lam CW, Wu AK. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang KJ, Su IJ, Theron M. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75:185. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu CM, Poon LL, Cheng VC. Initial viral load and the outcomes of SARS. CMAJ. 2004;171:1349. doi: 10.1503/cmaj.1040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hung IF, Cheng VC, Wu AK. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10:1550. doi: 10.3201/eid1009.040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung EC, Chim SS, Chan PK. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49:2108. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lau KK, Yu WC, Chu CM. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin M, Tseng HK, Trejaut JA. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:9. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng MH, Lau KM, Li L. Association of human-leukocyte-antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J Infect Dis. 2004;190:515. doi: 10.1086/421523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsieh YH, Chen CW, Schmitz SF. Candidate genes associated with susceptibility for SARS-coronavirus. Bull Math Biol. 2009 doi: 10.1007/s11538-009-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poon LL, Chan KH, Wong OK. Early diagnosis of SARS coronavirus infection by real time RT-PCR. J Clin Virol. 2003;28:233. doi: 10.1016/j.jcv.2003.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grant PR, Garson JA, Tedder RS. Detection of SARS coronavirus in plasma by real-time RT-PCR. N Engl J Med. 2003;349:2468. doi: 10.1056/NEJM200312183492522. [DOI] [PubMed] [Google Scholar]
- 66.Ng EK, Hui DS, Chan KC. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49:1976. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.So LK, Lau AC, Yam LY. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361:1615. doi: 10.1016/S0140-6736(03)13265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu CM, Cheng VC, Hung IF. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan KS, Lai ST, Chu CM. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multi-center retrospective matched cohort study. H K Med J. 2003;9:399. [PubMed] [Google Scholar]
- 70.Chiang CH, Chen HM, Shih JF. Management of hospital-acquired severe acute respiratory syndrome with different disease spectrum. J Chin Med Assoc. 2003;66:328. [PubMed] [Google Scholar]
- 71.Loutfy MR, Blatt LM, Siminovitch KA. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 72.Wong SF, Chow KM, Leung TN. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hon KL, Leung CW, Cheng WT. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ng CK, Chan JW, Kwan TL. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59:889. doi: 10.1136/thx.2004.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hui DS, Wong KT, Ko FW. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]


