Abstract
Infectious agents have intimately co-evolved with the host immune system, acquiring a portfolio of highly sophisticated mechanisms to modulate immunity. Among the common strategies developed by viruses, bacteria, protozoa, helminths, and fungi is the manipulation of the regulatory T cell network in order to favor pathogen survival and transmission. Treg activity also benefits the host in many circumstances by controlling immunopathogenic reactions to infection. Interestingly, some pathogens are able to directly induce the conversion of naive T cells into suppressive Foxp3-expressing Tregs, while others activate pre-existing natural Tregs, in both cases repressing pathogen-specific effector responses. However, Tregs can also act to promote immunity in certain settings, such as in initial stages of infection when effector cells must access the site of infection, and subsequently in ensuring generation of effector memory. Notably, there is little current information on whether infections selectively drive pathogen-specific Tregs, and if so whether these cells are also reactive to self-antigens. Further analysis of specificity, together with a clearer picture of the relative dynamics of Treg subsets over the course of disease, should lead to rational strategies for immune intervention to optimize immunity and eliminate infection.
Keywords: Bacteria, dendritic cell, fungi, helminth, hygiene hypothesis, infection, parasite, pathogen, protozoa, specificity
1. Introduction
Regulatory T cells are now recognized as an absolute requirement for healthy function of the mammalian immune system to forestall autoimmune pathology by self-reactive lymphocytes and to prevent deleterious reactions against extrinsic commensal and dietary antigens (Kim et al., 2007, Sakaguchi et al., 2008). Tregs also control immune responsiveness to infective pathogens (Belkaid and Tarbell, 2009), and in this context, their influence is not always benign. In this review, we survey our current knowledge of the role of Tregs in a wide range of infection settings and highlight the examples in which these cells are of critical importance in conferring susceptibility, dampening pathogenesis, and maintaining functional immunity.
The significance of Treg involvement in infectious episodes is not limited simply to how the host handles a particular pathogen; there is abundant evidence from infections of all types that adaptation or modulation of immune capacity resulting from infection can profoundly impact on bystander immune responses, in particular allergies, autoimmune diseases, and gastrointestinal disorders (Bach, 2002, Maizels, 2005). While this interaction is popularly termed the “Hygiene Hypothesis” (see Section 6 below), it is clearly highly context-dependent, and we also review the emerging evidence that some infectious agents in particular are able to alter the immune status of their host through the regulatory T cell compartment.
2. Regulatory T Cells
Regulatory T cells encompass several distinct phenotypes of immune system cells able to block or suppress immune reactivity in vivo and in vitro. While the most well-characterized Treg subset is a dedicated lineage selected in the thymus, there is peripheral conversion of potential effector cells into the Treg compartment, and evidence that additional T cell types engage transiently in regulatory activity or combine production of regulatory cytokines with more conventional effector products. The predominant Treg types are CD4+ and express either or both the surface IL-2Rα chain CD25 and the forkhead box transcription factor Foxp3 (Gavin et al., 2007). The CD4+CD25+Foxp3+ phenotype also commonly expresses the inhibitory receptor CTLA-4 (Alegre et al., 2001) as well as the GITR receptor which can activate both regulatory and nonregulatory T cells (Shimizu et al., 2002). In addition to Foxp3-expressing Tregs are other functional regulatory cells, which may produce IL-10 (termed Tr1) and IL-35 (Tr35) (Collison et al., 2010). In the case of Tr1, as IL-10 can also be produced by both Th1 (IFNγ+ (Jankovic et al., 2007)) and Th2 (IL-4+) effectors, definition of this subset is relatively fluid. Moreover, T cells producing TGF-β, originally named Th3, can also act in a regulatory capacity. Finally, while Tregs are generally found to be CD4+, CD8+ T cells can express Foxp3 (Nakagawa et al., 2010) and produce the same suppressive cytokines.
Foxp3+ Treg cells exist in two categories, which arise in fundamentally different ways. Thymic, or “natural,” regulatory T cells recognize self-antigens in the thymus and are imprinted with regulatory function before being released into the periphery. Thymic Tregs are a CD4+CD25+Foxp3+ phenotype, and deficiency of these cells results in fatal autoimmune inflammation; this subset remains committed to this function over time (Rubtsov et al., 2010). In addition, naive peripheral CD4+ T cells can be induced to adopt a regulatory function by initiating expression of Foxp3 (or indeed, TGF-β, IL-10, or IL-35). In particular, induced or “adaptive” Tregs can convert from CD4+Foxp3− to CD4+Foxp3+, as discussed further below, thereby expanding the range of Treg specificities to exogenous antigens (Bluestone and Abbas, 2003). However, induced Tregs are not irrevocably programmed and may revert to an effector phenotype, losing expression of CD25 and Foxp3 under certain conditions.
Early experiments with Tregs used CD25 as an accessible surface marker for cell transfer and in vivo antibody-mediated depletion with the antibody PC61 (developed by Lowenthal et al. (1985)). While landmark studies were achieved with these methods, it was recognized that effector populations can also be compromised by anti-CD25 antibody, and that a substantial minority of Foxp3+ Tregs do not, at any one time, express CD25. In recent years, it has been possible to better target Foxp3+ Tregs, through the construction of transgenic mice expressing the diphtheria toxin receptor (DTR) under the control of the Treg-specific Foxp3 promoter. Constructs described to date include the DEREG (Lahl et al., 2007) and Foxp3LuciDTR (Suffner et al., 2010) mice, both of which have transgenic BACs randomly inserted, and the Foxp3DTR mouse (Kim et al., 2007, Lund et al., 2008) containing a construct knocked-in to the Foxp3 locus on the X chromosome. Administration of diphtheria toxin (DTx) selectively depletes Tregs, although transiently and sparing cells which downregulate Foxp3 or (in the case of the BAC transgenics) suppress the randomly integrated locus.
3. Infections
Infectious agents have developed, over long evolutionary time, effective and often exquisite means of surviving in the host sufficiently long to assure transmission, and very often to establish long-term residence. Among the many strategies infectious organisms employ, exploiting the regulatory T cell compartment is doubtless one of the most effective. However, in the twists and turns of evolving host-pathogen co-adaption, some surprising interactions have developed: thus Tregs do not simply suppress immunity and forestall pathology, but also facilitate appropriate effector mechanisms and maintain long-term memory.
Some thought needs to be given to how infectious diseases of humans are appropriately modeled in laboratory rodents. Acute microbial infections in mice are not reflective of long-term human chronic infections, in which immunoregulation and re-setting of immune homeostasis are the norm. However, mouse models are invaluable for understanding early events, in particular the induction and activation of regulatory networks, and elegant transgenic constructs are available which permit tracking, manipulation, and gene-deletion within individual cell phenotypes.
While the application of these more sophisticated tools to infectious disease systems is still in its infancy, a substantial body of information has accumulated on the expression of Treg subsets, their functions in vitro, and the role of either CD25+ or Foxp3+ Tregs in the course of infection in vivo. We summarize in the following sections the data from the major infection systems, in humans and experimental models, while presenting more a complete annotation of infections under study in Table 3.1, Table 3.2, Table 3.3, Table 3.4, Table 3.5 for each of the taxonomic groupings.
Table 3.1.
Tregs in viral infections
| Retroviruses | ||
| FIV | Tregs promote progression and restrain anti-viral responses | Vahlenkamp et al., 2004, Mikkelsen et al., 2010 |
| Friend retrovirus | Expansion of Tregs in vivo and loss of tumor immunity | Iwashiro et al., 2001, Zelinskyy et al., 2006 |
| Tregs suppress CD8 antiviral immunity | Dittmer et al., 2004, Robertson et al., 2006, Zelinskyy et al., 2009a, Zelinskyy et al., 2009b | |
| Nonantigen-specific Tregs control pathology in RAG model | Antunes et al. (2008) | |
| HIV | Treg numbers correlate with viral load but decline in persistent viremia | Andersson et al., 2005, Baker et al., 2007, Nilsson et al., 2006, Tsunemi et al., 2005 |
| CD25+ Tregs maintain suppressive capacity in infection | Kinter et al., 2007a, Kinter et al., 2007b | |
| Tregs reduce activation of and inhibit infection of effector T cells | Chase et al., 2008, Moreno-Fernandez et al., 2011 | |
| gp120 binding to CD4 may activate Tregs T cells recognising protective HLA allele specificities not suppressed by Tregs | Becker et al., 2009, Elahi et al., 2011 | |
| LCMV | Blocks diabetes through Tregs | Diana et al. (2011) |
| Superantigen-mediated expansion of Tregs | Punkosdy et al. (2011) | |
| MAIDS | Tregs promote infection, ablation blocks | Beilharz et al. (2004) |
| MMTV | Tregs reduce viral load at outset, increase later. Superantigen-specific Tregs | Cabrera et al. (2008) |
| SIV | Foxp3 in both CD25− and CD25+ T cells correlates with high viremia | Boasso et al. (2007) |
| RNA viruses | ||
| Hepatitis C | Elevated functional Foxp3+ Tregs | Ebinuma et al. (2008) |
| Disease resolution associated with reduced Treg activity | Smyk-Pearson et al. (2008) | |
| Influenza A | CD25+ depletion raises CD8+ response | Haeryfar et al. (2005) |
| MHV coronavirus | Treg depletion can be fatal | Anghelina et al. (2009) |
| Tregs reduce demyelination | Trandem et al. (2010) | |
| Viral epitope-specific Tregs | Zhao et al. (2011) | |
| Rhinovirus in humans | Induce IL-35+Foxp3− Tregs via DCs | Seyerl et al. (2010) |
| RSV | Tregs dampen response and limit pathology but depletion does not change viral load | Fulton et al., 2010, Lee et al., 2010 |
| DNA viruses | ||
| CMV | CD25+ Tregs suppress CMV response | Aandahl et al. (2004) |
| EBV | CD8+ Tregs in active infection | Popescu et al. (2007) |
| Hepatitis B | CD4+FoxP3+ Treg numbers correlate with viral load and serum TGF-β | Barboza et al., 2007, Yang et al., 2007a |
| HSV-1 | Tregs expand in infection, restraining responsiveness and pathology | Suvas et al., 2003, Suvas et al., 2004 |
| Ocular pathology controlled by Tregs (and IL-10), including in vitro-generated viral-specific Tregs | Sarangi et al., 2008, Sehrawat et al., 2008 | |
| HSV-2 | Treg ablation results in loss of immunity through reduced effectors at site of infection | Lund et al. (2008) |
| Human papillomavirus 16 | CD25+ Tregs correlate with persistent infection | Molling et al. (2007) |
| Vaccinia | CD25+ depletion raises CD8+ response | Haeryfar et al. (2005) |
Table 3.2.
Tregs in bacterial infections
| Mycobacteria | ||
| Mycobacterium bovis BCG in mice | Elevated pulmonary Foxp3+ cells, bacterial load unchanged by anti-CD25 depletion | Quinn et al. (2006) |
| Mycobacterium tuberculosis in humans | Elevated FOXP3+ cells, inversely correlating with immunity, reduced after treatment | Chen et al., 2007b, Guyot-Revol et al., 2006, Li et al., 2007a, Qin et al., 2008 |
| Mycobacterium tuberculosis in mice | Tregs expand, increase bacterial load | Kursar et al., 2007, Ordway et al., 2007, Ordway et al., 2011 |
| Tregs delay priming and migration of effectors | Scott-Browne et al. (2007) | |
| Mtb-specific Tregs activated | Shafiani et al. (2010) | |
| Mycobacterium vaccae | Induce Tregs, block allergy | Zuany-Amorim et al. (2002) |
| Other intracellular | ||
| Brucella abortus (Gram −) | Tregs block protective immunity | Pasquali et al. (2010) |
| Chlamydia trachomatis | Tregs stimulated but no correlation with disease | Gall et al. (2011) |
| Listeria monocytogenes (Gram +) | Tregs suppress memory CD8+ T cells | Kursar et al. (2002) |
| No antigen-specific Tregs in vivo | Fontenot et al. (2005) | |
| Respiratory | ||
| Bordetella pertussis (Gram −) | Tr1 generation through filamentous hemagglutinin | McGuirk et al. (2002) |
| Gastrointestinal pathogens | ||
| Haemophilus ducreyi (Gram −) | Tregs enriched in lesions | Li et al. (2010) |
| Helicobacter pylori (Gram −) | Treg expansion in the mucosa, CD25 depletion reduces bacterial load but generates pathology | Lundgren et al., 2005, Rad et al., 2006, Raghavan et al., 2003 |
| Infection-related Tregs suppress airway allergy | Arnold et al. (2011) | |
| Salmonella enterica (Typhimurium; Gram −) | Treg depletion or anti-CTLA-4 boosts clearance and memory | Johanns et al. (2010) |
| Commensal bacteria | ||
| Bacteroides fragilis (Gram −) | Drives Treg expansion, through PSA binding to TLR2 | Round and Mazmanian, 2010, Round et al., 2011 |
| Bifidobacterium infantis (Gram +) | Induction of Tregs, bystander suppression of inflammation following mucosal S. typhimurium infection | O'Mahony et al. (2008) |
| Clostridium species (Gram +) | Mediates Treg induction through TGF-β, protects against DSS colitis | Atarashi et al. (2011) |
| Helicobacter hepaticus (Gram −) | Tr1-like IL-10-producing cells block gut inflammation | Kullberg et al. (2002) |
| Streptococcus pneumoniae (Gram +) | CD8+CD28+ suppressive Tregs producing IL-10 and TGF-β | Mertens et al. (2009) |
Table 3.3.
Tregs in protozoal infections
| Human malaria | ||
| Plasmodium falciparum | Elevated CD25+ and FOXP3+ in infection, correlate with parasite load, and in cord blood of newborns to infected mothers | Brustoski et al., 2006, Mackroth, 2011, Walther et al., 2005, Walther et al., 2009 |
| FOXP3+ numbers expand in severe malaria, decline following treatment | Minigo et al. (2009) | |
| Human patients have high FOXP3+ Tregs | Goncalves et al. (2010) but see Finney et al. (2009) | |
| Bystander FOXP3 (hi) induction in human T cells | Scholzen et al. (2009) | |
| Human placenta Treg induction | Bisseye et al. (2009) | |
| P. vivax | Elevated FOXP3+ in infection | Bueno et al., 2010, Goncalves et al., 2010, Jangpatarapongsa et al., 2008 |
| Murine malaria | ||
| P. berghei | CD25 depletion alleviates cerebral malaria (CM) | Amante et al., 2007, Vigario et al., 2007, Wu et al., 2010 |
| Foxp3+ depletion does not alter CM while expansion through IL-2/IL-2R complexes protects from CM | Haque et al., 2010, Steeg et al., 2009 | |
| P. chabaudi | Foxp3 overexpression compromises protection; Tregs are anti-inflammatory | Berretta et al., 2011, Cambos et al., 2008 |
| P. yoelii | Anti-CD25 prevents malaria immune evasion through TLR9 signaling | Hisaeda et al., 2004, Hisaeda et al., 2005, Hisaeda et al., 2008 |
| IL-10 and anti-CD25 in malaria | Chen et al., 2009a, Couper et al., 2007, Couper et al., 2008a | |
| Early CD25+ Treg expansion in susceptible strain | Wu et al. (2007) | |
| Leishmaniasis | ||
| L. braziliensis (cutaneous) | Human lesions have FOXP3+ | Campanelli et al. (2006) |
| L. donovani | CD40-low DCs induce Tregs, exacerbate infection | Martin et al. (2010) |
| Human lesions have high FOXP3+, abated with treatment | Ganguly et al. (2010) | |
| L. guyanensis (cutaneous) | Human lesions have high FOXP3+ | Bourreau et al., 2009a, Bourreau et al., 2009b |
| L. infantum (visceral) | Elevated Foxp3+CD103+ in infection | Rodrigues et al. (2009) |
| L. major | Tregs maintain low-level infection and protective immunity, require CD103 to access infection site and suppress | Belkaid et al., 2002, Suffia et al., 2005 |
| Treg depletion raises Th2 response and susceptibility; Tregs reactivate infection | Aseffa et al., 2002, Mendez et al., 2004 | |
| Toxoplasmosis | ||
| Toxoplasma gondii | Tregs reduce parasite-induced abortion in pregnant mice | Ge et al. (2008) |
| Trypanosomiasis | ||
| T. congolense | Foxp3+ Tregs suppress protective CD8+ NKT cells | Wei and Tabel (2008) |
| T. congolense | Natural Tregs suppress CD4+, CD8+, and macrophage inflammation | Guilliams et al. (2007) |
| T. cruzi | Increased FOXP3+ in human infection, and CD25+ Tregs prolong survival in mice | de Araujo et al., 2011, Mariano et al., 2008 |
Table 3.4.
Tregs in helminth infections
| Filarial nematodes | ||
| Brugia malayi | Induces Foxp3 expression, including in DO11.10 T cells | McSorley et al. (2008) |
| Brugia pahangi | CD25 depletion raises Th2 response | Gillan and Devaney (2005) |
| Litomosoides sigmodontis | Tregs maintain infection through CTLA-4 and inhibit allergy | Dittrich et al., 2008, Taylor et al., 2005, Taylor et al., 2007 |
| Onchocerca volvulus | TGF-β-producing clones from human infection site | Doetze et al. (2000) |
| Wuchereria bancrofti | Raised FOXP3+ T cells in infected patients | Babu et al. (2006) |
| Intestinal nematodes | ||
| Enterobius vermicularis | High FOXP3 expression in uninflamed mucosa of UC patient | Büning et al. (2008) |
| Heligmosomoides polygyrus | De novo induction of Tregs; Tregs reduce intestinal pathology, suppress Th2 response and bystander airway allergy | Finney et al., 2007, Grainger et al., 2010, Rausch et al., 2008, Rausch et al., 2009, Wilson et al., 2005 |
| Strongyloides ratti | Treg depletion reduces worm burden | Blankenhaus et al. (2011) |
| Strongyloides stercoralis | In HTLV-1 co-infection, excessive FOXP3+ Tregs, suppression of IL-5 and high worm burdens | Montes et al. (2009) |
| Toxocara canis | Tissue-migrating larvae induce Foxp3 in mice | Othman et al. (2010) |
| Trichinella spiralis | IL-10− Tregs restrain Th2 responses | Beiting et al. (2007) |
| Trichuris muris | IL-10− Tregs restrain Th2 responses | D'Elia et al. (2009) |
| Trematodes (flatworms) | ||
| Fasciola hepatica | Infection induces IL-10 and TGF-β from Tr1-like Tregs | Walsh et al. (2007) |
| Schistosoma haematobium | FOXP3+ Tregs correlate with infection intensity in children | Nausch et al. (2011) |
| Schistosoma japonicum | Egg antigens stimulate CD25+ suppression of airway allergy | Yang et al. (2007b) |
| Treg induction via TLR2 ligation to HSP60 peptide | Wang et al. (2009) | |
| Anti-CD25 treatment reduces worm load | Tang et al. (2011) | |
| Schistosoma mansoni | IL-10− Tregs elevated CD103+, dampen IL-4 responses to eggs | Baumgart et al. (2006) |
| IL-10+ CD25+ Tregs control pathology, dampen Th1 allowing Th2 to expand |
Hesse et al. (2004); McKee and Pearce (2004) |
|
| CD25+ Tregs expand through TLR2 to control pathology, upregulating CD103, CTLA4, and many other genes | Layland et al., 2007, Layland et al., 2010 | |
| Tregs induced by eggs, inhibit Th1 | Taylor et al. (2006) | |
| Foxp3 expression decreases following chemotherapeutic cure | Watanabe et al. (2007) | |
| Pathology patients have fewer CD25high Tregs | Teixeira-Carvalho et al. (2008) | |
| Cestodes (tapeworms) | ||
| Echinococcus multilocularis | Peritoneal T cells express high Foxp3 | Mejri et al. (2011) |
Table 3.5.
Tregs in fungal infections
| Aspergillus fumigatus TLR | Inflammation controlled by CD4+CD25+ Tregs | Montagnoli et al. (2006) |
| Candida albicans | Early Th17 promoted by Tregs, but later immunity suppressed; Tregs neutralized by TLR2 ligation | Montagnoli et al., 2002, Netea et al., 2004, Pandiyan et al., 2011 |
| Cordyceps sinensis | Increased Foxp3+ Tregs and reduced T1D in NOD mice | Shi et al. (2009) |
| Histoplasma capsulatum | Tregs suppress Th17 at site of infection | Kroetz and Deepe (2011) |
| Onychomycosis | Higher CD4+CD25+ cell numbers in patients | Kaya et al. (2009) |
| Paracoccidioides brasiliensis | Tregs control inflammation and limit fungal clearance; migration of Foxp3+ Tregs to lesions | Cavassani et al., 2006, Loures et al., 2010, Moreira et al., 2008 |
| Pneumocystis carinii | CD4+CD25+ Tregs suppress inflammation | Hori et al., 2002, McKinley et al., 2006 |
3.1. Viruses
Since the early 1900s, virus infections have been associated with immune suppression, variously attributed to functional impairment of lymphocytes, compromised function of antigen presenting cells, and the triggering of a suppressive T cell subset (Rouse and Horohov, 1986). Over recent years, a wide variety of viral infections has been examined for Treg activity (as detailed in Table 3.1), supporting the contention that suppression of antiviral effector cells may allow the establishment and maintenance of chronic viral infection (Li et al., 2008, Mills, 2004).
3.1.1. Retroviral infections and suppression of CD8+ effector function
Retroviral infections in both mice and humans can be influenced by Treg populations (Li et al., 2008, Rouse et al., 2006). In mice, infection with the chronic Friend retrovirus (FV) (Iwashiro et al., 2001) or the LP-BM5 murine leukemia virus mixture (which causes mouse AIDS) stimulated expansion of CD4+ Treg cells co-expressing CD25 or CD38, cell surface markers associated with regulatory cells (Antunes et al., 2008, Beilharz et al., 2004, Robertson et al., 2006, Zelinskyy et al., 2006). Treg expansion was associated with the detection of virus-specific CD8+ T cells that displayed an exhausted phenotype with low levels of effector cytokines and cytotoxic molecules (Dittmer et al., 2004, Zelinskyy et al., 2005).
The protective CD8+ T cell response could be experimentally inhibited by the transfer of virus-specific CD4+ T cells from naive or persistently infected mice to an acutely infected host (Dittmer et al., 2004, He et al., 2004); while both CD25+ and CD25− populations could mediate this effect, contact-dependent in vitro suppression of CD8+ T cell function was associated with the CD4+CD25+ subset (Robertson et al., 2006). Depletion of CD25+ T cells from persistently infected mice did not consistently improve the ability of CD8+ T cells to control virus loads, but treatment of FV-infected mice with anti-GITR resulted in rescue of CD8+ T cell dysfunction and reversal of retrovirus-induced immunosuppression (Dittmer et al., 2004, He et al., 2004). Similarly, LP-BM5 viral progression was retarded in mice treated with combined anti-CD25, -CTLA-4, and GITR antibodies (Beilharz et al., 2004). These results illustrate the important principle that in ongoing infection, immunity may not be restored simply by removing Tregs, if the resident effector population is anergized or exhausted. Rather, intervention to restimulate effector cells is also required.
The FV system also clearly displays a broader bystander suppression by Tregs, with CD8+ T cell antitumor responsiveness inhibited in mice receiving CD4+ cells from FV-infected mice, with TGF-β and CTLA-4 shown to act in vitro (Iwashiro et al., 2001). Normally, FV infection does not elicit immune-related pathology, except in the bone marrow of RAG1−/− mice receiving virus-specific CD4+ T cells. In this setting, pathology is suppressed in an antigen-noncognate manner by both polyclonal TCRβ-transgenic and wild-type CD4+CD25+Foxp3+ T cells (Antunes et al., 2008).
When DEREG mice (expressing a BAC-inserted Foxp3-promoter DTR construct) were depleted during the early, acute phase of Friend virus infection, they showed stronger and more multifunctional virus-specific CD8+ T cells, and > 10-fold lower viral loads, without evident cost to the host in terms of immunopathology (Zelinskyy et al., 2009a, Zelinskyy et al., 2009b). Interestingly, the transient Treg depletion that is achieved in the DEREG mouse model not only rescued CD4+ effector cells from functional exhaustion, but also had a lasting effect in reducing chronic virus loads (Dietze et al., 2011). Hence, transient depletion of Tregs could be a safe therapy for chronic viral infection.
An important retroviral pathogen is feline immunodeficiency virus (FIV), which expands functionally suppressive CD25+ T cells in chronically-infected cats (Vahlenkamp et al., 2004). In vivo depletion of CD25+ T cells in infected cats resulted in transient increases in both anti-viral and bystander responses (Mikkelsen et al., 2010), although depletion prior to infection did not alter the course of disease (Mikkelsen et al., 2011). In human retrovirus infection, Treg activity is largely inferred from phenotypic analysis of ex vivo lymphocytes, with reports showing a positive correlation between viral load and FOXP3+ Treg numbers (Andersson et al., 2005, Nilsson et al., 2006, Tsunemi et al., 2005). However, Tregs may impact on the course of HIV infection not only by impeding protective immunity but conversely by minimizing the pool of activated effector cells which are susceptible to virus infection (Eggena et al., 2005).
In T cells from HIV patients, both HIV-specific and bystander (CMV) in vitro responses are enhanced by depletion of CD4+CD25+ Tregs (Aandahl et al., 2004), and Tregs in vitro can suppress cytolytic capacity and cytokine secretion by HIV-specific CD4+ (Weiss et al., 2004) and CD8+ (Kinter et al., 2007a, Kinter et al., 2007b) effector T cells in vitro. The possibility that Tregs can also act to ameliorate infection was also raised by data showing that Treg inhibition correlated with lower levels of viremia (Kinter et al., 2004), and patients with low Treg numbers had greater peripheral T cell activation, a poor prognostic indicator for disease (Eggena et al., 2005). Later studies showed that molecules of the B7:CD28 family, programmed death-1 (PD-1) and CTLA-4, may maintain virus-specific T cell exhaustion typical of HIV infection (Kaufmann and Walker, 2009), as PD-1 expression correlated with viral load and disease progression in cohorts of HIV+ untreated patients (Day et al., 2006, Trautmann et al., 2006).
3.1.2. Expansion of Tregs following LCMV infection
Lymphocytic choriomeningitis virus (LCMV) is a natural murine RNA virus transmitted directly from mother to offspring, and different isolates cause infections of varying duration. Chronic, but not acute, infection of mice with LCMV results in a marked expansion of TGF-β-producing CD4+CD25+ Tregs (Filippi et al., 2009). More precisely, expansion occurs within a TCR Vβ5 CD4+Foxp3+ population, which derived from pre-existing “natural” Tregs, as there was no conversion of GFP−Foxp3− T cells transferred to mice immediately prior to infection (Punkosdy et al., 2011). This TCR reacts with the endogenous mouse mammary tumor provirus (MMTV) Mtv9 superantigen, one of several MMTVs which have segregated during inbreeding of laboratory mouse strains (Cohen and Varmus, 1979), and MMTV superantigen-specific Foxp3+ Tregs have been previously reported (Cabrera et al., 2008). The expansion of regulatory T cell mediated by endogenous retroviral superantigens provides a unique mechanism of immune-evasion following chronic LCMV infection.
Interestingly, LCMV is a potent inhibitor of type 1 autoimmune diabetes in mice (Filippi et al., 2009), an effect associated with the activity of Tregs (Diana et al., 2011, Filippi et al., 2011), as discussed below in Section 6.
3.1.3. Tregs and protective immunity to herpes simplex virus
Herpes simplex virus (HSV) is an acute cytolytic virus, immunity to which depends upon a protective CD8+ T cell response. However, HSV-1 infection heightened the suppressive function of CD4+CD25+ Tregs in mice (Suvas et al., 2003) and PC61-mediated depletion of CD25+ cells prior to infection amplified the virus-specific CD8+ response, whereas CD25+ Treg transfer had the opposite effect. Moreover, anti-CD25-mediated Treg depletion enhanced memory responses and protective immunity following primary infection with HSV or re-exposure to viral antigen following HSV antigen immunization or primary infection (Toka et al., 2004).
Depletion of Tregs using Foxp3DTR knock-in mice in a local HSV-2 infection resulted in increased viral loads in the mucosa and nervous system and fatal infection. Treg depletion, however, attenuated cellular trafficking to the site of infection and reduced inflammatory cytokine levels to a degree that significantly compromised protective immunity (Lund et al., 2008). In this instance, and in supporting studies ablating Tregs during LCMV infection of nonlymphoid tissue (Wherry et al., 2003), Tregs play an important role in controlling viral load as well as intensifying the cytokine milieu in secondary lymphoid organs.
3.1.4. Tregs benefit both host and pathogen in hepatitis C virus infection
The outcome of human hepatitis C virus (HCV) infection can range from complete control to viral persistence and associated liver disease (Rehermann, 2009); however, the development of therapeutic strategies for treatment have been hampered by difficulties in establishing in vitro and in vivo models of viral replication, so that currently all data pertain to infection in primate systems.
In HCV-infected patients, frequencies of peripheral CD4+CD25+ and Foxp3+ Tregs are elevated (Cabrera et al., 2004, Ebinuma et al., 2008, Sugimoto et al., 2003) and in vitro analysis indicated that CD4+CD25+ cells suppress virus-specific CD4+ and CD8+ responses through IL-10 and TGF-β secretion (Cabrera et al., 2004). Although other authors confirmed the suppressive activity of HCV patient-derived CD4+CD25+ cells, antibodies to these same mediators did not block the suppression of purified CD8+ effectors, suggesting action through a CD4+ intermediary (Boettler et al., 2005, Rushbrook et al., 2005). Significantly, HCV-associated Tregs were able to suppress influenza virus-specific CD8+ T cell function (Boettler et al., 2005). Comparison with a cohort recovering from acute HCV infection suggested a decline in CD4+CD25+ regulatory function (Boettler et al., 2005), while longitudinal studies in individual patients more convincingly concluded that spontaneous recovery from HCV infection is associated with the temporal loss of Foxp3+ Treg function (Smyk-Pearson et al., 2008).
Clear evidence has also been provided that HCV-antigen-specific Tregs evolve during infection. Foxp3+ Tregs can be isolated from HCV-positive PBMC stimulated with HCV peptides, with different peptides proving optimal for different patients (Li et al., 2007b), and epigenetic analysis of the Foxp3 locus indicating stable rather than transient commitment to the Treg phenotype (Li et al., 2009). Similarly, HCV peptides were used to expand Tregs from infected patients, some Foxp3+ Tregs reacting with HCV-specific class II-peptide tetramers (Ebinuma et al., 2008).
Although Tregs may impair immunity to HCV, they may also protect the patient from excessive pathology. Thus, liver inflammation is inversely correlated to CD4+CD25+ T cell numbers in chronic HCV infection (Cabrera et al., 2004). Moreover, CD25+ Tregs from patients with low pathological scores exerted more suppressive effects on HCV-specific CD4+ T cell responses than Tregs from patients with advanced clinical disease (Bolacchi et al., 2006), demonstrating that loss of Treg function can be correlated with organ-specific viral-induced inflammation and pathology.
3.2. Bacteria
Historically, most bacterial immunology focused on the acutely pathogenic species representing the most pressing threat to human health; more recently, research has also encompassed the commensal microbiome, particularly in the gut in which intense interactions occur with the immune system. It is appropriate to consider both pathogens and commensals in terms of Treg activity, in part because they form a biological continuum (with many commensals being opportunistic pathogens), and also because of the shared signaling pathways and specific receptors that are involved in their recognition. We summarize below the data from some of the principal bacterial systems, with additional details listed in Table 3.2.
3.2.1. Mycobacteria
Mycobacterium tuberculosis (Mtb) is present in two billion individuals worldwide and remains a major cause of morbidity and mortality around the world (Dye, 2006). Most infectious episodes are effectively resolved, but where elimination of the bacteria does not occur, Th1 immunity is impaired (Jo et al., 2003, Lienhardt et al., 2002).
In mice, Tregs expand in the lung and associated lymph nodes following Mtb infection (Kursar et al., 2007), and bacterial loads are 10-fold lower following depletion of Thy1.1+Foxp3+ cells in Thy1.1-wild-type:Thy1.2-Foxp3−/− mixed bone marrow chimeras (Scott-Browne et al., 2007). Conversely, co-transfer of CD4+CD25+ Tregs neutralizes immunity to infection mediated by effector CD4+CD25− T cells in RAG-1−/− mice (Kursar et al., 2007). Treg expansion was particularly rapid in mice infected with a hypervirulent strain of Mtb with the emergence of a CD4+CD25+CD223+Foxp3+IL-10+ regulatory T cell population in the lung (Ordway et al., 2007). The expansion of Mtb-specific Tregs was followed in vivo, using an Mtb-specific TCR transgenic mouse, P25; in a RAG-sufficient background, a subset of P25 Mtb-reactive T cells express Foxp3, possibly representing natural Tregs with dual specificity for this pathogen (Shafiani et al., 2010). The Foxp3+ pathogen-specific Tregs proliferated faster than the effector populations in the lung, delayed the infiltration of CD4+ and CD8+ populations, and caused a significant rise in bacterial titer (Shafiani et al., 2010).
The saprophytic species Mycobacterium vaccae may also have Treg-stimulating activity. While interest in this organism was initially focused as a possible immunogen against Mtb, it was also found to be beneficial in downregulating human atopic dermatitis (Arkwright and David, 2001). Treatment of mice with a heat-killed M. vaccae suspension prior to ovalbumin sensitization gave rise to a population of Ova-specific CD4+CD45RBlo regulatory T cells, which mediated inhibition of airway allergy through IL-10 and TGF-β (Zuany-Amorim et al., 2002).
In humans, the frequency of FOXP3+ cells is substantially higher in TB patients (Guyot-Revol et al., 2006, Li et al., 2007a) and declines following successful chemotherapy of infection (Chen et al., 2007b). In patients, GITR expression is also significantly raised in CD4+CD25+ T cells, which functionally suppress effector responses (Li et al., 2007a), while depletion of CD4+CD25+ Tregs restores in vitro responsiveness of peripheral T cells (Ribeiro-Rodrigues et al., 2006). Moreover, the number of CD4+CD25+Foxp3+ cells present in patients’ pleural fluid (PF) inversely correlates with the ability of PF CD4+CD25− T cells to mount a IFN-γ response to Mtb antigens (Chen et al., 2007b). CD4+CD25+Foxp3+ cells from healthy carriers multiplied in vitro in response to heat-killed Mtb, and the active principle shown to be the 19-kDa M. tuberculosis lipoprotein ManLAM (mannose-capped lipoarabinomannan), acting via the mannose receptor of human monocytes (Garg et al., 2008).
An important issue is how Treg activity will be influenced by vaccination. Following infection of BCG-vaccinated mice with naturally virulent strains of M. tuberculosis, initial effector responses declined while Treg activity increased, with pathology accentuating over time (Ordway et al., 2011). Whether regulatory T cell populations can be specifically depleted or modified to favor the outcome of mycobacterium vaccination remains to be determined.
3.2.2. Listeria monocytogenes
Regulatory T cells can also control the magnitude of a secondary response in infections where CD8+ T cells are important in controlling protective immunity against pathogens such as Listeria monocytogenes. In this instance, depletion of CD4+ T cells significantly enhanced the formation of a memory CD8+ T cell response following secondary infection or immunization. Anti-CD25 depletion and transfer experiments demonstrated that this suppressive activity was enriched within the CD4+CD25+ T cell population from naive or L. monocytogenes-infected mice (Kursar et al., 2002). Through the use of transgenic mice where all the T cells recognize Ova presented in the context of H-2Ab (OT-II), or where Foxp3 was coupled to a GFP reporter (Foxp3–GFP), it was possible to demonstrate that acute infection with L. monocytogenes expressing OVA was not associated with the induction of antigen-specific regulatory T cells (Fontenot et al., 2005), suggesting that downstream suppression of immunity was more likely via an interaction with CD8+ T cells than a direct antigen-specific regulation of CD4+ T cell function.
Foxp3+ Tregs also inhibit Listeria-specific CD8+ T cell responses in vivo; however, Foxp3+ Tregs were found to be less potent at suppressing effector responsiveness, and specific depletion of the Treg population in Foxp3-DTR mice did not alter bacterial clearance or the expansion and activation of virus-specific CD8+ T cells following infection of mice with L. monocytogenes (Ertelt et al., 2011). These findings highlight the importance of Tregs in controlling inflammatory responses in the steady state and raise the possibilities of this function being overcome following infection.
Infection with L. monocytogenes is more common, and more hazardous, in pregnancy. In this context, a recent study in mice reported that the physiological increase in Foxp3+ Tregs during allogeneic pregnancy was associated with greater susceptibility to Listeria (and Salmonella) infections, an effect attributable to IL-10 production by these cells (Rowe et al., 2011). Moreover, Treg depletion in pregnant Foxp3DTR mice restored normal levels of resistance to infection while reducing live births by 70%; hence, the extraordinary balance between infection and reproduction is managed to optimal effect by Foxp3+ Tregs.
3.2.3. Helicobacter pylori
Human Helicobacter pylori infection correlates with a higher number of regulatory T cells in the gastric mucosa (Lundgren et al., 2005, Rad et al., 2006), which are also found in H. pylori-induced gastric adenocarcinoma (Enarsson et al., 2006). The inability of the host to eradicate H. pylori infection can therefore be linked to Treg suppression of H. pylori-specific effector T cell responses in humans and mice (Lundgren et al., 2003, Raghavan et al., 2003). Accordingly, depletion of CD25+ T cells increased the gastric inflammatory response and reduced bacterial burden in infected mice, but also resulted in development of severe gastritis (Rad et al., 2006, Raghavan et al., 2003), although another laboratory reported no effect of depletion (Kaparakis et al., 2006). Tregs purified from gastric tumors were able to suppress H. pylori-specific effector responses in vitro, suggesting that antigen-specific regulatory T cells might contribute to tumor progression through bystander suppression, as noted above in Friend virus infection (Enarsson et al., 2006).
Studies on induction of Tregs during H. pylori infection have shown that gastric epithelial cells (GECs) exposed to this organism upregulate the PD1 ligand B7-H1, and that increased conversion of naive cells into Tregs is inhibited by anti-B7-H1 antibody (Beswick et al., 2007). In addition, GEC production of TGF-β both acts to induce Foxp3+ Tregs and to inhibit effector T cell responses in vitro (Beswick et al., 2011). The systemic impact of H. pylori on the generation of Tregs is so strong that infected mice are protected from airway allergic inflammation induced by ovalbumin, and CD4+CD25+ Tregs from infected mice can confer this protection on uninfected, allergen-sensitized animals (Arnold et al., 2011).
3.2.4. Bordetella pertussis
Bordetella pertussis infection is associated with a severe and protracted disease, which is often fatal in young children. Although the development of antigen-specific Th1 cells promotes recovery from infection and clearance of bacteria from the respiratory tract, these responses are suppressed in acute infections (McGuirk et al., 1998). One virulence factor implicated in this is filamentous hemagglutinin (FHA), and FHA-specific Tr1 clones have been generated from infected mice, expressing high levels of IL-10 but little IFN-γ (McGuirk et al., 2002).
The major virulence factor of B. pertussis is its toxin (PTx), which is widely used to enhance the incidence and severity of disease in murine experimental autoimmune encephalomyelitis (EAE). A single injection is reported to inhibit Tregs and promote Th17 responsiveness (Chen et al., 2007a). Most recently, however, it has been reported that weekly PTx administration causes expansion and persistence of peripheral CD4+CD25+Foxp3+ regulatory T cells and elevations in serum IL-10 and TGF-β (Weber et al., 2010). It will be interesting to ascertain if, in active infection, sustained release of pertussis toxin in fact promotes suppressive Tregs rather than proinflammatory effector responses.
3.2.5. Commensal microbes
The development of germ-free (GF) mice has allowed us to analyze the specific impact of certain commensal bacteria species on the immune system. GF mice appear to have site-specific differences in the phenotype and suppressive capacity of their CD4+CD25+ regulatory T cell population (Ostman et al., 2006). In particular, GF mice lack Foxp3+ Tregs in the colonic lamina propria, which are induced as a predominantly Helios− population when animals are colonized with defined commensals (Geuking et al., 2011). However, the generation of Foxp3DTR mice in specific-pathogen free (SPF) and GF conditions demonstrated that the suppressive activity of splenic and lymph node CD4+Foxp3+ Tregs was equivalent in both mice (Chinen et al., 2010). Treg depletion in either mice also resulted in equivalent systemic inflammatory responses; however, inflammation was much more severe in the small intestine of Treg-depleted SPF mice, reflecting the substantial load of nonself antigen represented by the commensal microbiota, and the critical role of Tregs in subduing reactivity to gut flora.
Earlier work which had established this principle includes the transfer of naive (CD45RBhigh) T cells into T cell-deficient mice, provoking massive gut inflammation, and its suppression by co-transfer of Tregs through IL-10, TGF-β, and CTLA-4 (Maloy et al., 2003). Similarly, transfer of naive T cells from an IL-10-deficient RAG−/− mouse enhanced inflammation induced following Helicobacter hepaticus infection, whereas co-transfer of IL-10-sufficient CD45RBlowCD4+ T cells, of either CD25+ or CD25− phenotype, from H. hepaticus-infected but not uninfected mice was most able to prevent disease (Kullberg et al., 2002). These studies were key steps toward the concept that regulatory IL-10-producing T cells are essential to prevent bacteria-induced colitis.
Normally asymptomatically resident within the colon, Bacteroides fragilis is a Gram-negative bacteria that has been detected within abscesses formed throughout the peritoneal cavity as a result of bowel perforation (Polk and Kasper, 1977). B. fragilis was found to protect animals from experimental colitis induced by H. hepaticus via a single microbial molecule (polysaccharide A, PSA) (Mazmanian et al., 2008). This molecule induces IL-10 production from T cells, suppresses proinflammatory IL-17 production, and was further shown to promote the differentiation of CD4+Foxp3+ regulatory T cells through TLR2 during protection from experimental colitis, as further discussed in Section 4.4 below (Round and Mazmanian, 2010). This microbial polysaccharide and B. fragilis were also shown protect against pathology in a mouse model of experimentally induced EAE, where both stimulated Foxp3+ regulatory T cell expansion in vivo (Ochoa-Reparaz et al., 2010a, Ochoa-Reparaz et al., 2010b).
A defined mix of 46 spore-forming Clostridium species, prominent and indigenous to the murine gastrointestinal tract, was also found to enhance TGF-β production and expand IL-10+Foxp3+Helios− regulatory T cells in the intestine of previously GF mice (Atarashi et al., 2011). Clostridial enrichment of the neonatal gut flora resulted in resistance to DSS-mediated colitis and reduced polyclonal IgE responsiveness to OVA–alum. A parallel probiotic effect has been found with Bifidobacterium infantis, which increases Foxp3+ Tregs and counters inflammation following Salmonella typhimurium infection (O'Mahony et al., 2008), while Lactobacillus reuteri evokes a similar Foxp3+ Treg expansion and mediates suppression of airway allergy in mice (Karimi et al., 2009).
3.3. Protozoa
Protozoa are single-celled organisms which include parasites of both extracellular and intracellular niches; the major global health problems from protozoal pathogens are caused by Plasmodium (malaria) and Leishmania species, along with human trypanosomes in South America and trypanosomes of livestock in Africa. These species are highlighted below, with further details given of Tregs in protozoal infections in Table 3.3.
3.3.1. Leishmania
One of the founding paradigms of T cell immunology emerged from research into infections of mice with Leishmania major, in which the progressive disease in BALB/c mice compared to C57BL/6 is linked to their dominant Th2 response to this parasite (Reiner and Locksley, 1995). C57BL/6 mice resolve infection after several weeks unless their Th1 response is compromised. However, IL-4R-deficient BALB/c are not resistant to all strains of L. major (Noben-Trauth et al., 1999), and other Leishmania species (which cause cutaneous or visceral forms of disease) elicit little immunity in any strain of mouse. In Leishmania tropica, a cutaneous leishmaniasis agent which is equally infective to BALB/c and C57BL/10, only the combined neutralization of TGF-β and IL-10 signaling was able to induce immune clearance of parasites (Anderson et al., 2008). The prominence of these two cytokines is repeated in other species, including the cutaneous L. major (Belkaid et al., 2001), and the visceral species Leishmania donovani (Murphy et al., 2001, Rodrigues et al., 1998) and Leishmania infantum (Rodrigues et al., 2009). In the latter, most recent, study, infection was associated with elevated levels of CD4+ Foxp3+ CD103+ Tregs, which contributed toward a high IL-10 profile (Rodrigues et al., 2009).
The role of IL-10 is particularly well documented in L. major infection, as for example in the C57BL/6 mouse, in which sterile immunity only takes effect if IL-10 is neutralized (Belkaid et al., 2001). Similarly, in IL-4Rα−/− BALB/c mice, IL-10R blockade is required for complete parasite elimination (Nagase et al., 2007). Importantly, IL-10 is derived primarily from CD25− T cells, including some co-expressing IFN-γ (Anderson et al., 2007), explaining why anti-CD25 depletion is less effective than anti-IL-10R in conferring immunity in IL-4Rα−/− mice (Nagase et al., 2007).
The modulatory effect of Tregs therefore depends critically on the genetic and immunological status of the host. Thus, anti-CD25 depletion of BALB/c mice resulted in enhanced Th2 responsiveness and greater susceptibility rather than resistance (Aseffa et al., 2002). The ability of CD4+CD25+ T cells to suppress both Th1 and Th2 responses to L. major was then shown in co-transfer experiments (Xu et al., 2003). Although equal suppression of both Th subsets would have no net effect on protection, it is interesting that BALB/c mice lacking CD103 are resistant to infection (Suffia et al., 2005), presumably because T regs cannot access or be retained at the infection site.
In wild-type C57BL/6 mice, CD25+ Tregs down-modulate immunity sufficiently to allow low-level persistence of parasites in the dermal site. In the absence of Tregs, parasites are eliminated but mice also lose their long-term immunity to reinfection (Belkaid et al., 2002). Because parasite persistence and reactivation of infection in humans are major issues, it is relevant to note that high-dose reinfection in mice can expand CD4+CD25+ Tregs thereby allowing latent L. major at a distal site to reactivate (Mendez et al., 2004). By co-transfer of allotype-marked CD4+CD25+ and CD4+CD25− T cells from naive mice into RAG-2−/− recipients prior to L. major infection, it was also established that the infection only stimulates pre-existing “natural” Tregs, with little conversion observed from CD4+CD25− to Foxp3+ Tregs (Suffia et al., 2006); these authors also showed that the Foxp3+ natural Treg population was reactive to L. major antigens, and indeed, they were able to propagate parasite-specific Treg clones that maintained this specificity for months in vi t ro.
Each of these factors appears to be at play in human Leishmaniasis. Cutaneous lesions caused by Leishmania braziliensis show elevated Foxp3+ Tregs which co-express CTLA-4 and GITR while producing both TGF-β and IL-10 (Campanelli et al., 2006), while an independent study on this infection found that IL-10 production (by both Tregs and monocytes) strongly correlated with lesion activity (Salhi et al., 2008). Similarly, in a related cutaneous species (Leishmania guyanensis), high IL-10 and Foxp3 expression were reported in patients with long-standing lesions who were unresponsive to chemotherapy (Bourreau et al., 2009a, Bourreau et al., 2009b).
3.3.2. Malaria
Malaria, caused by Plasmodium species, is one of the world's most prevalent lethal diseases, causing anemia (due to parasitism of erythrocytes) and cerebral inflammation (due to trapping of infected red cells in the vasculature). The complexity of both immunity and inflammation with the parasite is reflected in the dual roles of Tregs as protectors, in different settings, of both the host and the parasite (Finney et al., 2010, Scholzen et al., 2010), although as with Leishmania, IL‐10 (Couper et al., 2008a, Couper et al., 2008b) and TGF-β (Omer et al., 2003) are the critical regulators in malaria. Consistent with the latter study, a human malaria vaccine trial with healthy European volunteers found elevated serum TGF-β in individuals who did not respond to vaccination with inflammatory cytokines (Walther et al., 2005).
In endemic humans, many studies have reported elevated CD25+FOXP3+ cell numbers in Plasmodium falciparum malaria (Finney et al., 2009, Goncalves et al., 2010, Minigo et al., 2009, Walther et al., 2009) as well as Plasmodium vivax (Bueno et al., 2010, Goncalves et al., 2010, Jangpatarapongsa et al., 2008). However, although the frequencies of Tregs can vary significantly between individuals, the ratios of Treg:Th1 may not differ (Finney et al., 2009). Of further note is the suggestion that Tregs in humans repress development of malaria-specific T cell memory rather than act on inflammation itself (Walther et al., 2009); this study also implicated Tr1 (IL-10+IFN-γ+) regulatory cells which do not express FOXP3. Hence, there is currently little compelling evidence that FOXP3+ Tregs suppress immunity to malaria in endemic populations.
A prominent aspect of human malaria is its effect on infants born to infected mothers. In two recent studies, it has been reported that following delivery from infected mothers, cord blood lymphocytes show high IL-10 and low Th1 responsiveness to malaria antigens, which can be reversed by CD25+ T cell depletion (Bisseye et al., 2009, Brustoski et al., 2006). Prenatal exposure to P. falciparum antigens also correlated with greater frequency of CD4+CD25hi or CD25+CD127lo Tregs in newborns’ cord blood, able to suppress malaria antigen-specific IFN-γ production in vitro (Mackroth, 2011, Walther et al., 2009). It is interesting to consider whether these Tregs may persist and so determine the susceptibility of the child to malaria infection and disease.
Murine models of malaria infection reflect a major, but not exclusive, role for Tregs in determining infection outcome. The rodent malaria species Plasmodium yoelii is frequently studied in both susceptible (BALB/c) and resistant (DBA/2) mice. Within 3–4 days of infection, the susceptible BALB/c mice raise CD4+CD25+ Treg frequency and overall IL-10 production, suggesting a functional link with their poor protective Th1 response (Wu et al., 2007), and supporting an early finding that anti-CD25 depletion generated protective immunity to this parasite (Hisaeda et al., 2004). However, a subsequent study in C57BL/6 mice compared lethal (Py17XL) and nonlethal (Py17X, NL) strains of P. yoelii and found that both elicited similar, modest, rises in Foxp3+ Treg numbers and that in neither case did CD4+CD25+ cell depletion alter the course of infection (Couper et al., 2008b). In contrast, IL-10 from CD4+CD25−Foxp3− T cells following P. yoelii infection was the critical factor in impeding parasite clearance and ameliorating liver pathology following infection, with IL-10−/− mice surviving the otherwise lethal Py17XL infection. Nevertheless, a demonstration that Treg activation can suppress immunity to P. yoelii comes from mice co-infected with the helminth Heligmosomoides polygyrus (see below, Section 3.4.2); co-infected mice developed more severe malaria infections which were rescued by anti-CD25 antibody treatment (Tetsutani et al., 2009).
The best available mouse model for P. falciparum-mediated cerebral malaria (CM) is another rodent species, Plasmodium berghei in the C57BL/6 mouse, associated with parasite vascular adhesion and overproduction of Th1 inflammatory mediators within the brain. Perhaps counter-intuitively, anti-CD25 Treg depletion protects mice from CM, reducing parasite sequestration and also CD8+ T cell infiltration (Amante et al., 2007, Randall et al., 2008, Wu et al., 2010). Interestingly, the effect of depletion is time dependent (Vigario et al., 2007) suggesting that the action of Tregs could be to facilitate entry of effector cells into the CNS, as described above (see Section 3.1.3) in HSV-2 infections (Lund et al., 2008). An alternative explanation is that key effector populations for CM express CD25 after infection and are co-depleted by antibody treatment. In support of this, Treg ablation in DEREG mice showed a substantial population of CD25+Foxp3− T cells developing after infection, and no amelioration of CM disease (Haque et al., 2010, Steeg et al., 2009). However, when Treg numbers are experimentally boosted with IL-2/anti-IL-2 complexes, mice were fully protected from CM (Haque et al., 2010), arguing again that the action of Tregs depends critically on their proportions and activation state in vivo.
Other mouse strains are more resistant to P. berghei-induced CM, but in the BALB/c, anti-CD25-depletion had the opposite effect and accentuated CM symptoms (Nie et al., 2007). However, as in P. yoelii, Treg depletion had little effect on overall parasitemias or progression to death from fulminant infections (Wu et al., 2010). Clearly, regulation of the immune response to both human and murine malaria involves multiple cellular components, particularly at the level of tissue infiltration, and is greatly dependent upon dynamic and kinetic factors that have yet to be defined; while Tregs may not be uniquely responsible for susceptibility to infection, clearly it is essential to strike the appropriate balance with effector mechanisms for a health outcome to this potentially devastating infection (Hansen and Schofield, 2010, Scholzen et al., 2010).
3.3.3. Trypanosomes
The trypanosomes encompass two very different groups of parasites, as the African species (e.g., Trypanosoma brucei) are extracellular pathogens, which can cause disease in humans and livestock, while the South American species (Trypanosoma cruzi) has an intracellular niche in human phagocytes and smooth muscle cells. Immunosuppression has long been a prominent feature in African trypanosomiasis, and active suppressor cell populations were described in mice by the late George Roelants and colleagues (Roelants et al., 1979).
C57BL/6 mice, which escape lethality to Trypanosoma congolense infection through limitation of an early IFN-γ response, show expansion of IL-10 producing Foxp3+ Tregs. In this “trypanotolerant” strain, Tregs were able to downregulate classical activation of macrophages and limit tissue pathology resulting from the inflammatory immune response (Guilliams et al., 2007). This role of Tregs in limiting pathology, but allowing increased resistance following trypanosome infection, was also demonstrated following T. brucei infection. Treg expansion with the CD28 superagonist resulted in downregulation of inflammatory type 1 cytokines and the development of macrophages into the alternatively activated phenotype (Guilliams et al., 2008). Later studies demonstrated that anti-CD25 antibody treatment and effective depletion of natural Foxp3+ Tregs before T. congolense infection protects BALB/c mice against this normally lethal disease. Protection was reversed in CD25-depleted mice by administration of a specific inhibitor of inducible nitric oxide synthase (Wei and Tabel, 2008).
T. cruzi is the causative agent of Chagas’ disease in South America, and infection has again been associated with immunosuppression of humoral and cell-mediated immunity, in part attributable to the action of IL-10 and TGF-β which disable iNOS-mediated killing by infected macrophages (Gazzinelli et al., 1992). In patients, greater CD4+CD25+FOXP3+ Treg numbers are found in both asymptomatic carriers and those developing pathology due to parasites in the myocardium; however, only in healthy patients did Tregs produce IL-10, indicating that cardiomyopathy may results from insufficient production of this cytokine (de Araujo et al., 2011). In infected mice, similar phenotype Tregs were found to migrate to the heart, but depleting interventions with anti-CD25 increased mortality, while administration of anti-GITR antibody additionally increased myocarditis and tissue parasitism (Mariano et al., 2008).
3.4. Helminths
Helminths are multicellular worms comprised of three broad taxa, the Nematodes (round worms, including the model organism Caenorhabditis elegans), Trematodes (flukes), and Cestodes (tapeworms), each separated by approximately 500 million years of evolution. While taxonomically distant, the parasitic species share many immunological features which are likely to have co-evolved under similar selective pressure from the immune system of the host (Allen and Maizels, 2011).
Most helminth infections, in man and livestock, are long-term chronic infestations which are maintained in the population by repeated cycles of reinfection; hence protective immunity is slow to develop, and indeed, most helminth species are associated to some degree with a state of immune suppression. Classic studies demonstrated that peripheral blood T cells from Schistosome and filariasis-infected patients showed parasite antigen-specific hyporesponsiveness, as detailed below, which could be reversed by chemotherapeutic removal of the parasite burden (Cooper et al., 2000, Greene et al., 1985, Sartono et al., 1995).
A marked contrast from infections with microbial agents is seen for the role of IL-10 in helminth infections; while in viral, bacterial, and protozoal infections, IL-10 generally impairs resistance (Couper et al., 2008a, Moore et al., 2001), the role of IL-10 in Th2-dominated helminth infections is both complex and double-edged (Hoffmann et al., 2000a). For example, IL-10 is essential to protect against potentially fatal immunopathology in chronic schistosome infection, but it is equally necessary in the initial stages of infection to establish dominant (and generally protective) Th2 responses by suppressing competing Th1/Th17 activity. Similarly, IL-10 is required for Th2-mediated expulsion of adult Trichinella spiralis nematodes from the intestine, and yet acts to block immunity to their offspring, larvae which encyst in tissue musculature (Beiting et al., 2007, Helmby and Grencis, 2003). In human helminth infections, IL-10 acts more unequivocally as an immunoregulatory player, perhaps because patients are studied in the chronic, homeostatic phase rather than during the initial events of priming and Th subset selection.
3.4.1. Filarial nematodes
Human filarial nematodes include the causative agents of lymphatic filariasis (Brugia malayi, Brugia timori, and Wuchereria bancrofti) and onchocerciasis or river blindness (Onchocerca volvulus). In these long-lived infections, many infected patients are asymptomatic but carry large numbers of transmission stages (microfilariae, MF) in the blood (for lymphatic filariasis) or skin (in onchocerciasis). Typically, peripheral blood T cells from these patients fail to respond to parasite antigen challenge in vitro, and are hence termed hyporesponsive (Piessens et al., 1980, Yazdanbakhsh et al., 1993). Treg activity was presaged in this system by Piessens’ report on suppressor T cells in hyporesponsive MF+ patients (Piessens et al., 1982), and by later work showing that the hyporesponsiveness can be reversed, in vitro, with anti-IL-10 and TGF-β antibodies (King et al., 1993). Most recently, the link between Tregs and the human filarial infection has been firmly established with elevations of both natural and adaptive Treg numbers (Metenou et al., 2010). Moreover, in individuals who are more reactive to parasite infections, with low or zero circulating MF and immunopathological symptoms such as lymphoedema and elephantiasis, Treg activity is deficient (Babu et al., 2009b).
Additional evidence for Treg-like cells in human onchocerciasis came from analysis of T cells in the subcutaneous granulomas surrounding adult O. volvulus (Doetze et al., 2000), with CD4+ T cell clones from this tissue expressing IL-10 and TGF-β (Satoguina et al., 2002). At this time, their FOXP3 status was not determined. In lymphatic filariasis, asymptomatic carriers were found to express higher CTLA-4 levels (Steel and Nutman, 2003), with anti-CTLA-4 antibody also raising the cytokine responses of patients’ cells in vitro. Interestingly, CTLA-4 may act with PD-1 in filariasis patients to block protective Th1 and Th17 responses to tuberculosis (Babu et al., 2009a).
A particularly striking feature of human filarial infections is the extremely high levels of IgG4 antibodies, both parasite-specific and total, that are rapidly lost once parasites are removed by chemotherapy (Atmadja et al., 1995). Hyporesponsive patients show the maximal IgG4 levels alongside depressed IgE responses (Yazdanbakhsh et al., 1993), a relationship which can now be explained by the action of Tregs, as in vitro switching of B cells to the IgG4 isotype is promoted by IL-10 (Satoguina et al., 2005) as well as TGF-β and GITR ligation, although not CTLA-4 (Satoguina et al., 2008). Hence, circulating IgG4 levels in humans could be a marker not only for helminth infection but also for elevated Treg activity.
The conclusion that human filariasis activates Tregs is well supported by studies in animal models; although the mosquito-borne infective larvae of B. malayi are tolerated for less than 14 days in mice, the parasites induce a short-lived expansion in Foxp3+ Tregs, as occurs more strongly in mice transplanted with adult worms of the same species (McSorley et al., 2008). Dead parasites of either stage did not elicit this response, indicating that the presence or products of live filarial worms were responsible for stimulating Tregs. Moreover, bystander-specificity T cells (carrying the DO11.10 ovalbumin-specific TCR) were induced to express Foxp3 when transferred into BALB/c mice carrying either larval or adult B. malayi (McSorley et al., 2008).
Because human filariae cannot complete their infection cycle in mice, it is necessary to study related, rodent-compatible, species to ascertain the functional importance of Tregs in the natural context. As such, the model system of Litomosoides sigmodontis (Hoffmann et al., 2000b) has proven exceptionally informative. Very soon after infection, there is expansion of natural Tregs, as determined by BrdU uptake in vivo, followed by a second wave of inducible Tregs (Taylor et al., 2009), with the initial wave at least essential for parasite establishment. Transfer of cells from infected mice protected allergic recipients from allergic airway hypersensitivity, in a manner inhibited by blockade of TGF-β or anti-CD25 Treg depletion (Dittrich et al., 2008). One consequence of regulatory expansion is silencing of effector cell responses (an interesting parallel to hyporesponsiveness in humans), and the emergence of a Foxp3−GITR+CTLA4+ unresponsive CD4+ population (Taylor et al., 2005). Most significantly, intervention with depleting antibodies, using anti-CD25 in combination with either anti-GITR (Taylor et al., 2005), or anti-CTLA4 (Taylor et al., 2007) boosted responsiveness and elicited immune killing of worms. These studies were the first to demonstrate that interfering with Treg function (and re-stimulating hyporesponsive effectors through GITR ligation) can reverse susceptibility to a helminth infection.
3.4.2. Intestinal nematodes
Intestinal nematode infections (also termed geohelminths reflecting their fecal-oral transmission) are extraordinarily prevalent in humans in tropical countries, with approximately two billion cases in the world today (Hotez et al., 2008). Studies have not found significant increases in systemic Foxp3+ Treg frequencies, but qualitative changes are apparent, which may well be immunologically significant.
In areas hyperendemic for the intestinal helminth infections Ascaris lumbricoides and Trichuris suum, lymphocytes from infected children constitutively express high levels of IL-10 and TGF-β, while antigen-specific responses are inversely depressed (Turner et al., 2008). For example, CD4+CTLA4+ T cells are more numerous in children with intestinal helminths than uninfected subjects (García-Hernández et al., 2009). Functionally, peripheral T cells from geohelminth-infected children show depressed in vitro immune responses to malarial and mycobacterial antigens that are rescued by removal of the CD25high cells (Wammes et al., 2010).
In a less common infection, Strongyloides stercoralis, patients co-infected with HTLV-1 show exaggerated levels of circulating Foxp3+ T cells, reaching ∼ 18% of the total CD4+ T cell population (Montes et al., 2009), together with higher worm burdens, while IL-5 and eosinophilia were suppressed.
Research into mouse gastrointestinal parasites has employed several model systems, with the most information to date obtained from H. polygyrus, a relative of the human hookworms, which spends its entire parasitic phase within the gastrointestinal tract (Monroy and Enriquez, 1992). This species is particularly associated with immunosuppression, down-modulating responses to allergens, autoantigens, and other infectious organisms (reviewed by Maizels et al. (2011)). Early in infection, both the proportion and absolute numbers of Foxp3+ Treg cells expand in the mesenteric lymph nodes (Finney et al., 2007, Rausch et al., 2008), while within the Foxp3+ population, there is also increased expression of CD103, considered to be a marker of Treg activation (Huehn et al., 2004). CD25+ Tregs from H. polygyrus-infected mice are suppressive when transferred to uninfected recipients, as shown by inhibition of airway allergic inflammation (Wilson et al., 2005). CD8+ Tregs are also found to expand in the lamina propria (Metwali et al., 2006).
Remarkably, H. polygyrus attenuates colitis in IL-10-deficient mice (Elliott et al., 2004), although IL-10 is necessary for this helminth to protect normal mice from chemically induced colitis (Setiawan et al., 2007). In contrast, infection cannot block colitis in mice expressing a T cell-specific kinase-dead TGF-β receptor II (Ince et al., 2009), demonstrating that both IL-10 and TGF-β can be invoked by the regulatory pathways activated by the infection. The importance of TGF-β is emphasized, however, both by the finding that H. polygyrus secretes a functional mimic of this cytokine (see Section 4.1 below) and by the successful boosting of immunity to adult worms by in vivo administration of an inhibitor of TGF-β receptor kinase I (Grainger et al., 2010).
A recent study reported on H. polygyrus infection in DEREG mice, expressing DTR under a BAC transgene (Lahl et al., 2007); in this report, Foxp3-depleted mice showed heightened Th2 responses but similar infection levels (Rausch et al., 2009). It should be noted, however, that intestinal worms were enumerated at an early time point before genetically resistant mice expel most worms (Maizels et al., 2011), and this system will need further investigation.
As with H. polygyrus, many (but not all) mouse intestinal nematode infections cause an expansion of CD4+Foxp3+ Tregs; in the case of T. spiralis infections, this occurs in both mice (Beiting et al., 2007) and rats (Gruden-Movsesijan et al., 2010). In mice, anti-CD25 antibody-mediated Treg depletion does not reduce worm numbers, although treatment results in a heightened Th2 and intact IL-10 production by CD4+CD25− T cells (Beiting et al., 2007). In a separate study, anti-CTLA-4 antibody administration to infected mice did reduce muscle larval numbers (Furze et al., 2006), indicating that perhaps a CD25−CTLA‐4+IL-10+ Tr1-like population is in play.
Trichuris muris is, like H. polygyrus, a well-studied intestinal model and inhabits the cecum of mice (Cliffe and Grencis, 2004). Interestingly, different strains of T. muris survive for varying times in vivo, and the longest-lived isolate elicits the strongest Foxp3+ Treg response (D'Elia et al., 2009). This parasite also elicits a population of intestinal Foxp3− IL-35-producing suppressive T cells (Tr35 cells), which in vitro differentiate under the influence of IL-10 and IL-35 (Collison et al., 2010). An important role in limiting pathology has also been established in these infections, as anti-CD25- and anti-GITR-treated mice develop aggravated pathology, as well as lower worm numbers in the case of anti-GITR treatment (D'Elia et al., 2009).
3.4.3. Schistosomes
Schistosomes are trematode worms causing schistosomiasis (Bilharzia) in some 220 million people worldwide. Like other helminths, they form long-lived, chronic infections which are associated with a degree of parasite-specific immune suppression, deviation (e.g., to IgG4 in humans), and susceptibility to repeated reinfection. As with human filariasis (see Section 3.4.1 above), peripheral T cells from infected patients often fail to respond to parasite antigen challenge in vitro (Grogan et al., 1998), and two reports have charted Treg activity in human schistosomiasis. In Schistosoma mansoni (in which adult worms live in the mesenteric vasculature), CD4+CD25high T cell frequencies were inversely proportional to effector phenotype (CD25midHLA-DR+) cells, but not to parasite intensity; however, curative chemotherapy significantly reduced the frequency of Tregs using these markers (Watanabe et al., 2007). More recently, analysis of the urogenital parasite S. haematobium found a significant positive correlation between CD4dimCD25highCD127lowFoxp3+ T cells and parasite intensity in children at the age at which they are still susceptible to reinfection; interestingly in adults, the reverse was the case (Nausch et al., 2011).
More information is available from mice, for which S. mansoni is infective. The infection follows two phases, a Th1-dominated maturation period, during which skin-penetrating cercaria migrate to the lung and then the hepatic portal vasculature; and a later Th2-dominated stage which is provoked by egg release from adult worms (Pearce and MacDonald, 2002). Because eggs become lodged in the liver, this later stage is accompanied by severe granulomatous immunopathology that is moderated by IL-10-producing T cells, both Th2 and Treg, which become numerous at this time (Hesse et al., 2004, McKee and Pearce, 2004). While CD25+Foxp3+ Tregs are not the major contributor of IL-10, they dampen Th2 responses, with anti-CD25 depletion resulting in significantly enhanced IL-4 production (Baumgart et al., 2006). Moreover, anti-CD25 treatment increased egg destruction and aggravated liver pathology around the eggs, demonstrating a beneficial role for Tregs at this stage of the infection (Layland et al., 2007). Subsequent studies confirmed the Foxp3+ phenotype of Tregs surrounding the site of inflammation (Layland et al., 2010), and the interaction was more formally demonstrated by retroviral expression of Foxp3 in mice resulting in the suppression of liver granuloma formation (Singh et al., 2005).
Schistosome eggs produce a number of immunologically active substances, including an IL-4-inducing protein (IPSE or α-1 (Schramm et al., 2007)) and a ribonuclease, ω-1 (Everts et al., 2009, Steinfelder et al., 2009). While both can drive Th2 responses in vivo and in vitro, only ω-1 can induce Foxp3 in T cells, requiring the presence of DCs, TGF-β, and retinoic acid (Zaccone et al., 2011). Unrelated to this protein, an HSP60-derived peptide SJMHE1 from Schistosoma japonicum was shown to expand CD4+CD25+Foxp3+ T cell populations in vivo and in vitro, with such cells able to inhibit delayed-type hypersensitivity on transfer to mice 1 day prior to allergen sensitization (Wang et al., 2009).
3.5. Fungi
Fungal pathogens are found in a variety of niches and in different developmental forms; in addition, a number are commensals which can adapt opportunistically to immunodeficiency. One such example is oropharyngeal Candida albicans infection, immunity to which is compromised by the CD4+CD25+ IL10-producing Treg population that is deficient in TLR2−/− mice, and which when depleted in vivo with anti-CD25 antibody, results in improved resistance to infection (Netea et al., 2004). Interestingly, as observed for L. major infection (Belkaid et al., 2002), the CD4+CD25+ subset was also required to generate normal protective memory responses to infection, establishing a “protective tolerance” that restrains pathology while allowing a form of commensalism to persist (Montagnoli et al., 2002). Recent work has elucidated a fascinating dynamic in which, when confronted with an acute infection, Tregs promote Th17 responses to C. albicans, while at later time points act to restrain the same effector population from mediating inflammatory bowel disease (Pandiyan et al., 2011); thus early CD25 depletion resulted in diminished Th17 immunity and increased fungal burden, whereas transfer of CD25+ Tregs could prevent colitis in infected RAG mice caused by in vitro polarized Th17 cells.
Paracoccidioides brasiliensis is regarded as the most prevalent primary fungal pathogen of Latin America and is the causative agent of a systemic granulomatous disease in the host. CD4+CD25+CD103+CTLA-4+Foxp3+GITR+ Tregs are found in the lesions of infected patients (Cavassani et al., 2006). In a mouse model, adoptive transfer of CD4+CD25+ but not CD4+CD25− T cells from infected mice increased the fungal load in recipients, except in a CCR5−/− setting (Moreira et al., 2008). Mice lacking CCR5 had a reduced number of Tregs in the lungs, and did not exhibit suppressed T cell proliferation ex vivo following a more contained infection. CCR5−/− mice may have a generalized defect in egress of thymic Tregs, as also demonstrated by their greater resistance to another fungal pathogen, Histoplasma capsulatum (Kroetz and Deepe, 2011). The correlation between Treg activity and extent of fungal infection did not hold, however, in TLR4-deficient mice, which showed higher Foxp3+ Treg numbers and yet were able to control P. brasiliensis infection more efficiently (Loures et al., 2010); whether this reflects an early stimulatory role by Tregs as observed for C. albicans has not yet been tested.
Aspergillus fumigatus is a further fungal pathogen and a causative agent of airway hypersensitivity and allergy. Exposure of mice to Aspergillus conidia resulted in the early expansion, activation, and recruitment of CD4+CD25+ Tregs, which correlated with decreases in inflammation at this time point. Depletion of natural Tregs using cyclophosphamide or anti-CD25 reduced CD4+CD25+ T cell numbers, exacerbated inflammation, and decreased the survival of infected wild-type mice (Montagnoli et al., 2006). This work also highlighted the role of IDO, as well as IL-10 and CTLA-4, as a mediator feeding back to tolerize DCs and forestall hypersensitivity in the later stages of infection.
4. Treg Activation—a Common Immune-Evasion Strategy Achieved Through Diverse Routes
The evidence from the many and diverse infectious agents reviewed above is that Tregs often suppress protective immunity: examples can be given from the retrovirus (FV) model, through malaria to the helminth worms. However, where pathogens are reliant on Treg activity, this offers a therapeutic route to eliminate infection, which has been reproduced in a number of these same models (Hisaeda et al., 2004). Hence, identifying the pathway(s) for Treg activation (Figure 3.1 ) is crucial for future intervention strategies.
Figure 3.1.
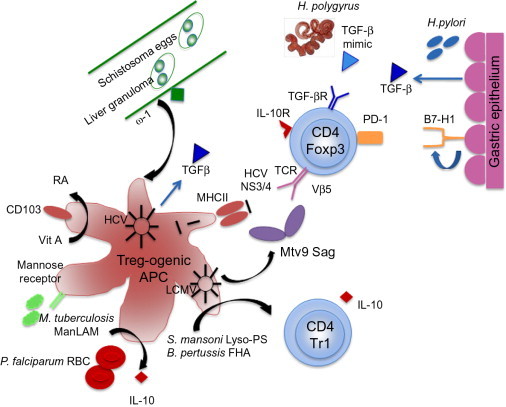
Pathways of Treg induction and activation in infection.
Treg activation can benefit both host and pathogen however. Most frequently, this is evident at the level of dampening pathology. T cell-mediated responses to HSV in the corneal stroma are a frequent cause of human blindness. Depletion of natural regulatory T cells was shown to enhance lesion formation and keratitis following HSV infection by impairing antiviral immunity and T cell migration to lesion sites (Suvas et al., 2004). Similarly, Tregs restrain intestinal pathology in infections with T. muris; in this system, anti-GITR antibody results in lower worm burdens, but incurs more intense gut pathology (D'Elia et al., 2009). In the long term (especially in chronic human infections), the key to a healthy status is the balance between controlling infection and limiting pathology—maintaining a recalibrated homeostasis in chronic infection.
4.1. Direct conversion of T cells into Tregs
A few examples are now established in which pathogen products directly activate, promote, or induce Tregs; clearly these are important proof-of-principle that the expansion of Tregs in vivo is not purely a homeostatic response that accompanies every effector expansion. In these instances, at least, we can surmise that pathogens have evolved to stimulate and exploit the host's down-modulatory Treg populations.
A recent example is the induction of host Tregs by H. polygyrus, which secretes a TGF-β-like mimic that activates the TGF-β signaling pathway (Grainger et al., 2010). H. polygyrus adult worm excretory-secretory products (HES), when added to naive Foxp3− murine T cells together with TCR ligation, induce de novo Foxp3. As this fails to occur in cells expressing the T cell-specific dominant negative TGF-β-RII, the interaction is directly between HES and the T cells in question. Foxp3+ T cells, whether induced by HES or mammalian TGF-β, are equally able to suppress airway allergy in recipient mice (Grainger et al., 2010). A similar activity was also found in secreted products of the related sheep parasite Teladorsagia circumcincta, indicating that some helminth parasites have evolved to exploit a key immunosuppressive pathway of their host. The helminth TGF-β mimic may be the biological equivalent of viral cytokine-like molecules, such as the EBV IL-10 homolog and other examples summarized elsewhere (Tortorella et al., 2000).
A very different mechanism is employed by Streptococcus pneumoniae which elaborates a zwitterionic polysaccharide able to directly cross-link the TCR of CD8+ T cells and switch them into a regulatory phenotype (Mertens et al., 2009). As with the H. polygyrus HES, this is a process which can by-pass any requirement for an APC population.
4.2. Induction of Tregs via DCs
Treg cells, like all T cells, require cognate APC interactions for their activation, and DCs are the major cell type responsible; consequently, it is not surprising that in the majority of systems studied, Treg generation involves the DC population. This is most clearly demonstrated where individual molecular components from pathogens are able to drive Treg differentiation through a DC pathway: for example, the FHA from B. pertussis modifies DC interactions with naive antigen-specific T cells, polarizing them to an IL-10-producing phenotype (McGuirk et al., 2002); similarly, the lysophosphatidylserine molecule from S. mansoni acts through DCs to induce IL-10+ Tr1 cells (van der Kleij et al., 2002). In the former case, the FHA binds TLR4 (Higgins et al., 2003) while the lyso-PS ligates TLR2, highlighting the regulatory face of the Toll ligand family as discussed in a following section.
Most recently, the induction of Foxp3 in naive T cells by DCs exposed to the S. mansoni egg antigen ω-1 has been described (Zaccone et al., 2011), providing a mechanistic pathway that may be followed in vivo when DCs from S. japonicum-infected mice promote Foxp3+ and IL-10+ Treg in vitro, inhibiting airway allergy in the process (Liu et al., 2011). Likewise, the Ac-TMP-1 protein released by adult Ancylostoma caninum hookworm skews DCs to induce both CD4+ and CD8+CD25+Foxp3+ T cells; both subsets expressed IL-10 while the CD4+ Tregs also produced TGF-β (Cuéllar et al., 2009). In addition, Anisakis simplex (a nematode from marine mammals), elaborates a homologue of MIF (macrophage migration inhibitory factor) which when injected into mice elicits increased numbers of Foxp3+ T cells (Park et al., 2009).
Many further examples of Treg generation by DCs in infectious settings employ whole organisms, or their secreted products. For example, bone marrow DCs exposed to live H. pylori bacteria are able to drive de novo induction of Tregs in vitro (Zhang et al., 2010), and expand Foxp3+ Tregs when adoptively transferred to mice shortly before infection (Kao et al., 2010). In a different system, secreted products of the nematode H. polygyrus were used to pulse DCs which preferentially induced functional CD4+CD25+IL-10+Foxp3− Tregs (Segura et al., 2007). In vivo, expansion of pro-regulatory DC subsets occurs, such as the predominant CD11cloCD103− DC subset in the mesenteric lymph nodes of H. polygyrus-infected mice which are potent inducers of Foxp3 in naive murine T cells (Smith et al., 2011).
In each of these systems, the mechanisms by which DCs induce Tregs are similar to those established in model systems in the absence of infection: TGF-β, IL-10, and retinoic acid are all implicated. For example, the induction of Foxp3+ Tregs by malaria-infected red blood cells is dependent on both TGF-β and IL-10 (Scholzen et al., 2009). Similarly, the Treg-inducing ability of Schistosome egg antigen and its component ω-1 requires target DCs to produce TGF-β (Zaccone et al., 2011).
4.3. Bystander induction by other cell types
Although not involving DCs, the strategy mounted by HCV to induce Tregs is similar to that described for several other pathogens above, except that infected human hepatocytes are induced to express TGF-β, which, using well-characterized pathways, is able to drive expression of Foxp3 and other regulatory markers (including CD25, CTLA-4, and LAP) in human CD4+ T cells (Hall et al., 2010). GECs are similarly stimulated by H. pylori to express TGF-β and B7H-1 to evoke Foxp3+ Tregs (Beswick et al., 2011).
In S. mansoni infection, regulatory B cells are able to induce Foxp3+ Tregs to infiltrate airways, suppressing allergic inflammation (Amu et al., 2010). B cells are also implicated in Helicobacter felix infection, in which TLR2 activation of B cells is required to induce IL-10-producing CD4+CD25+ regulatory T cells and control immunopathology (Sayi et al., 2011).
4.4. TLRs in Treg activation
Most pathogens are initially recognized by the innate immune system through one or more Toll-like receptors (TLRs), ligating to archetypal molecular species characteristic of particular microbe classes, and expressed by DCs and other sentinels of immunity (Medzhitov, 2007). Adaptive immune lymphocytes, including T cells, may also express TLRs, raising the question of whether in infection Tregs are either stimulated or inhibited by interacting with TLR ligands.
In general, TLR stimulation of DCs and other APCs is considered to be strongly proinflammatory and likely to overcome homeostatic Treg control. However, some pathogen TLR ligands (such as the TLR2-binding S. haematobium lysophosphatidylserine (van der Kleij et al., 2002)) drive human DCs to induce IL-10-secreting Tr1 cells, while S. typhimurium LPS-driven TLR4 ligation was reported to promote proliferation and suppressive activity of murine CD4+CD25+ T cells (Caramalho et al., 2003). Subsequently, numerous studies into pathogen-derived pro-regulatory TLR stimulation have been reported with some clear instances of direct effects on Tregs, rather than pathways routed through APC populations (Himmel et al., 2008, van Maren et al., 2008).
TLR2 emerges as a significant enhancer of Treg activity in the steady state (Sutmuller et al., 2006) as well as in the context of several infections, as indicated by the greater resistance of TLR2−/− mice to C. albicans (Bellocchio et al., 2004, Netea et al., 2004) and Yersinia enterocolitica (Sing et al., 2002). Likewise, in infections with the helminth S. mansoni, TLR2−/− mice, in which CD4+CD25+ Tregs do not expand, suffer aggravated liver pathology which can be rescued by transfer of wild-type schistosome-primed CD4+CD25+ T cells (Layland et al., 2007). An increase in Tregs occurred in TLR4- but not in TLR2-deficient mice following administration of the HSP-60-derived S. japonicum peptide SJMHE1, as bone marrow-derived macrophage and dendritic cells from TLR2−/− mice, but not wild-type mice, primed in vitro or in vivo were unable to induce Tregs in vitro (Wang et al., 2009).
Treg proliferation can be promoted by TCR signaling and TLR2 ligation with a synthetic agonist; however, in C. albicans infection, the effect of TLR2 ligation is to reverse CD4+CD25+ Treg suppression of anti-fungal responses and allow fungal outgrowth (Netea et al., 2004). Hence, TLR2+ Tregs transferred into TLR2−/− mice promote a 100-fold rise in C. albicans infection, which is prevented in the presence of the TLR2 ligand Pam-3-Cys (Sutmuller et al., 2006). It is important to note that later studies indicate that the effects of TLR2 expression on Tregs are largely to promote proliferation independently of APCs (Chen et al., 2009b), and that loss of suppressive activity may be relatively transient (Liu et al., 2006) or circumscribed in effect (Oberg et al., 2010, van Maren et al., 2011). Moreover, other TLR2 ligands can enhance Treg function (Zanin-Zhorov et al., 2006). The apparently contradictory role of TLR2 may be explained by its ability to heterodimerize with different partners (TLR1, TLR6, and TLR10), and the property of some pathogen-derived ligands to selectively stimulate an immunosuppressive, rather than an activating, signal (Depaolo et al., 2008).
Recently, TLR2 activation of B cells has been shown to be critical for microbial Treg induction by the PSA of the commensal bacterium B. fragilis (Round et al., 2011). These authors showed that wild-type bacteria, but not PSA-deficient organisms, stimulated IL-10-producing Foxp3+ Tregs in wild-type, but not in TLR2-deficient mice. Since induction was intact in both TLR1- and TLR6-deficient hosts, B. fragilis signaling does not appear to be mediated by heterodimers with either of these components, while the TLR2-dependent expression of IL-10 by purified T cells in the absence of APCs argues that the bacterial product acts directly on the T cell without requiring an intermediary population.
Other TLRs show similar involvement in both enhancement and inhibition of Tregs, depending upon the setting. Bacterial flagellin binding through TLR5, for example, was reported to promote human Tregs (Crellin et al., 2005). In other systems, the reversal of Treg suppression by potent synthetic ligands for TLR7 (Hackl et al., 2011, Van et al., 2011) and TLR8 (Peng et al., 2005) does not necessarily reflect the potential for more subtle pathogen-derived molecules to activate Tregs in different ways, and this will clearly be a fertile area for future research.
5. Antigen Specificity of Natural and Adaptive Tregs in Infection
Two central issues in regulatory T cell biology are the division between thymic (“natural”) and induced (“adaptive”) Tregs, and the nature of the antigen specificity of Tregs functional in any particular setting (Bluestone and Abbas, 2003, Rudensky, 2011). Although natural Tregs will have been selected in the thymus for self-reactivity, this does not preclude them recognizing exogenous ligands through cross-reactivity, mimicry, or dual specificity; conversely, induced Tregs in infection are not necessarily pathogen-specific, but may carry a third-party specificity having been activated as bystanders in a pro-regulatory cytokine environment. Indeed, molecular analysis of TCR usage among natural Tregs argues that they do not have a self-restricted repertoire (Pacholczyk et al., 2007). In any event, specificity is not essential for suppressive function, as once a regulatory cell has been triggered, their production of downregulatory cytokines and ability to tolerize DCs allow them to modify systemic reactions to bystander antigens, whether of pathogenic or nonpathogenic (e.g., allergen) origin.
A number of studies have addressed whether Foxp3+ T cell responses observed in infection represent stimulation of pre-existing natural/thymic Tregs or conversion of naive/effector T cells into adaptive Tregs in the periphery. De novo induction of Tregs did not occur among Foxp3-negative OVA-transgenic (OT-II) T cells adoptively transferred into mice prior to infection with Ova-expressing L. monocytogenes (Fontenot et al., 2005). In the L. major system, co-transfer of allotype-marked CD25+ and CD25− T cells demonstrated that Foxp3 expression remained entirely within the CD25+ population, and conversion from naive/effector cells did not occur (Suffia et al., 2006), while in L. sigmodontis infections, BrdU labeling showed early natural Treg proliferation in response to infection (Taylor et al., 2009). However, in H. polygyrus infections, conversion into induced Tregs was demonstrated in Foxp3-negative T cells from a Foxp3–GFPxDO11.10 F1 mouse when transferred to an infected wild-type recipient. When mice were given oral ovalbumin, up to 50% switched on Foxp3 expression (Grainger et al., 2010). This discrepancy may lie in the specific localization of the pathogen following infection in these models, as peripheral induction of Tregs has been shown to occur most efficiently in gut-associated lymphoid tissue (Sun et al., 2007). This highly regulated site is populated in the steady state by tolerogenic dendritic cell populations producing high levels of TGF-β (Coombes et al., 2007) or by pro-regulatory DCs in helminth infection (Smith et al., 2011).
A closely related issue is whether pathogen persistence depends on either or both thymic and/or induced Tregs. In L. sigmodontis infection, prior depletion of existing (thymic and noncognate induced) Tregs (with anti-CD25 antibody) amplified Th2 responses and reduced worm survival (Taylor et al., 2009). Remarkably, the effects of natural Treg depletion were not evident until some 60 days post-infection; this has been attributed to the early rapid proliferation of pre-existing Tregs on infection, which are able then to dominate the course of the ensuing response (Taylor et al., 2009).
An alternative approach is to identify whether Tregs specific for exogenous, pathogen-derived epitopes are generated in infection. For example, it was noted that following chronic LCMV infection, there is selective expansion of a Vβ5+Foxp3+ Treg population (Punkosdy et al., 2011). However, these Tregs evolved from a pre-existing pool and are found only in mouse strains carrying an endogenous Mtv9 superantigen-encoding provirus. Hence, LCMV represents a potentially unique case in which a viral Treg epitope is also encoded in the host genome, with specific Treg activation resulting in chronic infection.
A different transgenic TCR system involved the P25 TCR expressed by CD4+ T cells specific for an immunodominant M. tuberculosis peptide Ag85B240–254 presented by the class II molecule I-Ab. When P25 transgenic T cells (negative for Foxp3–GFP) were transferred to mice prior to infection, there was no conversion to Foxp3 expression despite an overall increase in the endogenous Treg population (Shafiani et al., 2010); hence the predominant Treg type in this infection could be considered natural Tregs. Interestingly, when donor P25 TCR transgenic mice were analyzed in detail, a population of splenic P25+Foxp3+ Tregs was observed, representing “dual specificity” natural Tregs. Purified P25+ Tregs from uninfected mice were then transferred to naive recipients and shown to delay the priming of effector T cells following Mtb infection (Shafiani et al., 2010). Hence, immunity to infection is impaired by Tregs which are both naturally arising and pathogen-specific. In L. major infection, it was previously reported that natural Tregs respond specifically to parasite antigen, as shown by propagation through repeated antigen stimulation (Suffia et al., 2006). As this was a nontransgenic, polyclonal TCR population, this observation implies that the “dual specificity” natural Tregs may be found more extensively than in the case of P25 alone.
Despite these illustrations of natural Treg involvement in microbial infections, there are few clear examples of the specificity of peripherally induced Tregs in infection setting. In RSV-infected mice, preferential binding of an MHC class II tetramer containing a defined epitope (M209) was observed to CD4+Foxp3+ Tregs (Liu et al., 2009); these cells downregulate virus-induced pathology when transferred to infected recipients (Liu et al., 2010), but as they emerge in a polyclonal environment, it is not established whether they represent dual specificity natural Tregs or have been selectively induced by infection. If Helios proves to be an authentic transcription factor for natural/thymic Tregs (Thornton et al., 2010), staining for this molecule would be a valuable adjunct to these analyses.
In addition to specificity, there remains a major unresolved question of whether natural or induced Tregs are more important in the dampening of immunity and control of pathogenesis. While the answer to this question will depend on the infection setting, the tissue site, and the kinetics of the response, it may also underestimate the complexity of immune regulation in vivo: in most cases, both types of Treg are likely to be necessary. An interesting perspective has emerged from studies in the filarial nematode L. sigmodontis indicating that early natural Tregs act to limit responsiveness (to the detriment of long-term protective immunity), before a second wave of induced Tregs come into play (Taylor et al., 2009).
6. Tregs and the Hygiene Hypothesis
One of the most significant implications of regulatory T cell activation by infectious agents may be the downregulation of immune responsiveness to other coincident antigens. The impact of this modulation may be either beneficial to the host, in suppressing responses to allergens, autoantigens, and commensals (Maizels, 2005), or detrimental, in compromising immunity to life-threatening infections such as malaria (Su et al., 2005).
Robust experimental and epidemiological evidence that infections can protect against allergies and other immunological over-reactions has been established across the board for infectious organisms from helminths (Cooper et al., 2003, Fleming and Cook, 2006, Maizels, 2005, Smits and Yazdanbakhsh, 2007), mycobacteria (Zuany-Amorim et al., 2002), and viruses (Filippi et al., 2009, Richer et al., 2008), extending also to probiotic and commensal bacteria (Feleszko et al., 2007, Karimi et al., 2009, Repa et al., 2003). It should be noted, however, that the same infections can result in poorer responses to childhood vaccination (Cooper et al., 1998, Cooper et al., 2001), as well as to co-infections with pathogens such as malaria (Hartgers et al., 2009).
The close link between helminth infections, Tregs, and suppression is one of the most active research areas in this regard (Maizels and Wiedermann, 2009). In mouse models, H. polygyrus was originally shown to generate CD4+CD25+ Tregs which, on transfer to allergen-sensitized recipients, protected them from airway allergy (Wilson et al., 2005). Similarly, L. sigmodontis nematode infection has been shown to inhibit airway allergy to a bystander antigen (Dittrich et al., 2008) and block development of autoimmune disease in diabetes-prone NOD mice, alongside expansion of both Th2 and CD4+CD25+Foxp3+ Treg cells (Hübner et al., 2009). These findings with helminth infections are remarkably similar to reports of viral infections such as LCMV and Coxsackie virus, involving the expansion of CD4+CD25+ T cells producing TGF-β and suppression of allergy and diabetes (Diana et al., 2011, Filippi et al., 2011).
Currently, several avenues are being explored for the therapeutic treatment of immunopathological conditions with certain parasite species associated with regulatory effects, such as the porcine intestinal worm Trichuris suis which has been reported to benefit patients with inflammatory bowel diseases (Summers et al., 2005, Summers et al., 2006). A further report on the remission of disease in MS patients with adventitious intestinal helminthiases (Correale and Farez, 2007) correlated these benefits of infection with enhanced Treg and TGF-β levels. These findings, together with an intriguing case report of an ulcerative colitis patient (Broadhurst et al., 2010) and reduced diabetes incidence in filariasis patients (Aravindhan et al., 2010), have heightened interest in helminth therapy of severe immunological dysfunction (Fleming and Fabry, 2007). However, not all trials utilizing this strategy have had positive outcomes (Bager et al., 2010), and it remains to be demonstrated that any beneficial effects in humans of helminth therapy are mediated by Tregs rather than parallel regulatory pathways which are likely to be activated by the same infections.
7. Conclusion
Any overview of the impact of Tregs across a diverse range of infectious organisms will inevitably highlight the individual features of each system, with unique niches, dynamics, and molecular interactions. Nevertheless, it is clear that Tregs are involved in the outcome of nearly every infectious episode studied, not necessarily in the central role, but invariably modifying the scale and mode of immunity. Moreover, in many cases from viruses to worms, their intervention is pivotal in differentiating healthy from pathogenic outcomes; we can now learn from these examples, and understand the exceptions, to design new strategies for the control of diseases.
Targeting Tregs for the therapy of infectious diseases is an attractive option in settings where the host mounts an immune response of low pathogenic potential, but which normally is muted and cannot attain the level or intensity required to eliminate the pathogen. More caution may be required if Tregs shelter the host from pathogenesis or contribute to immunity in nonintuitive ways such as facilitating tissue access for effector cells or maintaining low-level persistent antigen for immunological memory. As fundamental understanding of Treg function deepens, more precise targeting may become feasible, for example, by preventing de novo induction of Tregs in infection, by blocking specific molecular interactions (such as CTLA-4), and by interfering with key molecules required for Treg migration, including CD103, CCR4, and CCR5 (Moreira et al., 2008, Sather et al., 2007).
A key issue that requires advancing in all the infection systems under consideration, is that of the breadth and antigen specificity of Treg populations in infection. If there is global activation of the natural Treg compartment (e.g., as a physiological response to major inflammatory reactions), then Treg targeting is likely to unleash an unacceptable level of immunopathology if not autoimmunity. However, if the key factor in infection is the activity of a selective subset of pathogen-specific Tregs (or a small subset of natural Tregs which also react to a pathogen determinant), these could be ablated in a more restricted fashion.
Vaccination will, of course, remain our primary strategy to eradicate infectious diseases. Two very interesting perspectives on vaccination emerge from our survey of Tregs in infection. Firstly, the efficacy of vaccines against the major microbial infections is compromised in children harboring common helminth infections, most likely due to the higher level of Tregs in those individuals; hence anthelmintic and/or Treg-reducing interventions may be necessary if current vaccines are to achieve further effects in populations within endemic areas. Secondly, the question is raised of whether vaccines should be fine-tuned to minimize Treg activation. While empirically we have developed adjuvants which stimulate effector immunity, less consideration has been given to whether new vaccines (particularly to parasitic organisms in which vaccines have been notoriously inefficient) should be purged of Treg-stimulating specificities, or even of epitopes which stimulate pathogenic rather than protective effector responses.
Finally, we continue to learn much from those most accomplished immunologists, the successful organisms which can establish themselves in the human body. The complexity of the regulatory network in most infections remains to be defined, but will surely reveal many critically important features of the sequence and hierarchy through which immune suppression is established in vivo. In addition, at a molecular level, there is every expectation that new “drugs from bugs” will be developed that specifically enhance Treg development and activity, which should prove invaluable in the treatment of noninfectious immunopathologies such as allergy and autoimmunity.
Acknowledgments
The authors thank the Wellcome Trust for Programme Grant support.
References
- Aandahl E.M., Michaelsson J., Moretto W.J., Hecht F.M., Nixon D.F. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre M.L., Frauwirth K.A., Thompson C.B. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- Allen J.E., Maizels R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- Amante F.H., Stanley A.C., Randall L.M., Zhou Y., Haque A., McSweeney K., Waters A.P., Janse C.J., Good M.F., Hill G.R., Engwerda C.R. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am. J. Pathol. 2007;171:548–559. doi: 10.2353/ajpath.2007.061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amu S., Saunders S.P., Kronenberg M., Mangan N.E., Atzberger A., Fallon P.G. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 2010;125:1114–1124. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Anderson C.F., Oukka M., Kuchroo V.J., Sacks D. CD4+CD25–Foxp3– Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C.F., Lira R., Kamhawi S., Belkaid Y., Wynn T.A., Sacks D. IL-10 and TGF-β control the establishment of persistent and transmissible infections produced by Leishmania tropica in C57BL/6 mice. J. Immunol. 2008;180:4090–4097. doi: 10.4049/jimmunol.180.6.4090. [DOI] [PubMed] [Google Scholar]
- Andersson J., Boasso A., Nilsson J., Zhang R., Shire N.J., Lindback S., Shearer G.M., Chougnet C.A. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- Anghelina D., Zhao J., Trandem K., Perlman S. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology. 2009;385:358–367. doi: 10.1016/j.virol.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes I., Tolaini M., Kissenpfennig A., Iwashiro M., Kuribayashi K., Malissen B., Hasenkrug K., Kassiotis G. Retrovirus-specificity of regulatory T cells is neither present nor required in preventing retrovirus-induced bone marrow immune pathology. Immunity. 2008;29:782–794. doi: 10.1016/j.immuni.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindhan V., Mohan V., Surendar J., Rao M.M., Ranjani H., Kumaraswami V., Nutman T.B., Babu S. Decreased prevalence of lymphatic filariasis among subjects with type-1 diabetes. Am. J. Trop. Med. Hyg. 2010;83:1336–1339. doi: 10.4269/ajtmh.2010.10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkwright P.D., David T.J. Intradermal administration of a killed Mycobacterium vaccae suspension (SRL 172) is associated with improvement in atopic dermatitis in children with moderate-to-severe disease. J. Allergy Clin. Immunol. 2001;107:531–534. doi: 10.1067/mai.2001.113081. [DOI] [PubMed] [Google Scholar]
- Arnold I.C., Dehzad N., Reuter S., Martin H., Becher B., Taube C., Müller A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J. Clin. Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseffa A., Gumy A., Launois P., MacDonald H.R., Louis J.A., Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 2002;169:3232–3241. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmadja A.K., Atkinson R., Sartono E., Partono F., Yazdanbakhsh M., Maizels R.M. Differential decline in filarial-specific IgG1, IgG4 and IgE antibodies following diethylcarbamazine chemotherapy of Brugia malayi infected patients. J. Infect. Dis. 1995;172:1567–1572. doi: 10.1093/infdis/172.6.1567. [DOI] [PubMed] [Google Scholar]
- Babu S., Blauvelt C.P., Kumaraswami V., Nutman T.B. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: Implications for parasite persistence. J. Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- Babu S., Bhat S.Q., Kumar N.P., Jayantasri S., Rukmani S., Kumaran P., Gopi P.G., Kolappan C., Kumaraswami V., Nutman T.B. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J. Infect. Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu S., Bhat S.Q., Pavan Kumar N., Lipira A.B., Kumar S., Karthik C., Kumaraswami V., Nutman T.B. Filarial lymphedema is characterized by antigen-specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl. Trop. Dis. 2009;3:e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Bager P., Arnved J., Rønborg S., Wohlfahrt J., Poulsen L.K., Westergaard T., Petersen H.W., Kristensen B., Thamsborg S., Roepstorff A., Kapel C., Melbye M. Trichuris suis ova therapy for allergic rhinitis: A randomized, double-blind, placebo-controlled clinical trial. J. Allergy Clin. Immunol. 2010;125:123–130. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Baker C.A., Clark R., Ventura F., Jones N.G., Guzman D., Bangsberg D.R., Cao H. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin. Exp. Immunol. 2007;147:533–539. doi: 10.1111/j.1365-2249.2006.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza L., Salmen S., Goncalves L., Colmenares M., Peterson D., Montes H., Cartagirone R., Gutierrez Mdel C., Berrueta L. Antigen-induced regulatory T cells in HBV chronically infected patients. Virology. 2007;368:41–49. doi: 10.1016/j.virol.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Baumgart M., Tomkins F., Leng J., Hesse M. Naturally-occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 2006;176:5374–5387. doi: 10.4049/jimmunol.176.9.5374. [DOI] [PubMed] [Google Scholar]
- Becker C., Taube C., Bopp T., Michel K., Kubach J., Reuter S., Dehzad N., Neurath M.F., Reifenberg K., Schneider F.J., Schmitt E., Jonuleit H. Protection from graft-versus-host disease by HIV-1 envelope protein gp120-mediated activation of human CD4+CD25+ regulatory T cells. Blood. 2009;114:1263–1269. doi: 10.1182/blood-2009-02-206730. [DOI] [PubMed] [Google Scholar]
- Beilharz M.W., Sammels L.M., Paun A., Shaw K., van Eeden P., Watson M.W., Ashdown M.L. Timed ablation of regulatory CD4+ T cells can prevent murine AIDS progression. J. Immunol. 2004;172:4917–4925. doi: 10.4049/jimmunol.172.8.4917. [DOI] [PubMed] [Google Scholar]
- Beiting D.P., Gagliardo L.F., Hesse M., Bliss S.K., Meskill D., Appleton J.A. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-β. J. Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Hoffmann K.F., Mendez S., Kamhawi S., Udey M.C., Wynn T.A., Sacks D.L. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Piccirillo C.A., Mendez S., Shevach E.M., Sacks D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Bellocchio S., Montagnoli C., Bozza S., Gaziano R., Rossi G., Mambula S.S., Vecchi A., Mantovani A., Levitz S.M., Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- Berretta F., St-Pierre J., Piccirillo C.A., Stevenson M.M. IL-2 contributes to maintaining a balance between CD4+Foxp3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection. J. Immunol. 2011;186:4862–4871. doi: 10.4049/jimmunol.1003777. [DOI] [PubMed] [Google Scholar]
- Beswick E.J., Pinchuk I.V., Das S., Powell D.W., Reyes V.E. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+CD25+FoxP3+ regulatory T cells. Infect. Immun. 2007;75:4334–4341. doi: 10.1128/IAI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick E.J., Pinchuk I.V., Earley R.B., Schmitt D.A., Reyes V.E. Role of gastric epithelial cell-derived transforming growth factor beta in reduced CD4+ T cell proliferation and development of regulatory T cells during Helicobacter pylori infection. Infect. Immun. 2011;79:2737–2745. doi: 10.1128/IAI.01146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseye C., van der Sande M., Morgan W.D., Holder A.A., Pinder M., Ismaili J. Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4+CD25+forkhead box P3+ regulatory T cells and interleukin-10. Clin. Exp. Immunol. 2009;158:287–293. doi: 10.1111/j.1365-2249.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenhaus B., Klemm U., Eschbach M.L., Sparwasser T., Huehn J., Kuhl A.A., Loddenkemper C., Jacobs T., Breloer M. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J. Immunol. 2011;186:4295–4305. doi: 10.4049/jimmunol.1001920. [DOI] [PubMed] [Google Scholar]
- Bluestone J.A., Abbas A.K. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Boasso A., Vaccari M., Hryniewicz A., Fuchs D., Nacsa J., Cecchinato V., Andersson J., Franchini G., Shearer G.M., Chougnet C. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J. Virol. 2007;81:11593–11603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettler T., Spangenberg H.C., Neumann-Haefelin C., Panther E., Urbani S., Ferrari C., Blum H.E., von Weizsacker F., Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J. Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolacchi F., Sinistro A., Ciaprini C., Demin F., Capozzi M., Carducci F.C., Drapeau C.M., Rocchi G., Bergamini A. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin. Exp. Immunol. 2006;144:188–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourreau E., Ronet C., Darcissac E., Lise M.C., Sainte Marie D., Clity E., Tacchini-Cottier F., Couppie P., Launois P. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect. Immun. 2009;77:1465–1474. doi: 10.1128/IAI.01398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourreau E., Ronet C., Darsissac E., Lise M.C., Marie D.S., Clity E., Tacchini-Cottier F., Couppie P., Launois P. In leishmaniasis due to Leishmania guyanensis infection, distinct intralesional interleukin-10 and Foxp3 mRNA expression are associated with unresponsiveness to treatment. J. Infect. Dis. 2009;199:576–579. doi: 10.1086/596508. [DOI] [PubMed] [Google Scholar]
- Broadhurst M.J., Leung J.M., Kashyap V., McCune J.M., Mahadevan U., McKerrow J.H., Loke P. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med. 2010;2:60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- Brustoski K., Moller U., Kramer M., Hartgers F.C., Kremsner P.G., Krzych U., Luty A.J. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 2006;193:146–154. doi: 10.1086/498578. [DOI] [PubMed] [Google Scholar]
- Bueno L.L., Morais C.G., Araujo F.F., Gomes J.A., Correa-Oliveira R., Soares I.S., Lacerda M.V., Fujiwara R.T., Braga E.M. Plasmodium vivax: Induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 2010;5:e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büning J., Homann N., von Smolinski D., Borcherding F., Noack F., Stolte M., Kohl M., Lehnert H., Ludwig D. Helminths as governors of inflammatory bowel disease. Gut. 2008;57:1182–1183. doi: 10.1136/gut.2008.152355. [DOI] [PubMed] [Google Scholar]
- Cabrera R., Tu Z., Xu Y., Firpi R.J., Rosen H.R., Liu C., Nelson D.R. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- Cabrera G., Burzyn D., Mundinano J., Courreges M.C., Camicia G., Lorenzo D., Costa H., Ross S.R., Nepomnaschy I., Piazzon I. Early increases in superantigen-specific Foxp3+ regulatory T cells during mouse mammary tumor virus infection. J. Virol. 2008;82:7422–7431. doi: 10.1128/JVI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambos M., Bélanger B., Jacques A., Roulet A., Scorza T. Natural regulatory (CD4+CD25+FOXP+) T cells control the production of pro-inflammatory cytokines during Plasmodium chabaudi adami infection and do not contribute to immune evasion. Int. J. Parasitol. 2008;38:229–238. doi: 10.1016/j.ijpara.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Campanelli A.P., Roselino A.M., Cavassani K.A., Pereira M.S., Mortara R.A., Brodskyn C.I., Goncalves H.S., Belkaid Y., Barral-Netto M., Barral A., Silva J.S. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J. Infect. Dis. 2006;193:1313–1322. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- Caramalho I., Lopes-Carvalho T., Ostler D., Zelenay S., Haury M., Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavassani K.A., Campanelli A.P., Moreira A.P., Vancim J.O., Vitali L.M., Mamede R.C., Martinez R., Silva J.S. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J. Immunol. 2006;177:5811–5818. doi: 10.4049/jimmunol.177.9.5811. [DOI] [PubMed] [Google Scholar]
- Chase A.J., Yang H.C., Zhang H., Blankson J.N., Siliciano R.F. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J. Virol. 2008;82:8307–8315. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Howard O.M., Oppenheim J.J. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. J. Immunol. 2007;178:6123–6129. doi: 10.4049/jimmunol.178.10.6123. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhou B., Li M., Deng Q., Wu X., Le X., Wu C., Larmonier N., Zhang W., Zhang H., Wang H., Katsanis E. CD4+CD25+FoxP3+ regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 2007;123:50–59. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Chen G., Liu J., Wang Q.H., Wu Y., Feng H., Zheng W., Guo S.Y., Li D.M., Wang J.C., Cao Y.M. Effects of CD4+CD25+Foxp3+ regulatory T cells on early Plasmodium yoelii 17XL infection in BALB/c mice. Parasitology. 2009;136:1107–1120. doi: 10.1017/S0031182009990370. [DOI] [PubMed] [Google Scholar]
- Chen Q., Davidson T.S., Huter E.N., Shevach E.M. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J. Immunol. 2009;183:4458–4466. doi: 10.4049/jimmunol.0901465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen T., Volchkov P.Y., Chervonsky A.V., Rudensky A.Y. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J. Exp. Med. 2010;207:2323–2330. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe L.J., Grencis R.K. The Trichuris muris system: A paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- Cohen J.C., Varmus H.E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979;278:418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Collison L.W., Chaturvedi V., Henderson A.L., Giacomin P.R., Guy C., Bankoti J., Finkelstein D., Forbes K., Workman C.J., Brown S.A., Rehg J.E., Jones M.L. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.J., Espinel I., Paredes W., Guderian R.H., Nutman T.B. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: A possible role for interleukin-10. J. Infect. Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- Cooper P.J., Chico M.E., Losonsky G., Sandoval C., Espinel I., Sridhara R., Aguilar M., Guevara A., Guderian R.H., Levine M.M., Griffin G.E., Nutman T.B. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 2000;182:1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- Cooper P.J., Chico M., Sandoval C., Espinel I., Guevara A., Levine M.M., Griffin G.E., Nutman T.B. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.J., Chico M.E., Rodrigues L.C., Ordonez M., Strachan D., Griffin G.E., Nutman T.B. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J. Allergy Clin. Immunol. 2003;111:995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- Correale J., Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann. Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., de Souza J.B., Suffia I., Belkaid Y., Riley E.M. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J. Immunol. 2007;178:4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., Riley E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., Wilson M.S., Hafalla J.C., Belkaid Y., Kamanaka M., Flavell R.A., de Souza J.B., Riley E.M. IL-10 from CD4+CD25–Foxp3–CD127– adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin N.K., Garcia R.V., Hadisfar O., Allan S.E., Steiner T.S., Levings M.K. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- Cuéllar C., Wu W., Mendez S. The hookworm tissue inhibitor of metalloproteases (Ac-TMP-1) modifies dendritic cell function and induces generation of CD4 and CD8 suppressor T cells. PLoS Negl. Trop. Dis. 2009;3:e439. doi: 10.1371/journal.pntd.0000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., Mncube Z., Duraiswamy J. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- de Araujo F.F., Vitelli-Avelar D.M., Teixeira-Carvalho A., Antas P.R., Assis Silva Gomes J., Sathler-Avelar R., Otavio Costa Rocha M., Eloi-Santos S.M., Pinho R.T., Correa-Oliveira R., Martins-Filho O.A. Regulatory T cells phenotype in different clinical forms of Chagas' disease. PLoS Negl. Trop. Dis. 2011;5:e992. doi: 10.1371/journal.pntd.0000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia R., Behnke J.M., Bradley J.E., Else K.J. Regulatory T cells: A role in the control of helminth driven intestinal pathology and worm survival. J. Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaolo R.W., Tang F., Kim I., Han M., Levin N., Ciletti N., Lin A., Anderson D., Schneewind O., Jabri B. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–361. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana J., Brezar V., Beaudoin L., Dalod M., Mellor A., Tafuri A., von Herrath M., Boitard C., Mallone R., Lehuen A. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J. Exp. Med. 2011;208:729–745. doi: 10.1084/jem.20101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze K.K., Zelinskyy G., Gibbert K., Schimmer S., Francois S., Myers L., Sparwasser T., Hasenkrug K.J., Dittmer U. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc. Natl. Acad. Sci. USA. 2011;108:2420–2425. doi: 10.1073/pnas.1015148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer U., He H., Messer R.J., Schimmer S., Olbrich A.R.M., Ohlen C., Greenberg P.D., Stromnes I.M., Iwashiro M., Sakaguchi S., Evans L.H., Peterson K.E. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- Dittrich A.M., Erbacher A., Specht S., Diesner F., Krokowski M., Avagyan A., Stock P., Ahrens B., Hoffmann W.H., Hoerauf A., Hamelmann E. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J. Immunol. 2008;180:1792–1799. doi: 10.4049/jimmunol.180.3.1792. [DOI] [PubMed] [Google Scholar]
- Doetze A., Satoguina J., Burchard G., Rau T., Loliger C., Fleischer B., Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and transforming growth factor-β but not by a Th1 to Th2 shift. Int. Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- Ebinuma H., Nakamoto N., Li Y., Price D.A., Gostick E., Levine B.L., Tobias J., Kwok W.W., Chang K.M. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J. Virol. 2008;82:5043–5053. doi: 10.1128/JVI.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggena M.P., Barugahare B., Jones N., Okello M., Mutalya S., Kityo C., Mugyenyi P., Cao H. Depletion of regulatory T cells in HIV infection is associated with immune activation. J. Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- Elahi S., Dinges W.L., Lejarcegui N., Laing K.J., Collier A.C., Koelle D.M., McElrath M.J., Horton H. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med. 2011;17:989–995. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D.E., Setiawan T., Metwali A., Blum A., Urban J.F., Jr., Weinstock J.V. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- Enarsson K., Lundgren A., Kindlund B., Hermansson M., Roncador G., Banham A.H., Lundin B.S., Quiding-Jarbrink M. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin. Immunol. 2006;121:358–368. doi: 10.1016/j.clim.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ertelt J.M., Rowe J.H., Mysz M.A., Singh C., Roychowdhury M., Aguilera M.N., Way S.S. Foxp3+ regulatory T cells impede the priming of protective CD8+ T cells. J. Immunol. 2011;187:2569–2577. doi: 10.4049/jimmunol.1100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B., Perona-Wright G., Smits H.H., Hokke C.H., van der Ham A.J., Fitzsimmons C.M., Doenhoff M.J., van der Bosch J., Mohrs K., Haas H., Mohrs M., Yazdanbakhsh M. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleszko W., Jaworska J., Rha R.D., Steinhausen S., Avagyan A., Jaudszus A., Ahrens B., Groneberg D.A., Wahn U., Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin. Exp. Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- Filippi C.M., Estes E.A., Oldham J.E., von Herrath M.G. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J. Clin. Invest. 2009;119:1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi C.M., Ehrhardt K., Estes E.A., Larsson P., Oldham J.E., von Herrath M.G. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur. J. Immunol. 2011;41:1399–1409. doi: 10.1002/eji.200939841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney C.A.M., Taylor M.D., Wilson M.S., Maizels R.M. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney O.C., Nwakanma D., Conway D.J., Walther M., Riley E.M. Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur. J. Immunol. 2009;39:1288–1300. doi: 10.1002/eji.200839112. [DOI] [PubMed] [Google Scholar]
- Finney O.C., Riley E.M., Walther M. Regulatory T cells in malaria—friend or foe? Trends Immunol. 2010;31:63–70. doi: 10.1016/j.it.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Fleming J.O., Cook T.D. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67:2085–2086. doi: 10.1212/01.wnl.0000247663.40297.2d. [DOI] [PubMed] [Google Scholar]
- Fleming J., Fabry Z. The hygiene hypothesis and multiple sclerosis. Ann. Neurol. 2007;61:85–89. doi: 10.1002/ana.21092. [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Fulton R.B., Meyerholz D.K., Varga S.M. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 2010;185:2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furze R.C., Culley F.J., Selkirk M.E. Differential roles of the co-stimulatory molecules GITR and CTLA-4 in the immune response to Trichinella spiralis. Microbes Infect. 2006;8:2803–2810. doi: 10.1016/j.micinf.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Gall A., Horowitz A., Joof H., Natividad A., Tetteh K., Riley E., Bailey R.L., Mabey D.C., Holland M.J. Systemic effector and regulatory immune responses to chlamydial antigens in trachomatous trichiasis. Front Microbiol. 2011;2:10. doi: 10.3389/fmicb.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Mukhopadhyay D., Das N.K., Chaduvula M., Sadhu S., Chatterjee U., Rahman M., Goswami R.P., Guha S.K., Modak D., Mallik S., Gonju D. Enhanced lesional Foxp3 expression and peripheral anergic lymphocytes indicate a role for regulatory T cells in Indian post-kala-azar dermal leishmaniasis. J. Invest. Dermatol. 2010;130:1013–1022. doi: 10.1038/jid.2009.393. [DOI] [PubMed] [Google Scholar]
- García-Hernández M.H., Alvarado-Sánchez B., Calvo-Turrubiartes M.Z., Salgado-Bustamante M., Rodríguez-Pinal C.Y., Gámez-Lopez L.R., González-Amaro R., Portales-Pérez D.P. Regulatory T cells in children with intestinal parasite infection. Parasite Immunol. 2009;31:597–603. doi: 10.1111/j.1365-3024.2009.01149.x. [DOI] [PubMed] [Google Scholar]
- Garg A., Barnes P.F., Roy S., Quiroga M.F., Wu S., Garcia V.E., Krutzik S.R., Weis S.E., Vankayalapati R. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur. J. Immunol. 2008;38:459–469. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin M.A., Rasmussen J.P., Fontenot J.D., Vasta V., Manganiello V.C., Beavo J.A., Rudensky A.Y. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:936–940. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R.T., Oswald I.P., Hieny S., James S.L., Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur. J. Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- Ge Y.Y., Zhang L., Zhang G., Wu J.P., Tan M.J., Hu E., Liang Y.J., Wang Y. In pregnant mice, the infection of Toxoplasma gondii causes the decrease of CD4+CD25+-regulatory T cells. Parasite Immunol. 2008;30:471–481. doi: 10.1111/j.1365-3024.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- Geuking M.B., Cahenzli J., Lawson M.A., Ng D.C., Slack E., Hapfelmeier S., McCoy K.D., Macpherson A.J. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Gillan V., Devaney E. Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae. Infect. Immun. 2005;73:4034–4042. doi: 10.1128/IAI.73.7.4034-4042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves R.M., Salmazi K.C., Santos B.A., Bastos M.S., Rocha S.C., Boscardin S.B., Silber A.M., Kallas E.G., Ferreira M.U., Scopel K.K. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: Do different parasite species elicit similar host responses? Infect. Immun. 2010;78:4763–4772. doi: 10.1128/IAI.00578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J.R., Smith K.A., Hewitson J.P., McSorley H.J., Harcus Y., Filbey K.J., Finney C.A.M., Greenwood E.J.D., Knox D.P., Wilson M.S., Belkaid Y., Rudensky A.Y. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene B.M., Gbakima A.A., Albiuz E.J., Taylor H.R. Humoral and cellular immune responses to Onchocerca volvulus in humans. Rev. Infect. Dis. 1985;7:789–795. doi: 10.1093/clinids/7.6.789. [DOI] [PubMed] [Google Scholar]
- Grogan J.L., Kremsner P.G., Deelder A.M., Yazdanbakhsh M. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J. Infect. Dis. 1998;177:1433–1437. doi: 10.1086/517832. [DOI] [PubMed] [Google Scholar]
- Gruden-Movsesijan A., Ilic N., Mostarica-Stojkovic M., Stosic-Grujicic S., Milic M., Sofronic-Milosavljevic L. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol. 2010;32:450–459. doi: 10.1111/j.1365-3024.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- Guilliams M., Oldenhove G., Noel W., Herin M., Brys L., Loi P., Flamand V., Moser M., De Baetselier P., Beschin A. African trypanosomiasis: Naturally occurring regulatory T cells favor trypanotolerance by limiting pathology associated with sustained type 1 inflammation. J. Immunol. 2007;179:2748–2757. doi: 10.4049/jimmunol.179.5.2748. [DOI] [PubMed] [Google Scholar]
- Guilliams M., Bosschaerts T., Herin M., Hunig T., Loi P., Flamand V., De Baetselier P., Beschin A. Experimental expansion of the regulatory T cell population increases resistance to African trypanosomiasis. J. Infect. Dis. 2008;198:781–791. doi: 10.1086/590439. [DOI] [PubMed] [Google Scholar]
- Guyot-Revol V., Innes J.A., Hackforth S., Hinks T., Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 2006;173:803–810. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- Hackl D., Loschko J., Sparwasser T., Reindl W., Krug A.B. Activation of dendritic cells via TLR7 reduces Foxp3 expression and suppressive function in induced Tregs. Eur. J. Immunol. 2011;41:1334–1343. doi: 10.1002/eji.201041014. [DOI] [PubMed] [Google Scholar]
- Haeryfar S.M., DiPaolo R.J., Tscharke D.C., Bennink J.R., Yewdell J.W. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 2005;174:3344–3351. doi: 10.4049/jimmunol.174.6.3344. [DOI] [PubMed] [Google Scholar]
- Hall C.H.T., Kassel R., Tacke R.S., Hahn Y.S. HCV+ hepatocytes induce human regulatory CD4+ T cells through the production of TGF-β. PLoS One. 2010;5:e12154. doi: 10.1371/journal.pone.0012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D.S., Schofield L. Natural regulatory T cells in malaria: Host or parasite allies? PLoS Pathog. 2010;6:e1000771. doi: 10.1371/journal.ppat.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A., Best S.E., Amante F.H., Mustafah S., Desbarrieres L., de Labastida F., Sparwasser T., Hill G.R., Engwerda C.R. CD4+ natural regulatory T cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS Pathog. 2010;6:e1001221. doi: 10.1371/journal.ppat.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgers F.C., Obeng B.B., Kruize Y.C., Dijkhuis A., McCall M., Sauerwein R.W., Luty A.J., Boakye D.A., Yazdanbakhsh M. Responses to malarial antigens are altered in helminth-infected children. J. Infect. Dis. 2009;199:1528–1535. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- He H., Messer R.J., Sakaguchi S., Yang G., Robertson S.J., Hasenkrug K.J. Reduction of retrovirus-induced immunosuppression by in vivo modulation of T cells during acute infection. J. Virol. 2004;78:11641–11647. doi: 10.1128/JVI.78.21.11641-11647.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmby H., Grencis R.K. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur. J. Immunol. 2003;33:2382–2390. doi: 10.1002/eji.200324082. [DOI] [PubMed] [Google Scholar]
- Hesse M., Piccirillo C.A., Belkaid Y., Prufer J., Mentink-Kane M., Leusink M., Cheever A.W., Shevach E.M., Wynn T.A. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- Higgins S.C., Lavelle E.C., McCann C., Keogh B., McNeela E., Byrne P., O'Gorman B., Jarnicki A., McGuirk P., Mills K.H. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 2003;171:3119–3127. doi: 10.4049/jimmunol.171.6.3119. [DOI] [PubMed] [Google Scholar]
- Himmel M.E., Hardenberg G., Piccirillo C.A., Steiner T.S., Levings M.K. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisaeda H., Maekawa Y., Iwakawa D., Okada H., Himeno K., Kishihara K., Tsukumo S., Yasutomo K. Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nat. Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- Hisaeda H., Hamano S., Mitoma-Obata C., Tetsutani K., Imai T., Waldmann H., Himeno K., Yasutomo K. Resistance of regulatory T cells to glucocorticoid-induced [corrected] TNFR family-related protein (GITR) during Plasmodium yoelii infection. Eur. J. Immunol. 2005;35:3516–3524. doi: 10.1002/eji.200526073. [DOI] [PubMed] [Google Scholar]
- Hisaeda H., Tetsutani K., Imai T., Moriya C., Tu L., Hamano S., Duan X., Chou B., Ishida H., Aramaki A., Shen J., Ishii K.J. Malaria parasites require TLR9 signaling for immune evasion by activating regulatory T cells. J. Immunol. 2008;180:2496–2503. doi: 10.4049/jimmunol.180.4.2496. [DOI] [PubMed] [Google Scholar]
- Hoffmann K.F., Cheever A.W., Wynn T.A. IL-10 and the dangers of immune polarization: Excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- Hoffmann W.H., Petit G., Schulz-Key H., Taylor D.W., Bain O., Le Goff L. Litomosoides sigmodontis in mice: Reappraisal of an old model for filarial research. Parasitol. Today. 2000;16:387–389. doi: 10.1016/s0169-4758(00)01738-5. [DOI] [PubMed] [Google Scholar]
- Hori S., Carvalho T.L., Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: The great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner M.P., Stocker J.T., Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127:512–522. doi: 10.1111/j.1365-2567.2008.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehn J., Siegmund K., Lehmann J.C.U., Siewert C., Haubold U., Feuerer M., Debes G.F., Lauber J., Frey O., Przybylski G.K., Niesner U., de la Rosa M. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince M.N., Elliott D.E., Setiawan T., Metwali A., Blum A., Chen H.L., Urban J.F., Flavell R.A., Weinstock J.V. Role of T cell TGF-β signaling in intestinal cytokine responses and helminthic immune modulation. Eur. J. Immunol. 2009;39:1870–1878. doi: 10.1002/eji.200838956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashiro M., Messer R.J., Peterson K.E., Stromnes I.M., Sugie T., Hasenkrug K.J. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. USA. 2001;98:9226–9230. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangpatarapongsa K., Chootong P., Sattabongkot J., Chotivanich K., Sirichaisinthop J., Tungpradabkul S., Hisaeda H., Troye-Blomberg M., Cui L., Udomsangpetch R. Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur. J. Immunol. 2008;38:2697–2705. doi: 10.1002/eji.200838186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., Sher A. Conventional T-bet+Foxp3– Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E.K., Park J.K., Dockrell H.M. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr. Opin. Infect. Dis. 2003;16:205–210. doi: 10.1097/00001432-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Johanns T.M., Ertelt J.M., Rowe J.H., Way S.S. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010;6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J.Y., Zhang M., Miller M.J., Mills J.C., Wang B., Liu M., Eaton K.A., Zou W., Berndt B.E., Cole T.S., Takeuchi T., Owyang S.Y. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaparakis M., Laurie K.L., Wijburg O., Pedersen J., Pearse M., van Driel I.R., Gleeson P.A., Strugnell R.A. CD4+CD25+ regulatory T cells modulate the T-cell and antibody responses in Helicobacter-infected BALB/c mice. Infect. Immun. 2006;74:3519–3529. doi: 10.1128/IAI.01314-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi K., Inman M.D., Bienenstock J., Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am. J. Respir. Crit. Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- Kaufmann D.E., Walker B.D. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya T.I., Eskandari G., Guvenc U., Gunes G., Tursen U., Burak Cimen M.Y., Ikizoglu G. CD4+CD25+ Treg cells in patients with toenail onychomycosis. Arch. Dermatol. Res. 2009;301:725–729. doi: 10.1007/s00403-009-0941-y. [DOI] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- King C.L., Mahanty S., Kumaraswami V., Abrams J.S., Regunathan J., Jayaraman K., Ottesen E.A., Nutman T.B. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter A.L., Hennessey M., Bell A., Kern S., Lin Y., Daucher M., Planta M., McGlaughlin M., Jackson R., Ziegler S.F., Fauci A.S. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter A., McNally J., Riggin L., Jackson R., Roby G., Fauci A.S. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc. Natl. Acad. Sci. USA. 2007;104:3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter A.L., Horak R., Sion M., Riggin L., McNally J., Lin Y., Jackson R., O'Shea A., Roby G., Kovacs C., Connors M., Migueles S.A. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res. Hum. Retroviruses. 2007;23:438–450. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- Kroetz D.N., Deepe G.S., Jr. An aberrant thymus in CCR5−/− mice is coupled with an enhanced adaptive immune response in fungal infection. J. Immunol. 2011;186:5949–5955. doi: 10.4049/jimmunol.1003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg M.C., Jankovic D., Gorelick P.L., Caspar P., Letterio J.J., Cheever A.W., Sher A. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. J. Exp. Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar M., Bonhagen K., Fensterle J., Kohler A., Hurwitz R., Kamradt T., Kaufmann S.H.E., Mittrücker H.W. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 2002;196:1585–1592. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar M., Koch M., Mittrücker H.W., Nouailles G., Bonhagen K., Kamradt T., Kaufmann S.H.E. Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- Lahl K., Loddenkemper C., Drouin C., Freyer J., Arnason J., Eberl G., Hamann A., Wagner H., Huehn J., Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland L.E., Rad R., Wagner H., da Costa C.U. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur. J. Immunol. 2007;37:2174–2184. doi: 10.1002/eji.200737063. [DOI] [PubMed] [Google Scholar]
- Layland L.E., Mages J., Loddenkemper C., Hoerauf A., Wagner H., Lang R., da Costa C.U. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J. Immunol. 2010;184:713–724. doi: 10.4049/jimmunol.0901435. [DOI] [PubMed] [Google Scholar]
- Lee D.C.P., Harker J.A.E., Tregoning J.S., Atabani S.F., Johansson C., Schwarze J., Openshaw P.J.M. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J. Virol. 2010;84:8790–8798. doi: 10.1128/JVI.00796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lao S.H., Wu C.Y. Increased frequency of CD4+CD25high Treg cells inhibit BCG-specific induction of IFN-gamma by CD4+ T cells from TB patients. Tuberculosis (Edinb) 2007;87:526–534. doi: 10.1016/j.tube.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Li S., Jones K.L., Woollard D.J., Dromey J., Paukovics G., Plebanski M., Gowans E.J. Defining target antigens for CD25+ FOXP3+ IFN-γ– regulatory T cells in chronic hepatitis C virus infection. Immunol. Cell Biol. 2007;85:197–204. doi: 10.1038/sj.icb.7100020. [DOI] [PubMed] [Google Scholar]
- Li S., Gowans E.J., Chougnet C., Plebanski M., Dittmer U. Natural regulatory T cells and persistent viral infection. J. Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Floess S., Hamann A., Gaudieri S., Lucas A., Hellard M., Roberts S., Paukovic G., Plebanski M., Loveland B.E., Aitken C., Barry S. Analysis of FOXP3+ regulatory T cells that display apparent viral antigen specificity during chronic hepatitis C virus infection. PLoS Pathog. 2009;5:e1000707. doi: 10.1371/journal.ppat.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Tenner-Racz K., Racz P., Janowicz D.M., Fortney K.R., Katz B.P., Spinola S.M. Role played by CD4+FOXP3+ regulatory T cells in suppression of host responses to Haemophilus ducreyi during experimental infection of human volunteers. J. Infect. Dis. 2010;201:1839–1848. doi: 10.1086/652781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhardt C., Azzurri A., Amedei A., Fielding K., Sillah J., Sow O.Y., Bah B., Benagiano M., Diallo A., Manetti R., Manneh K., Gustafson P. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur. J. Immunol. 2002;32:1605–1613. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Liu H., Komai-Koma M., Xu D., Liew F.Y. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ruckwardt T.J., Chen M., Johnson T.R., Graham B.S. Characterization of respiratory syncytial virus M- and M2-specific CD4 T cells in a murine model. J. Virol. 2009;83:4934–4941. doi: 10.1128/JVI.02140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ruckwardt T.J., Chen M., Nicewonger J.D., Johnson T.R., Graham B.S. Epitope-specific regulatory CD4 T cells reduce virus-induced illness while preserving CD8 T-cell effector function at the site of infection. J. Virol. 2010;84:10501–10509. doi: 10.1128/JVI.00963-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.Y., Li L.Y., Yang X.Z., Li J., Zhong G., Wang J., Li L.J., Ji B., Wu Z.Q., Liu H., Yang X., Liu P.M. Adoptive transfer of DCs isolated from helminth-infected mice enhanced T regulatory cell responses in airway allergic inflammation. Parasite Immunol. 2011;33:525–534. doi: 10.1111/j.1365-3024.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- Loures F.V., Pina A., Felonato M., Araujo E.F., Leite K.R., Calich V.L. Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells. Infect. Immun. 2010;78:1078–1088. doi: 10.1128/IAI.01198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J.W., Corthesy P., Tougne C., Lees R., MacDonald H.R., Nabholz M. High and low affinity IL 2 receptors: Analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J. Immunol. 1985;135:3988–3994. [PubMed] [Google Scholar]
- Lund J.M., Hsing L., Pham T.T., Rudensky A.Y. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren A., Suri-Payer E., Enarsson K., Svennerholm A.-M., Lundin B.S. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 2003;71:1755–1762. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren A., Strömberg E., Sjöling A., Lindholm C., Enarsson K., Edebo A., Johnsson E., Suri-Payer E., Larsson P., Rudin A., Svennerholm A.-M., Lundin B.S. Mucosal FOXP3-expressing CD4+CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 2005;73:523–531. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackroth M.S., Malhotra I., Mungai P., Koech D., Muchiri E., King C.L. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186:2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- Maizels R.M. Infections and allergy—helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Wiedermann U. Immunoregulation by microbes and parasites in the control of allergy and autoimmunity. In: Rook G.A.W., editor. The Hygiene Hypothesis and Darwinian Medicine. Birkhäuser Verlag; Basel: 2009. pp. 45–75. [Google Scholar]
- Maizels R.M., Hewitson J.P., Murray J., Harcus Y., Dayer B., Filbey K.J., Grainger J.R., McSorley H.J., Reynolds L.A., Smith K.A. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp. Parasitol. 2011 doi: 10.1016/j.exppara.2011.08.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy K.J., Salaun L., Cahill R., Dougan G., Saunders N.J., Powrie F. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano F.S., Gutierrez F.R., Pavanelli W.R., Milanezi C.M., Cavassani K.A., Moreira A.P., Ferreira B.R., Cunha F.Q., Cardoso C.R., Silva J.S. The involvement of CD4+CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008;10:825–833. doi: 10.1016/j.micinf.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Martin S., Agarwal R., Murugaiyan G., Saha B. CD40 expression levels modulate regulatory T cells in Leishmania donovani infection. J. Immunol. 2010;185:551–559. doi: 10.4049/jimmunol.0902206. [DOI] [PubMed] [Google Scholar]
- Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McGuirk P., Mahon B.P., Griffin F., Mills K.H. Compartmentalization of T cell responses following respiratory infection with Bordetella pertussis: Hyporesponsiveness of lung T cells is associated with modulated expression of the co-stimulatory molecule CD28. Eur. J. Immunol. 1998;28:153–163. doi: 10.1002/(SICI)1521-4141(199801)28:01<153::AID-IMMU153>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- McGuirk P., McCann C., Mills K.H.G. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: A novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.S., Pearce E.J. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J. Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- McKinley L., Logar A.J., McAllister F., Zheng M., Steele C., Kolls J.K. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J. Immunol. 2006;177:6215–6226. doi: 10.4049/jimmunol.177.9.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley H.J., Harcus Y.M., Murray J., Taylor M.D., Maizels R.M. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite, Brugia malayi. J. Immunol. 2008;181:6456–6466. doi: 10.4049/jimmunol.181.9.6456. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Mejri N., Muller N., Hemphill A., Gottstein B. Intraperitoneal Echinococcus multilocularis infection in mice modulates peritoneal CD4+ and CD8+ regulatory T cell development. Parasitol. Int. 2011;60:45–53. doi: 10.1016/j.parint.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Mendez S., Reckling S.K., Piccirillo C.A., Sacks D., Belkaid Y. Role for CD4+ CD25+ regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J. Exp. Med. 2004;200:201–210. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J., Fabri M., Zingarelli A., Kubacki T., Meemboor S., Groneck L., Seeger J., Bessler M., Hafke H., Odenthal M., Bieler J.G., Kalka C. Streptococcus pneumoniae serotype 1 capsular polysaccharide induces CD8CD28 regulatory T lymphocytes by TCR crosslinking. PLoS Pathog. 2009;5:e1000596. doi: 10.1371/journal.ppat.1000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metenou S., Dembele B., Konate S., Dolo H., Coulibaly S.Y., Coulibaly Y.I., Diallo A.A., Soumaoro L., Coulibaly M.E., Sanogo D., Doumbia S.S., Traore S.F. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwali A., Setiawan T., Blum A.M., Urban J., Elliott D.E., Hang L., Weinstock J.V. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G253–G259. doi: 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- Mikkelsen S.R., Reckling S.K., Egan E.A., Dean G.A. In vivo depletion of CD4+CD25hi regulatory T cells is associated with improved antiviral responses in cats chronically infected with feline immunodeficiency virus. Virology. 2010;403:163–172. doi: 10.1016/j.virol.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen S.R., Long J.M., Zhang L., Galemore E.R., VandeWoude S., Dean G.A. Partial regulatory T cell depletion prior to acute feline immunodeficiency virus infection does not alter disease pathogenesis. PLoS ONE. 2011;6:e17183. doi: 10.1371/journal.pone.0017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.H.G. Regulatory T cells: Friend or foe in immunity to infection? Nat. Rev. Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- Minigo G., Woodberry T., Piera K.A., Salwati E., Tjitra E., Kenangalem E., Price R.N., Engwerda C.R., Anstey N.M., Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molling J.W., de Gruijl T.D., Glim J., Moreno M., Rozendaal L., Meijer C.J., van den Eertwegh A.J., Scheper R.J., von Blomberg M.E., Bontkes H.J. CD4+CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int. J. Cancer. 2007;121:1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- Monroy F.G., Enriquez F.J. Heligmosomoides polygyrus: A model for chronic gastrointestinal helminthiasis. Parasitol. Today. 1992;8:49–54. doi: 10.1016/0169-4758(92)90084-f. [DOI] [PubMed] [Google Scholar]
- Montagnoli C., Bacci A., Bozza S., Gaziano R., Mosci P., Sharpe A.H., Romani L. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 2002;169:6298–6308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- Montagnoli C., Fallarino F., Gaziano R., Bozza S., Bellocchio S., Zelante T., Kurup W.P., Pitzurra L., Puccetti P., Romani L. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J. Immunol. 2006;176:1712–1723. doi: 10.4049/jimmunol.176.3.1712. [DOI] [PubMed] [Google Scholar]
- Montes M., Sanchez C., Verdonck K., Lake J.E., Gonzalez E., Lopez G., Terashima A., Nolan T., Lewis D.E., Gotuzzo E., White A.C. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl. Trop. Dis. 2009;3:e456. doi: 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moreira A.P., Cavassani K.A., Massafera Tristao F.S., Campanelli A.P., Martinez R., Rossi M.A., Silva J.S. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J. Immunol. 2008;180:3049–3056. doi: 10.4049/jimmunol.180.5.3049. [DOI] [PubMed] [Google Scholar]
- Moreno-Fernandez M.E., Rueda C.M., Rusie L.K., Chougnet C.A. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood. 2011;117:5372–5380. doi: 10.1182/blood-2010-12-323162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.L., Wille U., Villegas E.N., Hunter C.A., Farrell J.P. IL-10 mediates susceptibility to Leishmania donovani infection. Eur. J. Immunol. 2001;31:2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Nagase H., Jones K.M., Anderson C.F., Noben-Trauth N. Despite increased CD4+Foxp3+ cells within the infection site, BALB/c IL-4 receptor-deficient mice reveal CD4+Foxp3-negative T cells as a source of IL-10 in Leishmania major susceptibility. J. Immunol. 2007;179:2435–2444. doi: 10.4049/jimmunol.179.4.2435. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Tsuruoka M., Ogura H., Okuyama Y., Arima Y., Hirano T., Murakami M. IL-6 positively regulates Foxp3+CD8+ T cells in vivo. Int. Immunol. 2010;22:129–139. doi: 10.1093/intimm/dxp119. [DOI] [PubMed] [Google Scholar]
- Nausch N., Midzi N., Mduluza T., Maizels R.M., Mutapi F. Regulatory and activated T cells in human Schistosoma haematobium infections. PLoS One. 2011;6:e16860. doi: 10.1371/journal.pone.0016860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Sutmuller R., Hermann C., Van der Graaf C.A.A., Van der Meer J.W.M., van Krieken J.H., Hartung T., Adema G., Kullberg B.J. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- Nie C.Q., Bernard N.J., Schofield L., Hansen D.S. CD4+ CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infect. Immun. 2007;75:2275–2282. doi: 10.1128/IAI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., Boasso A., Velilla P.A., Zhang R., Vaccari M., Franchini G., Shearer G.M., Andersson J., Chougnet C. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth N., Paul W.E., Sacks D.L. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 1999;162:6132–6140. [PubMed] [Google Scholar]
- Oberg H.H., Ly T.T., Ussat S., Meyer T., Kabelitz D., Wesch D. Differential but direct abolishment of human regulatory T cell suppressive capacity by various TLR2 ligands. J. Immunol. 2010;184:4733–4740. doi: 10.4049/jimmunol.0804279. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J., Mielcarz D.W., Ditrio L.E., Burroughs A.R., Begum-Haque S., Dasgupta S., Kasper D.L., Kasper L.H. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J., Mielcarz D.W., Wang Y., Begum-Haque S., Dasgupta S., Kasper D.L., Kasper L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- O'Mahony C., Scully P., O'Mahony D., Murphy S., O'Brien F., Lyons A., Sherlock G., MacSharry J., Kiely B., Shanahan F., O'Mahony L. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-κB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer F.M., de Souza J.B., Riley E.M. Differential induction of TGF-β regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J. Immunol. 2003;171:5430–5436. doi: 10.4049/jimmunol.171.10.5430. [DOI] [PubMed] [Google Scholar]
- Ordway D., Henao-Tamayo M., Harton M., Palanisamy G., Troudt J., Shanley C., Basaraba R.J., Orme I.M. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- Ordway D.J., Shang S., Henao-Tamayo M., Obregon-Henao A., Nold L., Caraway M., Shanley C.A., Basaraba R.J., Duncan C.G., Orme I.M. Mycobacterium bovis BCG mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clin. Vaccine Immunol. 2011;18:1527–1535. doi: 10.1128/CVI.05127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman S., Rask C., Wold A.E., Hultkrantz S., Telemo E. Impaired regulatory T cell function in germ-free mice. Eur. J. Immunol. 2006;36:2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- Othman A.A., El-Shourbagy S.H., Soliman R.H. Kinetics of Foxp3-expressing regulatory cells in experimental Toxocara canis infection. Exp. Parasitol. 2010;127:454–459. doi: 10.1016/j.exppara.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Pacholczyk R., Kern J., Singh N., Iwashima M., Kraj P., Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P., Conti H.R., Zheng L., Peterson A.C., Mathern D.R., Hernandez-Santos N., Edgerton M., Gaffen S.L., Lenardo M.J. CD4+CD25+Foxp3+ regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.K., Cho M.K., Park H.K., Lee K.H., Lee S.J., Choi S.H., Ock M.S., Jeong H.J., Lee M.H., Yu H.S. Macrophage migration inhibitory factor homologs of anisakis simplex suppress Th2 response in allergic airway inflammation model via CD4+CD25+Foxp3+ T cell recruitment. J Immunol. 2009;182:6907–6914. doi: 10.4049/jimmunol.0803533. [DOI] [PubMed] [Google Scholar]
- Pasquali P., Thornton A.M., Vendetti S., Pistoia C., Petrucci P., Tarantino M., Pesciaroli M., Ruggeri F., Battistoni A., Shevach E.M. CD4+CD25+ T regulatory cells limit effector T cells and favor the progression of brucellosis in BALB/c mice. Microbes Infect. 2010;12:3–10. doi: 10.1016/j.micinf.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Pearce E.J., MacDonald A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Peng G., Guo Z., Kiniwa Y., Voo K.S., Peng W., Fu T., Wang D.Y., Li Y., Wang H.Y., Wang R.F. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- Piessens W.F., McGreevy P.B., Piessens P.W., McGreevy M., Koiman I., Saroso J.S., Dennis D.T. Immune responses in human infections with Brugia malayi. Specific cellular unresponsiveness to filarial antigens. J. Clin. Invest. 1980;65:172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessens W.F., Partono F., Hoffman S.L., Ratiwayanto S., Piessens P.W., Palmieri J.R., Koiman I., Dennis D.T., Carney W.P. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N. Engl. J. Med. 1982;307:144–148. doi: 10.1056/NEJM198207153070302. [DOI] [PubMed] [Google Scholar]
- Polk B.F., Kasper D.L. Bacteroides fragilis subspecies in clinical isolates. Ann. Intern. Med. 1977;86:569–571. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- Popescu I., Macedo C., Abu-Elmagd K., Shapiro R., Hua Y., Thomson A.W., Morelli A.E., Storkus W.J., Metes D. EBV-specific CD8+ T cell reactivation in transplant patients results in expansion of CD8+ type-1 regulatory T cells. Am. J. Transplant. 2007;7:1215–1223. doi: 10.1111/j.1600-6143.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- Punkosdy G.A., Blain M., Glass D.D., Lozano M.M., O'Mara L., Dudley J.P., Ahmed R., Shevach E.M. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc. Natl. Acad. Sci. USA. 2011;108:3677–3682. doi: 10.1073/pnas.1100213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X.J., Shi H.Z., Liang Q.L., Huang L.Y., Yang H.B. CD4+CD25+ regulatory T lymphocytes in tuberculous pleural effusion. Chin. Med. J. (Engl) 2008;121:581–586. [PubMed] [Google Scholar]
- Quinn K.M., McHugh R.S., Rich F.J., Goldsack L.M., de Lisle G.W., Buddle B.M., Delahunt B., Kirman J.R. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol. Cell Biol. 2006;84:467–474. doi: 10.1111/j.1440-1711.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- Rad R., Brenner L., Bauer S., Schwendy S., Layland L., da Costa C.P., Reindl W., Dossumbekova A., Friedrich M., Saur D., Wagner H., Schmid R.M. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Raghavan S., Fredriksson M., Svennerholm A.M., Holmgren J., Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 2003;132:393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L.M., Amante F.H., McSweeney K.A., Zhou Y., Stanley A.C., Haque A., Jones M.K., Hill G.R., Boyle G.M., Engwerda C.R. Common strategies to prevent and modulate experimental cerebral malaria in mouse strains with different susceptibilities. Infect. Immun. 2008;76:3312–3320. doi: 10.1128/IAI.01475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch S., Huehn J., Kirchhoff D., Rzepecka J., Schnoeller C., Pillai S., Loddenkemper C., Scheffold A., Hamann A., Lucius R., Hartmann S. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect. Immun. 2008;76:1908–1919. doi: 10.1128/IAI.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch S., Huehn J., Loddenkemper C., Hepworth M.R., Klotz C., Sparwasser T., Hamann A., Lucius R., Hartmann S. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur. J. Immunol. 2009;39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistence. J. Clin. Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S.L., Locksley R.M. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Repa A., Grangette C., Daniel C., Hochreiter R., Hoffmann-Sommergruber K., Thalhamer J., Kraft D., Breiteneder H., Mercenier A., Wiedermann U. Mucosal co-application of lactic acid bacteria and allergen induces counter-regulatory immune responses in a murine model of birch pollen allergy. Vaccine. 2003;22:87–95. doi: 10.1016/s0264-410x(03)00528-0. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Rodrigues R., Resende Co T., Rojas R., Toossi Z., Dietze R., Boom W.H., Maciel E., Hirsch C.S. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin. Exp. Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer M.J., Straka N., Fang D., Shanina I., Horwitz M.S. Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-β. Diabetes. 2008;57:1302–1311. doi: 10.2337/db07-1460. [DOI] [PubMed] [Google Scholar]
- Robertson S.J., Messer R.J., Carmody A.B., Hasenkrug K.J. In vitro suppression of CD8+ T cell function by Friend virus-induced regulatory T cells. J. Immunol. 2006;176:3342–3349. doi: 10.4049/jimmunol.176.6.3342. [DOI] [PubMed] [Google Scholar]
- Rodrigues V., Jr., Santana da Silva J., Campos-Neto A. Transforming growth factor-β and immunosuppression in experimental visceral leishmaniasis. Infect. Immun. 1998;66:1233–1236. doi: 10.1128/iai.66.3.1233-1236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues O.R., Marques C., Soares-Clemente M., Ferronha M.H., Santos-Gomes G.M. Identification of regulatory T cells during experimental Leishmania infantum infection. Immunobiology. 2009;214:101–111. doi: 10.1016/j.imbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Roelants G.E., Pearson T.W., Mayor-Withey K.S., Lundin L.B. Immune depression in Trypanosoma congolense-infected mice. Adv. Exp. Med. Biol. 1979;114:661–666. doi: 10.1007/978-1-4615-9101-6_108. [DOI] [PubMed] [Google Scholar]
- Round J.L., Mazmanian S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., Mazmanian S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B.T., Horohov D.W. Immunosuppression in viral infections. Rev. Infect. Dis. 1986;8:850–873. doi: 10.1093/clinids/8.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B.T., Sarangi P.P., Suvas S. Regulatory T cells in virus infections. Immunol. Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Rowe J.H., Ertelt J.M., Aguilera M.N., Farrar M.A., Way S.S. Foxp3+ regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov Y.P., Niec R.E., Josefowicz S., Li L., Darce J., Mathis D., Benoist C., Rudensky A.Y. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushbrook S.M., Ward S.M., Unitt E., Vowler S.L., Lucas M., Klenerman P., Alexander G.J. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J. Virol. 2005;79:7852–7859. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Salhi A., Rodrigues V., Jr., Santoro F., Dessein H., Romano A., Castellano L.R., Sertorio M., Rafati S., Chevillard C., Prata A., Alcais A., Argiro L. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J. Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- Sarangi P.P., Sehrawat S., Suvas S., Rouse B.T. IL-10 and natural regulatory T cells: Two independent anti-inflammatory mechanisms in herpes simplex virus-induced ocular immunopathology. J. Immunol. 2008;180:6297–6306. doi: 10.4049/jimmunol.180.9.6297. [DOI] [PubMed] [Google Scholar]
- Sartono E., Kruize Y.C.M., Kurniawan A., van der Meide P.H., Partono F., Maizels R.M., Yazdanbakhsh M. Elevated cellular responses and interferon-γ release after long-term diethylcarbamazine treatment of patients with human lymphatic filariasis. J. Infect. Dis. 1995;171:1683–1687. doi: 10.1093/infdis/171.6.1683. [DOI] [PubMed] [Google Scholar]
- Sather B.D., Treuting P., Perdue N., Miazgowicz M., Fontenot J.D., Rudensky A.Y., Campbell D.J. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoguina J., Mempel M., Larbi J., Badusche M., Loliger C., Adjei O., Gachelin G., Fleischer B., Hoerauf A. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis) Microbes Infect. 2002;4:1291–1300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Satoguina J.S., Weyand E., Larbi J., Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: Role of IL-10. J. Immunol. 2005;174:4718–4726. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- Satoguina J.S., Adjobimey T., Arndts K., Hoch J., Oldenburg J., Layland L.E., Hoerauf A. Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR-L interaction, IL-10 and TGF-β. Eur. J. Immunol. 2008;38:3101–3113. doi: 10.1002/eji.200838193. [DOI] [PubMed] [Google Scholar]
- Sayi A., Kohler E., Toller I.M., Flavell R.A., Muller W., Roers A., Muller A. TLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J. Immunol. 2011;186:878–890. doi: 10.4049/jimmunol.1002269. [DOI] [PubMed] [Google Scholar]
- Scholzen A., Mittag D., Rogerson S.J., Cooke B.M., Plebanski M. Plasmodium falciparum-mediated induction of human CD25hiFoxp3hi CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFβ. PLoS Pathog. 2009;5:e1000543. doi: 10.1371/journal.ppat.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen A., Minigo G., Plebanski M. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol. 2010;26:16–25. doi: 10.1016/j.pt.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Schramm G., Mohrs K., Wodrich M., Doenhoff M.J., Pearce E.J., Haas H., Mohrs M. IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J. Immunol. 2007;178:6023–6027. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- Scott-Browne J.P., Shafiani S., Tucker-Heard G., Ishida-Tsubota K., Fontenot J.D., Rudensky A.Y., Bevan M.J., Urdahl K.B. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura M., Su Z., Piccirillo C., Stevenson M.M. Impairment of dendritic cell function by excretory-secretory products: A potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- Sehrawat S., Suvas S., Sarangi P.P., Suryawanshi A., Rouse B.T. In vitro-generated antigen-specific CD4+CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J. Virol. 2008;82:6838–6851. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan T., Metwali A., Blum A.M., Ince M.N., Urban J.F., Jr., Elliott D.E., Weinstock J.V. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyerl M., Kirchberger S., Majdic O., Seipelt J., Jindra C., Schrauf C., Stockl J. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. Eur. J. Immunol. 2010;40:321–329. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- Shafiani S., Tucker-Heard G., Kariyone A., Takatsu K., Urdahl K.B. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., Wang Z., Jin H., Chen Y.W., Wang Q., Qian Y. Immunoregulatory Cordyceps sinensis increases regulatory T cells to Th17 cell ratio and delays diabetes in NOD mice. Int. Immunopharmacol. 2009;9:582–586. doi: 10.1016/j.intimp.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Shimizu J., Yamazaki S., Takahashi T., Ishida Y., Sakaguchi S. Stimulation of CD25+CD4 + regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- Sing A., Rost D., Tvardovskaia N., Roggenkamp A., Wiedemann A., Kirschning C.J., Aepfelbacher M., Heesemann J. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 2002;196:1017–1024. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.P., Gerard H.C., Hudson A.P., Reddy T.R., Boros D.L. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology. 2005;114:410–417. doi: 10.1111/j.1365-2567.2004.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.A., Hochweller K., Hämmerling G.J., Boon L., Macdonald A.S., Maizels R.M. Chronic helminth infection mediates tolerance in vivo through dominance of CD11clo CD103– DC population. J. Immunol. 2011;186:7098–7109. doi: 10.4049/jimmunol.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H.H., Yazdanbakhsh M. Chronic helminth infections modulate allergen-specific immune responses: Protection against development of allergic disorders? Ann. Med. 2007;39:428–439. doi: 10.1080/07853890701436765. [DOI] [PubMed] [Google Scholar]
- Smyk-Pearson S., Golden-Mason L., Klarquist J., Burton J.R., Jr., Tester I.A., Wang C.C., Culbertson N., Vandenbark A.A., Rosen H.R. Functional suppression by FoxP3+CD4+CD25high regulatory T cells during acute hepatitis C virus infection. J. Infect. Dis. 2008;197:46–57. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]
- Steeg C., Adler G., Sparwasser T., Fleischer B., Jacobs T. Limited role of CD4+Foxp3+ regulatory T cells in the control of experimental cerebral malaria. J. Immunol. 2009;183:7014–7022. doi: 10.4049/jimmunol.0901422. [DOI] [PubMed] [Google Scholar]
- Steel C., Nutman T.B. CTLA-4 in filarial infections: Implications for a role in diminished T cell reactivity. J. Immunol. 2003;170:1930–1938. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- Steinfelder S., Andersen J.F., Cannons J.L., Feng C.G., Joshi M., Dwyer D., Caspar P., Schwartzberg P.L., Sher A., Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J. Exp. Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z., Segura M., Morgan K., Loredo-Osti J.C., Stevenson M.M. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect. Immun. 2005;73:3531–3539. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffia I., Reckling S.K., Salay G., Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- Suffia I.J., Reckling S.K., Piccirillo C.A., Goldszmid R.S., Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffner J., Hochweller K., Kuhnle M.C., Li X., Kroczek R.A., Garbi N., Hammerling G.J. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J. Immunol. 2010;184:1810–1820. doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Ikeda F., Stadanlick J., Nunes F.A., Alter H.J., Chang K.M. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Summers R.W., Elliott D.E., Weinstock J.V. Why Trichuris suis should prove safe for use in inflammatory bowel diseases. Inflamm. Bowel Dis. 2005;11:783–784. doi: 10.1097/01.mib.0000179316.50002.f3. [DOI] [PubMed] [Google Scholar]
- Summers R.W., Elliott D.E., Weinstock J.V. Therapeutic colonization with Trichuris suis. Arch. Pathol. Lab. Med. 2006;130:1753. doi: 10.5858/2006-130-1753b-IR. author reply 1753–1754. [DOI] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller R.P., den Brok M.H., Kramer M., Bennink E.J., Toonen L.W., Kullberg B.J., Joosten L.A., Akira S., Netea M.G., Adema G.J. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S., Kumaraguru U., Pack C.D., Lee S., Rouse B.T. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S., Azkur A.K., Kim B.S., Kumaraguru U., Rouse B.T. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- Tang C.L., Lei J.H., Wang T., Lu S.J., Guan F., Liu W.Q., Li Y.L. Effect of CD4+ CD25+ regulatory T cells on the immune evasion of Schistosoma japonicum. Parasitol. Res. 2011;108:477–480. doi: 10.1007/s00436-010-2089-2. [DOI] [PubMed] [Google Scholar]
- Taylor M., Le Goff L., Harris A., Malone E., Allen J.E., Maizels R.M. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- Taylor J.J., Mohrs M., Pearce E.J. Regulatory T cell responses develop in parallel to Th responses, and control the magnitude and phenotype of the Th effector population. J. Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- Taylor M.D., Harris A., Babayan S., Bain O., Culshaw A., Allen J.E., Maizels R.M. CTLA-4+ and CD4+CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- Taylor M.D., van der Werf N., Harris A., Graham A.L., Bain O., Allen J.E., Maizels R.M. Early recruitment of natural CD4+Foxp3+ regulatory T cells by infective larvae determines the outcome of filarial infection. Eur. J. Immunol. 2009;39:192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- Teixeira-Carvalho A., Martins-Filho O.A., Peruhype-Magalhaes V., Silveira-Lemos D., Malaquias L.C., Oliveira L.F., Silveira A.M., Gazzinelli A., Gazzinelli G., Correa-Oliveira R. Cytokines, chemokine receptors, CD4+CD25HIGH+ T-cells and clinical forms of human schistosomiasis. Acta Trop. 2008;108:139–149. doi: 10.1016/j.actatropica.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Tetsutani K., Ishiwata K., Ishida H., Tu L., Torii M., Hamano S., Himeno K., Hisaeda H. Concurrent infection with Heligmosomoides polygyrus suppresses anti-Plasmodium yoelii protection partially by induction of CD4+CD25+Foxp3+ Treg in mice. Eur. J. Immunol. 2009;39:2822–2830. doi: 10.1002/eji.200939433. [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Korty P.E., Tran D.Q., Wohlfert E.A., Murray P.E., Belkaid Y., Shevach E.M. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toka F.N., Suvas S., Rouse B.T. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J. Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella D., Gewurz B.E., Furman M.H., Schust D.J., Ploegh H.L. Viral subversion of the immune system. Annu. Rev. Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- Trandem K., Anghelina D., Zhao J., Perlman S. Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus. J. Immunol. 2010;184:4391–4400. doi: 10.4049/jimmunol.0903918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.R., Delwart E., Sepulveda H., Balderas R.S., Routy J.P., Haddad E.K. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Tsunemi S., Iwasaki T., Imado T., Higasa S., Kakishita E., Shirasaka T., Sano H. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS. 2005;19:879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- Turner J.D., Jackson J.A., Faulkner H., Behnke J., Else K., Kamgno J., Boussinesq M., Bradley J.E. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J. Infect. Dis. 2008;197:1204–1212. doi: 10.1086/586717. [DOI] [PubMed] [Google Scholar]
- Vahlenkamp T.W., Tompkins M.B., Tompkins W.A. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+CD25+ T regulatory cells. J. Immunol. 2004;172:4752–4761. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- van der Kleij D., Latz E., Brouwers J.F.H.M., Kruize Y.C.M., Schmitz M., Kurt-Jones E.A., Espevik T., de Jong E.C., Kapsenberg M.L., Golenbock D.T., Tielens A.G.M., Yazdanbakhsh M. A novel host-parasite lipid cross talk: Schistosomal lysophosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- van Maren W.W.C., Jacobs J.F., de Vries I.J., Nierkens S., Adema G.J. Toll-like receptor signalling on Tregs: To suppress or not to suppress? Immunology. 2008;124:445–452. doi: 10.1111/j.1365-2567.2008.02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maren W.W.C., Nierkens S., Toonen L.W., Bolscher J.M., Sutmuller R.P., Adema G.J. Multifaceted effects of synthetic TLR2 ligand and Legionella pneumophilia on Treg-mediated suppression of T cell activation. BMC Immunol. 2011;12:23. doi: 10.1186/1471-2172-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van L.P., Bardel E., Gregoire S., Vanoirbeek J., Schneider E., Dy M., Thieblemont N. Treatment with the TLR7 agonist R848 induces regulatory T-cell-mediated suppression of established asthma symptoms. Eur. J. Immunol. 2011;41:1992–1999. doi: 10.1002/eji.201040914. [DOI] [PubMed] [Google Scholar]
- Vigario A.M., Gorgette O., Dujardin H.C., Cruz T., Cazenave P.A., Six A., Bandeira A., Pied S. Regulatory CD4+ CD25+ Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. Int. J. Parasitol. 2007;37:963–973. doi: 10.1016/j.ijpara.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Walsh C.M., Smith P., Fallon P.G. Role for CTLA-4 but not CD25+ T cells during Schistosoma mansoni infection of mice. Parasite Immunol. 2007;29:293–308. doi: 10.1111/j.1365-3024.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- Walther M., Tongren J.E., Andrews L., Korbel D., King E., Fletcher H., Andersen R.F., Bejon P., Thompson F., Dunachie S.J., Edele F., de Souza J.B. Upregulation of TGF-β, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Walther M., Jeffries D., Finney O.C., Njie M., Ebonyi A., Deininger S., Lawrence E., Ngwa-Amambua A., Jayasooriya S., Cheeseman I.H., Gomez-Escobar N., Okebe J. Distinct roles for FOXP3+ and FOXP3– CD4+ T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wammes L.J., Hamid F., Wiria A.E., de Gier B., Sartono E., Maizels R.M., Luty A.J., Fillie Y., Brice G.T., Supali T., Smits H.H., Yazdanbakhsh M. Regulatory T cell in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur. J. Immunol. 2010;40:437–442. doi: 10.1002/eji.200939699. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhou S., Chi Y., Wen X., Hoellwarth J., He L., Liu F., Wu C., Dhesi S., Zhao J., Hu W., Su C. CD4+CD25+ Treg induction by an HSP60-derived peptide SJMHE1 from Schistosoma japonicum is TLR2 dependent. Eur. J. Immunol. 2009;39:3052–3065. doi: 10.1002/eji.200939335. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Mwinzi P.N., Black C.L., Muok E.M., Karanja D.M., Secor W.E., Colley D.G. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am. J. Trop. Med. Hyg. 2007;77:676–682. [PMC free article] [PubMed] [Google Scholar]
- Weber M.S., Benkhoucha M., Lehmann-Horn K., Hertzenberg D., Sellner J., Santiago-Raber M.L., Chofflon M., Hemmer B., Zamvil S.S., Lalive P.H. Repetitive pertussis toxin promotes development of regulatory T cells and prevents central nervous system autoimmune disease. PLoS One. 2010;5:e16009. doi: 10.1371/journal.pone.0016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Tabel H. Regulatory T cells prevent control of experimental African trypanosomiasis. J. Immunol. 2008;180:2514–2521. doi: 10.4049/jimmunol.180.4.2514. [DOI] [PubMed] [Google Scholar]
- Weiss L., Donkova-Petrini V., Caccavelli L., Balbo M., Carbonneil C., Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S., Taylor M., Balic A., Finney C.A.M., Lamb J.R., Maizels R.M. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang Q.H., Zheng L., Feng H., Liu J., Ma S.H., Cao Y.M. Plasmodium yoelii: Distinct CD4+CD25+ regulatory T cell responses during the early stages of infection in susceptible and resistant mice. Exp. Parasitol. 2007;115:301–304. doi: 10.1016/j.exppara.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Wu J.J., Chen G., Liu J., Wang T., Zheng W., Cao Y.M. Natural regulatory T cells mediate the development of cerebral malaria by modifying the pro-inflammatory response. Parasitol. Int. 2010;59:232–241. doi: 10.1016/j.parint.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Xu D., Liu H., Komai-Koma M., Campbell C., McSharry C., Alexander J., Liew F.Y. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- Yang G., Liu A., Xie Q., Guo T.B., Wan B., Zhou B., Zhang J.Z. Association of CD4+CD25+Foxp3+ regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int. Immunol. 2007;19:133–140. doi: 10.1093/intimm/dxl130. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhao J., Yang Y., Zhang L., Yang X., Zhu X., Ji M., Sun N., Su C. Schistosoma japonicum egg antigens modulate airway inflammation by producing CD4+CD25+ T cells in a murine model of asthma. Immunology. 2007;120:8–18. doi: 10.1111/j.1365-2567.2006.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh M., Paxton W.A., Kruize Y.C.M., Sartono E., Kurniawan A., van het Wout A., Selkirk M.E., Partono F., Maizels R.M. T cell responsiveness correlates differentially with antibody isotype levels in clinical and asymptomatic filariasis. J. Infect. Dis. 1993;167:925–931. doi: 10.1093/infdis/167.4.925. [DOI] [PubMed] [Google Scholar]
- Zaccone P., Burton O.T., Gibbs S.E., Miller N., Jones F.M., Schramm G., Haas H., Doenhoff M.J., Dunne D.W., Cooke A. The S. mansoni glycoprotein ω-1 induces Foxp3 expression in NOD mouse CD4+ T cells. Eur. J. Immunol. 2011;41:2709–2718. doi: 10.1002/eji.201141429. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A., Cahalon L., Tal G., Margalit R., Lider O., Cohen I.R. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J. Clin. Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zelinskyy G., Robertson S.J., Schimmer S., Messer R.J., Hasenkrug K.J., Dittmer U. CD8+ T-cell dysfunction due to cytolytic granule deficiency in persistent Friend retrovirus infection. J. Virol. 2005;79:10619–10626. doi: 10.1128/JVI.79.16.10619-10626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G., Kraft A.R., Schimmer S., Arndt T., Dittmer U. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur. J. Immunol. 2006;36:2658–2670. doi: 10.1002/eji.200636059. [DOI] [PubMed] [Google Scholar]
- Zelinskyy G., Dietze K., Sparwasser T., Dittmer U. Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog. 2009;5:e1000406. doi: 10.1371/journal.ppat.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G., Dietze K.K., Husecken Y.P., Schimmer S., Nair S., Werner T., Gibbert K., Kershaw O., Gruber A.D., Sparwasser T., Dittmer U. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009;114:3199–3207. doi: 10.1182/blood-2009-03-208736. [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu M., Luther J., Kao J.Y. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes. 2010;1:325–329. doi: 10.4161/gmic.1.5.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Fett C., Trandem K., Fleming E., Perlman S. IFN-γ- and IL-10-expressing virus epitope-specific Foxp3+ T reg cells in the central nervous system during encephalomyelitis. J. Exp. Med. 2011;208:1571–1577. doi: 10.1084/jem.20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuany-Amorim C., Sawicka E., Manlius C., Le Moine A., Brunet L.R., Kemeny D.M., Bowen G., Rook G., Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]


