SERUM TOTAL PROTEIN AND ALBUMIN
Commonly Indicated
In most ill patients, but especially those with known or suspected anemia, edema, ascites, coagulopathies, diarrhea, weight loss, and hepatic or renal disease.
Advantages
Technically easy to perform.
Disadvantages
Additional testing is required to establish cause of altered protein concentrations.
Analysis
Total protein can be estimated in fluid, serum, or plasma (ethylenediaminetetraacetic acid [EDTA] or heparinized) by a refractometer that measures total solids. Total protein and albumin can be measured in serum, urine, or fluid by spectrophotometric or dry reagent methods. Serum globulin concentration is calculated by subtracting the serum albumin from the serum total protein. (See comments under Artifacts.)
Normal Values
Listed in Table 12-1 . NOTE: Lower values are normal for perinates and very young animals. Values gradually increase until adulthood; the higher values are average normal values for adults. Both albumin and globulin tend to decline with advancing old age.
TABLE 12-1.
Normal Serum Total Protein and Albumin Values (g/dl)
| DOGS | CATS | |
|---|---|---|
| Plasma total protein | 6.0–7.8 | 6.0–7.5 |
| Serum total protein | 5.5–7.5 | 5.5–7.8 |
| Serum albumin | 2.5–4.0 | 2.5–4.0 |
| Serum globulin | 3.0–3.5 | 3.0–3.8 |
Danger Values
Albumin less than or equal to 1.0 g/dl can be associated with major fluid shifts in the body (may occur at higher serum albumin concentrations in patients with increased portal pressures). Patients with severe hypoalbuminemia are often deficient in antithrombin-III (AT III), thus also putting them at risk for pulmonary thromboembolism.
Artifacts
Falsely increased (refractometer): lipemia, hyperbilirubinemia, hemolysis, severe hyperglycemia, azotemia, hypernatremia, and hyperchloremia. Important: Certain methodologies that measure human albumin give falsely low values with canine albumin.
Drug Therapy That May Alter Protein Values
Hormonal changes generally have a slight effect on serum proteins, even though physical changes (e.g., body weight, muscle mass) may be marked. Hyperproteinemia may be caused by anabolic steroids, progesterone, insulin, and thyroid hormones in people. Prolonged, high-dose corticosteroid therapy can cause hyperproteinemia and hyperalbuminemia in normal dogs, but values return to normal within weeks after cessation of therapy (Moore et al, 1992). Hypoproteinemia may be due to estrogen; hypoalbuminemia may be due to anticonvulsants, acetaminophen, estrogens, and various antineoplastic agents in people.
Causes of Alteration in Plasma and Serum Protein
The serum total protein concentration is only important in that it allows calculation of serum globulin concentration.
Causes of Hyperalbuminemia
Dehydration and laboratory error are major causes.
Causes of Hypoalbuminemia
Concurrently evaluating serum globulin can sometimes help determine the cause of hypoalbuminemia. If both albumin and globulin values are decreased, hemorrhage, exudation from severe skin lesions, protein-losing enteropathy (PLE), and dilution are usually more important considerations (Table 12-2 ). Dilution usually causes mild decreases (albumin 2.1 to 2.4 g/dl), whereas PLE can cause moderate (1.5 to 2.0 g/dl) to severe (< 1.5 g/dl) hypoalbuminemia. PLE can be the result of primary intestinal disease or various causes of GI hemorrhage (e.g., mild-to-severe hypoalbuminemia was reported in approximately one third of over 40 dogs with Addison's disease [Langlais-Burgess, Lumsden, and Mackin, 1995], probably because of GI hemorrhage). Although both serum albumin and globulin are usually decreased in PLE, globulin concentration may be normal to increased in some cases.
TABLE 12-2.
Causes of Hypoalbuminemia in Dogs and Cats
|
Most common and important causes of serum albumin
Of very doubtful importance as a sole cause of serum albumin ≤2.0 g/dl. Probably more important as a contributing factor when there is another problem that results in hypoalbuminemia.
Can be important in very young animals or animals fed diets that are extremely restricted in protein for prolonged periods.
Hypoalbuminemia plus normal to increased globulins suggests decreased albumin production, increased loss, or sequestration (see TABLE 12-2, TABLE 12-3 ). Increased albumin loss occurs in glomerular disease (and may be severe; see Chapter 7).
TABLE 12-3.
Causes of Hyperglobulinemia in Dogs and Cats
NOTE: Effect of age should be considered when assessing globulin values (see Causes of Hypoglobulinemia in text).
Mild (4 to 5 g/dl).
Moderate (5 to 6 g/dl). 3
Severe (>6 g/dl).
Albuminuria as the result of glomerulopathy is rarely associated with significant globulin loss. Decreased production is due to chronic hepatic insufficiency or hyperglobulinemia. The latter may cause mild hypoalbuminemia, whereas chronic hepatic insufficiency can cause moderate to severe decreases. In hyperglobulinemia, albumin synthesis may be decreased (i.e., “down regulation”). When inflammation is associated with hypoalbuminemia and hyperglobulinemia, albumin is sometimes called a negative acute phase protein. Inadequate protein intake (including poorly digestible protein), maldigestion, and malabsorption are rare causes of mild hypoalbuminemia; however, occasionally they accompany other conditions causing hepatic insufficiency or increased protein loss. Significant hypoalbuminemia (i.e., albumin <2.1 g/dl) should never be attributed solely to decreased nutrition until hepatic insufficiency and protein-losing disorders have been eliminated by definitive tests (not just by history and physical examination). Decreased intake very rarely causes serum albumin concentrations less than 2.1 g/dl, except perhaps in very young animals.
Sequestration may occur in pleural or peritoneal cavities or subcutaneous (SC) tissues. Thus patients with effusion caused by hypoalbuminemia may further lower their serum albumin concentration via sequestration. Alternatively, sequestration can be secondary to increased hydrostatic pressure (e.g., portal hypertension, right-sided cardiac failure). Immune-mediated or infectious vasculopathies (e.g., endotoxemia and bacteremia, ehrlichiosis, Rocky Mountain spotted fever [RMSF]) also allow loss from the vascular compartment. Hypoalbuminemia as the result of sequestration or vasculopathy is usually mild.
A diagnostic approach to hypoalbuminemic patients is outlined in Figure 12-1 . A urinalysis (sometimes including urine protein:creatinine ratio; see Chapter 7) and measurement of serum bile acids (see Chapter 9) are indicated. Severe cutaneous exudative lesions may be diagnosed by physical examination, but the possibility of renal, hepatic, and alimentary disease should still be investigated. Hypercholesterolemia plus hypoalbuminemia suggests protein-losing nephropathy. Significant proteinuria indicates a diagnostic workup for protein-losing nephropathy (PLN) (see Chapter 7). Hypocholesterolemia plus hypoalbuminemia is suggestive of hepatic insufficiency or PLE. Hypoalbuminemia associated with hepatomegaly; microhepatia; neurologic signs; icterus; decreased blood urea nitrogen (BUN) with or without increased alanine aminotransferase (ALT), serum alkaline phosphatase (SAP), or both; or abnormal hepatic function test results (e.g., serum bile acids) requires a diagnostic workup for hepatic insufficiency (see Chapter 9). NOTE: ALT and SAP are normal in many patients with severe hepatic disease. A portosystemic shunt is more likely in young animals; however, congenital shunts can be diagnosed in animals more than 10 years old. Acquired hepatic disease is more common in adults and requires hepatic biopsy for diagnosis; however, some dogs less than 1 year old have severe, acquired hepatic disease. Hypoalbuminemia with normal liver function tests and absence of proteinuria or cutaneous lesions allows one to diagnose PLE by exclusion (see Chapter 9), even if feces are normal. If the patient has renal or hepatic disease and PLE is still a concern, then measurement of fecal alpha-1 protease inhibitor concentrations (Chapter 9) may allow diagnosis of PLE by inclusion. Intestinal biopsy may then provide a definitive diagnosis of which intestinal disease is causing PLE. Endoscopic biopsies are safer, but it is critical that excellent quality tissue samples be obtained (many endoscopically-obtained samples are nondiagnostic). Exploratory laparotomy is acceptable. If laparotomy is performed, hepatic biopsy should generally be performed along with intestinal biopsies. It is important to obtain biopsy specimens at several sites along the small intestine, even when no apparent gross lesions are found.
FIGURE 12-1.
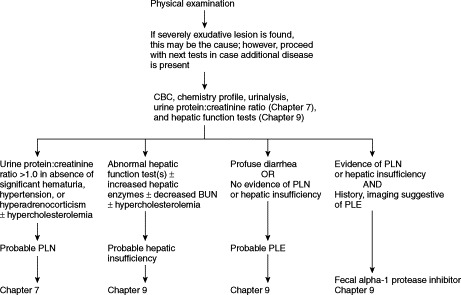
Diagnostic evaluation of hypoalbuminemia in dogs and cats when the serum albumin ≤2.0 g/dl. BUN, Blood urea nitrogen; CBC, complete blood count; HI, hepatic insufficiency; PLE, protein-losing enteropathy; PLN, protein-losing nephropathy.
Edematous SC fluid accumulations associated with hypoalbuminemia are usually transudates: PLN or PLE, chronic hepatic insufficiency, and immune-mediated or infectious vasculitis may be responsible. However, one should always sample fluid accumulations to be sure that they are in fact transudates, as opposed to unexpected modified transudates or exudates.
Causes of Altered Globulins
See following discussion of Protein Electrophoresis.
PROTEIN ELECTROPHORESIS
Occasionally Indicated
Protein electrophoresis is performed when hyperglobulinemia is not caused by hemoconcentration and either (1) the globulin concentration is high enough to make monoclonal gammopathy a reasonable possibility or (2) humoral immunodeficiencies are suspected. A pale-blue background on stained blood or bone marrow smears can represent increased plasma protein and may be an indication for protein electrophoresis.
Advantages
A useful screening test.
Disadvantages
Specific diagnosis is seldom obtained.
Although a specific diagnosis is seldom obtained from electrophoresis, electrophoretic patterns can be valuable when interpreted with clinical signs and other laboratory data. Electrophoresis is quantitative. Immunoelectrophoresis is qualitative, identifying specific proteins (e.g., immunoglobulins) but not detecting slight increases or decreases. Immunoelectrophoresis is the method of choice to detect urinary and serum Bence Jones protein, a monoclonal protein equivalent to immunoglobulin light chains which occasionally occurs in multiple myeloma and macroglobulinemia. A routine protein electrophoresis performed on a concentrated urine sample occasionally detects an isolated monoclonal peak in the urine (e.g., Bence Jones protein). Finding a urine electrophoresis pattern mimicking that of serum indicates a glomerular lesion substantial enough to allow leakage of most serum proteins, including the serum monoclonal heavy chain peak; therefore, it is not evidence of Bence Jones light chains. Canine Bence Jones proteinuria is only rarely detected by heat precipitation. Positive results for Bence Jones proteins by an acid precipitation screening test should be confirmed by concentrated urine electrophoresis or immunoelectrophoresis because of the possibility of false-positive results.
Analysis
Serum or urine may be analyzed, and it may be refrigerated or frozen.
ELECTROPHORESIS
Analysis
The cellulose acetate technique is the method of choice. Interpretation of electrophoretograms is based on densitometric measurements of intensity of staining of protein bands on cellulose acetate strips. The serum separates into four fractions: (1) albumin, (2) alpha (α) globulins, (3) beta (β) globulins, and (4) gamma (γ) globulins. Canine and feline α-, β-, and γ-globulins are usually divided into two subfractions each: α1, α2; β1, β2; and γ1, γ2 (Table 12-4 ). Normal-appearing electrophoretograms from dogs and cats are presented in FIGURE 12-2, FIGURE 12-3 .
Table 12-4.
Normal Values (Mean ± 1 SD) for Serum Protein Electrophoresis in Dogs and Cats
|
BREITSCHWERDT ET AL, 1987 |
KANEKO, 1980* |
|||
|---|---|---|---|---|
| DOGS | MEAN | LIMITS | MEAN | LIMITS |
| Total protein (g/dl) | 6.84 ± 0.66 | (6.0–7.6) | 6.10 ± 0.52 | (5.4–7.1) |
| Albumin† | 3.20 ± 0.34 | (2.72–3.67) | 2.91 ± 0.11 | (2.6–3.3) |
| α1-globulin | 0.33 ± 0.11 | (0.25–0.60) | 0.30 ± 0.03 | (0.2–0.5) |
| α2-globulin | 1.13 ± 0.25 | (0.72–1.40) | 0.62 ± 0.21 | (0.3–1.1) |
| β1-globulin | 0.74 ± 0.10 | (0.63–0.89) | 0.82 ± 0.23 | (0.7–1.3) |
| β2-globulin | 0.79 ± 0.14 | (0.59–0.96) | 0.89 ± 0.33 | (0.6–1.4) |
| γ1-globulin | 0.64 ± 0.15 | (0.49–0.83) | ||
| γ1-globulin | 0.80 ± 0.25 | (0.5–1.3) | ||
| γ2-globulin | 0.70 ± 0.14 | (0.4–0.9) | ||
| A:G ratio | 0.89 ± 0.10 | (0.79–1.02) | 0.83 ± 0.16 | (0.59–1.11) |
| CATS | TURNWALD, BARTA, 1989 | KANEKO, 1980* | ||
|---|---|---|---|---|
| Total protein (g/dl) | 7.66 ± 0.10 | (7.3–7.8) | 6.60 ± 0.70 | (5.4–7.8) |
| Albumin† | 3.41 ± 0.18 | (2.82–4.18) | 2.70 ± 0.17 | (2.1–3.9) |
| α1-globulin | 0.47 ± 0.03 | (0.30–0.64) | 0.70 ± 0.02 | (0.2–1.1) |
| α2-globulin | 0.55 ± 0.04 | (0.41–0.68) | 0.70 ± 0.02 | (0.4–0.9) |
| β1-globulin | 0.91 ± 0.06 | (0.77–1.25) | 0.70 ± 0.03 | (0.3–0.9) |
| β2-globulin | 0.40 ± 0.02 | (0.35–0.47) | 0.70 ± 0.02 | (0.6–1.0) |
| γ1-globulin | 1.92 ± 0.12 | (1.39–2.22) | ||
| γ1-globulin | 1.60 ± 0.77 | (0.30–2.50) | ||
| γ2-globulin | 1.70 ± 0.36 | (1.40–1.90) | ||
| A:G ratio | 0.80 ± 0.11 | (0.63–1.15) | 0.71 ± 0.20 | (0.45–1.19) |
Numbers do not add up to the total protein values and A:G ratios as given in the table.
Concentration of albumin is usually underestimated by electrophoresis compared with a chemical determination. Therefore the A:G ratio is usually higher by chemical determination than by electrophoretic determination.
FIGURE 12-2.
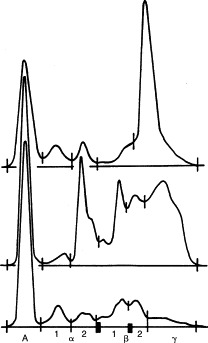
Electrophoretograms of different canine sera. Top, Monoclonal gammopathy (i.e., ehrlichiosis); middle, polyclonal gammopathy (i.e., blastomycosis, ehrlichiosis, dirofilariasis); bottom, normal.
FIGURE 12-3.
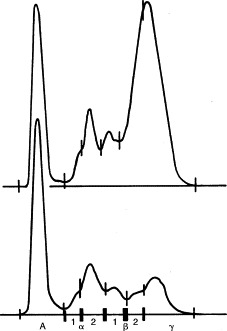
Electrophoretograms of feline sera. Top, Polyclonal gammopathy (i.e., feline infectious peritonitis [FIP]); bottom, normal.
Artifacts
Albumin concentration is usually underestimated by electrophoresis compared with a chemical determination. Therefore the albumin:globulin ratio (A:G) is usually higher by chemical determinations than by electrophoretic determination.
IMMUNOELECTROPHORESIS
Analysis
After electrophoresis in agar gel, polyclonal antiserum to specific proteins (including immunoglobulins) is added to a trough parallel with the separated serum proteins. The reagents are allowed to diffuse. To obtain quantitation of the individual immunoglobulins, radial immunodiffusion (RID), electroimmunodiffusion (i.e., rocket electrophoresis), or laser nephelometry is performed. These procedures, when available, can also be used to quantitate immunoglobulin subclasses.
Normal Values
Values vary among laboratories and the different techniques for quantitating individual immunoglobulins. Immunoglobulins migrate in the β2 and γ regions of electrophoresis. Average concentrations of immunoglobulin classes are listed in Table 12-5 .
TABLE 12-5.
Serum Immunoglobulin Concentrations in Dogs and Cats
|
MEAN CONCENTRATION (mg/dl) |
|||||
|---|---|---|---|---|---|
| PUPPY (2 WEEKS) | PUPPY (2 MONTHS) | ADULT MONGREL DOG | ADULT PUREBREED DOG | ADULT CAT | |
| IgA | Undetected | 30 | 79 | 83 | ND |
| IgG | 56 | 143 | 1445 | 925 | 2400 |
| IgM | 73 | 118 | 45 | 156 | ND |
ND, Not done.
NOTE: Normal values for puppies differ substantially from those for adults. Age-matched controls are recommended when submitting samples for immunoglobulin quantitation from young dogs because of great variation in reaching adult values among different antibody classes (Feldsburg, 1994). It is likely that breed-specific variations also occur.
Artifacts
Electrophoretic bands with high-intensity staining (e.g., albumin) are underestimated, and bands of low-intensity staining are overestimated. IgG migrates in β- and γ-globulin regions; therefore, IgG concentrations determined by RID are usually higher than the γ-globulin fraction determined by electrophoresis. This discrepancy increases when IgG hyperproduction occurs, such as in some myelomas, canine ehrlichiosis, feline infectious peritonitis [FIP], and other chronic infections.
Causes of Altered Electrophoretic Patterns
Diagnostic evaluation of patients with abnormal electrophoretograms is discussed under Causes of Hyperglobulinemia.
Causes of Hyperglobulinemia
Polyclonal hyperglobulinemias, also called gammopathies, have a broad-based peak encompassing β and γ regions and suggest persistent antigenic stimulation and inflammation (chronic bacterial, viral, fungal, protozoal, rickettsial, or parasitic disorders), neoplasia, or immune-mediated disease (see Table 12-3). The most common causes in dogs are cutaneous parasitism, pyoderma, dirofilariasis, and ehrlichiosis, depending on geographic location (see Figure 12-2 and Table 12-3). Increases are commonly in the β and γ regions. In cats the most common cause of severe polyclonal gammopathy is FIP (see Figure 12-3). Increases in feline globulins are commonly in the γ region. A concurrent mild decrease in albumin synthesis may occur in patients with hyperglobulinemia, perhaps to maintain oncotic pressure or viscosity. In hypoalbuminemic states, globulin may increase secondarily.
Monoclonal hyperglobulinemias have a narrow-based electrophoretic peak (i.e., “spike”) in the β or γ region, normally no wider than the albumin peak. Monoclonal immunoglobulin elevations are also called paraproteins or M proteins and are usually the result of lymphocyte and plasma cell neoplasias (e.g., multiple myeloma, macroglobulinemia, lymphosarcoma; see Table 12-3). Monoclonal or oligoclonal spikes are occasionally caused by infectious (e.g., ehrlichiosis; see Figure 12-2) or idiopathic disorders. Both multiple myeloma and ehrlichiosis can have monoclonal electrophoretic patterns and bone marrow plasmacytosis. In ehrlichiosis, however, the electrophoretic pattern is often a monoclonal or oligoclonal pattern superimposed on or arising within a broader-based globulin peak (see Figure 12-2, top). In such cases, examination of the stained electrophoretogram bands shows a clearly restricted monoclonal band with sharper edges within a paler, broader background band. In contrast, monoclonal spikes associated with neoplastic disorders are frequently accompanied by normal to decreased nonparaprotein globulin fractions, often reflecting impaired production of other immunoglobulins.
A suggested diagnostic approach to hyperglobulinemia is outlined in Figure 12-4 . In dogs with hyperglobulinemia and severe pruritic dermatitis, diagnostic evaluation may involve only a physical examination to identify fleas and ticks or skin scrapings to detect mites. Demodex canis mites are usually detected easily, whereas Sarcoptes scabiei are often difficult to find. For canine heartworm disease, the enzyme-linked immunosorbent assay (ELISA) antigen test is the preferred screening procedure. In areas endemic for ehrlichiosis or RMSF, titers are indicated (see Chapter 15), particularly if the patient has anemia, thrombocytopenia, leukopenia (or a combination thereof).
FIGURE 12-4.
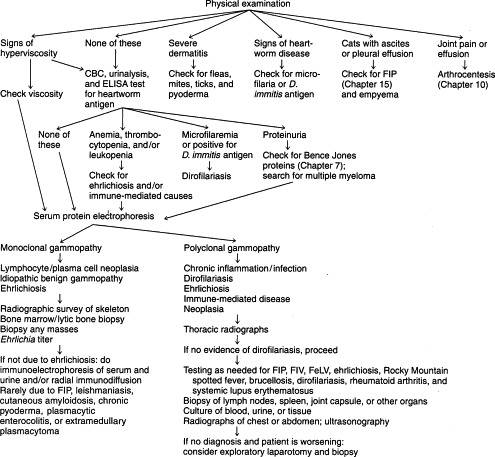
Diagnostic evaluation of moderate to severe hyperglobulinemia (globulin ≥5.0 g/dl) in dogs and cats. CBC, Complete blood count; ELISA, enzyme-linked immunosorbent assay; FIP, feline infectious peritonitis; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus.
Testing for other infectious disorders, such as canine brucellosis, blastomycosis, histoplasmosis, coccidioidomycosis, or feline cryptococcosis, is dictated by geographic location and other physical, laboratory, or radiographic abnormalities. The possibility of more than one cause of hyperglobulinemia should be considered. Gammopathies associated with immune-mediated disease and nonlymphocytic and plasmacytic neoplasia are usually mild and rarely require extensive evaluation of the gammopathy.
Feline corona virus (i.e., FIP) titers are generally not useful in cats with signs consistent with FIP. If an exudative effusion is present, fluid:serum γ-globulin ratios can be determined (see Chapter 15). Confirmatory testing for FIP can be performed on affected tissue biopsies, usually liver, using an immunohistochemical stain for FIP virus. Hyperglobulinemia can be the result of dirofilariasis in cats, but the disease is less common than in dogs. Hyperglobulinemia can also be present in chronic feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) infections.
If joint pain, stiff gait, or increased joint fluid volume accompanies hyperglobulinemia, radiographs and joint fluid analysis (see Chapter 10) are indicated. Rheumatoid factor (RF) testing, ANA testing, rickettsial titers, or a borreliosis titer (or a combination of these tests) may be helpful if the joint fluid is a nonseptic exudate.
If a diagnosis is not obtained at this stage, if a patient appears inappropriately ill, if the hyperglobulinemia seems excessive (e.g., globulin >5g/dl), or if any signs consistent with hyperviscosity are present (see Serum Viscosity), one should differentiate monoclonal from polyclonal gammopathies by serum protein electrophoresis. If monoclonal gammopathy is detected in a patient with multiple myeloma or lymphosarcoma, immunoelectrophoresis or quantitation of immunoglobulins by RID (or rocket electrophoresis) identifies the class of immunoglobulin composing the paraprotein. These tests also detect nonparaprotein immunoglobulin deficiencies, which are common in patients with lymphocytic or plasmacytic neoplasias (see Causes of Hypoglobulinemia).
Polyclonal gammopathies may be caused by infectious, immune-mediated, or neoplastic disorders. If the cause of polyclonal gammopathy is unknown, thoracic and abdominal imaging, various titers, and/or exploratory surgery plus biopsy may be indicated.
Monoclonal gammopathies are usually caused by lymphoproliferative (e.g., plasma cell) neoplasia. Evaluation of patients with monoclonal gammopathy may include skeletal radiographs, serum and urine immunoelectrophoresis, and bone marrow biopsy with cytologic and histologic evaluation. Diagnosis of multiple myeloma in dogs requires finding at least two of the following: lytic skeletal lesions, bone marrow plasmacytosis, Bence Jones proteinuria, or a monoclonal spike on serum protein electrophoresis. Skeletal lesions are uncommon in feline multiple myeloma. In areas endemic for ehrlichiosis, an Ehrlichia canis titer should be performed in dogs with a monoclonal gammopathy. Ehrlichiosis commonly causes proteinuria and bone marrow plasmacytosis, closely resembling multiple myeloma. FIP rarely causes monoclonal spikes. Serum viscosity can be measured.
Causes of Hypoglobulinemia
Newborn animals are physiologically hypogammaglobulinemic and have serum total protein concentrations 60% to 80% of adult values. Congenital combined or selective immunodeficiencies occur but are rarely diagnosed, probably because immunodeficient puppies or kittens rapidly succumb to infections. In dogs these infections are usually distemper or parvovirus, and they occur in the postnatal period after maternal immunity wanes. Immunodeficiency should be suspected when more than one pup in a properly cared for litter dies of infection in the first 2 to 6 months of life. Complete blood count (CBC), serum chemistry profile, serum protein electrophoresis, and immunoelectrophoresis are typical initial tests in such situations. Immunoglobulin quantitation by RID or rocket electrophoresis is recommended for confirmation and characterization of the type of immunoglobulin deficiency present.
Immunoglobulin quantitation techniques are more precise than qualitative methods (i.e., immunoelectrophoresis). Selective (single class or subclass) and partial immunoglobulin deficiencies may not be readily detectable via serum electrophoresis and immunoelectrophoresis; all immunoglobulin classes (and IgG subclasses when available) should be quantitated when a humoral immunodeficiency is suspected. Humoral immunodeficiency is usually detected by finding decreased IgG and IgA and normal to decreased IgM concentrations. Selective IgA deficiency and transient hypogammaglobulinemia occur in dogs; patients with selective IgA deficiency may show chronic problems involving mucosal immunity (e.g., antibiotic responsive enteropathy) or be asymptomatic. Serum electrophoresis in cases of selective or partial immunoglobulin deficiency may yield normal results or even show polyclonal elevations in globulins. Immunoelectrophoresis of normal canine serum may show a low or absent stainable IgA precipitation band because of IgA's relatively low concentration or the result of quality assurance problems involving antisera or technique. When selective IgA deficiency is suspected, the best diagnostic test is quantitative RID or rocket electrophoresis. IgA deficiency is sometimes accompanied by elevated concentrations of IgM or IgG.
The relationship between certain types of chronic inflammatory enteropathy (e.g., lymphocytic plasmacytic enteritis) and serum IgA deficiency is unclear at this time. It is likely that deficiency of local secretory IgA is not always reflected by serum IgA values. In addition, low concentrations of serum IgA have been associated with canine allergic and parasitic disorders, with return to normal after successful therapy. This finding suggests that those disease processes may lead to down regulation of IgA as an immunomodulation event, and low IgA probably has no causative role in the underlying chronic inflammatory bowel disease (Hill, Moriello, and DeBoer, 1995). Breed-specific normal ranges for immunoglobulins may provide a clearer picture of the role of selective IgA deficiency in chronic enteropathies.
The most common causes of hypoglobulinemia are external blood loss and PLE. Less common causes are PLN and hepatic insufficiency (see Chapters 7 and 9, respectively).In patients with paraproteinemias (e.g., multiple myeloma, macroglobulinemia, lymphosarcoma), remaining immunoglobulins are usually depressed. A complement measurement and evaluations of lymphocyte and phagocyte functions may also be indicated for patients with suspected congenital immunodeficiency (see Testing for Cellular Immunity).
SERUM VISCOSITY
Rarely Indicated
Monoclonal gammopathy, hyperglobulinemic patients with signs of hyperviscosity (i.e., poor tissue perfusion, dilated retinal vessels, retinal hemorrhage or retinal detachment, renal disease, central nervous system (CNS) dysfunction, bleeding problems), and monitoring of treatment of diseases causing hyperviscosity. Hyperviscosity may be suspected based on finding high serum protein (usually >10 g/dl) or on physical characteristics of serum (i.e., viscous). Polycythemia should be included as a cause of increased blood viscosity to be eliminated in patients showing clinical signs of hyperviscosity.
Advantages
Simple and diagnostically significant.
Analysis
Measured in serum with an Ostwald viscosimeter or a 0.1 ml capillary pipette. Time for a given volume of serum to flow from the pipette is compared with that for the same volume of water.
Normal Values
Relative viscosity, 1.4 to 1.8. (Relative viscosity = flow time of serum [seconds] divided by flow time of water [seconds].)
Artifacts
Volume depletion causing increased serum protein concentration may increase serum viscosity but is unlikely to be clinically significant. Markedly increased blood viscosity can interfere with tests using flow through devices (e.g., hematology autoanalyzers).
Drug Therapy That May Alter Serum Viscosity
Any drug causing volume depletion can increase serum viscosity.
Causes of Serum Hyperviscosity
A relative viscosity greater than or equal to 4 is abnormal in people and probably abnormal for dogs. Because of the relatively large size of IgM, it has the greatest potential to cause hyperviscosity. IgA (which can exist as a polymer or dimer) and very high concentrations of IgG can also cause hyperviscosity. Clinically significant serum hyperviscosity is almost invariably caused by lymphocyte and plasma cell neoplastic disorders (e.g., multiple myeloma, macroglobulinemia, lymphosarcoma; see Table 12-3). Hyperviscosity syndrome rarely occurs in monoclonal gammopathy caused by ehrlichiosis.
Diagnostic approach is described in Figure 12-4 under Monoclonal Gammopathy. Because lymphosarcoma and plasma cell myeloma are the major causes of serum hyperviscosity, aspiration of bone marrow, involved lymph nodes, masses, or other abnormal lymphoreticular organs is indicated. If results are equivocal, biopsy and histopathologic evaluation of bone marrow or involved tissues are indicated. Cytologic or histologic evaluation of spleen and liver occasionally helps diagnose lymphocytic or plasma-cytic neoplasia when samples from lymph nodes, bone marrow, or solid masses are not diagnostic.
CRYOPRECIPITATION
Cryoglobulins are usually monoclonal or complexed immunoglobulins that reversibly precipitate or gel at low temperatures but dissolve when heated. Cryoglobulins are rarely found in canine multiple myeloma and macroglobulinemia.
Rarely Indicated
Paraproteins that precipitate or gel when blood or serum is refrigerated at 4° C.
NOTE: Cryoglobulins are not cold agglutinins (i.e., antibodies binding antigen reversibly at temperatures <37° C).
Advantage
Detection of cryoglobulins is important, because failing to detect them may cause false-negative tests for hyperglobulinemia or monoclonal paraproteinemia in patients with clinical or laboratory evidence of hyperviscosity.
Analysis
The clinician should contact a reference lab about assaying for cryoglobulins.
Causes of Cryoglobulinemia
Macroglobulinemia or multiple myeloma of IgM and IgA classes may cause canine cryoglobulinemia. Finding cryoglobulinemia indicates diagnostic evaluation for lymphocyte and plasma cell neoplasia as described for monoclonal gammopathies.
ANTINUCLEAR ANTIBODY
Occasionally Indicated
Abnormalities suggestive of systemic lupus erythematosus (SLE), such as symmetric dermatitis principally distributed on the head and mucous membranes, hemolytic anemia, thrombocytopenia, nonseptic polyarthritis, myositis, proteinuria, or fever of unknown origin. Less common are neuromuscular, cardiac, or pulmonary abnormalities. The test can be used to monitor patients being treated for SLE.
Advantages
Simple and indicative of immune-mediated disease when positive with a relatively high titer in conjunction with compatible clinical abnormalities.
Disadvantages
Not a disease-specific test (i.e., many diseases besides SLE may be associated with a low titer; Table 12-6 ).
TABLE 12-6.
Causes of Increases Antinuclear Antibody Titer in Dogs and Cats
|
NOTE: ANA titer, if positive, in disorders without * or † is likely to be low.
Moderate to high titers.
Moderate titers.
Analysis
Measured in serum by indirect immunofluorescence testing (IIT). The result should be reported as the highest dilution of a patient's serum causing definite staining of nuclei. Several patterns of nuclear fluorescence are recognized: homogeneous (diffuse), rim (peripheral), speckled (fine or large speckles), and nucleolar.
Normal Values
Values vary among laboratories owing to different substrates, controls, and procedures used. Accuracy requires procedural consistency and experience. Fetal and newborn sera do not stain nuclei. Most veterinary laboratories use tissue culture monolayers of human epithelial-2 (HEp-2) cells as substrate, which allows improved discernment of fluorescent patterns of staining and more standardized procedural consistency. When this test method was used in a comparative study involving 112 canine serum samples, a significant positive titer was established at a screening dilution of 1:25 for greater than 95% specificity, whereas a minimum significant ANA titer of 1:100 was established as the corresponding significant titer using rat liver sections, which identified the identical group of ANA-positive dogs at the same specificity of greater than 95% (Hansson, Turnwald-Wigh, and Karlsson-Parra, 1996).
Drug Therapy That May Alter Antinuclear Antibody Titer
Anything decreasing antibody synthesis (e.g., cytotoxic drugs, chronic or high-dose corticosteroid therapy) can decrease the titer. Positive ANA titers have been attributed to treatment with griseofulvin, hydralazine, procainamide, sulfonamides, and tetracyclines. Positive ANA titers can occur in cats treated with propylthiouracil or methimazole (Peterson, Kintzer, and Hurvitz, 1988). Some of these cats develop drug-induced, immune-mediated hemolytic anemia (IMHA) and immune-mediated thrombocytopenia (IMT).
Artifacts
Improper reagent preparation, storage, or application; inadequate controls.
Causes of Increased Antinuclear Antibody Titer
A positive titer may occur in a number of infectious, inflammatory, and neoplastic disorders. A partial list of diseases (in addition to SLE, in which a positive ANA titer can be found) is given in Table 12-6. Normal dogs and cats can also have detectable ANA; however, these tend to be low titers. Positive titers obtained in disorders other than SLE are generally not markedly elevated; therefore, it is important to consider values established by the laboratory for low, moderate, and high titers. Equally important is consideration of other clinical and clinicopathologic changes consistent with a diagnosis of SLE.
Suggested criteria for diagnosis of canine and feline SLE are a positive ANA titer plus one or more of the following: skin or oral cavity lesions with histopathologic and immunopathologic changes consistent with SLE, polyarthritis, Coombs'-positive hemolytic anemia, idiopathic or IMT, PLN, myositis, or (particularly in cats) neurologic disturbances.
A positive ANA titer is the most important criterion for diagnosis of SLE, providing established clinical criteria are met and exclusionary diagnoses are not made (e.g., FeLV or FIP infection, cholangiohepatitis, rickettsial or systemic parasitic diseases). No well-established patterns of ANA fluorescence in cats and dogs distinguish SLE from other (i.e., non-SLE) immune-mediated diseases or other conditions associated with positive ANA titers (see Table 12-6). Although ANA titers in SLE are frequently higher than in other disorders, there can be some overlap. Magnitude of titer does not parallel severity of disease. Periodic ANA titers may be useful in monitoring a lupus patient's response to therapy.
A positive ANA titer can be an indication for performing additional tests to distinguish SLE from non-SLE disease: dermatitis favoring mucocutaneous junctions is an indication for biopsy, histopathologic examination, and immunohistochemical or direct immunofluorescent testing (DIT; see Immunostaining of Tissues); hemolytic anemia is an indication for an antiglobulin (i.e., Coombs') test (see Chapter 3) and tests for hemoparasites; swollen, painful joints are an indication for arthrocentesis, fluid analysis (see Chapter 10), and RF test (see later discussion).
LUPUS ERYTHEMATOSUS TEST
Occasionally Indicated
Suspected SLE (see Antinuclear Antibody).
Advantages
Specific; does not require species-specific reagents, therefore more widely available.
Disdvantages
Time-consuming and much less sensitive than the ANA test; requires very fresh blood sample.
Analysis
Depending on the laboratory, heparinized or clotted blood is used. Formation of LE-cells in vivo is rarely demonstrated in routinely stained bone marrow smears or in joint fluid from patients with polyarthritis (Color Plate 5A), but it is highly suggestive of SLE when present. A laboratory experienced in performing and interpreting LE cells is necessary.
Artifacts
LE cells must be differentiated from “tart” cells, which are neutrophils that have phagocytosed intact nuclei. The LE cell test is complement dependent, and low concentrations of complement, excessive heparin, or failure to use freshly drawn blood may cause false-negative results.
Drug Therapy Causing False-Negative Results
Steroid therapy alters test results. The LE cell test is more sensitive to effects of steroids than is an ANA titer.
Causes of Positive Lupus Erythematosus Cell Preparations
Ideally a minimum of three to four LE cells on a slide is necessary for a diagnosis of SLE. The test should be performed at least three times before results are considered negative. The test is specific for SLE but insensitive. Negative results are common in SLE patients that are ANA positive. Such patients may lack the particular autoantibodies to histone-DNA involved in LE cell formation but have other types of ANA detectable by immunofluorescence. Positive test results may rarely be obtained in other diseases (e.g., osteochondritis dissecans, nonimmunologic joint disease, neoplasia, disseminated intravascular coagulation). If a positive LE cell preparation is obtained, an ANA titer and tests to obtain other evidence of SLE are indicated (as described earlier under Antinuclear Antibody). An ANA titer is the preferred screening test for SLE.
ANTIGLOBULIN (COOMBS') TEST
See Chapter 3.
TESTS FOR IMMUNE-MEDIATED THROMBOCYTOPENIA (IMT)
Dogs and cats showing marked thrombocytopenia (<50,000/μl) may have IMT. This is typically a diagnosis of exclusion because no widely available test allows a definitive diagnosis of IMT. Measurement of platelet-associated antibodies and assay for antimegakaryocyte antibodies have been performed, but as of this writing, neither has been proven to have the desired sensitivity or specificity in clinical practice. A recently reported technique seems promising (Scott et al, 2002).
RHEUMATOID FACTOR TEST
Occasionally Indicated
Dogs, particularly small breeds, suspected of having rheumatoid arthritis (RA) because of lameness, heat, swelling, or pain of multiple joints, particularly peripheral joints. Crepitation and joint laxity may be detected in chronic cases. Nonspecific signs include anorexia, fever, depression, and reluctance to move and may precede clinically or radiographically detectable evidence of joint disease.
Disadvantages
Insensitive (i.e., many false-negative results) and not highly specific for RA in dogs because of relatively low titers.
Analysis
Serum is submitted refrigerated but unfrozen. The Rose-Waaler test is the recommended test for canine RF (autoantibody reacting against a patient's own IgG). Canine RFs are predominantly IgG but may also be mixed complexes of IgG, IgA, and IgM. Factors are detected by incubating rabbit IgG-sensitized sheep red blood cells (RBCs) with serial dilutions of the patient's serum (the rabbit Rose-Waaler test). If RF is present, agglutination occurs. Because canine sera often contain naturally occurring antibodies to sheep RBCs, a control must be used with unsensitized sheep RBCs. If agglutination to an equal or higher dilution appears in the control, natural antibodies must be absorbed from the sera before testing for RF. Latex agglutination tests for canine RF are not recommended because results are poorly reproducible and lack specificity. Latex agglutination titers may differ substantially from Rose-Waaler titers. Both tests are available through human laboratories; proper test controls are necessary.
Results
For the Rose-Waaler test in normal animals, the differential titer (difference between titers at which agglutination of unsensitized versus sensitized sheep RBCs occurs) should be less than eight (Barta, 1993). A titer against sensitized RBCs not corrected for the actual titer of natural antibodies to sheep RBCs can be misleading because some normal animals have higher titers of naturally occurring antibodies to sheep RBCs. Some laboratories perform the Rose-Waaler test by preabsorbing all test sera with sheep RBCs.In this case a titer of less than 16 is expected in normal dogs.
Artifacts
Serum submitted for RF testing should not be frozen, because RF activity (especially IgM) may be destroyed, causing a false-negative result. Canine RF tends to self-associate, forming multimeric complexes that significantly lower the detectable titer. Wide, sometimes negative, fluctuations in RF titer occur over time; these fluctuations do not appear to correlate with disease severity. False-positive results may occur in the Rose-Waaler test if a patient's serum has antibodies against sheep RBCs and appropriate controls or absorption of these antibodies is not performed.
Causes of an Increased Rheumatoid Factor Titer
Because RF is an antibody against Fc fragments of immunoglobulin molecules that become exposed only after antibody binds to antigen, any disease with long-standing immune complexes can eventually induce RF formation. In the Rose-Waaler test, a differential titer of greater than or equal to 1:8 is positive for RF (Barta, 1993). Between 40% and 75% of dogs with RA have a positive RF test result. Hence, a negative result does not eliminate RF. The RF test is rarely positive in normal dogs and occasionally positive in some patients with SLE, because RF may be a part of the SLE complex. Incidence of RF in other arthropathies and systemic diseases has not been adequately studied via the Rose-Waaler method, but other methods have shown RF (in titers comparable with those of patients with RA) in these arthropathies (Carter et al, 1989). Therefore a positive RF test result should never be the sole criterion for a diagnosis of canine RA.
RA is a progressive, erosive, immune-mediated polyarthritis that must be differentiated from other types of joint diseases, preferably before joint destruction occurs. Unfortunately no test for canine RF is highly reliable in making this distinction. Other routine tests indicated in making the diagnosis include joint radiographs and synovial fluid analysis. Septic polyarthritis often causes erosive lesions, especially involving larger joints, plus evidence of sepsis on routine blood and synovial fluid examinations (see Chapter 10), including culture. In contrast, RA typically causes erosive lesions of smaller peripheral joints before progressing to larger joints. Radiographic lesions may be lacking or inconclusive early in the course of RA. Furthermore, no distinguishing cytologic features can reliably differentiate among RA, SLE, and other types of immune-mediated joint disease that have similar joint fluid cytology. Histopathologic examination of synovium from affected joints is the most reliable means of diagnosing canine RA.
Histopathologic examination of synovium allows an early diagnosis and therapy in patients lacking classic radiographic changes. An ANA titer helps distinguish SLE from RA; but it is occasionally positive for RA. Finding both types of autoantibodies may represent the rare, combined occurrence of both SLE and RA (a so-called overlap syndrome) or merely the appearance of multiple autoantibodies in a patient with RA or SLE. In either case, clinical criteria are required for diagnosis.
IMMUNOSTAINING OF TISSUES
Immunofluorescent testing of tissues uses a fluorescent dye conjugated with an antibody (i.e., fluorescein isothiocyanate [FITC] reagent) that detects antigen, immunoglobulin, or complement. Several techniques are used for immunohistochemical staining involving enzyme-conjugated antibody in a chromogenic reaction system. The testing may be direct or indirect. In the direct test, tissues are assayed for deposits of antigen, immunoglobulins, or complement.
Occasionally Indicated
Suspected autoimmune skin disease or (rarely) diseases of internal organs (e.g., kidney) that may have an immunologic basis. In veterinary medicine, the test is used mainly on biopsy specimens of erosive or vesiculobullous cutaneous lesions.
Advantages
A definitive diagnosis can occasionally be made; formalin-fixed tissue can be used for some immunohistochemical procedures, allowing routine histopathology and immunostaining on sections from the same biopsy.
Disadvantages
Extreme care must be taken in tissue selection and preservation. The test result is markedly influenced by corticosteroids; some immunofluorescence procedures require a special fixative and transport medium or fresh, snap-frozen tissue.
Sample Preparation
Requirements for handling and submitting biopsied tissues vary according to the procedure and reagents used, so the laboratory should be contacted. In cutaneous diseases it is imperative that early primary lesions (i.e., vesicles, bullae, pustules) and a margin of uninvolved skin be obtained. Multiple specimens are recommended. Obtaining primary lesions may necessitate checking a patient several times daily to identify a suitable, newly erupting lesion. Ulcerated, crusted, or scarred lesions are worthless. Ideally, biopsy specimens should not be obtained from the planum nasale or foot pads if other primary lesions are available because positive staining occurs at the basement membrane of these sites in many normal dogs. Biopsy specimens for routine histopathology should be taken from the same, or at least a similar, lesion. Larger samples can be bisected to provide comparable material for both tests. The biopsy specimen should not remain attached to the wall of the fixative container because this may cause inadequate preservation and false-negative results.
Analysis
Tissue sections are prepared and tissue incubated with labeled antibodies to IgG, IgM, IgA, or C3 and examined by fluorescent microscopy for immunofluorescent assays or routine microscopy for immunohistochemical assays.
Normal Values
Healthy epidermis has no detectable deposits of immunoglobulins in the intercellular spaces or at the basement membrane. Nonspecific fluorescence of the stratum corneum should not be mistaken for deposits of immunoglobulins. Appropriate controls are necessary to detect nonspecific background staining.
Artifacts
Biopsy of inappropriate lesions produces false-negative results. Cutaneous bacterial infections may cause immunoglobulin deposits in pustules, which should not be mistaken for autoimmune disease.
Drug Therapy That May Affect Results
Corticosteroids and immunosuppressive drugs (e.g., cyclophosphamide) may cause negative results. If an animal is receiving short-acting corticosteroids, the drug should be withdrawn for at least 3 weeks before biopsy. A longer period (1 to 2 months) may be necessary if long-acting injectable corticosteroids have been used.
Causes of Positive Immunostaining
Immunostaining results must be interpreted in light of clinical and histopathologic findings. Immunoglobulin deposits can occur in other skin disorders, including mycosis fungoides, pyodermas, and acariasis. In these disorders, staining is usually patchy, focal, and present in pustules rather than in the adjacent tissues.
INDIRECT IMMUNOFLUORESCENT TESTING (IIT)
In the indirect test, serum is assayed for circulating autoantibodies to a specific tissue component, such as cutaneous basement membrane, intercellular cement substance, or renal glomerular basement membrane. The ANA test is another example of IIT discussed previously.
Rarely Indicated
As an adjunct test for suspected pemphigus, bullous pemphigoid, or immune-mediated glomerulonephritis.
Advantages
Noninvasive method to detect circulating autoantibodies to tissue, especially when biopsy of these organs is problematic or nondiagnostic.
Disadvantages
Significantly less sensitive than direct immunostaining, because a relatively large amount of antibody must be present to be detected. This test so rarely gives positive results in confirmed cases of autoimmune skin disease or immune-mediated glomerulonephritis that it is not recommended.
TESTING FOR CELLULAR IMMUNITY
Routinely available tests are used to screen suspected cases of immunodeficiency. These tests include serial CBC examinations (total lymphocyte and neutrophil numbers), serum immunoglobulins (see Causes of Hypoglobulinemia), and antibody titers to common viral vaccines such as distemper or parvovirus. Sustained lymphopenias (<1000/μl) are noted in some cases of severe combined immunodeficiency, and marked neutrophilias (>50,000/μl) are often observed in neutrophil function or chemotactic disorders (see Chapter 4). Routine histologic evaluation and immunophenotypic staining of lymph node tissue taken at biopsy are occasionally useful in pursuing a diagnosis of congenital or acquired immunodeficiency. Possible abnormal findings include depletion or altered distribution of B-lymphocyte or T-lymphocyte populations within the nodal architecture. Tests for cellular immunity include lymphocyte blastogenesis (i.e., transformation), assays for cell-mediated cytotoxicity, enumeration of T-lymphocyte and B-lymphocyte subpopulations, neutrophil function, and evaluation of leukocytic and erythrocytic histocompatibility antigens. If cellular immunity needs to be evaluated, the veterinarian should contact a college of veterinary medicine or large diagnostic laboratory to determine availability.
EVALUATION OF PHAGOCYTES
Clinically significant phagocytic function disorders tend to occur in purebreds (e.g., Irish setters, Weimaraners) or closely related crossbreds as a congenital or inherited defect. These animals show chronic or recurrent bacterial infections from a young age, along with markedly elevated neutrophil counts and general unthriftiness. Tests include phagocytic assays, bactericidal assays, chemotactic response, and testing for cell surface markers such as leukocyte adhesion molecules LFA-1 and Mo-1. The veterinarian should contact a college of veterinary medicine to determine availability.
ENUMERATION OF PROPORTIONS OF T AND B LYMPHOCYTES
Occasional Indications
Poor response to vaccinations, chronic unresponsive infections, persistently elevated or decreased lymphocyte counts, distinguishing between neoplastic and reactive lymphoproliferation by evaluating clonality.
Analysis
Studies are usually performed on peripheral blood lymphocytes but can also be performed on lymph node tissue obtained by biopsy. The clinician should consult individual laboratories for sample specification.
Causes of Altered Proportions of T and B Cells
Inherited defects of the immune system, lymphosarcoma, lymphocytic leukemia, and some viral infections (especially retroviruses such as FeLV, FIV, and other viruses infecting lymphoid cells) change the proportions of T and B cells.
EVALUATION OF LYMPHOCYTE FUNCTIONS IN VITRO
Occasional Indications
Poor vaccination response or recurrent infections. The lymphocyte transformation test is the most commonly used test for assessing functional capability of lymphocytes.
Disadvantages
The test is not readily available and is only a simulation of an in vivo situation; therefore, the results should be interpreted as an approximation only.
Analysis
Isolated lymphocytes are exposed to various substances that cause their activation and division. The proliferation response of stimulated cells is quantitated by their increased uptake of radionucleotide compared with control cells from healthy animals of the same species and similar age. These tests can detect intrinsic lymphocyte function defects and serum suppressive factors, which can be found in many acquired disease states. If evaluation of lymphocyte function testing in vitro is contemplated, the reader should contact the local or regional veterinary school immunology laboratory for additional information on availability of testing.
References
- Barta O. Rose-Waaler test. In: Barta O, editor. Veterinary clinical immunology laboratory. BARLAB; Blacksburg: 1993. [Google Scholar]
- Carter SD. Immune complexes and rheumatoid factors in canine arthritides. Ann Rheum Dis. 1989;48:185. doi: 10.1136/ard.48.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldsburg PJ. Overview of the immune system and immunodeficiency diseases. Vet Clin North Am. 1994;24:629. doi: 10.1016/s0195-5616(94)50076-7. [DOI] [PubMed] [Google Scholar]
- Hansson H, Turnwald-Wigh G, Karlsson-Parra A. Detection of antinuclear antibodies by indirect immunofluorescence in dog sera: comparison of rat liver tissue and human epithelial-2 cells as antigenic substrate. J Vet Intern Med. 1996;10:199. doi: 10.1111/j.1939-1676.1996.tb02050.x. [DOI] [PubMed] [Google Scholar]
- Heddle RJ, Rowley D. Dog immunoglobulins: immunochemical characterization of dog serum, saliva, colostrum, milk and small bowel fluid. Immunology. 1975;29:185. [PMC free article] [PubMed] [Google Scholar]
- Hill PB, Moriello KA, DeBoer DJ. Concentrations of total serum IgE, IgA, and IgG in atopic and parasitized dogs. Vet Immunol Immunopathol. 1995;44:105. doi: 10.1016/0165-2427(94)05298-7. [DOI] [PubMed] [Google Scholar]
- Kaneko JJ. Serum proteins and the dysproteinemias. In: Kaneko JJ, editor. Clinical biochemistry of domestic animals. ed 3. Academic Press; San Diego: 1980. [Google Scholar]
- Langlais-Burgess L, Lumsden JH, Mackin A. Concurrent hypoadrenocorticism and hypoalbuminemia in dogs: a retrospective study. J Am Anim Hosp Assoc. 1995;31:307. doi: 10.5326/15473317-31-4-307. [DOI] [PubMed] [Google Scholar]
- Moore GE, Mahaffey EA, Hoenig M. Hematologic and serum biochemical effects of long-term administration of anti-inflammatory doses of prednisone in dogs. Am J Vet Res. 1992;53:1033. [PubMed] [Google Scholar]
- Peterson M, Kintzer PP, Hurvitz A. Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med. 1988;2:150. doi: 10.1111/j.1939-1676.1988.tb02812.x. [DOI] [PubMed] [Google Scholar]
- Reimann KA. Immunologic profiles of cats with persistent naturally acquired feline leukemia virus infection. Am J Vet Res. 1986;47:1935. [PubMed] [Google Scholar]
- Reynolds HY, Johnson JS. Quantitation of canine immunoglobulins. J Immunol. 1970;105:698. [PubMed] [Google Scholar]
- Schultz RD, Adams LS. Immunologic methods for the detection of humoral and cellular immunity. Vet Clin North Am. 1978;8:721. doi: 10.1016/s0091-0279(78)50110-x. [DOI] [PubMed] [Google Scholar]
- Scott MA. Development of a sensitive immunoradiometric assay for detection of platelet surface- associated immunoglobulins in thrombocytopenic dogs. Am J Vet Res. 2002;63:124. doi: 10.2460/ajvr.2002.63.124. [DOI] [PubMed] [Google Scholar]
- Turnwald GH, Barta O. Immunologic and plasma protein disorders. In: Willard MD, Tvedten H, Turnwald GH, editors. Small animal clinical diagnosis by laboratory methods. WB Saunders; Philadelphia: 1989. [Google Scholar]
Ucited references
- Barta O, Pourciau SS. Electrophoresis. In: Barta O, editor. Laboratory techniques of veterinary clinical immunology. Charles C Thomas; Springfield, Ill: 1984. [Google Scholar]
- Collins L. Canine albumin results. Lab Med. 1999;30:293. [Google Scholar]
- Harrus S. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol. 1996;51:13. doi: 10.1016/0165-2427(95)05516-9. [DOI] [PubMed] [Google Scholar]
- Reynolds HY. Serum immunoglobulin levels in grey collies. Proc Soc Exp Biol Med. 1971;136:574. doi: 10.3181/00379727-136-35314. [DOI] [PubMed] [Google Scholar]
- Scott DW. Immune-mediated dermatoses in domestic animals: ten years after. II. Comp Cont Ed. 1987;9:539. [Google Scholar]
- Werner LL, Brown KA, Halliwell REW. Diagnosis of autoimmune skin disease in the dog: correlation between histopathologic, direct immunofluorescent and clinical findings. Vet Immunol Immunopathol. 1984;5:47. doi: 10.1016/0165-2427(83)90031-4. [DOI] [PubMed] [Google Scholar]


